Open Access Article
Open Access ArticleUnderstanding friction mechanisms of Si-DLC/steel interfaces under aqueous lubrication
J. L. Lanigan * and
R. Lewis
* and
R. Lewis
The University of Sheffield, Western Bank, Sheffield, S10 2TN, UK. E-mail: j.lanigan@sheffield.ac.uk
First published on 3rd April 2023
Abstract
A key driver in current research on lubricant formulation is the need to move away from older technology that is highly reliant on resources derived from industries associated with high carbon dioxide emissions. In this paper, the adoption of water based (or aqueous) lubrication is explored. This is in direct contrast with most lubricated systems that rely on oil or other petroleum products. In nature, most known biological systems employ aqueous lubrication for tribological contacts, such as those found in cartilage and more widely in mammalian joints including hips and knees giving friction coefficients as low as μ < 0.002. This is achieved very effectively without the presence of an oil or grease as a base lubricant. In most engineering applications, however, oils and greases are used to achieve desired low friction levels. While effective, this comes with the associated higher costs and carbon footprint of using petroleum derived products. In recent years, certain engineering applications have shifted to aqueous lubrication, a notable example of which is stern tube bearings in maritime applications. These are typically low pressure contacts though. Depending on speed of travel this can range from 100–400 MPa. The research detailed in this paper explores the viability of high pressure contacts lubricated with novel aqueous packages and what effects this shift may have on friction and wear profiles of the system. The work reported herein demonstrates that with some modifications, effective lubrication can be achieved using aqueous lubricant packages.
1. Introduction
Prior to recent years, the development of some effective (oil based) lubricant packages were, it can be argued, a serendipitous development. A typical lubricant package for automotive lubrication (such as the piston ring and liner) would be composed of a base oil, typically a mineral oil. This is used as the base fluid into which additional additives are added. Some key additives for this application include anti-wear additives like zinc di-alkyl diphosphate (ZDDP), friction modifiers including molybdenum disulphide (MoS2), viscosity modifiers, anti-oxidants and corrosion inhibitors.1 These tailored packages are designed to maintain effective running of the system by preventing catastrophic wear of components while maintaining desired friction levels, in addition to preventing particulate build up within the engine. These all combine to maximise the lifetime of the system. Developed over 80 years ago, cost effective, oil-soluble additives seemed to be the cornerstone of successful lubrication. These additives, developed by equal parts chance and luck, suffer in that the long term consequence of using these chemicals was largely unexplored, instead being rapidly adopted on a large scale. In the past few decades the detrimental impact of certain additives has been observed on a large scale.This ranges from the sourcing and processing of the required raw materials, like zinc. Zinc has a climate change impact of 2600 kg CO2-eq./t as well as the potential to cause acidification (from mining) and eutrophication (from release into the environment).2 In addition to this, reliance on petroleum derived products like the base oil is clearly associated with high carbon emissions. Finally, the breakdown products within the engine from some additives release phosphorus and zinc. These species are able to bind to, or physically block, access to the ‘noble metals’ (often composed of platinum) used in catalytic converters in the exhaust.3 This renders them ineffective, thus decreasing their efficiency and in turn causing higher release rates of noxious gasses including carbon monoxide, and nitrous oxides.3
Automotive oil additives used in engines are currently used to reduce wear, control friction and prevent many other aspects of oil ageing (including acid ageing).
There is a clear need to move towards novel environmentally friendly solutions – this has become ever more apparent in recent years as effects of emission became apparent. One way in which this could be resolved is to move away from traditional lubricants (typically mineral oil, or other high intensity derived bio-oils) and towards tribosystems inspired by nature.
The key drivers for this research are two-fold; one aspect being driven by legislation designed to reduce emissions from automotive and maritime vehicles and more importantly to reduce the global reliance on fossil fuels.
A paradigm change is possible within the area of lubrication that, although in its infancy, shows much potential. Aqueous based lubricated contacts are a recent development, tailored to certain industries, where they have shown great promise. The possibility of moving away from petro-chemical derived lubricants, or the main current alternative being presented, bio-lubricants, which typically rely on fast growing crops to generated plant hydrocarbons.
There are some drawbacks associated with the use of water as a lubricant base fluid. This includes how chemically reactive water can be if not properly formulated. This could lead to aggravated wear rates of corrosion prone surfaces. This is a limitation when thinking about the common tribo-contacts requiring effective lubrication. Typically, metal surfaces (such as steel) do not have good wear characteristics when in water, due to corrosion problems and reduced lubricant viscosity (when compared to fully formulated lubricant packages).
Part of a step change in solutions to this is to employ novel technology such as thin film coatings with age old lubricant solutions, namely water. These thin-film coated tribo-pairs (as opposed to the classic steel/steel system) would benefit from water lubrication, rather than suffer from higher wear in this type of environment. To be fully effective, the water would need to have additives formulated in (as is the case with motor oil, to reduce wear rates and oil ageing). This is the current tribological challenge, selecting appropriate additives to facilitate a shift away from oil lubricated systems with effective, more environmentally friendly additives.
Having considered a wide range of a family of thin film coatings fitting broadly under the category of diamond-like carbon, one is currently seen as the front runner.4 Si-DLCs, have several benefits for this application. Doping DLCs with Si has been shown to increase failure-resistant capability.5 It has also been demonstrated to give low friction values in water.6 Diamond-like carbon coatings containing silicon and often oxygen, are known to be inherently low friction in aqueous environments.5,7,8 This behaviour indicates that Si-DLC coated parts could be used in certain applications instead of steel components.
There has been a large body of research on the low friction mechanism of Si-DLCs whilst under both aqueous and dry lubrication.8,9 It is noteworthy that even in unlubricated contacts, the presence of trace atmospheric water (from laboratory air) is able to lower friction noticeably.8 There is ongoing research into the mechanistic insight into how this low friction is achieved, current theories include generation of colloidal silica or a sol–gel low friction film generated in situ.10,11
This paper explores the low frictional behaviours of Si-DLC with various formulated water-lubricated contacts, and establishes how these behaviours can be controlled, or curtailed where necessary.
2. Background
Herein, a tribo-pair was analysed to assess the viability of this approach. The system's tribology was assessed with a pin-on-disc tribometer with DLC coated discs. After testing, friction transducer data was analysed to examine the effects of additives on the tribopairs tested. Ex situ surface analysis was conducted to gain a detailed understanding of the wear processes at the interfaces and how this could be enhanced or attenuated, depending on the findings.Silicon-doped diamond-like carbon films, or ‘Si-DLC’, exhibit interesting tribochemical behaviours when compared to most other doped DLCs. The non-lubricated tribological behaviour of Si-doped DLCs is where initial interest was generated around the coating.8,12–15 This is because Si-DLCs typically exhibit far lower coefficients of friction when compared to other types of DLCs, in unlubricated contacts. This behaviour is attributed to the formation of silicon rich oxide species.8,12,13,15,16
These research finds are key as the presence of any oxide rich layer at a surface immediately makes the upper layers far more like a traditional steel/steel tribocouple, making the move from traditional steel–steel, oil lubrication contacts a far easier gap to bridge.
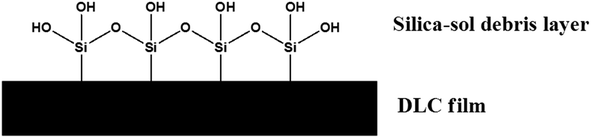
Oxides species on Si-DLC make the surface much more similar to a steel component when compared to a non-Si containing DLC. These species are rich in alcohol (or –OH) bonds which are also recognised as potentially being able to reduce friction.17,18 OH groups have the ability to form hydrogen bonds, these transient interactions are also present in water.
It therefore follows that Si-DLCs will form favourable interactions when water is employed as a lubricant, and that these interactions are much stronger than those found in less reactive, novel surface coatings.
Although no mechanism for the reduced friction in dry environments has been completely settled upon, the most likely factors are either:
• inclusion of additional alcohol groups/polar species,
• or production of a low shear strength, wear derived layer of SiOx debris.
Both of these species will inevitable form in a dry contact due to oxidation from ambient air. A representative schematic of the films upper surface is shown in Fig. 1.
To fully establish the role of the alcohol rich debris that forms from the coating, a series of experiments were planned to control the formation of such species. With effective lubricant tailoring, oxidation attenuation should be possible. Lubricant acidification, a factor effecting traditional automotive engine components enhance tribochemical wear. These can be effectively reduced with a well-tailored lubricant packages.
3. Experimental details
3.1 Test apparatus/experimental details
A unidirectional, pin-on-disc tribometer or “High Speed Pin-On-Disc (HSPOD)” tester was employed for testing. For all experiments at least two repeats were conducted, to allow for calculation of experimental deviation.The disc was rotated against a fixed-in-place ball bearing. The tribometer consists of a fixed arm that is perpendicular to the rotating disc. The tribometer is instrumented to measure friction force via a transducer rod on the load arm which translated the friction force to the load cell. This data was recorded through a LabVIEW based data acquisition system. The motor rotates the sample disc, which is screwed onto the sample tray at the desired speed and the friction produced against the ball bearing measured through the transducer rod. The friction coefficients of the rotating disc on the pin were measured and calculated by the LabVIEW based data acquisition system, available on the attached PC (Fig. 2).
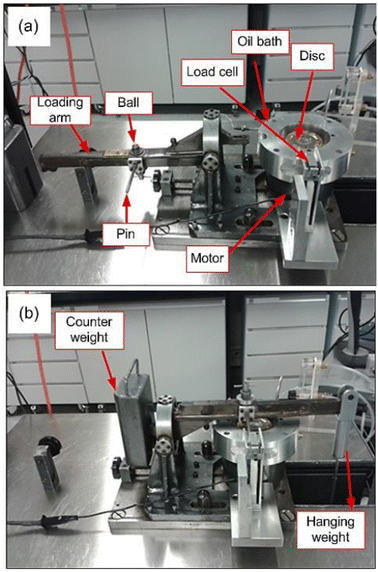 | ||
| Fig. 2 Labelled image of the pin-on-disc tribometer, as given in ref. 9. | ||
After testing, friction data was processed and specimens were taken for surface sensitive analysis.
3.2 Test specimens
The DLC used was a commercial sample produced by Sulzer Sorevi (now a part of Oerlikon) using the Plasma Enhanced (PE) Physical Vapour Deposition (PVD) technique. The coating was produced using lower temperature plasma; the substrate is negatively biased by 500 V with the chamber acting as the electrode. A hot cathode auxiliary system is also employed to enhance plasma generation. The process typically takes place at 10−3 mbar. The coating contains interlayers (Ti, Si), which are employed to improve coating adhesion to the substrate. The substrate is first cleaned by argon ion etching before any deposition commences. An initial titanium layer is deposited to enhance bonding between the coating and substrate, followed by silicon based interlayer. This graded interface shifts the bonding from metallic, at the steel, to carbide which enables better coating adhesion. After interlayer deposition the bulk DLC is deposited.The coating has been characterised by the manufacturer using Elastic Recoil Detection Analysis (ERDA), which is a technique able to detect hydrogen. As shown in Table 1, the coating has 14% Si and 7% O. This gives the unworn coating a Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O ratio of 2
O ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, this is a useful reference point to monitor wear processes from.
1, this is a useful reference point to monitor wear processes from.
| Coating/Element% | C | H | O | Si | Ar |
|---|---|---|---|---|---|
| Si-doped DLC | 55.6 | 34 | 7 | 14 | 0.4 |
3.3 Additive selection
Current lubricant oils have several common features (although specific applications like those in maritime may have additional requirements). With this in mind, key additives were listed from common oil lubricant packages, these were evaluated by their function.Following this, water-soluble ‘greener’ analogues were selected, based on prior tribology experience, guided by information in the scientific literature. Table 2 shows this process.
| Additive/role | Commonly used in oil or grease lubricants | Water soluble candidates with similar abilities |
|---|---|---|
| Anti-corrosions | Film formers (ZDDP) are able to reduce corrosion | Phosphate buffered saline (or similar pH buffer/acidity regulator) |
| Anti-wear | ZDDP (polyphosphate glass former) | Component able to generate polyphosphate film or P, S film in water |
| Anti-oxidant | MoDTC/ZDDP are dependent on oxidation (and thus control oxidation rates) for formation of their active components | Anti-oxidant species |
| EP additives (older technologies) | Sulfur containing organics, typically aromatic sulfur compounds (oil soluble), currently being phased out due to catalytic converter issues | S containing aqueous additives (with known, low toxicity profiles) |
| Friction modifiers | Various: glycerol mono oleate (ester), long chain fatty acids, MoS2, WS2, graphite, ethoxylated fatty amines | Aqueous lubricants – shorter chain fatty acids, viscosity modifiers |
| Film formers | ||
| Viscosity index modifiers | Anti-wax additives/oil soluble polymers – typically used to lower viscosity | Star polymers/aqueous polymers – in this application would be used to increase viscosity |
| Anti-foam agents | Detergents (typically “over based”) to act as reservoir of alkaline material to combat oil ageing/acidification | Detergent (opportunity for different ‘payload’ as pH is accounted for) |
In addition, the aqueous additive candidates were then checked against key factors including:
• cost,
• low/no toxicity,
• potential environmental impact upon release and impact of production,
• wider availability,
• presence of key functional groups crucial to the known tribology of iron.
Four lubricants solutions were formulated for testing. These were selected using knowledge of traditional oil based additives and translated to water soluble candidates.
A pH buffer was included to prevent lubricant acidification and reduce corrosive wear (Phosphate Buffered Saline (PBS), used predominately for biological systems, which typically operate at neutral pH ∼7) was used to assess if attack of the ferrous surface could be inhibited by acidification control.
Potential film-forming additives were also tested. Alongside PBS which is a source of phosphate (like the ubiquitous ZDDP oil additive) the molecule ‘cysteine’ was selected, these are explored in more detail later. The rationale for selecting ‘cysteine’ was that it is an amino acid found in the human body and more widely in most living organisms – the assumption was made this would be acceptable to most legislative bodies as banning this would be troublesome for a variety of industry.
These compounds were selected as they have the potential to prevent oxidative damage, or create Fe–S bonds (like common oil soluble extreme-pressure additives), respectively. It is noted that both the additives are found in nature and have excellent, low toxicity profiles.
The lubricant blends were created by dissolving PBS tablets (available from sigma Aldrich) in deionised water (DI), creating a phosphate buffered saline (PBS) solution. For reference, PBS typically is composed of the following dissolved minerals (Table 3).
| Salt | Concentration (g L−1) |
|---|---|
| NaCl | 8.0 |
| KCl | 0.2 |
| Na2HPO4 | 1.42 |
| KH2PO4 | 0.24 |
A more complex lubricant that contained additional additives is detailed below. A final lubricant blend was added to verify experimental findings and will be explored in more depth later. This final blend contained the non-ionic surfactant “Tween 80”. This species is similar to classical detergent molecules as found in oil based engine additives, commonly as over-based detergents. These species are able to form a micelle (or protective pocket) around a suspension in solution.
In traditional uses detergent species are pre-loaded with a core of (basic, or alkaline) calcium oxide, to counter the generation of combustion species that generate acid in the engine. The benefit of working with water based additives is that acidity is easier to control with a water soluble pH buffer.
In this instance, the inclusion of such a micelle former was important in order to explore the role of wear debris on friction. That is to the say, in the aqueous system the micelles are not required to have a core of alkaline chemicals to counter acidification. Instead they are ‘empty’ and ready to ‘capture’ wear debris generated from tribocontacts. Information on the lubricant formulation is given in Table 4.
| Lubricant | Additive 1 | Additive 2 | Additive 3 | Comment |
|---|---|---|---|---|
| Deionized (DI) water (benchmark) | — | — | — | Reference lubricant |
| PBS/pH controlled DI water | Phosphate buffered saline (pH control/source of phosphorous) | — | — | Maintains system pH at ∼7 |
| Formulated additive package | Phosphate buffered saline (pH control/source of phosphorous) | Melatonin anti-oxidant | Cysteine sulfur containing species (EP analogue) | pH control with additional anti-oxidant (melatonin) and a source of sulphur (replacing EP style additives) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Additional additive tested | ||||
| ‘Tween 80’ was additionally tested to explore the effects of removal of wear debris | Phosphate buffered saline (pH control/source of phosphorous) | (5 wt%) ‘Tween 80’ (micelle former, captures wear debris) | — | pH controlled system, potential to trap wear particles/debris in micelles |
3.4 Selected lubricant additives
In comparison, water can be acidic, typically having a pH of 6 (DI water). The higher pH of DI water is due to removal of mineral ions. These mineral ions are replaced with carbon dioxide, which then generates acid via formation of carbonic acid to resort the osmotic balance of dissolve solutes in the DI water.
That is to say that it does not contain elements that are being phased out of future use, including: phosphorous and some metals.
Oxidation is a key factor in the wear of both metals and DLCs. In metals, oxidative wear is curtailed by the presence of ZDDP and MoDTC, which preferentially oxidize, helping to form an anti-wear film.
With respect to DLC, carbon is known to oxidise when given a long enough time (the rate of oxidation is influenced by a variety of factors including heat and pressure). This process in a tribological setting is sped up markedly under the pressures associated intended applications.
Melatonin is an organic compound found widely in nature where is it is a potent anti-oxidant. Melatonin is able to scavenge reactive oxygen species (ROS), which is useful as oxygen is a prominent cause of tribochemical wear. Melatonin is also a powerful free-radical scavenger, able to trap radical oxygen species including OH˙, O˙2−. Unlike other commonly encountered anti-oxidants (such as vitamin C) melatonin cannot act as ‘pro-oxidant’, having a well-established reaction mechanism resulting in stable products that cause no damage. This was a key motivator for its inclusion in the aqueous package.
For a comparison, commonly used oil additives are compared below in Table 5, with Table 6 showing the novel candidates. Table 5 shows traditional additives with certain functional groups highlighted (Zn, P and Mo), these are crucial elements from additives like ZDDP and MoDTC. In addition, additives used in wider applications, like detergents are shown for completion.
In Table 6 water soluble additives are shown that should act in a similar fashion to traditional additives are shown, again with their key functional groups highlighted.
Cysteine was added to the lubricant blend as it is a water soluble source of sulphur. Sulphur is an important element in terms of its tribology. Sulphur is well known as an extreme pressure additive. EP additives are able to release sulphur under high pressure, typically at a metal tribocontact. This reactive sulphur then goes on to rapidly bond with a metal or ferrous alloy surface, thus preventing metal-on-metal contact.
The mechanism by which EP additives are believed to work is dependent on the ease of cleavage of the C–S bonds in the additive molecule and the strength of the Fe–S bond formed. This results in a protective FeS2 layer being formed.20 The benefit of using a thiol (–SH group) is that they are similar to older EP components like “alkyl polysulfides”, with the general formula R–(S)–R (where ‘R’ denotes a long carbon chain).21
Traditional EP additives have already been largely phased out of usage, due to issues with their safety profiles and compatibility with certain components. The authors would stress that the sulphur containing compound use here, cysteine, is an amino acid found in humans, essential to protein synthesis. It is unlikely to be phased out or banned by legislative bodies for this reason.
Outside of this, its biological role is not relevant to this system as there are no other biologically active/responsive species present.
Micelles are able to capture water-insoluble species as they have a hydrophobic (water-repelling) core.22 Tween 80 was selected for this reason as it was hypothesized that it would be capable of removing wear debris from solution, in a similar fashion to that of other detergent species in oil packages. It has the added benefit of also being a ‘NOCH’ additive.
3.5 Test methods
Tests were ran for 50 minutes. Test conditions are tabulated in Table 7.| Running conditions | Value | Comment |
|---|---|---|
| Load (g) | 500 | |
| Maximum hertzian pressure (GPa) | 1 | 1 GPa is reachable by marine engines23 and within automotive operating conditions for the cam and follower interface24 |
| Running speed (m s−1) | 5 | Selected running speed typical for HSPOD testing for tribological applications and novel materials25,26 |
To ensure the disc and the ball were always submerged in the lubricant, 35–40 ml of lubricant were injected via syringe into the sample tray. The tribometer has a lubricant circulation pipe, once the sample tray is rotating with the lubricant added, the flow produced in the tray helps to circulate the lubricant back into the contact.
3.6 Post-test surface analysis
4. Results
4.1 Friction profiles
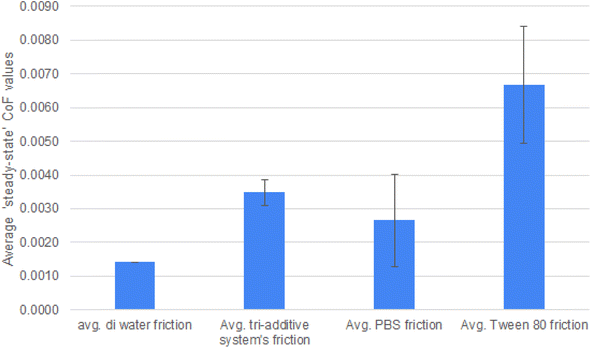
Individual friction traces of the lubricant systems are given in Fig. 3. As mentioned in the experimental section, a total of three experiments were carried out to allow for calculation of standard deviation. For clarity, averaged data with deviation is shown separately, in Fig. 5, as steady state friction values. The lowest friction value (μ = 0.02) was seen with deionised water.Testing with PBS as the pH buffer has a notable effect on friction, with it starting at a much higher level at 0.06. After the initial running period, the friction coefficient drops to 0.04. The clear changes in behaviour indicate that the additives are having effects on the tribology of the system. The pH buffer prevents the system reaching the low friction it normally would with water. It is noted that the friction trace fluctuates throughout the testing. This indicates a dynamic process is taking place at the interface, which gives useful insight into the mechanisms influencing friction.
With the triple additive lubricant package, the system appears to reach a steady state after 40 minutes at μ = 0.07. These higher friction values would usually be associated with the formation of a tribofilm.
The difference between testing in DI and testing with Tween 80 is notable. The friction of each system is 0.02 at the end of testing in DI, to above 0.06 at the end of testing with PBS. This confirms Tween 80 is not acting to reduce friction in any manner. Instead, Tween 80 raises friction, confirming that the wear debris is crucial to the low friction behaviour on the surface of Si-DLCs.
| Steady state (last 20 min) | Average DI water friction | Average tri-additive system's friction | Average PBS friction | Average Tween 80 friction | Average steel/steel under aqueous lubrication |
|---|---|---|---|---|---|
| CoF, μ | 0.0014 | 0.0035 | 0.0027 | 0.0067 | 0.0887 |
| ± | 0.0004 | 0.0014 | 0.0017 | 0.0052 | 0.0076 |
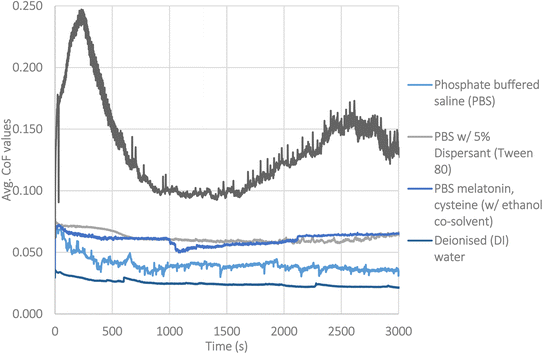 | ||
| Fig. 3 Friction traces of the different lubricants, one trace from each is shown, for clarity. Fig. 5 shows the averaged data from repeats. | ||
4.2 Surface analysis
SiOx species are detected by XPS. This species is likely only present in large quantities at the upper-surface and is evidence of ambient oxidation of the coating, even prior to testing. This conclusion can be drawn as ERDA data from the coating is provided by the manufacturer, and gives the Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O ratio as 2
O ratio as 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Whereas, XPS (which only examines the uppermost surface) gives an Si
1. Whereas, XPS (which only examines the uppermost surface) gives an Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O ratio of 1
O ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. These types of species are often cited as precursor materials for sol–gel creation (Table 9).
1. These types of species are often cited as precursor materials for sol–gel creation (Table 9).
| Attribution | Position (eV) | % Concentration |
|---|---|---|
| C–Si–O | 100.3 | 36.3 |
| SiOx | 101.2 | 51.4 |
| Si–C | 99.6 | 12.3 |
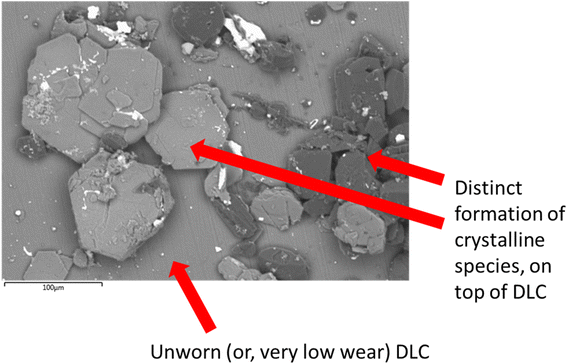 | ||
| Fig. 7 SEM image of crystalline materials on the DLCs surface. Derived from the PBS, cysteine and melatonin system, potentially from counter body wearing or film formation. | ||
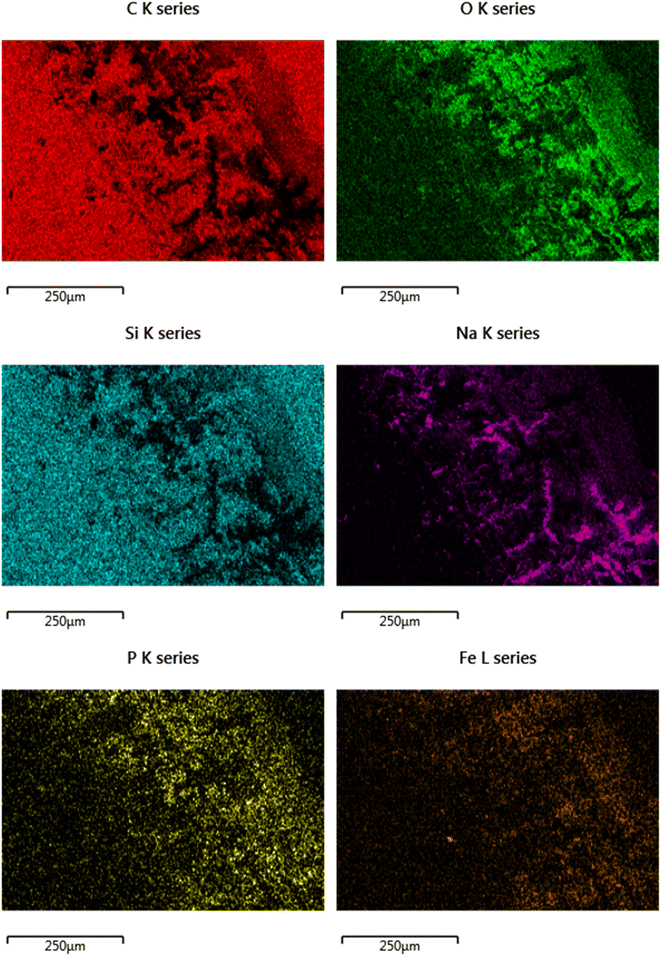
There is also a build-up of elements from the phosphate buffered saline, specifically Na and P. Also there is Fe noted in the contact area, which could either be from wearing of the substrate, or the metallic counter body (pin).
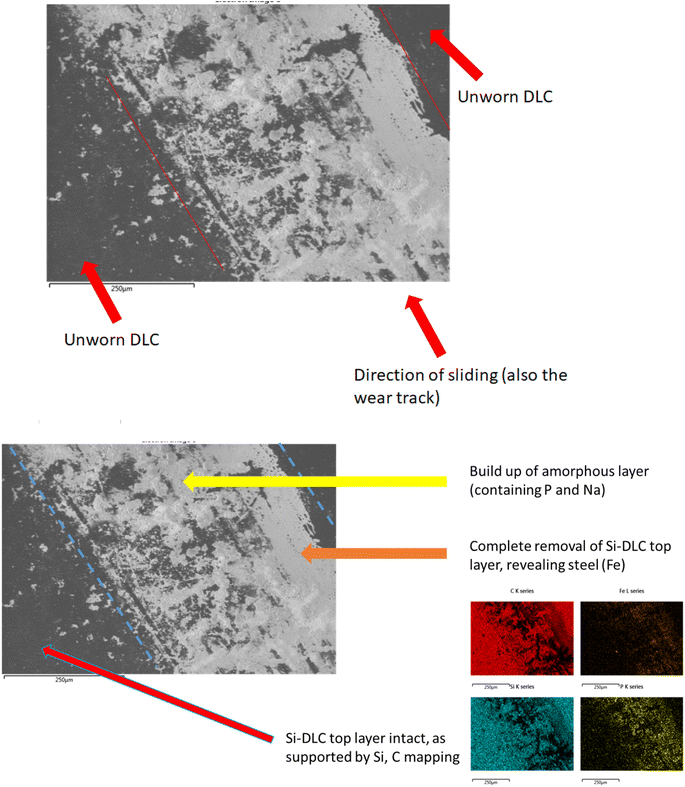
The formation of a crystalline, patchy tribofilm is observed in the SEM images taken from the novel lubricant system, this is shown in Fig. 8 and 9 below. The ‘patchy’ nature of the surface observed is noted.
The worn area is not well defined (likely due to a low wear rate) on this sample and as such is not labelled. The highly polished surface is visible (Fig. 8) with a crystalline structure appearing to grow on top of this. Compared with the DI tested sample, optically assessed (via SEM images) wear processes were far less noticeable with the novel lubricant package (Fig. 6).
The contact point was, however, easily identifiable from the surface growth of crystals on the DLC, located where the steel pin was in contact with the plate.
SEM/EDX chemical maps (Fig. 8 and 9) show that Si is still detected in the image (Si is a component of Si-DLC and used as a proxy for wear processes). Where the large crystalline structures (labelled with a red arrow) are present – Si is not detected in the chemical map. This is because the crystalline deposits (marked by the arrow) are blocking the electron beam's penetration to the coating beneath. This is directly opposed to wear related removal.
Rather, it is indicative of the build-up of a tribo-film. This is confirmed by areas of the image of the DLC remaining smooth – even after wear testing. If high wearing had occurred, it would be observed visually (as in Fig. 6). Si is noted in the chemical maps from both the samples, but in different ways.
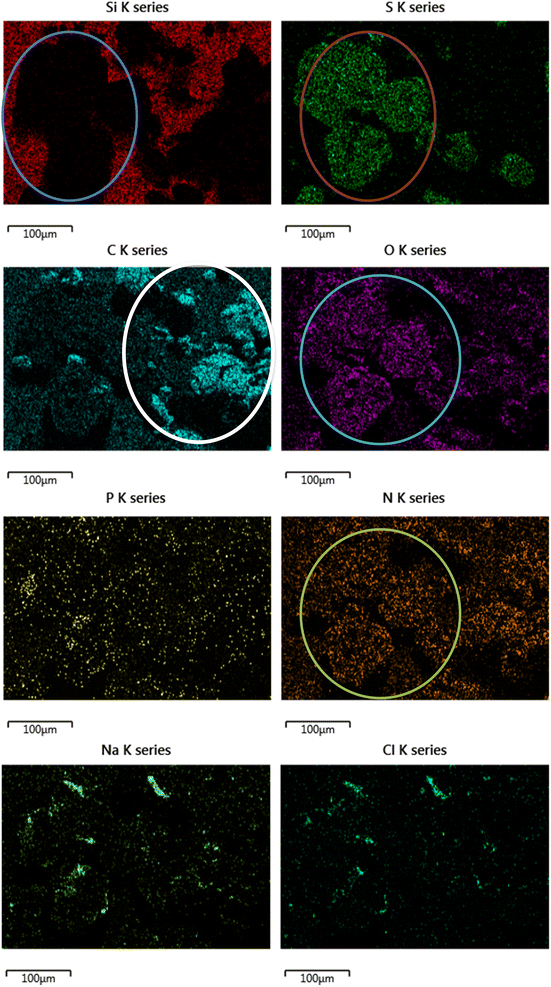
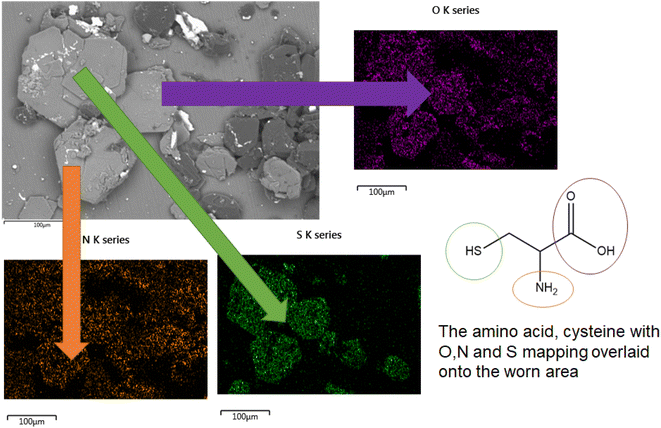
The direct overlap of low levels of Si and high levels of S matches perfectly in Fig. 9, confirming growth on top of the DLC. There is a marked build-up of S and O (again in Fig. 9) the only source of sulphur in the lubricant package is from the amino acid cysteine, confirming it has deposited on the surface.
This would firmly indicate cysteine has formed a film on top of the diamond-like carbon coating. This is confirmed as the (greyscale image, Fig. 8) electron image shows a predominately undamaged surface.
These images can be overlaid on the standard SEM and compared with the structure of key additives, as shown below in Fig. 9. The Si signal in Fig. 9 appears occluded from detection by the growth of large crystals on the top surface. The C signal is difficult to accurately attribute here, as carbon is present in the coating as well as many of the additives, as such it is included, but not labelled on the worn area image analysed.
5. Discussion
5.1 Friction assessment and wear process insight
The friction behaviour of silicon doped coatings has been explored previously in the literature. Other researchers have established there is a marked difference between the coating's behaviour under oil lubrication when compared with water lubricated, or dry sliding.33 When sliding in oil, the coating behaves much more like a ‘standard’ DLC, as it cannot react with water/form low friction hydrogen bonds as easily.Many authors note the presence of silicon rich oxide debris, SiOx particles or silica-sol type particles within the wear scar of the tested coating.13,15,34,35 Typically for silica-sol–gels to form, chemical literature on the subject states that an acid or base is required to catalyse the process. The catalyst also influences the size and shape of the sol–gels formed.36,37
The lowest friction profile is obtained when the Si-DLC is tested in DI water. When examining the friction profile generated from the DI system, it is important to note that the lubricant is slightly acidic. This acidity must also increase with wear processes, as various ions are released into the system, including the Lewis acids Fe2+ and Fe3+.
From the images of the tests conducted in deionised water it seems likely that the DLC plate would undergo several wear processes including cavitation and that his likely contributes to the total wear. Articles in the literature indicate that in this system the wear may be accelerate by cavitation once the DLC has been completely delaminated.38 This is seen in the contact area.
For the tri-additive (or formulated) aqueous lubricant the wear rate appears to be very low as assessed by the SEM image. Here it appears that the lubricant additives are able to negate a large amount of wear. This can be attributed to a combination of pH control by the buffering agent potentially aided by the formation of thin films on the surface of the DLC.39
5.2 Influence of pH
For all the other lubricants, PBS is added to control the pH of the system. Acidity is maintained at a pH value of approximately 7.4. It is therefore clear that pH is highly important with regards to the low friction behaviour of Si-DLC in aqueous environments. Typically, it seems to be the case that an acidic pH will create a silica-sol–gel layer, which then facilitate low friction.When the buffer PBS is tested alone, the friction trace increases (compared to de-ionised water) and the signal continually fluctuates. The fluctuation of the friction trace is clearly related to the PBS buffer resisting pH changes from acid released by wear processes from the interface, which is a dynamic process. The other friction traces do not show this on-going dynamic fluctuation as the additives present prevent this type of behaviour, this will be discussed below.
The only steel–steel tribopair, tested as a benchmark, gives the highest friction at all times. The friction trace shows far more variability with time – this was as expected as the conditions at the contact when lubricated with water would facilitate accelerated wear processes through the formation of iron oxides.
The majority of the work focuses on the DLC plate with a steel pin as a counter body. The relative size of the components mean that the DLC plate has a greater surface area exposed to the lubricant. Therefore, wear of the steel pin which will likely also generate iron oxide wear particles will be reduced, but not eliminated.
Within this system the pH was controlled for all lubricant packages except for the de-ionised water. The testing of phosphate-buffered saline and its subsequent inclusion in all the other additive packages should maintain a neutral pH of 7.4, due to the nature of buffer solutions.
The de-ionised water, when lubricating the DLC-steel contact gives the lowest and steadiest friction values throughout the test duration of μ = 0.0014. Due to the slightly acidic pH of de-ionised water it is likely that this facilitates the formation of a silica-sol low friction layer. Silica-sol–gel layers formation is known to be influenced by both pH and the presence of Lewis acids.40 Lewis acids in the form of iron oxide wear debris from the metal pin are present in all the systems examined.
This is in agreement with literature reports that Si-doped DLCs can form silica-sol, low friction layers at high humidity levels.41 Additional, in a study on Si-doped DLC against a steel ball, it has previously been shown that the composition of the debris formed enabled the conclusion that the low, stabilized friction coefficient was intimately related to the formation of the silicon-rich oxide debris.42
This further supports the hypothesis that wear processes which generate Lewis acids such as iron oxides can accelerate wear processes in the absence of a pH buffer.43 The pH buffer PBS is known to positively influence the tribology of Si-DLCs in a variety of applications.44 In the absence of a buffer solution the formation of silicon rich wear debris that are known to give low coefficients of friction in these types of system are highly likely.
This gives further insight into the other systems tested.
When the pH is controlled by the PSB buffer, there is an increase in the friction values throughout testing (μ = 0.0027) and the friction trace shows slightly more variation throughout the test. In this system as well as the pH being controlled, additional elements are incorporated into the system – it is difficult to separate the role of the pH from the addition of these elements. However, the CoF is still lower than the next two systems which gives some insight into what may be happening.
As the PBS CoF value for the Si-DLC/steel tribopair is still relatively low, it seems feasible that despite the control of the acidity, wear processes are still able to generate silica rich oxide debris that are able to reduce the CoF values.
The next two systems (PBS w 5% tween 80 and the tri-additive system) will be considered together, as their friction values are almost exactly the same throughout the duration of the testing.
Firstly, the role of Tween 80 (or ‘polysorbate 80) must be considered. Tween 80 is a water soluble surfactant and emulsifier that is able to form micelles. Micelles are relevant to tribology and are incorporated into automotive engines lubricant formulations, where they are able to ‘catch’ wear debris within the micelle. As the friction is higher (0.0067) than just PBS alone or de-ionised water the authors believe that Tween 80 is able to form micelles to a certain extent in this system and that these are able to catch wear generated oxide debris. This would explain the relative rise in friction when compared to the other systems.
For the final system consisting of the three additives again pH will be controlled by the PBS buffer. However, when taking the SEM/EDX images into account it is apparent that a film has formed that will also be influencing the friction compared to the other systems. This will be discussed later.
5.3 Influence of novel additives
In the formulated lubricant, testing melatonin and cysteine (in PBS solution) provides higher friction values, with far less signal fluctuation. This was expected as melatonin is a well-known and potent anti-oxidant. Therefore, in this system, not only is pH being controlled but also oxidation of the coating is curtailed. This severely limits the formation of oxides of Si, and in turn –OH terminated surfaces. The high friction trace seen here is indicative of the formation of a protective tribolayer, akin to ZDDP deployed in car engines. This is supported by SEM images of crystal growth composed of S, O and N; all of which are elements derived from cysteine. This firmly suggests cysteine has the ability to form a tribofilm in this instance. This is as expected as cysteine has several reactive groups including two chelators groups, the thiol group and the carboxyl group. These groups are known to be surface active towards metals and metalloids.5.4 Effect of micelle formers on friction behaviour
There is a clear difference between the friction trace when comparing PBS alone with PBS blended with 5% Tween 80.Tween 80 is known to form micelles in water at low concentrations. These micelles are able to capture various species, as Tween 80 core is able to ‘mop-up’ wear debris. The core of Tween 80 is densely packed, while the more hydrophilic moiety forms strong interactions between the surfactant and water molecules.22
As discussed, the PBS solution gives low and unsteady friction throughout. Inclusion of Tween 80 causes the friction trace to become much higher and also steadier. With Tween 80 able to form micelles and capture wear debris, this effectively removes the low friction species from the interface, and friction increase as a result. This is as opposed to building up of a wear resistant tribofilm that generates high friction values.
It has been reported that micelle forming species are able to inhibit cavitation derived wear processes in specific solvents. For the Tween 80 (polysorbate) tested system it possibly the case here that not only is the additive able to capture wear debris but also form a thin film that inhibits cavitation.45,46 However, this relationship is highly system dependant.
It has also been reported that Tween 80 is known for its ability to not confound friction data collection through other means. It has previously been noted that while the ‘Tween’ series of surfactants (of which there are several) can effect friction values, Tween 80 has an insignificant effect in experiments.47
5.5 Mechanistic insight
The lowest friction was found in DI water, due to acid catalysed formation of a sol–gel type species. This conclusion is drawn as once the silica wear debris is removed from the interface when testing with Tween 80, friction rises and also that the behaviour is pH dependant.The highest friction was found with the melatonin, cysteine and PBS containing lubricant. Derivatives elements from cysteine can be seen to form crystalline structures on the coated Si-DLC plate. These crystals prevent the formation of low friction layer, but appear to reduce wear on the plate.
Si DLCs are known to oxidise in most environments containing traces of water. This effectively more –OH terminated surfaces and oxygen species. Including melatonin as a protective agent against oxidative wear processes in the lubricant mitigated this behaviour.
Sol–gels are known to influence friction. Concentrated dispersions of colloidal silica particles in water can behave like soft solids. Under large stresses they can accommodate any rate of shear through internal slip layers. The mechanism for localization results from release of water into fractures which let the solid regions flow past each other.48 These dispersions have a dynamic yield stress; after which they accept any rate of flow with only moderate resistance.
5.6 Relationship between pH and silica/Si-doped surfaces
It is know that pH causes stress corrosion of silica surfaces.49 This process is relevant as it initiates at the Si–O–Si bond, which is known to be present in the coating tested. A stepwise process occurs whereby:(1) A water molecule attaches to a bridging Si–O–Si bond, via hydrogen bonding.
(2) Then a concerted reaction occurs in which H+ is transferred to O. This then causes rupture of hydrogen bond, generating Si–O–H groups.49
(3) This effect is also seen in amorphous silica, where again the strained Si–O–Si bridges preferentially react with water to form a silica-gel.50
Furthermore, quartz rocks have been observed to weaken by formation and shearing of silica gel layers. These gels have been identified to be comprised of very fine particles of amorphous silica in water.51 This clearly identifies the propensity of Si–O–Si and more general silica composites to decompose under certain conditions.
There is also much literature covering the creation of colloidal silica particles and sol–gels. Experimentally it has been noted that monodispersed, spherical silica particles are not typically generated in acid-catalysed reactions. In such environments the resultant species are gel structures.37
Again, acidity is noted as causing the differences observed between some silica-sol–gels. Condensation of the Si–OH groups occurs more rapidly when the reaction takes place in acid. This resulting in the formation of larger particles.52
Colloidal silica particles can become covered with a layer of carbon chains, this demonstrates that the particles become sterically stabilized and lipophilic.53 This type of behaviour explains why silica-sol–gels adhere to the detergents micelle, rather than migrating to the bulk water where they would still potentially reduce friction.
6. Conclusions
The tribological behaviour of Si-DLC/steel pairs were tested in aqueous systems with different additives, to examine their effect on friction and surface activity. This was with the overarching goal of assessing the viability of aqueous lubrication in combination with coated surfaces.The findings are in line with other literature sources on the low friction behaviour of Si-DLC in water. The use of compounds able to remove wear debris helps establish the low friction mechanism that can exist within the contact. A novel water based lubricant package was able to confer tribofilm forming properties upon the system.
• pH plays a clear role in the tribological behaviour of silicon doped DLCs in non-buffered aqueous environments, confirming that generation of acid or base fundamentally changes the friction of the system.
• This behaviour can be prevented by control of pH by using a buffer (PBS), which maintains a near neutral pH and results in raised CoFs when compared to water alone.
• The friction of Si-DLC against steel in water can be controlled with relative ease, giving the ability to tailor the tribology of the system to an application.
• Testing conducted in deionised water alone gave the lowest CoF, possibly due to higher wear rates (not quantitatively assessed) or from the formation of postulated silica-sol–gel easy-shear lubricant species.
• The inclusion of the micelle former species ‘Tween 80’ firmly indicates that solid debris is directly related to friction. As, upon its addition the CoF raises notably.
• Water soluble species appear to produce surface layers/crystalline structures on Si-DLC surfaces.
• Compounds have been identified that may be viable as additives for fully formulated aqueous lubricant packages.
In this instance, the biological buffer PBS, and two small molecules, cysteine (source of S) and melatonin (an anti-oxidant) are seen to raise friction dramatically, with SEM confirming crystal growth on the surface of the sample. This suggests that Si-DLCs could be useful in biological/maritime systems where low friction is required and also helps identify novel tribofilm forming agents.
International shipping is a large and growing source of greenhouse gas emissions. The EU is attempting to reduce these emissions and has put in place EU-wide measures to enact this.54 Issues they are aiming to resolve include reduction on reliance of heavily sulphated marine oils (these are distinctly different to the amino acid used herein), reducing ocean acidification and reducing carbon emissions. The combination of newer coating technology enables the move award from mineral/vegetable and synthetic oils (which are sourced or created at a high carbon cost). Here they can effectively be replaced with water as the base fluid, when suitable additives are included.
Author contributions
All data collection, analysis and original draft writing was conducted by the lead author. Supervision and draft review was undertaken by the 2nd author.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The author would like to dedicate this to the late Prof Anne Neville of the University of Leeds. The University of Sheffield Institutional Open Access Fund provided funding for open access publication fees.References
- H. Spikes, Tribol. Lett., 2004, 17, 469–489 CrossRef CAS.
- E. Van Genderen, M. Wildnauer, N. Santero and N. Sidi, Int. J. Life Cycle Assess., 2016, 21, 1580–1593 CrossRef CAS.
- D. D. Beck, J. W. Sommers and C. L. DiMaggio, Appl. Catal., B, 1997, 11, 257–272 CrossRef CAS.
- J. L. Lanigan, Tribochemical analysis of si-doped and non-doped diamond-like carbon for application within the internal combustion engine, Doctoral dissertation, University of Leeds, 2015.
- X. Wu, M. Suzuki, T. Ohana and A. Tanaka, Diamond Relat. Mater., 2008, 17, 7–12 CrossRef CAS.
- T. Ohana, M. Suzuki, T. Nakamura, A. Tanaka and Y. Koga, Diamond Relat. Mater., 2006, 15, 962–966 CrossRef CAS.
- J. Lanigan, H. M. Freeman, C. Wang, M. B. Ward, A. Morina, A. Neville and R. Brydson, RSC Adv., 2017, 7, 43600–43610 RSC.
- A. Tanaka, M. Suzuki and T. Ohana, Tribol. Lett., 2004, 17, 917–924 CrossRef CAS.
- A. Erdemir and C. Donnet, J. Phys. D: Appl. Phys., 2006, 39, R311 CrossRef CAS.
- A. Dorner-Reisel, Z. B. Kavaklioglu, S. Svoboda and J. Engemann, J. Appl. Chem., 2016, 2016 DOI:10.1155/2016/1307691.
- F. Zhao, H. X. Li, L. Ji, Y. F. Mo, W. L. Quan, W. Du, H. D. Zhou and J. M. Chen, Surf. Coat. Technol., 2009, 203, 981–985 CrossRef CAS.
- K. Oguri and T. Arai, J. Mater. Res., 1992, 7(6), 1313–1316 CrossRef CAS.
- D.-C. Pham, H.-S. Ahn, J.-E. Oh and E.-S. Yoon, J. Mech. Sci. Technol., 2007, 21, 1083–1089 CrossRef.
- N. Moolsradoo, Adv. Mater. Sci. Eng., 2011, 2011 Search PubMed.
- M.-G. Kim, K.-R. Lee and K. Y. Eun, Surf. Coat. Technol., 1999, 112, 204–209 CrossRef CAS.
- M. Ikeyama, S. Nakao, Y. Miyagawa and S. Miyagawa, Surf. Coat. Technol., 2005, 191, 38–42 CrossRef CAS.
- F. G. Sen, X. Meng-Burany, M. J. Lukitsch, Y. Qi and A. T. Alpas, Surf. Coat. Technol., 2013, 215, 340–349 CrossRef CAS.
- M. Kano, Y. Yasuda, Y. Okamoto, Y. Mabuchi, T. Hamada, T. Ueno, J. Ye, S. Konishi, S. Takeshima, J. M. Martin, M. I. D. Bouchet and T. Le Mogne, Tribol. Lett., 2005, 18, 245–251 CrossRef CAS.
- R. M. Torrente-Rodríguez, H. Lukas, J. Tu, J. Min, Y. Yang, C. Xu, H. B. Rossiter and W. Gao, Matter, 2020, 3, 1981–1998 CrossRef PubMed.
- E. S. Forbes, Tribology, 1970, 3, 145–152 CrossRef CAS.
- R. D. Evans, H. P. Nixon, C. V. Darragh, J. Y. Howe and D. W. Coffey, Tribol. Int., 2007, 40, 1649–1654 CrossRef CAS.
- R. A. Karjiban, M. Basri, M. B. A. Rahman and A. B. Salleh, APCBEE Proc., 2012, 3, 287–297 CrossRef.
- E. Gatete, H. M. Ndiritu and R. Kiplimo, 2018.
- M. Muraki and D. Dong, Lubr. Sci., 2005, 17, 185–196 CrossRef CAS.
- Q. B. Nguyen, Y. H. M. Sim, M. Gupta and C. Y. H. Lim, Tribol. Int., 2015, 82, 464–471 CrossRef CAS.
- V. Chaudhary, P. K. Bajpai and S. Maheshwari, Fibers Polym., 2018, 19, 403–415 CrossRef CAS.
- S. Hofmann, Surf. Interface Anal., 1986, 9, 3–20 CrossRef CAS.
- H. Liu and T. J. Webster, Biomaterials, 2007, 28, 354–369 CrossRef CAS PubMed.
- M. Veres, M. Koós, S. Tóth, M. Füle, I. Pócsik, A. Tóth, M. Mohai and I. Bertóti, Diamond Relat. Mater., 2005, 14(3–7), 1051–1056 CrossRef CAS.
- W.-J. Wu and M.-H. Hon, Surf. Coat. Technol., 1999, 111, 134–140 CrossRef CAS.
- G. B. D. Briggs, High Resolution XPS of Organic Polymers, Wiley, Chichester, 1992 Search PubMed.
- D. Briggs, Surface analysis of Polymers by XPS and static SIMS, OUP, Cambridge, 1998 Search PubMed.
- J. Lanigan, PhD thesis, University of Leeds, 2015.
- J. Lanigan, H. Zhao, A. Morina and A. Neville, Tribol. Int., 2015, 82, 431–442 CrossRef CAS.
- X. Wu, T. Ohana, A. Tanaka, T. Kubo, H. Nanao, I. Minami and S. Mori, Diamond Relat. Mater., 2008, 17, 147–153 CrossRef CAS.
- L. L. Hench and J. K. West, Chem. Rev., 1990, 90, 33–72 CrossRef CAS.
- I. A. Rahman and V. Padavettan, J. Nanomater., 2012, 2012, 15 Search PubMed.
- F. Cheng and S. Jiang, Appl. Surf. Sci., 2014, 292, 16–26 CrossRef CAS.
- T. N. Viten’ko and Y. M. Gumnitskii, J. Water Chem. Technol., 2007, 29, 231–237 CrossRef.
- C. Milea, C. Bogatu and A. Duta, Bull. Transilv. Univ. Bras., 2011, 4, 53 Search PubMed.
- Y. Liu, A. Erdemir and E. I. Meletis, Surf. Coat. Technol., 1997, 94–95, 463–468 CrossRef CAS.
- M.-G. Kim, K.-R. Lee and K. Y. Eun, Surf. Coat. Technol., 1999, 112, 204–209 CrossRef CAS.
- E. Vrancken and J. M. Campagne, PATAI'S Chemistry of Functional Groups, 2009 Search PubMed.
- L. K. Randeniya, A. Bendavid, P. J. Martin, M. S. Amin, E. W. Preston, F. S. Magdon Ismail and S. Coe, Acta Biomater., 2009, 5, 1791–1797 CrossRef CAS PubMed.
- I. K. Karathanassis, K. Trickett, P. Koukouvinis, J. Wang, R. Barbour and M. Gavaises, Sci. Rep., 2018, 8, 14968 CrossRef CAS PubMed.
- F. Burlon, D. Micheli, R. Furlanetto, M. Simonato and V. Cucit, Energy Procedia, 2016, 101, 718–725 CrossRef.
- M. Graca, J. H. H. Bongaerts, J. R. Stokes and S. Granick, J. Colloid Interface Sci., 2007, 315, 662–670 CrossRef CAS PubMed.
- J. Persello, A. Magnin, J. Chang, J. Piau and B. Cabane, J. Rheol., 1994, 38, 1845–1870 CrossRef CAS.
- T. A. Michalske and S. W. Freiman, Nature, 1982, 295, 511–512 CrossRef CAS.
- Y. Nakamura, J. Muto, H. Nagahama, I. Shimizu, T. Miura and I. Arakawa, Geophys. Res. Lett., 2012, 39(21), L21303 CrossRef.
- D. L. Goldsby and T. E. Tullis, Geophys. Res. Lett., 2002, 29, 25 CrossRef.
- C. R. Silva and C. Airoldi, J. Colloid Interface Sci., 1997, 195, 381–387 CrossRef CAS PubMed.
- S. Coenen and C. G. de Kruif, J. Colloid Interface Sci., 1988, 124, 104–110 CrossRef CAS.
- EU, Directive (Eu) 2016/802 of the European Parliament and of the Council L 132/58, 2016 Search PubMed.
| This journal is © The Royal Society of Chemistry 2023 |