DOI:
10.1039/D3RA00737E
(Paper)
RSC Adv., 2023,
13, 11311-11323
Magneto-structural studies on a number of doubly end-on cyanate and azide bridged dinuclear nickel(II) complexes with {N3O} donor Schiff base ligands†
Received
3rd February 2023
, Accepted 24th March 2023
First published on 11th April 2023
Abstract
Two new doubly μ1,1-N3 bridged (1 and 3) and six new doubly μ1,1-NCO bridged NiII complexes (2, 4–8) with six different N3O donor Schiff base ligands have been synthesized and magneto-structurally characterized. All these neutral complex molecules are isostructural and constitute edge sharing bioctahedral structures. Magnetic studies revealed that all these complexes exhibit ferromagnetic interaction through bridging pseudohalides with ferromagnetic coupling constant J being significantly higher for azide-bridged complexes than that of the cyanate analogues. This is consistent with the literature reported data and also the presence of polarizable π systems and two different N and O donor atoms in cyanate ion, rendering it a poor magnetic coupler in comparison to azide analogues. Although, the magneto-structurally characterized doubly μ1,1-N3 bridged NiII complexes are abundant, only few such complexes with μ1,1-bridging NCO− ions are reported in the literature. Remarkably, addition of these six new examples in this ever-growing series of doubly μ1,1-NCO bridged systems gives us an opportunity to analyse the precise magneto-structural correlation in this system, showing a general trend in which the J value increases with an increase in bridging angles. Therefore, the high degree of structural and magnetic resemblances by inclusion of six new examples in this series is the major achievement of the present work. An elaborate DFT study was performed resulting in magneto-structural correlation showing that nature and value of the J-parameter is defined not only by Ni–Nb–Ni bond angles, but an important role is also played by the Ni1–Ni2–Nb–Xt dihedral angle (Nb and Xt are bridging N and terminal N or O atom of bridging ligands, respectively).
Introduction
Di- and poly-nuclear transition metal complexes with organic ligands have been widely studied because of their applications in various fields including bioinorganic chemistry and magnetic materials.1–7 In such metal complexes, the paramagnetic centres are either bridged by endogenous bridges such as phenolate ion or exogenous bridging ligands viz. hydroxide, alkoxide, carboxylate and pseudohalide groups or by a combination of both.8–10 Among the different bridging ligands, pseudohalides particularly azide ions, are the most investigated ones due to their versatile coordination abilities that lead to huge structural diversities and remarkable magnetic properties.11 In this regard, pseudohalide-bridged NiII complexes have attracted considerable attention in the field of molecular magnetism because of the presence of significant magnetic anisotropy in NiII ions, associated with the second order spin–orbit coupling, and because of the occurrence of possible high spin ground states through ferromagnetic interactions among NiII ions, mediated by the bridging pseudohalides, which are the essential criteria for displaying single-molecule magnetic behaviour.12–16 It is well known that the end-to-end (EE, μ1,3) bridging mode usually propagates antiferromagnetic (AF) interaction, while the end-on (EO, μ1,1) bridging azide ion transmits ferromagnetic (F) coupling between NiII ions, indicating that the introduction of more and more EO bridging azide ions is prerequisite for developing better molecule-based magnetic materials.11 Unlike azide ion, other heteroatomic pseudohalides, particularly cyanate ion, with polarizable π systems and two different N and O donor atoms, which can coordinate the metal ions through either of the heteroatoms, or both, are less versatile than the azide ion.17–23 Therefore, in contrast to the vast chemistry of azide-bridged complexes, magneto-structurally characterised compounds with cyanate ion are very limited.17–23 Consequently, unlike azide-bridged analogues, a sophisticated magneto-structural correlation is yet to be established for cyanate-bridged NiII systems, and therefore, it requires further attention.24,25 However, going through the only few examples available in the literature, one can assess that cyanate ion with EO bridging mode usually propagates ferromagnetic interaction between NiII centres in a similar way that azide ion does, but weakly, while a mixed immersion has been observed through EE bridging mode.17–23
Although it is clear that the EO bridging mode of pseudohalides is crucial for developing better molecule-based magnetic materials with high spin ground state, it is difficult to control the bridging motif of the pseudohalides in the resulting complexes as it is influenced by several factors including nature of the organic ligands. Among several organic ligands, Schiff-base ligands have been extensively utilised for the development of a large numbers of NiII clusters with interesting structures and magnetic properties because of their synthetic simplicity and tuneable stereo-electronic properties by incorporating different amine and carbonyl counterparts.26 Our recent studies disclosed that Schiff base ligands with N3O donor sets derived from condensation of salicylaldehyde or its derivative with N,N-dimethyldipropylenetriamine are capable to produce doubly EO azide or cyanate bridged ferromagnetically coupled dinuclear NiII complexes.20,22,23 Therefore, we anticipated that a large numbers of EO cyanate-bridged NiII complexes can be synthesised using different carbonyl components of the Schiff base ligands derived from this triamine, which not only add few more examples to the only limited members of such complexes reported in the literature, but it is a possibility to establish precise magneto-structural corelation in this system. Consequently, in this present endeavour, a series of tetradentate N3O donor Schiff base (HL1–HL6, Scheme 1), derived from the condensation reaction of N,N-dimethyldipropylenetriamine with different carbonyl components, namely 2-hydroxy-3-methoxy-5-methylbenzaldehyde, 2-hydroxy-3-methoxybenzaldehyde, 5-nitrosalicylaldehyde, 5-bromo-3-methoxysalicylaldehyde, 4-(diethylamino)salicylaldehyde and 2-hydroxy-1-naphthaldehyde, have been utilised to prepare closely related EO cyanate-bridged NiII complexes. Furthermore, two EO azide-bridged NiII complexes with Schiff base HL1 and HL2 were produced to compare its magnetic transmitting ability with analogues EO cyanate-bridged NiII complexes. Herein, the structures and magnetic properties of eight new complexes [Ni2(L1)2(μ1,1-N3)2] (1), [Ni2(L1)2(μ1,1-NCO)2] (2), [Ni2(L2)2(μ1,1-N3)2]·2CH3CN (3), [Ni2(L2)2(μ1,1-NCO)2] (4), [Ni2(L3)2(μ1,1-NCO)2] (5), [Ni2(L4)2(μ1,1-NCO)2] (6), [Ni2(L5)2(μ1,1-NCO)2] (7) and [Ni2(L6)2(μ1,1-NCO)2] (8) have been reported, and an emphasis was also given to establish magneto-structural correlation in these systems, that is yet to be established for cyanate-bridged NiII complexes. Moreover, the elaborated DFT study was performed for in-depth understating of the magnetic interaction in these systems.
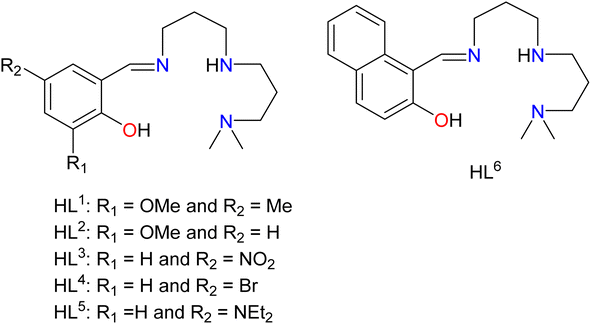 |
| | Scheme 1 Drawing of the ligands used in the present study. | |
Experimental section
Materials and physical measurements
Chemicals such as nickel(II) nitrate hexahydrate, 2-hydroxy-3-methoxybenzaldehyde, 5-nitrosalicylaldehyde, 5-bromo-3-methoxysalicylaldehyde, 2-hydroxy-1-naphthaldehyde, 4-(diethylamino)salicylaldehyde and N,N-dimethyldipropylenetriamine were commercially available reagent or analytical grade chemicals and used as received. 2-Hydroxy-3-methoxy-5-methylbenzaldehyde was synthesized following a literature reported method.27,28 Solvents were of reagent grade and used without further purification.
Elemental analyses for carbon, hydrogen and nitrogen were carried out in a PerkinElmer 240C elemental analyser. FTIR spectra of the complexes in the range 4000 to 400 cm−1 were measured in a Thermo Scientific Nicolet iS5 FTIR spectrometer using a universal ATR sampling accessory. Direct current (dc) magnetic susceptibility measurements were performed on a Quantum Design MPMS-XL-5 magnetometer under a static magnetic field of 10 kOe in the temperature range of 2 to 300 K. The field dependent magnetization data were collected in the range of dc magnetic field 0–50 kOe at 5 K. All magnetic data were corrected for the sample holder and for the diamagnetic contribution of the samples.
Synthesis of Schiff base ligands HL1–HL6
Ligands HL1–HL6 were prepared adopting conventional Schiff base condensation method in which N,N-dimethyldipropylenetriamine was allowed to react with 2-hydroxy-3-methoxy-5-methylbenzaldehyde, 2-hydroxy-3-methoxybenzaldehyde, 5-nitrosalicylaldehyde, 5-bromo-3-methoxysalicylaldehyde, 2-hydroxy-1-naphthaldehyde or 4-(diethylamino)salicylaldehyde in methanol under reflux to afford tetradentate {N3O} donor Schiff base ligands HL1–HL,6 respectively. In a typical reaction, N,N-dimethyldipropylenetriamine (0.5 mmol, 80 mg) and 2-hydroxy-3-methoxy-5-methylbenzaldehyde (0.5 mmol, 83 mg) in 20 mL methanol were heated to reflux for ca. 1 h, and thereafter allowed to cool at room temperature. The dark-orange methanol solution of HL1 was subsequently used for the synthesis of complexes 1 and 2. Similarly, Schiff base ligands HL2–HL6 were prepared, when condensation reactions were carried out using 2-hydroxy-3-methoxybenzaldehyde, 5-nitrosalicylaldehyde, 5-bromo-3-methoxysalicylaldehyde, 4-(diethylamino)salicylaldehyde or 2-hydroxy-1-naphthaldehyde in place of 2-hydroxy-3-methoxy-5-methylbenzaldehyde and afterward directly used for the synthesis of complexes 3–8.
Synthesis of [Ni2(L1)2(μ1,1-N3)2] (1)
Ni(NO3)2·6H2O (145 mg, 0.5 mmol), dissolved in 10 mL of methanol, was added to a 20 mL of methanol solution of Schiff base HL1 (0.5 mmol) with stirring. A 10 mL methanol solution of sodium azide (NaN3) (0.5 mmol, 33 mg) was added to the reaction mixture with constant starring. The resulting dark-green solution was thereafter allowed to reflux for ca. 30 min. The reaction mixture was then filtered and kept at ambient temperature for slow evaporation. Crops of dark-green crystals, suitable for X-ray diffraction study, were developed over a period of one week. The crystals were filtered off and washed with cold methanol and dried in air to obtain analytically pure complex 1. Yield: 175 mg (86%). Anal. calcd for C34H56N12Ni2O4: C 50.15%, H 6.93%, N 20.64%. Found: C 50.34%, H 7.04%, N 20.47%. Selected FTIR bands (cm−1): 1621 s (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); 2051 s (
N); 2051 s ( ); 3161 w (νN–H); 1400–1610 cm−1 (νC
); 3161 w (νN–H); 1400–1610 cm−1 (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C).
C).
Synthesis of [Ni2(L1)2(μ1,1-NCO)2] (2)
Complex 2 was prepared adopting a very similar method as described for 1 with only exception that NaNCO was used in place of NaN3. Colour: dark-green. Yield: 141 mg (69%). Anal. calcd for C36H56N8Ni2O6: C 53.10%, H 6.93%, N 13.76%. Found: C 53.24%, H 7.08%, N 13.52%. Selected FTIR bands (cm−1): 1617 s (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); 2166 s (νNCO−); 3155 w (νN–H); 1405–1615 cm−1 (νC
N); 2166 s (νNCO−); 3155 w (νN–H); 1405–1615 cm−1 (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C).
C).
Synthesis of [Ni2(L2)2(μ1,1-N3)2]·2CH3CN (3)
Complex 3 was prepared applying a very similar methodology as described for 1, but ligand HL2 was used instead of ligand HL1 and a methanol/acetonitrile (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) solvent mixture was used for the synthesis. Colour: dark-green. Yield: 180 mg (83%). Anal. calcd for C36H58N14Ni2O4: C 49.80%, H 6.73%, N 22.58%. Found: C 49.94%, H 6.88%, N 22.37%. Selected FTIR bands (cm−1): 1620 s (νC
1, v/v) solvent mixture was used for the synthesis. Colour: dark-green. Yield: 180 mg (83%). Anal. calcd for C36H58N14Ni2O4: C 49.80%, H 6.73%, N 22.58%. Found: C 49.94%, H 6.88%, N 22.37%. Selected FTIR bands (cm−1): 1620 s (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); 2052 s (
N); 2052 s ( ); 3163 w (νN–H); 1402–1610 cm−1 (νC
); 3163 w (νN–H); 1402–1610 cm−1 (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C).
C).
Synthesis of [Ni2(L2)2(μ1,1-NCO)2] (4)
Complex 4 was synthesised adopting the same methodology applied for the synthesis of 2, but ligand HL2 was used instead of ligand HL1. Colour: dark-green. Yield: 138 mg (70%). Anal. calcd for C34H52N8Ni2O6: C 51.94%, H 6.67%, N 14.25%. Found: C 52.05%, H 6.69%, N 14.11%. Found: C 53.24%, H 7.08%, N 13.52%. Selected FTIR bands (cm−1): 1624 s (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); 2171 s (νNCO−); 3165 w (νN–H); 1410–1615 cm−1 (νC
N); 2171 s (νNCO−); 3165 w (νN–H); 1410–1615 cm−1 (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C).
C).
Synthesis of [Ni2(L3)2(μ1,1-NCO)2] (5)
Complex 5 was synthesised following an identical methodology that applied for the synthesis of 2, but ligand HL3 was used instead of ligand HL1. Colour: dark-green. Yield: 155 mg (76%). Anal. calcd for C32H46N10Ni2O8: C 47.09%, H 5.68%, N 17.16%. Found: C 47.24%, H 5.78%, N 17.00%. Found: C 53.24%, H 7.08%, N 13.52%. Selected FTIR bands (cm−1): 1301 s and 1594 m (νNO2); 1624 m (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); 2173 s (νNCO−); 3211 w (νN–H); 1405–1610 cm−1 (νC
N); 2173 s (νNCO−); 3211 w (νN–H); 1405–1610 cm−1 (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C).
C).
Synthesis of [Ni2(L4)2(μ1,1-NCO)2] (6)
Complex 6 was prepared following an identical procedure as described for 2 with only exception that ligand HL4 was used in place of ligand HL1. Colour: dark-green. Yield: 194 mg (82%). Anal. calcd for C68H100Br4N16Ni4O12: C 43.26%, H 5.34%, N 11.87%. Found: C 43.01%, H 5.38%, N 11.72%. Selected FTIR bands (cm−1): 1621 s (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); 2172 s (νNCO−); 3172 w (νN–H); 1400–1605 cm−1 (νC
N); 2172 s (νNCO−); 3172 w (νN–H); 1400–1605 cm−1 (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C).
C).
Synthesis of [Ni2(L5)2(μ1,1-NCO)2] (7)
Complex 7 was prepared applying a very similar methodology as described for 2, but ligand HL5 was used instead of ligand HL1. Colour: dark-green. Yield: 122 mg (59%). Anal. calcd for C40H52N8Ni2O4: C 58.14%, H 6.34%, N 13.56%. Found: C 57.81%, H 6.49%, N 13.37%. Selected FTIR bands (cm−1): 1584 s (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); 2172 s (νNCO−); 3134 w (νN–H); 1410–1620 cm−1 (νC
N); 2172 s (νNCO−); 3134 w (νN–H); 1410–1620 cm−1 (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C).
C).
Synthesis of [Ni2(L6)2(μ1,1-NCO)2] (8)
Complex 8 was synthesised adopting the same methodology applied for the synthesis of 2, but ligand HL6 was used instead of ligand HL1. Colour: dark-green. Yield: 132 mg (61%). Anal. calcd for C40H66N10Ni2O4: C 55.32%, H 7.66%, N 16.13%. Found: C 55.24%, H 7.89%, N 15.81%. Selected FTIR bands (cm−1): 1613 s (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); 2173 s (νNCO−); 3166 w (νN–H); 1400–1600 cm−1 (νC
N); 2173 s (νNCO−); 3166 w (νN–H); 1400–1600 cm−1 (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C).
C).
X-ray crystallography
Single crystal X-ray diffraction data of complexes 1–8 were collected on a Bruker Kappa Apex-II CCD diffractometer equipped with a fine focus sealed tube of monochromated Mo-Ka radiation (λ = 0.71073 Å). The collected data frames were processed with SAINT-plus programme, and finally the intensity data were corrected by applying multi-scan absorption correction method implemented in SADABS programme.29 The structures were solved by the direct method (SHELXS) and refined by full-matrix least squares technique based on F2 using SHELX-2018 programme.30 All the non-hydrogen atoms were refined with anisotropic thermal parameters. Whereas hydrogen atoms attached to carbon atoms were included in geometrically idealised positions and refined with a fixed thermal parameter (1.2 or 1.5 times) with respect to their parent atoms, the secondary amine protons were located on the difference Fourier map and refined freely. Other details of crystallographic data and structure refinement parameters are placed in Table 1. Crystal structures were generated through the programme MERCURY-4.3.1 and POV-ray software.
Table 1 Crystallographic data and structural refinement parameters of 1–8
| |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
| Empirical formula |
C34H56N12Ni2O4 |
C36H56N8Ni2O6 |
C36H58N14Ni2O4 |
C34H52N8Ni2O6 |
C32H46N10Ni2O8 |
C68H100Br4N16Ni4O12 |
C40H52N8Ni2O4 |
C40H66N10Ni2O4 |
| Formula weight (g mol−1) |
814.32 |
814.30 |
868.38 |
786.25 |
816.21 |
1888.11 |
826.31 |
868.44 |
| Temperature (K) |
150 |
150 |
298 |
150 |
150 |
150 |
150 |
150 |
| Crystal system |
Monoclinic |
Monoclinic |
Monoclinic |
Monoclinic |
Monoclinic |
Monoclinic |
Monoclinic |
Monoclinic |
| Space group |
P21/n |
P21/c |
P21/c |
P21/c |
P21/c |
P21/c |
P21/c |
P21/n |
| a (Å) |
14.9397(9) |
11.0841(5) |
11.8236(3) |
10.4277(8) |
11.489(4) |
22.299(4) |
9.2184(7) |
14.1830(7) |
| b (Å) |
16.9091(9) |
14.7030(7) |
18.2885(4) |
19.5733(14) |
15.3326(10) |
19.031(3) |
18.3850(12) |
10.8572(5) |
| c (Å) |
15.1735(9) |
11.4961(5) |
10.8047(2) |
18.0962(13) |
10.892(4) |
9.0106(17) |
11.5306(7) |
15.1649(7) |
| α (deg) |
90 |
90 |
90 |
90 |
90 |
90 |
90 |
90 |
| β (deg) |
91.850(2) |
95.432(2) |
115.053(1) |
95.491(2) |
108.741(11) |
93.165(7) |
96.090(3) |
112.574(2) |
| γ (deg) |
90 |
90 |
90 |
90 |
90 |
90 |
90 |
90 |
| Volume (Å3) |
3831.1(4) |
1865.10(15) |
2116.55(8) |
3676.6(5) |
1764.8(10) |
3818.1(12) |
1943.2(2) |
2085.1(3) |
| Z |
4 |
4 |
4 |
4 |
2 |
2 |
2 |
2 |
| Dcalc (g cm−3) |
1.412 |
1.450 |
1.363 |
1.420 |
1.536 |
1.642 |
1.142 |
1.338 |
| μ (mm−1) |
1.037 |
1.066 |
0.944 |
1.079 |
1.132 |
3.134 |
1.021 |
0.924 |
| F(000) |
1728 |
864 |
920 |
1664 |
856 |
1936 |
872 |
928 |
| θ range (deg) |
1.804–27.132 |
1.846–26.393 |
1.90–27.60 |
2.221–29.123 |
1.872–28.122 |
1.829–26.679 |
2.222–29.186 |
2.374–27.152 |
| Reflections collected |
37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 738 738 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 195 195 |
34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 956 956 |
35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 838 838 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 536 536 |
95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 335 335 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 183 183 |
4829 |
| Independent reflections (Rint) |
8448 (0.0603) |
3821 (0.0722) |
4895 (0.0303) |
9861 (0.0757) |
4254 (0.0621) |
7898 (0.0678) |
5231 (0.0468) |
4829 (0.0600) |
| Observed reflections [I > 2σ(I)] |
6049 |
2669 |
4171 |
6450 |
3282 |
6651 |
3912 |
4113 |
| Restraints/parameters |
1/485 |
1/253 |
0/260 |
0/476 |
0/241 |
0/483 |
0/250 |
0/262 |
| Goodness-of-fit on F2 |
1.004 |
1.021 |
1.050 |
1.038 |
1.022 |
1.161 |
1.089 |
1.045 |
| Final R indices [I > 2σ(I)] |
R1 = 0.0423 |
R1 = 0.0391 |
R1 = 0.0286 |
R1 = 0.0456 |
R1 = 0.0384 |
R1 = 0.0484 |
R1 = 0.0453 |
R1 = 0.0334 |
| wR2 = 0.0994 |
wR2 = 0.0805 |
wR2 = 0.0705 |
wR2 = 0.0913 |
wR2 = 0.0755 |
wR2 = 0.1323 |
wR2 = 0.0881 |
wR2 = 0.0823 |
| R Indices (all data) |
R1 = 0.0708 |
R1 = 0.0719 |
R1 = 0.0366 |
R1 = 0.0848 |
R1 = 0.0582 |
R1 = 0.0602 |
R1 = 0.0702 |
R1 = 0.0454 |
| wR2 = 0.1123 |
wR2 = 0.0935 |
wR2 = 0.0738 |
wR2 = 0.1080 |
wR2 = 0.0822 |
wR2 = 0.1380 |
wR2 = 0.0966 |
wR2 = 0.0878 |
| Largest diff. peak/hole (e Å−3) |
0.967/−0.562 |
0.384/−0.386 |
0.490/–0.222 |
0.837/–0.625 |
0.383/–0.460 |
1.178/−1.035 |
0.388/−0.342 |
0.342/−0.386 |
Results and discussion
Syntheses and general characterization
Tetradentate {N3O}-donor Schiff base ligands (HL1–HL6) were synthesised by condensation reaction of N,N-dimethyldipropylenetriamine with 2-hydroxy-3-methoxy-5-methylbenzaldehyde, 2-hydroxy-3-methoxybenzaldehyde, 5-nitrosalicylaldehyde, 5-bromo-3-methoxysalicylaldehyde, 4-(diethylamino)salicylaldehyde or 2-hydroxy-1-naphthaldehyde in methanol, respectively (Scheme 1). Thereafter, eight new dark-green binuclear pseudohalide-bridged complexes, [Ni2(L1)2(μ1,1-N3)2] (1), [Ni2(L1)2(μ1,1-NCO)2] (2), [Ni2(L2)2(μ1,1-N3)2]·2CH3CN (3), [Ni2(L2)2(μ1,1-NCO)2] (4), [Ni2(L3)2(μ1,1-NCO)2] (5), [Ni2(L4)2(μ1,1-NCO)2] (6), [Ni2(L5)2(μ1,1-NCO)2] (7) and [Ni2(L6)2(μ1,1-NCO)2] (8) were resynthesized in high yield through one-pot reaction of in situ generated Schiff base ligand, Ni(NO3)2·6H2O and pseudohalide salts in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio in presence of triethylamine base in methanol or methanol/acetonitrile under reflux. All the compounds were routinely characterised by elemental analysis and IR spectroscopy.
1 molar ratio in presence of triethylamine base in methanol or methanol/acetonitrile under reflux. All the compounds were routinely characterised by elemental analysis and IR spectroscopy.
The IR spectra of all the complexes (Fig. S1†) disclose characteristic azomethine C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching bond of the Schiff base ligands in the range 1584–1624 cm−1. All of them also consist of N–H stretching band of the secondary amine group of the Schiff base ligands in the range 3134–3211 cm−1. Most importantly, the IR spectra of complexes 1 and 3 display a very strong absorption band at around 2052 cm−1 which is attributed to the asymmetric stretching frequency of azide ion, which is consistent with EO azide-bridged NiII systems. Similarly, IR bands for the asymmetric stretching vibrations of EO cyanate bridges in complexes 2, 4–8 were observed in the range 2166–2173 cm−1, which are usual for EO cyanate-bridges. Moreover, broadening/bifurcation of the stretching band of cyanate group in 2 and 6 is consistent with the presence of two crystallographically independent complex molecules (vide infra). The stretching frequency in the range of 1400–1620 cm−1 are assigned to phenyl (C
N stretching bond of the Schiff base ligands in the range 1584–1624 cm−1. All of them also consist of N–H stretching band of the secondary amine group of the Schiff base ligands in the range 3134–3211 cm−1. Most importantly, the IR spectra of complexes 1 and 3 display a very strong absorption band at around 2052 cm−1 which is attributed to the asymmetric stretching frequency of azide ion, which is consistent with EO azide-bridged NiII systems. Similarly, IR bands for the asymmetric stretching vibrations of EO cyanate bridges in complexes 2, 4–8 were observed in the range 2166–2173 cm−1, which are usual for EO cyanate-bridges. Moreover, broadening/bifurcation of the stretching band of cyanate group in 2 and 6 is consistent with the presence of two crystallographically independent complex molecules (vide infra). The stretching frequency in the range of 1400–1620 cm−1 are assigned to phenyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) ring stretching for all the complexes. Additional bands at 1301 and 1594 cm−1 in 5 have been assigned to symmetric and asymmetric stretching vibrations of nitro group of the Schiff base ligand, respectively.
C) ring stretching for all the complexes. Additional bands at 1301 and 1594 cm−1 in 5 have been assigned to symmetric and asymmetric stretching vibrations of nitro group of the Schiff base ligand, respectively.
Description of crystal structures
X-ray crystallography reveals that all the complexes are isostructural with formulas [Ni2(L1)2(μ1,1-N3)2] (1), [Ni2(L1)2(μ1,1-NCO)2] (2), [Ni2(L2)2(μ1,1-N3)2]·2CH3CN (3), [Ni2(L2)2(μ1,1-NCO)2] (4), [Ni2(L3)2(μ1,1-NCO)2] (5), [Ni2(L4)2(μ1,1-NCO)2] (6), [Ni2(L5)2(μ1,1-NCO)2] (7) and [Ni2(L6)2(μ1,1-NCO)2] (8), and they crystallized uniformly in the monoclinic unit cell but in either space group P21/n (1 and 7) or P21/c (2−6 and 8). The structures of all the complexes are eventually similar in that the neutral dinuclear complex molecules are constructed by doubly EO bridged N3− ions in 1 and 3 (Fig. 1) and NCO− ions in 2 and 4−8 (Fig. 2). Whereas a full set of dinuclear complex molecule in 1 and two halves of the crystallographically independent centro-symmetrical dinuclear complex molecules in 2 and 6 are present in the asymmetric units, only a half of the centro-symmetrical dinuclear complex molecule constitutes the asymmetric units of 4, 5, 7 and 8. Additionally, only in 3, the complex molecules crystallized with acetonitrile solvent molecules. However, in all the complexes, the NiII centres are hexa-coordinate with a distorted octahedral geometry in which each of the NiII ion is surrounded by three N donor atoms and one phenolate-O atom of the deprotonated Schiff base ligands and two N-atoms either from the EO bridging azide ions (in 1 and 3) or cyanate ions (in 2 and 4–8). The EO bridging pseudohalide ions share the common edges, leading to the edged-sharing bioctahedral structures (see Fig. 1 and 2). In all these complexes, three N-atoms (imine, secondary amine and tertiary amine N-atoms) from the triamine part of the deprotonated Schiff base ligands coordinate the NiII centres in a facial configuration. The Ni–N bond lengths of imine, secondary amine and tertiary amine of the Schiff base ligands span in the range 2.046(2) to 2.239(2) Å in 1–8, which are comparable to those found in closely related NiII complexes reported in the literature (Table 2).17–23,31–51 The central {NiN2Ni} core in 1 and 3 is planar with the metal–metal separation and the Ni–N–Ni bridging angles are 3.355(1) and 3.374(1) Å, and 101.00(8)°–101.99(8)°, respectively. Similarly, the metal–metal separations (3.327(1)–3.235(1) Å) in the central {NiN2Ni} core structures in 2–8 are very similar as that found in 1 and 3, but the Ni–N–Ni bridging angles in the range 96.79(9)–99.87(8)° are slightly lower than that found in 1 and 3. The bridging region is slightly asymmetric as can be seen from the Ni–N bond distances of 2.120(2)–2.148(1) (shorter) and 2.197(2)–2.212(2) Å (longer) of the bridging azide ions in 1 and 3 and two sets the Ni–N bond distances (2.091(4)–2.134(2) Å and 2.221(4)–2.2650(18) Å) of the bridging cyanate ions in 2–8. Invariably, in these complexes, secondary amine hydrogen atom establishes moderately strong intramolecular hydrogen bonding interactions with the phenolate oxygen atom within the dinuclear unit with the donor–acceptor distances varying in the range 2.914–3.099 Å, to provide the solid-state stability of these compounds. Additional stability in the solid state is appeared from the different weak intermolecular interactions, such as C–H⋯N/O, π⋯π and C–H⋯π in these complexes (Fig. S2–S9†).
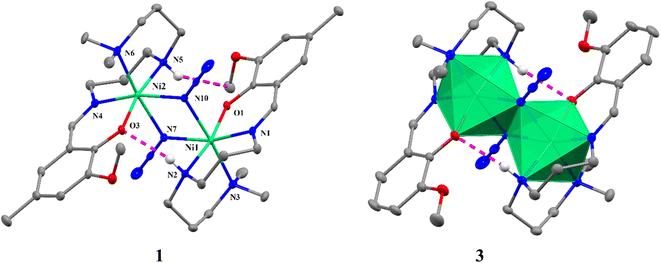 |
| | Fig. 1 Crystal structures of doubly μ1,1-azido-bridged dinuclear NiII complexes 1 and 3 showing selected atom numbering scheme (left) and local polyhedral around NiII centre (right). Ellipsoids are drawn at the 30% probability level. The intramolecular H-bonds are shown as dashed lines. | |
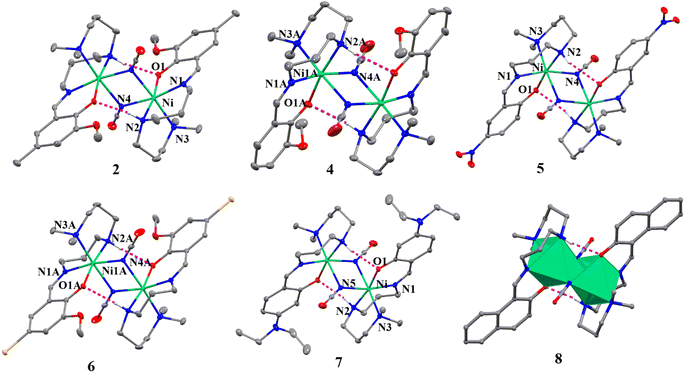 |
| | Fig. 2 Crystal structures of dinuclear NiII complexes 2 and 4–8 with selected atom numbering scheme. Ellipsoids are drawn at the 30% probability level. Hydrogen atoms are omitted for clarity of the picture. The noncovalent interactions are shown as dashed lines. | |
Table 2 Selected bond lengths in Å for 1–8
| Bond |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
| NiII–N (imine) |
2.050(2) |
2.068(2) |
2.060(1) |
2.059(2) |
2.077(2) |
2.075(4) |
2.059(2) |
2.052(2) |
| 2.046(2) |
|
|
2.066(2) |
|
2.059(4) |
|
|
| NiII–N (secondary amine) |
2.109(2) |
2.106(2) |
2.123(1) |
2.117(2) |
2.102(2) |
2.124(4) |
2.122(2) |
2.113(2) |
| 2.109(2) |
|
|
2.107(2) |
|
2.117(4) |
|
|
| NiII–N (tertiary amine) |
2.198(2) |
2.233(2) |
2.148(1) |
2.239(2) |
2.205(2) |
2.225(4) |
2.225(2) |
2.202(2) |
| 2.202(2) |
|
|
2.189(2) |
|
2.227(4) |
|
|
| NiII–O (phenolate) |
2.002(2) |
1.992(2) |
2.013(1) |
2.001(2) |
2.069(2) |
2.020(3) |
2.003(1) |
2.003(2) |
| 2.005(2) |
|
|
2.001(2) |
|
2.011(3) |
|
|
| NiII–N (pseudohalide) |
2.136(2) |
2.134(2) |
2.148(1) |
2.114(2) |
2.101(2) |
2.118(4) |
2.114(2) |
2.112(2) |
| 2.212(2) |
2.223(2) |
2.209(1) |
2.248(2) |
2.243(2) |
2.221(4) |
2.265(2) |
2.247(2) |
| 2.197(2) |
|
|
2.115(2) |
|
2.091(4) |
|
|
| 2.120(2) |
|
|
2.240(2) |
|
2.235(4) |
|
|
| Ni⋯Ni |
3.355(1) |
3.258(1) |
3.374(1) |
3.286(1) |
3.327(1) |
3.299(1) |
3.292(1) |
3.292(1) |
| |
|
|
3.298(1) |
|
3.235(1) |
|
|
| Ni–N–Ni bridging angle |
101.00(8) |
96.79(9) |
101.48(5) |
98.23(8) |
99.87(8) |
99.0(2) |
97.43(7) |
98.04(7) |
| 101.99(8) |
|
|
97.93(8) |
|
96.8(2) |
|
|
Magnetic studies
The temperature dependence of direct current (dc) magnetic susceptibility measurements was carried out on crystalline samples of 1–8 in the temperature range 2–300 K under a constant applied dc field of 10 kOe and the magnetic data were plotted as a function of χT vs. T (Fig. 3 and 4; χ being the molar magnetic susceptibility per dimer), while the molar magnetization as a function of applied dc magnetic field (M/μB per f.u. vs. H) for these complexes are depicted in Fig. S10.† At room temperature, the χT values are in most cases slightly higher than the theoretical value of 2.0 emu mol−1 K for two non-interacting NiII ions with S = 1 and g = 2, consistent with the presence of spin–orbit coupling in the NiII ion. As the temperature is lowered, the χT products increase continuously with decreasing temperature to attain maxima in all the cases and thereafter decrease sharply until 2 K. The magnetic behaviour of these samples is typical for an intramolecular ferromagnetic interaction between two NiII ions together with the influence of intermolecular interactions and/or zero-field splitting (ZFS) of ground state (S = 2), particularly at lower temperature. The saturation magnetization values of about 4 M/μB per f.u. also suggest the ferromagnetic interaction between the NiII centres, leading to the S = 2 ground state in these systems. Therefore, the magnetic susceptibility data can nicely be modelled with a full-matrix diagonalisation approach assuming an isotropic spin exchange Hamiltonian (−2JŜ1·Ŝ2), Zeeman interaction (gNi, average) and zero-field splitting (D)/intermolecular interaction (zJ′) to extract the different numerical parameters using the PHI programme.52 The best fittings of the magnetic data yielded J = +36.8(5) cm−1, gNi = 2.14(1), and D = +7.79(4) cm−1 for complex 1 and J = +34.48(2) cm−1, gNi = 2.07(1) and D = +13.61(1) cm−1 for complex 3, while the numerical parameters for complexes 2 and 4−8 are assembled in Table 3.
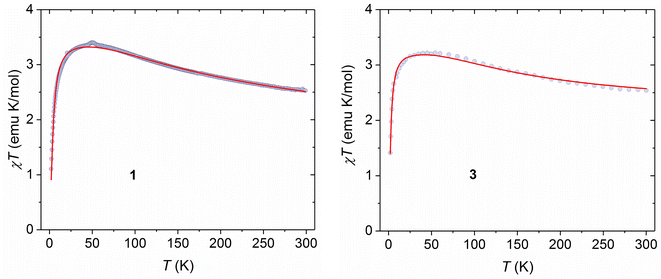 |
| | Fig. 3 Temperature dependence of χT products measured at 10 kOe dc field for 1 and 3. Symbols and solid lines represent experimental and best fitted data. | |
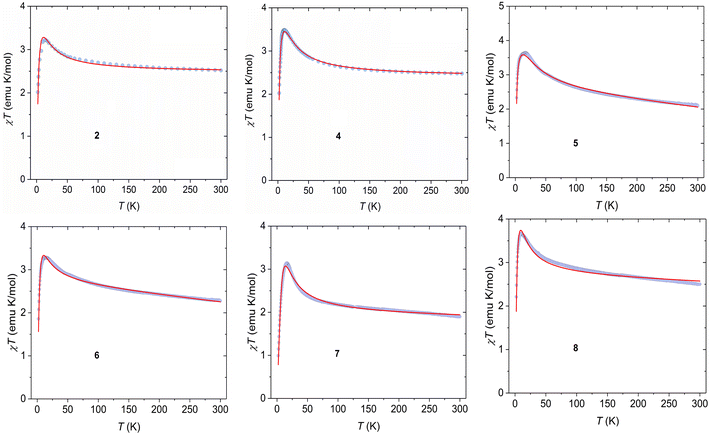 |
| | Fig. 4 Temperature dependence of χT products measured at 10 kOe dc field for 2 and 4–8. Symbols and solid lines represent experimental and best fitted data. | |
Table 3 Selected structural and magnetic data of doubly end-on cyanato bridged NiII dimers (following Ĥ = −2JŜ1·Ŝ2 convention)a
| Compounds |
J (cm−1) |
D (cm−1) |
g |
M–N (Å) |
M–N–M (°) |
Dihedral angles (°) between two Ni1–Ni2–Nb–Xt planesb |
Ref. |
| L9 = [N,N′-bis(2-pyridinylmethylene)]propane-1,3-diamine; L10 = N,N′-bis(pyridin-2-yl)benzylidene]ethane-1,2-diamine; terpy = terpyridine; L10, L11 and L12 = tetradentate N3O donor Schiff base ligands. As defined in the text and average values are reported. |
| 2 |
+4.17(8) |
+9.11(2) |
2.22(1) |
2.134, 2.223 |
96.79(9) |
158.05 |
This work |
| 4 |
+5.75(3) |
+10.80(2) |
2.18(1) |
2.114, 2.248 2.115, 2.240 |
98.23(8) |
174.77 |
This work |
| 97.93(8) |
162.72 |
| 5 |
+7.80(9) |
+2.00(3) |
2.238(2) |
2.101, 2.243 |
99.87(8) |
168.46 |
This work |
| 6 |
+9.1(1) |
+2.84(2) |
2.141(2) |
2.118, 2.221 2.091, 2.235 |
99.0(2) |
165.25 |
This work |
| 96.8(2) |
177.81 |
| 7 |
+4.6(2) |
+7.63(7) |
1.973(6) |
2.114, 2.265 |
97.43(7) |
178.98 |
This work |
| 8 |
+4.7(2) |
+2.34(2) |
2.315(7) |
2.112, 2.247 |
98.04(7) |
174.55 |
This work |
| [Ni2(L9)2(μ1,1-NCO)2](ClO4)2 |
+1.7 |
+3.6 |
2.23 |
2.125, 2.090 |
94.4, 95.8 |
177.86 |
19 |
| [Ni2(L10)2(μ1,1-NCO)2](ClO4)2 |
+2.8 |
+3.6 |
2.23 |
2.099, 2.115 |
95.33, 95.79 |
175.41 |
19 |
| [Ni2(terpy)2(μ1,1-NCO)2(H2O)2](PF6)2 |
+4.59 |
−12.32 |
2.23 |
2.044, 2.195 |
97.7 |
175.10 |
20 |
| [Ni2(L11)2(μ1,1-NCO)2] |
+6.85 |
+7.18 |
2.19 |
2.107, 2.244 2.115, 2.280 |
97.84, 98.74 |
171.68 |
21 |
| [Ni2(L12)2(μ1,1-NCO)2] |
+5.31 |
+5.00 |
2.19 |
2.225, 2.201 |
98.47 |
172.73 |
22 |
| [Ni2(L13)2(μ1,1-NCO)2] |
+3.70 |
+8.05 |
2.28 |
2.119, 2.205 |
97.45, 97.84 |
172.94 |
23 |
It is now well studied that the sign and magnitude of the exchange interaction (J) among the paramagnetic centres is governed by several structural parameters in which the bridging angle plays the most important role in determining the sign and strength of magnetic interactions. The extensive theoretical works by Ruiz et al.24 showed that the doubly μ1,1-azido-bridged dinuclear NiII systems experience ferromagnetic interactions through the bridging Ni–N–Ni angles (θ) studied in between 80° and 115° in which J value increases with increasing angles and reaches the maximum at around 104°, and thereafter decreases with increasing θ. Interestingly, all the reported μ1,1-azido-bridged dinuclear NiII systems exhibit ferromagnetic interactions with only one exception where a very low bridging angle of 90.4° mediates the antiferromagnetic interaction between NiII centres.37 The Ni–N–Ni bridging angles found in 1 and 3 are comparable (101.00(8)° and 101.99(8)° for 1 and 101.48(5)° for 3), and are close to the angle where the strong ferromagnetic interaction is expected between two NiII ions as shown in the theoretical study,24,53 and thus moderately strong J values (36.8(5) and 34.48(2) cm−1 for 1 and 3, respectively) found in these systems are in good agreement with the theoretical calculations. However, unlike theoretical prediction, a large number of magnetically characterized μ1,1-azido-bridged dinuclear NiII systems do not display a clear relationship of J values with the Ni–N–Ni bridging angles as a small variation of the Ni–N–Ni angles in a narrow range of 98°–103.9° display a wide range of J values from 1.91 to 43.9 cm−1.31–51 This can be further confirmed by the fact that the present bridging angles of 101.00(8)° and 101.99(8) ° for 1 and 101.48(5)° for 3 are comparable to the value of 101.0° in [Ni2(L7)2(μ1,1-N3)2(N3)2] (L7 = a tridentate quinoline-based ligand)40 and 101.79° (average angle) in [Ni2(L8)2(N3)2(μ1,1-N3)2]·CH3OH (L8 = N,N-dimethyl-N′-(pyrid-2-ylmethyl)-ethylenediamine),54 yet they display significantly different J values of 6.1 and 1.91 cm−1, respectively. In contrast, the magnetic coupling constants of the present systems are nicely matched with other reported compound [Ni(pepci)(μ1,1-N3)2]2 (where pepci = N′-(2-pyridin-2-ylethyl)pyridine-2-carbaldimine)47 that displayed very similar bridging angle of 101.6° with J value of 36.3 cm−1. Moreover, the present J values are significantly different from our previous report with similar ligand system, derived from the condensation of salicylaldehyde and N,N-dimethyldipropylenetriamine, in which J value and average bridging angle are found to be 10 cm−1 and 101.35°, respectively.
In contrast to extensively rich magneto-structurally characterized μ1,1-N3 bridged NiII systems, only few doubly μ1,1-NCO bridged dinuclear NiII complexes are available in the literature.19–23 Therefore, the addition of six new examples in this series enriches the field of doubly μ1,1-NCO bridged dinuclear NiII compounds. As can be seen from Table 3 that the magnetic coupling constants are weak compared to azide-bridged systems, which is because of the presence of polarizable π systems and two different N and O donor atoms in cyanate ion, rendering to be a poor magnetic coupler in comparison to azide analogue. The magnetic and relevant crystallographic data of all doubly μ1,1-NCO bridged dinuclear NiII systems as given in Table 3 show the variation of J values in a narrow range of 1.7–9.1 cm−1.19–23 Remarkably, unlike azide-bridged systems, the cyanate analogues follow a general trend in which J value increases with increase in bridging angles (Fig. 5, left), similar to theoretical prediction that has been conducted by Ruiz et al. for azide analogues.24 The possibility of more precise correlation cannot be ruled out if simultaneous influence of different structural parameters take into account in correlation studies. For example, other than the bridging angle, the deviation of the bridging azide or cyanate ligand from Ni2N2 plane could influence the magnetic interaction between the paramagnetic centres as with increase in deviation from Ni2N2 plane orbitals overlap is expected to be reduced, leading to a weaker magnetic coupling via super-exchange mechanism. Although a great correlation is not observed when experimental J values are plotted as a function of Ni1–Ni2–Nb–Xt dihedral angles (Nb and Xt are bridging N and terminal N or O atom of bridging ligands, respectively) (Fig. 5, right), the increasing trend of J values with dihedral angles could not be ruled out, further confirming that the simultaneous influence of different structural parameters are involved in magnetic communication.
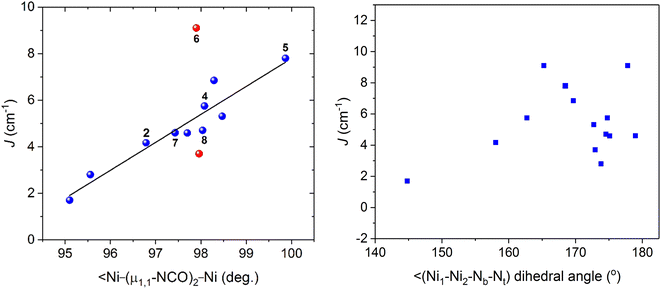 |
| | Fig. 5 Magneto-structural correlation data showing dependence of J values with Ni–(μ1,1-NCO)2–Ni bridging angles. The red symbols indicates that these data have been excluded from this correlation. The data have been taken from Table 3 and the data of the present work are denoted by numbers. | |
Theoretical calculations
The variation of the magnetic interaction in presented cyanate and azide bridged NiII complexes motivated us to investigate these molecular systems thoroughly by the Density Functional Theory (DFT) using freely available ORCA 5.0 software.55 The isotropic exchange interaction J is usually calculated with the help of the broken-symmetry DFT calculations by comparing the energies of the high-spin (HS,↑↑) and broken-symmetry spin states (BS, ↑↓), Δ = εBS − εHS. The Δ-parameter is then used to calculate J with the help of Ruiz56 or Yamaguchi57 formulas:
| JR = Δ/[(S1 + S2)(S1 + S2 + 1)] |
herein, we decided to apply two DFT functionals, namely well-known B3LYP hybrid functional58 and M06-2X hybrid meta-GGA functional.59 First one provided good performance for many polynuclear 3d-metal complexes,60 while the second functional was benchmarked as one of the best for thiocyanate and cyanate double bridged binuclear NiII complexes.61 All calculations were performed with ZORA relativistic approximation62 and relativistic analogues of Ahlrichs basis sets,63 ZORA-def2-TZVP for Ni, ZORA-def2-TZVP(-f) for all non-metals except for C and H for which ZORA-def2-SVP was used. First, dinuclear molecular fragments of 1–8 were extracted from experimental X-ray data while the positions of the hydrogen atoms were normalized with the help of Mercury software.64 The respective results of DFT calculations are presented in Fig. 6 and Table S1† as JRB3LYP, JYB3LYP, JRM062X and JYM062X. It is evident that the best match with the experimental J-parameters were achieved for JRB3LYP and JYM062X and both approaches provided similar results as is also depicted in Fig. 6 (right) for Ni–Nb–Ni angle dependence, where Nb is the bridging atom of N3 or NCO ligand.
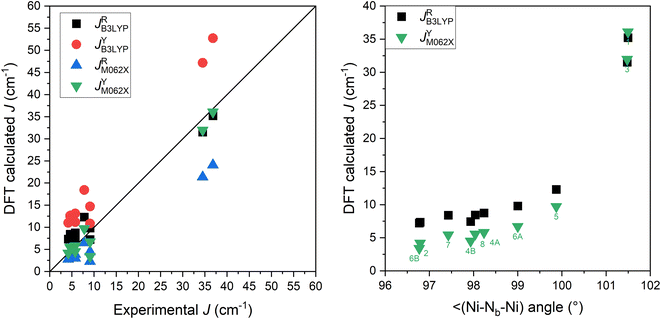 |
| | Fig. 6 The comparison of DFT calculated and experimental values of J in complexes 1–8 (left). The Ni–Nb–Ni angle dependence of selected DFT J-values: JRB3LYP and JYM062X (right). | |
As discussed in previous section, the Ni–Nb–Ni angle is not unique parameter to evaluate the magnetic interactions in these dinuclear complexes. Indeed, the search in the Cambridge Crystallographic Database (CSD)65 revealed not only large deviation of Ni–Nb–Ni angle in azido and cyanate bridged complexes, but also the significant span of dihedral angles defined as Ni1–Ni2–Nb-Xt, where Xt is terminal atom of pseudohalide ligand (Xt is N for azide and O for cyanate) – Fig. S11 and S12.† This inspired us to further explore the magneto-structural correlation in simple model compounds [Ni2(μ1,1-N3)2(NH3)8]2+ and [Ni2(μ1,1-NCO)2(NH3)8]2+ – Fig. 7. The good performance of M06-2X functional motivated us to use it both for geometry optimization and calculations of JYM062X. First, we studied dependence of J on Ni–Nb–Ni angle ranging from 75 to 115° without restricting dihedral angles (Fig. 8 left, empty symbols).
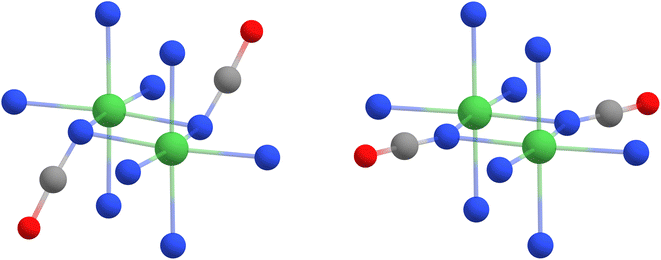 |
| | Fig. 7 The comparison of DFT geometries using M06-2X functional obtained for model complex [Ni2(μ1,1-NCO)2(NH3)8]2+ for Ni–Nb–Ni bond angles equal to 100° (left) and similar calculation in which Ni1–Ni2–Nb–Xt dihedral angles were fixed to 180° (right). The nickel atoms are green, nitrogen atoms are blue, carbon atoms are grey, and the hydrogen atoms were omitted for clarity. | |
 |
| | Fig. 8 Magneto-structural correlation for model complexes [Ni2(μ1,1-N3)2(NH3)8]2+ and [Ni2(μ1,1-NCO)2(NH3)8]2+ calculated with M06-2X functional. | |
It was found that dihedral angles Ni1–Ni2–Nb–Xt can reach values down to ∼100° (the optimized molecular geometries are provided as XYZ coordinates in ESI†). These calculations showed that strongest ferromagnetic exchange is provided for Ni–Nb–Ni angles close to 100° and that stronger ferromagnetic exchange is induced by N3 ligand. For Ni–Nb–Ni angles below ∼90°, the antiferromagnetic exchange is expected. Second, the dihedral angles Ni1–Ni2–Nb–Xt were fixed to value 180° and again Ni–Nb–Ni angle was varied similarly to previous case. Interestingly, it led to dramatic threefold increase of the ferromagnetic exchange for N3-bridged complexes reaching value up to J = 28 cm−1 for Ni–Nb–Ni angle equal to 100°. In case of NCO-bridged complexes twofold increase was observed (Fig. 8 left, full symbols). Finally, the impact of dihedral angle is demonstrated in Fig. 8 (right), where the computational protocol was applied for fixed Ni–Nb–Ni = 100° and variation of dihedral angle resulted in consequential changes of the isotropic exchange.
Conclusion
We have synthesized and structurally characterized two new doubly μ1,1-N3 bridged and six new doubly μ1,1-NCO bridged NiII complexes with the six different N3O donor Schiff base ligands. All these neutral complex molecules are isostructural and construct edge sharing bioctahedral structures. Magnetic studies revealed that all these complexes exhibit ferromagnetic interaction through bridging pseudohalides with ferromagnetic coupling constant J being significantly higher for azide-bridged complexes than that of the cyanate analogues, which is consistent with the literature reported data and also the presence of polarizable π systems and two different N and O donor atoms in cyanate ion, rendering it to be a poor magnetic coupler in comparison to azide analogues. Although, the magneto-structurally characterized doubly μ1,1-N3 bridged NiII complexes are abundant, only few such complexes with doubly μ1,1-NCO bridged systems are known in the literature. Interestingly, inclusion of these six new examples in this ever-growing series of doubly μ1,1-NCO bridged systems gives us an opportunity to analyse the precise magneto-structural correlation in this system, showing a general trend in which J value increases with increase in bridging angles, similar to theoretical prediction that has been conducted by Ruiz et al. for azide analogues. Therefore, the unprecedented high degree of structural and magnetic resemblances by inclusion of six new examples in this series is the major achievement of the present study. Moreover, very detailed DFT study fully confirmed that N3-ligand is prominent to NCO-ligand in promoting ferromagnetic exchange and that accurate magneto-structural correlation of such NiII dinuclear complexes is two-dimensional, based on Ni–Nb–Ni bond angles and Ni1–Ni2–Nb–Xt dihedral angles.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
A. P. gratefully acknowledges the financial support of this work by the Government of West Bengal through the Department of Science & Technology and Biotechnology, Kolkata, India (Sanction No. 331/ST/P/S&T/15G-8/2018, dated-19.06.2019). P. B. gratefully acknowledges the financial support of this work by the project CICECO-Aveiro Institute of Materials, UIDB/50011/2020, UIDP/50011/2020 & LA/P/0006/2020, financed by national funds through the FCT/MCTES (PIDDAC). R. H. acknowledges the financial support from the institutional sources of the Department of Inorganic Chemistry, Palacký University Olomouc, Czech Republic.
References
- G. A. Craig and M. Murrie, Chem. Soc. Rev., 2015, 44, 2135–2147 RSC.
- G. E. Kostakis, A. M. Ako and A. K. Powell, Chem. Soc. Rev., 2010, 39, 2238–2271 RSC.
- M. Andruh, Chem. Commun., 2018, 54, 3559–3577 RSC.
- M. Mondal, S. Ghosh, S. Maity, S. Giri and A. Ghosh, Inorg. Chem. Front., 2020, 7, 247–259 RSC.
- X.-Y. Wang, Z.-M. Wang and S. Gao, Chem. Commun., 2008, 281–294 RSC.
- M. Sarkar, R. Clérac, C. Mathonière, N. G. R. Hearns, V. Bertolasi and D. Ray, Inorg. Chem., 2011, 50, 3922–3933 CrossRef CAS PubMed.
- K. Aoki, K. Otsubo, Y. Yoshida, Y. Kimura, K. Sugimoto and H. Kitagawa, Inorg. Chem., 2021, 60, 16029–16034 CrossRef CAS PubMed.
- N. C. Jana, Z. Jagličić, P. Brandão, S. Adak, A. Saha and A. Panja, New J. Chem., 2021, 45, 7602–7613 RSC.
- S. Khan, T. Dutta, M. Cortijo, R. González-Prieto, M. G. B. Drew, R. M. Gomila, A. Frontera and S. Chattopadhyay, CrystEngComm, 2021, 23, 1942–1952 RSC.
-
(a) S. Sasmal, S. Hazra, P. Kundu, S. Dutta, G. Rajaraman, E. C. Sañudo and S. Mohanta, Inorg. Chem., 2011, 50, 7257–7267 CrossRef CAS PubMed;
(b) P. Bhunia, S. Maity, J. Mayans and A. Ghosh, New J. Chem., 2022, 46, 4363–4372 RSC;
(c) H. S. Jena, S. Goswami, S. Sanda, S. Parshamoni, S. Biswas and S. Konar, Dalton Trans., 2014, 43, 16996–16999 RSC.
- A. Escuer, J. Esteban, S. P. Perlepes and T. C. Stamatatos, Coord. Chem. Rev., 2014, 275, 87–129 CrossRef CAS.
- L. Botana, J. Ruiz, A. J. Mota, A. Rodríguez-Diéguez, J. M. Seco, I. Oyarzabal and E. Colacio, Dalton Trans., 2014, 43, 13509–13524 RSC.
- R. Boča, Coord. Chem. Rev., 2004, 248, 757–815 CrossRef.
- J. Miklovič, D. Valigura, R. Boča and J. Titiš, Dalton Trans., 2015, 44, 12484–12487 RSC.
- R. Maurice, Inorg. Chem., 2021, 60, 6306–6318 CrossRef CAS PubMed.
- B. Drahoš and R. Herchel, Dalton Trans., 2022, 51, 18033–18044 RSC.
- A. Escuer, R. Vicente, M. S. E. Fallah, X. Solans and M. Font-Bardia, J. Chem. Soc., Dalton Trans., 1996, 1013 RSC.
- Z. Mahendrasinh, S. Ankita, S. B. Kumar, A. Escuer and E. Suresh, Inorg. Chim. Acta, 2011, 375, 333–337 CrossRef CAS.
- M. Habib, T. K. Karmakar, G. Aromí, J. Ribas-Ariño, H.-K. Fun, S. Chantrapromma and S. K. Chandra, Inorg. Chem., 2008, 47, 4109–4117 CrossRef CAS PubMed.
- M. I. Arriortua, R. Cortes, J. L. Mesa, L. Lezama, T. Rojo and G. Villeneuve, Transition Met. Chem., 1988, 13, 371–374 CrossRef CAS.
- A. Panja, N. C. Jana, S. Adak, P. Brandão, L. Dlháň, J. Titiš and R. Boča, New J. Chem., 2017, 41, 3143–3153 RSC.
- M. Patra, P. Brandão, A. P. Pikul, S. Adak and A. Panja, ChemistrySelect, 2020, 5, 12924–12931 CrossRef CAS.
- S. Adak, Y.-C. Sun, N. C. Jana, P. Brandão, X.-Y. Wang and A. Panja, CrystEngComm, 2021, 23, 3371–3382 RSC.
- E. Ruiz, J. Cano, S. Alvarez and P. Alemany, J. Am. Chem. Soc., 1998, 120, 11122–11129 CrossRef CAS.
- F. F. de Biani, E. Ruiz, J. Cano, J. J. Novoa and S. Alvarez, Inorg. Chem., 2000, 39, 3221–3229 CrossRef PubMed.
-
(a) P. S. Perlepe, L. Cunha-Silva, K. J. Gagnon, S. J. Teat, C. Lampropoulos, A. Escuer and T. C. Stamatatos, Inorg. Chem., 2016, 55, 1270–1277 CrossRef CAS PubMed;
(b) M. K. Mahish, L. M. Carrella, A. Patra, D. Saren, E. Zangrando, P. Vojtíšek, E. Rentschler and S. C. Manna, New J. Chem., 2022, 46, 16899–16906 RSC;
(c) A. Panja, S. Adak, P. Brandão, Ľ. Dlháň and R. Boča, Eur. J. Inorg. Chem., 2020, 2362–2371 CrossRef CAS.
- A. Panja, Z. Jagličić, R. Herchel, P. Brandão, K. Pramanik and N. C. Jana, New J. Chem., 2022, 46, 5627–5637 RSC.
- A. Panja, Z. Jagličić, R. Herchel, P. Brandão, K. Pramanik and N. C. Jana, New J. Chem., 2022, 46, 13546–13557 RSC.
- G. M. Sheldrick, SADABS, University of Göttingen, Germany, 1996 Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112 CrossRef CAS PubMed.
- S. S. Massoud, F. R. Louka, Y. K. Obaid, R. Vicente, J. Ribas, R. C. Fischerc and F. A. Mautner, Dalton Trans., 2013, 42, 3968–3978 RSC.
- M. G. Barandika, R. Cortés, L. Lezama, M. K. Urtiaga, M. I. Arriortua and T. Rojo, J. Chem. Soc., Dalton Trans., 1999, 2971–2976 RSC.
- P. Mukherjee, M. G. B. Drew, C. J. Gómez-García and A. Ghosh, Inorg. Chem., 2009, 48, 5848–5860 CrossRef CAS PubMed.
- S. K. Dey, N. Mondal, M. S. E. Fallah, R. Vicente, A. Escuer, X. Solans, M. Font-Bardía, T. Matsushita, V. Gramlich and S. Mitra, Inorg. Chem., 2004, 43, 2427–2434 CrossRef CAS PubMed.
- S. Sarkar, A. Mondal, M. S. E. Fallah, J. Ribas, D. Chopra, H. Stoeckli-Evans and K. K. Rajak, Polyhedron, 2006, 25, 25–30 CrossRef CAS.
- S. Nandi, D. Bannerjee, J.-S. Wu, T.-H. Lu, A. M. Z. Slawin, J. D. Woollins, J. Ribas and C. Sinha, Eur. J. Inorg. Chem., 2009, 3972–3981 CrossRef CAS.
- P. Chaudhuri, R. Wagner, S. Khanra and T. Weyhermüller, Dalton Trans., 2006, 4962–4968 RSC.
- A. Bhattacharyya, P. K. Bhaumik, M. Das, A. Bauzá, P. P. Jana, K. Harms, A. Frontera and S. Chattopadhyay, Polyhedron, 2015, 101, 257–269 CrossRef CAS.
- M. Č. Romanović, B. R. Čobeljić, A. Pevec, I. Turel, V. Spasojević, A. A. Tsaturyan, I. N. Shcherbakov, K. K. Anđelković, M. Milenković, D. Radanović and M. R. Milenković, Polyhedron, 2017, 128, 30–37 CrossRef.
- P. Ghorai, P. Brandão, S. Benmansour, C. J. Gómez García and A. Saha, Polyhedron, 2020, 188, 114708 CrossRef CAS.
- P. P. Chakrabarty, S. Giri, D. Schollmeyer, H. Sakiyama, M. Mikuriya, A. Sarkar and S. Saha, Polyhedron, 2015, 89, 49–54 CrossRef.
- H.-Z. Kou, S. Hishiya and O. Sato, Inorg. Chim. Acta, 2008, 361, 2396–2406 CrossRef CAS.
- S. Sarkar, A. Mondal, A. Banerjee, D. Chopra, J. Ribas and K. K. Rajak, Polyhedron, 2006, 25, 2284–2288 CrossRef CAS.
- S. Sarkar, A. Datta, A. Mondal, D. Chopra, J. Ribas, K. K. Rajak, S. M. Sairam and S. K Pati, J. Phys. Chem. B, 2006, 110, 12–15 CrossRef CAS PubMed.
- X.-J. Lin, Z. Shen, Y. Song, H.-J. Xu, Y.-Z. Li and X.-Z. You, Inorg. Chim. Acta, 2005, 358, 1963–1969 CrossRef CAS.
- S. Sain, S. Bid, A. Usman, H.-K. Fun, G. Aromíc, X. Solans and S. K. Chandra, Inorg. Chim. Acta, 2005, 358, 3362–3368 CrossRef CAS.
- R. Cortes, J. I. Ruiz de Larramendi, L. Lezama, T. Rojo, K. Urtiaga and M. I. Arriortua, J. Chem. Soc., Dalton trans., 1992, 2723–2728 RSC.
- R. Vicente, A. Escuer, J. Ribas, M. S. E. Fallah, X. Solans and M. Font-Bardia, Inorg. Chem., 1993, 32, 1920–1924 CrossRef CAS.
- J. Ribas, M. Monfort, C. Diaz, C. Bastos and X. Solans, Inorg. Chem., 1994, 33, 484–489 CrossRef CAS.
- A. Escuer, R. Vicente, J. Ribas and X. Solans, Inorg. Chem., 1995, 34, 1793–1798 CrossRef CAS.
- A. Donmez, G. Oylumluoglu, M. B. Coban, C. Kocak, M. Aygun and H. Kara, J. Mol. Struct., 2017, 1149, 569–575 CrossRef CAS.
- N. F. Chilton, R. P. Anderson, L. D. Turner, A. Soncini and K. S. Murray, J. Comput. Chem., 2013, 34, 1164–1175 CrossRef CAS PubMed.
- G. Manca, J. Cano and E. Ruiz, Inorg. Chem., 2009, 48, 3139–3144 CrossRef CAS PubMed.
- H.-D. Bian, W. Gu, Q. Yu, S.-P. Yan, D.-Z. Liao, Z.-H. Jiang and P. Cheng, Polyhedron, 2005, 24, 2002–2008 CrossRef CAS.
- F. Neese, Wiley Interdiscip.
Rev.: Comput. Mol. Sci., 2022, 12, e1606 Search PubMed.
- E. Ruiz, J. Cano, S. Alvarez and P. Alemany, J. Comput. Chem., 1999, 20, 1391–1400 CrossRef CAS.
- T. Soda, Y. Kitagawa, T. Onishi, Y. Takano, Y. Shigeta, H. Nagao, Y. Yoshioka and K. Yamaguchi, Chem. Phys. Lett., 2000, 319, 223–230 CrossRef CAS.
-
(a) A. D. Becke, Phys. Rev. A, 1988, 38, 3098–3100 CrossRef CAS PubMed;
(b) C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785–789 CrossRef CAS PubMed;
(c) P. J. Stephens, F. J. Devlin, C. F. Chabalowski and M. J. Frisch, J. Phys. Chem., 2002, 98, 11623–11627 CrossRef.
- Y. Zhao and D. G. Truhlar, Theor. Chem. Acc., 2008, 120, 215–241 Search PubMed.
-
(a) L. Havlíček, R. Herchel, I. Nemec and P. Neugebauer, Polyhedron, 2022, 223, 115962 CrossRef;
(b) A. Bhanja, L. Smythe, R. Herchel, I. Nemec, M. Murrie and D. Ray, Dalton Trans., 2021, 50, 5023–5035 RSC;
(c) B. Rybníčková, J. Kuchár, P. Antal and R. Herchel, Inorganica Chim. Acta, 2020, 509, 119689 CrossRef;
(d) R. Herchel, I. Nemec, M. Machata and Z. Trávníček, Inorg. Chem., 2015, 54, 8625–8638 CrossRef CAS PubMed.
- M. Zlatar, F. Vlahović, D. Mitić, M. Zlatović and M. Gruden, J. Serbian Chem. Soc., 2020, 85, 1577–1590 CrossRef CAS.
- C. van Wüllen, J. Chem. Phys., 1998, 109, 392–399 CrossRef.
- F. Weigend and R. Ahlrichs, Phys. Chem. Chem. Phys., 2005, 7, 3297–3305 RSC.
- C. F. Macrae, I. Sovago, S. J. Cottrell, P. T. A. Galek, P. McCabe, E. Pidcock, M. Platings, G. P. Shields, J. S. Stevens, M. Towler and P. A. Wood, J. Appl. Crystallogr., 2020, 53, 226–235 CrossRef CAS PubMed.
- C. R. Groom, I. J. Bruno, M. P. Lightfoot and S. C. Ward, Acta. Crystallogr. B. Struct., 2016, 72, 171–179 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1–S12, Table S1† and XYZ coordinated of model complexes used for DFT calculations. CCDC 2238074–2238081 for complexes 1–8. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3ra00737e. |
|
| This journal is © The Royal Society of Chemistry 2023 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article a,
Marko Jagodič
a,
Marko Jagodič b,
Paula Brandão
b,
Paula Brandão c,
Moumita Patraa,
Radovan Herchel
c,
Moumita Patraa,
Radovan Herchel d,
Zvonko Jagličić
d,
Zvonko Jagličić be and
Anangamohan Panja
be and
Anangamohan Panja *af
*af
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); 2051 s (
N); 2051 s ( ); 3161 w (νN–H); 1400–1610 cm−1 (νC
); 3161 w (νN–H); 1400–1610 cm−1 (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C).
C).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); 2166 s (νNCO−); 3155 w (νN–H); 1405–1615 cm−1 (νC
N); 2166 s (νNCO−); 3155 w (νN–H); 1405–1615 cm−1 (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C).
C).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) solvent mixture was used for the synthesis. Colour: dark-green. Yield: 180 mg (83%). Anal. calcd for C36H58N14Ni2O4: C 49.80%, H 6.73%, N 22.58%. Found: C 49.94%, H 6.88%, N 22.37%. Selected FTIR bands (cm−1): 1620 s (νC
1, v/v) solvent mixture was used for the synthesis. Colour: dark-green. Yield: 180 mg (83%). Anal. calcd for C36H58N14Ni2O4: C 49.80%, H 6.73%, N 22.58%. Found: C 49.94%, H 6.88%, N 22.37%. Selected FTIR bands (cm−1): 1620 s (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); 2052 s (
N); 2052 s ( ); 3163 w (νN–H); 1402–1610 cm−1 (νC
); 3163 w (νN–H); 1402–1610 cm−1 (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C).
C).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); 2171 s (νNCO−); 3165 w (νN–H); 1410–1615 cm−1 (νC
N); 2171 s (νNCO−); 3165 w (νN–H); 1410–1615 cm−1 (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C).
C).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); 2173 s (νNCO−); 3211 w (νN–H); 1405–1610 cm−1 (νC
N); 2173 s (νNCO−); 3211 w (νN–H); 1405–1610 cm−1 (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C).
C).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); 2172 s (νNCO−); 3172 w (νN–H); 1400–1605 cm−1 (νC
N); 2172 s (νNCO−); 3172 w (νN–H); 1400–1605 cm−1 (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C).
C).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); 2172 s (νNCO−); 3134 w (νN–H); 1410–1620 cm−1 (νC
N); 2172 s (νNCO−); 3134 w (νN–H); 1410–1620 cm−1 (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C).
C).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); 2173 s (νNCO−); 3166 w (νN–H); 1400–1600 cm−1 (νC
N); 2173 s (νNCO−); 3166 w (νN–H); 1400–1600 cm−1 (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C).
C).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 738
738![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 195
195![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 956
956![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 838
838![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 536
536![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 335
335![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 183
183![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio in presence of triethylamine base in methanol or methanol/acetonitrile under reflux. All the compounds were routinely characterised by elemental analysis and IR spectroscopy.
1 molar ratio in presence of triethylamine base in methanol or methanol/acetonitrile under reflux. All the compounds were routinely characterised by elemental analysis and IR spectroscopy.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching bond of the Schiff base ligands in the range 1584–1624 cm−1. All of them also consist of N–H stretching band of the secondary amine group of the Schiff base ligands in the range 3134–3211 cm−1. Most importantly, the IR spectra of complexes 1 and 3 display a very strong absorption band at around 2052 cm−1 which is attributed to the asymmetric stretching frequency of azide ion, which is consistent with EO azide-bridged NiII systems. Similarly, IR bands for the asymmetric stretching vibrations of EO cyanate bridges in complexes 2, 4–8 were observed in the range 2166–2173 cm−1, which are usual for EO cyanate-bridges. Moreover, broadening/bifurcation of the stretching band of cyanate group in 2 and 6 is consistent with the presence of two crystallographically independent complex molecules (vide infra). The stretching frequency in the range of 1400–1620 cm−1 are assigned to phenyl (C
N stretching bond of the Schiff base ligands in the range 1584–1624 cm−1. All of them also consist of N–H stretching band of the secondary amine group of the Schiff base ligands in the range 3134–3211 cm−1. Most importantly, the IR spectra of complexes 1 and 3 display a very strong absorption band at around 2052 cm−1 which is attributed to the asymmetric stretching frequency of azide ion, which is consistent with EO azide-bridged NiII systems. Similarly, IR bands for the asymmetric stretching vibrations of EO cyanate bridges in complexes 2, 4–8 were observed in the range 2166–2173 cm−1, which are usual for EO cyanate-bridges. Moreover, broadening/bifurcation of the stretching band of cyanate group in 2 and 6 is consistent with the presence of two crystallographically independent complex molecules (vide infra). The stretching frequency in the range of 1400–1620 cm−1 are assigned to phenyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) ring stretching for all the complexes. Additional bands at 1301 and 1594 cm−1 in 5 have been assigned to symmetric and asymmetric stretching vibrations of nitro group of the Schiff base ligand, respectively.
C) ring stretching for all the complexes. Additional bands at 1301 and 1594 cm−1 in 5 have been assigned to symmetric and asymmetric stretching vibrations of nitro group of the Schiff base ligand, respectively.








