Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Stabilization of copper nanoparticles onto the double Schiff-base-functionalized ZSM-5 for A3 coupling reaction catalysis aimed under mild conditions†
Leila Mohammadia,
Mojtaba Hosseinifard *b,
Mohammad Reza Vaezi*a and
Sadegh Rostamniac
*b,
Mohammad Reza Vaezi*a and
Sadegh Rostamniac
aDepartment of Nano Technology and Advanced Materials, Materials and Energy Research Center, Karaj, Iran. E-mail: l.mohammadi3790@gmail.com; m_r_vaezi@merc.ac.ir
bDepartment of Energy, Materials and Energy Research Center, Karaj, Iran. E-mail: m.hosseini@merc.ac.ir
cOrganic and Nano Group (ONG), Department of Chemistry, Iran University of Science and Technology (IUST), PO BOX 16846-13114, Tehran, Iran. E-mail: rostamnia@iust.ac.ir
First published on 7th February 2023
Abstract
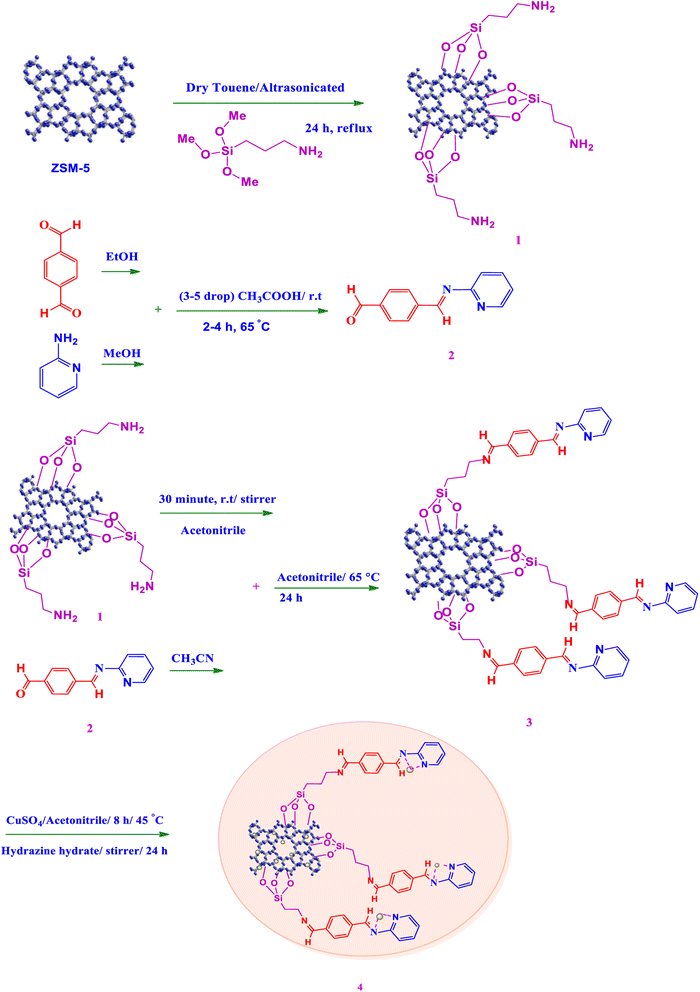
In this research a highly efficient and heterogeneous catalyst of ZSM-5@APTMS@(E)-4-((pyridin-2-ylimino)methyl) benzaldehyde@Cu-NPs was synthesized for upgrading the A3 coupling reaction for the synthesis of propargylamines under mild conditions. Rational tuning of the microenvironment of metallic NPs to improve efficiency and reusability in catalytic performances is of significance for scalable applications. Firstly, ZSM-5 was immobilized with APTMS (3-aminopropyltrimethoxysilane) and further modified with (E)-4-((pyridin-2-ylimino)methyl)benzaldehyde. Subsequently, the amine-activated zeolite@(E)-4-((pyridin-2-ylimino)methyl)benzaldehyde was applied to increase the stabilization of Cu metal nanoparticles. The catalyst was treated with hydrazine to reduce Cu(II) to Cu(0), which led to active metal sites. The results of catalytic performance in comparison with different parts of catalysis indicate high efficiency due to the stabilization of copper nanoparticles onto the newly synthesized support of MOF modified with nitrogen aromatic groups. The addition of N-rich organic ligand through modification alters the electronic structure of the final composite in favor of the progress of the A3-coupling reaction. Moreover, the proposed catalyst showed no reduction in the catalytic performance up to four cycles, and a minor loss of efficiency occurs after the seventh cycle. In addition, the catalyst was effectively recycled up to 7 times without leaching of Cu-NPs.
1. Introduction
Multi-component responses (MCRs) are focused on synthetic processes involving three or more well-defined reactants to create an item that contains noteworthy fragments of all reactants, exquisitely all atoms.1–5 In addition, this class of responses offers a higher level of atomic productivity due to time-saving isolation, as well as the filtration of synthetic intermediates.6–8 Lately, a multitude of MCRs, A3-type coupling reactions, involving amines and aldehydes by terminal alkynes, directly in a one-pot process, as a robust, valuable, and specific method for a set of complex molecules by noteworthy biological properties through diverse elementary as well as remarkable pioneers has been efficiently developed.9–13 Propargylamines which are advantageous platforms, due to economical, easy availability, and adaptability, have been effectively developed in research as compatibility scaffolds for chemical methods and key intermediate drugs to combine biological-active nitrogenous scaffolds including, polyfunctional amino compounds, oxotremorine analogs, β-lactams, as well as pesticides, insecticides, and also medicinal substances for the treatment of Alzheimer's and Parkinson's diseases.14–17Recently, coordinate expansion of terminal alkynes on C–N double bonds arranged from either amines and aldehydes or imines in a one-pot strategy through C–H activated of alkynes through noble transition-metal-catalyzed through homogeneous composites such as Au,18 Cu,19,20 Ag,21 Zn,22,23 Ni,24 Fe,25 Hg,26 Co,27 Ir,28 Ru,29 In,30 Zr,31 Re,32 and polyoxovanadate,33 as well heterogeneous backed (Ag(I), Au(III), Cu(II) and (Cu(I), Ag(I), and Au(I)34 were effectively used to catalyze the A3 coupling reaction.35–39 Copper metal, as one of the most widely utilized metals, has been effectively applied in A3-coupling reactions due to its economical availability, and a high potential for reactivity. Nowadays, proficient and versatile solid catalysts such as silicon dioxide, activated charcoal, ZSM-5, Santa Barbara Amorphous-15 (SBA-15),40 and also Cu(II)41 complexes by virtue of large surface area caused to effectively adsorb more dynamic location like dynamic metal antecedents and exhibit magnificent catalytic activity are successfully applied to form propargylamine in a one-pot process, which has been more widely preferred to avoid problems such as the accumulation of active sites, toxic metals, and non-recyclable catalysts to minimize risks in biological products.42–45
The preparation of organic compounds in green aquatic solvents or solvent-free conditions is a hot topic in today's scientific community.46,47 Accomplishments within the field of green chemistry have opened up new prospects for more prominent impacts on the efficiency and performance of chemicals, whereas reducing their adverse effects, also facilitating the safety and benefits of incorporating mild conditions, as well expanding the scope of various organic reactions.48–50
ZSM-5 zeolites belong to the pentasil zeolite family, which are crystalline aluminosilicates with periodic arrangements of cages and channels, which have been widely applied as catalysts within the petrochemical industry, adsorbents, and ion exchangers due to their tunable acidity, large surface area, unique shape, high selective stability, as well as uniform micro-porous.51–54 Among various zeolites (zeolite Y, beta and mordenite, and ZSM-5), the ZSM-5 catalyst is more valuable for particular aromatic constructions. Medium-pore zeolites like ZSM-5 exhibit increased steric hindrance to reactant molecules within the zeolite pores, resulting in higher aromatics yields and less coke deposition.55–58 MFI-type zeolites are generally synthesized in the presence of organic templates, owing to high surface area, interesting channel structure, thermal steadiness, corrosiveness, shape selectivity, and properties of porous ZSM-5; recently, there has been tremendous interest in zeolite-supported heterogeneous catalysts.59–61
Here, we have outlined a newly synthesized heterogeneous ZSM-5@APTMS@(terephthalaldehyde/2-aminopyridine)@Cu-NPs catalyst, which has been efficiently used to perform ternary coupling reactions (A3-coupling reaction) under mild conditions to successfully promote the preparation of a series of propargylamines with high performance yield.
One reason for the high regeneration performance of the proposed catalyst could be its heightened water resistance as medium-pore zeolites such as ZSM-5 exhibit increased steric hindrance to reactant molecules within the zeolite pores, resulting in higher aromatics yields and less coke deposition, which preserves its structure from destruction by water molecules. Thus, the designed catalysts exhibit superior catalytic performance due to the modulation of the microenvironment of Cu NPs. Also, ZSM-5@APTMS@(E)-4-((pyridin-2-ylimino)methyl) benzaldehyde as the catalyst support and Cu nanoparticles as the anchored transition-metal, since the amino group can be firmly attached to the metal nanoparticles. The purpose of ZSM-5 modification was to prepare nitrogen-rich support by providing amino groups to immobilize Cu nanoparticles into it, which led to the high loading of Cu nanoparticles without a noteworthy loss in the leaching of Cu-NPs. Moreover, the proposed catalyst showed excellent recyclability for up to 7 cycles.
2. Experimental
2.1. Materials and methods
All materials and reagents used in this work were acquired from Merck and Sigma-Aldrich companies and used without further purification.2.2. Synthesis of the E(-4-((pyridin-2-ylimino)methyl)benzaldehyde
In order to prepare the compound E(-4-((pyridin-2-ylimino) methyl) benzaldehyde, the condensation of 2-aminopyridine within terephthalaldehyde was performed based on a report by Gutha et al.62 In a round bottom flask, (0.2 g) of 2-aminopyridine dissolved in ethanol (25 ml) and (2.81 g) terephthalaldehyde dissolved in methanol (40 ml) were mixed, then acetic acid (3–5 drops) was added dropwise to the flask with stirring at room temperature. Afterward, the reaction mixture was reacted at 65 °C for 2 to 4 h. Then, the reaction mixture was poured over ice (frozen and crushed deionized water) until it was cooled. At this step, yellow sediments were accumulated that were collected by filtration with a Buchner funnel under vacuum. The compound was crystallized using water (Fig. 1).2.3. Synthesis of amine-functionalized ZSM-5 (ZSM-5@APTS, ZSM-5@NH2)
A round dry bottom flask was charged with ZSM-5 (a type of MFI zeolite) (1.0 g) and dry toluene (60 ml), then it was dispersed under ultrasonic irradiation for 30 minutes. Afterward, the mixture was completely dispersed and functionalized with APTMS (3-aminopropyltrimethoxysilane) (1.1 ml), diluted in dry toluene (2–3 ml) by adding it dropwise to the flask at room temperature. Afterward, the flask was equipped with a condenser and heated at 80 °C for 24 h. After the reaction was complete, the synthesized amine-functionalized ZSM-5 was filtered, deionized with dry toluene three times, and dried in a vacuum oven at 55 °C for 12 h.2.4. Synthesis of ZSM-5@APTS@E(-4-((pyridin-2-ylimino)methyl)benzaldehyde
Zeolite-NH2 (0.5 g), prepared in the previous step was dispersed in acetonitrile (CH3CN) (25 ml) under stirring for 30 minutes at room temperature. Next, (E(-4-((pyridin-2-ylimino(methyl)benzaldehyde) (0.15 g) was completely dissolved in acetonitrile (15 ml) and charged to the mixture and stirred at room temperature for 15 minutes. Then, this mixture was stirred at 75 °C for 24 h. After the reaction time was over. Then, the obtained product was centrifuged, washed with acetonitrile twice, and dried in a vacuum oven at 60 °C for 12 h to provide ZSM-5@APTS@E(-4-((pyridin-2-ylimino)methyl)benzaldehyde.2.5. Immobilization of Cu-NPs on ZSM-5@APTS@E(-4-((pyridin-2-ylimino)methyl)benzaldehyde
In a round bottom flask, amine-functionalized ZSM-5@(2-aminopyridine/terephthalaldehyde) (0.2 g) in deionized water (50 ml) was added and stirred at room temperature. Afterward, a specified amount of copper(II) sulfate (CuSO4) (0.04 g) was dissolved in deionized water (10 ml) until clear and was added to the flask under stirring at 40–45 °C for 8 h. In order to reduce Cu(II) to Cu(0), the temperature should be lowered to the ambient temperature. The prepared hydrazine hydrate was completely homogeneous (3 drops of hydrazine hydrate in 3 ml of deionized water) (0.3 ml) and was added to the flask and stirred at room temperature for 24 h, then, Cu(II) was reduced to Cu (0) with hydrazine hydrate. In the end, the Cu-catalyst was separated easily using a 9000 rpm centrifuge, washed once with acetonitrile, and dried in a vacuum oven at 35 °C for 12 h (Scheme 1).2.6. General procedure for the preparation of the propargylamines
To investigate the catalytic performance of the proposed catalyst, the progress of the A3-coupling preparation of propargylamines from the corresponding aldehydes (1 mmol), phenylacetylene (1.1 mmol), and the secondary amines (1 mmol), potassium carbonate as a base (K2CO3) (2 mmol), solvent water H2O (3 ml) and newly synthesized ZSM-5@APTS@(2-aminopyridine/terephthalaldehyde)@Cu-NPs (0.025 g) catalyst were added. The optimum conditions were monitored (Table 2). Purification of the synthesized propargylamines was performed by plate-chromatography. The chemical structure of the synthesized propargylamines was probed by 1H NMR and 13C NMR (ESI) (Fig. S1†).3. Results and discussion
3.1. Catalyst preparation
Following our previous attempts to develop facile and sustainable methodologies for developing various organic reactions,63–67 in this report, we introduce a highly efficient and recyclable copper-based catalyst for the promotion of the A3-coupling reaction for the preparation of the medicinally important propargylamine derivatives from an aldehyde, an amine, and a terminal alkyne as the starting materials. First, the ZSM-5 was modified with APTMS (3-aminopropyltrimethoxysilane) and further functionalized with (4-pyridine-2-(ylimino)methyl)benzaldehyde). Subsequently, the amine-activated zeolite@(4-pyridin-2-(ylimino)methyl)benzaldehyde) was applied to increase the stabilization of Cu metal nanoparticles. The catalyst was reduced by hydrazine to reduce Cu(II) to Cu(0) which led to active metal sites (Scheme 1). Finally, the manufactured ZSM-5@APTMS@(4-pyridin-2-(ylimino)methyl)benzaldehyde)/Cu-NPs was employed as a catalyst for the A3-coupling preparation of a series of propargylamines (Fig. 2).3.2. Spectroscopic characterization of ZSM-5@APTS@terephthalaldehyde/2-aminopyridine@Cu-NPs catalyst
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) and the bands corresponding at 1156 cm−1 and 1155 cm−1 are assumed to be of (C–O) groups, which have distinct functions in the structures. The presence of an asymmetric stretching vibration band around 1225 cm−1 confirms the structure of zeolite in the final synthesized catalyst, as well as the bands in the regions of 1100 cm−1 and 1400 cm−1 are associated with internal tetrahedral asymmetric, stretching vibrations and bending vibration, individually. A band at 1400 cm−1 corresponds to the bending of the N–H group. The peak at 3439 cm−1 illustrates branching related to the stretching mode of NH2 groups, which was modified successfully. Thus, there is strong evidence for the functionalization of ZSM-5. The peak emerging at 1532 cm−1 is associated with the stretching vibration of C–N in amine in the ZSM-5@APTMS compound. These outcomes confirm the fruitful fixation of APTMS on the surface of the ZSM-5 substrate. Other characteristic peaks of various parts of the composite overlapped, and other characterization methods were used to prove the formation of the catalyst (Fig. 3A).
C) and the bands corresponding at 1156 cm−1 and 1155 cm−1 are assumed to be of (C–O) groups, which have distinct functions in the structures. The presence of an asymmetric stretching vibration band around 1225 cm−1 confirms the structure of zeolite in the final synthesized catalyst, as well as the bands in the regions of 1100 cm−1 and 1400 cm−1 are associated with internal tetrahedral asymmetric, stretching vibrations and bending vibration, individually. A band at 1400 cm−1 corresponds to the bending of the N–H group. The peak at 3439 cm−1 illustrates branching related to the stretching mode of NH2 groups, which was modified successfully. Thus, there is strong evidence for the functionalization of ZSM-5. The peak emerging at 1532 cm−1 is associated with the stretching vibration of C–N in amine in the ZSM-5@APTMS compound. These outcomes confirm the fruitful fixation of APTMS on the surface of the ZSM-5 substrate. Other characteristic peaks of various parts of the composite overlapped, and other characterization methods were used to prove the formation of the catalyst (Fig. 3A).
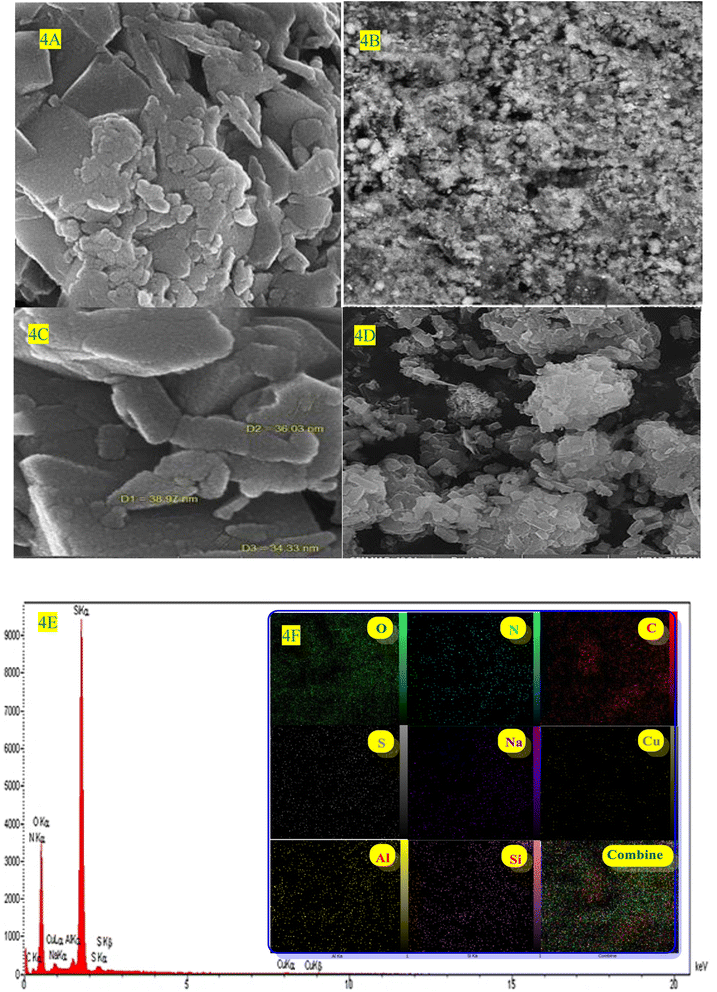 | ||
| Fig. 4 (A–D) the SEM image, (E) EDS, and (F) the mapping spectra of ZSM-5@ATPS@(2-aminopyridine-terephthalaldehyde)@Cu-NPs. | ||
In the XRD pattern of the composition of ZSM-5@APTMS@(2-aminopyridine-terephthalaldehyde) with Cu-NPs, new peaks appeared, which corresponded to the standard Bragg reflections (111), (200), (220) of the Cu-NPs, in accordance with the JCPDS card (No. 71-4610),68,69 proving their successful formation.
In this context, we used hydrazine hydrate as a reductant, which results in the formation of bigger NPs compared to other reductants such as NaBH4, hoping to form Cu-NPs over the surface of the modified ZSM-5. The TEM image exhibits the presence of well-dispersed Cu-NPs over the surface of ZSM-5 (Fig. 5A and B). SEM, EDS, and elemental mapping analysis were used to investigate the elemental composition and spatial distribution of elements in the final composite (Fig. 4A–F, and inset images). SEM-EDS analysis confirmed the presence of Al, S, Si, Na, O, C, N, and Cu in ZSM-5@APTMS@(2-aminopyridine/terephthalaldehyde)@Cu-NPs with 7.42 wt% loading of copper NPs and SEM-elemental mapping exhibits uniform elemental dispersion in the sample matrix, proving its successful formation (Fig. 4A–D).
 | ||
| Fig. 5 (A and B) The transmission electron microscope (TEM) images of ZSM-5@APTMS@(2-aminopyridine/terephthalaldehyde)@Cu-NPs. | ||
The morphology and surface structure of ZSM-5@APTMS@(2-aminopyridine/terephthalaldehyde)@Cu-NPs were investigated by SEM and TEM techniques (Fig. 5A and B, respectively). According to Fig. 4A–D, the SEM image shows the nanoparticles with a diameter starting at approximately 34.33–38.97 nm.
Elemental composition and spatial elemental distribution of ZSM-5@APTMS@(2-aminopyridine/terephthalaldehyde)@Cu-NPs were investigated by EDS and mapping techniques (Fig. 4E). According to the EDS data, the atomic ratio of Si/Al is equal to 39, which confirmed the successful immobilization of copper (Cu) nanoparticles in the newly synthesized ZSM-5@APTMS@(2-aminopyridine/terephthalaldehyde)@Cu-NPs catalyst. ZSM-5 catalyst with a high silicon-to-aluminum ratio (Si/Al > 10), indicates an increase in the crystal size, as well as an increase in the surface area, which improves the physicochemical properties of the catalyst.
The transmission electron microscope (TEM) images of the newly synthesized catalyst ZSM-5@APTMS@(2-aminopyridine/terephthalaldehyde)@Cu-NPs illustrate hierarchies of the modified ZSM-5 nanocrystals with intra- and inter-crystalline porous structures (Fig. 5). Low-magnification TEM images confirmed that ZSM-5-modified zeolite@Cu had uniform crystallites of sizes 50 and 100 nm, which is in perfect agreement with the obtained SEM images. Notably, bright spots were observed relative to intra-crystalline mesoporous, many brighter areas correspond to the presented mesoporous and are recognizable.
4. Application. Catalytic performance
After careful characterization of the synthesized ZSM-5/APTMS/(4-pyridine-2-(ylimino)methyl)benzaldehyde)/Cu-NPs, we evaluated their catalytic performance for the A3-coupling preparation of a series of propargylamine products. To this end, we chose the reaction of benzaldehyde, piperidine, and phenylacetylene as the model reaction. We also examined the effects of the reaction time and temperature, the type of solvent, and the catalyst dosage on the progress of the reaction, and the results are depicted in Table 1. To find the best solvent, the progress of the A3-coupling reaction of the model reaction in the presence of the proposed catalyst in various solvents, including water, toluene, DMSO, DMF, CH2Cl2, MeCN, a mixture of EtOH and H2O, EtOH, tetrahydrofuran, and solvent-free conditions were monitored. Despite the remarkable performance of our proposed method in solvent-free conditions, further studies were carried out with H2O as the solvent due to its green nature and ease of employment. Studies on the effect of the temperature on the progress of the reaction showed that the best results were obtained at 60 °C, and a further increase in the temperature did not increase the yield of the reaction. We monitored the progress of the reaction by the thin-layer chromatography technique, and we observed that at 60 °C in H2O, the reaction reached equilibrium after 120 min. The ideal amount of the catalyst was obtained by tracking the progress of the reaction at various catalyst dosages. The results of this study indicated that 25 mg of the proposed catalyst was sufficient for the optimal progress of the A3-coupling reaction. So, based on these studies, 25 mg of ZSM-5/APTMS/(4-pyridine-2-(ylimino)methyl) benzaldehyde)/Cu-NPs catalyst at 60 °C in 3 ml H2O as green solvent and base (K2CO3) (2 mmol) after 120 min gave the highest product yields. In addition, the reaction processes with different types of derivatives aldehydes (with electron-donating and electron-withdrawing groups) and terminal alkynes with different second-type amines were studied (Table 1).| Entrance | Catalyst (mg) | Solvent | Base | T (°C) | Time (min) | Yield (%) |
|---|---|---|---|---|---|---|
| a Reaction conditions: 1 mmol of morpholine, 1 mmol of benzaldehyde, 1.1 mmol of phenylacetylene, H2O (3 ml), K2CO3 (2 mmol), and catalyst (25 mg). | ||||||
| 1 | — | H2O | NaOH | 95 | 250 | 8 |
| 2 | 5 | H2O | KOH | 95 | 240 | 35 |
| 3 | 10 | H2O | Mg(OH)2 | 90 | 240 | 55 |
| 4 | 15 | H2O | Ca(OH)2 | 85 | 200 | 65 |
| 5 | 18 | H2O | K2CO3 | 80 | 120 | 70 |
| 6 | 20 | H2O | K2CO3 | 80 | 120 | 80 |
| 7 | 25 | H2O | K2CO3 | 75 | 120 | 96 |
| 8 | 25 | H2O | K2CO3 | 70 | 120 | 95 |
| 9 | 25 | H2O | K2CO3 | 65 | 120 | 92 |
| 10 | 25 | H2O | K2CO3 | 60 | 120 | 98 |
| 11 | 30 | H2O | K2CO3 | 60 | 120 | 92 |
| 12 | 25 | — | K2CO3 | 100 | 300 | 94 |
| 13 | 25 | H2O | K2CO3 | — | 300 | 10 |
| 14 | 25 | H2O | — | 90 | 180 | 73 |
| 15 | 25 | PhCH3 | K2CO3 | 100 | 180 | 60 |
| 16 | 25 | DMSO | K2CO3 | 100 | 200 | 62 |
| 17 | 25 | DMF | K2CO3 | 100 | 200 | 65 |
| 18 | 25 | CH2Cl2 | K2CO3 | 45 | 200 | 30 |
| 19 | 25 | MeCN | K2CO3 | 75 | 200 | 30 |
| 20 | 25 | EtOH/H2O | K2CO3 | — | 200 | 60 |
| 21 | 25 | EtOH | K2CO3 | 78 | 200 | 80 |
| 22 | 25 | THF | K2CO3 | 66 | 150 | 35 |
After obtaining the optimum condition, we tested the generality of our proposed method by synthesizing different propargylamines from various precursors. In this context, diverse aldehydes and amines reacted with the phenylacetylene in the presence of the proposed catalyst under the optimum conditions. Table 2 summarizes the outcomes of this study. As this table indicates, the reaction proceeds in excellent yields in the presence of aromatic aldehydes containing electron donating or withdrawing groups in the aromatic ring's ortho or para positions. However, employing an aliphatic aldehyde resulted in lower yields. We used three different secondary amines in this reaction, and as shown in Table 2, the change in the amine did not affect the yield of the reaction. These results indicate the excellent performance of the ZSM-5/APTMS/(4-pyridine-2-(ylimino)methyl)benzaldehyde)/Cu-NPs as a catalyst for the promotion of the A3-coupling preparation of the propargylamines.
| Entrance | Aldehyde | Amine | Product | Time (min) | Yield (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1 mmol of morpholine, 1 mmol of benzaldehyde, and 1.1 mmol of phenylacetylene, H2O (3 ml), K2CO3 (2 mmol), and catalyst (25 mg). | |||||
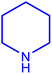
| 1 |  |
 |
 |
120 | 99 |
| 2 |  |
 |
 |
120 | 98 |
| 3 |  |
 |
 |
120 | 91 |
| 4 |  |
 |
 |
120 | 90 |
| 5 |  |
 |
 |
120 | 93 |
| 6 |  |
 |
 |
120 | 95 |
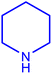
| 7 |  |
 |
 |
120 | 92 |
| 8 |  |
 |
 |
120 | 91 |
| 9 |  |
 |
 |
120 | 92 |
| 10 |  |
 |
 |
120 | 94 |
| 11 |  |
 |
 |
120 | 90 |
| 12 |  |
 |
 |
120 | 95 |
| 13 |  |
 |
 |
120 | 93 |
| 14 | 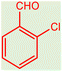 |
 |
 |
120 | 92 |
| 15 |  |
 |
 |
120 | 94 |
| 16 |  |
 |
 |
120 | 93 |
| 17 |  |
 |
 |
120 | 95 |
| 18 |  |
 |
 |
120 | 96 |
| 19 |  |
 |
 |
120 | 97 |
| 20 |  |
 |
 |
120 | 85 |
5. The proposed mechanism
The plausible mechanism was performed through the C–H activation of alkyne by the efficient newly synthesized copper-ZSM-5 catalyst to establish copper acetylide, which was performed to develop A3 coupling reactions. The first reaction was expected to activate the C–H bond through copper nanoparticles to develop a copper acetylide intermediate.70 Following, the prepared copper acetylide intermediate was reacted with the immonium ion, which was provided via nucleophilic attack of the secondary amine on the electrophilic carbon of the aldehyde group with the elimination of a water molecule, in order to provide the formation-desired propargylamine. ZSM-5/APTMS/(E)-4-((pyridin-2-ylimino) methyl) benzaldehyde@Cu-NPs was used as a catalyst for the synthesis of propargylamines by the stabilization of copper nanoparticles on the ZSM-5 support. Copper nanoparticles, through their coordination effect, have instantaneous nucleation and slow growth. The ZSM-5/APTMS/(E)-4-((pyridin-2-ylimino)methyl)benzaldehyde@Cu-NPs catalyst was applied to perform the response in order to perform aromatic and aliphatic aldehydes and various amines (Fig. 6).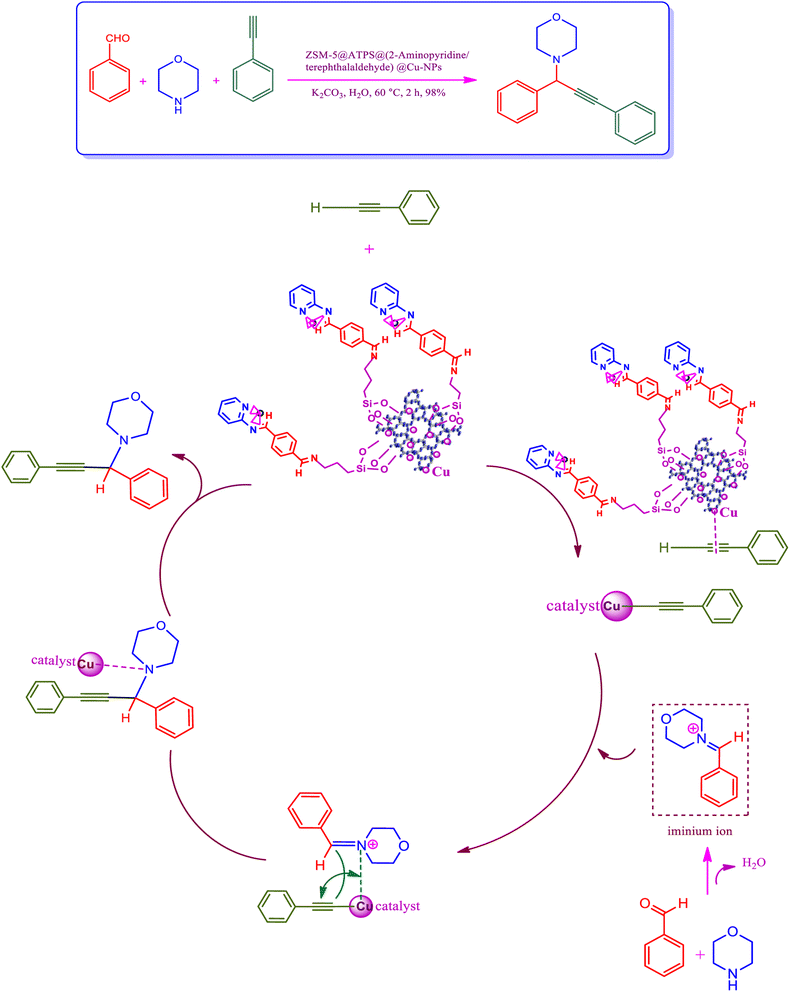 | ||
| Fig. 6 The proposed mechanism to provide propargylamines in the presence of ZSM-5/APTMS/(4-pyridine-2-(ylimino)methyl)benzaldehyde)/Cu-NPs. | ||
6. Comparison of each catalyst sections in the final catalyst
To check whether the modulation of the microenvironment around the Cu NPs affected the promotion of the A3-coupling reaction, we monitored the formation of all the synthesized propargylamine derivatives in the presence of Cu NPs decorated ZSM-5, ZSM-5@ATPMS, and ZSM-5/APTMS/(4-pyridine-2-(ylimino)methyl)benzaldehyde)/Cu-NPs. These tests were performed at the optimum conditions (25 mg catalyst, 60 °C, H2O, 120 min) to compare the results. Table S1† indicates that each step successfully modified the electronic structure of the chemical surrounding the Cu NPs, resulting in the improvement of the yield of the reaction. Based on this study, ZSM-5@ATPMS and ZSM-5/APTMS/(4-pyridine-2-(ylimino)methyl)benzaldehyde)/Cu-NPs is the optimum catalyst for promoting the preparation of propargylamines via the A3-coupling reaction, the data are shown in Table S1.† According to the results obtained, the participation of ligands and organic linkers, i.e. (4-pyridin-2-(ylimino)methyl)benzaldehyde), and APTMS (3-amino-propyltriethoxysilane), as well as stabilization of Cu nanoparticles in the synthesis and design of the structure of the final mesoporous zeolite catalyst can successfully emerge in the preparation of a high yield product.7. Comparison of the catalyst with other catalysts in the desired reaction
Table 3 compares the catalytic performance of ZSM-5/APTMS/(4-pyridine-2-(ylimino)methyl)benzaldehyde)/Cu-NPs with some of the reported catalysts from the literature for the three-component preparation of propargylamines. This table indicates that our proposed catalyst exhibits one of the highest reported yields. This could be due to the careful modification of the electronic structure of the ZM-5 via the modification process with nitrogen-rich ligands. The presence of APTMS and (E)-4-((pyridin-2-ylimino)methyl)benzaldehyde alters the electronic structure of ZSM-5, leading to the boosting of the catalytic ability of the Cu NPs in the final composite.| Entrance | Catalyst | Amount of catalyst (% mol) | Solvent | T (°C) | Time (h) | Yield (%) | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Cu/Al/oxide mesoporous | 0.12 | Toluene | 90 | 22 | 94 | 71 |
| 2 | Cu@N-rGo | — | — | 70 | 8 | 76 | 72 |
| 3 | Cu@PMO-IL | 0.15 mol | CHCl3 | 60 | 24 | 97 | 73 |
| 4 | CuO/ZnO/Al2O3 nanocatalyst | 0.05 g | — | 80 | 1.5 | 94 | 74 |
| 5 | CuI/HTNT-5 | 0.02 g | — | 80 | 1.5 | 96 | 75 |
| 6 | CuI supported Amberlyst A-21 in N2 atmosphere | 10 mol% | Neat | 98 | 8 | 85 | 76 |
| 7 | Eggshell-Cu(II)-salophen complex | 0.2 g | — | 80 | 4 | 95 | 77 |
| 8 | CuNPs/TiO2 | 0.5 mol | — | 70 | 7 | 90 | 78 |
| 9 | Cu(OH)x–Fe3O4 | 0.1 mol | — | 120 | 3 | >99 | 79 |
| 10 | CuI–USY zeolite catalyst | 0.020 g | — | 80 | 15 | 80 | 80 |
| 11 | Silica-CHDA-Cu | 0.020 g | — | 80 | 12 | 92 | 38 |
| 12 | PS-PEG-BPy-CuBr2 in N2 atmosphere | 0.05 mol% Cu | — | 110 | 1 | 88 | 81 |
| 13 | CuO NPs | 8 | Toluene | 90 | 5 | 87 | 82 |
| 14 | Cu-MCM-41 | 40 mg | — | 90–100 | 1.5 | 93 | 83 |
| 15 | [AQ2Cu(II)] | 5 | — | 100 | 1 | 90 | 84 |
| 16 | Fe3O4@TiO2/Cu2O | (0.01 g) | — | 100 | 1 | 95 | 85 |
| 17 | ZSM-5@APTMS@E(-4-((pyridin-2-ylimino)methyl)benzaldehyde@Cu-NPs | 25 mg | K2CO3·H2O | 60 | 2 | 98 | This work |
8. Reusability
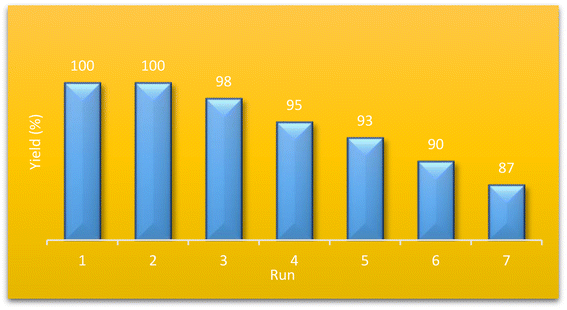
The ability of the catalyst to be used several times in the same reaction is a defining factor for its industrial applications. To investigate the recyclability of our proposed catalyst, we separated the catalyst from the reaction medium by filtration and washed it with ethyl acetate several times for purification. Then, we applied the recycled catalyst in the model reaction for seven cycles and evaluated its performance. As shown in Fig. 7, no decrease in the catalyst performance was observed for four cycles, and even after seven runs, its catalytic performance was still above 90 percent of its first use. This excellent reusability could be due to the high resistance nature of the ZSM-5 in the aqueous medium. Moreover, the modification of the ZSM-5 filled the pores of the MOF, limiting water molecules from accessing the zirconium clusters and increasing their stability. The FT-IR spectrum of recycled catalyst assigned that the catalyst has fully maintained the properties of the catalyst after seven times of use Fig. 8.8.1. ICP-OES analysis
According to the ICP-OES analysis results, the loading of Cu(0) for the fresh catalyst is 0.9. It is mentioned that the ICP analysis of the activities of the recycled catalyst in order to estimate the reusability indicated a very small reduction of less than 0.03% in the amount of copper (Cu) leaching from the synthesized catalyst after seven cycles of recycling. The Cu loading of the catalyst was measured using ICP analysis (Table 4).| Entrance | Catalyst | (mol%) Cu |
|---|---|---|
| 1 | Order 1 | 0.9 |
| 2 | Order 2 | 0.88 |
| 3 | Order 3 | 0.85 |
| 4 | Order 4 | 0.8 |
| 5 | Order 5 | 0.78 |
| 6 | Order 6 | 0.76 |
| 7 | Order 7 | 0.75 |
9. Conclusion
In this study, ZSM-5 was chosen as a support for the heterogenization of the Cu NPs due to its high potential for surface area, and inherent structural resistance. The modification of ZSM-5 was carried out by a step-by-step strategy, in which a series of N-rich organic compounds were coordinated to the NH2 groups of the organic ligand of the ZSM-5. The resulting catalyst was used to promote the A3-coupling reaction, which showed superior performance. The results of this study indicate that such high efficiency is a result of the modulation of the microenvironment of the copper NPs. The proposed catalyst exhibited superior recycling performance due to the inherent resistance of the ZSM-5 and the induced resistance due to its high surface area, interesting channel structure, thermal steadiness, corrosiveness, shape selectivity, and porous properties of ZSM-5.Author contributions
L. M. synthesized the final catalyst ZSM-5@(4-pyridine-2-(ylimino)methyl)benzaldehyde)/Cu-NPs catalyst, more synthesized, identified the propargylamine desired. L. M. prepared the manuscript. M. R. V (supervisor or project), M. H (supervisor or project), and S. R. (advisor) reviewed the manuscript.Conflicts of interest
The authors declare no competing interests.Acknowledgements
The authors would like to thank the Materials and Energy Research Center (Grant No: 9911940) for the financial support of this project.References
- M. Kidwai, V. Bansal, A. Kumar and S. Mozumdar, Green Chem., 2007, 9, 742–745 RSC.
- K. K. Das, S. Manna and S. Panda, Chem. Commun., 2021, 57, 441–459 RSC.
- C. de Graaff, E. Ruijter and R. V. Orru, Chem. Soc. Rev., 2012, 10, 3969–4009 RSC.
- B. V. Rokade, J. Barker and P. J. Guiry, Chem. Soc. Rev., 2019, 48, 4766–4790 RSC.
- M. Nasrollahzadeh, M. Sajjadi, F. Ghorbannezhad and S. M. Sajadi, Chem. Record., 2018, 18, 1409–1473 CrossRef CAS PubMed.
- L. H. Choudhury and T. Parvin, Tetrahedron, 2011, 67, 8213 CrossRef CAS PubMed.
- T. Zarganes-Tzitzikas, A. L. Chandgude and A. Dömling, Chem. Rec., 2015, 15, 981–996 CrossRef CAS PubMed.
- P. S. Nunes, H. D. A. Vidal and A. G. Corrêa, Org. Biomol. Chem., 2020, 18, 7751–7773 RSC.
- Y. Liu, Arkivoc, 2014, 1, 1–20 Search PubMed.
- K. Lauder, A. Toscani, N. Scalacci and D. Castagnolo, Chem. Rev., 2017, 117, 14091–14200 CrossRef CAS PubMed.
- Y. Gu, Green Chem., 2012, 14, 2091–2128 RSC.
- M. Syamala, Org. Prep. Proced. Int., 2009, 41, 1–68 CrossRef CAS.
- Z.-J. Yang, Q.-T. Gong, Y. Wang, Y. Yu, Y.-H. Liu, N. Wang and X.-Q. Yu, Mol. Catal., 2020, 491, 110983 CrossRef CAS.
- B. S. Iyengar, W. A. Remers and W. T. Bradner, J. med. chem., 1986, 29, 1864–1868 CrossRef CAS PubMed.
- S. Dragoni, V. Porcari, M. Valoti, M. Travagli and D. Castagnolo, J. Pharm. Pharmacol., 2006, 58, 561–565 CrossRef CAS PubMed.
- Y. Kleiner, O. Bar-Am, T. Amit, A. Berdichevski, E. Liani, G. Maor, I. Reiter, M. B. Youdim and O. Binah, J. Cardiovasc. Pharmacol., 2008, 52, 268–277 CrossRef CAS PubMed.
- I. Matsuda, J. Sakakibara and H. Nagashima, Tetrahedron Lett., 1991, 32, 7431–7434 CrossRef CAS.
- G. Villaverde, A. Corma, M. Iglesias and F. Sánchez, ACS Catal., 2012, 2, 399–406 CrossRef CAS.
- H. Li, J. Liu, B. Yan and Y. Li, Tetrahedron Lett., 2009, 50, 2353–2357 CrossRef CAS.
- V. A. Peshkov, O. P. Pereshivko and E. V. Van der Eycken, Chem. Soc. Rev., 2012, 41, 3790–3807 RSC.
- A. Mariconda, M. Sirignano, C. Costabile and P. Longo, Mol. Catal., 2020, 480, 110570 CrossRef.
- C. Mukhopadhyay and S. Rana, Catal. Commun., 2009, 11, 285–289 CrossRef CAS.
- N. P. Eagalapati, A. Rajack and Y. Murthy, J. Mol. Catal. A: Chem., 2014, 381, 126–131 CrossRef CAS.
- K. Namitharan and K. Pitchumani, Nickel-Catalyzed Solvent-Free Three-Component Coupling of Aldehyde, Alkyne and Amine. Wiley Online Library: 2010 Search PubMed.
- X. Huo, J. Liu, B. Wang, H. Zhang, Z. Yang, X. She and P. Xi, J. Mater. Chem. A, 2013, 1, 651–656 RSC.
- L. Pin-Hua and W. Lei, Chin. J. Chem., 2005, 23, 1076–1080 CrossRef.
- W.-W. Chen, H.-P. Bi and C.-J. Li, Synlett, 2010, 2010, 475–479 CrossRef.
- S. Sakaguchi, T. Mizuta, M. Furuwan, T. Kubo and Y. Ishii, Chem. Commun., 2004, 14, 1638–1639 RSC.
- E. RyanáBonfield, Org. Biomol. Chem., 2007, 5, 435–437 RSC.
- T. L. da Silva, R. S. Rambo, D. da Silveira Rampon, C. S. Radatz, E. V. Benvenutti, D. Russowsky and P. H. Schneider, J. Mol. Catal. A: Chem., 2015, 399, 71–78 CrossRef.
- L. C. Akullian, M. L. Snapper and A. H. Hoveyda, Angew. Chem., 2003, 115, 4376–4379 CrossRef.
- Y. Kuninobu, P. Yu and K. Takai, Chem. Lett., 2007, 36, 1162–1163 CrossRef CAS.
- H. Naslhajian, M. Amini, S. M. Farnia and J. Janczak, Mol. Catal., 2020, 483, 110769 CrossRef CAS.
- L. Ma, X. Shi, X. Li and D. Shi, Org. Chem. Front., 2018, 5, 3515–3519 RSC.
- Z. Li, C. Wei, L. Chen, R. S. Varma and C. J. Li, Tetrahedron Lett., 2004, 45, 2443–2446 CrossRef CAS.
- S. B. Park and H. Alper, Chem. Commun., 2005, 10, 1315–1317 RSC.
- M. L. Kantam, B. V. Prakash, C. R. Reddy and B. Sreedhar, Synlett, 2005, 2005, 2329–2332 CrossRef.
- P. Li and L. Wang, Tetrahedron, 2007, 63, 5455–5459 CrossRef CAS.
- B. Sreedhar, P. S. Reddy, C. V. Krishna and P. V. Babu, Tetrahedron Lett., 2007, 48, 7882–7886 CrossRef CAS.
- D. S. Patel, Metal Nanoparticles Decorated on Carbonaceous Solid Supports: Towards the Development of Heterogeneous Catalysts. Maharaja Sayajirao University of Baroda (India), 2021 Search PubMed.
- S. Pandey and T. Mandal, Eur. J. Inorg. Chem., 2021, 2021, 1763–1769 CrossRef CAS.
- N. Pal and A. Bhaumik, RSC Adv., 2015, 5, 24363–24391 RSC.
- H. Li, Q. Pan, Y. Ma, X. Guan, M. Xue, Q. Fang, Y. Yan, V. Valtchev and S. Qiu, J. Am. Chem. Soc., 2016, 138, 14783–14788 CrossRef CAS PubMed.
- Y.-B. Huang, J. Liang, X.-S. Wang and R. Cao, Chem. Soc. Rev., 2017, 46, 126–157 RSC.
- L. V. Chopda and P. N. Dave, ChemistrySelect, 2020, 5, 5552–5572 CrossRef CAS.
- S. G. Newman and K. F. Jensen, Green Chem., 2013, 15, 1456–1472 RSC.
- V. Polshettiwar, C. Len and A. Fihri, Coord. Chem. Rev., 2009, 253, 2599–2626 CrossRef CAS.
- T. Baran, I. Sargin, M. Kaya and A. Menteş, J. Mol. Catal. A: Chem., 2016, 420, 216–221 CrossRef CAS.
- P. R. Boruah, P. S. Gehlot, A. Kumar and D. Sarma, Mol. Catal., 2018, 461, 54–59 CrossRef CAS.
- S. Saneinezhad, L. Mohammadi, V. Zadsirjan, F. F. Bamoharram and M. M. Heravi, Sci. Rep., 2020, 10, 1–26 CrossRef PubMed.
- R. Kore, R. Srivastava and B. Satpati, Chem. – Eur. J., 2014, 20, 11511–11521 CrossRef CAS PubMed.
- G. Kokotailo, S. Lawton, D. Olson and W. Meier, Nature, 1978, 272, 437–438 CrossRef CAS.
- R. J. Argauer and G. R. Landolt, Crystalline zeolite ZSM-5 and method of preparing the same. Google Patents: 1972 Search PubMed.
- C. Colella and A. F. Gualtieri, Microporous Mesoporous Mater., 2007, 105, 213–221 CrossRef CAS.
- A. Mastinu, A. Kumar, G. Maccarinelli, S. A. Bonini, M. Premoli, F. Aria, A. Gianoncelli and M. Memo, Molecules, 2019, 24, 1517 CrossRef CAS PubMed.
- K. S. Triantafyllidis, L. Nalbandian, P. N. Trikalitis, A. K. Ladavos, T. Mavromoustakos and C. P. Nicolaides, Microporous Mesoporous Mater., 2004, 75, 89–100 CrossRef CAS.
- S. Sang, F. Chang, Z. Liu, C. He, Y. He and L. Xu, Catal. Today, 2004, 93, 729–734 CrossRef.
- O. Singh, A. Agrawal, N. Dhiman, B. P. Vempatapu, K. Chiang, S. Tripathi and B. Sarkar, Renewable Energy, 2021, 179, 2124–2135 CrossRef CAS.
- D. P. Serrano, J. A. Melero, G. Morales, J. Iglesias and P. Pizarro, Catal. Rev., 2018, 60, 1–70 CrossRef CAS.
- I. Díaz, E. Kokkoli, O. Terasaki and M. Tsapatsis, Chem. Mater., 2004, 16, 5226–5232 CrossRef.
- C. Pagis, A. R. Morgado Prates, D. Farrusseng, N. Bats and A. Tuel, Chem. Mater., 2016, 28, 5205–5223 CrossRef CAS.
- Y. Gutha and V. S. Munagapati, Int. J. Biol. Macromol., 2016, 93, 408–417 CrossRef CAS PubMed.
- W. Zhang, R. Taheri-Ledari, M. Saeidirad, F. S. Qazi, A. Kashtiaray, F. Ganjali, Y. Tian and A. Maleki, J. Environ. Chem. Eng., 2022, 108836 CrossRef CAS.
- S. Babaee, M. Zarei, H. Sepehrmansourie, M. A. Zolfigol and S. Rostamnia, ACS Omega, 2020, 5, 6240–6249 CrossRef CAS PubMed.
- S. Rostamnia and E. Doustkhah, Tetrahedron Lett., 2014, 55, 2508–2512 CrossRef CAS.
- H. Alamgholiloo, R. Mohammadi, S. Rostamnia and M. Shokouhimehr, J. Environ. Chem. Eng., 2021, 9, 105486 CrossRef CAS.
- E. Doustkhah, S. Rostamnia, B. Gholipour, B. Zeynizadeh, A. Baghban and R. Luque, Mol. Catal., 2017, 434, 7–15 CrossRef CAS.
- A. Taghizadeh and K. Rad-Moghadam, J. Cleaner Prod., 2018, 198, 1105–1119 CrossRef CAS.
- A. S. Nagpure, N. M. Mohture and A. Kayarkar, Inorg. Chem. Commun., 2022, 146, 110118 CrossRef CAS.
- R. R. Julian, J. A. May, B. M. Stoltz and J. Beauchamp, J. Am. Chem. Soc., 2003, 125, 4478–4486 CrossRef CAS PubMed.
- J. Dulle, K. Thirunavukkarasu, M. C. Mittelmeijer-Hazeleger, D. V. Andreeva, N. R. Shiju and G. Rothenberg, Green Chem., 2013, 15, 1238–1243 RSC.
- V. V. Kumar, R. Rajmohan, P. Vairaprakash, M. Mariappan and S. P. Anthony, Dalton Trans., 2017, 46, 11704–11714 RSC.
- M. Gholinejad, B. Karimi, A. Aminianfar and M. Khorasani, ChemPlusChem, 2015, 80, 1573–1579 CrossRef CAS PubMed.
- Z. Sotoudehnia, J. Albadi and A. R. Momeni, Appl. Organomet. Chem., 2019, 33, e4625 CrossRef.
- B. R. Reddy, P. V. G. Reddy, M. V. Shankar and B. N. Reddy, Asian J. Org. Chem., 2017, 6, 712–719 CrossRef CAS.
- G. Bosica and R. Abdilla, J. Mol. Catal. A: Chem., 2017, 426, 542–549 CrossRef CAS.
- M. Bakherad, A. Keivanloo, A. H. Amin, R. Doosti and O. Hoseini, Iran. J. Catal., 2016, 6, 325–332 CAS.
- M. J. Albaladejo, F. Alonso, Y. Moglie and M. Yus, Eur. J. Org. Chem., 2012, 2012, 3093–3104 CrossRef CAS.
- M. J. Aliaga, D. J. Ramón and M. Yus, Org. Biomol. Chem., 2010, 8, 43–46 RSC.
- M. K. Patil, M. Keller, B. M. Reddy, P. Pale and J. Sommer, Copper zeolites as green catalysts for multicomponent reactions of aldehydes, terminal alkynes and amines: An efficient and green synthesis of propargylamines. Wiley Online Library: 2008 Search PubMed.
- S. Yan, S. Pan, T. Osako and Y. Uozumi, ACS Sustainable Chem. Eng., 2019, 7, 9097–9102 CrossRef CAS.
- M. Nasrollahzadeh, S. M. Sajadi and A. Rostami-Vartooni, J. Colloid Interface Sci., 2015, 459, 183–188 CrossRef CAS PubMed.
- M. Abdollahi-Alibeik and A. Moaddeli, RSC Adv., 2014, 4, 39759–39766 RSC.
- H. Sharghi, A. Khoshnood and M. M. Doroodmand, J. Iran. Chem. Soc., 2012, 9, 231–250 CrossRef CAS.
- A. Elhampour and F. Nemati, J. Chin. Chem. Soc., 2016, 63, 653–659 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ra07700k |
| This journal is © The Royal Society of Chemistry 2023 |