Open Access Article
Open Access ArticleResearch progress of yolk–shell structured nanoparticles and their application in catalysis
Meiyu Siab,
Feng Lin *a,
Huailan Nia,
Shanshan Wangb,
Yaning Luac and
Xiangyan Meng*a
*a,
Huailan Nia,
Shanshan Wangb,
Yaning Luac and
Xiangyan Meng*a
aDepartment of Chemistry and Chemical Engineering, Heze University, Heze 274015, Shandong Province, China. E-mail: linfeng@hezeu.edu.cn; mengxiangyan@hezeu.edu.cn
bSchool of Marine Science and Technology, Harbin Institute of Technology at Weihai, Weihai 264209, Shandong Province, China
cSchool of Chemistry and Chemical Engineering, University of Jinan, Jinan 250022, Shandong Province, China
First published on 12th January 2023
Abstract
Yolk–shell nanoparticles (YSNs) have attracted a broad interest in the field of catalysis due to their unique structure and properties. The hollow structure of YSNs brings high porosity and specific surface areas which is conducive to the catalytic reactions. The flexible tailorability and functionality of both the cores and shells allow a rational design of the catalyst and may have synergistic effect which will improve the catalytic performance. Herein, an overview of the research progress with respect to the synthesis and catalytic applications of YSNs is provided. The major strategies for the synthesis of YSNs are presented, including hard template method, soft template method, ship-in-a-bottle method, galvanic replacement method, Kirkendall diffusion method as well as the Ostwald ripening method. Moreover, we discuss in detail the recent progress of YSNs in catalytic applications including chemical catalysis, photocatalysis and electrocatalysis. Finally, the future research and development of YSNs are prospected.
1. Introduction
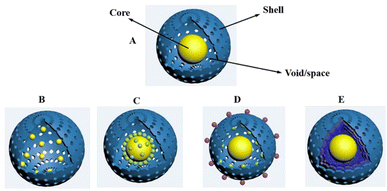
With the continuous research on materials science, nanomaterials with unique structure and function have attracted more and more attention, in which the yolk–shell structured nanoparticles (YSNs), or so-called rattle-typed structured nanoparticles become one of the most popular nanomaterials. The unique yolk–shell structure endows YSNs with excellent physical and chemical properties, showing great potential in many different fields, such as lithium-ion batteries, drug delivery, nanoreactor and catalysis. Indeed, the yolk–shell structure represents a special core–shell structure with movable core and functional shell, which can be more precisely described as core@void@shell structure. Compared with the conventional core–shell structure, the yolk–shell structure has lower density, larger space, higher specific surface area as well as stronger loading capacity.1 The hard outer shell structure not only protects the inner core from agglomeration, but also allows the selective adsorption and enrichment of various reactants, promoting the reactions. The core of YSNs can move freely inside the cavity, which allows a full contact with the reactant and maximum exert its function. In addition, the cavity of YSNs will provide a confined space and can be also used as a storage or reaction chamber. According to the structure of core and shell, YSNs can be roughly divided into the following five different types:2 (i) single core encapsulated in shell; (ii) multiple cores encapsulated in shell; (iii) core-satellite–shell structure; (iv) single yolk in shell with multiple cores loaded on shell surface; (v) single core within multiple shells (Fig. 1). Till now, many different synthetic strategies have been developed for the fabrication of YSNs. Among those, the hard template method is considered to be the simplest and effective approach, in which a layer of template material will be first coated on a hard-core surface, followed by coating with the target shell material, and YSNs will be obtained after sacrificing the template layer. The soft template method is quite similar with that of the hard template method, except for using vesicles formed by the self-assembly of amphoteric molecules (including surfactants and block copolymers) as the sacrificial template, which can easily be removed afterwards. Besides, a series of self-template approaches have been developed without the use of additional structural guiding template, such as the galvanic replacement method, Kirkendall diffusion method and Ostwald ripening method.3The development of high efficiency, low cost and environmentally friendly catalyst has been considered to be the main desire in the field of catalysis. Due to its unique structure and properties, YSNs have practical significance in nanocatalysis, magnetic assisted catalysis, integrated catalysis and so on.3 On the one hand, the hollow structure of YSNs brings it with high porosity and specific surface areas which is conducive to the catalysis reactions. On the other hand, the different composition of the core and shell may have synergistic effect which will improve its catalytic activity and selectivity. Moreover, the combination of the properties of core and shell can greatly extend the application fields, for example, magnetic materials@catalytic materials can be used for many times while maintaining their activity,4 improving their recycling efficiency and adapting to green and sustainable development.
Herein, we introduce in detail the synthesis and catalytic application of YSNs. Firstly, we give a comprehensive overview of the synthesis strategies: hard template method, soft template method, ship-in-a-bottle method, galvanic replacement method, Kirkendall diffusion method as well as Ostwald ripening method. All strategies are very effective and have their own set of advantages that will lead to YSNs with the certain composition and structure. Then, we focus on the state of the art of YSNs in catalysis applications, including chemical catalysis, photocatalysis and electrocatalysis. At last, the future research and development of YSNs are prospected.
