 Open Access Article
Open Access ArticleIron/iron oxide-based magneto-electrochemical sensors/biosensors for ensuring food safety: recent progress and challenges in environmental protection
Mina Adampourezare*a,
Mohammad Hasanzadeh *bc,
Mohammad-Ali Hoseinpourefeizia and
Farzad Seidi
*bc,
Mohammad-Ali Hoseinpourefeizia and
Farzad Seidi d
d
aDepartment of Biology, Faculty of Natural Science, University of Tabriz, Tabriz, Iran. E-mail: adampourezare@gmail.com
bPharmaceutical Analysis Research Center, Tabriz University of Medical Sciences, Tabriz, Iran. E-mail: hasanzadehm@tbzmed.ac.ir
cNutrition Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
dJiangsu Co-Innovation Center for Efficient Processing and Utilization of Forest Resources and International Innovation Center for Forest Chemicals and Materials, Nanjing Forestry University, Nanjing, 210037, China
First published on 4th May 2023
Abstract
Foodborne diseases have arisen due to the globalization of industry and the increase in urban population, which has led to increased demand for food and has ultimately endangered the quality of food. Foodborne diseases have caused some of the most common public health problems and led to significant social and economic issues worldwide. Food quality and safety are affected by microbial contaminants, growth-promoting feed additives (β-agonists and antibiotics), food allergens, and toxins in different stages from harvesting to storage and marketing of products. Electrochemical biosensors, due to their reduced size and portability, low cost, and low consumption of reagents and samples, can quickly provide valuable quantitative and qualitative information about food contamination. In this regard, using nanomaterials can increase the sensitivity of the assessment. Magnetic nanoparticle (MNP)-based biosensors, especially, are receiving significant attention due to their low-cost production, physicochemical stability, biocompatibility, and eco-friendly catalytic characteristics, along with magnetic, biological, chemical and electronic sensing features. Here, we provide a review on the application of iron-based magnetic nanoparticles in the electrochemical sensing of food contamination. The types of nanomaterials used in order to improve the methods and increase the sensitivity of the methods have been discussed. Then, we stated the advantages and limitations of each method and tried to state the research gaps for each platform/method. Finally, the role of microfluidic and smartphone-based methods in the rapid detection of food contamination is stated. Then, various techniques like label-free and labelled regimes for the sensitive monitoring of food contamination were surveyed. Next, the critical role of antibody, aptamer, peptide, enzyme, DNA, cells and so on for the construction of specific bioreceptors for individual and simultaneous recognition by electrochemical methods for food contamination were discussed. Finally, integration of novel technologies such as microfluidic and smartphones for the identification of food contaminations were investigated. It is important to point out that, in the last part of each sub-section, attained results of different reports for each strategy were compared and advantages/limitations were mentioned.
1. Introduction
As the world's population grows, food safety is identified as one of the most vital human preferences. The industrialization of food and agriculture is a strategy that guarantees sustained access to food.1 However, food quality and safety are affected by microbial pollutants (such as bacterial pathogens, fungal, and viral contaminants), chemical contaminants (such as pesticides, veterinary drugs including antimicrobials, hormones, and growth-promoting), physical contaminants (such as pieces of plastic, metal, hair, stone, or plant stalks), growth promoting feed additives (β-agonists and antibiotics), food allergens, and toxins2,3 in different stages from harvesting to storage and marketing of products. On the other hand, profiteers are always trying to endanger the health of society because they are looking for different strategies to make more profit by reducing production costs through fraud. Foodborne diseases have arisen due to the globalization of industry and the increase in urban population, which has led to increased demand for food and has ultimately endangered the quality of food. Foodborne diseases have caused some of the most common public health problems and led to significant social and economic issues worldwide.4,5 Unfortunately, millions of people die from unhealthy foods every year.1 Due to the increase in the quantity and variety of food contaminants, the demand for rapid analysis of food samples has increased.Current methods for assessing food safety, such as cell culture, microbiological techniques, and chemical assays, are time consuming and require sample pretreatment steps.6–8 Also; these methods are expensive and insensitive and require considerable scientific expertise.9,10 Powerful analysis techniques such as high-performance liquid chromatography and gas chromatography combined with mass spectrometry detection are also time-consuming and require accurate and expensive tools.11–14 Thus, the growing demand for easy and simple analytical methods that can quickly provide valuable quantitative and qualitative information increases the interest of scientists in using electrochemical biosensors due to their reduced size and portability, low cost and low consumption of reagents and samples.15–19
Biosensing is performed based on an immobilized biological recognition element on the surface of a signal transducer for monitoring of target analytes. Considering the important role of biomolecule immobilization in biosensing evaluation, substrate materials such as the surface of electrodes should be modified with materials with suitable functional groups that effectively attach to target molecules with high binding strength, excellent long-term stability, biocompatibility, and high activity.20 In this context, using nanomaterials lead to increase the surface-to-volume ratio and detection sensitivity.21 In this context, magnetic nanoparticles (MNPs)-based biosensors are receiving significant attention due to their low-cost production, physicochemical stability, biocompatibility, and eco-friendly characteristics,22 catalytic, magnetic, biological, chemical, and electronic sensing features.23–26
MNPs may show many benefits like low toxicity, controllable size, and shape, coating or modification routes, larger surface areas to the volume ratio, capability of promoting quicker electron transfer kinetic between the electrodes and higher catalytic efficiencies.27 Iron oxide nanoparticles of size between 1 nm and 100 nm are mostly used. The most common iron oxide nanoparticles include magnetite (Fe3O4) and maghemite (γ-Fe2O3) nanoparticles. Among these iron oxide nanoparticles, magnetite nanoparticles (MNPs) are extensively studied due to their unique properties.28 Iron-based magnetic nanoparticles by increasing the surface area to immobilize biomolecules, lead to a decrease in the detection limit and play an important role in the monitoring analytes, especially in complex matrices. They eliminate the need for sample pretreatment using centrifuge or chromatography, thus reducing the reaction time, which may indicate poor mass transfer to the biosensor or physical blockage of the biosensor surface by non-specific adsorption.29 Most of iron-based magnetic materials (MNPs), especially iron oxides, are biocompatible and non-genotoxic; so, they can be applied widely in biosensing devices for simple adsorption of biomolecules, functionalized or encapsulated in polymers, metal or silica NPs, carbon-based materials to enhance the biocompatibility and increase the functionalities.30 They can be easily functionalized with different surface functionalities including hydroxyl, aldehyde, carboxyl, amine, thiol or tosyl, and further biomolecule modification (such as antibodies, oligonucleotides, proteins). These features make them a valuable case for the recognizing a variety of targets with high specificity.31
Various types of electrochemical tests can be used to evaluate these targets, such as cyclic voltammetry (CV), differential pulse voltammetry (DPV), square wave voltammetry (SWV), chronoamperometry (CA), electrochemical impedance spectroscopy (EIS), linear sweep voltammetry (LSV). Also, different types of electrodes can be used such as glassy carbon electrode (GCE), screen-printed carbon electrode (SPCE), indium tin oxide electrode (ITO), magnetic glassy carbon electrode (MGCE), streptavidin modified screen printed electrode (STE), gold electrode (GE), screen-printed gold electrode (SPCAu), and etc.32
Different types of chemical bonds such as hydrogen, van der Waals, covalent, electrostatic, and dipole ion bonds can be formed between different nanomaterials, as well as between nanomaterials with the electrode surface on one side and target molecules on the other side.33–49
In this review article, application of magnetic biosensors for the electrochemical recognition of different types of food contamination, such as microbial contamination, allergen, pesticide, genetically modified organisms, growth-promoting feed additives (β-agonists and antibiotics), toxin, other contaminants (estradiol, melamine, bisphenol, heavy metal, and etc.) have been investigated. Then, the role of various technique like label-free and labelled regime for the sensitive monitoring of food contamination were surveyed. Next, the critical role of antibody, aptamer, peptide, enzyme, DNA, cell and so on for the construction of specific bioreceptors for the individual and simultaneous recognition by electrochemical method of food contamination were discussed. Finally, integration of novel technologies such as microfluidic and smartphones for the identification of food contaminations were investigated. It is important to point out that, on the last pant of each sub-section attained results of different report for each strategy were compared and advantage and limitation were mentioned.
Table 1 ref. 50–175 summarizes the types of food contamination studied using the electrochemical sensors based on iron magnetic beads.
| Type of contamination | Real sample | Analyte | Nanomaterial | Range of concentration | Limit of detection | Ref. |
|---|---|---|---|---|---|---|
| Microbial | Water and apple juice | Escherichia coli (E. coli), Staphylococcus aureus (S. aureus) and Salmonella typhimurium (S. typhi) | magnetite nanoparticles and antimicrobial peptide melittin (MLT) | 1 CFU mL−1 in potable water and 3.5 CFU mL−1 in apple juice | 50 | |
| Milk | Salmonella typhimurium | magnetic-gold core/shell nanoparticles (Fe@Au) and CdS NCs | 0.1 to 500 cells per mL | 13 cells per mL | 51 | |
| — | Gram-negative bacterial quorum signaling molecules | Fe3O4@SiO2 | 2.5 × 10−9 mol L−1 to 1.0 × 10−7 mol L−1 | 8 × 10−10 mol L−1 | 52 | |
| — | Escherichia coli O157:H7 and Salmonella typhimurium | Magnetite bead | 102–106 cfu mL−1 | 2.05 × 103 CFU g−1 and 1.04 × 103 cfu mL−1 for E. coli O157:H7 and S. typhimurium | 62 | |
| Food/water | E. coli O157:H7 | Magnetite nanoparticle | 101 to 106 CFU mL | — | 63 | |
| Listeria | Magnetic nanoparticles (MNPs) gold nanoparticles (AuNPs) | 1.6 × 102 CFU mL−1 | 1.6 × 102 CFU mL−1 | 65 | ||
| Seafood | Vibrio parahaemolyticus | MoS2 nanosheets | 10–106 CFU mL−1 | 5.74 CFU mL−1 | 66 | |
| Escherichia coli O157:H7 and Staphylococcus aureus | Alumina nanoporous | — | 102 CFU mL−1 | 67 | ||
| Allergen | Ovalbumin | Magnetic particles functionalized with specific anti-ovalbumin immunoglobulin G | 11 to 222 nM | 5 nM | 75 | |
| Foodstuffs | Shrimp and fish | Fe3O4–SiO2-fluorescein isothiocyanate (FITC)-lipidosome nanoparticles | 1.6 × 103 to 1.6 × 1010 cells per mL | 0.03 μg mL−1 (for shrimp) and 0.16 ng mL−1 (for fish) | 63 | |
| Potatoes | Acrylamide | Iron magnetic-chitosan-hemoglobin nanoparticles | 10 to 171 nmol L−1 | 0.06 nmol L−1 | 76 | |
| Wine sample | Egg allergen ovalbumin | Poly(diallyldimethylammonium chloride), GO, magnetic beads | 0.01 to 10 pg mL−1 | 0.2 fg mL−1 | 78 | |
| Pesticide | — | Mesotrione | Yttrium iron garnet (Y3Fe5O12; YIG) and graphitic carbon nitride (GCN) nanoparticles | — | 950 pM | 83 |
| Sudan I | Magnetic Fe3O4 nanoparticles | 0.01 to 1 μM and 1 to 20 μM | 0.001 μM | 84 | ||
| Food sample | Dichlorodiphenyltrichloroethane (DDT) | Magnetic Fe3O4 and polydopamine | 1 × 10−11 to 1 × 10−3 mol L−1 | 6 × 10−12 mol L−1 | 85 | |
| Vegetable sample | Acephate, trichlorfon | Fe3O4@carboxyl-functionalized multiwalled carbon nanotubes/chitosan nanocomposite | 1.0 × 10−4 to 1.0 × 10−10, 1.0 × 10−5 to 1.0 × 10−11 M | 6.81 × 10−11 M for acephate and 8.94 × 10−12 M for trichlorfon | 86 | |
| Methyl parathion | (SiO2NPs) and CNTs | 0.3–20.0 μM and 20.0–150.0 μM | 0.092 μM | 99 | ||
| Genetically modified organisms (GMO) | — | Soybean gene | Core–shell Fe3O4@Au magnetic nanoparticles | 0.1 to 10.0 nM | 0.02 nM | 105 |
| GMO | MON810 maize | — | Magnetic core–shell Fe3O4@Au | 0.25–2.5 nM | 0.15 nM | 106 |
| GMO | Tomato | Cauliflower mosaic virus 35S (CaMV35S) gene | cMWCNTs and Au@Ag–Fe3O4 | 1 × 10−16 M to 1 × 10−10 M | 1.26 × 10−17 M | 107 |
| GMO | Maize | Maize taxon-specific (HMGA gene) | Fe3O4@Au | 0.5 to 5 nM | 90 pM | 108 |
| Growth promoting feed additives (β-agonists) | — | Ractopamine (a β-adrenergic agonist) | Iron oxide magnetic nanoparticles/graphene oxide (Fe3O4/rGO) | 0.05–10 and 10–100 μM | 13 nM | 92 |
| Antibiotic | — | Streptomycin | Graphene composite–Fe3O4–AuNPs- porous carbon nanorods (PCNR) | 0.05–200 ng mL−1 | 0.028 ng mL−1 | 139 |
| — | Streptomycin | Au@MWCNTs–Fe3O4/NP-PtTi | 0.05 to 100 ng. mL−1 | 7.8 pg. mL−1 | 138 | |
| — | Penicillin | NP-PtTi/GR-Fe3O4/MWCNT-Fe3O4 | 0.05 to 100 ng mL−1 | 25.3 pg mL−1 | 137 | |
| Milk | Oxytetracycline | Fe3O4@mesoporous carbon foe | 0.005 to 1.0 ng mL−1 | 0.027 pg mL−1 | 136 | |
| — | Chloramphenicol | Magnetite Fe3O4 nanoparticles | 0.09–47 μM | 0.09 μM | 129 | |
| Milk | Tetracycline | Carboxyl-Fe3O4 nanoparticle (MNPs) and chitosan (CS) as linker | 0.08 to 1 ng mL−1 | 0.0321 ng mL−1 | 130 | |
| — | Tetracycline | Magnetic nanoparticles modified with tetraethylorthosilicate and trimethoxysilyl propyl methacrylate | — | — | 131 | |
| — | Kanamycin | Ag@Fe3O4 NPs | 0.050 to 16 ng mL−1 | 15 pg mL−1 | 132 | |
| Chloramphenicol | Fe3O4 magnetic nanoparticles | 0.09 to 47 μM | 0.09 μM | 133 | ||
| In drug, milk, honey and blood serum sample | Tetracycline | Fe3O4 magnetic nanoparticles and oleic acid | 1.0 × 10−12–1.0 × 10−7 M and 3.0 × 10−13 M | 2.9 × 10−11 M | 134 | |
| — | Kanamycin | Fe3O4 nanoparticles, multi-walled carbon nanotubes (MWCNTs) | 1.0 × 10−10 mol L−1 to 1.0 × 10−6 mol L−1 | 2.3 × 10−11 mol L−1 | 135 | |
| Food | Tetracycline and streptogramin | 9.22 and 6.33 ng mL−1 | 141 | |||
| Toxin | Food sample | Tetrodotoxin | Fe3O4–Au modified by poly-(ethylenimine) (BPEI) functionalized graphene nanosheets | 0.01–100 ng mL | — | 142 |
| Corn sample | Aflatoxin B1 | CdS–Fe3O4 nanocomposites | 0.01 ng mL−1 to 80 ng mL−1 | 5.0 pg mL−1−1 | 147 | |
| Cereal sample | Aflatoxin B1 | Au–Fe3O4 | 0.05 to 5 ng mL−1 | 0.07 ng mL−1 | 148 | |
| Fish | Scombrotoxin (histamine) | Histamine magnetic-MIP, 2-vinyl pyridine | — | 1.6 × 10−6 mg L−1 | 100 | |
| Cow milk | Aflatoxin M1 | Fe3O4/PANi | 6–60 ng L−1 | 1.98 ng L−1 | 149 | |
| Milk | Staphylococcal enterotoxin B | Polyaniline nano-gold composite and 1,2-dimethyl-3-butylimidazolium hexafluorophosphate ionic liquid, magnetosomes (Fe3O4) | 0.05 to 5 ng mL−1 | 0.017 ng mL−1 | 150 | |
| Nucleotide | fish meat, tea and soft drinks | Xanthine and theophylline | Fe3O4/SWCNTs | 4.0 nM–300.0 μM and 0.1–300.0 μM for Xanthine and Theophylline | — | 169 |
| Heavy metal | — | Cd(II) and Pb(II) | Carboxyl iron oxide nanoparticle | 10−100 ppb | 0.90 and 0.60 ppb | 172 |
| Local tap water | Zn(II), Cd(II), and Pb(II) | Bismuth plating and iron oxide nanoparticle/graphene | 1–100 μg L−1 | 0.11, 0.08, and 0.07 ppb | 173 | |
| Environmental water | Cd(II) and Pb(II) | Polyamidoamine dendrimer, iron oxide nanoparticle | 0.5 to 80 ng mL−1 | 0.21 and 0.17 ppb | 174 | |
| River water and soybean | Cd(II), Pb(II), Cu(II), and Hg(II) | Fluorinated multiwalled carbon nanotube (MWCNT), iron oxide nanoparticle | 0.5–30.0, 0.5–30.0, 0.5–30.0, and 0.5–20.0 μM for Cd2+, Pb2+, Cu2+, and Hg2+, respectively | 0.05, 0.08, 0.02, and 0.05 nM | 175 |
2. Iron and iron-oxide magnetic nanoparticle-based electrochemical biosensors for the detection of microbial contamination in food sample
Foodborne illnesses caused by microbial pathogens represent serious health problems and even death. Therefore, for early diagnosis and effective treatment, it is critical the detection of a specific bacteria strain, especially at very low concentrations (fM or zM).34 The statistics and food poisoning caused by five species of bacteria in 2000 in the United States and the resulting adult medical costs ($6.9 billion) and decrease in productivity and premature child death incidents can show the actual facts and dimensions of the food health challenge.35The most reported cases of gastrointestinal disease in the United States in 2013 were associated with Campylobacter spp., with mortality rate of 15.6%.36 In 2012, the largest report of foodborne illness in the EU was related to the outbreak of norovirus, which affected 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 950 people.37 The most susceptible populations to food-borne diseases include the very young, the elderly, the immune-compromised,38 solid organ transplant patients,39 and patients with immunodeficiency or undergoing immunosuppressive therapy.40 The other prominent pathogens responsible for food outbreaks include viral pathogens such as the avian influenza viruses and SARS coronavirus, and parasites such as numerous protozoa and parasitic worms.41 Thus, the detection of pathogenic bacteria is crucial.40
950 people.37 The most susceptible populations to food-borne diseases include the very young, the elderly, the immune-compromised,38 solid organ transplant patients,39 and patients with immunodeficiency or undergoing immunosuppressive therapy.40 The other prominent pathogens responsible for food outbreaks include viral pathogens such as the avian influenza viruses and SARS coronavirus, and parasites such as numerous protozoa and parasitic worms.41 Thus, the detection of pathogenic bacteria is crucial.40
Common methods used as the gold standard for detecting bacterial contaminants in foods are including cell culture with analysis of colony-forming units,42 enzyme-linked immunosorbent assays (ELISA),43,44 polymerase chain reaction (PCR),45 and immune-chromatographic lateral flow assays (strip test).46 These methods are usually time-consuming, complex, and expensive.47,48 Therefore, they do not fulfill many industrial requirements of food processing, efficient quality control and shelf-life evaluation.49 Therefore, substantial efforts have been made to develop new technologies that can meet such stringent requirements, and electrochemical biosensors could possibly overcome the limitations of these methods. In this subsection application of various type of magneto-electrochemical sensors and biosensor for the recognition of microbial food contaminations was investigated. Also, the role of detection method/technique and magnetic nanoparticle or nanocomposite for the enhance of sensors performance were discussed. Finally, advances and limitation of different strategies were surveyed.
For example, Wilson and coworkers50 proposed a novel electrochemical method based on magnetite nanoparticles, antimicrobial peptide melittin (MLT) and using silver screen-printed interdigitated electrodes for the detection of Staphylococcus aureus (S. aureus), Escherichia coli (E. coli), and Salmonella Typhimurium in water and apple juice. In this method, MLT as a biorecognition element was immobilized on the surface of MNPs-carboxylated particles, MLT-MNPs-carboxylated particles (Fig. 1A). After the capture of bacteria by MLT-MNPs and magnetic separation, measurements were performed by electrochemical impedance spectroscopy (EIS). This approach can detect E. coli with LOD of 1 CFU mL−1 in potable water and 3.5 CFU mL−1 in apple juice, within 25 min. Also, Freitas et al.51 reported a labeled electrochemical sandwich immunosensor for detecting Salmonella typhimurium in milk using magnetic-gold core/shell nanoparticles (Fe@Au) and CdS NCs. In this method, after the synthesis of magnetic-gold core/shell nanoparticles (Fe@Au) and functionalization with 2-mercaptoethanol and 12-mercaptododecanoic acid (ME:MDDA) monolayer, anti-Salmonella Typhimurium antibody was immobilized on the surface of Fe@Au/ME:MDDA. After capture of Salmonella Typhimurium by Fe@Au/ME:MDDA/Ab1, CdS-Ab2 conjugate served for electrochemical measurement. The linear range of used concentration was from 0.1 to 500 cells per mL. The LOD was 13 cells per mL and analysis time was less than 1 h. A novel electrochemical sensor based on Fe3O4@SiO2-MIP was proposed to detect of Gram-negative bacterial quorum signaling molecules (AHLs).52 In this report, after synthesis of Fe3O4@SiO2-MIP and deposition onto a magnetic carbon paste electrode (MGCE) surface, the measurement was performed by differential pulse voltammetry (DPV). The LOD was about 8 × 10−10 mol L−1 with a linear range of 2.5 × 10−9 mol L−1 to 1.0 × 10−7 mol L−1.
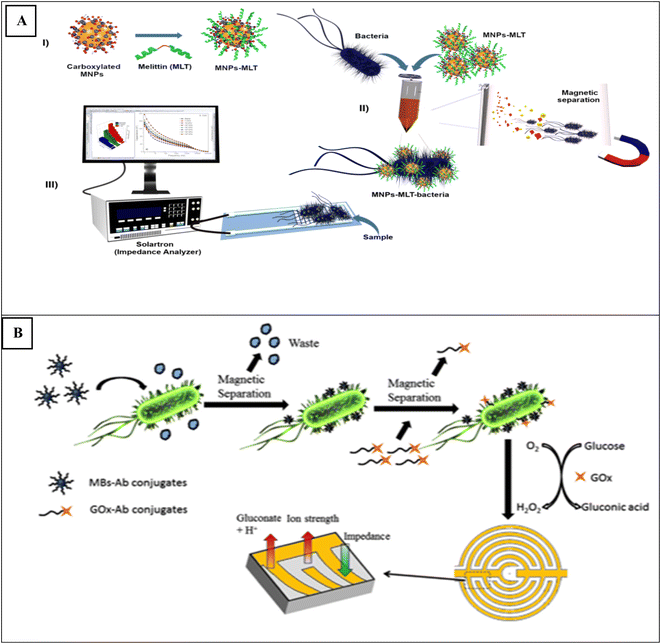 | ||
| Fig. 1 (A) Illustrates the application of carboxylated magnetic nanoparticles (MNPs) for the identification of MLTs [this figure has been adapted/reproduced from ref. 50 with permission from Elsevier, copyright 2022]. (B) MBs-based immunosensor towards recognition on of bacteria using MBs–Ab–cell–Ab–Gox [this figure has been adapted/reproduced from ref. 62 with permission from Elsevier, copyright 2022]. | ||
Since the standard methods of identifying bacteria require several days, the use of immuno-magnetic separation (IMP) methods is an efficient method for extracting and concentrating the target cells. So, iron based magnetic particles with micro- and nanometer scale functionalized with antibody are used to separate the target cells. Target cells are separated from the sample matrix using a magnetic field. Then, the target cells that attached to the magnetic particles can be separated from magnetic particles by increasing the concentration of the reaction medium. This method is easier compared to centrifugation, filtration, or capture of target on an immune-functionalized surface. Adsorption efficiency is generally increased using magnetic particles due to more surface area available for target binding. The use of screen-printed carbon electrodes (SPCE) or molecular imprinting polymer (MIP) electrodes reduces the consumption of samples and reagents. Also, due to faster diffusion, it reduces the response time and makes the sensor more economical.53,54 In addition to the mentioned advantages, these methods have some limitation. Immunosensors, in particular, are based on the antigen-antibody reaction. The high cost and low stability of monoclonal antibodies limit their use, which may limit detecting of microorganisms in harsh environments.55,56 Compared to DNA and antibody-based biosensors, biosensors based on antimicrobial proteins (AMP) have low production cost and high stability in harsh environments and can be produced in high scales.57–59
One of the advantages of molecular imprinting is specificity and selectivity for target molecules. They are resistant to environmental conditions, simplicity and high sensitivity, low production cost, fast production, biocompatibility, and long life are their other features. Another advantage of them, especially in the case of small molecules, is their universality, considering that it is difficult to detect small molecules in matrices of molecular diversity and complexity, the use of molecular imprint polymer in this situation facilitates the selection of the desired molecule.60 They are stable thermally and can be autoclaved. Compared to enzymes, antibodies and molecular receptors, their production cost is very low. These devices also have some disadvantages, such as the low diffusion of materials in molecular imprint polymers, which makes their regeneration difficult. The slow response is another disadvantage of these devices, which takes more than 40 minutes and is effectively the same for both 2D and 3D polymers.61
Also, Xu et al.62 reported an impedimetric immunosensor based on the use of iron magnetic beads for detection of Escherichia coli O157:H7 and Salmonella Typhimurium in food sample using a screen-printed interdigitated microelectrode (SP-IDME) (Fig. 1B). MBs coated by Streptavidin and functionalized with biotinylated antibodies (Ab) used to capture to the target analyte. The MBs–Ab–cell complexes were labeled with glucose oxidase (GOx)–Ab conjugates. The MBs–Ab–cell–Ab–GOx biomass in presence glucose solution using an enzymatic reaction produced gluconic acid that with increasing of the ion strength of the solution, causes decreasing the impedance of the solution measured on the SP-IDME. the concentration range was 102–106 cfu mL−1. The LODs were 2.05 × 103 CFU g−1 and 1.04 × 103 cfu mL−1 for E. coli O157:H7 and S. Typhimurium, respectively.
An electrochemical immunosensor using antibody-functionalized magnetic nanoparticles (MNPs), gold nanoparticle (AuNP)-conjugated lead sulfide (PbS) nanoparticles (as electrochemical reporter) via oligonucleotide linkage were designed by Wang et al.63 for detection of E. coli O157:H7 in food/water by screen-printed carbon electrode (SPCE). In this method, polyclonal anti-E. coli O157:H7 antibodies were bonded to the target bacterial cells which were captured and separated from the sample by antibody functionalized MNPs. The measurement was performed by square wave anodic stripping voltammetry (SWASV). The concentration range was 101 to 106 CFU mL−1 with a signal-to-noise ratio ranging from 2.77 to 4.31. Duration = 1 h from sample processing to final readout.
In conclusion of this subsection, it is important to point out that, there is a lack of various types of apta-sensors and genosensors based on the magnetic beads for determining different types of bacteria. Therefore, most of the sensors and biosensors developed in this sector have used MIP and SPE electrodes. In general, only one protein-based electrochemical biosensor with 3.5 CFU mL−1 sensitivity has been reported, and the majority of studies have used antibodies. Also, electrochemical biosensor based on bacteria-specific glycoconjugate and iron magnetic nanoparticles were not used to detection of bacteria in food sample, till now. Additionally, different types of other nanomaterials such as carbon-based nanomaterials (including MWCNTs, SWCNTs, GO, GQDs and rGO) and metallic nanomaterials (such as Ni, Ag NPs, CuNPs ZnS, and AuNPs) have not been used yet in this area.
Until now, various methods based on biosensors have been used for the sensitive detection of foodborne pathogens, such as electrochemical and optical, and mass-based biosensors. The working principles of these methods are well known and have been used to quantify food pathogens with high sensitivity and specificity. Due to the complexity of food samples, these methods often require expensive equipment. Therefore, researchers are looking for easy, fast and sensitive methods to detect pathogens in food samples. This requires further development and adaptation of new technologies to facilitate the detection of foodborne pathogens. In recent years, microfluidic systems have been used as a powerful tool for diagnostic applications.64 For the example, Chen et al. reported a microfluidic biosensor integrating with electrochemical impedance analysis, urease catalysis for the detection of Listeria.65 In this report, the Listeria cells, magnetic nanoparticles (MNPs) functionalized with the anti-Listeria polyclonal antibodies and gold nanoparticles (AuNPs) functionalized with urease were incubated in a fluidic separation chip with active mixing to form the MNP-Listeria-AuNP-urease sandwich complexes. By applying a high gradient magnetic field, this complex was captured by the chip. This complex was resuspended by injecting urea. Then urea was hydrolyzed by urease on the complex to ammonium and carbonate ions. These ions were transferred into the microfluidic detection chip. The detection chip contained a microelectrode to measure the impedance that determined the amount of the Listeria cells. The separation chip had ∼93% capture efficiency of the Listeria cells with duration of 30 min. The LOD of chip was as low as 1.6 × 102 CFU mL−1 and detection time was reduced from original ∼2 h to current ∼1 h. Jiang et al. fabricated a label-free electrochemical aptasensor based on a thread microfluidic for detection of Vibrio parahaemolyticus in seafood. MoS2 nanosheets was used to increase the sensitivity of electrochemical measurement. Dynamic detection range was from 10–106 CFU mL−1 and a LOD was 5.74 CFU mL−1. Duration = 30 min.66
Tan et al. developed a multiplex PDMS microfluidic immunosensor based on electrochemical impedance using specific antibody immobilized on alumina nanoporous membrane via self-assembled (3-glycidoxypropyl) trimethoxysilane (GPMS) silane for rapid detection of foodborne pathogens Escherichia coli O157:H7 and Staphylococcus aureus.67 The impedance spectrum of detection ranging was from 1 Hz to 100 kHz. Duration was 2 h. Detection sensitivity was of 102 CFU mL−1.
The advantages of these devices are being small and portable and improving sensitivity and specificity. In addition, all test steps such as sample pretreatment and separation and chemical reactions and real-time quantification can be performed in a single microfluidic platform. However, due to the complexity of food matrices, the detection of food pathogens by microfluidic methods is still challenging. Various ligands are used to detect bacteria in food, which are combined with bacterial surface biomarkers, for example, bacterial surface antigens.68
In summary, smartphones have great potential to achieve on-site detection of foodborne pathogens due to their advantages such as small size, high accessibility.69 Therefore, the use of microfluidic and smartphone systems and lab on chip methods along with electrochemical methods based on iron magnetic nanoparticles and using immunosensor, aptasensors, enzyme sensors, and cell sensors can be very effective in detecting pathogens at the cell level.70 Therefore, the design of biosensors that can detect several bacteria simultaneously is one of the interests of researchers. The use of iron magnetic beads functionalized with antibodies and electrochemical methods in combination with microfluidics to isolate pathogenic bacteria is a suitable solution for quick and sensitive detection of these pathogenic agents in food samples. Also, the use of magnetic beads functionalized with different antibodies that are specific to different bacteria can be used to detect several pathogenic agents at the same time.
3. Iron and iron-oxide magnetic nanoparticle-based electrochemical biosensors for the detection of allergen contamination in food sample
Food allergens are another food component that can cause an abnormal clinical response related to food consumption.71,72 Food allergens are common proteins or glycoproteins found in all food products, and some people have an allergic reaction to these proteins. Allergenic responses to food are mainly type I, which refers to susceptible immediate or severe reactions mediated by allergen-specific immunoglobulin E (IgE). The well-known plant and animal food allergens include milk, fish, crustaceans, mollusks, and sulfites, peanuts, nuts, gluten, lupin, soybeans, celery, mustard, and sesame seeds.73 These substances are often present at very low levels, making their determination very difficult. Because allergens are harmful to the health of sensitive people, in some countries, there are laws regarding the declaration of potential allergens on food labels. Also, the venture of cross-contamination in food processing and storage is another problem that determines the exact determination of allergens.74 Electrochemical-based methods could possibly be effective in detecting food allergens. Purushothama and coworkers75 reported a sandwich-type electrochemical immunosensor for the detection of ovalbumin (a water-soluble phosphoglycoprotein) based on magnetic beads, in which dual mode-recognition using immunoreaction and enzyme-linked amplification are applied by MNPs functionalized with specific anti-ovalbumin immunoglobulin G/ovalbumin molecules and anti-ovalbumin (as secondary antibodies) conjugated with the enzyme horseradish peroxidase was applied as label tag. The electrochemical signal was measured by linear sweep voltammetry (LSV) technique. The linear range of concentration was attained as 11 to 222 nM, with LOD of 5 nM. Also, Jiang et al.58 developed a cell-based electrochemical sensor (cytosensor) using rat basophilic leukemia cell (RBL-2H3) for detecting allergens in foodstuffs using Fe3O4–SiO2-fluorescein isothiocyanate (FITC)-lipidosome nanoparticles on magnetic glassy carbon electrode (MGCE). In the method, electrochemical assay was performed by the cationic magnetic fluorescent nanoparticles (CMFNP) transfected into RBL-2H3 cells activated by an allergen antigen. The limit of detection was attained 3.3 × 10−4 ng mL−1. This sensor showed a good relation with the logarithmic value of cell numbers, ranging from 1.6 × 103 to 1.6 × 1010 cells per mL. Navarro and coworkers76 proposed a sensitive electrochemical method for detecting acrylamide in thermally processed potatoes using iron magnetic-chitosan-hemoglobin nanoparticles on a simple carbon paste electrode. The acrylamide concentrations range was 10–171 nmol L−1. The limit of detection was attained 0.06 nmol L−1. Electrode surface is passivated by hemoglobin and acrylamide leading to the reduction of the electrochemical signal of reduction. Peak was measured.Microfluidics integrated with electrochemical methods can also be used to detect allergens in food. For example, Jiang et al.77 developed a novel cell co-culture model electrochemical microfluidic chip for qualitative and quantitative analysis of food allergen. This device was able to perform microfluidic cell culture, food allergen-induced cell morphological changes, and cell metabolism measurements. Allergic response of RBL-2H3 mast cells and ANA-1 macrophages (within a cell co-culture model) to the antigen stimulus was visible. The microfluidic chip was fabricated from two cell cultivation channels which contained 4 gold electrodes. The cell-secreted inflammatory cytokines by exocytosis were measured by enzyme-linked immune sorbent assay (ELISA) and cell impedance changes were detected using cell-based electrochemical assay.
Baldo et al.78 developed a sandwich-based immunoassay electrochemical microfluidic device (DEμD) for the detection of egg allergen ovalbumin using GO-Ab1-OVA-Ab2-MB-HRP in wine samples. In this method, first electrodes were modified with poly(diallyldimethylammonium chloride) solution (PDDA) and GO and then Ab1 was immobilized on GO. A polyclonal anti-OVA antibody (Ab2) attached to magnetic beads (MBs) and labeled with horseradish peroxidase (HRP) was used for the assay of ovalbumin. The linear range of concentration was from 0.01 to 10 pg mL−1. The LOD was 0.2 fg mL−1. The integration of biosensors with smartphones enables the development of powerful analysis platforms for food evaluation. For the example, Lin et al.79 developed a smartphone based electrochemical method for point-of use detection of five major food antigens in milk, eggs, peanuts, hazelnuts, and wheat using exogenous antigen testing (iEAT). The system was consisted of a kit for allergen extraction, an electrode chip, and a pocket-size detector. First, allergen was extracted using an immunomagnetic kit contains magnetic beads functionalized with Ab1. This kit captured allergen and then captured allergen was labeled with a second antibody conjugated with an oxidizing enzyme (horseradish peroxidase (HRP)). Then, 3,3′,5,5′-tetramethylbenzidine (TMB) was used as chromogenic electron mediator of horseradish peroxidase. The electrical current was measured by a miniaturized electronic device.
In conclusion, iron magnetic particles with micro- or nanometer scale functionalized with specific antibody or protein of the target allergen are used to separate allergenic molecules or cells. Allergenic molecules or cells are separated from the sample matrix using a magnetic field. Then, allergenic molecules or cells attached to the magnetic particles can be separated from magnetic particles by increasing the concentration of the reaction medium. The use of protein is more economical than the using antibody due to the synthesis in high quantities and low cost of protein.60 On the other hand, the use of RBL-2H3 mast cells to detect allergens due to their excellent exotoxic response to antigens has a high potential for use in biosensors. In compared to other cells attached to IgE antibodies (basophils and activated eosinophils) that trigger some intracellular events such as the release of chemicals mediators inside the cell such as histamine, and serotonin and cellular degranulation, RBL-2H3 mast cells have low exotoxic response.80
It is important to point out that, there is a lack of different types of aptasensors, genosensors, peptide-based sensors, and enzymatic sensors based on the use of magnetic beads to determine different types of allergens. Other types of nanomaterials such as carbon-based nanomaterials (including MWCNTs, SWCNTs, GO, GQDs, and rGO) and metallic nanomaterials (such as Ni, Bi2O3, MoS2, Ag NPs, CuNPs, ZnS, and AuNPs) have not been used. Also, magnetic beads have not been used yet for electrochemical detection of different types of food allergens, such as egg, milk, peanut, wheat, fish, soybean, and so on. In general, there is little report about the use of magnetic beads and electrochemical techniques for the detection of food allergens. The concentration range used in these studies has been reported from 10 to 222 nmol L−1 and the LOD is 0.06 and 5 nmol.
In this subsection, we first discussed about the types of allergens in food, then examples were discussed about allergen detection methods with electrochemical methods and the use of magnetic beads. Also, the abbreviations of nanomaterials that can be used to evaluate allergens are stated. In this subsection, we concluded that the integration of electrochemical methods with the microfluidic system can accelerate and facilitate the detection method. We also concluded that the integration of biosensors with smartphones allows us to have a strong analysis platform for detecting food allergens. Development of new solutions to evaluate several allergens simultaneously, along with microfluidic methods and smartphones, are other solutions to increase detection efficiency.
Iron and iron-oxide magnetic nanoparticle-based electrochemical biosensors for detection of pesticide contamination in food sample.
The growing human population and the need to increase food production have forced the agricultural community to use pesticides to control plant diseases and pests and increase yields.81 Residues of these chemicals are released into the environment and cause significant pollution of terrestrial ecosystems and food poisoning. Pesticide may also be absorbed through the gastrointestinal tract or even the skin. Foods contaminated with these substances are one of the main ways to transfer pesticides. These substances, even in low concentrations, cause severe problems for human health. Consequently, detecting the roots of pesticides in food (such as cereals, vegetables, fruit, and various meats) is one of the most necessary steps in regulating and monitoring their levels.82 Therefore, developing a sensitive and user-friendly sensor device is essential to quantify trace levels of pesticide and herbicide residues in food samples. Therefore, in this part of review, various type of magneto-electrochemical (bio)sensors for the monitoring of pesticides were investigated. Also, advantages and limitations of methods/materials on the sensing efficiency were critically evaluated.
A smartphone-based electrochemical technique83 was designed based on yttrium iron garnet (Y3Fe5O12; YIG) and graphitic carbon nitride (GCN) nanoparticles for the detection of mesotrione (MTO) (herbicide used in agriculture) in fruits and vegetables on GCN sheets via a calcination method. A limit of detection 950 pM for MTO was attained. Yin and coworkers84 developed an electrochemical method using magnetic Fe3O4 nanoparticles to detect Sudan I in food samples. CV and DPV techniques were applied for electrochemical measurements. With concentration range of 0.01–1 μM and 1–20 μM, respectively. The LOD was attained 0.001 μM.
Miao et al.85 reported an electrochemical method using Fe3O4 and polydopamine molecularly imprinted polymer magnetic nanoparticles (PDA@Fe3O4 MIP MNPs) to detect of dichlorodiphenyltrichloroethane (DDT) (a kind of insecticide) in food samples. A linear correlation was showed between the charge transfer resistance (Rct) and concentration the 4,4′-DDT in linear range of 1 × 10−11 to 1 × 10−3 mol L−1. The LOD was 6 × 10−12 mol L−1.
Interestingly, Tang et al.86 reported a biomimetic imprinted electrochemical sensor based on Fe3O4@carboxyl-functionalized multi-walled carbon nanotubes/chitosan nanocomposite layer to detect multi-pesticide residues (acephate and trichlorfon) in vegetable samples. The reactions were recorded using DPV and CV. The linear concentrations range for acephate was from 1.0 × 10−4 to 1.0 × 10−10 M and for trichlorfon was 1.0 × 10−5 to 1.0 × 10−11 M. The low limits of detection were attained 8.94 × 10−12 M for trichlorfon and 6.81 × 10−11 M for acephate.
Also, Luo et al.87 designed an acetylcholinesterase biosensor using acetylcholinesterase/core–shell magnetic mesoporous hollow carbon spheres/glassy carbon electrode (AChE/Fe3O4@MHCS/GCE) for the detection Malathion in pears. To show the role of Fe3O4 in the performance of the sensor, a sensor without Fe3O4 (AChE/MHCS/GCE) was also prepared for comparison. The Malathion concentration ranges attained by AChE/MHCS/GCE sensor were 0.01 to 600 ppb which LOD was attained as 0.0148 ppb (incubation time = 10 min). The malathion concentration ranges in AChE/Fe3O4@MHCS/GCE sensor were 0.01–600 ppb. The LOD was attained of 0.0182 ppb (incubation time = 12 min). These researchers concluded that Fe3O4 increases the stability of the sensor.
In addition, Aruna and coworkers88 prepared an electrochemical nanosensor based on α-Fe2O3–CdO using screen print electrode (SPE) for the detection of chloridazon (CLZ) in Coriandrum sativum leaves. The prepared sensor responded properly to CLZ in the presence of foreign substances. Electrochemical measurements were performed by CV technique. The linear concentration range used for CLZ was from 0.1 to 36.00 μg mL−1 which LOD was 0.059 μg mL−1.
Rodrigues et al.89 designed an acetylcholinesterase enzyme biosensor based on AChE/chitosan/Fe3O4 on a screen-printed electrode for the detection of malathion in Tomato Sauce and Pond Water. In this report, acetylcholinesterase enzyme was immobilized on magnetic iron nanoparticles by glutaraldehyde and a mixture of the pesticide malathion and acetylthiocholine was applied to assay function of sensor. The linear concentration range of this sensor obtained for malathion was from 0.5 to 20 nmol L−1 and the limit of detection was 0.3 μg mL−1.
In summary, different types of biosensors based on enzyme, aptamer and molecularly imprinted polymers can be used to detection of pesticides. The use of iron based magnetic nanoparticles in the designed sensors causes the surface area and the stability increasing of the sensor. The advantage of increasing the surface area is that it increases recognition sites and improves the ability of binding of candidate analyte to the receptor.
One of the great advantages of electrochemical methods is the possibility of using screen-printed electrodes as disposed sensor, which have low manufacturing costs and low sample consumption, and it is possible to modify the surface.90,91 One of the limitations of SPE is that it is not possible to detect several biological molecules using specific antigens. Also, the surface of the electrode is destroyed during the washing process.92 The advantages of using magnetic molecularly imprinted polymer (MMIP) are the easy separation of samples using a magnetic field without the use of centrifugation or filtration, good biocompatibility, low toxicity, and strong paramagnetic properties, which is used to simplify the SPE process and increase the sensitivity of the electrochemical sensor.93–96 However, these devices also have some limitation, such as the low diffusion of materials in molecular imprint polymers, which makes their regeneration difficult. The slow response is another disadvantage of these devices, which takes more than 40 minutes and is effectively the same for both 2D and 3D polymers.61
Finally, the important point is that there is a lack of various types of aptasensors and genosensors, peptide-based sensors and enzymatic methods based on using magnetic beads to determine different types of pesticides. Also, the use of other types of nanomaterials such as metallic nanomaterials (such as Ni, Bi2O3, MoS2, Ag NPs, CuNPs, ZnS and AuNPs) has not been yet reported. Additionally, other types of pesticides (such as chlorpyriphos and carbofura in rice, imidacloprid, deltamethrin, isocarbophos, and phorate in tea leave, acetamiprid, parathion-methyl in fruit, organochlorine, insecticides, fungicides, herbicides and etc.) that are used to control plant diseases have not been studied using electrochemical methods and the use of iron magnetic beads. Also, most studies have used MIP and SPE electrodes. Magnetic beads are not used in microfluidic systems and sandwich methods used to detect pesticides. The linear concentration range obtained in these studies is from 1.0 × 10−4 to 1.0 × 10−11 M, and the LOD is reported for all type of pesticides is from 6.81 × 10−11 M to 8.94 × 10−12 M.
Improved analysis time and cost reduction are other benefits of using biosensors in the field of food analysis. The use of biosensors on a microfluidic or lab-on-a-chip platform reduces the amount of sample consumption and the necessary chemical reagents and directly eliminates the cost of sample preparation and the need for a laboratory.97 Also, multiplex analyte detection can be improved time. For the example, Islam et al.98 reported a label-free electrochemical-based CE–AD microfluidic platform for the detection of multiplex triazine herbicides (sample mixture contained simazine, atrazine and ametryn) in soil and drinking water. Duration was 1.25 min without any pretreatment.
Yao et al.99 designed a microfluidic device containing microdroplets of silica nanoparticles (SiO2NPs) and CNTs based on electrochemical sensors for the detection of methyl parathion in practical samples of cabbage and tomato. Surface area and electrocatalytic activity of glassy carbon electrodes (GCEs) were enhanced by modification of h-CNT-μPs and Nafion (h-CNT-μPs/Nafion/GCEs). The concentration ranges were from 0.3–20.0 μM and 20.0–150.0 μM with the limit of detection was 0.092 μM using Nafion (h-CNT-μPs/Nafion/GCEs).
As a result, in this subsection, different types of insecticides that cause food contamination are discussed and different types of nanomaterials that can be used in future studies are mentioned. There are few studies on the integration of detection methods based on electrochemical and microfluidic methods and smartphone. It is hoped that with the advancement of technology and the increase of more studies, we will see more successes in the field of integration of methods and evaluation of multiple analytes simultaneously.
4. Iron and iron-oxide magnetic nanoparticle-based electrochemical biosensors for the detection of genetically modified organisms' contamination in food sample
With the advancement of genetic engineering in the production of transgenic plants and animals, a great change has taken place in the field of agriculture and has contributed to food security and climate change. In recent years, the global level of biotechnology products has increased 110 times. Soybean (Glycine max L.), for example, is the most important genetically modified crop and widely used as an ingredient in many foods worldwide. In 2016, this product accounted for 83% of the global biotechnology products.100 There has been much scientific and public debate about the dangers of genetically modified organisms (GMO) in the food chain. In the European Union, all transgenic products require a license before entering to the market. According to regulations, food products containing transgenic substances above 0.9% must be labeled.101 Therefore, to ensure the law execution, it is necessary to adopt methods that allow the evaluation of food quality and the quantification of genetically modified organisms. DNA-based techniques such as PCR and real-time PCR are considered the gold standard and reference methods for GMO assay due to their specificity, sensitivity and high reliability. However, these methods are high cost and time-consuming. Electrochemical-based methods using biosensors are a suitable alternative to molecular methods due to their low cost, easy monitoring, and portability.102–104An electrochemical method105 based on core–shell Fe3O4@Au magnetic nanoparticles (MNPs) was developed for the assay of gene sequence in soybean using an aptamer-target gene-aptamer sandwich architecture. In this method, after immobilization of aminated capture probes on Fe3O4@Au and hybridization with Roundup Ready (RR)-target and FITC-tagged signaling probe, anti-FITC-POD antibodies were added. Enzyme labeling is employed to produce amplified catalytic signals. The chronoamperometry technique was performed by screen-printed carbon electrodes (SPCE). The linear range was 0.1–10.0 nM with LOD of 0.02 nM for the event-specific (RR). According to the results of this report, the proposal method can detect genetically modified organisms (GMOs) in food.
Also, Freitas and coworkers106 developed a sandwich type chronoamperometric genosensor based on magnetic core–shell Fe3O4@Au nanoparticles (MNPs) for detecting GMO in MON810 maize on SPE. In this dual mode recognition based on aptasensor and enzymatic sensor, an aminated DNA capture probe covalently linked to a carboxylate self-assembled monolayer and a fluorescein isothiocyanate (FITC) signaling probe were used in a sandwich assay format. Enzymatic labeling was performed with anti-FITC-peroxidase. The developed sensor had a limit of detection of 0.15 nM and linear range of 0.25–2.5 nM.
Interestingly, Ye and et al.107 designed a sandwich-type labeled electrochemical genosensor for the detection of cauliflower mosaic virus 35S (CaMV35S) gene sequence in tomato samples using cMWCNTs-modified GCE which Au@Ag–Fe3O4 was applied as label of signal DNA probe (sDNA). In this method, after immobilization of the thiolated probes on GCE modified with cMWCNTs, target probes were hybridized with thiolated probes. Then Au@Ag–Fe3O4 were used as labels. The peroxides-like activity of Au@Ag–Fe3O4 nanoparticle caused the conversion of H2O2 to H2O and enhanced electrocatalytic activity of Au@Ag–Fe3O4 nanoparticle and caused enhanced electrochemical response. Genosensor designed by these researchers showed a limit of detection 1.26 × 10−17 M. Also, the linear concentration range was 1 × 10−16 to 1 × 10−10 M.
Sousa and coworkers108 developed a chronoamperometric sensor for detection of maize taxon-specific (HMGA gene) using Fe3O4@Au nanoparticles as nanosized platform that HMGA DNA probes was covalently immobilized onto carboxylated self-assembled monolayers on nanoparticles (Fig. 2). DNA target was hybridized with DNA probes using sandwich type format and fluorescein isothiocyanate was used as DNA signaling tag. For this aim, the hybridization reaction was labelled with enzymes and the peroxidase activity linked to the nanoplatform located on gold surface was registered by the chronoamperometric measurement. The linear concentration range of developed magneto-genosensor was from 0.5 to 5 nM which along with LOD = of 90 pM.
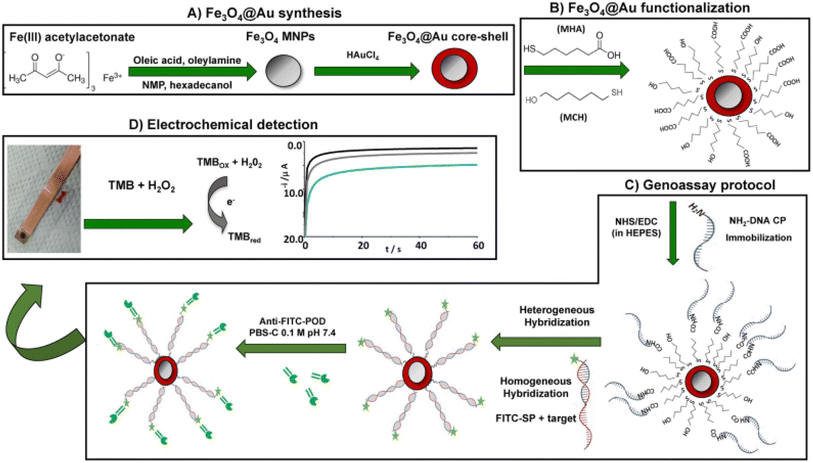 | ||
| Fig. 2 Schematic illustration of EC genosensor based on core–shell of Fe3O4@Au MNPs for the targeting the endogenous maize gene [this figure has been adapted/reproduced from ref. 108 with permission from Elsevier, copyright 2022]. | ||
Due to their superparamagnetic properties, good biocompatibility, low toxicity and ease of preparation, iron based magnetic nanoparticles are widely used in biosensors structure. Iron based magnetic nanoparticles are easily functionalized with various organic and biological molecules such as aptamers, polymers, enzymes, and antibodies and form signal tags. Therefore, the use of iron based magnetic nanoparticles together with electrochemical methods increases sensitivity and bonding strength of the target to the solid support.107,109
The covalent immobilization of the probe sequence allows the DNA to be easily attached to the electrode surface from its end, increasing structural flexibility and hybridization efficiency.
In electrochemical genosensors used in recent studies detection of GMO, in addition to using the inherent electrochemistry of nucleic acids for the detection of the hybridization event, electroactive labels such as methylene violet, methylene blue, osmium and cobalt complexes, anthraquinone compounds have also been used to increase the sensitivity of the analysis. These tags are attached to guanine bases in the DNA molecule and are used in GMO genetic sensors. However, enzyme tags are a suitable tool in GMO bioassays due to the amplification of the intrinsic catalytic signal which one enzyme molecule can have many catalytic turns. Thus, many marker molecules generate per hybridization event.110 Streptavidin-biotin, avidin-biotin, and fluorescein-anti-Fab-fluorescein compounds also have been used to bind enzyme to DNA sequences. Alkaline phosphatase,111,112 catalase,113 glucose oxidase and horseradish peroxidase114 are the most commonly used enzymes. Recently, Liao and coworkers114 have used a two-enzyme approach, which includes an enzymatic cascade reaction with two different enzymes. Sandwich hybridization is usually applied to prevent labeling of target sequence.111,112,115 In this type of assay, two types of probes are used: a probe sequence that is complementary to the target sequence and binds to the electrode surface and a labeled signaling sequence that hybridizes with the rest of the target. In recent studies, GMO sensors have been used only for pure solutions of synthetic oligonucleotides. The implementation of the gene-sensor with a real sample is complicated due to the presence of large amounts of non-complementary nucleic acids, proteins, organic molecules, salts, etc. Therefore, analytical devices are facing this big challenge. Therefore, real food samples have not been evaluated with these electrochemical gene-sensors.116 The linear concentration range used in these studies is reported to be 1.0 × 10−16 to 1.0 × 10−10 M, and the LOD is 1.26 × 10−17 M.
In general, in the studies conducted for GMO, reported DNA sensors targeted NOS, PAT, 35S and PAT as target gene sequences. But in electrochemical methods based on Fe2O3, 35S and event-specific genosensors have been reported, and PAT, 35S and PAT as target gene sequences have not been reported.
As a result, in the past years, immunosensors, microfluidics, cell sensor, sandwich method and multiplex detection of analytes based on electrochemical methods and the use of iron nanoparticles have not been studied to detect GMO in food products. Most of the studies have used other nanomaterials. Also, there is no report of smartphone technology in revealing GMO.
5. Iron and iron-oxide magnetic nanoparticle-based electrochemical biosensors for detection of growth-promoting feed additives (β-agonists and antibiotics) contamination in food sample
Although veterinary drugs such as antimicrobials, growth stimulants, and hormones used to treat animal diseases play a crucial role in curing animal diseases and increasing the effectiveness of animal foods but their residues in foods have serious adverse effects on human health.117 Several cases of pathogen resistance to antibiotics in humans due to the consumption of meat treated with these antibiotics during animal husbandry. Also, β-agonists have some health-threatening effects such as cardiac arrhythmias and stress.118 The β-agonists are one of the most famous growth stimulants and consists of phenylethanolamines with different functional groups on aromatic rings such as resorcinol, aniline, or phenol.119 β-Agonists are used to treat lung disease and asthma.120 Today, they are used illegally as animal feed to help muscle growth, improve protein accumulation and reduce fat deposition,121 and increase growth rate in livestock in the agricultural industry. These compounds can endanger the consumer' s health due to their slow metabolism and accumulation in animal tissues. Therefore, its use in the daily feed of animals is banned in the European Union, China, and many other countries. However, they are still used in some countries.122–124 So, we need accurate methods for their monitoring in food samples. In this subsection the critical role of MMPs and various bioreceptors for the identification of β-agonists and antibiotics were discussed. Arporn and coworkers125 proposed an electrochemical method based on the use of iron oxide magnetic nanoparticles/graphene oxide (Fe3O4/rGO) for the detection of ractopamine (β-adrenergic agonist). The linear concentration ranges were 0.05–10 and 10–100 μM, and the detection limit was 13 nM.The use of antibiotics in veterinary medicine has led to the problem of antibiotic resistance in human pathogens, which has led to widespread debate. Although the main cause of antibiotic resistance in human pathogens is related to the misuse of antibiotics in human medicine, the use of antibiotics in animals is another cause of antibiotic resistance in human pathogens. Antibiotics are mainly used for three purposes in animals: (i) therapeutic use for the treatment of sick animals in a short period with doses of antibiotics over the minimum inhibitory concentration, (ii) preventive use to prevent infection in animals, including moderate to high doses of antibiotics, (iii) as a growth stimulant to improve feed use and production that is given to entire herds over long periods of low-level treatment.126 The use of antibiotics in animals suppresses subclinical infections and facilitate microbial metabolites that reduce growth and prevent the consumption of nutrients by the microbial agent. Although the use of antibiotics in animals contributes to their health and significantly increases livestock production, these substances have been banned in the European Union due to the antibiotic resistance of human pathogens. Therefore, in recent years, the use of electrochemical methods for the detection of antibiotics in livestock products has attracted much attention of scientific.127,128
Recently, Giribabu et al.129 reported an electrochemical method based on magnetite Fe3O4 nanoparticles for detecting chloramphenicol in real samples. The concentration range was 0.09–47 μM, with LOD of 0.09 μM. Moreover, the fabricated sensor can detect 4-nitrophenol, thiamphenicol, and 4-nitrobenzamide.
Interestingly, Liu et al.130 proposed a dual-recognition electrochemical immunosensor and enzyme-linked immunoassay-based analysis using gold electrode modified with carboxyl-Fe3O4 nanoparticle (MNPs)/chitosan (CS) for the detection of tetracycline in milk samples. The DPV technique was applied to assay immobilization event of anti-tetracycline monoclonal antibody (Ab) on the modified electrode surface and binding of tetracycline to Ab. In this study, the linear range of concentration was 0.08–1 ng mL−1, and the detection limit was 0.0321 ng mL−1.
Also, Aquino et al.131 proposed a magnetic-MIP for the detection tetracycline using electrochemical and HPLC-UV method. In this study, magnetic nanoparticles modified with tetraethylorthosilicate and trimethoxysilyl propyl methacrylate were coated with MIP selective tetracycline. Finally, polymerization of the MIP towards resulted in a core@shell material. An imprinting factor of 3.5 was obtained for the polymer synthesized with acrylic acid as a functional monomer.
A simple label-free electrochemical immunosensor based on Ag@Fe3O4 nanoparticles and thionine mixed graphene sheet (TH-GS) (TH-GS/GA/Ag@Fe3O4 nanoparticles) was prepared for detection of kanamycin in pork meat sample by Yu et al.132. In this report, Ag@Fe3O4 nanoparticles were employed as the support for immobilizing antibody of kanamycin on a TH-GS/GA pretreated GCE which thionine was utilized as an electron transfer mediator. Using SWV technique, the LOD of this biosensor was 15 pg mL−1, and linear range was from 0.050 to 16 ng mL−1. The duration was 3 min.
Giribabu et al.133 have developed an electrochemical method using Fe3O4/GCE as an electrochemical platform for recognition of chloramphenicol (CAP) by cyclic voltammetry and square wave voltammetry. The concentration range was used from 0.09 to 47 μM. The LOD was attained 0.09 μM.
Jahanbani et al.134 improved an electrochemical aptasensor method based on oleic acid (OA)/anti-TET, and Fe3O4/OA/anti-TET using modified carbon paste electrode (CPE) for the detection of tetracycline (TET), in drug, milk, honey and blood serum samples. In this method, a tetracycline-binding aptamer (5′-NH2-CGT ACG GAA TTC GCT AGCCCC CCG GCA GGC CAC GGC TTG GGT TGG TCC CACTGC GCG TGG ATC CGA GCT CCA CGT G-3′) is adopted to recognize TET. In this report, the results of TET detection using two designed electrodes were compared. The linear range for TET using CPE/OA/anti-TET based aptasensors was 1.0 × 10−12–1.0 × 10−7 M, and the detection limit was attained 3.0 × 10−13 M by EIS method. The linear range for TET with the CPE/OA/anti-TET aptasensor was 1.0 × 10−10 to 1.0 × 10−7 M and the detection limit was attained 2.9 × 10−11 M.
Long and coworkers135 proposed a magnetic imprinted electrochemical sensor based on magnetic multi-walled carbon nanotubes (MWCNTs)/Fe3O4 nanoparticles/methacrylic acid (MAA) for the detection of kanamycin in complicated matrixes. In this report, a linear correlation was observed between the response of peak currents the magnetic imprinted electrochemical sensor which negative logarithm of kanamycin concentrations ranging from 1.0 × 10−10 mol L−1 to 1.0 × 10−6 mol L−1. The LOD was 2.3 × 10−11 mol L−1.
Song et al.136 designed an electrochemical aptasensor based on Fe3O4@mesoporous carbon for detection of oxytetracycline (OTC) in the milk samples. In this method, the OTC-targeted aptamer (5'-CGTA CGGA ATTC GCTA GCCG AGGC ACAG TCGC TGGT GCCT ACCT GGTT GCCG TTGT GTGG ATCC GAGC TCCA CGTG-3') was immobilized on the Fe3O4@mesoporous carbon nanocomposites by forming an amide bond. After adding OTC, a Gquadruplex structure was formed between OTC and aptamer strands. Using this method, the low detection limit was obtained as 0.027 pg mL−1, and the linear range of OTC concentration was from 0.005 to 1.0 ng mL−1.
Guo et al.136 developed a label-free electrochemical aptasensor based on graphene, iron oxide, multi-walled carbon nanotubes, nanoporous platinum and titanium (NP–PtTi/GR-Fe3O4/MWCNT-Fe3O4) for the detection of penicillin in food sample. In this method, the penicillin -targeted aptamer (5′-NH2-TGG TTG TTC CTG GTT TCG TTT TTG TCA GTT TGT AT-3′) was immobilized on the NP-PtTi/GR-Fe3O4/MWCNT-Fe3O4 using bonds of Pt–NH2 between NP-PtTi and the NH2 functional groups of the aptamer at 5′ end. After adding penicillin (target analyte), a specific connection was formed between penicillin and aptamer strands. Interference studies using kanamycin sulfate, neomycin sulfate and HCG showed the current signal produced by penicillin was much stronger than those produced by kanamycin sulphate, neomycin sulphate and HCG. Therefore, designed aptamer represented a special selectivity to penicillin. Using EIS technique this biosensor shows LOD of 25.3 pg mL−1 with linear 0.05 to 100 ng mL−1.
Similarly, Yin and coworkers138 proposed an electrochemical biosensor method using specific aptamer (NH2-TAG GGA ATT CGT CGA CGG ATC CGG GGT CTG GTG TTC TGC TTT GTT CTG TCG GGT CGT CTG CAG GTC GAC GCA TGC GCC G-3′) immobilized on Au@MWCNTs–Fe3O4/NP-PtTi-GCE for detecting streptomycin. Using this biosensor, the low detection limit was obtained as 7.8 pg mL−1, and the linear range of penicillin concentration was from 0.05 to 100 ng mL−1.
Yin et al.139 developed a labeled electrochemical aptasensor based on graphene composite–Fe3O4–AuNPs-porous carbon nanorods (PCNR) for detection of streptomycin antibiotic in food. In this strategy, the penicillin-targeted aptamer (5-TAG GGA ATT CGT CGA CGG ATC CGG GGT CTG GTG TTC TGC TTT GTT CTG TCG GGT CGT CTG CAG GTC GAC GCA TGC GCC G-Thiol-3) was immobilized on the graphene composite–Fe3O4–AuNPs-PCNR using chemical bonds of AuNPs-SH2 between AuNPs and the surface of electrode modified by modified SH functionalize groups of the aptamer at 3′ end sample. Using this method, the low detection limit was obtained as 0.028 ng mL−1, and the linear range of penicillin concentration was from 0.05–200 ng mL−1.
Superparamagnetic properties, good biocompatibility, low toxicity and ease of preparation have turned iron magnetic nanomaterials into a valuable tool that are used in different types of biosensors, especially electrochemical biosensors, to detect different types of growth-promoting food additives based on aptamers, enzymes or synthetic polymers and antibodies.
As a result, immunosensors, aptasensors and enzyme-based methods have been used in the studies conducted using electrochemical methods based on iron magnetic nanoparticles to detect beta agonists and antibiotics. To increase the detection sensitivity, other nanomaterials (such as rGO, chitosan, tetraethylorthosilicate and trimethoxysilyl propyl methacrylate, graphene sheet, MWCNTs, mesoporous carbon, nanoporous platinum and titanium, AuNPs, porous carbon nanorods (PCNR)) have been used together with iron materials. Multi-channel electrochemical devices based on magnetic beads to detect several analytes were reported. The same method can also be based on iron magnetic beads.
In recent studies, different enzymes or receptors (such as horseradish peroxidase [HRP], penicillinase [PCN], and glucose oxidase [GOx]) labeled with different nanomaterials such as carbonaceous nanomaterials, colloidal gold tags, and magnetic nanoparticles have been used to detect antibiotics in food using electrochemical methods. Different types of bioreceptors such as immune-sensor, apta-sensor, molecular imprinted polymer (MIP)-biosensor based on electrochemical methods were used in the detections. Also, different types of nanoparticles such as carbon-based nanomaterials, carbon nanotubes, and metal nanoparticles have been used in these studies.140 It seems that the integration of the aforementioned methods with microfluidic devices and smartphone technology can save time and cost of evaluation and at the same time provide the evaluation of multiple analytes in a food sample.
Kling et al.141 developed a microfluidic platform integrated with electrochemical method enzyme-mediated readout using glucose oxidase [GOx] for the simultaneous detection of multiplexed assays in food samples. In this study, tetracycline and streptogramin were detected. To evaluate multiple samples simultaneously, each sample was immobilized in a specific section of this network and readout was performed with a single electrochemical cell in the downstream channel. To evaluate multiple samples simultaneously, each sample was immobilized in a specific section of the chip and readout was performed with a single electrochemical cell. Immobilization sections had a very low volume of 680 nL. The limits of detection (LOD) were determined 9.22 and 6.33 ng mL−1 for pristinamycin and tetracycline, respectively.
The use of smartphone technology for the final detection of analyte in combination with microfluidic chip and electrochemical methods for antibiotic detection can improve the detection sensitivity.
In this subsection, different types of growth enhancing additives that can play a role in food contamination were discussed and different types of nanomaterials that can be used in the electrochemical detection of these compounds were mentioned. Also, various examples have been given for a better understanding of the problem and the advantages and limitations of each case have been explained. The integration of electrochemistry methods with microfluidics and smart phones and the simultaneous evaluation of analyte gen were discussed at the same time.
6. Iron and iron-oxide magnetic nanoparticle-based electrochemical biosensors for detection of toxin contamination in food sample
Pathogenic bacteria can produce toxins, or toxigenic species. Food products contaminated with bacteria toxin cause a high hazard for human health and food safety. There are two classes of bacterial toxins including endotoxins and exotoxins. Endotoxins (lipopolysaccharides) are located on the outer surface of bacteria and exotoxins (proteins or polypeptides) are usually secreted by bacteria or are released only by lysis of the bacterial cell.142 Exotoxins are the main cause of food contamination and their poison causes many foodborne diseases. Detection of toxins and their sub-products in food spoiled by microorganisms is very necessary to establish safety in food.143 Viruses are another type of pathogenic microorganisms.144 Mycotoxins are natural toxic secondary metabolites that are produced by filamentous fungi and consist of more than 500 different subclasses, and usually result from improper storage of food, mainly agricultural crops and animal feed. Other categories of toxins are phycotoxins, which are byproducts of toxic microalgae and are found primarily in seafood, as well as cyanotoxins produced by cyanobacteria.145 In this subsection various types of (bio)sensors for the recognition of toxins using iron-based magnetic nanoparticles and their composite were surveyed.Shang et al.146 proposed a sandwich-type electrochemiluminescence sensor using Fe3O4–Au modified by poly-(ethylenimine) (BPEI) functionalized graphene nanosheets and Ab1 bound on Fe3O4–Au for the detection tetrodotoxin in food sample. In this method, after capturing tetrodotoxin by Ab1, the subsequently bond of luminol-AuNP-Ab2 conjugate served for ECL measurement. Luminol capped gold nanocomposites (luminol-AuNPs) served as the signal tag. The linear range was obtained as 0.01–100 ng mL−1.
Liu et al.147 proposed a label-free photoelectrochemical (PEC) platform for detection of aflatoxin B1 (AFB1) in corn samples based on CdS–Fe3O4 nanocomposites/anti-Antiaflatoxin B1 using a SPE. By this sensor, the linear concentration range of aflatoxin B1 (AFB1) was determined to be 0.01–80 ng mL−1 and LOD was 5.0 pg mL−1.
Also, Chauhan et al.148 developed a sandwich type electrochemical immunosensor based on Au–Fe3O4 nanostructure/monoclonal anti-aflatoxin antibody (aAFB1)/4-aminothiophenol/gold-coated quartz crystal electrode for the detection of AFB1 in corn flake samples. In this report, first, quartz crystal electrode was treated 4-ATP for SAM formation. Then, the monoclonal anti-aflatoxin B1 (aAFB1) antibody activated with EDC and NHS. BSA was applied for blocking nonspecific sites of fabricated immune-electrodes. Then, fabricated BSA/aAFB1/4-ATP/Au immune-electrodes were exposed to AFB1. Finally, secondary rabbit-immunoglobulin antibody (r-IgG)-Cys-Au-Fe3O4 was interacted with AFB1. Both competitive and non-competitive strategies were employed. The competitive mode showed a higher linear range (0.05 to 5 ng mL−1) than the non-competitive one (0.5 to 5 ng mL−1), and LOD 0.07 ng mL−1.
Interestingly, an electrochemical method based on magnetic-MIP/poly (2-vinyl pyridine) was proposed for the detection of scombrotoxin (histamine) in fish by Hassan et al.100. In this report, LOD was about 1.6 × 10−6 mg L−1, much lower than the index for fish spoilage (50 mg kg−1) accordingly to the legislation.
Nguyen et al.149 proposed a label-free electrochemical biosensor based on Fe3O4 incorporated polyaniline (Fe3O4/PANi) film as a signal amplification element for the detection of AFM1 in cow milk. In this report, immobilized aptamers on Fe3O4/PANi have been employed as an affinity capture reagent for AFM1 sensing. Using SWV technique the linear range to AFM1 determination was from 6–60 ng L−1, LOD = 0.98 ng L−1.
Also, a novel electrochemical immunosensor was developed based on Fe3O4/polyaniline nano-gold composite/1,2-dimethyl-3-butylimidazolium hexafluorophosphate ionic liquid for the detection of staphylococcal enterotoxin B in milk sample.150 The anti-enterotoxin B/Fe3O4 was formed a nanostructure smooth and dense film on the surface of a gold electrode. Linear response of sensor for the various concentrations of analyte was from 0.05 to 5 ng mL−1 and LOD was 0.017 ng mL−1.
In conclusion of this subsection, only aptasensors and immunosensors have been used detection of bacterial toxin. Also, peptide and enzymatic biosensors have not been reported till now. The nanomaterials used in these studies are including AuNPs, CdS, and polyaniline. There is no report of other nanomaterials such as metallic nanomaterials (such as Ni, Bi2O3, MoS2, Ag NPs, CuNPs, ZnS) and carbon nanomaterial. Also, a few bacterial toxins have been studied using these methods. In general, fewer researchers have studied the detection of bacterial toxins using electro-analysis by based iron magnetic nanomaterials.
In summary, integration of electrochemical enzyme sensors and aptasensor and immunosensors with microfluidic devices and using smartphone technology and nanotechnology also using cell sensors based on morphological changes of the cell surface can reduce analysis time and increase detection sensitivity. Also, the design of multi-channel microfluidics to detect several analytes at the same time can overcome the time limitation.
Panini et al.151 developed a platform based on microfluidic immunoassay for the detection of zearalenone in feedstuffs samples using electrochemical analysis. In this report, magnetic beads 3-aminopropyl-modified were functionalized with anti-ZEA monoclonal antibodies. Then between ZEA in feedstuffs sample and ZEA-horseradish peroxidase (HPR) conjugated there was a competition to bind to the anti-ZEA antibody in the presence of hydrogen peroxide (H2O2), HPR catalyzed the oxidation of 4-tert-butylcatechol (4-BC). The detection limit was 0.41 μg kg−1.
Lillehoj et al.152 developed a lab-on-a-chip (LOC) technique on poly(dimethylsiloxane) (PDMS) based on electrochemical method with enzymatic amplification using a dually-labeled aptamer probe for detection of botulinum neurotoxin. Aptamer was labelled with fluorescein (as reporting) and biotin (as anchoring) tags. In the presence of botulinum neurotoxin, the conformation aptamer changed and the antibody bound to the reporter and generated an electrochemical current signal through its HRP moiety. This method had a detection limit of 1 pg of botulinum neurotoxin type A (in a 1 mL sample) within 15 min.
Hao et al.153 developed an electrochemical immunosensor using smartphone detection based on polyamindoamine dendrimer (PD)-multi-walled carbon nanotube (MWCNT) nanocomposite containing carboxymethyl cellulose (CMC) for detection of zearalenone residual in feed ingredients and agricultural food. The linear range was 1–105 pg mL−1 and LOD was 0.15 pg mL−1.
In this subsection, the types of toxins found in food samples were investigated and the types of biosensors available for the detection of toxins based on electrochemical methods and using magnetic beads and the integration of these methods with microfluid devices were described.
7. Iron and iron-oxide magnetic nanoparticle-based electrochemical biosensors for the detection of other contaminants (estradiol, melamine, bisphenol, heavy metal, etc.) in food sample
Due to population growth and changing diet, and demand for nutritious foods, the trend towards foods of animal origin has increased. In order to meet nutritional needs, food manufacturers use veterinary drugs such as hormones and hormone-like anabolic agents such as estradiol, progesterone, testosterone, melengestrol acetate, propionate, zeranol, trenbolone acetate and bovine somatotropin to enhance the growth of farm animals and increase body division and improve body weight and increase feed conversion efficiency and productivity. Residues of these hormones in animal-derived foods, if beyond tolerance, act as a risk factor for potential public health problems.154,155Xanthine (3,7-dihydropurine-2,6-dione) is a biological compound with a purine structure found in all living organisms, including humans body cells, animals, and plants.156 Xanthine is produced by the synthesis and breakdown of the metabolic process of purine, and under the influence of the enzyme xanthine oxidase is converted to uric acid, which is broken down in the blood and excreted by the kidneys.157 Incomplete excretion of uric acid causes hyperuricemia, which is one of the most common causes of gout,158 diabetes,159 cerebral ischemia,160 and cardiovascular disease.161 Therefore, foods containing low purine are an excellent option to reduce the risk of these diseases.162 Also, it was offered as a powerful drug for treatment of asthma and bronchitis.163,164 Theophylline found in nuts, brewed tea, cola, and guarana also is a derivative of xanthine and is suggested as drug for respiratory diseases and asthma.165 In this regard, it is necessary to develop appropriate and cost-effective methods for detection of xanthine. Traditional methods for xanthine detection are high-pressure liquid chromatography,166 capillary column gas chromatography,167 fluorometric mass spectrometric.168 Despite their high sensitivity and selectivity, these methods have limitation such as costly equipment, time-consuming, and require an experienced operator and used to pro-treatment before analysis. Electrochemical sensors are excellent option for the recognition of xanthine.
In this regard, Emamian et al.169 designed an electrochemical platform based on Fe3O4/SWCNTs composite for the determination of xanthine and theophylline in fish meat, tea, and soft drinks. In this report, the applied concentration ranges were from 4.0 nM to 300 μM and from 0.1 to 300 μM for xanthine and theophylline, respectively.
Heavy metal (such as Hg2+, Pb2+, Zn2+, Cd2+, As3+, Cu2+, Ni2+, Cr2+, and etc.) is another class of food pollution. Anthropogenic or natural sources are the main source of their entry into environment. These metals may enter the soil or groundwater and accumulate in food webs that cause serious hazards to human health. Plants, especially green leafy plants, are the main source of food consumed around the world. Plants, especially green leafy plants, are the main source of food consumed around the world, which strongly collect heavy metals in themselves and cause great harm to human health. For example, exposure to cadmium and mercury causes kidney and lungs damage, respectively.170,171 So, for this purpose screening of heavy metals concentration in food samples is necessary.
Nor et al.172 designed an electrochemical sensor method based on carboxyl iron oxide nanoparticles (IONPs) for detecting Cd(II) and Pb(II) in seawater samples. In this report, the indium tin oxide (ITO) electrode functionalized with 3-aminopropyltriethoxysilane (APTES) was modified by carboxyl iron oxide nanoparticles. Using SWV technique these were determined in the linear of 10–100 ppb. The limits of detection of Cd(II) and Pb(II) ions were attained 0.90 and 0.60 ppb, respectively.
In general, iron oxide nanoparticles along with other nanomaterials such as bismuth plating,173 magnetic nanoparticles functionalized with polyamidoamine dendrimer,174 and fluorinated multiwalled carbon nanotube (MWCNT)175 using carbon-based electrodes, such as GCE176–178 and CPE179,180 have been used to identify heavy metals such as Zn(II), Cd(II), Pb(II) Cu(II), and Hg(II). In these researches, the detection limit has been achieved in the range of 0.02 to 0.21 ppb for the various type of candidate ions.
In conclusion, there is a lack of different types of magneto-electrochemical biosensors to determine hormonal and heavy metal contaminations. Also, other types of nanomaterials such as carbon-based nanomaterials (including MWCNTs, GO, GQDs, and rGO) and metallic nanomaterials (such as Ni, Bi2O3, MoS2, Ag NPs, CuNPs, ZnS, and AuNPs) have not been used yet. In general, there is very minor report on the use of magnetic beads and electrochemical methods for the detection of hormonal and heavy metal contamination in food sample.
Due to the strong electronegativity, some heavy metals such as Mn cannot be detected electrochemically by traditional electrodes because when the potential is negative, the solution hydrolyzes and produces bubbles. To overcome this limitation, lab-on-a chip sensor was developed. In example, researcher.181 developed a lab-on-a chip sensor for electrochemical detection Mn and Zn by anodic stripping voltammetry with a bismuth working electrode. Due to the negative potential window of bismuth and its low toxicity compared to the mercury electrode, it reduces the effects of hydrolysis of the solution containing heavy metals.
Scientists182 developed a lab chip sensor for the detection of lead (Pb(II)) in nature. The chip consists of microfluidic channels, silver working electrode, bismuth electrodes, a reference electrode. The square-wave anodic stripping voltammetry (SWASV) was applied for measurement. The concentration range was 1–1000 ppb and the LOD was 0.55 ppb. Duration 300 s.
Also, reserchers183 developed a 3D microfluidic electrochemical biosensor using screen-printed electrode (SPE) modified with Mn2O3 for the detection of Cd(II) and Pb(II) linear ranges were from 0.5 to 8 and 10 to 100 μg L−1 for Cd(II) and Pb(II), respectively. The LOD was 0.5 μg L−1 for Cd(II) and 0.2 μg L−1 for Pb(II).
Microfluidic technology enables portability and electrochemical method ensures accurate analysis. However, there are still many shortcomings in the existing technology. For example, the use of this technology has a limited scope compared to the colorimetric method, because only certain types of ions can be detected electrochemically. One limitation of the microfluidic chip analysis system is that it does not provide pretreatment, which prevents its practicality. We hope that this problem will be solved in the near future and the technology of electrochemical microfluidic detection will be developed with the current knowledge for detection of different types of ions and hormones will be used with further expansion.
In this subsection, we examined hormonal and heavy metal pollution using electrochemical methods and magnetic beads, and the advantages and limitations of this method were examined. The integration of electrochemical method with microfluidic devices for detection of heavy metals was also discussed. The use of smartphone technology in the final detection of these analytes is currently not used. In general, microfluidic devices and smartphones have been used very little to detect hormonal contamination and heavy metals.
8. Conclusion and future perspective
In this study, we tried to describe different types of food contaminants and draw attention to the serious health risks caused by these contaminants and highlight recent trends and new studies for the detection of these substances by iron magnetic beads and electrochemical methods. A combination of carbon, organic and metallic nanoparticles due to their excellent electrical conductivity to modify the electrode surface to create a high surface to stabilize different types of bioreceptors such as antibody, probe and aptamer, and also to strengthen the electrical signal together with iron magnetic beads can be used. Pathogenic bacteria, antibiotics and toxins are by far the most widely analyzed pollutants using several bioreceptors such as aptasensors and immunosensors and cell electrochemical sensors. Despite the many recent reports, there are still some issues that need immediate study and attention. For example, different types of allergens such as ingredients in eggs and milk, peanut, wheat, fish, soybean are responsible for different types of allergens, which unfortunately have received little attention. Another topic of recent concern is the genetic manipulation of food products, which must be addressed to prevent health consequences and meet the expectations of consumers who are aware of their rights to consume healthier and organic food products. Regarding the modified genes of food products, only a few of the modified genes have been revealed by electrochemical methods, and no aptasensors and genosensors have been designed for most of the manipulated genes.There are few reports about the evaluation of hormones and phenolic compounds and heavy metals by electrochemical methods and using magnetic beads (MBs). In general, by reviewing recent studies on food contamination detection methods, it is concluded that for the rapid evaluation of food contamination, methods must be devised that can detect several contaminating agents at the same time. For example, the launch of multi-channel electrochemical chips or the use of integrated methods such as the integration of microfluidic devices or smartphone technology with electrochemical methods and the use of nanomaterials, a combination of carbon and metal materials, which increase the detection sensitivity and reduce the evaluation time, are suitable methods for the evaluation of several analytes at the same time.
Considering the serious problems of food-related diseases, it is important to find new methods that can detect these contaminations even at very low concentrations under simple conditions. In this regard, the use of nanotechnology to design highly sensitive biosensors to evaluate food contamination has a promising future. Due to their superparamagnetic properties, good biocompatibility, low toxicity and ease of preparation, iron magnetic nanoparticles are widely used in biosensors. Iron magnetic nanoparticles are easily functionalized with various organic and biological molecules such as aptamers, polymers, enzymes, and antibodies and form signal tags. Therefore, the use of iron magnetic nanoparticles together with electromagnetic methods increases sensitivity and bonding strength of the target to the solid support.108,109 Due to the physicochemical characteristics of iron magnetic nanomaterials, it seems that in the future, their use along with electrochemical methods can be very effective in identifying food contamination. One of the great advantages of electrochemical methods is the possibility of using screen-printed electrodes, which have low manufacturing costs and low sample consumption, and it is possible to modify the surface.90,91 One of the disadvantages of screen-printed electrodes is that it is not possible to detect several biological molecules using specific antigens. Therefore, it affects its kinetics. Also, the surface of the electrode is destroyed during the washing process.92 The use of iron magnetic beads that have a large specific surface area and functional groups to connect different ligands solves this problem. One of the disadvantages of magnetic beads is that the size of the synthesized particles is probably not uniform and it affects the quality of their performance.90,92 The advantages of using magnetic molecularly imprinted polymer (MMIP) are the easy separation of samples using a magnetic field without the use of centrifugation or filtration, good biocompatibility, low toxicity, and strong paramagnetic properties, which is used to simplify the screen-printing electrode (SPE) process and increase the sensitivity of the electrochemical sensor.93–96 In conclusion, designing a method that combines the advantages of the methods mentioned above can overcome the disadvantages of these methods. In this review article, application of magnetic biosensors for the electrochemical recognition of different types of food contamination, such as microbial contamination, allergen, pesticide, genetically modified organisms, growth-promoting feed additives (β-agonists and antibiotics), toxin, other contaminants (estradiol, melamine, bisphenol, heavy metal, and etc.) have been investigated.
Finally, the advantages of using combined methods such as the integration of electrochemical methods and microfluidic devices and the use of smartphones in the final detection of the desired analyte and the use of methods that can reveal several contaminants at the same time or detect contamination from the cell surface without the need for sample pretreatment. It is one of the future developments in the field of detecting contamination in food.
The main limitation of the methods discussed in this article is the lack of use of real samples in the detection stages. Most of the methods discussed in this article are performed using synthetic analytes and are unable to detect the target analyte from real samples in complex matrices that contain other substances in addition to the target analyte. Also, the analysis and detection of several analytes at the same time cannot be separated and detected with these methods. Another issue is related to non-specific absorption, where other substances than the target analyte bind to the bioreceptors, causing background signals, to prevent amplification of background signals, proper washing steps and surface blocking should be performed. Therefore, research should be focused to increase the sensitivity and selectivity of nanosensors and lifetime-based detection methods. In the future, the use of hybrid nanocomposites such as iron oxide composites with graphene and carbon dots or nuclear structures with metal will provide tools for designing advanced biosensors. Also, the use of iron oxide nanomaterials as labels on biomolecules compared to traditional labels and the integration of iron-based electrochemical biosensors with microfluidic sensors have shown promising results.
Conflicts of interest
There is no conflict of interest.Acknowledgements
We thank University of Tabriz and Tabriz University of Medical Sciences for instrumental supporting of this research.References
- A. Bahrami, Z. Moaddabdoost Baboli, K. Schimmel, S. M. Jafari and L. Williams, Trends Food Sci. Technol., 2020, 96, 61–78 CrossRef CAS
.
- L. iang, M. M. Hassan, S. Ali, H. Li, R. Sheng and Q. Chen, Trends Food Sci. Technol., 2021, 112, 225–240 CrossRef
.
- Y. Cao, T. Feng, J. Xu and C. Xue, Biosens. Bioelectron., 2019, 141, 111447 CrossRef CAS PubMed
.
- J. C. Buzby and T. Roberts, Gastroenterology, 2009, 136, 1851–1862 CrossRef PubMed
.
- A. M. Shonhiwa, G. Ntshoe, V. Essel, J. Thomas and K. McCarthy, Int. J. Infect. Dis., 2019, 79, 2013–2017 Search PubMed
.
- E. Lewis, J. Hudson, N. Cook, J. Barnes and E. Haynes, J. Microbiol. Methods, 2020, 105840 CrossRef CAS PubMed
.
- G. Lopez-Campos, J. V. Martínez-Suárez, M. Aguado-Urda and V. López-Alonso, SpringerBriefs in Food, Health, and Nutrition, 2012, pp. 13–32 Search PubMed
.
- D. Sharma, A. Nagpal, Y. B. Pakade and J. K. Katnoria, Talanta, 2010, 82, 1077–1089 CrossRef CAS PubMed
.
- O. Ahumada and J. R. Villalobos, Eur. J. Oper. Res., 2009, 196, 1–20 CrossRef
.
- J. Spink, D. L. Ortega, C. Chen and F. Wu, Trends Food Sci. Technol., 2017, 62, 215–220 CrossRef CAS
.
- D. Rojas, F. Della Pelle, M. Del Carlo, E. Fratini, A. Escarpa and D. Compagnone, Microchim. Acta, 2019, 189, 1–9 Search PubMed
.
- F. Della Pelle, D. Rojas, A. Scroccarello, M. Del Carlo, G. Ferraro, C. Di Mattia, M. Martuscelli, A. Escarpa and D. Compagnone, Sens. Actuators, B, 2019, 296, 126651 CrossRef CAS
.
- F. Della Pelle, D. Rojas, A. Scroccarello and M. Del Carlo, Microchim. Acta, 2020, 187, 1–13 CrossRef PubMed
.
- A. Scroccarello, F. Della Pelle, G. Ferraro, E. Fratini and F. Tempera, Talanta, 2021, 222, 121682 CrossRef CAS PubMed
.
- D. Rojas, F. Della Pelle, M. Del Carlo, D. Compagnone and A. Escarpa, Electrochem. Commun., 2020, 115, 106718 CrossRef CAS
.
- Y. Zeng, Z. Zhu, D. Du and Y. Lin, J. Electroanal. Chem., 2016, 781, 147–154 CrossRef CAS
.
- N. Yadav, A. Mishra and J. Narang, Evaluation Technologies for Food Quality, 2019, pp. 793–815 Search PubMed
.
- A. Vasilescu and J. L. Marty, Trends Anal. Chem., 2016, 60–70 CrossRef CAS
.
- G. K. Mishra, A. Barfidokht, F. Tehrani and R. K. Mishra, Foods, 2018, 7(9), 141 CrossRef PubMed
.
- S. K. Vashist, E. Lam, S. Hrapovic, K. B. Male and J. H. T. Luong, Chem. Rev., 2014, 114, 11083–11130 CrossRef CAS PubMed
.
- M. Hasanzadeh, A. Bahrami and M. Alizadeh, RSC Adv., 2013, 3, 24237–24246 RSC
.
- I. Koh and L. Josephson, Sensors, 2009, 9, 8130–8145 CrossRef CAS PubMed
.
- J. Ma, S. Guo, X. Guo and H. Ge, Appl. Surf.
Sci., 2015, 353, 1117–1125 CrossRef CAS
.
- A. Walcarius, S. D. Minteer, J. Wang, Y. Lin and A. Merkoci, J. Mater. Chem. B, 2013, 1, 4878–4908 RSC
.
- I. M. Hsing, Y. Xu and W. Zhao, Electroanalysis, 2007, 19, 755–768 CrossRef CAS
.
- S. Krishnan and C. Walgama, Am. Chem. Soc., 2013, 85(23), 11420–11426 CAS
.
- M. Hasanzadeh, N. Shadjou and M. de la Guardia, Trends Anal. Chem., 2015, 72, 1–9 CrossRef CAS
.
- U. Schwertmann and R. Cornell, Iron oxides in the laboratory, VCH Publ. Inc., New York, 1991 Search PubMed
.
- I. M. Hsing, Y. Xu and W. Zhao, Electroanalysis, 2007, 19, 755–768 CrossRef CAS
.
- L. Stanciu, Y. H. Won and M. Ganesana, Sensors, 2009, 9, 2976–2999 CrossRef CAS PubMed
.
- M. Pastucha, Z. Farka, K. Lacina, Z. Mikušova and P. Skladal, Microchim. Acta, 2019, 186, 312 CrossRef PubMed
.
- T. Kavetskyy, M. Alipour, O. Smutok and O. Mushynska, Microchem. J., 2021, 167, 106320 CrossRef CAS
.
- M. Yar and K. Ayub, Microporous Mesoporous Mater., 2020, 300, 110146 CrossRef CAS
.
- G. A. Zelada-Guillén, S. V. Bhosale, J. Riu and F. X. Rius, Anal. Chem., 2010, 82(22), 9254–9260 CrossRef PubMed
.
- S. Akhtar, M. Sarker and A. Hossain, Crit. Rev. Microbiol., 2014, 40(4), 348–359 CrossRef CAS PubMed
.
- H. Lee and Y. Yoon, Food Sci. Anim. Resour., 2021, 41(1), 1–7 CrossRef PubMed
.
- World Health Organization [WHO], WHO estimates of the global burden of foodborne diseases, 2020 Search PubMed
.
- Centers for Disease Control and Prevention [CDC], Surveillance for foodborne disease outbreaks, United States, 2011, annual report, US Department of Health and Human Services, CDC, Atlanta, GA, USA, 2014 Search PubMed
.
- Centers for Disease Control and Prevention [CDC], Surveillance for foodborne disease outbreaks, United States, 2017, annual report, US Department of Health and Human Services, CDC, Atlanta, GA, USA, 2019 Search PubMed
.
- Z. Altintas, M. Akgun, G. Kokturk and Y. Uludag, Biosens. Bioelectron., 2018, 100, 541–548 CrossRef CAS PubMed
.
- D. G. Newell, M. Koopmans, L. Verhoef, E. Duizer, A. Aidara-Kane, H. Sprong and M. Opsteegh, Int. J. Food Microbiol., 2010, 139, S3–S15 CrossRef PubMed
.
- O. Lazcka, F. J. D. Campo and F. X. Muñoz, Biosens. Bioelectron., 2007, 22, 1205–1217 CrossRef CAS PubMed
.
- R. Ali, K. Al-Achkar, A. Al-Mariri and M. Safi, Egypt J. Med. Hum. Genet., 2014, 15, 293–298 CrossRef
.
- J. A. Jordan and M. B. Durso, J. Mol. Diagn., 2005, 7, 575–581 CrossRef CAS PubMed
.
- E. Galikowska, D. Kunikowska, E. Tokarska-Pietrzak, H. Dziadziuszko, J. M. Los and P. Golec, Eur. J. Clin. Microbiol. Infect. Dis., 2011, 30, 1067–1073 CrossRef CAS PubMed
.
- J. W. Lee, J. Y. Jung and S. K. Lim, Forensic Sci. Int. Genet., 2018, 33, 155–160 CrossRef CAS PubMed
.
- U. Sharaha, E. Rodriguez-Diaz, K. Riesenberg, I. J. Bigio, M. Huleihel and A. Salman, Anal. Chem., 2017, 89, 8782–8790 CrossRef CAS PubMed
.
- L. Richter, M. Janczuk-Richter, J. Niedziółka-Jönsson, J. Paczesny and R. Hołyst, Drug Discov. Today, 2018, 23, 448–455 CrossRef CAS PubMed
.
- N. Magan, A. Pavlou and I. Chrysanthakis, Sens. Actuators, B, 2001, 72, 28–34 CrossRef CAS
.
- D. Wilsona, E. M. Materóna, G. Ibáñez-Redína, R. C. Fariac and D. S. Correab, Talanta, 2019, 194, 611–618 CrossRef PubMed
.
- M. Freitas, S. Viswanathan, H. P. A. Nouws, M. B. P. P. Oliveira and C. Delerue-Matos, Biosens. Bioelectron., 2013, S0956–S5663 Search PubMed
.
- H. Jiang, D. Jiang, J. Shao and X. Sun, Biosens. Bioelectron., 2016, 15(75), 411–419 CrossRef PubMed
.
- E. B. Setterington and E. C. Alocilja, Biosensors, 2012, 2, 15–31 CrossRef CAS PubMed
.
- H. Jiang, D. Jiang, J. Shao and X. Sun, Biosens. Bioelectron., 2015, 75(15), 11–19 Search PubMed
.
- Y. Li, R. Afrasiabi, F. Fathi, N. Wang, C. Xiang and R. Love, Biosens. Bioelectron., 2014, 58, 193–199 CrossRef CAS PubMed
.
- K. Niemirowicz, I. Swiecicka, A. Z. Wilczewska, K. H. Markiewicz and U. S. A. Kułakowska, Colloids Surf. B Biointerfaces, 2015, 131, 29–38 CrossRef CAS PubMed
.
- V. Templier, A. Roux, Y. Roupioz and T. Livache, Trends Anal. Chem., 2016, 79, 71–79 CrossRef CAS
.
- L. T. Nguyen, E. F. Haney and H. J. Vogel, Trends Biotechnol., 2011, 29, 464–472 CrossRef CAS PubMed
.
- J. M. Ageitos, A. Sánchez-Pérez, P. Calo-Mata and T. G. Villa, Biochem. Pharmacol., 2017, 133, 117–138 CrossRef CAS PubMed
.
- J. J. BelBruno, Chem. Rev., 2019, 119, 94–119 CrossRef CAS PubMed
.
- S. A. Piletsky and A. P. Turner, Electroanalysis, 2002, 14(5), 317–323 CrossRef CAS
.
- M. Xu, R. Wang and Y. Li, Talanta, 2016, 148, 200–208 CrossRef CAS PubMed
.
- Y. Wang, P. A. Fewins and E. C. Alocilja, IEEE Sensor. J., 2015, 15, 4692–4699 CAS
.
- K. Kant, M. A. Shahbazi, V. Priy Dave, T. Anh Ngo, V. A. Chidambara and Q. T. Linh, Biotechnol. Adv., 2017, 36(4), 1003–1024 CrossRef PubMed
.
- Q. Chen, D. Wang, G. Cai, Y. Xiong, Y. Li, M. Wang, H. Huo and J. Lin, Biosens. Bioelectron., 2016, 86, 770–776 CrossRef CAS PubMed
.
- H. Jiang, Z. Sun, Q. Guo and X. Weng, Biosens. Bioelectron., 2021, 182, 113191 CrossRef CAS PubMed
.
- F. Tan, P. H. M. Leung, Z. Liu, Y. Zhang, L. Xiao, W. Ye and X. Zhang, Sens. Actuators, B, 2011, 159, 328–335 CrossRef CAS
.
- K. Kant, M. A. Shahbazi, V. Priy Dave, T. Anh Ngo, V. A. Chidambara and Q. T. Linh, Biotechnol. Adv., 2017, 4(36), 3–24 Search PubMed
.
- T. Yang, Z. Luoad, T. Bewal and L. Li, Food Chem., 2022, 394(15), 133534 CrossRef CAS PubMed
.
- K. Kant, M. A. Shahbazi, V. P. Dave, T. A. Ngo, V. A. Chidambara, Q. T. Linh and D. D. Bang, Biotechnol. Adv., 2017, 36(4), 100–102 Search PubMed
.
- A. Cianferoni and J. M. Spergel, Allergol. Int., 2009, 58, 457–466 CrossRef CAS PubMed
.
- A. W. Burks, M. Tang, S. Sicherer, A. Muraro, P. A. Eigenmann, M. Ebisawa, A. Fiocchi, W. Chiang, K. Beyer, R. Wood, J. Hourihane, S. M. Jones, G. Lack and H. A. Sampson, ICON: food allergy, J. Allergy Clin. Immunol., 2012, 129, 906–920 CrossRef PubMed
.
- A. Vasilescu, G. Nunes, A. Hayat, U. Latif and J. Marty, Sensors, 2016, 16(11), 1863 CrossRef PubMed
.
- M. Prado, I. Ortea, S. Vial and J. Rivas, Crit. Rev. Food Sci. Nutr., 2016, 56(15), 2511–2542 CrossRef CAS PubMed
.
- M. Cadkov, R. Metelka, L. Holubová, D. Horák, V. Dvořáková, Z. Bílková and L. Korecká, Anal. Biochem., 2015, 484, 4–8 CrossRef PubMed
.
- K. M. Navarro, J. C. Silva and M. V. Ossick, Anal. Lett., 2020, 54(7), 1180–1192 CrossRef
.
- H. Jiang, D. Jiang, P. Zhu, F. Pi, J. Ji, C. Sun, J. Sun and X. Sun, Biosens. Bioelectron., 2016, 83, 126–133 CrossRef CAS PubMed
.
- T. A. Baldo, C. A. Proença and F. da Silva Felix, Talanta, 2021, 232, 122447 CrossRef CAS PubMed
.
- H. Y. Lin, C. H. Huang, J. Park, D. Pathania and C. M. Castro, ACS Nano, 2017, 11, 10062–10069 CrossRef CAS PubMed
.
- D. Jiang, P. Zhu, H. Jiang, J. Ji, X. Sun, W. Gu and G. Zhang, Biosens. Bioelectron., 2015, 70, 482–490 CrossRef CAS PubMed
.
- L. Zheng, F. Seidi, Y. Liu, W. Wu and H. Xiao, Eur. Polym. J., 2022, 111432 CrossRef CAS
.
- M. L. Xu, Y. Gao, X. X. Han and B. Zhao, J. Agric. Food Chem., 2017, 65, 6719–6726 CrossRef CAS PubMed
.
- U. Rajaji, S. Chinnapaiyan, S. M. Chen, M. Govindasamy, J. I. de Oliveira Filho, W. Khushaim and V. Mani, ACS Appl. Mater. Interfaces, 2021, 13, 24865–24876 CrossRef CAS PubMed
.
- H. Yin, Y. Zhou, X. Meng, T. Tang, S. Ai and L. Zhu, Food Chem., 2011, 127, 1348–1353 CrossRef CAS PubMed
.
- J. Miao, A. Liu, L. Wu, M. Yu, W. Wei and S. Liu, Anal. Chim. Acta, 2019, 1095, 82–92 CrossRef PubMed
.
- Q. Tang, X. Shi, X. Hou, J. Zhou and Z. Xu, Analyst, 2014, 2–26 Search PubMed
.
- R. Luo, Z. Feng, G. Shen, Y. Xiu, Y. Zhou, X. Niu and H. Wang, Sensors, 2018, 18, 4429 CrossRef PubMed
.
- P. Aruna, P. Reddy, P. P. Sandhya, N. V. S. S. Seshagiri Rao and N. Y. Sreedhar, Biointerface Res. Appl. Chem., 2022, 12(5), 5772–5784 CAS
.
- N. F. Marinho Rodrigues, S. Y. Neto, R. de Cássia Silva Luz, F. S. Damos and H. Yamanaka, Biosensors, 2018, 8(16) Search PubMed
.
- F. Ricci, G. Volpe, L. Micheli and G. Palleschi, Anal. Chim. Acta, 2007, 605, 111–129 CrossRef CAS PubMed
.
- P. Fanjul-Bolado, D. Hernández-Santosa, P. J. Lamas-Ardisanaa, A. MartínPerníab and A. Costa-Garcíaa, Electrochim. Acta, 2008, 53, 3635–3642 CrossRef CAS
.
- F. Ricci, G. Adornetto and G. Palleschi, Electrochim. Acta, 2012, 84, 74–83 CrossRef CAS
.
- L. Zhu, D. Pan, L. Ding, F. Tang, Q. L. Zhang, Q. Liu and S. Z. Yao, Talanta, 2010, 80, 1873–1880 CrossRef CAS PubMed
.
- Z. C. Zhang, L. M. Zhang, L. Chen, L. G. Chen and Q. H. Wan, Biotechnol. Prog., 2006, 22, 514–518 CrossRef CAS PubMed
.
- Q. Q. Gai, F. Qu, Z. J. Liu, R. J. Dai and Y. K. Zhang, J. Chromatogr., A, 2010, 1217, 5035–5042 CrossRef CAS PubMed
.
- T. Jing, H. R. Du, Q. Dai, H. Xia, J. W. Niu, Q. L. Hao, S. R. Mei and Y. K. Zhou, Biosens. Bioelectron., 2010, 26, 301–306 CrossRef CAS PubMed
.
- J. Bunney, S. Williamson, D. Atkin, M. Jeanneret and D. Cozzolino, Curr. Res. Nutr. Food Sci., 2017, 5(3), 183–195 Search PubMed
.
- K. Islam, R. Chand, D. Han and Y. S. Kim, Bull. Environ. Contam. Toxicol., 2015, 94, 41–45 CrossRef CAS PubMed
.
- A. Kling, C. Chatelle, L. Armbrecht, E. Qelibari, J. Kieninger, C. Dincer, W. Weber and G. Urban, Anal. Chem., 2016, 88, 10036–10043 CrossRef CAS PubMed
.
- A. Hassan, L. Sappia, S. L. Moura, F. H. M. Ali, W. A. Moselhy and M. del Pilar, Talanta, 2018, 194, 997–1004 CrossRef PubMed
.
- ISAAA, Global Status of Commercialized Biotech/GM Crops, ISAAA Brief, 2016 Search PubMed
.
- M. A. Arugula, Y. Zhang and A. I. Simonian, Anal. Chem., 2013, 86(1), 119–129 CrossRef PubMed
.
- S. Kamle and S. Ali, Gene, 2013, 522(2), 123–132 CrossRef CAS PubMed
.
- A. Plácido, J. Amaral, J. Costa, T. Fernandes, M. Oliveira, C. Delerue-Matos and I. Mafra, Novel Strategies for Genetically Modified Organism Detection, Regulation and Public Health, Waltham, MA, USA, 2016, pp. 119–131 Search PubMed
.
- A. Plácido, C. Pereira, A. Guedes, M. F. Barroso, R. M. Castro, N. d. los-Santos-Álvarez and C. D. Matos, Biosens. Bioelectron., 2018 Search PubMed
.
- M. C. Castro Freitas, M. M. Sá Couto, M. F. Barroso and C. Pereira, ACS Sens., 2016, 14, 156–163 Search PubMed
.
- Y. Ye, S. Mao, S. He, X. Xu, X. Cao, Z. Wei and S. Gunasekaran, Talanta, 2020, 206, 120205 CrossRef CAS PubMed
.
- J. B. Sousaa, J. R. Jesusc, L. C. Silvad, C. Pereirae, N. de-los-Santos-Álvarezf, R. A. S. Fonsecad, R. Miranda-Castrof, C. Delerue-Matosb, J. Ribeiro Santos Júniora and M. Fátima Barroso, Talanta, 2020, 206, 120220 CrossRef PubMed
.
- A. Akbarzadeh, M. Samiei and S. Davaran, Nanoscale Res. Lett., 2012, 7, 144 CrossRef PubMed
.
- E. Palecek and M. Fojta, Talanta, 2007, 74, 276–290 CrossRef CAS PubMed
.
- G. Carpini, F. Lucarelli, G. Marrazza and M. Mascini, Biosens. Bioelectron., 2004, 20, 167–175 CrossRef CAS PubMed
.
- F. Lucarelli, G. Marrazza and M. Mascini, Biosens. Bioelectron., 2005, 20, 2001–2009 CrossRef CAS PubMed
.
- J. Wang, Q. Sheng, N. Tian, L. Chen, Z. Xu and J. Zheng, J. Appl. Electrochem., 2009, 39, 935–945 CrossRef CAS
.
- W. C. Liao, M. C. Chuang and J. a. A. Ho, Biosens. Bioelectron., 2013, 50, 414–420 CrossRef CAS PubMed
.
- X. Jiang, K. Chen and H. Han, Biosens. Bioelectron., 2011, 28, 464–468 CrossRef CAS PubMed
.
- J. Tosar, G. Branas and J. Laiz, Biosens. Bioelectron., 2010, 26, 1205–1217 CrossRef CAS PubMed
.
- D. Wu, D. Du and Y. Lin, Trends Anal. Chem., 2016, 83, 95–101 CrossRef CAS
.
- C. Wenk, Asian-Australas. J. Anim. Sci., 2000, 13(1), 86–95 CrossRef CAS
.
- H. Xiong, C. Guo, P. Liu, W. Xu, X. Zhang and S. Wang, Anal. Chem., 2014, 86(10), 4729–4738 CrossRef CAS PubMed
.
- W. McK. Jefferies, Cortisol and immunity, Med. Hypotheses, 1991, 34, 198–208 CrossRef CAS PubMed
.
- M. J. Yaeger, K. Mullin and S. M. Ensley, Vet. Pathol., 2012, 49, 569–573 CrossRef CAS PubMed
.
- T. Peng, A. L. Royer, Y. Guitton, B. L. Bizec and G. Dervilly-Pinel, Metabolomics, 2017, 13, 77–91 CrossRef
.
- G. Brambilla, T. Cenci, F. Franconi, R. Galarini, A. Macrı`, F. Rondoni, M. Strozzi and A. Loizzo, Toxicol. Lett., 2000, 114, 47–53 CrossRef CAS PubMed
.
- H. Zhang, G. Liu and C. Chai, Sensor. Actuator. B Chem., 2012, 168, 103–110 CrossRef CAS
.
- Y. Poo-arporn, S. Pakapongpan, N. Chanlek and R. P. Poo-Arporn, Sens. Actuators, B, 2018, 284, 164–171 CrossRef
.
- M. D. Barton, Nutr. Res. Rev., 2000, 13, 279–299 CrossRef CAS PubMed
.
- H. Gaskins, C. Collier and D. Anderson, Anim. Biotechnol., 2002, 13(1), 29–42 CrossRef CAS PubMed
.
- K. Brown, R. R. Uwiera, M. L. Kalmokoff and S. P. Brooks, Int. J. Antimicrob. Agents, 2017, 49(1), 12–24 CrossRef CAS PubMed
.
- K. Giribabu, S. C. Jang, Y. Haldorai and M. Rethinasabapathy, Carbon Lett., 2017, 23, 38–47 Search PubMed
.
- X. Liu, S. Zheng, Y. Hu, Z. Li, F. Luo and Z. He, Food Anal. Methods, 2016, 56, 128–139 Search PubMed
.
- C. Pizan-Aquino, A. Wong, L. Aviles-Felix, S. Khan, G. Picasso and M. D. P. T. Sotomayor, Mater. Chem. Phys., 2020, 245, 122777 CrossRef CAS
.
- S. Yu, Q. Wei, B. Du, D. Wu, H. Li, L. Yan, H. Ma and Y. Zhang, Biosens. Bioelectron., 2013, 48, 224–229 CrossRef CAS PubMed
.
- K. Giribabu, S. C. Jang, Y. Haldorai, M. Rethinasabapathy and S. Yeong Oh, Carbon Lett., 2017, 23, 38–47 Search PubMed
.
- S. Jahanbani and A. Benvidi, Biosens. Bioelectron., 2016, 85, 553–562 CrossRef CAS PubMed
.
- F. Long, Z. Zhang, Z. Yang, J. Zeng and Y. Jiang, J. Electroanal. Chem., 2015, 755, 7–14 CrossRef CAS
.
- Y. Song, F. Duan, S. Zhang, J. Yue Tian, Z. Zhang, Z. W. Wang, C. S. Liu, W. M. Xu and M. Du, J. Mater. Chem. A, 2013, 1–3 Search PubMed
.
- W. Guoa, A. Umarb, M. A. Alsaiari, L. Wang and M. Pei, Microchem. J., 2020, 158, 105270 CrossRef
.
- J. Yin, X. Qin, Q. Wang and Y. Yin, RSC Adv., 2016, 6, 39401–39408 RSC
.
- J. Yin, W. Guo, X. Qin, J. Zhao, M. Pei and F. Ding, Sens. Actuators, B, 2017, 241, 151–159 CrossRef CAS
.
- A. Joshi and K. H. Kim, Biosens. Bioelectron., 2020, 153, 112046 CrossRef CAS PubMed
.
- A. Kling, C. Chatelle, L. Armbrecht, E. Qelibari and J. Kieninger, Anal. Chem., 2016, 88, 10036–10043 CrossRef CAS PubMed
.
- K. Todar, The mechanisms of bacterial pathogenicity, in Todar, 2nd edn, 2009 Search PubMed
.
- S. S. Arnon, R. Schechter, T. V. Inglesby, D. A. Henderson, J. G. Bartlett, M. S. Ascher, E. Eitzen, A. D. Fine, J. Hauer and M. Layton, Jama, Consensus Statement, 2001, vol. 285, pp. 1059–1070 Search PubMed
.
- R. H. Wang, J. J. Zhao, T. S. Jiang, Y. M. Kwon, H. G. Lu, P. R. Jiao, M. Liao and Y. B. Li, J. Virol Methods, 2013, 189, 362–369 CrossRef CAS PubMed
.
- M. Campàs, D. Garibo and B. Prieto-Simón, Analyst, 2012, 137(5), 1055–1067 RSC
.
- F. Shang, Y. Liu, S. Wang, Y. Hu and Z. Guo, Electroanalysis, 2017, 29, 1–9 CrossRef
.
- Y. Liu, T. Yan, Y. Li, W. Cao, X. Pang, D. Wu and W. Qin, RSC Adv., 2015, 5, 19581 RSC
.
- R. Chauhan and T. Basu, J. Nanomater., 2015, 15, 2015 Search PubMed
.
- B. H. Nguyen, L. D. Tran, Q. P. Do and H. Le Nguyen, Mater. Sci. Eng. C, 2013, 33, 2229–2234 CrossRef CAS PubMed
.
- L. Wu, B. Gao, F. Zhang, X. Sun, Y. Zhang and Z. Li, Talanta, 2013, 106, 360–366 CrossRef CAS PubMed
.
- N. V. Panini, E. Salinas, G. A. Messina and J. Raba, Food Chem., 2011, 125, 791–796 CrossRef CAS
.
- P. B. Lillehoj, F. Weib and C. M. Ho, Lab Chip, 2010, 10, 2265–2270 RSC
.
- W. Hao, Y. Ge, M. Qu, Y. Wen, H. Liang, M. Li and C. Chen, J. Food Compos. Anal., 2022, 107, 104360 CrossRef
.
- B. B. Hirpessa, B. H. Ulusoy and C. Hecer, J. Food Qual., 2020, 12, 12 Search PubMed
.
- K. E. Nachman and T. J. Smith, Curr. Environ. Health Rep., 2015, 2(1), 1–14 CrossRef CAS PubMed
.
- G. V. Waeg, F. Niklasson and C. H. D. Verdier, Pediatr. Res., 1985, 19, 780 Search PubMed
.
- D. A. Kostić, D. S. Dimitrijević and G. S. Stojanović, J. Chem., 2015, 1–8 Search PubMed
.
- K. Y. Kim, H. R. Schumacher and E. Hunsche, Clin. Therapeut., 2003, 25, 1593–1617 CrossRef PubMed
.
- S. N. Davis and G. Lastra-Gonzalez, J. Clin. Endocrinol. Metab., 2008, 93(8), E2 CrossRef
.
- T. Ono, R. Tsuruta and M. Fujita, Brain Res., 2009, 1305, 158–167 CrossRef CAS PubMed
.
- R. H. Nelson, Primary Care: Clin. Off. Pract., 2013, 40(1), 195–211 CrossRef PubMed
.
- S. Hayman and W. Marcason, J. Am. Diet Assoc., 2009, 109, 1652 CrossRef PubMed
.
- R. S. Pagliarussi, L. A. Freitas and J. K. Bastos, J. Sep. Sci., 2002, 25, 371 CrossRef CAS
.
- C. Persson, I. Erjefalt and J. A. Karlsson, Basic Clin. Pharmacol. Toxicol., 1981, 49, 317 CAS
.
- F. Belliardo, A. Martelli and M. G. Valle, Unters. Forsch., 1985, 180, 398 CrossRef CAS PubMed
.
- R. Kock, B. Delvoux and H. Greiling, Clin. Chem. Lab. Med., 1993, 31, 303–310 CAS
.
- R. S. Pagliarussi, L. A. P. Freitas and J. K. Bastos, J. Sep. Sci., 2002, 25, 371–374 CrossRef CAS
.
- R. Olojo, R. Xia and J. Abramson, Anal. Biochem., 2005, 339, 338–344 CrossRef CAS PubMed
.
- R. Emamian, M. Ebrahimi and H. Karimi-Maleh, J. Electrochem. Soc., 2018, 165(14), B762 CrossRef CAS
.
- N. K. Srivastava and C. B. Majumder, J. Hazard. Mater., 2008, 151, 1–8 CrossRef CAS PubMed
.
- M. Tytła, Int. J. Environ. Res. Public Health, 2019, 16, 2430 CrossRef PubMed
.
- N. Mohamad Nor, S. Arivalakan, N. D. Zakaria, N. Nilamani, Z. Lockman and K. A. Razak, ACS Omega, 2022, 7(4), 3823–3833 CrossRef CAS PubMed
.
- S. Lee, J. Oh, D. Kim and Y. Piao, Talanta, 2016, 160, 528–536 CrossRef CAS PubMed
.
- B. Maleki, M. Baghayeri, M. Ghanei-Motlagh, F. Mohammadi Zonoz, A. Amiri, F. Hajizadeh, A. Hosseinifar and E. Esmaeilnezhad, Measurement, 2019, 140, 81–88 CrossRef
.
- W. Wu, M. Jia, Z. Zhang, X. Chen, Q. Zhang, W. Zhang, P. Li and L. Chen, Ecotoxicol. Environ. Saf., 2019, 175, 243–250 CrossRef CAS PubMed
.
- F. Leroux, E. Raymundo-Pinero, J.-M. Fedelec, F. Beguin and J. Mater, Chem, 2006, 16, 2074 CAS
.
- A. Sayari and S. Hamoudi, Chem. Mater., 2001, 13, 3151 CrossRef CAS
.
- L. Yang, Y. Qi, X. Yuan, J. Shen and K. Jiman, J. Mol. Catal. Chem., 2005, 229, 199 CrossRef CAS
.
- A. Lapkin, B. Bozkaya, T. Mays and L. Orillo, Catal. Today, 2003, 81, 611 CrossRef CAS
.
- A. Kukovecz, Z. Balogi, Z. Konya, M. Toba, P. Lentz, S.-I. Niwa, F. Mizukami, A. Molnar, J. B. Nagy and I. Kiricsi, Appl. Catal., A, 2002, 228, 83 CrossRef CAS
.
- E. B. Bahadır and M. K. Sezgintürk, Electrochemical biosensors for hormone analyses, Biosens. Bioelectron, 2015, 68, 62–71 CrossRef PubMed
.
- M. Hasanzadeh, A. Bahrami, M. Alizadeh and N. Shadjou, RSC Adv., 2013, 3, 24237–24246 RSC
.
- J. Wang, Electroanalysis, 2005, 17, 7–14 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2023 |
