Open Access Article
Open Access ArticleSimultaneous determination of phenols in the four main original plants of the famous traditional Chinese medicine Shihu by pressurized capillary electrochromatography
Xiao-Quan Liuac,
Chao-Feng Qin†
ac,
Nai-Dong Chen *abc,
Jing-Wen Hao*ab,
Shi-Tang Maab,
Min Zhangab,
Yu Songd,
Yun-Jiang Minab,
Ya-Qin Buac and
Sheng Liac
*abc,
Jing-Wen Hao*ab,
Shi-Tang Maab,
Min Zhangab,
Yu Songd,
Yun-Jiang Minab,
Ya-Qin Buac and
Sheng Liac
aCollege of Biotechnology and Pharmaceutical Engineering, West Anhui University, Lu'an City 237012, Anhui Province, P. R. China. E-mail: 2004cnd@163.com; haojingwen2018@163.com
bAnhui Engineering Laboratory for Conservation and Sustainable Utilization of Traditional Chinese Medicine Resource, Lu'an City 237012, Anhui Province, P. R. China
cCollege of Pharmacy, Anhui University of Chinese Medicine, No.1 Qianjian Road, Hefei City, 230012, Anhui Province, P. R. China
dCollege of Pharmacy, Xinxiang Medical University, Xinxiang City 453003, Henan Province, P. R. China
First published on 27th June 2023
Abstract
A rapid pressurized capillary electrochromatography (pCEC) method has been established for the simultaneous analysis of 11 phenols in the four main original plants of the famous traditional Chinese medicine (TCM) Shihu. The effects of wavelength, mobile phase, flow rate, pH value, concentration of buffer, and applied voltage were systematically studied. The investigated 11 phenols could be isolated in 35 min on a reversed-phase EP-100-20/45-3-C18 capillary column using the established method. To apply the established pCEC method, all phenols except tristin (11) were detected in the four Dendrobium plants. A total of 10 components were detected in D. huoshanense, 6 components in D. nobile, 3 components in D. chrysotoxum, and 4 components in D. fimbriatum. The consistent evaluation revealed that the similarities among the four original plants of Shihu were 38.2–86.0% based on the 11 polyphenols and 92.5–97.7% based on the pCEC fingerprints. These further suggested that the components of the four original plants of TCM Shihu might be significantly different. Further investigation should be conducted to confirm and evaluate if the four species could be used as the same medicine with the same amount according to Chinese Pharmacopoeia (ChP).
1. Introduction
Dendrobium is one of the biggest genera of the Orchidaceae family with more than 1100 species worldwide, among which 74 species and 2 varieties are distributed in China. Most Dendrobium plants are used as the famous traditional Chinese medicine (TCM) Shihu for a very long time.1 Dendrobium huoshanense C. Z. Tang and S. J. Cheng, Dendrobium nobile Lindl, Dendrobium chrysotoxum Lindl, and Dendrobium fimbriatum Hook are the most important original plants of the TCM Shihu owing to their similar and prominent biological activities.2,3 The stems of the four different herbs are believed to produce the same functions, such as the treatment of stomach, salivary, and ophthalmic disorders, and thus can be used as the same medicine based on Chinese Pharmacopoeia (ChP). The phytochemical documents indicated that Shihu is rich in polysaccharides, polyphenols, and alkaloids.4–6 These metabolites exhibited multiple pharmacological properties, including the immunoenhancement of the body and the special curative effect on the human eye, pharynx, lung, stomach, intestine, kidney, and the cardiovascular system,7–9 and thus were the main bioactive components of Shihu. One medicine from multiple origins was common in herbs besides Shihu. This demands the chemical compositions, at least the main bioactive components from the different original plants are the same, so that they may be used instead of the other in the clinic. The rationality of using the four Dendrobium plants as one medicine needs to be clarified. As the major active constituents of Shihu, polyphenols are also rich in many other natural sources, such as crude extracts of fruits, herbs, vegetables, grains, and nuts. In short, phenols are increasingly attracting considerable interest in most fields, including the food industry, and significant efforts have been spent to develop and enhance the analytical methods for their detection including bioassay,10 ELISA,11,12 and chromatographic methods, such as high-performance liquid chromatography (HPLC), gas chromatography with mass spectrometry (GC-MS), and liquid chromatography with mass spectrometry (LC-MS).13–18 Compared with the previous analytical techniques, pressurized capillary electrochromatography (pCEC)19,20 is a potential separation system in which a mobile phase is driven by both a pressurized flow and an electro-osmotic flow (EOF). This setup is especially compatible with the isolation of highly polar compounds, for example, polyphenols.21 To our knowledge, few references had ever mentioned the application of the pCEC technique for the analysis of polyphenols in natural medicines and food. Zhu used pressurized capillary electrochromatography for determining three isoflavones from Pueraria lobata.23 Derazshamshir A. used capillary electrochromatography to determine catecholamines (CAs) and their metabolites.24 Zhao used pressurized capillary electrochromatography to determine costunolide and dehydrocostus lactone in Maren Runchang Wan.25 The method we established simultaneously determined 11 compounds, which is more than the sample of compounds measured by others using this method.26 The accuracy of the method we established is also better than that of others, and the results showed that the RSD value was less than 1.84%, and the method's accuracy was adequate.27Based on the above foundation, a fast pCEC method was established for the simultaneous determination of the phenols in the major original plants of Shihu. In order to isolate and simultaneously quantify the phenols in D. huoshanense, D. nobile, D. chrysotoxum and D. fimbriatum, the proposed method was then effectively used and verified. The consistency of the four Dendrobium plants was then evaluated based on the composition of polyphenols and the pCEC fingerprints of their methanol extracts to explore the logic of the use of four different Dendrobium species as the same medicine and thus providing references for other multiple-origin plants.
2. Materials and methods
2.1. Chemicals and reagents
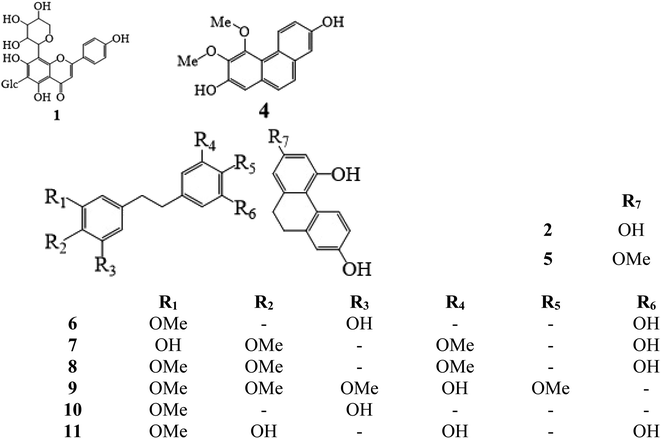
Methanol and acetonitrile (chromatographic grade) were purchased from Tedia High-Purity Solvent Co., Ltd. (USA). Secondary distilled water was made in the laboratory using a Milli-Q Plus purification system (Millipore, Billerica, MA, USA). Ammonium acetate, formic acid, potassium dihydrogen phosphate, borax, and sodium hydroxide were bought from Aladdin Reagent Co., Ltd. (Shanghai, China). Standards of schaftoside (1) 2,4,7-trihydroxy-9,10-dihydrophenanthrene (2), dihydroresveratrol (3), nudol (4), coelonin (5), batatasin III (6), gigantol (7), 3′-hydroxy-3,4,5′-trimethoxybibenzy (8), erianin (9), dihydropinosylvin methyl ether (10), and tristin (11) were obtained from Sichuan Weikeqi Biological Technology Co., Ltd. (Sichuan, Chengdu, China). The structures of these standards are shown in Fig. 1.2.2. Sample preparation and purification procedures
D. huoshanense, D. nobile, D. chrysotoxum, and D. fimbriatum samples were collected on November 14, 2020, in Huoshan County, Anhui Province, China, and identified by Pro. Nai-Fu Chen, West Anhui University, China. The samples were prepared as follows: the fresh stems were freeze-dried at −50 °C to constant weight, then separately powdered using a blender and screened using a 200 mesh sieve. 2.0 g of each powdered sample was extracted with 100 mL 90% methanol 3 times. By decompressing and concentrating the filtrate to dryness, the methanol extracts of 4 Dendrobium plants were produced.A CNWBOND LC-C18 SPE cartridge (2 g 10 mL; ANPEL Laboratory Technologies Inc., Shanghai, China) was used for solid-phase extraction according to our previous study;2 the detailed steps were as follows: the cartridge was activated using 50 mL of methanol followed by 30 mL of Milli-Q water. An accurate 10 mL of the sample solution was injected into the activated and conditioned SPE cartridge. The elution was performed with 50 mL of 60% methanol solution (the required substance is in the elution), concentrated eluent to 1 mL under reduced pressure to prepare the purified sample.
2.3. Preparation of standard solutions
Appropriate amounts of the substances were mixed and dissolved in methanol. Diluted working standard solutions were prepared as follows: (1) 0.11 mg mL−1, (2) 0.12 mg mL−1, (3) 0.21 mg mL−1, (4) 0.67 mg mL−1, (5) 0.32 mg mL−1, (6) 0.13 mg mL−1, (7) 0.33 mg mL−1, (8) 0.15 mg mL−1, (9) 0.25 mg mL−1, (10) 0.10 mg mL−1, (11) 0.12 mg mL−1. All the solutions were stored at 4 °C.2.4. Instruments and analytical conditions
The prepared standard phenolic compounds were analyzed using a TriSep™ 2100 pCEC system (Unimicro Technologies Co., Ltd., Pleasanton, CA, USA) comprising two high-pressure infusion modules, a microfluidic module, a high-voltage power module (±30 kV), and a variable-wavelength UV detector (190–800 nm). Chromatographic separation was performed on an EP-100-20/45-3-C18 (Electropak™, C18, ODS, 3 μm, effective length 20 cm × 100 μm i.d.). In this experiment, the wavelength and chromatographic conditions were optimized. The detection wavelength was selected over a range of 190–400 nm. The optimization of chromatographic conditions includes the composition of mobile phases, mobile phase concentration, pH, flow rate, and voltage. In order to better realize the effective separation of chromatographic peaks in the sample, ACN–borax buffer, ACN–phosphate buffer, ACN–ammonium acetate buffer, and ACN–formic acid buffer were selected for comparison. In our experiment, the effect of buffer concentrations on the separation of the 11 analytes was investigated using 0.0 mM, 2.5 mM, 5.0 mM, 7.5 mM, and 10.0 mM, 12.5 mM borax buffer solution in ACN. In pCEC, the pH value of the mobile phase is an important factor to maintain EOF. As such, we compared the resolution of the objective chemicals isolated using the 10.0 mM borax buffer solution in ACN at pH = 7, 8, 9, 10, and 11. The effect of the pCEC flow rate on the resolution of the analytes was investigated by various flow rates of 0.02–0.06 mL min−1. In addition, the influence of applied voltage (from −2.5 kV to +10 kV) was chosen for further optimization so as to shorten the analysis time and prevent peak broadening.Column EP-100-20/45-3-C18 (Electropak™, C18, ODS, 3 μm, effective length 20 cm × 100 μm i.d.); detection wavelength 280 nm; the voltage applied to the column +5 kV; flow rate 0.03 mL min−1; ACN/10.0 mM borax buffer (containing 0.1 mol L−1 NaOH, pH = 9) as the mobile phase, using gradient elution mode as follows 0 min–10% ACN; 6 min–10% ACN; 7 min–30% ACN; 15 min–30% ACN; 16 min–40% ACN; 20 min–40% ACN; 24 min–80% ACN; 29 min–80% ACN; 31 min–100% ACN; 35 min–100% ACN; the analysis time of each sample was 35 minutes, and fifteen more minutes were needed to equilibrate the column at the end, the injection volume was 3 μL (the volume of the sampling loop was 0.05 μL).
To perform pCEC's response surface methodology optimization, design expert software version 13 was used. Similarity analysis (SA) was performed on the basis of the relative retention time (RRT) and relative peak area (RPA) using the professional software Similarity Evaluation System for Chromatographic Fingerprint of Traditional Chinese Medicine (2004A).
2.5. Method validation
The developed pCEC method was validated by linearity, sensitivity, precision, recovery, stability, and specificity, in accordance with the ICH Q2(R2) guidelines.22In order to create calibration curves, a minimum of eight calibration concentration levels were used, and the peak area (x) was plotted as a function of the corresponding mass (y, ng) of the working solution. The limit of detection (LOD) and quantification (LOQ) were the lowest concentrations of target analytes that could be detected and measured at a signal-to-noise ratio (S/N) of 3 and 10, respectively. The peak area was determined, a precision indicator known as the relative standard deviation (RSD) was computed and the stability was performed using a single standard solution sampled at 0, 2, 4, 8, 12, and 24 h. To evaluate the accuracy of the method, three different concentration levels (75%, 100%, and 125%) of the standard solutions were added to the samples with known content for the recovery tests. Three parallel samples were extracted for each level. Specificity evaluation was performed by injecting separately 3 μL solution of the mixed working standards, sample and blank into the chromatographic system.
3. Results and discussion
3.1. Optimization of pCEC conditions
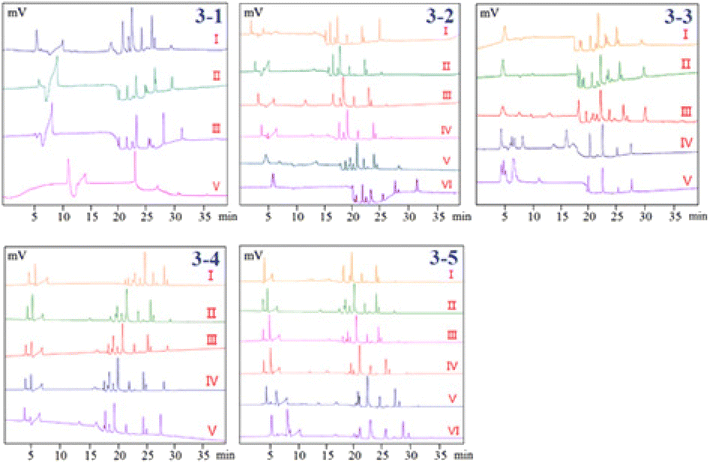
The resolution of the investigated phenols will be affected by the change in the buffer concentration since it will alter the EOF of the pCEC system. Therefore, our experiments systematically investigated the effects of the borax buffer concentration (0.0 mM, 2.5 mM, 5.0 mM, 7.5 mM, 10.0 mM, and 12.5 mM of borax in ACN at pH 9.0) on the separation system. The results showed that the resolutions and RTs of the analytes were slightly increased with buffer concentration rising from 0.0 mM to 12.5 mM. Fig. 3-2 shows that the separation resolution became better as the borax concentration increased, but a longer analysis time was needed and the chromatographic column was very easy to be blocked at 12.5 mM borax. In this case, 10 mM was chosen as the optimum concentration of borax for further optimization.
| Analyte | Calibration curve | Liner range (μg mL−1) | LOD (μg mL−1) | LOQ (μg mL−1) | Precision (RSD) | Recovery (%) | Recovery (RSD) | Stability (RSD) |
|---|---|---|---|---|---|---|---|---|
| a Schaftoside (1), 2,4,7-trihydroxy-9,10-dihydrophenanthrene (2), dihydroresveratrol (3), nudol (4), coelonin (5), batatasin III (6), gigantol (7), 3′-hydroxy-3,4,5′-trimethoxybibenzy (8), erianin (9), dihydropinosylvin methyl ether (10), and tristin (11). | ||||||||
| 1 | y = 502.44x + 106.33 | 0.19–17.11 | 0.05 | 0.18 | 0.85 | 95.69 | 1.15 | 1.72 |
| 2 | y = 778.02x + 149.19 | 0.21–18.42 | 0.03 | 0.11 | 0.63 | 90.64 | 1.57 | 0.66 |
| 3 | y = 652.68x + 252.38 | 0.37–33.16 | 0.04 | 0.14 | 1.62 | 97.85 | 0.74 | 1.52 |
| 4 | y = 149.55x − 281.58 | 5.30–105.26 | 0.08 | 0.27 | 1.44 | 94.72 | 1.24 | 1.20 |
| 5 | y = 675.55x + 17.219 | 0.56–50.00 | 0.02 | 0.06 | 1.84 | 96.68 | 1.63 | 1.56 |
| 6 | y = 875.38x − 232.15 | 0.49–20.72 | 0.02 | 0.05 | 1.22 | 91.37 | 1.38 | 1.67 |
| 7 | y = 1269.6x − 675.23 | 0.59–52.63 | 0.01 | 0.03 | 0.64 | 96.35 | 0.94 | 0.58 |
| 8 | y = 768.73x − 101.34 | 0.55–23.16 | 0.02 | 0.06 | 1.78 | 92.14 | 0.86 | 1.47 |
| 9 | y = 197.19x − 287.03 | 1.99–6.14 | 0.07 | 0.22 | 0.53 | 93.66 | 0.71 | 0.66 |
| 10 | y = 1640.5x − 123.02 | 0.18–15.79 | 0.01 | 0.03 | 0.69 | 95.42 | 1.52 | 0.76 |
| 11 | y = 433.83x + 50.197 | 0.44–18.42 | 0.03 | 0.10 | 1.32 | 94.33 | 1.46 | 1.41 |
3.2. Method validation of the established pCEC method
The optimized chromatographic method was validated according to the validation principles stated in the ICH guidelines “validation of analytical procedures: text and methodology Q2 (R2)” (ICH 2022). Key validation parameters evaluated include linearity, sensitivity, precision, recovery, stability, and specificity.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 under the optimal pCEC circumstances. The LOD was obtained by the consecutive analysis of spiked matrices with decreasing quantities of each phenolic standard until S/N = 3
1 under the optimal pCEC circumstances. The LOD was obtained by the consecutive analysis of spiked matrices with decreasing quantities of each phenolic standard until S/N = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was attained. Relevant results from Table 1 indicated that the proposed method was highly sensitive.
1 was attained. Relevant results from Table 1 indicated that the proposed method was highly sensitive.Overall, the experimental results suggested that the developed pCEC method could be utilized to simultaneously quantify all the investigated 11 phenols and was quick, reliable, sensitive, and accurate.
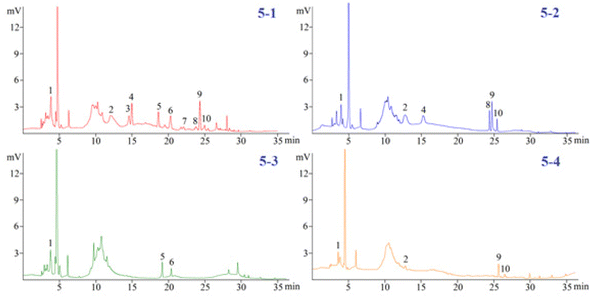
3.3. Detection of polyphenols in four Dendrobium plants
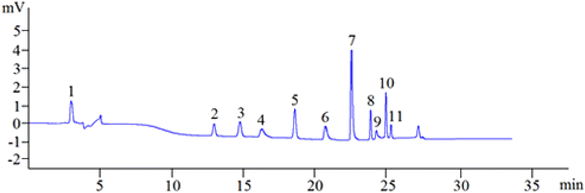
3.3.1.1. Location of the chromatographic peak of the 11 phenolic compounds. For better qualitative analysis of phenolic compounds, the chromatographic peak positions of each of the 11 phenolic compounds were further investigated by a TriSep™ 2100 pCEC system on an EP-100-20/45-3-C18 column. The chromatographic peak position was identified by the relative retention time (RRT), which was calculated according to the following equation:
| ΔtR,is = tR,i − tR,s |
3.3.1.2. The internal standards experiment. For qualitative analysis, an internal standard method was used to analyze the polyphenols in four Dendrobium plants. Specifically, polyphenol compounds as internal standards were added into the sample solution in turn to determine the position of each compound in the sample, comparing the changes in the target peak area and retention time in the sample. The results showed that the peak area corresponding to each compound increased and there was no significant difference in the retention time. The detailed data are shown in Table 2. It is worth noting that the introduction of internal standards in both samples and calibration standards improved the precision and accuracy of the qualitative analysis.
| Times | RT1 | RT2 | RT3 | RT4 | RT5 | RT6 | RT7 | RT8 | RT9 | RT10 | RT11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a RT = retention time. | |||||||||||
| 1 | 0.0981 | 0.0879 | 0.0763 | 0.0695 | 0.0901 | 0.0833 | 0.0930 | 0.0865 | 0.0782 | 0.0858 | 0.0923 |
| 2 | 0.0971 | 0.0895 | 0.0814 | 0.0734 | 0.0897 | 0.0849 | 0.0942 | 0.0859 | 0.0813 | 0.0849 | 0.0897 |
| 3 | 0.0902 | 0.0916 | 0.0759 | 0.0725 | 0.0924 | 0.0867 | 0.0915 | 0.0798 | 0.0801 | 0.0835 | 0.0874 |
| 4 | 0.0973 | 0.0883 | 0.0817 | 0.0682 | 0.0896 | 0.0851 | 0.0889 | 0.0834 | 0.0796 | 0.0861 | 0.0916 |
| 5 | 0.0981 | 0.0876 | 0.0815 | 0.0713 | 0.0923 | 0.0865 | 0.0872 | 0.0876 | 0.0853 | 0.0842 | 0.0925 |
| 6 | 0.0986 | 0.0849 | 0.0796 | 0.0688 | 0.0915 | 0.0904 | 0.0856 | 0.0843 | 0.0794 | 0.0797 | 0.0893 |
| Average | 0.0966 | 0.0883 | 0.0794 | 0.0706 | 0.0909 | 0.0862 | 0.0901 | 0.0846 | 0.0807 | 0.0840 | 0.0905 |
| RSD | 3.28% | 2.51% | 3.36% | 2.98% | 1.42% | 2.81% | 3.76% | 3.30% | 3.09% | 2.78% | 2.22% |
| Compounds | D. huoshanense | D. nobile | D. chrysotoxum | D. fimbriatum |
|---|---|---|---|---|
| a Schaftoside (1), 2,4,7-trihydroxy-9,10-dihydrophenanthrene (2), dihydroresveratrol (3), nudol (4), coelonin (5), batatasin III (6), gigantol (7), 3′-hydroxy-3,4,5′-trimethoxybibenzy (8), erianin (9), dihydropinosylvin methyl ether (10), and tristin (11). | ||||
| 1 | 11.38 ± 0.22 | 8.57 ± 0.38 | 6.16 ± 0.28 | 3.81 ± 0.08 |
| 2 | 12.72 ± 1.10 | 10.46 ± 0.38 | — | 2.83 ± 0.11 |
| 3 | 5.46 ± 0.50 | — | — | — |
| 4 | 9.06 ± 0.36 | 7.10 ± 0.30 | — | — |
| 5 | 6.16 ± 0.66 | — | 5.13 ± 0.11 | — |
| 6 | 5.84 ± 0.24 | — | 2.83 ± 0.13 | — |
| 7 | 2.48 ± 0.14 | — | — | — |
| 8 | 3.30 ± 0.04 | 6.73 ± 0.24 | — | — |
| 9 | 13.73 ± 0.82 | 11.08 ± 1.24 | — | 7.60 ± 0.45 |
| 10 | 3.70 ± 0.17 | 3.97 ± 0.20 | — | 1.76 ± 0.01 |
| 11 | — | — | — | — |
3.4. Consistency evaluation
| D. huoshanense | D. nobile | D. chrysotoxum | D. fimbriatum | |
|---|---|---|---|---|
| D. huoshanense | 1 | 0.823 | 0.456 | 0.795 |
| D. nobile | 1 | 0.382 | 0.860 | |
| D. chrysotoxum | 1 | 0.497 | ||
| D. fimbriatum | 1 |
| D. huoshanense | D. nobile | D. chrysotoxum | D. fimbriatum | |
|---|---|---|---|---|
| D. huoshanense | 1 | 0.977 | 0.951 | 0.959 |
| D. nobile | 1 | 0.970 | 0.943 | |
| D. chrysotoxum | 1 | 0.925 | ||
| D. fimbriatum | 1 |
4. Conclusions
We developed and assessed a pCEC technique for the rapid simultaneous detection of 11 typical phenolic compounds in 35 min. Systematic research was performed on pCEC circumstances. The established approach demonstrated excellent linearity, accuracy, and sensitivity. Compared with the reported studies by Zhu, our proposed method provided a shorter analysis time. This suggested approach made it possible to identify more phenolic compounds, and it offers an accurate and cost-effective alternative for phenolic compound analysis with a wide range of potential applications.2 We employed the conventional approach in the circumstances of our experiment involving a total of 10 phenolic compounds including schaftoside, 2,4,7-trihydroxy-9,10-dihydrophenanthrene, dihydroresveratrol, nudol, coelonin, batatasin III, gigantol, 3′-hydroxy-3,4,5′-trimethoxybibenzy, erianin, and dihydropinosylvin methyl ether were detected in D. huoshanense. This is the first report of the systematic and simultaneous identification and quantification of multiple phenolic compounds in 4 Dendrobium plants using pCEC, and this methodology could provide this plant with a new tool for quick quality control. This thorough examination of the composition of four Dendrobium plants will serve as the foundation for additional studies into the functions of numerous discovered substances and may help in the design and formulation of nutraceutical and cosmetic products in the future.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was granted by Natural Science Foundation of Anhui Scientific Research and Innovation Team of Quality Evaluation and Improvement of Traditional Chinese Medicine (No. 2022AH010090) and the Project of Anhui Rural Revitalization of Traditional Chinese Medicine Industry Collaborative Technology Service Center (GXXT-2022-079); The Provincial Level Nature Science Foundation of Anhui Education Department (KJ2019A0628, KJ2019A0617, KJ2019A0626, KJ2021A0957, KJ2020A0634, KJ2020A0632); School Level Projects of West Anhui University (WGKQ2021030); The Open Fund of Anhui Engineering Laboratory for Conservation and Sustainable Utilization of Traditional Chinese Medicine Resources, (TCMRPSU-2022-06); The Project from Anhui Province Rural Revitalization Collaborative Technology Service Center. The authors would like to thank the anonymous reviewers for their invaluable suggestions that helped improve the manuscript.References
- N. D. Chen, H. Chen, J. Li and H. Yu, J. Mol. Struct., 2015, 1086, 255–265 CrossRef CAS.
- A. L. Zhu, J. W. Hao, L. Liu, N. D. Chen, G. L. Wang, X. Liu, Q. Li, H. M. Xu and W. H. Yang, Front. Nutr., 2021, 8, 771078 CrossRef PubMed.
- National Pharmacopoeia Committee, Pharmacopoeia of the People's Republic of China One, China Medical Science and Technology Press, Beijing, China, 2020, p. 135 Search PubMed.
- Y. J. Fan, T. Jiang and Z. Chun, Plant Physiol. Biochem., 2021, 162, 656–666 CrossRef CAS PubMed.
- M. S. Zhang, H. L. Ling and G. Wang, et al., Nat. Prod. Res., 2022, 36, 5393–5399 CrossRef CAS PubMed.
- X. L. Fu, S. Chen and X. T. Wang, et al., Biochem. Biophys. Rep., 2021, 26, 100877 CAS.
- Z. Niu, J. Pan, Q. Xue, S. Zhu, W. Liu and X. Ding, Acta Pharm. Sin. B, 2018, 8, 466–477 CrossRef PubMed.
- M. Li, Y. He, C. Peng, X. F. Xie and G. Y. Hu, Oncol. Lett., 2018, 16, 5006–5012 Search PubMed.
- Q. Zeng, C. H. Ko, W. S. Siu, K. K. Li, C. W. Wong, X. Q. Han, L. Yang, C. B. Lau, J. M. Hu and P. C. Leung, Chin. J. Nat. Med., 2018, 16, 481–489 CAS.
- Y. Bai, F. Du, Y. Bai and H. Liu, Anal. Methods, 2010, 2, 1867–1873 RSC.
- R. Bari and J. D. G. Jones, Plant Mol. Biol., 2009, 69, 473–488 CrossRef CAS PubMed.
- A. Santner, C. V. Li and M. Estelle, Nat. Chem. Biol., 2009, 5, 301–307 CrossRef CAS PubMed.
- S. D. S. Chiwocha, S. R. Abrams, S. J. Ambrose, A. J. Cutler, M. Loewen, A. R. S. Ross and A. R. Kermode, Plant J., 2003, 35, 405–417 CrossRef CAS PubMed.
- D. Rao, Y. D. Hu and R. X. Zhao, Int. J. Anal. Chem., 2022, 2022, 9510598 Search PubMed.
- C. Wu, S. Gui, Y. Huang, Y. Dai, Q. Shun, K. Huang, S. Tao and G. Wei, Anal. Methods, 2016, 8, 3802–3808 RSC.
- Y. D. Yuan, M. Y. Yu, B. Zhang, X. Liu and J. C. Zhang, PLoS One, 2019, 14, 1–16 Search PubMed.
- X. Y. Huang and Y. I. Yin, Southwest Chin. J. Agric. Sci., 2007, 20, 735–738 Search PubMed.
- J. Liu, T. He and Z. Chun, Zhongguo Zhongyao Zazhi, 2009, 34, 2853–2856 CAS.
- Q. M. Chen, Q. Liu, L. Shen, Y. Wang and C. Yan, Chin. J. Chromatogr., 2018, 36, 388–394 CAS.
- P. Li, S. P. Li, F. Q. Yang and Y. T. Wang, J. Sep. Sci., 2007, 30, 900–905 CrossRef CAS PubMed.
- S. Liu, W. Chen, L. Qu, Y. Gai and X. Jiang, Anal. Bioanal. Chem., 2013, 405, 1257–1266 CrossRef CAS PubMed.
- ICH Q2(R2) Validation of Analytical Procedures, https://www.ema.europa.eu/en/ich-q2r2-validation-analytical-procedures, accessed on 18 August 2022 Search PubMed.
- J. Q. Zhu, H. Cheng and M. M. Zhou, et al., J. Pharm. Biomed. Anal., 2022, 212, 114592 CrossRef CAS PubMed.
- A. Derazshamshir and S. I. Aşir, et al., J. Chromatogr. Sci., 2019, 57, 758–765 CAS.
- Y. Y. Zhao, L. J. Li and W. Y. Huang, et al., Chin. J. Anal. Lab., 2016, 35, 150–152 CAS.
- L. S. Yan, Z. H. Wang and G. A. Luo, et al., Chem. J. Chin. Univ., 2004, 25, 827–830 CAS.
- W. L. Zhou, W. B. Kan and Y. H. Wang, et al., J. Instrum. Anal., 2015, 34, 321–327 CAS.
Footnote |
| † This author contribution equal. |
| This journal is © The Royal Society of Chemistry 2023 |