Open Access Article
Open Access ArticlePredicting the speciation of ionizable antibiotic ciprofloxacin by biochars with varying carbonization degrees†
Guowei Shiabc,
Yasong Liab,
Yaci Liuab and
Lin Wu *abd
*abd
aFujian Provincial Key Laboratory of Water Cycling and Eco-Geological Processes, Xiamen 361021, China. E-mail: wulin_shs@mail.cgs.gov.cn; Fax: +86-311-67598661; Tel: +86-311-67598598
bChina Geological Survey, Hebei Province Key Laboratory of Groundwater Contamination and Remediation, Institute of Hydrogeology and Environmental Geology, Chinese Academy of Geological Sciences, Shijiazhuang 050061, China
cChina University of Geosciences (Beijing), Beijing 100083, China
dNorth China University of Water Resources and Electric Power, Zhengzhou 450046, China
First published on 29th March 2023
Abstract
Sorption mechanisms of ionizable organic pollutants by biochars and approaches for the prediction of sorption are still unclear. In this study, batch experiments were conducted to explore the sorption mechanisms of woodchip-derived biochars prepared at 200–700 °C (referred as WC200–WC700) for cationic, zwitterionic and anionic species of ciprofloxacin (referred as CIP+, CIP± and CIP−, respectively). The results revealed that the sorption affinity of WC200 for different CIP species was in the order of CIP± > CIP+ > CIP−, while that of WC300–WC700 remained the order of CIP+ > CIP± > CIP−. WC200 exhibited a strong sorption ability, which could be attributed to hydrogen bonding and electrostatic attraction with CIP+, electrostatic attraction with CIP±, and charge-assisted hydrogen bonding with CIP−. Pore filling and π–π interactions contributed to the sorption of WC300–WC700 for CIP+, CIP± and CIP−. Rising temperature facilitated CIP sorption to WC400 as verified by site energy distribution analysis. Proposed models including the proportion of the three CIP species and sorbent aromaticity index (H/C) can quantitatively predict CIP sorption to biochars with varying carbonization degrees. These findings are vital to elucidating the sorption behaviors of ionizable antibiotics to biochars and exploring potential sorbents for environmental remediation.
1. Introduction
In recent years, the residue of antibiotics in the aquatic and soil environments has caused many serious environmental problems. As a typical fluoroquinolone antibiotic, ciprofloxacin (CIP) has a wide spectrum of antibacterial properties and is extensively used in disease treatment and livestock breeding.1 However, most of the CIP is excreted to sewage systems in the form of urine and feces because only a small part of the drugs is absorbed by humans or animals.2 It was reported that CIP has been detected in water bodies and sediments in several regions of the world.3,4 For instance, the concentration of CIP in the water body of Firozabad Ditch in Iran reached 656.8 ng L−1,5 and that in the sediment of the Haihe River in China was up to 1290 ng g−1.6 The heavy use of drugs can cause some problems such as antibiotic abuse and emergence of resistance genes.7 Due to the existence and potential toxicity of CIP in the environment, it has been regarded as a high-risk pollutant and its pollution control is urgent.8Biochar, as an environment-friendly carbonaceous material with abundant pore structures and surface functional groups, has gradually become a research hotspot.9,10 The application of biochar in sewage treatment, efficient removal, and functional composite materials has become frontier research topics.11 It can effectively control the migration of pollutants in soil and groundwater through sorption-locking and display an excellent performance in environmental remediation.12–14 Certain studies have proven that the addition of hardwood-derived biochars to agricultural soils can reduce the pore water concentration of sulfamethazine and control its migration in soils.15 In addition, due to strong catalytic properties and compatibility coupled with other materials of biochar, it has been used as a mediator for the degradation of pollutants in sewage treatment. For instance, the higher removal efficiency of oxytetracycline by periodate activated with manganese oxide modified biochar was observed, which provided a promising idea for tackling water pollution.16 Biochar's applications for global climate mitigation, salinity and drought stress amelioration, and biofuel production are also of great concern.17
It has been reported that biochar can effectively sorb CIP and the precursor materials of biochar include rice straw, bagasse, sludge, bamboo, urban solid waste, marine algae, etc.1,18–21 However, report on CIP sorption by biochar derived from woodchip, a biomass with high content of lignin and cellulose, is rare. Compared with other plant materials, woody biochars have been of particular interest since the carbon content in woods is retained in a greater percentage.22 Also, woodchips are common plant waste in nature, with low cost and wide source. Woodchip-derived biochars played an effective role in removing ionizable organic pollutants including tetracycline, levofloxacin, sulfamethazine and ibuprofen,23–25 which exhibited a broad application prospect in environmental restoration.26
Moreover, sorption of ionizable antibiotics was expected to be highly affected by sorbate species and the sorption mechanisms dynamically changed with speciation.27 For instance, sorption of CIP with different dissociated species by titanate nanotubes was studied through both experimental and theoretical calculations. The results showed that CIP species at various pH exhibited significantly different sorption favorability.28 Mechanisms relevant to CIP sorption by biochars were summarized based on previous studies, including electrostatic interaction, pore filling, π–π interactions and hydrogen bonding,19,29 but the effect of speciation on CIP sorption by biochars was still unclear. Also, few models can be used to quantitatively predict the sorption of different CIP species by biochars. Furthermore, previous studies focused on thermodynamic analysis to explore the influence of temperature on sorption, while a deep understanding of site energy distribution of CIP sorption was still lacking.
Therefore, in this study, woodchip-derived biochars were produced by oxygen-limited pyrolysis at 200–700 °C. A series of batch experiments were employed to investigate the sorption behaviors of CIP by biochars. The purposes of this study were to explore the sorption affinity and mechanisms of different CIP species to biochars, and establish a practical model to predict CIP sorption. Furthermore, the effect of temperature on sorption was evaluated by thermodynamics and site energy distribution analysis. This study reveals the speciation and thermodynamic behaviors of CIP sorption by biochars, and provides a novel approach for the sorption prediction of ionizable antibiotics.
2. Materials and methods
2.1 Materials and reagents
Ciprofloxacin (≥98%) was purchased from Shanghai ANPEL Experimental Technology Co., Ltd. It is an amphoteric antibiotic that exhibits cationic, zwitterionic and anionic species of CIP at various pH (Fig. S1†). HCl, NaOH and NaCl (analytically pure) were obtained from Beijing Chemical Works. Acetonitrile (Thermo Fisher Scientific, Shanghai) and potassium dihydrogen phosphate (≥99.5%, Macklin, Shanghai) were chromatographically pure, and deionized water was used in the experiments.2.2 Preparation and characterization of biochars
Biochars were produced by the oxygen-limited temperature control pyrolysis method. Woodchips were collected from the field of Quanzhou, Fujian Province of China. Firstly, the surface impurities of woodchips were removed by rinsing with water three times, and then dried in a blast drying box at 80 °C for 12 h. Secondly, the pretreated woodchips were sealed in a crucible and placed into a muffle furnace, pyrolyzed at 200, 300, 400, 500, 600, and 700 °C for 6 h, respectively. Finally, the generated biochars were sieved through a 0.3 mm sieve and loaded into a brown bottle for further use. The biochars obtained at 200–700 °C were labeled as WC200–WC700. The temperature range of 200–700 °C was chosen to explore the evolution of sorption mechanisms of biochars with varying carbonization degrees.Elemental composition (C, H, N) of biochars was determined using the elemental analyzer (Vario EL Cube, Elementar, Germany), and the O content was calculated by mass balance. The surface functional groups were analyzed by Fourier transform infrared spectrometer (iS10 FTIR spectrometer, USA) in the range of 4000–400 cm−1 region at 25 °C. The pore size distribution and specific surface area of biochars were measured with the N2 sorption–desorption method at a relative pressure of 0.99 by a surface area analyzer (Micromeritics ASAP 2460, USA). Surface morphologies of biochars were observed using scanning electron microscopy (SU8020, Hitachi, Japan). Surface charge properties of biochars under different pH conditions were obtained by a zeta potentiometer (Malvern Instruments Ltd, U.K.).
2.3 Batch sorption experiments
Batch experiments were carried out to study the sorption of CIP to biochars. To investigate the effect of pH on sorption, certain dosages of biochars (1 g L−1 for WC200 and WC300, 0.5 g L−1 for WC400 and WC500, 0.25 g L−1 for WC600 and WC700) were weighed in brown glass bottles. Next, 20 mL of CIP solution with the initial concentration of 1, 5, 10, 15, 20, 30 and 40 mg L−1 was placed (0.01 mol L−1 of NaCl background solution). The initial pH was adjusted to 3.0, 5.8, 7.2, 8.6 and 10.0 with 0.1 M HCl or NaOH according to the proportion of different CIP species. Subsequently, all the samples were kept to the thermostatic shaker with a speed of 175 rpm for 96 h (the equilibrium time obtained from the kinetic experiment) at 298 K. After reaching equilibrium, the samples were centrifuged for 10 min at 3000 rpm and then the supernatants were passed through the membrane (0.45 μm) for analysis. To explore the influence of temperature on sorption, WC200, WC400 and WC600 were selected as representatives of low, medium and high pyrolysis temperature biochars, respectively. The concentration of CIP was repeated as above and the initial solution pH was adjusted to 3.0. The shaker temperatures were controlled at 298 K, 308 K, and 318 K, respectively. All experiments were performed in duplicate and control samples (without biochar) were considered to observe the natural losses (volatilization, degradation and photolysis) of CIP during the experimental process.CIP concentration was analyzed by high performance liquid chromatography (HPLC, Shimadzu, LC-2030C, Japan) with a Shim-pack GIST C18 column (4.6 mm × 250 mm) at the detection wavelength of 278 nm. The mobile phase A and B were potassium dihydrogen phosphate (0.01 mol L−1, pH was adjusted to 3.0 with H3PO4) and acetonitrile, respectively, with a volume ratio of 77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23. The flow rate was 1.0 mL min−1 and the injection volume was 10 μL. The retention time was 5 min and the column temperature was 40 °C.
23. The flow rate was 1.0 mL min−1 and the injection volume was 10 μL. The retention time was 5 min and the column temperature was 40 °C.
2.4 Data analysis
The Langmuir (eqn (1)) and Freundlich (eqn (2)) models were applied to fit the sorption isotherm data.
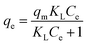 | (1) |
| qe = KFCeN | (2) |
Sorption coefficient Kd (L g−1) was obtained by eqn (3), and energy change and the endothermic or exothermic situation of the sorption process were judged by the thermodynamic models (eqn (4) and (5)):
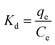 | (3) |
ΔG = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd Kd
| (4) |
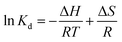 | (5) |
Sorption capacity of the heterogeneous surface was obtained by the integral equation as follows:
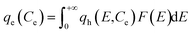 | (6) |
The relationship between Ce and E* was described as:
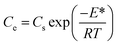 | (7) |
The frequency of site energy distribution F(E*) was calculated by differentiating q(E*) to E*.
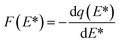 | (8) |
According to the eqn (1), (7) and (8), the generalized Langmuir model (eqn (9)) could be obtained to describe the sorption energy distribution:
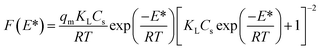 | (9) |
3. Results and discussion
3.1 Properties of woodchip-derived biochars
Pyrolysis temperature is a crucial factor dominating the structures and properties of biochars.30,31 With the increase of pyrolysis temperature, the content of “black carbon” gradually increased, while the non-carbonated organic matter decreased correspondingly,32 resulting in the marked increase of carbon content from WC200 to WC700 (49.2% → 87.9%) (Table 1). However, the content of hydrogen and oxygen reduced (5.78% → 1.33% for H and 43.1% → 4.03% for O) owing to the destruction of some surface functional groups at higher temperatures.33 The element ratios of H/C, O/C and (O + N)/C were reflective of the aromaticity, polarity and hydrophobicity of biochars, respectively.34 Consequently, the hydrophobicity and aromaticity increased while the polarity decreased from WC200 to WC700.| Sample | C (%) | H (%) | Oa (%) | O/Cb | H/Cc | (O + N)/Cd | SSA (m2 g−1) | Vtotal (cm3g−1) | Average pore width (nm) |
|---|---|---|---|---|---|---|---|---|---|
| a O content was calculated by mass balance.b Atomic ratio of oxygen to carbon.c Atomic ratio of hydrogen to carbon.d Atomic ratio of oxygen and nitrogen to carbon. | |||||||||
| WC200 | 49.2 | 5.78 | 43.1 | 0.658 | 1.41 | 0.662 | 0.920 | 0.00309 | 13.4 |
| WC300 | 68.0 | 4.83 | 24.9 | 0.274 | 0.853 | 0.279 | 1.27 | 0.00288 | 9.03 |
| WC400 | 75.5 | 3.78 | 18.1 | 0.180 | 0.600 | 0.184 | 5.61 | 0.00620 | 4.42 |
| WC500 | 80.0 | 2.95 | 12.7 | 0.119 | 0.442 | 0.124 | 170 | 0.0954 | 2.25 |
| WC600 | 87.9 | 2.26 | 4.33 | 0.0370 | 0.308 | 0.0410 | 367 | 0.180 | 1.96 |
| WC700 | 87.9 | 1.33 | 4.03 | 0.0344 | 0.182 | 0.0380 | 271 | 0.147 | 2.17 |
FTIR spectra of WC200–WC700 was shown in Fig. 1a. The hydroxyl peak was notably weakened from WC200 to WC300 owing to the volatilization of water molecules, and then increasingly disappeared by rising the pyrolysis temperature.35 Similarly, the –CH2 was gradually reduced and then vanished approximately at 500 °C. Moreover, the sorption peak strength of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O–C tended to flatten, while C–H bond peak became significantly stronger, implying an increase in aromaticity. This conclusion fit well with the elemental analysis results. According to Fig. 1b, the pH at the point zero charge (pHpzc) of biochars was estimated to be approximately 2.0. The zeta potential was negative when pH exceeded 2.0 and then gradually decreased as pH increased. Solution pH in this study ranged from 3.0 to 10.0, so the surfaces of the biochars were negatively charged.
O and C–O–C tended to flatten, while C–H bond peak became significantly stronger, implying an increase in aromaticity. This conclusion fit well with the elemental analysis results. According to Fig. 1b, the pH at the point zero charge (pHpzc) of biochars was estimated to be approximately 2.0. The zeta potential was negative when pH exceeded 2.0 and then gradually decreased as pH increased. Solution pH in this study ranged from 3.0 to 10.0, so the surfaces of the biochars were negatively charged.
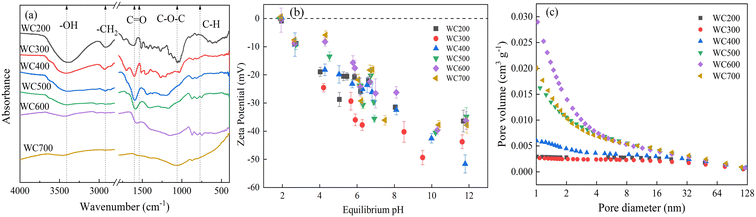 | ||
| Fig. 1 FTIR spectra (a), zeta potential at different equilibrium pH (b), and pore size distribution (c) of the woodchip-derived biochars at 200–700 °C (WC200–WC700). | ||
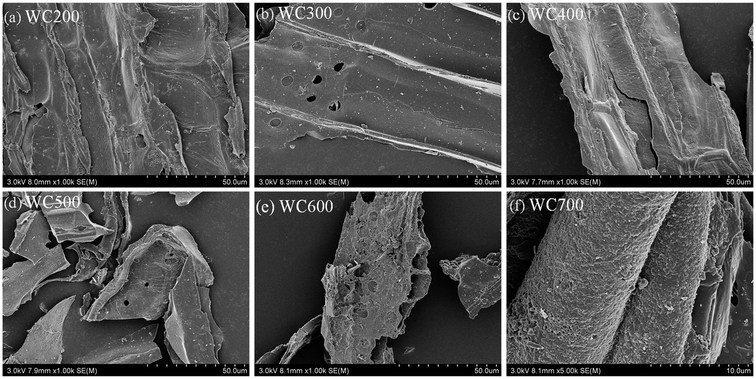
The specific surface area (SSA) and pore volume also increased as pyrolysis temperature rose (Fig. 1c and Table 1), which was caused by the release of volatile substances during pyrolysis and the formation of fiber tubular structures with the increase of temperature.36 The SSA of WC500 suddenly increased by two orders of magnitude (5.61 → 170 m2 g−1) because the amorphous carbon content decreased and high temperature promoted the formation of holes. The development of pore structure and the formation of micropores caused by high temperature led to a significant increase in SSA.37 More interestingly, the SSA and pore volume of WC700 were reduced, and the pore size was slightly increased compared with WC600, suggesting that the increasing temperature resulted in the destruction and blockage of some pore structures.38 Surface morphologies of WC200–WC700 were presented in SEM images (Fig. 2). The surfaces of WC200–WC400 were relatively smooth and the morphological structures were more obvious. The occurrence of micropores was observed on the surface of WC300, but some pores were blocked. When the pyrolysis temperature reached 500 °C, the non-carbonated organic matter was obviously destroyed. As the pyrolysis temperature was beyond 600 °C, biochars were composed of highly concentrated aromatic structures.
3.2 Sorption isotherms of CIP by biochars
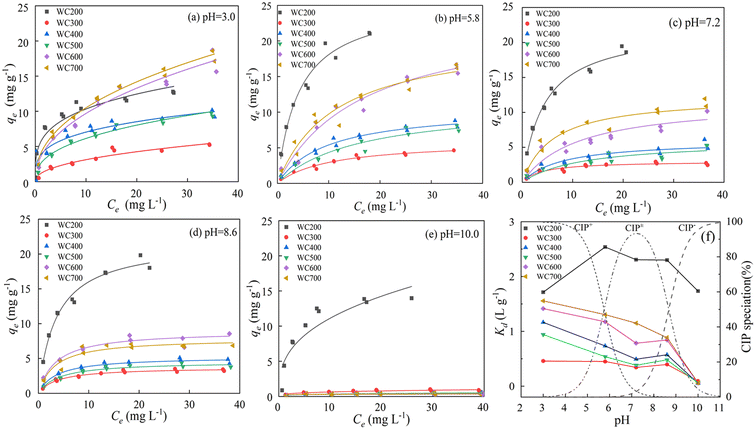
Isotherm data was well-fitted to the Langmuir model, depicting that CIP molecules mainly developed a single-molecular layer coverage on the biochar surfaces.21 The CIP–biochar sorption isotherms under certain pH conditions were presented in Fig. 3a–e, and the fitting parameters were summarized in Table 2. The maximum sorption amount of 21.15 mg g−1 was observed for WC200 at pH 5.8. Sorption affinity of the woodchip-derived biochars at 200–700 °C displayed a similar tendency that a gradual increase in qe was exhibited as Ce increased. Pyrolysis temperature was the main factor governing sorption capacity. The Kd values of biochars were ranked as WC200 > WC700 > WC600 > WC400 > WC500 > WC300 (Fig. 3f). WC200 exhibited a strong sorption performance compared to the high-temperature biochars. This conclusion was similar to the previous study that the sorption capacity of 250 °C biochar for sulfamethoxazole was higher than high-temperature biochars.39| pH | Sample | Langmuir | Freundlich | ||||||
|---|---|---|---|---|---|---|---|---|---|
| qm (mg g−1) | KL (L mg−1) | R2 | KF (mg(1−N) LN g−1) | KF-SSAa (mg(1−N) LN m−2) | KF-Ob (mg(1−N) LN g−1) | N | R2 | ||
| a Specific surface area normalized Freundlich sorption constant.b Oxygen content normalized Freundlich sorption constant. | |||||||||
| 3.0 | WC200 | 12.2 | 1.03 | 0.958 | 5.55 | 2.53 | 0.0539 | 0.270 | 0.911 |
| WC300 | 6.46 | 0.109 | 0.941 | 1.12 | 0.877 | 0.0450 | 0.446 | 0.927 | |
| WC400 | 10.1 | 0.316 | 0.813 | 3.74 | 0.411 | 0.128 | 0.275 | 0.835 | |
| WC500 | 11.0 | 0.156 | 0.983 | 2.53 | 0.0149 | 0.199 | 0.386 | 0.961 | |
| WC600 | 19.6 | 0.093 | 0.950 | 3.40 | 0.00927 | 0.786 | 0.455 | 0.957 | |
| WC700 | 21.6 | 0.107 | 0.971 | 3.88 | 0.0143 | 0.962 | 0.433 | 0.988 | |
| 5.8 | WC200 | 25.7 | 0.236 | 0.974 | 6.35 | 6.91 | 0.147 | 0.431 | 0.955 |
| WC300 | 5.13 | 0.165 | 0.812 | 0.993 | 0.956 | 0.0490 | 0.447 | 0.859 | |
| WC400 | 10.7 | 0.102 | 0.977 | 1.77 | 0.316 | 0.0979 | 0.452 | 0.952 | |
| WC500 | 12.6 | 0.046 | 0.864 | 1.27 | 0.00649 | 0.0870 | 0.519 | 0.893 | |
| WC600 | 23.9 | 0.060 | 0.951 | 2.46 | 0.00669 | 0.567 | 0.542 | 0.933 | |
| WC700 | 20.8 | 0.088 | 0.948 | 3.01 | 0.0111 | 0.746 | 0.481 | 0.944 | |
| 7.2 | WC200 | 22.2 | 0.231 | 0.981 | 5.64 | 6.14 | 0.131 | 0.409 | 0.966 |
| WC300 | 3.02 | 0.264 | 0.911 | 0.967 | 1.05 | 0.0389 | 0.311 | 0.843 | |
| WC400 | 6.01 | 0.127 | 0.876 | 1.19 | 0.212 | 0.0660 | 0.418 | 0.904 | |
| WC500 | 6.01 | 0.083 | 0.822 | 0.912 | 0.00536 | 0.0719 | 0.458 | 0.885 | |
| WC600 | 11.7 | 0.090 | 0.851 | 1.91 | 0.00521 | 0.442 | 0.444 | 0.923 | |
| WC700 | 12.3 | 0.164 | 0.916 | 2.74 | 0.0100 | 0.679 | 0.397 | 0.925 | |
| 8.6 | WC200 | 21.8 | 0.277 | 0.980 | 6.28 | 6.83 | 0.136 | 0.376 | 0.948 |
| WC300 | 3.73 | 0.266 | 0.970 | 1.21 | 0.950 | 0.0487 | 0.306 | 0.878 | |
| WC400 | 5.24 | 0.280 | 0.960 | 1.79 | 0.319 | 0.0990 | 0.292 | 0.853 | |
| WC500 | 4.53 | 0.252 | 0.947 | 1.50 | 0.00881 | 0.118 | 0.294 | 0.816 | |
| WC600 | 8.96 | 0.286 | 0.943 | 3.04 | 0.00827 | 0.701 | 0.291 | 0.863 | |
| WC700 | 8.01 | 0.313 | 0.822 | 2.82 | 0.0104 | 0.698 | 0.268 | 0.773 | |
| 10 | WC200 | 16.3 | 0.312 | 0.965 | 4.87 | 5.29 | 0.113 | 0.358 | 0.842 |
| WC300 | 1.07 | 0.199 | 0.993 | 0.236 | 0.249 | 0.0128 | 0.424 | 0.894 | |
| WC400 | 0.583 | 0.200 | 0.965 | 0.132 | 0.0295 | 0.00916 | 0.417 | 0.840 | |
| WC500 | 0.314 | 0.507 | 0.858 | 0.185 | 0.00109 | 0.0146 | 0.279 | 0.798 | |
| WC600 | 0.592 | 0.193 | 0.806 | 0.152 | 0.000630 | 0.0534 | 0.397 | 0.357 | |
| WC700 | 0.798 | 0.159 | 0.928 | 0.119 | 0.000822 | 0.0554 | 0.571 | 0.879 | |
The pH of solution has a crucial impact on the sorption process of ionic organic pollutants.40,41 For WC300–WC700, the values of Kd were negatively correlated with pH and the variation of that showed a similar trend (Fig. 3f). Specifically, the Kd values decreased significantly with the increase in pH from 3.0 to 7.2. At pH 7.2–8.6, the Kd values of WC300–WC600 increased slightly and that of WC700 still decreased. When pH rose above 8.6, the sorption was apparently affected by pH and the sorption capacity decreased drastically. In contrast with WC300–WC700, the increased solution pH resulted in the increased and then decreased sorption capacity of WC200. WC200 displayed an excellent sorption performance under near-neutral conditions (5.8 < pH < 8.6). The results demonstrated that the sorption of WC200 for different CIP species ranked as CIP± > CIP+ > CIP−, while that of WC300–WC700 was in the order of CIP+ > CIP± > CIP−.
3.3 Dynamic changes in sorption mechanisms
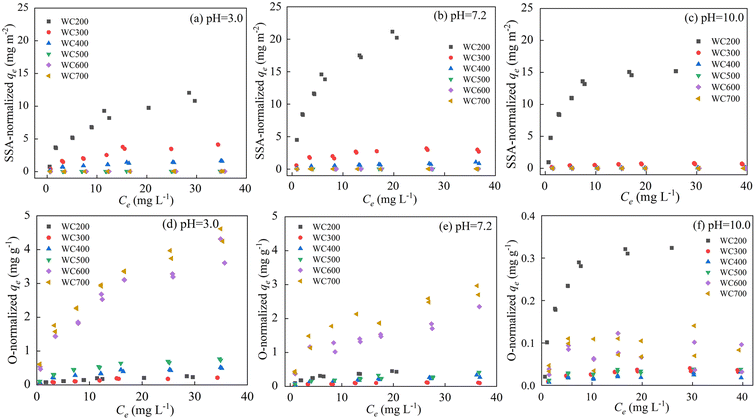
Properties of biochar have a great influence on sorption mechanism. To identify the effect of SSA and oxygen content on sorption, SSA-normalized and oxygen content-normalized sorption isotherms were investigated (Fig. 4). SAA-normalized KF (KF-SAA) of WC200 was significantly greater than other biochars (Table 2), indicating that polarity had a great contribution to its sorption. This result was comparable to the previous research that the sorption capacity per unit SSA of 300 °C biochar for N-acyl homoserine lactones was higher than that of 600 °C biochar.42 A previous study showed that some oxygen-containing groups on the biochar surfaces such as –COOH and –OH could become the main hydrogen-bonding sorption positions for ionic organic pollutants.43 Based on the elemental and FTIR analysis, WC200 had the highest H and O content and the –OH stretching vibration peak was the most intense. These oxygen-containing groups promoted sorption by acting with polar functional groups such as –COOH on CIP through hydrogen bonding. Moreover, the electrostatic attraction between CIP and WC200 was strong due to more negatively charged functional groups of WC200. Thus, polarity dominated the sorption of CIP to WC200 through electrostatic attraction and hydrogen bonding.In addition, the oxygen content-normalized KF (KF-O) values of WC600 and WC700 were apparently higher than low-temperature biochars, which meant that high-temperature biochars had higher aromaticity and then provided more π–π interaction sites.44 For example, the benzene rings of CIP could become electron receptors on account of the strong electron attraction of F atoms, while some aromatic structures on the surfaces of biochars can be used as electron donors. Thus, sorption was promoted by π–π interactions. Moreover, high-temperature biochars contained larger SSA and abundant pore structures which provided more sorption sites and CIP could be adsorbed to biochars through pore-filling.35
For WC300–WC700, they exhibited higher sorption for CIP+ than CIP± and CIP− owing to the electrostatic attraction between the negatively charged biochars and CIP+. As pH increased, –COOH gradually dissociated and the proportion of CIP± increased, causing the weakening of electrostatic attraction. It was worth noting that the sorption changed slightly as pH rose from 7.2 to 8.6, indicating the effect of electrostatic interactions on CIP± sorption was small. When pH exceeded 8.6, CIP− became the main form of CIP in the solution and the sorption was hindered due to the strong electrostatic repulsion. Similarly, titanate nanotubes showed higher sorption for CIP+ than CIP± and CIP−, which could result from the transition from electrostatic attraction to repulsion.28
The sorption capacity of WC200 for CIP increased rapidly over the pH range of 3.0 to 5.8. It was because CIP had smaller solubility and stronger hydrophobicity as pH increased, resulting in more sorption of CIP molecules to the surfaces of WC200 through electrostatic attraction and hydrogen bonding. Furthermore, the result showed the optimal sorption capacity and less variation at pH 5.8–8.6, illustrating the sorption was insensitive to this pH range while CIP± was dominant. When pH exceeded 8.6, a gradual decrease in sorption was attributed to the strong electrostatic repulsion and the weakening of the hydrophobic effect. Notably, WC200 still maintained a certain sorption amount for CIP− compared with other biochars (Fig. 4f), which could be associated with the formation of negative charge-assisted hydrogen bonding (–CAHB). Specifically, neutral CIP was released because CIP− captured H+ in water molecules, and then hydrogen bonding was formed between neutral CIP and WC200. The hypothesis was similar to the formation of –CAHB on sulfonamides sorption under alkaline conditions.39,40 Specifically, the proposed possible sorption mechanisms of CIP+, CIP± and CIP− by woodchip-derived biochars were exhibited in Fig. 5.
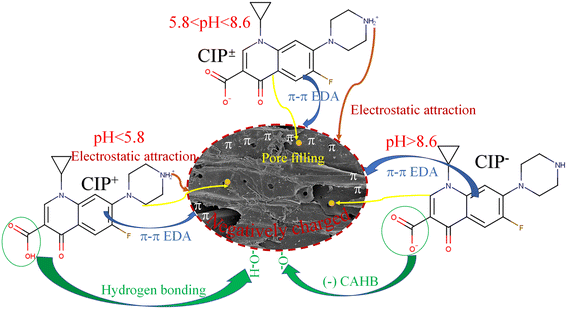 | ||
| Fig. 5 Proposed possible sorption mechanisms of cationic CIP (CIP+), zwitterionic CIP (CIP±) and anionic CIP (CIP−) by woodchip-derived biochars under varied pH conditions. | ||
3.4 Thermodynamics and site energy distribution
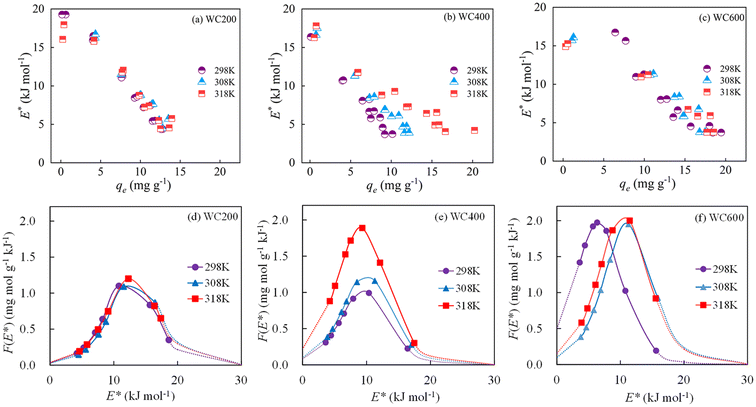
According to Fig. S2 and Table S1,† the sorption increased to varied degrees with the rise of the reaction temperature. This meant that more CIP molecules diffused onto the surfaces of biochars at higher temperatures. Usually, the viscosity of water gradually reduced as temperature rose, causing the decrease of the resistance on solid–liquid boundary layer. Specifically, the effects of temperature on the sorption of WC400 and WC600 were more obvious than WC200. This difference could be explained that the pores of the medium–high temperature biochars were gradually exposed and the biochar surfaces were activated with increasing temperature.45 It was assumed that pore filling was more sensitive to temperature than hydrogen bonding. According to Table S2,† all the values of ΔG were negative (−2.31 to −0.38 kJ mol−1), indicating that CIP sorption was spontaneous and thermodynamics favorable. This was in accordance with a previously reported result about CIP sorption.46 The positive value of ΔH (4.54–20.9 kJ mol−1) showed that CIP sorption to biochar was an endothermic process. Moreover, a gradual increase in sorption randomness was further proved by the positive values of ΔS (0.019–0.074 kJ mol−1 K−1).According to Fig. 6a–c, the value of site energy E* versus CIP loading for three kinds of biochars presented a similar trend. With the increase in CIP loading, E* acutely decreased, indicating that CIP preferentially took up the high-energy sorption points, and then moved to the low-energy sorption sites on biochars. The results were similar to the energy change of tetracycline sorption to biochars.47 The curves of site energy distribution for CIP sorption also exhibited similar features (Fig. 6d–f). With the increase in sorption energy, F(E*) increased to achieve a peak, and then dramatically decreased in the range of experimental data. The peak value of curve (F(E*)) represented the maximum distribution frequency at the specific site energy referred as 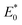 and the area below the curve could be deemed to be the number of sorption sites.48,49 It was observed that the area under the curve of WC400 gradually enlarged with increasing temperature. This phenomenon could be interpreted that more high-energy sorption sites were obtained at higher temperatures, which promoted the sorption affinity between CIP and biochar. Furthermore, WC600 exhibited a higher F(E*) value compared with WC200 and WC400, suggesting there were more high-energy sites for CIP sorption to WC600.
and the area below the curve could be deemed to be the number of sorption sites.48,49 It was observed that the area under the curve of WC400 gradually enlarged with increasing temperature. This phenomenon could be interpreted that more high-energy sorption sites were obtained at higher temperatures, which promoted the sorption affinity between CIP and biochar. Furthermore, WC600 exhibited a higher F(E*) value compared with WC200 and WC400, suggesting there were more high-energy sites for CIP sorption to WC600.
Moreover, the right side of 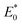 in the energy distribution was defined as the high-energy area and the left side was the low-energy area.50 Therefore, CIP sorption was concentrated on low-energy and middle-energy site areas according to Fig. 6d–f. Noticeably,
in the energy distribution was defined as the high-energy area and the left side was the low-energy area.50 Therefore, CIP sorption was concentrated on low-energy and middle-energy site areas according to Fig. 6d–f. Noticeably, 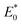 of WC600 increased when temperature rose from 298 K to 308 K, which could be due to the enhancement of π–π interactions. It could be elucidated that increasing temperature made π-acceptors and π-donors of biochar surfaces more activated, causing a slight reinforcement of sorption.47 Less movement of
of WC600 increased when temperature rose from 298 K to 308 K, which could be due to the enhancement of π–π interactions. It could be elucidated that increasing temperature made π-acceptors and π-donors of biochar surfaces more activated, causing a slight reinforcement of sorption.47 Less movement of 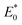 in energy distribution curve of WC200 resulted from the insensitivity of hydrogen bonding to temperature, which was consistent with the results of thermodynamic studies. As for WC400, although
in energy distribution curve of WC200 resulted from the insensitivity of hydrogen bonding to temperature, which was consistent with the results of thermodynamic studies. As for WC400, although 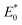 changed slightly, the increase in F(E*) value could be due to the exposure of the sorption sites as temperature increased.
changed slightly, the increase in F(E*) value could be due to the exposure of the sorption sites as temperature increased.
3.5 Prediction of CIP sorption to biochars
Due to the different sorption behaviors of CIP species to biochars, two improved Freundlich models were proposed to predict CIP sorption affected by speciation. H/C, O/H and SSA, representing the aromaticity, polarity and specific surface area of biochars, respectively, were considered as the important parameters affecting sorption.51 For WC300–WC700, H/C showed a preferable linear relationship with KF and N from Freundlich model compared with SSA and O/C, thus it was chosen as the sorbent parameter to predict sorption. The improved Freundlich model was proposed as:| qe = (aH/C + b)Ce(cH/C+d) | (10) |
The speciation model could be described as:52,53
| Kd = Kd+α+ + Kd±α± + Kd−α− | (11) |
According to the eqn (10), (11) and (3), two modified Freundlich models (eqn (12) and (13)) could be obtained to predict CIP sorption.
| Kd = KF+CwN+−1α+ + KF±CwN±−1α± + KF−CwN−−1α− | (12) |
| Kd = (a1H/C + b1)Cw(c1H/C+d1)α+ + (a2H/C + b2)Cw(c2H/C+d2)α± + (a3H/C + b3)Cw(c3H/C+d3)α− | (13) |
The proposed model parameters were calculated by Microsoft Excel solver tool according to the experiment data on CIP sorption and then the predicted log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd values of CIP to WC200 and WC300–WC700 were obtained from eqn (12) and (13), respectively.
Kd values of CIP to WC200 and WC300–WC700 were obtained from eqn (12) and (13), respectively.
The prediction models performed excellently in depicting the sorption for different species of CIP by biochars (Fig. 7a and b). Specifically, most deviations between the modeled and measured Kd values of CIP to WC200 and WC300–WC700 were less than 0.2 and 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log unit, respectively. However, some modeled Kd values of CIP− to WC300–WC700 were lower than experimental values in this study, with deviations more than 0.5
log unit, respectively. However, some modeled Kd values of CIP− to WC300–WC700 were lower than experimental values in this study, with deviations more than 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log unit. It was speculated that this underestimation may be due to the strong electrostatic repulsion between CIP− and biochars. Furthermore, some data regarding CIP sorption reported in other literature were collected to verify the practicality of the model,19,54–56 and the results showed that the prediction effect was good, with deviations between measured and modeled Kd values within 0.5
log unit. It was speculated that this underestimation may be due to the strong electrostatic repulsion between CIP− and biochars. Furthermore, some data regarding CIP sorption reported in other literature were collected to verify the practicality of the model,19,54–56 and the results showed that the prediction effect was good, with deviations between measured and modeled Kd values within 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log unit (Fig. 7b). These deviations may be ascribed to the pH change during the sorption process, causing the slight change of the proportion of different CIP species in the solution. Overall, the deviations between the measured and modeled values of Kd for all the data were less than 1
log unit (Fig. 7b). These deviations may be ascribed to the pH change during the sorption process, causing the slight change of the proportion of different CIP species in the solution. Overall, the deviations between the measured and modeled values of Kd for all the data were less than 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log unit. The established models can be well applied to predict the sorption of ionizable antibiotic CIP to biochars.
log unit. The established models can be well applied to predict the sorption of ionizable antibiotic CIP to biochars.
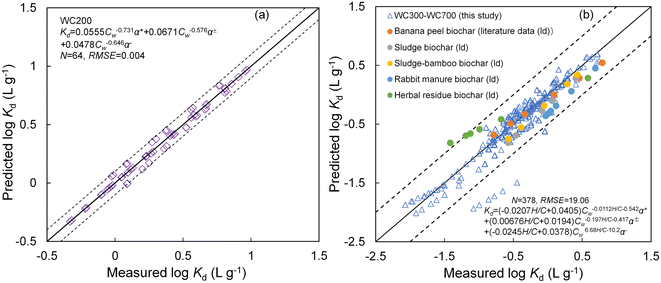 | ||
Fig. 7 Modeled and measured log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd values of CIP to woodchip-derived biochar at 200 °C (WC200) (a) and comparison between the measured and modeled log Kd values of CIP to woodchip-derived biochar at 200 °C (WC200) (a) and comparison between the measured and modeled log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd values of CIP to woodchip-derived biochars at 300–700 °C (WC300–WC700) and other biochars reported in literature: banana peel-derived biochar at 750 °C,54 sludge and sludge-bamboo derived biochars at 700 °C,19 rabbit manure biochar at 400 °C,55 and herbal residue-derived biochar at 800 °C (b).56 The 1 Kd values of CIP to woodchip-derived biochars at 300–700 °C (WC300–WC700) and other biochars reported in literature: banana peel-derived biochar at 750 °C,54 sludge and sludge-bamboo derived biochars at 700 °C,19 rabbit manure biochar at 400 °C,55 and herbal residue-derived biochar at 800 °C (b).56 The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 line is represented by the solid lines, and 0.2 (a) and 0.5 (b) log unit deviations are represented by the dotted lines. H/C indicates the aromaticity of biochars, and α+, α± and α− indicates the percentage of CIP+, CIP± and CIP− in solution, respectively (%). N represents the number of fitting points. RMSE denotes the root mean squared error. 1 line is represented by the solid lines, and 0.2 (a) and 0.5 (b) log unit deviations are represented by the dotted lines. H/C indicates the aromaticity of biochars, and α+, α± and α− indicates the percentage of CIP+, CIP± and CIP− in solution, respectively (%). N represents the number of fitting points. RMSE denotes the root mean squared error. | ||
It is noting that the models are more suitable for application in water environment, which can quantitatively predict the sorption behavior of CIP at the biochar–water interface. When biochar is added to the soil, the soil may cause changes in the physical and chemical properties of biochars by clogging the pores of biochars.57 This will affect the sorption prediction accuracy. Thus, it is necessary to further analyze the influence of actual environmental conditions, such as soil components and its inherent properties, on sorption and continuously improve the accuracy of the prediction mode.
4. Conclusions
Sorption mechanisms dynamically changed with pyrolysis temperature of biochar and CIP speciation. Sorption affinity of WC200 for different forms of CIP decreased as CIP± > CIP+ > CIP−, while that of WC300–WC700 followed the order of CIP+ > CIP± > CIP−. Sorption thermodynamics implied that the sorption process was spontaneous and endothermic. The analysis of site energy distribution suggested that rising temperature caused the increase in the site energy and its maximum occurring frequency, leading to the marked enhancement of CIP sorption by WC400. It can be speculated that high-temperature biochars (WC600 or WC700) could be appropriately amended to strongly acidic water environment to immobilize CIP owing to their higher sorption for CIP+ at pH 3.0, while WC200 could be used to remove CIP at a wider pH range. Sorption of CIP to WC200–WC700 and other kinds of biochars with varying carbonization degrees could be well predicted by the proposed models regarding sorbate speciation and sorbent aromaticity, with most deviations of sorption coefficient (Kd) within 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log unit. These findings revealed the difference in sorption mechanism of biochars with varying carbonization degrees for ionizable antibiotics.
log unit. These findings revealed the difference in sorption mechanism of biochars with varying carbonization degrees for ionizable antibiotics.
Author contributions
Guowei Shi: formal analysis, investigation, visualization, writing – original draft. Yasong Li: conceptualization, methodology, resources, validation. Yaci Liu: data curation, writing – review & editing. Lin Wu: funding acquisition, project administration, supervision.Conflicts of interest
We declare that we have no conflicts of interest to this work.Acknowledgements
This work was funded by the National Natural Science Foundation of China (41902259), the Natural Science Foundation of Hebei Province of China (D2020504006) and China Geological Survey project (DD20190303).References
- T. B. Nguyen, Q. M. Truong, C. W. Chen, W. H. Chen and C. D. Dong, Bioresour. Technol., 2022, 351, 127043 CrossRef CAS PubMed.
- Q. Sun, M. Li, C. Ma, X. Chen, X. Xie and C. Yu, Environ. Pollut., 2016, 208, 371–381 CrossRef CAS PubMed.
- H. Zhang, Y. Zhou, Y. Huang, L. Wu, X. Liu and Y. Luo, Chemosphere, 2016, 152, 229–237 CrossRef CAS PubMed.
- N. Gottschall, E. Topp, C. Metcalfe, M. Edwards, M. Payne, S. Kleywegt, P. Russell and D. R. Lapen, Chemosphere, 2012, 87, 194–203 CrossRef CAS PubMed.
- R. Mirzaei, M. Yunesian, S. Nasseri, M. Gholami, E. Jalilzadeh, S. Shoeibi and A. Mesdaghinia, Sci. Total Environ., 2018, 619, 446–459 CrossRef PubMed.
- L. Zhou, G. Ying, J. Zhao, J. Yang, L. Wang, B. Yang and S. Liu, Environ. Pollut., 2011, 159, 1877–1885 CrossRef CAS PubMed.
- S. M. Zainab, M. Junaid, N. Xu and R. N. Malik, Water Res., 2020, 187, 116455 CrossRef CAS PubMed.
- S. Ganesan, M. Amirthalingam, P. Arivalagan, S. Govindan, S. Palanisamy, A. P. Lingassamy and V. K. Ponnusamy, J. Environ. Manage., 2019, 245, 409–417 CrossRef CAS PubMed.
- A. N. Ngigi, Y. S. Ok and S. Thiele-Bruhn, J. Hazard. Mater., 2019, 364, 663–670 CrossRef CAS PubMed.
- B. Zhao, H. Xu, F. Ma, T. Zhang and X. Nan, RSC Adv., 2019, 9, 5218–5223 RSC.
- F. Qin, J. Li, C. Zhang, G. Zeng, D. Huang, X. Tan, D. Qin and H. Tan, Sci. Total Environ., 2022, 818, 151774 CrossRef CAS PubMed.
- F. Lian and B. Xing, Environ. Sci. Technol., 2017, 51, 13517–13532 CrossRef CAS PubMed.
- A. Zielińska and P. Oleszczuk, Environ. Sci. Pollut. Res., 2016, 23, 21822–21832 CrossRef PubMed.
- Y. Sun, T. Wang, L. Bai, C. Han and X. Sun, J. Environ. Chem. Eng., 2022, 10, 108292 CrossRef CAS.
- M. Teixido, C. Hurtado, J. J. Pignatello, J. L. Beltran, M. Granados and J. Peccia, Environ. Sci. Technol., 2013, 47, 6197–6205 CrossRef CAS PubMed.
- G. Fang, J. Li, C. Zhang, F. Qin, H. Luo, C. Huang, D. Qin and Z. Ouyang, Environ. Pollut., 2022, 300, 118939 CrossRef CAS PubMed.
- P. Wu, B. P. Singh, H. Wang, Z. Jia, Y. Wang and W. Chen, Biochar, 2023, 5, 6 CrossRef.
- X. Zheng, X. He, H. Peng, J. Wen and S. Lv, Bioresour. Technol., 2021, 334, 125238 CrossRef CAS PubMed.
- J. Li, G. Yu, L. Pan, C. Li, F. You and Y. Wang, Environ. Sci. Pollut. Res., 2020, 27, 22806–22817 CrossRef CAS PubMed.
- K. Luo, Y. Pang, Q. Yang, D. Wang, X. Li, L. Wang, M. Lei and J. Liu, Chemosphere, 2019, 231, 495–501 CrossRef CAS PubMed.
- Y. Ma, M. Li, P. Li, L. Yang, L. Wu, F. Gao, X. Qi and Z. Zhang, Bioresour. Technol., 2021, 319, 10 CrossRef PubMed.
- N. Boraah, S. Chakma and P. Kaushal, J. Environ. Chem. Eng., 2022, 10, 107825 CrossRef CAS.
- S. Z. Yi, B. Gao, Y. Y. Sun, J. C. Wu, X. Q. Shi, B. J. Wu and X. Hu, Chemosphere, 2016, 150, 694–701 CrossRef CAS PubMed.
- P. Liu, W. Liu, H. Jiang, J. Chen, W. Li and H. Yu, Bioresour. Technol., 2012, 121, 235–240 CrossRef CAS PubMed.
- L. Li, D. Zou, Z. Xiao, X. Zeng, L. Zhang, L. Jiang, A. Wang, D. Ge, G. Zhang and F. Liu, J. Cleaner Prod., 2019, 210, 1324–1342 CrossRef CAS.
- X. Zhou, L. Shi, T. B. Moghaddam, M. Chen, S. Wu and X. Yuan, J. Hazard. Mater., 2022, 425, 128003 CrossRef CAS PubMed.
- M. B. Ahmed, J. L. Zhou, H. H. Ngo, W. Guo, M. A. H. Johir and K. Sornalingam, Chem. Eng. J., 2017, 311, 348–358 CrossRef CAS.
- H. Ji, T. Wang, T. Huang, B. Lai and W. Liu, J. Cleaner Prod., 2021, 278, 123924 CrossRef CAS.
- T.-B. Nguyen, Q.-M. Truong, C.-W. Chen, W.-H. Chen and C.-D. Dong, Bioresour. Technol., 2022, 351, 127043 CrossRef CAS PubMed.
- J. A. Ippolito, L. Cui, C. Kammann, N. Wrage-Mönnig, J. M. Estavillo, T. Fuertes-Mendizabal, M. L. Cayuela, G. Sigua, J. Novak, K. Spokas and N. Borchard, Biochar, 2020, 2, 421–438 CrossRef.
- J. Z. Lima, A. P. Ogura, L. C. M. d. Silva, I. M. R. Nauerth, V. G. S. Rodrigues, E. L. G. Espíndola and J. P. Marques, J. Environ. Chem. Eng., 2022, 10, 108192 CrossRef CAS.
- G. Sigmund, H. Sun, T. Hofmann and M. Kah, Environ. Sci. Technol., 2016, 50, 3641–3648 CrossRef CAS PubMed.
- X. Fan, X. Wang, B. Zhao, J. Wan, J. Tang and X. Guo, J. Environ. Chem. Eng., 2022, 10, 107328 CrossRef CAS.
- Q. Fang, B. Chen, Y. Lin and Y. Guan, Environ. Sci. Technol., 2014, 48, 279–288 CrossRef CAS PubMed.
- M. Ahmad, S. S. Lee, A. U. Rajapaksha, M. Vithanage, M. Zhang, J. S. Cho, S. E. Lee and Y. S. Ok, Bioresour. Technol., 2013, 143, 615–622 CrossRef CAS PubMed.
- X. Tan, Y. Liu, G. Zeng, X. Wang, X. Hu, Y. Gu and Z. Yang, Chemosphere, 2015, 125, 70–85 CrossRef CAS PubMed.
- Y. Sun, G. Yang, L. Zhang and Z. Sun, Process Saf. Environ. Prot., 2017, 107, 281–288 CrossRef CAS.
- K. Weber and P. Quicker, Fuel, 2018, 217, 240–261 CrossRef CAS.
- F. Lian, B. Sun, Z. Song, L. Zhu, X. Qi and B. Xing, Chem. Eng. J., 2014, 248, 128–134 CrossRef CAS.
- C. Peiris, S. R. Gunatilake, T. E. Mlsna, D. Mohan and M. Vithanage, Bioresour. Technol., 2017, 246, 150–159 CrossRef CAS PubMed.
- M. Kah, G. Sigmund, F. Xiao and T. Hofmann, Water Res., 2017, 124, 673–692 CrossRef CAS PubMed.
- H. Sheng, Y. Yin, L. Xiang, Z. Wang, J. D. Harindintwali, J. Cheng, J. Ge, L. Zhang, X. Jiang, X. Yu and F. Wang, Chemosphere, 2022, 299, 134446 CrossRef CAS PubMed.
- J. Ni, J. J. Pignatello and B. Xing, Environ. Sci. Technol., 2011, 45, 9240–9248 CrossRef CAS PubMed.
- F. Xiao and J. J. Pignatello, Water Res., 2015, 80, 179–188 CrossRef CAS PubMed.
- R. Han, W. Zou, H. Li, Y. Li and J. Shi, J. Hazard. Mater., 2006, 137, 934–942 CrossRef CAS.
- J. Zhao, G. Liang, X. Zhang, X. Cai, R. Li, X. Xie and Z. Wang, Sci. Total Environ., 2019, 688, 1205–1215 CrossRef CAS PubMed.
- Y. Zhou, Y. He, Y. He, X. Liu, B. Xu, J. Yu, C. Dai, A. Huang, Y. Pang and L. Luo, Sci. Total Environ., 2019, 650, 2260–2266 CrossRef CAS PubMed.
- J. Liu, B. Zhou, H. Zhang, J. Ma, B. Mu and W. Zhang, Bioresour. Technol., 2019, 294, 122152 CrossRef CAS PubMed.
- X. Shen, X. Guo, M. Zhang, S. Tao and X. Wang, Environ. Sci. Technol., 2015, 49, 4894–4902 CrossRef CAS PubMed.
- X. Li, F. Lei, B. Li and E. Bi, J. Environ. Qual., 2021, 50, 706–716 CrossRef CAS PubMed.
- G. Sigmund, M. Gharasoo, T. Hüffer and T. Hofmann, Environ. Sci. Technol., 2020, 54, 4583–4591 CrossRef CAS PubMed.
- Z. Chen, X. Xiao, B. Xing and B. Chen, Environ. Pollut., 2019, 248, 48–56 CrossRef CAS PubMed.
- M. Teixidó, J. J. Pignatello, J. L. Beltrán, M. Granados and J. Peccia, Environ. Sci. Technol., 2011, 45, 10020–10027 CrossRef PubMed.
- M. Patel, R. Kumar, C. U. Pittman and D. Mohan, Environ. Res., 2021, 201, 111218 CrossRef CAS PubMed.
- W. Huang, J. Chen and J. Zhang, Environ. Technol., 2020, 41, 1380–1390 CrossRef CAS PubMed.
- J. Shang, X. Kong, L. He, W. Li and Q. Liao, Int. J. Environ. Sci. Technol., 2016, 13, 2449–2458 CrossRef CAS.
- Y. Liu and J. Chen, Chemosphere, 2022, 292, 133427 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra00122a |
| This journal is © The Royal Society of Chemistry 2023 |