Open Access Article
Open Access Article2,1-Benzothiazine – (quinolin/thiophen)yl hydrazone frameworks as new monoamine oxidase inhibitory agents; synthesis, in vitro and in silico investigation†
Noman Javid‡
ab,
Saquib Jalil‡c,
Rubina Munir *d,
Muhammad Zia-ur-Rehmane,
Amna Sahard,
Sara Arshadd and
Jamshed Iqbal
*d,
Muhammad Zia-ur-Rehmane,
Amna Sahard,
Sara Arshadd and
Jamshed Iqbal *c
*c
aSchool of Chemistry, University of the Punjab, Lahore, 54590, Pakistan
bChemistry Department (C-Block), Forman Christian College, Ferozepur Road Lahore, Pakistan
cCentre for Advanced Drug Research, COMSATS University Islamabad, Abbottabad Campus, Abbottabad, 22060, Pakistan. E-mail: drjamshed@cuiatd.edu.pk; Fax: +92-992-383441; Tel: +92-992-383591-96
dDepartment of Chemistry, Kinnaird College for Women, Lahore 54000, Pakistan. E-mail: organist94@gmail.com; rubina.munir@kinnaird.edu.pk
eApplied Chemistry Research Centre, PCSIR Laboratories Complex, Lahore, 54600, Pakistan
First published on 9th January 2023
Abstract
Two series of new 2,1-benzothiazine derivatives have been synthesized by condensation of 4-hydrazono-1-methyl-3,4-dihydro-1H-benzo[c][1,2]thiazine 2,2-dioxide (5) with 2-chloroquinoline-3-carbaldehydes and acetylthiophenes to acquire new heteroaryl ethylidenes 7(a–f) and 9(a–k) in excellent yields. After characterization by FTIR, 1H NMR, 13C NMR and elemental analyses, the newly synthesized analogues were investigated against monoamine oxidase enzymes (MAO A and MAO B). The titled compounds exhibited activity in the lower micromolar range among which 9e was the most potent compound against MAO A with IC50 of 1.04 ± 0.01 μM whereas 9h proved to be the most potent derivative against MAO B with an IC50 value of 1.03 ± 0.17 μM. Furthermore, in vitro results were further endorsed by molecular docking studies to determine the interaction between the potent compounds and the enzyme active site. These newly synthesized compounds represent promising hits for the development of safer and potent lead molecules for therapeutic use against depression and other neurological diseases.
Introduction
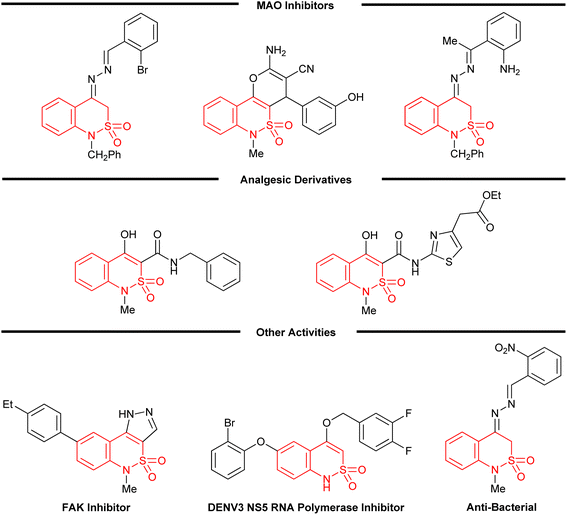
Human monoamine oxidase (hMAO) is a flavin adenine dinucleotide (FAD) enzyme present in outer mitochondrial membrane which is responsible for the metabolism of different biogenic amines along with some neuro-transmitters.1 To date, two types of MAOs have been identified (MAO A and MAO B) which are accountable for the metabolism of different neurotransmitters like dopamine, norepinephrine, serotonin and epinephrine found in central and peripheral tissues.2 As a result of degradation of amines, hydrogen peroxide (H2O2) and reactive oxygen species (ROS) are produced which are responsible for the death of neural cells.3 Neurological disorders (Alzheimer's and Parkinson disease) and depression originate due to unnecessary concentration of monoamine oxidase (MAO) which decreases the level of monoaminergic transmitters in the brain. The use of monoamine oxidase inhibitors (MAOI) can control the concentration of monoamine oxidase by inhibiting the excessive amount of oxidases and thus enhancing the concentration of monoaminergic transmitters.4One of the primary aims of synthetic organic chemistry is the development of new, selective, and potent drug molecules. Different classes of compounds have been identified as MAOIs by various researchers among which heterocyclic organic compounds are investigated to be more effective.5 Among different heterocyclic compounds, benzothiazine ring systems are one of the supreme targeted areas for the synthetic and clinical interest in the field of pharmacokinetics as they act as a skeleton with a rich bio-activity profile.6–8 A number of biological potentials have marked benzothiazine based derivatives interesting as antifungal,9,10 anti-bacterial,10,11 anti-malarial,12 anti-oxidant,13 anti-hypertensive,14 anti-neoplastic,15 anti-viral,16,17 and cardio-protective18 agents. 1,2-Benzothiazines have experimentally proven to be effective as analgesic agents and different candidates have been tested to examine their respective strength as painkillers and anti-inflammatory pills.19,20 The most overwhelming potential is exhibited by the Oxicams in this category which constitute 1,2-benzothiazine motif as the main structural feature.20,21 2,1-Benzothiazine also known as benzo[c][1,2]thiazine is considered to be the bioisostere of 1,2-benzothiazine.22 Different compounds bearing 2,1-benzothiazine framework have been documented as anti-psychotic,22 anti-inflammatory,23 anti-cancer,24 and analgesic agents.25 These have also been investigated for their lipoxygenase and RNA polymerase inhibitory potential (Fig. 1).26,27
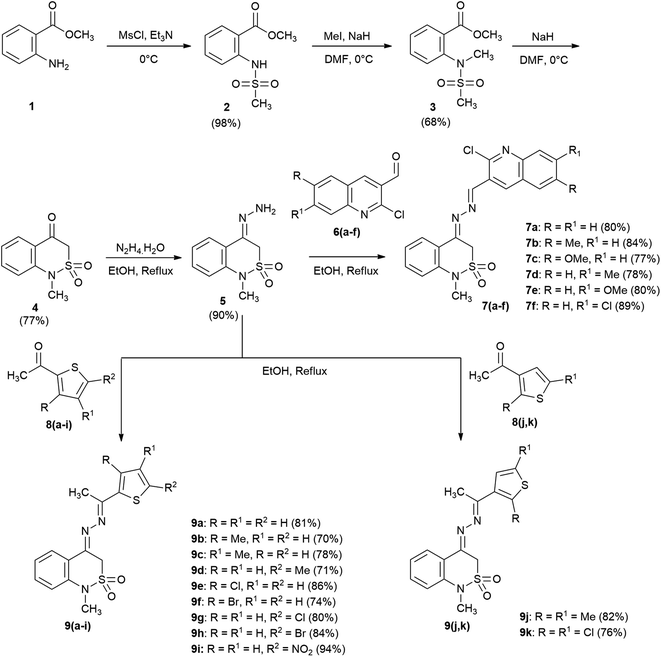
In continuation of our efforts to search for new bioactive synthetic compounds,28–30 we herein report the synthesis of new 2,1-benzothiazine 2,2-dioxide analogues and evaluation of their monoamine oxidase (MAO A and MAO B) inhibitory potential. To validate the results, in silico docking studies have been conducted to assess the binding interaction of the synthesized compounds inside the active site of enzyme.
Experimental
All the chemicals were purchased from Merck and Sigma Aldrich through their indigenous suppliers and were used as received. Melting points were taken by open capillary method on Gallenkamp melting point apparatus and are uncorrected. 1H NMR spectra and 13C NMR spectra (300 and 75 MHz respectively) were recorded in DMSO-d6 on Brucker Avance NMR instrument. Chemical shifts δ are reported in ppm with reference to tetramethylsilane. FTIR spectral data was obtained on an Agilent Technologies Cary 630 FTIR spectrophotometer. Elemental analyses were investigated in LECO 630-200-200 TruSpec CHNS microanalyzer and the values are established to be within ± 0.4% of the calculated results. Compounds 2–4 and 6 were synthesized by procedures reported in literature.29,31Synthesis of 4-hydrazono-1-methyl-3,4-dihydro-1H-benzo[c][1,2]thiazine 2,2-dioxide (5)
A solution of 1-methyl-1H-benzo[c][1,2]thiazin-4(3H)-one 2,2-dioxide 4 (25 mmol; 5.275 g) and 64% hydrazine monohydrate (75 mmol; 5.86 g, 5.7 mL) in absolute ethanol (50 mL) was heated under reflux conditions for 8 hours. The progress of reaction was monitored by TLC until the reactant spot disappeared. After completion of the reaction (TLC monitoring with eluting solvent n-hexane – ethyl acetate (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3)), excess solvent was removed on rotavapor and the concentrated solution was allowed to stand overnight after neutralizing it using 5N HCl solution. The product was obtained as yellow crystals which were filtered, washed with cold ethanol, and dried.
3)), excess solvent was removed on rotavapor and the concentrated solution was allowed to stand overnight after neutralizing it using 5N HCl solution. The product was obtained as yellow crystals which were filtered, washed with cold ethanol, and dried.
Yellow crystalline solid; mp 139–140 °C (Lit mp 139–141 °C);31 yield: 90%; IR (![[small nu, Greek, macron]](https://www.rsc.org/images/entities/char_e0ce.gif) cm−1; neat): 3388, 3310 (N–H), 3070, 2954 (C–H), 1640 (C
cm−1; neat): 3388, 3310 (N–H), 3070, 2954 (C–H), 1640 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1320 & 1118 (S
N), 1320 & 1118 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR (DMSO-d6, 300 MHz) δ: 3.23 (s, 3H; N–CH3), 4.48 (s, 2H; –CH2–), 7.07 (s, 2H; –NH2), 7.14 (td, 1H; J = 7.5 Hz, 1.2 Hz; Ar–H), 7.20 (dd, 1H, J = 8.1 Hz, 0.9 Hz; Ar–H), 7.32 (td, 1H; J = 8.7 Hz, 1.8 Hz; Ar–H), 7.95 (dd, 1H; J = 8.1 Hz, 1.5 Hz; Ar–H) ppm. 13C NMR (DMSO-d6, 75 MHz) δ: 34.7 (N–CH3), 47.8 (–CH2–), 120.9, 124.2, 124.7, 125.7, 129.2, 130.5, 139.9 ppm. Anal. Calcd. for C9H11N3O2S: C, 47.99; H, 4.92; N, 18.65; S, 14.23%. Found: C, 48.13; H, 4.98; N, 18.82; S, 14.39%.
O). 1H NMR (DMSO-d6, 300 MHz) δ: 3.23 (s, 3H; N–CH3), 4.48 (s, 2H; –CH2–), 7.07 (s, 2H; –NH2), 7.14 (td, 1H; J = 7.5 Hz, 1.2 Hz; Ar–H), 7.20 (dd, 1H, J = 8.1 Hz, 0.9 Hz; Ar–H), 7.32 (td, 1H; J = 8.7 Hz, 1.8 Hz; Ar–H), 7.95 (dd, 1H; J = 8.1 Hz, 1.5 Hz; Ar–H) ppm. 13C NMR (DMSO-d6, 75 MHz) δ: 34.7 (N–CH3), 47.8 (–CH2–), 120.9, 124.2, 124.7, 125.7, 129.2, 130.5, 139.9 ppm. Anal. Calcd. for C9H11N3O2S: C, 47.99; H, 4.92; N, 18.65; S, 14.23%. Found: C, 48.13; H, 4.98; N, 18.82; S, 14.39%.
General procedure for the synthesis of 1-methyl-4-((1-(heteroaryl)ethylidene)hydrazono)-3,4-dihydro-1H-2,1-benzothiazine 2,2-dioxides (7,9)
To a solution of 4-hydrazono-1-methyl-3,4-dihydro-1H-benzo[c][1,2]thiazine 2,2-dioxide 5 (1 mmol; 0.225 g) in distilled methanol (15 mL) was added 2-chloroquinoline-3-carbaldehyde (6a) (1 mmol; 0.192 g) and o-phosphoric acid (2–3 drops). The resulting mixture was refluxed until the formation of precipitates in the flask which were filtered, dried and recrystallized from absolute ethanol to obtain pure product (7a).The same procedure was adopted for the condensation of 5 with substituted 2-chloroquinoline-3-carbaldehydes 6(b–f) and substituted acetylthiophenes 8(a–k) to achieve the derivatives 7(b–f) and 9(a–k).
![[small nu, Greek, macron]](https://www.rsc.org/images/entities/char_e0ce.gif) cm−1; neat): 3106, 2980 (C–H), 1586 (C
cm−1; neat): 3106, 2980 (C–H), 1586 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1329 & 1143 (S
N), 1329 & 1143 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 732 (C–Cl). 1H NMR (DMSO-d6, 300 MHz) δ: 3.34 (s, 3H; N–CH3), 5.25 (s, 2H; –SO2CH2–), 6.77 (s, 1H; –SO2CH
O), 732 (C–Cl). 1H NMR (DMSO-d6, 300 MHz) δ: 3.34 (s, 3H; N–CH3), 5.25 (s, 2H; –SO2CH2–), 6.77 (s, 1H; –SO2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 7.20–7.40 (m, 2H; Ar–H), 7.52 (d, 1H; J = 8.1 Hz; Ar–H), 7.60–7.66 (m, 2H, Ar–H), 7.83–7.87 (m, 1H; Ar–H), 7.95 (d, 1H; J = 7.8 Hz; Ar–H), 8.37 (d, 1H; J = 8.1 Hz; Ar–H), 8.61, 8.69 (2s, 1H; Ar–H), 8.80, 8.95 (2s, 1H; N
), 7.20–7.40 (m, 2H; Ar–H), 7.52 (d, 1H; J = 8.1 Hz; Ar–H), 7.60–7.66 (m, 2H, Ar–H), 7.83–7.87 (m, 1H; Ar–H), 7.95 (d, 1H; J = 7.8 Hz; Ar–H), 8.37 (d, 1H; J = 8.1 Hz; Ar–H), 8.61, 8.69 (2s, 1H; Ar–H), 8.80, 8.95 (2s, 1H; N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 10.94 (s, 1H; NH) ppm. 13C NMR (DMSO-d6, 75 MHz) δ: 29.6 (N–CH3, enamine), 33.0 (N–CH3, imine), 50.1 (SO2CH2), 95.1 (SO2CH), 115.6, 116.5, 117.5, 119.4, 119.8, 122.6, 122.8, 124.1, 125.8, 129.3, 131.4, 132.3, 135.0, 139.3, 140.7, 143.3, 145.2, 155.9, 161.5 ppm. Anal. Calcd. for C19H15ClN4O2S: C, 57.21; H, 3.79; N, 14.05; S, 8.04%. Found: C, 57.37; H, 3.81; N, 14.20; S, 8.11%.
CH), 10.94 (s, 1H; NH) ppm. 13C NMR (DMSO-d6, 75 MHz) δ: 29.6 (N–CH3, enamine), 33.0 (N–CH3, imine), 50.1 (SO2CH2), 95.1 (SO2CH), 115.6, 116.5, 117.5, 119.4, 119.8, 122.6, 122.8, 124.1, 125.8, 129.3, 131.4, 132.3, 135.0, 139.3, 140.7, 143.3, 145.2, 155.9, 161.5 ppm. Anal. Calcd. for C19H15ClN4O2S: C, 57.21; H, 3.79; N, 14.05; S, 8.04%. Found: C, 57.37; H, 3.81; N, 14.20; S, 8.11%.![[small nu, Greek, macron]](https://www.rsc.org/images/entities/char_e0ce.gif) cm−1; neat): 3059, 2982 (C–H), 1584 (C
cm−1; neat): 3059, 2982 (C–H), 1584 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1328 & 1154 (S
N), 1328 & 1154 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 748 (C–Cl). 1H NMR (DMSO-d6, 300 MHz) δ: 2.50 (s, 3H; Ar–CH3), 3.35 (s, 3H; N–CH3), 5.29 (s, 2H; –SO2CH2–), 6.89 (s, 1H; –SO2CH
O), 748 (C–Cl). 1H NMR (DMSO-d6, 300 MHz) δ: 2.50 (s, 3H; Ar–CH3), 3.35 (s, 3H; N–CH3), 5.29 (s, 2H; –SO2CH2–), 6.89 (s, 1H; –SO2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 7.28–7.41 (m, 2H; Ar–H), 7.64–7.74 (m, 2H, Ar–H), 7.82–7.93 (m, 2H; Ar–H), 8.39 (d, 1H; J = 7.2 Hz; Ar–H), 8.72, 8.92 (2s, 1H; Ar–H), 9.08, 9.27 (2s, 1H; N
), 7.28–7.41 (m, 2H; Ar–H), 7.64–7.74 (m, 2H, Ar–H), 7.82–7.93 (m, 2H; Ar–H), 8.39 (d, 1H; J = 7.2 Hz; Ar–H), 8.72, 8.92 (2s, 1H; Ar–H), 9.08, 9.27 (2s, 1H; N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 11.13 (s, 1H; NH) ppm. 13C NMR (DMSO-d6, 75 MHz) δ: 21.6 (Ar-CH3), 29.6 (N–CH3, enamine), 33.0 (N–CH3, imine), 50.3 (SO2CH2), 95.8 (SO2CH), 116.4, 117.5, 119.7, 122.6, 125.1, 126.5, 127.6, 127.9, 132.4, 134.1, 135.4, 137.8, 139.7, 140.6, 143.4, 145.1, 146.0, 147.8, 156.8. Anal. Calcd. For C20H17ClN4O2S: C, 58.18; H, 4.15; N, 13.57; S, 7.77%. Found: C, 58.06; H, 4.03; N, 13.43; S, 7.63%.
CH), 11.13 (s, 1H; NH) ppm. 13C NMR (DMSO-d6, 75 MHz) δ: 21.6 (Ar-CH3), 29.6 (N–CH3, enamine), 33.0 (N–CH3, imine), 50.3 (SO2CH2), 95.8 (SO2CH), 116.4, 117.5, 119.7, 122.6, 125.1, 126.5, 127.6, 127.9, 132.4, 134.1, 135.4, 137.8, 139.7, 140.6, 143.4, 145.1, 146.0, 147.8, 156.8. Anal. Calcd. For C20H17ClN4O2S: C, 58.18; H, 4.15; N, 13.57; S, 7.77%. Found: C, 58.06; H, 4.03; N, 13.43; S, 7.63%.![[small nu, Greek, macron]](https://www.rsc.org/images/entities/char_e0ce.gif) cm−1; neat): 3106, 2950 (C–H), 1585 (C
cm−1; neat): 3106, 2950 (C–H), 1585 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1327 & 1147 (S
N), 1327 & 1147 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 732 (C–Cl). 1H NMR (DMSO-d6, 300 MHz) δ:3.34 (s, 3H; N–CH3), 3.92 (s, 3H; –OCH3), 5.29 (s, 2H; –SO2CH2–), 6.86 (s, 1H; –SO2CH
O), 732 (C–Cl). 1H NMR (DMSO-d6, 300 MHz) δ:3.34 (s, 3H; N–CH3), 3.92 (s, 3H; –OCH3), 5.29 (s, 2H; –SO2CH2–), 6.86 (s, 1H; –SO2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 7.34–7.41 (m, 1H; Ar–H), 7.45 (dd, 1H; J = 9.0 Hz, 2.7 Hz; Ar–H), 7.58–7.68 (m, 2H, Ar–H), 7.85 (d, 1H; J = 9.3 Hz, Ar–H), 7.95 (dd, 1H; J = 8.1 Hz, 0.9 Hz; Ar–H), 8.40 (dd, 1H; J = 7.8 Hz, 1.5 Hz; Ar–H), 8.73, 8.93 (2s, 1H; Ar–H), 9.08, 9.27 (2s, 1H; N
), 7.34–7.41 (m, 1H; Ar–H), 7.45 (dd, 1H; J = 9.0 Hz, 2.7 Hz; Ar–H), 7.58–7.68 (m, 2H, Ar–H), 7.85 (d, 1H; J = 9.3 Hz, Ar–H), 7.95 (dd, 1H; J = 8.1 Hz, 0.9 Hz; Ar–H), 8.40 (dd, 1H; J = 7.8 Hz, 1.5 Hz; Ar–H), 8.73, 8.93 (2s, 1H; Ar–H), 9.08, 9.27 (2s, 1H; N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 11.15 (s, 1H; NH) ppm. 13C NMR (DMSO-d6, 75 MHz) δ: 29.6 (N–CH3, enamine), 32.9 (N–CH3, imine), 50.2 (SO2CH2), 56.1 (-OCH3), 95.7 (SO2CH), 106.8, 116.3, 117.5, 119.7, 122.6, 124.5, 125.1, 126.7, 128.9, 129.5, 132.4, 134.7, 139.7, 140.6, 143.5, 145.1, 146.1, 158.4 ppm. Anal. Calcd. for C20H17ClN4O3S: C, 56.01; H, 4.00; N, 13.06; S, 7.48%. Found: C, 56.20; H, 4.10; N, 13.10; S, 7.52%.
CH), 11.15 (s, 1H; NH) ppm. 13C NMR (DMSO-d6, 75 MHz) δ: 29.6 (N–CH3, enamine), 32.9 (N–CH3, imine), 50.2 (SO2CH2), 56.1 (-OCH3), 95.7 (SO2CH), 106.8, 116.3, 117.5, 119.7, 122.6, 124.5, 125.1, 126.7, 128.9, 129.5, 132.4, 134.7, 139.7, 140.6, 143.5, 145.1, 146.1, 158.4 ppm. Anal. Calcd. for C20H17ClN4O3S: C, 56.01; H, 4.00; N, 13.06; S, 7.48%. Found: C, 56.20; H, 4.10; N, 13.10; S, 7.52%.![[small nu, Greek, macron]](https://www.rsc.org/images/entities/char_e0ce.gif) cm−1; neat): 3106, 2927 (C–H), 1662 (C
cm−1; neat): 3106, 2927 (C–H), 1662 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1330 & 1149 (S
N), 1330 & 1149 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 750 (C–Cl). 1H NMR (DMSO-d6, 300 MHz) δ: 2.52 (s, 3H; Ar–CH3), 3.34 (s, 3H; N–CH3), 5.29 (s, 2H; –SO2CH2–), 6.88 (s, 1H; –SO2CH
O), 750 (C–Cl). 1H NMR (DMSO-d6, 300 MHz) δ: 2.52 (s, 3H; Ar–CH3), 3.34 (s, 3H; N–CH3), 5.29 (s, 2H; –SO2CH2–), 6.88 (s, 1H; –SO2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 7.04–7.14 (m, 1H; Ar–H), 7.20–7.41 (m, 1H; Ar–H), 7.51–7.73 (m, 2H, Ar–H), 7.95 (d, 1H; J = 7.2 Hz; Ar–H), 8.08 (d, 1H; J = 8.4 Hz, 2.7 Hz; Ar–H), 8.36 (d, 1H; J = 8.4 Hz; Ar–H), 8.74, 8.93 (2s, 1H; Ar–H), 9.15, 9.28 (2s, 1H; N
), 7.04–7.14 (m, 1H; Ar–H), 7.20–7.41 (m, 1H; Ar–H), 7.51–7.73 (m, 2H, Ar–H), 7.95 (d, 1H; J = 7.2 Hz; Ar–H), 8.08 (d, 1H; J = 8.4 Hz, 2.7 Hz; Ar–H), 8.36 (d, 1H; J = 8.4 Hz; Ar–H), 8.74, 8.93 (2s, 1H; Ar–H), 9.15, 9.28 (2s, 1H; N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 11.12 (s, 1H; NH) ppm. 13C NMR (DMSO-d6, 75 MHz) δ: 22.1 (Ar-CH3), 29.6 (N–CH3, enamine), 32.9 (N–CH3, imine), 50.1 (SO2CH2), 95.7 (SO2CH), 116.4, 117.7, 119.8, 122.6, 124.1, 125.7, 130.4, 130.7, 133.5, 135.8, 138.8, 139.9, 140.7, 142.5, 143.4, 145.1, 148.6, 155.7, 156.9, 161.2 ppm. Anal. Calcd. for C20H17ClN4O2S: C, 58.18; H, 4.15; N, 13.57; S, 7.77%. Found: C, 58.24; H, 4.21; N, 13.63; S, 7.89%.
CH), 11.12 (s, 1H; NH) ppm. 13C NMR (DMSO-d6, 75 MHz) δ: 22.1 (Ar-CH3), 29.6 (N–CH3, enamine), 32.9 (N–CH3, imine), 50.1 (SO2CH2), 95.7 (SO2CH), 116.4, 117.7, 119.8, 122.6, 124.1, 125.7, 130.4, 130.7, 133.5, 135.8, 138.8, 139.9, 140.7, 142.5, 143.4, 145.1, 148.6, 155.7, 156.9, 161.2 ppm. Anal. Calcd. for C20H17ClN4O2S: C, 58.18; H, 4.15; N, 13.57; S, 7.77%. Found: C, 58.24; H, 4.21; N, 13.63; S, 7.89%.![[small nu, Greek, macron]](https://www.rsc.org/images/entities/char_e0ce.gif) cm−1; neat): 3080, 2920 (C–H), 1653 (C
cm−1; neat): 3080, 2920 (C–H), 1653 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1338 & 1148 (S
N), 1338 & 1148 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1025 (C–O–C). 1H NMR (DMSO-d6, 300 MHz) δ: 3.33 (s, 3H; N–CH3), 3.93 (s, 3H; –OCH3), 5.23 (s, 2H; –SO2CH2–), 6.71 (s, 1H; –SO2CH
O), 1025 (C–O–C). 1H NMR (DMSO-d6, 300 MHz) δ: 3.33 (s, 3H; N–CH3), 3.93 (s, 3H; –OCH3), 5.23 (s, 2H; –SO2CH2–), 6.71 (s, 1H; –SO2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 6.84–6.91 (m, 1H; Ar–H),7.31–7.39 (m, 1H; Ar–H), 7.56–7.65 (m, 1H; Ar–H), 7.76 (dd, 1H; J = 8.7 Hz, 3.0 Hz; Ar–H), 7.94 (d, 1H; J = 8.1 Hz; Ar–H), 8.09 (d, 1H; J = 8.7 Hz; Ar–H), 8.35 (d, 1H; J = 8.1 Hz, 1.5 Hz; Ar–H), 8.60, 8.77 (2s, 1H; Ar–H), 9.11, 9.27 (s, 1H; N
), 6.84–6.91 (m, 1H; Ar–H),7.31–7.39 (m, 1H; Ar–H), 7.56–7.65 (m, 1H; Ar–H), 7.76 (dd, 1H; J = 8.7 Hz, 3.0 Hz; Ar–H), 7.94 (d, 1H; J = 8.1 Hz; Ar–H), 8.09 (d, 1H; J = 8.7 Hz; Ar–H), 8.35 (d, 1H; J = 8.1 Hz, 1.5 Hz; Ar–H), 8.60, 8.77 (2s, 1H; Ar–H), 9.11, 9.27 (s, 1H; N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 11.86 (s, 1H; NH) ppm. 13C NMR (DMSO-d6, 75 MHz) δ: 29.5 (N–CH3, enamine), 33.0 (N–CH3, imine), 50.0 (SO2CH2), 56.0 (–OCH3), 98.3 (SO2CH), 112.5, 113.8, 116.5, 117.4, 119.7, 121.5, 122.3, 124.1, 130.9, 131.6, 133.4, 140.6, 141.3, 142.6, 143.2, 145.3, 155.4, 157.0, 161.8 ppm. Anal. Calcd. for C20H17ClN4O3S: C, 56.01; H, 4.00; N, 13.06; S, 7.48%. Found: C, 56.13; H, 4.16; N, 13.12; S, 7.60%.
CH), 11.86 (s, 1H; NH) ppm. 13C NMR (DMSO-d6, 75 MHz) δ: 29.5 (N–CH3, enamine), 33.0 (N–CH3, imine), 50.0 (SO2CH2), 56.0 (–OCH3), 98.3 (SO2CH), 112.5, 113.8, 116.5, 117.4, 119.7, 121.5, 122.3, 124.1, 130.9, 131.6, 133.4, 140.6, 141.3, 142.6, 143.2, 145.3, 155.4, 157.0, 161.8 ppm. Anal. Calcd. for C20H17ClN4O3S: C, 56.01; H, 4.00; N, 13.06; S, 7.48%. Found: C, 56.13; H, 4.16; N, 13.12; S, 7.60%.![[small nu, Greek, macron]](https://www.rsc.org/images/entities/char_e0ce.gif) cm−1; neat): 3106, 2980 (C–H), 1659 (C
cm−1; neat): 3106, 2980 (C–H), 1659 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1322 & 1150 (S
N), 1322 & 1150 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 766 (C–Cl). 1H NMR (DMSO-d6, 300 MHz) δ:3.35 (s, 3H; N–CH3), 5.27 (s, 2H; –SO2CH2-), 6.88 (s, 1H; –SO2CH
O), 766 (C–Cl). 1H NMR (DMSO-d6, 300 MHz) δ:3.35 (s, 3H; N–CH3), 5.27 (s, 2H; –SO2CH2-), 6.88 (s, 1H; –SO2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 7.21–7.37 (m, 2H; Ar–H), 7.60–7.71 (m, 1H, Ar–H), 7.92 (d, 1H; J = 7.8 Hz; Ar–H), 7.99 (s, 1H; Ar–H), 8.19 (d, 1H; J = 9.0 Hz; Ar–H), 8.35 (d, 1H; J = 8.1 Hz, 1.5 Hz; Ar–H), 8.70, 8.89 (2s, 1H; Ar–H), 9.19, 9.33 (s, 1H; N
), 7.21–7.37 (m, 2H; Ar–H), 7.60–7.71 (m, 1H, Ar–H), 7.92 (d, 1H; J = 7.8 Hz; Ar–H), 7.99 (s, 1H; Ar–H), 8.19 (d, 1H; J = 9.0 Hz; Ar–H), 8.35 (d, 1H; J = 8.1 Hz, 1.5 Hz; Ar–H), 8.70, 8.89 (2s, 1H; Ar–H), 9.19, 9.33 (s, 1H; N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 11.16 (s, 1H; NH) ppm. 13C NMR (DMSO-d6, 75 MHz) δ: 29.6 (N–CH3, enamine), 32.8 (N–CH3, imine), 50.3 (SO2CH2), 96.0 (SO2CH), 116.3, 117.5, 119.7, 122.6, 125.0, 126.1, 126.2, 127.1, 128.9, 131.1, 135.9, 136.5, 140.6, 145.0, 147.5, 149.9, 157.2, 161.3 ppm. Anal. Calcd. for C19H14Cl2N4O2S: C, 52.67; H, 3.26; N, 12.93; S, 7.40%. Found: C, 52.73; H, 3.40; N, 13.09; S, 7.58%.
CH), 11.16 (s, 1H; NH) ppm. 13C NMR (DMSO-d6, 75 MHz) δ: 29.6 (N–CH3, enamine), 32.8 (N–CH3, imine), 50.3 (SO2CH2), 96.0 (SO2CH), 116.3, 117.5, 119.7, 122.6, 125.0, 126.1, 126.2, 127.1, 128.9, 131.1, 135.9, 136.5, 140.6, 145.0, 147.5, 149.9, 157.2, 161.3 ppm. Anal. Calcd. for C19H14Cl2N4O2S: C, 52.67; H, 3.26; N, 12.93; S, 7.40%. Found: C, 52.73; H, 3.40; N, 13.09; S, 7.58%.![[small nu, Greek, macron]](https://www.rsc.org/images/entities/char_e0ce.gif) cm−1; neat): 3090, 2999 (C–H), 1584 (C
cm−1; neat): 3090, 2999 (C–H), 1584 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1331 & 1150 (S
N), 1331 & 1150 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR (DMSO-d6, 300 MHz) δ:2.52 (s, 3H; –CH3), 3.33 (s, 3H; N–CH3), 4.95 (s, 2H; –CH2-), 7.19 (t, 1H; J = 4.5 Hz; Ar–H), 7.28 (t, 1H; J = 7.5 Hz; Ar–H), 7.35 (d, 1H; J = 8.4 Hz; Ar–H), 7.60 (t, 1H; J = 8.1 Hz; Ar–H), 7.74 (d, 1H; J = 3.9 Hz; Ar–H), 7.77 (d, 1H; J = 5.1 Hz; Ar–H), 8.36 (d, 1H; J = 8.1 Hz; Ar–H) ppm. 13C NMR (DMSO-d6, 75 MHz) δ: 15.9 (-CH3), 33.3 (N–CH3), 49.8 (–CH2–), 120.1, 122.7, 124.3, 126.4, 128.6, 130.8, 131.4, 133.2, 142.9, 143.3, 151.9, 160.7 ppm. Anal. Calcd. for C15H15N3O2S2: C, 54.03; H, 4.53; N, 12.60; S, 19.23%. Found: C, 54.19; H, 4.71; N, 12.72; S, 19.41%.
O). 1H NMR (DMSO-d6, 300 MHz) δ:2.52 (s, 3H; –CH3), 3.33 (s, 3H; N–CH3), 4.95 (s, 2H; –CH2-), 7.19 (t, 1H; J = 4.5 Hz; Ar–H), 7.28 (t, 1H; J = 7.5 Hz; Ar–H), 7.35 (d, 1H; J = 8.4 Hz; Ar–H), 7.60 (t, 1H; J = 8.1 Hz; Ar–H), 7.74 (d, 1H; J = 3.9 Hz; Ar–H), 7.77 (d, 1H; J = 5.1 Hz; Ar–H), 8.36 (d, 1H; J = 8.1 Hz; Ar–H) ppm. 13C NMR (DMSO-d6, 75 MHz) δ: 15.9 (-CH3), 33.3 (N–CH3), 49.8 (–CH2–), 120.1, 122.7, 124.3, 126.4, 128.6, 130.8, 131.4, 133.2, 142.9, 143.3, 151.9, 160.7 ppm. Anal. Calcd. for C15H15N3O2S2: C, 54.03; H, 4.53; N, 12.60; S, 19.23%. Found: C, 54.19; H, 4.71; N, 12.72; S, 19.41%.![[small nu, Greek, macron]](https://www.rsc.org/images/entities/char_e0ce.gif) cm−1; neat): 3094, 2970 (C–H), 1584 (C
cm−1; neat): 3094, 2970 (C–H), 1584 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1329 & 1144 (S
N), 1329 & 1144 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR (DMSO-d6, 300 MHz) δ:2.53 (s, 3H; –CH3), 2.54 (s, 3H; –CH3), 3.33 (s, 3H; N–CH3), 4.94 (s, 2H; –CH2–), 7.06 (d, 1H; J = 5.1 Hz; Ar–H), 7.28 (td, 1H; J = 7.5 Hz, 1.2 Hz; Ar–H), 7.35 (d, 1H; J = 7.5 Hz; Ar–H), 7.59 (td, 1H; J = 7.8 Hz, 1.8 Hz; Ar–H), 7.65 (d, 1H; J = 5.1 Hz; Ar–H), 8.36 (dd, 1H; J = 7.8 Hz, 1.5 Hz; Ar–H) ppm. 13C NMR (DMSO-d6, 75 MHz) δ: 17.9 (–CH3), 18.2 (–CH3), 33.4 (N–CH3), 50.2 (–CH2–), 120.1, 123.0, 124.4, 126.4, 128.6, 133.0, 133.5, 136.4, 140.3, 142.8, 151.5, 161.7 ppm. Anal. Calcd. for C16H17N3O2S2: C, 55.31; H, 4.93; N, 12.09; S, 18.46%. Found: C, 55.29; H, 4.87; N, 12.01; S, 18.38%.
O). 1H NMR (DMSO-d6, 300 MHz) δ:2.53 (s, 3H; –CH3), 2.54 (s, 3H; –CH3), 3.33 (s, 3H; N–CH3), 4.94 (s, 2H; –CH2–), 7.06 (d, 1H; J = 5.1 Hz; Ar–H), 7.28 (td, 1H; J = 7.5 Hz, 1.2 Hz; Ar–H), 7.35 (d, 1H; J = 7.5 Hz; Ar–H), 7.59 (td, 1H; J = 7.8 Hz, 1.8 Hz; Ar–H), 7.65 (d, 1H; J = 5.1 Hz; Ar–H), 8.36 (dd, 1H; J = 7.8 Hz, 1.5 Hz; Ar–H) ppm. 13C NMR (DMSO-d6, 75 MHz) δ: 17.9 (–CH3), 18.2 (–CH3), 33.4 (N–CH3), 50.2 (–CH2–), 120.1, 123.0, 124.4, 126.4, 128.6, 133.0, 133.5, 136.4, 140.3, 142.8, 151.5, 161.7 ppm. Anal. Calcd. for C16H17N3O2S2: C, 55.31; H, 4.93; N, 12.09; S, 18.46%. Found: C, 55.29; H, 4.87; N, 12.01; S, 18.38%.![[small nu, Greek, macron]](https://www.rsc.org/images/entities/char_e0ce.gif) cm−1; neat): 3080, 2950 (C–H), 1590 (C
cm−1; neat): 3080, 2950 (C–H), 1590 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1324 & 1143 (S
N), 1324 & 1143 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR (DMSO-d6, 300 MHz) δ: 2.49 (s, 3H; –CH3), 2.57 (s, 3H; –CH3), 3.32 (s, 3H; N–CH3), 4.98 (s, 2H; –CH2–), 7.28 (td, 1H; J = 7.5 Hz, 0.9 Hz; Ar–H), 7.33–7.38 (m, 2H; Ar–H), 7.58–7.65 (m, 2H; Ar–H), 8.36 (dd, 1H; J = 8.1 Hz, 1.5 Hz; Ar–H) ppm. 13C NMR (DMSO-d6, 75 MHz) δ: 15.9 (–CH3), 23.4 (–CH3), 33.3 (N–CH3), 49.8 (–CH2–), 120.1, 122.6, 124.4, 126.6, 127.4, 132.3, 133.1, 137.0, 142.8, 143.1, 151.8, 160.7 ppm. Anal. Calcd. for C16H17N3O2S2: C, 55.31; H, 4.93; N, 12.09; S, 18.46%. Found: C, 55.37; H, 4.99; N, 12.11; S, 18.50%.
O). 1H NMR (DMSO-d6, 300 MHz) δ: 2.49 (s, 3H; –CH3), 2.57 (s, 3H; –CH3), 3.32 (s, 3H; N–CH3), 4.98 (s, 2H; –CH2–), 7.28 (td, 1H; J = 7.5 Hz, 0.9 Hz; Ar–H), 7.33–7.38 (m, 2H; Ar–H), 7.58–7.65 (m, 2H; Ar–H), 8.36 (dd, 1H; J = 8.1 Hz, 1.5 Hz; Ar–H) ppm. 13C NMR (DMSO-d6, 75 MHz) δ: 15.9 (–CH3), 23.4 (–CH3), 33.3 (N–CH3), 49.8 (–CH2–), 120.1, 122.6, 124.4, 126.6, 127.4, 132.3, 133.1, 137.0, 142.8, 143.1, 151.8, 160.7 ppm. Anal. Calcd. for C16H17N3O2S2: C, 55.31; H, 4.93; N, 12.09; S, 18.46%. Found: C, 55.37; H, 4.99; N, 12.11; S, 18.50%.![[small nu, Greek, macron]](https://www.rsc.org/images/entities/char_e0ce.gif) cm−1; neat): 3080, 2978 (C–H), 1585 (C
cm−1; neat): 3080, 2978 (C–H), 1585 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1325 & 1150 (S
N), 1325 & 1150 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR (DMSO-d6, 300 MHz) δ: 2.47 (s, 3H; –CH3), 2.49 (s, 3H; –CH3), 3.33 (s, 3H; N–CH3), 4.94 (s, 2H; –CH2–), 6.89 (dd, 1H; J = 3.6 Hz, 1.2 Hz; Ar–H), 7.27 (td, 1H; J = 7.5 Hz, 0.9 Hz; Ar–H), 7.34 (d, 1H; J = 8.4 Hz; Ar–H), 7.55 (d, 1H; J = 3.9 Hz; Ar–H), 7.59 (td, 1H; J = 8.1 Hz, 1.5 Hz; Ar–H), 8.35 (dd, 1H; J = 8.1 Hz, 1.5 Hz; Ar–H) ppm. 13C NMR (DMSO-d6, 75 MHz) δ: 15.4 (–CH3), 15.9 (–CH3), 33.3 (N–CH3), 49.7 (–CH2–), 120.0, 122.8, 124.3, 126.4, 127.1, 131.2, 133.1, 141.0, 142.8, 145.6, 151.8, 160.9 ppm. Anal. Calcd. for C16H17N3O2S2: C, 55.31; H, 4.93; N, 12.09; S, 18.46%. Found: C, 55.43; H, 5.05; N, 12.21; S, 18.60%.
O). 1H NMR (DMSO-d6, 300 MHz) δ: 2.47 (s, 3H; –CH3), 2.49 (s, 3H; –CH3), 3.33 (s, 3H; N–CH3), 4.94 (s, 2H; –CH2–), 6.89 (dd, 1H; J = 3.6 Hz, 1.2 Hz; Ar–H), 7.27 (td, 1H; J = 7.5 Hz, 0.9 Hz; Ar–H), 7.34 (d, 1H; J = 8.4 Hz; Ar–H), 7.55 (d, 1H; J = 3.9 Hz; Ar–H), 7.59 (td, 1H; J = 8.1 Hz, 1.5 Hz; Ar–H), 8.35 (dd, 1H; J = 8.1 Hz, 1.5 Hz; Ar–H) ppm. 13C NMR (DMSO-d6, 75 MHz) δ: 15.4 (–CH3), 15.9 (–CH3), 33.3 (N–CH3), 49.7 (–CH2–), 120.0, 122.8, 124.3, 126.4, 127.1, 131.2, 133.1, 141.0, 142.8, 145.6, 151.8, 160.9 ppm. Anal. Calcd. for C16H17N3O2S2: C, 55.31; H, 4.93; N, 12.09; S, 18.46%. Found: C, 55.43; H, 5.05; N, 12.21; S, 18.60%.![[small nu, Greek, macron]](https://www.rsc.org/images/entities/char_e0ce.gif) cm−1; neat): 3105, 2975 (C–H), 1586 (C
cm−1; neat): 3105, 2975 (C–H), 1586 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1325 & 1144 (S
N), 1325 & 1144 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR (DMSO-d6, 300 MHz) δ: 2.60 (s, 3H; –CH3), 3.33 (s, 3H; N–CH3), 4.92 (s, 2H; –CH2–), 7.19 (d, 1H; J = 5.4 Hz; Ar–H), 7.29 (t, 1H; J = 7.8 Hz; Ar–H), 7.36 (d, 1H; J = 8.4 Hz; Ar–H), 7.61 (td, 1H; J = 8.1 Hz, 1.2 Hz; Ar–H), 7.85 (d, 1H; J = 5.1 Hz; Ar–H), 8.36 (dd, 1H; J = 8.1 Hz, 1.2 Hz; Ar–H) ppm. 13C NMR (DMSO-d6, 75 MHz) δ: 17.8 (–CH3), 33.4 (N–CH3), 50.1 (–CH2–), 120.1, 122.6, 124.4, 125.5, 126.5, 130.4, 130.8, 133.4, 135.5, 143.0, 152.4, 159.4 ppm. Anal. Calcd. for C15H14ClN3O2S2: C, 48.97; H, 3.84; N, 11.42; S, 17.43%. Found: C, 49.11; H, 4.00; N, 11.66; S, 17.59%.
O). 1H NMR (DMSO-d6, 300 MHz) δ: 2.60 (s, 3H; –CH3), 3.33 (s, 3H; N–CH3), 4.92 (s, 2H; –CH2–), 7.19 (d, 1H; J = 5.4 Hz; Ar–H), 7.29 (t, 1H; J = 7.8 Hz; Ar–H), 7.36 (d, 1H; J = 8.4 Hz; Ar–H), 7.61 (td, 1H; J = 8.1 Hz, 1.2 Hz; Ar–H), 7.85 (d, 1H; J = 5.1 Hz; Ar–H), 8.36 (dd, 1H; J = 8.1 Hz, 1.2 Hz; Ar–H) ppm. 13C NMR (DMSO-d6, 75 MHz) δ: 17.8 (–CH3), 33.4 (N–CH3), 50.1 (–CH2–), 120.1, 122.6, 124.4, 125.5, 126.5, 130.4, 130.8, 133.4, 135.5, 143.0, 152.4, 159.4 ppm. Anal. Calcd. for C15H14ClN3O2S2: C, 48.97; H, 3.84; N, 11.42; S, 17.43%. Found: C, 49.11; H, 4.00; N, 11.66; S, 17.59%.![[small nu, Greek, macron]](https://www.rsc.org/images/entities/char_e0ce.gif) cm−1; neat): 3080, 2978 (C–H), 1585 (C
cm−1; neat): 3080, 2978 (C–H), 1585 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1332 & 1150 (S
N), 1332 & 1150 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR (DMSO-d6, 300 MHz) δ: 2.62 (s, 3H; –CH3), 3.33 (s, 3H; N–CH3), 4.94 (s, 2H; –CH2–), 7.25 (d, 1H; J = 5.4 Hz; Ar–H), 7.28 (t, 1H; J = 7.5 Hz; Ar–H), 7.36 (d, 1H; J = 8.4 Hz; Ar–H), 7.61 (td, 1H; J = 8.1 Hz, 1.2 Hz; Ar–H), 7.83 (d, 1H; J = 5.4 Hz; Ar–H), 8.36 (dd, 1H; J = 8.1 Hz, 1.5 Hz; Ar–H) ppm. 13C NMR (DMSO-d6, 75 MHz) δ: 17.9 (–CH3), 33.4 (N–CH3), 50.2 (–CH2–), 111.3, 120.1, 122.6, 124.4, 126.6, 130.9, 133.4, 133.6, 137.1, 143.0, 152.3, 159.5 ppm. Anal. Calcd. for C15H14BrN3O2S2: C, 43.69; H, 3.42; N, 10.19; S, 15.55%. Found: C, 43.71; H, 3.50; N, 10.33; S, 15.73%.
O). 1H NMR (DMSO-d6, 300 MHz) δ: 2.62 (s, 3H; –CH3), 3.33 (s, 3H; N–CH3), 4.94 (s, 2H; –CH2–), 7.25 (d, 1H; J = 5.4 Hz; Ar–H), 7.28 (t, 1H; J = 7.5 Hz; Ar–H), 7.36 (d, 1H; J = 8.4 Hz; Ar–H), 7.61 (td, 1H; J = 8.1 Hz, 1.2 Hz; Ar–H), 7.83 (d, 1H; J = 5.4 Hz; Ar–H), 8.36 (dd, 1H; J = 8.1 Hz, 1.5 Hz; Ar–H) ppm. 13C NMR (DMSO-d6, 75 MHz) δ: 17.9 (–CH3), 33.4 (N–CH3), 50.2 (–CH2–), 111.3, 120.1, 122.6, 124.4, 126.6, 130.9, 133.4, 133.6, 137.1, 143.0, 152.3, 159.5 ppm. Anal. Calcd. for C15H14BrN3O2S2: C, 43.69; H, 3.42; N, 10.19; S, 15.55%. Found: C, 43.71; H, 3.50; N, 10.33; S, 15.73%.![[small nu, Greek, macron]](https://www.rsc.org/images/entities/char_e0ce.gif) cm−1; neat): 3100, 2923 (C–H), 1585 (C
cm−1; neat): 3100, 2923 (C–H), 1585 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1328 & 1148 (S
N), 1328 & 1148 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 761 (C–Cl). 1H NMR (DMSO-d6, 300 MHz) δ: 2.46 (s, 3H; –CH3), 3.33 (s, 3H; N–CH3), 4.93 (s, 2H; –CH2-), 7.25–7.30 (m, 2H; Ar–H), 7.35 (d, 1H; J = 8.4 Hz; Ar–H), 7.58–7.63 (m, 2H; Ar–H), 8.35 (d, 1H; J = 7.5 Hz; Ar–H) ppm. 13C NMR (DMSO-d6, 75 MHz) δ: 15.0 (–CH3), 33.3 (N–CH3), 49.9 (–CH2–), 120.1, 122.6, 124.5, 126.5, 128.5, 130.6, 131.6, 132.9, 138.9, 142.3, 152.5, 160.3 ppm. Anal. Calcd. for C15H14ClN3O2S2: C, 48.97; H, 3.84; N, 11.42; S, 17.43%. Found: C, 48.89; H, 3.76; N, 11.38; S, 17.31%.
O); 761 (C–Cl). 1H NMR (DMSO-d6, 300 MHz) δ: 2.46 (s, 3H; –CH3), 3.33 (s, 3H; N–CH3), 4.93 (s, 2H; –CH2-), 7.25–7.30 (m, 2H; Ar–H), 7.35 (d, 1H; J = 8.4 Hz; Ar–H), 7.58–7.63 (m, 2H; Ar–H), 8.35 (d, 1H; J = 7.5 Hz; Ar–H) ppm. 13C NMR (DMSO-d6, 75 MHz) δ: 15.0 (–CH3), 33.3 (N–CH3), 49.9 (–CH2–), 120.1, 122.6, 124.5, 126.5, 128.5, 130.6, 131.6, 132.9, 138.9, 142.3, 152.5, 160.3 ppm. Anal. Calcd. for C15H14ClN3O2S2: C, 48.97; H, 3.84; N, 11.42; S, 17.43%. Found: C, 48.89; H, 3.76; N, 11.38; S, 17.31%.![[small nu, Greek, macron]](https://www.rsc.org/images/entities/char_e0ce.gif) cm−1; neat): 3080, 2978 (C–H), 1587 (C
cm−1; neat): 3080, 2978 (C–H), 1587 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1325 & 1148 (S
N), 1325 & 1148 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR (DMSO-d6, 300 MHz) δ: 2.47 (s, 3H; –CH3), 3.33 (s, 3H; N–CH3), 4.93 (s, 2H; –CH2–), 7.27 (t, 1H; J = 7.5 Hz; Ar–H), 7.36–7.40 (m, 2H; Ar–H), 7.61–7.68 (m, 2H; Ar–H), 8.35 (d, 1H; J = 7.8 Hz; Ar–H) ppm. 13C NMR (DMSO-d6, 75 MHz) δ: 15.2 (–CH3), 33.3 (N–CH3), 49.9 (–CH2–), 117.7, 120.0, 122.7, 124.3, 126.5, 131.4, 131.9, 133.3, 134.4, 143.0, 152.5, 160.2 ppm. Anal. Calcd. for C15H14BrN3O2S2: C, 43.69; H, 3.42; N, 10.19; S, 15.55%. Found: C, 43.77; H, 3.54; N, 10.27; S, 15.69%.
O). 1H NMR (DMSO-d6, 300 MHz) δ: 2.47 (s, 3H; –CH3), 3.33 (s, 3H; N–CH3), 4.93 (s, 2H; –CH2–), 7.27 (t, 1H; J = 7.5 Hz; Ar–H), 7.36–7.40 (m, 2H; Ar–H), 7.61–7.68 (m, 2H; Ar–H), 8.35 (d, 1H; J = 7.8 Hz; Ar–H) ppm. 13C NMR (DMSO-d6, 75 MHz) δ: 15.2 (–CH3), 33.3 (N–CH3), 49.9 (–CH2–), 117.7, 120.0, 122.7, 124.3, 126.5, 131.4, 131.9, 133.3, 134.4, 143.0, 152.5, 160.2 ppm. Anal. Calcd. for C15H14BrN3O2S2: C, 43.69; H, 3.42; N, 10.19; S, 15.55%. Found: C, 43.77; H, 3.54; N, 10.27; S, 15.69%.![[small nu, Greek, macron]](https://www.rsc.org/images/entities/char_e0ce.gif) cm−1; neat): 3089, 2934 (C–H), 1585 (C
cm−1; neat): 3089, 2934 (C–H), 1585 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1325 & 1150 (S
N), 1325 & 1150 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR (DMSO-d6, 300 MHz) δ: 2.64 (s, 3H; –CH3), 3.34 (s, 3H; N–CH3), 4.97 (s, 2H; –CH2–), 7.29 (t, 1H; J = 7.5 Hz; Ar–H), 7.36 (d, 1H; J = 7.5 Hz; Ar–H), 7.63 (t, 1H; J = 8.1 Hz; Ar–H), 7.80 (d, 1H; J = 4.5 Hz; Ar–H), 8.19 (d, 1H; J = 4.5 Hz; Ar–H), 8.36 (d, 1H; J = 7.8 Hz; Ar–H) ppm. 13C NMR (DMSO-d6, 75 MHz) δ: 15.3 (–CH3), 33.2 (N–CH3), 50.2 (–CH2–), 120.2, 121.9, 124.4, 127.6, 129.0, 131.9, 134.1, 137.1, 143.7, 149.8, 152.8, 159.6 ppm. Anal. Calcd. for C15H14N4O4S2: C, 47.61; H, 3.73; N, 14.81; S, 16.95%. Found: C, 47.69; H, 3.81; N, 14.85; S, 17.10%.
O). 1H NMR (DMSO-d6, 300 MHz) δ: 2.64 (s, 3H; –CH3), 3.34 (s, 3H; N–CH3), 4.97 (s, 2H; –CH2–), 7.29 (t, 1H; J = 7.5 Hz; Ar–H), 7.36 (d, 1H; J = 7.5 Hz; Ar–H), 7.63 (t, 1H; J = 8.1 Hz; Ar–H), 7.80 (d, 1H; J = 4.5 Hz; Ar–H), 8.19 (d, 1H; J = 4.5 Hz; Ar–H), 8.36 (d, 1H; J = 7.8 Hz; Ar–H) ppm. 13C NMR (DMSO-d6, 75 MHz) δ: 15.3 (–CH3), 33.2 (N–CH3), 50.2 (–CH2–), 120.2, 121.9, 124.4, 127.6, 129.0, 131.9, 134.1, 137.1, 143.7, 149.8, 152.8, 159.6 ppm. Anal. Calcd. for C15H14N4O4S2: C, 47.61; H, 3.73; N, 14.81; S, 16.95%. Found: C, 47.69; H, 3.81; N, 14.85; S, 17.10%.![[small nu, Greek, macron]](https://www.rsc.org/images/entities/char_e0ce.gif) cm−1; neat): 3095, 2919 (C–H), 1594 (C
cm−1; neat): 3095, 2919 (C–H), 1594 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1327 & 1146 (S
N), 1327 & 1146 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR (DMSO-d6, 300 MHz) δ: 2.40–2.42 (2s, 6H; –CH3), 2.61 (s, 3H; –CH3), 3.32 (s, 3H; N–CH3), 4.90 (s, 2H; –CH2–), 7.12 (s, 1H; Ar–H), 7.28 (t, 1H; J = 7.5 Hz; Ar–H), 7.34 (d, 1H; J = 8.4 Hz; Ar–H), 7.58 (td, 1H; J = 8.1 Hz, 1.2 Hz; Ar–H), 8.35 (dd, 1H; J = 8.1 Hz, 1.2 Hz; Ar–H) ppm. 13C NMR (DMSO-d6, 75 MHz) δ: 15.1 (–CH3), 16.8 (–CH3), 18.2 (–CH3), 33.4 (N–CH3), 50.3 (–CH2–), 120.1, 123.1, 124.4, 126.4, 127.3, 132.9, 135.0, 135.3, 139.3, 142.8, 150.8, 162.5 ppm. Anal. Calcd. for C17H19N3O2S2: C, 56.48; H, 5.30; N, 11.62; S, 17.74%. Found: C, 56.56; H, 5.45; N, 11.70; S, 17.86%.
O). 1H NMR (DMSO-d6, 300 MHz) δ: 2.40–2.42 (2s, 6H; –CH3), 2.61 (s, 3H; –CH3), 3.32 (s, 3H; N–CH3), 4.90 (s, 2H; –CH2–), 7.12 (s, 1H; Ar–H), 7.28 (t, 1H; J = 7.5 Hz; Ar–H), 7.34 (d, 1H; J = 8.4 Hz; Ar–H), 7.58 (td, 1H; J = 8.1 Hz, 1.2 Hz; Ar–H), 8.35 (dd, 1H; J = 8.1 Hz, 1.2 Hz; Ar–H) ppm. 13C NMR (DMSO-d6, 75 MHz) δ: 15.1 (–CH3), 16.8 (–CH3), 18.2 (–CH3), 33.4 (N–CH3), 50.3 (–CH2–), 120.1, 123.1, 124.4, 126.4, 127.3, 132.9, 135.0, 135.3, 139.3, 142.8, 150.8, 162.5 ppm. Anal. Calcd. for C17H19N3O2S2: C, 56.48; H, 5.30; N, 11.62; S, 17.74%. Found: C, 56.56; H, 5.45; N, 11.70; S, 17.86%.![[small nu, Greek, macron]](https://www.rsc.org/images/entities/char_e0ce.gif) cm−1; neat): 3080, 2978 (C–H), 1585 (C
cm−1; neat): 3080, 2978 (C–H), 1585 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1325 & 1150 (S
N), 1325 & 1150 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR (DMSO-d6, 300 MHz) δ: 2.43 (s, 3H; –CH3), 3.32 (s, 3H; N–CH3), 4.94 (s, 2H; –CH2–), 7.28 (t, 1H; J = 7.5 Hz; Ar–H), 7.35 (d, 1H; J = 8.4 Hz; Ar–H), 7.60 (t, 1H; J = 7.8 Hz; Ar–H), 7.69 (s, 1H; Ar–H), 8.34 (d, 1H; J = 8.1 Hz; Ar–H) ppm. 13C NMR (DMSO-d6, 75 MHz) δ: 17.8 (–CH3), 33.3 (N–CH3), 50.2 (–CH2–), 120.1, 122.6, 124.3, 125.6, 126.6, 128.6, 133.3, 136.5, 142.9, 143.0, 151.6, 158.6 ppm. Anal. Calcd. for C15H13Cl2N3O2S2: C, 44.78; H, 3.26; N, 10.44; S, 15.94%. Found: C, 44.90; H, 3.42; N, 10.60; S, 16.08%.
O). 1H NMR (DMSO-d6, 300 MHz) δ: 2.43 (s, 3H; –CH3), 3.32 (s, 3H; N–CH3), 4.94 (s, 2H; –CH2–), 7.28 (t, 1H; J = 7.5 Hz; Ar–H), 7.35 (d, 1H; J = 8.4 Hz; Ar–H), 7.60 (t, 1H; J = 7.8 Hz; Ar–H), 7.69 (s, 1H; Ar–H), 8.34 (d, 1H; J = 8.1 Hz; Ar–H) ppm. 13C NMR (DMSO-d6, 75 MHz) δ: 17.8 (–CH3), 33.3 (N–CH3), 50.2 (–CH2–), 120.1, 122.6, 124.3, 125.6, 126.6, 128.6, 133.3, 136.5, 142.9, 143.0, 151.6, 158.6 ppm. Anal. Calcd. for C15H13Cl2N3O2S2: C, 44.78; H, 3.26; N, 10.44; S, 15.94%. Found: C, 44.90; H, 3.42; N, 10.60; S, 16.08%.Biological activity
Docking studies
In order to look into the protein–ligand interactions of the newly synthesized analogues, docking studies were carried out against human monoamine oxidase A and B with PDB-ID 2Z5Y and 2V5Z, respectively, using Molecular Operating Environment (MOE 2014) software package. MAOs showed three functional areas in the active site, i.e., the aromatic-cage (formed from Tyr435, Tyr398, and FAD), the substrate-cavity, and the entrance-cavity. Once the protein was prepared, the cognate ligand was a docked to validate the docking protocol by reproducing the experimentally determined orientation within an RMSD value of 1.5 Å.Results and discussion
Chemistry
The titled 2,1-benzothiazine derivatives 7(a–f) and 9(a–k) were achieved by practicing the synthetic route presented in Scheme 1. The N-sulphonylated ester 2 obtained by solvent-free N-mesylation of methyl anthranilate 1 upon N-methylation lead to the formation of ester 3.29 Subsequent base catalyzed ring closure of the ester 3 resulted in the formation of ketone 4 with 2,1-benzothiazine framework which was further condensed with hydrazine monohydrate to yield hydrazone 5 in excellent yield. Finally, the acid catalyzed reaction of hydrazone 5 with (un)substituted 2-chloroquinoline carbaldehydes 6(a–f) and methyl-thiophenyl ketones 8(a–k) in methanol resulted in the formation of new titled compounds 7(a–f) and 9(a–k) correspondingly in reasonably good yields.The necessary techniques like FTIR, 1H NMR, 13C NMR and elemental analyses were used to characterize the synthesized compounds. After determination of functional groups from the FTIR spectra and confirmation of composition from elemental analyses, the synthesized compounds were studied by NMR spectroscopy. The 1H NMR spectra of the compounds 9(a–k) exhibited three conspicuous singlet peaks referring to –SO2CH2– methylene protons (4.90 and 4.98 ppm), N–CH3 (near 3.33 ppm) and methyl protons of ketone (near 2.50 ppm). The aromatic proton signals on the other hand exhibited chemical shifts in a range 6.89–8.36 ppm with multiplicities depending upon the environment of the corresponding hydrogen atoms. In 13C NMR spectra of these compounds, N–CH3 and –SO2CH2–methylene carbon showed signals around 33.3 ppm and 50.0 ppm respectively. The aromatic and imine (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) signals appeared in aromatic region i.e., 111.3–162.5 ppm. The 1H NMR spectra of compounds 7(a–f) too were in agreement with the proposed structures showing N–CH3 singlet near 3.33 ppm and aromatic signals between 6.84 and 8.93 ppm. However, these spectra exhibited duplicate signals for few protons including N
C) signals appeared in aromatic region i.e., 111.3–162.5 ppm. The 1H NMR spectra of compounds 7(a–f) too were in agreement with the proposed structures showing N–CH3 singlet near 3.33 ppm and aromatic signals between 6.84 and 8.93 ppm. However, these spectra exhibited duplicate signals for few protons including N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, H-4 of quinoline ring and –SO2CH2– protons. The presence of a deshielded singlet around 10.94–11.86 ppm referring to NH proton indicated the formation of tautomers in the solution i.e., imine and enamine forms (Fig. 2). The imine form showed the singlet for methylene protons (–SO2CH2–) around 5.25 ppm while enamine form exhibited the singlet peak for NH around 10.94–11.86 ppm and methine (SO2CH
CH, H-4 of quinoline ring and –SO2CH2– protons. The presence of a deshielded singlet around 10.94–11.86 ppm referring to NH proton indicated the formation of tautomers in the solution i.e., imine and enamine forms (Fig. 2). The imine form showed the singlet for methylene protons (–SO2CH2–) around 5.25 ppm while enamine form exhibited the singlet peak for NH around 10.94–11.86 ppm and methine (SO2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ) singlet around 6.71–6.89 ppm. The N
) singlet around 6.71–6.89 ppm. The N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH proton due to neighbouring isomeric system, appeared as two discrete singlets for the two forms in a range 8.80–9.33 ppm. Conversely, 13C NMR spectra too, showed duplicated signals for few carbon atoms. The imine form gave signal for N–CH3 near 33.0 ppm however the enamine form exhibited this signal around 29.6 ppm. Methylene (SO2CH2) carbon atom showed up near 50.2 ppm for imine form while enamine form gave signal of methine (SO2CH
CH proton due to neighbouring isomeric system, appeared as two discrete singlets for the two forms in a range 8.80–9.33 ppm. Conversely, 13C NMR spectra too, showed duplicated signals for few carbon atoms. The imine form gave signal for N–CH3 near 33.0 ppm however the enamine form exhibited this signal around 29.6 ppm. Methylene (SO2CH2) carbon atom showed up near 50.2 ppm for imine form while enamine form gave signal of methine (SO2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ) carbon atom near 95.0 ppm. The N
) carbon atom near 95.0 ppm. The N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH signal appeared near 150.0 ppm in aromatic region with other aromatic carbon atoms (106.8–161.8 ppm).
CH signal appeared near 150.0 ppm in aromatic region with other aromatic carbon atoms (106.8–161.8 ppm).
Monoamine oxidase activity
| Entry | Code | MAO A | MAO B |
|---|---|---|---|
| IC 50 (μM) & % inhibition | |||
| a Percentage inhibition.b IC50.c Positive control. | |||
| 1 | 7a | 38.8a | 43.6a |
| 2 | 7b | 2.32 ± 0.91b | 1.43 ± 0.89b |
| 3 | 7c | 2.10 ± 0.09b | 35.75a |
| 4 | 7d | 1.82 ± 0.24b | 1.05 ± 0.66b |
| 5 | 7e | 3.43 ± 0.52b | 2.22 ± 0.94a |
| 6 | 7f | 2.03 ± 0.91a | 1.21 ± 0.17 b |
| 7 | 9a | 2.09 ± 0.89a | 1.98 ± 0.14 b |
| 8 | 9b | 38.5a | 2.38 ± 0.19 b |
| 9 | 9c | 39.1a | 3.83 ± 0.48 b |
| 10 | 9d | 1.27 ± 0.10 b | 31.09a |
| 11 | 9e | 1.04 ± 0.01 b | 41.93a |
| 12 | 9f | 41.6a | 3.82 ± 0.37 b |
| 13 | 9g | 1.52 ± 0.56 b | 1.25 ± 0.28 b |
| 14 | 9h | 28.4a | 1.03 ± 0.17 b |
| 15 | 9i | 39.8a | 1.21 ± 0.13 b |
| 16 | 9j | 26.3a | 2.61 ± 0.37 b |
| 17 | 9k | 2.48 ± 0.70 b | 2.58 ± 0.48 b |
| 19 | Clorgylinec | 0.0045 ± 0.03 | 61.35 ± 1.13 |
| 20 | Deprenylc | 67.25 ± 1.02 | 0.0196 ± 0.001 |
The unsubstituted compound 7a showed less activity toward both isoforms, while the introduction of the methyl group i.e., compound 7b and methoxy group i.e., compound 7c at position 6 on quinoline ring made these derivatives potent inhibitor against MAO A (with IC50 values 2.10 ± 0.09 and 2.32 ± 0.91 μM respectively) as well as against MAO B (IC50 values 1.43 ± 0.89 μM, however, less activity for methoxy substituted analogue). Furthermore, the presence of methyl i.e., compound 7d, methoxy i.e., compound 7e and chloro group i.e., compound 7f at position 7 on same ring showed activities 1.82 ± 0.24 μM, 3.43 ± 0.5 μM and 2.03 ± 0.9 μM, respectively on MAO A whereas inhibition against MAO B was observed 1.05 ± 0.66 μM, 2.22 ± 0.94 μM and 1.21 ± 0.17 μM, respectively (Fig. 5).
In 2nd series, the introduction of chloro-substituent at thiophene ring led the compound 9e to be the most potent derivative against MAO A having IC50 1.04 ± 0.01 μM. The presence of methyl group at position 5 on same ring showed activity 1.27 ± 0.10 μM whereas the introduction of chloro group at same position showed nearly same IC50 value 1.52 ± 0.56 μM toward MAO A. The introduction of bromo- (9g), nitro- (9h) and chloro- (9i) at the same position exhibited inhibition of MAO B with IC50 values 1.03 ± 0.17 μM, 1.21 ± 0.13 μM and 1.25 ± 0.28 μM respectively.
Docking studies
The newly synthesized compound 4-((1-(3-chlorothiophen-2-yl)ethylidene)hydrazono)-1-methyl-3,4-dihydro-1H-benzo[c][1,2]thiazine 2,2-dioxide (9e) showed activity toward MAO A in micromolar range (IC50 = 0.04 ± 0.01 μM). The presence of the two oxygen atoms in thiazine 2,2-dioxide were found to make hydrogen bond interactions with the amino acid Tyr69 and Ala68 same as already reported.32–34 Furthermore, the benzene ring of the inhibitor was found to form π–π interactions with the amino acid residues Tyr444 and Tyr407 aromatic rings. Moreover, the presence of halogen i.e., chloro-group at thiophene ring and the methyl group exhibited π–alkyl interactions with the amino acid residues Ile180, Leu337 and Ile335, respectively. Similarly, the presence of chlorine at position 3 of thiophene and sulfur in thiophene ring made halogens and π–sulfur interaction with the amino acid residues Phe208 and Phe352 respectively, comparable to the reference ligand interactions. Hence 9e interacts with other residues as well as with those observed in case of reference cognate ligand. The putative binding mode of compound 9e is shown in Fig. 6.4-((1-(5-Bromothiophen-2-yl)ethylidene)hydrazono)-1-methyl-3,4-dihydro-1H-benzo[c][1,2]thiazine 2,2-dioxide (9h) showed highest inhibitory activity against MAO B in micromolar range with IC50 value 1.03 ± 0.17 μM. The presence of the oxygen at thiazine 2,2-dioxide was found to show hydrogen bond interaction with the amino acid residue Tyr60, Ser59 and Lys296. The presence of benzene ring made π–sigma interaction with the amino acid Tyr398. The presence of bromo group at thiophene ring allowed π–alkyl interaction with the amino acid residue Ile199. 5-Bromothiophene ring made π–alkyl and π–sulfur interaction with amino acid residues Leu171 and Cys172, respectively. The presence of methyl group made π–alkyl interaction with Tyr435 as reported previously.32–34 While discussing the type of interaction displayed by reference ligand, the synthesized inhibitor (9h) has displayed same kind of interactions at the active site of the enzyme. The putative binding mode of compound 9h is shown in Fig. 7.
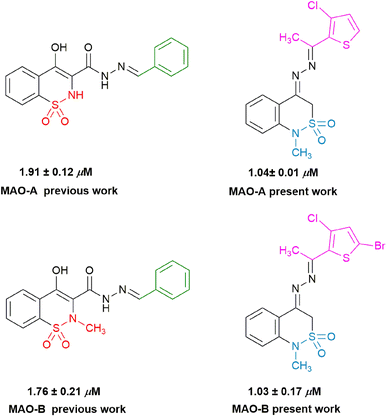
Previously reported work and current study
If we compare our work with previously or already reported monoamine oxidase inhibitors, it was well known that already reported analogues of benzylidenethiazine-3-carbohydrazide 1,1-dioxide showed distinguished inhibition against monoamine oxidases. Whereas present study showed that the derivatives of benzylidenethiazine 2,2-dioxide showed better results than already reported derivatives (Fig. 8). The introduction of different groups (–F, –Cl, –Br, –NO2,–CH3,–OCH3) in basic pharmacophore made it more potent and selective towards the targeted enzymes.Conclusion
A library of 1-methyl-3,4-dihydro-1H-benzo[c][1,2]thiazine 2,2-dioxides with different substituent was synthesized and investigated against MAOs. All the synthesized compounds showed MAO inhibition activity in the lower micromolar range. Compound 9e, having an IC50 value of 1.04 ± 0.01 μM, was the most potent MAO A inhibitor, while compound 9h, with an IC50 value of 1.03 ± 0.17 μM, was the most active MAO B inhibitor. Furthermore, the docking studies further verified the binding site interactions of the inhibitors. Moreover, the pivotal role played by this target in AD and PD pathogenesis suggests that these potent compounds may act as promising new chemical entity in the design of multi-target-directed-ligands.Author contributions
Noman Javid: conceptualization, methodology, formal analysis, validation. Saquib Jalil: investigation (bioactivity), writing – original draft preparation (bioactivity part), software. Rubina Munir: supervision, resources (synthesis), data curation, writing – original draft preparation (synthesis), project administration. Muhammad Zia-ur-Rehman: reviewing and editing, software. Amna Sahar: investigation (synthesis). Sara Arshad: investigation (synthesis). Jamshed Iqbal: supervision, resources (bioactivity and docking), funding acquisition. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
The authors declare that they have no significant conflict of interest.Acknowledgements
Rubina Munir is grateful to Chemistry Department, Kinnaird College for Women, for providing facilities for synthetic lab work. The authors gratefully acknowledge the financial support for this research provided by the Higher Education Commission of Pakistan (HEC) via NRPU project No. 20-15846/NRPU/R&D/HEC/2021, German-Pakistani Research Collaboration Programme and Equipment Grant funded by DAAD, Germany.References
- S. Carradori and R. Silvestri, J. Med. Chem., 2015, 58, 6717–6732 CrossRef CAS PubMed.
- P. Sharma, M. Singh and B. Mathew, ChemistrySelect, 2021, 6, 1404–1429 CrossRef CAS.
- M. Bajda, N. Guzior, M. Ignasik and B. Malawska, Curr. Med. Chem., 2011, 18, 4949–4975 CrossRef CAS PubMed.
- L. Pisani, M. Catto, F. Leonetti, O. Nicolotti, A. Stefanachi, F. Campagna and A. Carotti, Curr. Med. Chem., 2011, 18, 4568–4587 CrossRef CAS.
- D. Mousseau and G. B. Baker, Curr. Top. Med. Chem., 2012, 12, 2163–2176 CrossRef CAS PubMed.
- C. Brown and R. M. Davidson, Adv. Heterocycl. Chem., 1985, 38, 135–176 CrossRef CAS.
- J. G. Lombardino and D. E. Kuhla, Adv. Heterocycl. Chem., 1981, 28, 73–126 CrossRef CAS.
- J. G. Lombardino, E. H. Wiseman and W. M. McLamore, J. Med. Chem., 1971, 14, 1171–1175 CrossRef CAS PubMed.
- F. Schiaffella, A. Macchiarulo, L. Milanese, A. Vecchiarelli and R. Fringuelli, Bioorg. Med. Chem., 2006, 14, 5196–5203 CrossRef CAS PubMed.
- N. Ahmad, M. Zia-ur-Rehman, H. L. Siddiqui, M. F. Ullah and M. Parvez, Eur. J. Med. Chem., 2011, 46, 2368–2377 CrossRef CAS PubMed.
- V. Cecchetti, A. Fravolini, P. G. Pagella, A. Savino and O. Tabarrini, J. Med. Chem., 1993, 36, 3449–3454 CrossRef CAS PubMed.
- A. Barazarte, N. Gamboa, J. Rodrigues, R. Atencio, T. González and J. Charris, J. Chem. Res., 2009, 2009, 17–19 CrossRef.
- M. Ahmad, H. L. Siddiqui, M. Zia-ur-Rehman and M. Parvez, J. Med. Chem., 2010, 45, 698–704 CAS.
- V. Cecchetti, F. Schiaffella, O. Tabarrini and A. Fravolini, Bioorg. Med. Chem. Lett., 2000, 10, 465–468 CrossRef CAS PubMed.
- Y. P. Gong, R. Z. Wan and Z. P. Liu, Expert Opin. Ther. Pat., 2018, 28, 167–171 CrossRef CAS PubMed.
- H. Khalid, S. Shahid, S. Tariq, B. Ijaz, U. A. Ashfaq and M. Ahmad, Clin. Exp. Pharmacol. Physiol., 2021, 48, 1653–1661 CrossRef CAS PubMed.
- S. Aslam, M. Ahmad, M. Zia-ur-Rehman, C. Montero, M. Detorio, M. Parvez and R. F. Schinazi, Arch. Pharm. Sci. Res., 2014, 37, 1380–1393 CrossRef CAS PubMed.
- G. V. Mokrov, Arch. Pharm., 2021, 355, 210–428 Search PubMed.
- I. V. Ukrainets, A. A. Burian, V. N. Baumer, S. V. Shishkina, L. V. Sidorenko, I. A. Tugaibei, N. I. Voloshchuk and P. S. Bondarenko, Sci. Pharm., 2018, 86, 21 CrossRef PubMed.
- B. M. Szczęśniak-Sięga, B. Wiatrak, Ż. Czyżnikowska, J. Janczak, R. J. Wiglusz and J. Maniewska, Bioorg. Chem., 2021, 106, 104–476 CrossRef.
- R. K. Dudek-Wicher, B. M. Szczęśniak-Sięga, R. J. Wiglusz, J. Janczak, M. Bartoszewicz and A. F. Junka, Molecules, 2020, 25, 3503 CrossRef CAS PubMed.
- S. Ahmad, S. Zaib, S. Jalil, M. Shafiq, M. Ahmad, S. Sultan, M. Iqbal, S. Aslam and J. Iqbal, Bioorg. Chem., 2018, 80, 498–510 CrossRef CAS.
- I. V. Ukrainets, G. M. Hamza, A. A. Burian, S. V. Shishkina, N. I. Voloshchuk and O. V. Malchenko, Sci. Pharm., 2018, 86, 9 CrossRef PubMed.
- N. Tomita, Y. Hayashi, S. Suzuki, Y. Oomori, Y. Aramaki, Y. Matsushita, M. Iwatani, H. Iwata, A. Okabe, Y. Awazu and O. Isono, Bioorg. Med. Chem. Lett., 2013, 23, 1779–1785 CrossRef CAS PubMed.
- I. V. Ukrainets, L. A. Petrushova, S. P. Dzyubenko and G. Sim, Chem. Heterocycl. Compd., 2014, 50, 103–110 CrossRef CAS.
- S. Mir, A. M. Dar and B. A. Dar, Mini-Rev. Org. Chem., 2020, 17, 148–157 CrossRef CAS.
- R. Cannalire, D. Tarantino, A. Astolfi, M. L. Barreca, S. Sabatini, S. Massari, O. Tabarrini, M. Milani, G. Querat, E. Mastrangelo and G. Manfroni, Eur. J. Med. Chem., 2018, 143, 1667–1676 CrossRef CAS PubMed.
- N. Javid, R. Munir, F. Chaudhry, A. Imran, S. Zaib, A. Muzaffar and J. Iqbal, Bioorg. Chem., 2020, 99, 103–852 CrossRef PubMed.
- A. Mahmood, R. Munir, M. Zia-ur-Rehman, N. Javid, S. J. A. Shah, L. Noreen, T. A. Sindhu and J. Iqbal, ACS Omega, 2021, 6, 25062–25075 CrossRef CAS PubMed.
- R. Munir, M. Zia-ur-Rehman, S. Murtaza, S. Zaib, N. Javid, S. J. Awan, K. Iftikhar, M. M. Athar and I. Khan, Molecules, 2021, 26, 656 CrossRef CAS PubMed.
- M. Shafiq, M. Zia-ur-Rehman, I. U. Khan, M. N. Arshad and S. A. Khan, J. Chil. Chem. Soc., 2011, 56, 527–531 CrossRef CAS.
- N. T. Tzvetkov, S. Hinz, P. Küppers, M. Gastreich and C. E. Müller, J. Med. Chem., 2014, 57, 6679–6703 CrossRef CAS PubMed.
- R. M. Geha, K. Chen, J. Wouters, F. Ooms and J. C. Shih, J. Biol. Chem., 2002, 277, 17209–17216 CrossRef CAS.
- A. Fierro, A. Montecinos, C. Gómez-Molina, G. Núñez, M. Aldeco, D. E. Edmondson, M. Vilches-Herrera, S. Lühr, P. Iturriaga-Vásquez, and M. Reyes-Parada, An integrated view of the molecular recognition and toxinology. From analytical procedures to biomedical applications, InTech—Open Access Publisher, Rijeka, 2013, pp. 405–431 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ra07045f |
| ‡ The authors have equal contribution. |
| This journal is © The Royal Society of Chemistry 2023 |