Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Hydrogen evolution reaction following the Slater–Pauling curve: acceleration of rate processes induced from dipole interaction between protons and ferromagnetic catalysts†
Masao Morishita *a,
Yuki Okumuraa,
Ramu Fukushimaa,
Hiroaki Yamamotoa and
Hidefumi Yanagitab
*a,
Yuki Okumuraa,
Ramu Fukushimaa,
Hiroaki Yamamotoa and
Hidefumi Yanagitab
aDepartment of Chemical Engineering and Materials Science, University of Hyogo, 2167 Shosha, Himeji 671-2201, Japan
bSanalloy Industry Co., Ltd., 290-44 Takahashi, Fukusaki-cho, Kanzaki 679-2216, Japan. E-mail: morisita@eng.u-hyogo.ac.jp
First published on 25th April 2023
Abstract
Developing new concepts to design noble-metal-free catalysts is necessary to achieve the hydrogen economy and reduce global CO2 emissions. Here, we provide novel insights into the design of catalysts with internal magnetic fields by investigating the relationship between the hydrogen evolution reaction (HER) and the Slater–Pauling rule. This rule states that adding an element to a metal reduces the alloy's saturation magnetization by an amount proportional to the number of valence electrons outside the d shell of the added element. We observed that rapid hydrogen evolution occurred when the magnetic moment of the catalyst was high, as predicted by the Slater–Pauling rule. Numerical simulation of the dipole interaction revealed a critical distance, rC, at which the proton trajectory changes from a Brownian random walk to a close-approach orbit towards the ferromagnetic catalyst. The calculated rC was proportional to the magnetic moment, consistent with the experimental data. Interestingly, rC was proportional to the number of protons contributing to the HER and accurately reflected the migration length for the proton dissociation and hydration and the O–H bond length in water. The magnetic dipole interaction between the nuclear spin of the proton and the electronic spin of the magnetic catalyst is verified for the first time. The findings of this study will open a new direction in catalyst design aided by an internal magnetic field.
Introduction
To reduce CO2 emissions, the production of hydrogen fuel instead of fossil fuels is critical.1 Although Pt-based catalysts are being widely used for hydrogen production,2–8 Pt is one of the most expensive noble metals. One strategy to achieve the hydrogen economy is to develop inexpensive catalysts with pertinent activity to replace Pt. In 1845, Faraday9 had an insight that magnetic forces affect electrolytes. However, his insight was greatly overlooked for the next 150 years. Recently, the use of magnetic fields has been highlighted as an effective strategy for improving the performance of catalysts for the oxygen evolution reaction (OER)10–16 and hydrogen evolution reaction (HER)17–21 during water splitting,10–15,17–21 and for enhancing power generation with fuel cells.16The oxygen molecule, O2(g), has a triplet spin configuration. Catalysts with spin selectivity22 are known to improve OER efficiency by effectively inducing a triplet spin configuration, thereby decreasing the OER overpotential.10–15 The spin-selective reaction that accelerates OER consists of the following three steps:10–16 (I) spin-selective conduction in the catalyst acting as a spin filter;11–15 (II) spin transport from the catalyst to O2(g) to configure the triplet spin state;12–16 and (III) dissociation of triplet O2(g).10
Step (I) has been validated experimentally.11–15 TiO2 electrodes coated with self-assembled DNA,11 chiral paramagnetic CoOx films,14 and ferrite oxides13 were shown to act as spin filters for OER. Concerning step (II), Gracia12 used transition theory to describe the tunnelling phenomenon as the mechanism of catalyst-to-O2(g) spin transport required to reach the spin-parallel triplet state. In addition, the spin-selective emission of β rays (i.e., electron beams) was observed during the β decay of radionuclide 60Co.23 Regarding step (III), first-principles calculations verified that the triplet spin state is advantageous for the dissociation process.10
Exposure to an external magnetic field is found to increase the electric current generated during the HER.17–21 Water is composed of the diamagnetic H2O(l) molecule and charged ions, i.e., proton (H+(aq)), hydroxide (OH−(aq)), and hydronium (H3O+(aq)) ions. Under an external magnetic field, as is well-known in the field of magnetohydrodynamics (MHD), an increase in the hydrogen evolution rate results from convection due to the Lorenz force17–21 and from enhanced electrical conductance due to the magneto-resistive effect.20,21 Nonetheless, the interaction between the nuclear spin of the proton and the electronic spin of the metal catalyst remains unclear.9–22
Tungsten carbide (WC) has 10 valence electrons, 5d6 from W and 2p4 from C, making it similar to Pt (5d10). In 1973, Levy and Boudart24 proposed that WC exhibited singular catalytic activity similar to Pt. Since then, many researchers have investigated this theory using spectroscopy,25–27 first principles calculations,28,29 and experiments to measure catalytic activity.24,30–35 The catalytic activities of WC and its composites have only been observed under applied external voltage,30–35 and the predicted intrinsic catalytic activity of mono-WC under voltage-free operation has not yet been verified.
Bennett et al.25 reported that magnetic properties are one of the intrinsic differences between WC and Pt. WC is non-magnetic, whereas Pt exhibits high magnetic susceptibility. In our previous study,36 an HER catalyst was developed by doping tungsten carbide (WC) lattice with ferromagnetic Co nanocrystals. The resulting alloy was used for the catalytic hydrolysis of ammonium borane (NH3BH3), a material known as a high-capacity hydrogen-storage compound.37–43 The activity of the novel carbide was 30% higher than Pt nanoparticles under the same conditions. We hypothesised that the enhanced catalytic activity was attributed to the synergistic effect of the WC matrix promoting hydrolytic cleavage of NH3BH3 and the ferromagnetic Co crystals interacting with the nucleus spin of the protons. In the present study, we aimed to verify the interaction between the nucleus proton and magnetic substances from both experimentation and numerical simulation. The relationship between the rate of HER and the magnetic moment of a catalyst was investigated considering the Slater–Pauling rule.44,45 This rule states that adding an element to a metal reduces the alloy's saturation magnetization by an amount proportional to the number of valence electrons outside the d shell of the added element. To understand the relationship between HER and the Slater–Pauling rule,44,45 the dipole interaction44 between the proton nucleus spin and magnetic substances was simulated based on the electromagnetism for the first time.
An application of HER catalysts is the generation of hydrogen fuel from NH3BH3. In its stable crystal form, NH3BH3 contains 19.6 wt% hydrogen,37–43 and is being investigated for efficient transportation of hydrogen-based fuel for portable fuel-cell systems. Previous studies investigated the HER by hydrolysis over 10 wt% Co (ref. 39) or 2 wt% Pt (ref. 40) (both supported by Al2O3) and found that the HER in the latter was significantly faster than that in the former. A similar HER in NH3BH3(aq) catalysed by Ni nanoparticles (NPs) supported by a zeolite molecular sieve was observed.42 The atomic configuration in the AB molecule was investigated by neutron diffraction.38 The chemical bonding states for AB were investigated by first principles calculations41 and soft X-ray adsorption spectroscopy.43 The standard enthalpy of formation, 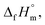 at 298.15 K was determined by combustion calorimetry.37 The catalytic design of selecting metals and alloys based on thermodynamic cycles for hydrolysis of AB have not previously been investigated. In the present study, a new strategy was developed to enhance catalytic performance and investigate the effect of an internal magnetic field on the thermodynamic mechanism of the hydrolysis of AB.
at 298.15 K was determined by combustion calorimetry.37 The catalytic design of selecting metals and alloys based on thermodynamic cycles for hydrolysis of AB have not previously been investigated. In the present study, a new strategy was developed to enhance catalytic performance and investigate the effect of an internal magnetic field on the thermodynamic mechanism of the hydrolysis of AB.
Methods
Sample preparation
Cobalt(II) acetate tetrahydrate [Co(C2H3O2)2·4H2O, 99%, Kojundo Chemical Laboratory Co., Ltd., Saitama], iron(III) nitrate nonahydrate [Fe(NO3)2·9H2O, 99.9%, Kojundo Chemical Laboratory Co., Ltd., Saitama], and nickel(II) acetate tetrahydrate [Ni(C2H3O2)2·4H2O, 99%, Kanto Chemical Co., Inc., Tokyo], were used as the starting materials. Thirteen catalyst compositions were prepared with various compositions using the following procedure. For each sample, the requisite amount of Co(C2H3O2)2·4H2O, Fe(NO3)2·9H2O, and Ni(C2H3O2)2·4H2O were separately dissolved in 100 ml deionized water at 363 K contained in alumina crucibles.For all samples (except 1, 7, and 13), the corresponding precursor solutions were mixed to form homogeneous aqueous solutions with a total volume of 200 ml. The homogeneous mixtures of metal complexes were prepared by calcination at about 500 K. These mixtures were then thermally decomposed under O2 at 773 K for 2 h to obtain homogeneous oxide-containing components. After thermal decomposition, powder samples were produced by reducing oxide-containing components with H2 at 1073 K for 2 h followed by cooling with 20 K min−1. Using an electron probe microanalysis system (JXA-8530FPlus, Co., JOEL Ltd, Tokyo) with a 15 kV accelerating voltage, the homogeneous chemical compositions of the samples were confirmed via X-ray images. Particle sizes and morphologies were compared by scanning electron microscopy (SEM).
The SA of the samples was determined by the Brunauer–Emmett–Teller method using nitrogen physisorption isotherms at 77 K, obtained with a sorption and porosity analyzer (BELSORP mini, MicrotracBEL Corp). The SA of the sample powders was about 2 m2 g−1, as shown in Table 1.
| Sample | Co/mol% | Fe/mol% | Ni/mol% | Econca | MFerro/μBb | SSA/(m2 g−1) | Structure | Nv | Md/eV |
|---|---|---|---|---|---|---|---|---|---|
| a The Econc values for Fe (3d6 and 4s2), Co (3d7 and 4s2), and Ni (3d8 and 4s2) are 8, 9, and 10, respectively. The Slater–Pauling rule44,45 was used to calculate the Econc of alloys by averaging the Econc of the elemental components.b The MFerro values for the samples (except 1, 7, and 13) were estimated by interpolating the Slater–Pauling curve.c Two phase equilibrium region composed of fcc and bcc solid solutions in the Co–Fe binary system.46d Single phase region composed of hcp solid solutions in the Co–Fe binary system.46e Single phase region composed of hcp solid solutions in the Co–Ni binary system.46 | |||||||||
| 1 | 0 | 100 | 0 | 8.00 | 2.20 | 2.01 | bcc | 2.66 | 0.81 |
| 2 | 50 | 50 | 0 | 8.50 | 2.29 | 1.92 | bcc | 2.19 | 0.43 |
| 3 | 65 | 35 | 0 | 8.65 | 2.23 | 2.27 | bcc | 2.04 | 0.32 |
| 4 | 85 | 15 | 0 | 8.85 | 1.87 | 1.71 | fccc | 1.85 | 0.17 |
| 5 | 92 | 8 | 0 | 8.92 | 1.82 | 1.94 | fcc | 1.79 | 0.12 |
| 6 | 96 | 4 | 0 | 8.96 | 1.76 | 1.94 | fccd | 1.75 | 0.09 |
| 7 | 100 | 0 | 0 | 9.00 | 1.70 | 1.93 | hcp | 1.71 | 0.06 |
| 8 | 96 | 0 | 4 | 9.04 | 1.66 | 1.92 | hcp + fcce | 1.67 | 0.09 |
| 9 | 92 | 0 | 8 | 9.08 | 1.59 | 1.86 | hcp + fcce | 1.63 | 0.13 |
| 10 | 85 | 0 | 15 | 9.15 | 1.55 | 1.91 | hcp + fcce | 1.55 | 0.18 |
| 11 | 65 | 0 | 35 | 9.35 | 1.37 | 1.85 | fcc | 1.28 | 0.27 |
| 12 | 50 | 0 | 50 | 9.50 | 1.18 | 1.47 | fcc | 1.19 | 0.48 |
| 13 | 0 | 0 | 100 | 10.0 | 0.60 | 1.93 | fcc | 0.66 | 0.89 |
HER analysis
For HER tests, 20 mg of each sample was placed in a glass test tube containing 1 ml of H2O. Separately, solutions of NH3BH3(aq) were made by dissolving 0.5 mmol NH3BH3(cr) in 1.5 ml H2O. This solution was then mixed with each sample to initiate hydrolysis and hydrogen evolution. The volume of evolved hydrogen (VHER) was measured as a function of time t, and the hydrogen evolution rate (RHER) was determined from the slope of the VHER vs. t curve. From this, it was determined that VHER increased linearly while excess unreacted NH3BH3(aq) remained. The used aqueous solution was decanted after the first VHER measurement, while the ferromagnetic samples were held back inside the test tube with an external magnet placed outside the glass test tube. After draining, the second and third VHER measurements were conducted similarly to the first. After completing the third measurement, fresh water was added to the ferromagnetic samples, which were then drained. After drying the samples in a dryer overnight, a fourth measurement was performed in the same way. The fourth VHER measurement was similar to the first three. Because the produced hydrogen gas appeared to reduce the sample surface, the VHER obtained in all four was averaged and used as the total VHER. During the HER test, the AB solution was stirred with a rotating magnetic stirrer.Results and discussion
Hydrogen evolution following the Slater–Pauling magnetic rule
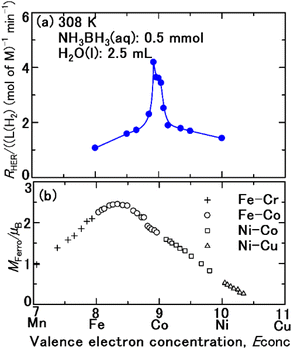
The structures of the samples determined from XRD are summarized in Table 1. The phases were consistent with the phase diagrams46 of the Co–Fe and Co–Ni binary systems. However, the phases composed in 85 mol% Co–15 mol% Fe (no. 4), 96 mol% Co–4 mol% Fe (no. 6), 96 mol% Co–4 mol% Ni (no. 8), 96 mol% Co–8 mol% Ni (no. 9), 85 mol% Co–15 mol% Ni (no. 10) were different from the equilibrium phase diagrams46 as shown in Fig. S1–S5 (ESI†). In no. 4, the high temperature fcc solid solution47 was only formed due to rapid cooling (20 K min−1), which prevented the formation of the equilibrium bcc solid solution.46 In no. 6, the high temperature fcc solid solution47 remained due to rapid cooling, which prevented the formation of the equilibrium hcp solid solution.48 In no. 8–10, the high fcc solid solution47 remained partly mixed with the equilibrium hcp solid solution48 resulting from rapid cooling. However, the meta stable fcc solid solutions were in the ferromagnetic phase.Fig. 1a and b compare the plots of RHER for the hydrolysis of ammonium borane (NH3BH3)36 as a function of the valence electron concentration Econc, of the catalyst and the Slater–Pauling curve.44,45 The composition, Econc, magnetic moment, SSA, and crystal structure of Fe, Co, Ni, and their binary alloys are shown in Table 1. The perpendicular axis of the Slater–Pauling curve44,45 is the magnetic moment MFerro with the units of Bohr magneton. The MFerro of pure Fe, Co, and Ni, as well as their alloys, varies with Econc. The Co–Fe alloy, with an Econc of 8.33, has the highest MFerro. The RHER increases with Econc, beginning with Fe (Econc = 8), and reaching a maximum for the 92Co–8Fe (mol%) alloy with Econc = 8.92. As Econc continues to increase, RHER decreases. Specifically, RHER varies according to the Slater–Pauling curve, and a magnetic-moment-induced increase in RHER is observed.
To clarify the reason for that the magnetic-moment increase in RHER, the first of all, the thermodynamic cycle of hydrolysis of AB was discussed. Table 2 shows the thermodynamic cycle of the hydrolysis of AB where the standard enthalpies of formation,  at 298.15 K of the standard substances of NH3BH3(cr),37 H2O(l),49 orthoboric acid (B(OH)3(aq)),49 ammonium (NH4+(aq)),49 metaboric acid (BO2−(aq)),50 and H2(g)49 are summarised in Table S1.† Eqn (I) shows the hydration reaction of NH3BH3(cr), where the thermodynamic value is unknown. Eqn (II) shows the HER of the hydrolysis of NH3BH3(aq). Eqn (III), rewritten as the sum of eqn (I) and (II), indicates the HER from the initial substance of NH3BH3(cr). Eqn (IV) shows the formation of NH4+(aq). Eqn (V) shows the formation of BO2−(aq). Finally, eqn (VI), rewritten as the sum of eqn (III)–(V), shows the final state of the hydrolysis Since eqn (IV)–(VI) are spontaneous reactions, the HER is given by eqn (III). As the standard entropy,
at 298.15 K of the standard substances of NH3BH3(cr),37 H2O(l),49 orthoboric acid (B(OH)3(aq)),49 ammonium (NH4+(aq)),49 metaboric acid (BO2−(aq)),50 and H2(g)49 are summarised in Table S1.† Eqn (I) shows the hydration reaction of NH3BH3(cr), where the thermodynamic value is unknown. Eqn (II) shows the HER of the hydrolysis of NH3BH3(aq). Eqn (III), rewritten as the sum of eqn (I) and (II), indicates the HER from the initial substance of NH3BH3(cr). Eqn (IV) shows the formation of NH4+(aq). Eqn (V) shows the formation of BO2−(aq). Finally, eqn (VI), rewritten as the sum of eqn (III)–(V), shows the final state of the hydrolysis Since eqn (IV)–(VI) are spontaneous reactions, the HER is given by eqn (III). As the standard entropy,  of NH3BH3(cr) has not yet been measured, the standard entropy of reaction, ΔrS°, and the standard Gibbs energy of reaction, ΔrG°, are unknown. However, ΔrG° is more negative than ΔrH° as the HER increases ΔrS°. Therefore, when a driving energy is applied corresponding to the hydrogen overpotential of metals, the HER reaches equilibrium, as defined by eqn (VI) via eqn (III).
of NH3BH3(cr) has not yet been measured, the standard entropy of reaction, ΔrS°, and the standard Gibbs energy of reaction, ΔrG°, are unknown. However, ΔrG° is more negative than ΔrH° as the HER increases ΔrS°. Therefore, when a driving energy is applied corresponding to the hydrogen overpotential of metals, the HER reaches equilibrium, as defined by eqn (VI) via eqn (III).
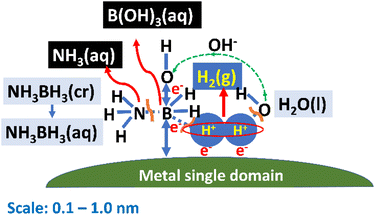
Next, we sought to understand the HER mechanism using the Slater–Pauling rule.44,45 Fig. 2 depicts a schematic illustration of the catalytic HER over a magnetic metal single domain, which is relevant for Fe, Co, and Ni pure metals and their alloys. The HER is carried out through the following steps: (1) NH3BH3 molecules collide during Brownian motion51 and are adsorbed on the single domain. (2) The B–N bonds in NH3BH3(aq) are broken to form the stable NH3(aq) molecule and transition-state species BH3(aq) to reach equilibrium. (3) Short-lived BH3(aq) releases three quasi-stable protons, and the B atom coordinates the three OH−(aq) ions released from the surrounding H2O(l) molecule to form a stable B(OH)3 molecule. H2O(l) also releases three protons. (4) A sufficient number of protons from BH3(aq) and H2O(l) assemble at the single domain owing to the magnetic interaction between their nuclear spins and the electronic spin of the single domain. (5) Hydrogen molecules are produced when protons accept electrons (e−). (6) One e− is released, along with a proton, during the decomposition of BH3, while the other e− is released from OH−(aq) during coordination to form B(OH)3(aq). In other words, the source of charge transfer is the B atom adsorbed on the metal single domain. It is likely that an attractive dipole interaction44 occurs directly between a proton and ferromagnetic single domain when the nuclear and electronic spins of the catalyst are aligned in parallel. As a result, the magnetic force of a ferromagnetic material interacts with protons followed by H2 (g) evolution. An attractive dipole interaction44 was studied by a numerical simulation in the next section.
The thermodynamic cycle shown in Table 2 can be re-written as an electrochemical cycle, given by
| Anode: NH3NH3(aq) + 3H2O(l) = NH3(aq) + B(OH)3(aq) + 6H+(aq) + 6e− | (1) |
| Cathode: 6H+(aq) + 6e− = 3H2(g) | (2) |
Therefore, the cathodic protection mechanism prevents the surface of the sample from corrosion, which is further evidence for HER following the Slater–Pauling magnetic rule.44
The maximum MFerro in the Slater–Pauling curve is at Econc = 8.33, whereas the maximum RHER is at Econc = 8.92. That is, the HER drop was observed in the samples including 15 mol% Fe (no. 4), 35 mol% Fe (no. 3), 50 mol% Fe (no. 2), and pristine Fe (no. 1). The cause of the HER drop was examined. The NH3BH3(aq) solution was alkaline (pH 8.53). The electric potential (Eh)–pH diagram52 indicates that Fe2O3(cr) is stable. Therefore, the aqueous ion equilibrated with Fe2O3(cr) is ferric hydroxide ion Fe3+(aq), which appears with an orange colour. Fig. 3 shows the aqueous solution after first series of the hydrolysis of AB over the sample 85 mol%–15 mol% Fe (no. 4). Orange colour development was clearly observed. The same colour development occurred with other samples including 35 mol% Fe (no. 3), 50 mol% Fe (no. 2), and pristine Fe (no. 1). Consequently, the corrosion of samples with over 15 mol% Fe resulted in overriding of the cathodic protection (eqn (2)).
 | ||
| Fig. 3 Appearance of 85 mol% Co–15 mol% Fe sample after hydrolysis of ammonia borane. The aqueous solution shows orange colour indicating that Fe3+ aqueous ion was formed. | ||
It is well known that d-electron vacancies53 have control on the corrosion of transition metals and alloys. d-electron vacancies53 capture the electrons of OH−(aq), resulting in adsorption of the radical oxygen atoms. The alloys with high Fe content have many electron vacancies in the 3d band. The samples with over 15 mol% Fe (no. 1–4) had many d-electron vacancies, which adsorb the radical oxygen atoms to form a carrion product, Fe2O3. This product overrides the cathodic protection (eqn (2)) during the hydrolysis of AB, which was concluded to be the reason for the HER drop.
The quantitative contents of the d-electron vacancies53 were defined as the Nv values.54 The phase stabilities of the super heat resistant alloys are estimated in terms of the Nv values. The alloys with excess Nv values form harmful σ phases, as was determined by multiple regression analysis54 of the experimental data. The optimum alloy compositions are simulated to be less than the critical Nv value, known as PHACOMP (Phase Computation).54 The d-orbital level parameter, Md, was suggested based on the molecular orbital calculations to update the Nv values considering the alloying effect.55 The Nv (ref. 54) and Md (ref. 55) values for the samples are shown in Table 1.54,55 The critical value of Nv was 1.85 and Md was 0.17 eV for 85 mol% Co–8 mol% Fe (no. 4), which was determined to prevent corrosion. When values less than the critical values are used, HER is actively caused by the cathodic protection of the surface from corrosion.
Numerical simulation of the dipole interaction between proton and magnetic catalyst
A hypothesis that an attractive dipole interaction occurs directly between a proton and ferromagnetic single domain when its nuclear and the electronic spin of the catalyst are aligned in parallel based on that HER follows the Slater–Pauling rule. In this section, the dipole interaction44 was directly investigated by numerical simulation.Fig. 4 depicts a schematic of the most fundamental model.44 The potential energy resulting from this magnetic dipole interaction Umag, is defined as follows.
 | (3) |
 | (4) |
 | ||
| Fig. 4 Schematic of the dipole interaction between a proton and ferromagnetic nano sphere single domain.56–59 | ||
The acceleration, a, of the proton is determined by dividing F by the mass of the proton mP (1.67262171 × 10−27 kg).60
 | (5) |
The present model includes a Co single domain sphere with an assumed diameter, d, of 60 nm to correlate it with the same domain in the WC lattice used in our previous study.36 The magnetic moment per Co atom44,45 is 1.7μB, and the number of moles, n, in this Co single domain is 1.6945 × 10−17 based on the density of hcp Co (8.9 Mg m−3).61 Therefore, the number of atoms, N, is 1.0205 × 107. The magnetic moment of the Co single domain is N × 1.7μB per Co atom;44,45 hence, MFerro equals 2.0211 × 10−22 Wb m. The MP of a proton44 is 6.33 × 10−33 Wb m.
These MFerro and MP values were used in eqn (3) to simulate a as a function of r, and the resulting plot is shown in Fig. 5. When r is 2.33375 μm, a is 9.807 ms−2, consistent with the gravitational acceleration, g.60 Because g was obtained at the appropriate distance, the present simulation accurately estimated the magnetic dipole interaction.
 | ||
| Fig. 5 Acceleration, a, acting on a proton as a function of the distance r from a Co single domain with a diameter of 60 nm under the magnetic potential energy Umag acting on a proton. | ||
To confirm that the proton is attracted to the magnetic force of the ferromagnetic single domain, its hydration was subsequently investigated. The hydration enthalpy ΔH+ad is −260.7 ± 2.5 (kcal mol−1),62,63 i.e., −1.811 [aJ (H+ad)−1]62,63 per proton. With the Brownian motion,29 a proton dissociates from H3O+(aq) and is then hydrated by another H2O(l) molecule.64–66 Fig. 6 shows schematic structures for H3O+(aq) and H2O(l) molecules, and a proton migrating between them. The migration length lm of the dissociation and hydration is 0.03–0.08 nm.64 Moreover, the O–H bond length in the H2O(l) molecule is 0.097 nm.64
 | ||
| Fig. 6 Schematic of the structures of hydronium ion, H3O+(aq), and H2O(l) molecules, and migration length of proton between them.62–64 | ||
Fig. 7 shows the effect of Umag of a Co single domain on a proton as a function of r (in the range of 0.03–0.06 nm) as described by eqn (3). With decreasing r, Umag shifts to negative values. Remarkably, at 0.044725 nm, Umag becomes equal to −1.811 [aJ (H+ad)−1],62,63 which is the ΔH+ad. The r value at which Umag is equal to ΔH+ad is the critical distance rc, exactly. Therefore, as shown in Fig. 7, when r < rc (0.044725 nm), Umag becomes deeper than ΔH+ad, causing a proton to be attracted to the Co single domain. Specifically, protons assemble on the Co single domain, and hydrogen gas rapidly evolves. (A) Initially, a proton accepts an electron to form a hydrogen atom, followed by adsorption (Volmer mechanism2,3). (B) Subsequently, another proton accepts another electron and aggregates with a hydrogen atom to form a dimer molecule, and a hydrogen gas molecule H2(g) desorbs (Heyrovsky mechanism2,4). (C) Selectively, two hydrogen atoms aggregate to form a dimer molecule, and a hydrogen gas molecule H2(g) dissociates (Tafel mechanism5,6).
In contrast, when r is greater than rc, Umag becomes shallower than ΔH+ad, causing a proton to move towards an H2O molecule and form H3O+(aq) in Brownian motion. In conclusion, rc is the exact distance at which the trajectory of a proton changes from a random walk caused by Brownian motion to an approach orbit towards the Co single domain. Moreover, the calculated rc value (0.044725 nm) is within the range of lm of proton dehydration and rehydration (0.03–0.08 nm64,66) and the O–H bond length (0.097 nm (ref. 62) in water. The effective particle number, Neff, of the proton, of which diameter, lP, is 8.751 × 10−7 nm,67 contributing to the frequency factor, A, in the Arrhenius equation68 is likely to be proportional to rc.
The rc values for other single domains with the same compositions as the samples (Table 1) were calculated in the same way. Additionally, the MFerro values were estimated by interpolating the Slater–Pauling curve. Fig. 8 depicts the calculated rc as a function of Econc, which shows that Econc is optimal when rc is 8.5. Theoretically, the contribution of Neff to RHER is the highest at Econc = 8.5. However, as shown in Fig. 1, the maximum RHER is obtained when Econc is 8.92. Due to excess Fe, the formation of Fe2O3, resulting from excess 3d electron vacancies as above described, inhibited by the Heyrovsky2,4 and Tafel mechanisms,5,6 which are mediated by the Volmer mechanism.2,3
The auxiliary data for the present numerical simulation were summarized in Table 3.
The singular catalytic activity of the Co nanocrystal doped WC in our previous study36 was discussed. The valence electron number of WC and Pt are the same. However, their magnetic properties are different and Pt has a higher magnetic susceptibility because WC is non-magnetic. Singular WC does not show catalytic activity. In our previous study,36 WC was doped with 60 nm diameter ferro-magnetic Co crystals to introduce an ordered-spin configuration, which showed a RHER value even higher than that of the Pt nanoparticles during the hydrolysis of AB. A hypothesis for the enhanced catalytic activity was attributed to the synergistic effect of the WC matrix promoting hydrolytic cleavage of NH3BH3 and the ferromagnetic Co crystals interacting with the nucleus spin of the protons. The present verified attractive dipole interaction between protons and Co is evidence for the hypothesis of singular catalytic activity of WC arising from an internal magnetic field.
It was previously hypothesised that a synergetic effect of WC breaking NH3BH3(aq) to form protons and the antiparallel alignment of the nuclear spins of protons and electronic spins in a single domain to increase the magnetic entropy.36,69,70 Therefore, the 1s electronic spin of the hydrogen atoms absorbed by Pd (ref. 71) and adsorbed on Gd (ref. 72) induce disorder of their electron spin polarisation, thereby increasing the entropy of the system. By the above-described mechanism for HER, it is concluded the dipole attractive interaction between proton and magnetic catalyst enhances HER. After donating electrons by the Volmer mechanism,2,3 electronic spins of that hydrogen atoms are likely to be aligned against spins of Co. This problem should be further investigated by molecular orbital calculations.
AB is hopeful hydrogen fuel.37–43 The HER by hydrolysis over the 10 wt% Co nano particle supported on the SiO2 nano particle (Co/SiO2) was investigated by Xu and Chandra.39 In order to compare HER property of the Co particle obtained in the present study with one of Co/SiO2 by Xu and Chandra,39 their normalised hydrogen evolution rate per unit area (m−2), RNHER, was evaluated. Table 4 shows their RNHER data. The experimental conditions for Co/SiO2 were 50 percent more conc. in the concentration of AB, 25 times larger in SSA (=52 m−2 g−1), and 83 percent smaller in mass (3.4 mg) than ones for the present Co particle. Considering difference in the experimental conditions, the intrinsic RNHER data appears to be similar.
| RNHER/(H2 mmol min−1 m−2) | n(AB)/mmol | V(H2O)/mL | Remarks | |
|---|---|---|---|---|
| a SSA of the Co nano was 52 m−2 g−1, and its mass was 3.4 mg.b SSA was hypothetically estimated as volume ratio of the Co nano-crystal against WC matrix. | ||||
| Co particle | 1.28 | 0.5 | 2.5 | This study |
| Co(cr)/SiO2 | 0.70a | 3.2 | 10 | Ref. 39 |
| Co(cr) in WC | 31.9b | 0.5 | 2.5 | Ref. 36 |
The atomic configuration in the AB molecule was investigated by the neutron diffraction by Klooster et al.38 The chemical bonding states for AB were investigated by the first principles calculation by Banu et al.,41 and soft X-ray adsorption spectroscopy by Niibe et al.43 The  datum at 298.15 K was determined by the combustion calorimetry by Shaulov.37 The effect of an internal magnetic field cooperating the cathodic protection on the thermodynamic mechanism of the hydrolysis of AB has been suggested in the present study for the first time.
datum at 298.15 K was determined by the combustion calorimetry by Shaulov.37 The effect of an internal magnetic field cooperating the cathodic protection on the thermodynamic mechanism of the hydrolysis of AB has been suggested in the present study for the first time.
In our previous study for the Co doped WC,36 the activation energy determined by the Arrhenius plots was found to be well consistent with the electrochemical hydrogen over potential of Co.73 This means hydrogen gas H2(g) is evolved over the Co crystals via Heyrovsky2,4 and Tafel mechanisms.5,6 The RNHER datum of the Co crystals in the WC matrix was compared with that of the Co particle in the present study. Where the ratio of SSA of the fcc Co36 nanocrystals against the WC matrix were hypothetically assumed to be as same as the ratio of their volumes.61,74 Their volumes were calculated from their densities (Table 3). Table 4 shows their RNHER data. The RNHER of the Co crystal was found to be 25 times higher than that of the Co particle in the present study. This means that WC, of which electrons density of states are similar to Pt, adsorbs much amount of AB followed by decomposing the B–N bonding, and makes much protons dissociating from AB. Much protons are aligned on the Co crystals by the attractive dipole interaction, and 25 times higher RNHER is concluded to be caused.
The parameters used in the present simulation are an extremely small scale power functions as, e.g., MFerro/(Wb m) = 2.0211 × 10−22, and MP/(Wb m) = 6.33 × 10−33 (Table 3) 44 the obtained rc datum reflects the structure parameters for H3O+(aq) and H2O(l) molecules, and a proton migration (Fig. 6), indicating that the present numerical simulation was done with highly accuracy. Although a very fundamental model based on the sphere single domain magnetic structure64–66 was adopted, this is a novel finding and likely to give impact to general science due to that proton in aqueous solution are associated with various phenomena in nature. In the future, the dipole interactions in the complicated magnetic force lines from the magnetic substances composed of the multi domain should be investigated.
Conclusions
The current study clarified the magnetic dipole interactions between the nuclear spin of a proton and the electronic spin of a magnetic catalyst for the first time. The conclusions are as follows: (1) the HER rate varied in accordance with the Slater–Pauling rule, resulting in a rapid rate of hydrogen evolution for catalysts with a high magnetic moment. (2) Numerical simulation of the magnetic dipole interaction revealed a critical distance at which the proton, as H3O+(aq), changes its path from a Brownian random walk trajectory to a close-approach orbit towards the ferromagnetic catalyst, in the same order as the migration length of the dissociation and hydration of the proton and O–H bond length in water. Consequently, this study provides novel insights into noble-metal-free catalyst design from the viewpoint of the internal magnetic field.Conflicts of interest
There are no conflicts to declare.Author contributions
M. M. conceived the idea and wrote the paper; Y. O. and R. F. synthesized the materials and conducted the hydrogen evolution tests and SA measurements; H. Y. reviewed and validated the work shown in the paper; and H. Y. rendered helpful discussions.Acknowledgements
This work was supported by an academic research grant from the Hyogo Science and Technology Association and special research grant for hydrogen energy from the University of Hyogo.References
- International Energy Agency, Global Hydrogen Review 2022, https://www.iea.org/reports/global-hydrogen-review-2022, September 2022 Search PubMed.
- N. M. Marković, B. N. Grgur and P. N. Ross, Temperature-dependent hydrogen electrochemistry on platinum low-index single-crystal surfaces in acid solutions, J. Phys. Chem. B, 1997, 101, 5405–5413 CrossRef
.
- N. M. Marković and P. N. Ross Jr, Surface science studies of model fuel cell electrocatalysts, Surf. Sci. Rep., 2002, 45, 117–229 CrossRef
.
- M. C. Tavares, S. A. S. Machado and L. H. Mazo, Study of hydrogen evolution reaction in acid medium on Pt microelectrodes, Electrochim. Acta, 2001, 46, 4359–4369 CrossRef CAS
.
- J. Barber, S. Morin and B. E. Conway, Specificity of the kinetics of H2 evolution to the structure of single-crystal Pt surfaces, and the relation between opd and upd H, J. Electroanal. Chem., 1998, 446, 125–138 CrossRef CAS
.
- K. Kunimatsu, T. Senzaki, M. Tsushima and M. Osawa, A combined surface-enhanced infrared and electrochemical kinetics study of hydrogen adsorption and evolution on a Pt electrode, Chem. Phys. Lett., 2005, 401, 451–454 CrossRef CAS
.
- I. Hamada and O. Sugino, Towards first-principles understanding of hydrogen evolution reaction at the platinum electrode, J. Surf. Sci. Soc. Jpn., 2013, 34, 638–643 CrossRef CAS
.
- W. Karim, C. Spreafico, A. Kleibert, J. Gobrecht, J. VandeVondele, Y. Ekinci and J. A. V. Bokhoven, Catalyst support effects on hydrogen spillover, Nature, 2017, 541, 68–71 CrossRef CAS PubMed
.
- M. Faraday, Faraday's Diary, Bell and Sons, London, 2018, vol. IV, pp. 288–290 Search PubMed
.
- S. Chrétien and H. Metiu, O2 evolution on a clean partially reduced rutile TiO2 (110) surface and on the same surface precovered with Au1 and Au2: the importance of spin conservation, J. Chem. Phys., 2008, 129, 074705 CrossRef PubMed
.
- W. Mtangi, V. Kiran, C. Fontanesi and R. Naaman, Role of the electron spin polarization in water splitting, J. Phys. Chem. Lett., 2015, 6, 4916–4922 CrossRef CAS PubMed
.
- J. Gracia, Spin dependent interactions catalyse the oxygen electrochemistry, Phys. Chem. Chem. Phys., 2017, 19, 20451–20456 RSC
.
- F. A. Garcés-Pineda, M. Blasco-Ahicart, D. Nieto-Castro, N. López and J. R. Galán-Mascarós, Direct magnetic enhancement of electrocatalytic water oxidation in alkaline media, Nat. Energy, 2019, 4, 519–525 CrossRef
.
- S. Ghosh, B. P. Bloom, Y. Lu, D. Lamont and D. H. Waldeck, Increasing the efficiency of water splitting through spin polarization using cobalt oxide thin film catalysts, J. Phys. Chem. C, 2020, 124, 22610–22618 CrossRef CAS
.
- Z. Bian, K. Kato, T. Ogoshi, Z. Cui, B. Sa, Y. Tsutsui, S. Seki and M. Suda, Hybrid chiral MoS2 layers for spin-polarized charge transport and spin-dependent electrocatalytic applications, Adv. Sci., 2022, 9, 2201063 CrossRef CAS PubMed
.
- C. Biz, M. Fianchini and J. Gracia, Catalysis meets spintronics; spin potentials associated with open-shell orbital configurations enhance the activity of Pt3Co nanostructures for oxygen reduction: a density functional theory study, ACS Appl. Nano Mater., 2020, 3, 506–515 CrossRef CAS
.
- O. Lioubashevski, E. Katz and I. Willner, Magnetic field effects on electrochemical processes: a theoretical hydrodynamic model, J. Phys. Chem. B, 2004, 108, 5778–5784 CrossRef CAS
.
- L. Elias and A. C. Hegde, Effect of magnetic field on HER of water electrolysis on Ni–W alloy, Electrocatalysis, 2017, 8, 375–382 CrossRef CAS
.
- N. Bidin, S. Azni, S. Islam, M. Abdullah, M. F. S. Ahmad, G. Krishnan, A. R. Johari, M. A. A. Bakar, N. S. Sahidan, N. Musa, N. M. Salebi, N. Razali and M. Sanagi, The effect of magnetic and optic field in
water electrolysis, Int. J. Hydrogen Energy, 2017, 42, 16325–16332 CrossRef CAS
.
- W. Zhou, M. Chen, M. Guo, A. Hong, T. Yu, X. Luo, C. Yuan, W. Lei and S. Wang, Magnetic enhancement for hydrogen evolution reaction on ferromagnetic MoS2 catalyst, Nano Lett., 2020, 20, 2923–2930 CrossRef CAS PubMed
.
- J. Saha, R. Ball and C. Subramaniam, Premagnetized carbon-catalyst interface delivering 650% enhancement in electrocatalytic kinetics of hydrogen evolution reaction, ACS Sustainable Chem. Eng., 2021, 9, 7792–7802 CrossRef CAS
.
- R. Naaman and D. H. Waldeck, Chiral-induced spin selectivity effect, J. Phys. Chem. Lett., 2012, 3, 2178–2187 CrossRef CAS PubMed
.
- C. S. Wu, E. Ambler, R. W. Hayward, R. D. D. Hoppes and R. P. Hudson, Experimental test of parity conservation in beta decay, Phys. Rev., 1957, 105, 1413–1415 CrossRef CAS
.
- R. B. Levy and M. Boudart, Platinum-like behavior of tungsten carbide in surface catalysis, Science, 1973, 181, 547–549 CrossRef CAS PubMed
.
- L. H. Bennett, J. R. Cuthill, A. J. McAlister and N. E. Erickson, Electronic structure and catalytic behavior of tungsten carbide, Science, 1974, 184, 563–565 CrossRef CAS PubMed
.
- J. E. Houston, G. E. Laramore and R. L. Park, Surface electronic properties of tungsten, tungsten carbide, and platinum, Science, 1974, 185, 258–260 CrossRef CAS PubMed
.
- L. H. Bennett, J. R. Cuthill, A. J. McAlister and N. E. Erickson, Electronic and catalytic properties of tungsten carbide, Science, 1975, 187, 858–859 CrossRef CAS PubMed
.
- L. F. Mattheiss and D. R. Hamann, Bulk and surface electronic structure of hexagonal WC, Phys. Rev. B, 1984, 15, 1731–1738 CrossRef
.
- X. Zhang, B. Grabowski, C. Freysoldt, F. Körmann and J. Neugebauer, Accurate electronic free energies of the 3d, 4d, and 5d transition metals at high temperature, Phys. Rev. B, 2017, 95, 165126 CrossRef
.
- D. V. Esposite, S. T. Hunt, A. L. Stottlemyer, K. D. Dobson, B. E. McCandless, R. W. Birkmire and J. G. Chen, Low-cost hydrogen-evolution catalysts based on monolayer platinum on tungsten monocarbide substrates, Angew. Chem., Int. Ed., 2010, 49, 9859–9862 CrossRef PubMed
.
- J. Zhang, J. Chen, Y. Jiang, F. Zhou, G. Wang, R. Wang and R. Wang, Tungsten carbide encapsulated in nitrogen-doped carbon with iron/cobalt electrocatalyst for oxygen reduction reaction, Appl. Surf. Sci., 2016, 389, 157–164 CrossRef CAS
.
- D. J. Ham, R. Ganesan and J. S. Lee, Tungsten carbide microsphere as an electrode for cathodic hydrogen evolution from water, Int. J. Hydrogen Energy, 2008, 33, 6865–6873 CrossRef CAS
.
- W. Zheng, L. Wang, F. Deng, S. A. Stephen, A. Gilles, A. K. Prasad, S. G. Advani, Y. Yan and D. G. Vlachos, Durable and self-hydrating tungsten carbide-based composite polymer electrolyte membrane fuel cells, Nat. Commun., 2017, 8, 418 CrossRef PubMed
.
- C. Ma, J. Sheng, N. Brandon, C. Zhang and G. Li, Preparation of tungsten carbide-supported nano Platinum catalyst and its electrocatalytic activity for hydrogen evolution, Int. J. Hydrogen Energy, 2007, 32, 2824–2829 CrossRef CAS
.
- P. Xiao, X. Ge, H. Wang, A. Liu, A. Fisher and X. Wang, Novel molybdenum carbide-tungsten carbide composite nanowires and their electrochemical activation for efficient and stable hydrogen evolution, Adv. Funct. Mater., 2015, 25, 1520–1526 CrossRef CAS
.
- M. Morishita, A. Nozaki, H. Yamamoto, N. Fukumuro, M. Mori, K. Araki, F. Sakamoto, A. Nakamura and H. Yanagita, Catalytic activity of Co-nanocrystal-doped tungsten carbide arising from an internal magnetic field, RSC Adv., 2021, 11, 14063–14070 RSC
.
- Y. K. Shaulov, G. O. Shmyreva and S. Tubyanskaya, Heat of combustion of ammonia borane, Zh. Fiz. Khim., 1966, 40, 122–124 CAS
.
- W. T. Klooster, T. F. Koetzle, P. E. M. Siegbahn, T. B. Richardson and R. H. Crabtree, Study of the N−H⋯H−B Dihydrogen Bond Including the Crystal Structure of BH3NH3 by Neutron Diffraction, J. Am. Chem. Soc., 1999, 121, 6337–6343 CrossRef CAS
.
- Q. Xu and M. Chandra, Catalytic activities of non-noble metals for hydrogen generation from aqueous ammonia-borane at room temperature, J. Power Sources, 2006, 163, 364–370 CrossRef CAS
.
- M. Chandra and Q. Xu, Room temperature hydrogen generation from aqueous ammonia-borane using noble metal nano-clusters as highly active catalysts, J. Power Sources, 2007, 168, 135–142 CrossRef CAS
.
- T. Banu, T. Debnath, T. Ash and A. K. Das, Hydrolysis of ammonia borane and metal amidoboranes: a comparative study, J. Chem. Phys., 2015, 143, 194305 CrossRef PubMed
.
- C. Wang, J. Tuninetti, Z. Wang, C. Zheng, R. Ciganda, L. Salmon, S. Moya, J. Ruiz and D. Astruc, Hydrolysis of ammonia-borane over Bi/ZIF-8 nanocatalyst: high efficiency, mechanism, and controlled hydrogen release, J. Am. Chem. Soc., 2017, 139, 11610–11615 CrossRef CAS PubMed
.
- M. Niibe, Y. Haruyama, A. Heya and S. Ito, Soft X-ray Absorption/Emission Spectroscopy and Atomic Hydrogen Irradiation Effect of AmmoniaBorane, e-J. Surf. Sci. Nanotechnol., 2022, 20, 226–231 CrossRef
.
- S. Chikazumi, Physics of Magnetism, John Wiley & Sons, Inc., New York, 1964, pp. 5–73 Search PubMed
.
- C. Kittel, Introduction to Solid State Physics, John Wiley & Sons, Inc., New York, 1986, p. 597 Search PubMed
.
- In Binary Alloy Phase Diagrams, ed. T. B. Massalski, H. Okamoto, P. R. Subramanian and L. Kacprzak, ASM International, 1990, p. 1186 Search PubMed
.
- L. Marick, Variation of Resistance and Structure of Cobalt with Temperature and a discussion of its photoelectric emission, Phys. Rev., 1936, 49, 831–839 CrossRef
.
- A. Taylor and R. W. Floyd, Prediction Measurements of Lattice Parameters of Non-Cubic Crystals, Acta Crystallogr., 1950, 13, 285–289 CrossRef
.
- H. Gamsjäger, J. Bugajski, T. Gajda, R. J. Lemire and W. Preis, Chemical Thermodynamics of Nickel, Nuclear Energy Agency, Organization for Economic Co-operation, Development, in Chemical Thermodynamics, North Holland Elsevier Science Publisher B. V., Amsterdam, The Netherlands, 2005, vol. 6, pp. 43–52 Search PubMed
.
- D. D. Wagman, W. H. Evans, V. B. Parker, R. H. Schumm, I. Halow, S. M. Bailey, K. L. Churney and R. L. Nuttall, The NBS tables of chemical thermodynamic properties: selected values for inorganic and C1 and C2 organic substances in SI units, J. Phys. Chem. Ref. Data, 1982, 11(suppl. 2), 123 Search PubMed
.
- A. Einstein, Zur Theorie der Brownschen Bewegung, Ann. Phys., 1905, 17, 371–381 Search PubMed
.
- N. Takeno, Atlas of Eh-pH Diagrams, https://www.gsj.jp/data/openfile/no0419/openfile419j.pdf, 2005 Search PubMed.
- R. W. Revie and H. H. Uhlig, Corrosion and Corrosion Control: An Introduction to Corrosion Science and Engineering, Wiley Interscience, Inc., Hoboken New Jersey, 4th edn, 2008, pp. 102–108 Search PubMed
.
- R. G. Barrows and J. B. Newkirk, A modified system for predicting σ formation, Metall. Mater. Trans. B, 1972, 3, 2889–2893 CrossRef CAS
.
- M. Morinaga, A Quantum Approach to Alloy Design: An Exploration of Material Design and Development Based Upon Alloy Design Theory and Atomization Energy Method, Elsevier Inc., Amsterdam, 2019, 23, 50 Search PubMed
.
- J. Frenkel and J. Dorfman, Spontaneous and Induced Magnetisation in Ferromagnetic Bodies, Nature, 1930, 126, 274–275 CrossRef
.
- C. Kittel, Theory of the Structure of Ferromagnetic Domains in Films and Small Particles, Phys. Rev., 1946, 70, 965–971 CrossRef CAS
.
- L. Neél, Properties for Ferromagnetic Cubic Grains, Compt. Rend., 1947, 224, 1488–1490 Search PubMed
.
- E. C. Stoner and E. P. Wohlfarth, Interpretation of High Coercivity in Ferromagnetic Materials, Nature, 1947, 160, 650–651 CrossRef
.
- In Chronological Scientific Tables, National Astronomical Observatory, Jpn., 2007354–356 Search PubMed
.
- S. Nagasaki, Metals Handbook, Japan Institute of Metals, 1993, p. 10 Search PubMed
.
- H. F. Halliwell and S. C. Nyburg, Enthalpy of hydration of the proton, Trans. Faraday Soc., 1963, 59, 1126–1140 RSC
.
- H. Ohtaki and M. Tanaka, Thermodynamics and rate theory for analytical chemist II, Jpn. Anal., 1969, 18, 400–414 CrossRef
.
- B. E. Conway, J. O. Bockris and H. Linton, Proton conductance and the existence of the H3O• ion, J. Chem. Phys., 1956, 24, 834–850 CrossRef CAS
.
- M. D. Newton, Ab initio studies of the hydrated H3O+ ion. II. The energetics of proton motion in higher hydrates (n = 3–5), J. Chem. Phys., 1977, 67, 5535–5546 CrossRef CAS
.
- K. Ando and J. T. Hynes, Molecular mechanism of HCl acid ionization in water: ab initio potential energy surfaces and Monte Carlo simulations, J. Phys. Chem. B, 1997, 101, 10464–10478 CrossRef CAS
.
- CODATA Value: proton rms charge radius (nist.gov), https://physics.nist.gov/cgi-bin/cuu/Value?rp|search_for=radius+proton.
- D. Kondepudi, Introduction to Modern Thermodynamics, Wiley, West Sussex, 2008, pp. 266–269 Search PubMed
.
- M. Morishita, T. Abe, A. Nozaki, I. Ohnuma and K. Kamon, Calorimetric study of Nd2Fe14B: heat capacity, standard Gibbs energy of formation and magnetic entropy, Thermochim. Acta, 2020, 690, 178672 CrossRef CAS
.
- M. Morishita, T. Abe, H. Yamamoto, A. Nozaki and S. Kimura, Thermodynamic and Magnetic Properties for Dy2Fe14B Determined by Heat Capacity Measurement from Very Low to High Temperatures and Solution Calorimetry, Thermochim. Acta, 2023, 721, 179410 CrossRef CAS
.
- R. J. Miller and C. B. Satterthwaite, Electronic model for the reverse isotope effect in superconducting Pd-H(D), Phys. Rev. Lett., 1975, 34, 144–148 CrossRef CAS
.
- A. Cerri, D. Mauri and M. Landolt, Surface magnetization and hydrogen chemisorption on Gd: a spin-polarized photoemission study, Phys. Rev. B, 1983, 27, 6526–6529 CrossRef CAS
.
- H. Ezaki, M. Morinaga and S. Watanabe, Hydrogen overpotential for transition metal and alloys, and its interpretation using an electronic model, Electrochim. Acta, 1993, 38, 557–564 CrossRef CAS
.
- Encyclopedia Chimica, Kyoritsu Shippan Co., LTD, Tokyo, 1975, vol. 5, p. 699 Search PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ra07865a |
| This journal is © The Royal Society of Chemistry 2023 |