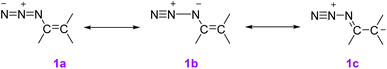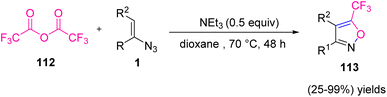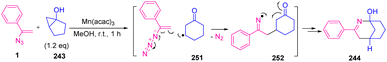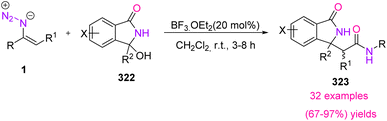Open Access Article
Open Access ArticleVinyl azides in organic synthesis: an overview
Fateme Gholamia,
Faeze Yousefnejada,
Bagher Larijanib and
Mohammad Mahdavi *b
*b
aSchool of Chemistry, College of Science, University of Tehran, Tehran, Iran
bEndocrinology and Metabolism Research Center, Endocrinology and Metabolism Clinical Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran. E-mail: momahdavi@tums.ac.ir
First published on 4th January 2023
Abstract
Among organic azides, vinyl azides have attracted significant attention, because of their unique properties in organic synthesis, which led to reports of many types of research on this versatile conjugated azide in recent years. This magical precursor can also be converted into intermediates such as iminyl radicals, 2H-azirines, iminyl metal complexes, nitrilium ions, and iminyl ions, making this compound useful in heterocycle synthesis.
Introduction
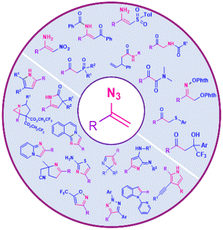
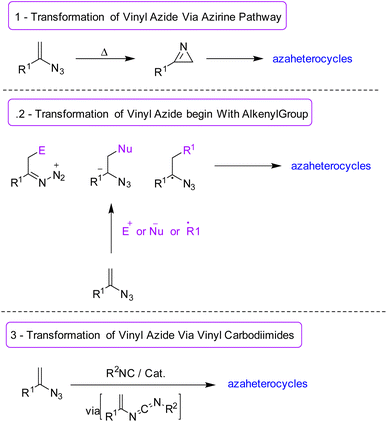
Vinyl azides are versatile building blocks in which a double bond is attached directly to the azide group.1 One reason for the great importance of vinyl azides in organic chemistry is the application of this compound to the synthesis of a large number of nitrogen-containing heterocycles, such as pyrazoles, pyrroles, imidazoles, thiazoles, triazoles, pyridines, quinolines, isoquinolines, and imidazo[1,2-a]pyridines (Fig. 1).2,3There are different types of cyclization pathway for vinyl azides, like the azirine pathway, carbodiimide pathway, and initial alkenyl group, which can be seen in Scheme 1.4
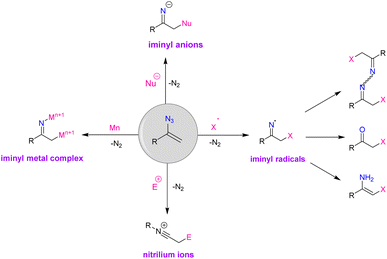
The versatility of vinyl azides in organic synthesis has long been known. Photolysis, thermolysis, cycloaddition, and reaction with nucleophiles and electrophiles are common processes that vinyl azides can go through. The double bond in this compound also causes the azide to be more reactive with other functional groups. It is found that the addition of an external radical species causes the production of an iminyl radical that undergoes numerous transformations (Fig. 2). α-Substituted vinyl azides have been utilized as radical acceptors to produce a variety of molecules, including α-trifluoro methylated ketones, α-trifluoromethyl azines, keto sulfones, α-azido styrene, cyclic β-amino ketones, N-unprotected enamines, enaminones, β-keto phosphine oxides, unsymmetrical ketones, and 2-aryacetophenones.5
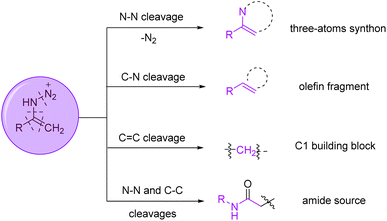
It has also been observed that vinyl azides can serve as multipurpose precursors for the synthesis of various units through diverse cleavages. Units generated from different cleavages are shown in Fig. 3.2
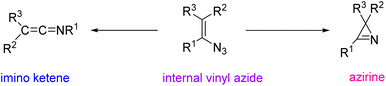
Vinyl azide decomposition has been the subject of several investigations. Thermal decomposition of vinyl azides gives different products (depending on substituents): in most cases, internal vinyl azides give azirine intermediates.6 It is found that thermolysis and photolysis of internal vinyl azides produce azirines in good yields, and sometimes with a small amount of iminoketenes (Scheme 2). In rare cases, ketene imines are major products of this type of reaction.1
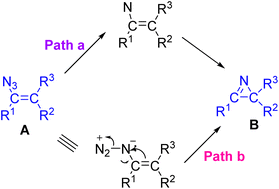
In Scheme 3 two mechanistic pathways for the transformation of vinyl azide to azirine (A → B) are shown. The vinyl azides can either release nitrogen to generate vinyl nitrene and then cyclize to the azirine (path a), or can decompose with simultaneous ring closure to generate B directly (pathway b).7
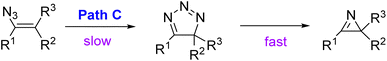
Smolinsky, who first pyrolyzed vinyl azides to azirines, considered the possibility that vinyl azides would decompose to azirine via an unstable isotriazole intermediate (path C) (Scheme 4).7
A computational study on vinyl azide decomposition was also done by da Silva et al. in 2014. Electronic structural calculations showed that the decomposition of the s-cis conformer of vinyl azide leads to the generation of ketenimine via a single-step conversion: s-cis-CH2CHN3 → CH2CNH + N2 while the transformation of the s-trans conformer to acetonitrile happens in two steps: s-trans-CH2CHN3 → cyc-CH2NCH + N2 → CH3CN + N2.6
Results of their calculations indicate that the s-cis (øCCNN = 0.0°) and s-trans (øCCNN = 180.0°) conformers of vinyl azide have analogous relative energetic stabilities. Furthermore, in an experimental microwave study, the s-cis conformer was declared to be a bit more stable than the s-trans by 0.460 kcal mol−1.8 Because of the very small energy difference between these conformers, fast interconversion is anticipated.6
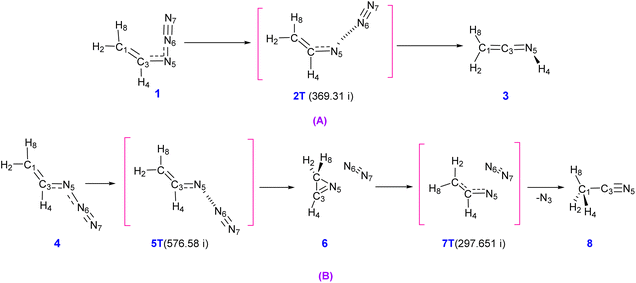
According to the calculations, the decomposition of vinyl azides in the singlet state can go through two different reaction pathways, as shown in Scheme 5, beginning from s-cis or s-trans-vinyl azide. Ketenimine (CH2CNH) is generated through single-step conversion of the s-cis conformer. The transition state for the s-cis-CH2CHN3 → CH2CNH reaction (2T) is 37.78 kcal mol−1 (47.92 kcal mol−1 at MP2/6-311++G(d,p) level) higher in energy than s-cis-CH2CHN3 (1). Meanwhile, conversion of the s-trans conformer to acetonitrile happens in two steps: s-trans-CH2CHN3 → cyc-CH2NCH + N2 → CH3CN + N2. The transition state between s-trans-CH2CHN3 and cyc-CH2NCH-(2H-azirine) (5T) is 33.90 kcal mol−1 (41.89 kcal mol−1 at MP2/6-311++G(d,p) level) higher than s-trans-CH2CHN3, while the transition state between acetonitrile and 2H-azirine (7T) is 49.93 kcal mol−1 (68.55 kcal mol−1 at MP2/6-311++G(d,p) level) higher than 3H-azirine (Fig. 4).6
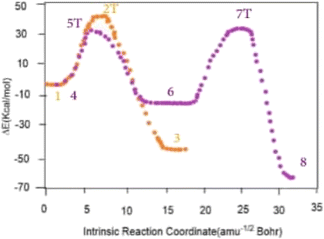 | ||
| Fig. 4 Full energy profile of the IRC path calculated for the decomposition of s-cis-vinyl azide (orange points) and s-trans-vinyl azide (purple points) at B3LYP/6-311++G(d,p) level. | ||
From the analysis of da Silva and coworker it can be deduced that N2, 2H-azirine, acetonitrile and ketenimine are generated from the decomposition of vinyl azide in the singlet state. It is also known that the decomposition mechanism of organic azide includes a nitrene species corresponding to a TS localized at a point of intersystem crossing (ISC) between singlet (1S) and triplet (3S) surfaces.6
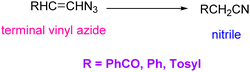
Vinyl azides can also be good precursors for the synthesis of nitrile compounds.9 In the synthesis of nitrile from terminal vinyl azide, the decomposition of vinyl azide first produces iminoketene; then, the latter product is tautomerized to the more stable nitrile (Scheme 6).1
Vinyl nitrene is also proposed as an intermediate for the chemistry of vinyl azides. The former compound can be singlet or triplet (Fig. 5). They can also act as a three-atom C–C–N synthon in cycloaddition reactions.10
In an investigation of the stability of vinyl azides, it is important to pay attention to the point that the azide group in vinyl azide increases the electron density at the β carbon atom. This means, that canonical structure 1C makes an extreme contribution to the overall stabilization of azide with the result that the order of the N2–N bond that cleaves on thermolysis is less than 1.5 (Scheme 7). Gerrit Labbe and coworkers showed that vinyl azides exhibit moderate energies of activation and low entropies of activation, consistent with the nitrene pathway.7,11
Todeschini and coworker, in 1978, compared the net charge of N1 in cis and trans forms of vinyl azides. The net charge understood on N1 (−0.388 in the trans form and −0.396 in the cis form) and on N3 (−0.299 in trans and −0.294 in cis) confirm the suggestion that the resonance form of covalently bonded azides (R–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N+
N+![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N−) is more stable in the trans conformation, while (R–N
N−) is more stable in the trans conformation, while (R–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N+
N+![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N) is more stable in the cis conformation.12
N) is more stable in the cis conformation.12
Formation of 5-membered heterocycles
In 2015, Donthiri et al. proposed a novel strategy for the synthesis of substituted 1H-pyrroles 3 through the copper-catalyzed C(sp3)–H functionalization of ketones 2 with vinyl azides 1. Their method efficiently accessed a series of 2,3,5-trisubstituted-1H-pyrroles in fair to excellent yields (50–93%).13 Pyrroles are considered as specific heterocycles due to their application in biological systems and their remedial activities. Pharmaceuticals such as potent blockers for potassium-competitive acid, anti-tumor agents, the leading cholesterol-lowering drug Lipitor, and a wide range of natural products are constituted with a pyrrole core structure.14,15 Pyrroles also serve as useful building blocks in the synthesis of bioactive compounds (Scheme 8).16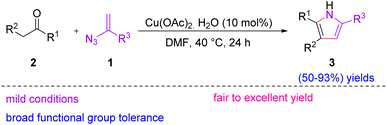 | ||
| Scheme 8 Synthesis of substituted 1H-pyrroles 3 through copper-catalyzed C(sp3)–H functionalization of ketones 2. | ||
To explain the mechanism of this reaction, deoxy benzoin 2 in the presence of Cu(II) first generated a radical intermediate 4, and Cu(II) transformed into Cu(I). Intermediate 4 in the presence of vinyl azides 1, gave iminyl radical intermediate 5. Next, the latter compound instantly underwent a radical addition and gave Cu(II) complex intermediate 6. In the final step, abstraction of Cu(II) followed by [1,3]-H shift, gave the final product 3 (Scheme 9).13
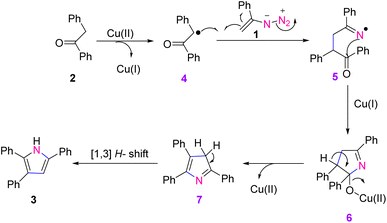 | ||
| Scheme 9 Mechanism for the synthesis of substituted 1H-pyrroles 3 through copper-catalyzed C(sp3)–H functionalization of ketones 2. | ||
In 2019, Jiang et al. synthesized substituted pyrroles 9 and 11 by utilizing vinyl azides 1 and terminal alkynes 8 and 10 in the presence of a nano copper catalyst. By using this strategy, the research group synthesized 2,5-disubstituted pyrroles 11 in poor to very good yields (13–83%) and 2,3,4-trisubstituted pyrroles 9 in fair to very good yields (50–82%).17 In this project, Jiang and coworkers observed switchable reactions by varying the substituent R of the terminal alkyne. This transition-metal-catalyzed C–C and C–N bond formation is a necessary tool in organic synthesis, allowing for the construction of basis backbones which are popular building blocks of active molecules in the material sciences (Scheme 10).18,19
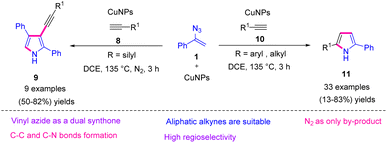 | ||
| Scheme 10 Synthesis of substituted pyrroles 9 and 11 through switchable reactivity between vinyl azides 1 and terminal alkynes 8 and 10. | ||
To illustrate the mechanism, the vinyl azides 1 underwent thermal decomposition to generate 2H-azirine 12. In pathway A for the generation of the 2,5-disubstituted pyrrole 11, 2H-azirine first underwent an SET reduction with CuNPs to give iminyl Cu(II) intermediate 13. This C-radical was trapped by terminal alkyne 10 through intermolecular addition to give intermediate 14. Then, the radical was captured by intramolecular copper(II) to generate copper adduct 15, which underwent reductive elimination to reproduce the active copper and gave the 3H-pyrroles. Eventually, 2,5-disubstituted pyrrole 11 was produced through the isomerization of the 3H-pyrrole (Scheme 11).
