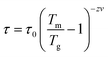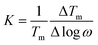Open Access Article
Open Access ArticleMagnetic clusters and ferromagnetic spin glass in the novel hexagonal perovskite 12R-Ba4SbMn3O12†
Xiaohui Yana,
Junkun Wua,
Xiuyun Lei*a,
Lunhua Hebcd,
Wenbin Guo*a,
Xiaojun Kuang ae and
Congling Yin
ae and
Congling Yin *a
*a
aMOE Key Laboratory of New Processing Technology for Nonferrous Metal and Materials, Guangxi Key Laboratory of Optic and Electronic Materials and Devices, College of Materials Science and Engineering, Guilin University of Technology, Guilin 541004, P. R. China. E-mail: xylei@glut.edu.cn; guo0281@163.com; congling.yin@glut.edu.cn
bSpallat. Neutron Source Sci. Ctr, Dongguan 523803, P. R. China
cChinese Acad Sci, Inst Phys, Beijing Natl Lab Condensed Matter Phys, Beijing 100190, P. R. China
dSongshan Lake Mat Lab, Dongguan 523808, P. R. China
eCollege of Chemistry and Bioengineering, Guilin University of Technology, Guilin 541004, P. R. China
First published on 11th April 2023
Abstract
A 12-layer hexagonal perovskite Ba4SbMn3O12 (space group: R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m; a = 5.72733(3) Å, and c = 28.1770(3) Å) has been synthesized by high-temperature solid-state reactions and studied using powder X-ray and neutron diffraction and magnetization measurements. This 12R polytype structure contains one corner-sharing (Sb, Mn)O6 octahedron and a trimer of face-sharing MnO6 octahedra per formula unit. Ba4SbMn3O12 displays a paramagnetic state of the Mn3 magnetic cluster at 100–200 K, which partially disassociates into individual Mn ions at 250–300 K. The ferromagnetic interaction between these Mn3 clusters is mainly mediated by Mn3+ at the M1 site, leading to dynamic ferromagnetic clusters below TD = ∼70 K and ferromagnetic spin freezing transition at Tg = ∼11.5 K. The stability of Mn3 magnetic clusters in the 12R polytypes is related to the intracluster Mn–Mn distance.
m; a = 5.72733(3) Å, and c = 28.1770(3) Å) has been synthesized by high-temperature solid-state reactions and studied using powder X-ray and neutron diffraction and magnetization measurements. This 12R polytype structure contains one corner-sharing (Sb, Mn)O6 octahedron and a trimer of face-sharing MnO6 octahedra per formula unit. Ba4SbMn3O12 displays a paramagnetic state of the Mn3 magnetic cluster at 100–200 K, which partially disassociates into individual Mn ions at 250–300 K. The ferromagnetic interaction between these Mn3 clusters is mainly mediated by Mn3+ at the M1 site, leading to dynamic ferromagnetic clusters below TD = ∼70 K and ferromagnetic spin freezing transition at Tg = ∼11.5 K. The stability of Mn3 magnetic clusters in the 12R polytypes is related to the intracluster Mn–Mn distance.
1 Introduction
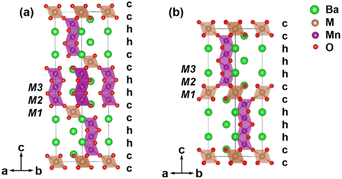
Manganese oxides have been the focus of materials research for many decades owing to their technological applications as heterogeneous catalysts,1 Li-ion batteries,2,3 SOFC electrode materials,4 CMR materials,5 and quantum materials.6,7 This has motivated the investigation of the hexagonal perovskite BaMnO3−δ system, which shows a diversity of crystal structures and magnetic properties.8–12 A series of BaMnO3−δ polytypes (2H → 15R → 8H → 6H → 10H → 4H) have been found as the oxygen vacancy concentration δ increases.8,11 Further perovskite polytypes may be produced when Mn and Ba are replaced by other cations.Many Mn-substituted BaMxMn1−xO3 systems have been investigated, for instance, with M = Ca,13 In,14,15 Sn,16,17 Sb,18 Ti,19 Fe,20 Ru,21 Ir,22 Nb,23 and rare earth Ln.24–26 This led to the discovery of a series of M-cation ordered 12R perovskites Ba4M1+xMn3−xO12 (M = Ti4+ and Sn4+),17,19 Ba4MMn3O11.5 (M = In3+ and Y3+)14,27 and Ba4MMn3O12 (M = Nb5+, Ce4+, and Pr4+).23,26 The 12R perovskite Ba4MMn3O12 contains close-packed BaO3 layers in a (cchh)3 sequence and accommodates the M and Mn ions in the corner-sharing octahedral centre M1 and face-sharing octahedral centres M2 and M3 respectively (Fig. 1a). Partial disorder of M and Mn elements occurs on the M2 site in case of M = Ti4+ and Sn4+,17,19 and oxygen vacancies are present for the M = In3+ and Y3+ case.14,27
Polymorphs of BaMnO3−δ have long-range antiferromagnetic (AFM) order of all the Mn spins, with TN = 220–270 K.10,11 However, the long-range magnetic order is suppressed in the 12R perovskites upon low-level nonmagnetic doping, and the substituted M ions plays a key role in the magnetic behaviour of these materials. Ba4YMn3O11.5,27 Ba4Sn1.1Mn2.9O12 (ref. 17) and Ba4CeMn3O12 (ref. 24) undergo an AFM transition at TN = 4–6 K, while Ba4InMn3O11.5 (ref. 14) and Ba4Ti2Mn2O12 (ref. 19) display no magnetic order, and Ba4NbMn3O12 shows a ferrimagnetic transition at TC = 42 K (ref. 23) (Table S1†). Furthermore, the Nb, Ti and Sn-doped 12R polytype show meta-magnetism, with linear Mn3 magnetic clusters arranged in a triangular lattice, leading to magnetic frustrations.17,23,28 Therefore, the 12R perovskite materials are potential quantum materials, such as quantum spin liquid and spin ice. The exploration of new 12R perovskite BaMxMn1−xO3 is of great concern.
In the BaSb1−xMnxO3 system, two perovskite polymorphs 6H Ba3MnSb2O9 (ref. 29) and 10H Ba5Sb1−xMn4+xO15−δ have been identified. The Ba5Sb1−xMn4+xO15−δ has close-packed BaO3 layers in (cchhh)2 sequences and features tetramers of face-sharing MnO6 octahedra (Fig. 1b).18 Regarding the close relationship between the 10H and 12R polymorphs, one wonders whether the 12R perovskites BaSb1−xMnxO3 can exit. If the 12R BaSb1−xMnxO3 is stabilized, it would be a good material with magnetic clusters and frustration. Motived by such ideas, we started a series of investigations on the BaSb1−xMnxO3 materials. Herein we report the synthesis, crystal structure, and magnetic properties of 12R Ba4SbMn3O12 perovskite.
2 Experimental
2.1 Synthesis
The polycrystalline Ba4SbMn3O12 was synthesized from BaCO3 (99.5%, Aladdin), MnCO3 (99.95%, Aladdin), and Sb2O3 (99.9%, Aladdin) reagents with solid-state reaction methods. These starting materials were thoroughly mixed with alcohol in an agate mortar and pestle and heated in a platinum crucible at 1173 K for 10 hours to decompose the carbonates. The materials were then reground, pressed into pellets (20 ton cm−2), placed on platinum crucibles, and sintered at 1573 K for 36 hours with heating and cooling rates of 5 K min−1. At several intermediate steps, the materials were re-ground and re-pressed, and powder X-ray diffraction (XRD) was performed at each step to follow the reaction to completion.2.2 Characterization
The phase purity of the samples was characterized by XRD using a PANalytical X'Pert Pro powder diffractometer with Cu Kα radiation at 40 kV and 40 mA. High-quality XRD data were collected on the 2θ range 5–120° at room temperature (RT) for Rietveld analysis carried out using the Topas Academic software.30 Bond valence sum (BVS) was calculated using standard parameters with Brown and Altermatt's method.31 Low-temperature XRD data were collected in the 5–298 K range with an interval of 10 K, using a Rigaku SmartLab X-ray diffractometer with Cu Kα radiation at 45 kV and 200 mA.Time of flight (TOF) neutron powder diffraction (NPD) data were collected from a 5 g powder sample of Ba4SbMn3O12 using the General-Purpose Powder Diffractometer (GPPD) at the China Scattering Neutron Source (CSNS, Dongguan, China). Scanning electron microscopy (SEM) imaging and X-ray energy dispersive spectroscopy (EDS) elemental analysis were carried out using a GeminiSEM 300 (ZEISS, Germany) scanning electron microscope with an Ultim Max (Oxford, U.K.) EDS spectrometer.
Magnetic measurements were performed on Quantum Design's MPMS-3 Superconducting Quantum Interference Device (SQUID) magnetometer. Direct-current (dc) magnetic susceptibility was recorded in a magnetic field of 1000 Oe while heating the sample from 2 K to 300 K after zero-field cooling (ZFC) and field cooling (FC). Alternating-current (ac) magnetization was measured with an ac amplitude of μ0hac = 10 Oe at frequencies of 3 ≤ f ≤ 250 Hz in the temperature range 5–15 K. Isothermal field-dependent magnetization was performed at the applied field from −6 to 6 T and temperature of 2 K, 5 K, 15 K, 20 K, 30 K, 40 K, 50 K, 60 K and 100 K.
3 Results and discussions
3.1 Phase formation
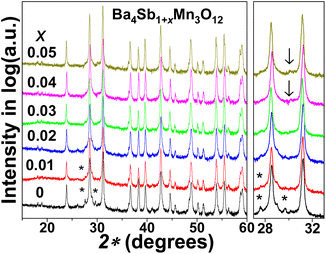
The initial Ba4SbMn3O12 samples consisted of mixed 12R perovskite phase and Mn-rich phases such as 8H BaMnO3−δ (marked with asterisks), due to the loss of antimony oxides during the sintering process (melting point of Sb2O5 and Sb2O3 are ∼655 K and ∼928 K).32 The Mn-rich phase was reduced through extra Sb doping. The 12R perovskite phase was isolated as a single phase at the nominal composition of Ba4Sb1.03Mn3O12, while beyond this Sb doping level other impurity reflections were observed, as marked as arrows in Fig. 2.The chemical composition of the nominal Ba4Sb1.03Mn3O12 sample is most likely to be consistent with the stoichiometric perovskite of Ba4SbMn3O12, which is reasonably supported by the Ba![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sb
Sb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn ratio of 4.00(20)
Mn ratio of 4.00(20)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.01(4)
1.01(4)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.73(12) from the EDS elemental analysis (Fig. 3) and the following Rietveld refinements.
2.73(12) from the EDS elemental analysis (Fig. 3) and the following Rietveld refinements.
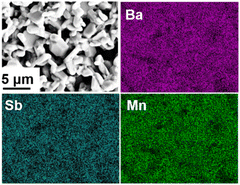 | ||
| Fig. 3 SEM image and EDS elemental mapping image on the Ba4SbMn3O12 sample, indicating the homogeneous distribution of elements. | ||
3.2 Crystal structure
The light oxygen atoms are not well located with X-ray diffraction in the presence of heavy elements such as barium. Neutron diffraction (ND) is more sensitive to oxygen because the neutron scattering length of oxygen (5.083 fm) is comparable to that of Ba (5.07 fm).33 Therefore, to find the accurate oxygen position, neutron diffraction data were collected on the Ba4SbMn3O12 sample. The Rietveld refinements against the XRD and NPD data simultaneously were performed, based on the structural model of 12R Ba4NbMn3O12 (Fig. 1a). During the refinements, the lattice parameters, the atomic coordinates, and the thermal displacement parameters (Beq) were varied freely. Anisotropic peak broadening was described with the Stephen model. In the first step, the occupancies of Sb and Mn at M1, M2, and M3 sites were refined with the total occupancies on each site subject to unity. A negative value of Sb on the M3 site was found, excluding the presence of Sb at the M3 site. In the following refinement, the occupancies of Sb and Mn were refined only at M1 and M2 sites, with the total ratio of Sb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn constrained as 1
Mn constrained as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
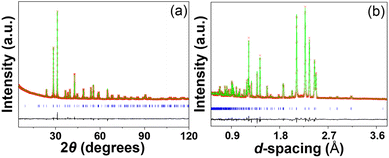
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. In the second step, the oxygen occupancies were refined, and no vacancies were found at the oxygen sites within the refinement error. Therefore, the occupancies at both O1 and O2 sites were set as unity. The final refinement rapidly converged at the Rwp parameters of 5.50% and 4.32% for XRD and NPD data respectively (Fig. 4a and b). The refined structural parameters and selected bond distances and angles are summarized in Tables 1 and 2.
3. In the second step, the oxygen occupancies were refined, and no vacancies were found at the oxygen sites within the refinement error. Therefore, the occupancies at both O1 and O2 sites were set as unity. The final refinement rapidly converged at the Rwp parameters of 5.50% and 4.32% for XRD and NPD data respectively (Fig. 4a and b). The refined structural parameters and selected bond distances and angles are summarized in Tables 1 and 2.
| Atom | Site | x, y, z | Occupancy | Beq | BVS |
|---|---|---|---|---|---|
a a = 5.7273(1) Å, and c = 28.1770(3) Å, space group: R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m. m. |
|||||
| Ba1 | 6c | 0, 0, 0.1288(1) | 1 | 0.1(1) | 2.22 |
| Ba2 | 6c | 0, 0, 0.2860(1) | 1 | 0.7(2) | 2.42 |
| (Sb/Mn)M1 | 3a | 0, 0, 0 | 0.84/0.16(1) | 1.9(1) | 4.94/2.96 |
| (Mn/Sb)M2 | 6c | 0, 0, 0.4110(1) | 0.92/0.08(1) | 0.5(1) | 3.47/5.24 |
| MnM3 | 3b | 0, 0, 0.5 | 1 | 0.5(2) | 3.89 |
| O1 | 18h | 0.4834(1), 0.5165(1), 0.1236(1) | 1 | 0.7(1) | |
| O2 | 18h | 0.4994(2), 0.5006(2), 0.2928(1) | 1 | 1.1(2) | |
| Bond length (Å) | Bond length (Å) | Bond angle (degree) |
|---|---|---|
| (Sb/Mn)M1–O2 (×6) 2.014 (2) | MnM3–O1 (×6) 1.921 (1) | (Sb/Mn)M1–O2–MnM2 177.97 (7) |
| (Mn/Sb)M2–O1 (×3) 1.972 (2) | MnM2–MnM3 2.506 (2) | MnM2–O1–MnM3 80.14 (5) |
| (Mn/Sb)M2–O2 (×3) 1.953 (2) |
In the crystal structure of 12R Ba4SbMn3O12, Sb and Mn atoms are mixed on two crystallographic independent sites M1 and M2, with occupancy of 84% Sb/16% Mn and 8% Sb/92% Mn respectively. The BVS results listed in Table 1 show that the formal Mn charges are segregated, with Sb5+ and Mn3+ (which have similar ionic radii of 0.60 and 0.645 Å (ref. 34) respectively) at the corner-sharing M1 site, mixed Mn3+, Mn4+ and Sb5+ at the M2 site, and Mn4+ at the M3 site. Based on the charge neutrality, this corresponds to the chemical formula Ba4(Sb10.845+Mn10.163+)(Sb20.085+Mn20.423+Mn20.54+)2(Mn34+)O12, where Mn1 corresponds to Mn at site M1, etc. This chemical formula has an average charge of Mn2 of +3.54, essentially identical to the BVS of 3.47.
The cationic distributions in Ba4SbMn3O12 are different from those of the Sn, Y, and Nb-analogue.17,23,27,35 In Ba4Sn1.1Mn2.9O12, Sn4+ and Mn4+ are mixed on the corner-sharing M1 (Sn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn ratio of 0.88
Mn ratio of 0.88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.12) and face-sharing M2 (Sn
0.12) and face-sharing M2 (Sn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn ratio of 0.11
Mn ratio of 0.11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.89) sites.17 In Ba4YMn3O11.5 and Ba4NbMn3O12, the substituted Y3+/Nb5+ and Mn4+(and Mn3+) ions are well separated over the M1 and M2 sites respectively. The structure of Ba4SbMn3O12 contains a higher proportion of magnetic Mn3+ cations (∼16%) at the M1 sites that interconnect the Mn3O12 trimers of face-sharing octahedra, which has a strong impact on the magnetic properties as discussed in the following sections.
0.89) sites.17 In Ba4YMn3O11.5 and Ba4NbMn3O12, the substituted Y3+/Nb5+ and Mn4+(and Mn3+) ions are well separated over the M1 and M2 sites respectively. The structure of Ba4SbMn3O12 contains a higher proportion of magnetic Mn3+ cations (∼16%) at the M1 sites that interconnect the Mn3O12 trimers of face-sharing octahedra, which has a strong impact on the magnetic properties as discussed in the following sections.
It should be noted about the distortion of the outer MnO6 octahedra in the trimers. The MnM2 atom shifts toward the c-BaO3 layers, forming three short Mn–O bonds (∼1.95 Å) and three long ones (∼1.97 Å), while the inner MnM3O6 octahedron has no distortion constrained by its local site symmetry (D3d). This leads to a short Mn–Mn distance dMn–Mn of ∼2.5 Å inside the Mn3O12 trimer, which is comparable with that in the elemental α-Mn.36 This phenomenon could indicate a degree of d-orbital overlap between neighboring Mn ions forming Mn3 magnetic clusters.
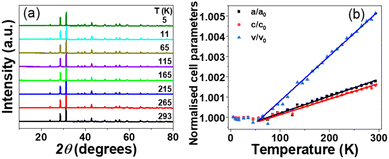
Phase stability of Ba4SbMn3O12 was investigated by low-temperature XRD (Fig. 5a). No phase transition was observed in the range of 5–298 K. The lattice parameters and cell volume keep constant below ∼70 K and increase linearly as temperature increases above 70 K, showing thermal expansion coefficient of αa = 7.9 × 10−6 K−1, αc = 7.0 × 10−6 K−1 and αV = 2.2 × 10−5 K−1.
3.3 Magnetic properties
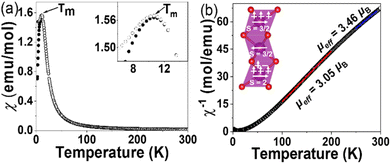
The dc magnetization data for Ba4SbMn3O12 (Fig. 6a) were corrected against two background terms: the susceptibility of the capsule determined as −5.6 × 10−6 emu with a blank measurement, and the diamagnetic susceptibility of Ba4SbMn3O12, which can be estimated as −2.91 × 10−4 emu f.u.−1 (or equally −1.00 × 10−4 emu per mol Mn4+) using Pascal's constant.37 The susceptibilities are nearly constant above 150 K and increase as the temperature goes down, which is typical for localized spin systems. A broad peak in magnetic susceptibilities (χdc) appears at around Tm = 10.5 K, indicating a magnetic phase transition. Ferromagnetic components are present below TC as suggested by the divergent ZFC and FC susceptibilities.The susceptibilities between 100 and 200 K can be well fitted based on the equation χ = C/(T − θ), yielding the Weiss temperature θcw of ∼43.4 K and C ∼ 1.16 emu K mol−1 (Fig. 6b). This corresponds to a paramagnetic moment μeff of ∼3.05μB per mol Mn or ∼5.28μB per mol Mn3 cluster. The former is significantly smaller than the expected value of 4.24μB calculated from spin only Mn3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn4+ moment in the proportion 1
Mn4+ moment in the proportion 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and this contradicts the paramagnetic states of individual Mn spins. The latter value is essentially identical to the expected values of ∼4.89μB, as estimated with the most possible Mn3 cluster of Ba4SbMn3O12 containing one terminal Mn3+ and two Mn4+ ions (Fig. 6b inset). This observation indicates the stabilization of Mn3 magnetic clusters in a paramagnetic state at 100–200 K. Ferro- or ferri-magnetic interactions occur between the Mn3 clusters as indicated by the positive θcw ∼ 43.4 K.
2, and this contradicts the paramagnetic states of individual Mn spins. The latter value is essentially identical to the expected values of ∼4.89μB, as estimated with the most possible Mn3 cluster of Ba4SbMn3O12 containing one terminal Mn3+ and two Mn4+ ions (Fig. 6b inset). This observation indicates the stabilization of Mn3 magnetic clusters in a paramagnetic state at 100–200 K. Ferro- or ferri-magnetic interactions occur between the Mn3 clusters as indicated by the positive θcw ∼ 43.4 K.
The Curie–Weiss fits of magnetic susceptibility at 250–300 K range leads to an μeff = 3.45(1)μB per mol Mn or μeff = 5.98μB per mol Mn3 cluster equally. This result is larger than the expected value of Mn3 clusters and smaller than that of Mn ions. This phenomenon indicates the Mn3 magnetic cluster is partially destroyed, leading to the coexistence of individual Mn ions and Mn3 clusters in this temperature range, although an exclusively paramagnetic state of Mn ions requires a higher temperature.
Similar Mn3 magnetic clusters have been evidenced with different stability in 12R Sn- and Nb-doped analogs.17,23 Compared with that in Ba4SbMn3O12 (dMn–Mn = ∼2.506 Å), the Mn3 cluster in Ba4NbMn3O12 (dMn–Mn = ∼2.469 Å) is more stable without dissociation at RT,23 while the Mn3 cluster in Ba4Sn1.1Mn2.9O12 (dMn–Mn = ∼2.523 Å) is unstable at RT and split into Mn ions.17 The stability of Mn3 clusters can be associated with the intra-cluster dMn–Mn. A smaller Mn–Mn distance would mediate stronger direct exchange interactions via a greater overlap between the t2g orbitals of Mn ions, leading to greater stability of Mn3 clusters in these 12R perovskites.
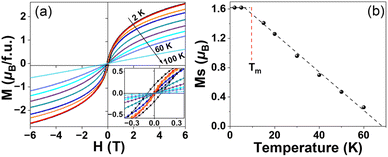
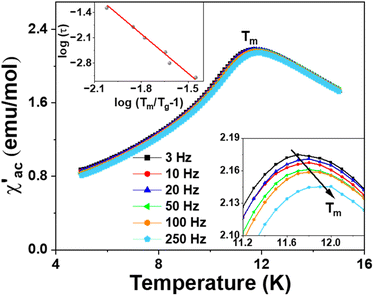
Isothermal M–H data were performed at 2–100 K to study the magnetic ground states of Ba4SbMn3O12 (Fig. 7a). Linear M–H dependence at 100 K is consistent with the paramagnetic states of Mn3 clusters. Magnetic hysteresis and saturation are observed at 2–60 K. In contrast with the isotropic magnetization at 15–60 K, small magnetic anisotropy is observed with a coercive field of 100 mT and 12 mT at 2 K and 5 K respectively. This indicates Ba4SbMn3O12 displays a ferro- or ferri-magnetic state below 60 K. The saturated moment Ms can be estimated with the intercept from the linear M–H fit at the high field range. Being a constant of ∼1.61μB at 2–5 K, the Ms decreases linearly at increasing temperatures above TC and disappears at TD ∼ 70 K from extrapolation (Fig. 7b). Here, TD represents the temperature dynamical ferromagnetic clusters initially form, while TC represents the spin-freezing temperature. This critical linear behavior is different from the saturation behavior in typical long-range ferromagnet SrFe0.5Co0.5O3,38 and indicates the nature of ferromagnetic spin glass.39 The TD/TC of about 6 keeps with those observed in other spin glass systems.40,41
The spin-glass behavior is further studied by the ac susceptibility, and the real (and imaginary) component of the ac susceptibility χ′ (and χ′′) versus temperature at different frequencies is plotted in Fig. 8 (and Fig. S1†). The peak in dc susceptibility at Tm ≈ 10.5 K appears to be a frequency-dependent peak in the ac susceptibilities. The temperature Tm at the broad peak maximum shifts to higher values as the frequency increases (Fig. 8 bottom inset). The frequency dependence of Tm can be well fitted to a critical power law (Fig. 8 top inset),41,42 with the best power-law fit,
Another important factor for characterizing a magnetic glassy transition is the frequency shift K, which gives a relative variation in the peak temperature Tm with the angular frequency and is defined as follows:
The frequency shift K on Ba4SbMn3O12 is estimated to be about 0.016, much smaller than the values for super-paramagnets and close to those observed in spin glasses. This indicates the magnetic state of Ba4SbMn3O12 is a spin glass rather than a super-paramagnet.
The magnetic saturation and frustration blew TC may arise from the partial occupancy of Mn3+ at the M1 sites, as observed in Ba5Sb1−xMn4+xO15−δ systems.18 The dominant magnetic super-exchange interactions between Mn3 magnetic clusters are associated with the near-linear M1–O–M2 connections in the 12R structure type (see Table 2). The hexagonal average lattice symmetry is incompatible with long-range orbital order, but when Mn3+ ions are present at the M1 sites, local orbital order concerning the Mn3+ and Mn4+ ions at the six neighboring M2 sites is likely in such a highly connected manganite network. This gives rise to a mixture of strong ferromagnetic MnM13+–O–MnM24+ and antiferromagnetic MnM13+–O–MnM23+ interactions (both types are found in long-range orbitally ordered and spin-ordered manganites such as La0.5Ca0.5MnO3 and LaMnO3 (ref. 44)).
According to the SbM15+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MnM13+ ratio of ∼5.25, we can assume each Mn3 trimer connects with six Sb5+ or five Sb5+ and one Mn3+ on the adjacent M1 site, while each MnM13+ has six Mn3 trimers as neighbors. As a result, about 96% of Mn3 clusters assemble into isolated superclusters of (Mn3)6Mn3+ via Mn3+ on the M1 site, while minor (∼4%) Mn3 clusters are isolated with six adjacent Sb5+ ions. The local (Mn3)6Mn3+ supercluster possesses three kinds of MnM2–O–MnM13+–O–MnM2 linear bridge related to the charge state of terminal Mn ions. The charge symmetric bridges MnM24+–O–MnM13+–O–MnM24+ and MnM23+–O–MnM13+–O–MnM23+ would mediate a parallel alignment between Mn3 clusters spins, while the charge asymmetric bridge MnM23+–O–MnM13+–O–MnM24+ would lead to an antiparallel alignment. As Sb5+, Mn3+, and Mn4+ mixes on the M2 site at the ratio of 0.08
MnM13+ ratio of ∼5.25, we can assume each Mn3 trimer connects with six Sb5+ or five Sb5+ and one Mn3+ on the adjacent M1 site, while each MnM13+ has six Mn3 trimers as neighbors. As a result, about 96% of Mn3 clusters assemble into isolated superclusters of (Mn3)6Mn3+ via Mn3+ on the M1 site, while minor (∼4%) Mn3 clusters are isolated with six adjacent Sb5+ ions. The local (Mn3)6Mn3+ supercluster possesses three kinds of MnM2–O–MnM13+–O–MnM2 linear bridge related to the charge state of terminal Mn ions. The charge symmetric bridges MnM24+–O–MnM13+–O–MnM24+ and MnM23+–O–MnM13+–O–MnM23+ would mediate a parallel alignment between Mn3 clusters spins, while the charge asymmetric bridge MnM23+–O–MnM13+–O–MnM24+ would lead to an antiparallel alignment. As Sb5+, Mn3+, and Mn4+ mixes on the M2 site at the ratio of 0.08![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.42
0.42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 (see Table 1), the MnM2–O–MnM13+–O–MnM2 bridges in the (Mn3)6Mn3+ supercluster consist of 42.64% charge-symmetric, 42% charge-asymmetric, and 15.36% diamagnetic Sb5+ ions containing ones. Therefore, these charge-symmetric MnM2–O–MnM13+–O–MnM2 bridges would mediate 40.9% of all Mn3 cluster spins frozen in a parallel arrangement in the magnetic ground state of 12R Ba4SbMn3O12, and leads to expected saturated moments of 1.63μB, which is essentially identical with the observed Ms of 1.61μB at 2–5 K.
0.5 (see Table 1), the MnM2–O–MnM13+–O–MnM2 bridges in the (Mn3)6Mn3+ supercluster consist of 42.64% charge-symmetric, 42% charge-asymmetric, and 15.36% diamagnetic Sb5+ ions containing ones. Therefore, these charge-symmetric MnM2–O–MnM13+–O–MnM2 bridges would mediate 40.9% of all Mn3 cluster spins frozen in a parallel arrangement in the magnetic ground state of 12R Ba4SbMn3O12, and leads to expected saturated moments of 1.63μB, which is essentially identical with the observed Ms of 1.61μB at 2–5 K.
The presence of a significant proportion of Mn3+ at the M1 sites in Ba4SbMn3O12 appears to be essential for the pronounced ferromagnetism. This can be indicated by Ba4Sn1.1Mn2.9O12, which hosts Mn4+ and Sn4+ on the M1 site and displays weak ferromagnetism with Ms of ∼0.09μB at 2 K. Compared with the magnetic Mn4 cluster with all spin canceled, the Mn3 cluster has net spins and also contributes to ferromagnetism. This explains the reduced ferromagnetism of 10H Ba5Sb1−xMn4+xO15 with higher content of Mn3+ (∼24–36%) on the M1 site compared with that of 12R Ba4SbMn3O12. Further studies of magnetic order using theoretical calculation will be needed to account more fully for the spin ordering phenomena.
4 Conclusions
The new 12R perovskite Ba4SbMn3O12 has been synthesized by high-temperature solid-state reactions. The material has an ordered arrangement of Sb and Mn cations, and trimers of face-sharing MnO6 octahedra. The paramagnetic state of Ba4SbMn3O12 at 100–200 K can be described with the Mn3 magnetic clusters, which partially disassociate into individual Mn ions at 250–300 K. The stability of Mn3 magnetic clusters in the 12R polytypes is related to the intracluster Mn–Mn distance. The ferromagnetic interaction between these Mn3 clusters is mainly mediated by Mn3+ at the M1 site, leading to dynamic ferromagnetic clusters below TD = ∼70 K and ferromagnetic spin freezing transition at Tg = ∼11.5 K. Ba4SbMn3O12 is another rare example of a periodical lattice that shows cluster magnetism due to the cationic order beyond orbital molecules.45Conflicts of interest
There are no conflicts to declare.Acknowledgements
The National Natural Science Foundation of China (No. 51662013, 22161014, 21850410458), Guangxi Natural Science Foundation (No. 2020GXNSFAA297220, 2019GXNSFGA245006, AD19245097), and the Foundation of Guilin University of Technology (No. GUTQDJJ2018115) are acknowledged for their financial support.Notes and references
- Q. Feng, H. Kanoh and K. Ooi, J. Mater. Chem., 1999, 9, 319–333 RSC.
- P. Strobel and C. Mouget, Mater. Res. Bull., 1993, 28, 93–100 CrossRef CAS.
- A. R. Armstrong and P. G. Bruce, Nature, 1996, 381, 499–500 CrossRef CAS.
- M. Mogensen, K. V. Jensen, M. J. Jorgensen and S. Primdahl, Solid State Ionics, 2002, 150, 123–129 CrossRef CAS.
- Y. Tokura, Rep. Prog. Phys., 2006, 69, 797–851 CrossRef CAS.
- A. J. Browne, A. Krajewska and A. S. Gibbs, J. Mater. Chem. C, 2021, 9, 11640–11654 RSC.
- L. T. Nguyen and R. Cava, Chem. Rev., 2020, 121, 2935–2965 CrossRef PubMed.
- T. Negas and R. S. Roth, J. Solid State Chem., 1971, 3, 323–339 CrossRef CAS.
- E. J. Cussen and P. D. Battle, Chem. Mater., 2000, 12, 831–838 CrossRef CAS.
- J. J. Adkin and M. A. Hayward, J. Solid State Chem., 2006, 179, 70–76 CrossRef CAS.
- J. J. Adkin and M. A. Hayward, Chem. Mater., 2007, 19, 755–762 CrossRef CAS.
- J. Varignon and P. Ghosez, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 87, 140403 CrossRef.
- N. Floros, C. Michel, M. Hervieu and B. Raveau, Chem. Mater., 2000, 12, 3197–3201 CrossRef CAS.
- N. Créon, C. Michel, M. Hervieu, A. Maignan and B. Raveau, Solid State Sci., 2003, 5, 243–248 CrossRef.
- C. L. Yin, G. B. Li, T. N. Jin, L. P. You, J. Tao, J. W. Richardson, C. K. Loong, J. L. Sun, F. H. Liao and J. H. Lin, Chem. Mater., 2008, 20, 2110–2116 CrossRef CAS.
- C. L. Yin, G. B. Li, T. N. Jin, J. Tao, J. W. Richardson, C. K. Loong, F. H. Liao and J. H. Lin, J. Alloys Compd., 2010, 489, 152–156 CrossRef CAS.
- J. Wu, X. Yan, W. Guo, X. Wang, C. Yin and X. Kuang, RSC Adv., 2021, 11, 40235 RSC.
- C. Yin, G. Li, W. A. Kockelmann, F. Liao, J. P. Attfield and J. Lin, Chem. Mater., 2010, 22, 3269–3276 CrossRef CAS.
- G. M. Keith, C. A. Kirk, K. Sarma, N. M. Alford, E. J. Cussen, M. J. Rosseinsky and D. C. Sinclair, Chem. Mater., 2004, 16, 2007–2015 CrossRef CAS.
- L. Miranda, D. C. Sinclair, M. Hernando, A. Varela, A. Wattiaux, K. Boulahya, J. M. Gonzalez-Calbet and M. Parras, Chem. Mater., 2009, 21, 5272–5283 CrossRef CAS.
- C. L. Yin, G. B. Li, W. A. Kockelmann, J. H. Lin and J. P. Attfield, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 80, 094420 CrossRef.
- N. A. Jordan and P. D. Battle, J. Mater. Chem., 2003, 13, 2220–2226 RSC.
- L. T. Nguyen, T. Kong and R. J. Cava, Mater. Res. Express, 2019, 6, 056108 CrossRef CAS.
- M. A. Macias, O. Mentre, C. Pirovano, P. Roussel, S. Colis and G. H. Gauthier, New J. Chem., 2015, 39, 829–835 RSC.
- C. L. Yin, G. F. Tian, G. B. Li, F. H. Liao and J. H. Lin, RSC Adv., 2017, 7, 33869 RSC.
- A. F. Fuentes, K. Boulahya and U. Amador, J. Solid State Chem., 2004, 177, 714–720 CrossRef CAS.
- X. J. Kuang, C. Bridges, M. Allix, J. B. Claridge, H. Hughes and M. J. Rosseinsky, Chem. Mater., 2006, 18, 5130–5136 CrossRef CAS.
- S. V. Streltsov and D. I. Khomskii, JETP Lett., 2018, 108, 686–690 CrossRef CAS.
- Y. S. Doi, Y. Hinatsu and K. Ohoyama, J. Phys.: Condens. Matter, 2004, 16, 8923–8935 CrossRef CAS.
- A. Coelho, Coelho Software, 2012 Search PubMed.
- I. D. Brown and D. Altermatt, Acta Crystallogr., 1985, 41B, 244–247 Search PubMed.
- P. Patnaik, Handbook of Inorganic Chemicals, McGraw-Hill Companies, Inc., New York, 2002 Search PubMed.
- V. F. Sears, J. Neutron Res., 1992, 3, 26–37 CrossRef.
- R. D. Shannon, Acta Crystallogr., 1976, 32A, 751–767 CrossRef.
- X. J. Kuang, H. Zhu, M. Allix, C. A. Bridges, M. J. Rosseinsky and Y. X. Li, J. Mater. Chem., 2012, 22, 8103–8109 RSC.
- J. A. Oberteuffer and J. A. Ibers, Acta Crystallogr., 1970, 26B, 1499–1504 CrossRef.
- G. A. Bain and J. F. Berry, J. Chem. Educ., 2008, 85, 532–536 CrossRef CAS.
- C. Yin, Q. Liu, R. Decourt, M. Pollet, E. Gaudin and O. Toulemonde, J. Solid State Chem., 2011, 184, 3228–3231 CrossRef CAS.
- J. Crangle, Solid State Magnetism, Edward Arnold, 1991 Search PubMed.
- K. Binder and A. P. Young, Rev. Mod. Phys., 1986, 86, 801–963 CrossRef.
- J. A. Mydosh, Spin Glasses: An Experimental Introduction, Taylor & Francis, London, 1993 Search PubMed.
- S. Bedanta and W. Kleemann, J. Phys. D: Appl. Phys., 2009, 42, 013001 CrossRef.
- P. C. Hohenberg and B. I. Halperin, Rev. Mod. Phys., 1977, 49, 435–479 CrossRef CAS.
- E. Dagotto, T. Hotta and A. Moreo, Phys. Rep., 2001, 344, 1–153 CrossRef CAS.
- J. P. Attfield, APL Mater., 2015, 3, 041510 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2212931. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3ra00607g |
| This journal is © The Royal Society of Chemistry 2023 |