Open Access Article
Open Access ArticleHollow nanotube arrays of nickle–cobalt metal sulfide for high energy density supercapacitors†
Ding Shen a,
MingYue Liab,
Yaohan Liua,
Xiaofan Fua,
Haoran Yua,
Wei Dong
a,
MingYue Liab,
Yaohan Liua,
Xiaofan Fua,
Haoran Yua,
Wei Dong *a and
ShaoBin Yang*a
*a and
ShaoBin Yang*a
aCollege of Materials Science and Engineering, Liaoning Technical University, Fuxin, Liaoning 123000, China. E-mail: lgddongwei@163.com; lgdysb@163.com
bInstitute of Engineering Technology and Natural Science, Belgorod State University, Belgorod 308015, Belgorod Oblast, Russia
First published on 14th February 2023
Abstract
High energy density is still difficult to achieve using existing metal sulfides because of their low specific capacitance. To improve capacitance, a series of nickel and cobalt metal sulfides with different Ni/Co ratios were synthesized by a two-step hydrothermal method. Using the combining method of experimental research and first-principles calculation, the morphology, structural stability, electronic structure and electrochemical properties of metal sulfides were investigated systematically. The results show that the morphology of metal sulfides gradually grows from two-dimensional structure to nanotube arrays, and finally to nanorod arrays, as the Ni/Co ratios decrease. Among them, the NC24 sample with the Ni/Co ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 is a hollow nanotube array composed of NiCo2S4, which shows excellent electrochemical performance. The specific capacity of the NC24 sample reaches 1527C g−1 at 1 A g−1, and the capacity retention is 93.81% at 10 A g−1 after 2000 cycles. Furthermore, a symmetrical supercapacitor assembled from the NiCo2S4 nanotube array shows a high energy density of 67.5 W h kg−1. This strategy develops a nanotube array of metal sulfides and expands its application in a high energy density supercapacitor.
2 is a hollow nanotube array composed of NiCo2S4, which shows excellent electrochemical performance. The specific capacity of the NC24 sample reaches 1527C g−1 at 1 A g−1, and the capacity retention is 93.81% at 10 A g−1 after 2000 cycles. Furthermore, a symmetrical supercapacitor assembled from the NiCo2S4 nanotube array shows a high energy density of 67.5 W h kg−1. This strategy develops a nanotube array of metal sulfides and expands its application in a high energy density supercapacitor.
1. Introduction
Due to the rapid development of society, the consumption of traditional fossil energy is huge and increasing gradually, which leads to a serious shortage of fossil energy and the aggravation of carbon dioxide pollution.1–3 The development of new energy sources such as solar energy, wind energy and tidal energy is one of the main approaches to solve the above problem. However, these new energies can not be used directly on a large scale because of their randomness and instability. It is necessary to develop efficient energy conversion and storage devices.4,5 As one of the energy storage devices, the supercapacitor has many advantages, such as high power density, fast charge and discharge speed, long cycle life, etc., so supercapacitors and their electrode materials have been of wide concern for many researchers.6–9However, the large-scale application of supercapacitor is seriously hindered by the low energy density, compared with batteries. The approach to improve the energy density of supercapacitor is to increase the specific capacitance and voltage window of electrode materials, according to the formula E = 1/2CV2.10 Therefore, electrode materials such as transition metal oxides, hydroxides and transition metal sulfides have become an important research field because of their high specific capacitance.11,12 Transition metal sulfides have more flexible crystal structure and better electrical conductivity, compared with metal oxides and hydroxides.13–15 Moreover, the valence range of Ni and Co in transition metal sulfides is wide, which is beneficial to the reversible redox reaction of nickle cobalt metal sulfides during the charge and discharge process.16 Considering these characteristics, nickle–cobalt metal sulfides are considered to be one of the most promising electrode materials for supercapacitor.17
At present, many nickle–cobalt metal sulfides with various morphologies and nanostructures, such as zero-dimensional nanoparticles, one-dimensional nanotubes and nanorods, two-dimensional nanoflakes, three-dimensional sea urchins and flowers, etc., are synthesized by chemical deposition, electrodeposition, solvothermal and sol–gel methods, respectively.
For example, Zhao et al.18 prepared three-dimensional flaky NiCoxSy materials by chemical deposition. The NiCoxSy materials have rich mesoporous structure and good electrical conductivity, which is beneficial to improve their capacitance properties. Therefore, the specific capacitance of the NiCoxSy materials is 1196 F g−1 when the current density is 1 A g−1 and the specific capacitance retention is 97.5% after 4000 cycles. When the current density increases to 20 A g−1, the specific capacitance of the NiCoxSy materials maintains 61.7% of the capacitance at 1 A g−1, showing good structural stability and rate performance. Similarly, Shinde et al.19 synthesized nanosheets-like NiCo2S4 materials by chemical deposition method. The nanosheets-like structure helps to improve the capacity of the electrode material, and the maximum capacitance can reach 1072 F g−1. Shi et al.20 prepared a three-dimensional hollow sea urchin-like NiCo2S4 material by solvothermal method. Due to the unique structure of both external sea urchin-like morphology and internal hollow structure, NiCo2S4 materials show high specific capacitance and rate performance. The specific capacitance of NiCo2S4 material is 1398 F g−1 at 1 A g−1, and it still reaches 1110 F g−1 at 10 A g−1. Tang et al.21 prepared a three-dimensional flower-like NiCo2S4 material by first chemical precipitation and then annealing treatment. The crystal structure of the NiCo2S4 material modified by polyethylene glycol is pure and the specific surface area is larger, thus the electrochemical performance is significantly improved. The specific capacitance of the modified NiCo2S4 material is 2199 F g−1 at 1 A g−1, and the specific capacitance retention is 82.0% after 1000 cycles at 10 A g−1. Jiang et al.22 prepared a three-dimensional pine cone structure Ni–Co–S4 nanomaterial by a one-step solvothermal method. The specific capacitance of the Ni–Co–S4 material is 2215 F g−1 at 0.5 A g−1, and the specific capacitance retention is close to 90.2% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, showing good cycling performance. Wang et al.23 successfully prepared a two-dimensional nanorod array NiCo2S4 material with a nanorod as the backbone and nanowires as the branch by using the hydrothermal method. The NiCo2S4 nanorod array has good electrochemical performance with a specific capacitance of 3093 F g−1 at 5 A g−1 and 2130 F g−1 at 30 A g−1. In addition, the introduction of graphene,24 carbon nanotubes,25 graphite-like carbon nitride,26 carbon fiber,27 activated carbon28 or polyaniline29 into NiCo2S4 metal sulfides could also significantly improve the electrochemical performance, which have attracted wide attention.
000 cycles, showing good cycling performance. Wang et al.23 successfully prepared a two-dimensional nanorod array NiCo2S4 material with a nanorod as the backbone and nanowires as the branch by using the hydrothermal method. The NiCo2S4 nanorod array has good electrochemical performance with a specific capacitance of 3093 F g−1 at 5 A g−1 and 2130 F g−1 at 30 A g−1. In addition, the introduction of graphene,24 carbon nanotubes,25 graphite-like carbon nitride,26 carbon fiber,27 activated carbon28 or polyaniline29 into NiCo2S4 metal sulfides could also significantly improve the electrochemical performance, which have attracted wide attention.
The above results show that the structure and morphology of metal sulfide NiCo2S4 have a significant effect on its capacitance properties. However, it is impossible to directly compare and investigate the structure and morphology of NiCo2S4 material and their effects on electrochemical performance, due to different preparation methods or different synthesis conditions. Meanwhile, the interaction between Ni and Co in NiCo2S4 metal sulfides and the mechanism of improving the electrochemical performance have not been fully revealed, compared with Ni3S4 or Co3S4. In this paper, a series of nickle and cobalt metal sulfides were synthesized by a two-step hydrothermal method by adjusting the Ni/Co ratios and the effects of the Ni/Co ratios on the composition, morphology and capacitance properties of metal sulfides were investigated. Meanwhile, the formation energies of metal sulfides with different Ni/Co ratios and the electronic structures of typical metal sulfides, such as Ni3S4, NiCo2S4 and Co3S4, are calculated by the first principles.
2. Experimental and theoretical details
2.1. Synthesis of metal sulfides
A series of nickle–cobalt metal sulfides were synthesized by a two-step hydrothermal method, including the first synthesis of precursors and the second synthesis of nickle–cobalt metal sulphide by Na2S. Firstly, seven groups of NiCl2·6H2O and CoCl2·6H2O were weighed, according to the molar ratio of Ni to Co of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 5
0, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 4
1, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 3
2, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 2
3, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 1
4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and 0
5 and 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, and then they were dissolved in deionized water of 40 mL, respectively. Then seven groups of 0.07 g urea were dissolved in 35 mL deionized water, respectively. The above two solutions were uniformly mixed and added into a Teflon-lined stainless steel autoclave. Then, the pre-cleaned nickle foam sheet (2.5 × 3 cm2) was also put into the autoclave. The autoclave was kept at 120°C for 6 h, then cooled to room temperature. Then, the foamed nickle sheet with metal sulfide precursors was taken out and put into an ultrasonic cleaner for 2 min, then washed alternately with deionized water and ethanol many times.
6, and then they were dissolved in deionized water of 40 mL, respectively. Then seven groups of 0.07 g urea were dissolved in 35 mL deionized water, respectively. The above two solutions were uniformly mixed and added into a Teflon-lined stainless steel autoclave. Then, the pre-cleaned nickle foam sheet (2.5 × 3 cm2) was also put into the autoclave. The autoclave was kept at 120°C for 6 h, then cooled to room temperature. Then, the foamed nickle sheet with metal sulfide precursors was taken out and put into an ultrasonic cleaner for 2 min, then washed alternately with deionized water and ethanol many times.
The second step, seven groups of 0.72 g Na2S were weighed and dissolved in deionized water of 75 mL, in which the Na2S was used to sulfurize the precursors to prepare nickle–cobalt metal sulfides. And then put into the Teflon-lined stainless steel autoclave. The nickle foam sheet with metal sulfide precursors prepared in the previous step was put into the seven groups of an autoclave. These autoclaves were kept at 160°C for 6 h, and then cooled to room temperature. The nickle foam sheets with metal sulfides were taken out and washed alternately with deionized water and ethanol for many times. Finally, the metal sulfides with different Ni/Co ratios were obtained by drying at 60°C for 10 h, which were labeled as NC60, NC51, NC42, NC33, NC24, NC15 and NC06, respectively.
2.2. Characterization of morphology and structure
The phase analysis was carried out by powder X-ray diffractometer (XRD) with Cu tube, λ = 1.54182 nm (XRD-6100 Shimadzu). The morphological analysis was performed with the scanning electron microscope (SEM, JEOL 7600). The chemical properties and composition of the surface were measured by the X-ray photoelectron spectrometer (XPS, Thermo Fischer). The chemical states of cobalt, nickle and sulfur were measured by XPS with Al kα radiation (1486.6 eV) and binding energies were calibrated by C 1s (284.6 eV). The high resolution images were tested by transmission electron microscope (TEM, Tecnai G2).2.3. Characterization of electrochemical performance
The electrochemical performance of metal sulfides was carried out using an electrochemical workstation (CHI- 660E, Chenhua) in 6.0 mol L−1 KOH electrolyte. Using nickle foam loaded with metallic sulfides (∼4.7 mg cm−2) as working electrode, platinum plate as counter electrode and Hg/HgO electrode as reference electrode, a three-electrode system was assembled for the galvanostatic charge–discharge test, cyclic voltammetry test and AC impedance spectrum test. The specific capacitance (Cs), specific capacity (C) and theoretical specific capacity (C′) of the electrode can be calculated by the following formula:30| Cs = I × Δt/(m × ΔV) | (1) |
| C = I × Δt/m | (2) |
| C′ = nF/m | (3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 485C mol−1).
485C mol−1).
The cyclic voltammetry curve was tested in the voltage window of 0–0.54 V and the scanning rate of 2, 5, 10, 15, 20, 25, 30 and 50 mV s−1, respectively. The AC impedance spectrum is measured with an AC amplitude of 5 mV and a frequency range of 10−2 to 105 Hz. Selecting a pair of foam nickle of loaded NC24 sample with excellent electrochemical performance as working electrodes, a symmetrical supercapacitor was assembled for energy density, power density and cycle life testing. The energy density (E) and power density (P) can be calculated by the formula (4):
| E = Ccell × ΔV2/7.2, P = 3600 × E/Δt | (4) |
2.4. First-principles theoretical calculation
The formation energies of metal sulfides with different Ni/Co ratios and the electronic structures of typical metal sulfides, such as Ni3S4, NiCo2S4 and Co3S4, were calculated by CASTEP31 based on density functional theory(DFT). In the process of calculation, the interaction between nuclei and valence electrons is described by Ultra-soft pseudopotential32 and the electron exchange correlation can be carried out using PBE functional with generalized gradient approximation(GGA).33 After testing, the energy cut-off for the plane-wave expansion is set to 500 eV, the atomic total energy convergence is set to 1.0 × 10−5 eV per atom, and the force convergence is set to 0.2 eV nm−1. Due to the different crystal structures of metal sulfides, the Brillouin-zone integral is sampled by Monkhorst–Pack grid K points at an interval of about 0.02 Å−1.34In order to investigate the thermodynamic stability of metal sulfides, the formation energy (ΔEf)35 is defined as:
| ΔEf = [E(NixCoyS4) − xE(Ni) − yE(Co) − 4E(S)]/(x + y + 4) | (5) |
3. Results and discussion
3.1. Structural characterizations
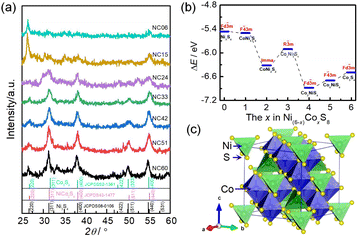
In order to analyze the effect of the Ni/Co ratios on the structure of metal sulfides, all metal sulfides samples were analyzed by XRD and the results are shown in Fig. 1. From Fig. 1a, it can be found that the single nickle sulfide sample (NC60) has obvious diffraction peaks near 26.6, 31.4, 38.0, 50.1 and 54.7°. These diffraction peaks correspond to the standard JCPDS card of Ni3S4 (no. 08-0106), which indicates that the NC60 sample is mainly composed of cubic Ni3S4. Similarly, the diffraction peaks of a single cobalt sulfide sample (NC06) near 26.7, 31.7, 38.1, 47.5, 50.0 and 54.9°correspond to the standard JCPDS card of Co3S4 (no. 02-1361), which indicates that the NC06 sample is mainly composed of cubic phase Co3S4. For NC24 sample, the diffraction peaks appears at near 26.7, 31.5, 38.2, 50.3 and 55.2°, corresponding to the standard JCPDS card of NiCo2S4 (no. 43-1477). Therefore, combined with the XRD patterns of nickle–cobalt sulfides and the molar ratio of Ni and Co in the reactant raw materials, NC51 sample is composed of NiCo2S4 and Ni3S4, while NC33, NC24 and NC15 samples are composed of NiCo2S4 and Co3S4.In order to investigate the thermodynamic structural stability of metal sulfides with different Ni/Co ratios, the formation energies (ΔEf) of metal sulfides with different Ni/Co ratios are calculated by the first-principle calculation, and the results are shown in Fig. 1b. It can be seen from Fig. 1b that the value ΔEf of metal sulfides with different Ni/Co ratios are all negative, indicating that these metal sulfides are thermodynamically stable. Among them, the value of ΔEf of NiCo2S4 with space group Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m is the lowest, indicating that the thermodynamic structure of NiCo2S4 is the most stable. This also explains why the metal sulfides synthesized with different Ni/Co ratios are mainly composed of cubic NiCo2S4. The schematic of crystal structure of NiCo2S4 is shown in Fig. 1c. NiCo2S4 is cubic with space group Fd
m is the lowest, indicating that the thermodynamic structure of NiCo2S4 is the most stable. This also explains why the metal sulfides synthesized with different Ni/Co ratios are mainly composed of cubic NiCo2S4. The schematic of crystal structure of NiCo2S4 is shown in Fig. 1c. NiCo2S4 is cubic with space group Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, in which Co atoms are bonded with six equivalent S atoms to form a CoS6 octahedron, and Ni atoms are bonded with four equivalent S atoms to form a NiS4 tetrahedron.36,37
m, in which Co atoms are bonded with six equivalent S atoms to form a CoS6 octahedron, and Ni atoms are bonded with four equivalent S atoms to form a NiS4 tetrahedron.36,37
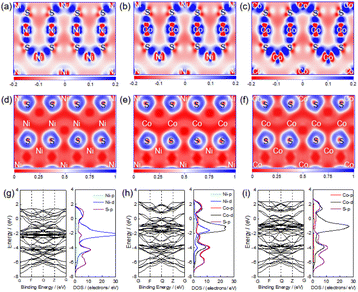
In order to further understand the electronic properties of metal sulfides, typical metal sulfides, such as Ni3S4, NiCo2S4 and Co3S4, were selected for structural optimization and electronic properties by first-principle calculation, and the results are shown in Fig. 2. Fig. 2a–c shows the charge density difference (CDF) of Ni3S4, NiCo2S4 and Co3S4. From Fig. 2a–c, it can be seen that the Ni or Co metal atoms lose electrons and the S atoms gain electrons, indicating that the electrons of Ni or Co metal atoms transfer to S atoms in Ni3S4, NiCo2S4 and Co3S4. Fig. 2d–f shows the electronic local function (ELF) of Ni3S4, NiCo2S4 and Co3S4, in which the value range of ELF is 0–1, as shown in the icon. As known, when the ELF value is 0.75–1, it is mainly a covalent bond, 0.5–0.75 is mainly a metal bond, and 0–0.5 is mainly a strong ionic bond. As can be seen from Fig. 2d–f, the Ni–S bond or Co–S bond in Ni3S4, NiCo2S4 and Co3S4 are all ionic bonds with certain covalent bond properties according the ELF value. Fig. 2g–i shows the energy band structure (EBS) and density of states (DOS) of Ni3S4, NiCo2S4 and Co3S4. From the EBS in Fig. 2g–i, it can be seen that Ni3S4, NiCo2S4 and Co3S4 all have electronic states near the Fermi level (0 eV), which shows that they all have good conductivity. The experimental results show that the electronic conductivity of NiCo2S4 is 1.25 × 106 s m−1 at room temperature,38 which is in agreement with the theoretical calculation results. From the DOS near the Fermi level in Fig. 2g–i, it can be seen that the electronic state of Ni3S4 is mainly contributed by the electrons of Ni-3d and S-3p, while the electronic state of Co3S4 is mainly contributed by the electrons of Co-3d and S-3p, and the electronic states of NiCo2S4 are mainly contributed by electrons of Ni-3d, Co-3d, Co-4p and S-3p. The synergistic effect between Co and Ni in NiCo2S4 crystal leads to the change of electronic structure, especially the electrons transfer of Co-4p, which results in the shortening of Co–S bond from 0.226 nm in Co3S4 crystal to 0.214 nm, which indicates that the chemical bond of Co–S bond in NiCo2S4 crystal is shorter and the interaction is stronger. However, the change of Ni–S bond in NiCo2S4 crystal is not obvious compared with that in Ni3S4 crystal, which is between 0.219–0.220 nm.
In order to analyze the elemental composition and valence distribution of NC24 samples in detail, the NC24 samples were analyzed by X-ray photoelectron spectra (XPS) and the results are shown in Fig. 3. As can be seen from Fig. 3a, the full spectra of NC24 sample shows that it contains Ni, Co, S, C and O elements, in which the molar ratio of Ni, Co and S is 0.96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.84
1.84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. That is to say, the chemical formula of NC24 sample is Ni0.96Co1.84S4, which is close to its stoichiometric ratio of 1
4. That is to say, the chemical formula of NC24 sample is Ni0.96Co1.84S4, which is close to its stoichiometric ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. It is also confirmed that the NC24 sample is composed of NiCo2S4, which is consistent with the results of XRD analysis in this paper. In addition, the O element may come from the slight oxidation on NC24 sample surface. Fig. 3b shows a high-resolution XPS spectra of Ni 2p. It can be seen from Fig. 3b that Ni 2p1/2 and Ni 2p3/2 are fitted by two peaks respectively, and the peaks of binding energy at 852.5 eV and 872.9 eV correspond to Ni2+, binding energy at 855.3 eV and 874.9 eV belonged to Ni3+, binding energy at 860.8 eV and 879.7 eV are satellite peaks, marked as “Sat.” Fig. 3c is a high-resolution XPS spectra of Co 2p. Similarly, the peaks of binding energy at 780.7 eV and 796.2 eV correspond to Co2+, binding energy at 778.1 eV and 793.0 eV belonged to Co3+, binding energy at 782.8 eV and 800.1 eV are satellite peaks. The weak satellite peak indicates that most of the Co elements in NC24 sample exist in Co2+ state.39 Fig. 3d is a high-resolution XPS spectra of S 2p, showing obvious main peaks and satellite peaks. The peaks of binding energy at 160.9 eV and 161.9 eV correspond to S 2p3/2 and S 2p1/2, respectively. The former is attributed to S2− related to sulfur vacancy, and the latter to metal–sulfur bond (Ni–S and Co–S bond). The above results show that there are Ni2+, Ni3+, Co2+, Co3+ and S2− in the NC24 sample, which is consistent with the reports in the40 literature.
4. It is also confirmed that the NC24 sample is composed of NiCo2S4, which is consistent with the results of XRD analysis in this paper. In addition, the O element may come from the slight oxidation on NC24 sample surface. Fig. 3b shows a high-resolution XPS spectra of Ni 2p. It can be seen from Fig. 3b that Ni 2p1/2 and Ni 2p3/2 are fitted by two peaks respectively, and the peaks of binding energy at 852.5 eV and 872.9 eV correspond to Ni2+, binding energy at 855.3 eV and 874.9 eV belonged to Ni3+, binding energy at 860.8 eV and 879.7 eV are satellite peaks, marked as “Sat.” Fig. 3c is a high-resolution XPS spectra of Co 2p. Similarly, the peaks of binding energy at 780.7 eV and 796.2 eV correspond to Co2+, binding energy at 778.1 eV and 793.0 eV belonged to Co3+, binding energy at 782.8 eV and 800.1 eV are satellite peaks. The weak satellite peak indicates that most of the Co elements in NC24 sample exist in Co2+ state.39 Fig. 3d is a high-resolution XPS spectra of S 2p, showing obvious main peaks and satellite peaks. The peaks of binding energy at 160.9 eV and 161.9 eV correspond to S 2p3/2 and S 2p1/2, respectively. The former is attributed to S2− related to sulfur vacancy, and the latter to metal–sulfur bond (Ni–S and Co–S bond). The above results show that there are Ni2+, Ni3+, Co2+, Co3+ and S2− in the NC24 sample, which is consistent with the reports in the40 literature.
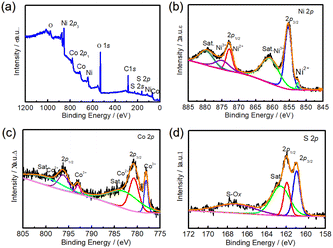 | ||
| Fig. 3 XPS spectra of the NC24 sample. (a) Full spectrum. (b) High-resolution XPS spectra of Ni 2p. (c) High-resolution XPS spectra of Co 2p. (d) High-resolution XPS spectra of S 2p. | ||
3.2. Morphological characterizations
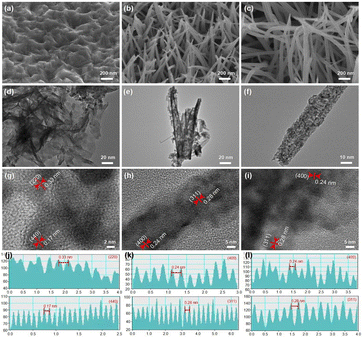
The effect of the Ni/Co ratios on the morphology of metal sulfides was observed by SEM and TEM, the typical morphology and structure of NC60, NC24 and NC06 are shown in Fig. 4 and the morphologies of other metal sulfides were observed by SEM as shown in the ESI Fig. S1,† the NC60 sample shows a two-dimensional uneven morphology. As the Ni/Co ratios decrease, the morphology of metal sulfides gradually grows from two-dimensional structure to nanotube arrays, in which NC24 samples have regular vertical morphology. When the Ni/Co ratio continues to decline, the nanotube arrays began to converge gradually for NC15 sample and the nanotube arrays grow into a nanorod arrays for NC06 sample, showing a messily intertwined morphology. This shows that the Ni/Co ratio has a significant effect on the morphology of nickle–cobalt metal sulfides.It is found in Fig. 4d that the NC60 sample is a thin sheet composed of disordered flakes. The lattice spacing of 0.33 nm and 0.17 nm in the HRTEM image are consistent with the (220) and (440) planes of Ni3S4, respectively. As shown in Fig. 4e, the interior of the NiCo2S4 nanotube of NC24 samples is hollow, which provides a fast path for ion diffusion in the electrolyte. It is measured that the diameter of NiCo2S4 nanotubes is about 30 nm and the wall of the nanotubes is partially surrounded by thin nano flakes, which greatly increases the electroactive site. The lattice spacing of 0.28 nm and 0.24 nm belongs to the (311) and (400) planes of NiCo2S4, respectively. As shown in Fig. 4f, the NC06 sample shows a nanorod morphology and the lattice spacing of 0.28 nm and 0.24 nm corresponds to the (311) and (400) planes of Co3S4, respectively, which is consistent with the results of XRD analysis in Fig. 2.
3.3. Electrochemical performances
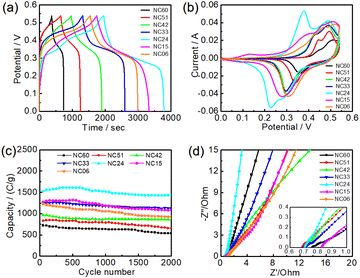
The electrochemical performances of metal sulfides with different Ni/Co ratios are shown in Fig. 5. According to formula (1), the specific capacities of NC60, NC51, NC42, NC33, NC24, NC15 and NC06 samples are calculated to be 721, 828, 964, 1244, 1527, 1270 and 1230C g−1, respectively, from Fig. 5a. This shows that specific capacity of the NC06 sample is higher than that of NC60 samples. Meanwhile, with the decrease of Ni/Co ratio, the specific capacity of metal sulfides increased at first and then decreased, in which the specific capacity of NC24 sample composed mainly of NiCo2S4 reached the maximum value of 1527C g−1.Fig. 5b shows the cyclic voltammetry (CV) curves of metal sulfides. There are obvious redox peaks in these cyclic voltammetry curves, which indicates that all metal sulfides undergo redox reactions during the charge and discharge process. This is due to the change of the valence of Ni2+/Ni3+, Co2+/Co3+ and Co3+/Co4+ in the metal sulfides electrode materials, which belongs to pseudo-capacitance characteristics.41
Cycle performance is an important parameter to evaluate the electrochemical performance of supercapacitor. Fig. 5c shows the cycle performance curve of metal sulfides at the current density of 1 A g−1. After 2000 cycles, the samples of NC60, NC51, NC42, NC33, NC24, NC15 and NC06 maintained 74.12%, 79.17%, 89.08%, 90.60%, 93.81%, 85.30% and 75.30% of the first specific capacity, respectively. This shows that the structural stability and cycle performance of NC24 samples mainly composed of NiCo2S4 are the best. In particular, the specific capacity of the NC24 sample increases gradually before 500 cycles, and begins to decline gradually after 500 cycles. This change phenomenon also exists in Ni–Co–P material for supercapacitors,42 which may be due to the fact that the infiltration and diffusion of electrolyte is a time-consuming process before all the electrochemical activities of electrode materials can be excited.
The measured electrochemical impedance spectra are shown in Fig. 5d, in which a slanted line at low frequency and a semicircle at high frequency region. It can be seen from Fig. 5d that the equivalent series resistance Rs intercept of curve on the x-axis43 of the NC51, NC42, NC33 and NC24 samples are 0.68–0.71 Ω, lower than that of other samples. Furthermore, the NC24 sample shows the maximum linear slope at low frequency, which showing a good diffusion behavior of ion in the electrode material. This may be related to the fact that the NiCo2S4 material with hollow nano-array is beneficial to the diffusion of the electrolyte.
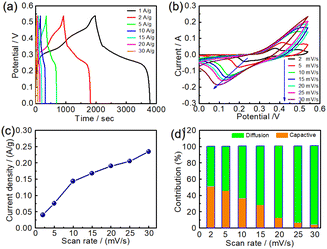
Because of the excellent electrochemical performance of NC24 samples composed of NiCo2S4, the constant current charge–discharge tests of NC24 samples were carried out at a current density of 1, 2, 5, 10, 15, 20 and 30 A g−1, respectively. The relationship between the current density and specific capacity is shown in Fig. 6a. As can be seen from Fig. 6a, the specific capacity values of NC24 samples at different current densities are 1527, 1485, 1428, 1327, 1244, 1154 and 982C g−1, respectively, which indicates that NC24 samples have good rate performance, and the specific capacity at the current density of 30 A g−1 maintains 64.36% of the specific capacity at the current density of 1 A g−1.
In order to further investigate the charge storage mechanism of NC24 sample, the cyclic voltammetry curves of NC24 sample were measured in the range of 2–30 mV s−1 and the results are shown in Fig. 6b. At a lower scanning rate of 2 mV s−1, a pair of significant redox peaks are observed near 0.37 and 0.22 V, which indicates that the specific capacity is mainly attributed to the following typical reaction process:44,45
| NiCo2S4 + OH− + H2O ↔ NiS(4−4x)OH + 2CoS2xOH + e− | (6) |
| CoS2xOH + OH− ↔ CoS2xO + H2O + e− | (7) |
| CoS2x + OH− ↔ CoS2xOH + e− | (8) |
| NiS(4−4x)+ OH− ↔ NiS(4−4x)OH + e− | (9) |
According to the electrochemical reaction mechanism of electrode materials,46–48 the rapid reversible electrochemical reactions occur in the redox pairs of Ni2+/Ni3+, Co2+/Co3+ and Co3+/Co4+ during the charging process of NiCo2S4. So nickel and cobalt ions in NiCo2S4 can be turned into an oxidation state of +4. Considering the total charges passed is 4 mol per 1 mol NiCo2S4, the calculated theoretical pesudocapacitance of NiCo2S4 is 1267C g−1, according to the theoretical capacitance calculation formula (3). It should be mentioned that the capacity of the N24 sample has exceeded the theoretical capacity of NiCo2S4, which may be caused by the synergistic effect of pseudo-capacitance and electric double-layer capacitance formed by hollow structure.49,50
The relationship between the different scanning rates (ν) and the current density (i) at reduction peak is obtained from Fig. 6b, as shown in Fig. 6c. And the charge–discharge mechanism can be qualitatively evaluated by the formula:
| i = aVb | (10) |
Fig. 6d further shows the contribution rates of diffusion and surface capacitance in the redox process of NiCo2S4 at different scanning speeds. With the increase of scanning rate, the ion diffusion contribution of redox reaction of NiCo2S4 electrode increases, which shows that metal sulfides have some electrochemical properties of the battery.53
There are two important indexes to evaluate the application prospect of supercapacitors, namely, energy density and power density. Under the current density of 10 A g−1 and the window voltage of 1.5 V, the specific capacitance of the symmetrical supercapacitor assembled by NiCo2S4 electrode is 176.7 F g−1. Therefore, according to formula (3), the symmetrical supercapacitor device shows a high energy density of 67.5 W h kg−1 at a power density of 795.3 W kg−1. These values are higher than those reported in the existing literature about NiCo2S4 metal sulfides and their composites for supercapacitors, as shown in Table 1.
| Materials | Morphology | Specific capacitance | Cycling performance | Energy density | Ref. |
|---|---|---|---|---|---|
| NiCoxSy | Flaky | 1196 F g−1 at 1 A g−1 | 97.5% after 4000 cycles | — | 18 |
| NiCo2S4 | Urchin-like hollow | 1398 F g−1 at 1 A g−1 | 74.4% after 5000 cycles | 39.3 W h kg−1 at 749.6 W kg−1 | 20 |
| NiCo2S4 | Flower-like | 2199 F g−1 at 1 A g−1 | 80.0% after 3000 cycles | 38.2 W h kg−1 at 400.0 W kg−1 | 21 |
| Ni–Co–S4 | Pine cone | 2215 F g−1 at 1 A g−1 | 90.2% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
36.6 W h kg−1 at 850.0 W kg−1 | 22 |
| NiCo2S4 | Nanorod array | 3093 F g−1 at 5 A g−1 | 41.7% after 2000 cycles | 39.3 W h kg−1 at 800.0 W kg−1 | 23 |
| NiCo2S4/DG | Nanosheet | 1137 F g−1 at 2 A g−1 | 7.1% after 5000 cycles | 25.6 W h kg−1 at 8000.0 W kg−1 | 54 |
| NiCo2S4/3DGF | Nanowire array | 1455 F g−1 at 1.3 A g−1 | 96.0% after 3000 cycles | — | 55 |
| NiCo2S4@NiS/CoS | Nanoflake | 2551 F g−1 at 2 A g−1 | 86.4% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
58.4 W h kg−1 at 8002.4 W kg−1 | 56 |
| NiCo2S4/PANI/CNTs | Hierarchy | 1290 mF cm−2 at 2 mA cm−2 | 80.1% after 5000 cycles | 83.3 μW h cm−2 at 420 μW cm−2 | 57 |
| NiCo2S4 | Hollow nanotube array | 1527C g−1 at 1 A g−1 | 93.8% after 2000 cycles | 67.5 W h kg−1 at 795.3 W kg−1 | This work |
Above all, a series of nickle–cobalt metal sulfides were synthesized by a two-step hydrothermal method and the Ni/Co ratios of nickel–cobalt metal sulfides has an important influence on the morphology, composition and structure, as well as their electrochemical properties. The NC60 and NC06 samples is composed of single phase Ni3S4 and Co3S4, respectively. The NC24 sample is composed of NiCo2S4, and other samples are two-phase mixtures. Among them, the NC24 sample composed of NiCo2S4 has excellent electrochemical performance. This is due to the fact that the metal sulfide NiCo2S4 with hollow nanotube arrays has rapid ion diffusion properties, which is confirmed by AC impedance spectroscopy. The above results demonstrate that the assembled symmetrical devices have a promising applications for high energy density supercapacitor.
4. Conclusions
In brief, a series of nickle–cobalt metal sulfides with different Ni/Co ratios were synthesized by a two-step hydrothermal method. The experimental results show that nickle–cobalt metal sulfides are mainly composed of NiCo2S4, which can be confirmed by the conclusion that NiCo2S4 phase is the most stable in thermodynamics calculated by the first principle. Furthermore, the synergistic effect between Co and Ni in NiCo2S4 phase leads to the change of electronic structure and makes the Co–S bond stronger. As the Ni/Co ratios decrease, the morphology of metal sulfides gradually grows from two-dimensional structure to nanotube arrays, and finally to nanorod arrays. Among them, the NC24 sample with the Ni/Co ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 is NiCo2S4 hollow nanotube array, showing excellent electrochemical performance. The specific capacity of the NiCo2S4 nanotube array is as high as 1527C g−1 at 1 A g−1 and the specific capacity maintained 93.81% of the initial capacity after 2000 cycles at 10 A g−1. The symmetrical supercapacitor assembled by the NiCo2S4 nanotube array show a high energy density of 67.5 W h kg−1. Thus, the reported strategies may attract widespread interest in the research electrode materials for high energy density supercapacitor.
2 is NiCo2S4 hollow nanotube array, showing excellent electrochemical performance. The specific capacity of the NiCo2S4 nanotube array is as high as 1527C g−1 at 1 A g−1 and the specific capacity maintained 93.81% of the initial capacity after 2000 cycles at 10 A g−1. The symmetrical supercapacitor assembled by the NiCo2S4 nanotube array show a high energy density of 67.5 W h kg−1. Thus, the reported strategies may attract widespread interest in the research electrode materials for high energy density supercapacitor.
Author contributions
Conceptualization, Shen D.; methodology, Yang S. B.; and Dong W., investigation, Li M. Y.; data curation, Liu Y. H.; writing—original draft preparation, Fu X. F.; writing—review and editing, Yu H. R.; project administration, All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial supports from the National Natural Science Foundation of China (Grant No. 51874167, 21808095, 51774175), China Postdoctoral Science Foundation Funded Project (2018M641707), Discipline Innovation Team of Liaoning Technical University (LNTU20TD-09) are greatly acknowledged.References
- X. Chu, F. Meng, T. Deng, Y. Lu, O. Bondarchuk, M. Sui, M. Feng, H. Li and W. Zhang, Nanoscale, 2020, 12, 5669–5677 RSC.
- H. Huang, Y. Tang, L. Xu, S. Tang and Y. Du, ACS Appl. Mater. Interfaces, 2014, 6, 10248–10257 CrossRef CAS PubMed.
- S. Wang, Z. Xiao, S. Zhai, G. Wang, W. Niu, L. Qin and Q. An, Electrochim. Acta, 2020, 343, 136106 CrossRef CAS.
- J. Wang, X. Zhang, Z. Li, Y. Ma and L. Ma, J. Power Sources, 2020, 451, 227794 CrossRef CAS.
- Y. Tian, H. Huang, G. Liu, R. Bi and L. Zhang, Chem. Commun., 2019, 55, 3243–3246 RSC.
- S. Wang, Z. Xiao, S. Zhai, G. Wang, Q. An and D. Yang, Electrochim. Acta, 2019, 309, 197–208 CrossRef CAS.
- X. Zhang, A. Wu, X. Wang, C. Tian, R. An and H. Fu, J. Mater. Chem. A, 2018, 6, 17905–17914 RSC.
- M. Gao, W. K. Wang, X. Zhang, J. Jiang and H. Q. Yu, J. Phys. Chem. C, 2018, 122, 25174–25182 CrossRef CAS.
- R. Zhao, D. Cui, J. Dai, J. Xiang and F. Wu, Sustainable Mater. Technol., 2020, 24, 00151 Search PubMed.
- W. Zhao, Y. Zhu, L. Zhang, Y. Xie and X. Ye, J. Alloys Compd., 2019, 787, 1–8 CrossRef CAS.
- W. He, C. Wang, H. Li, X. Deng, X. Xu and T. Zhai, Adv. Energy Mater., 2017, 7, 1700983 CrossRef.
- Z. Peng, L. Gong, J. Huang, Y. Wang, L. Tan and Y. Chen, Carbon, 2019, 153, 531–538 CrossRef CAS.
- W. Yang, R. Zhao, J. Xiang, S. Loy, Y. Di, J. Li, M. Li, D. Ma and F. Wu, J. Colloid Interface Sci., 2022, 626, 866–878 CrossRef CAS PubMed.
- D. Zha, Y. Fu, L. Zhang, J. Zhu and X. Wang, J. Power Sources, 2018, 378, 31–39 CrossRef CAS.
- X. Yu, M. Wang, A. Gagnoud, Y. Fautrelle, Z. Ren and X. Li, Mater. Des, 2018, 145, 135–143 CrossRef CAS.
- Y. Y. Chen, P. Dhaiveegan, M. Michalska and J. Y. Lin, Electrochim. Acta, 2018, 274, 208–216 CrossRef CAS.
- C. Liu and X. Wu, Mater. Res. Bull., 2018, 103, 55–62 CrossRef CAS.
- F. Zhao, W. Huang and D. Zhou, J. Alloys Compd., 2018, 755, 15–23 CrossRef CAS.
- S. K. Shinde, M. B. Jalak, G. S. Ghodake, N. C. Maile, V. S. Kumbhar, D. S. Lee, V. J. Fulari and D. Y. Kim, Appl. Surf. Sci., 2019, 466, 822–829 CrossRef CAS.
- Z. Shi, X. Shen, Z. Zhang, X. Wang, N. Gao, Z. Xu, X. Chen and X. Liu, J. Colloid Interface Sci., 2021, 604, 292–300 CrossRef CAS PubMed.
- J. Tang, W. Huang, X. Lv and Q. Shi, Nanotechnology, 2020, 32, 085604 CrossRef PubMed.
- J. Jiang, Y. Sun, Y. Chen, X. Hu, L. Zhu, H. Chen and S. Han, Mater. Sci., 2019, 54, 11936–11950 CAS.
- S. Wang, P. Zhang and C. Liu, Colloids Surf., A, 2021, 616, 126334 CrossRef CAS.
- J. Xiao, H. Tong, F. Jin, D. Gong, X. Chen, Y. Wu, Y. Zhou, L. Shen and X. Zhang, J. Power Sources, 2022, 518, 230763 CrossRef CAS.
- H. Chen, X. Ma and P. K. Shen, Chem. Eng. J., 2019, 364, 167–176 CrossRef CAS.
- R. Peymanfar and S. Ghorbanian-Gezaforodi, Nanotechnology, 2020, 31, 495202 CrossRef CAS PubMed.
- X. Xiong, G. Waller, D. Ding, D. Chen, B. Rainwater, B. Zhao, Z. Wang and M. Liu, Nano Energy, 2015, 16, 71–80 CrossRef CAS.
- M. M. Baig and I. H. Gul, J. Energy Storage, 2021, 37, 102477 CrossRef.
- Y. Wang, J. Zhong, F. Ding, Q. Zhao, Z. Zhang, X. Liu, Y. Liu, H. Rao, P. Zou and X. Wang, New J. Chem., 2018, 42, 9398–9409 RSC.
- K. Xu, W. Li, Q. Liu, B. Li, X. Liu, L. An, Z. Chen, R. Zou and J. Hu, J. Mater. Chem. A, 2014, 2, 4795–4802 RSC.
- S. J. Clark, M. D. Segall, C. J. Pickard, P. J. Hasnip, M. J. Probert, K. Refson and M. Payne, Z. Kristallogr. - Cryst. Mater., 2005, 220, 567–570 CrossRef CAS.
- D. Vanderbilt, Phys. Rev. B: Condens. Matter Mater. Phys., 1990, 41, 7892–7895 CrossRef PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS PubMed.
- Y. Pan, Y. Wang, M. Ye, R. Qu, H. Zhong, Z. Song, X. Peng, D. Yu, J. Yang, J. Shi and J. Lu, Chem. Mater., 2016, 28, 2100–2109 CrossRef CAS.
- X. Shi, S. L. Bernasek and A. Selloni, J. Phys. Chem. C, 2016, 120, 14892–14898 CrossRef CAS.
- Y. Gao and K. Huang, Chem.–Asian J., 2017, 12, 1969–1984 CrossRef CAS PubMed.
- M. Dakshana and S. Meyvel, Vacuum, 2021, 192, 110499 CrossRef CAS.
- C. Xia, P. Li, A. N. Gandi, U. Schwingenschlögl and H. N. Alshareef, Chem. Mater., 2015, 27, 6482–6485 CrossRef CAS.
- H. Wan, J. Jiang, J. Yu, K. Xu, L. Miao, L. Zhang, H. Chen and Y. Ruan, CrystEngComm, 2013, 15, 7649–7651 RSC.
- Y. Liu, J. Zhang, S. Wang, K. Wang, Z. Chen and Q. Xu, New J. Chem., 2014, 38, 4045–4048 RSC.
- Y. Ruan, J. Jiang, H. Wan, X. Ji, L. Miao, L. Peng, B. Zhang, L. Lv and J. Liu, J. Power Sources, 2016, 301, 122–130 CrossRef CAS.
- X. Zhang, A. Wu, X. Wang, C. Tian, R. An and H. Fu, J. Mater. Chem. A, 2018, 6, 17905–17914 RSC.
- L. Mei, T. Yang, C. Xu, M. Zhang, L. Chen, Q. Li and T. Wang, Nano Energy, 2014, 3, 36–45 CrossRef CAS.
- T. Liu, Y. Zheng, W. Zhao, L. Cui and J. Liu, J. Colloid Interface Sci., 2019, 556, 743–752 CrossRef CAS PubMed.
- J. Zhao, Y. Wang, Y. Qian, H. Jin, X. Tang, Z. Huang, L. Lou, Q. Zhang, Y. Lei and S. Wang, Adv. Funct. Mater., 2023, 33, 2210238 CrossRef CAS.
- J. Yan, L. Mao, H. Duan, D. Zhu, Y. Lv, L. Li, L. Gan and M. Liu, Chin. Chem. Lett., 2022, 33, 2681–2686 CrossRef CAS.
- X. Zheng, L. Mao, Z. Song, W. Du, D. Zhu, Y. Lv, L. Li, L. Gan and M. Liu, J. Mater. Chem. A, 2022, 10, 611–621 RSC.
- Z. Song, L. Mao, l. Ruhimann, Y. Lv, D. Zhu, L. Li, L. Gan and M. Liu, Adv. Funct. Mater., 2022, 32, 2208049 CrossRef CAS.
- X. Yu, M. Wang, A. Gagnoud, Y. Fautrelle, Z. Ren and X. Li, Mater. Des., 2018, 145, 135–143 CrossRef CAS.
- G. Xiang, Y. Meng, G. Qu, J. Yin, B. Teng, Q. Wei and X. Xu, Sci. Bull., 2020, 65, 443–451 CrossRef CAS PubMed.
- F. Lu, M. Zhou, W. Li, Q. Weng, C. Li, Y. Xue, X. Jiang, X. Zeng, Y. Bando and D. Golberg, Nano Energy, 2016, 26, 313–323 CrossRef CAS.
- M. K. Sahoo and G. Rao, Electrochim. Acta, 2018, 268, 139–149 CrossRef CAS.
- H. Xuan, Y. Guan, X. Han, X. Liang, Z. Xie, P. Han and Y. Wu, Electrochim. Acta, 2020, 335, 135691 CrossRef CAS.
- C. Jing, X. Guo, L. Xia, Y. Chen, X. Wang, X. Liu, B. Dong, F. Dong, S. Li and Y. Zhang, Chem. Eng. J., 2019, 379, 122305 CrossRef.
- Z. Kang, Y. Li, Y. Yu, Q. Liao, Z. Zhang, H. Guo, S. Zhang, J. Wu, H. Si, X. Zhang and Y. Zhang, J. Mater. Sci., 2018, 53, 10292–10301 CrossRef CAS.
- Y. Chen, L. Wang, H. Gan, Y. Jiang, J. Feng, J. Liu and X. Shi, J. Energy Storage, 2022, 47, 103626 Search PubMed.
- X. Cheng, D. Wang, H. Ke, Y. Li, Y. Cai and Q. Wei, Comp. Commun., 2022, 30, 101073 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ra07624a |
| This journal is © The Royal Society of Chemistry 2023 |