Open Access Article
Open Access ArticleTotal synthesis of bi-magnolignan†
Si-Yuan Lu,
Hong-Mei Wang,
Na Feng* and
Ai-Jun Ma *
*
School of Biotechnology and Health Sciences, Wuyi University, Jiangmen, Guangdong 529020, People's Republic of China. E-mail: wyuchemfn@126.com; maaijun@wyu.edu.cn
First published on 16th March 2023
Abstract
Bi-magnolignan, isolated from the leaves of Magnolia officinalis, has shown excellent physiological activity against tumor cells. An efficient strategy for the first total synthesis of bi-magnolignan is reported. The bi-dibenzofuran skeleton was constructed via functional group interconversions of commercially available materials 1,2,4-trimethoxybenzene and 4-allylanisole. Then, the dibenzofuran skeleton was afforded by subsequent Suzuki coupling and intramolecular dehydration. The total synthesis of natural product was accomplished through FeCl3 catalyzed oxidative coupling.
The bark of Magnolia officinalis Rehder & E. Wilson, known as “Houpo” or “Houpu” in Chinese, is a traditional herbal medicine that has long been used in Chinese and Japanese medicine for the treatment of anxiety, asthma, depression, gastrointestinal disorders, and headache.1 To date, at least 255 different ingredients have been isolated from the cones, bark, flowers, and leaves of the genus Magnolia, such as lignans, alkaloids, coumarins, and flavonoids.2 Among these ingredients, magnolol (2) and honokiol (3) are considered as the two principal compounds and the main active constituents which have been shown to possess potent anti-oxidative,3 anti-anxiety,4 anti-inflammatory,5 and anti-cancer6 activities (Fig. 1) widespread interest in the ingredients of these bioactive materials led to the isolation of its ingredients.
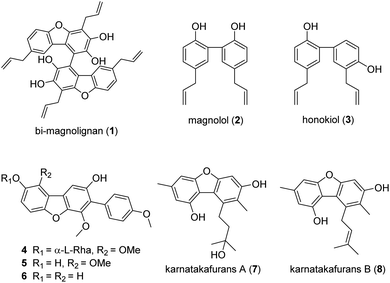 | ||
| Fig. 1 Bi-magnolignan (1) and representative members of Magnolia officinalis and dibenzofuran-containing natural products. | ||
Recently, a new lignan named bi-magnolignan (1) (Fig. 1) was isolated from the leaves of Magnolia officinalis by Ma and co-workers.7 The structure of bi-magnolignan has been characterized as a bi-dibenzofuran skeleton, formed by two identical monomers connected by a C–C bond on the benzene ring. Many dibenzofuran-containing products show a range of biological activities,8 such as kehokorins A(4)–C(6) and karnatakafurans A(7) and B(8).9 Notably, there are two hydroxyl and allyl groups on each benzene ring of bi-magnolignan, similar to honokiol and magnolol, whose potent activities are attributed to the presence of the hydroxyl and allylic groups on a biphenolic moiety.10 However, the spatial conformation of bi-magnolignan is completely different to that of honokiol and it has a larger molecular volume than honokiol. Generally, molecules with small molecular volume have more potential targets in the body, potentially causing toxic off-target side effects; however, the bi-dibenzofuran skeleton with a large molecular volume may result in fewer toxic side effects. The target of bi-magnolignan is completely different from that of magnolia officinalis phenols, and bi-magnolignan has better target specificity. The experimental data show that it has good antineoplastic effects and strong inhibitory activity against tumor cells of various tissues, with IC50 values ranging from 0.4 to 7.5 μM (after 48 h), while honokiol has values ranging from 18.8 to 56.4 μM (after 72 h).7 Research by Ma and co-workers shows that bi-magnolignan can also induce tumor cell apoptosis, while it has little toxic side effects on normal cells. Furthermore, bi-magnolignan has active hydroxyl and allyl groups, which can be further used to link other groups or react with other reagents to obtain a new structure of Magnolia officinalis derivatives.
The derivatives of bi-magnolignan have diverse structures and have high potential application value. Given the potential as an antitumor drug lead candidate, we were attracted to attempt the total synthesis of bi-magnolignan.
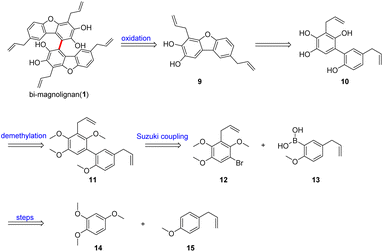
A retrosynthetic analysis of bi-magnolignan is shown in Scheme 1. Considering its symmetry, we envisioned that it should be accessible via oxidative coupling of its monomer 9, which could be generated through intramolecular dehydration of 10 to construct the dibenzofuran framework. Further disconnection led to compound 11 via a Suzuki coupling of 12 and 13 followed by demethylation. In turn, 12 and 13 are accessible from commercially available 1,2,4-trimethoxybenzene (14) and 4-allylanisole (15) in few steps.
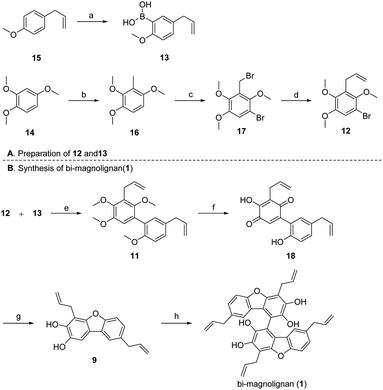
As outlined in Scheme 2, our strategy for the total synthesis began with the preparation of 12 and 13. According to Denton's protocol,11 directed ortho lithiation of 15 followed by trimethylborate and hydrolysis of the resulting arylboronate ester with aqueous hydrochloric acid afforded 13 in 63% isolated yield. The methylation of 14 with MeI afforded 16 in excellent yield (99%) on a decagram scale. Subsequently, we envisioned bromination of both the benzyl and the benzene in one pot. Fortunately, we were delighted to find that the reaction performed with NBS and AIBN afforded 17 in 91% yield after refluxing for 8 h in CCl4. Benzyl bromide 17 was then reacted with vinylmagnesium bromide in THF at −20 °C to afford 12 in good yield (83%).
With 12 and 13 in hand, we turned our attention to the construction of the desired C–C bond. Suzuki coupling between 12 and 13 using Pd(PPh3)4 and K2CO3 afforded 11 in 84% yield on a gram scale. However, subsequent demethylation with BBr3 failed to afford the desired compound 10, giving benzoquinone 18 instead with a yield of 89%. This may be due to the instability of 10, which contains many hydroxyl groups on the same benzene meaning that it is easily oxidized. Fortunately, we were delighted to find that the original conditions developed by Högberg12 and co-workers under sulfuric acid and hydroquinone provided the desired product 9 in an acceptable yield (55%), thus we successfully furnished the dibenzofuran skeleton. After screening various conditions for oxidative coupling of phenols, such as Koga's copper–amine complex CuCl(OH)·TMEDA, CuCl, DDQ, MnO2 and FeCl3, we found that the FeCl3 catalyzed oxidative coupling of aromatic nuclei, developed by Wang and co-workers,13 was suitable for the last step, however, the yield is low or no reaction under other conditions. Finally, the synthesis of the natural product was completed using FeCl3 and m-CPBA with a yield of 56%. The NMR data of the synthetic sample of bi-magnolignan were consistent with those reported in the literature.
Conclusions
In summary, we have developed the first total synthesis of bi-magnolignan in eight steps from commercially available starting materials. This provides a synthetic strategy for the oxidative coupling of natural products in dimer form, especially those lignans who share analogous structural frameworks. In addition, the salt of bi-magnolignan and its derivatives have shown considerable antitumor activity. The present work may facilitate larger-scale preparation and further biological studies of bi-magnolignan.Author contributions
A.-J. M. conceived and directed the project. S.-Y. L. performed the experiments. H.-M. W. and N. F. participated in substrate synthesis and discussions. S.-Y. L. and A.-J. M. wrote the manuscript and ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Guangdong Province Rural Revitalization Strategy Special Fund (No. Jiangke [2021] 183), the Department of Education of Guangdong Province (2020KCXTD036 and 2021KQNCX101), the Hong Kong–Macao Joint Research and Development Fund of Wuyi University (2019WGALH12 and 2021WGALH12), the Jiangmen City Science and Technology Basic Research Project (2021030102630004945) and the Innovations in Graduate Education Program (YJSSFJD-21–01). We thank Alison McGonagle, PhD, from Liwen Bianji (Edanz) (https://www.liwenbianji.cn) for editing the English text of a draft of this manuscript.Notes and references
- M. Poivre and P. Duez, Biological activity and toxicity of the Chinese herb Magnolia officinalis Rehder & E. Wilson (Houpo) and its constituents, J. Zhejiang Univ., Sci., B, 2017, 18, 194–214 CrossRef PubMed.
- W. Cui, Y. Wang, Q. Chen, W. Sun, L. Cai, Y. Tan, K. S. Kim, K. H. Kim and Y. H. Kim, Magnolia extract (BL153) ameliorates kidney damage in a high fat diet-induced obesity mouse model, Oxid. Med. Cell. Longevity, 2013, 2013, 367040 Search PubMed.
- (a) Y. M. Lee, G. Hsiao, H. R. Chen, Y. C. Chen, J. R. Sheu and M. H. Yen, Magnolol reduces myocardial ischemia/reperfusion injury via neutrophil inhibition in rats, Eur. J. Pharmacol., 2001, 422, 159–167 CrossRef CAS PubMed; (b) H. S. Cui, L. S. Huang, D. E. Sok, J. Shin, B. M. Kwon, U. J. Youn and K. Bae, Protective action of honokiol, administered orally, against oxidative stress in brain of mice challenged with NMDA, Phytomedicine, 2007, 14, 696–700 CrossRef CAS PubMed.
- (a) H. Kuribara, E. Kishi, N. Hattori, M. Okada and Y. Maruyama, The anxiolytic effect of two oriental herbal drugs in Japan attributed to honokiol from magnolia bark, J. Pharm. Pharmacol., 2000, 52, 1425–1429 CrossRef CAS PubMed; (b) Y. Maruyama, H. Kuribara, M. Morita, M. Yuzurihara and S. T. Weintrau, Identification of magnolol and honokiol as anxiolytic agents in extracts of saiboku-to, an oriental herbal medicine, J. Nat. Prod., 1998, 61, 135–138 CrossRef CAS PubMed.
- (a) J. P. Wang, T. F. Ho, L. C. Chang and C. C. Chen, Anti-inflammatory effect of magnolol, isolated from Magnolia officinalis, on A23187-induced pleurisy in mice, J. Pharm. Pharmacol., 1995, 47, 857–860 CrossRef CAS PubMed; (b) J. S. Kang, K. H. Lee, M. H. Han, H. Lee, J. M. Ahn, S. B. Han, G. Han, K. Lee, S. K. Park and H. M. Kim, Antiinflammatory activity of methanol extract isolated from stem bark of Magnolia Kobus, Phytother. Res.c, 2008, 22, 883–888 CrossRef PubMed.
- (a) J. Hu, L. J. Chen, L. Liu, X. Chen, P. L. Chen, G. Yang, W. L. Hou, M. H. Tang, F. Zhang, X. H. Wang, X. Zhao and Y. Q. Wei, Liposomal honokiol, a potent anti-angiogenesis agent, in combination with radiotherapy produces a synergistic antitumor efficacy without increasing toxicity, Exp. Mol. Med., 2008, 40, 617–628 CrossRef CAS PubMed; (b) H. B. Li, X. Yi, J. M. Gao, X. X. Ying, H. Q. Guan and J. C. Li, Magnolol-induced H460 cells death via autophagy but not apoptosis, Arch. Pharmacal Res., 2007, 30, 1566–1574 CrossRef CAS PubMed.
- W. Z. Ma, Z. Y. Yang, Z. Long, J. J. Du, K. Q. Guo, W. J. Lin, Y. F. Shen, C. M. Wei, Z. Wang, J. H. Chen, Q. Y. Lin and F. M. Zhang, Extraction of bi-magnolignan from Magnolia officinalis, CN Pat., CN115466237, 2022 Search PubMed.
- Z. Liu and R. C Larock, Synthesis of Carbazoles and Dibenzofurans via Cross-Coupling of o-Iodoanilines and o-Iodophenols with Silylaryl Triflates and Subsequent Pd-Catalyzed Cyclization, Tetrahedron, 2007, 63, 347–355 CrossRef CAS PubMed.
- (a) K. Kaniwa, T. Ohtsuki, Y. Yamamoto and M. Ishibashi, Kehokorins A–C, novel cytotoxic dibenzofurans isolated from the myxomycete Trichia favoginea var. persimilis, Tetrahedron Lett., 2006, 47, 1505–1508 CrossRef CAS; (b) S. Manniche, K. Sprogøe, P. W. Dalsgaard, C. Christophersen and T. O. Larsen, Karnatakafurans A and B: two dibenzofurans isolated from the Fungus Aspergillus karnatakaensis, J. Nat. Prod., 2004, 67, 2111 CrossRef CAS PubMed.
- (a) T. Esumi, G. Makado, H. Zhai, Y. Shimizu, Y. Mitsumoto and Y. Fukuyama, Efficient synthesis and structure-activity relationship of honokiol, a neurotrophic biphenyl-type neolignane, Bioorg. Med. Chem. Lett., 2004, 14, 2621–2625 CrossRef CAS PubMed; (b) Z. L. Kong, S. C. Tzeng and Y. C. Liu, Cytotoxic neolignans: an SAR study, Bioorg. Med. Chem. Lett., 2005, 15, 163–166 CrossRef CAS PubMed.
- R. M. Denton and J. T. Scragg, A strategy for the synthesis of the fargenone/fargenin family of natural products: synthesis of the tricyclic core, Org. Biomol. Chem., 2012, 10, 5629 RSC.
- H. E. Högberg, The formation of dibenzofurans in acid-catalysed quinone–phenol reactions and quinone oligomerisations: evidence for quinone hemiacetal intermediates, J. Chem. Soc., Perkin Trans. 1, 1979, 11, 2517–2520 RSC.
- K. Wang, M. Lü, A. Yu, X. Zhu and Q. Wang, Iron(III) chloride catalyzed oxidative coupling of aromatic nuclei, J. Org. Chem., 2009, 74, 935 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra01121f |
| This journal is © The Royal Society of Chemistry 2023 |