2. Synthetic strategies for YSNs
2.1 Hard template
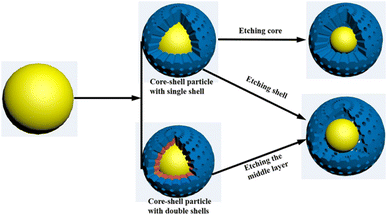
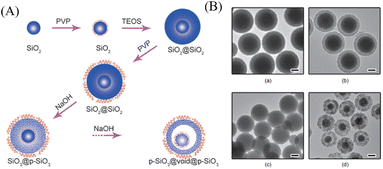
Hard template method is one of the most universal methods for preparing almost all types of YSNs materials.5,6 As shown in Fig. 2, in this approach, the pre-synthesized core is first coated with one or two layers of hard template to form a core–shell or sandwich-like structure, respectively. Then, the template (core, shell, or middle layer) can be selectively removed by calcination, chemical etching or solvent dissolution to create a cavity between the core and outer-layer shell.2.2 Soft template method
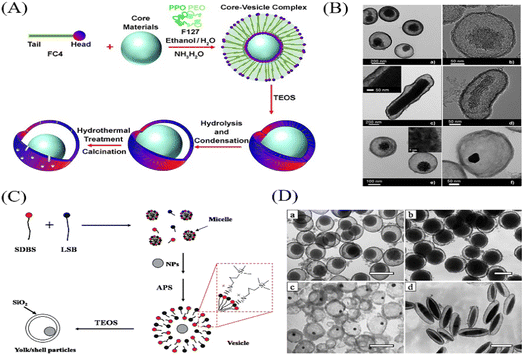
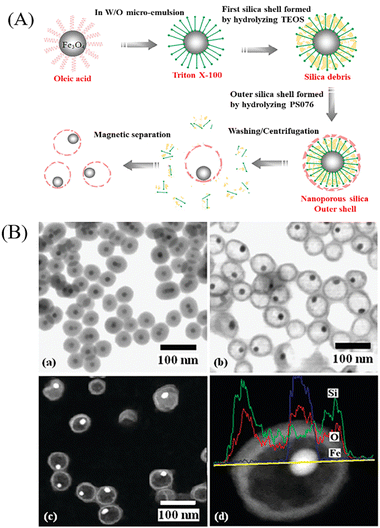
Compared with hard template method, the soft template method is a simple, facile and efficient approach for the preparation of inorganic multi-functional YSNs,18 which overcomes the difficulties related with selective etching, such as multiple steps and accurate control of etchants. When noble metal cores are dispersed in soft template mixture, the soft templates attach to the cores to give well-defined assemblies under certain conditions, which can be regarded as absorbed surfactant layers or microemulsion for the preparation of YSNs, and soft template can be easily removed through washing or calcination.2.3 “Ship-in-a-bottle” method
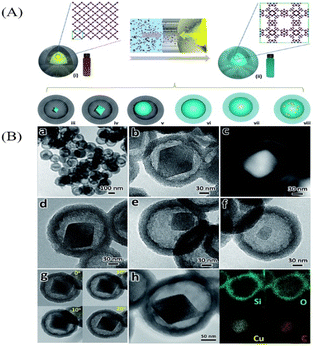
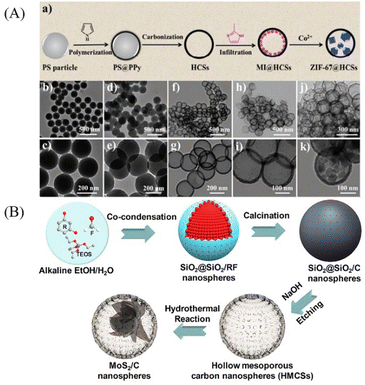
In contrast to the hard template method which can be roughly defined as “from inside to outside”, the “ship-in-a-bottle” method is totally opposite which is actually “from outside to inside”.24 The “ship-in-a-bottle” method includes normally three steps: firstly, the hollow shell as nanoreactor is pre-synthesized. Secondly, loading the core precursors into the hollow shell. Thirdly, the core particles are formed in the hollow cavity through chemical reaction or self-assembly process. In this method, the hollow nanoparticles which act as the nanoreactors will provide a space confinement effect and restrict the growth of core particles inside hollow cavities. According to the formation mechanism, the “ship-in-a-bottle” method can be divided into confined enrichment method and seed-mediated growth method.Following this method, Xiue and co-workers prepared hollow mesoporous carbon spheres (HMCSs) serve as nanoreactors to confine the growth of MoS2 nanosheets (Fig. 10B).28 Dai and co-workers also prepared HPW@Hollow S-1 YSNs successfully by this method,29 Na2WO4 and Na2HPO4 are smaller than the pore size of silicalite-1 (S-1), so they can penetrate into the cavities of the hollow nanosphere and assemble to form phosphotungstic acid (HPW) nanoparticles which will be confined within the Hollow S-1. Recently, MoSe2@HCNS,30 ZIF-67@CNCs31 and MxPy@CNCs32 were also successfully prepared by the confined enrichment method.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
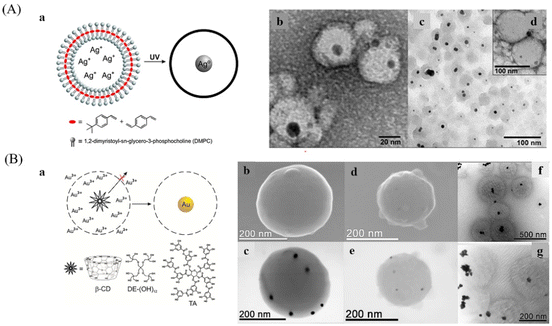
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of tert-butylstyrene and divinylbenzene) and a photoinitiator (2,2-dimethoxy-2-phenylacetophenone, DMPA) in the bilayer and silver ions in the aqueous core are prepared by hydrating a mixture of lipids and monomers with the aqueous solution of silver nitrate followed by extrusion,33 then UV light is used to initiate the polymerization and Ag nanoparticles were formed inside the nanocapsules (Fig. 11A). A similar method was used to prepare Au nanoparticles inside hollow nanocapsules by the reduction of Au3+ that pre-trapped in the hollow cavities with reducing agents, such as tannic acid, β-cyclodextrin, and polyether dendrimer,34 which can be easily fragmented by acid hydrolysis and removed (Fig. 11B).
1 mixture of tert-butylstyrene and divinylbenzene) and a photoinitiator (2,2-dimethoxy-2-phenylacetophenone, DMPA) in the bilayer and silver ions in the aqueous core are prepared by hydrating a mixture of lipids and monomers with the aqueous solution of silver nitrate followed by extrusion,33 then UV light is used to initiate the polymerization and Ag nanoparticles were formed inside the nanocapsules (Fig. 11A). A similar method was used to prepare Au nanoparticles inside hollow nanocapsules by the reduction of Au3+ that pre-trapped in the hollow cavities with reducing agents, such as tannic acid, β-cyclodextrin, and polyether dendrimer,34 which can be easily fragmented by acid hydrolysis and removed (Fig. 11B).