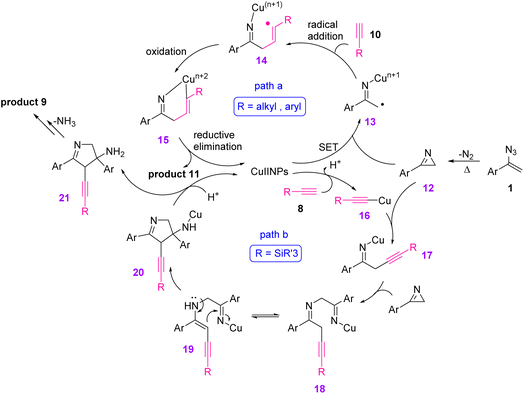 | ||
| Scheme 11 Mechanism for the Cu-catalyzed synthesis of pyrroles 9 and 11 from vinyl azides 1 and alkyne. | ||
In path B for the synthesis of 2,3,4-trisubstituted pyrrole 9, CuNPs first reacted with the terminal alkyne 8 to generate copper acetylide 16. Then, 2H-azirine underwent a nucleophilic ring opening with copper acetylide 16, giving iminyl copper intermediate 17, which was protonated to generate the NH imine. In the next step, the iminyl copper intermediate 17 reacted with another 2H-azirine, generating intermediates 18 and 19, which are keto–enol tautomers. The intramolecular cyclization of 18 generated intermediate 20, which underwent protonolysis to release intermediate 21 along with copper. Further elimination of NH3 and isomerization produced the desired product 9 (Scheme 11).17
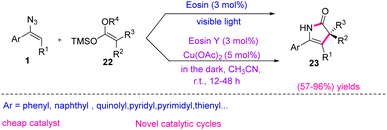
In 2018, Liu et al. utilized a copper-catalyzed dark reaction with eosin Y to produce ene-γ lactams 23 (Scheme 12). Eosin Y is a common organo-photocatalyst in visible-light photo-redox processes. It showed excellent catalytic activities for thermal redox reactions in the presence of a catalytic amount of Cu(OAc)2. With this catalytic system, ketene silyl acetals 22 and vinyl azides 1 combined through [3 + 2]-cycloadducts, resulting in the production of ene-γ-lactams 23 in fair to excellent yields (57–96%).20 Ene-γ-lactams are important synthons as they are broadly used in the synthesis and key structural elements of many bioactive natural products and medicinally relevant compounds.21 The electron-rich enamine scaffolds of ene-γ-lactams, as highly versatile intermediates, could take part in a hetero-Diels–Alder22 reaction or [3 + 2]-cyclization with α,β-unsaturated carbonyl compounds, producing tetrahydro-pyranopyrazole or enantioenriched bicyclic γ-lactams. Ene-γ-lactams could also react with electrophilic aldehydes to produce fused bicycles.23
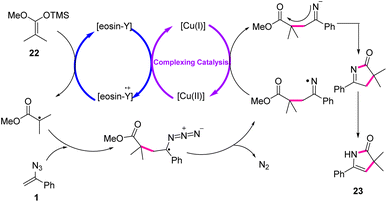
To explain the mechanism, the initial reaction between Cu(OAc)2 and eosin Y would give an eosin Y radical cation, which would be capable of oxidizing the ketene silyl acetal through single electron transfer (SET) to α-ester radicals. Addition of α-ester radicals to vinyl azides 1 would give the iminyl radical and dinitrogen. The resulting iminyl radicals should afterward be reduced by low-valent Cu(I), thus generating iminyl anions and regenerating Cu(II). Eventually, the intramolecular nucleophilic substitution of iminyl anion to ester after isomerization produced final products 23 (Scheme 13).20
In 2019, Guo et al. reported Ni-catalyzed ring-opening/radical addition/ring-closing of cycloketone oxime esters and vinyl azides (Scheme 14). Their protocol resulted in fast access to cyano-alkylated 3,4-dihydro-2H-pyrroles 25 (five-membered ring) in fair to good yields (56–77%).24
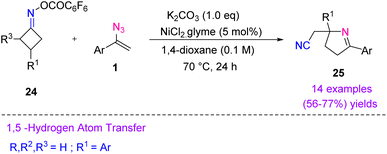 | ||
| Scheme 14 Synthesis of cyano-alkylated 3,4-dihydro-2H-pyrroles 25 through reaction of vinyl azides 1 and cycloketone oxime esters 24. | ||
To describe the mechanism, Ni-mediated single-electron reduction of cyclobutanone oxime ester 24 first generated iminyl radical 26, which underwent C–C bond cleavage and led to the formation of cyano-alkyl radical 27. The latter radical reacted with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of vinyl azide, producing iminyl radical 28 by releasing N2. For aryl vinyl azides, iminyl radical 28 proceeded through 1,5-H-transfer to generate radical 29. Oxidation, cyclization, and then deprotonation of 29 gave desired product 25 (Scheme 15).24
C bond of vinyl azide, producing iminyl radical 28 by releasing N2. For aryl vinyl azides, iminyl radical 28 proceeded through 1,5-H-transfer to generate radical 29. Oxidation, cyclization, and then deprotonation of 29 gave desired product 25 (Scheme 15).24
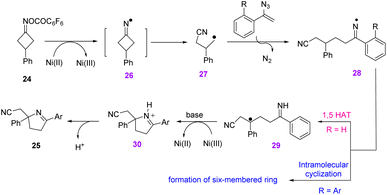 | ||
| Scheme 15 Mechanism for the synthesis of cyano-alkylated 3,4-dihydro-2H-pyrroles 25 from vinyl azide 1. | ||
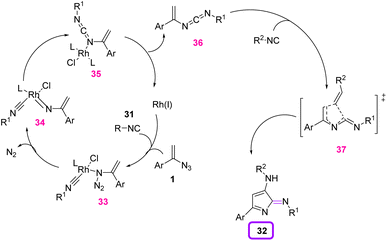
In 2019, Zhang et al. achieved novel 2H-pyrrol-2-imine derivatives 32 in a one-pot process through a rhodium-catalyzed reaction of vinyl azides 1 and two equivalent amounts of isocyanide 31 (Scheme 16). Their method offered facile access to 3-amino-5-aryl-2H-pyrrol-2-imines bearing different substitutions on the nitrogen in poor to excellent yields (16–96%).25 2H-Pyrrole is a significant structural motif present in a broad range of natural products and biologically active compounds.26,27
A plausible mechanism consists of two processes: Rh(I)-catalyzed cross-coupling of vinyl azides 1 with the first isocyanide 31, and the cyclization of the vinyl carbodiimide with the second isocyanide. Vinyl carbodiimide 36 was first generated through an Rh-nitrene pathway with the release of N2. In the next step, thermal cyclization of the second isocyanide generated 2H-pyrrol-2-imine 32 as the product (Scheme 17).25
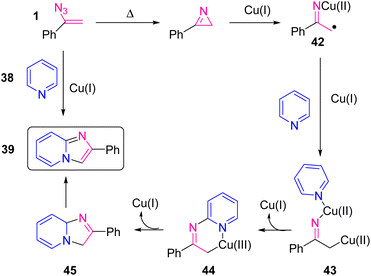
In 2014, Bairagi et al. came up with a new approach to synthesize imidazole heterocycles (Scheme 18). In this study, by utilizing copper-catalyzed C–H functionalization of pyridine and isoquinoline, the research group synthesized derivatives of 39 in poor to good yields (29–74%) and 41 in poor to good yields (48–76%).28 The development of an efficient strategy for the synthesis of azaheterocycles via direct functionalization of C–H bonds utilizing transition metal catalysts is a highly important subject in organic chemistry. Imidazo[1,2-a]pyridines 39 are aza heterocycles that have received a lot of attention due to their diverse biological activities.29,30
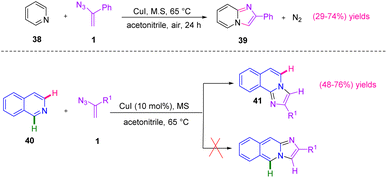 | ||
| Scheme 18 Cu-catalyzed synthesis of imidazo[1,2-a]pyridines 39 and imidazole[2,1-a]isoquinoline 41 derivatives. | ||
Scheme 19 provides plausible information about the mechanism of the reaction. Vinyl azides 1 first underwent thermal decomposition to generate the 2H-azirine, which gave iminylcopper(II) radical intermediate 42 in the presence of Cu(I), with homolytic cleavage of the C–N bond. Then intermediate 42 generated intermediate 43. Oxidative cyclization of 43 gave Cu(III) complex 44, and reductive elimination followed by oxidation produced the final product.28
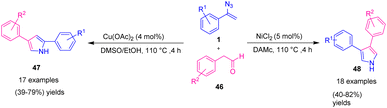
In 2012, Jiao et al. developed an innovative and effective method to synthesize 2,4-disubstituted pyrrole 47 and 3,4-disubstituted pyrrole 48 by copper and nickel catalysts (Scheme 20).31 Pyrroles are a significant class of heterocyclic compound; they are also building blocks in many natural products,32 synthetic pharmaceutical compounds,15 and material science.33 Compared with previous acidic or basic conditions for poly-substituted pyrrole synthesis, the conditions were mild, neutral, and did not involve any additive. They resulted in the synthesis of 2,4-disubstituted pyrrole in poor to good yields (39–79%) and 3,4-disubstituted pyrrole in poor to very good yields (40–82%).31
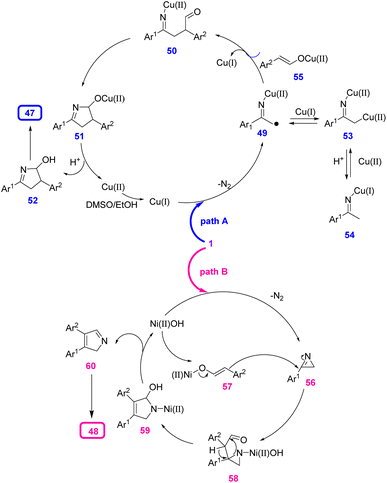
For the Cu-catalyzed formation of 2,4-disubstituted pyrrole 47 (path A), Cu(OAc)2 might first be reduced by DMSO/EtOH to generate Cu(I) or through disproportionation to generate Cu(I). In the next step, radical intermediates 49 were produced through a denitrogenative decomposition of the vinyl azides 1. Then, the radical coupling of intermediate 49 with enol tautomers of the phenylacetaldehydes produced intermediates 50, which underwent nucleophilic attack on the aldehyde, leading to the addition of intermediates 51. Subsequent protonation gave intermediates 52, and dehydration of intermediates 52 produced 2,4-disubstituted pyrroles 47 (Scheme 21).
 | ||
| Scheme 21 Proposed mechanism for the synthesis of 2,4- and 3,4-disubstituted pyrroles 47 and 48 from vinyl azides 1. | ||
For the Ni-catalyzed production of 3,4-disubstituted pyrrole 48 (path B), NiCl2 first promoted the decomposition of vinyl azides 1 to give 2H-azirines 56, which could not be generated in the presence of a Cu catalyst. In the next step, nucleophilic attack by the enol tautomers of phenylacetaldehydes generated intermediates 58. Ring-opening of intermediates 58 gave five-membered species 59. β-OH elimination gave the 2H-pyrroles 60 and Ni complexes, which reacted with the aldehyde to regenerate enol intermediate 57. In the final step, 3,4-disubstituted pyrroles 48 were produced through tautomerization of intermediates 60 (Scheme 21).31
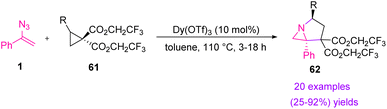
The field of donor–acceptor cyclopropane chemistry has seen significant resurgence since the early work of Wenkert, Danishefsky, and Reissig. In 2016, Kerr et al. utilized donor–acceptor cyclopropanes and (1-azidovinyl) benzene 1 to synthesize 62 (Scheme 22). Under the influence of Lewis acid and heat, donor/acceptor cyclopropanes underwent an annulation reaction with (1-azidovinyl)benzene and produced an unusual azabicyclic scaffold of 62 in poor to excellent yield (25–92%).34
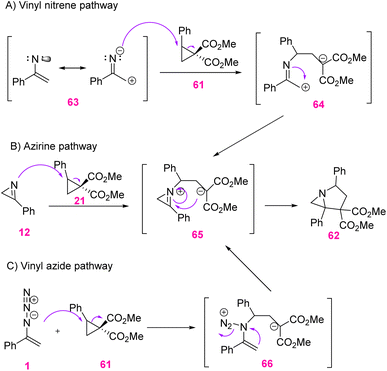
Three possible mechanistic explanations for the observed transformation are shown in Scheme 23. Option A involves vinyl nitrene formation by the thermolysis of vinyl azides 1. Nitrene 63 may be assumed to behave as a dipolar species in the Lewis-acid-mediated ring opening of cyclopropane 61 to generate 64. Conversion of 64 to the more stable iminium ion 65 established a Mannich-style ring closure to produce 62. Option B involved an azirine formation by the thermolysis of the vinyl azide through a putative nitrene 63. Ring opening of the cyclopropane by nucleophilic nitrogen gave the same intermediate 65. Eventually, option C has the vinyl azide, itself, nucleophilically opening cyclopropane 61 to give intermediate 66.34
 | ||
| Scheme 23 Mechanism for the annulation reaction of donor–donor–acceptor cyclopropanes 61 with vinyl azides 1. | ||
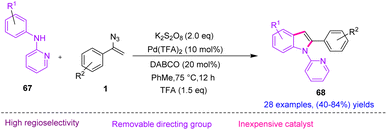
In 2018, Cui et al. synthesized diverse 2-arylindole 68 derivatives with the Pd(II)-catalyzed cyclization of anilines with vinyl azides 1 (Scheme 24).35 Indoles are a key structural unit in a large number of synthetic drugs and are widely found in natural products.36 Indole and its derivatives can also be used in the field of skeletal modification. In this study, the research group developed a palladium-catalyzed highly regioselective cyclization reaction of vinyl azide and aniline by utilizing pyridine as a directing group to produce 2-arylindole derivatives in poor to very good yields (40–84%).35
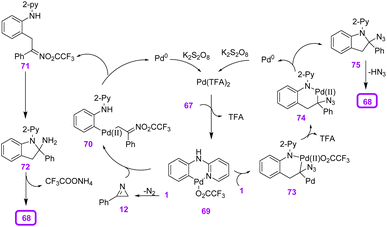
To explain the plausible mechanism of this reaction, a chelation-assisted C–H bond activation first took place via a concerted metalation–deprotonation process to generate the six-membered cyclopalladated intermediate 69. In the next step, two pathways were probably involved in the following transformation. (1) Palladacycle 69 underwent migratory insertion with vinyl azide, and then release of TFA from 73 gave intermediate 74. Then, oxidative cyclization generated indoline intermediate 75, which transformed to product 68 via the elimination of HN3. Meantime, the catalytically active Pd(II) was regenerated by oxidation of Pd(0). Decomposition of vinyl azide gave 2H-azirine 12 by the acceleration of DABCO, which underwent migratory insertion into palladacycle 69 to generate intermediate 70. In the next step, reductive elimination of 70 produced intermediate 71 with simultaneous formation of Pd(0), which could be reoxidized to the active catalytic Pd(II). In the final step, an intramolecular nucleophilic addition of intermediate 71 resulted in intermediate 72, which underwent further deamination, generating product 68 (Scheme 25).35
 | ||
| Scheme 25 Mechanism for the synthesis of 2-arylindole 68 through the Pd-catalyzed reaction of vinyl azides 1. | ||
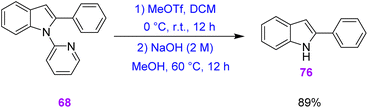
Finally, they removed the 2-pyridyl directing group from compound 68. 2-Arylindole 68 was treated with methyl trifluoromethanesulfonate (MeOTf) to generate a pyridinium intermediate and NH-free indole 76 in 89% yield (Scheme 26).35
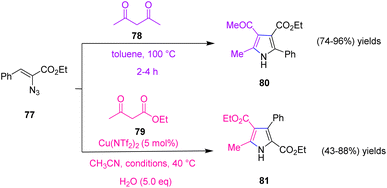
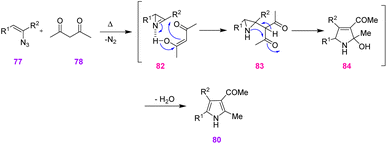
In 2008, Narasaka et al. reached various derivatives of poly-substituted N–H pyrroles through the reaction of vinyl azides and 1,3-dicarbonyl compounds. They proposed two synthetic methods for the synthesis of tetra- and tri-substituted N–H pyrroles that resulted in the production of scaffolds of 80 with good to excellent yields (74–96%) and scaffolds of 81 in poor to very good yields (43–88%). In method (I) they used the reaction of vinyl azide 77 with 1,3-dicarbonyl 78 compounds that proceed through 1,2-addition of 1,3-dicarbonyl compounds to 2H-azirin, the research group used Cu(II)-catalyzed synthesis of pyrroles from vinyl azide 77 and ethyl acetoacetate 79 via the 1,4-addition reaction (Scheme 27).37
In method (I) they designed a plan to use vinyl azides 77 as precursors of 2H-azirines. When a mixture of vinyl azides 77 and acetylacetone 78 was heated in toluene at 85–100 °C (depending on its derivatives), substituted pyrrole was obtained in good to excellent yields (74–96%) (Scheme 28).37
 | ||
| Scheme 28 Synthesis of pyrroles 80 and 81 from vinyl azides 77 and 1,3-dicarbonyl compounds 77 in toluene. | ||
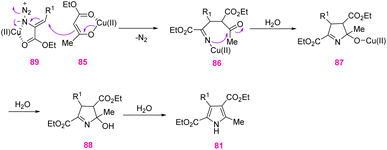
In method (II) since the Cu-catalytic reaction was done at 40–60 °C, it was improbable that a 2H-azirine intermediate would be found in the reaction course. The reaction may be started by the 1,4-addition of copper enolate 85 to vinyl azide 77, the internal nitrogen of which is coordinated to copper. Simultaneous removal of dinitrogen obtained alkylidene amino copper 86 that underwent intramolecular nucleophilic attack on the carbonyl group, producing pyrrole with the extrusion of water (Scheme 29).37
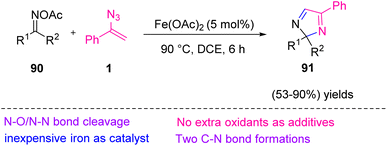
In 2017, Jiang et al. proposed an approach to produce 2H-imidazoles 91 from oxime acetates 90 and vinyl azides 1 under redox-neutral conditions (Scheme 30). They developed a novel method for the synthesis of 2H-imidazoles via iron-catalyzed [3 + 2]-annulation of oxime acetates with vinyl azides that resulted in a synthesis of scaffolds of 91 in fair to excellent yields (53–90%).38 2H-Imidazoles are significant motifs found in a broad range of natural products39 as well as in organic synthesis. These compounds are used for detection of diradical intermediates40 and could also be used as a chiral auxiliary for organic synthesis reactions.41 Oximes and their derivatives can be prepared by the reaction of hydroxylamine hydrochloride, ketone, and acetic anhydride.38
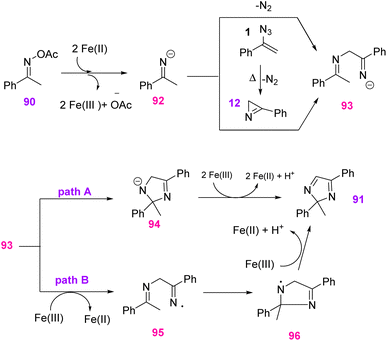
To explain the possible mechanism, imine anion intermediate 92 was first generated through the reduction of oxime acetate 90 by Fe(II) with a two-step, single-electron-transfer operation. Then, nucleophilic attack may happen between anion intermediate 92 and 2H-azirine or the Michael addition–elimination of 92 to vinyl azides 1 to generate intermediate 93. After that, intermediate 94 was generated through intramolecular cyclization of 93. Eventually, oxidative dehydrogenation of 94 by Fe(III) generated product 91 (path A). There was another approach in which intermediate 93 was first oxidized by Fe(III) to generate imine radical intermediate 95, which cyclized to intermediate 96. In the final step, the desired product was obtained via a sequential oxidation deprotonation process (path B) (Scheme 31).38
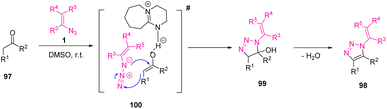
In 2017, Ramachary et al. reported the synthesis of fully decorated N-vinyl-1,2,3-triazoles 98 scaffolds in good to excellent yields (50–95%) through an organocatalytic vinyl azide-carbonyl [3 + 2]-cycloaddition.42 Triazoles have an important role in pharmaceutical chemistry and their drug properties mainly depend on their aromatic or aliphatic substitution.43 Tazobactam, solithromycin, cefatrizine, and rufinamide are some examples of 1,2,3-triazole-based drugs (Scheme 32).44
Reaction of the aryl acetaldehydes/aryl acetones/deoxybenzoines with catalyst DBU in DMSO (solvent) gave the stable enolate that on in situ treatment with vinyl-azides 1 produced 1,2,3-triazolines by [3 + 2]-cycloaddition (Scheme 33).42
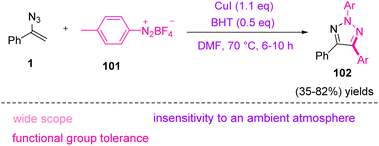
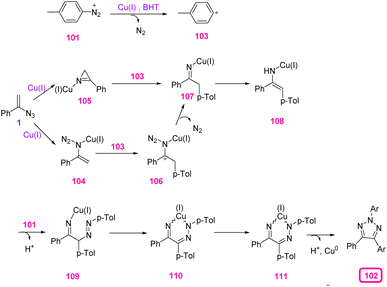
In 2017, Tang et al. synthesized N2-substituted 1,2,3-triazoles 102 through copper(I)-mediated carbo-amination of vinyl azides 1 by aryldiazonium salts 101 (Scheme 34). By this strategy, Tang and coworkers succeeded in the synthesis of diverse N2-substituted 1,2,3-triazoles in poor to very good yields (35–82%).45 1,2,3-Triazoles have played an important role in material science, pharmacological development, and chemical biology due to their privileged chemical structures and properties as five-membered heterocycles. 1,2,3-Triazoles have also received interest as chelates,46 auxiliaries for C–H activation,47 and systematic carbene precursors.
To illustrate the mechanism of this reaction, the aryl cation 103 was first delivered from aryldiazonium cation 101 with the release of nitrogen under acidic conditions. Copper-chelated complex 104 that was generated from vinyl azides 1 was attacked by aryl cation 103 to generate iminyl metal ions 106, which could further generate iminyl copper(I) intermediate 107 with the release of dinitrogen. Then, C–N bond cleavage of 105 that was produced from the decomposition of vinyl azide with the aid of Cu(I) generated intermediate 107 in the presence of aryl cation 103. Then, tautomerization of 107 gave enamide intermediate 108, which underwent addition with aryldiazonium cation 101 to generate complex 109. Copper-chelate complex 109 could isomerize to give intermediate 110. Direct reductive elimination of intermediate 109 produced desired product 102 (Scheme 35).45
In 2020, Weng et al. developed a novel approach for the synthesis of poly-substituted 5-trifluoromethyl isoxazole 113 and its derivatives (Scheme 36). The research group, through denitrogenative cyclization of vinyl azides with trifluoroacetic anhydride, synthesized diverse 5-trifluoromethyl isoxazoles in poor to quantitative yields (25–99%).48 Nitrogen- and oxygen-containing heterocycles, due to the exhibition of biological and pharmaceutical activities, such as antimicrobial, antibiotic, anti-inflammatory, and anticancer activities have been receiving a lot of attention in the past few years.49,50
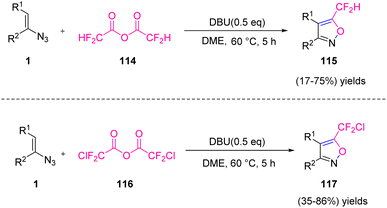
In the same year, Weng et al. synthesized 5-difluoromethyl isoxazoles 115 and 5-chlorodifluoromethyl isoxazole 117 via the reaction between vinyl azides 1 and difluoroacetic anhydride 114 or chlorodifluoroacetic anhydride 116 (Scheme 37). Their method involved sequential difluoroacetylation of vinyl azide, followed by deprotonation, cyclization, and dinitrogen elimination, providing a suitable synthesis of 5-difluoromethyl in poor to good yields (19–75%) and 5-chlorodifluoromethyl isoxazole in poor to very good yields (35–86%).51 Among organo-fluorine molecules, difluoromethylated compounds, especially difluoromethylated heterocyclic structures, play a significant and unique role in agricultural and medicinal chemistry.52 Difluoromethyl (–CF2H) behaves in an isopolar and isosteric way to the hydroxyl (–OH) group and can act as a hydrogen donor via hydrogen bonding.53
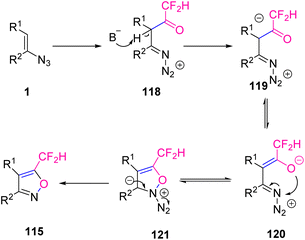
In Scheme 38, difluoroacetylation of vinyl azides 1 with difluoroacetic anhydride 114 first generated intermediate 118, which underwent deprotonation with a base to give intermediate 119. Isomerization of 119 to an alkoxy anion 120 followed by a 5-endo cyclization gave intermediate 121. In a final step, intermediate 121 underwent a dinitrogen elimination to produce desired product 115.51
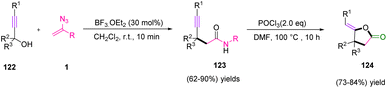
In 2015, Bi et al. introduced a new strategy to synthesize dihydrofuran-2(3H)-ones 124 via the synthesis of 4-ynamides 123 (Scheme 39). The nucleophilic addition of vinyl azides 1 to propargylic alcohols 122 in the presence of BF3·Et2O catalysis is an efficient method that produced 4-ynamides in fair to excellent yields (62–90%). Then, a Vilsmeier intramolecular cyclization of 4-ynamides 123 resulted in good to very good yields (73–84%) of dihydrofuran-2(3H)-ones 124, which was the first report of the conversion of alkyne to dihydrofurane-2(3H)-ones by the use of a Vilsmeier reagent.54
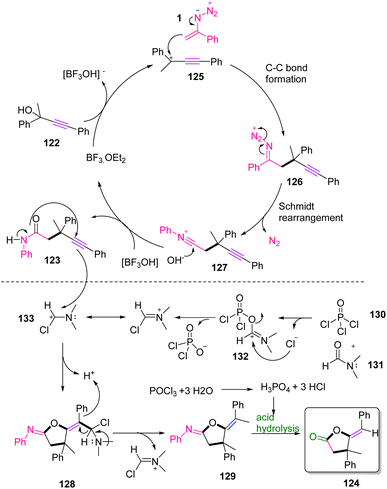
In a plausible mechanism, electrophilic attack of BF3·H2O on the –OH group of propargylic alcohol 122 first facilitated the generation of propargylic carbocation intermediate 125, which stimulated the nucleophilic addition of vinyl azides 1 to generate intermediate 126. Then, intermediate 126 underwent Schmidt rearrangement to give nitrilium intermediate 127. After that, nitrilium intermediate 127 extracted –OH from [BF3OH]− and rearranged to the final product. The Vilsmeier reagent helping intramolecular cyclization involves the production of a Vilsmeier species from the Vilsmeier reagent. Then, the nucleophilic attack of 4-ynamide 123 on Vilsmeier species occurred. Releasing the Vilsmeier species by displacement with a proton produced 129 and eventually hydrolytic cleavage of 129 afforded final product 124 (Scheme 40).54
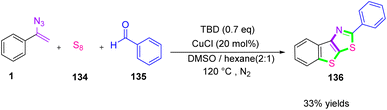
In 2019, Jiang et al. synthesized bis-S-heterocycles 136 via copper-catalyzed three-component tandem cyclization by the use of S8 134 as a sulfur source (Scheme 41). In this protocol, the research team used S8 as two sulfur atom donors for thiophene and thiazole rings, respectively. Organic sulfides are an important class of compound that have been broadly used in biomedical fields. In recent years, many drugs that are listed as best-selling drugs contain sulfur.55
For example, cefmenoxime,56 a bis-S-heterocyclic, is an antibiotic with a high curative effect on special kinds of bacteria, and nocodazole,57 a thiophene structure, is an anticancer drug that can inhibit cancer cell division.
The research team in this work employed a concise synthetic mode to pick up the thiophene and thiazole ring in a one-pot method, in which S8 was utilized as a two-sulfur donor.55
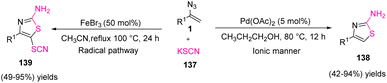
In 2015, Yu et al. reported selective access to 4-substituted 2-aminothiazoles 138 and 4-substituted 5-thiocyano-2-aminothiazoles 139 from potassium thiocyanate 137 and vinyl azides 1 switched by palladium and iron catalysts (Scheme 42). Yu and co-workers constructed diverse derivatives of 4-substituted 2-aminothiazoles in poor to excellent yields (42–94%) and 5-thiocyano-2-aminothiazoles in poor to excellent yields (49–95%).58 2-Aminothiazoles and their derivatives are one of the most significant aza-heterocycles broadly found in pharmaceutical compounds and natural products. The wide spectrum of biological activities shown by this structure include antimicrobial,59 anticancer,60 antiviral and antiprion, and anti-inflammatory effects. The most widely utilized synthetic method to gain 2-aminothiazoles is the Hantzsch cyclocondensation of thiourea and α-halo carbonyl compounds.61 Many other methods to synthesize 2-aminothiazoles have also been expanded.58
For the Pd(OAc)2-catalyzed reaction (path A), the first coordination of palladium(II) to the azide group gave palladium(II)–azide complex 140, expanding the electrophilicity of pendant olefin. The nucleophilic attack of potassium thiocyanate sent N2 out to give intermediate 141, and its intramolecular nucleophilic attack on the cyano group generated intermediate 142. Protonation of intermediate 142 and then aromatization would produce 4-substituted 2-aminothiazoles 138. But for the FeBr3-catalyzed reaction, one-electron oxidation of the thiocyanate anion by iron(III) bromide first generated a thiocyanate radical with the release of reduced iron(II) species. Vinyl azide was reduced by iron(II) to provide an iminyl iron(III) radical with the removal of molecular nitrogen. Thiocyanate radicals added to radical 144 to generate alkylidenamino iron(III) 145, which underwent an intramolecular nucleophilic assault to give intermediate 146. Then, 1,5-H migration of 146 gave 2-aminothiazole intermediate 147, which can be converted to 4-substituted 2-aminothiazoles via protonation. Thiocyanate radicals attacked the electron-rich site of 2-aminothiazole 147 to generate radical 148, which was oxidized by iron(III) to generate cation 149. Deprotonation of cation 149 gave the target 4-substituted 5-thiocyano-2-aminothiazoles 139 with the regeneration of iron(III) (Scheme 43).58
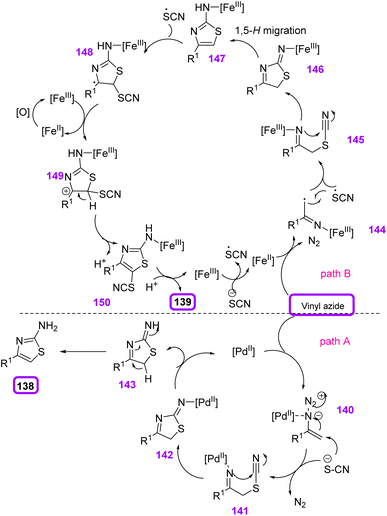 | ||
| Scheme 43 Mechanism for iron- and palladium-catalyzed reaction of vinyl azides 1 and potassium thiocyanate 137. | ||
Table 1 was prepared to provide a summary of the strategies the research groups utilized to synthesize five-membered heterocycle derivatives in which the catalyst, solvent, temperature, yields, and MW irradiation are compared.
| Catalyst (mol%) | Solvent | Temperature | MW irradiation | Yields | Reference | |
|---|---|---|---|---|---|---|
| Cu(OAc)2 (10 mol%) | DMF | 40 °C | ✗ | 50–93% | Donthiri13 | |
| CuNPs (30 mol%) | DCE | 135 °C | ✗ | 50–83% | Jiang17 | |
| Eosin Y (3 mol%) | CH3CN | r.t. | ✓ | 57–97% | Liu20 | |
| Eosin Y (3 mol%) | CH3CN | r.t. | ✗ | 57–97% | ||
| Cu(OAc)2 (5 mol%) | ||||||
| NiCl2·glyme (5 mol%) | 1,4-Dioxane | 70 °C | ✗ | 56–77% | Guo24 | |
| Rh[(Cod)Cl]2 (2.5 mol%) | 1,4-Dioxane | r.t. to 120 °C | ✗ | 16–96% | Zhang25 | |
| DPPE (5 mol%) | ||||||
| CuI (10 mol%) | Acetonitrile | 65 °C | ✗ | 48–76% | Bairagi28 | |
| NiCl2 (5 mol%) | DAMc | 110 °C | ✗ | 40–82% | Jiao31 | |
| Cu(OAc)2 (4 mol%) | DMSO/EtOH | 110 °C | ✗ | 39–79% | ||
| Dy(OTf)3 (10 mol%) | Toluene | 110 °C | ✗ | 25–92% | Kerr34 | |
| Pd(TFA) (10 mol%) | Toluene | 75 °C | ✗ | 40–84% | Cui35 | |
| No cat. | Toluene | 100 °C | ✗ | 74–96% | Narasaka37 | |
| Cu(NTf2)2 (5 mol%) | Acetonitrile/H2O | 40 °C | ✗ | 43–88% | ||
| Fe(OAc)2 (5 mol%) | DCE | 90 °C | ✗ | 53–90% | Jiang38 | |
| DBU (10 mol%) | DMSO | r.t. | ✗ | 53–95% | Ramachary42 | |
| Taking advantage of non-catalytic amount of CuI | DMF | 70 °C | ✗ | 35–82% | Tang45 | |
| No cat. | Dioxane | 70 °C | ✗ | 25–99% | Weng48 | |
| No cat. | DME | 60 °C | ✗ | 17–75% | Weng51 | |
| No cat. | DME | 60 °C | ✗ | 35–86% | ||
| BF3·OEt2 (30 mol%) | CH2Cl2 | r.t. | ✗ | 62–90% | Bi54 | |
| CuI (20 mol%) | DMSO/hexene | 120 °C | ✗ | 33% | Jiang55 | |
| Pd(OAc)2 (5 mol%) | CH3CH2CH2OH | 80 °C | ✗ | 42–94% | Yu58 | |
| FeBr3 (50 mol%) | CH3CN | 80 °C | ✗ | 49–95% | ||
Formation of 6-membered heterocycles
In 2018, Guo et al. approached a great method to synthesize substituted phenanthridines 153 (Scheme 44). Guo's research group, by using metal-free, visible-light-promoted decarboxylative radical cyclization of N-acryloxy phthalimides 152 and vinyl azides 151 synthesized diverse phenanthridines in poor to very good yields (32–80%).62 Phenanthridines are in a significant class of alkaloids that show remarkable biological activities and optoelectronic properties, so many efforts have been devoted to developing efficient strategies for the synthesis of these motifs.63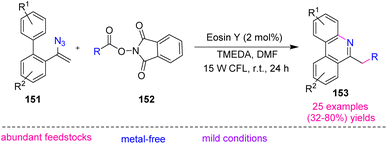 | ||
| Scheme 44 Synthesis of substituted phenanthridines 153 through the reaction of N-acryloxy 152 phthalimides and vinyl azides 151. | ||
Photoexcitation of eosin Y by visible light generated excited eosin Y*. Oxidative quenching of eosin Y* by single electron transfer (SET) to an NHP ester gave radical anion 154 along with eosin Y.+. A phthalimide anion and the corresponding alkyl radical 155, were generated through fragmentation of CO2 from 154. Then, the phthalimide anion and alkyl radical 155 added to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of 152, furnishing iminyl radical 156 by releasing N2. Iminyl radical 156 underwent an intramolecular cyclization to give intermediate 157, which was oxidized by eosin Y.+ to generate the corresponding carbocation 158. In the last step, deprotonation of intermediate 158 gave the desired product 153 (Scheme 45).62
C bond of 152, furnishing iminyl radical 156 by releasing N2. Iminyl radical 156 underwent an intramolecular cyclization to give intermediate 157, which was oxidized by eosin Y.+ to generate the corresponding carbocation 158. In the last step, deprotonation of intermediate 158 gave the desired product 153 (Scheme 45).62
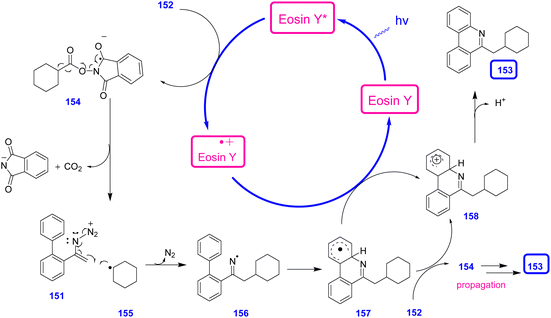 | ||
| Scheme 45 Mechanism for the synthesis of substituted phenanthridines 153 through the reaction of N-acryloxy phthalimides and vinyl azides 151. | ||
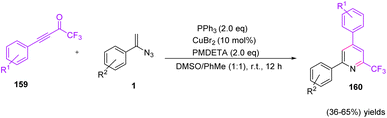
In 2021, Wang et al. reported a novel method to synthesize 2,4-diaryl-6-trifluoromethylated pyridines 160 through copper-catalyzed cyclization of CF3-ynones 159 and vinyl azides 1 (Scheme 46). Their procedure for this transformation led to the production of diverse 2,4-diaryl-6-trifluoromethylated pyridines in poor to fair yields (36–65%).64 Pyridine derivatives are versatile precursors of different topoisomerase I inhibitors and anticancer agents.65 The pyridine skeletons containing trifluoromethyl motifs, like 2-trifluoromethylated pyridines, have emerged as core units in an increasing number of important agrochemicals and drugs.66
To explain the mechanism of Wang and coworkers' methods, the reaction of vinyl azides 1 with PPh3 first gave vinyl iminophosphorane 161 through the Staudinger–Meyer reaction. Then, 161 was trapped by electrophilic CF3-ynone through copper-accelerated 1,4-addition to give intermediate 162, which would further give intermediate 163 and the corresponding resonance 164 while PPh3 and H2O were present in the reaction system. Finally, 2,4-diaryl-6-trifluoromethypiridine 160 was generated through cascade intramolecular cyclization and dehydration processes with the assistance of PMEDTA (path A). Meanwhile, 1,2-addition from intermediate 161 and CF3-ynone 159 could proceed as well to form the iminophosphorane 166, which further delivered intermediate 167 and the corresponding resonance 168. Then, intramolecular hydroamination on the alkyne moiety resulted in cyclic intermediate 168 with the aid of Cu(II) species, and dehydration gave 2,6-diaryl-4-trifluoromethylpyridine compound 170 (path B) (Scheme 47).64
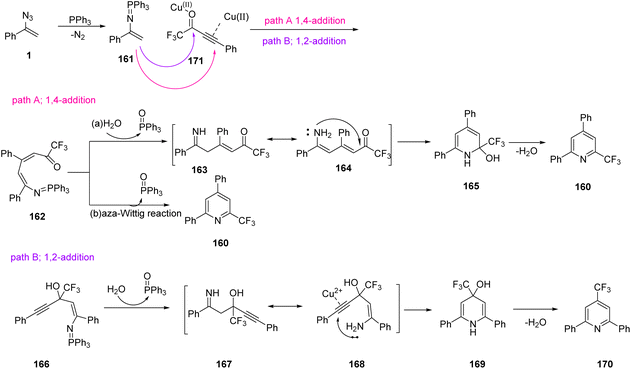 | ||
| Scheme 47 Mechanism for the copper-catalyzed synthesis of 2,4-diaryl-6-trifluoromethylated pyridines 160. | ||
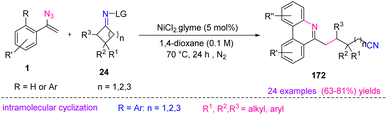
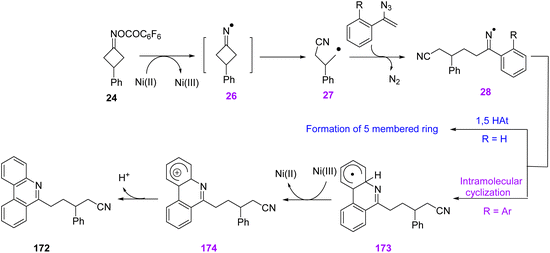
In 2019, Guo et al. proposed a new method by utilizing cycloketone oxime esters 24 and vinyl azides 1 to produce substituted phenanthridines 172 (Scheme 48). This reaction proceeded under mild and redox-neutral conditions with a wide substrate scope that resulted in the synthesis of diverse phenanthridines in fair to very good yields (63–81%).24
In the plausible mechanism, Ni-mediated single-electron reduction of cyclobutanone oxime ester first generated iminyl radical 26, which underwent C–C bond cleavage producing cyanoalkyl radical 27. In the next step, radical 27, added to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of vinyl azide, generated iminyl radical 28 by releasing N2. For biaryl vinyl azides, iminyl radical 28 underwent oxidative cyclization to afford the final product 172 (Scheme 49).24
C bond of vinyl azide, generated iminyl radical 28 by releasing N2. For biaryl vinyl azides, iminyl radical 28 underwent oxidative cyclization to afford the final product 172 (Scheme 49).24
 | ||
| Scheme 49 Mechanism for the nickel-catalyzed reaction of cycloketone oxime esters 24 and vinyl azides 1. | ||
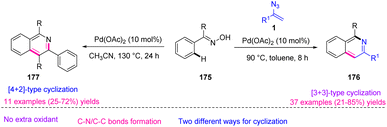
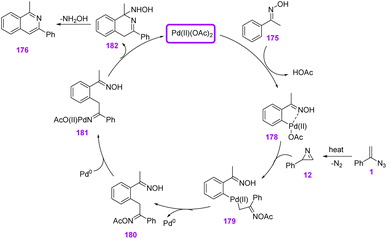
In 2016, Jiang et al. attempted to synthesize isoquinoline derivatives 176 and 177 via palladium-catalyzed C–H functionalization of aromatic oxime 175 (Scheme 50). With this strategy, through the Pd(II)-catalyzed cyclization reaction of aromatic oxime 175 and vinyl azides 1, they synthesized diverse isoquinolines in poor to very good yields (25–85%). Oximes are fascinating building blocks in synthetic chemistry because of their easy preparation, efficient reactivity, and harmless byproducts.67
In the possible mechanism, an oxime directing group ortho-C–H bond cleavage by Pd(II) first took place to form palladacycle intermediate 178. The thermal decomposition of vinyl azides 1 occurred to generate 2H-azirines that underwent migratory insertion into palladacycle 178 to generate intermediate 179. After that, the reductive elimination of 179 gave intermediate 180 and released the Pd species, that underwent further oxidative addition across the C–N bond to prepare imido-Pd species 181. Intramolecular condensation of 181 regenerated Pd(II) and gave 182 that concomitantly released hydroxylamine to form product 176 (Scheme 51).67
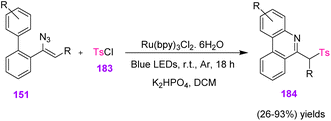
In 2018, Zhou et al. found a one-pot approach to obtain derivatives of 6-(sulfonylmethyl) phenanthridines 184 (Scheme 52). They used visible-light-induced sulfonylation/cyclization of vinyl azides 151 and developed a strategy to synthesize 6-(sulfonylmethyl)-phenanthridines 184 in poor to excellent yields (26–93%). Zhou's research group also utilized readily available sulfonyl chloride 183 as a sulfonylation reagent.68
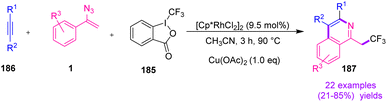
In 2016, Liu et al. synthesized diverse trifluoromethyl isoquinolines 187 by utilizing multicomponent cascade synthesis from alkynes 186 and vinyl azides 1 (Scheme 53). The research group used Togni's reagent 185 as a CF3 radical supplier; they also used the Rh(III)–Cu(II) bimetallic system for their methods that resulted in the production of trifluoromethyl isoquinolines in poor to very good yields (21–85%).69 The trifluoromethyl group is highly important among fluorine functional groups because of its remarkable potential for modulating a molecule's chemical, physical, and biochemical properties.70 Togni's reagent has been utilized to produce trifluoromethylate compounds, usually in the presence of Cu salts as catalysts.69
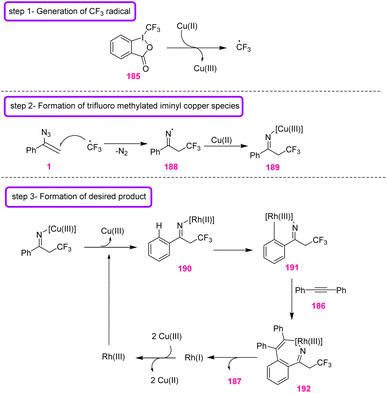
The mechanism of this reaction was proposed as depicted in Scheme 54. In step 1, CF3 radicals may first be generated from Togni's reagent in the presence of Cu(II). The CF3 radical may be added to vinyl azides 1 to generate hypothetical radical intermediate 188, which could then be trapped by Cu(II) to generate 189 (step 2). Intermediate 189 would react with Rh(III) through an iminyl rhodium intermediate 190 to give rhodacyclic intermediate 191. Insertion of alkyne 186 generated intermediate 192, which underwent reductive elimination to produce 187, along with the Rh(I) species. In the final step, a redox reaction between Rh(I) and Cu(III) regenerated the Rh(III) species.69
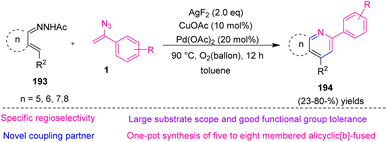
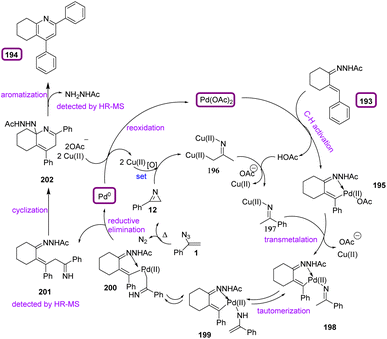
In 2022, Jiang et al. proposed an approach to produce alicyclic[b]-fused pyridine 194 via C(sp2)–H activation of α,β-unsaturated N-acetyl hydrazones 193 with vinyl azides 1 (Scheme 55). This strategy resulted in the production of diverse alicyclic[b]-fused pyridines in poor to very good yields (23–80%). Alicyclic[b]-fused pyridine scaffolds exist everywhere in natural products, functional materials, ligands, and pharmaceuticals. For example, tacrine that contains a cyclohexane[b]-fused pyridine structural skeleton has shown remedial potential in Alzheimer's disease.
Various substance scopes and specific regioselectivities were shown in this reaction. As regards the broad application of alicyclic[b]-fused pyridine, it is expected this Pd-catalyzed regioselective C–H activation will gain wide synthetic usage.71
In the plausible mechanism of the reaction, Pd(II)-catalyzed C–H activation with α,β-unsaturated hydrazone first formed intermediate 195, along with the release of HOAc. Pyrolysis of vinyl azides 1 generated 2H-azirines which underwent C–N bond cleavage and reduction with Cu(I) to yield Cu(II) aza-enolate 196 species. Next, protonation with HOAc gave iminyl cooper species 197. Also, trans-metalation with intermediate 195 generated iminyl palladium species 198, which underwent tautomerization to form Pd(II) complex 199. Afterward, the reductive elimination of 200 provided intermediate 201 and Pd(0) species. The Pd(II) catalyst was reproduced through reoxidation with Pd(0) by the Ag/Cu oxidant. Then, intramolecular condensation and subsequent aromatization with the release of NH2NHAc produced the pyridine product 194 (Scheme 56).71
 | ||
| Scheme 56 Mechanism for the palladium-catalyzed synthesis of alicyclic[b]-fused pyridine 194 via C(sp2)–H activation. | ||
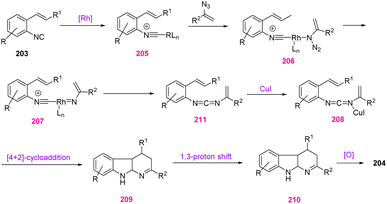
In 2020, Zhao et al. reported a one-pot and novel method to obtain α-carbolines 204 through rhodium/copper-catalyzed coupling-cyclization of O-alkenyl aryl isocyanides 203 with vinyl azides 1 (Scheme 57). In this method, the reactive vinyl carbodiimides, produced in situ from the coupling reaction of vinyl azides 1 with O-alkenyl aryl isocyanides 203, underwent intermolecular [4 + 2]-cycloaddition and provided a new method for the synthesis of poly-substituted α-carbolines in poor to excellent yields (40–74%). Among different functionalized aryl isocyanides, O-alkenyl aryl isocyanides have appeared as valuable precursors for the efficient synthesis of different fused azaheterocycles in the past decade.72
 | ||
| Scheme 57 Rhodium/copper-catalyzed coupling-cyclization of O-alkenyl aryl isocyanides 203 with vinyl azides. | ||
The proposed mechanism of this reaction is depicted in Scheme 58. First, aryl isocyanide 203 coordinated with [Cp*RhCl2]2 to generate intermediate 205, which coordinated with vinyl azides 1 to give 206. Then, intermediate 206 released N2 to give nitrene intermediate 207, which underwent migratory insertion, and rhodium was released to produce vinyl carbodiimide 211. In the next step, intermediate 208, which was activated by copper, underwent an intermolecular [4 + 2]-cycloaddition to give intermediate 209. In the last step, α-carbolines 204 were produced through a 1,3-H shift and oxidative aromatization process.72
 | ||
| Scheme 58 Mechanism for rhodium/copper-catalyzed coupling-cyclization of O-alkenyl aryl isocyanides with vinyl azides. | ||
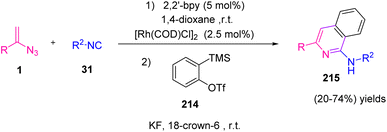
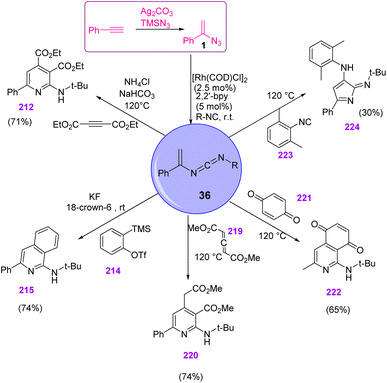
In 2018, Zhang et al. developed a new and efficient method by utilizing vinyl azides 1, isonitriles 31, and alkynes 213 in the presence of Rh as catalyst to synthesize various derivatives of pyridine and isoquinoline in poor to very good yields (17–80%) (Scheme 59).4
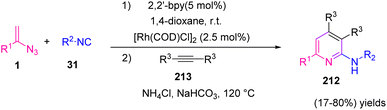 | ||
| Scheme 59 Rhodium-catalyzed synthesis of pyridine derivatives 212 by utilizing vinyl azides and isonitrile. | ||
The cascade cyclization was further developed from alkynes to benzynes 214, which afforded aminoisoquinoline 215 as the product in poor to good yields (20–74%) (Scheme 60).4
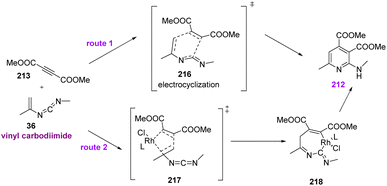
After the generation of vinyl carbodiimide 36, from the reaction of vinyl azides 1 and isonitrile 31 two pathways were possible: direct electrocyclization or Rh(I)-catalyzed oxidative/cyclization/reductive elimination (Scheme 61).4
This research group also used vinyl carbodiimides for the synthesis of different heterocycles. Other popular cyclic building blocks were employed to construct different azaheterocycles. When vinyl carbodiimide reacted with an allene 219, aminopyridine 220 was obtained in 74% yield. The reaction of vinyl carbodiimide with benzoquinone 221 led to the formation of aminoisoquinoline-5,8-dione 222 in 65% yield. The vinyl carbodiimide intermediate 36 could also go through an α,α-insertion reaction with another isocyanide to produce pyrrole-2-imine 224 with 30% yield (Scheme 62).4
In 2016, Gua et al. synthesized substituted phenanthridines 226 and 228 through the copper-catalyzed oxidative cyclization of vinyl azides 151 with the benzylic Csp3–H bond in poor to good yields (40–70%) and (34–56%) (Scheme 63).73 Toluene 225 and its derivatives are important chemical starting materials that are widely utilized as solvents in organic synthesis and the chemical industry.74 Phenanthridines are an important class of alkaloids that, due to their remarkable biological activities and optoelectronic properties, have attracted the interest of chemists. The research group, in this protocol, presented a cyclization process involving the capture of an iminyl radical by the intramolecular aryl ring, which provided a distinctive idea for constructing the phenanthridine framework.73
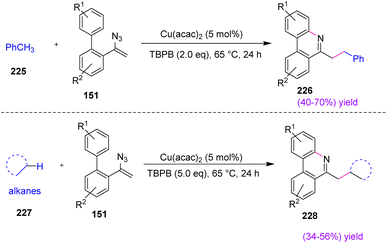 | ||
| Scheme 63 Synthesis of substituted phenanthridines 226 and 228 through the copper-catalyzed oxidative cyclization of vinyl azides. | ||
To illustrate the mechanism, metal-mediated single electron transfer (SET) or thermal homolytic cleavage or oxidation of TBPB first yielded the tert-butoxy radical. Subsequent hydrogen abstraction from toluene by the tert-butoxy radical created benzyl radical 290. Then, radical 230 added to the double bond of vinyl azide, producing an iminyl radical with the release of dinitrogen. After that, radical 230 underwent an intramolecular cyclization to create radical 231, which was converted to the corresponding carbocation by Cu(II) via single-electron oxidation with subsequent loss of H+, regenerating Cu(I) and producing desired product 226 (Scheme 64).73
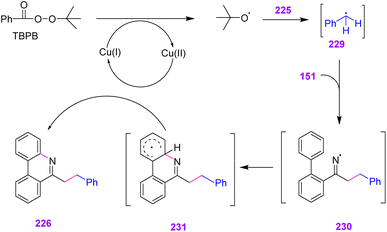 | ||
| Scheme 64 Mechanism for the synthesis of substituted phenanthridines 226 through copper-catalyzed oxidative cyclization of vinyl azides. | ||
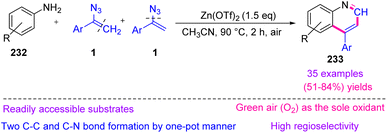
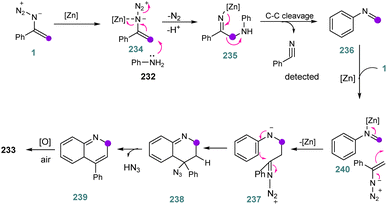
In 2018, Jiang et al. synthesized 4-substituted quinolines 233 with vinyl azides 1 as dual synthons through C–N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond cleavage in fair to very good yields (51–84%). In this protocol, vinyl azides acted as dual synthons through C
C bond cleavage in fair to very good yields (51–84%). In this protocol, vinyl azides acted as dual synthons through C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C–N bond cleavage with the formation of two C
C and C–N bond cleavage with the formation of two C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds and one C
C bonds and one C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond (Scheme 65).2 As a new strategy to further develop MCRs, the dual-synthon approach, which uses one reactant to obtain multiple fragments, has attracted a lot of attention.75
N bond (Scheme 65).2 As a new strategy to further develop MCRs, the dual-synthon approach, which uses one reactant to obtain multiple fragments, has attracted a lot of attention.75
The mechanism of this reaction can be seen in Scheme 66. First, intermediate 234 was generated by the coordination of zinc to the azide, enhancing the electrophilicity of the olefin. Then, a nucleophilic attack by the aniline produced intermediate 235. After that, intermediate 235 generated imine intermediate 236 through C–C bond cleavage. In the next step, intermediate 236 underwent an intramolecular cyclization with vinyl azides 1 to generate intermediate 238. Intermediate 238 converted to 239 with the elimination of HN3. Eventually, aromatization of 239 by O2 in air obtained desired product 233.2
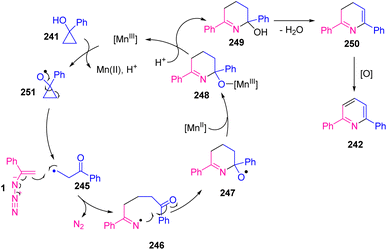
In 2009, Wang et al. developed a new method to synthesize diverse substituted pyridines 242 in poor to very good yields (11–84%) and 2-azabicyclo[3,3,1]non-2-en-1-ols 244 in poor to excellent yields (28–91%). This study focused on the use of cyclopropanols 241 as precursors of β-carbonyl radicals and reactions of vinyl azides. In this protocol, utilization of Mn(acac)3 was found to be essential because it might play a dual role in the oxidation of cyclopropanol and dihydropyridine 250 (Scheme 67).76
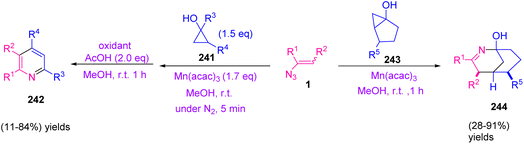 | ||
| Scheme 67 Synthesis of substituted pyridines 242 and 2-azabicyclo[3,3,1]non-2-en-1-ols 244 via vinyl azides 1. | ||
The reaction was initiated by the addition of β-keto radical 245, which was produced by one-electron oxidation of 241 by Mn(III) to vinyl azide, generating iminyl radical 246 with the release of dinitrogen. Cyclization of iminyl radical 246 to an intramolecular carbonyl group would generate alkoxy radical 247 that can be reduced by Mn(II) and then protonated to give tetrahydropyridine 249. Dehydration of 249 and further oxidation producing dihydropyridine 250 would produce desired pyridine 242 (Scheme 68).76
Treatment of chiral bicyclic cyclopropanol 243 with vinyl azides 1 provided racemic 244. Generation of ring-expanded β-keto radical 251 from bicyclic cyclopropnol followed by radical addition of 251 to vinyl azides 1 was probable in the reaction mechanism (Scheme 69).76
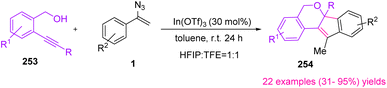
In 2015, Liu et al. utilized Lewis-acid-catalyzed polycyclization of internal alkynols 253 and vinyl azides 1 to synthesize pyran-based indeno[1,2-c]isochromene 254 scaffolds (Scheme 70). With this strategy, the research group synthesized 22 examples of this compound in poor to excellent yields (31–95%).77 Tetrahydropyran rings are common in a wide array of biologically and pharmacologically relevant natural products. In Fig. 6 some examples of natural products containing octahydrocyclopenta[b]pyran motif are shown.78
Initially, the triple bond of 253 coordinated with In(OTf)3, promoting the electrophilicity of the alkyne. Then, the hydroxyl group added to the electron-deficient alkyne, giving vinylindium species 256. After that, 256 was trapped by the N-unsubstituted imine, which gave intermediate 257. Then, carbocyclization of 257 generated 258. Acid-promoted cleavage of the carbon–metal bond, and elimination led to final product 254 (Scheme 71).77
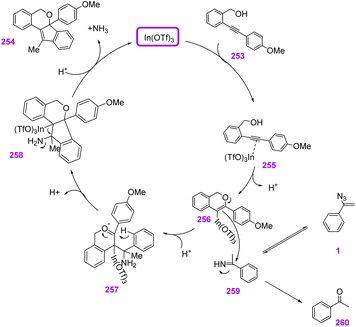 | ||
| Scheme 71 Mechanism for the synthesis of pyran-based indeno[1,2-c]isochromene 254 scaffolds via vinyl azides. | ||
Table 2 provides a summary of the strategies the researchers utilized to synthesize six-membered heterocycles derivatives in which the catalyst, solvent, temperature, yields, and MW irradiation have been compared.
| Catalyst (mol%) | Solvent | Temperature | MW irradiation | Yields | Reference |
|---|---|---|---|---|---|
| Eosin Y (2 mol%) | DMF | r.t. | ✓ | 32–80% | Guo62 |
| CuBr2 (10 mol%) | DMSO/PhMe | r.t. | ✗ | 36–65% | Wang64 |
| NiCl2·glyme (5 mol%) | 1,4-Dioxane | 70 °C | ✗ | 63–81% | Guo24 |
| Pd(OAc)2 (10 mol%) | Toluene | 90 °C | ✗ | 25–85% | Jiang67 |
| Ru(bpy)3Cl2·H2O (2 mol%) | DCM | r.t. | ✓ | 26–93% | Zhou68 |
| [CP*RhCl2]2 (9.5 mol%) | CH3CN | 90 °C | ✗ | 21–85% | Liu69 |
| Pd(OAc)2 (20 mol%) | Toluene | 90 °C | ✗ | 23–80% | Jiang71 |
| Cu(OAc) (10 mol%) | |||||
| [Rh(COD)Cl]2 (2.5 mol%) CuI (5 mol%) | Toluene | 120 °C | ✗ | 40–74% | Zhao72 |
| [Rh(COD)Cl]2 (2.5 mol%) | 1,4-Dioxane | r.t. | ✗ | 17–80% | Zhang4 |
| Cu(acac)2 (5 mol%) | Toluene | 65 °C | ✗ | 40–70% | Gua73 |
| Taking advantage of non-catalytic amount of Zn (OTf)2 | CH3CN | 90 °C | ✗ | 51–84% | Jiang2 |
| Taking advantage of non-catalytic amount of Mn(acac)3 | MeOH | r.t. | ✗ | 33–84% | Wang76 |
| Mn(acac)3 (5 mol%) | MeOH | r.t. | ✗ | 28–91% | |
| In(OTf)3 (30 mol%) | Toluene | r.t. | ✗ | 31–95% | Liu77 |
Others
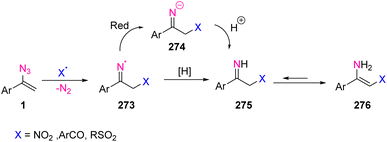
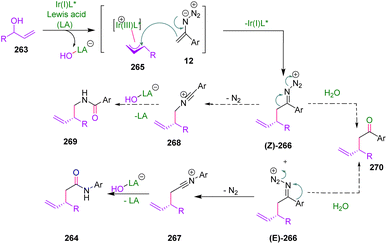
In 2020, Mukherjee et al. developed an efficient method for the enantioselective α-alkylation of amides using vinyl azides 1 as an enolate surrogate (Scheme 72). By this strategy, Mukherjee and coworkers succeeded in synthesizing derivatives of 264 in poor to good yields (40–75%). Among the unstabilized enolates utilized as nucleophiles in iridium-catalyzed asymmetric allylic alkylation reactions, amide enolates are the least explored. In this study, vinyl azides 1 were employed as amide enolate surrogates in Ir-catalyzed asymmetric allylic alkylation with branched allylic alcohols 263 as the allylic electrophile.79In the possible mechanism, the nucleophilic addition of vinyl azides 1 to the more substituted terminal of an electrophilic π-allyl-Ir complex was expected to give branched imino diazonium ion 266, which can generate a mixture of (E)- and (Z)-isomers. Schmidt-type rearrangement of (E)-266 through 1,2-aryl migration followed by hydration of the resulting nitrilium ion 267 would then furnish the α-functionalized acetamide 264. A competitive pathway involving (Z)-266 favored 1,2-alkyl migration to generate an isomeric nitrilium ion 268 and finally N-homo-allylbenzamide derivatives 269. Controlling the geometry of 266 was critical to ensure the desired aryl migration. Another competitive pathway contained the hydrolysis of imino-diazonium ion 266 to the α-allyl ketone 270. The suppression of the latter two pathways has been the key to the success of this method (Scheme 73).79
 | ||
| Scheme 73 Mechanism for the enantioselective α-allylic alkylation (AAA) of amide 264 through vinyl azides 1. | ||
In 2021, O. Terent'ev et al. reported the photo-redox-catalyzed synthesis of N-unsubstituted enamino sulfones 272 from vinyl azides 1 and sulfinates 271 (Scheme 74). The research group used ethanol as solvent and eosin Y as photocatalyst in combination with nitrobenzene as an electron shuttle. In this strategy, elimination of N2 from vinyl azides allowed the energetically favorable production of enamine derivatives in poor to fair yields (38–68%).80
 | ||
| Scheme 74 Photo-redox-catalyzed synthesis of N-unsubstituted enamino sulfones 272 through vinyl azides 1. | ||
Formation of N-unsubstituted enamine derivatives through radical addition to vinyl azides 1 is depicted in Scheme 75.80
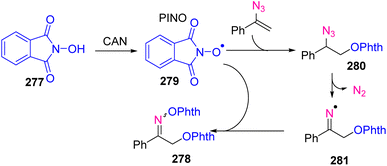
In 2020, O. Terent'ev et al. reported a cerium(IV) ammonium nitrate promoted synthesis of O-phthalimide oximes 278 from N-hydroxy phthalimide 277 and vinyl azides 1 (Scheme 76). The disclosed protocol was based on the radical transformation of vinyl azides with the release of nitrogen and the formation of iminyl N-radicals that led to the formation of O-phthalimide oximes in fair to very good yields (54–88%).81 N-oxyl radicals are broadly used in biological and organic chemistry and material science.82 In organic synthesis, more stable nitroxyl radicals are utilized as a catalyst for the oxidation of alcohol and free radical scavengers.83,84
The plausible mechanism started with the formation of a PINO radical 279 from NHPI 277 under the action of CAN, followed by addition to the terminal carbon of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of vinyl azides 1. Nitrogen elimination from 280 occurred with the generation of iminyl radical 281. In the last step, radical 281 was intercepted by the PINO radical to form the final product 278 (Scheme 77).81
C bond of vinyl azides 1. Nitrogen elimination from 280 occurred with the generation of iminyl radical 281. In the last step, radical 281 was intercepted by the PINO radical to form the final product 278 (Scheme 77).81
In 2020, Fan et al. proposed a new method to synthesize α-amido ketone 283 through the cascade reaction of carboxylic acid 282 with vinyl azides 1 under catalyst-free conditions (Scheme 78). This strategy led to the formation of diverse α-amido ketones in fair to very good yields (58–88%).85 α-Amido ketone derivatives have attracted attention due to their being not only necessary motifs of a plethora of pharmaceutically active compounds but also essential intermediates that are broadly utilized in organic synthesis.86
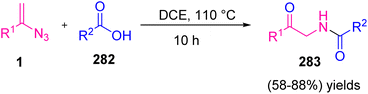 | ||
| Scheme 78 Synthesis of α-amido ketones 283 through the reaction of carboxylic acid 282 with vinyl azides 1. | ||
To explain the mechanism, the vinyl azides 1 were first decomposed under the reaction conditions to generate an azirine intermediate 12. Then, 12 reacted with carboxylic acid 282 to generate an azirine 284. Eventually, the unstable aziridine 284 through a thermal rearrangement produced α-amido ketone 283 (Scheme 79).85
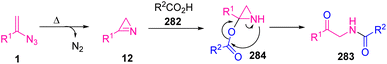 | ||
| Scheme 79 Mechanism for the synthesis of α-amido ketones 283 through the reaction of carboxylic acid with vinyl azides. | ||
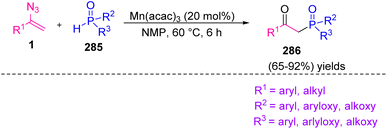
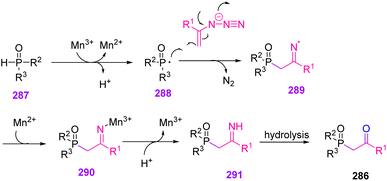
In 2017, Yu et al. synthesized β-keto phosphine oxides 286 through Mn(III)-catalyzed phosphorylation of vinyl azides 1 in fair to excellent yields (65–92%).87 Organophosphorus compounds play an important role in material science, organic chemistry, and pharmaceuticals.88 In recent years, protocols between organo-phosphorous radicals and radical acceptors have been expanded for producing organo-phosphorous compounds, especially for the production of β-keto phosphine oxides (Scheme 80).89,90
The mechanism of this reaction might be initiated by the addition of phosphine radical 288, produced by one-electron oxidation of 287 by Mn(III) to vinyl aizide, generating iminyl radical 289 with the release of N2. Sequentially, the produced iminyl radical 289 was reduced by Mn(II) and then protonated to generate imine intermediate 291. The hydrolysis of 291 would produce the desired β-keto phosphonate or β-keto phosphine oxides 286 (Scheme 81).87
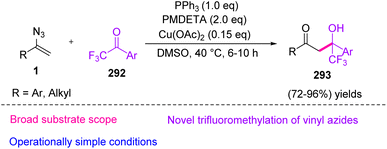
In 2019, Tang et al. proposed a new method to synthesize β-hydroxy-trifluoromethyl ketone 293 (Scheme 82) through the copper-catalyzed aldol reaction of vinyl azides 1 with trifluoromethyl ketone 292 in good to excellent yields (72–96%).91 The trifluoromethyl group (CF3) appears to have wide applications in pharmaceuticals, such as mefloquine (antimalaria),92 efavirenz (anti-HIV),93 and sorafenib (anti-cancer).94
In the possible mechanism, vinyl azides 1 first complexed with Cu(II) to give an N-diazo enamine copper 294, which comfortably trapped electrophile 292 by nucleophilic addition to generate intermediate 296 with concomitant release of N2 under standard conditions (path a). Another plausible active intermediate 295, produced from 294 in the presence of PPh3 and trace H2O in the reaction vessel, underwent nucleophilic addition with trifluoromethyl ketone to give intermediate 296 (path b). Protonation of imine copper 296 with H2O could further afford imine 297, with the release of a Cu2+ ion. Eventually, the hydrolysis reaction of 297 produced final product 293 (Scheme 83).91
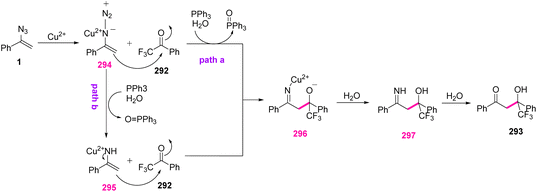 | ||
| Scheme 83 Mechanism for the copper-catalyzed aldol reaction of vinyl azides with trifluoromethyl ketone 292. | ||
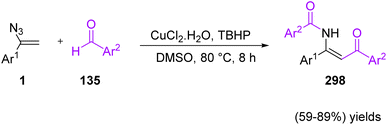
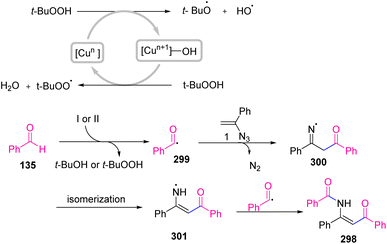
In 2022, Wang et al. developed a CuCl2·2H2O/TBHP-mediated method for the synthesis of β-enaminones 298 via a coupling reaction of vinyl azides 1 with aldehydes 135 (Scheme 84). By using this protocol, Wang and co-workers succeeded in synthesizing diverse β-enaminones in fair to very good yields (59–89%).95 β-Enaminones are important pharmaceutically active compounds. They are also versatile and extremely attractive intermediates utilized in the preparation of pharmaceutical targets and various heterocyclic compounds.96 β-Enaminones are also used as N,O-bidentate ligands in transition-metal-catalyzed reactions and organoboron complexes.97
The mechanism of this reaction can be seen in Scheme 85. First, TBHP underwent a single electron transfer (SET) to give t-BuO˙ radical and/or t-BuOO˙ radical by the action of copper ions. Then, benzoyl radical 299 was generated via the processes of deprotonation and oxidation of benzaldehyde 135 in the vicinity of the t-BuO˙ radical and/or t-BuOO˙ radical, which then attacked the terminal carbon of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of α-phenyl vinyl azide, affording iminyl radical 300 with the release of nitrogen. The generated radical 300 then underwent isomerization to produce enaminyl radical 301, which recombined with radical 299 to give final product 298.95
C bond of α-phenyl vinyl azide, affording iminyl radical 300 with the release of nitrogen. The generated radical 300 then underwent isomerization to produce enaminyl radical 301, which recombined with radical 299 to give final product 298.95
 | ||
| Scheme 85 Mechanism for the synthesis of β-enaminones 298 via a coupling reaction of vinyl azides with aldehydes. | ||
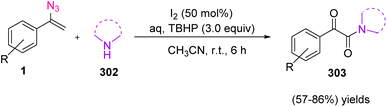
In 2022, Dandela et al. published the iodine-TBHP mediated synthesis of α-ketoamide 303 through the reaction of vinyl azides 1 and amines 302 in fair to very good yields (57–86%). This reaction can be seen in Scheme 86.5 α-Ketoamides and their derivatives are present in a variety of natural products, biologically active molecules such as antitumor, anti-IBD,98 antiviral,99 anti-HIV,100 and antibacterial drugs, and functional materials. The α-ketoamide moieties are also present in diverse pharmacologically interesting compounds, as shown in Fig. 7.5
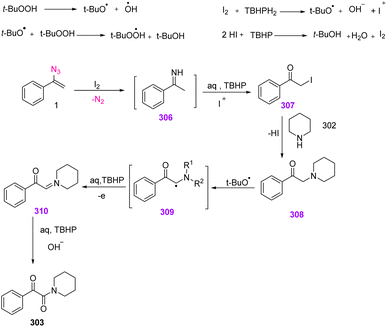
In the possible mechanism, in the presence of I2, N-unsubstituted imine 306 would first be generated from α-aryl vinyl azides, with the release of N2. Then, aq. TBHP would cause the hydrolysis of imine intermediate 306. In the next step, iodinated intermediate 307 could be generated. The nucleophilic substitution of amine to intermediate 307 gave α-aminoketone 308 which was converted to intermediate 309. In the presence of aq. TBHP 309 converted to intermediate 310. Eventually, this iminium intermediate oxidized to produce final product 303 (Scheme 87).5
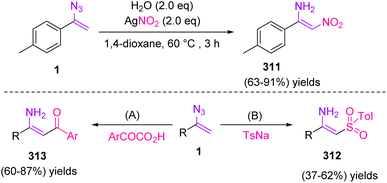
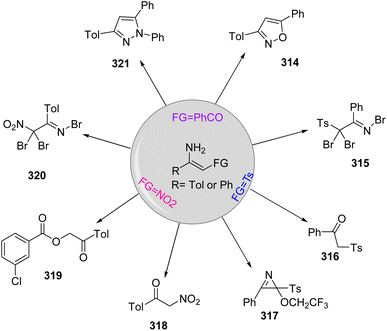
In 2017, Bi et al. synthesized N-unprotected enamines 311, 312, and 313 through radical enamination of vinyl azides 1 in fair to excellent yields (63–91%), poor to fair yields (37–62%), and fair to very good yields (60–87%), respectively (Scheme 88). In this work, the research group focused on establishing the relationship between enamines and vinyl azides. It was found that an electron-withdrawing-group-generable radical induced enamination of vinyl azides, which resulted in different types of β-functionalized N-unprotected enamines. The research team eventually found this goal using acyl, nitro, and sulfonyl radicals, thereby providing a general way to access different types of β-functionalized N-unprotected enamines.101
To evaluate the scalability of this strategy, Bi and coworkers further transformed the β-functionalized primary enamines to many bioactive molecules as useful building blocks according to Scheme 89.101
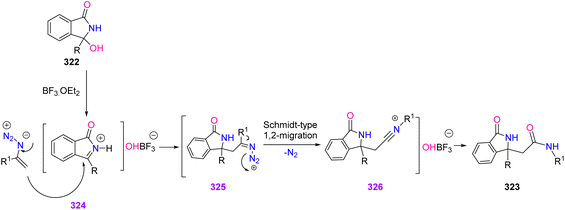
In 2019, Singh et al. developed a novel method for the synthesis of 3-oxoisoindoline-1-acetamides 323 (Scheme 90). This reaction progressed at ambient temperature with a broad range of 3-hydroxy isoindole-1-ones and vinyl azides and resulted in the production of 3-oxoisoindoline-1-actamideds in fair to excellent yields (67–97%).102 In modern pharmaceuticals and biologically active compounds, the amide functionality is omnipresent. It has also been found that small organic molecules containing methylene amide linkages are in many bioactive compounds, drug leads, and chemical probes.103,104 On the other hand, isoindolinones are the key structural unit present in numerous synthetically useful or naturally bioactive compounds, as shown in Fig. 8.105
In the mechanism of this reaction, N-acyl iminium salt intermediate 324 was first produced from 3-aryl-3-hydroxyisoindolineones, when BF3·Et2O was in the reaction vessel. The nucleophilic addition of vinyl azides to intermediate 324 generated iminodiazonium intermediate 325. Then, intermediate 325 underwent Schmidt-type 1,2-migration, with the release of dinitrogen, to generate nitrilium ion intermediate 326. Eventually, hydrolysis of this intermediate 326 gave product 323 (Scheme 91).102
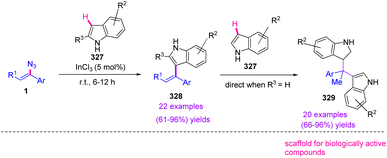
In 2020, Szpilman et al. reported the indium(III)-catalyzed reaction of indole 327 and vinyl azides 1, which led to the production of derivatives of 328 in fair to excellent yields (61–96%). In this strategy, another indole also reacted with 328 and produced 329 in fair to excellent yields (66–96%) (Scheme 92). This is the first displacement of the azide group by a carbon nucleophile while keeping the vinyl part. Extraordinarily, the substitution of azide on the sp2 carbon with the retention of the alkene system has not yet been disclosed. It would also be an attractive type of reactivity and would pioneer many new possibilities in chemistry.106
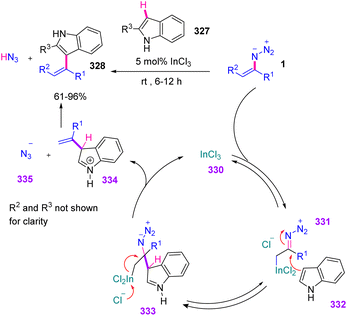
In such a process, it would be necessary to reversibly attach an electrophilic catalyst, e.g., a Lewis acid, at the α-position of vinyl azides 1, generating an electrophilic diazoimine species 331. The initial step would allow a nucleophile-like indole to attack the azide-bearing carbon in 331 with the generation of 333. E2 elimination of indium trichloride and azide anion 335 would lead to the generation of charged species 334 and close the catalytic cycle. This step would likely be irreversible. In the final step, the azide anion would abstract a proton from 334 to generate the product vinyl indole 328 and hydrazoic acid (Scheme 93).106
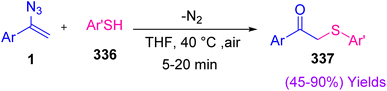
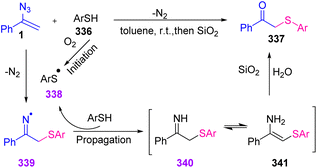
In 2021, Dong Xu et al. reported an efficient synthesis of β-keto sulfides 337 through an aryl-thiol azide coupling reaction (Scheme 94). Diverse β-keto sulfides were produced through this method in poor to excellent yields (45–90%). Thiyl radicals have been well suited and broadly used in organic synthesis, but the reaction of vinyl azide with thiyl radical is rare.107
For the first time in 1997, Montevecchi and coworkers studied the reaction of thiols 336 and vinyl azides 1. They found that the reaction of α-phenyl vinyl azides with aryl thiols 336 was fast, producing β-keto sulfides 337 almost quantitatively. In this study, the authors proposed a radical-chain mechanism. First, aryl thiyl radical 338, produced through the spontaneous oxidation of aryl thiol by oxygen, added to β-vinylic carbon of α-phenyl vinyl azides with subsequent nitrogen extrusion to generate β-sulfanyliminyl radical 339. Hydrogen abstraction from the next aryl thiol reproduced thiyl radical 338 and generated intermediate imine 340 and its tautomer 341 that were both hydrolyzed to desired product 337 (Scheme 95).107
 | ||
| Scheme 95 Mechanism for the synthesis of β-keto sulfides 337 through an aryl–thiol azide coupling reaction. | ||
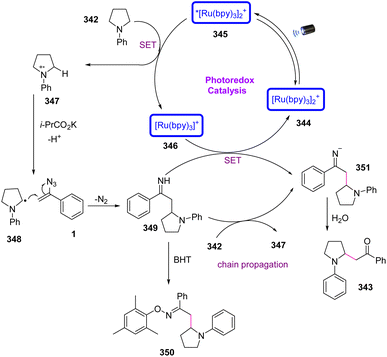
In 2019, Fie Xu et al. developed an efficient method to obtain cyclic β-amino ketones 343 via visible-light photo-redox catalysis in poor to very good yields (18–82%).108 β-Amino ketones are versatile synthetic building blocks in organic chemistry that can be converted into a range of beneficial and valuable derivatives containing β-amino-alcohols. β-Amino carbonyl derivatives are attractive as key synthetic intermediates of a wide range of drugs and biologically active natural products (Scheme 96).109
To illustrate the mechanism of this reaction, the single-electron transfer from the amine to visible-light-excited photocatalyst *Ru(bpy)32+ 345 (*RuII/RuI = 0.84 V) started the first catalytic cycle and prepared electron-rich Ru(bpy)3+ 346 and amine radical cation 347. Then, the amine radical cation 347 reacting with the base generated α-amino radical 348. The C–H bonds adjacent to the nitrogen atom in intermediate 347 underwent deprotonation by i-PrC O2K to generate α-amino radical 348. Radical addition of α-amino radical 348 to vinyl azide gave iminyl radical 349, which was reduced by the reductive photocatalyst to generate imine anion intermediate 351. In the final step, hydrolysis of the imine anion 351 produced β-amino ketone 343 (Scheme 97).108
 | ||
| Scheme 97 Mechanism for the synthesis of β-amino ketones 343 via visible-light photo-redox catalysis. | ||
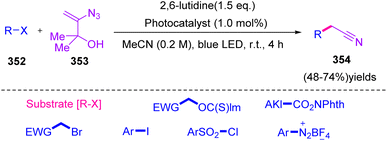
In 2019, Donald et al. proposed a novel strategy for radical cyanomethylation through vinyl azides 1 and produced diverse derivatives of 354 in fair to good yields (48–74%) (Scheme 98).110 Nitrile groups are present in the structures of many pharmaceuticals and bioactive natural products.111 Nitriles are also widely utilized as a directing group in C–H activation chemistry112 and as versatile synthetic intermediates, especially as precursor to heterocycles113 and functionalities at the carboxylic acid oxidation level.110
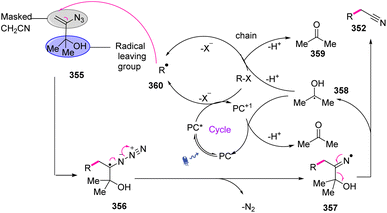
3-Azido-2-methylbut-3-en-2-ol 353 was considered an appropriate tool to achieve the cyanomethylation of radicals due to it encompassing two key design elements: (I) vinyl azides 1 that can act as masked cyanomethyl groups, and (II) dimethyl carbinol as a latent radical leaving group. With radical generation from a substrate through the oxidative quenching of an excited-state photo-redox catalyst (PC* → PC1+), it was anticipated that reagent 355 would intercept open-shell species to initiate a cascade process via radical addition to the olefin, producing adduct 356 that would release dinitrogen to produce iminyl radical 357. In the next step, fragmentation of iminyl radical 357 via α-C–C bond cleavage and ejection of the 2-hydroxypropyl radical 358 was expected to produce nitrile functionality (Scheme 99).110
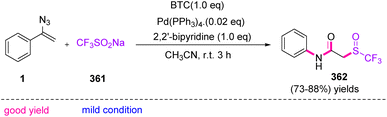
In 2022, Tang et al. synthesized N-aryl-(trifluoromethyl sulfinyl) acetamides 362 in good to very good yields (73–88%), through S-triggered Schmidt-type rearrangement of vinyl azides 1 (Scheme 100).114 The trifluoromethylthio (CF3S) functional group is very common in the structures of agrochemical115 compounds, such as fipronil, toltrazuril, triflorex, and cefazafur, and medicinal compounds, improving physio-chemical properties and pharmacokinetics, owing to their electron-negativity and excellent lipophilicity.116
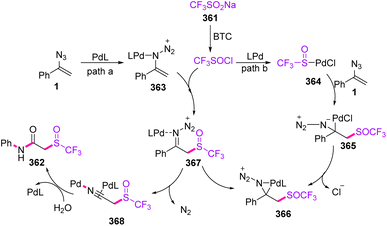
As depicted in Scheme 101, a Pd(0) catalyst first activated vinyl azides 1 to give metal complex 363 via path a. Addition of CF3SOCl, in situ generated from the reaction of CF3SO2Na with BTC, to the palladium-activated intermediate 363 happened to form the C–S bond, giving imine species 367. On the other hand, path b suggested that the PdL complex might be inserted into the CF3SO–Cl bond through oxidative addition to give intermediate 364. Then, substrate 1 coordinated to metal complex 364 for the synthesis of 365, proceeding to intramolecular migratory insertion to generate species 366. This complex 366 could be isomerized into intermediate 367. Then, intermediate 367 underwent Schmidt-type 1,2-phenyl migration together with extrusion of N2 to give nitrilium ion 368. In the final step, trace H2O in the solvent intercepted the reactive intermediate 368 to complete the process via hydration that gave the desired product 362.114
Table 3 was prepared to provide a summary of the strategies researchers have utilized to synthesize other compounds through vinyl azides, in which catalyst, solvent, temperature, yield, and MW irradiation are compared.
| Catalyst (mol%) | Solvent | Temperature | MW irradiation | Yield | Reference |
|---|---|---|---|---|---|
| Ir[(COD)Cl]2 (3 mol%) | THF | 50 °C | ✗ | 40–75% | Mukherjee79 |
| Eosin Y (1–10 mol%) | EtOH | r.t. | ✓ | 38–68% | O. Terent'ev80 |
| Taking advantage of non-catalytic amount of CAN | MeOH | r.t. | ✗ | 54–88% | O. Terent'ev81 |
| No cat. | DCE | 110 °C | ✗ | 58–88% | Fan85 |
| Mn(acac)3 (20 mol%) | NMP | 60 °C | ✗ | 65–92% | Yu87 |
| Cu(OAc)2 (15 mol%) | DMSO | 40 °C | ✗ | 72–96% | Tang91 |
| CuCl2·H2O (10 mol%) | DMSO | 80 °C | ✗ | 59–89% | Wang95 |
| I2 (50 mol%) | CH3CN | r.t. | ✗ | 57–86% | Dandela5 |
| Taking advantage of non-catalytic amount of Ag NO2 | 1,4-Dioxane | 60 °C | ✗ | 63–91% | Bi101 |
| Ag NO3 (20 mol%) | NMP | 60 °C | ✗ | 37–62% | |
| CuI (10 mol%) | CH3CN | 70 °C | ✗ | 60–87% | |
| BF3·OEt2 (20 mol%) | CH2Cl2 | r.t. | ✗ | 67–97% | Singh102 |
| InCl3 (5 mol%) | DCM | r.t. | ✗ | 61–96% | Szpilman106 |
| No cat. | THF | 40 °C | ✗ | 45–90% | Dong107 |
| Ru(bpy)(PF6)2 (3 mol%) | DMF | −10 °C | ✓ | 18–82% | Fie108 |
| Photocatalyst (1 mol%) | CH3CN | r.t. | ✓ | 48–74% | Donald110 |
| Pd(PPh3)4 (2 mol%) | CH3CN | r.t. | ✗ | 73–88% | Tang114 |
Conclusion
Vinyl azides as versatile synthons are applied for the synthesis of different types of compounds, such as cyclic, heterocyclic, and non-cyclic compounds. N-heterocycles are a group of nitrogen-containing compounds with a broad range of interesting biological and pharmaceutical applications. Due to their high and diverse reactivity, vinyl azides are promising candidates as precursors for the production of N-heterocycles. It is our belief that utilizing this three-atom synthon in new protocols for the synthesis of heterocyclic compounds and other pharmaceutical compounds will continue to progress in coming years.Conflicts of interest
There are no conflicts to declare.Notes and references
- G. Smolinsky and C. Pryde, J. Org. Chem., 1968, 33, 2411–2416 CrossRef CAS.
- J. Cen, J. Li, Y. Zhang, Z. Zhu, S. Yang and H. Jiang, Org. Lett., 2018, 20, 4434–4438 CrossRef CAS.
- K. V. Sajna and K. K. Swamy, J. Org. Chem., 2012, 77, 8712–8722 CrossRef CAS PubMed.
- Z. Li, T. Huo, L. Li, S. Feng, Q. Wang, Z. Zhang, S. Pang, Z. Zhang, P. Wang and Z. Zhang, Org. Lett., 2018, 20, 7762–7766 CrossRef CAS PubMed.
- S. Bhukta, R. Chatterjee and R. Dandela, Org. Biomol. Chem., 2022, 20, 3907–3912 RSC.
- D. J. Duarte, M. S. Miranda and J. C. Esteves da Silva, J. Phys. Chem. A, 2014, 118, 5038–5045 CrossRef CAS.
- G. L'Abbe and G. Mathys, J. Org. Chem., 1974, 39, 1778–1780 CrossRef.
- R. G. Ford, J. Mol. Spectrosc., 1977, 65, 273–279 CrossRef CAS.
- C. Lin, Y. Shen, B. Huang, Y. Liu and S. Cui, J. Org. Chem., 2017, 82, 3950–3956 CrossRef CAS PubMed.
- N. Thirupathi, C. H. Tung and Z. Xu, Adv. Synth. Catal., 2018, 360, 3585–3589 CrossRef CAS.
- G. L'abbe, Chem. Rev., 1968, 69, 345–363 CrossRef.
- G. Favini and R. Todeschini, J. Mol. Struct., 1978, 50, 191–193 CrossRef CAS.
- R. R. Donthiri, Org. Biomol. Chem., 2015, 13, 10113–10116 RSC.
- R. B. Thompson, FASEB J., 2001, 15, 1671–1676 CrossRef CAS PubMed.
- A. Fürstner, Angew. Chem., Int. Ed., 2003, 42, 3582–3603 CrossRef PubMed.
- H. Nishida, A. Hasuoka, Y. Arikawa, O. Kurasawa, K. Hirase, N. Inatomi, Y. Hori, F. Sato, N. Tarui, A. Imanishi and M. Kondo, Bioorg. Med. Chem., 2012, 20, 3925–3938 CrossRef CAS PubMed.
- J. Cen, Y. Wu, J. Li, L. Huang, W. Wu, Z. Zhu, S. Yang and H. Jiang, Org. Lett., 2019, 21, 2090–2094 CrossRef CAS PubMed.
- R. Chinchilla and C. Nájera, Chem. Rev., 2007, 107, 874–922 CrossRef CAS PubMed.
- K. Huang, C. L. Sun and Z. J. Shi, Chem. Soc. Rev., 2011, 40, 2435–2452 RSC.
- W. L. Lei, K. W. Feng, T. Wang, L. Z. Wu and Q. Liu, Org. Lett., 2018, 20, 7220–7224 CrossRef CAS PubMed.
- R. H. Feling, G. O. Buchanan, T. J. Mincer, C. A. Kauffman, P. R. Jensen and W. Fenical, Angew. Chem., Int. Ed., 2003, 42, 355–357 CrossRef CAS PubMed.
- G. I. Ioannou, T. Montagnon, D. Kalaitzakis, S. A. Pergantis and G. Vassilikogiannakis, ChemPhotoChem, 2018, 2, 860–864 CrossRef CAS PubMed.
- D. Kalaitzakis, M. Sofiadis, M. Triantafyllakis, K. Daskalakis and G. Vassilikogiannakis, Org. Lett., 2018, 20, 1146–1149 CrossRef CAS PubMed.
- Y. Q. Tang, J. C. Yang, L. Wang, M. Fan and L. N. Guo, Org. Lett., 2019, 21, 5178–5182 CrossRef CAS PubMed.
- Y. Wang, Z. Li, H. Zhao and Z. Zhang, Synth., 2019, 51, 3250–3258 CrossRef CAS.
- P. I. Hernández, D. Moreno, A. A. Javier, T. Torroba, R. Pérez-Tomás and R. Quesada, Chem. Commun., 2012, 48, 1556–1558 RSC.
- S. A. Parikh, H. Kantarjian, A. Schimmer, W. Walsh, E. Asatiani, K. El-Shami, E. Winton and S. Verstovsek, Clin. Lymphoma, Myeloma Leuk., 2010, 10, 285–289 CrossRef CAS PubMed.
- R. R. Donthiri, V. Pappula, N. N. K. Reddy, D. Bairagi and S. Adimurthy, J. Org. Chem., 2014, 79, 11277–11284 CrossRef CAS PubMed.
- C. Enguehard-Gueiffier and A. Gueiffier, Mini-Rev. Med. Chem., 2007, 7, 888–899 CrossRef CAS PubMed.
- K. C. Rupert, J. R. Henry, J. H. Dodd, S. A. Wadsworth, D. E. Cavender, G. C. Olini, B. Fahmy and J. J. Siekierka, Bioorg. Med. Chem. Lett., 2003, 13, 347–350 CrossRef CAS PubMed.
- F. Chen, T. Shen, Y. Cui and N. Jiao, Org. Lett., 2012, 14, 4926–4929 CrossRef CAS PubMed.
- C. T. Walsh, S. Garneau-Tsodikova and A. R. Howard-Jones, Nat. Prod. Rep., 2006, 23, 517–531 RSC.
- P. Novák, K. Müller, K. S. V. Santhanam and O. Haas, Chem. Rev., 1997, 97, 207–281 CrossRef PubMed.
- J. E. Curiel Tejeda, L. C. Irwin and M. A. Kerr, Org. Lett., 2016, 18, 4738–4741 CrossRef CAS PubMed.
- L. Jie, L. Wang, D. Xiong, Z. Yang, D. Zhao and X. Cui, J. Org. Chem., 2018, 83, 10974–10984 CrossRef CAS PubMed.
- L. Li, Z. Chen, X. Zhang and Y. Jia, Chem. Rev., 2018, 118, 3752–3832 CrossRef CAS PubMed.
- S. Chiba, Y. F. Wang, G. Lapointe and K. Narasaka, Org. Lett., 2008, 10, 313–316 CrossRef CAS PubMed.
- Z. Zhu, X. Tang, J. Li, X. Li, W. Wu, G. Deng and H. Jiang, Org. Lett., 2017, 19, 1370–1373 CrossRef CAS PubMed.
- K. J. Shaw, P. W. Erhardt, A. A. Hagedom III, C. A. Pease, W. R. Ingebretsen and J. R. Wiggins, J. Med. Chem., 1992, 35, 1267–1272 CrossRef CAS PubMed.
- M. Kozaki, A. Isoyama and K. Okada, Tetrahedron Lett., 2006, 47, 5375–5378 CrossRef CAS.
- E. J. Corey, D. H. Lee and S. Sarshar, Tetrahedron: Asymmetry, 1995, 6, 3–6 CrossRef CAS.
- D. B. Ramachary, G. S. Reddy, S. Peraka and J. Gujral, ChemCatChem, 2017, 9, 263–267 CrossRef CAS.
- T. Tsuritani, H. Mizuno, N. Nonoyama, S. Kii, A. Akao, K. Sato, N. Yasuda and T. Mase, Org. Process Res. Dev., 2009, 13, 1407–1412 CrossRef CAS.
- B. Gopalan and K. K. Balasubramanian, Click React. Org. Synth., 2016, 25–76 CAS.
- Z. Liu, H. Ji, W. Gao, G. Zhu, L. Tong, F. Lei and B. Tang, Chem. Commun., 2017, 53, 6259–6262 RSC.
- R. Cai, W. Yan, M. G. Bologna, K. de Silva, Z. Ma, H. O. Finklea, J. L. Petersen, M. Li and X. Shi, Org. Chem. Front., 2015, 2, 141–144 RSC.
- Q. Gu, H. H. Al Mamari, K. Graczyk, E. Diers and L. Ackermann, Angew. Chem., Int. Ed., 2014, 53, 3868–3871 CrossRef CAS PubMed.
- W. Wu, Q. Chen, Y. Tian, Y. Xu, Y. Huang, Y. You and Z. Weng, Org. Chem. Front., 2020, 7, 1878–1883 RSC.
- A. Sysak and B. Obmińska-Mrukowicz, Eur. J. Med. Chem., 2017, 137, 292–309 CrossRef CAS PubMed.
- C. Lamberth, J. Heterocycl. Chem., 2018, 55, 2035–2045 CrossRef CAS.
- J. Lin, W. Wu and Z. Weng, Tetrahedron Lett., 2020, 61, 152672 CrossRef CAS.
- F. Giornal, S. Pazenok, L. Rodefeld, N. Lui, J. P. Vors and F. R. Leroux, J. Fluorine Chem., 2013, 152, 2–11 CrossRef CAS.
- N. A. Meanwell, J. Med. Chem., 2011, 54, 2529–2591 CrossRef CAS PubMed.
- Z. Zhang, R. K. Kumar, G. Li, D. Wu and X. Bi, Org. Lett., 2015, 17, 6190–6193 CrossRef CAS PubMed.
- P. Zhou, Y. Huang, W. Wu, W. Yu, J. Li, Z. Zhu and H. Jiang, Org. Biomol. Chem., 2019, 17, 3424–3432 RSC.
- K. Okonogi, M. Kuno, M. Kida and S. Mitsuhashi, Antimicrob. Agents Chemother., 1981, 20, 171–175 CrossRef CAS PubMed.
- M. L. Zhu, C. M. Horbinski, M. Garzotto, D. Z. Qian, T. M. Beer and N. Kyprianou, Cancer Res., 2010, 70, 7992–8002 CrossRef CAS PubMed.
- B. Chen, S. Guo, X. Guo, G. Zhang and Y. Yu, Org. Lett., 2015, 17, 4698–4701 CrossRef CAS PubMed.
- S. Annadurai, R. Martinez, D. J. Canney, T. Eidem, P. M. Dunman and M. Abou-Gharbia, Bioorg. Med. Chem. Lett., 2012, 22, 7719–7725 CrossRef CAS.
- B. Smith, H. H. Chang, F. Medda, V. Gokhale, J. Dietrich, A. Davis, E. J. Meuillet and C. Hulme, Bioorg. Med. Chem. Lett., 2012, 22, 3567–3570 CrossRef CAS.
- S. Arutyunyan and A. Nefzi, J. Comb. Chem., 2010, 12, 315–317 CrossRef CAS.
- J. C. Yang, J. Y. Zhang, J. J. Zhang, X. H. Duan and L. N. Guo, J. Org. Chem., 2018, 83, 1598–1605 CrossRef CAS.
- B. Zhang and A. Studer, Chem. Soc. Rev., 2015, 44, 3505–3521 RSC.
- J. Wang, D. Ba, M. Yang, G. Cheng and L. Wang, J. Org. Chem., 2021, 86, 6423–6432 CrossRef CAS PubMed.
- E. Kiselev, K. Agama, Y. Pommier and M. Cushman, J. Med. Chem., 2012, 55, 1682–1697 CrossRef CAS PubMed.
- P. Schlagenhauf, M. Adamcova, L. Regep, M. T. Schaerer and H. G. Rhein, Malar. J., 2010, 9, 1–15 CrossRef PubMed.
- Z. Zhu, X. Tang, X. Li, W. Wu, G. Deng and H. Jiang, J. Org. Chem., 2016, 81, 1401–1409 CrossRef CAS PubMed.
- L. L. Mao, D. G. Zheng, X. H. Zhu, A. X. Zhou and S. D. Yang, Org. Chem. Front., 2018, 5, 232–236 RSC.
- K. Liu, S. Chen, X. G. Li and P. N. Liu, J. Org. Chem., 2016, 81, 265–270 CrossRef CAS PubMed.
- K. Muller, C. Faeh and F. Diederich, Science, 2007, 317, 1881–1886 CrossRef PubMed.
- B. Nie, W. Wu, C. Jin, Q. Ren, J. Zhang, Y. Zhang and H. Jiang, J. Org. Chem., 2022, 87, 159–171 CrossRef CAS PubMed.
- M. Yang, X. H. Meng, Z. Wang, Y. Gong and Y. L. Zhao, Org. Chem. Front., 2020, 7, 3493–3498 RSC.
- J. C. Yang, J. J. Zhang and L. N. Guo, Org. Biomol. Chem., 2016, 14, 9806–9813 RSC.
- C. S. Yeung and V. M. Dong, Chem. Rev., 2011, 111, 1215–1292 CrossRef CAS PubMed.
- U. K. Das, L. J. Shimon and D. Milstein, Chem. Commun., 2017, 53, 13133–13136 RSC.
- Y. F. Wang and S. Chiba, J. Am. Chem. Soc., 2009, 131, 12570–12572 CrossRef CAS PubMed.
- H. X. Siyang, X. Y. Ji, X. R. Wu, X. Y. Wu and P. N. Liu, Org. Lett., 2015, 17, 5220–5223 CrossRef CAS PubMed.
- G. Chianese, E. Fattorusso, O. Taglialatela-Scafati, G. Bavestrello, B. Calcinai, H. A. Dien, A. Ligresti and V. Di Marzo, Steroids, 2011, 76, 998–1002 CrossRef CAS PubMed.
- A. Chakrabarty and S. Mukherjee, Org. Lett., 2020, 22, 7752–7756 CrossRef CAS PubMed.
- O. M. Mulina, A. I. Ilovaisky, T. Opatz and A. O. Terent'ev, Tetrahedron Lett., 2021, 64, 152737 CrossRef CAS.
- S. A. Paveliev, L. S. Alimkhanova, A. V. Sergeeva and A. O. Terent'ev, Tetrahedron Lett., 2020, 61, 152533 CrossRef CAS.
- E. G. Bagryanskaya and S. R. Marque, Chem. Rev., 2014, 114, 5011–5056 CrossRef CAS PubMed.
- L. Tebben and A. Studer, Angew. Chem., Int. Ed., 2011, 50, 5034–5068 CrossRef CAS PubMed.
- R. Ciriminna and M. Pagliaro, Org. Process Res. Dev., 2010, 14, 245–251 CrossRef CAS.
- C. Gao, Q. Zhou, L. Yang, X. Zhang and X. Fan, J. Org. Chem., 2020, 85, 13710–13720 CrossRef CAS PubMed.
- A. Białas, J. Grembecka, D. Krowarsch, J. Otlewski, J. Potempa and A. Mucha, J. Med. Chem., 2006, 49, 1744–1753 CrossRef PubMed.
- P. Tang, C. Zhang, E. Chen, B. Chen, W. Chen and Y. Yu, Tetrahedron Lett., 2017, 58, 2157–2161 CrossRef CAS.
- C. Queffelec, M. Petit, P. Janvier, D. A. Knight and B. Bujoli, Chem. Rev., 2012, 112, 3777–3807 CrossRef CAS PubMed.
- P. Zhang, L. Zhang, Y. Gao, J. Xu, H. Fang, G. Tang and Y. Zhao, Chem. Commun., 2015, 51, 7839–7842 RSC.
- W. Wei and J. X. Ji, Angew. Chem., 2011, 123, 9263–9265 CrossRef.
- Z. Liu, Z. Zhang, G. Zhu, Y. Zhou, L. Yang, W. Gao, L. Tong and B. Tang, Org. Lett., 2019, 21, 7324–7328 CrossRef CAS PubMed.
- M. Müller, C. M. Orben, N. Schützenmeister, M. Schmidt, A. Leonov, U. M. Reinscheid, B. Dittrich and C. Griesinger, Angew. Chem., Int. Ed., 2013, 52, 6047–6049 CrossRef PubMed.
- J. W. Corbett, S. S. Ko, J. D. Rodgers, L. A. Gearhart, N. A. Magnus, L. T. Bacheler, S. Diamond, S. Jeffrey, R. M. Klabe, B. C. Cordova and S. Garber, J. Med. Chem., 2000, 43, 2019–2030 CrossRef CAS PubMed.
- G. M. Keating and A. Santoro, Drugs, 2009, 69, 223–240 CrossRef CAS PubMed.
- Y. Zhang, M. Luo, Y. Zhang, K. Cheng, Y. Li, C. Qi, R. Shen and H. Wang, Org. Biomol. Chem., 2022, 20, 1952–1957 RSC.
- L. Deng, X. Cao, Y. Liu and J. P. Wan, J. Org. Chem., 2019, 84, 14179–14186 CrossRef CAS PubMed.
- P. A. Vigato, V. Peruzzo and S. Tamburini, Coord. Chem. Rev., 2009, 253, 1099–1201 CrossRef CAS.
- A. G. Montalban, E. Boman, C. D. Chang, S. C. Ceide, R. Dahl, D. Dalesandro, N. G. Delaet, E. Erb, J. T. Ernst, A. Gibbs and J. Kahl, Bioorg. Med. Chem. Lett., 2010, 20, 4819–4824 CrossRef CAS PubMed.
- C. Steuer, C. Gege, W. Fischl, K. H. Heinonen, R. Bartenschlager and C. D. Klein, Bioorg. Med. Chem., 2011, 19, 4067–4074 CrossRef CAS PubMed.
- Y. Kim, A. C. G. Kankanamalage, V. C. Damalanka, P. M. Weerawarna, W. C. Groutas and K. O. Chang, Antiviral Res., 2016, 125, 84–91 CrossRef CAS PubMed.
- Y. Ning, X. F. Zhao, Y. B. Wu and X. Bi, Org. Lett., 2017, 19, 6240–6243 CrossRef CAS PubMed.
- D. Kumar Das, V. K. Kannaujiya, M. M. Sadhu, S. K. Ray and V. K. Singh, J. Org. Chem., 2019, 84, 15865–15876 CrossRef CAS PubMed.
- C. J. Gerry and S. L. Schreiber, Nat. Rev. Drug Discovery, 2018, 17, 333–352 CrossRef CAS PubMed.
- L. Arzel, D. Dubreuil, F. Dénès, V. Silvestre, M. Mathe-Allainmat and J. Lebreton, J. Org. Chem., 2016, 81, 10742–10758 CrossRef CAS PubMed.
- K. Speck and T. Magauer, Beilstein J. Org. Chem., 2013, 9, 2048–2078 CrossRef PubMed.
- A. A. More and A. M. Szpilman, Org. Lett., 2020, 22, 3759–3764 CrossRef CAS PubMed.
- Y. Wang, Y. J. Wang, X. C. Liang, M. H. Shen, H. D. Xu and D. Xu, Org. Biomol. Chem., 2021, 19, 5169–5176 RSC.
- J. T. Xu, G. Q. Xu, Z. Y. Wang and P. F. Xu, J. Org. Chem., 2019, 84, 14760–14769 CrossRef CAS PubMed.
- E. Buchdunger, U. Trinks, H. Mett, U. Regenass, M. Müller, T. Meyer, E. McGlynn, L. A. Pinna, P. Traxler and N. B. Lydon, Proc. Natl. Acad. Sci. U. S. A., 1994, 91, 2334–2338 CrossRef CAS PubMed.
- J. R. Donald and S. L Berrell, Chem. Sci., 2019, 10, 5832–5836 RSC.
- F. F. Fleming, L. Yao, P. C. Ravikumar, L. Funk and B. C. Shook, J. Med. Chem., 2010, 53, 7902–7917 CrossRef CAS PubMed.
- B. Heller and M. Hapke, Chem. Soc. Rev., 2007, 36, 1085–1094 RSC.
- P. J. Lindsay-Scott and P. T. Gallagher, Tetrahedron Lett., 2017, 58, 2629–2635 CrossRef CAS.
- Z. Liu, T. Yu, L. Li, W. Fu, X. Gan, H. Chen, W. Gao and B. Tang, Org. Chem. Front., 2022, 9, 1241–1246 RSC.
- D. O'Hagan, Chem. Soc. Rev., 2008, 37, 308–319 RSC.
- F. Leroux, P. Jeschke and M. Schlosser, Chem. Rev., 2005, 105, 827–856 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |