Open Access Article
Open Access ArticleCopper-catalyzed Sonogashira reactions: advances and perspectives since 2014
Koduvarathodi Vadakkethil Arundhathi
,
Palemkunnummal Vaishnavi
,
Thaipparambil Aneeja
and
Gopinathan Anilkumar
 *
*
School of Chemical Sciences, Mahatma Gandhi University, Priyadarsini Hills P.O, Kottayam, Kerala 686560, India. E-mail: anilgi1@yahoo.com; anil@mgu.ac.in; Fax: +91-481-2731036
First published on 7th February 2023
Abstract
Transition metal catalyzed Sonogashira coupling reaction has evolved as an efficient pathway for the construction of C–C bonds. Initially, palladium cooperating with copper was considered as the efficient catalytic system for this reaction. However, nowadays there have been astonishing progress in copper catalyzed Sonogashira coupling reactions. Copper catalyzed reactions have attained significant attention owing to the cost effective and environmentally benign characteristics of this metal compared to palladium. This review summarizes the recent developments in copper catalyzed Sonogashira coupling covering literature from 2014 to 2021.
1 Introduction
Transition metal-catalyzed coupling reactions are becoming an indispensable tool in organic synthesis.1 Transition metals are widely used in catalysis due to their incomplete “d orbitals” which promote them to easily accept and donate electrons to other atoms or molecules. Due to this profitable property of transition metals, several transition metals are used as catalysts in different organic reactions.2–5 These types of reactions are widely used in industrial applications to synthesize medicine, fertilizers, and pesticides.6Using different transition metals as catalysts, coupling reactions between aryl halides (Cl, –Br, –I, –OTf) and terminal acetylene were introduced.7–9 This created a new era in synthetic organic chemistry. In 1972 Heck introduced the formation of the substituted olefinic compound through the addition of organopalladium with olefin.10 This discovery inspired the scientific community and motivated them to exploit palladium.11 Afterward, different scientists such as Stille, Hiyama, Negishi, and Suzuki proposed novel cross-coupling reactions using palladium as a catalyst and aryl halides as the initial precursor.12–15 Later in 1975, as a continuation of his work, Heck proposed that monosubstituted acetylenes are transformed into disubstituted acetylenes by reaction with aryl, heterocyclic, or vinylic bromides or iodides at 100 °C in the presence of a basic amine and a diacetatobis(triphenylphosphine)palladium(II) catalyst.16 In the same year, Sonogashira forwarded a new mechanism for a similar coupling using copper as a co-catalyst and palladium as a catalyst which created a straightforward formation of C–C bonds under mild conditions.17
Palladium as a metal is expensive and there is a high probability to lose the catalyst at the end of the catalytic cycle. Furthermore, the product's probable Pd contamination limits its application in bioactive compounds and raises environmental and economic concerns in large-scale synthesis and industry. These issues create economic barriers to coupling reactions using palladium. On behalf of that Sonogashira coupling devoid of palladium metal gives out a profitable way in organic synthesis. The green chemistry aspect in modern synthetic chemistry leads to promoting the use of less toxic first-row transition metals as catalysts in organic reactions.18,19 Due to its low toxicity, easy commercial availability at a reasonable cost, and complimentary reactivity compared with heavier late d-block metals, copper catalyzed reactions have significant economic and operational benefits.20–22
All over the world, several pieces of literature are reported under palladium-free copper-catalyzed Sonogashira coupling. In 2014, Thomas et al. published a review that covers the progress in copper-catalyzed Sonogashira coupling reactions up to 2014.23 Herein, we summarize the recent works in copper-catalyzed Sonogashira coupling reactions covering the literature from 2014 to 2021.
For better understanding, this review is categorized into two sections as copper catalyzed Sonogashira reaction using homogeneous and heterogeneous catalyst.
2 Homogeneous copper catalysts
2.1 Cu–nitrogen complexes
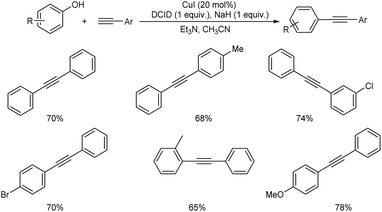
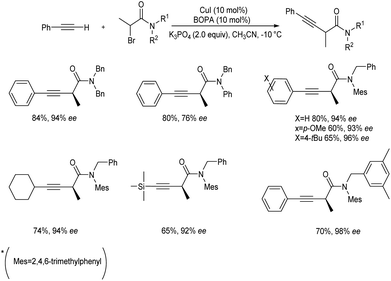
Hosseini and co-workers proposed a copper-catalyzed Sonogashira cross-coupling reaction in presence of dichloroimidazolidinedione (DCID).24 This method involves the reaction between substituted phenols and phenyl acetylenes. Phenols with electron-donating groups in para, ortho, and meta position and disubstituted phenols efficiently gave the desired product in good yields. Phenols with electron-withdrawing group are less efficient and achieved the desired product in lower yield. This paper provides the first application of air-stable, less expensive DCID in Sonogashira cross-coupling (Scheme 1).Mo et al. proposed enantioselective Sonogashira type coupling of alkynes with α-bromoamides catalyzed by copper.25 This reaction enabled the enantioselective coupling between α-halo amides with unfunctionalized terminal alkynes and as a result β,γ-alkynyl amides were generated. This is a simple method for synthesising synthetically valuable alkynyl amides from two widely available starting materials in a highly enantioselective way. They identified that bisoxazoline diphenylaniline (BOPA) was the efficient ligand for this method. The optimal reaction condition includes 1.0 equiv. of N-benzyl-2-bromo-N-mesitylpropanamide and 2.0 equiv. of ethynylbenzene at −10 °C in CH3CN, catalyzed by 10 mol% CuI and 10 mol% iPr-BOPA. The steric requirement of R2 is a critical parameter in boosting the efficiency of the cross-coupling process (Scheme 2). The mechanistic pathway of the abovementioned reaction is depicted (Scheme 3). In the presence of base, the Cu(I)I undergoes ligand exchange with L, resulting in the tridentate complex Cu(I)L. Following an interaction with RC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH in the presence of K3PO4, [LCu(I)(C
CH in the presence of K3PO4, [LCu(I)(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR)]− K+ is formed (A). The species (A) which could reduce the α-bromoamide to provide a copper(II) species LCu(II)(C
CR)]− K+ is formed (A). The species (A) which could reduce the α-bromoamide to provide a copper(II) species LCu(II)(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR) with a simultaneous formation of an alkyl radical (B). Afterward, there are two possible pathways for the formation of the desired product. In path a, the alkyl radical reacts with copper(II), delivering a copper(III) complex (C), which undergoes reductive elimination to form the product and the catalyst gets regenerated. In path b, the alkyl radical could undergo direct out-of-cage bond formation with the copper(II) species to generate the same product.
CR) with a simultaneous formation of an alkyl radical (B). Afterward, there are two possible pathways for the formation of the desired product. In path a, the alkyl radical reacts with copper(II), delivering a copper(III) complex (C), which undergoes reductive elimination to form the product and the catalyst gets regenerated. In path b, the alkyl radical could undergo direct out-of-cage bond formation with the copper(II) species to generate the same product.
 | ||
| Scheme 2 Enantioselective Sonogashira type coupling of alkynes with α-bromoamides catalyzed by copper. | ||
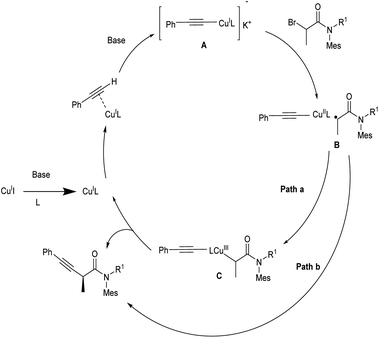 | ||
| Scheme 3 Proposed mechanism of copper catalyzed Sonogashira coupling of alkynes with α-bromoamides (this figure has been reproduced from ref. 25 with permission from John Wiley and Sons copyright 2020). | ||
CuI-catalyzed Sonogashira reaction for the synthesis of 1H-imidazo[2,1-a]isoquinoline derivatives was proposed by Gang and co-workers.26 They developed a CuI/o-phen catalyzed tandem Sonogashira coupling/hydroamination method in which 2-(2-bromophenyl)imidazoles react with terminal alkynes to give imidazo[2,1-a]isoquinoline-based fused polycyclic compounds in good yields. The electron-donating groups (e.g., –Me, –Et, –OMe), as well as the electron-withdrawing groups (e.g., –F and –Cl) in the benzene moieties, were tolerated well to achieve the desired imidazo[2,1-a]isoquinoline derivatives in good yields. Under the same reaction conditions, cyclopropyl acetylene was also tolerant, yielding 3-cyclopropylimidazo[2,1-a]isoquinolines in 90% yield (Scheme 4).
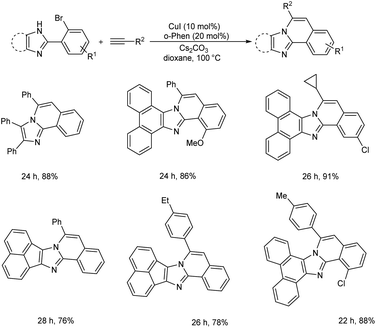 | ||
| Scheme 4 Copper catalyzed Sonogashira reaction for the synthesis of 1H-imidazo[2,1-a]isoquinoline derivatives. | ||
Copper associated with nitrogen-based ligands as a catalyst for Sonogashira coupling was widely exploited. Hajipour et al. established an efficient Sonogashira cross-coupling reaction of phenylacetylene with different aryl halides using monobenzylnicotinium chloride and copper(I) chloride as the catalytic system.27 Monobenzyl nicotinium chloride, a quaternary ammonium salt with a coordinating centre, is a key component of this catalytic system because it improves the efficiency of Cu(I) species during the reaction. This method was found suitable to different varieties of internal alkynes and obtained the desired product in moderate to good yields. The effectiveness of this catalytic system was compared to that of a copper-based catalyst made from dibenzylnicotinium chloride, which lacks the N donor active site, and which has lower activity due to the lack of a coordination site (Scheme 5).
Yamane et al. worked on copper-catalyzed functionalized tertiary-alkylative Sonogashira type couplings via copper acetylide at room temperature.28 There have been several reports on Sonogashira coupling, however many of them have used aryl or alkenyl halides as coupling partners. Sonogashira coupling is hence inappropriate for alkyl loadings, particularly tertiary alkyl groups. They discovered that at ambient temperature, copper catalyst is effective for forming a quaternary carbon with an alkynyl group from a terminal alkyne and an α-bromocarbonyl molecule. Copper acetylide was discovered to be a critical intermediate in control tests. This approach can be used to make a variety of congested alkynylated nitrogen compounds. Surprisingly, the multidentate ligands for copper salts were TPMA (tris(2-pyridylmethyl) amine), PMDETA (N,N,N′,N′,N′′-pentamethyldiethylenetriamine) and Me6TREN (tris[2-(dimethylamino)ethyl] amine), which are all incredibly efficient for providing an atom transfer radical addition type of reaction whereas bidentate ligands like 1,10-phenanthroline led to a Sonogashira coupled end product (Scheme 6).
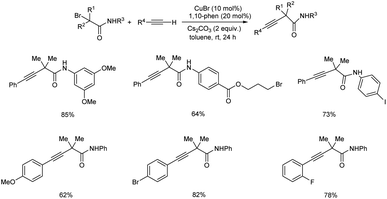 | ||
| Scheme 6 Reaction between a terminal alkyne and an α-bromocarbonyl compound to form a quaternary carbon having alkynyl group at room temperature. | ||
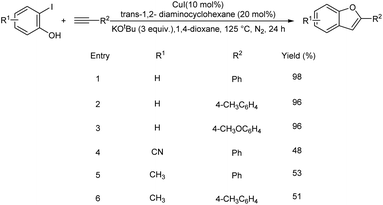
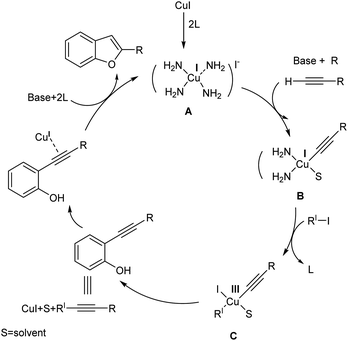
An efficient one-pot strategy for the synthesis of 2-arylbenzofuran derivatives via Cu-catalyzed tandem Sonogashira coupling-cyclization was accomplished by Anilkumar et al.29 In comparison to the traditional Pd-catalyzed techniques, this protocol is shown to be cost effective and has a broad substrate scope. This protocol was found efficient towards the direct synthesis of corsifuran C, a physiologically active natural molecule. This methodology also provides an easy access to the family of benzofuran-based heterocyclic compounds using less reactive bromophenols as starting material. This method was found suitable to 2-iodophenols with electron-withdrawing and electron-releasing groups (Scheme 7).
Based on observations, they proposed a plausible mechanism for tandem Sonogashira-cyclization reaction. The mechanism begins with the addition of phenylacetylene to the Cu–ligand complex A. Further, a copper–acetylide complex B is formed by adding alkyne to this complex, followed by the oxidative addition of aryl halide to B resulting in the formation of complex C. The high-energy Cu(III) complex is subsequently reductively eliminated to release the Sonogashira product, which then undergoes the permitted 5-endo-dig cyclization process to yield benzofuran in the presence of base (Scheme 8).
 | ||
| Scheme 8 Proposed mechanism for the one-pot synthesis of benzofurans (this figure has been reproduced from ref. 29 with permission from John Wiley and Sons copyright 2019). | ||
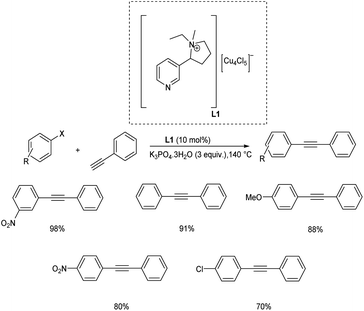
Wang and co-workers utilized triazine triazole conjugates (TT) as ligand system for copper catalyzed Sonogashira and decarboxylative cross-coupling reaction.30 For the copper-catalyzed Sonogashira reaction, TT conjugates were identified as effective N,N′ donor ligands. The electron-donating groups on heterocyclic rings were found to be adverse for enhancing the catalytic efficiency of TT ligands in ligand effect tests. In the Sonogashira reaction, an economical catalyst system was designed for diverse electron-poor and electron-rich aryl iodides and aryl/alkyl alkynes, yielding the desired products in moderate to good yields (Scheme 9).
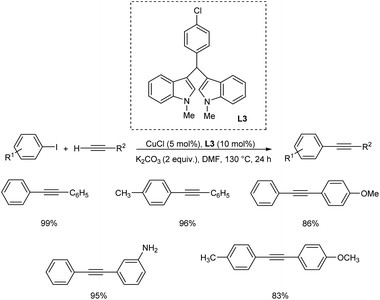
For copper catalyzed Sonogashira reactions, BIMs (bisindoles) were utilized as novel N,N′ donor ligands.31 The ligand screening revealed that neither electron-withdrawing groups nor electron-donating groups boosted cross-coupling product yields any further. The most active ligand for Cu-catalyzed Sonogashira reactions was discovered to have a chlorobenzyl unit, according to ligand screening tests. To obtain outstanding yields, copper (5 mol%) and ligand loading (10 mol%) were effectively applied to a variety of variably substituted aryl iodides for coupling to both alkyl- and aryl-substituted terminal alkynes (Scheme 10).
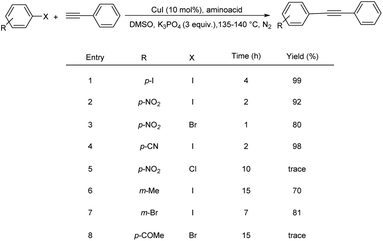
Hajipour and co-workers discovered methionine as a green and efficient promoter for copper-catalyzed Sonogashira cross-coupling reactions. CuI-catalyzed Sonogashira cross-coupling of different aryl halides with phenylacetylene was carried out in the presence of amino acids as ecologically friendly ligands to obtain the appropriate internal alkynes.32 Surprisingly, this palladium-free coupling reaction worked well in the presence of L-methionine as ligand. The solvent system was also found to play a crucial role in this reaction, with a large impact on product formation and reaction rate. Among the various solvents, DMSO was identified as the best solvent in this reaction. Among the two amino acids methionine and proline, it is found that reaction with methionine gave excellent yield. Substrate scope studies revealed that electron-withdrawing aryl halides gave comparatively higher yield than electron-donating ones (Scheme 11).
2.2 Cu–NHC complexes
Domyati et al. worked on Sonogashira-type cross-coupling reactions catalyzed by copper complexes of pincer N-heterocyclic carbenes. N-Heterocyclic carbene (NHC) copper complexes have recently got a lot of interest in catalysis, although their applications have been limited to neutral complexes with monodentate ligands.33 They described the synthesis and complete characterization of a cationic Cu–pincer bis (NHC) complex with bulky tert-butyl wingtips that acts as a catalyst for the coupling of aryl iodides and phenylacetylene, as well as its analogues with tiny alkyl substituents. The mechanistic analyses show that coupling yields are higher in air than in argon, implying a mechanism other than the traditional Sonogashira process. When they used a radical trap in their controlled investigations, the air-assisted cross-coupling reaction came to a full stop, indicating the existence of a radical in the mechanism. Oxygen reactivity and radical trap experiments support this theory. This is the first report of Sonogashira-type cross-coupling reactions catalyzed by well-defined copper catalysts under oxidative conditions. Further mechanistic studies are presently underway to elucidate the structure and reactivity of Cu-oxygen adducts, as well as the subtleties of homo-coupling suppression (Scheme 12).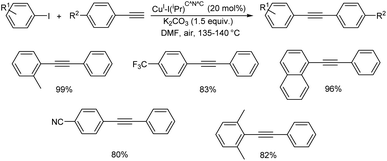 | ||
| Scheme 12 The coupling of aryl iodides and phenylacetylene derivatives catalyzed by Cu–pincer NHC complex. | ||
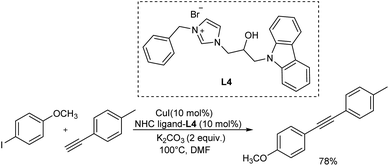
Girase et al. established a palladium-free Cu/carbazole-based N-heterocyclic carbene catalyzed homo, hetero coupling of alkynes at ambient temperature.34 They optimised the reaction conditions using 4-iodoanisole and 4-methyl phenylacetylene as the model substrates and the optimized condition includes 10 mol% of CuI and 10 mol% of NHC ligand -L1 in the basic medium of 2 equiv. K2CO3 at 100 °C dissolved in a DMF medium. However, the same ligand system at a lower temperature condition at 60 °C provided a lower yield (Scheme 13).
2.3 Cu–phosphorous complexes
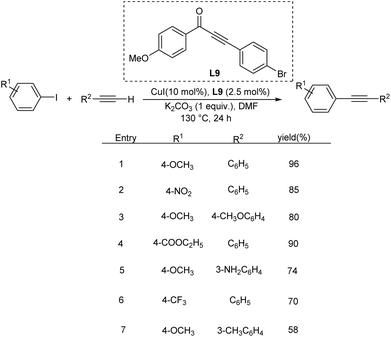
Xu et al. proposed an efficient catalytic system (Cu(OTf)2/L1 (L1 = (R)-(−)-1,1′-binaphthyl-2,2′-diyl hydrogenphosphate)) towards Sonogashira cross coupling reactions.35 The system needed a low copper loading of about 4 mol% for the cross-coupling between aromatic or aliphatic terminal alkynes and aryl iodides. The optimised reaction condition for the Sonogashira coupling using this novel catalytic system include 4 mol% of Cu(OTf)2 and 10 mol% of phosphate ligand at 130 °C for 16 h. Iodobenzenes with electron-donating groups are more efficient in this reaction than electron-withdrawing ones. When comparing the substrate scope of alkynes, aryl alkynes produced a better yield whereas aliphatic alkynes provided the required product in low yield (Scheme 14).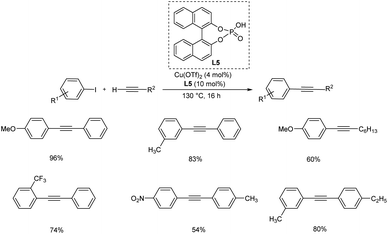 | ||
| Scheme 14 Sonogashira reaction between aryl iodides and terminal alkynes in presence of Cu(OTf)2 and phosphate ligand. | ||
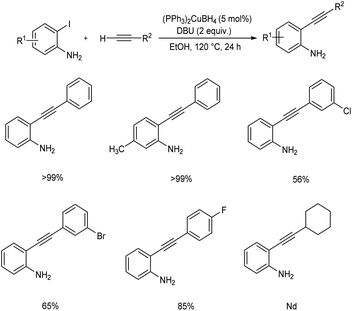
Chen et al. reported the synthesis of 2-ethynylaniline derivatives through Sonogashira cross-coupling of o-iodoanilines with terminal alkynes.36 The optimised condition for this reaction includes (PPh3)2CuBH4 (5 mol%) as catalyst, DBU (2 equiv.) as base at 120 °C for 24 h under air. Substrate scope studies revealed that o-iodoanilines with methyl, trifluoromethyl, and fluorine substituents afforded the desired product in 99 or >99% yields. Phenylacetylenes with electron-donating and electron-withdrawing groups tolerated well in this reaction. Moreover, this method was found applicable to 3-ethynylthiophene and obtained the desired product in 84% yield. Terminal aliphatic alkynes were failed to achieve the required product via this method (Scheme 15).
2.4 Cu–carbon complexes
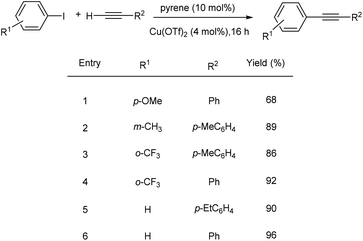
Xu et al. proposed copper-catalyzed C(sp2)–C(sp) Sonogashira-type cross-coupling reactions accelerated by polycyclic aromatic hydrocarbons.37 Sonogashira-type cross-coupling reactions have been established employing a very effective catalytic system based on Cu(OTf)2 catalyst and polycyclic aromatic hydrocarbon ligand (pyrene) (Scheme 16). Because it costs around a tenth of what similar catalyst systems do, this catalytic system is suitable for both industrial and academic contexts. The methodology established has been used to make several fluorescent diynes. Aryl iodides containing electron-donating or electron-withdrawing groups react rapidly with arylalkynes, resulting in good to exceptional yields of desired products.2.5 Cu–nitrogen and phosphorous based complexes
Cao and team proposed a copper-catalyzed Sonogashira coupling reaction of alkyl halides with terminal alkynes. This reaction efficiently provides a flexible tool for the production of substituted alkynes.38 Under mild reaction conditions, a novel proline-based N,N,P-ligand is used to facilitate the transformation. The exact reaction was carried out between 1-bromo-1-phenyl ethane and phenylacetylene in the presence of CuOAc (10 mol%), N,N,P-ligand (12 mol%) and Cs2CO3 (2 equiv.) in Et2O at room temperature and obtained the desired product in 94% yield. The alkyl halides with electron-withdrawing groups at meta and para positions of the phenyl ring provided a better yield when compared to the ortho-substituted phenyl ring. Terminal alkynes with aromatic rings provided a better yield than aliphatic terminal alkynes (Scheme 17).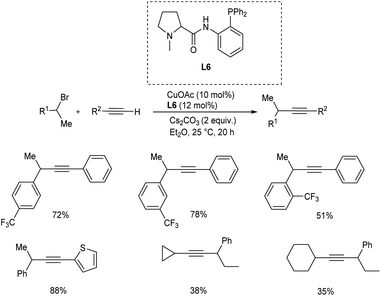 | ||
| Scheme 17 Copper catalyzed Sonogashira coupling of alkyl halides with terminal alkynes using copper salt and proline-based N,N,P-ligand. | ||
Zhang et al. reported a copper/chiral cinchona alkaloid-based N,N,P-ligand catalyst for highly regio-, chemo-, and enantioselective oxidative cross-coupling of unactivated C(sp3)–H bonds with terminal alkynes.39 The team investigated the viability of a detachable guiding amide group for sequential N-oxidation, as well as asymmetric copper-catalyzed Sonogashira-type coupling of unactivated C–H bonds with terminal alkynes. The use of N-fluoroamide as a mild oxidant was employed for efficiently generating site-selective alkyl radical species and for preventing Glaser homocoupling. The reaction was performed in a basic THF solvent medium between the N-fluoro amide and a terminal alkyne in the presence of 10 mol% CuI/chiral cinchona alkaloid-based N,N,P-ligand catalyst at room temperature. Aryl alkynes, as well as hetero aryl alkynes with electron-donating and electron-withdrawing groups were tolerated well in this reaction. The reaction was also found suitable to silyl alkyne and achieved the required product in of 78% yield with 97% enantioselectivity (Scheme 18).
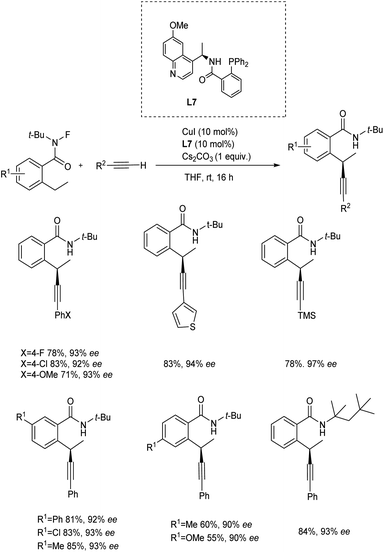 | ||
| Scheme 18 A copper/chiral cinchona alkaloid-based N,N,P-ligand catalyzed oxidative cross-coupling of unactivated C(sp3)–H bonds with terminal alkynes. | ||
Dong and co-workers demonstrated a widespread stereoconvergent Sonogashira C(sp3)–C(sp) cross-coupling of terminal alkynes and racemic alkyl halides enabled by radical-involved alkynylation with a chiral cinchona alkaloid-based P,N-ligand.40 This study underlines the significance of radical species in the development of enantioconvergent transformations. Because of the more difficult oxidative addition (OA) and the possibility of β-H elimination, Sonogashira C(sp3)–C(sp) cross-coupling is more difficult than C(sp2)–C(sp) cross-coupling. The reaction was carried out between the racemic 1-phenyl ethyl bromide and terminal alkyne in presence of 5 mol% of CuTC (copper thiophene 2-carboxylate), 7.5 mol% of chiral cinchona alkaloid-based P,N-ligand and 2 equiv. of Cs2CO3 in Et2O at room temperature. A diverse range of substituted aryl alkynes, including those with monosubstituted phenyl rings containing electron-donating or electron-withdrawing groups at various locations (ortho, meta, or para), a disubstituted phenyl ring, a naphthalene ring, a ferrocene ring and heteroaryl alkynes performed well in this reaction. On analysing the substrate scope of racemic alkyl halides, secondary benzylic bromides have the ability to produce perfect chemo selective products than primary bromides and all chlorides (Scheme 19).
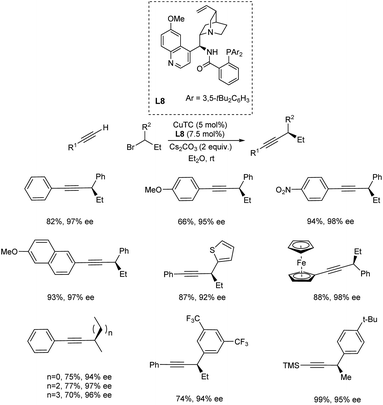 | ||
| Scheme 19 Copper catalyzed stereoconvergent Sonogashira C(sp3)–C(sp) cross-coupling of terminal alkynes and racemic alkyl halides. | ||
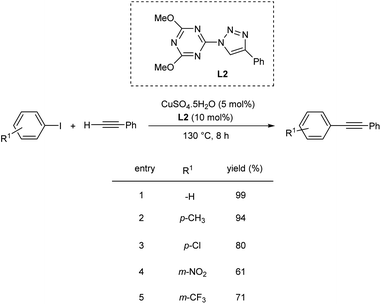
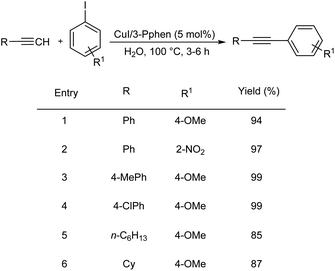
Yu et al. proposed a Cu-catalyzed Sonogashira coupling of aryl iodides with terminal alkynes in water.41 They carried out the initial reaction using phenylacetylene and 4-iodoanisole as the model substrates and the optimized condition includes CuI (5 mol%), 3-Pphen (5 mol%), and K2CO3 (2 equiv.) in water at 100 °C. Various electron-rich, electron-neutral, and electron-deficient aryl iodides were coupled with phenylacetylene to produce diaryl acetylenes in good yield. Alkynes other than phenylacetylene also reacted well with 4-iodoanisole and afforded the required product in good yield. However, aryl bromides are less efficient in these conditions and gave only 9% yield of the desired product (Scheme 20).
2.6 Ligand-free Sonogashira coupling
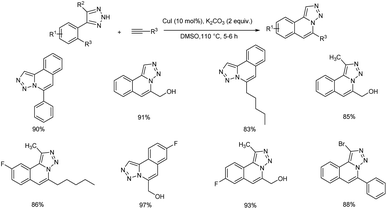
A copper-catalyzed tandem Sonogashira coupling/regioselective 6-endo cyclization was established for the synthesis of [1,2,3] triazolo [5,1-a]isoquinoline derivatives.42 This NH-triazole directed-annulation technique yielded the matching N-fused heterocycles with acceptable functional group tolerance. A large variety of [1,2,3] triazolo-[5,1-a]isoquinoline derivatives were obtained in good to excellent yield using this NH-triazole directed-annulation technique (Scheme 21).Dhiman and co-workers formulated a copper catalyzed one-pot, atom-economical multicomponent reaction of 2-bromoindole-3-carbaldehydes, alkynes and ammonia to give a series of 3-aryl-γ-carboline derivatives in good to excellent yield.43 This method involves the imine production followed by copper-catalyzed directed Sonogashira coupling and intramolecular hydroamination reactions. This multicomponent technique involves mild reaction conditions and exhibit good functional group compatibility. The suggested methodology's atom-economical and operationally simple characteristics are extremely desirable green attributes in the field of current synthetic chemistry. Arylacetylenes with electron-donating substituents gave higher yield of the desired product compared to the electron-withdrawing ones (Scheme 22).
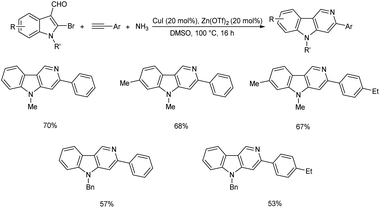 | ||
| Scheme 22 Copper catalyzed multicomponent reaction of 2-bromoindole-3-carbaldehydes, alkynes and ammonia. | ||
2.7 Miscellaneous
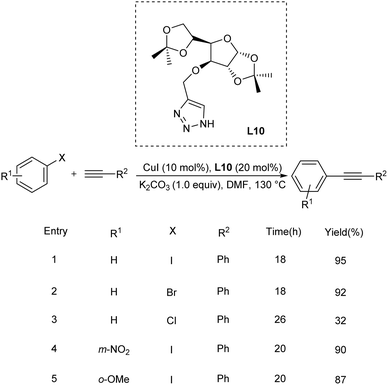
Wang et al. used functionalized α,β-ynones as efficient ligand for Cu catalyzed Sonogashira-type cross-coupling reaction.44 Usually Csp2–Csp cross coupling requires considerable excess of copper catalyst and N, O donor ligands under the typical reaction condition. In this method, they utilized α,β-ynones as an efficient ligand system and found that substituted alkynes, at low-mol percent catalyst system demonstrated adequate activity and tolerance. From their studies, it was identified that the most efficient ligand is the one with bromo- and methoxy-groups, which catalyzed Sonogashira-type reactions with 1.0 mol% copper. The coupling reactions are compatible with a wide range of functional groups and achieved good yields of products (Scheme 23).Sideways homocoupling reactions have been identified as the major shortcomings in copper catalysed Sonogashira reaction. To overcome this limitation, Tiwari in 2021, introduced a novel methodology utilising glycosyl triazoles (L10) as the ligand.45 Their studies revealed that at high temperature (130 °C), L10-copper catalysed Sonogashira reaction did not form any homocoupling product under air atmosphere. The major highlight of this method is that it avoids the use of inert gas which is essential for copper catalysed Sonogashira reaction to completely exclude oxygen. At 130 °C, excellent product yields were obtained for a variety of substrates including aliphatic and aromatic terminal alkynes and variously substituted aromatic halides such as 9-bromo noscapine. Surprisingly, the same catalytic system at room temperature was found efficient in Glaser coupling including homocoupling and heterocoupling of variety of aliphatic and aromatic alkynes (Scheme 24).
3 Heterogeneous Sonogashira coupling
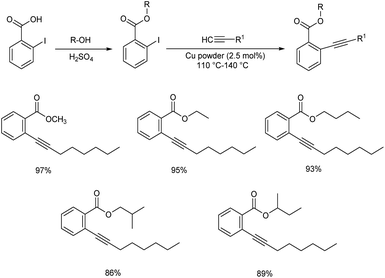
An efficient copper catalyzed Sonogashira coupling reaction and simulation studies were formulated by Ali and co-workers.46 They utilized Cu powder as a catalyst in 1-substitution of alkyl-2-iodobenzoates with 1-octynes under solvent, co-catalyst, and base-free conditions. The reaction went successfully, yielding the required products in moderate to excellent amounts (84–97%). The formed compounds can be used in anti-cizmatics, anti-fobic disorder and inhibition of aspulvinone dimethylallyltransferase to control Alzheimer's disease. Major advantages of this method include eco-friendly and ligand-free, co-catalyst-free, and base-free characteristics (Scheme 25).Fath and co-workers developed a simple and effective method for producing a GO-supported copper(I) complex catalyst for enhancing the rate of Sonogashira coupling between aryl halides and phenylacetylene.47 The organosilane functionalized graphene oxide was utilized as a stabiliser in this method. The [Cu(PPh3)3Cl] complex was successfully fixed onto the graphene oxide surface through coordination interaction with organosilane ligand spacers. The optimised condition for the reaction between aryl halides and phenylacetylene was employed at a temperature of 80 °C in the presence of K2CO3 in water. The Sonogashira reaction of activated aryl iodide and aryl bromide with various substituents were reacted well to afford the required product in high yields. Their studies revealed that aryl iodides and bromides are better substrates than aryl chlorides for diarylacetylene production (Scheme 26).
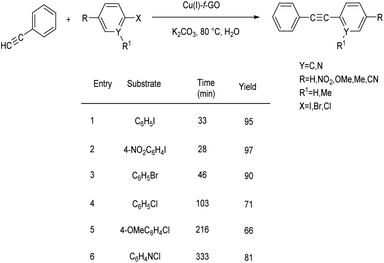 | ||
| Scheme 26 Water based GO-supported copper(I) complex catalyzed Sonogashira coupling between aryl halides with phenylacetylene. | ||
Shanmugam et al. mentioned a visible light initiated single metal heterogeneous Sonogashira coupling.48 They demonstrated a CO2 enhanced photo redox Sonogashira coupling of terminal alkynes and aryl halides catalyzed by Cu2O truncated nanocubes (Cu2O TNCs) at room temperature (Scheme 27). The increased yield under CO2 conditions is most likely due to two things. First, because an O2 environment is known to stimulate the synthesis of Glaser homocoupling products, removing molecular O2 can decrease the homocoupling products. Second, the addition of CO2 to the surface of Cu2O TNCs likely promotes the formation of surface-bound Cu1-phenylacetylide and increases its excited-state lifetime, resulting in more efficient single electron transfer to aryl halides. Cu2O nanocrystals with different morphologies could catalyse the reaction and provide the desired product in good yields whereas Cu2O nanoparticles are not able to catalyse the above-mentioned reaction. From the substrate cope studies, it is clear that differently substituted aryl iodides and phenylacetylenes were tolerated well in this reaction. However, aryl bromides gave comparatively lower yield of the desired product. The mechanistic pathway indicated that CO2 promotes the synthesis of light-absorbing heterogeneous surface-bound Cu1-phenylacetylide (λmax = 472 nm), which then undergoes single-electron transfer with aryl iodides/bromides to create Csp2–Csp bonds of the Sonogashira coupling (Scheme 28).
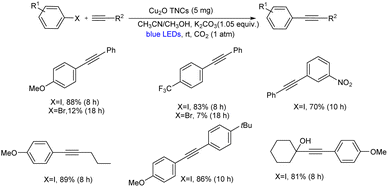 | ||
| Scheme 27 Cu2O truncated nanocubes catalyzed visible light initiated single metal heterogeneous Sonogashira coupling of terminal alkynes and aryl halides. | ||
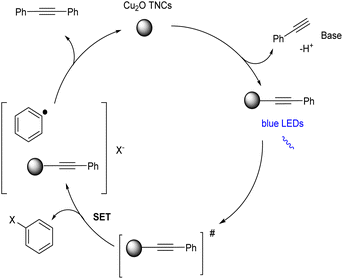 | ||
| Scheme 28 Proposed mechanistic pathway of photo redox/Cu catalyzed Sonogashira coupling of terminal alkynes and aryl halides (this figure has been reproduced from ref. 48 with permission from John Wiley and Sons copyright 2019). | ||
4 Conclusion
Sonogashira coupling reaction is one of the famous and extremely used synthetic procedures for the formation of aryl acetylenes, which are present in many pharmaceuticals and natural products. The journey in this field begins years back even though new innovations are still going on by modifying the reaction conditions. In this review we summarized the reported data in the field of palladium-free copper catalyzed Sonogashira coupling reactions around the last eight years. Copper catalysed Sonogashira coupling devoid of palladium metal creates a new impulse in synthetic organic chemistry through the replacement of an expensive transition metal catalyst with an another one which is comparatively cheap, safe and affordable.Homogeneous Sonogashira coupling reactions are widely studied and reported. Heterogeneous copper catalyzed Sonogashira coupling reactions with a high recyclability of catalyst can introduce the catalyst into more commercialisation aspects and provide a more sustainable environment, and this area needs much focus in future. Despite nitrogen and phosphorous-based ligands, synthesis of other ligand systems that can modify the stability of catalysts to a high extent of time should be a matter of interest in this field of research. Some of the works reported under ligand-free, copper-catalyzed Sonogashira systems offer new avenues in pharmaceutical and industrial applications. Further, mechanistic studies are necessary for the modifications of the ongoing catalytic systems. The scientific community should focus on green and eco-friendly copper-based catalysts with better regio- and stereoselectivity.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
TA thanks the Council of Scientific and Industrial Research (CSIR New Delhi) for the award of senior research fellowship.References
- A. D. Meijere, S. Brase and M. Oestreich. Metal catalyzed cross-coupling reactions and more, John Wiley & Sons, 2013 Search PubMed.
- J. Takaya, Chem. Sci., 2021, 12, 1964–1981 RSC.
- J. K. Kochi, J. Organomet. Chem., 2002, 653, 11–19 CrossRef CAS.
- B. Su, Z. C. Cao and Z. J. Shi, Acc. Chem. Res., 2015, 48, 886–896 CrossRef CAS PubMed.
- D. M. Miller, G. R. Buettener and S. D. Aust, Free Radical Biol. Med., 1990, 8, 95–108 CrossRef CAS PubMed.
- L. V. Budarin, P. S. Shuttleworth, J. H. Clark and R. Luque, Curr. Org. Synth., 2010, 7, 614–627 CrossRef.
- M. B. Marulasiddeshwara and P. R. Kumar, Mater. Today: Proc., 2018, 5, 20811–20818 CAS.
- X. Wang, Y. Song, J. Qu and Y. Luo, Organometallics, 2017, 36, 1042–1048 CrossRef CAS.
- M. Alami, F. Ferri and G. Linstrumelle, Tetrahedron Lett., 1993, 34, 6403–6406 CrossRef CAS.
- R. F. Heck and J. P. Nolley Jr, J. Org. Chem., 1972, 37, 2320–2322 CrossRef CAS.
- H. A. Dieck and F. R. Heck, J. Organomet. Chem., 1975, 3, 259–263 CrossRef.
- J. K. Stille, Angew. Chem., Int. Ed., 1986, 25, 508–524 CrossRef.
- Y. Hatanaka and T. Hiyama, J. Org. Chem., 1988, 53, 918–920 CrossRef CAS.
- A. O. King, N. Okukado and E. Negishi, J. Chem. Soc., Chem. Commun., 1977, 19, 683–684 RSC.
- N. Miyaura, K. Yamada and A. Suzuki, Tetrahedron Lett., 1979, 20, 3437–3440 CrossRef.
- A. H. Dieck and R. F. Heck, J. Organomet. Chem., 1975, 93, 259–263 CrossRef.
- K. Sonogashira, Y. Tohda and N. Hagihara, Tetrahedron Lett., 1975, 16, 4467–4470 CrossRef.
- R. A. Jagtap and B. Punji, Asian J. Org. Chem., 2019, 9, 326–342 CrossRef.
- E. M. Beck and M. J. Gaunt, Top. Curr. Chem., 2010, 292, 85–121 CrossRef CAS PubMed.
- I. P. Beletskaya and A. P. Cheprakov, Coord. Chem. Rev., 2004, 248, 2337–2364 CrossRef CAS.
- L. J. Cheng and N. P. Mankad, Chem. Soc. Rev., 2020, 49, 8036–8064 RSC.
- I. P. Beletskaya and A. P. Cheprakov, Organometallics, 2012, 31, 7753–7808 CrossRef CAS.
- A. M. Thomas, A. Sujatha and G. Anilkumar, RSC Adv., 2014, 4, 21688–21698 RSC.
- N. Hosseini, J. Mokhtari and I. Yavari, ChemistrySelect, 2021, 6, 5198–5202 CrossRef CAS.
- X. Mo, B. Chen and G. Zhang, Angew. Chem., 2020, 132, 14102–14106 CrossRef.
- M. Y. Gang, J. Q. Liu and X. S. Wang, Tetrahedron, 2017, 73, 4698–4705 CrossRef CAS.
- A. R. Hajipour, E. Boostani and F. Mohammadsaleh, RSC Adv., 2015, 5, 94369–94374 RSC.
- Y. Yamane, N. Miwa and T. Nishikata, ACS Catal., 2017, 7, 6872–6876 CrossRef CAS.
- A. M. Thomas, S. Asha, R. Menon and G. Anilkumar, ChemistrySelect, 2019, 4, 5544–5547 CrossRef CAS.
- Z. Wang, X. Wang, H. Sun, Z. Zhu, G. Zhang, W. Zhang and Z. Gao, ChemistrySelect, 2016, 1, 391–395 CrossRef CAS.
- X. Wang, Z. Wang, Y. Wu, Y. Luo, G. Zhang, Y. Jian, H. Sun, W. Zhang and Z. Gao, Appl. Organomet. Chem., 2016, 30, 831–834 CrossRef CAS.
- A. R. Hajipour and F. Mohammadsaleh, Appl. Organomet. Chem., 2015, 29, 787–792 CrossRef CAS.
- D. Domyati, R. Latifi and L. Tahsini, J. Organomet. Chem., 2018, 860, 98–105 CrossRef CAS.
- T. R. Girase, S. Bhilare, S. S. M. Bandaru, N. Chrysochos, C. Schulzke, Y. S. Sanghvi and A. R. Kapdi, Asian J. Org. Chem., 2020, 9, 274–291 CrossRef.
- W. Xu, B. Yu, H. Sun, G. Zhang, W. Zhang and Z. Gao, Appl. Organomet. Chem., 2015, 29, 301–304 CrossRef CAS.
- X. Chen and X. Y. Zhou, Synthesis, 2022, 54, A–H Search PubMed.
- W. Xu, B. Yu, H. Sun, G. Zhang, W. Zhang and Z. Gao, Appl. Organomet. Chem., 2015, 29, 353–356 CrossRef CAS.
- Y. X. Cao, X. Y. Dong, J. Yung, S. P. Jiang, S. Zhou, Z. L. Li, G. Q. Chen and X. Y. Liu, Adv. Synth. Catal., 2020, 362, 2280–2284 CrossRef CAS.
- Z. H. Zhang, X. Y. Dong, X. Y. Du, Q. S. Gu, Z. L. Li and X. Y. Liu, Nat. Commun., 2019, 10, 1–10 CrossRef PubMed.
- X. Y. Dong, Y. F. Zhang, C. L. Ma, Q. S. Gu, F. L. Wang, Z. L. Li, S. P. Jiang and X. Y. Liu, Nat. Chem., 2019, 11, 1158–1166 CrossRef CAS PubMed.
- A. Y. Mitrofanov and I. P. Beletskaya, Mendeleev Commun., 2019, 29, 378–379 CrossRef CAS.
- M. Fan, Y. Liu, Q. Hu, L. Jia and Y. Chen, Eur. J. Org. Chem., 2016, 33, 5470–5473 CrossRef.
- S. Dhiman, S. Rhodes, D. Kumar, A. Kumar and M. Jha, ChemistrySelect, 2017, 2, 8922–8926 CrossRef CAS.
- X. Wang, Z. Wang, Z. Xie, G. Zhang, W. Zhang and Z. Gao, RSC Adv., 2016, 6, 109296–109300 RSC.
- N. Mishra, S. K. Singh, A. S. Singh, A. K. Agrahari and V. K. Tiwari, J. Organomet. Chem., 2022, 86, 17884–17895 Search PubMed.
- I. Ali, D. Nighot, M. N. Lone and A. Jain, Synth. Commun., 2017, 47, 1175–1184 CrossRef CAS.
- R. H. Fath and S. F. Hoseini, Appl. Organomet. Chem., 2018, 32, e3964 CrossRef.
- M. Shanmugam, A. Sagadevan, V. P. Charpe, V. K. K. Pampana and K. C. Hwang, ChemSusChem, 2020, 13, 287–292 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |