Open Access Article
Open Access ArticleA non-enzymatic electrochemical sensor based on nitrogen-doped mesoporous carbon@hydroxyl-functionalized ionic liquid composites modified electrode for the detection of fenitrothion†
Xinsheng Liua,
Yutong Lib,
Wenli Qiaob,
Mengjun Changb and
Yonghong Li *bc
*bc
aSchool of Basic Medical Sciences, Ningxia Medical University, Yinchuan 750004, P. R. China. Fax: +86-951-6980139; Tel: +86-951-6980139
bSchool of Public Health, Ningxia Medical University, Yinchuan 750004, P. R. China. E-mail: yonghongli2012@163.com
cKey Laboratory of Environmental Factors and Chronic Disease Control, Yinchuan 750004, P. R. China
First published on 27th April 2023
Abstract
The overuse of organophosphorus pesticides (OPs) results in severe environmental pollution and food safety issues. Fenitrothion (FNT) is a typical derivative of OPs, so rapid and sensitive detection of FNT plays an important role in environmental protection and public health. An FNT non-enzymatic electrochemical sensor based on nitrogen-doped mesoporous carbon@functionalized ionic liquid composites (N-CMK-3@IL) was constructed in this work. The surface topography and electrochemical properties of the sensor were investigated by scanning electron microscopy (SEM), cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS), respectively. Because N-CMK-3@IL composites could improve the conductivity and increase the active surface area of the modified electrode, the sensor exhibited good electrocatalytic activity to FNT. Under the optimal experimental conditions, a good linear relationship for FNT was obtained in the range of 0.5–100 ng mL−1, and the detection limit was 0.1 ng mL−1 (S/N = 3). The sensor was successfully applied for the detection of FNT in vegetable samples.
1. Introduction
Organophosphorus pesticides (OPs) are widely used in crops to improve their quality and yield. The overuse of OPs can cause various environmental and ecological problems through soil, atmosphere and water.1 OPs can cause a variety of central nervous system symptoms to humans by inhibiting acetylcholinesterase and accumulating acetylcholine. OPs may result in serious gestational hypertension, teratogenesis, pulmonary edema, and respiratory paralysis.2 Therefore, the rapid and sensitive detection of organophosphorus pesticides is of great importance for environmental protection and public health.Various techniques, such as high-performance liquid chromatography (HPLC), gas chromatography coupled to mass spectrometry (GC-MS) and electrochemical methods, have been developed to detect Ops.3–9 However, the traditional detection methods need expensive equipment and time-consuming sample pretreatment. With the advantages of simplicity, low cost, quick response, and high sensitivity, the electrochemical methods are regarded as promising techniques for the analysis of OPs.10–12 The Ops electrochemical sensors contain enzymatic and non-enzymatic sensors in this field. Because enzymes are biological substances, the enzymatic sensors are affected by pH value, temperature, humidity, and toxic chemicals, which can lead to instability, complex storage and transportation. In order to overcome these limitations, non-enzymatic sensors have attracted increasing interest due to their great sensitivity, quick response, good stability and reproducibility. Fenitrothion (FNT) is a typical derivative of OPs, it can be directly analyzed by the electrochemical methods due to the nitro group in its structure. Therefore, it is possible to develop a non-enzymatic electrochemical sensor for the accurate and quick analysis of FNT.
Ordered mesoporous materials with unique properties have been widely studied in the non-enzymatic electrochemical field. Among them, ordered mesoporous carbon owns adjustable pore structure and large specific surface area, exhibiting good electrical properties and chemical stability.13–15 In addition, ordered mesoporous carbon doped with nitrogen atoms can improve its affinity and provide more electroactive sites.16 These superior electrochemical properties make it possible to improve the performance of electrochemical sensors. Meanwhile, ionic liquids exhibit high viscosity, good conductivity, as well as wide electrochemical window.17,18 Apart from the common characteristics of ionic liquids, hydroxyl-functionalized ionic liquids possess strong hydrogen bonds and high surface tension.
In this work, a non-enzymatic FNT electrochemical sensor based on nitrogen-doped mesoporous carbon@hydroxyl-functionalized ionic liquid composites modified glassy carbon electrode was firstly prepared by drop-casting method. The surface morphologies and structural characterization of nitrogen-doped mesoporous carbon@hydroxyl-functionalized ionic liquid composites was performed using scanning electron microscopy (SEM) and X-ray photoelectron spectroscopy (XPS). The electrochemical properties of the modified electrodes were studied by cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS). The constructed sensor showed good electrocatalytic activity and ultra-sensitive response to FNT. Furthermore, the fabricated sensor was successfully applied to analyze FNT in vegetable samples. The proposed sensor offered the advantages such as simple preparation process, high sensitivity and stability for the determination of FNT.
2. Experimental
2.1. Reagents and materials
Nitrogen-doped mesoporous carbon (N-CMK-3, particle diameter: 1 μm) was acquired from Nanjing XFNANO Materials Technology Co. Ltd. Ionic liquid 1-hydroxyethyl-3-methylimidazolium tetrafluoroborate ([HOEtMlm]BF4) was purchased from Qingdao OLIKE New Material Technology Co. Ltd. Fenitrothion (FNT) was obtained from Sigma-Aldrich Co., Ltd. 0.1 M phosphate buffer (PBS) was prepared with potassium dihydrogen phosphate (KH2PO4) and disodium hydrogen phosphate (Na2HPO4). Ultrapure water used in the experiment was provided by the Heal Force EASY50 water purification system (Shanghai Canrex Analytical Instrument Co., Ltd.).2.2. Apparatus
The electrochemical measurement (containing electrochemical impedance spectroscopy) was completed on the electrochemical workstation (CHI660E, Shanghai Chenhua Instrument Co., Ltd.) and PARSTAT 4000 electrochemical workstation (Princeton, USA). The modified glassy carbon electrode as the working electrode, a platinum wire as the auxiliary electrode, and a saturated calomel electrode (3 M KCl) as the reference electrode formed a three-electrode system. The Hitachi S-3400N scanning electron microscope was used to explore the surface morphologies of the modified electrode (Tokyo, Japan). The X-ray photoelectron spectroscopy was obtained with X-ray photoelectron spectrometer (America Thermo Scientific ESCALAB Xi+).2.3. Electrode preparation
Nitrogen-doped mesoporous carbon@hydroxyl-functionalized ionic liquid composites (N-CMK-3@IL) were obtained by dispersing ultrasonically appropriate N-CMK-3 into the ionic liquid ([HOEtMlm]BF4) solution for 30 min. The bare glassy carbon electrode (GCE) was polished into mirror surface with 0.3, and 0.05 μm alumina powder, and then cleaned by ultrasonic with 95% ethanol and ultrapure water, respectively. Finally, the glassy carbon electrode was dried by nitrogen. The nitrogen-doped mesoporous carbon@hydroxyl-functionalized ionic liquid composites modified glassy carbon electrode (N-CMK-3@IL/GCE) was prepared by dropping 7.0 μL N-CMK-3@IL composites onto glassy carbon electrode and dried under an infrared lamp.For comparison, GCE and ionic liquid modified GCE (IL/GCE) were constructed in the similar procedure. The bare GCE was polished with alumina powder, and dried after ultrasonic cleaning. Then, 7.0 μL ionic liquid solution was applied to the GCE surface and dried in the infrared lamp. The obtained electrode was denoted as IL/GCE.
2.4. Analytical procedure
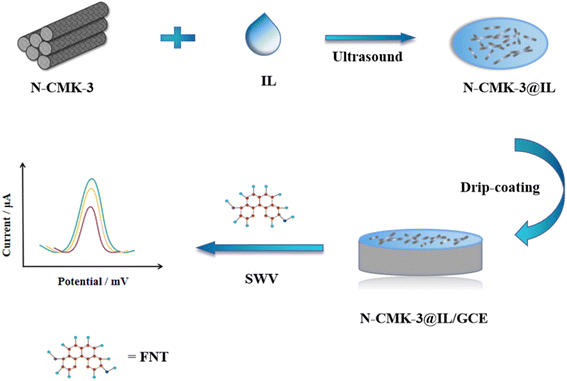
Cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) were utilized to investigate the electrochemical properties of the prepared sensors in 0.1 M KCl and 5 mM K3[Fe(CN)6]/K4[Fe(CN)6] solution. The electrochemical behavior of FNT was studied by square wave voltammetry (SWV) in the range of −1.2 to −0.3 V with the accumulation time of 60 s. Scheme 1 shows the fabrication procedure of N-CMK-3@IL/GCE and the electrochemical sensing process of FNT.3. Results and discussion
3.1. Surface morphologies and structural characterization
The surface morphologies of different modified electrodes were studied by scanning electron microscopy (SEM). As shown in Fig. 1a, bare GCE exhibited a smooth surface. On the surface of N-CMK-3/GCE (exhibited in Fig. 1b), N-CMK-3 was scattered in clusters. Fig. 1c displayed that N-CMK-3 was uniformly distributed and wrapped in ionic liquid on the surface of N-CMK-3@IL/GCE (shown in Fig. 1c) due to the high viscosity of the ionic liquid.XPS analysis plays a significant role in characterizing functional groups on the surface of the composite materials. Fig. 2 records the XPS spectra of N-CMK-3@IL composites. As shown in Fig. 2A, N-CMK-3@IL composites contained C 1s, N 1s, O 1s, F 1s and N 1s transitions. It could be acquired by Elemental Analyzer that the amount of N in the composites was 11.07%. According to the C1s spectrum (exhibited in Fig. 2B), four deconvolution peaks could be observed at about 284.5 eV (C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 285.4 eV (C–N), 286.2 eV (C–O), and 288.6 eV (O–C
C), 285.4 eV (C–N), 286.2 eV (C–O), and 288.6 eV (O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), respectively. The formation of C–N verified the N atoms were doped into the N-CMK-3. As observed in Fig. 1c, the N 1s spectrum was fitted with two peaks at about 399.5 eV (pyrrolic-N) and 401.5 eV (graphitic-N), individually. The N-CMK-3@IL composites were beneficial for facilitating the electrochemical properties.
O), respectively. The formation of C–N verified the N atoms were doped into the N-CMK-3. As observed in Fig. 1c, the N 1s spectrum was fitted with two peaks at about 399.5 eV (pyrrolic-N) and 401.5 eV (graphitic-N), individually. The N-CMK-3@IL composites were beneficial for facilitating the electrochemical properties.
 | ||
| Fig. 2 XPS spectra of N-CMK-3@IL composites. (A) General spectrum (B) C 1s spectrum (C) N 1s spectrum. | ||
3.2. CV and EIS characterization
Fig. 3 shows CVs of different electrodes in 0.1 M KCl and 5 mM [Fe(CN)6]3−/4− solution. A pair of small redox peaks with the peak potential difference (ΔEp) of 140 mV can be observed on GCE (curve a). When the ionic liquid was modified on the surface of GCE (IL/GCE, curve b), the response current of the sensor was increased and the ΔEp was reduced to 104 mV. The results exhibited that IL/GCE owned better electrochemical properties compared with GCE. The peak current on N-CMK-3@IL composites modified electrode (curve c) further increased with the ΔEp of 100 mV. There are several possible explanations for this result. On the one hand, N-CMK-3@IL composites have larger specific surface areas and can provide more electroactive sites. On the other hand, the composites can promote electron transfer rate between the analyte and the modified electrode.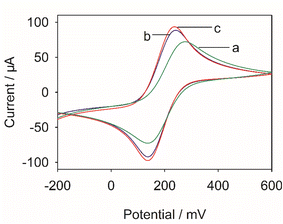 | ||
| Fig. 3 CVs of (a) GCE, (b) IL/GCE, (c) N-CMK-3@IL/GCE in 0.1 M KCl and 5 mM [Fe(CN)6]3−/4− solution. | ||
Electrochemical impedance spectroscopy (EIS) was further employed to study the interfacial charge transfer properties. In EIS, the semicircle part in the high-frequency region represents the charge transfer resistance (Rct). The larger the semicircle, the greater the Rct value. Fig. 4 exhibits the Nyquist plots of the different modified electrodes. As shown in the figure, the bare GCE displayed the highest resistance (curve a). However, after the GCE was modified with functional ionic liquid, the Rct value of the modified electrode decreased significantly due to the excellent conductivity of ionic liquid. Compared with IL/GCE, the Rct value of N-CMK-3@IL/GCE were further reduced. This could be attributed to the synergistic effect of N-CMK-3@IL composites in enhancing the conductivity of the sensor.
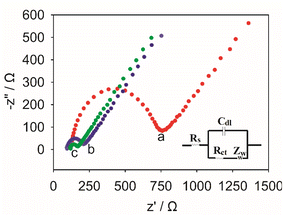 | ||
| Fig. 4 EIS of (a) GCE, (b) IL/GCE, (c) N-CMK-3@IL/GCE in 0.1 M KCl and 5 mM [Fe(CN)6]3−/4− solution. | ||
3.3. Surface area study
As shown in Fig. 5, the effective surface area of different modified electrodes was studied in K3[Fe(CN)6]/K4[Fe(CN)6] solution. According to the Randles–Sevcik equation,19 the relationship between peak current (Ipa) and scan rate (ν) is as follows:| Ipa = (2.69 × 105)n3/2AC0DR1/2ν1/2 | (1) |
 | ||
| Fig. 5 CVs of (A) GCE, (B) IL/GCE, (C) N-CMK-3@IL/GCE in 0.1 M KCl and 5 mM [Fe(CN)6]3−/4−solution with different scan rates (from inner to outer: 25, 50, 100, 150, 200, 250 mV s−1). | ||
3.4. Electrochemical behavior of FNT
Fig. 6 exhibits the SWVs of 20 ng mL−1 FNT at different modified electrodes. There was a small reduction peak of FNT on GCE (Curve a). Compared to GCE, the current on IL/GCE (Curve b) increased obviously. The phenomenon indicated that the hydroxyl-functionalized ionic liquid was successfully modified on the GCE and effectively promoted electron transfer rate. Besides, the peak current of FNT further increased on N-CMK-3@IL/GCE (curve c), indicating that N-CMK-3@IL composites could improve the conductivity of the sensor and increase the adsorption capacity for FNT.3.5. Effect of scan rate
The effect of scan rate on FNT response was investigated by CV. Fig. 7 shows the CVs of 20 ng mL−1 FNT on N-CMK-3@IL/GCE in the scan rate range of 25 to 250 mV s−1. It can be observed that a pair of REDOX peaks and a reduction peak appeared in the reaction process, which is consistent with previous reports.2 The possible reaction mechanism of FNT was exhibited in Scheme S1.† The irreversible reduction peak was attributed to reduction of the nitro group to the corresponding hydroxylamine group (reaction 1), and the reversible peaks were ascribed to the redox of nitroso-compound and hydroxylamine (reaction 2). The peak current increased with the scan rate, and their relationship conformed to the following equation: I (μA) = 0.0868 v (mV s−1) + 7.2317 (r2 = 0.9908), indicating that the reaction of fenitrothion on N-CMK-3@IL/GCE was an adsorption-controlled electrochemical process. | ||
| Fig. 7 (A) CVs of 20 ng mL−1 FNT on N-CMK-3@IL/GCE with different scan rates (from inner to outer: 25, 50, 100, 150, 200, 250 mV s−1). (B) The relationship between peak current and scan rate. | ||
3.6. Optimization of experimental factors
 | ||
| Fig. 8 (A) SWVs of 20 ng mL−1 FNT on N-CMK-3@IL/GCE in different pH solutions (from a to f: pH from 4.0 to 9.0). (B) Effect of pH value on the peak current and peak potential of FNT. | ||
According to Fig. 8B, the peak potential of FNT shifted negatively as the pH value increased, showing a good linear relationship: E(V) = −0.0373 pH − 0.4698 (r2 = 0.9949) with the slope was −37.3 mV pH−1, which was consistent with the previous report.20
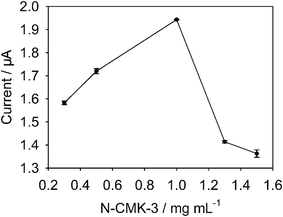
The effect of N-CMK-3 content (from 0.3 to 1.5 mg mL−1) on FNT current was represented in Fig. 10. The result suggested that the maximum peak current of FNT occurred at 1 mg mL−1 N-CMK-3. This phenomenon can be explained by the following reasons: The N-CMK-3 with pore structure could provide large specific surface areas, and the doping nitrogen atoms could offer more active centers. However, too much N-CMK-3 also lead to large background current. Therefore, 1 mg mL−1 of N-CMK-3@IL composite was utilized for the preparation of the sensor.
3.7. Determination of FNT
The SWVs of FNT with different concentrations on N-CMK-3@IL/GCE were shown in Fig. 13. The peak current of FNT varied linearly with its concentration in the range of 0.5–100 ng mL−1. The regression equation was: I (μA) = 0.0321 c (ng mL−1) + 0.7521 (r2 = 0.9947), with the detection limit of 0.1 ng mL−1 (S/N = 3). Table 1 compares the analytical parameters of different FNT sensors.21–24 The N-CMK-3@IL/GCE in this work exhibited excellent electrochemical performance with lower detection limit and wider linear range. | ||
| Fig. 13 (A) SWVs for various concentrations of FNT (from a to h: 0, 0.1, 0.5, 1, 5, 10, 50, 100 ng mL−1 FNT). (B) The calibration graph of FNT. | ||
| Modified electrode | Method | Linear range (ng mL−1) | Limit of detection (ng mL−1) | Reference |
|---|---|---|---|---|
| a CeO2: cerium(IV) oxide.b rGO: reduced graphene oxide.c GCE: glassy carbon electrode.d DPA: poly(E)-1-(4-((4-(phenylamino)phenyl)diazenyl)phenyl)ethanone.e PGE: pencil graphite electrode.f ZrO2: zirconia nanoparticles.g MoS2-Au: molybdenum sulfide-Au.h AuE: gold electrode. | ||||
| CeO2a/rGOb/GCEc | SWV | 5.54–554.4 | 0.832 | 21 |
| RGO/DPAd/PGEe | SWV | 27.72–529.51 | 0.97 | 22 |
| Nano-TiO2/Nafion/GCE | DPV | 55.45–1.109 × 103 | 24.95 | 23 |
| ZrO2f/RGO/MoS2-Aug/AuEh | SWV | 4.99–5.99 × 103 | 2.2 | 24 |
| N-CMK-3@IL/GCE | SWV | 0.5–100 | 0.1 | This work |
3.8. Reproducibility, stability, and specificity
The reproducibility of N-CMK-3@IL/GCE was discussed in 0.1 M pH 7.0 PBS containing 20 ng mL−1. The FNT response current on six electrodes prepared in the same process showed good reproducibility with a relative standard deviation (RSD) of 1.11%. After one-month storage at 4 °C, the response signal of FNT maintained 93.62% of the initial current, exhibiting excellent stability of the sensor.The anti-interference ability of N-CMK-3@IL/GCE was also explored. As shown in Table S1,† the interference experiments exhibited that 1000-fold concentration of Ca2+, Cd2+, Fe2+, K+, Zn2+, Cl−, NO3−, SO42−, 100-fold concentration of dopamine (DA), ascorbic acid (AA), uric acid (UA), glucose, 10-fold concentration of p-nitrophenol and nitrobenzene have little effects on the response signals of FNT, suggesting the good selectivity of the sensor.
3.9. Sample analysis
The feasibility of N-CMK-3@IL/GCE in practice application was investigated by the standard addition method. The lettuce, cabbage, and greens were purchased from the local market. The samples were first ground, then filtered, and the supernatant was extracted. Before measurement the obtained supernatant was diluted 50 times with 0.1 M pH 7.0 PBS. The detection results are shown in Table 2. The recoveries varied in the range of 96.9–104.9%, proving that the sensor based on N-CMK-3@IL/GCE has good application prospects.| Cabbage | Lettuce | Greens | |
|---|---|---|---|
| a Three different measurements were made for each sample. | |||
| Added/ng mL−1 | 5.00 | 5.00 | 5.00 |
| Detected/ng mL−1 | 4.95 | 5.24 | 4.84 |
| Recovery/% | 99.1 | 104.9 | 96.9 |
| RSD/% | 1.99 | 2.42 | 0.87 |
4. Conclusion
In summary, a non-enzymatic FNT sensor was successfully prepared based on nitrogen-doped mesoporous carbon@hydroxyl-functionalized ionic liquid composites. Due to the unique porous structure of nitrogen-doped mesoporous carbon and the superior electrical conductivity of the functionalized ionic liquid, the sensor showed good adsorption and catalytic performance toward FNT. The sensor exhibited low detection limit, good stability and selectivity. The proposed method could be successfully applied to detect FNT in vegetable samples.Author contributions
Xinsheng Liu: writing—original draft preparation, and visualization; Yutong Li: software, investigation and data curation; Wenli Qiao: methodology, software, validation; Mengjun Chang: software, investigation, and visualization; Yonghong Li: writing—review & editing and supervision.Conflicts of interest
The authors declare that they have no competing interests.Acknowledgements
This research was supported by the of Ningxia, China (No. 2022AAC03147).References
- R. R. Shi, W. T. Zou, Z. L. Zhao, G. Q. Wang, M. Guo, S. Y. Ai, Q. Zhou, F. C. Zhao and Z. Y. Yang, Development of a sensitive phage-mimotope and horseradish peroxidase based electrochemical immunosensor for detection of O,O-dimethyl organophosphorus pesticides, Biosens. Bioelectron., 2022, 218, 114748 CrossRef CAS PubMed.
- A. Özcan, D. Topçuoğulları and A. A. Özcan, Fenitrothion sensing with reduced graphene oxide decorated fumed silica nanocomposite modified glassy carbon electrode, Sens. Actuators, B, 2019, 284, 179–185 CrossRef.
- S. M. H. Sereshti, Three-dimensional graphene aerogel-supported iron oxide nanoparticles as an efficient adsorbent for magnetic solid phase extraction of organophosphorus pesticide residues in fruit juices followed by gas chromatographic determination, J. Chromatogr. A, 2016, 1443, 43–53 CrossRef PubMed.
- A. M. Filho, F. N. D. Santos and P. A. D. P. Pereira, Development, validation and application of a methodology based on solid-phase micro extraction followed by gas chromatography coupled to mass spectrometry (SPME/GC-MS) for the determination of pesticide residues in mangoes, Talanta, 2010, 81, 346–354 CrossRef PubMed.
- C. C. Yu, D. Y. Hao, Q. Chu, T. Wang, S. N. Liu, T. Lan, F. H. Wang and C. P. Pan, A one adsorbent QuEChERS method coupled with LC-MS/MS for simultaneous determination of 10 organophosphorus pesticide residues in tea, Food Chem., 2020, 321, 126657 CrossRef CAS PubMed.
- M. Bhattu, M. Verma and D. Kathuria, Recent advancements in the detection of organophosphate pesticides: a review, Anal. Methods, 2021, 13, 4390–4428 RSC.
- G. D. Liu and Y. H. Lin, Electrochemical sensor for organophosphate pesticides and nerve agents using zirconia nanoparticles as selective sorbents, Anal. Chem., 2005, 77, 5894–5901 CrossRef CAS PubMed.
- J. H. Wu, Q. T. Yang, Q. Li, H. Y. Li and F. Li, Two-dimensional MnO2 nanozyme-mediated homogeneous electrochemical detection of organophosphate pesticides without the interference of H2O2 and Color, Anal. Chem., 2021, 93, 4084–4091 CrossRef CAS PubMed.
- K. Jangid, R. P. Sahu, S. Sakib, I. Zhitomirsky and I. K. Puri, Surface-Modified Metal Oxides for Ultrasensitive Electrochemical Detection of Organophosphates, Heavy Metals, and Nutrients, ACS Appl. Nano Mater., 2022, 5, 17183–17193 CrossRef CAS.
- L. S. Porto, L. F. Ferreira, W. T. P. dos Santos and A. C. Pereira, Determination of organophosphorus compounds in water and food samples using a non-enzymatic electrochemical sensor based on silver nanoparticles and carbon nanotubes nanocomposite coupled with batch injection analysis, Talanta, 2022, 246, 123477 CrossRef CAS PubMed.
- M. Ganesan, R. K. Devi, A. H. Liao, K. Y. Lee, G. Gopalakrishnan and H. C. Chuang, 3D-flower-like porous neodymium molybdate nanostructure for trace level detection of organophosphorus pesticide in food samples, Food Chem., 2022, 396, 133722 CrossRef CAS PubMed.
- H. Ehzari, M. Safari, M. Samimi, M. Shamsipur and M. B. Gholivand, A highly sensitive electrochemical biosensor for chlorpyrifos pesticide detection using the adsorbent nanomatrix contain the human serum albumin and the Pd:CdTe quantum dots, Microchem. J., 2022, 179, 107424 CrossRef CAS.
- D. X. Nie, P. Li, D. Zhang, T. S. Zhou, Y. Liang and G. Y. Shi, Simultaneous determination of nitroaromatic compounds in water using capillary electrophoresis with amperometric detection on an electrode modified with a mesoporous nano-structured carbon material, Electrophoresis, 2010, 31, 2981–2988 CrossRef CAS PubMed.
- H. Dai, Y. Y. Lin, G. F. Xu, L. S. Gong, C. P. Yang, X. L. Ma and G. N. Chen, Cathodic electrochemiluminescence of luminol using polyaniline/ordered mesoporous carbon (CMK-3) hybrid modified electrode for signal amplification, Electrochim. Acta., 2012, 78, 508–514 CrossRef CAS.
- H. W. Wang, Y. Q. Liu, G. X. Hu, Y. J. Ye, L. L. Pan, P. J. Zhu and S. Yao, Ultrasensitive electrochemical sensor for determination of trace carbadox with ordered mesoporous carbon/GCE, J. Electroanal. Chem., 2020, 857, 113736 CrossRef CAS.
- S. H. Zhou, H. B. Xu, J. Y. Liu, Y. J. Wei, X. H. Ma, Z. M. Han and H. L. Chen, Simplified synthesis of N-doped carbon nanotube arrayed mesoporous carbon for electrochemical detection of amitrole, Chem. Phys., 2021, 542, 111074 CrossRef CAS.
- H. Bagheri, A. Afkhami, H. Khoshsafar, M. Rezaei, S. J. Sabounchei and M. Sarlakifar, Simultaneous electrochemical sensing of thallium, lead and mercury using a novel ionic liquid/graphene modified electrode, Anal. Chim. Acta., 2015, 870, 56–66 CrossRef CAS PubMed.
- H. J. Gao, T. T. Kan, S. Y. Zhao, Y. X. Qian, X. Y. Cheng, W. L. Wu, X. D. Wang and L. Q. Zheng, Removal of anionic azo dyes from aqueous solution by functional ionic liquid cross-linked polymer, J. Hazard. Mater., 2013, 261, 83–90 CrossRef CAS PubMed.
- Y. Li, Y. T. Li, Y. Wang, G. D. Ma, X. S. Liu, Y. H. Li and J. Soar, Application of zeolitic imidazolate frameworks (ZIF-8)/ionic liquid composites modified nano-carbon paste electrode as sensor for electroanalytical sensing of 1-hydroxypyrene, Microchem. J., 2020, 159, 105433 CrossRef CAS.
- M. Govindasamy, U. Rajaji, S. M. Chen, S. Kumaravel, T. W. Chen, F. M. A. Al-Hemaid, M. A. Ali and M. S. Elshikh, Detection of Pesticide Residues (Fenitrothion) in Fruit samples based on Niobium Carbide@Molybdenum Nanocomposite: An Electrocatalytic Approach, Anal. Chim. Acta., 2018, 1030, 52–60 CrossRef CAS PubMed.
- A. A. Ensafi, R. Noroozi, N. Zandi-Atashbar and B. Rezaei, Cerium(IV) oxide decorated on reduced graphene oxide, a selective and sensitive electrochemical sensor for fenitrothion determination, Sens. Actuators, B, 2017, 245, 980–987 CrossRef CAS.
- O. Surucu, G. Bolat and S. Abaci, Electrochemical behavior and voltammetric detection of fenitrothion based on a pencil graphite electrode modified with reduced graphene oxide (RGO)/poly(E)-1-(4-((4-(phenylamino)phenyl)diazenyl)phenyl)ethanone (DPA) composite film, Talanta, 2017, 168, 113–120 CrossRef CAS PubMed.
- A. Kumaravel and M. Chandrasekaran, A biocompatible nano TiO2/Nafion composite modified glassy carbon electrode for the detection of fenitrothion, J. Electroanal. Chem., 2011, 650, 163–170 CrossRef CAS.
- P. P. Qi, J. Wang, X. Wang, X. Y. Wang, Z. W. Wang, H. Xu, S. S. Di, Q. Wang and X. Q. Wang, Sensitive determination of fenitrothion in water samples based on an electrochemical sensor layered reduced graphene oxide, molybdenum sulfide (MoS2)-Au and zirconia films, Electrochim. Acta, 2018, 292, 667–675 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra01011b |
| This journal is © The Royal Society of Chemistry 2023 |