2.4 Galvanic replacement
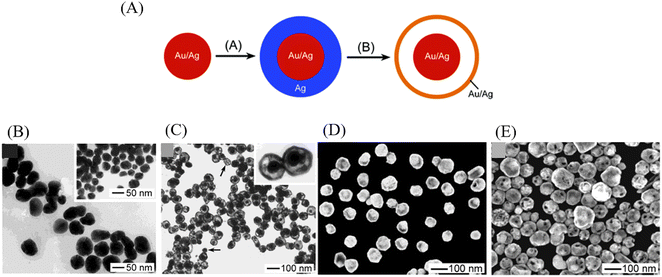
Galvanic replacement is a remarkably efficient and facile route to synthesis YSNs with controllable nanostructure, especially for noble metal and metal oxides. The galvanic replacement is mainly focus on the replacement reaction between two metals with different electrical potential, in which the metal with strong activity is used as reducing agent to replace the less active metal from its salt solution. Typically, the core particles coated with anode metal are placed in a cathode salt solution where the dissolving of the anode metal surface and the deposition of the cathode metal on the anode metal surface happens simultaneously, leading to a void between the cathode metal and anode metal.24 And in fact, the spacing of the voids rely on reaction conditions which could eventually expand towards the center, resulting in a hollow structure. For instance, Sun and co-workers used this method preparing YSNs consisting of Au/Ag alloy core and Au/Ag alloy shell. Two major details can be listed as follows: (1) electroless deposition of a conformal coating of silver on the surface of an Au/Ag alloy nanoparticle; (2) reaction of the resultant particle with an aqueous HAuCl4 solution to replace the coating of silver into Au/Ag alloy shell larger in size.35 The size of the core, the thickness of the shell and the avoid between the core and shell depend primarily on the amount of HAuCl4 added to the reaction mixture (Fig. 12). Similarly, via this approach, a Pd@Cu core shell nanocube can be changed into Pb@AuxCu1−x yolk–shell nanocage, with the further addition of HAuCl4, the Cu shell can be dissolved from the corners toward the interior, after which the cavity will be increasingly enlarged.362.5 Kirkendall diffusion method
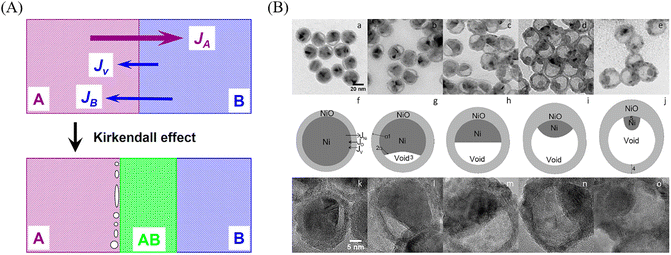
The Kirkendall diffusion is another classical method for YSNs synthesis, which is based on the ion-exchange. However, instead of making use of the different electrical potential, the Kirkendall diffusion occurs at the boundary of two different metals when an imbalance in their diffusion rates appears, leaving vacancies at the material side with the faster diffusion rate. Similar to the galvanic replacement, this approach is also appropriate for the preparation of composites with metal and alloy. As illustrated in Fig. 13A, owing to faster diffusion rate of metal A into B than that of B into A, the alloy (AB) grows in the direction of the faster-moving species (A), which lead to unfilled voids and gaps left in the zone of the faster diffusing component and coalesce into large pores,37 thus resulting in the YSNs. For example, Cui and co-workers have prepared a kind of yolk–shell Bi@C nanostructures via this approach, and the prepared Bi@C YSNs have been proved to be an excellent solid catalyst on the thermal decomposition of cyclotrimethylenetrinitramine.38 Railsback et al. demonstrated the transformation of Ni nanoparticles with different size to hollow or porous NiO through the method of Kirkendall diffusion, Ni diffuse across the Ni/NiO interface and vacancies are formed at the interface and diffuse to the void. The void nucleates when vacancies supersaturate, and the core becomes a small nanoball toward the end of reaction (Fig. 13B).392.6 Ostwald ripening
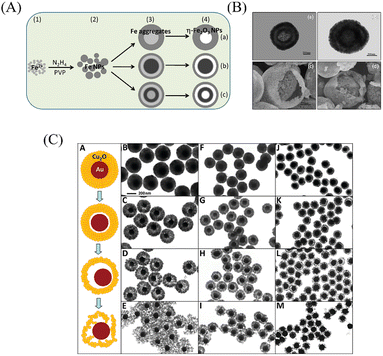
Ostwald ripening is a physical phenomenon that refers to the smaller crystal particles produced in the solution will gradually dissolve into the surrounding medium due to their large curvature and high interface energy,40 and then redeposit onto larger crystalline or sol particles, which further increase the size of the larger crystal particles. This process has recently been widely used in the preparation of YSNs. For example, Zhong et al. prepared hollow and yolk–shell structure η-Fe2O3 nanoparticles through the Ostwald ripening process. First, hydrazine hydrate is added into the mixture of Fe3O4 and poly(vinyl-pyrrolidone) (PVP) solution, then followed by heating, washing and calcinating,41 after which the products are collected. By controlling the amount of PVP and reaction time, η-Fe2O3 YSNs with spherical, egg-like, olivary elliptical and shuttle-like structures41 can be easily obtained (Fig. 14A and B). Li et al. obtained Au@Cu2O rattle-like YSNs,42 via the controllable growth of polycrystalline Cu2O shell surrounding Au core and the followed the Ostwald ripening of Cu2O shell (Fig. 14C).3. Catalytic applications of YSNs
3.1 Chemical catalysis
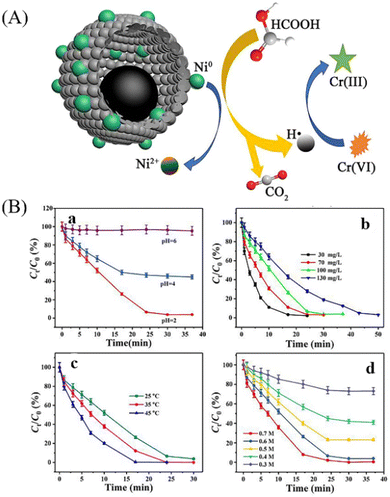
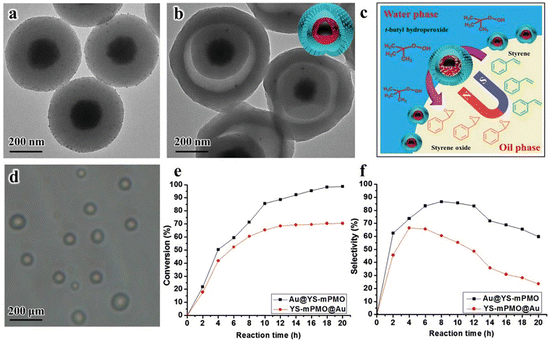
Due to the unique properties of YSNs, such as low density, movable core, void space between the core and shell, and their readily tailorability and functionality in both the cores and shells, YSNs materials have been widely used as catalysts in many reactions.43–55 In most of the cases, the movable core of YSNs often serve as catalyst, while the outer shell can not only control the diffusion of the reactants and products, but also provide a confinement effect which prevent particles agglomeration and improve the catalytic performance.Lv et al. reported the use of MOF derived Ni@carbon YSNs as reusable catalysts for reduction of Cr(VI). The results show that Ni@carbon450 exhibit excellent catalytic performance in the presence of HCOOH, and can completely reduce highly toxic Cr(VI) to non-toxic Cr within 30 min, and due to protection effect of the carbon shell, Ni@carbon450 displayed good stability and high catalytic activity after 10 cycles. As described in Fig. 15A, HCOOH and Cr(VI) were firstly adsorbed on the surface of Ni@carbon450 by electrostatic attraction and then Cr(VI) entered the interior of YSNs to reach the active sites. The dehydrogenation decomposition of HCOOH produced H2 which will be enriched on the surface of the catalyst, and Ni will promote the production of H˙, which can readily induce the reduction of Cr(VI) to Cr(III).56 Fig. 15B shows that pH value and temperature has also a great influence on the catalytic performance. Yu et al. used Fe3O4@RF (resorcinol formaldehyde)@Au-void@Ys-mPMO (yolk–shell magnetic periodic mesoporous organosilica) to synthesize Au@YS-mPMO with amphiphilic shell, high surface area (393 m2 g−1), tunable intermediate hollow space (150–156 nm) and high superparamagnetism (34.4–37.1 cmu g−1),57 which can be used not only as a solid emulsifier to disperse styrene in water, but also as an interface catalyst to convert styrene into styrene oxide with excellent conversion and selectivity of 97.5% and 83.3% respectively (Fig. 16). Dai and co-workers successfully synthesized CeO2@Pt-Beta YSNs as novel catalysts to improve the H2 production by LT-ESR (Low Temperature-Ethanol Steam Reforming) reaction. Compared with Pt-Beta and CeO2–Pt-Beta catalysts, CeO2@Pt-Beta catalysts show better catalytic performance and excellent stability, the conversion of ethanol is 100% and the selectivity of hydrogen can reach 67%.58 It is proposed that the ethanol will first enter the interior of the YSNs, then interact with Pt to dehydrogenate to acetaldehyde which will readily decompose into CH4 and CO. At the same time, SAR (CH3CHO + 3H2O → 2CO2 + 5H2) reactions promote the production of H2, and CeO2 cores also promotes the WGS (CO + H2O → CO2 + H2) and ETD (C2H5OH → CH3CHO + H2) reaction, resulting in more H2. Finally, H2 and CO2 are purified from the mixed gas by the selectivity of Pt shell.
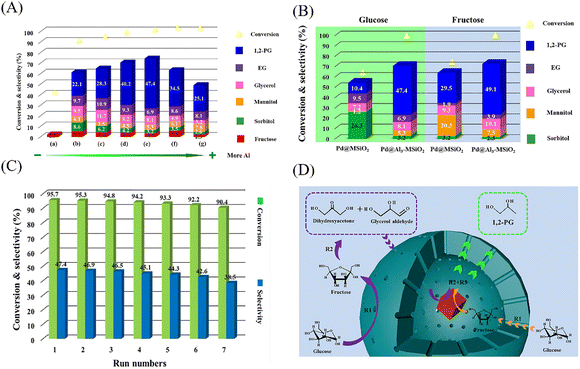
Lv et al. reported the superior selectivity (47.4%) of Pd@Al3–MSiO2 YSNs in the hydrogenolysis of glucose to 1,2-propylene glycol (1,2-PG) (Fig. 17A and B). After even 7 times of recycling, the selectivity of 1,2-PG still could reach 38.5% and the conversion of glucose was 90.4% (Fig. 17C).59 It is proposed that Al atoms can enter mesoporous SiO2 to form 4-coordinated Al species, which will provide more Lewis acid sites. On the one hand, glucose will be isomerized into fructose in the pores of the shell, and then fructose undergoes retro-aldol condensation in the hollow cavity of the YSNs to generate C3 precursor of 1,2-PG (Fig. 17D). Liu et al. prepared YSNs with Ag nanoparticles wrapped in porous Tanus polymer shells composed of hypercrosslinked polystyrene (xPS) and acrylic acid (PAA) brush lining. The porous outer layer of xPS will be beneficial for the diffusion of cationic dyes, and PAA with carboxyl groups show great affinity for cationic dyes, making them easier to be enriched in the cavity of YSNs.60 The catalytic experiment shows that, under the same experimental conditions, the catalytic performance of Ag@PAA-xPS is much better than that of Ag@xPS, indicating the important role of PAA in the catalyst. Li and coworkers prepared (Pd/C)@Tp(2,4,6-trihydroxybenzene-1,3,5-tricarbaldehyde)Pa(phenylenediamine) COFs(covalent organic frameworks) YSNs for catalyzing the Suzuki reaction. The cut off efficiency of the catalyst for aryl benzene was 100%. When the Pd loading was only as 0.05 mol%, the conversion for aryl benzene was as high as 82%.50 In addition, the catalyst could be easily recovered by filtration and reused without any deactivation. The Pd nanoparticles immobilized on the carbon core release Pd atoms to undergo oxidative addition with aryl benzene, and then react with phenylboric acid to produce intermediates (Ar1-Pd-Ar2). Then Pd atoms are captured by the parent Pd core, which catalyzes the completion of the Suzuki reaction. Acharya et al. synthesized Au@carbon YSNs which can efficiently catalyze the reduction of 2-amino-4-nitrophenol (NP) to 2,4-diaminophenol (AP).61 Within 60 min, the deep yellow solution gradually faded, the absorbance at 443 nm (NP) of the UV-Vis absorption spectrum gradually decreased, and a new absorption peak appeared at 320 nm (AP).
3.2 Photocatalysis
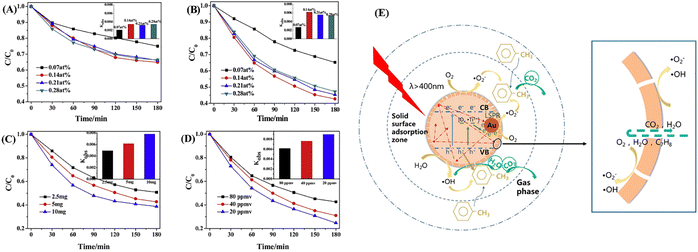
Photocatalysis has been regarded as one of the most promising green technologies in chemical conversion, which is driven by the active electrons and holes that generated through photoexcitation. Photocatalysts play the most vital role in photocatalysis, thus the research and development of highly efficient photocatalysts is pivotal to the development of photocatalytic technology.16 Due to the unique architecture and flexible compositions, YSNs can not only enhance light scattering in the hollow space and provide a large specific surface area to create sufficient active sites, but also allow the enrichment of the reactant and provide a homogeneous reaction environment, which minimizes environmental effects in catalytic reactions, achieving improved catalytic performance. In recent years, there are many studies on the preparation of metal oxide@metal oxide yolk–shell structure for photocatalysis. Besides, because Au, Ag, Pd and other noble metal nanoparticles show strong spectral adsorption in the UV-vis band, noble metal@oxide yolk–shell structure show better light absorption and enhance the separation efficiency of electron–hole pairs. In addition, the magnetic materials such as Fe3O4 wrapped in semiconductor metal oxide results in magnetic separation performance that can be easily recycled under the action of external magnetic field.4Volatile organic compounds (VOCs) such as formaldehyde, benzene, xylene and lipids often come from the furniture, industrial facilities and construction products and so on.62 They are not only toxic substances, but also are photochemical reactive with other air pollutants, resulting in secondary pollution, which will cause great harm to environmental quality and human health. Yue et al. prepared Au@TiO2 YSNs for photocatalytic degradation of gaseous toluene under visible light. The degradation rate of gaseous toluene with Au@TiO2 YSNs was 1.63 times higher than of Au@TiO2 core–shell nanospheres catalyst and the removal rate of gaseous toluene reached 57% when the concentration of Au@TiO2 YSNs catalyst was only 0.14 wt% within 3 h,62 and the catalyst dosage of Au@TiO2YSNs and C7H8 concentration are also explored as factors affecting the degradation efficiency of gaseous toluene (Fig. 18A–D). It is proposed that h+, OH˙, ˙O2−, and Ti3+ produced in Au@TiO2 YSNs are the main active components during the reaction. Meanwhile, the separation efficiency of e−/h+ pairs was improved by unique yolk–shell structure and localized surface plasmon resonance (LSPR) effect of Au cores, leading to excellent photocatalytic performance (Fig. 18E).
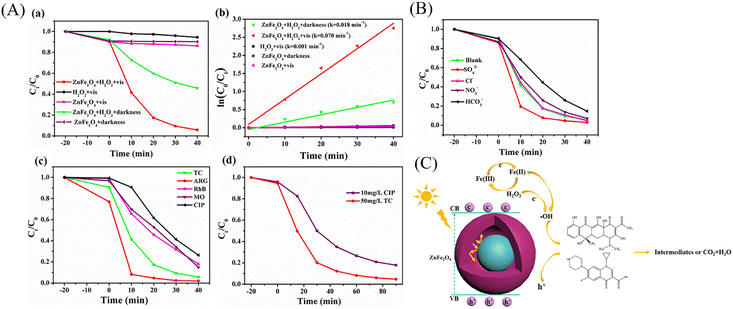
YSNs can also be used as photocatalyst for the degradation of organic pollutants in water, such as dyes,63–75 pesticides76 and antibiotics.77,78 For example, Wang et al. reported the preparation of Fe3O4@ns-TiO2/Ag/g-C3N4 YSNs which exhibited superior photocatalytic performance for degradation of methyl orange. The reaction rate constant is 0.12723, which is 2.36 times of Fe3O4@ns-TiO2 and 3.64 times of commercial P25.74 The combination with g-C3N4 and the LSPR effect of Ag extend the light response range of TiO2 to the visible region. Ag located between TiO2 and g-C3N4 can serve as an electron transfer bridge to effectively promote the separation of electron–hole pairs. Xiang and co-workers have prepared ZnFe2O4@void@ZnFe2O4 YSNs with excellent tetracycline (TC) photo-Fenton degradation (Fig. 19A and C). Moreover, they found that the presence of SO42− and Cl− could increase the degradation rate of TC due to the production of radicals (Cl˙ and ˙SO42−) which retarded the combination of electrons and holes (Fig. 19B).77
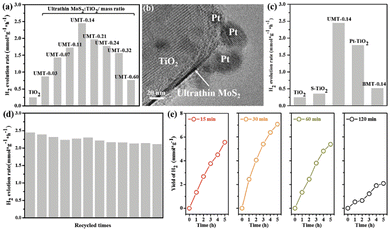
Photocatalytic decomposition of water is one of the most promising and environmentally friendly approach for the production of hydrogen, which is most ideal pollution-free green energy sources in the 21st century. Wang and co-workers fabricated UMT (ultrathin MoS2 decorated yolk–shell TiO2) YSNs, in which the ultrathin MoS2 sheets are evenly embedded on TiO2. When the loading amount of ultrathin MoS2 is 0.14 wt%, the photocatalytic hydrogen evolution rate (HER) is 2443 μm g−1 h−1, which is 100% and 470% of pristine TiO2 (247 μ mol g−1 h−1) and BMT (bulk MoS2/TiO2) (513μ mol g−1 h−1).79 They deposited Pt in UMT-0.14 YSNs (Pt/UMT-0.14), and found that Pt was reduced and grown at the edge of ultrathin MoS2, which indicates that a large number of photoelectrons exist in ultrathin MoS2. In addition, they observed that UMT-0.14 still shows stable HER performance after 12 cycles, and long sulfurization time led to a significant decrease in hydrogen production (Fig. 20). The significant enhancement of HER is attributed to the fact that the ultrathin MoS2 sheets provide sufficient channels to accelerate the transfer of photogenerated electrons between the TiO2 and ultrathin MoS2, which improve the carrier density (1.97 × 1022 cm−3) and reduces the transfer resistance. Similarly, Zhang et al. prepared Cu@Cu2O YSNS with controllable morphology by changing the concentration of OTAC (octadecyl trimethylammonium chloride).80 The photocatalytic hydrogen production rate was 97 times higher than that of pure TiO2, and showed excellent long-term durability.
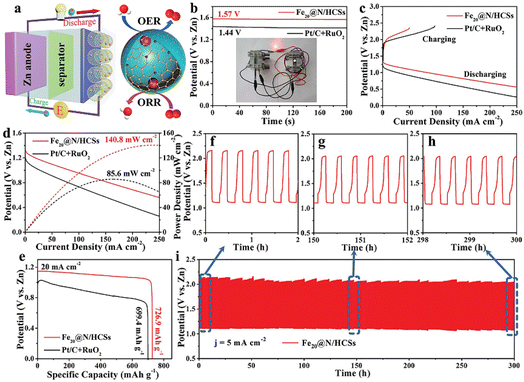
3.3 Electrocatalysis
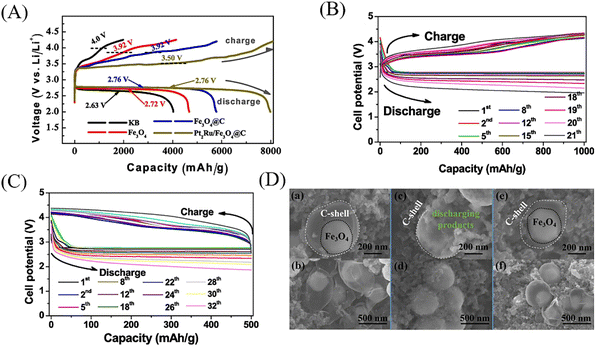
With the development of mobile electronics and electric vehicles, lithium-ion batteries with high energy density, high safety, low cost and long-life periods are highly desirable.81 YSNs with nanoscale void space and functional shells can act as a barrier to prevent the aggregation of the encapsulated electroactive NPs. Moreover, it will offer sufficient space to accommodate the huge volume variation of NPs during the charge/discharge process, which can affect the insertion and detachment of lithium ions and improve the cycling performance. Li et al. prepared Fe3O4@C–PtRu YSNs as catalysts to enhance the performance of lithium–O2 battery. When Fe3O4@C–PtRu catalyst is used in the cathode of lithium–O2 battery, the electrochemical performance of the battery has been greatly improved. The battery has a high reversible capacity of 7996 mA h g−1 at 200 mA g−1, and the limited capacities of 21 and 32 cycles at 200 mA g−1 is 1000 and 500 mA h g−1 respectively (Fig. 21A–C).82 As shown in Fig. 21D, Li2O2 discharge products were deposited in the cavity of Fe3O4@C–PtRu YSNs after discharge. After recharging, the deposited products disappear, and the Fe3O4 core can be observed again, which indicates that the internal void between the shell and core can overcome the volume change in the recharging/discharging process (2Li + O2 ↔ Li2O2) and maintain a good shape of the yolk–shell structure, thereby improving the performance of lithium–O2 batteries. Wang and co-workers reported that the addition of Fe2O@N/HCSs into the cathode of Zn–air battery can achieve high open circuit voltage (1.57 V), high power density (140.8 mW cm−2), high specific capacity (726.9 mA h g−1) and excellent long-term cycle performance (300 h) compared with commercial Pt/C + RuO2 (Fig. 22).83 Similarly, Kun et al. reported the use of NiCO2Px/rGO YSNs as bidirectional catalysts in the sold–liquid process of advanced lithium–sulfur battery. They claimed that the unique yolk–shell structure exposes more catalytic active sites, which ensure more effective contact between catalysts and polysulfides, and provides sufficient space for sulfur loading and volume variation during the buffer cycle. As a result, the sulfur cathode assembled with NiCO2Px/rGO has a high capacity of 1238.7 mA h g−1 at 0.1 C and still maintains a stable discharge capacity of 561.1 mA h g−1 after 400 cycles.84 Finally, they further studied the nucleation and dissolution of Li2S, the discharging and charging profiles of Li2S8 on different surfaces and electrodes confirmed that NiCO2Px/rGO was an excellent bidirectional catalyst in redox conversion (Li2Sx ↔ Li2S).Electrolytic water is also an environmentally friendly way to produce hydrogen at present. However, the oxygen evolution reaction (OER) involved in electrolytic water is considered to be the main bottleneck to for hydrogen production, since the formation of O–O bond in OER is a 4e− process with slow kinetics and requires high overpotential to reach its reaction barrier.85–87
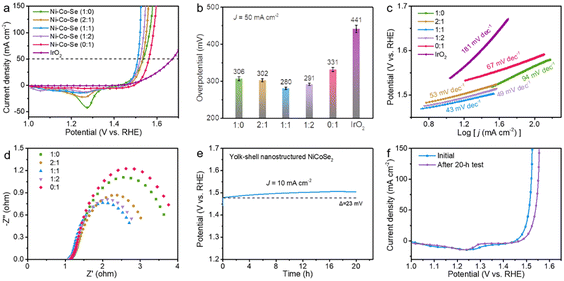
Wang et al. designed Fe2O@N/HCSs YSNs with Fe–Nx active sites as electrocatalysts, which showed excellent ORR (half-wave potential E1/2 = 0.850 V, Tafel plots = 58.7 mV dec−1 and limited current density JL = 5.750 mA cm−2) and OER (η10 = 289 mV, Tafel plots = 52.4 mV dec−1) activity and stability (high relative current of 87.0% after 30 h electrolysis).83 It is proposed that confinement effect of YSNs with could improve the OER in the hollow cavity and reduced the reaction delay caused by material agglomeration. Besides, Fe3O4 nanoparticles embedded in hollow carbon spheres could improve the electrical conductivity of the materials and enhanced the effective distribution of Fe–Nx active sites, thus leading to excellent ORR and OER properties. Similarly, Gan et al. synthesized a series of NiCoSe2@Se YSNs based on Kirkendall effect. When the molar ratio of Ni to Co is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, NiCoSe2@Se YSNs showed the best OER performance (highest current density at 1.0–1.65 V, low overpotential of 249 mV, lowest contact resistance and charge transfer impedance, small Tafel slope of 43 mV dec−1 and tiny decay of 23 mV over 20 h electrolysis) (Fig. 23).88 They believed that the metal properties of selenide and the high conductivity of Ni enable raid electron transfer between electrode and electrolyte, the wide and hollow annular channel in the unique yolk–shell nanostructure is conducive to the penetration and transportation of electrolyte ions and the exposure of active centers.
1, NiCoSe2@Se YSNs showed the best OER performance (highest current density at 1.0–1.65 V, low overpotential of 249 mV, lowest contact resistance and charge transfer impedance, small Tafel slope of 43 mV dec−1 and tiny decay of 23 mV over 20 h electrolysis) (Fig. 23).88 They believed that the metal properties of selenide and the high conductivity of Ni enable raid electron transfer between electrode and electrolyte, the wide and hollow annular channel in the unique yolk–shell nanostructure is conducive to the penetration and transportation of electrolyte ions and the exposure of active centers.
4. Conclusions
In this review, we summarize the synthetic methods of YSNs and their applications in the field of chemical catalysis, photocatalysis and electrocatalysis. Despite many different synthetic methods have been developed, there are still many challenges need to be overcome. For instance, for removing the sacrificial layers in hard template method, environment-friendly dissolving or etching agents is urgent desired to avoid using the harmful dissolving solvents such as hydrofluoric acid. In addition, the soft template method cannot well control the uniformity of YSNs. Moreover, it is still lack of a common set of approaches for the fabrication of YSNs, most of which involve multiple steps and work successfully only in specific situations. Exploring more effective, environmentally friendly, universal and low-cost synthetic methods is still our primary research objective. In order to meet the requirements of practical applications, the core and shell of yolk–shell structure need to be selectively functionalized, and YSNs with more complex structure and compositions are required. And a better control of the structure, morphology and function of YSNs deserves further investigation. Finally, more effort should be made to improve the recyclability of YSNs.When YSNs being used as catalyst, the porosity of the shell can be further adjusted to regulate the diffusion rate of reactants, so as to better control the catalytic performance. Similarly, precise localization of the active sites in YSNs can also improve the catalytic performance. For example, due to the continuous movement of yolk core particles in a photocatalytic process, where complex charge transfer and separation are required, a rational design of the charge transfer mechanism between yolk and shell will be a key future research direction. Meanwhile, in the core and shell region, the spatial separation of photogenerated electrons and holes is very difficult which requires a new breakthrough. In addition, yolk–shell nanostructures are expected to be used as electrocatalysts to improve battery performance in the near future, especially when commercial processes are established. Nevertheless, the application of YSNs in more research fields needs to be explored.
Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work is supported by the Natural Science Foundation of Shandong Province of China (No. ZR2022MB136) and the Scientific Research Fund of Heze University (No. XY20BS18).References
- C. A. H. Price, T. R. Reina and J. Liu, J. Energy Chem., 2021, 57, 304–324 CrossRef CAS.
- X. Sun, J. Han and R. Guo, Front. Chem., 2020, 8, 1135–1143 Search PubMed.
- G. M. Ziarani, P. Mofatehnia and F. Mohajer, et al., RSC Adv., 2020, 10, 30094–30109 RSC.
- D. Du, W. Shi and L. Wang, Appl. Catal. B Environ., 2017, 200, 484–492 CrossRef CAS.
- M. Wang, Y. Boyjoo and J. Pan, Chin. J. Catal., 2017, 38, 970–990 CrossRef CAS.
- J. He, L. Luo and Y. Chen, Adv. Mater., 2017, 29, 1702707–1702711 CrossRef PubMed.
- J. Lee, J. C. Park and H. Song, Adv. Mater., 2008, 20, 1523–1528 CrossRef CAS.
- W. S. Choi, H. Y. Koo and D. Y. Kim, Langmuir, 2008, 24, 4633–4636 CrossRef CAS PubMed.
- L. Luo, W. S. Lo and X. Si, et al., J. Am. Chem. Soc., 2019, 141, 20365–20370 CrossRef CAS PubMed.
- Y. Zhu, E. Kockrick and T. Ikoma, Chem. Mater., 2009, 21, 2547–2553 CrossRef CAS.
- J. Yang, Y. Wang and S. Chou, Nano Energy, 2015, 18, 133–142 CrossRef CAS.
- P. M. Arnal, M. Comotti and F. Schüth, Angew. Chem., Int. Ed., 2006, 45, 8224–8227 CrossRef CAS PubMed.
- W. Qingqing and L. Wei, Chem.–Eur J., 2018, 24, 15663–15668 CrossRef PubMed.
- C. Dong, L. Li and F. Tang, Adv. Mater., 2009, 21, 3804–3807 CrossRef.
- Q. Zhang, T. Zhang and J. Ge, Nano Lett., 2008, 8, 2867–2871 CrossRef CAS PubMed.
- R. Purbia and S. Paria, Nanoscale, 2015, 7, 19789–19873 RSC.
- Q. Zhang, J. Ge and J. Goebl, Nano Res., 2009, 2, 583–591 CrossRef CAS.
- L. S. Lin, J. Song and H. H. Yang, Adv. Mater., 2018, 30, 1701–4639 Search PubMed.
- J. Liu, S. Z. Qiao and S. Budi Hartono, Angew. Chem., Int. Ed., 2010, 49, 4981–4985 CrossRef CAS PubMed.
- X. J. Wu and D. Xu, J. Am. Chem. Soc., 2009, 131, 2774–2775 CrossRef CAS PubMed.
- K. A. Dahlberg and J. W. Schwank, Chem. Mater., 2012, 24, 2635–2644 CrossRef CAS.
- W. J. Liu, Z. C. Zhang and W. D. He, et al., J. Solid State Chem., 2006, 179, 1253–1258 CrossRef CAS.
- X. Zhang, L. Clime and H. Roberge, J. Phys. Chem. C, 2011, 115, 1436–1443 CrossRef CAS.
- R. Purbia and S. Paria, Nanoscale, 2015, 7, 19789–19873 RSC.
- Z. Huang, L. L. Fan and B. Chen, J. Mater. Chem. A, 2021, 9, 3976–3984 RSC.
- L. Qi, J. Guo and Z. Hai, Small, 2019, 15, 1804874–1804881 CrossRef PubMed.
- Z. A. Qiao, P. Zhang and S. H. Chai, J. Am. Chem. Soc., 2014, 136, 112601–112607 Search PubMed.
- Z. Xiue and C. Ming, ACS Nano, 2017, 11, 8429–8436 CrossRef PubMed.
- C. Dai, A. Zhang and J. Li, Chem. Commun., 2014, 50, 4846–4848 RSC.
- L. Hui, G. Hong and B. Liu, Adv. Funct. Mater., 2018, 1707480–1707488 Search PubMed.
- X. Lu, A. Liu and Y. Zhang, ACS Appl. Energy Mater., 2020, 3, 11153–11163 CrossRef CAS.
- W. Li, R. Zhao and K. Zhou, J. Mater. Chem. A, 2019, 7, 8443–8450 RSC.
- S. N. Shmakov and E. Pinkhassik, Chem. Commun., 2010, 46, 7346–7348 RSC.
- S. N. Shmakov, Y. Jia and E. Pinkhassik, Chem. Mater., 2014, 26, 1126–1132 CrossRef CAS.
- Y. Sun, B. Wiley and Z. Y. Li, J. Am. Chem. Soc., 2004, 126, 9399–9406 CrossRef CAS PubMed.
- S. Xie, M. Jin and J. Tao, Chem.–Eur. J., 2012, 18, 14974–14980 CrossRef CAS PubMed.
- W. Wang, M. Dahl and Y. Yin, Chem. Mater., 2013, 25, 1179–1189 CrossRef CAS.
- C. M. Cui, X. H. Guo, Y. M. Geng, T. T. Dang, G. Xie, S. P. Chen and F. Q. Zhao, Chem. Commun., 2015, 51, 9276–9279 RSC.
- J. G. Railsback, A. C. Johnston-Peck and J. Wang, ACS Nano, 2010, 4, 1913–1920 CrossRef CAS PubMed.
- Y. A. Chen, Y. T. Wang and H. S. Moon, RSC Adv., 2021, 11, 12288–12305 RSC.
- J. Zhong, C. Cao and H. Liu, Ind. Eng. Chem. Res., 2013, 52, 1303–1308 CrossRef CAS.
- Z. Li, D. A. Blom and W. Hui, Chem. Mater., 2011, 23, 4587–4598 CrossRef.
- J. Sun, J. Hu and J. Han, Langmuir, 2019, 35, 1–30 CrossRef PubMed.
- X. Du, C. Zhao and Y. Luan, J. Mater. Chem. A, 2017, 5, 21560–21569 RSC.
- Y. Zhuang, S. Yuan and J. Liu, Chem. Eng. J., 2019, 379, 122262–122270 CrossRef.
- Q. C. Do, D. G. Kim and S. O. Ko, Environ. Res., 2019, 171, 92–100 CrossRef CAS PubMed.
- G. Yuan, J. Sun and X. Sun, Colloids Surf., A, 2021, 623, 126665–126672 CrossRef CAS.
- A. Acharya, A. Kumar and I. S. Lee, Bull. Korean Chem. Soc., 2021, 42, 915–918 CrossRef CAS.
- S. Gao, L. Zhang and H. Yu, Carbon, 2021, 175, 307–311 CrossRef CAS.
- Y. Li, B. Pei and J. Chen, J. Colloid Interface Sci., 2021, 591, 273–280 CrossRef CAS PubMed.
- Y. Guo, L. Feng and C. Wu, ACS Appl. Mater. Interfaces, 2019, 11, 33978–33986 CrossRef CAS PubMed.
- R. Cai, H. Jin and D. Yang, Nano Energy, 2020, 71, 104542–104548 CrossRef CAS.
- F. Shaik, ChemNanoMat, 2020, 6(10), 1449–1473 CrossRef CAS.
- Y. Lu, D. Guo and Y. Ruan, J. CO2 Util., 2018, 24, 190–199 CrossRef CAS.
- S. Prabu and K. Y. Chiang, J. Colloid Interface Sci., 2021, 604, 584–595 CrossRef CAS PubMed.
- Z. Lv, X. Tan and C. Wang, Chem. Eng. J., 2020, 389, 123428–123436 CrossRef CAS.
- L. Yu, P. Pan and Y. Zhang, Small, 2019, 1805465–1805473 CrossRef PubMed.
- R. Dai, Z. Zheng and C. Lian, Int. J. Energy Res., 2019, 43, 2075–2085 CrossRef CAS.
- M. Lv, Y. Zhang and Q. Xin, Chem. Eng. J., 2020, 396, 125274–125285 CrossRef CAS.
- S. Liu, Y. Lin and W. Guo, Chin. J. Polym. Sci., 2020, 38, 847–852 CrossRef CAS.
- A. Acharya, A. Kumar and I. S. Lee, Bull. Korean Chem. Soc., 2021, 42, 915–918 CrossRef CAS.
- Y. Wang, C. Yang and A. Chen, Appl. Catal. B Environ., 2019, 251, 57–65 CrossRef CAS.
- M. Ma, Y. Yang and W. Li, J. Alloys Compd., 2019, 810, 151807–151816 CrossRef CAS.
- H. Sun, Q. He and P. She, J. Colloid Interface Sci., 2017, 505, 884–891 CrossRef CAS PubMed.
- Y. Zhang, Y. Zhao and J. Li, Inorg. Chem. Front., 2018, 5(12), 3082–3090 RSC.
- Y. Zhang, L. Wang and M. Yang, Appl. Surf. Sci., 2019, 466, 515–524 CrossRef CAS.
- J. Zhao, S. Tian and H. Shi, Adv. Mater. Interfac., 2019, 6, 1801933–1801945 CrossRef CAS.
- J. Feng, J. Liu and X. Cheng, Adv. Sci., 2018, 5, 1700376–1700382 CrossRef PubMed.
- J. Lu, L. Lan and X. T. Liu, Front. Chem. Sci. Eng., 2019, 13, 665–671 CrossRef CAS.
- Q. Li, F. Wang and L. Sun, Nano-Micro Lett., 2017,(9), 131–139 Search PubMed.
- Z. A. Jian, B. Wl and B. Hl, Adv. Powder Technol., 2019, 30, 1965–1975 CrossRef.
- C. Rui, J. Lu and S. Liu, J. Mater. Sci., 2018, 53, 1–10 CrossRef.
- Z. Zhao, J. Liu and M. Qin, J. Nanosci. Nanotechnol., 2019, 20, 3140–3147 CrossRef PubMed.
- Y. Lv, L. Yue and I. M. Khan, Nano Res., 2021, 14, 2363–2371 CrossRef CAS.
- M. Farbod and L. Sharif, Phys. B, 2021, 613, 412729–412736 CrossRef CAS.
- I. Khan, N. Sun and Y. Wang, Mater. Res. Bull., 2020, 127, 110857–110863 CrossRef CAS.
- Y. Xiang, Y. Huang and B. Xiao, Appl. Surf. Sci., 2020, 513, 145820–145831 CrossRef CAS.
- Y. Xing, S. Tang and X. Du, Nano Res., 2020, 14, 1–6 Search PubMed.
- W. Wang, S. Zhu and Y. Cao, Adv. Funct. Mater., 2019, 29, 1901958–1901968 CrossRef.
- H. Zhang, Y. Ma and Z. Liu, Colloids Surf., A, 2021, 629, 127451–127460 CrossRef CAS.
- G. D. Moon, Nanomaterials, 2020, 10, 675–698 CrossRef CAS PubMed.
- J. Li, L. Chen and R. Ye, Appl. Surf. Sci., 2020, 507, 145103–145109 CrossRef CAS.
- B. Wang, Y. Ye and L. Xu, Adv. Funct. Mater., 2020, 30, 2005834–2005841 CrossRef CAS.
- Z. Kun, Z. ZX and R. ZW, ChemElectroChem, 2021, 8, 1605–1611 CrossRef.
- M. Yang, D. Wu and D. Cheng, Int. J. Hydrogen Energy, 2019, 44, 6525–6534 CrossRef CAS.
- G. Mei, Electrochim. Acta, 2018, 290, 82–89 CrossRef CAS.
- A. Annamalai, D. V. Shinde and J. Buha, J. Mater. Chem. A, 2021, 9, 10385–10392 RSC.
- L. Lv, Y. Gan and H. Wan, Int. J. Hydrogen Energy, 2021, 46, 28387–28396 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |