Open Access Article
Open Access ArticleDependence of 1H-NMR T1 relaxation time of trimethylglycine betaine deep eutectic solvents on the molar composition and on the presence of water†‡
Chiara Allegretti a,
Paola D'Arrigo
a,
Paola D'Arrigo *ab,
Francesco G. Gatti
*ab,
Francesco G. Gatti *a,
Letizia A. M. Rossato
*a,
Letizia A. M. Rossato a and
Eleonora Ruffini
a and
Eleonora Ruffini a
a
aDepartment of Chemistry, Materials and Chemical Engineering “Giulio Natta”, Politecnico di Milano, P.zza Leonardo da Vinci 32, Milano, 20133, Italy. E-mail: paola.darrigo@polimi.it; francesco.gatti@polimi.it
bIstituto di Scienze e Tecnologie Chimiche “Giulio Natta” – Consiglio Nazionale delle Ricerche (SCITEC-CNR), Via Luigi Mancinelli 7, Milano, 20131, Italy
First published on 19th January 2023
Abstract
1H-NMR spin lattice relaxation times (T1), measured by inversion recovery technique, allowed to establish the stoichiometric coefficient (ratio between the H-bond acceptor and H-bond donor) of a series of trimethylglycine betaine/diol based deep eutectic solvents (DESs); ethylene glycol, triethylene glycol and 1,3-propandiol were selected as H-bond donors. The maximum amount of water tolerated by the DES, before its complete hydration, was determined as well. Finally, the method was validated comparing the eutectic composition of the betaine/glycol system with that determined by means of differential scanning calorimetry analysis; the stoichiometric coefficients were identical.
The term eutectic was coined by Guthrie in 1884 from the old Greek eutecticos, easy meltdown, to describe the melting point depression of a mixture with respect to its pure components.1 The term deep eutectic solvent (DES) was used for the first time in the seminal work of Abbott in 2003,2 where the adjective “deep” was meant to describe a much lower melting temperature than the ideal mixture of its components, typically two. Ever since many others DESs were discovered, and nowadays this new class of solvents has become extremely popular, especially in green chemistry. Later in 2011, Choi introduced the term natural DES (NADES), to describe the so called third liquid phase in microbial mammalian and plant cells, besides water phase and membrane lipids phase.3,4
Even though the scientific community is still debating on a full comprehension of the nature of these solvents,5 during the last decades, the number of DES-based applications in a wide range of fields has rapidly grown.6–9
In addition to their melting/freezing point, these solvents are characterized by many other physicochemical properties such as viscosity, density, ionic conductivity, surface tension, vapour pressure and refractive index.10 Some of these observables (ionic conductivity, solvent polarity and viscosity) vary significantly with the molar composition of the mixture.10,11 Noteworthy, very recently, it was found that the presence of small amounts of water (embedded water) might play an important role in promoting the DES formation.12
At nanoscopic level, the combination of a hydrogen bond acceptor HBA (usually solid) with a hydrogen bond donor HBD (either liquid or solid) leads to the formation of an HBA⋯HBD supramolecule, self-assembled by means of a cluster of H-bonds, and accordingly the mixture becomes liquid at eutectic temperature only at a definite HBA/HBD molar ratio (eutectic composition). However, it is not rare to find in literature different stoichiometric coefficients for the same DES. For instance, the formation of trimethylglycine betaine/ethylene glycol DES has been reported with three different stoichiometries: the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 was used for the biocatalyzed synthesis of phospholipids in water,13 whereas for the purification of gasoline a ratio of 1
2 was used for the biocatalyzed synthesis of phospholipids in water,13 whereas for the purification of gasoline a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 was preferred,14 the same composition was later found by Paiva,15 instead, for the extraction of palmitic acid from palm oil, a mixture with more glycol (1
3 was preferred,14 the same composition was later found by Paiva,15 instead, for the extraction of palmitic acid from palm oil, a mixture with more glycol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) was applied.16 Lastly, it is not always clear which is the maximum amount of water tolerated before that the H-bond disruption of the supramolecule occurs, in any case, the water added, typically does not exceed the 30–50% in weight.17
4) was applied.16 Lastly, it is not always clear which is the maximum amount of water tolerated before that the H-bond disruption of the supramolecule occurs, in any case, the water added, typically does not exceed the 30–50% in weight.17
Prompted by the above considerations, the following study aims: (i) to verify if and how the 1H-NMR spin-lattice relaxation time (T1) of a set of selected DESs changes with the HBD molar fraction (X), and (ii) to ascertain how such a suggested correlation might be modified by progressive additions of water.
Our choice of the longitudinal relaxation time constant T1 as an observable potentially informative of DES-supramolecule formation is based on its well-known dependence on the molecular tumbling motion (random molecular rotations and diffusion movements) in a liquid.18,19 Thus, it is reasonable to think that larger and more rigid is an organic molecule, slower is its tumbling in solution. Indeed, without going too much in details of the complex spin-lattice relaxation theory, T1 depends, among many other variables, on the molecular size and therefore on the molecular weight. Now, such relationship hints our tentative conjecture that the DES-supramolecule formed at the eutectic point might be characterized by a lower tumbling rate than its HBD component and conceivably by a specific value of T1.
In the frame of our ongoing research work13 we focused our attention on the trimethylglycine betaine/diol based DESs. In Fig. 1 the selected HBD diols to be combined with the HBA trimethylglycine betaine (Gb) are shown: ethylene glycol (D1), triethylene glycol (D2) and 1,3-propandiol (D3), for more details on sample's preparation see the experimental part. However, the moisture content of diols was checked by Karl Fischer titration (<1.5% in weight) before mixing with Gb.
The T1 were measured by inversion recovery method at 75 °C, to avoid detrimental effects of viscosity on the linewidth (i.e., spectral resolution) of the 1H-NMR spectra. In addition, at this temperature we could widen the number of observable HBA/HBD mixtures, especially those with molar fraction X (X = [diol]/([diol] + [Gb])) lower than the eutectic mixture (infra eutectic region), which otherwise at room temperature would precipitate.
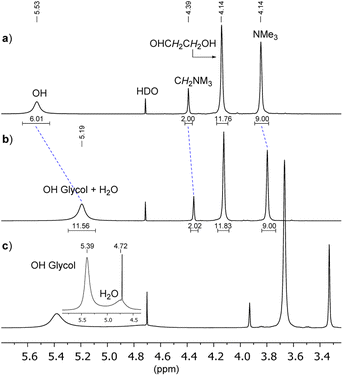
In Fig. 2a we show the 1H-NMR spectra of Gb/D1 DES (molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, at 75 °C, external lock on D2O in a coaxial tube). The OH resonance (broad singlet, chemical shift δ = 5.53 ppm) is clearly well separated from the other signals, moreover, using a long relaxation delay (d = 20 s), the integrals result fully consistent with the ethylene glycol molar fraction of the mixture (XD1 ≈ 0.75). Then, by adding 3 eq. of water to the freshly prepared DES, the OH signal appears significantly shielded (δ from 5.53 to 5.19 ppm) indicating a partial disruption of the H-bond self-assembled supramolecule (Fig. 2b), whereas the chemical shift of the other signals did not change so much.
3, at 75 °C, external lock on D2O in a coaxial tube). The OH resonance (broad singlet, chemical shift δ = 5.53 ppm) is clearly well separated from the other signals, moreover, using a long relaxation delay (d = 20 s), the integrals result fully consistent with the ethylene glycol molar fraction of the mixture (XD1 ≈ 0.75). Then, by adding 3 eq. of water to the freshly prepared DES, the OH signal appears significantly shielded (δ from 5.53 to 5.19 ppm) indicating a partial disruption of the H-bond self-assembled supramolecule (Fig. 2b), whereas the chemical shift of the other signals did not change so much.
Since both OH protons of DES and of water at 75 °C are under rapid chemical exchange, the observed chemical shift is the weighted average of the chemical shifts of the two species. Indeed, by lowering the temperature at 29 °C it was possible to discriminate the different nature of OHs (5.39 ppm vs. 4.72 ppm, Fig. 2c).
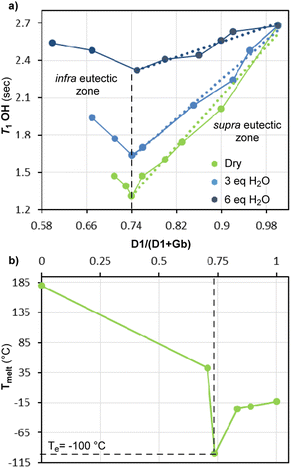
Now, to begin with aim (i), the measured T1 of OH proton in Gb/D1 mixtures appears to change with the molar fraction (XD1) as shown in Fig. 3a. Indeed, the diagram T1 versus XD1 exhibits a minimum (T1 = 1.26 s) in correspondence of the molar composition Gb/D1 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (XD1 ≈ 0.75), somehow reminding the customary solid–liquid phase diagram of binary eutectic mixtures. However, this point should correspond to the formation of the Gb(D1)3 supramolecule, in which the lowest mobility of OH is most likely due to the formation of stronger H-bonds in the newly self-assembled supramolecule. Besides, in the ultra-eutectic region, the relaxation time increases linearly as the XD1 increases (linear regression equation: T1 = −2.46 + 5.0 XD1, R2 = 0.98) reaching the upper value of neat D1 (technical glycol T1 = 2.63 s vs. T1 = 2.11 s of pure and anhydrous glycol,20 purity ≥99.8%, the two values are different likely for the moisture, lit.21 H2O T1 = 9.11 s at 75 °C).
3 (XD1 ≈ 0.75), somehow reminding the customary solid–liquid phase diagram of binary eutectic mixtures. However, this point should correspond to the formation of the Gb(D1)3 supramolecule, in which the lowest mobility of OH is most likely due to the formation of stronger H-bonds in the newly self-assembled supramolecule. Besides, in the ultra-eutectic region, the relaxation time increases linearly as the XD1 increases (linear regression equation: T1 = −2.46 + 5.0 XD1, R2 = 0.98) reaching the upper value of neat D1 (technical glycol T1 = 2.63 s vs. T1 = 2.11 s of pure and anhydrous glycol,20 purity ≥99.8%, the two values are different likely for the moisture, lit.21 H2O T1 = 9.11 s at 75 °C).
As initial approximation, we reckon that the T1 observed in this region of the diagram is the molar average of the eutectic point and neat glycol values. However, quite recently, it was shown by IR and Raman spectroscopy that ethylene glycol is in equilibrium with its H-bond self-assembled dimer,22 thus it is not unreasonable to think that the measured T1 might be arise also from other supramolecular species present in solution.
On the other hand, in the infra-eutectic region of the diagram, the relaxation time becomes longer as XD1 decreases. However, since the leftmost point of this region was determined from a mixture with XD1 ≈ 0.71, not much different from that of the eutectic composition (XD1 ≈ 0.75), the correlation between the small increments of T1 (from 1.26 s to 1.42 s) and the progressive dissociation of DES supramolecule was not reliable.
Finally, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 eutectic stoichiometry found by OH T1 measurements (data in agreement with that reported by Paiva15) was confirmed by differential scanning calorimetry (DSC) analysis (Fig. 3b), and a eutectic temperature (Te) of −100 °C was determined.
3 eutectic stoichiometry found by OH T1 measurements (data in agreement with that reported by Paiva15) was confirmed by differential scanning calorimetry (DSC) analysis (Fig. 3b), and a eutectic temperature (Te) of −100 °C was determined.
Intrigued by the net influence of DES formation on the OH bonding donor mobility, the relaxation times of the remaining proton signals were analysed as well (see ESI‡); however, the variations of T1 were not anymore significantly traceable to a neat formation of Gb(D1)3, mainly for two reasons: (i) these hydrogens are not involved in strong non-covalent interactions and therefore their mobility is less influenced by the formation of the H-bond self-assembled supramolecule; (ii) the chemical shift of these signals may change with X, and in some mixtures partial overlap of signals occurred, making the T1 measure less accurate and reliable, and consequentially not anymore diagnostic of DES formation.
Concerning aim (ii), the relaxation time measurements of the Gb/D1 mixtures were repeated on samples containing three and then six equivalents of water with respect to the HBA Gb (Fig. 3a, blue and blue-navy solid lines, respectively). The T1 diagrams of the wet mixtures have a similar shape to that of the “dry” system (H2O < 1.5% in weight), but there are two discernible differences. First, by adding H2O the T1 value at the eutectic point becomes longer, in agreement with recently published studies,23 secondly, the slopes of both regions of the diagram decrease remarkably (for instance in the ultra-eutectic region T1 = 1.35 + 3.95 XD1 with 3 eq. of H2O and T1 = 1.14 + 1.51 XD1 with 6 eq. of H2O, R2 = 0.98 and R2 = 0.94, respectively).
These observations suggest that the addition of water has the beneficial effect of reducing the viscosity, while the eutecticity of the system is partially conserved; however, it is conceivable that higher concentrations of water promote the complete dissociation of the eutectic supramolecule Gb(D1)3 in the individual hydrated components.
Lastly, we repeated the T1 measurements for the Gb/D2 and Gb/D3 mixtures (Fig. 4a and b), and analogously to that seen for the Gb/D1 system, the OH mobility of each diol decreased linearly to a minimum value in the correspondence of the eutectic point. More precisely, for the Gb/D2 system the T1 of triethylene glycol decreased from 2.03 s to 1.35 s, (T1 = 1.30 − 3.29 XD2, R2 = 0.98), suggesting that the formation of DES-supramolecule occurs most likely with the Gb(D2)4 stoichiometry (ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, XD3 ≈ 0.80), such eutectic composition fully agreed with the one reported in literature.24 While for Gb/D3, the formation of DES-supramolecule was achieved by mixing Gb with D3 in a ratio of 1
4, XD3 ≈ 0.80), such eutectic composition fully agreed with the one reported in literature.24 While for Gb/D3, the formation of DES-supramolecule was achieved by mixing Gb with D3 in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (XD3 ≈ 0.75, Gb(D3)3), indeed at such molar composition the OH relaxation time reached the lowest value (i.e., T1 = 1.11 s with T1 = 0.25 − 1.78 XD3, R2 = 0.99). Even in this case, the eutectic composition determined by T1 measurements resulted in full agreement with the literature data.24
3 (XD3 ≈ 0.75, Gb(D3)3), indeed at such molar composition the OH relaxation time reached the lowest value (i.e., T1 = 1.11 s with T1 = 0.25 − 1.78 XD3, R2 = 0.99). Even in this case, the eutectic composition determined by T1 measurements resulted in full agreement with the literature data.24
In conclusion, we have shown that T1 measurements allow to establish the appropriate stoichiometry to which the trimethylglycine betaine and the diol form the corresponding DES. The T1 values were determined by inversion recovery method on a standard NMR instrumentation using an automated system; the eutectic compositions were validated by DSC analysis. All in all, the presented methodology compares well in terms of simplicity and time consuming with other analytical methods, especially if it will be updated with the new rapid T1 estimation technique, recently reported.25
Lastly, our study shows that the trimethylglycine betaine/glycol DES can tolerate a maximum of 15% in weight of water at 75 °C, indeed, using a higher amount of water (26% in weight), the ethylene glycol seems no longer H-bonded to the glycine betaine.
Author contributions
P. D'Arrigo and F. G. Gatti conceived and designed the experiments. C. Allegretti, L. A. M. Rossato and E. Ruffini performed experiments and analysed data. P. D'Arrigo and F. G. Gatti prepared the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We express our gratitude to Prof. Franca Castiglione (Politecnico of Milan) for fruitful comments and discussion of results. L. A. M. R. acknowledges MIUR for PhD grant (XXXVI Research Doctorate Cycle).Notes and references
- F. Guthrie, Phylosof. Mag. Ser., 1884, 5, 462–482 CrossRef.
- A. P. Abbott, G. Capper, D. L. Davies, R. K. Raasheed and V. Tambyrajah, Chem. Commun., 2003, 99, 70–71 RSC.
- Y. H. Choi, J. van Spronsen, Y. Dai, M. Verberne, F. Hollmann, I. W. C. E. Arends, G. Witkamp and R. Verpoorte, Plant Physiol., 2011, 156, 1701–1705 CrossRef CAS PubMed.
- A. Triolo, F. Lo Celso, M. Brehm, V. Di Lisio and O. Russina, J. Mol. Liq., 2021, 331, 115750 CrossRef CAS.
- M. A. R. Martins, S. P. Pinho and J. A. Coutinho, J. Sol. Chem., 2019, 48, 962–982 CrossRef CAS.
- E. L. Smith, A. P. Abbott and K. S. Ryder, Chem. Rev., 2014, 114, 11060–11081 CrossRef CAS PubMed.
- F. M. Perna, P. Vitale and V. Capriati, Curr. Opin. Green Sustain. Chem., 2020, 21, 27–33 CrossRef.
- C. Allegretti, E. Bellinetto, P. D'Arrigo, G. Griffini, S. Marzorati, L. A. M. Rossato, E. Ruffini, L. Schiavi, S. Serra, A. Strini, D. Tessaro and S. Turri, Fermentation, 2022, 8(4), 151 CrossRef CAS.
- C. Allegretti, E. Bellinetto, P. D'Arrigo, M. Ferro, G. Griffini, L. A. M. Rossato, E. Ruffini, L. Schiavi, S. Serra, A. Strini and S. Turri, Molecules, 2022, 27(24), 8879 CrossRef CAS PubMed.
- B. Hansen, S. Spittle, B. Chen, D. Poe, Y. Zhang, J. M. Klein, A. Horton, L. Adhikari, T. Zelovich, B. W. Doherty, B. Gurkan, E. J. Maginn, A. Ragauskas, M. Dadmun, T. A. Zawodzinski, G. A. Baker, M. E. Tuckerman, R. F. Savinell and J. R. Sangoro, Chem. Soc. Rev., 2021, 121, 1232–1285 CrossRef CAS PubMed.
- Q. Zang, K. De Oliveira Vigier, S. Royer and F. Jerome, Chem. Soc. Rev., 2012, 41, 7108–7146 RSC.
- C. Ma, A. Laaskonen, C. Liu, X. Lu and X. Ji, Chem. Soc. Rev., 2018, 47, 8685–8720 RSC.
- C. Allegretti, F. G. Gatti, S. Marzorati, L. A. M. Rossato, S. Serra, A. Strini and P. D'Arrigo, Catalysts, 2021, 11, 655 CrossRef CAS.
- K. Zagajski Kučan, M. Perković, K. Cmrk, D. Načinović and M. Rogošić, ChemistrySelect, 2018, 3, 12582–12590 CrossRef.
- L. A. Rodrigues, M. Cardeira, I. C. Leonardo, F. B. Gaspar, I. R. Redovniković, A. R. C. Duarte, A. Paiva and A. A. Matias, J. Mol. Liq., 2021, 335, 116201 CrossRef CAS.
- K. Mulia, D. Adam, I. Zahrina and E. A. Krisanti, Int. J. Technol., 2018, 2, 335–344 CrossRef.
- Y. Dai, G.-J. Witkamp, R. Verpoorte and Y. H. Choi, Food Chem., 2015, 187, 14–19 CrossRef CAS PubMed.
- N. Bloembergen, E. M. Purcell and R. V. Pounds, Phys. Rev., 1948, 73, 679–712 CrossRef CAS.
- M. L. Levitt, Spin Dynamics, Wiley, 2nd edn, 2008 Search PubMed.
- We couldn’t find a measure of T1 at 75 °C, but the value was extrapolated from T1= 13.89 − 0.1075T + 2.116 10−4 T2, W. M. Spees, S.-K. Song, J. R. Garbow, J. J. Neil and J.-J. H. Ackerman, Magn. Reson. Med., 2012, 68, 319–324 CrossRef CAS PubMed.
- K. Krynicki, Physica, 1966, 32, 167–178 CrossRef CAS.
- F. Kollipost, K. E. Otto and M. A. Suhm, Angew. Chem., Int. Ed., 2016, 55, 4591–4595 CrossRef CAS PubMed.
- M. E. Di Pietro, M. Tortora, C. Bottari, G. C. Dugoni, R. V. Pivato, B. Rossi, M. Paolantoni and A. Mele, ACS Sustainable Chem. Eng., 2021, 9, 12262–12273 CrossRef CAS.
- Y. Hsieh, Y. Li, Z. Pan, Z. Chen, J. Lu, J. Yuan, Z. Zhu and J. Zhang, Ultrason. Sonochem., 2020, 63, 104915 CrossRef PubMed.
- R. Wei, C. L. Dickson, D. Uhrín and G. C. Lloyd-Jones, J. Org. Chem., 2021, 86, 9023–9029 CrossRef CAS PubMed.
Footnotes |
| † The trimethylglycine betaine and diols were used as received by the suppliers without any further treatment. Trimethylglycine betaine (1.17 g, 1.0 mmol) was mixed with diol (see the ESI‡ for the molar ratio) and stirred at 75 °C until the reaction mixture appeared completely homogeneous, then, it was left to stir for others 2 hours. The freshly prepared mixtures were submitted to NMR analysis. All NMR experiments were carried out on an Avance 400 Bruker instrument at 75 °C or at 29 °C, using an automation routine (IconNMR software). 1H-NMR T1 relaxation times were measured using the Bruker library inversion recovery pulse program (Topspin software, version 2.5). D2O was used as external lock in coaxial tube (5 mm), and the chemical shift calibration was done on the HDO residual signal (δ = 4.71 ppm). Acquisition and processing parameters: number of scans = 1; relaxation delay d1 = 20 s; dummy scans ds = 2; variable delay list (s): 0.1, 0.2, 0.3, 0.5, 0.7, 1.0, 1.4, 1.8, 2.3, 2.8, 3.4, 4.1, 5.0, 7.0, 9.0, 12.0, 16.0, 20.0; line broadening lb = 2 Hz. T1 fittings were obtained using Dynamic Center software, version 2.7.4 (see ESI‡). |
| ‡ Electronic supplementary information (ESI) available: Copies of 1H-NMR spectra and T1 fitting. See DOI: https://doi.org/10.1039/d2ra08082f |
| This journal is © The Royal Society of Chemistry 2023 |