Open Access Article
Open Access ArticleQuadrangulosides A–F: new pregnane glycosides from Caralluma quadrangula†
Ghada A. Ismaila,
Mohamed E. Mostafa *b,
Ahmed Y. Mubarakcd,
AbelAziz M. Dawidara and
Mamdouh Abdel-Mogiba
*b,
Ahmed Y. Mubarakcd,
AbelAziz M. Dawidara and
Mamdouh Abdel-Mogiba
aChemistry Department, Faculty of Science, Mansoura University, Mansoura, 35516, Egypt
bPlant Protection Research Institute, Agriculture Research Center, 12618, Egypt. E-mail: melhosieny80@gmail.com
cChemistry Department, Faculty of Applied Sciences and Humanities, Amran University, Yemen
dFaculty of Medicine, 21 September University of Medicals and Applied Sciences, Yemen
First published on 17th April 2023
Abstract
The phytochemical investigation of Caralluma quadrangula aerial parts yielded six new pregnane glycosides, quadrangulosides A–F (1–6), in addition to nine known pregnane glycosides and three known flavone glycosides. Structures of isolated phyto-constituents were elucidated via spectroscopic 1D-, 2D-NMR and spectrometric ESI-MS spectra.
Introduction
The genus Caralluma comprises a group of tender, perennial, succulent plants belonging to the family Apocynaceae, subfamily Asclepiodoidae.1 Caralluma classified to 2500 species which are distributed throughout Africa and Asia.2 Caralluma quadrangula (Forssk.) is a wild medicinal succulent leafless perennial, edible herb that grows in hilly and stony regions.3–6 Some of the Arabian folklore uses of C. quadrangula include treatment of liver diseases, diabetes, hypertension, snake venom, scorpion bite, cancer, tuberculosis, skin disorders, fever, inflammation, rheumatic arthritis, ulcers, leprosy and as antiseptics and disinfectants,7 freckles, vitiligo, melisma, and to fulfill hunger and thirst.3,8 Several pregrane glycosides including acylated boucerosides9 and russeliosides,10 in addition to the flavonoid luteolin 4′-O-β-D-neohesperidoside10 were elucidated previously from Caralluma quadrangula.11 Pregnane glycosides, flavone glycosides, terpenoids, and sterols are the most frequently bioactive constituents characterize the genus Caralluma with significant therapeutic activities8 such as antioxidant, antidiabetic, anticancer, antimicrobial, anti-malarial, anti-inflammatory, and antieczema.4,7 We aimed in this article to attain further investigation of this important species.Results and discussion
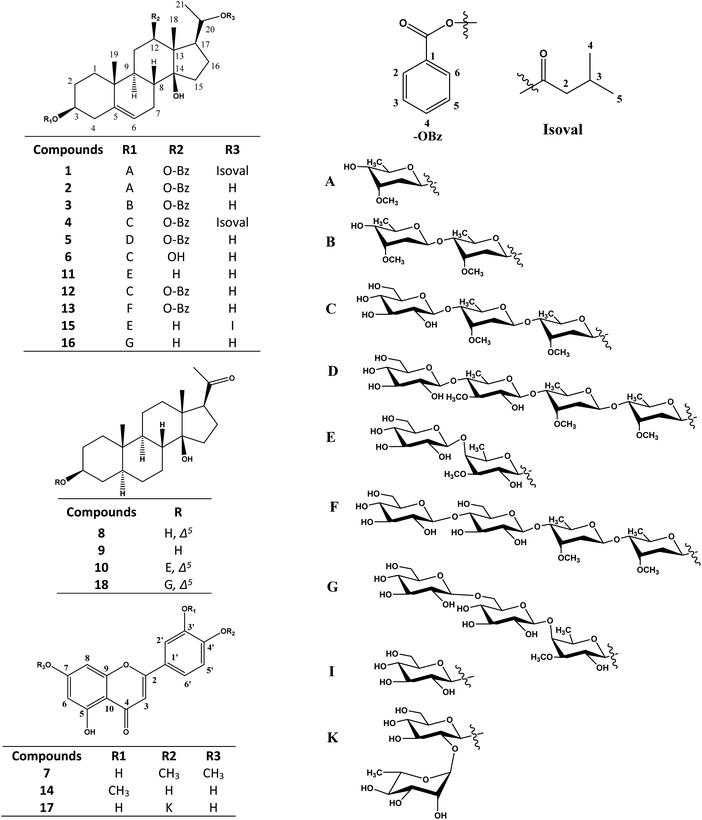
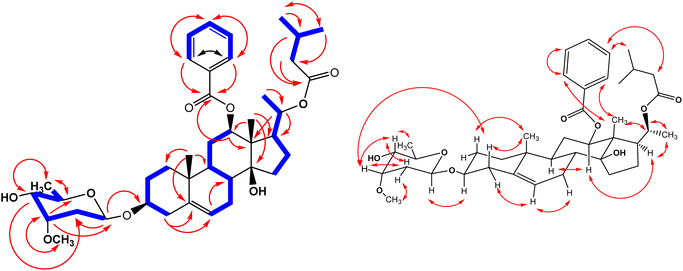
Application of various chromatographic separations for the methanolic extract of C. quadrangula aerial parts afforded fifteen pregnane glycosides and three flavone glycosides. Structure elucidation of these compounds were performed using extensive spectroscopic (1H, 13C, APT, H,H COSY, HSQC, HMBC and NOESY NMR) data and spectrometric (ESI MS) analyses. The new pregnane glycosides quadrangulosides A–F (1–6) as well as the two compounds (12, 13) are possessing the boucerin aglycon, compounds (11, 15, 16) having the calogenin aglycon, compounds (8, 10, 18) having the ketocalogenin skeleton and compound (9) having the 5α-dihydrocalogenin structure (Fig. 1).Compound (1) was isolated from the CHCl3 fraction as a white amorphous powder and exhibited molecular formula C40H58O9 based on negative mode ESIMS (m/z 699.4245 [M + H2O − H]−). l3C NMR spectrum (Tables 1 and 2) has confirmed its molecular formula by indicating the presence of 40 carbon signals that were assigned, by APT experiments, to six methyl, one methoxy, nine methylene, seventeen methine groups and seven quaternary carbon atoms. 1H and 13C-NMR data of (1) (Table 1) displayed resonances for a pregnane glycoside with two singlet signals characteristic for two angular methyl groups appeared at δH 1.17 (δC 9.74) and 0.99 (δC 19.49) assigned for CH3-18 and CH3-19, respectively, in addition to, an olefinic proton signal at δH 5.43 (brs, 1H, H-6) (δC 121.62) located at C-5/C-6 and showed long range correlation in HMBC spectrum with C-5 (δC 139.60), C-10 (δC 29.84) and C-8 (δC 37.41) characteristic for the Δ5 pregnanes type. 10 13C and APT NMR spectra also displayed three resonances at δC 77.54, 73.69 and 78.38 characteristic for oxygenated methine carbons and correlated in the HSQC spectrum with protons at δH 3.55 (m, 1H, W1/2 23.4 Hz), 4.82 (dd, 1H, 4.3, 12.0 Hz) and 4.91 (dq, 1H, 12.0, 6.0 Hz) which attributed to H-3, H-12 and H-20, respectively. In addition to another oxygenated quaternary carbon at δC 86.61 was given for C-14 identical with the C/D-cis junction of boucerin polyoxypregnane.12–15 The 1H and 13C NMR spectra of (1) displayed characteristic resonances for two carbonyl carbon esters at (δC 166.56 and 172.61) corresponding to benzoyl and isovaleroyl groups, respectively (see Table 1), which were further confirmed by comparing with the previously published spectra.10,11,13–15 The esterification of C-12-OH of boucerin with benzoic acid was approved by the long ranged HMBC correlation of H-12 proton at δH 4.82 (dd, 1H, 4.3, 12.0 Hz) with δC 166.56, while the ester linkage at C-20-OH showed a long-range correlation between carbonyl groups at δC 172.61 of isovaleroyl moiety and H-20 δH 4.91, (dq, 1H, 12.0, 6.0 Hz) (Fig. 2).12,14,16–19
| Position | 1 | 2 | 3 | 4 | 5 | 6 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1H | 13C | 1H | 13C | 1H | 13C | 1H | 13C | 1H | 13C | 1H | 13C | |
| 1 | 1.14/1.84, m, 2H | 37.35 | 1.15/1.85, m, 2H | 38.42 | 1.17/1.84, m, 2H | 38.40 | 1.14/1.83, m, 2H | 38.31 | 1.14/1.83, m, 2H | 38.38 | 1.13/1.89, m, 2H | 38.32 |
| 2 | 1.96/1.56, m, 2H | 29.54 | 1.91/1.52, m, 2H | 30.66 | 1.90/1.51, m, 2H | 30.65 | 1.90/1.53, m, 2H | 30.64 | 1.89/1.52, m, 2H | 30.64 | 1.90/1.54, m, 2H | 30.67 |
| 3 | 3.55, m, 1H, W1/2 23.4 | 77.54 | 3.52, tt, 1H, 11.2, 4.6 | 79.06 | 3.51, tt, 1H, 11.4, 4.4 | 79.06 | 3.50, tt, 1H, 11.4, 4.4 | 79.04 | 3.51, m, 1H, W1/2 22.7 | 79.02 | 3.50, m, 1H, W1/2 20.3 | 79.07 |
| 4 | 2.37/2.23, m, 2H | 38.73 | 2.18/2.37, m, 2H | 39.77 | 2.17/2.37, m, 2H | 39.76 | 2.16/2.35, m, 2H | 39.75 | 2.16/2.36, m, 2H | 39.72 | 2.17/2.34, m, 2H | 39.72 |
| 5 | — | 139.60 | — | 140.33 | — | 140.49 | — | 140.50 | — | 140.44 | — | 140.43 |
| 6 | 5.43, brs, 1H | 121.62 | 5.47, brd, 1H, 5.2 | 123.09 | 5.46, brd, 1H, 5.1 | 123.08 | 5.47, d, 1H, 5.0 | 123.00 | 5.46, brd, 1H, 4.9 | 123.10 | 5.44, brd, 1H, 5.0 | 123.17 |
| 7 | 1.88, 2.25, m, 2H | 27.15 | 1.89/2.25, m, 2H | 28.23 | 1.89/2.29, m, 2H | 28.22 | 1.87/2.26, m, 2H | 28.15 | 1.88/2.25, m, 2H | 28.20 | 1.87/2.21, m, 2H | 28.33 |
| 8 | 1.76, m, 1H | 37.41 | 1.87, m, 1H | 37.51 | 1.88, m, 1H | 37.50 | 1.91, m, 1H | 37.98 | 1.87, m, 1H | 37.45 | 1.77, m, 1H | 38.11 |
| 9 | 1.33, m, 1H | 43.29 | 1.36, m, 1H | 44.74 | 1.36, m, 1H | 44.73 | 1.35, m, 1H | 44.63 | 1.35, m, 1H | 44.67 | 1.20, m, 1H | 45.14 |
| 10 | — | 38.73 | — | 38.37 | — | 38.36 | — | 38.45 | — | 38.33 | — | 38.43 |
| 11 | 1.57/1.87, m, 2H | 26.25 | 1.64/1.83, m, 2H | 26.85 | 1.66/1.84, m, 2H | 26.85 | 1.65/1.81, m, 2H | 27.31 | 1.65/1.84, m, 2H | 26.84 | 1.45/1.63, m, 2H | 29.83 |
| 12 | 4.82, dd, 1H, 4.3, 12.0 | 78.38 | 4.81 dd, 1H, 4.3, 12.0 | 79.77 | 4.81 dd, 1H, 4.3, 11.9 | 79.76 | 4.88 dd, 1H, 4.2, 12.5 | 79.72 | 4.81, dd, 1H, 4.1, 12.0 | 79.70 | 3.27, m, 1H | 76.83 |
| 13 | — | 51.87 | — | 53.88 | — | 53.87 | — | 53.26 | — | 53.81 | — | 54.39 |
| 14 | — | 86.61 | — | 86.81 | — | 86.81 | — | 87.35 | — | 86.80 | — | 87.07 |
| 15 | 1.81/1.68, m, 2H | 32.39 | 1.84/1.68, m, 2H | 33.46 | 1.84/1.68, m, 2H | 33.45 | 1.82/1.69, m, 2H | 32.91 | 1.83/1.67, m, 2H | 33.42 | 1.62, m, 2H | 33.76 |
| 16 | 1.95/1.66, m, 2H | 25.33 | 1.98/1.67, m, 2H | 26.66 | 1.98/1.67, m, 2H | 26.66 | 1.83/1.59, m, 2H | 25.89 | 1.96/1.63, m, 2H | 26.65 | 1.87/1.61, m, 2H | 27.13 |
| 17 | 2.10, m, 1H | 49.89 | 1.92, m, 1H | 53.61 | 1.91, m, 1H | 53.61 | 2.08, m, 1H | 51.23 | 1.92, m, 1H | 53.57 | 2.09, m, 1H | 54.91 |
| 18 | 1.17, s, 3H | 9.74 | 1.38, s, 3H | 11.27 | 1.38, s, 3H | 11.26 | 1.15, s, 003H | 10.28 | 1.38, s, 3H | 11.27 | 1.08, s, 3H | 9.59 |
| 19 | 1.00, s, 3H | 19.49 | 1.05, s, 3H | 19.83 | 1.04, s, 3H | 19.83 | 1.04, s, 3H | 19.82 | 1.04, s, 3H | 19.85 | 1.03, s, 3H | 19.92 |
| 20 | 4.91, dq, 1H, 12.0, 6.0 | 73.69 | 3.81, m, 1H | 71.76 | 3.82, m, 1H | 71.75 | 4.93, m, 1H | 74.87 | 3.81, m, 1H | 71.71 | 3.76, m, 1H | 71.25 |
| 21 | 1.13, d, 3H, 6.3 | 19.37 | 1.18, d, 3H, 6.5 | 23.04 | 1.18, d, 3H, 6.6 | 23.04 | 1.09, d, 3H, 6.1 | 19.42 | 1.18, d, 3H, 6.3 | 23.05 | 1.20, d, 3H, 6.0 | 22.97 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||||
| Benzoyl – 12 | ||||||||||||
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) OO OO |
— | 166.56 | — | 168.03 | — | 168.02 | — | 167.93 | — | 167.95 | ||
| 1 | — | 130.81 | — | 132.14 | — | 131.85 | — | 132.00 | — | 131.82 | ||
| 2, 6 | 8.10, brd, 2H, 7.5 | 129.81 | 8.08, m, 2H | 130.61 | 8.08, m, 2H | 130.62 | 8.10, m, 2H | 130.66 | 8.07, brd, 2H, 7.5 | 130.63 | ||
| 3, 5 | 7.45, t, 2H, 7.5 | 128.48 | 7.49, m, 2H | 129.59 | 7.49, m, 2H | 129.60 | 7.51, m, 2H | 129.59 | 7.49, t, 2H, 7.5 | 129.61 | ||
| 4 | 7.59, t, 1H, 7.5 | 133.19 | 7.61, m, 1H | 134.25 | 7.61, m, 1H | 134.26 | 7.64, m, 1H | 134.33 | 7.61, t, 1H, 7.5 | 134.28 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||||
| Isovaleroyl – 20 | ||||||||||||
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) OO OO |
— | 172.61 | — | 174.29 | ||||||||
| 2 | 2.06, m, 2H | 44.06 | 2.02, m, 2H | 44.95 | ||||||||
| 3 | 2.02, m, 1H | 25.64 | 1.94, m, 1H | 26.67 | ||||||||
| 4 | 0.86, d, 3H, 6.1 | 22.49 | 0.83, d, 3H, 6.5 | 22.69 | ||||||||
| 5 | 0.90, d, 3H, 6.1 | 22.59 | 0.88, d, 3H, 6.5 | 22.77 | ||||||||
| Position | 1 | 2 | 3 | 4 | 5 | 6 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1H | 13C | 1H | 13C | 1H | 13C | 1H | 13C | 1H | 13C | 1H | 13C | |
| Cym | ||||||||||||
| 1′ | 4.78, brd, 1H, 9.5 | 95.68 | 4.86, dd, 1H, 9.7, 2.1 | 97.28 | 4.86, dd, 1H, 9.8, 2.1 | 97.27 | 4.86, dd, 1H, 9.9, 1.7 | 97.27 | 4.86, dd, 1H, 9.5, 2.0 | 97.23 | 4.86, d, 1H, 9.6, 1.3 | 97.22 |
| 2′ | 1.58/2.21, m, 2H | 34.19 | 1.54/2.15, m, 2H | 35.95 | 1.54/2.07, m, 2H | 36.63 | 1.62/2.14, m, 2H | 36.39 | 1.57/2.13, m, 2H | 36.33 | 1.56/2.07, m, 2H | 36.62 |
| 3′ | 3.63, m, 1H | 77.64 | 3.59, q, 1H, 3.3 | 79.20 | 3.60, q, 1H, 3.1 | 79.18 | 3.84, q, 1H, 3.3 | 78.58 | 3.84, m, 1H | 78.51 | 3.85, m, 1H | 78.56 |
| 4′ | 3.22, m, 1H | 72.64 | 3.17, dd, 1H, 9.5, 3.3 | 74.48 | 3.23, dd, 1H, 3.0, 9.6 | 83.81 | 3.34, dd, 1H, 3.3, 9.7 | 83.76 | 3.25, m, 1H | 83.81 | 3.24, m, 1H | 83.76 |
| 5′ | 3.60, m, 1H | 70.90 | 3.72, dq, 1H, 9.6, 6.3 | 71.45 | 3.80, m, 1H | 69.99 | 3.80, dq, 1H, 9.7, 6.3 | 69.98 | 3.81, m, 1H | 70.03 | 3.80, m, 1H | 69.98 |
| 6′ | 1.28, d, 3H, 6.4 | 18.42 | 1.22, d, 3H, 6.3 | 18.67 | 1.20, d, 3H, 6.2 | 18.49 | 1.19, d, 3H, 6.3 | 18.50 | 1.19, d, 3H, 6.3 | 18.52 | 1.19, d, 3H, 5.9 | 18.50 |
| 3′-OCH3 | 3.43, s, 3H | 57.38 | 3.43, s, 3H | 58.08 | 3.42, s, 3H | 58.09 | 3.43, s, 3H | 58.47 | 3.42, s, 3H | 58.41 | 3.43, s, 3H | 58.44 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||||
| Cym | ||||||||||||
| 1′′ | 4.77, dd, 1H, 9.7, 2.0 | 101.21 | 4.80, dd, 1H, 9.7, 1.6 | 101.16 | 4.79, d, 1H, 9.8, 2.1 | 101.11 | 4.80, d, 1H, 9.7, 1.2 | 101.19 | ||||
| 2′′ | 1.57/2.23, m, 2H | 35.61 | 1.56/2.06, m, 2H | 36.66 | 1.57/2.07, m, 2H | 36.55 | 1.61/2.14, m, 2H | 36.36 | ||||
| 3′′ | 3.85, q, 1H, 3.3 | 78.57 | 3.93, m, 1H, 3.1 | 78.70 | 3.85, m, 1H | 78.62 | 3.93, q 1H, 3.4 | 78.69 | ||||
| 4′′ | 3.17, dd, 1H, 3.3 9.7 | 74.46 | 3.23, dd, 1H, 3.2, 9.7 | 83.82 | 3.23, m, 1H | 84.08 | 3.35, m, 1H | 83.83 | ||||
| 5′′ | 3.72, dq, 1H, 9.7, 6.3 | 71.31 | 3.88, m, 1H | 70.12 | 3.86, m, 1H | 69.94 | 3.88, m, 1H | 70.12 | ||||
| 6′′ | 1.23, d, 3H, 6.2 | 18.73 | 1.30, d, 3H, 6.3 | 18.69 | 1.30, d, 3H, 6.1 | 18.68 | 1.30, d, 3H, 6.4 | 18.68 | ||||
| 3′′-OCH3 | 3.43, s, 3H | 58.43 | 3.45, s, 3H | 58.54 | 3.43, s, 3H | 58.56 | 3.46, s, 3H | 58.54 | ||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||||
| Thev | ||||||||||||
| 1′′′′ | 4.34, d, 1H, 7.6 | 105.98 | ||||||||||
| 2′′′′ | 3.30, m, 1H | 74.88 | ||||||||||
| 3′′′′ | 3.20, m, 1H | 85.98 | ||||||||||
| 4′′′′ | 3.37, brd, 1H, 2.7 | 82.69 | ||||||||||
| 5′′′′ | 3.48, m, 1H | 72.49 | ||||||||||
| 6′′′′ | 1.37, d, 3H, 6.1 | 18.48 | ||||||||||
| 3′′′′-OCH3 | 3.63, s, 3H | 61.23 | ||||||||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||||
| Glc | ||||||||||||
| 1′′′ | 4.34, d, 1H, 7.8 | 106.21 | 4.42, d, 1H, 7.7 | 104.28 | 4.34, d, 1H, 7.6 | 106.25 | ||||||
| 2′′′ | 3.20, dd, 1H, 7.8, 9.3 | 75.30 | 3.20, m, 1H | 75.65 | 3.21, m, 1H | 75.29 | ||||||
| 3′′′ | 3.34, m, 1H | 77.94 | 3.36, m, 1H | 77.93 | 3.34, m, 1H | 78.01 | ||||||
| 4′′′ | 3.26, m, 1H | 71.79 | 3.24 m, 1H | 71.81 | 3.25, m, 1H | 71.77 | ||||||
| 5′′′ | 3.27, m, 1H | 78.01 | 3.26, m, 1H | 78.36 | 3.26, m, 1H | 77.92 | ||||||
| 6′′′ a | 3.64, dd, 1H, 5.8, 11.8 | 63.00 | 3.65, dd, 1H, 6.3, 12 | 63.08 | 3.65, dd, 1H, 5.8, 11.8 | 62.96 | ||||||
| 6′′′ b | 3.88, dd, 1H, 2.0, 11.8 | 3.85, d, 1H, 6.3 | 3.89, dd, 1H, 2.0, 11.8 | |||||||||
The β-configuration of the benzoyl group at C-12 (δH 4.82) was confirmed by NOESY cross-correlation (Fig. 2) with H-9 (δH 1.33) also, large coupling constant of H-12 (dd, 1H, 4.3, 12.0 Hz) has proved the α (axial)-orientation of this proton.10,12,17,20 The relative configuration at C-17 was determined through the correlation between H-17 (δH 2.05) and H-12 (δH 4.82) suggesting the β-configuration of the side chain and α-orientation of H-17.4,14,21 Stereochemical assignment of C-20 was established to be S, from the NOESY cross-peaks between H-17/H-21, H-20/H-18 and H-20/H-21 as well as the absence of any correlation between H-l8 and H-21, along with comparing the spectral data with the related compounds russeleosides A–D, penicillosides A–C and russeleosides E–H.17,19,22 So, the aglycone moiety was established as 12β-O-benzoyl-20-O-isovaleroyl-3β,12β,14β,20β-tetrahydroxy-(20S)-pregn-5-ene.12,16,19,22
1H- and 13C-NMR spectra of (1) (Table 2) displayed an anomeric proton signal at 4.78 (brd 1H, 9.5 Hz) (δC 95.68) together with signals for methyl δH 1.28 (d, 3H, 6.4 Hz) (δC 95.68), one methoxy group 3.43 (s, 3H) (δC 57.38) and one methylene carbon (δC 34.19) characteristic for one 3-O-methyl-2,6-deoxyhexopyranose. Detailed examination of H–H COSY, HSQC, HMBC and NOESY spectra identified the sugar as cymarose and the anomeric proton coupling constant (brd, 1H, 9.5 Hz) suggested its β-D form. The strong 3J HMBC correlation between anomeric proton of cymaropyranose unit (δH 4.78) and the aglycone C-3 (δC 77.54) revealed the glycosylation site at C-3.20
By comparing the aforementioned data, with the previously published closely related pregnane glycosides, compound (1) was confirmed as 12β-O-benzoyl-20-O-isovaleroyl-3β,12β,14β,20β-tetrahydroxy-(20S)-pregn-5-ene 3-O-β-D-cymaropyranoside, that we named quadranguloside A.
Compound (2) was separated from the CHCl3 fraction as an amorphous solid with a molecular formula established C35H50O8 based on the sodium adduct ion at m/z 311.1833 for [M + H + Na]2+ in the positive ESI MS spectrum. The 1H and 13C-NMR data of (2) (Tables 1 and 2) were closely related to those of (1), as all the observed signals are typically matched those of quadranguloside A, except the absence of the isovaleroyl group, indicating a free hydroxyl group at C-20. The signal at δC 71.76 was attributed to the HO–C-20 with its proton appeared as multiplet at δH 3.81 ppm. The benzoyl group exist at C-12, as deduced from comparing their spectral data and also by HMBC correlations. The β-orientation of side chain at C-17 and α-configuration of H-17 was determined in similar way as in 1. Thus compound (2) was identified as 12β-O-benzoyl-3β,12β,14β,20-tetrahydroxy-pregn-5-ene 3-O-β-D-cymaropyranoside, that we named quadranguloside B.
Compound (3) was obtained from the CHCI3 fraction as a white powder. Examination of negative ionization ESI MS of (3) showed an deprotonated intense ion [M + HCOOH − H]− at m/z 787.4901 and [M + Cl]− at m/z 777.4920, corresponding to the molecular formulas C42H62O11. Careful examination of 1H and 13C NMR data of (3) displayed good similarity to those of (2), with the signals of the isovaleroyl group no longer present. In addition to the presence of two anomeric protons instead of one in (2), which appeared at δH 4.86 (dd, 1H, 2.1, 9.8 Hz) (δC 97.27) and 4.77 (dd, 1H, 2.0, 9.7 Hz), (δC 101.21). The additional sugar moiety was identified as cymarose. Both anomeric protons were appeared as β-configuration from their large coupling constants (≈10 Hz). HMBC experiment showed direct evidences for the linkage sites between sugar moieties and the aglycone which showed correlation between H-1cym-I–C-3 and H-1cym-II–C-4cym-I.
The aforementioned results revealed that (3) could be identified as 12β-O-benzoy-l3β,12β,14β,20-tetrahydroxy-pregn-5-ene 3-O-β-D-cymaropyranose(1→4)-β-D-cymaropyranoside, (named quadranguloside C).
Compound (4) was obtained from butanol fraction as a white amorphous residue, showed a negative ESI MS [M − 3H2O − H]− ion at m/z 933.5948, consistent with molecular formula C53H80O17. 1H and 13C-NMR signals of the aglycone moiety of (4) were undistinguishable from those of (1) with the same (isovaleroyl and benzoyl) acyl groups at the same positions as in (1), defining the aglycone unit as 12β-O-benzoyl-20-O-isovaleroyl-3β,12β,14β,20β-tetrahydroxy-(20S)-pregn-5-ene. 1H-NMR signals showed three anomeric protons characteristic to a trisaccharide glycoside at δH 4.86 (dd, 1H, 9.9, 1.7 Hz), 4.80 (dd, 1H, 9.7, 1.6 Hz) and 4.34 (d, 1H, 7.8 Hz), with their corresponding carbons resonances at δC 97.27, 101.16 and 106.21, respectively indicating that compound (4) having two cymarose and one glucose units, in agreement with 2D NMR (HSQC, HMBC, COSY) data. The sugar sequence of attachment was established from the 3J HMBC spectrum between the terminal D-glucose H-1′′′ (δH 4.34) and C-4′′ of the middle D-cymarose (δC 83.82) and the H-1′′ of the latter (δH 4.80) and C-4′ of inner D-cymarose (δC 83.76), furthermore long-range correlation between H-1′ (δH 4.86) and C-3 (δC 79.04) of the aglycone has confirmed that D-cymarose was the inner sugar moiety and C-3 was the only site of glycosylation. The β-configuration of sugar parts was deduced from the large JH1,H2 coupling constant (7–9 Hz) of the anomeric protons.9,12 On the basis of extensive (HSQC, HMBC and COSY) NMR data the sugar parts were identified and the linkage sites (H-1cym I–C-3, H-1cym II–C-4cym I, H-1glc–C-4cym II) were confirmed which were identical with the data published previously in literature.12,23 Therefore, the sugar chain was identified as β-D-glucopyranosyl-(1→4)-β-D-cymaropyranosyl-(1→4)-β-D-cymaropyranoside. Based on these results, (4) was established as the new quadranguloside D, 12β-O-benzoyl-20-O-isovaleroyl-3β,12β,14β,20β-tetrahydroxy-(20S)-pregn-5-ene-3-O-β-D-glucopyranosyl-(1→4)-β-D-cymaropyranosyl-(1→4)-β-D-cymaropyranoside.
Compound (5), an amorphous solid, showed a negative [M + 2HCOOH − 2H]− ion at m/z 557.1984 identical with molecular formula C55H84O20. The aglycone moiety of (5) was proved to be 12β-O-benzoyl-3β,12β,14β,20-tetrahydroxy-pregn-5-ene by comparison of its NMR data (Table 1) with those of (2). Analysis of the 1H and 13C-NMR data (Table 2) of (5) has confirmed the presence of four anomeric protons at δH 4.86 (dd, 1H, 9.5, 2.0 Hz) (δC 97.23), 4.79 (d, 1H, 9.8, 2.1 Hz) (δC 101.11), 4.34 (d, 1H, 7.6 Hz) (δC 105.98) and 4.42 (d, 1H, 7.7 Hz) (δC 104.28). Careful analysis of HMBC data concluded each monosaccharide spin system. The anomeric protons of the 3-O-methyl-2,6-dideoxyhexopyranoses appeared at δH 4.86 and 4.79 were established as two cymaroses in addition to, one 3-O-methyl-6-deoxyhexopyranose with anomeric proton at δH 4.34 was deduced as thevetose and a terminal sugar unit with anomeric proton appeared at δH 4.42 identified as glucopyranose. All the sugars moieties were assigned a β-conformations based on their coupling constants.24 The anomeric proton signal at δH 4.86 showed HMBC correlation with C-3 of the aglycone at δC 79.02 suggesting the glycosylation linkage at C-3. The second cymarose anomeric proton at δH 4.79 exhibited a clear correlation with δC 83.81 (C-4′). Further, 3J HMBC correlations between the anomeric proton of thevetose at δH 4.34 and δC 84.08 (C-4′′) and a correlation of the terminal glucopyranose anomeric proton at δH 4.42 with δC 82.69 (C-4′′′) were established. Therefore, compound (5) was identified as 12β-O-benzoyl-3β,12β,14β,20-tetrahydroxy-pregn-5-ene-3-O-β-D-glucopyranosyl-(1→4)-β-D-thevetopyranosyl-(1→4)-β-D-cymaropyranosyl-(1→4)-β-D-cymaropyranoside, that we named quadranguloside E.
Compound (6), white amorphous solid, gave in the ESI MS spectrum a deprotonated [M + HCOOH − H]− ion at m/z 845.5329 and [M + Cl]− ion at m/z 835.4434, corresponding to C41H68O15. 1H and 13C-NMR spectra (Tables 1 and 2) of (6) clearly displayed a close similarity to those of (4). Investigation of 1D and 2D NMR spectra of (6) showed the same aglycone unit without the benzoyl and isovaleroyl acyl groups, only two free hydroxyl groups at C-12 and C-20. The signals at δC 76.83 and 71.25 were attributed to hydroxylated C-12 and C-20, respectively. So, the aglycon of (6) was boucerin.24 Thus compound (6) was assigned as boucerin 3-O-β-D-glucopyranosyl-(1→4)-β-D-cymaropyranosyl-(1→4)-β-D-cymaropyranoside, that we named quadranguloside F.
Beside the new quadranguloside A–F (1–6), known previously published compounds were isolated and identified from C. quadrangula as arabincoside C (12), arabincoside D (13),23 cis-polyoxypregnanes boucerin derivatives, isolated from the ethyl acetate fraction, russelioside C (11), russelioside B (15) and russelioside D (16), calogenin derivatives, that were identified previously from C. russeliana,4 3β,14-dihydroxy-14β-pregn-5-en-20-one (8),25 arabincoside A (10) and arabincoside B (18),23 ketocalogenin derivatives, and 3β,14-dihydroxy-5α,14β-pregnan-20-one (9),26 a 5α-dihydrocalogenin derivative and 5,3′-dihydroxy-7,4′-dimethoxyflavone (7),27 chrysoeriol (14),28 luteolin 4′-O-β-D-neohesperidoside (17),29 flavone glycosides. These isolated compounds were identified by comparing their 1D and 2D NMR spectral data with those previously reported in the literature. It is worthwhile mentioned that all the above mentioned compounds except (17) are reported for the first time from C. quadrangula.
Experimental
General
Optical rotations were assessed on WXG-4. UV polarimeter, and the spectra were recorded on Ati-Unicam-UV/Visible Vision. NMR spectra were performed on a JEOL 500 MHz and Bruker 400 MHz using deuterated (MeOH-d4 or CDCl3) solvents for NMR measurements, Mansoura University, Egypt. LC-DAD/ESI-MS analysis, A XEVO-TQD#QCA423 HPLC system coupled via an ESI interface consists of Waters ACQUITY FTN and controlled using MSD Trap Control Version 1.71.2686 software, Ain Shams University. Column chromatography (CC) was processed on silica gel F254 (230–400 mesh), Sephadex LH-20 and polyamide 6. PTLC were carried out using 0.25 thickness silica gel (Kieselgel 60, GF 254). Chloroform, petroleum ether (60–80), methanol (MeOH), ethyl acetate (EtOAc) and anhydrous sodium sulphate were purchased from Adwic Company Mansoura, Egypt.Plant material
C. quadrangula (Forssk.) aerial parts were obtained in June 2021 from Amran Governorate, Yemen and were dried in shade. Botanical identification was made by Dr Hassan M. Ibrahim, head of the herbarium, Biological Science Department, Faculty of Science, Sana'a University. A herbarium specimen was deposited in the herbarium of Biological Science Department, Faculty of Science, Sana'a University in special collection of the third author.Extraction and isolation
C. quadrangula powdered aerial parts (1395 g) were extracted using MeOH (8 × 15 L), evaporated to give 750.63 g of brown residue and partitioned successively with CHCl3 (4 × 2 L), ethyl acetate (4 × 3 L) and n-butanol (5 × 3 L) to yield 31.56, 57.71 and 228.62 g, respectively.The chloroform fraction (31.56 g) was separated over silica gel CC using pet. ether![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate and CHCl3
ethyl acetate and CHCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH as eluent with gradient increasing polarities. The sub-fractions were monitored using TLC after p-anisaldehyde sulfuric acid reagent spraying. Fractions with similar pattern gathered to give three main sub-fractions. Sub-fraction I was purified on PTLC plates using CHCl3
MeOH as eluent with gradient increasing polarities. The sub-fractions were monitored using TLC after p-anisaldehyde sulfuric acid reagent spraying. Fractions with similar pattern gathered to give three main sub-fractions. Sub-fraction I was purified on PTLC plates using CHCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH (98
MeOH (98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) as eluent to give compound (1) (50 mg, Rf = 0.33). Fraction II was further purified on PTLC using CHCl3
2) as eluent to give compound (1) (50 mg, Rf = 0.33). Fraction II was further purified on PTLC using CHCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH (96
MeOH (96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) to yield, compound (2) (30 mg, Rf = 0.56) and (3) (24 mg, Rf = 0.39). Sub-fraction III was also purified on PTLC using CHCl3
4) to yield, compound (2) (30 mg, Rf = 0.56) and (3) (24 mg, Rf = 0.39). Sub-fraction III was also purified on PTLC using CHCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH (99
MeOH (99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to yield, (7) (30 mg, Rf = 0.82), (8) and (9) (24 mg, Rf = 0.39).
1) to yield, (7) (30 mg, Rf = 0.82), (8) and (9) (24 mg, Rf = 0.39).
Ethyl acetate fraction (42.71 g) was chromatographed on silica gel CC with CHCl3 and MeOH with increasing amount of MeOH (1–100%) as eluent. Similar collected fractions were gathered as indicated from their TLC profiling, to give ten sub-fractions. Sub-fraction I was further purified on PTLC plates using CHCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH
MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (85
H2O (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) as eluent to give compound (5) (40 mg, Rf = 0.40), (6) (10 mg, Rf = 0.53), (10) (40 mg, Rf = 0.26) and (12) (30 mg, Rf = 0.46). Sub-fraction (II) purified by PTLC silica gel plates (CHCl3
5) as eluent to give compound (5) (40 mg, Rf = 0.40), (6) (10 mg, Rf = 0.53), (10) (40 mg, Rf = 0.26) and (12) (30 mg, Rf = 0.46). Sub-fraction (II) purified by PTLC silica gel plates (CHCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH
MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O) (82
H2O) (82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) to give compound (11) (35 mg, Rf = 0.4) and (13) (40 mg, Rf = 0.63). Sub-fraction (III) was chromatographed on silica gel PTLC plates CHCl3
7) to give compound (11) (35 mg, Rf = 0.4) and (13) (40 mg, Rf = 0.63). Sub-fraction (III) was chromatographed on silica gel PTLC plates CHCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH
MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (92
H2O (92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) to yield compounds (4) (10 mg, Rf = 0.28) and decreasing polarity to (95
4) to yield compounds (4) (10 mg, Rf = 0.28) and decreasing polarity to (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) to give compound (14) (30 mg, Rf = 0.35).
5) to give compound (14) (30 mg, Rf = 0.35).
Butanol fraction (65.0 g) was chromatographed over polyamide S6 column using mixture of dist. H2O, (dist. H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol) and (methanol
methanol) and (methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ammonia) as eluent, with increasing polarities. The collected sub-fractions were examined by TLC and gave four main sub-fractions. Sub-reaction I was further purified by PTLC using (CHCl3
ammonia) as eluent, with increasing polarities. The collected sub-fractions were examined by TLC and gave four main sub-fractions. Sub-reaction I was further purified by PTLC using (CHCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH
MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O) (75
H2O) (75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26
26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) to yield compound (15), (16) (40 mg, Rf = 0.48) and (17) (30 mg, Rf = 0.29). Sub-fraction II purified using PTLC silica gel plates (CHCl3
5) to yield compound (15), (16) (40 mg, Rf = 0.48) and (17) (30 mg, Rf = 0.29). Sub-fraction II purified using PTLC silica gel plates (CHCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH
MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O) (80
H2O) (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) to give compound (18) (40 mg, Rf = 0.62).
3) to give compound (18) (40 mg, Rf = 0.62).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 253 (4.71), sh 282 (4.62); see Table 1 for 1H, 13C NMR (500 MHz, 125 MHz, CDCl3) of aglycone moiety; and Table 2 for 1H, 13C NMR of sugar moiety.
ε): 253 (4.71), sh 282 (4.62); see Table 1 for 1H, 13C NMR (500 MHz, 125 MHz, CDCl3) of aglycone moiety; and Table 2 for 1H, 13C NMR of sugar moiety.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 248 (4.82), sh 274 (4.71); see Table 1 for 1H, 13C NMR (500 MHz, 125 MHz, CDCl3) of aglycone moiety; and Table 2 for 1H, 13C NMR of sugar moiety.
ε): 248 (4.82), sh 274 (4.71); see Table 1 for 1H, 13C NMR (500 MHz, 125 MHz, CDCl3) of aglycone moiety; and Table 2 for 1H, 13C NMR of sugar moiety.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 253 (4.76), sh 274 (4.69); see Table 1 for 1H, 13C NMR (500 MHz, 125 MHz, CDCl3) of aglycone moiety; and Table 2 for 1H, 13C NMR of sugar moiety.
ε): 253 (4.76), sh 274 (4.69); see Table 1 for 1H, 13C NMR (500 MHz, 125 MHz, CDCl3) of aglycone moiety; and Table 2 for 1H, 13C NMR of sugar moiety.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 249 (4.87), sh 278 (4.78); see Table 1 for 1H, 13C NMR (500 MHz, 125 MHz, CD3OD) of aglycone moiety; and Table 2 for 1H, 13C NMR of sugar moieties.
ε): 249 (4.87), sh 278 (4.78); see Table 1 for 1H, 13C NMR (500 MHz, 125 MHz, CD3OD) of aglycone moiety; and Table 2 for 1H, 13C NMR of sugar moieties.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 252 (4.31), sh 287 (4.23); see Table 1 for 1H, 13C NMR (500 MHz, 125 MHz, CD3OD) of aglycone moiety; and Table 2 for 1H, 13C NMR of sugar moieties.
ε): 252 (4.31), sh 287 (4.23); see Table 1 for 1H, 13C NMR (500 MHz, 125 MHz, CD3OD) of aglycone moiety; and Table 2 for 1H, 13C NMR of sugar moieties.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 237 (4.76), sh 273 (4.50); see Table 1 for 1H, 13C NMR (500 MHz, 125 MHz, CD3OD) of aglycone moiety; and Table 2 for 1H, 13C NMR of sugar moieties.
ε): 237 (4.76), sh 273 (4.50); see Table 1 for 1H, 13C NMR (500 MHz, 125 MHz, CD3OD) of aglycone moiety; and Table 2 for 1H, 13C NMR of sugar moieties.Conclusions
A unique new series of polyhydroxylated pregnane glycosides (1–6) were identified for the first time from Caralluma quadrangula by means of extensive chromatographic and spectral analyses.Author contributions
Ghada A. Ismail performed the chemistry experiments, AbelAziz M. Dawidar, Mamdouh Abdel-Mogib and Mohamed E. Mostafa performed experimental planning and data analysis. Ahmed Y. Mubarak collected the plant materials. Conceptualization and supervision performed by AbelAziz M. Dawidar and Mamdouh Abdel-Mogib. The manuscript was written by Ghada A. Ismail, Mohamed E. Mostafa and Ahmed Y. Mubarak. All authors reviewed the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors is gratefully acknowledged Dr Hassan M. Ibrahim Faculty of Science, Sana'a University, Yemen for the plant identification.Notes and references
- H. Ch. Dutt, S. Singh, B. Avula, I. A. Khan and Y. S. Bedi, J. Med. Food, 2012, 15, 108 CrossRef CAS PubMed
.
- M. Adnan, S. Jan, S. Mussarat, A. Tariq, S. Begum, A. Afroz and Z. Kh. A. Shinwari, J. Pharm. Pharmacol., 2014, 66, 1351 CrossRef CAS PubMed
.
- J. A. N. Al-Mahweety, S. H. S. Azzam and A. Alyahawi, Univers. J. Pharm. Res., 2020, 52, 1 Search PubMed
.
- A. I. Hamed, R. Ben Said, M. A. Ben Aissa, I. B. Abdel-Farid, B. Kontek, M. Kowalczyk, W. Oleszek, A. Stochmal, I. Kowalska and B. Olas, Biomed. Pharmacother., 2022, 150, 1 CrossRef PubMed
.
- M. A. Al-Yahya, E. Adbel-sattar and E. Guittt, J. Nat. Prod., 2000, 63, 1451 CrossRef CAS PubMed
.
- R. Ben Said, M. A. Ben Aissa, S. Rahali, B. Tangour, M. Kowalczyk, W. Oleszek, A. Stochmal and A. I. Hamed, Nat. Prod. Res., 2023, 37, 136 CrossRef CAS PubMed
.
- R. A. El-Shiekh, A. Salama, A. K. Al-Mokaddem, A. Bader and E. A. Abdel-Sattar, Steroids, 2021, 165, 108759 CrossRef CAS PubMed
.
- A. Abba, D. A. Alzahrani, S. S. Yaradua and E. J. Albokhari, J. Appl. Bot. Food Qual., 2021, 94, 148 CAS
.
- H. M. Abdallah, A.-M. M. Osman, H. Almehdar and E. Abdel-Sattar, Phytochemistry, 2013, 88, 54 CrossRef CAS PubMed
.
- R. A. El-Shiekh, M. Hassan, R. A. Hashem and E. Abdel-Sattar, Antibiotics, 2021, 10, 811 CrossRef CAS PubMed
.
- R. Ben Said, M. A. Ben Aissa, S. Rahali, B. Tangour, M. Kowalczyk, W. Oleszek, A. Stochmal and A. I. Hamed, Nat. Prod. Res., 2023, 37, 136 CrossRef CAS PubMed
.
- Y. S. Tsai, C. T. Chen, H. C. Yeh, H. T. Li and C. Y. Chen, Chem. Nat. Compd., 2019, 55, 334 CrossRef CAS
.
- S. Malladi, V. N. Ratnakaram, K. Suresh Babu and T. Pullaiah, Int. J. Chem. Sci., 2017, 15, 339 Search PubMed
.
- E. A. Abdel-Sattar, O. S. S. Al-Hawshabi, A. A. Shalabi, A. M. El Halawany and M. R. Meselhy, Tetrahedron, 2022, 119, 1 CrossRef
.
- T. Tanaka, S. Tsukamoto and K. Hayashi, Phytochemistry, 1990, 29, 229 CrossRef CAS
.
- A. M. El Sayed, M. M. Basyoni, S. H. ElGayed, A. A. El-Badry and E. Abdel-Sattar, Nat. Prod. Res., 2020, 34, 311 CrossRef CAS PubMed
.
- E. Abdel-Sattar, F. M. Harraz, S. M. Al-ansari, S. El-Mekkawy, Ch. Ichino, H. Kiyohara, A. Ishiyama, K. Otoguro, S. Omura and H. Yamada, Phytochemistry, 2008, 69, 2180 CrossRef CAS PubMed
.
- E. Abdel-Sattar, A. A. Ahmed, M. A. F. Hegazy, M. A. Farag and M. A. A. Al-Yahya, Phytochemistry, 2007, 68, 1459 CrossRef CAS PubMed
.
- A. Braca, A. Bader, I. Morelli, R. Scarpato, G. Turchi, C. Pizza and N. Tommasi, Tetrahedron, 2002, 58, 5837 CrossRef CAS
.
- A. F. Halim and A. T. Khalil, Phytochemistry, 1996, 42, 1135 CrossRef CAS PubMed
.
- D. Deepak, A. Khare and M. P. Khare, Phytochemistry, 1989, 28, 3255 CrossRef CAS
.
- E. Abdel-Sattar, M. R. Meselhy and M. A.-A. Al-Yahya, Planta Med., 2002, 68, 430 CrossRef CAS PubMed
.
- K. Yoshikawa, K. Matsuchika, S. Arihara, H.-C. Chang and J.-D. Wang, Chem. Pharm. Bull., 1998, 46, 1239 CrossRef CAS PubMed
.
- J. J. Chen, Z. X. Zhang, J. Zhou and B. T. Li, J. Nat. Prod., 1999, 62, 829 CrossRef CAS PubMed
.
- P. Rasoanaivo, N. Kaneda, A. D. Kinghorn and N. R. Farnsworth, J. Nat. Prod., 1991, 54, 1672 CrossRef CAS
.
- A. Plaza, P. Sonia, A. Perrone, A. Hamed, C. Pizza and G. Bifulco, Tetrahedron, 2004, 60, 12201 CrossRef CAS
.
- E. Abdel-Sattar, M. A.-A. Al-Yahya, N. Nakamura and M. Hattori, Phytochemistry, 2001, 57, 1213 CrossRef CAS PubMed
.
- P. K. Agrawal, Phytochemistry, 1992, 31, 3307 CrossRef CAS PubMed
.
- A. K. Kipchakbaeva, B. K. Eskalieva, G. S. Burasheva and H. A. Aisa, Chem. Nat. Compd., 2019, 55, 131 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra01103h |
| This journal is © The Royal Society of Chemistry 2023 |