Open Access Article
Open Access ArticlePreparation of graphitized carbon by the corn straw liquefaction method
Shuo Daia,
Yao Fua,
Peng Hea,
Li Mu *a,
Pengfei Liub and
Yuyan Zhangc
*a,
Pengfei Liub and
Yuyan Zhangc
aCollege of Food Science and Engineering, Changchun University, Changchun, Jilin 130022, China. E-mail: mul@ccu.edu.cn
bSchool of Chemical Engineering in Changchun University of Technology, Changchun, Jilin 130023, China
cNorth China Electric Power University, Beijing, 071003, China
First published on 18th January 2023
Abstract
Corn straw-based graphitized carbon was prepared by carbonization and a catalyzed graphitization method using corn straw as the raw material and catalytic liquefaction technology. A mixture of polyethylene glycol (PEG200) compounded with glycerol in the mass ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 was used as the liquefied agent, meanwhile 0.3 g of hydroxydiethylidene glycolic acid acted as the liquefied catalyst. The liquefied products were treated via carbonization and graphitization processes to form graphitized carbon. The graphitized carbon showed better graphitization, a microscopic lamellar structure, and smaller defects, when the carbonized temperature was 600 °C, graphitization temperature 850 °C, and the catalyst was ferric acetylacetonate at a concentration of 7.0 mmol g−1. The corn straw-based graphitized carbon yield reached 22.20%.
3 was used as the liquefied agent, meanwhile 0.3 g of hydroxydiethylidene glycolic acid acted as the liquefied catalyst. The liquefied products were treated via carbonization and graphitization processes to form graphitized carbon. The graphitized carbon showed better graphitization, a microscopic lamellar structure, and smaller defects, when the carbonized temperature was 600 °C, graphitization temperature 850 °C, and the catalyst was ferric acetylacetonate at a concentration of 7.0 mmol g−1. The corn straw-based graphitized carbon yield reached 22.20%.
1 Introduction
As a renewable biomass resource, corn straw is widely distributed in nature and has abundant resources. The main components of corn straw are cellulose, hemicellulose, and lignin,1,2 and is a kind of energy material that human beings have been using since long ago; however, how to deal with straw crops reasonably has become one of the hot spots to be solved among current environmental pollution problems. The preparation of graphite materials from corn straw can realize the high value utilization of corn straw to a certain extent.At present, the main technologies for the liquefaction method of corn straw are microwave chemical liquefaction,3,4 catalyzed liquefaction,5,6 hydrothermal liquefaction,7–10 rapid pyrolytic liquefaction,11 liquefaction by enzymatic fermentation,12–14 and super/near-critical technology.15–17 There are many studies using various liquefaction technologies to process corn straw to obtain direct products, such as bio-oil, ethanol, resins, and polyurethane, but the liquefaction technology seems to be face certain barriers. On the one hand, some liquefied reactions require the catalysts to promote the reaction progress and get more favorable factors to prepare the corresponding products by increasing the liquefaction rate. In this case, strong acids and bases, such as concentrated sulfuric acid, are used as catalysts, which inevitably requires consideration of the impact of the use of strong corrosive reagents in experimental operations, waste liquid disposal, and the environment. On the other hand, when a relatively mild catalyst is added to the reaction system, the liquefaction effect is reduced or even greatly diminished compared to the former. Therefore, how to balance these two differentiated situations through improvement of the experimental conditions and technical means to be able to use more moderate conditions and reagents to prepare higher quality, high-performance products will inevitably be a subject for future long-term investigation and exploration.
In the experiment, polyethylene glycol (PEG200) compounded with glycerol in the mass ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 was used as the liquefied agent for experimental investigation of the liquefaction process of corn straw. After corn straw is liquefied, the liquefied products are used as raw materials for graphitization for two main reasons. One is that corn straw contains hemicellulose and lignin, which are unfavorable for the formation of thin graphene materials.18 Being liquefied, the three major components of corn straw are degraded to varying degrees, which is beneficial for the recombination of graphitized carbon. Second, the normal biomass will be degassed and dehydrated during the carbonization process, while the remaining parts that are difficult to be liquefied during the liquefaction process will be shaped into a stronger carbon skeleton after carbonization, and the graphitization effect will be better. In this study, graphitic carbon materials were prepared from inexpensive woody biomass feedstock corn straw using combinatorial technological innovation to provide the base material for the exfoliation of high-quality graphene.
3 was used as the liquefied agent for experimental investigation of the liquefaction process of corn straw. After corn straw is liquefied, the liquefied products are used as raw materials for graphitization for two main reasons. One is that corn straw contains hemicellulose and lignin, which are unfavorable for the formation of thin graphene materials.18 Being liquefied, the three major components of corn straw are degraded to varying degrees, which is beneficial for the recombination of graphitized carbon. Second, the normal biomass will be degassed and dehydrated during the carbonization process, while the remaining parts that are difficult to be liquefied during the liquefaction process will be shaped into a stronger carbon skeleton after carbonization, and the graphitization effect will be better. In this study, graphitic carbon materials were prepared from inexpensive woody biomass feedstock corn straw using combinatorial technological innovation to provide the base material for the exfoliation of high-quality graphene.
2 Materials and instruments
2.1 Experimental materials and reagents
Corn straw powder was provided by Jilin COFCO Biochemical Energy Sales Co. Ltd, China. Polyethylene glycol 200 (PEG 200) was obtained from Tianjin Guangfu Fine Chemical Research Institute. Glycerol (GL) and anhydrous ethanol were ordered from Shandong Jinan Mingliang Chemical Co. Ltd. Hydroxyethylene bisphosphate (HEDP), ferric acetylacetonate, ferrocene, ferric oxalate, and ferric citrate were ordered from Shanghai Macklin Biochemical Co., Ltd.2.2 Experimental instruments
An electronic analytical balance (Huazhi Electronic Technology Co., Ltd, China) was used to weigh the materials and samples. A vacuum drying oven (Shanghai Xinmiao Medical Equipment Co., Ltd, China) was used to remove water from the materials. A tube furnace (Luoyang Pure Green Furnace Co., Ltd, China) was used to heat the materials and samples.Powder XRD patterns were obtained using a BRUKER diffractometer with Cu radiation (λ = 0.15418 nm). Scanning electron microscopy (SEM) was done on a JSM-7610F electron microscope. Raman spectroscopy was performed on a LabRAM HR Evolution system. Fourier transform infrared spectroscopy (FTIR) was done on a NICOLET IS 10 system.
Brunauer–Emmer–Teller (BET) analysis was performed with a Quantachrome® ASiQwin™, Quantachrome Instruments v2.02 with nitrogen (N2) gas used as an absorptive for the determination of the surface area.
3 Experimental methods and mechanisms
3.1 Principle of the liquefaction of the polyhydroxy alcohols
The direct liquefaction process of corn straw is a complex reaction process, consisting of the alcoholysis reaction with cellulose, hemicellulose, and lignin used as the reaction substances, the phenolic condensation reaction of liquefaction products, the hydroxylaldehyde condensation reaction, and the esterification condensation reaction. However, under relatively mild reaction conditions, the liquefaction reaction has some priority over the condensation reaction of the liquefaction products.193.2 Corn straw liquefaction process
The liquefaction process of corn straw was the first step in our experiments, and its liquefied products were the raw materials for the subsequent carbonization and graphitization processes. This study focused on investigating the feasibility of preparing graphitic carbon materials from corn straw after liquefaction and the degree of graphitization of the products. Since the solid–liquid ratio factor in the liquefaction process has a large influence on the state of the graphitized products, the liquefaction experimental conditions were referred to the relevant literature,20 and only the solid–liquid ratio was initially investigated for its influence on the liquefied yield. The solid–liquid ratio was then analyzed as a subsequent factor affecting the degree of graphitization.First, 10.0 g of corn straw powder was weighed and placed into a high-pressure reactor, with liquefied agent added that was compounded by polyethylene glycol (PEG200) and glycerol in a mass ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, with 0.3 g of hydroxyethylene bisphosphate also added as the catalyst in to the high-pressure reactor. Then, the high-pressure reactor was tightened and put into a preheated vacuum drying oven for the liquefaction reaction, with a reaction temperature of 140 °C and reaction time of 5 h. Then, the high-pressure reactor was cooled to room temperature after finishing the experimental reaction.
3, with 0.3 g of hydroxyethylene bisphosphate also added as the catalyst in to the high-pressure reactor. Then, the high-pressure reactor was tightened and put into a preheated vacuum drying oven for the liquefaction reaction, with a reaction temperature of 140 °C and reaction time of 5 h. Then, the high-pressure reactor was cooled to room temperature after finishing the experimental reaction.
Eqn (1) was used to calculate the liquefaction yield of corn straw.
 | (1) |
3.3 Principle of preparing graphitized carbon from the liquefaction products
Corn straw liquefied products as raw material underwent carbonization and graphitization with an iron-based catalyst and suitable reaction temperature and the system was blown by N2 gas. The iron-based catalysts decompose into the liquid phase, and through the carburization of the iron-based catalyst flow from the interstices to encapsulate the carbon element in corn straw to form layers between the carbon and polymers with multiple groups.21,22 The dissolved carbon gradually forms fixed graphitized carbon during the annealing process to room temperature, and at the same time, the iron elements agglomerate on and around the graphite carbon surface due to cooling to appear as irregular agglomerates. Then, the graphitized product was washed with hydrochloric acid solution to remove the iron elements, until finally the corn straw-based graphitized carbon is obtained (Fig. 1).3.4 Carbonization of the liquefied products
The liquefied products of corn straw were weighed and placed in a corundum crucible, then the crucible was put into the middle section of the tube furnace, in which the liquefied products were changed, under the reaction temperatures of 400 °C, 500 °C, 600 °C, at a temperature increase rate of 5 °C min−1, while the process of reaction was aiding by blowing N2 gas in to the tube furnace. When reaction was terminated, the temperature was kept at a constant set temperature for 1.0 h. The obtained calcination product was soaked in 20 wt% anhydrous ethanol, ground, and then filtered by extraction. The carbonized liquefied products were obtained after drying at 80 °C for 12.0 h.Eqn (2) was used to calculate the carbonization yield.
 | (2) |
3.5 Graphitization of the carbonized products
First, 1.0 g of carbonized products was weighed, and mixed respectively with 7.0 mmol of the iron-based catalyst, which was one of the four different iron-based catalysts, namely ferric acetylacetonate, ferrocene, ferric oxalate, and ferric citrate. According to the carbonization method in Section 3.4, when the reaction temperature was 850 °C, it was held there for one hour, and then the calcination products were removed by grinding, soaking in hydrochloric acid solution, followed by ultrasonication for 1 h to remove the iron element. Graphitized carbon was obtained after drying at 80 °C for 12.0 h, and calculated according to the following equation.
 | (3) |
4 Results and discussion
4.1 Effect of the solid–liquid ratio on the liquefied corn straw
According to the liquefaction method of corn straw outlined in Section 3.2, with the increase in the solid–liquid ratio, the liquefaction yield of corn straw showed an increasing trend. When the solid–liquid ratio increased from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to 1
2 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, the liquefaction yield of corn straw reached a maximum of 24.71% as shown in Fig. 2.
3, the liquefaction yield of corn straw reached a maximum of 24.71% as shown in Fig. 2.
 | ||
Fig. 2 Photograph of the mixed liquefaction products of corn straw with solid–liquid ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–1 1–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (a). Effect of the solid–liquid ratio on the liquefaction yield of corn straw (b). 4 (a). Effect of the solid–liquid ratio on the liquefaction yield of corn straw (b). | ||
4.2 Effect of the solid–liquid ratio on the carbonization of the liquefied products
After the liquefied products of corn straw were treated in line with the processes described in Sections 3.2 and 3.4, with the increase in the solid–liquid ratio, the carbonization yield overall decreased, as seen in Fig. 3, because when the solid–liquid ratio increased, there would be more of the liquid part of the mixed liquefaction products and less of the solid carbon products after the liquefied products of corn straw were heated and charred, so the carbonization yield would drop. | ||
Fig. 3 Photograph of the completely carbonized product with a solid–liquid ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (a). Effect of the solid–liquid ratio on the carbonization yield (b). 2 (a). Effect of the solid–liquid ratio on the carbonization yield (b). | ||
4.3 Single-factor experiment on graphitization
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 1
0, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, and the residues were detected by X-ray diffraction and Raman spectroscopy. The resulting chromatograms are shown in Fig. 4.
4, and the residues were detected by X-ray diffraction and Raman spectroscopy. The resulting chromatograms are shown in Fig. 4.
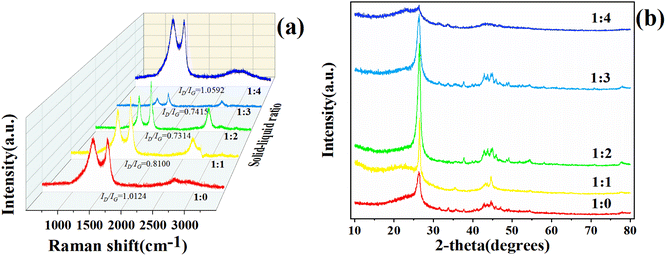 | ||
| Fig. 4 Raman spectra of the residues in different solid–liquid ratios (a). X-ray diffraction patterns of the residues in different solid–liquid ratios (b). | ||
It was found that degree of graphitization of the residues were different for the different solid–liquid ratios. The first peak around 1000 to 1400 cm−1 was the D peak, and the peak around 1400 to 1600 cm−1 was the G peak. The ID/IG value was used to indicate the degree of graphitization,23 whereby the smaller the ratio of ID/IG, the better the graphitization degree. When the solid–liquid ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, ID/IG was 0.7314, which was the smallest, with the graphitized degree obvious from Fig. 4(a).
2, ID/IG was 0.7314, which was the smallest, with the graphitized degree obvious from Fig. 4(a).
The characteristic peaks of the X-ray diffraction of the residues appearing around 25°–28°, corresponding to the (002) crystal plane of the sample. When the solid–liquid ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, the spectrum of the residues (Fig. 4(b)) had the sharpest (002) crystalline peak with a 2θ of 26.38°, which showed the best graphitization degree. The above data reflected by X-ray diffraction corresponded to the Raman spectra. Therefore, the most suitable solid–liquid ratio of the liquefied products for graphitization could be determined as 1
2, the spectrum of the residues (Fig. 4(b)) had the sharpest (002) crystalline peak with a 2θ of 26.38°, which showed the best graphitization degree. The above data reflected by X-ray diffraction corresponded to the Raman spectra. Therefore, the most suitable solid–liquid ratio of the liquefied products for graphitization could be determined as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.
2.
 | ||
| Fig. 5 Raman spectra of the residues in different catalyst concentrations (a). X-ray diffraction patterns of the residues in different catalyst concentrations (b). | ||
It was shown that with the increase in the catalyst concentration, the ID/IG values tended to decrease and then increase, as shown in Fig. 5(a). When the catalyst was 7.0 mmol g−1, ID/IG was 0.7314, which was the smallest and the graphitized degree was the best. When the catalyst concentration was 9.0 mmol g−1, the D and G peaks were higher, but the ID/IG values were larger and greater than 1. This means the graphitized degree was poor and defective, which might be due to the catalyst concentration reaching the supersaturated state and having a negative effect during characterization.
As shown in Fig. 5(b), when the catalyst concentration was 1.0 mmol g−1, the catalytic effect was not obvious. With the increase in the catalyst concentration, the graphitized carbon showed X-ray diffraction peaks around 25°–28°. When the catalyst concentration was 7.0 mmol g−1, the (002) crystalline peak of the residuals was the sharpest, so the optimal catalyst concentration for graphitization could be determined as 7.0 mmol g−1.
The graphitization catalysts were changed to ferric acetylacetonate, ferrocene, ferric oxalate and ferric citrate under the conditions of 1.0 g of the carbon of the corn straw liquefaction product, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 solid–liquid ratio of corn straw liquefaction products, 7.0 mmol g−1 catalyst, and 1.0 h of holding time at 850 °C for graphitization. Analytical graphs of the Raman spectra and X-ray diffraction were obtained under the corresponding catalysts, respectively.
2 solid–liquid ratio of corn straw liquefaction products, 7.0 mmol g−1 catalyst, and 1.0 h of holding time at 850 °C for graphitization. Analytical graphs of the Raman spectra and X-ray diffraction were obtained under the corresponding catalysts, respectively.
The ID/IG values of graphitized carbon catalyzed by the ferric oxalate, ferrocene, and ferric citrate catalysts were larger and all greater than the value of 1, indicating that its graphitized degree was poor and the graphitized carbon defects were large. In the system with ferric acetylacetonate as a catalyst, the ID/IG value was 0.7314 in Fig. 7(a). From Fig. 7(b), graphitized carbon characteristic peaks on the X-ray diffraction patterns are not shown, while the catalyst was one of the three catalysts, i.e. ferric oxalate, ferrocene, and ferric citrate, corresponding to the data reflected by the Raman spectra. Therefore ferric acetylacetonate as the graphitization catalyst had a better effect than the others.
 | ||
| Fig. 7 Raman spectra of graphitized carbon obtained with different catalysts (a). X-ray diffraction patterns of graphitized carbon obtained with different graphitization catalysts (b). | ||
The isotherms and pore-size distribution curves for the adsorption and desorption of the corn straw-based graphitized carbon obtained from the four iron-based catalysts are shown in Fig. 8. The curves coincided with type IV adsorption isotherm curves, with capillary coalescence present in the high-pressure region, while the adsorption–desorption curves did not overlap, indicating the presence of some mesopores. The H3-type hysteresis loop was also evident in the diagram, indicating that the materials obtained were in the form of loose flakes or granules, which was consistent with the SEM analysis. This indicated that the biochar produced was a mesoporous material. As can also be seen in Fig. 8(b), the pores of the material were mostly below 3.9 nm.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 and 1
0 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the graphitized carbon yields were 22.89% and 22.60%, respectively. Although the two values were more than 22.20%, which corresponded to a solid–liquid ratio of 1
1, the graphitized carbon yields were 22.89% and 22.60%, respectively. Although the two values were more than 22.20%, which corresponded to a solid–liquid ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, it could be found that the graphitization degree was not obvious by analysis of a higher ID/IG value.
2, it could be found that the graphitization degree was not obvious by analysis of a higher ID/IG value.
4.4 Structural characterization of the residues
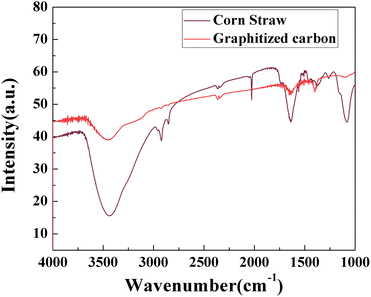
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–O–C, C–OH and the stretching vibration of C
O, C–O–C, C–OH and the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C of the graphite crystal sp2 structure, respectively. The intensity of the O–H stretching vibration peak near 3500 cm−1 was significantly weaker because the hydroxyl groups in the corn straw were re-polymerized by phosphoric acid and polyols to form water molecules (H2O) or other small molecules at the late stage of liquefaction. After graphitization, the absorption waveform state converged to the characteristic peak of graphene. Hydrogen bonds were probably formed, which are favorable for counteracting the van der Waals forces between the graphene layers.
C of the graphite crystal sp2 structure, respectively. The intensity of the O–H stretching vibration peak near 3500 cm−1 was significantly weaker because the hydroxyl groups in the corn straw were re-polymerized by phosphoric acid and polyols to form water molecules (H2O) or other small molecules at the late stage of liquefaction. After graphitization, the absorption waveform state converged to the characteristic peak of graphene. Hydrogen bonds were probably formed, which are favorable for counteracting the van der Waals forces between the graphene layers.
5 Conclusion
This study utilized a combination of liquefaction–carbonization–graphitization technology innovation through qualitative and quantitative analysis, a solid–liquid ratio of corn straw powder and liquefied agent of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, carbonization temperature of 600 °C, graphitization temperature of 850 °C, ferric acetylacetonate as the graphitization catalyst, and the catalyst concentration was 7.0 mmol g−1. Corn straw-based graphitized carbon showing a better graphitization degree, microscopic lamellar structure, and smaller defects was successfully prepared. The resulting proposed mechanism of the graphitized carbon process from corn straw provides a practical pathway for the preparation and utilization of corn straw to high-quality materials.
2, carbonization temperature of 600 °C, graphitization temperature of 850 °C, ferric acetylacetonate as the graphitization catalyst, and the catalyst concentration was 7.0 mmol g−1. Corn straw-based graphitized carbon showing a better graphitization degree, microscopic lamellar structure, and smaller defects was successfully prepared. The resulting proposed mechanism of the graphitized carbon process from corn straw provides a practical pathway for the preparation and utilization of corn straw to high-quality materials.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.Conflicts of interest
We declare that we have no conflicts of interest to this work. We do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.Acknowledgements
This work was supported by Jilin Province Science and Technology Department (grant number 20220101074JC); Climbing Project Fund of Changchun University (grant number ZKP202121).References
- H. Chang, Biomass conversion and utilization, Science Press, Beijing, 2019 Search PubMed.
- H. Miao, J. Phys.: Conf. Ser., 2022, 2152, 012053 CrossRef.
- X. Zhu, G. Li and T. Qin, J. Nanjing For. Univ., 2014, 38, 157–162 CAS.
- X. Li, X. Li, W. Li, T. Lei, J. Shi, Z. Wang and X. Duan, J. Beihua Univ., 2018, 19, 108–113 Search PubMed.
- B. Wei, F. Chen, Z. Li and Y. Cheng, Chem. Ind. For. Prod., 2014, 34, 53–59 CAS.
- R. Briones, L. Torres, Y. Saravia, L. Serrano and J. Labidi, Ind. Crops Prod., 2015, 78, 19–28 CrossRef CAS.
- T. H. Pedersen, I. Grigoras, J. Hoffmann, S. S. Toor, I. M. Daraban, C. U. Jensen, S. Iversen, R. Madsen, M. Glasius and K. R. Arturi, Appl. Energy, 2016, 162, 1034–1041 CrossRef CAS.
- P.-G. Duan, S.-K. Yang, Y.-P. Xu, F. Wang, D. Zhao, Y.-J. Weng and X.-L. Shi, Energy, 2018, 155, 734–745 CrossRef CAS.
- J. R. Collett, J. M. Billing, P. A. Meyer, A. J. Schmidt, A. B. Remington, E. R. Hawley, B. A. Hofstad, E. A. Panisko, Z. Dai and T. R. Hart, Appl. Energy, 2019, 233, 840–853 CrossRef.
- H. Jiang, F. Deng, Y. Luo, Z. Xie, Y. Chen, P. Zhou, X. Liu and D. Li, J. Cleaner Prod., 2022, 353, 131682 CrossRef CAS.
- W. Zhang, Z. Wang, T. Ge, C. Yang, W. Song, S. Li and R. Ma, Korean J. Chem. Eng., 2022, 39, 1240–1247 CrossRef CAS.
- B. N. Tochi, Z. Wang, S.-Y. Xu and W. Zhang, Pak. J. Nutr., 2009, 8, 1184–1189 CrossRef CAS.
- R. Zeng, X.-Y. Yin, T. Ruan, Q. Hu, Y.-L. Hou, Z.-Y. Zuo, H. Huang and Z.-H. Yang, Bioengineering, 2016, 3, 13 CrossRef PubMed.
- B. Zhang, Y. Gao, L. Zhang and Y. Zhou, J. Integr. Plant Biol., 2021, 63, 251–272 CrossRef PubMed.
- X. Li, X. a. Xie, C. Zheng and Y. Li, Trans. Chin. Soc. Agric. Eng., 2011, 27, 119–124 Search PubMed.
- C.-Y. Zheng, X.-A. Xie, H.-X. Tao, L.-S. Zheng and Y. Li, J. Fuel Chem. Technol., 2012, 40, 526–532 CAS.
- J. Sun, Y. L. Wang, X. A. Xie, L. I. Wei, L. I. Lu, L. I. Yan, D. Fan and X. Wei, J. Fuel Chem. Technol., 2017, 45(6), 660–668 CAS.
- N. Singh, M. Sharma, D. Mondal, D. A. Maru and K. Prasad, Mater. Sci. Energy Technol., 2021, 4, 100–106 CAS.
- Q. Chen, J. Gu, K. Sun, C. Zhang and S. Tian, Polym. Mater.: Sci. Eng., 2013, 29, 125–128 CAS.
- D. Y. Qi, Preparation and properties of biomass-based polyether polyols(D), Changchun University of Technology, 2020 Search PubMed.
- T. Yelverton, A. T. Brashear, D. G. Nash, J. E. Brown and P. Burnette, Fuel, 2020, 264, 116774 CrossRef CAS.
- X. Wang, A. Dong, Y. Hu, J. Qian and S. Huang, Chem. Commun., 2020 Search PubMed.
- A. Jorio, R. Saito, G. Dresselhaus and M. S. Dresselhaus, Raman Spectroscopy in Graphene Related Systems [M], Wiley-VCH, 2011 Search PubMed.
- W. Lei, The designed synthesis electrochemical performances of crystaline nanocarbon-based composites, Jilin University, 2013 Search PubMed.
- F. Gu, J. Ma and C. Che., J. Chin. Cereals Oils Assoc., 2022, 37, 240–245 Search PubMed.
| This journal is © The Royal Society of Chemistry 2023 |