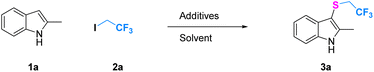Open Access Article
Open Access ArticleDirect fluoroalkylthiolation of indoles with iodofluoroethane enabled by Na2S2O4†
Nianhua Yu,
Jianjian Huang and
Faqiang Leng *
*
School of Pharmaceutical Sciences, Capital Medical University, Beijing 100069, P. R. China. E-mail: fqleng@ccmu.edu.cn
First published on 3rd January 2023
Abstract
In this paper, we report an efficient approach for the direct fluoroalkylthiolation of indoles with iodofluoroethane in the presence of Na2S2O4. In this work, we employed readily available iodofluoroethane and Na2S2O4 as fluoroalkylthiolation reagents, featuring mild conditions and a wide range of indole substrates. In addition, fluoroalkylthiolated 2,3′-biindole derivatives can also be prepared by this method.
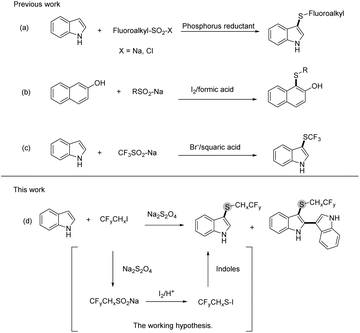
Fluoroalkylthio groups, owing to their unique properties such as high lipophilicity and strong electron-withdrawing effects, have been viewed as vital building blocks, which are widely applied to design and develop pharmaceutical agents and agricultural chemicals.1–4 A considerable amount of effort has been expended on the development of fluoroalkylthio group introduction method, particularly for devising novel fluoroalkylthiolation reagents.5,6 With regard to electrophilic CF3S-donors, trifluoromethanesulfinate CF3SO2–X(Na, Cl) was often used for trifluoromethylthiolation in the presence of reducing agents.7,8 For the reductive reagents, different phosphorus agents (PPh3,9 PCl3,10 and PMe3 (ref. 11)) were evaluated in the deoxygenation of sulfinate (Scheme 1a).12,13 Although many methods have been devised to fluoroalkylthiolation, some of these protocols are limited by reaction conditions such as air-sensitive fluoroalkylthiolation reagents and toxic phosphorus reductants. Therefore, the development of a sustainable and effective approach for fluoroalkylthiolation is still of great importance.
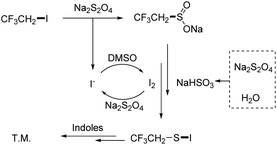
In the study of the reduction transformation, we have seen phosphorus reductants as the major player; however, the construction of novel reducing agents to replace phosphorus reductants was seldom described. Previous studies on the fluoroalkylthiolation mechanism have explained that the transformation was governed by the formation of Rf–S–Cl/Br/I intermediates.9,14–16 For this reason, halogen was commonly involved in all developed fluoroalkylthiolation systems. The cooperation of halogen and proton was found as the reducing system in the deoxygenation of sulfoxides;17,18 by contrast, the halogen-induced deoxygenation of fluoroalkyl-SO2–X is relatively difficult (Scheme 1b).19–21 Jiang and Yi group have reported that fluoroalkylsulfenylation of nucleophiles with Rf-SO2–X was promoted by the collaboration of cetyltrimethyl ammonium bromide and squaric acid (Scheme 1c).22 Our previous work has also found that a small amount of trifluoroethylthiolated product was formed by the CHxFyCH2I/Na2S2O4 system without using phosphine reductants.23 As a deduction, we envisioned that the reactive species CF3CH2S–I could also be conceivably produced by the collaboration of CHxFyCH2I and Na2S2O4, via the deoxygenation of R–SO2–X with self-generated I2/acid (Scheme 1d). In addition, iodofluoroethane and Na2S2O4 are inexpensive and readily available compounds, which are viewed as the idea source of fluoroalkylthio groups.
The reaction conditions were optimized for the trifluoroethylthiolation of 2-methyl indole with CF3CH2I and Na2S2O4 (Table 1). First, the solvent effect was examined, it was found that DMSO gave a better result (Table 1 entries 1–4). Considering the poor solubility Na2S2O4, the mixed solvent system was investigated. The yield of desired product will slightly increase by addition of little water, but the reaction will be terminated by large amount of water (ESI-Table S1†). The third mixture solvents were investigated further, and we found that the yield of resulting products varied with the change of solvent component. The solvent consisting of DMSO/CH3CN/H2O = 1/2/1 in volume gave the best result in yields of 55% (entry 5, ESI Table S1†). Using this solvent, optimisation of the additives was carried out, a variety of sulfinate additives including Na2S2O5, Na2S2O3, and thiourea dioxide were screened (entries 7 and 9–11, ESI Table S2†). It was identified that Na2S2O4 was the most effective reagent for trifluoroethylthiolation. We next examined the quantity of Na2S2O4 (entries 12–16), and it was unexpected to find that the slightly high loading amounts of Na2S2O4 result in a dramatic influence on the yield. The observation revealed that 9.0 equiv. Na2S2O4 presents the best effect for this reaction with a yield of 99%. Finally, the effects of other additives, temperature and time were also tested (see ESI†). However, no better yield was gained. Gratifyingly, the optimum reaction conditions were obtained as follows: 2-methyl indole (1.0 equiv.) with CF3CH2I (3.0 equiv.), Na2S2O4 (9.0 equiv.), DMSO/CH3CN/H2O = 1/2/1 (3 ml), 80 °C, and 4 h.
| Entry | Additives | Solvent | Yielda (%) |
|---|---|---|---|
| a Reaction conditions: 2-methyl-1H-indole (0.3 mmol), CF3CH2I (0.9 mmol), Na2S2O4 (0.9 mmol), solvent (ratio of volume), 80 °C for 4 h, 19F-NMR yields using PhCF3 as an internal standard.b Na2S2O4 (5.0 equiv.).c Na2S2O4 (7.0 equiv.).d Na2S2O4 (9.0 equiv.).e Isolated yield. | |||
| 1 | Na2S2O4 | DMSO | 9 |
| 2 | Na2S2O4 | H2O | n.d. |
| 3 | Na2S2O4 | CH3CN | n.d. |
| 4 | Na2S2O4 | DMF | Trace |
| 5 | Na2S2O4 | DMSO/H2O (1/1) | 14 |
| 6 | Na2S2O4 | DMSO/CH3CN/H2O (1/1/1) | 45 |
| 7 | Na2S2O4 | DMSO/CH3CN/H2O (1/2/1) | 55 |
| 8 | Na2S2O4 | DMSO/CH3CN/H2O (1/3/1) | 23 |
| 9 | Na2S2O5 | DMSO/CH3CN/H2O (1/2/1) | Trace |
| 10 | Na2S2O3 | DMSO/CH3CN/H2O (1/2/1) | Trace |
| 11 | Thiourea dioxide | DMSO/CH3CN/H2O (1/2/1) | 15 |
| 12b | Na2S2O4 | DMSO/CH3CN/H2O (1/2/1) | 61 |
| 13c | Na2S2O4 | DMSO/CH3CN/H2O (1/2/1) | 83 |
| 14d | Na2S2O4 | DMSO/CH3CN/H2O (1/2/1) | 99 (90)e |
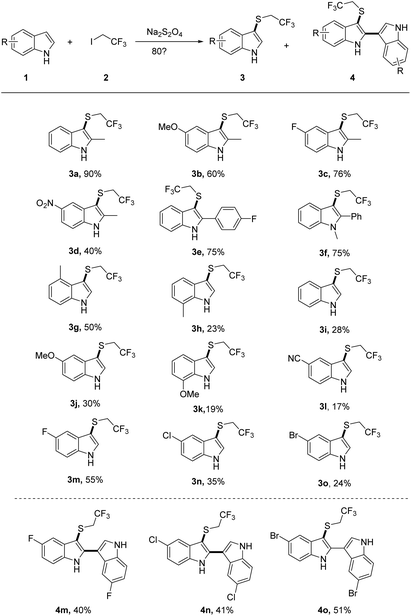
We first investigated the substrate scope of indoles 1 with CF3CH2I 2a (Scheme 2). Diverse functional groups of substituted indoles including methyl, methoxyl, halogen, nitro and nitrile (3 h) functional groups can all be well survived, affording the desired product with moderate to good yields. It was worth mentioning that 2-methyl-5-nitro-1H-indole with a strong withdrawing group (–NO2) gave a moderate yield of 40% of desired product (3d). The electron poor aryl group was also well-tolerated, with the formation of the desired indoles 3e in 75% yields. The reactivity of halogen substituted indoles showed a slightly decreasing tendency with the following order, F (3m) > Cl (3n) > Br (3o). Notably, the 2-position-substituted indoles have a higher yield than one unsubstituted.
To gain more insights into trifluoroethylthiolation of 2-position unsubstituted indoles, we carefully examined the reaction of halogen (F, Cl, and Br)-substituted indoles and CF3CH2I (Scheme 2). Interestingly, we found that the additional product of 3-trifluoroethylthiolated 2,3′-biindoles (4m, 4n, 4o) were formed with a moderate yield, probably because halogen(I) was created in the course of sulfination, thereby leading to the formation of 2,3′-biindoles.24,25 From further evaluation of the reaction, we found that it has less effects to improve the yield of 4 if one increases the loading of indoles.
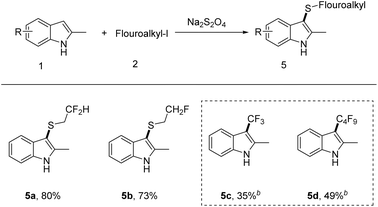
Next, we turned our attention to investigate the scope of iodofluoroalkane 2 (Scheme 3). The monofluoro/difluoro-iodoethane can readily undergo transformation, delivering the corresponding products in yields of 80 and 73%, respectively. Unfortunately, the reaction failed with perfluoroalkyl iodide, which only led to perfluoroalkylation products (5c and 5d).
According to the experiment results (ESI Fig. S1†) and the previous report,19–21 a possible reaction pathway was depicted in Scheme 4. The sulfination of CF3CH2I yielded CF3CH2SO2Na with Na2S2O4, accompanied by the formation of I− ions. Meanwhile, the rest of Na2S2O4 heated with water decomposed to NaHSO3. Then, CF3CH2SO2Na was deoxygenated in the presence of NaHSO3/I2, which could subsequently be converted into CF3CH2S–I. Finally, the desired product was formed by the reaction of CF3CH2S–I of indoles.
Conclusions
In summary, the novel fluoroethylthiolation protocol has been demonstrated establishing iodofluoroethane/Na2S2O4 as the powerful tool for fluoroethylthiolation of indoles and construction of 2,3′-biindoles. Various indole derivatives underwent smoothly by this mild and simple system. We proposed a possible mechanism of fluoroethylthiolation. The protocol paves an alternative route for the incorporation of fluoroethylthiol groups along the way toward the development of metal- and phosphorus-free systems.Author contributions
N. Yu and J. Huang prepared and characterized the fluoroalkylthiolated indoles. F. Leng conceived the idea of fluoroalkylthiolation synthesis, analysed the results and coordinated the work.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from Beijing Natural Science Foundation (2194070) and R&D Program of Beijing Municipal Education Commission (KM202210025025) are gratefully acknowledged.Notes and references
- B. M. Johnson, Y.-Z. Shu, X. Zhuo and N. A. Meanwell, J. Med. Chem., 2020, 63, 6315–6386 CrossRef CAS PubMed.
- J. Xin, T. Chen and P. Tang, Org. Lett., 2022, 24, 2035–2039 CrossRef CAS PubMed.
- P. Xiao, X. Pannecoucke, J.-P. Bouillon and S. Couve-Bonnaire, Chem. Soc. Rev., 2021, 50, 6094–6151 RSC.
- J. Yan, H. Tang, E. J. R. Kuek, X. Shi, C. Liu, M. Zhang, J. L. Piper, S. Duan and J. Wu, Nat. Commun., 2021, 12, 7214 CrossRef CAS.
- X.-H. Xu, K. Matsuzaki and N. Shibata, Chem. Rev., 2015, 115, 731–764 CrossRef CAS PubMed.
- M. Hamzehloo, A. Hosseinian, S. Ebrahimiasl, A. Monfared and E. Vessally, J. Fluorine Chem., 2019, 224, 52–60 CrossRef CAS.
- L. Jiang, J. Qian, W. Yi, G. Lu, C. Cai and W. Zhang, Angew. Chem., Int. Ed., 2015, 54, 14965–14969 CrossRef CAS.
- Q. Yan, L. Jiang, W. Yi, Q. Liu and W. Zhang, Adv. Synth. Catal., 2017, 359, 2471–2480 CrossRef CAS.
- M.-J. Bu, G.-P. Lu and C. Cai, Org. Chem. Front., 2017, 4, 266–270 RSC.
- D.-W. Sun, X. Jiang, M. Jiang, Y. Lin and J.-T. Liu, Eur. J. Org. Chem., 2017, 2017, 3505–3511 CrossRef CAS.
- H. Chachignon, M. Maeno, H. Kondo, N. Shibata and D. Cahard, Org. Lett., 2016, 18, 2467–2470 CrossRef CAS.
- A. Ghosh, M. Lecomte, S.-H. Kim-Lee and A. T. Radosevich, Angew. Chem., Int. Ed., 2019, 58, 2864–2869 CrossRef CAS PubMed.
- F. Xiao, H. Xie, S. Liu and G.-J. Deng, Adv. Synth. Catal., 2014, 356, 364–368 CrossRef CAS.
- T. Cellnik and A. R. Healy, J. Org. Chem., 2022, 87, 6454–6458 CrossRef CAS PubMed.
- Y. Xiao, Y. Jia, J. Huang, X. Li, Z. Zhou, J. Zhang, M. Jiang, X. Zhou, Z.-X. Jiang and Z. Yang, Adv. Synth. Catal., 2022, 364, 738–743 CrossRef CAS.
- X. Zhao, A. Wei, T. Li, Z. Su, J. Chen and K. Lu, Org. Chem. Front., 2017, 4, 232–235 RSC.
- F. Wang, G.-P. Lu and Y. Lin, Tetrahedron Lett., 2021, 70, 153015 CrossRef CAS.
- M. Abbasi, M. R. Mohammadizadeh and Z. Moradi, Tetrahedron Lett., 2015, 56, 6610–6613 CrossRef CAS.
- D. Equbal, R. Singh, S. Malik, A. G. Lavekar and A. K. Sinha, J. Org. Chem., 2019, 84, 2660–2675 CrossRef CAS.
- X.-l. He, S. Majumder, J. Wu, C. D. Jin, S.-R. Guo, Z.-p. Guo and M. Yang, Org. Chem. Front., 2019, 6, 2435–2440 RSC.
- F. Xiao, S. Chen, J. Tian, H. Huang, Y. Liu and G.-J. Deng, Green Chem., 2016, 18, 1538–1546 RSC.
- H. Xiang, J. Liu, J. Wang, L. Jiang and W. Yi, Org. Lett., 2022, 24, 181–185 CrossRef CAS.
- F. Leng, J. Huang, N. Yu and G. Wang, Tetrahedron Lett., 2021, 85, 153488 CrossRef CAS.
- Y.-X. Li, K.-G. Ji, H.-X. Wang, S. Ali and Y.-M. Liang, J. Org. Chem., 2011, 76, 744–747 CrossRef CAS PubMed.
- P. Huang, X. Peng, D. Hu, H. Liao, S. Tang and L. Liu, Org. Biomol. Chem., 2017, 15, 9622–9629 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ra07430c |
| This journal is © The Royal Society of Chemistry 2023 |