Open Access Article
Open Access ArticleEnrichment of the flavonoid fraction from Eucommia ulmoides leaves by a liquid antisolvent precipitation method and evaluation of antioxidant activities in vitro and in vivo
Mingfang Wu *ab,
Qianli Zhuangab,
Junkai Linab,
Yaya Penga,
Fei Luoab,
Zixuan Liuab,
Umar Farooqc and
Qian Zhangd
*ab,
Qianli Zhuangab,
Junkai Linab,
Yaya Penga,
Fei Luoab,
Zixuan Liuab,
Umar Farooqc and
Qian Zhangd
aSchool of Biological and Chemical Engineering, Zhejiang University of Science and Technology, 318 Liuhe Road, Hangzhou, 310023, Zhejiang, China. E-mail: 120068@zust.edu.cn; Tel: +86 571 8507 0340
bKey Laboratory of Agricultural Products Chemical and Biological Processing Technology of Zhejiang Province, Hangzhou, 310023, Zhejiang, China
cCarlo Genetics Inc., Manitoba, R5H 1C1, Canada
dCollege of Chemistry, Chemical Engineering and Resource Utilization, Northeast Forestry University, Harbin, 150040, Heilongjiang, China
First published on 12th June 2023
Abstract
Eucommia ulmoides leaves originate from the dry leaves of the Eucommia ulmoides plant. Flavonoids are the main functional components of Eucommia ulmoides leaves. Some flavonoids such as rutin, kaempferol and quercetin are rich in Eucommia ulmoides, and they have outstanding antioxidant efficacy. However, the poor water solubility significantly affects the bioavailability of flavonoids. In this study, we used a liquid antisolvent precipitation (LAP) method to enrich the main flavonoid fractions in Eucommia ulmoides leaves, and prepared nanoparticles by the LAP method to increase flavonoids' solubility and antioxidant properties. The technological parameters were optimized by Box–Behnken Design (BBD) software and were displayed as follows: (1) total flavonoids (TFs) concentration: 83 mg mL−1; (2) antisolvent–solvent ratio: 11; (3) deposition temperature: 27 °C. Under optimal processing conditions, the purity and recovery rate of TFs were 88.32% ± 2.54% and 88.08% ± 2.13%, respectively. In vitro experiments showed that the radical scavenging IC50 values for DPPH, ABTS, hydroxyl radicals and superoxide anions were 16.72 ± 1.07, 10.76 ± 0.13, 227.68 ± 18.23 and 335.86 ± 15.98 μg mL−1, respectively. In vivo studies showed that the obtained purified flavonoid (PF) (100, 200, 400 mg kg−1) treatment is able to improve CCl4-induced liver and kidney damage through adjusting, superoxide dismutase (SOD), catalase (CAT), glutathione (GSH), glutathione peroxidase (GSH-Px) and malondialdehyde (MDA) levels. These results demonstrated that the LAP method is capable of extracting TFs from Eucommia ulmoides leaves with high bioaccessibility.
1. Introduction
Eucommia ulmoides Oliv., a member of the Eucommia ulmoides family, is a large woody plant mainly growing in China.1 Eucommia ulmoides has multiple pharmacological effects on hypertension, diabetes, vertigo, chronic kidney diseases and other diseases.2 According to the Chinese Pharmacopoeia (National Commission of Chinese Pharmacopoeia, 2020), the medicinal parts of Eucommia ulmoides are Eucommia ulmoides's bark and leaves. It is worth noting that Eucommia ulmoides is a deciduous tree which can produce large amounts of deciduous leaves as the biological resources.3 The active ingredients in Eucommia ulmoides leaves are polyphenols, sterols, terpenes, phenylpropanoids, polysaccharides and flavonoids.4,5 Currently, multiple flavonoid components such as rutin, kaempferol and quercetin have been detected in Eucommia ulmoides leaves. Their chemical structures were shown in Fig. 1A. Flavonoids have a variety of biological functions, such as anti-cancer, anti-aging, and preventing neurodegenerative diseases.6–8 Importantly, the free radical removal ability of flavonoids make them the ideal resource for natural antioxidants. Mechanisms have been found to be responsible for antioxidant activities of rutin,9 kaempferol10 and quercetin11 by directly scavenging ROS and increasing the production of glutathione (GSH). In particularly, recent studies demonstrated that kaempferol is capable of maintaining oxidant–antioxidant status through inhibiting the MAPK/AGE-RAGE pathways,12 and upregulating the levels of NRF-2 and HO-1.10 Xu et al., reported that quercetin can alleviate oxidative stress by improving Nrf2-ARE pathway or activating PI3K/AKT pathway.11 In addition, the potentially toxicological properties including acute toxicity, carcinogenicity and mutagenicity of rutin, kaempferol and quercetin were also presented in Fig. 1B by referring to the PubChem database, International Agency for Research on Cancer (IARC) classification, and existing study (https://ncats.nih.gov/expertise/preclinical/pubchem).13At present, flavonoids extraction is widely used in food, medicine, cosmetics and other industries. However, the chemical components in Eucommia ulmoides leaves are very complex, making the purification process very difficult. The traditional method of flavonoids purification is silica gel column chromatography.14,15 This method is widely used, but with cumbersome operation, long process cycle, and residual toxic reagents.16,17 High-performance countercurrent chromatography (HPCCC) and high-speed countercurrent chromatography (HSCCC) have been reported to be used to purify flavonoids, but the amount of phytochemicals obtained is not enough to meet the rapid development of pharmaceutical and health products.18–21 Macroporous adsorption resin has good adsorption properties and analytical properties and is widely used, but its high organic residues and unsatisfactory purification effect are still need to be solved in the food and pharmaceutical industry.22–24
In order to improve the content of medicinal components in the extract, attention focused on the purification methods of Eucommia ulmoides leaves.
Recently, new isolation and purification techniques have been developed with significant advantages over conventional methods. These techniques include supercritical CO2 extraction method, membrane separation technique, metal ion complex purification, and recrystallization technique.25,26 Among them, recrystallization technique can be a purification technology with potential application value because of its simple and easy operation.
Liquid antisolvent precipitation (LAP) method is to make it saturated with a chemical in a mixed system containing solvent and antisolvent, to precipitate a substance with high solubility.27 In recent years, LAP technology has been widely used in changing the crystal form such as reducing particle size and improving water solubility. For example, Yu et al.28 produced Daidzein microparticles by LAP method. The minimum average particle size of was 181 ± 2 nm. Wu et al.29 used the LAP method to improve the water solubility of silibinin, with 180.81 ± 5.32 μg mL−1 in artificial gastric juice. This method has the advantages of simple preparation process, low cost, easy operation and high yield. However, few reports about LAP application in purification of the active ingredient of Eucommia ulmoides, but none considering purification of flavonoids in Eucommia ulmoides leaves until now.
Recently, our research team reported on the use of microwave-assisted micelle solution to extract total flavonoids in Eucommia ulmoides leaves, with an extraction rate of 1.45%.30 In this study, we continued the previous work by using the LAP method to purify crude flavonoids (CFs) from Eucommia ulmoides leaves, to simultaneously obtain purified flavonoid (PF) particles, and explore the effect of micronized flavonoids on antioxidant activity. We used methanol as the solvent and deionized water as the antisolvent. The influencing factors in the purification process are the concentration of TFs, ratio of antisolvent to solvent, deposition time and temperature. The optimal purification process was obtained by optimizing the factor conditions. In addition, the extracted micronized flavonoids components were studied for antioxidant activity in vitro and in vivo. The schematic diagram of the purification process is shown in Fig. 2.
2. Materials and methods
2.1 Plant and materials
Fresh Eucommia ulmoides leaves were purchased from Bozhou Chinese herb market (Anhui, PR China). The Eucommia ulmoides leaves were dried in a cool and ventilated place at room temperature for 20 days, and then stored in a dry place until use.2.2 Chemicals and reagents
Sodium dodecyl benzene sulfonate (SDBS, analytical purity) was acquired from Wuxi Ya Tai United Chemical Co., Ltd. (Jiangsu, PR China). 1,1-Diphenyl-2-picryl-hydrazyl (DPPH) and 2,2′-azinobis-(3-ethyl benzthiazoline-6-sulfonic acid) (ABTS) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Ascorbic acid (Vc) and butylated hydroxy toluene (BHT) were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).2.3 Extraction of TFs
TFs in Eucommia ulmoides leaves were extracted by microwave ultrasonic combined with alkaline sodium dodecyl benzene sulfonate solution.30,31 Briefly, 6 g of dried powdered Eucommia ulmoides leaves were fully mixed with 1.5% sodium dodecyl benzene sulfonate solution 300 mL in a round bottom flask and soaked for 6 h at room temperature. The mixture system was placed in the microwave ultrasonic extraction device for an extraction time of 30 min at a microwave power of 700 W. The filtrate and the filter residue were separated by centrifugation. The filtrate was then stored until the next purification step.2.4 Purification of TFs
In the above formula, y is the predicted response value, β0 is the coefficient constant, βi is the linear coefficient, βii is the quadratic equation coefficient, and βij is the interaction coefficient. Three different independent variables are defined as X1, X2 and X3.
2.5 Determination of the main flavonoids
2.6 Characterization of morphology
The morphology of the extracted and purified total flavonoids samples was examined by SEM (Quanta 200, FEI, Hillsboro, OR, USA). The dry samples were fixed on the silicon wafer and sputtered with gold to a thickness of about 150 nm.2.7 Antioxidant activity in vitro
| RSA(%) = (A0 − A1/A0) × 100% | (1) |
| ˙OH scavenging activity (%) = [1 − (A1 − A2)/A0] × 100% | (2) |
| O2˙− scavenging activity (%) = [1 − (A1 − A2)/A0] × 100% | (3) |
2.8 In vivo testing antioxidant activity of PFs
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2 mL kg−1 in olive oil, i.p.) on day 2 and day 3. Group III was administrated with vitamin E (100 mg kg−1 in 0.3% CMC-Na, p.o.) daily for 5 days and CCl4 (1 mL kg−1 in olive oil, i.p.) on day 2 and day 3. Group IV, Group V and Group VI were low dose (100 mg kg−1), middle dose (200 mg kg−1) and high dose (400 mg kg−1) of PFs, respectively.38 Group IV–VI (test groups) were treated with different concentrations of PFs in 0.3% CMC-Na by oral gavage for 5 days and CCl4–olive oil (1
1, 2 mL kg−1 in olive oil, i.p.) on day 2 and day 3. Group III was administrated with vitamin E (100 mg kg−1 in 0.3% CMC-Na, p.o.) daily for 5 days and CCl4 (1 mL kg−1 in olive oil, i.p.) on day 2 and day 3. Group IV, Group V and Group VI were low dose (100 mg kg−1), middle dose (200 mg kg−1) and high dose (400 mg kg−1) of PFs, respectively.38 Group IV–VI (test groups) were treated with different concentrations of PFs in 0.3% CMC-Na by oral gavage for 5 days and CCl4–olive oil (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2 mL kg−1) on day 2 and 3. On the 6th day, all the rats were sacrificed under anesthesia, and kidneys and livers were obtained by dissection. The liver and kidney homogenates (10.0%, w/v) were prepared with phosphate buffer solution (50 mM, pH 7.4). The supernatants were obtained for biochemical detection by centrifugation at 5000 rpm and 4 °C for 15 min.
1, 2 mL kg−1) on day 2 and 3. On the 6th day, all the rats were sacrificed under anesthesia, and kidneys and livers were obtained by dissection. The liver and kidney homogenates (10.0%, w/v) were prepared with phosphate buffer solution (50 mM, pH 7.4). The supernatants were obtained for biochemical detection by centrifugation at 5000 rpm and 4 °C for 15 min.2.9 Statistical analysis
Statistical analysis of data using GraphPad Prism 8.0 software. The data are shown as mean ± SD. Total variation of data was estimated by t-test and one-way ANOVA, p < 0.05 can be considered statistically significant (*p < 0.05, **p < 0.01, and ***p < 0.001).3. Results and discussion
3.1 Optimization of purification process for PFs
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v antisolvent to solvent ratio; 25 °C deposition temperature; 3 min deposition time) was used to purify the sample. As shown in Fig. 3A, the purity and recovery rate of TFs gradually increased (78.11 ± 2.22, 82.51 ± 1.13 and 84.13 ± 2.02%; 81.22 ± 1.73, 83.53 ± 1.47 and 85.73 ± 1.54%) as the content of TFs increased from 50.00 mg mL−1 to 70.00 mg mL−1. However, when the concentration of TFs increased to 90 mg mL−1, there was a decrease in the purity and recovery rate of TFs (85.23 ± 0.44%; 84.33 ± 1.42%). The phenomenon may be due to more TFs reached supersaturation as the increase of TFs concentration. The purity and recovery rate of TFs decreased after reaching the maximum value, which may be related to the precipitation of more impurity component content.
1, v/v antisolvent to solvent ratio; 25 °C deposition temperature; 3 min deposition time) was used to purify the sample. As shown in Fig. 3A, the purity and recovery rate of TFs gradually increased (78.11 ± 2.22, 82.51 ± 1.13 and 84.13 ± 2.02%; 81.22 ± 1.73, 83.53 ± 1.47 and 85.73 ± 1.54%) as the content of TFs increased from 50.00 mg mL−1 to 70.00 mg mL−1. However, when the concentration of TFs increased to 90 mg mL−1, there was a decrease in the purity and recovery rate of TFs (85.23 ± 0.44%; 84.33 ± 1.42%). The phenomenon may be due to more TFs reached supersaturation as the increase of TFs concentration. The purity and recovery rate of TFs decreased after reaching the maximum value, which may be related to the precipitation of more impurity component content.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 10
1, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 15
1, 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 20
1, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 25
1 and 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the sample was purified at 80.00 mg mL−1 TFs concentration, 25 °C deposition temperature and 3 min deposition time. The results (Fig. 3B) displayed that the purity and recovery rate of TFs increased with the increase of volume ratio of antisolvent to solvent from 5
1), the sample was purified at 80.00 mg mL−1 TFs concentration, 25 °C deposition temperature and 3 min deposition time. The results (Fig. 3B) displayed that the purity and recovery rate of TFs increased with the increase of volume ratio of antisolvent to solvent from 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 10
1 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. It could be attributed to supersaturation of LFs in the mixed system with the addition of antisolvent. However, the purity and recovery rate of the TFs did not change significantly when the antisolvent–solvent volume ratio increased from 10
1. It could be attributed to supersaturation of LFs in the mixed system with the addition of antisolvent. However, the purity and recovery rate of the TFs did not change significantly when the antisolvent–solvent volume ratio increased from 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 25
1 to 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. It may be due to the substantial nucleation rate of TFs and the increased other components with the increasing antisolvent–solvent volume ratio. Nevertheless, constant increase of the antisolvent–solvent volume ratio can lead to the similar nucleation rate of the TFs and the other components.
1. It may be due to the substantial nucleation rate of TFs and the increased other components with the increasing antisolvent–solvent volume ratio. Nevertheless, constant increase of the antisolvent–solvent volume ratio can lead to the similar nucleation rate of the TFs and the other components.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio of antisolvent to solvent and 3 min deposition time. The purity of TFs rose with the increase of deposition temperature, but the purity of TFs slightly decreased when the temperature exceed 25 °C (Fig. 3C). The reason is that the solubility of TFs decreases when the deposition temperature is low, making the saturated solubility of TFs in the solvent system larger and therefore easier to precipitate. Meanwhile, the precipitation of the impurity component causes a decrease in the purity of the TFs. With the gradual increase of the deposition temperature, the solubility of TFs increases, so the TFs are not easy to precipitate and thereby leading to a decreased recovery.
1 volume ratio of antisolvent to solvent and 3 min deposition time. The purity of TFs rose with the increase of deposition temperature, but the purity of TFs slightly decreased when the temperature exceed 25 °C (Fig. 3C). The reason is that the solubility of TFs decreases when the deposition temperature is low, making the saturated solubility of TFs in the solvent system larger and therefore easier to precipitate. Meanwhile, the precipitation of the impurity component causes a decrease in the purity of the TFs. With the gradual increase of the deposition temperature, the solubility of TFs increases, so the TFs are not easy to precipitate and thereby leading to a decreased recovery.Under the conditions of different deposition time (1.00, 3.00, 5.00, 7.00 and 9.00 min), the sample was purified at 80.00 mg mL−1 TFs concentration, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio of antisolvent to solvent and 25 °C deposition temperature. The purity of TFs tended to decrease as the deposition time increased from 1 min to 5 min (Fig. 3D). The reason is that at the deposition time of 1 min, the TFs are more supersaturated than other impurity components, and the nucleation rate is higher than the other components. Over time, other impurities continuously precipitate to reduce the purity of TFs. There is no significant difference in the recovery of TFs with time changes, so time has little effect on the recovery of TFs.
1 volume ratio of antisolvent to solvent and 25 °C deposition temperature. The purity of TFs tended to decrease as the deposition time increased from 1 min to 5 min (Fig. 3D). The reason is that at the deposition time of 1 min, the TFs are more supersaturated than other impurity components, and the nucleation rate is higher than the other components. Over time, other impurities continuously precipitate to reduce the purity of TFs. There is no significant difference in the recovery of TFs with time changes, so time has little effect on the recovery of TFs.
3.2 Optimization of purification technology of TFs by BBD software
According to the univariate results, the deposition time has little effect on the purity and recovery rate of TFs. Therefore, TFs concentration, antisolvent to solvent volume ratio and deposition temperature were selected as independent variables for the BBD design. The interactions of various factors (the concentration of TFs: X1, antisolvent–solvent ratio: X2 and deposition temperature: X3) in the quadratic polynomial model for the purification of TFs was further explored. The experimental design and experimental results are shown in Table 1. Experimental data were fitted to secondary multiple regression by Design-Expert 8.0.6 software. The results of analysis of variance for regression equation purity and recovery rate are given in Table 2 and Table 3. The “Lack of fit P value” for purity and recovery was 0.70 and 0.65 were both >0.05, respectively, revealed that the quadratic regression model fitted with the actual situation. The R2 values for the TFs purity and recovery equations were 0.95 and 0.98, respectively, with the correlation coefficients both close to 1. The correction determination coefficient Radj2 was 0.90 and 0.96, respectively, suggesting that a good linear correlation between the independent variables. The quadratic polynomial equation of the correlation between the response variables and the test variables is as follows:| Y(purity) = 88.44 + 1.18X1 + 1.21X2 + 0.82X3 + 0.33X1X2 + 0.012X1X3 − 0.16X2X3 – 2.28X12 − 3.24X22 − 1.91X32 |
| Y(recovery rate) = 87.69 + 1.09X1 + 1.82X2 + 1.51X3 + 0.017X1X2 + 0.2X1X3 + 0.38X2X3 − 1.35X12 − 2.03X22 − 1.06X32 |
| No. | Box–Behnken design | ||||
|---|---|---|---|---|---|
| X1 | X2 | X3 | Y1 | Y2 | |
| a Abbreviations: TFs, total flavonoids; LAP, liquid antisolvent precipitation. X1 is TFs concentration (mg mL−1), X2 is volume ratio of antisolvent to solvent (v/v), X3 is deposition temperature (°C), Y1 is purity (%), Y2 is recovery rate (%). | |||||
| 1 | 70.00 | 5.00 | 25.00 | 82.54 | 80.11 |
| 2 | 90.00 | 5.00 | 25.00 | 83.78 | 82.03 |
| 3 | 70.00 | 15.00 | 25.00 | 83.81 | 84.15 |
| 4 | 90.00 | 15.00 | 25.00 | 86.37 | 86.14 |
| 5 | 70.00 | 10.00 | 20.00 | 82.76 | 82.11 |
| 6 | 90.00 | 10.00 | 20.00 | 85.55 | 84.12 |
| 7 | 70.00 | 10.00 | 30.00 | 84.62 | 84.35 |
| 8 | 90.00 | 10.00 | 30.00 | 87.46 | 87.16 |
| 9 | 80.00 | 5.00 | 20.00 | 83.03 | 79.61 |
| 10 | 80.00 | 15.00 | 20.00 | 86.26 | 82.07 |
| 11 | 80.00 | 5.00 | 30.00 | 84.74 | 82.26 |
| 12 | 80.00 | 15.00 | 30.00 | 87.34 | 86.22 |
| 13 | 80.00 | 10.00 | 25.00 | 89.26 | 88.11 |
| 14 | 80.00 | 10.00 | 25.00 | 87.12 | 88.43 |
| 15 | 80.00 | 10.00 | 25.00 | 88.43 | 87.91 |
| 16 | 80.00 | 10.00 | 25.00 | 88.95 | 87.15 |
| 17 | 80.00 | 10.00 | 25.00 | 88.42 | 86.87 |
| Source | Sum of squares | Degrees of freedom | Mean square | F-value | P-value |
|---|---|---|---|---|---|
| Model | 77.34 | 9 | 8.59 | 16.26 | 0.00070 |
| X1 | 11.12 | 1 | 11.12 | 21.03 | 0.0025 |
| X2 | 11.74 | 1 | 11.74 | 22.21 | 0.0022 |
| X3 | 5.38 | 1 | 5.38 | 10.18 | 0.015 |
| X1X2 | 8.52 | 1 | 0.44 | 0.82 | 0.39 |
| X1X3 | 0.00063 | 1 | 0.00063 | 0.0012 | 0.97 |
| X2X3 | 0.099 | 1 | 0.099 | 0.19 | 0.68 |
| X12 | 21.85 | 1 | 21.85 | 41.35 | 0.00040 |
| X22 | 17.40 | 1 | 17.40 | 32.93 | 0.00070 |
| X32 | 4.74 | 1 | 4.47 | 8.96 | 0.020 |
| Residual | 3.70 | 7 | 0.53 | — | — |
| Lack of fit | 1.02 | 3 | 0.34 | 0.51 | 0.70 |
| Pure error | 2.68 | 4 | 0.67 | — | — |
| Cor total | 81.04 | 16 | — | — | — |
| Credibility analysis of the regression equations | |||
|---|---|---|---|
| R2 | Adjust R2 | Predicted R2 | Adequacy precision |
| 0.95 | 0.90 | 0.75 | 11.43 |
| Source | Sum of squares | Degrees of freedom | Mean square | F-value | P-value |
|---|---|---|---|---|---|
| Model | 128.86 | 9 | 14.32 | 40.05 | <0.0001 |
| X1 | 9.53 | 1 | 9.53 | 26.65 | 0.0013 |
| X2 | 26.54 | 1 | 26.54 | 74.22 | <0.0001 |
| X3 | 18.24 | 1 | 18.24 | 51.02 | 0.0002 |
| X1X2 | 0.0012 | 1 | 0.0012 | 0.0034 | 0.96 |
| X1X3 | 0.16 | 1 | 0.16 | 0.45 | 0.53 |
| X2X3 | 0.56 | 1 | 0.56 | 1.57 | 0.25 |
| X12 | 7.63 | 1 | 7.63 | 21.33 | 0.0024 |
| X22 | 44.22 | 1 | 44.22 | 123.68 | <0.0001 |
| X32 | 15.41 | 1 | 15.41 | 43.11 | 0.0003 |
| Residual | 2.50 | 7 | 0.36 | — | — |
| Lack of fit | 0.77 | 3 | 0.26 | 0.59 | 0.65 |
| Pure error | 1.74 | 4 | 0.43 | — | — |
| Cor total | 131.37 | 16 | — | — | — |
| Credibility analysis of the regression equations | |||
|---|---|---|---|
| R2 | Adjust R2 | Predicted R2 | Adequacy precision |
| 0.98 | 0.96 | 0.89 | 17.69 |
The 3D surface maps obtained according to the above formula are shown in Fig. 4. With the TFs purified by LAP, the significant factors of the purity analysis were ranked as: antisolvent–solvent ratio > TFs concentration > deposition temperature, and that of the significant factors of the recovery rate analysis were ranked as: antisolvent–solvent ratio > deposition temperature > TFs concentration. The best conditions for TFs purification by LAP were predicted by BBD software: the TFs concentration of 83.00 mg mL−1, antisolvent–solvent ratio of 11, and deposition temperature of 27 °C. Under the conditions of the point prediction, the purity and recovery rate of the final PFs were 88.93% and 88.53%, respectively. Three replicate experiments were performed according to the optimal purification conditions. The average purity and recovery rates of the PFs were 88.32% ± 2.54% and 88.08% ± 2.13%, respectively, indicating that the test results match the model well. The parameters of LAP purified TFs optimized by response surface analysis were more reliable and feasible.
3.3 The content of TF and main flavonoid compounds
The peak-out position of the main flavonoids was determined by comparing the HPLC chromatogram with the standard. Fig. 5 shows the HPLC profiles of reference standards, PF and CF. The peaks in Fig. 5A are the standard rutin, kaempferol and quercetin. The suitability tests of the HPLC method including linearity, reproducibility, precision, detection limit, recovery, and stability have been carried out before the extracts were determined. The limits of detection of rutin, kaempferol and quercetin were 0.95, 1.60, and 2.80 μg mL−1, respectively. The linear regression equations of rutin, kaempferol and quercetin were y = 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 008.49x – 115.99 (R2 = 0.999), y = 14
008.49x – 115.99 (R2 = 0.999), y = 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 244.22x – 5117.00 (R2 = 0.999), and y = 12
244.22x – 5117.00 (R2 = 0.999), and y = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 740.97x – 2888.90 (R2 = 0.999), respectively.
740.97x – 2888.90 (R2 = 0.999), respectively.
 | ||
| Fig. 5 HPLC chromatogram: (A) reference standards of three flavonoids, (B) the main flavonoids in PFs, and (C) CFs. | ||
In previous studies, the actual yield and purity of total flavonoids obtained by microwave extraction were 1.45% and 55.31%, respectively.30 The purification method of LAP method increased the purity of the total flavonoid up to 88.32% under the optimal process conditions (Table 4). In addition, rutin, kaempferol and quercetin increased at approximately 1.31 times, 1.87 times, and 1.91 times in PF, respectively.
| Samples | Rutin (%) | Kaempferol (%) | Quercetin (%) | Total flavonoids (%) |
|---|---|---|---|---|
| a Data presents as mean ± standard deviation. | ||||
| CFs | 20.61 ± 1.21 | 0.82 ± 0.09 | 1.22 ± 0.12 | 55.31 ± 3.12 |
| PFs | 27.14 ± 1.14 | 1.51 ± 0.21 | 2.32 ± 0.24 | 88.32 ± 2.54 |
The improved purity of the main flavonoids is because the flavonoids were firstly dissolved in methanol during the purification process. In addition, other impurities in methanol solution cannot be recrystallized when the solvent was added into deionized water.
3.4 Morphology analysis
As shown in Fig. 6, the morphological characteristics of CFs and PFs were examined by SEM. Fig. 6A showed the morphology of lyophilized CFs powder obtained from Eucommia ulmoides leaves treated by microwave extraction. The lyophilized CFs powder has a large particle size (approximately 0.9–21.0 μm) and presents irregular block particles. The morphology of the lyophilized PFs powder purified by the LAP method was shown in Fig. 6B. The particles are uniformly distributed spherical, with a particle size of about 500 nm. This result indicates that the PF particle size produced by the LAP method is smaller than the CF particle size, which is due to the slow recrystallization of total flavonoids after nucleation in the water–methanol system. The particle size distribution of the PF was shown in Fig. 6C, with an average particle size of 505.3 nm, and it is consistent with the SEM. TFs was purified by LAP method to obtain PFs powder with smaller particle size. Due to the TFs particle size change, smaller PFs particle sizes were expected to increase the degree of dispersion in the aqueous phase, thus contributing to its bioavailability.39,40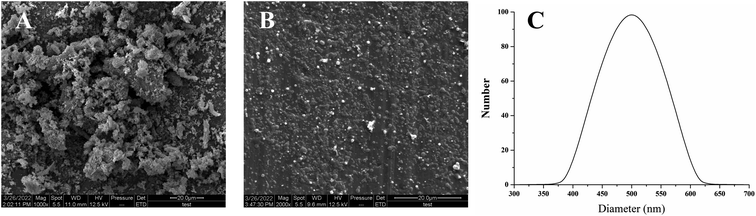 | ||
| Fig. 6 SEM images and particle size distribution of sample morphology: (A) CFs; and (B) PFs; (C) particle size distribution. | ||
3.5 Effects of PFs on in vitro antioxidant activities
Antioxidants from natural sources have been shown to have protective functions of oxidative damage and are associated with decrease the risk of chronic diseases.41 The conventional method for evaluating the free radical scavenging ability of antioxidants is UV/visible absorption spectrophotometer. The DPPH and ABTS can be purchased from merchants, easy to operate results credible. Therefore, DPPH and ABTS radical scavenging methods have been widely used to evaluate the radical scavenging activity of plant-derived antioxidant material samples.The free radical scavenging activity of PFs and CFs in vitro were investigated by DPPH, ABTS, ˙OH, O2˙− and FRAP methods in this study. The positive control resistance as a reference showed as ascorbic acid (Vc) consistent with concentrations of BHT. Fig. 7A showed the scavenging activity of different samples to DPPH radicals at different concentrations. The ability of PFs to scavenge DPPH free radicals is closely related to its concentration. The concentrations of Vc, BHT, PFs and CFs scavenging inhibition DPPH 50% free radical activity (IC50), as shown in Table 5, were 4.15 ± 0.06, 6.58 ± 0.11, 16.72 ± 1.07 and 19.13 ± 0.23, respectively. Among them, the IC50 value of PFs was lower than that of CFs and showed significant variability (p < 0.05). The reason is that the PFs obtained after purification by LAP method are more pure than the CFs, thus bringing with the enhanced free radical scavenging ability.
| Sample | DPPH (IC50) μg mL−1 | ABTS (IC50) μg mL−1 | ˙OH (IC50) μg mL−1 | O2˙− (IC50) μg mL−1 | FRAP (SRP) μg−1 |
|---|---|---|---|---|---|
| a Data presents as mean ± standard deviation. Abbreviations: Vc, ascorbic acid; BHT, butylated hydroxy toluene; PFs, purified flavonoids. SRP: slope of trend line in reducing power assay (μg−1). IC50: the IC50 value represents the concentration when free radical scavenging inhibits 50% (μg mL−1).b Positive control.c p < 0.05 compared to CFs. | |||||
| Vcb | 4.15 ± 0.06 | 3.76 ± 0.09 | — | 164.27 ± 9.72 | 0.0113 ± 0.0034 |
| BHTb | 6.58 ± 0.11 | 5.13 ± 0.10 | 114.25 ± 12.63 | — | 0.0080 ± 0.0015 |
| PFs | 16.72 ± 1.07 | 10.76 ± 0.13 | 227.68 ± 18.23 | 335.86 ± 15.98 | 0.0091 ± 0.0018 |
| CFs | 19.13 ± 0.23 | 13.12 ± 0.11 | 243.22 ± 8.625 | 377.52 ± 10.23 | 0.0083 ± 0.0012 |
The ABTS analysis result is shown in Fig. 7B, and free RSA gradually decreased in the following order: ascorbic acid > BHT > PFs > CFs. The IC50 value of ABTS free radical scavenging ability of PFs and CFs were 10.76 ± 0.13 μg mL−1 and 13.12 ± 0.11 μg mL−1, respectively. The IC50 values significantly change between PFs and CFs (p < 0.05).
˙OH is one of the strongest oxidants that can react unselectively with surrounding chemicals, including a variety of organic compounds and redox sensitive elements.42 The scavenging activity of different concentrations of samples to ˙OH were shown in Fig. 7C, where BHT is a positive control. The free radical scavenging activity of PFs and CFs on ˙OH increased dose-dependently in the measured range from 50 μg mL−1 to 250 μg mL−1. The IC50 value of ˙OH free radical scavenging ability of PFs and CFs were 227.68 ± 18.23 μg mL−1 and 243.22 ± 8.625 μg mL−1, respectively. In conclusion, the PFs showed better ˙OH free radical scavenging activity compared with CFs.
Under physiological conditions, O2˙− is the product of constant production during normal cell metabolism, but excessively accumulated concentrations will damage biofilm, tissue and organic systems.43 Fig. 7D showed the radical scavenging activity of O2˙− at different concentrations of PFs and CFs, with Vc as a positive control group. When the sample concentration was 300 μg mL−1, the RSA of PFs, CFs and Vc were 47.32 ± 2.61%, 42.41 ± 2.33% and 77.93 ± 3.54%, respectively. Although the radical scavenging activity of PFs (IC50 value of 335.86 mg mL−1) was lower than the control Vc (IC50 value of 164.27 mg mL−1), the radical scavenging activity of PFs increased with the PFs concentration throughout the experimental concentration range. The O2˙−-radical scavenging activity of PFs was always higher than that of CFs, and the IC50 of PFs was significantly different from the IC50 of CFs (p < 0.05).
The FRAP has been used as an important assessment of the antioxidant capacity of natural antioxidants. In the FRAP assay, the absorbance of Vc, PFs, BHT and CFs increased with the sample concentration. As shown in Fig. 7E, ascorbic acid showed a significantly stronger antioxidant capacity than PFs, BHT, and CFs (p < 0.05), whereas there is no significant difference in antioxidant capacity among the PF, CFs and BHT sample groups (p > 0.05). The PFs, CFs and BHT had equal antioxidant capabilities in the FRAP assay. Meanwhile, the SRP value showed an increased concentration dependence along with the sample concentration.
3.6 Antioxidant enzymes activities and MDA levels in the liver and kidney in vivo
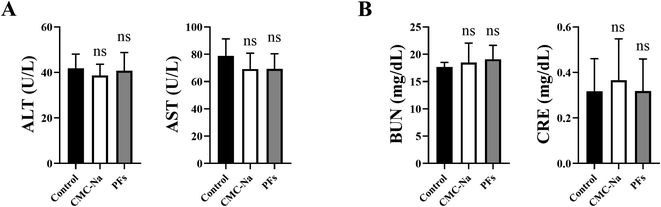
Reactive oxygen species (ROS) are produced by oxidative stress reactions in organisms at the core of aging-related diseases. The living organisms already has an antioxidant defense system that includes the presence of non-enzymatic antioxidants, such as GSH, uric acid, vitamin C, and enzymes such as SOD, CAT and GSH-Px. Free radicals are known to cause damage to the liver, and many antioxidants have proven to protect the liver against hepatotoxicants.44 In vivo experiments with PFs containing 88.32% ± 2.54% flavonoids, including rutin, kaempferol and quercetin were 27.14% ± 1.14%, 1.51% ± 0.21% and 2.32% ± 0.24, respectively.CCl4 is a classical toxicant that causes oxidative stress mediated toxicity.45 Therefore, CCl4 was used to establish the oxidative stress model in the study. The antioxidant activity of PFs was investigated in rats. The in vivo antioxidant activity test results were shown in Table 6. Experimental results showed that SOD, CAT, and GSH-Px activities in the CCl4-intoxicated group (Group II) was significantly reduced when compared to the blank control group (Group I) (p < 0.05) in the liver. Furthermore, the SOD, CAT, GSH and GSH-Px activities in the CCl4-intoxicated group (Group II) was significantly decreased compared to the control group (p < 0.05) in the kidney. The MDA levels in the liver and kidney have also increased significantly. When the PFs (100, 200, and 400 mg kg−1 body weight) were given along with CCl4, the PFs showed the ability to significantly increase the levels of SOD, CAT, GSH and GSH-Px (p < 0.05). Furthermore, the liver and kidney MDA levels decreased significantly as compared to the CCl4-intoxicated group (p < 0.05). The reason is that PFs can restore the activity of these antioxidant enzymes/or activate the enzyme activity in the damage caused by CCl4. There was no significant difference in enzyme activity in the three doses of PFs (100, 200 and 400 mg kg−1 body weight) and the vitamin E group (100 mg kg−1 body weight) at SOD, CAT, GSH and GSH-Px (p > 0.05). Moreover, the three dose groups of PFs (100, 200, and 400 mg kg−1 body weight) differ significantly from the blank control group at MDA levels in the liver (p < 0.05). Vitamin E (Group III) in the positive control group showed elevated SOD, CAT, and GSH-Px levels and a significant decrease in liver and kidney MDA levels (p < 0.05). In addition, we evaluated the potential adverse effects of PFs on rat liver and kidney by blood biochemical indicators. As shown in Fig. 8, compared to other two groups, we observed that 400 mg kg−1 PFs did not significantly alter serum levels of ALT, AST, BUN and CRE under a two-week administration, indicating that the current dose of PFs is safe for rat liver and kidney.
| No. | Liver | Kidney | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SOD | CAT | GSH | GSH-Px | MDA | SOD | CAT | GSH | GSH-Px | MDA | |
| a Data presents as mean ± standard deviation. Group I (control group) was treated with CMC-Na and olive oil. Group II (CCl4-induced damage group) was treated with CMC-Na and CCl4. Group III (positive control group) was treated with vitamin E and CCl4. Group IV (test group I) was treated low dose of PFs and CCl4. Group V (test group II) was middle dose of PFs and CCl4. Group VI (test group III) was High dose of PFs and CCl4. Vitamin E and PFs suspended in 0.3% (w/v) CMC-Na was administrated by oral gavage; CCl4 dissolved in olive oil was intraperitoneally injected. Abbreviations: SOD: Ssuperoxide dismutase, U mg−1 protein; CAT: catalase, U mg−1 protein; GSH: glutathione, mg GSH per g protein; GSH-Px: glutathione peroxidase, U mg−1 protein; MDA: malondialdehyde, nmol MDA per mg protein.b p < 0.05 compared to Group I.c p < 0.05 compared to Group II.d p < 0.05 compared to Group III.e p > 0.05 comparison among Group IV, Group V and Group VI. | ||||||||||
| Group I | 207.41 ± 8.73c | 42.52 ± 3.52c,d | 1.31 ± 0.35 | 1187.64 ± 106.32c,d | 6.92 ± 0.53c,d | 201.62 ± 4.31c | 38.44 ± 4.12c | 2.21 ± 0.13c | 323.24 ± 32.52c | 4.35 ± 0.42c,d |
| Group II | 157.32 ± 9.34b,d | 11.32 ± 2.73b,d | 0.87 ± 0.12 | 452.32 ± 58.74b,d | 10.45 ± 1.12b,d | 146.35 ± 5.32b,d | 10.45 ± 1.52b,d | 1.04 ± 0.21b,d | 235.21 ± 24.62b,d | 7.98 ± 0.86b,d |
| Group III | 184.82 ± 7.24c | 20.14 ± 4.32b,c | 1.24 ± 0.31 | 745.35 ± 66.24b,c | 1.45 ± 0.42b,c | 197.61 ± 7.23c | 30.24 ± 4.62c | 2.04 ± 0.16c | 346.71 ± 47.63c | 2.45 ± 0.32b,c |
| Group IV | 188.72 ± 11.45c,e | 27.64 ± 4.63b,c,e | 1.37 ± 0.25c,e | 703.25 ± 73.51b,c,e | 7.12 ± 0.84c,d,e | 195.14 ± 10.42c,e | 22.41 ± 2.85b,c,e | 2.56 ± 0.32c,e | 346.26 ± 32.51c,e | 3.03 ± 0.21b,c,e |
| Group V | 193.22 ± 7.23c,e | 28.72 ± 3.85b,c,e | 1.43 ± 0.31c,e | 668.47 ± 76.33b,c,e | 7.25 ± 0.48c,d,e | 194.21 ± 7.54c,e | 21.48 ± 3.27b,c,e | 2.77 ± 0.42c,e | 312.50 ± 25.62c,e | 2.56 ± 0.52b,c,e |
| Group VI | 196.85 ± 10.22c,e | 26.17 ± 2.52b,c,e | 1.48 ± 0.24c,e | 721.51 ± 83.52b,c,e | 7.72 ± 1.04c,d,e | 194.51 ± 6.26c,e | 25.24 ± 1.85b,c,e | 2.64 ± 0.35c,e | 334.52 ± 31.42c,e | 2.22 ± 0.32b,c,e |
ROS is a metabolite of various cells and plays an important role in the pathogenesis of various serious diseases.46 Recently natural antioxidants have been shown to have antioxidant or free radical removal activity.47 Briefly, antioxidants provide protective effects by removing numerous ROS (including peroxyl radicals, hydroxyl radicals, superoxide anions and hypochlorite acids). In particular, the multiple antioxidant-related activities of flavonoids in cardiovascular diseases, anti-cancer, liver preservation, anti-bacterial, anti-viral and anti-inflammatory have been widely described.48 CCl4 as a potent liver toxin as an important drug research substance is widely used in the study of liver disease caused by liver toxins. CCl4 is metabolized in vivo as a ·CCl3 free radical by cytochrome P450, further involved in oxidation to form trichloromethyl peroxide free radicals (CCl3O2˙). CCl3O2˙ radicals covalently bind to cellular macromolecules and biofilms, triggering lipid peroxidation, resulting in increased MDA levels, and reductions in SOD, CAT, GSH and GSH-Px.
In vivo studies in rats induced by carbon tetrachloride (CCl4) showed that PFs can effectively improve antioxidant enzymes (SOD, CAT and GSH-Px) activities, reducing the substance of lipid peroxide reaction product (MDA) in the liver and kidney.
4. Conclusions
In conclusion, a new technology for purifying flavonoids from Eucommia ulmoides leaves by LAP was proposed in this study. The results based on the scavenging activity of DPPH, ABTS, and O2˙− radicals showed that PFs have better scavenging ability than CFs. In vivo antioxidant studies showed that PFs purified by LAP method exhibited outstanding antioxidant activity in SD rats, which protected the liver and kidney from oxidative stress. Therefore, the LAP method may have great potential value and is expected to be a new technique for the purification Eucommia ulmoides leaves of total flavonoids.Author contributions
Data curation, Yaya Peng; Formal analysis, Yaya Peng and Fei Luo; Investigation, Fei Luo; Methodology, Mingfang Wu; Project administration, Qian Zhang; Software, Qianli Zhuang; Supervision, Mingfang Wu; Writing–original draft, Qianli Zhuang and Umar Farooq; Writing–review & editing, Mingfang Wu.Conflicts of interest
No potential conflict of interest was reported by the authors.Acknowledgements
This work was supported by the University-Industry Collaborative Education Program (grant number: 202102242031) and Fundamental Research Funds for the Zhejiang University of Science and Technology (grant number: 2023QN030).References
- Z. Wang, S. Peng, M. Peng, Z. She, Q. Yang and T. Huang, Ann. Transl. Med., 2020, 8, 1004 CrossRef PubMed.
- Z. J. Ding, C. Liang, X. Wang, X. Yao, R. H. Yang, Z. S. Zhang, J. J. He, H. Y. Du, D. Fang and Q. Li, J. Evidence-Based Complementary Altern. Med., 2020, 2020, 6432173 Search PubMed.
- C. Y. Wang, L. Tang, J. W. He, J. Li and Y. Z. Wang, Am. J. Chin. Med., 2019, 47, 259–300 CrossRef CAS.
- M. F. Peng, S. Tian, Y. G. Song, C. X. Li, M. S. Miao, Z. Ren and M. Li, J. Ethnopharmacol., 2021, 273, 113947 CrossRef CAS PubMed.
- S. Zhang, Z. Yu, J. Xia, X. Zhang, K. Liu, A. Sik and M. Jin, Food Funct., 2020, 11, 1425–1440 RSC.
- M. Ayaz, A. Sadiq, M. Junaid, F. Ullah, M. Ovais, I. Ullah, J. Ahmed and M. Shahid, Front. Aging Neurosci., 2019, 11, 155 CrossRef CAS PubMed.
- G. Li, K. Ding, Y. Qiao, L. Zhang, L. Zheng, T. Pan and L. Zhang, Molecules, 2020, 25, 5628 CrossRef CAS PubMed.
- H. Khan, H. Ullah, M. Martorell, S. E. Valdes, T. Belwal, S. Tejada, A. Sureda and M. A. Kamal, Semin. Cancer Biol., 2021, 69, 200–211 CrossRef CAS.
- R. Chen, H. Hassan, C. Rawlinson and D. Morgan, J. Exp. Stroke Transl. Med., 2021, 13, 1–12 Search PubMed.
- M. Kluska, M. Juszczak, J. Zuchowski, A. Stochmal and K. Wozniak, Molecules, 2022, 27, 333 CrossRef CAS PubMed.
- D. Xu, M. J. Hu, Y. Q. Wang and Y. L. Cui, Molecules, 2019, 24, 1123 CrossRef CAS PubMed.
- K. Suchal, S. Malik, S. I. Khan, R. K. Malhotra, S. N. Goyal, J. Bhatia, S. Ojha and D. S. Arya, Int. J. Mol. Sci., 2017, 18, 1001 CrossRef PubMed.
- K. S. Al-Numair, C. Veeramani, M. A. Alsaif and G. Chandramohan, Pharm. Biol., 2015, 53, 1372–1378 CrossRef CAS PubMed.
- J. Chen, J. Wang, R. Wang, B. Xian, C. Ren, Q. Liu, Q. Wu and J. Pei, BMC Plant Biol., 2020, 20, 353 CrossRef CAS PubMed.
- L. A. Paje, J. Choi, H. D. Lee, J. Kim, A. R. Yu, M. J. Bae, P. J. L. Geraldino and S. Lee, Heliyon, 2022, 8, e09046 CrossRef CAS PubMed.
- J. Pu, H. Wang, C. Huang, C. Bo, B. Gong and J. Ou, J. Chromatogr. A, 2022, 1668, 462914 CrossRef CAS PubMed.
- T. Chen, X. Yang, N. Wang, H. Li, J. Zhao and Y. Li, J. Sep. Sci., 2018, 41, 3660–3668 CrossRef CAS PubMed.
- Z. Li, X. Chen, L. Qiu, Y. Wang and Z. Zhou, Nanomaterials, 2020, 10, 1322 CrossRef PubMed.
- Y. Xu, X. Lv, G. Yang, J. Zhan, M. Li, T. Long, C. T. Ho and S. Li, Food Chem., 2019, 292, 160–165 CrossRef CAS PubMed.
- S. V. Luca, M. E. Czerwinska, A. Miron, A. C. Aprotosoaie, L. Marcourt, J. L. Wolfender, S. Granica and K. Skalicka-Wozniak, J. Pharm. Biomed. Anal., 2019, 166, 295–303 CrossRef CAS PubMed.
- L. Li, J. Zhao, T. Yang and B. Sun, Food Res. Int., 2022, 153, 110956 CrossRef CAS PubMed.
- M. S. Che Zain, S. Y. Lee, C. Y. Teo and K. Shaari, Molecules, 2020, 25, 778 CrossRef PubMed.
- X. Wang, J. Su, X. Chu, X. Zhang, Q. Kan, R. Liu and X. Fu, Molecules, 2021, 26 Search PubMed.
- S. Zhu, J. Xu, B. Wang, J. Xie, G. Ying, J. Li, Z. Cheng, J. Li and K. Chen, J. Colloid Interface Sci., 2022, 625, 158–168 CrossRef CAS PubMed.
- N. Munoz-Almagro, M. Prodanov, P. J. Wilde, M. Villamiel and A. Montilla, Food Chem., 2020, 318, 126476 CrossRef CAS PubMed.
- M. C. Wei, C. S. Wang, R. M. Liou and Y. C. Yang, Food Chem., 2022, 369, 130929 CrossRef CAS PubMed.
- C. H. M. Camargos and C. A. Rezende, Int. J. Biol. Macromol., 2021, 193, 647–660 CrossRef CAS.
- G. Yu, H. Zhu, Y. Huang, X. Zhang, L. Sun, Y. Wang and X. Xia, Ultrason. Sonochem., 2021, 79, 105772 CrossRef CAS.
- W. Wu, L. Wang and S. Wang, Colloids Surf. B Biointerfaces, 2021, 198, 111474 CrossRef CAS.
- M. Wu, P. Liu, S. Wang, C. Zhong and X. Zhao, Foods, 2021, 10 Search PubMed.
- Y. Jiang, S. Wang, M. Yu, D. Wu, J. Lei, W. Li, Y. He and W. Gang, ACS Omega, 2021, 6, 10505 CrossRef CAS PubMed.
- X. Yao, L. Zhu, Y. Chen, J. Tian and Y. Wang, Food Chem., 2013, 139, 59–66 CrossRef CAS PubMed.
- J. Zhang, D. Wang, Y. Wu, W. Li, Y. Hu, G. Zhao, C. Fu, S. Fu and L. Zou, J. Agric. Food Chem., 2018, 66, 4923–4932 CrossRef CAS PubMed.
- U. Sarker and S. Oba, Sci. Rep., 2020, 10, 18617 CrossRef CAS PubMed.
- F. Ullah, N. Iqbal, M. Ayaz, A. Sadiq, I. Ullah, S. Ahmad and M. Imran, Pak. J. Pharm. Sci., 2017, 30, 761–766 CAS.
- C. Lu, H. Li, C. Li, B. Chen and Y. Shen, Food Chem. Toxicol., 2018, 119, 368–374 CrossRef CAS PubMed.
- F. Younsi, S. Mehdi, O. Aissi, N. Rahali, R. Jaouadi, M. Boussaid and C. Messaoud, Chem. Biodivers., 2017, 14 Search PubMed.
- P. Li, J. Jia, D. Zhang, J. Xie, X. Xu and D. Wei, Food Funct., 2014, 5, 50–56 RSC.
- M. U. K. Sahibzada, M. Zahoor, A. Sadiq, F. Ur Rehman, A. M. Al-Mohaimeed, M. Shahid, S. Naz and R. Ullah, Saudi J. Biol. Sci., 2021, 28, 327–332 CrossRef CAS.
- C. C. Sun, H. Su, G. D. Zheng, W. J. Wang, E. Yuan and Q. F. Zhang, Food Chem., 2020, 330, 127245 CrossRef CAS.
- A. Garcia-Sanchez, A. G. Miranda-Diaz and E. G. Cardona-Munoz, Oxid. Med. Cell. Longev., 2020, 2020, 2082145 Search PubMed.
- S. Gligorovski, R. Strekowski, S. Barbati and D. Vione, Chem. Rev., 2015, 115, 13051–13092 CrossRef CAS PubMed.
- M. Szeliga, Antioxidants, 2020, 9, 1203 CrossRef CAS PubMed.
- S. Li, J. Zhou, S. Xu, J. Li, J. Liu, Y. Lu, J. Shi, S. Zhou and Q. Wu, Biomed. Pharmacother., 2019, 117, 109073 CrossRef CAS PubMed.
- M. Manubolu, L. Goodla, S. Ravilla, J. Thanasekaran, P. Dutta, K. Malmlof and V. R. Obulum, J. Ethnopharmacol., 2014, 153, 744–752 CrossRef CAS.
- Y. J. Suzuki, Antioxidants, 2019, 8, 91 CrossRef.
- C. G. Pereira, L. Barreira, S. Bijttebier, L. Pieters, C. Marques, T. F. Santos, M. J. Rodrigues, J. Varela and L. Custodio, Sci. Rep., 2018, 8, 4689 CrossRef PubMed.
- A. Ullah, S. Munir, S. L. Badshah, N. Khan, L. Ghani, B. G. Poulson, A. H. Emwas and M. Jaremko, Molecules, 2020, 25, 5243 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |