Open Access Article
Open Access ArticleA review on development of metal–organic framework-derived bifunctional electrocatalysts for oxygen electrodes in metal–air batteries
Najla Javed
a,
Tayyaba Noor
 *a,
Naseem Iqbal
*a,
Naseem Iqbal
 b and
Salman Raza Naqvi
a
b and
Salman Raza Naqvi
a
aSchool of Chemical and Materials Engineering (SCME), National University of Sciences and Technology (NUST), H-12 Campus, Islamabad 44000, Pakistan. E-mail: tayyaba.noor@scme.nust.edu.pk; Tel: +92 51 9085 5121
bU.S.-Pakistan Center for Advanced Studies in Energy (USPCAS-E), National University of Sciences and Technology (NUST), Islamabad 44000, Pakistan
First published on 16th January 2023
Abstract
Worldwide demand for oil, coal, and natural gas has increased recently because of odd weather patterns and economies recovering from the pandemic. By using these fuels at an astonishing rate, their reserves are running low with each passing decade. Increased reliance on these sources is contributing significantly to both global warming and power shortage problems. It is vital to highlight and focus on using renewable energy sources for power production and storage. This review aims to discuss one of the cutting-edge technologies, metal–air batteries, which are currently being researched for energy storage applications. A battery that employs an external cathode of ambient air and an anode constructed of pure metal in which an electrolyte can be aqueous or aprotic electrolyte is termed as a metal–air battery (MAB). Due to their reportedly higher energy density, MABs are frequently hailed as the electrochemical energy storage of the future for applications like grid storage or electric car energy storage. The demand of the upcoming energy storage technologies can be satisfied by these MABs. The usage of metal–organic frameworks (MOFs) in metal–air batteries as a bi-functional electrocatalyst has been widely studied in the last decade. Metal ions or arrays bound to organic ligands to create one, two, or three-dimensional structures make up the family of molecules known as MOFs. They are a subclass of coordination polymers; metal nodes and organic linkers form different classes of these porous materials. Because of their modular design, they offer excellent synthetic tunability, enabling precise chemical and structural control that is highly desirable in electrode materials of MABs.
1. Introduction
Increased fuel demand and its consumption is associated with environmental pollution and health issues due to greenhouse gas emission into the atmosphere. Fossil fuels, which are limited energy sources and release harmful gases into the atmosphere like carbon dioxide and methane, are a major contributor to global warming and the annual rise in Earth's temperature. Excess global warming is disturbing the whole ecosystem: marine life, wildlife, humans etc. The amount of water in the world's oceans and seas is rising owing to melting of glaciers, sea ice and polar ice sheets brought on by warmer temperatures. Extreme financial setbacks might result from extreme weather. In addition to rising house and health insurance costs for customers, food and energy prices are also rising. Factors including declining tourism and industrial profitability, rising energy, food, and water needs, disaster cleanup, and border tensions have an impact on governments and then on our economy.1–3 This has sparked considerable research into renewable and ecologically suitable energy sources. Effective energy storage systems must be created to put new sustainable energy sources in use efficiently. By offering sustainable power sources and fuel diversity, renewable energy sources improve energy security, lower the possibility of fuel leaks, and decrease the requirement for imported fuels. The nation's natural resources are also protected using these green energy sources. High demand can be reduced via energy storage, and any cost savings might be passed on to consumers. Energy storage can assist in meeting peak energy demands in highly populated places, reducing grid load and price increases.4,5Environmental degradation outweighs fossil fuels against renewable energy sources. Systems for storing energy can aid in the switching to renewable energy sources from fossil fuels. Due to the negative environmental effects of greenhouse gases (GHG) created by fossil fuels, electric power generation is altering drastically all over the world. By implementing energy storage systems (ESSs), the unpredictable daily and seasonal variations in electrical energy consumption can be addressed, reducing the additional GHG emissions into the atmosphere. Mechanical, electrochemical, chemical, thermal, and other energy storage methods are all possible. When there is an imbalance between supply and demand, energy storage systems (ESS) offer a way to increase the effectiveness of electrical systems.4,6,7 The most extensively used options for dealing with concerns related to oil shortage, upsurging fossil fuel consumption, and global warming are renewable energy sources including hydro power, photovoltaics, and wind turbines. Strongly reliant on weather resources with sporadic and variable characteristics are solar and wind energy. With benefits like quick response time, sustained power delivery, and regional independence, battery energy storage systems have gained widespread acceptance among the possible methods to solve this problem of power shortage.8
Nickel–cadmium, lead-acid, sodium–sulfur metal–air, lithium-ion, and other types of energy storage batteries are commonly employed for this purpose since they compared to engines and turbines, exhibit faster charge and discharge characteristics.9,10 One of the best ways to store energy is using batteries, which come in a variety of shapes and sizes. The primary focus of this review article will be metal–air batteries, which harness the energy of atmospheric oxygen to create power. This has the benefit that the oxygen does not need to be preserved inside the battery, unlike a standard alkaline or Li-ion battery (LIB). The theoretical specific energy of these metal–air batteries is noticeably (3–30 times) greater than that of Li-ion batteries. Because safer aqueous electrolytes are employed, these batteries are also intrinsically safe in that they won't catch on fire or explode while in use. Due to their comparably high energy density, flexible metal–air batteries have attracted a lot of attention lately and may compete with them for use in wearable or roll-up electronic devices. Flexible nonaqueous LIBs, aqueous zinc-ion batteries (ZIBs), and aqueous aluminum–air (AlBs) batteries are a few examples of metal–air batteries that have recently been produced.11,12
Researchers are keen to find cleaner energy technologies for storing and converting energy, including the smart grid, due to the effects of global warming and the shifting price of oil. The creation of portable electronics has also been advancing, necessitating the use of power sources with steadily increasing energy and power densities. LIBs, which have a higher energy density than more traditional power sources like Ni-MH batteries, are projected to be able to meet these demands. However, electrode materials with a complex chemistry have a restriction on the highest energy density of modern lithium-ion batteries, making them unsuitable for use in actual electric car applications. Metal–air batteries have generated a lot of interest as a potential alternative due to their incredibly high energy density in comparison to traditional rechargeable batteries.13,14 For Li–air batteries, it is possible to prevent unfavorable reactions and issues with electrolyte deterioration brought on by organic solvents by employing aqueous lithium salt solutions as the electrolytes. Aqueous electrolytes have a small potential window, which restricts their use in Li-based batteries. When employing high concentration hydrate-melt or water-in-salt (WiS) electrolytes, for example, one can get aqueous electrolytes to operate at high voltages by eliminating free water molecules from the electrolyte.15
We will also go over the application of MOFs and their derivatives which are being researched as prospective electrode materials because of their vast specific surface areas, numerous modular and programmable topologies, and enormous internal pore volume in metal–air batteries. Due to their tunable pore widths and metal ions with redox activities, huge specific surface areas and enormous internal pore volumes have made MOFs gain a lot of interest recently as electrode materials with high energy capacities.16,17 An open framework that may be permeable characterizes the structure of MOFs (porous materials). This type of MOF was initially recognized and described by the Yaghi team in 1995.18 A coordination bond between a key core metal ion and a bolstering organic ligand self-assembles in this organic–inorganic hybrid material. The MOF has significantly changed over time, beginning with its first direct synthesis, and ending with its current broad utilization. When compared to weak bonds like hydrogen bonds and van der Waals bonds, MOFs' coordination bond energy is much larger (usually 60–350 kJ mol−1). MOFs are stable, as a result they can create permanent pores.19
Furthermore, MOFs' structure, as well as their physical and chemical characteristics, are highly designable. Researchers have developed more than 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MOFs since the MOF was coined; this numerical figure is continually rising. This is due to the fact that these various center-metal ions can combine with a wide range of organic ligands to generate a vast variety of MOF compounds.20 Zhang et al. created the PCN-128W metal–organic framework in 2015 with the use of zirconium salt and chromophoric linker. The emission maximum shifts from 470 to 538 nm, and the hue of PCN-128W reversibly changes from white to yellow. This piezofluorochromic behavior is quite fascinating. Fluorescence spectroscopy was used to track the stepwise fluorescence change, and it showed that consecutive compression gradually shifted the emission maximum. To get profound understanding of the piezofluorochromic process, structural studies are conducted on both the white and yellow phases.21
000 MOFs since the MOF was coined; this numerical figure is continually rising. This is due to the fact that these various center-metal ions can combine with a wide range of organic ligands to generate a vast variety of MOF compounds.20 Zhang et al. created the PCN-128W metal–organic framework in 2015 with the use of zirconium salt and chromophoric linker. The emission maximum shifts from 470 to 538 nm, and the hue of PCN-128W reversibly changes from white to yellow. This piezofluorochromic behavior is quite fascinating. Fluorescence spectroscopy was used to track the stepwise fluorescence change, and it showed that consecutive compression gradually shifted the emission maximum. To get profound understanding of the piezofluorochromic process, structural studies are conducted on both the white and yellow phases.21
Recently, there has been a lot of interest in rechargeable sodium–air batteries (SABs), which have a high theoretical specific capacity, a high energy density, a low price, and no bad environmental consequences. Based on the electrolytes utilized in the system, SABs are categorized into two classes: non-aqueous and aqueous/hybrid. The electrochemical performance of non-aqueous SABs is constrained by the insolubility of solid discharge products like Na2O and Na2O2, which impedes long-term operation by fouling the air electrode. The potential of carbon nanostructures produced from MOF as non-noble metal-based oxygen electrocatalysts for metal–air batteries was investigated by Yuqi Wu et al. For the first time, hybrid sodium–air batteries (SABs) with N-doped carbon nanotubes (MOF-NCNTs) (OER) have shown improved electrocatalytic activity and stability for the oxygen reduction reaction (ORR) and oxygen evolution process.22,23
The sole purpose of this review is to provide the most recent developments in MOF-based materials utilized in MABs. A lot of significant factors that have a major impact on how active water splitting reactions are discussed along with how to construct catalysts. In addition to a summary of the major challenges in the areas of photocatalytic and electrocatalytic water splitting, some information on recent developments in the production of MOF-based catalysts has also been included. Future research objectives are listed, with an emphasis on achieving the required. For identifying and comprehending the factors influencing catalytic activity, MOF functionality and framework correlations are being developed. This paper highlights recent developments in this rapidly growing field and offers some suggestions for constructing extremely effective photo- and electrocatalysts for water splitting/redox reactions that are based on electrode made of MOFs which take place inside MABs.24,25
1.1. What are metal–air batteries and what is their working mechanism?
In MABs, the metal anode which is composed mainly of Li, Zn, Al, or Na is inserted first. The liquid or solid electrolyte and the air cathode are then added. This is true for mechanically rechargeable MABs, where the electrolyte and metal plate are occasionally replaced after being used up during discharge. The solid-state MAB, which is typically a gel-polymer based on PVA for Zn–air batteries and has an electrolyte in the solid phase. The electrically rechargeable MAB, it utilizes a breathing carbon-based or metallic porous foil cathode and supports the essential bifunctional electrocatalyst via a gas diffusion layer (GDL), has an open battery design. Such batteries with aqueous system are ideal for metals like Zn, Fe, Al, Cd, etc. Zinc–air batteries have a particularly promising future as an alternative energy storage device. Aluminium air batteries (AlBs) have a substantial energy density greater than ZABs, even though aluminum corrodes more readily than zinc in alkaline conditions. Additional benefits of Zn include its abundance, affordable, environmentally friendly, low equilibrium potential, flat discharge voltage, and long-time span of usability. These batteries' primary advantage is that oxygen reduction reactions can be catalyzed by non-noble metals and have a potential specific energy density of 1084 W h kg−1.26,27One of the distinguishing features of metal–air batteries is their open cell structure, which uses oxygen gas from the air as the cathode material. Traditional rechargeable batteries have a closed system for their cell arrangement. These batteries come in countless variations based on different metal types, and additional cell components are needed because of their unique reaction kinetics. Based on their electrolytes, these batteries are typically divided into two categories, the first is a cell structure that is impervious to moisture and operates with an aqueous electrolyte. The alternative approach makes use of an electrolyte and aprotic solvents and is water sensitive, but moisture damages this type of system.28,29 The two main chemical reactions in metal–air batteries are the oxygen evolution reaction (OER) during charging and the oxygen reduction reaction (ORR) during discharging.30
The creation of extremely effective bifunctional electrocatalysts that perform with outstanding chemical stability in electrolytes containing oxygen is the main problem in the development of metal–air battery technology. Although precious metals like Pt, Ru, and Ir are known to be efficient electrocatalysts for ORR and OER, their high cost has hindered their widespread accessibility. Recent studies have demonstrated the efficiency and affordability of carbon nanomaterials as platinum alternatives for ORR, including carbon nanotubes, porous carbon, carbon nitride, and N-doped graphene. The surface polarity and electrical properties of nitrogen can be modified by co-doping it with another heteroatom, such as S or P, which increases ORR catalytic activity. However, the OER activity of the bulk of these metal-free catalysts is subpar. The most efficient OER electrocatalysts to date, according to some research, are constructed using transition metal hydroxides and oxides.31,32
A feasible method for using air as an electrode for batteries has long been desired by the battery industry. Theoretically, air is a material with no cost. If there were no obstacles to enhancing the practical specific energy, specific power, and cycle life of these batteries which commonly comprise earth-abundant components like Zn, Al, Mg, Si, C, Mn, as mentioned before their market price would be far lower.33 Despite the existence of metal–air batteries for more than a century, their application has been constrained by the performance and stability of the electrodes, electrolyte, and cell design. Recently, aqueous metal–air batteries have undergone significant advancements due to the necessary components that assist the enhancement of long-term stability and the demonstration of superior electrochemical performance.34–36
Table 1 compares energy density and open potential circuit of different MABs and showcasing that AlB has the highest value for energy density whereas iron air batteries have lowest. Not only does this iron air battery also has lowest potential value where cadmium air batteries having highest which is 3.10 volts. Batteries use chemical energy to store electrical energy. Because they have a theoretically higher density than lithium-ion batteries, metal–air batteries are being researched. An M-denoted metal anode is oxidized, releasing metal ions (M+), which travel through the electrolyte to the cathode and react with oxygen to form metal oxides as shown in the equations below.36,38 The metal on the anode oxidizes during discharge to release electrons, and the liberated metal ions mix with the hydroxide ions to form metal hydroxide:
| M + nOH− → M(OH)n + ne− | (1) |
| Battery type | Open circuit potential (volts) | Energy density (W h L−1) | Energy density (W h kg−1) |
|---|---|---|---|
| Zn–air | 1.68 | 5960 | 1045 |
| Li–air | 2.96 | 6102 | 3458 |
| Fe–air | 1.35 | 1431 | 763 |
| Mg–air | 3.08 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
3991 |
| Ca–air | 3.10 | 9960 | 2980 |
| Al–air | 2.71 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
4116 |
In the next step hydroxide ions are created when oxygen molecules from the atmosphere react with water on the cathode, incoming electrons, and water. Where M could be any metal (for example, Zn, Al, or Mg). The liberated electrons participate in the oxygen reduction reaction at the cathode after travelling through the external circuit (ORR)
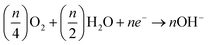 | (2) |
The generated hydroxide ions pass via the separator from the cathode to the anode to complete the current loop. Combining eqn (1) and (2) results in the following generic reaction:
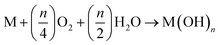 | (3) |
Zn, Al, and Mg are the only air batteries that can currently be recharged electrically in aqueous electrolyte; nevertheless, metal anodes cannot be transformed from ions to metals in the electrolyte, necessitating manual recharge of metal–air batteries. One of the essential parts of a metal–air battery is the air cathode, and reliable air electrodes guarantee that the battery operates effectively and consistently. An effective air electrode should typically have quick OH-migration, alkaline condition, good electrochemical stability.39 Oxygen evolution and reduction exhibit considerable electrochemical activity in rechargeable batteries as well as primary metal–air batteries (especially under high potentials). The design of air electrodes is hindered by these contradicting and difficult requirements. According to the literature, the development of air electrodes with improved catalytic activity, longer life, and lower production costs has thus been the main emphasis of metal–air battery research over the past 50 years.40,41
We have discussed almost all metal–air batteries in this section but, in this paper, our primary focus will be on the most common ones which are lithium and zinc air batteries.
1.2. Lithium air batteries
If a workable device could be created, the rechargeable LIBs, which has the greatest energy density among any rechargeable battery, might revolutionize energy storage. As a technology for energy storage, LABs have generated interest due to their high energy and power density. By surpassing the energy capabilities of traditional LIBs, these batteries are anticipated to revolutionize both the electrochemical energy storage systems and the automotive sector (for usage in electric and hybrid vehicles). Non-aqueous (aprotic) solvents, aqueous solvents, and hybrid (non-aqueous/aqueous/aqueous) solvents are the basic types of Li–air batteries as shown in Fig. 1 below it basically represents overall configuration of these batteries. Li salts are dissolved in all solid-state electrolytes. The anode and cathode materials in each of the four systems are formed of lithium metal and oxygen gas, respectively. Nevertheless, the type of electrolyte utilized affects how they react. Non-aqueous LIBs have porous cathodes where oxygen is reduced and converted to solid Li2O2. As a result, the main constraints limiting the capacity of this battery system are the solid product obstruction and/or passivation of active surfaces at the porous cathode. The configuration of the non-aqueous electrolyte system is similar to that of traditional LIBs.42,43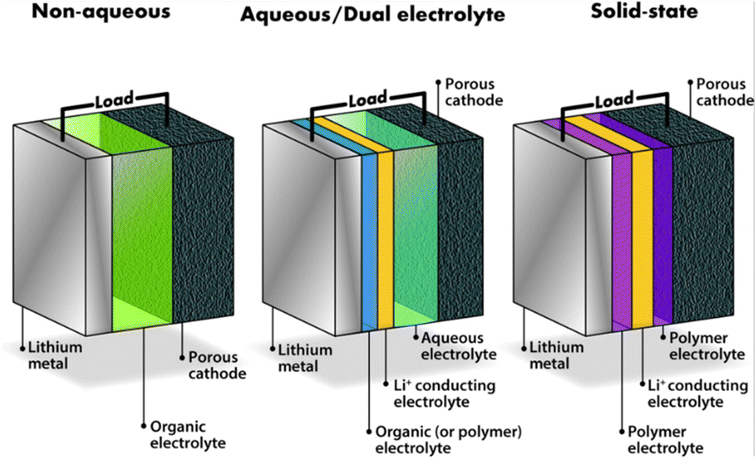 | ||
| Fig. 1 Schematic cell configurations for the three main types of Li–air battery. Copy with permission from ref. 42. Copyrights 2015, Wiley Online Library. | ||
Like standard LIBs, the non-aqueous electrolyte system is configured. Anodes composed of carbon or alloy materials, cathodes constructed of Li metal oxides or phosphates, and electrolytes formed of aprotic solvents in which Li salt is dissolved are all used in conventional batteries. Because oxygen gas acts as the cathode in Li–air batteries, porous carbon materials and catalyst composites must be considered. Since anodes are the lithium source in these batteries, this metal must be employed as the anode here. The primary difference between the two systems is that Li–air batteries need an open system. Additional parts, including air-dehydration membranes, are needed for an open system. Li2O2 is a precipitate that forms when Li+ ions near the air cathode's surface in Li–air batteries interact with oxide ions.42,44 Compared to previous rechargeable battery systems like nickel–cadmium or lead-acid, LIBs and LABs batteries offer a higher energy density, which has led to their significant success in the field of portable devices.45
Significant research is currently being conducted on them with the goal of storing energy produced by electric vehicles as well as renewable energy sources like solar and wind energy. Due to issues with energy, power, cycle life, cost, and safety, Li-ion batteries are not frequently employed for these later applications.46,47 For instance, the highest current capacity of insertion-oxide cathodes is 250 mA h g−1, and liquid electrolytes currently on the market typically dissolve above 4.3 V. New battery chemistries in addition to the next generation of electrochemical energy storage devices will require Li-ion technology. Due to its high specific gravity, lithium metal is the most promising anode material for high energy density batteries. During battery discharge, a solid product that forms in the porous cathode is strongly related to the multi-phase transport mechanisms that move electrons in the solid materials and dissolved oxygen and lithium ions (Li+) in the liquid electrolyte. For increasing battery capacity and design, particularly under high-rate situations, one must have a thorough understanding of the physics underlying transport phenomena as well as an accurate assessment of efficient transport attributes.48,49
The transport governing equations for porous-average level macroscopic continuum models are presented below. The simplest net reaction envisioned for the aprotic battery is a two-electron process:
| 2Li + O2 → Li2O2 | (4) |
| Li2O2 → 2Li + O2 | (5) |
The basic processes in an aqueous cathode electrolyte are:
The chemistry of the all-solid-state battery is most likely equivalent to the aprotic electrolyte battery, despite not yet being known. Due to its advantageous energy density and durability, LIBs are the ideal option for energy storage in portable devices. For usage as power sources for electric vehicles and smart grids, batteries must have a long lifespan, a high energy density, continuous safety performance, and a low price.50 Electrolyte additives have greatly improved in their ability to handle the problems with conventional electrolytes that lead to substandard cycle performance and unwanted gas generation because of advancements in electrode materials. The capacity of the cathode and anode materials, as well as the operating voltage, have a major impact on the energy density of LIBs. Reversible battery operation requires long-term electrode–electrolyte interfacial stability, even if high-energy-density LIBs can be created utilizing high-capacity electrode materials and a high operating voltage. Electrolyte additives' formation of electrochemically resistant interfacial layers and preservation of the interfacial structures of electrodes for high-performance LIBs will be advantageous for high-capacity electrodes to achieve electrochemical reversibility.51
Large pore volumes, clearly defined pores, and evenly dispersed catalysts are characteristics of materials generated from MOF that are beneficial for large-scale transportation, redox processes involving oxygen discharged products (e.g., lithium oxide). One such substance, a cage-type graphitic porous carbon-Co3O4 (GPC-Co3O4) polyhedron made from core–shell MOFs (ZIF-8@ZIF-67), provided low charge potentials and an exceptional cycle life when utilized as a cathode substance for lithium batteries. This was made feasible thanks to the advantages of the graphitic carbon component, which improves conductivity, and the cage-type and mesoporous structures, which can bind to Li2O2.52 Graphene/graphene-tube nanocomposites, cobalt–manganese oxide, Co9S8@carbon porous nanocages, Fe2O3/carbon, and ZnO/ZnFe2O4/C nanocages are also listed as cathode materials for LABs.53
Li2O2 may cause the carbon components of materials made from MOFs to break down, resulting in low cycle stability. To help in the development of suitable MOF-derived materials for use in real-world LABs, it would be wonderful to have a deeper knowledge of the fundamental chemistry of LABs. Due to their incredibly high theoretical capacities and energy densities, rechargeable LIBs and LABs are one of the most promising power sources for the upcoming generation of electric vehicles. The technology has gone through several revisions over the years, but significant problems still need to be resolved before it can be successfully marketed. A few concerns that need to be resolved include its practicality, round-trip effectiveness, and riding time. The advantages of this type of battery are discussed in this review. The battery's electrolyte and electrode optimization are considered at the system level of design. This study also offers viewpoints on how to achieve the necessary battery performance to satisfy the criteria for commercial feasibility.54,55
1.3. Zinc air batteries
A separator, a catalytic active layer, a gas diffusion layer (GDL), and an air electrode that serves as the cathode make up zinc–air batteries. Oxygen cannot be used as a liquid at atmospheric pressure due to its extremely low solubility; rather, it must be employed as a gas. The catalyst then aids in reducing oxygen to hydroxyl ions in the alkaline electrolyte by using the electrons produced by the oxidation of zinc metal as the anode process. Due to the fact that the catalyst, electrolyte, and oxygen are all in three phases simultaneously, we refer to this process as a “three-phase reaction” (gas).56 Zinc is oxidized with oxygen from the air in metal–air batteries, such as non-rechargeable zinc–air batteries and zinc–air fuel cells, to generate power (mechanically rechargeable). These batteries are made at quite low prices and have great energy densities. From the comparatively small button cells used in hearing aids to the massive batteries used in grid-scale energy storage, batteries come in a variety of shapes and sizes. Additional battery sizes include those used in mercury-powered film cameras and larger batteries for digital cameras. Interior and exterior of the cell, the porous carbon electrode can absorb atmospheric oxygen.48,57These electrochemical processes taking place in the alkaline solution between the anode and cathode, which constitute the entire discharge process, are explained. How a zinc–air battery works and how each electrode reacts. An electrolyte-saturated porous anode made of zinc particles forms after discharge. To generate zinc hydroxide and release electrons to flow to the cathode, oxygen from the air and the cathode combine to make hydroxyl ions. Water is reintroduced into the electrolyte as the zincate converts to zinc oxide. The water is not used since the hydroxyl and water recycled at the cathode from the anode.58
The red circle in Fig. 2 above designates the position of this three-phase interaction. In zinc–air batteries, oxygen absorption is facilitated by this shape. To finish the reaction, hydroxyl ions in the cell go from the zinc anode to the air cathode. Theoretically, the reactions generate 1.65 volts, but in practical cells, this is decreased to 1.35 to 1.4 volts. The oxygen reduction reaction (ORR) requires a significant overpotential in order to produce hydroxyl ions, as shown by the polarization curve.60,61 As a result, the actual zinc–air cell's operational voltage and open-circuit potential (OCV) are both substantially lower than 1.65 V. A higher potential is required for charging when the reverse process (the oxygen evolution reaction) is considered (blue line). It is clear from the preceding summary that using the ORR has advantages and disadvantages for zinc–air cells. The positive point enables this cell to have a higher energy density despite the lack of oxygen active material. The subject has already been discussed. The zinc–air cell experiences a considerable potential loss because of the negative point, which affects the cell's power density. As a result, many studies have concentrated on how to create novel catalysts and alter the designs of the air electrodes to lessen the significant overpotential in the cathode reaction.62
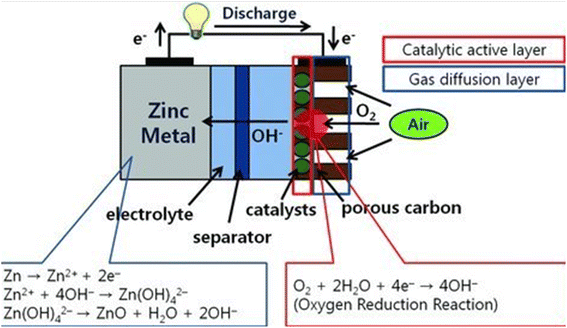 | ||
| Fig. 2 The zinc–air battery's operating concept and each electrode's reaction. In the air cathode, a three-phase reaction comprising liquid electrolyte, solid catalysts, and gaseous oxygen takes place in the red circle. Copy with permission from ref. 59. Copyrights 2010, Wiley Online Library. | ||
In a primary zinc–air cell, the zinc anode is not significant since old zinc metal can be spontaneously replenished with new metal through electromechanical charging. To make a zinc–air battery that can be electrically recharged, the electrochemical behavior of the zinc anode must be altered. The creation of a secondary zinc–air battery may be hampered by other issues even if these two primary limitations of zinc–air batteries are ignored.60,63 In zinc–air batteries, the anode's active component is pure zinc metal, which oxidizes during discharge. Due to this, most of the research has concentrated on making the air electrode rather than the zinc anode. According to the equation Zn + 2H2O → Zn(OH)2 + H2, zinc corrodes at a slower pace than aluminum does in an alkaline solution, but hydrogen gas is still produced during this corrosion process (hydrogen evolution reaction, or HER). Considering zinc metal's electrochemistry in the alkaline electrolyte as opposed to just its electrochemistry in the gas phase makes more sense. The hydrogen evolution process (HER), which is not intended to take place during discharge, has been identified as the most critical area of study. It is envisioned that to gradually increase capacity of these batteries zinc metal should be utilized entirely during the process of discharging.58,64
Electrolytes regulate the ion transport behavior in ZABs. The most advanced electrolytes for flexible ZABs are hydrogels because of their cohesive flexibility and capacity to transfer ions diffusively like liquids. The local segmental movements of polymer chains, which are responsive to temperature variations, are correlated with the ionic conductivity of hydrogel electrolytes. The hydrogel electrolytes also run the risk of becoming less flexible and conductible at below-freezing temperatures because they are aqueous in nature. Recent studies have used low-molecular vicinal alcohols to generate organ hydrogels that can function at subzero temperatures by replacing some of the water in aqueous hydrogels. However, ORR/OER and the ionic conductivity of gel electrolytes are always degraded (or poisoned) by the addition of organic molecules. But the concentrated alkaline solute utilized in ZABs, enables the use of the solutions' natural capacity to collaborate to lessen the impacts of low temperatures. The introduction of potent alkaline solutions to hydrogel electrolytes can adversely compromise the structural integrity of polymer backbones. The interactions between the terminal groups on these hydrogel electrolytes' polymer backbones, the electrolyte ions, and the water are what keep them from freezing. Although this has not yet been verified, hydrogel electrolyte backbones for ZABs can adjust to low temperatures.63,65
Aqueous zinc–air batteries are particularly commendable when working in high-temperature environments due to their inherent safety. However, the possibility of their functioning at high temperatures hasn't been studied very much. Systematically examining the practicability of high-temperature zinc–air batteries is done here. To determine the positive and negative influences of temperature, the impacts on the aqueous electrolyte, zinc anode, and air cathode are separated. Particularly, parasitic hydrogen evolution process, which is known as the primary bottleneck, intensifies at high temperatures and causes a drop in anode Faraday efficiency. Additionally, zinc–air batteries show that cycling is possible at 80 °C. This research shows how zinc–air batteries may provide energy storage at high temperatures and directs future research into improved batteries toward challenging operating environments. Traditionally, the Zn–air battery's performance-limiting electrode has been the air cathode. They are made by uniformly loading the proper electrocatalysts over porous gas diffusion layers that have been coated with polytetrafluoroethylene (GDLs). Water retention (hydrophilic) and water repulsion (hydrophobic) characteristics in good GDLs are balanced. They have many gas-electrolyte-electrode triple-phase boundaries, a high current density of up to >1 A cm−2 and allow O2 gas to enter the system quickly. The primary factor accelerating the ORR and OER and, thus, affecting the total battery performance is electrocatalysts placed on GDLs (e.g., power density, energy efficiency and cycle life). You can manually or electrically recharge zinc air batteries. Zn–air batteries that are mechanically rechargeable can be recharged by replacing the anode and electrolyte with fresh ones. They are main rechargeable batteries that merely require ORR electrocatalysts to serve as the cathode.66,67
1.4. Other metal–air batteries
Due to incentives to create affordable, ecologically friendly, and reliable rechargeable batteries. When compared to an aluminium or zinc air battery, the iron–air battery emits less energy, but it has the same charge and discharge characteristics and uses an excessive amount of electrode material. The projected operational voltage and capacity of an iron–air battery are lower than those of an aluminium or zinc battery, thus this is accurate. However, compared to zinc–air batteries or aluminum–air batteries, iron–air batteries are less likely to be used as primary batteries because the iron negative electrode's expected capacity and working voltage are lower than those of zinc and aluminium. Iron–air batteries also have an abundance of electrode material. Iron's electrode response is also more difficult in several ways than zinc's, as this article has explained.68,69 Due to this, commercial iron–air primary batteries are now extremely rare, and almost all of them feature a zinc negative electrode. The electrode reaction of iron in an alkaline electrolyte totally occurs in the solid state without engaging the deposition–dissolution process because the discharge products from iron, such as iron hydroxides and iron oxides, are not highly soluble. Because of this iron feature, a negative electrode's tendency to change form over cycles is bad for primary batteries but good for secondary batteries. This battery system has been perceived as a supplementary battery with more sophisticated technologies as a result.70Although still under development, Mg–air and Al–air batteries are generating a lot of scientific attention. In terms of weight and price per amp hour, aluminium is less expensive than zinc, the fifth most abundant element on earth. Due to its lightness and toughness, aluminium is also well-known as a building and manufacturing material and is frequently used in industries like automation and construction. Due to the advancement of commercial alloys, metal–air cells may be able to compete with established energy accumulating methods.71 The main problem is that an oxide coating spontaneously forms on the electrode surface when Al is exposed to air or aqueous solutions. The active dissolving of aluminium is significantly decreased by the presence of this protective oxide coating, which also positively affects the anode's corrosion potential. The amount of extractable energy is significantly decreased by this alteration. Al quickly loses its attraction as a potential component of an energetic anode. Although it still has certain limitations, such as anode corrosion, slow discharge products, a high rate of self-discharge, cell irreversibility, and shelf life, the rechargeable Al–air battery is one of the most promising technologies. By swapping out the current Al electrode, the battery system might be able to provide rapid mechanical recharging if electrochemical recharge is not available.72
2. MOF as a bifunctional electrocatalyst
A catalytic substance that has two catalytic functions one is catalyzation of cathodic reaction and the other one is anodic reaction and may therefore be referred as a bifunctional catalyst, also known as a dual-function catalyst.73 Electrocatalysts, a category of catalyst that takes part in electrochemical reactions, speed up the rates of oxidation and reduction in electrochemical cells or MABs. Electrocatalytic materials, which are not consumed during the reaction process, accelerate electrochemical reactions by interacting with reagents to change the reaction pathways and reduce the activation barrier. The majority of conventional single layer electrodes that have been created to date have substantial mass transport resistance and have subpar performance. The design of the double layer electrode also affects the reaction rate, most conventional single layer electrodes created to date have substantial mass transport resistance and have subpar performance. For flow batteries, a double-layer electrode has a backing layer and a catalyst layer. Therefore, by altering the electrocatalysts on the electrode surface, the electrochemical reaction rate can be managed. The OER and the ORR, which are the subjects of our review work, are two separate types of reactions that these bifunctional catalysts catalyze.74The catalysed oxygen evolution and oxygen reduction processes are crucial components of numerous energy conversion and storage systems (OER and ORR, respectively). Over the past ten years, they have evolved into viable choices to meet the market's increasing demand for renewable energy. It is anticipated that developing (bi)functional, inexpensive non-noble metal or metal-free electrocatalysts will boost useable energy density and significantly lower production costs. The amount of surface area and unique pore characteristics of mesoporous materials have a substantial impact on how reactive they are to electrochemical processes. As a result of the fact that the effectiveness of the electrochemical processes occurring at the electrodes during the charging and discharging activities directly determines how well these storage devices perform generally.75,76
The above Fig. 3 illustrates the oxygen reduction reaction's stoichiometries, which vary depending on the medium. To form molecular oxygen in OER, several proton/electron-coupled processes are necessary. Theoretically, a higher energy density might be achieved by using 4e− ORR rather than 2e−. The properties of the electrode surface and any extra catalysts affect the complicated ORR path. The various critical steps of the multielectron reactions known as the ORR and the OER involve many reaction intermediates. The oxygen reduction reaction, sometimes referred to as the reduction half reaction, is a chemical process that turns oxygen (O2) into water or hydrogen peroxide. Oxygen consumption reaction.78 Electrocatalyst activity, affordability, toughness, and stability are crucial for practical application. Exceptional electrical conductivity, superior chemical stability in a range of electrolytes, and a strong catalyst support with a large surface area are also necessary. Metal oxide nanostructures can be regarded as an essential electrocatalytic material because of its very distinctive optical, electrical, and molecular properties, easily functionalize nature, exceptional resistance to alkaline corrosion in an electrochemical environment, and other attributes.79
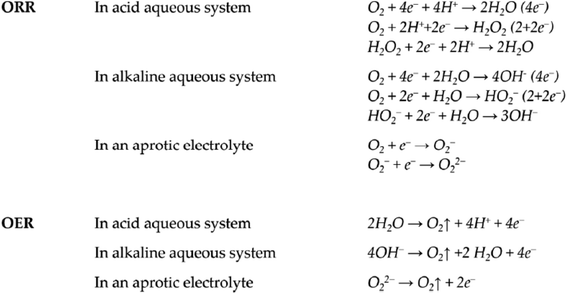 | ||
| Fig. 3 Oxygen evolution reaction (OER) and oxygen reduction reaction (ORR) equations in various electrolytes. Copyrights 2019 from ref. 77. | ||
Table 2 above shows that a high Tafel slope corresponds to a large band-gap energy, which results in a high overpotential since a lot of energy is required to produce activity and vice versa. The rate-determining phase of the electrochemical reaction is similarly described by the Tafel slope. The graph representing the relationship between the current produced in an electrochemical cell and the electrode potential of a specific metal is called a Tafel plot, which is frequently logarithmic. OER is a vital phase in energy conversion and storage, particularly in water electrolysis. Presently, the OER process makes extensive use of precious metals and their oxide complexes (Ir, Ru, IrO2, and RuO2) as cutting-edge catalysts to lower energy consumption and improve energy conversion efficiency.81 OER which provides the electrons needed for electrochemical conversion cycles between renewable electricity and chemical fuels, is significantly reliant on these energy storage devices. In a metal–air battery, OER occurs on the cathode, and its activity and stability can be readily assessed. Electrocatalysts are necessary for MAB device charging and discharging as well as for critical electron transport, chemical bond formation, and rupturing.82
| Materials | Electrolyte | Over potential for 10 mA cm−2 V−1 | Tafel slope mV−1 dec−1 |
|---|---|---|---|
| RuO2 | 1 M KOH | 0.3 | 42 |
| MnCo-G | KOH | 0.33 | 48 |
| CoP | 1 M KOH | 0.36 | 66 |
| CoMoO4 | 1 M KOH | 0.31 | 56 |
| NiFe LDH | 1 M KOH | 0.33 | 41 |
| CoFe LDH | 0.1 M KOH | 0.36 | 49 |
| Co3S4@MoS2 | 1 M KOH | 0.33 | 56 |
| CuCo2O4/N-rGO | 1 M KOH | 0.36 | 64 |
Electrocatalysts can be used to increase the activity and stability of ORR and OER. Noble metals are effective oxygen evolution catalysts. However, they are unable to be reversible, which is required for all applications involving energy storage because only one sort of reaction is the focus of their high activity. Reversible MABs and other energy storage technologies may be used more widely as a result of the identification of bifunctional catalysts active in ORR and OER. There is a constantly expanding interest in the creation of high-performance stable catalysts because these combinations and structures serve as the foundation for hybrid compositions, spinels, non-noble (mixed) transition metal oxides, and oxides of carbon.81,83
In OER, several proton/electron-coupled reactions are required to produce molecular oxygen. To enhance efficiency for practical applications, a full reduction is used. Furthermore, the slowness of OER and ORR reactions is well recognized. For OER and ORR to be beneficial for energy storage devices like MABs, where OER occurs, it is imperative to keep in mind that oxygen molecules must renew when charging. A full water splitting process that utilizes a ground-based, dual-purpose electrocatalyst as both a cathode and anode. The development of inexpensive, effective, stable, and bifunctional electrocatalysts has attracted a lot of scientific interest, as have air battery-based energy storage technologies.84 The oxygen reduction reaction is a crucial electrochemical process for generating energy in fuel cells (ORR). Recently, this reaction has been enhanced by several study teams employing size-selected clusters. Fuel cells and metal–air batteries both rely on the ORR. Although Pt-based nanoparticles exhibit excellent ORR activities, their high cost and limited shelf life preclude them from being widely used.85,86
The most difficult holdback of these advancements is the slow kinetics of electrochemical half-reactions like the (ORR) and (OER), which lowers the overall output efficiency throughout the process. Noble metal-based catalysts are frequently used to overcome these issues (such as Pt/C catalysts for ORR and Ru/Ir-based catalysts for OER), but their poor methanol tolerance, high cost, and low stability severely limit their future potential. The primary goal is to create a bi-functional catalyst with high catalytic activity and low-cost characteristics for the two distinct processes. A single active site catalyst is often unable to provide strong bifunctional catalytic activity because ORR and OER use distinct routes. The hybrid or composite of Co2P/CoP is thus expected to demonstrate the enhanced bifunctional ORR/OER activity. CoP and Co2P can coexist thanks to an improved three-dimensional self-supported electrocatalyst made of nitrogen-doped carbon nanotubes (NCNT), which works by phosphorylating molecules at low temperatures first. The resulting three-dimensional carbon network structure also enables rapid charge transfer. Additionally, the active sites of the carbon foam (CF) are greatly increased by hollow structural architecture.87 With only low overpotentials of 133 and 289 mV respectively, CoP/Co2P/NCNT@CF displayed outstanding catalytic activity for the oxygen evolution reaction and hydrogen evolution reaction in alkaline media. The developed catalysts also have exceptional long-term stability; they continue to have significant catalytic activity even after 20 hours of continuous operation.88
2.1. MOF types and derivatives
A special class of hybrid porous crystalline materials was developed in the 1990s by fusing organic linkers with inorganic metal centers. This novel family of porous materials, sometimes referred to as porous inorganic–organic hybrid materials (PCPs), is most frequently referred to as metal–organic frameworks (MOFs). For decades, scientists have worked to incorporate various metal centers and valuable organic molecules into porous materials to change their physical and chemical properties. Due to their adaptability in terms of their chemistries and structural makeup MOFs have captivated the interest of scientists, engineers, and technologists ever since their discovery.89 The development of synthesis techniques has been the focus of countless studies and research publications since it directly affects how MOFs crystallize, which determines their characteristics and functional performance. MOFs can have multiple topologies for their frameworks, pore patterns, and sizes by selecting various metal centers and organic linkers. By chemically functionalizing linkers and subsequently applying modifications, their chemical characteristics can be altered.90,91One of the most common ligands utilized is the substance 1,4-benzenedicarboxylic acid, often known as “struts” or “linkers” (BDC). Metals are often thought of as electron donors, whereas ligands are thought of as Lewis bases and acids. Among other things, ligands are classified according to their charge, size (bulk), kind of coordinating atom or atoms, quantity of electrons supplied to the metal, cone angle and other characteristics (denticity or hapticity), where cone angle is a measure of ligand magnitude.92 There are numerous alternate ranking systems for ligands and metal ions; one of them places emphasis on the “hardness” of the ligand. Metal ions prefer certain ligands over others when binding. Strong-field ligands bind metal ions in accordance with the Aufbau principle, while weak-field ligands bind complexes in accordance with Hund's rule. In the process of joining with the ligands, the metal creates new HOMO and LUMO structures that specify the properties and reactivity of the final complex as well as the configuration of the metal's five d-orbitals (which may be filled, or partially filled with electrons).93,94
Many different processes can be used to produce carbon materials, including physical or chemical carbonization from carbon, direct carbonization from organic precursors, the use of zeolites and mesoporous silica as template materials, high temperature solvothermal and hydrothermal processes, electrical arc techniques, and chemical vapor decomposition (CVD) procedures. Direct carbonization of organic precursors is the most popular technique for creating nano-porous carbons (NPCs) due to its adaptability and simplicity. These NPC materials do, however, have several shortcomings, including limited surface areas, disorganized patterns, and unequal sizes, which will significantly restrict their applications.95
Researchers studying fuel cells and metal–air batteries have recently become interested in non-noble metal oxygen reduction reaction (ORR) catalysts made by carbonizing metal–organic framework (MOF) due to their distinct inherent advantages, such as high catalytic activity, low cost, simple synthesis, and good adaptability. In contrast to the typical high-active noble metal investigations, this study carefully investigates recent advancements on non-noble metal ORR catalysts produced by various MOFs carbonization in diverse metal centers, including Fe, Co, Cu, Ni, Mn, and Mo. The topologically structured zeolite-like MOF (ZIF) and the Materials of Institute Lavoisier (MIL) with a significant specific surface area are two examples of the innovative MOFs with distinctive properties that have been created (5900 m2 g−1).96 MOFs have advanced to the point that they are now active areas of research in many areas. The enormous surface area, plentiful active centers, and adaptable structure of MOFs have made them the subject of intense research in recent years. However, it cannot be effectively used in water electrolysis due to its low conductivity and inaccessible active sites. Lei Y., et al.97 developed a self-supporting electrocatalyst by growing a MOF derivative on the surface of iron foam (IF) using a self-template partial transformation approach. The production of several catalysts with various morphologies was also accomplished by varying the Ni concentration and reaction duration. The resulting NiFe-LDH@Fe-MOF/IF-2 exhibits excellent oxygen evolution reaction (OER) activity in an alkaline electrolyte with the required overpotentials of 183, 237, and 257 mV because of its distinct hierarchical structure, large active area, and abundant active sites, strong combination of highly active amorphous NiFe-LDH and Fe-MOF, high conductivity, and stability of the MOFs array in situ. Using a straightforward ultrasonic process, bimetal–organic framework nanosheets wrapped around polypyrrole nanotubes (NiCo-MOF@PNTs) were effectively produced. PNTs may effectively prevent the aggregation of NiCo-MOF nanosheets and boost the electronic conductivity of NiCo-MOF in NiCo-MOF@PNTs nanocomposites. The unique structural architecture of NiCo-MOF@PNTs enables their improved electrochemical performance. The assembled NiCo-MOF@PNTs/AC asymmetric supercapacitor, which displays a high energy density of 41.2 W h kg−1 at a power density of 375 W kg−1, and the excellent cycling stability with a capacitance retention of 79.1% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, demonstrates the potential of the NiCo-MOF@PNTs nanocomposites as supercapacitor electrode materials.98
000 cycles, demonstrates the potential of the NiCo-MOF@PNTs nanocomposites as supercapacitor electrode materials.98
Scientists have learned that carbon compounds produced from metal–organic frameworks (MOFs) can overcome these limitations as research has progressed. Carbon materials made from MOFs include high porosity (up to 90%), an incredible amount of surface area (nearly 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m2 g−1), tunable pore widths, excellent cycle lifetime, and other advantages. The efficiency of different applications has been the subject of numerous significant studies in the past. For instance, MOFs are suitable for energy-related electrocatalysts depending on the choice of coordination metal due to their high surface areas and atomic metal sites. The electrocatalytic processes of oxygen reduction, carbon dioxide reduction, and water splitting have all extensively used MOFs HER and OER as shown also on Fig. 4.
000 m2 g−1), tunable pore widths, excellent cycle lifetime, and other advantages. The efficiency of different applications has been the subject of numerous significant studies in the past. For instance, MOFs are suitable for energy-related electrocatalysts depending on the choice of coordination metal due to their high surface areas and atomic metal sites. The electrocatalytic processes of oxygen reduction, carbon dioxide reduction, and water splitting have all extensively used MOFs HER and OER as shown also on Fig. 4.
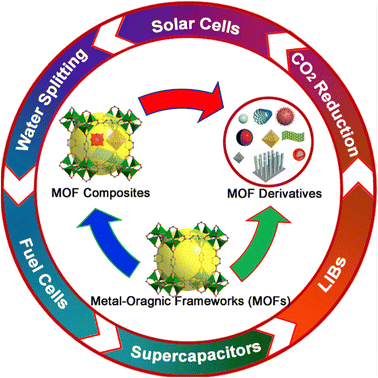 | ||
| Fig. 4 Showing use of MOFs derivatives and composites in different electrochemical cells and processes. Copy with permission from ref. 99. Copyrights 2017, ScienceDirect. | ||
Since electrode materials are an essential part of lithium-ion batteries, they have been the subject of in-depth research. Due to their high porosity and controlled structural characteristics, MOFs make the greatest choices for electrode materials in lithium-ion batteries.100,101
2.2. MOF composites
Large-surface MOFs make good structural hosts since they can hold a lot of functional guests. Because pure MOFs have limitations. For addressing their shortcomings, MOF composites have been researched and created. Since most MOFs composites just not only maintain the porous architectures of MOF precursor but also display superior electrical conductivity brought on by the carbon component, they have a greater probability of success as electrode materials. Overall, the development of materials linked to MOFs, such as MOF composites, and electrode materials made from MOFs, has been an intriguing cross-disciplinary area where opportunities and challenges coexist. There are countless opportunities for the discovery of potential electrode materials for various battery systems when electroactive sites are included in the designable MOF components. Ion transportation and electrolyte penetration are straightforward processes since MOFs are naturally porous.102 The usage of MOFs in electrochemical processes as well as the expansion of the use of novel advanced materials are both supported by these composites. These composites can improve the intrinsic properties and functionality of MOFs, and these qualities and functioning of MOFs can be improved by 0D materials, which have a tiny size and high surface energy. 0D MOF composites can be created in a variety of methods, but most of them include employing MOFs as carriers for already-formed NPs or as carriers by themselves that use templates to create NPs on their surfaces.103,104Metal oxides created from MOFs have proven to be acceptable candidates for the construction of efficient and well-established cathode catalysts, which are necessary to deal with the slow redox reactions in Li–air batteries. Since electrochemical processes on the cathode are the basis for evaluating Li–O2 battery performance, high energy density nanostructured enhanced cathode materials have been produced. A new, self-standing catalytic framework was created utilizing thermal annealing following the 3D printing of a cobalt-based metal–organic framework (Co-MOF). Because the insulating Li2O2 particles are enclosed within carefully regulated holes and incorporate Co-based electrocatalysts, the unique structure successfully deposits insulating Li2O2 particles during charging and then expedites their breakdown. Due to its exceptional conductivity and crucial mechanical stability, the carbon framework itself is a superb conducting matrix. By transforming a porous matrix into a self-standing catalytic structure, specific energy with a high energy density of up to 798 W h kg−1 cell can be significantly increased.105
The scheme in Fig. 5 shows the shape size and types of different MOFs dimension-wise. 0D MOF composites which are also discussed later in the review can be synthesized utilizing the one-pot approach, and by following this simple and effective process, it is possible to improve catalytic performances while also lowering the cost of large-scale production.107,108
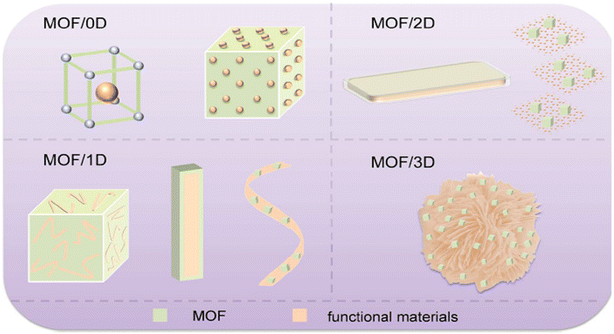 | ||
| Fig. 5 MOF/composite materials, such as MOF/0D, MOF/1D, MOF/2D, and MOF/3D, are illustrated schematically. Copyrights 2019 from ref. 106. | ||
3. MOF/1 dimension
As illustrated on the Scheme in Fig. 5 above, materials with MOFs surrounding them, the term “MOF/1D materials” refers to MOFs with one dimensional nanobelt materials generated on carbon fabric as well as MOFs with one dimensional material on their outer surface. Utilizing polymerization in place, MOFs and 1D nanobelt oxidation can be chemically coupled in situ. The self-template technique allows for the construction of 1D composites from 1D components. In order to initiate the synthesis of MOFs without surface modification, it can also be employed as a template to dissolve metal ions in solvents. In addition to being beneficial for generating MOFs like ZIF-8 powder, the spontaneous phase segregation approach is also suitable to a wide range of supported semiconductor 1D material applications. These substances are tiny and have a high surface energy.109Due to its persistent porosity, high surface chemistry, and adjustable pore sizes, MOFs have drawn a lot of interest in the fields of catalysis, gas adsorption, separation, and storage over the past 20 years. The creation of new MOF applications in fields including electrical catalysis, bio-imaging, drug transport, and better features as compared to pure MOFs is facilitated by the addition of functional species or matrix materials to MOFs. This results from interactions between the MOF structures and the functional species or matrix. Even though the creation, chemical alteration, and potential applications of MOFs have all been covered previously, there is growing interest in the creation and use of their composites. In order to close this gap, the creation, characteristics, and applications of MOF composites are discussed in this work. Processing methods, maximizing composite qualities, and potential applications have all been discussed as prospects and problems in upcoming sections.110
CNTs@Mn-MOF was created using an effective hydrothermal method, which may be able to compensate for the drawbacks of traditional Mn-based electrode materials. The method also shows how 2D materials can be encased in MOFs to form MOF/2D materials as well as how MOFs can be grown on 2D nanosheet materials. MOFs are frequently combined with 2D nanosheet materials, including graphene oxide, using solvothermal techniques (GO). In the case of MOF/3D composites, which contain cellular structures with a cubic and core–shell design. Layer-by-layer (LBL) assembly is a versatile sequential technique that permits precise control of the MOF shell thickness. It can be used to create three-dimensional composites like three-dimensional core–shell composites.111
3.1. MOF/nanorod composites
MNPs (metal nanoparticles) are frequently utilized in electronics, energy conversion, storage, electrochemistry, and catalytic applications because of their wide variety of forms, sizes, crystallinity, and practicality. The development of various nanostructured electrode materials using MOFs, either directly or as in composite form, are a promising technique to improve the electrochemical characteristics for practical usage.112Combining MNPs and MOFs results in MNP@MOF composites, which have better capabilities and unique functionalities. It has also been attempted to homogenize MOFs and metal oxides into core–shell nanostructures, especially those having semiconductor and magnetic characteristics. Metal oxide NPs and MOF can be combined using a variety of techniques, including the synthetic yolk–shell method, the liquid phase epitaxy method, and the gas phase in titration approach.113,114 Co oxides were enclosed in an appropriate hollow nanostructure, as illustrated in Fig. 6a, according to Zeng et al.106 This resulted in a distinctive yolk–shell nanoreactor that hasn't received much research but will successfully address the previous building challenges. Particularly, the porous outer layer of the nanostructure can prevent nearby Co oxide NPs from accumulating in the hollow nanostructure and protect the contained catalyst from unfavorable environmental conditions without obstructing the movement of the reactants and products. A microenvironment for the oxidation of SO4 can be created in the nanoreactor's interior chamber, which also serves to protect exposed catalytic sites and make them accessible to reactants.115,116
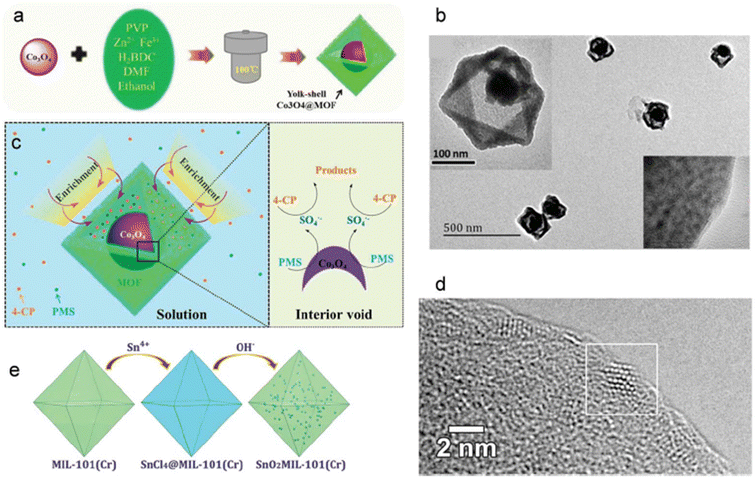 | ||
| Fig. 6 (a) The method of creating nanocomposites with yolk–shell (b) TEM pictures, of yolk–shell (c) potential process for 4-CP to degrade in the yoke–shell nanoreactor (d) on the surface of ZIF-8 tiny ZnO NPs are visible in the HRTEM image. (e) An illustration of the SnO2@MIL-101(Cr). Copyrights 2019 from ref. 106. | ||
Zeng et al. described a one-pot solvothermal method to make yolk–shell Co3O4@MOF hollow-centered nanostructures. The octahedral MOF-5 shell served as a link between the structure and the catalytically active Co3O4 core. They mainly showed how useful they might be as a nanoreactor for SR-AOPs. A gas phase method was also used to mix ZnO with a MOF. Fig. 5d shows the high-resolution transmission electron microscopy image of the tiny ZnO NPs on the surface of ZIF-8 (HRTEM). Co3O4@MOF is one metal-oxide-NP@MOF core–shell nanocomposites that has so far garnered attention. Here, we demonstrate one of the earliest instances of iron oxide particles being successfully integrated into porous MOFs. MOFs feature a lot of active sites and a lot of surface area, which have led to extensive research on them for organic and electrochemical catalytic processes. Research is increasingly pointing to the utilization of “meta-NP@MOF” systems in the fields of catalytic activity and gas storage, where the incorporation of metal NPs into MOFs could be particularly beneficial.117,118
3.2. MOF/core–shell composites
It was possible to make core–shell composites via solvothermal Fe3O4 synthesis. Cai et al.120 presented a general technique for the rational design and synthesis of novel magnetic core-and-shell porous core–shell structures. A few Fe3O4@MOF core–shell composites with distinctive MOF shells were created using an LBL controlled process. Solvothermal techniques were used to create Fe3O4 nanospheres. Fe3O4@-HKUST-1 number 153 core–shell nanospheres with a similar structure were created. An LBL assembly procedure was used to produce magnetized Fe3O4@MIL-100 core–shell composites.119 By using LBL assembly, we were able to create core–shell Fe3O4@MOF microspheres. Additionally, the Fe3O4 particles were measured using HKUST-1.120 Iterative circular growth is used to build PS@MOF core–shell microspheres with a potential shell thickness for the LBL construction process.121 In order to create hollow PS@MOF microspheres, the polystyrene cores from the prefabricated PS@MOF core–shell composites were successfully removed using DMF.1224. MOFs application in energy storage systems
The remarkable performance of MOF composites, which are made of two intriguing materials, and their electrochemical applications include catalysis, sensors, supercapacitors, batteries, and other electrochemical devices. MOFs emphasize each MOF's unique properties and encourage the development of electrochemical applications because they are adaptable and constructed of unusual materials. Despite the fact that 3D functional materials made with MOFs have hardly ever been explored or mentioned, they offer a variety of applications in electrochemistry with dimensions ranging from 0 to 2. Based on the various application types and dimensions, this section analyses and deconstructs MOF composites.1234.1. MOF composites for catalysis
Over the past few years, there has been a sharp and unpredictable rise in the global demand for and consumption of energy. Burning non-renewable fossil fuels like coal and oil accounts for the majority of global energy use, dramatically increasing atmospheric CO2 emissions as well as creating other severe environmental issues. Significant efforts are being undertaken to address the problem of energy scarcity on a global scale. One such attempt is the search for clean, renewable energy sources to replace petroleum-based fuels. Despite their high cost, Pt catalysts are frequently used in electrochemistry. As a result, from an economic and environmental perspective, non-Pt catalysts such MOF composites are gaining popularity. The oxygen evolution process (OER) and hydrogen evolution reaction are both electro catalyzed regularly using MOF composites (HER), and oxygen reduction reaction (ORR).124Hydrogen-based fuel cells, another form of electrolytic cell, also experience ORR at the cathode. The two-electron transfer reaction is an example of a catalytic intermediate that is widely used (HER). The two-step reaction, which needs energy in acidic environments, depends on H. The second stage produces H2 by either mixing two H* or H* with a proton. Thus, the overpotential of the HER is decreased by active electrocatalysts such MOF composites. The electrochemical oxidation of water results in the generation of oxygen, or OER. Two water molecules can be oxidized in acidic or neutral electrolytes to yield four protons and one oxygen molecule.124
Fig. 7 represents synthesis of bifunctional electrocatalysts. Fig. 7a shows use of various materials drop cast on a glassy carbon electrode, 10 mM Fe (CN)63/4 cyclic voltammograms in 1 M KCl were obtained. Cu-MOF in the following concentrations: (GO 8 wt%), (GO 6 wt%), (GO 4 wt%), (GO 2 wt%), (GO 5%), Cu-MOF, and (GO 6 wt%) bare glassy carbon electrode.
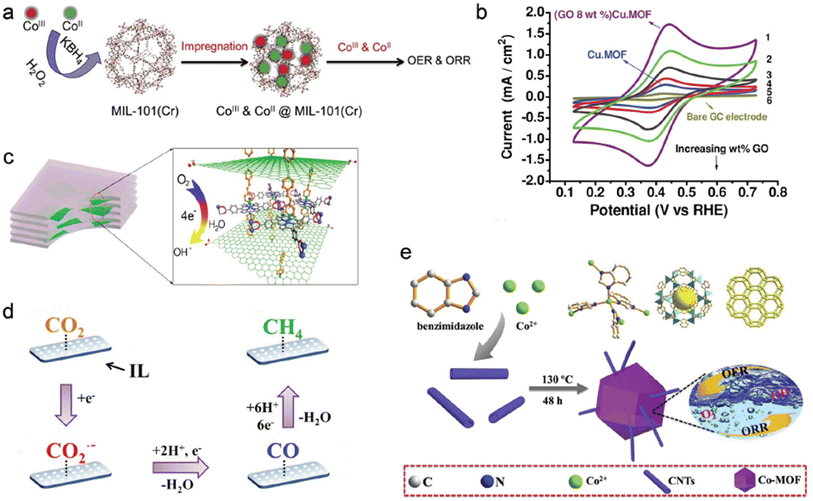 | ||
| Fig. 7 (a) An example of the bifunctional electrocatalyst's design and preparation. (b) The catalysts' electrochemical activity, sorption surface areas, and surface areas. (c) A enlarged view of the layers inside the (G-dye-FeP)n MOF's framework and graphene sheets (d) an electrochemical procedure on the Zn-MOF/CP cathode in ionic liquids that might convert CO2 to CH4 (e) Co-MOF@CNTs catalyst synthesis. Copyrights 2019 from ref. 106. | ||
In contrast, OH can oxidize in basic electrolytes to create H2O and O2. Due to their exceptional electrocatalytic efficiency and stability, MOF composite electrocatalysts have lately seen an increase in OER activity. A great lot of interest has also been generated by electrocatalysts in ORR for technologies like metal–air batteries and fuel cells. Additionally, the nano-CuS concentrations affected the ORR's Eonset (onset potential). As the amount of nano-CuS was increased, its porosity shrank, and its electrical conductivity was more than 109 times greater than that of bare Cu-MOF.125 These values were all exceeded by the Eonset values of nano-CuS and bare Cu-BTC. However, when superabundant nano-CuS was introduced to or stacked on top of CuS (56 weight percent) @ Cu-BTC. These results demonstrated a substantial relationship between the materials' porosity and electrical conductivity and the ORR electrocatalytic performance of composite electrocatalysts.126,127
When compared to pure nano-CuS and bare MOF, the electrocatalytic performance of the nano-CuS@Cu-BTC composite dramatically enhanced in terms of starting voltage, the number of electrons transported, and the density of the kinetic current. These outcomes are the result of interactions between nano-CuS, which increases electrical conductivity, and CuBTC, which improves porosity. He and his colleagues also developed Co/MIL-101(Cr), which was used as a bi-functional electrocatalyst by employing an oxidative or reductive method following the impregnation.128 They found that the surface's shifting CoII and CoIII composition significantly affected the electrocatalyst's OER and ORR activities. However, neither the BET's surface area nor the overall amount of Co on it had any impact on how well it performed. As the catalyst's OER activity increased. More O2 will also be preferentially absorbed as the amount of CoII on the electrocatalyst surface increases, and in a basic electrolyte, electrons will be transferred to oxygen to produce hydro-oxide.129,130
4.2. MOF composites for batteries
Batteries, which are a proven commercial technology, have a much wider range of applications than supercapacitors and fuel cells. Batteries, which are autonomous devices that transform chemical energy into electricity, may power a variety of items. In these metal–air batteries MOFs are incorporated and that is what will be discussed in this section starting with use of MOFs in LIB.From Table 3 it can be estimated that the composites made with Fe-MOF and RGO (5%) materials had the highest initial coulombic efficiency. This is because the coating of the Fe-RGO MOF is thick enough to prevent direct contact between the electrode and electrolyte. The battery's performance was improved by combining SnO2's high specific capacity with MIL-101(Cr), which acts as a protective shell for electrochemical stability. Liu and coworkers developed 2D Fe-MOF/RGO composites for reversible Li+ storage.132
| MOF composite | Functional materials | MOF | Battery type | Current density/Ca | Cycle number |
|---|---|---|---|---|---|
| a 1C = 1675 mA g−1 | |||||
| Fe-MOF/RGO | RGO | Fe-MOF | LIBs | 0.3 | 200 |
| Fe3O4@MOF | Fe2O3 | HKUST-1 | LIBs | 0.06 | 100 |
| S@MOF-525 (Cu) | S | MOF525 (Cu) | Li–S | 0.5 | 200 |
| SnO2@MIL101 (Cr) | SnO2 | MIL-101 (Cr) | LIBs | 0.1 | 100 |
| S@S-ZIF-8@CNTs | CNTs, S | ZIF-8 | Li–S | 0.1 | 100 |
| MOF-5@CNTs | CNTs | MOF-5 | Li–S | 0.5 | 50 |
| S@HKUST-1/CNT | CNT | HKUST-1 | Li–S | 2 | 40 |
A 3D core–shell composite of Fe3O4@MOF for usage as anode materials in LIBs has been demonstrated by Feng et al.133 Fe3O4@MOF displayed exceptional reversible capacity with a high discharge capacity of 1001.5 mA h g−1 and a high charge capacity of 993.7 mA h g−1 in the 100th cycle. These results were significantly higher than the comparable capacities for pure Fe3O4 of 696 mA g−1 and 993.7 mA g−1. After 70 cycles, the current density increased from 0.1 to 2 A g−1, and the reversible capacity reached 429 mA h g−1. Since the HKUST-1 buffer layer reduces the volume expansion of Fe3O4, Fe3O4@MOF displays better discharging–charging behaviors than pure Fe3O4 during all cycles.133 Building transition-metal oxides as the cathode elements of potential Li–O2 batteries is now more practical. Transition metal carbides (TMCs) have recently attracted a great deal of interest due to their outstanding surface physicochemical properties, unique thermal and electrical conduction properties, and other features. Molybdenum carbides have garnered a lot of interest because of their outstanding d-band electronic structures and good electrical conductivity. By utilizing MoC catalysts, Pt-based ORR electrocatalyst activity and durability were dramatically improved. Particularly, heteroatom-doped CNTs doped with metals and nitrogen displayed improved catalytic activity, making them effective MoCx conductive materials.134
4.3. Use of MOFs in supercapacitors
Flexible energy storage devices are needed to meet the power requirements of new flexible and wearable technology. Due to their superior energy storage properties, such as high-power density and long cycle life, electrochemical capacitors (also known as supercapacitors or ultracapacitors) and metal-ion batteries (such as lithium-ion batteries, sodium-ion batteries, etc.) have attracted a lot of attention recently. Metal-ion batteries' lack of flexibility is a significant barrier to their use in wearable technology. They cannot be used in safe on-body device applications because of their low power density and flammability caused by organic electrolytes. However, since aqueous electrolytes can be used to create supercapacitors, they are a safer option for wearable applications. The notable characteristics of MOFs, such as their substantial surface area, three-dimensional porous architecture, permeability to foreign substances, structural adaptability, etc., make them innovative possibilities as electrode materials.135Even while pure MOFs have poor intrinsic conductivity, this can be improved by creating composites with other electronically conducting materials. Due to their high energy and power densities, MOF-based electrodes hold great promise for flexible and wearable supercapacitors. In order to create flexible supercapacitors, new advancements in the field of MOF-based composite electrodes are the main focus of this paper. Due to their considerable tunability, which includes beneficial porosity features, tunable chemical compositions, programmable crystal structures, and a variety of geometric morphologies, metal–organic frameworks (MOFs) have received a lot of attention for supercapacitor applications.136
4.4. Use of MOFs in hydrogen fuel cells
The use of MOF-based technologies can hasten hydrogen's integration into the energy mix since MOFs are very adaptable and provide a highly selective medium for hydrogen impurities. MOFs can purify hydrogen by eliminating byproducts like methane, nitrogen, carbon dioxide, and carbon monoxide from mixtures of hydrogen. MOFs, for instance, can enable the production of low cost, low carbon, blue hydrogen by removing CO2 from current steam reforming processes. The development of cutting-edge solutions that solve significant technological issues in the hydrogen value chain is made possible by the breakthrough adsorbents known as MOFs.137Independent of source, MOF-based hydrogen purification can also produce the ultra-pure hydrogen supply needed by fuel cell vehicles for final usage. High volumetric and gravimetric capacity hydrogen storage solutions are being developed thanks to the combination of near-infinite pore chemistries and record-breaking porosities. A game-changer for hydrogen transportation, large volumes of hydrogen may now be securely stored and delivered at far lower pressures, improving the range of cars running on H2. In order to unlock the hydrogen economy and facilitate the switch to cleaner energy sources, MOFs are a crucial component.138
5. Limitations and challenges faced by MOFs and metal–air batteries
Due to a lack of lithium supply and security concerns, the development of LIBs devices with high weight-to-energy and volume-to-energy ratios has been delayed. Other types of metal-ion batteries are of great interest (MIBs). In recent years, interest in sodium-ion batteries (SIBs) as prospective replacements has increased due to their electrochemical mechanism and inherent sodium enrichment. Potassium-ion batteries (PIBs) have also drawn more attention as a viable new generation of energy storage technologies because of their global availability and strong potassium-ion transport properties. ZABs on the other hand due to their non-toxic metallic zinc, naturally available, high ionic conductivity in the safer aqueous electrolyte, and other characteristics, zinc-ion batteries (ZIBs) are among all metal-ion batteries regarded as the most desirable option. But still there are a lot of pros and cons related to these batteries and MOFs. By combining MOF with other beneficial materials, MOF composites, the electrochemical performance of MIBs can be greatly enhanced.1395.1. Challenges faced by MOFs
Batteries and supercapacitors have profited from recent developments in the study of energy storage devices based on MOF and its composites, (particularly when various functional materials are linked such as carbonaceous materials). The two most significant aspects of MOF composites are their shape and electrochemistry. Due to their outstanding capacity, supernormal cycling stability, high conductivity, strong mechanical property, fantastic rate property, and spectacular electronic features, MOF composites have been proven to be promising materials for EES applications. Because of the beneficial interactions between MOF and functional materials, EES devices have much better electrochemical characteristics than traditional EES devices. But in the actual world, there are still certain challenges.140Some of the major difficulties and problems in MOFs are listed below:
• During charging and discharging, MOF may irrevocably break down or change into an amorphous phase. Aqueous, acidic, and alkaline conditions can all lead to MOF disintegration and framework collapse. It has seriously impeded its widespread use in EES devices.
• Since the significant charge transfer resistance that results from many MOF's low conductivity is detrimental to rate capacity and cycle stability, it is challenging to overcome this problem. This imposes various limitations on the development and use of MOF composites.
• Although MOF/MOF composite electrode materials offer useful features, it is not apparent how exactly these structural changes impact the electrochemical process. Understanding their workings is essential to creating high-quality, inexpensive, and easily made electrode materials.
• One of the major deterrents is cost hindering the widespread use of MOF/MOF composite electrodes. Large-scale manufacture has become much more difficult due to the employment of pricy ligands and labor-intensive fabric cationic processes.
• A lot of MOFs have low tap density and poor electrical conductivity, which may limit their applicability.
Despite the difficulties and problems listed above, MOF composites can be improved in terms of their electrochemical properties by addressing these difficulties and problems.141
5.2. Challenges faced by MA batteries
Since the battery's active metal reacts with oxygen drawn from the environment rather than having to be stored there, MA batteries' primary advantage is that their volume and weight are decreased. Furthermore, theoretical specific energies that are significantly higher than those measured for cutting-edge lithium-ion batteries are attainable. It is yet unclear how the stronger environmental interactions of the MA batteries compare to those of closed batteries.142 The following are some of the main issues with the MA battery system that require significant attention and continue to exist: | ||
| Fig. 8 Processes of dendrite development, passivation, and corrosion at a metal anode. Copy from ref. 148, Copyrights 2019. | ||
5.2.3.1 Passivation layer. An electrode that was passivated could not be discharged further because an insulating coating on its surface prevented the discharge product from migrating. LiOH, ZnO, and Al2O3 served as the corresponding systems' passivation layers in MABs. A non-conductive layer will be generated on the metal surface from soluble species that were produced at the air cathode. This layer, which is non-conductive, raises the cell's internal electrical resistance and inhibits metal breakdown. The most effective way to prevent the production of passivation layers is to employ porous electrodes.149
5.2.3.2 Dendrite formation and deformation. While the metal electrode cycles in an alkaline electrolyte, the metal anode releases metal ions during discharge and the metal ions re-deposit on the anode during charging. The metal electrode will afterwards progressively change form and develop irregular thicknesses on its surface. When the irregular shape builds into dendrites during numerous charge and discharge cycles, the battery system becomes unstable or swift route. To reduce dendritic development and deformation, many methods have been tried. Using non-reaction additives in the zinc electrode or electrolyte, for instance, and covering the zinc metal alloying lithium with sodium, Mg, Al has been demonstrated to successfully suppress the growth Li's dendritic.
In conclusion, dendritic structures form in ZABs, LABs, and FABs, passivation layers appear in these materials, and corrosion can occur in any of these materials. Several strategies are needed to resolve these issues.150
• Despite having higher ionic radii, sodium has a lower diffusion rate and a greater volume variance, which reduces rate capability and cycling stability. For any battery system, it is essential to find suitable electrode materials with the necessary electrochemical (redox and catalytic activities), physical (electrical and ionic conductivities), and structural and chemical innovations.
• Nickel has the potential to be an OER catalyst, however it is challenging to use in bifunctional catalysts because of its limitations. Due to the conversion of Ni3+ to Ni2+ and the formation of NiO impurities during high temperature heat treatment, which is frequently utilized in the production of catalysts, nickel ions have a tendency to migrate inside crystal structures, catalysts lose some of their capacity to carry out two tasks at once as a result of the limited reversibility of this structural change. Iron addition in the nickel oxide structure is one approach to these stability issues. Nickel–iron batteries have a lower energy density and specific power, or, to put it another way, are less efficient. The cells charge up slowly and discharge it gradually (cannot supply sudden large power spikes). This means that in order to attain the output of a “normal” lead-acid based power system, more batteries and solar panels would be required. As a result of their high hydrogen production, daily gas is necessary to provide the desired performance. Because hydrogen gas is explosive, proper ventilation is crucial. Ni–Fe batteries may endure such treatments without being harmed, although their performance will undoubtedly suffer. Weekly inspections and top-offs are recommended for Ni–Fe batteries. Additionally, the electrolyte solution must occasionally be entirely replenished, which is a nasty and labor-intensive process.151
• Last but not the least air electrode's extremely low efficiency is a serious problem for the iron/air and zinc/air batteries and is also susceptible to damage during charge at practical current densities. The stability of the air catalyst in the cathode and the conductivity of the electrolyte continue to pose problems. Researchers from all over the world are delving into these and other problems to realize the full potential of metal–air batteries.152
6. Conclusion and future perspectives
For energy-related technologies like MABs, research into MOF and COF applications has rapidly increased in recent years. One or more of the benefits that account for this is the potential of the elements and structures that support electrocatalytic activities to functionalize with semiconducting substrates like graphene, carbon nanotubes, and MXenes.153 The loaded metal nanoparticles accumulate as the temperature rises during pyrolysis because the carbon catalyst is unable to produce balanced nanostructures. Co3O4 nanosheets were assembled into 2D micro-assemblies using vacancy engineering methods by Hou et al.154 By altering the annealing temperature, changes were made to the oxygen and metal ion vacancy contents. It was discovered that the components' synergistic effects improved the Li–O2 battery's electrochemical performance. The annealing temperature raises the amount of oxygen vacancies in Co3O4 from 200 °C to 400 °C, with the biggest flaws appearing at 300 °C. By generating the active sites that speed up ORR during the discharge process, these faults also create railroads for O2 to travel across 2D micro-assembled Co3O4 nanosheets, increasing cycle stability.Despite the challenges faced by MOF composites, the following strategies have been devised to overcome them and improve their electrochemical properties:
• Low molecular weight functional materials and several redox active sites can function to a great extent when the right proportions of organic ligands, metallic ions, and ions are used.
• By fabricating MOF membranes using certain treatment techniques, such as pressing and phase change, it is possible to overcome their inherent sensitivity and processability restriction without endowing them with primitive characteristics. These restrictions can also be removed by carefully choosing organic ligands and metallic ions.
• Creating enhanced MOF composites by mixing MOF with novel conductive additives is one way to solve this issue. Composite materials embody the benefits of each component while also offsetting some of the drawbacks of the single component. A solution to this issue is also indicated by the development of conductive MOF, even though the materials are still in their infancy.70
• Field measurement, theoretical computation, and simulation must be used to conduct a thorough investigation into the electrochemical mechanism. Additionally, sophisticated characterization tools are crucial.
• The reduced rate capacity, high overpotential, and poor cycle stability of today's MOF and COF catalysts are mostly due to low OER activity, whether it be in ZAB systems or Li–air batteries. In order to give MOFs and COFs distinct ORR and OER performance for the advancement of this type of material in the future, a “structure-oriented” strategy will be developed.155
• Since so many of the tens of thousands of created MOFs and COFs are unstable in organic or aqueous electrolytes, it is challenging to understand the electrochemical process and they cannot be scaled for use in MABs. Therefore, the entire efficiency of electrochemical devices is determined by the stable design of this type of air cathode. Designing water-stable MOF and COF materials begins with the production of “water-in-salt” electrolytes.156
• It is frequently possible to use a variety of metal ions, carboxylate linkers containing azoles, and hydrophobic ligands to boost structural stability. Because of their remarkable conductivity, catalysts must be utilized as electrochemical components. Unfortunately, MOFs and COFs cannot be used in electrochemical devices like MABs because of their weak conductivity and confined ability to degrade the discharge products. An efficient strategy for raising MAB performance is to carefully develop.157 In MOF and COF materials, there is better intrinsic conductivity as well as bifunctional ORR and OER capabilities. Conjugated organic linkers, conductive carbon materials/substrates such as graphene, carbon nanotubes, and foam nickel, as well as the development of p-type doping systems are further techniques to somewhat increase the conductivity of the framework materials.158
• Using 3D-printed MOFs to create framework cathodes offers a creative, alternative way to increase the specific energy used in Li–O2 batteries. Metal dust was applied to porous carbon to create an oxygen electrode for LABs. However, throughout the reversible liquid–gas–solid cycles, nanoparticles are susceptible to losing their bond. This problem was addressed by an electrocatalyst constructed from a carbon matrix with stereoscopic Ru nanoparticles dispersed throughout (Ru-MOF). Still after finding solution there is still risk.159 With current densities of 500 mA g−1 and potentials of 0.2/0.7 V, a Ru/carbon electrocatalyst design achieved sustained charge–discharge cycling capability with 800 cycles over 107 days at ambient temperature. It opens up a new opportunity for the oxygen electrode of lithium–oxygen batteries (LOBs).160
• A nickel oxide-based catalyst that was created on graphene oxide by pyrolysis to produce metal oxide nanoparticles was examined and tested for redox reactions by Liu et al.175 When the metal oxide was coupled directly to the graphene oxide, further advantages included improved conductivity for the ORR and metal active sites are dispersed uniformly across the graphene sheets. The performance of the OER activity outperformed that of other cobalt and platinum-based catalysts and Ni2+ was found to be the essential component in both the ORR and the OER. The conductivity was enhanced by graphene oxide, but the Ni-oxide catalyst's ORR activity was only marginal.
• It is necessary to enhance the creation and analysis of creative two-dimensional MOFs. One of the best materials for enabling intercalation may be a two-dimensional MOF. Superior cycle stability and better electrochemical properties can be found in two-dimensional MOFs with appropriately interacting metal clusters and connectors.161 Additionally, by combining two-dimensional MOF with additional two-dimensional functional materials, novel heterogeneous structures with intriguing properties can be created (such MXene).162,163
• A method for catalyst regeneration has been developed that separates monovalent metal ions with a selected cation and a thermally regenerating metal–organic framework (MOF). Crosslinked MOF-808 with functionalized poly(N-isopropylacrylamide-co-acryloylamidobenzo-18-crown-6) (pNCE/MOF-808) preferentially adsorbs Na+ and K+ while leaving Li+ behind. The size-match effect, ion dehydration effect, and stability constant of the cation-benzo-18-crown-6 complex are responsible for this. It exhibits a high capacity for adsorption of 1.04 mmol g−1 and excellent selectivity up to 29.4 for Na+/Li+ and 34.4 for K+/Li+ in the mixed solution including 0.5 M LiCl, 0.5 M NaCl, and 0.5 M KCl. Furthermore, at low temperatures (like 45 °C) in water, pNCE/MOF-808 with adsorbed Na+ and K+ ions could be effectively regenerated. Additionally, adding functional groups to MOFs enhances their adsorption properties and allows for the selective binding of desired ions. Due to the high binding of the added thiol, Li et al. produced a thiol-functionalized MIL-68 with Hg2+ selectivity. The addition of functional groups to MOFs also improves their adsorption abilities and permits the selective binding of selected ions. However, the bulk of ion adsorbent regeneration reported up to this point has required concentrated salt solution, acid/base, or, which invariably results in secondary pollution, making it essential to identify a environmentally friendly regeneration method.164
6.1. Conclusion
The construction of MOF depends solely on the type of organic linker used. Each MOF formulation can store more metal ions by utilizing organic linkers for MIBs that contain a lot of redox active sites. The advantage of MOF that may successfully restrict the polysulfide's functional organic ligands is that they can prevent the shuttle effect of sol trisulfide on LSBs. Making hybrid matrix films for LOBs requires the appropriate organic connectors. These layers prevent deposits from accumulating in the battery as a result of carbon dioxide and water entering. Adsorbent MOFs are given the ability to regenerate under stimuli by the addition of stimuli-responsive components. Because the physical surroundings, such as temperature, can be altered to modify how the material operates, electrical or magnetic conditions, stimuli-responsive MOFs have come under increased scrutiny. Due to their ease of use, components that respond to light and temperature are frequently utilized.165,166The fabrication of a conductive MOF and its electrochemical characteristics are enhanced by the use of suitable organic connections for SCs. In conclusion, by using functional components to further improve the electrochemical behavior of virgin MOF, MOF composites have a significant impact on the field of EES. Research on MOF composites has recently evolved swiftly, despite certain persistent difficulties, and the development of innovative functional materials. The research presented in this review provides a solid platform for future study and is crucial for comprehending how MOF composites are being employed as potential energy storage materials.167,168 The electrochemical reaction for air cathodes consists of numerous electron transfer processes and the formation of a few transitional products. The development of MOF and COF catalysts in general may become more complicated because the actual catalytic centre and catalysis may be obscured and the disparity between catalytic activity and catalyst specifications may grow. These methods may enable the precise computational synthesis of MOF and COF catalysts.169
A number of developments have been made and were investigated here for the MOF and COF materials use as fundamental applications in MABs, especially as a cathode catalyst.153,170 According to past study, the development of new MABs devices that fully leverage the structural and compositional properties of MOF and COF materials would improve the market prospects for future energy. For instance, recent developments in catalysts for N2 electroreduction processes have made it possible to develop a unique class of Zn–N2 aqueous batteries.171,172 Though there is currently little knowledge about it, nitrogen reduction reaction (NRR) of MOF materials is highly anticipated in future. If MABs are selected as the intended applications, in order to indicate catalyst's useful performance, considerable battery research is also required. On the basis of similar working theories, it can be said that MOF materials could be potential candidates for usage in more MABs, such as sodium–air batteries, aluminum–air batteries, magnesium–air batteries, supercapacitors etc. The COF and MOF derivatives created for ZAB systems and Li–air batteries, however, have been included into the batteries on several occasions, particularly as cathode catalysts. Using pure MOFs and COFs directly in MABs, on the other hand, has received much less attention.173,174
Conflicts of interest
The authors declare no known competing conflict of interests.Acknowledgements
The author would like to acknowledge Higher Education Commission of Pakistan (HEC) for the financial support under Project No. CPEC-CRG-149.References
- A. Dehghani-Sanij, et al., Study of energy storage systems and environmental challenges of batteries, Renewable Sustainable Energy Rev., 2019, 104, 192–208 CrossRef.
- F. Martins, et al., Analysis of fossil fuel energy consumption and environmental impacts in European countries, Energies, 2019, 12(6), 964 CrossRef CAS.
- X. Lei, et al., Flexible lithium–air battery in ambient air with an in situ formed gel electrolyte, Angew. Chem., Int. Ed., 2018, 57(49), 16131–16135 CrossRef CAS PubMed.
- L. D. Harvey, Global warming, Routledge, 2018 Search PubMed.
- L. J. Al-Ghussain, Global warming: Review on driving forces and mitigation, Environ. Prog. Sustainable Energy, 2019, 38(1), 13–21 CrossRef CAS.
- A. Kalair, et al., Role of energy storage systems in energy transition from fossil fuels to renewables, Energy Storage, 2021, 3(1), e135 CrossRef.
- N. S. Diffenbaugh and M. Burke, Global warming has increased global economic inequality, Proc. Natl. Acad. Sci. U.S.A., 2019, 116(20), 9808–9813 CrossRef CAS PubMed.
- N. Khan, et al., Review of energy storage and transportation of energy, Energy Storage, 2019, 1(3), e49 CrossRef.
- C. Zhang, et al., Energy storage system: Current studies on batteries and power condition system, Renewable Sustainable Energy Rev., 2018, 82, 3091–3106 CrossRef CAS.
- Y. Yang, et al., Battery energy storage system size determination in renewable energy systems: A review, Renewable Sustainable Energy Rev., 2018, 91, 109–125 CrossRef.
- X. Zhang, et al., Recent progress in rechargeable alkali metal–air batteries, Green Energy, 2016, 1(1), 4–17 CrossRef.
- T. Kim, et al., Lithium-ion batteries: outlook on present, future, and hybridized technologies, J. Mater. Chem. A, 2019, 7(7), 2942–2964 RSC.
- L. Pietrosemoli and C. Rodríguez-Monroy, The Venezuelan energy crisis: Renewable energies in the transition towards sustainability, Renewable Sustainable Energy Rev.s, 2019, 105, 415–426 CrossRef.
- G. J. May, A. Davidson and B. J. Monahov, Lead batteries for utility energy storage: A review, J. Energy Storage, 2018, 15, 145–157 CrossRef.
- R. A Mainar and O. Leonet, et al., Alkaline aqueous electrolytes for secondary zinc–air batteries: an overview, Int. J. Energy Res., 2016, 40(8), 1032–1049 CrossRef.
- L. Jiang, et al., Building aqueous K-ion batteries for energy storage, Nat. Energy, 2019, 4(6), 495–503 CrossRef CAS.
- G. Crabtree, E. Kócs and L. Trahey, The energy-storage frontier: Lithium-ion batteries and beyond, MRS Bull., 2015, 40(12), 1067–1078 CrossRef CAS.
- O. M. Yaghi, G. Li and H. Li, Selective binding and removal of guests in a microporous metal–organic framework, Nature, 1995, 378, 703–706 CrossRef CAS.
- D. Larcher and J.-M. Tarascon, Towards greener and more sustainable batteries for electrical energy storage, Nat. Chem., 2015, 7(1), 19–29 CrossRef CAS PubMed.
- Y.-S. Wei, et al., Metal–organic framework-based catalysts with single metal sites, Chem. Rev., 2020, 120(21), 12089–12174 CrossRef CAS PubMed.
- Q. Zhang, et al., Piezofluorochromic metal–organic framework: A microscissor lift, J. Am. Chem. Soc., 2015, 137(32), 10064–10067 CrossRef CAS PubMed.
- Y. Wu, et al., A metal-organic framework-derived bifunctional catalyst for hybrid sodium-air batteries, Appl. Catal., B, 2019, 241, 407–414 CrossRef CAS.
- A. K. Rohit, K. P. Devi and S. J. Rangnekar, An overview of energy storage and its importance in Indian renewable energy sector: Part I–Technologies and Comparison, J. Energy Storage, 2017, 13, 10–23 CrossRef.
- N. Zaman, N. Iqbal and T. J. Noor, Advances and challenges of MOF derived carbon-based electrocatalysts and photocatalyst for water splitting: A review, Arabian J. Chem., 2022, 103906 CrossRef CAS.
- B. Diouf and R. Pode, Potential of lithium-ion batteries in renewable energy, Renewable Energy, 2015, 76, 375–380 CrossRef.
- A. G. Olabi, et al., Metal-air batteries—a review, Energies, 2021, 14(21), 7373 CrossRef CAS.
- X. Liu, et al., Current progresses and future prospects on aluminium–air batteries, Int. Mater. Rev., 2021, 1–31 CrossRef.
- A. Pierini, S. Brutti and E. Bodo, Reactive pathways toward parasitic release of singlet oxygen in metal-air batteries, npj Comput. Mater., 2021, 7(1), 1–8 CrossRef.
- H. Yang, et al., Excellent performance of aluminium anode based on dithiothreitol additives for alkaline aluminium/air batteries, J. Power Sources, 2020, 452, 227785 CrossRef CAS.
- H. S. Hirsh, et al., Sodium-ion batteries paving the way for grid energy storage, Adv. Energy Mater., 2020, 10(32), 2001274 CrossRef CAS.
- H. J. Qiu, et al., Metal and nonmetal codoped 3D nanoporous graphene for efficient bifunctional electrocatalysis and rechargeable Zn–air batteries, Adv. Mater., 2019, 31(19), 1900843 CrossRef PubMed.
- J. Zhang, et al., Zinc–air batteries: are they ready for prime time?, Chem. Sci., 2019, 10(39), 8924–8929 RSC.
- S. Zhang, et al., Quasi-solid-state electrolyte for rechargeable high-temperature molten salt iron-air battery, Energy Storage Mater., 2021, 35, 142–147 CrossRef.
- M. Salado and E. Lizundia, Advances, challenges and environmental impacts in metal-air battery electrolytes, Mater. Today Energy, 2022, 101064 CrossRef CAS.
- D. Zhang, et al., Nanostructured arrays for metal–ion battery and metal–air battery applications, J. Power Sources, 2021, 493, 229722 CrossRef CAS.
- Z. Moghadam, M. Shabani-Nooshabadi and M. J. Behpour, Electrochemical performance of aluminium alloy in strong alkaline media by urea and thiourea as inhibitor for aluminium-air batteries, J. Mol. Liq., 2017, 242, 971–978 CrossRef CAS.
- F. Nadeem, et al., Comparative review of energy storage systems, their roles, and impacts on future power systems, IEEE Access, 2018, 7, 4555–4585 Search PubMed.
- G. Houchins, V. Pande and V. Viswanathan, Mechanism for singlet oxygen production in Li-ion and metal–air batteries, ACS Energy Lett., 2020, 5(6), 1893–1899 CrossRef CAS.
- C. Niu, et al., Balancing interfacial reactions to achieve long cycle life in high-energy lithium metal batteries, Nat. Energy, 2021, 6(7), 723–732 CrossRef CAS.
- J. Pan, et al., Recent progress on transition metal oxides as bifunctional catalysts for lithium-air and zinc-air batteries, Batteries Supercaps, 2019, 2(4), 336–347 CrossRef CAS.
- Y.-J. Wang, et al., A review of carbon-composited materials as air-electrode bifunctional electrocatalysts for metal–air batteries, Electrochem. Energy Rev., 2018, 1(1), 1–34 CrossRef CAS.
- L. Grande, et al., The lithium/air battery: still an emerging system or a practical reality?, Adv. Mater., 2015, 27(5), 784–800 CrossRef CAS PubMed.
- T. Liu, et al., Protecting the lithium metal anode for a safe flexible lithium-air battery in ambient air, Angew. Chem., 2019, 131(50), 18408–18413 CrossRef.
- Q. Liu, et al., Aqueous metal-air batteries: Fundamentals and applications, Energy Storage Mater., 2020, 27, 478–505 CrossRef.
- J. Pan, et al., A lithium–air battery stably working at high temperature with high rate performance, Small, 2018, 14(6), 1703454 CrossRef PubMed.
- A. Manthiram and L. Li, Hybrid and Aqueous Lithium-Air Batteries, Adv. Energy Mater., 2015, 5(4), 1401302 CrossRef.
- M. Zhao, et al., Activating inert metallic compounds for high-rate lithium–sulfur batteries through in situ etching of extrinsic metal, Angew. Chem., Int. Ed., 2019, 58(12), 3779–3783 CrossRef CAS PubMed.
- J. Yuan, J.-S. Yu and B. J. Sundén, Review on mechanisms and continuum models of multi-phase transport phenomena in porous structures of non-aqueous Li-Air batteries, J. Power Sources, 2015, 278, 352–369 CrossRef CAS.
- L. Ma, et al., Emerging metal single atoms in electrocatalysts and batteries, Adv. Funct. Mater., 2020, 30(42), 2003870 CrossRef CAS.
- P. Tan, et al., Advances and challenges in lithium-air batteries, Appl. Energy, 2017, 204, 780–806 CrossRef.
- K. Kim, et al., Electrolyte-additive-driven interfacial engineering for high-capacity electrodes in lithium-ion batteries: promise and challenges, ACS Energy Lett., 2020, 5(5), 1537–1553 CrossRef CAS.
- V. Shrivastav, et al., Metal-organic frameworks (MOFs) and their composites as electrodes for lithium battery applications: Novel means for alternative energy storage, Coord. Chem. Rev., 2019, 393, 48–78 CrossRef CAS.
- M. Zhong, et al., Synthesis of MOF-derived nanostructures and their applications as anodes in lithium and sodium ion batteries, Coord. Chem. Rev., 2019, 388, 172–201 CrossRef CAS.
- Y. Liu, et al., Fabrication and performance of all-solid-state Li–air battery with SWCNTs/LAGP cathode, ACS Appl. Mater. Interfaces, 2015, 7(31), 17307–17310 CrossRef CAS PubMed.
- P. Kurzweil, Lithium battery energy storage: State of the art including lithium–air and lithium–sulfur systems, Electrochemical energy storage for renewable sources and grid balancing, 2015, pp. 269–307 Search PubMed.
- W. Zang, et al., Single Co atoms anchored in porous N-doped carbon for efficient zinc− air battery cathodes, ACS Catal., 2018, 8(10), 8961–8969 CrossRef CAS.
- D. Jiao, et al., Test factors affecting the performance of zinc–air battery, J. Energy Chem., 2020, 44, 1–7 CrossRef.
- K. Wang, et al., Advanced rechargeable zinc-air battery with parameter optimization, Appl. Energy, 2018, 225, 848–856 CrossRef CAS.
- J. S. Lee, et al., Metal-Air Batteries: Metal–Air Batteries with High Energy Density: Li–Air versus Zn–Air, Adv. Energy Mater., 2011, 1(1), 2 CrossRef.
- S. Suren and S. J. Kheawhom, Development of a high energy density flexible zinc-air battery, J. Electrochem. Soc., 2016, 163(6), A846 CrossRef CAS.
- A. R. Mainar, et al., An overview of progress in electrolytes for secondary zinc-air batteries and other storage systems based on zinc, J. Energy Storage, 2018, 15, 304–328 CrossRef.
- L. Ma, et al., Super-stretchable zinc–air batteries based on an alkaline-tolerant dual-network hydrogel electrolyte, Adv. Energy Mater., 2019, 9(12), 1803046 CrossRef.
- Z. Pei, et al., A Flexible Rechargeable Zinc–Air Battery with Excellent Low-Temperature Adaptability, Angew. Chem., Int. Ed., 2020, 59(12), 4793–4799 CrossRef CAS PubMed.
- H. Miao, et al., All-solid-state flexible zinc-air battery with polyacrylamide alkaline gel electrolyte, J. Power Sources, 2020, 450, 227653 CrossRef CAS.
- S. Wang, et al., Recent progress in cobalt-based carbon materials as oxygen electrocatalysts for zinc-air battery applications, Mater. Today Energy, 2021, 20, 100659 CrossRef CAS.
- J. Fu, et al., Electrically rechargeable zinc–air batteries: progress, challenges, and perspectives, Adv. Mater., 2017, 29(7), 1604685 CrossRef PubMed.
- H. Cheng, et al., Cu Co Bimetallic Oxide Quantum Dot Decorated Nitrogen-Doped Carbon Nanotubes: A High-Efficiency Bifunctional Oxygen Electrode for Zn–Air Batteries, Adv. Funct. Mater., 2017, 27(30), 1701833 CrossRef.
- J. Pan, et al., Advanced architectures and relatives of air electrodes in Zn–air batteries, Adv. Sci., 2018, 5(4), 1700691 CrossRef PubMed.
- S. Clark, A. Latz and B. J. C. Horstmann, Rational development of neutral aqueous electrolytes for zinc–air batteries, ChemSusChem, 2017, 10(23), 4735–4747 CrossRef CAS PubMed.
- M. Deyab and Q. Mohsen, Improved battery capacity and cycle life in iron-air batteries with ionic liquid, Renewable Sustainable Energy Rev., 2021, 139, 110729 CrossRef CAS.
- M. Pino, et al., Performance of commercial aluminium alloys as anodes in gelled electrolyte aluminium-air batteries, J. Power Sources, 2015, 299, 195–201 CrossRef CAS.
- M. Pino, et al., Carbon treated commercial aluminium alloys as anodes for aluminium-air batteries in sodium chloride electrolyte, J. Power Sources, 2016, 326, 296–302 CrossRef CAS.
- M. Hartmann and S. Elangovan, Catalysis with microporous aluminophosphates and silicoaluminophosphates containing transition metals. Advances in Nanoporous Materials, 2010. vol. 1: pp. 237–312 Search PubMed.
- L.-W. Chen and H.-W. Liang, Ir-based bifunctional electrocatalysts for overall water splitting, Catal. Sci. Technol., 2021, 11, 4673–4689 RSC.
- Z. F. Huang, et al., Design of efficient bifunctional oxygen reduction/evolution electrocatalyst: recent advances and perspectives, Adv. Energy Mater., 2017, 7(23), 1700544 CrossRef.
- Z. Wang, et al., Fe, Cu-coordinated ZIF-derived carbon framework for efficient oxygen reduction reaction and zinc–air batteries, Adv. Funct. Mater., 2018, 28(39), 1802596 CrossRef.
- T. Priamushko, R. Guillet-Nicolas and F. Kleitz, Mesoporous nanocast electrocatalysts for oxygen reduction and oxygen evolution reactions, Inorganics, 2019, 7(8), 98 CrossRef CAS.
- S. Li, et al., Nanostructured Co-based bifunctional electrocatalysts for energy conversion and storage: current status and perspectives, J. Mater. Chem. A, 2019, 7(32), 18674–18707 RSC.
- J. P. Zhu, X. H. Wang and X. X. Zuo, The application of metal-organic frameworks in electrode materials for lithium–ion and lithium–sulfur batteries, R. Soc. Open Sci., 2019, 6(7), 190634 CrossRef CAS PubMed.
- J. Bao, et al., Two-dimensional Mn-Co LDH/Graphene composite towards high-performance water splitting, Catalysts, 2018, 8(9), 350 CrossRef.
- Q. Xue, et al., Mn 3 O 4 nanoparticles on layer-structured Ti 3 C 2 MXene towards the oxygen reduction reaction and zinc–air batteries, J. Mater. Chem. A, 2017, 5(39), 20818–20823 RSC.
- B. Y. Xia, et al., A metal–organic framework-derived bifunctional oxygen electrocatalyst, Appl. Catal., B, 2016, 1(1), 1–8 Search PubMed.
- M. Kuang, et al., CuCo hybrid oxides as bifunctional electrocatalyst for efficient water splitting, Adv. Funct. Mater., 2016, 26(46), 8555–8561 CrossRef CAS.
- H. Huang, et al., Clusters Induced Electron Redistribution to Tune Oxygen Reduction Activity of Transition Metal Single-Atom for Metal–Air Batteries, Angew. Chem., 2022, 134(12), e202116068 Search PubMed.
- A. Gómez-Marín and J. Feliu, Oxygen reduction on platinum single crystal electrodes, Encycl. Interfacial Chem., 2018, 820–830 Search PubMed.
- Y. Jing and Z. Zhou, Computational insights into oxygen reduction reaction and initial Li2O2 nucleation on pristine and N-doped graphene in Li–O2 batteries, ACS Catal., 2015, 5(7), 4309–4317 CrossRef CAS.
- W. Gong, et al., Core@ shell MOFs derived Co2P/CoP@ NPGC as a highly-active bifunctional electrocatalyst for ORR/OER, J. Ind. Eng. Chem., 2022, 106, 492–502 CrossRef CAS.
- L. Zhang, et al., Construction of CoP/Co2P Coexisting Bifunctional Self-Supporting Electrocatalysts for High-Efficiency Oxygen Evolution and Hydrogen Evolution, ACS Omega, 2022, 7(15), 12846–12855 CrossRef CAS PubMed.
- Q. Wang and D. Astruc, State of the art and prospects in metal–organic framework (MOF)-based and MOF-derived nanocatalysis, Chem. Rev., 2019, 120(2), 1438–1511 CrossRef PubMed.
- Y. Li, et al., MOF-derived metal oxide composites for advanced electrochemical energy storage, Small, 2018, 14(25), 1704435 CrossRef PubMed.
- K. Y. Zou and Z. X. Li, Controllable Syntheses of MOF-Derived Materials, Chem. - Eur. J., 2018, 24(25), 6506–6518 CrossRef CAS PubMed.
- H. Konnerth, et al., Metal-organic framework (MOF)-derived catalysts for fine chemical production, Coord. Chem. Rev., 2020, 416, 213319 CrossRef CAS.
- W. Yang, et al., Applications of metal–organic-framework-derived carbon materials, Adv. Mater., 2019, 31(6), 1804740 Search PubMed.
- Z. Liang, et al., Metal-organic framework-derived materials for electrochemical energy applications, EnergyChem, 2019, 1(1), 100001 CrossRef.
- T. Saeed, et al., Structure, nomenclature and viable synthesis of micro/nanoscale metal–organic frameworks and their remarkable applications in adsorption of organic pollutants, Microchem. J., 2020, 159, 105579 CrossRef CAS.
- C. Li, et al., Recent advances in carbonized non-noble metal–organic frameworks for electrochemical catalyst of oxygen reduction reaction, Rare Met., 2021, 40(10), 2657–2689 CrossRef CAS.
- L. Ye, et al., A self-supporting electrode with in situ partial transformation of Fe-MOF into amorphous NiFe-LDH for efficient oxygen evolution reaction, Appl. Surf. Sci., 2021, 556, 149781 CrossRef CAS.
- Y. Liu, et al., NiCo-MOF nanosheets wrapping polypyrrole nanotubes for high-performance supercapacitors, Appl. Surf. Sci., 2020, 507, 145089 CrossRef CAS.
- H. Wang, et al., Metal-organic frameworks for energy applications, Chem, 2017, 2(1), 52–80 CAS.
- Y. Peng, et al., Hybridization of MOFs and COFs: a new strategy for construction of MOF@ COF core–shell hybrid materials, Adv. Mater., 2018, 30(3), 1705454 CrossRef PubMed.
- Q. Shao, J. Yang and X. Huang, The design of water oxidation electrocatalysts from nanoscale metal–organic frameworks, Chem. - Eur. J., 2018, 24(57), 15143–15155 CrossRef CAS PubMed.
- J. M. Goncalves, et al., Recent trends and perspectives in electrochemical sensors based on MOF-derived materials, J. Mater. Chem. C, 2021, 9(28), 8718–8745 RSC.
- J. Yu, et al., Nanoparticle/MOF composites: preparations and applications, Mater. Horiz., 2017, 4(4), 557–569 RSC.
- S. Feng, et al., A nanoscale Nd-based metal-organic framework electrochemical sensor for rapid detection of Rhodamine B, J. Solid State Chem., 2021, 303, 122508 CrossRef CAS.
- P. Falcaro, et al., Application of metal and metal oxide nanoparticles@ MOFs, Coord. Chem. Rev., 2016, 307, 237–254 CrossRef CAS.
- Y. Xue, et al., Metal–organic framework composites and their electrochemical applications, J. Mater. Chem. A, 2019, 7(13), 7301–7327 RSC.
- T. Wu, et al., Application of QD-MOF composites for photocatalysis: Energy production and environmental remediation, Coord. Chem. Rev., 2020, 403, 213097 CrossRef CAS.
- L. S. Xie, G. Skorupskii and M. Dincă, Electrically conductive metal–organic frameworks, Chem. Rev., 2020, 120(16), 8536–8580 CrossRef CAS PubMed.
- M. Witman, et al., In silico design and screening of hypothetical MOF-74 analogs and their experimental synthesis, Chem. Sci., 2016, 7(9), 6263–6272 RSC.
- Y. Du, et al., Metal-organic frameworks with different dimensionalities: An ideal host platform for enzyme@ MOF composites, Coord. Chem. Rev., 2022, 454, 214327 CrossRef CAS.
- M. Kalaj, et al., Nylon–MOF composites through postsynthetic polymerization, Angew. Chem., 2019, 131(8), 2358–2362 CrossRef.
- Q. Li and S. Sun, Recent advances in the organic solution phase synthesis of metal nanoparticles and their electrocatalysis for energy conversion reactions, Nano Energy, 2016, 29, 178–197 CrossRef CAS.
- R. Rahimi, et al., Synthesis, characterization, and photocurrent generation of a new nanocomposite based Cu–TCPP MOF and ZnO nanorod, RSC Adv., 2015, 5(58), 46624–46631 RSC.
- Z. Li, et al., Polyaniline-Coated MOFs Nanorod Arrays for Efficient Evaporation-Driven Electricity Generation and Solar Steam Desalination, Adv. Sci., 2021, 8(7), 2004552 CrossRef CAS PubMed.
- D. Yin, et al., CuO nanorod arrays formed directly on Cu foil from MOFs as superior binder-free anode material for lithium-ion batteries, ACS Energy Lett., 2017, 2(7), 1564–1570 CrossRef CAS.
- Y. Chen, et al., Enhanced oxygen reduction with single-atomic-site iron catalysts for a zinc-air battery and hydrogen-air fuel cell, Nat. Commun., 2018, 9(1), 1–12 CrossRef PubMed.
- W. Xie, et al., MOF-derived CoFe2O4 nanorods anchored in MXene nanosheets for all pseudocapacitive flexible supercapacitors with superior energy storage, Appl. Surf. Sci., 2020, 534, 147584 CrossRef CAS.
- M. Yang, et al., N-doped FeP nanorods derived from Fe-MOFs as bifunctional electrocatalysts for overall water splitting, Appl. Surf. Sci., 2020, 507, 145096 CrossRef CAS.
- M. B. Poudel, G. P. Awasthi and H. J. Kim, Novel insight into the adsorption of Cr (VI) and Pb (II) ions by MOF derived Co-Al layered double hydroxide@ hematite nanorods on 3D porous carbon nanofiber network, Chem. Eng. J., 2021, 417, 129312 CrossRef CAS.
- M. Cai, et al., One-step construction of hydrophobic MOFs@ COFs core–shell composites for heterogeneous selective catalysis, Adv. Sci., 2019, 6(8), 1802365 CrossRef PubMed.
- L. Chen, et al., The function of metal–organic frameworks in the application of MOF-based composites, Nanoscale Adv., 2020, 2(7), 2628–2647 RSC.
- R. Wang, et al., Metal−organic frameworks self-templated cubic hollow Co/N/C@ MnO2 composites for electromagnetic wave absorption, Carbon, 2020, 156, 378–388 CrossRef CAS.
- C. C. Hou and Q. Xu, Metal–organic frameworks for energy, Adv. Energy Mater., 2019, 9(23), 1801307 CrossRef.
- L. Chen and Q. Xu, Metal-organic framework composites for catalysis, Matter, 2019, 1(1), 57–89 CrossRef.
- D. H. Hong, et al., MOF-on-MOF architectures: applications in separation, catalysis, and sensing, Bull. Korean Chem. Soc., 2021, 42(7), 956–969 CrossRef CAS.
- G. Li, et al., Metal–organic frameworks encapsulating active nanoparticles as emerging composites for catalysis: recent progress and perspectives, Adv. Mater., 2018, 30(51), 1800702 CrossRef PubMed.
- N. Pan, et al., Conductive MOFs as bifunctional oxygen electrocatalysts for all-solid-state Zn–air batteries, Chem. Commun., 2020, 56(88), 13615–13618 RSC.
- W. Xiang, et al., Nanoparticle/metal–organic framework composites for catalytic applications: current status and perspective, Molecules, 2017, 22(12), 2103 CrossRef PubMed.
- H. Liu, et al., Well-Dispersed and Size-Controlled Supported Metal Oxide Nanoparticles Derived from MOF Composites and Further Application in Catalysis, Small, 2015, 11(26), 3130–3134 CrossRef CAS PubMed.
- N. Huang, et al., Flexible and Hierarchical Metal–Organic Framework Composites for High-Performance Catalysis, Angew. Chem., 2018, 130(29), 9054–9058 CrossRef.
- K. Liu, et al., Materials for lithium-ion battery safety, Sci. Adv., 2018, 4(6), eaas9820 CrossRef PubMed.
- K.-G. Liu, et al., Metal-organic framework composites as green/sustainable catalysts, Coord. Chem. Rev., 2021, 436, 213827 CrossRef CAS.
- X. S. Gao, D. Y. Yan and C. Feng, Synthesis and electrochemical properties of Fe3O4@ MOF core–shell microspheres as an anode for lithium ion battery application, Appl. Surf. Sci., 2017, 405, 52–59 CrossRef.
- M. K. Sahoo, A. K. Samantara and J. Behera, Impact of Iron in Three-Dimensional Co-MOF for Electrocatalytic Water Oxidation, Inorg. Chem. Commun., 2021, 61(1), 62–72 CrossRef PubMed.
- A. Mohanty, et al., An extensive review on three dimension architectural Metal-Organic Frameworks towards supercapacitor application, J. Power Sources, 2021, 488, 229444 CrossRef CAS.
- S. Tan, et al., A new strategy for the improvement of direct use of MOFs as supercapacitor electrodes, Mater. Lett., 2019, 251, 102–105 CrossRef CAS.
- H. Zhao, et al., The opportunity of metal–organic frameworks and covalent organic frameworks in lithium (ion) batteries and fuel cells, Energy Storage Mater., 2020, 33, 360–381 CrossRef.
- H. A. Patel, et al., Superacidity in Nafion/MOF hybrid membranes retains water at low humidity to enhance proton conduction for fuel cells, ACS Appl. Mater. Interfaces, 2016, 8(45), 30687–30691 CrossRef CAS PubMed.
- J. S. Lee, et al., Composites of a Prussian Blue Analogue and Gelatin-Derived Nitrogen-Doped Carbon-Supported Porous Spinel Oxides as Electrocatalysts for a Zn–Air Battery, Adv. Energy Mater., 2016, 6(22), 1601052 CrossRef.
- M. Shen, et al., MOFs based on the application and challenges of perovskite solar cells, Iscience, 2021, 24(9), 103069 CrossRef PubMed.
- D. U. Lee, et al., Recent progress and perspectives on bi-functional oxygen electrocatalysts for advanced rechargeable metal–air batteries, J. Mater. Chem. A, 2016, 4(19), 7107–7134 RSC.
- M.-X. Wu, et al., Sparks from different worlds: Collaboration of MOFs and COFs, Coord. Chem. Rev., 2021, 430, 213735 CrossRef CAS.
- M. Park, et al., Material design and engineering of next-generation flow-battery technologies, Nat. Rev. Mater., 2016, 2(1), 1–18 Search PubMed.
- K. Xu, et al., Engineering hierarchical MOFs-derived Fe–N–C nanostructure with improved oxygen reduction activity for zinc-air battery: the role of iron oxide, Mater. Today Energy, 2020, 18, 100500 CrossRef CAS.
- W.-Z. Cheng, et al., Bifunctional iron-phtalocyanine metal–organic framework catalyst for ORR, OER and rechargeable zinc–air battery, Rare Met., 2020, 39(7), 815–823 CrossRef CAS.
- M. M. Dinesh, et al., Water soluble graphene as electrolyte additive in magnesium-air battery system, J. Power Sources, 2015, 276, 32–38 CrossRef.
- B. J. Hopkins, Y. Shao-Horn and D. P. Hart, Suppressing corrosion in primary aluminum–air batteries via oil displacement, Science, 2018, 362(6415), 658–661 CrossRef CAS PubMed.
- C. Wang, et al., Recent progress of metal–air batteries—a mini review, Appl. Sci., 2019, 9(14), 2787 CrossRef CAS.
- Q. Li, et al., Dendrites issues and advances in Zn anode for aqueous rechargeable Zn-based batteries, EcoMat, 2020, 2(3), e12035 CAS.
- W. Lu, et al., Inhibition of zinc dendrite growth in zinc-based batteries, ChemSusChem, 2018, 11(23), 3996–4006 CrossRef CAS PubMed.
- G. Fu, et al., Hierarchically mesoporous nickel-iron nitride as a cost-efficient and highly durable electrocatalyst for Zn-air battery, Nano Energy, 2017, 39, 77–85 CrossRef CAS.
- N. Xiao, et al., Potassium superoxide: a unique alternative for metal–air batteries, Acc. Chem. Res., 2018, 51(9), 2335–2343 CrossRef CAS PubMed.
- S. Ren, et al., Bifunctional electrocatalysts for Zn–air batteries: recent developments and future perspectives, J. Mater. Chem. A, 2020, 8(13), 6144–6182 RSC.
- Y. Hou, C. Hou, Y. Zhai, H. Li, T. Chen, Y. Fan, H. Wang and W. Wang, Enhancing the electrocatalytic activity of 2D micro-assembly Co3O4 nanosheets for Li–O2 batteries by tuning oxygen vacancies and Co3+/Co2+ ratio, Electrochim. Acta, 2019, 324(1), 134884 CrossRef CAS.
- L. Zhang, et al., Design and fabrication of non-noble metal catalyst-based air-cathodes for metal-air battery, Can. J. Chem. Eng., 2019, 97(12), 2984–2993 CrossRef CAS.
- X. Ma, et al., When MOFs meet wood: From opportunities toward applications, Chem, 2022, 8(9), 2342–2361 CAS.
- S. Navalón and H. García, MOFs as photocatalysts: applications in separations and catalysis, 2018, pp. 477–501 Search PubMed.
- R. Zhao, et al., Constructing mesoporous adsorption channels and MOF–polymer interfaces in electrospun composite fibers for effective removal of emerging organic contaminants, ACS Appl. Mater. Interfaces, 2020, 13(1), 755–764 CrossRef PubMed.
- Q.-Q. Huang, et al., Tunable electrical conductivity of a new 3D MOFs: Cu-TATAB, Inorg. Chem. Commun., 2019, 105, 119–124 CrossRef CAS.
- G. Fu, et al., Ni3Fe-N doped carbon sheets as a bifunctional electrocatalyst for air cathodes, Adv. Energy Mater., 2017, 7(1), 1601172 CrossRef.
- P. Tan, et al., Flexible Zn–and Li–air batteries: recent advances, challenges, and future perspectives, Energy Environ. Sci., 2017, 10(10), 2056–2080 RSC.
- A. H. Mashhadzadeh, et al., Metal–Organic Framework (MOF) through the lens of molecular dynamics simulation: current status and future perspective, J. Compos. Sci., 2020, 4(2), 75 CrossRef CAS.
- S. Jang, et al., Application of various metal-organic frameworks (MOFs) as catalysts for air and water pollution environmental remediation, Catalysts, 2020, 10(2), 195 CrossRef CAS.
- S. Zhang, et al., Thermally regenerable metal-organic framework with high monovalent metal ion selectivity, Chem. Eng. J., 2021, 405, 127037 CrossRef CAS.
- X. Peng, et al., Flexible metal–air batteries: An overview, SmartMat, 2021, 2(2), 123–126 CrossRef.
- L. Ye, et al., Recent advances in flexible fiber-shaped metal-air batteries, Energy Storage Mater., 2020, 28, 364–374 CrossRef.
- D. Yu, et al., Dual-sites coordination engineering of single atom catalysts for flexible metal–air batteries, Adv. Energy Mater., 2021, 11(30), 2101242 CrossRef CAS.
- J. Zhao, H. Hu and M. Wu, N-Doped-carbon/cobalt-nanoparticle/N-doped-carbon multi-layer sandwich nanohybrids derived from cobalt MOFs having 3D molecular structures as bifunctional electrocatalysts for on-chip solid-state Zn–air batteries, Nanoscale, 2020, 12(6), 3750–3762 RSC.
- A. L. Semrau, et al., Surface-mounted metal–organic frameworks: Past, present, and future perspectives, Langmuir, 2021, 37(23), 6847–6863 CrossRef CAS PubMed.
- G. A. Elia, et al., An overview and future perspectives of aluminum batteries, Adv. Mater., 2016, 28(35), 7564–7579 CrossRef CAS PubMed.
- A. Varzi, et al., Current status and future perspectives of lithium metal batteries, J. Power Sources, 2020, 480, 228803 CrossRef CAS.
- A. Worku, D. Ayele and N. Habtu, Recent advances and future perspectives in engineering of bifunctional electrocatalysts for rechargeable zinc–air batteries, Mater. Today Adv., 2021, 9, 100116 CrossRef CAS.
- D. Yang, et al., Electrode materials for rechargeable zinc-ion and zinc-air batteries: current status and future perspectives, Electrochem. Energy Rev., 2019, 2(3), 395–427 CrossRef CAS.
- W. Fang, et al., Recent progress and future perspectives of flexible Zn-Air batteries, J. Alloys Compd., 2021, 869, 158918 CrossRef CAS.
- X. Liu, W. Liu, M. Ko, M. Park, M.G. Kim, P. Oh, S. Chae, S. Park, A. Casimir, G. Wu and J. Chu, Metal (Ni, Co)-metal oxides/graphene nanocomposites as multifunctional electrocatalysts, Adv. Funct. Mater., 2015, 25(36), 5799–5808 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |