Open Access Article
Open Access ArticleAg-embedded manganese oxide octahedral molecular sieve (Ag-OMS-2) nano-rods as efficient heterogeneous catalysts for hydration of nitriles to amides in aqueous solution†
Farimah Mazloom Kalimani and
Alireza Khorshidi *
*
Department of Chemistry, Faculty of Sciences, University of Guilan, 41335-1914, Rasht, Iran. E-mail: khorshidi@guilan.ac.ir; Fax: +98-1333367262; Tel: +98-9113397159
First published on 1st March 2023
Abstract
Silver-embedded manganese oxide octahedral molecular sieve (Ag-OMS-2) nano-rods were synthesized using a pre-incorporation approach, and unambiguously characterized by transmission electron microscopy (TEM), field emission scanning electron microscopy (FESEM), energy dispersive X-ray spectroscopy (EDS), X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), X-ray photoelectron spectroscopy (XPS), and thermogravimetric analysis (TGA). A highly uniform distribution of Ag nanoparticles embedded in the porous structure of OMS-2, was found to be in favor of high catalytic activity of the composite for hydration of nitriles to corresponding amides in aqueous solution. By using a catalyst dosage of 30 mg per mmol of substrate, in the temperature range of 80–100 °C, and reaction times of 4–9 h, excellent yields (73–96%) of the desired amides (13 examples) were obtained. Also, the catalyst was easy to recycle, and showed a slight decrease in efficiency after six consecutive runs.
Introduction
One of the well-known classic reactions in the organic synthesis and chemical industries is hydration of nitriles to their corresponding amides. These are important structural units in the production of pharmaceuticals, plastics, fertilizers, detergents and lubricants.1,2 Traditional catalytic systems were established by employing homogeneous strong acid and base catalysts. Nevertheless, these classic methods have some disadvantages, including over hydrolysis of amides into carboxylic acids, and generation of a massive amount of salts after neutralization of the catalysts.3 It is well known that transition metals can enhance the rate of the hydration step via coordination with the CN bonds.4 In this regard, there are some reports on the activation of nitriles by homogeneous transition-metal complexes including Cu,5,6 Co,7 Mo,8 Rh,9,10 and Ir.11 However, these are associated with some drawbacks, including difficulties in the catalyst separation from the products, use of organic solvents, high cost of ligands, and complexity in recovery and reuse of the catalyst.12–15 The growing interest in developing eco-friendly and practical methods for organic syntheses has drawn attention to heterogeneous transition-metal catalysts in aqueous medium under neutral conditions, which offers benefits such as high activity, ease of handling, and recovery.16–19 In the past decades, several heterogeneous catalysts such as nanorod MnO2, Ru/MnO2, CeO2, and Co3O4 have been shown to exhibit significant catalytic activity toward hydration of nitriles.20–23 Single-atom catalysts (SACs) have received increasing attention in recent years due to having the advantages of both homogeneous and heterogeneous catalysts, such as isolated active sites, stability, and easy separation. This category of catalysts exposes good activity and selectivity in various oxidation, reduction, and coupling reactions.24–26 Although water is an ideal solvent for the hydration of nitriles from environmental and practical points of view, a vast majority of the catalysts are reported in organic media or in the presence of only small amounts of water.15,27–31 Therefore, development of environmentally benign and safe processes is still in the focus of attention. Due to salient features like multivalent Mn species (+2, +3, and +4), high surface area, porous structure, easy release of lattice oxygen, and cheapness, octahedral manganese oxide molecular sieve (cryptomelane, K-OMS-2)32 has been widely used in heterogeneous catalysis,33 energy storage,34 sensors,35 rechargeable batteries,36 and wastewater treatment.37–39 OMS-2 has a one-dimensional 0.46 nm × 0.46 nm tunnel structure, with MnO6 octahedra as basic building units linked by edges or corners, to construct double MnO6 chains.40 Presence of K+ ions and slight amounts of water molecules inside the tunnels, is necessary to balance the charge and support the structure.41 Alkali or transition metal cations can be incorporated into the channels of OMS-2. These metal-modified OMS-2 samples have shown more reactive sites and improved catalytic properties.42Silver is a cheaper precious metal compared to other noble metals (Au and Pt) and has antimicrobial,43–45 optical scattering,46,47 and electrical conductivity48,49 properties. In some cases, Ag has shown a better catalytic oxidation performance than common transition metal oxides.41,50 Indeed, various researchers have studied the hydration of nitriles to amides by Ag NPs. Nevertheless, most of the reactions based on Ag catalysts, are associated with serious drawbacks including high reaction temperatures, need for an inert atmosphere, large amounts of Ag NPs, and catalyst recycling issues.3,16,17,19,50,51 In order to improve the catalytic activity of cryptomelane, K+ ions can be partially replaced by Ag+ during the synthesis.52 In this regard, Yadav et al.53 reported the catalytic performance of Ag doped OMS-2 (Ag-OMS-2) for hydrogenation of nitrobenzene to aniline. The weak acidic and basic sites of the catalyst resulted in the stop of hydrogenation at the azobenzene stage as an intermediate product. Moreover, Dong et al.54 prepared Ag-OMS-2 by various methods and studied the effect of preparation method on the oxidation of ethyl acetate and formaldehyde. Chen et al.42 revealed the high catalytic performance of Ag-OMS-2 nanorods synthesized by a solid-state approach in CO oxidation, and attributed it to the enhanced oxygen activation and CO absorption in the presence of Ag+ in the tunnel structure.
Herein, we report the Ag-embedded OMS-2 nano-rods as a high-performance catalyst for conversion of a broad range of nitriles (homo/hetero aromatic and aliphatic) to primary amides in water as a green solvent.
Experimental
Synthesis of Ag-OMS-2
Silver-embedded manganese oxide octahedral molecular sieve nano-rods (Ag-OMS-2) were prepared according to a previous report with minor modifications.54 In a typical run, 3.66 mmol of silver nitrate was dissolved in a solution of 10.6 mmol of potassium permanganate in 25 mL of deionized water. Meanwhile, a second solution was prepared by adding 1.7 mL of concentrated nitric acid to a solution of 16.4 mmol of manganese sulfate in 8.5 mL of deionized water. The first solution was added dropwise to the second solution under vigorous stirring, and the resulting dark brown suspension was refluxed at 110 °C for 24 h. After filtration, the precipitate was washed with distilled water until neutral pH and dried at 120 °C for 12 h. Then the precipitate was dispersed in 25 mL of distilled water and treated with a freshly prepared solution of NaBH4 (20 mL, 50 mM) for 1 h to ensure complete reduction of silver ions. Finally, the resulting Ag-OMS-2 was filtered and oven-dried at 100 °C overnight.Hydration of nitriles
A round-bottomed flask was charged with Ag-OMS-2 (0.03 g), water (4.0 mL) and nitrile compound (1.0 mmol), and vigorously stirred at 90 °C for 4 h. After completion of the reaction (monitored by TLC (n-hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc 2
EtOAc 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4)), the catalyst was separated by centrifugation and the solution was extracted with dichloromethane (3 × 5 mL). The collected organic phase was dried over anhydrous Na2SO4. The solvent was then evacuated by rotary evaporation and the resulting amides were characterized by 1H-NMR spectra in comparison with authentic samples. Column chromatography on silica gel with dichloromethane as eluent was applied when appropriate.
4)), the catalyst was separated by centrifugation and the solution was extracted with dichloromethane (3 × 5 mL). The collected organic phase was dried over anhydrous Na2SO4. The solvent was then evacuated by rotary evaporation and the resulting amides were characterized by 1H-NMR spectra in comparison with authentic samples. Column chromatography on silica gel with dichloromethane as eluent was applied when appropriate.
Characterizations
Powder X-ray diffraction (XRD) patterns were recorded using a PANalytical X'Pert Pro MPD diffractometer with Cu Kα radiation (λ = 0.15404 nm). Surface morphology of the samples was observed by a scanning electron microscope (SEM) (MIRA3 TESCAN-XMU) equipped with EDX facilities. Transmission electron microscopy (TEM) was performed by using a Philips CM120 instrument. Fourier-transform infrared (FT-IR) spectra were recorded in the range of 400–4000 cm−1 on a Bruker Alpha spectrometer. Oxidation states of the catalyst elements were studied by X-ray photoelectron spectroscopy (XPS), using a Bestec spectrometer with an Al Kα X-ray anode source under a pressure of 10−10 mbar. The charging effect was calibrated by adjusting the binding energy of C 1s to 248.6 eV. Thermogravimetric analysis (TGA) was carried out by a TA SDT-Q600 device to study the thermal stability of the catalyst. The sample was heated from room temperature to 900 °C under flowing N2 (ramp 10 °C min−1).Results and discussion
Characterization of the Ag-OMS-2
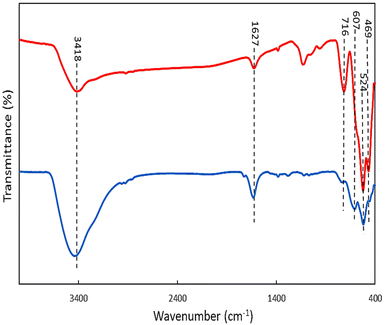
Fig. 1 compares the FTIR spectra of OMS-2 and Ag-OMS-2 samples. Both displayed characteristic bands of the Mn–O vibrations of octahedral MnO6 species in the range of 400–800 cm−1.42,55 Replacement of K+ with Ag+ resulted in a slight change in the intensity and position of the band at 716 cm−1, probably due to the Ag–O–Mn oxygen-bridged bond.41 Also, the absorption band intensity at 1634 cm−1 increased compared to the undoped OMS-2, indicating that more hydroxyl groups were present in the structure, due to the increased surface defects and more sites to absorb water molecules.56Fig. 2 shows the XRD patterns of the synthesized samples. The main diffraction peaks at 2θ 12.2, 17.9, 28.4, 37.2, 41.6, 49.5, and 59.9° corresponding to the (101), (002), (301), (211), (310), (114), and (600) crystal faces, are the characteristic peaks of K-OMS-2 (Fig. 2, middle, JCPDS card number 29-1020).57 After incorporation of silver species, there was no significant change in the tunnel framework of OMS-2 nanorods, and diffraction peaks attributable to hexagonal phase of metallic Ag were appeared at 2θ 38.2, 44.7, 64.9, and 77.7° corresponding to the (111), (311), (220), and (200) planes) (JCPDS card number 04-783).43 However, decrease in the intensity of the peaks in the XRD pattern of Ag-OMS-2 (Fig. 2, up) may address some structural changes upon reduction of Ag+, despite the smaller size of Ag+ (126 pm) compared to K+ (138 pm).33 The standard XRD pattern of Ag is also included for comparison (Fig. 2, bottom).58
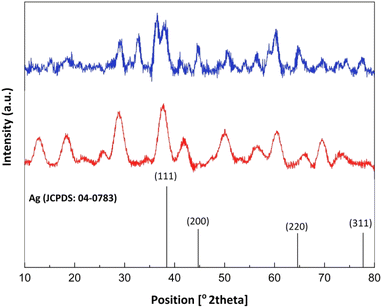 | ||
| Fig. 2 XRD patterns of OMS-2 (up), Ag-OMS-2 (middle) and hexagonal Ag (JCPDS card no. 04-0783, bottom). | ||
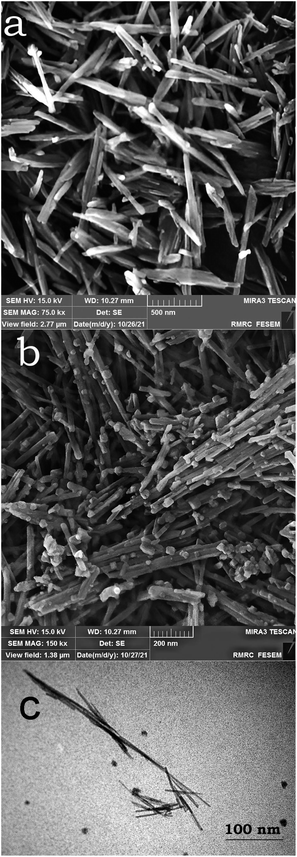
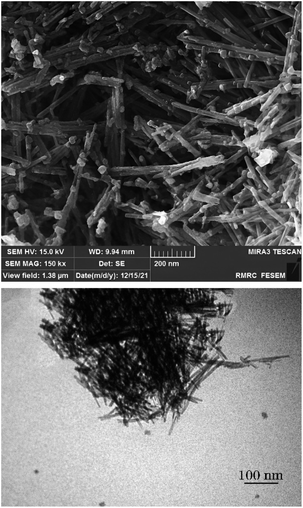
Fig. 3 shows the FESEM image of OMS-2 (a), and FESEM and TEM images of Ag-OMS-2 (b). Both samples exhibited the typical morphology of cryptomelane nanorods. Ag-OMS-2 nanoparticles were appeared as needle-like nanorods ranging from 20 to 30 nm in diameter, and 100 to 300 nm in length. There were no significant structural defects compared to the unmodified OMS-2. The spherical particles ranging from 30 to 50 nm can be attributed to Mn2O3 or very large silver particles.42,54 As a result of incorporation and in situ reduction of Ag+ ions inside the tunnels of OMS-2, it is hard to capture the metallic Ag particles, even with TEM imaging (Fig. 3c).
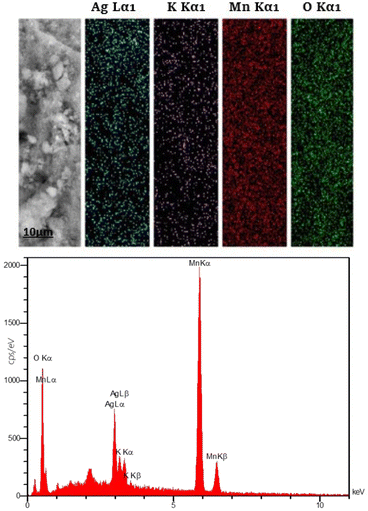
The EDS mapping images of the constituent elements of Ag-OMS-2 including Ag, K, Mn, and O are shown in Fig. 4. Clearly, a uniform dispersion of Ag species in the sampled area is visible. Also, presence of K+ in the modified sample indicates that potassium has not been completely substituted by Ag, and K+ still co-exists in the catalyst structure.
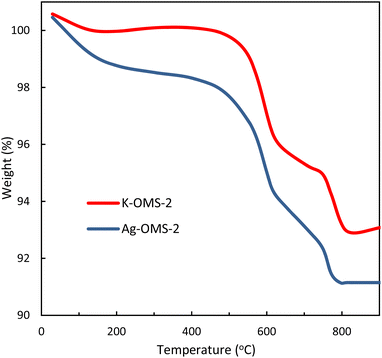
Thermal stability of the K-OMS-2 and Ag-OMS-2 samples were also studied by using TGA technique in the temperature range of 25–900 °C (Fig. 5). Probably, the first weight loss (∼4%) occurred in the range of 100–300 °C is due to the removal of adsorbed water, carbon dioxide, and some physisorbed oxygen.59,60 From the comparison of the two curves, it can be inferred that the amount of water adsorbed in the Ag-OMS-2 tunnels is more than that of the K-OMS-2, as is clearly visible in the first stage. The second gradual weight loss in the range of 300–450 °C is attributed to the chemically sorbed active oxygen species.60,61 Also, a remarkable weight loss (∼4.5%) in the range of 450–650 °C is attributed to the evolution of the structural oxygen in the OMS-2 tunnel structure. The weight loss at temperatures above 650 °C can be attributed to the structural collapse of OMS-2.56,62
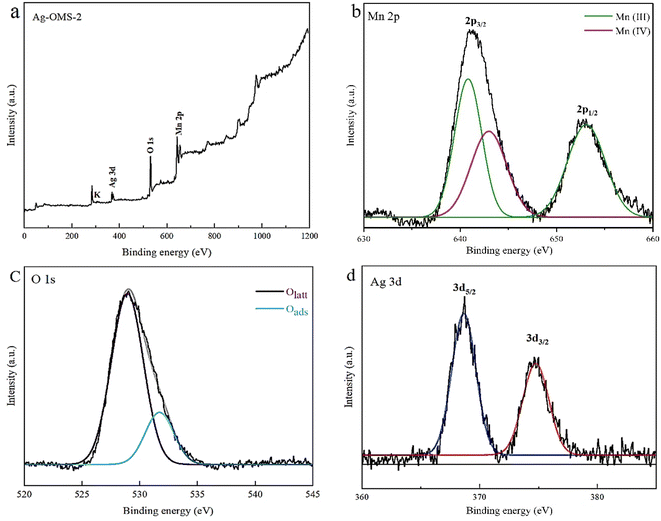
In order to clearly determine the oxidation state of silver in the Ag-OMS-2 sample, XPS analysis was performed. Fig. 6 shows the XPS spectrum of Ag-OMS-2, and clearly consists of the anticipated elements of O, Mn, Ag and K. As shown in Fig. 6b, the broad Mn 2p3/2 band exposes the spin–orbit splitting pair peaks at binding energies of 641.3 and 642.6 eV, which correspond to surface Mn3+ and Mn4+ species, respectively. The existence of mixed valence of Mn in the structure of OMS-2 causes electron transfer. Also, the Mn 2p1/2 peak located at BE = 654 eV is assignable to Mn3+.50 The molar ratio of Mn3+ to Mn4+ in Ag-OMS-2 was 2.84, calculated by CasaXPS software ver. 2.3.25. Fig. 6c shows the O 1s spectrum of the sample, which was fitted with two components. The peak at BE = 529.1 eV is attributed to the lattice oxygen (Olatt) and that at BE = 531 eV is attributed to the surface adsorbed oxygen (Oads).63 In the case of the Ag 3d XPS spectrum, two peaks located at the binding energies of 374.7 and 368.6 eV are ascribable to Ag 3d3/2 and Ag 3d5/2 states. These are in favour of the presence of silver as reduced Ag0 species.54
Catalytic hydration of nitriles using Ag-OMS-2
Hydration of nitriles is one of the most important chemical processes to provide primary amides, which are important precursors in production of raw materials necessary for the chemical and pharmaceutical industries. In our study, hydration of benzonitrile to benzamide was selected as a model reaction to optimize the reaction conditions including temperature, catalyst loading, choice of solvent and reaction time. Initially, it was found that the amount of the catalyst has an important effect on the reaction yield. Increasing the amount of the catalyst from 10 to 30 mg, drastically increased the yield, due to the increase of the available active sites for the substrates to be converted. In the absence of the catalyst, however, no reaction occurred under the abovementioned conditions (Table 1, entries 1–5). When the reaction was carried out in presence of K-OMS-2 as catalyst, the product yield was negligible. This demonstrates a synergistic effect between silver nanoparticles and OMS-2 support (Table 1, entry 6). With regard to the solvent type, it was found that water is the best choice in terms of the reaction time and yield. Fortunately, this is in favour of the green chemistry principals. Protic solvents, including ethanol and methanol, resulted in lower yields than expected, even in companion with water (Table 1, entries 7–10). Aprotic solvents, such as toluene and THF, on the other hand, led to a further decrease in the reaction efficiency (Table 1, entries 11 and 12). It was also found that the reaction is very time consuming, even in presence of the Ag-OMS-2 catalyst. However, the conversion was increased upon elevation of temperature, and a maximum yield of 93% was obtained at 80 °C. Higher temperatures, on the other hand, had no significant positive effect on the reaction yield (Table 1, entries 13–16).| Entry | Solvent | Catalyst amountb (mg) | Temperature (°C) | Time (h) | Yield (%) |
|---|---|---|---|---|---|
| a Reaction conditions: Ag-OMS-2 as catalyst, solvent (4.0 mL), benzonitrile (1.0 mmol).b 4.87 mol% of Ag.c K-OMS-2 was used as catalyst. | |||||
| 1 | Water | 0 | 80 | 4 | N.R. |
| 2 | Water | 10 | 80 | 4 | 15 |
| 3 | Water | 20 | 80 | 4 | 68 |
| 4 | Water | 30 | 80 | 4 | 93 |
| 5 | Water | 40 | 80 | 4 | 96 |
| 6c | Water | 30 | 80 | 4 | Trace |
| 7 | Methanol | 30 | 80 | 4 | N.R. |
| 8 | Ethanol | 30 | 80 | 4 | 20 |
| 9 | Methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (1 water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
30 | 80 | 4 | 48 |
| 10 | Methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (1 water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
30 | 80 | 4 | 70 |
| 11 | Toluene | 30 | 80 | 4 | Trace |
| 12 | THF | 30 | 80 | 4 | Trace |
| 13 | Water | 30 | 25 | 4 | 11 |
| 14 | Water | 30 | 40 | 4 | 28 |
| 15 | Water | 30 | 60 | 4 | 66 |
| 16 | Water | 30 | 100 | 4 | 95 |
| 17 | Water | 30 | 80 | 1 | 20 |
| 18 | Water | 30 | 80 | 2 | 42 |
| 19 | Water | 30 | 80 | 3 | 70 |
To study the generality and scope of the reaction, Ag-OMS-2 was applied to a wide range of nitriles (Table 2). Benzonitrile derivatives with electron-withdrawing (Table 2, entries 2–7) or electron-donating groups (Table 2, entries 8 and 9) exhibited comparable reactivity in terms of yield of the final product. Electron-withdrawing substituents, however, resulted in slightly more yields as a result of making the nitrile carbon more susceptible to nucleophilic attack by an activated water molecule.64 As expected, ortho-substituted benzonitriles produced lower yields than meta- and para-substituted analogues (Table 2, entries 2–4), probably due to the steric hindrance effects. Aliphatic substrates such as acetonitrile and acrylonitrile, on the other hand (Table 2, entries 10 and 11), required more times and temperatures to convert to corresponding amides due to the increased electron density of nitrile carbon compared to aromatic nitriles. Heterocyclic amides are also an important class of mediators in medicinal chemistry. For example, nicotinamide is a form of vitamin B3 and is used as a food and drug supplement in treating pellagra and skin inflammations.65 Nevertheless, hydration of their nitrile precursors is somehow difficult as a result of the strong tendency of nitrile substrates toward metal centres.66 Interestingly, our devised protocol resulted in excellent conversion of heteroaromatic nitriles containing S and N heteroatoms to their corresponding amides in a rather short time (Table 2, entries 12 and 13). According to these results, Ag-OMS-2 can be considered as an appropriate catalyst for the hydration of nitriles. It should be noted that, previous reports on Ag NPs as catalyst for the title reaction, require high temperatures (up to 140 °C) and large amounts of silver metal.
| Entry | Substrate | Product | Temp. (°C) | Time (h) | Isolated yield (%) |
|---|---|---|---|---|---|
| a Reaction conditions: nitrile (1.0 mmol), Ag-OMS-2 (30 mg), H2O (4 mL), 80 °C. The products were isolated by either recrystallization or column chromatography, and confirmed by 1H NMR. | |||||
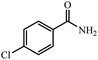
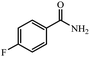
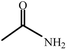
| 1 | 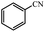 |
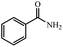 |
80 | 4 | 93 |
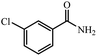
| 2 | 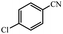 |
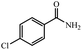 |
80 | 4 | 95 |
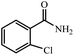
| 3 | 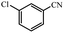 |
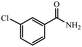 |
80 | 4 | 91 |
| 4 | 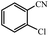 |
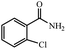 |
80 | 4 | 89 |
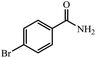
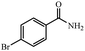
| 5 | 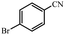 |
 |
80 | 4 | 92 |
| 6 | 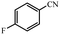 |
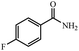 |
80 | 4 | 97 |
| 7 | 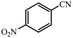 |
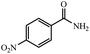 |
80 | 4 | 96 |
| 8 | 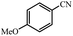 |
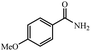 |
80 | 4 | 88 |
| 9 | 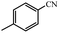 |
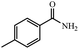 |
80 | 4 | 90 |
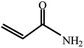
| 10 | CH3CN | 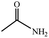 |
100 | 9 | 70 |
| 11 | 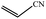 |
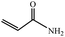 |
100 | 9 | 73 |
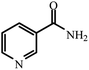
| 12 | 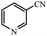 |
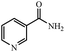 |
80 | 4 | 95 |
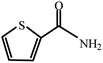
| 13 | 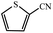 |
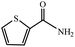 |
80 | 4 | 92 |
Recovery and reuse of the Ag-OMS-2 catalyst was also tested. After each run, the filtered catalyst was Soxhlet-extracted with methanol for 1 h, and then oven-dried for 3 h. After six consecutive cycles, a reasonable decrease in efficiency of the catalyst was observed (Fig. 7). A hot filtration test was performed to investigate the leaching of silver from the OMS-2 support into the solution.
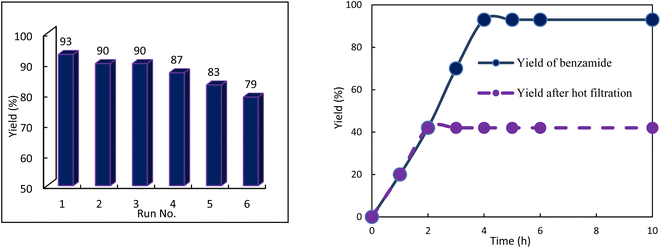 | ||
| Fig. 7 (Left) Reusability of the Ag-OMS-2 for hydration of benzonitrile. (Right) The yield of benzamide in presence of Ag-OMS-2, and after its removal halfway. | ||
The experiment was carried out for the hydration of benzonitrile, and the catalyst was removed from the reaction liquor 2 h after the start of the reaction at 80 °C. The filtered solution was allowed to react until the end of the period (4 h) and no change in the product yield was observed during the last 2 h (Fig. 7). ICP analysis of the supernatant also confirmed the absence of silver ions. Thus, it can be concluded that silver was not leached out during the hydration reaction.
In addition, FESEM and TEM images of the catalyst recovered after the 6th run are presented in Fig. 8. As can be seen, the catalyst retained its morphology and structure.
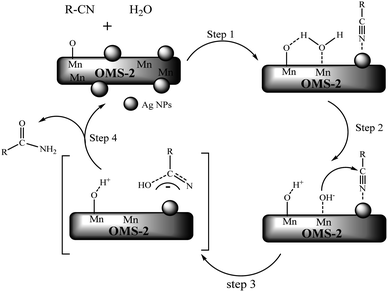
A possible reaction mechanism for nitrile hydration over the Ag-OMS-2 is proposed in Fig. 9. Initially, nitrile is coordinated through the interaction of nitrogen with the surface of Ag NPs, as they carry a positive surface charge.50 The active oxygen sites belonging to the OMS-2 structure (as Brønsted bases) effectively activate water molecules via Mn⋯O–H–OH interactions to form the OHδ− species (step 1). Nucleophilic addition of hydroxyl to the nitrile leads to the formation of a negatively charged transition state (steps 2 and 3). After hydrogen abstraction from Mn–O⋯H by nitrogen, active oxygen sites regenerate on the metal oxide. Finally, the resulting hydrated nitrile species exchange ligand with the solvent, and amide is produced (step 4).
Table 3 compares our results with some of the other catalytic systems reported for hydration of nitriles. Some of these systems have serious limitations such as need for additives, use of organic solvents, prolonged reaction times, elevated temperatures, high loads of catalyst, and need for a neutral atmosphere.
| Entry | Catalyst | Reaction conditions | Catalyst loadinga | Yield (%) | Ref. |
|---|---|---|---|---|---|
| a Mol% of the active ingredient relative to the nitrile substrate. | |||||
| 1 | [(IPr)Au(NTf2)] | H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) THF (1 THF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 140 °C, MW, 2 h 1), 140 °C, MW, 2 h |
2 mol% | 99 | 15 |
| 2 | Ag NPs@mPMF | H2O, 90 °C, in air, 7 h | 5 mol% | 97 | 64 |
| 3 | Ag–PAAS | H2O, 90 °C, in air, 9 h | 3 mol% | 90 | 67 |
| 4 | Ag/Fe3O4 | H2O, 150 °C, 6 h | 3 mol% | 99 | 68 |
| 5 | Ag-CIN-1 | H2O, 100 °C, 3 h | 50 mg | 96 | 2 |
| 6 | Ni NPs/HT | H2O, 120 °C, 10 h | 50 mg | 85 | 69 |
| 7 | Activated clay/C@Fe2O3 | H2O, KOH, 60 °C, 4 h | 25 mg | 81 | 70 |
| 8 | [RuH2(PPh3)4] | H2O, 100 °C, 24 h | 5 mol% | 62 | 71 |
| 9 | Ni(II) complex | H2O, iPrOH, 70 °C, 6 h | 2–10 mol% | 91 | 29 |
| 10 | Ru/chitin | H2O, N2 atmosphere, 120 °C, 20 h | 2.3 mol% | 97 | 72 |
| 11 | Ag/SiO2 | H2O, 140 °C, 3 h | 45 mg | 98 | 3 |
| 12 | Ag-OMS-2 | H2O, 80 °C, 4 h | 4.87 mol% | 93 | This work |
Conclusions
Hydration of a broad range of nitriles to their corresponding amides was achieved by using Ag-OMS-2 nanoparticles as a heterogeneous catalyst under neutral conditions in water. According to the proposed mechanism, the observed efficiency can be attributed to the synergistic effect of silver with OMS-2 scaffold. Highlights of the present protocol include thermal stability of the catalyst, ease of catalyst recycling and work-up, and eco-friendly nature of the process as a result of using water as both a solvent and a hydrating agent.Characterization data for the amide products
93% yield (112.6 mg); white solid; mp 124–126 °C; IR (KBr, cm−1): 3371, 3173, 1660, 1576, 1501, 1403, 1184, 1120, 786, 634; 1H NMR (250 MHz, CDCl3): δ 7.79 (d, J = 7.76 Hz, 2H), 7.40–7.51 (m, 1H), 7.25 (s, 2H), 6.27 (s, NH, 2H).
95% yield (147.8 mg); white solid; mp 179–181 °C; IR (KBr, cm−1): 3431, 3280, 1656, 1626, 1410, 1346, 1203, 1092, 791, 697; 1H NMR (250 MHz, CDCl3): δ 7.73 (d, J = 6.5 Hz, 2H), 7.41 (d, J = 5.75 Hz, 2H), 5.80 (br, NH, 2H, exchange with solvent).
91% yield (141.5 mg); white solid; mp 132–133 °C; IR (KBr, cm−1): 3355, 3199, 1676, 1616, 1435, 1325, 1302, 1102, 775; 1H NMR (250 MHz, CDCl3): δ 8.18 (s, 1H), 7.77–7.80 (d, J = 7.5 Hz, 1H), 7.58–7.63 (d, J = 12.5 Hz, 1H), 7.18–7.21 (t, J = 2.5 Hz, 1H), 5.88 (s, 2H).
89% yield (138.4 mg); white solid; mp 142–145 °C; IR (KBr, cm−1): 3392, 3198, 1645, 1620, 1460, 1403, 1216, 1119, 839, 768, 634; 1H NMR (250 MHz, CDCl3): δ 8.09 (d, J = 15.5 Hz, 1H), 7.46–7.54 (m, 1H), 7.09–7.30 (m, 1H), 6.68 (s, 1H), 6.17 (s, NH, 1H).
92% yield (184 mg); white solid; mp 192–193 °C; IR (KBr, cm−1): 3352, 3164, 1654, 1620, 1407, 1279, 1014, 792; 1H NMR (250 MHz, CDCl3): δ 7.87 (d, J = 7.5 Hz, 2H), 7.67 (d, J = 4.25 Hz, 2H), 6.05 (br, NH, 2H, exchange with solvent).
97% yield (134.9 mg); white solid; mp 155–157 °C; IR (KBr, cm−1): 3335, 3169, 2932, 1666, 1618, 1405, 1215, 1112, 780; 1H NMR (250 MHz, CDCl3): δ 7.83 (t, J = 4.25 Hz, 2H), 7.11–7.21 (m, 2H), 6.06 (s, NH, 2H).
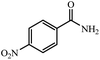
96% yield (159.4 mg); yellow solid; mp 197–199 °C; IR (KBr, cm−1): 3474, 3350, 3166, 1676, 1599, 1524, 1410, 1344, 1139, 1111, 1007, 864, 763, 707, 658, 607; 1H NMR (250 MHz, CDCl3) δ 8.30 (d, J = 8.28 Hz, 2H), 7.96 (d, J = 8.25 Hz, 2H), 7.26 (br, NH, 2H, exchange with solvent).
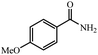
88% yield (133 mg); white solid; mp 166–167 °C; IR (KBr, cm−1): 3391, 3169, 1647, 1615, 1571, 1423, 1393, 1251, 1180, 1023, 850, 811, 649; 1H NMR (250 MHz, CDCl3): δ 7.76 (d, J = 7.0 Hz, 2H), 6.91 (d, J = 7.0 Hz, 2H), 5.92 (s, NH, 2H), 3.84 (s, 3H).
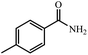
90% yield (121.6 mg); white solid; mp 157–159 °C; IR (KBr, cm−1): 3343, 3165, 1671, 1616, 1568, 1412, 1287, 1119, 840, 793, 672; 1H NMR (250 MHz, CDCl3): δ 7.69 (d, J = 7.75 Hz, 2H), 7.23 (d, J = 7.75 Hz, 2H), 5.96 (br, NH, 2H, exchange with solvent), 2.40 (s, 3H).
70% yield (41.3 mg); white solid; mp 79–80 °C; IR (KBr, cm−1): 3512, 3323, 2962, 1369, 1109, 996, 813, 644; 1H NMR (250 MHz, DMSO-d6): δ 6.60 (s, 2H), 1.73 (s, 3H).
73% yield (51.8 mg); white solid; mp 84–87 °C; IR (KBr, cm−1): 3466, 3355, 2864, 2337, 1629, 1509, 1390, 1107, 1021, 1135, 711; 1H NMR (250 MHz, DMSO-d6): δ 6.15–6.79 (m, 2H), 5.34 (s, NH, 2H).
95% yield (116 mg); white solid; mp 126–128 °C; IR (KBr, cm−1): 3354, 3156, 2915, 1666, 1606, 1399, 1192, 1089, 607; 1H NMR (250 MHz, DMSO-d6): δ 9.07 (d, J = 12.5 Hz, 1H), 8.60–8.70 (m, 1H), 8.14–8.28 (m, 1H), 7.58 (s, 1H), 7.28–7.34 (m, 1H).
92% yield (116.9 mg); yellow solid; mp 176–178 °C; IR (KBr, cm−1): 3304, 3078, 1612, 1538, 1404, 1345, 1278, 1228, 1086, 931, 832, 689; 1H NMR (250 MHz, CDCl3): δ 7.84 (t, J = 5.5 Hz, 1H), 7.57 (d, J = 7.0 Hz, 1H), 7.14 (t, J = 7.0 Hz, 1H), 5.66 (br, 2H, exchange with solvent).
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Partial support of this study by Research Council of University of Guilan is gratefully acknowledged.References
- N. S. Thirukovela, R. Balaboina, S. Kankala, R. Vadde and C. S. Vasam, Tetrahedron, 2019, 75, 2637–2641 CrossRef CAS.
- N. Salam, S. K. Kundu, R. A. Molla, P. Mondal, A. Bhaumik and S. M. Islam, RSC Adv., 2014, 4, 47593–47604 RSC.
- K. I. Shimizu, N. Imaiida, K. Sawabe and A. Satsuma, Appl. Catal., A, 2012, 421–422, 114–120 CrossRef CAS.
- V. Cadierno, J. Francos and J. Gimeno, Chem.–Eur. J., 2008, 14, 6601–6605 CrossRef CAS PubMed.
- A. Ishizuka, Y. Nakazaki and T. Oshiki, Chem. Lett., 2009, 38, 360–361 CrossRef CAS.
- T. J. Ahmed, S. M. M. Knapp and D. R. Tyler, Coord. Chem. Rev., 2011, 255, 949–974 CrossRef CAS.
- J. H. Kim, J. Britten and J. Chin, J. Am. Chem. Soc., 1993, 115, 3618–3622 CrossRef CAS.
- K. L. Breno, M. D. Pluth and D. R. Tyler, Organometallics, 2003, 22, 1203–1211 CrossRef CAS.
- A. Goto, K. Endo and S. Saito, Angew. Chem., Int. Ed., 2008, 47, 3607–3609 CrossRef CAS PubMed.
- M. C. K. B. Djoman and A. N. Ajjou, Tetrahedron Lett., 2000, 41, 4845–4849 CrossRef CAS.
- H. Takaya, K. Yoshida, K. Isozaki, H. Terai and S. I. Murahashi, Angew. Chem., Int. Ed., 2003, 42, 3302–3304 CrossRef CAS PubMed.
- Í. Ferrer, J. Rich, X. Fontrodona, M. Rodríguez and I. Romero, Dalton Trans., 2013, 42, 13461–13469 RSC.
- E. Tomás-Mendivil, F. J. Suárez, J. Díeza and V. Cadierno, Chem. Commun., 2014, 50, 9661–9664 RSC.
- P. Crochet and V. Cadierno, Dalton Trans., 2014, 43, 12447–12462 RSC.
- R. S. Ramón, N. Marion and S. P. Nolan, Chem.–Eur. J., 2009, 15, 8695–8697 CrossRef PubMed.
- T. Mitsudome, et al., Chem. Commun., 2009, 3258–3260 RSC.
- E. L. Downs and D. R. Tyler, Coord. Chem. Rev., 2014, 280, 28–37 CrossRef CAS.
- S. Kumar and P. Das, New J. Chem., 2013, 37, 2987–2990 RSC.
- A. Y. Kim, H. S. Bae, S. Park, S. Park and K. H. Park, Catal. Lett., 2011, 141, 685–690 CrossRef CAS.
- H. Wang, et al., Ind. Eng. Chem. Res., 2019, 58, 17319–17324 CrossRef CAS.
- M. A. Hussain, et al., J. Ind. Eng. Chem., 2021, 99, 187–195 CrossRef CAS.
- M. Tamura, A. Satsuma and K. I. Shimizu, Catal. Sci. Technol., 2013, 3, 1386–1393 RSC.
- Y. Gangarajula and B. Gopal, Chem. Lett., 2012, 41, 101–103 CrossRef CAS.
- S. Ding, M. J. Hülsey, H. An, Q. He, H. Asakura, M. Gao and N. Yan, CCS Chem., 2021, 10, 1814–1822 CrossRef.
- M. J. Hülsey, S. Baskaran, S. Ding, S. Wang, H. Asakura, S. Furukawa and N. Yan, CCS Chem., 2022, 10, 3296–3308 CrossRef.
- M. J. Hülsey, V. Fung, X. Hou, J. Wu and N. Yan, Angew. Chem., 2022, 40, e202208237 Search PubMed.
- S. I. Murahashi, S. Sasao, E. Saito and T. Naota, Tetrahedron, 1993, 39, 8805–8826 CrossRef.
- S. Murahashi, S. Sasao, E. Saito and T. Naota, J. Org. Chem., 1992, 9, 2521–2523 CrossRef.
- K. Singh, A. Sarbajna, I. Dutta, P. Pandey and J. K. Bera, Chem.–Eur. J., 2017, 23, 7761–7771 CrossRef CAS PubMed.
- T. Oshiki, H. Yamashita, K. Sawada, M. Utsunomiya, K. Takahashi and K. Takai, Organometallics, 2005, 26, 6287–6290 CrossRef.
- E. S. Kim, H. S. Kim and J. N. Kim, Tetrahedron Lett., 2009, 24, 2973–2975 CrossRef.
- A. Iyer, H. Galindo, S. Sithambaram, C. King'ondu, C. H. Chen and S. L. Suib, Appl. Catal., A, 2010, 375, 295–302 CrossRef CAS.
- S. Sithambaram, R. Kumar, Y. C. Son and S. L. Suib, J. Catal., 2008, 253, 269–277 CrossRef CAS.
- S. Rasul, S. Suzuki, S. Yamaguchi and M. Miyayama, Electrochim. Acta, 2013, 110, 247–252 CrossRef CAS.
- M. Nogami, T. Maeda and T. Uma, Sens. Actuators, B, 2009, 137, 603–607 CrossRef CAS.
- H. Zhang, et al., ACS Sustainable Chem. Eng., 2017, 5, 6727–6735 CrossRef CAS.
- W. Hou, S. Wang, X. Bi, X. Meng, P. Zhao and X. Liu, Chin. Chem. Lett., 2021, 32, 2513–2518 CrossRef CAS.
- M. Yao, M. Xie, S. Zhang, J. Yuan, L. Zhao and R. S. Zhao, Sep. Purif. Technol., 2022, 302, 122145 CrossRef CAS.
- P. Ye, Q. Zou, L. An, Y. Wei, A. Xu and X. Li, J. Colloid Interface Sci., 2019, 535, 481–490 CrossRef CAS PubMed.
- S. L. Suib, J. Mater. Chem., 2008, 18, 1623–1631 RSC.
- J. Fu, N. Dong, Q. Ye, S. Cheng, T. Kang and H. Dai, New J. Chem., 2018, 42, 18117–18127 RSC.
- J. Chen, J. Li, H. Li, X. Huang and W. Shen, Microporous Mesoporous Mater., 2008, 116, 586–592 CrossRef CAS ..
- S. K. Kailasa, T. J. Park, J. V. Rohit and J. R. Koduru, Nanopart. Pharmacother., 2019, 461–484 CAS.
- V. K. Sharma, R. A. Yngard and Y. Lin, Adv. Colloid Interface Sci., 2009, 1–2, 83–96 CrossRef PubMed.
- N. Durán, M. Durán, M. B. De Jesus, A. B. Seabra, W. J. Fávaro and G. Nakazato, Nanomedicine, 2016, 3, 789–799 CrossRef PubMed.
- D. D. Evanoff Jr and G. Chumanov, ChemPhysChem, 2005, 7, 1221–1231 CrossRef PubMed.
- I. A. Wani, S. Khatoon, A. Ganguly, J. Ahmed, A. K. Ganguli and T. Ahmad, Mater. Res. Bull., 2010, 8, 1033–1038 CrossRef.
- P. C. Ma, B. Z. Tang and J. K. Kim, Carbon, 2008, 11, 1497–1505 CrossRef.
- H. Wei and H. Eilers, Thin Solid Films, 2008, 2, 575–581 CrossRef.
- J. Liu, L. Ke, L. Sun, F. Pan, X. Yuan and D. Xia, J. Environ. Chem. Eng., 2021, 9, 106199 CrossRef CAS ..
- K. Kawai, H. Kawakami, T. Narushima and T. Yonezawa, J. Nanopart. Res., 2015, 17, 1–9 CrossRef CAS.
- M. Özacar, A. S. Poyraz, H. C. Genuino, C. H. Kuo, Y. Meng and S. L. Suib, Appl. Catal., A, 2013, 462–463, 64–74 CrossRef.
- G. D. Yadav and R. K. Mewada, Chem. Eng. J., 2013, 221, 500–511 CrossRef CAS.
- N. Dong, J. Fu, Q. Ye, M. Chen, Z. Fu and H. Dai, Catal. Surv. Asia, 2020, 24, 259–268 CrossRef CAS.
- C. M. Julien, M. Massot and C. Poinsignon, Spectrochim. Acta, Part A, 2004, 60, 689–700 CrossRef CAS PubMed.
- J. Xu, J. Li, Q. Yang, Y. Xiong and C. Chen, Electrochim. Acta, 2017, 251, 672–680 CrossRef CAS.
- N. Dong, M. Chen, Q. Ye, D. Zhang and H. Dai, J. Environ. Sci., 2022, 112, 258–268 CrossRef CAS PubMed.
- S. Ansari, A. Khorshidi and S. Shariati, RSC Adv., 2020, 10, 3554–3565 RSC ..
- R. Wang and J. Li, Environ. Sci. Technol., 2010, 11, 4282–4287 CrossRef PubMed.
- J. Li, R. Wang and J. Hao, J. Phys. Chem. C, 2010, 23, 10544–10550 CrossRef.
- G. Qiu, H. Huang, S. Dharmarathna, E. Benbow, L. Stafford and S. L. Suib, Chem. Mater., 2011, 17, 3892–3901 CrossRef.
- H. Sun, S. Chen, P. Wang and X. Quan, Chem. Eng. J., 2011, 178, 191–196 CrossRef CAS.
- F. Gao, X. Tang, H. Yi, C. Chu, N. Li, J. Li and S. Zhao, Chem. Eng. J., 2017, 322, 525–537 CrossRef CAS ..
- K. Ghosh, A. Iqubal, R. A. Molla, A. Mishra and S. M. Islam, Catal. Sci. Technol., 2015, 5, 1606–1622 RSC.
- S. C. Roy, P. Dutta, L. N. Nandy, S. K. Roy, P. Samuel, S. M. Pillai and M. Ravindranathan, Appl. Catal., A, 2005, 290, 175–180 CrossRef CAS ..
- Silver Nanoparticles, ed. D. Pozo Perez, In-Teh Olajnica 19/2, 32000 Vukovar, Croatia, 2010 Search PubMed.
- J. Li, G. Tang, Y. Wang, Y. Wang, Z. Li and H. Li, New J. Chem., 2016, 40, 358–364 RSC.
- H. Woo, K. Lee, S. Park and K. H. Park, Molecules, 2014, 19, 699–712 CrossRef PubMed.
- T. Subramanian and K. Pitchumani, Catal. Commun., 2012, 29, 109–113 CrossRef CAS.
- T. Rahman, G. Borah and P. K. Gogoi, J. Chem. Sci., 2021, 133, 20–22 CrossRef.
- R. García-Álvarez, J. Francos, E. Tomás-Mendivil, P. Crochet and V. Cadierno, J. Organomet. Chem., 2014, 771, 93–104 CrossRef.
- A. Matsuoka, et al., RSC Adv., 2015, 5, 12152–12160 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra00292f |
| This journal is © The Royal Society of Chemistry 2023 |