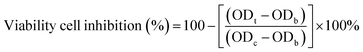Open Access Article
Open Access ArticleN,2,6-Trisubstituted 1H-benzimidazole derivatives as a new scaffold of antimicrobial and anticancer agents: design, synthesis, in vitro evaluation, and in silico studies†
Em Canh Pham *a,
Tuong Vi Le Thibh,
Huong Ha Ly Hongc,
Bich Ngoc Vo Thic,
Long B. Vong*de,
Thao Thanh Vuf,
Duy Duc Vog,
Ngoc Vi Tran Nguyenh,
Khanh Nguyen Bao Le*h and
Tuyen Ngoc Truong
*a,
Tuong Vi Le Thibh,
Huong Ha Ly Hongc,
Bich Ngoc Vo Thic,
Long B. Vong*de,
Thao Thanh Vuf,
Duy Duc Vog,
Ngoc Vi Tran Nguyenh,
Khanh Nguyen Bao Le*h and
Tuyen Ngoc Truong *h
*h
aDepartment of Medicinal Chemistry, Faculty of Pharmacy, Hong Bang International University, 700000 Ho Chi Minh City, Vietnam. E-mail: canhem112009@gmail.com; empc@hiu.vn
bDepartment of Pharmacology – Clinical Pharmacy, Faculty of Pharmacy, City Children's Hospital, 700000 Ho Chi Minh City, Vietnam
cDepartment of Pharmacognosy & Botany, Faculty of Pharmacy, Hong Bang International University, 700000 Ho Chi Minh City, Vietnam
dSchool of Biomedical Engineering, International University, 700000 Ho Chi Minh City, Vietnam. E-mail: vblong@hcmiu.edu.vn
eVietnam National University Ho Chi Minh City (VNU-HCM), Ho Chi Minh 700000, Vietnam
fDepartment of Microbiology – Parasitology, Faculty of Pharmacy, University of Medicine and Pharmacy at Ho Chi Minh City, 700000 Ho Chi Minh City, Vietnam
gUppsala University, Sweden, Tra Vinh University, Vietnam
hDepartment of Organic Chemistry, Faculty of Pharmacy, University of Medicine and Pharmacy at Ho Chi Minh City, 700000 Ho Chi Minh City, Vietnam. E-mail: lnbkhanh@ump.edu.vn; truongtuyen@ump.edu.vn
First published on 23rd December 2022
Abstract
Compounds containing benzimidazole moiety occupy privileged chemical space for discovering new bioactive substances. In continuation of our recent work, 69 benzimidazole derivatives were designed and synthesized with good to excellent yields of 46–99% using efficient synthesis protocol i.e. sodium metabisulfite catalyzed condensation of aromatic aldehydes with o-phenylenediamines to form 2-arylbenzimidazole derivatives followed by N-alkylation by conventional heating or microwave irradiation for diversification. Potent antibacterial compounds against MSSA and MRSA were discovered such as benzimidazole compounds 3k (2-(4-nitrophenyl), N-benzyl), 3l (2-(4-chlorophenyl), N-(4-chlorobenzyl)), 4c (2-(4-chlorophenyl), 6-methyl, N-benzyl), 4g (2-(4-nitrophenyl), 6-methyl, N-benzyl), and 4j (2-(4-nitrophenyl), 6-methyl, N-(4-chlorobenzyl)) with MIC of 4–16 μg mL−1. In addition, compound 4c showed good antimicrobial activities (MIC = 16 μg mL−1) against the bacteria strains Escherichia coli and Streptococcus faecalis. Moreover, compounds 3k, 3l, 4c, 4g, and 4j have been found to kill HepG2, MDA-MB-231, MCF7, RMS, and C26 cancer cells with low μM IC50 (2.39–10.95). These compounds showed comparable drug-like properties as ciprofloxacin, fluconazole, and paclitaxel in computational ADMET profiling. Finally, docking studies were used to assess potential protein targets responsible for their biological activities. Especially, we found that DHFR is a promising target both in silico and in vitro with compound 4c having IC50 of 2.35 μM.
1. Introduction
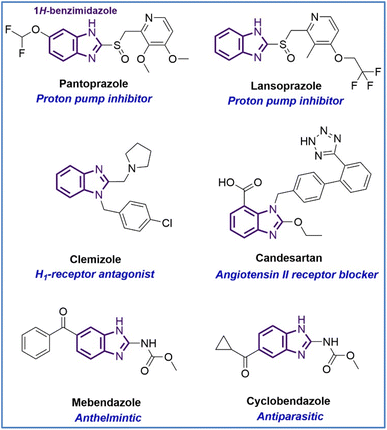
Heterocyclic compounds, which are present in a large number of biologically active synthetic and natural substances including many drugs, are of interest to pharmaceutical chemists for designing new potential bioactive compounds with a wide range of biological activities.1,2 Benzimidazole is a naturally occurring bicyclic compound consisting of fused benzene and imidazole ring and is an integral part of the structure of vitamin B12. Moreover, benzimidazole derivatives have showed anticancer,1,3–5 antimicrobial,4,6–8 anti-inflammation,9 antiviral,10 antihypertensive,11 antihistamine,12 antitubercular,13 antiulcer,14 analgesic,15 anthelmintic,16 antiprotozoal,17 antiamoebic,18 anticonvulsant,19 antiparasitic.20 In addition, benzimidazole scaffold presents in core structure of a vast list of important drugs such as antiulcer (omeprazole, lansoprazole, rabeprazole, pantoprazole), antihistamines (astemizole, clemizole, and emedastine), antihypertensives (telmisartan, candesartan, and azilsartan), anthelmintics (thiabendazole, parbendazole, mebendazole, albendazole, cambendazole, and flubendazole), antiviral (maribavir), antiparasitic (cyclobendazole, luxabendazole, and cambendazole), antidiabetic (rivoglitazone), analgesic (clonitrazene), especially antifungal (systemic fungicide, e.g. benomyl) and anticancer (antimitotic agent, e.g. nocodazole, PARP inhibitor, e.g. veliparib).21 Furthermore, the potency of drugs like carbendazim,22 and dovitinib containing benzimidazole moiety has been recognized against various types of cancer cell lines (Fig. 1).231H-Benzimidazole structures with different substituents at positions C-2 and C-5/6 can be synthesized by different methods. However, the most efficient syntheses are the condensation of o-phenylenediamines with carboxylic acids (or their derivatives such as nitriles, chlorides, and orthoesters) in the presence of an acid or with aldehydes using sodium metabisulfite (Na2S2O5).1,5 In addition, N-1 substituent 1H-benzimidazole derivatives can be introduced by N-alkylation with substituted halides in the presence of a base.24 Our study highlights the use of the green and environmentally-friendly chemical method as using microwaves in the whole synthesis process of 1H-benzimidazole derivatives.
Rationale and structure-based design of new antimicrobial and anticancer agents: Structure–activity relationship studies of the benzimidazole ring system suggested that the N-1, C-2, and C-6 positions are important for biological activities.25,26 Especially, the N-1 position can increase anticancer activity when attached to different substituents, for example, benzyl groups similar to clemizole and candesartan drugs. As part of our ongoing research, we were interested in designing N-substituted benzimidazoles which were presented in many biologically active compounds.24,27 Our designed derivatives and Dovitinib anticancer drug, Benomyl antifungal drug, and antibacterial derivatives of Dokla et al., 2020 (MIC on E. coli strain of 2 μg mL−1) share three common essential structural features: a planar benzimidazole moiety, C-2 aromatic substitution, and N-1 substitution.28 Moreover, the C-6 position with different substituents such as –H and –CH3 were designed in order to examine their effects on antimicrobial and anticancer activities (Fig. 2).
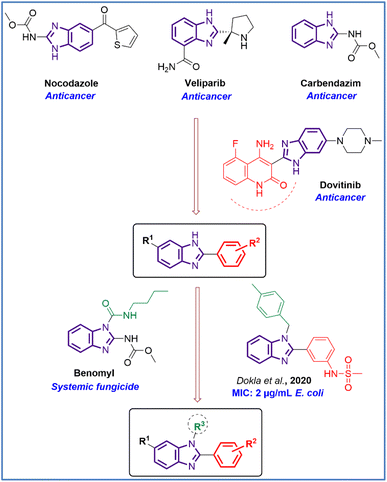 | ||
| Fig. 2 Rational study design of N,2,6-trisubstituted 1H-benzimidazole derivatives (MIC – minimal inhibitory concentration). | ||
Mechanistically, one pharmacological activity can be linked to one or more different receptors.2,29 A receptor may also be involved in different biological activities. Furthermore, the mechanism of action on the cell membrane and the inhibition of important enzymes present in both microbial and cancer cells may confer dual antibacterial, antifungal, and antitumor effects. A good example is dihydrofolate reductase (DHFR) which is a potential receptor for both antitumor and antimicrobial activities.21,30 This could be due to the similarity of DHFR from bacteria, fungi, and the cancer cell line. Therefore, the in silico studies were the potential approach to confirm the ligand–target interaction in many different receptors. In recent years there has been significant progress to improve the receptor flexibility in docking,31–33 in silico studies are able to rank the compound potency or precisely predict the target after having experimental in vitro results.
The development of antibiotic resistance in microorganisms, as well as cancer resistance, has resulted in research and development in search of new antibiotics and anticancer drugs to maintain an effective drug supply at all times. It is important to find out newer, safer, and more effective antibiotics and anticancer drugs with multiple effects, especially showing good anticancer and anti-microbial activities. This is very beneficial for cancer patients due to their weakened immunity and susceptibility to microbial attack. Therefore, the purpose of this study is to synthesize novel N,2,6-trisubstituted 1H-benzimidazole derivatives with various substituents at positions N-1, C-2, and C-6 and evaluation of their antibacterial, antifungal, and anticancer activities in continuation of our recent study.4
2. Results and discussion
2.1. Chemistry
The benzene-1,2-diamine derivatives with a 4-H or 4-CH3 group are the starting material for the preparation of N,2,6-trisubstituted 1H-benzimidazole derivatives. The process of synthetic research consists of two steps (Scheme 1). Firstly, a series of 2,6-disubstituted 1H-benzimidazole derivatives (1a–1w and 2a–2w) have been synthesized by condensing benzene-1,2-diamine derivatives with substituted aromatic aldehydes using conventional heating and microwave-assisted methods. Forty-six derivatives have been synthesized in good to excellent yields with the reflux method (75 to 93%) and excellent yields with the microwave-assisted method (90 to 99%). The reaction time has been dramatically reduced, as using conventional heating the reaction is carried out in 6–12 h compared with 10–15 min heating in the microwave. In addition, the reaction yield has increased ranging between 6 to 17% with microwave assistance (Table 1). Secondly, a series of N,2,6-trisubstituted 1H-benzimidazole derivatives (3a–3l and 4a–4k) have been synthesized by reacting 2,6-disubstituted 1H-benzimidazole derivatives with substituted halides using conventional heating and microwave-assisted methods. Compounds 3a–3l showed about 2 times higher yields than compounds 4a–4k. Twenty-three derivatives have been synthesized in moderate to good yields with the reflux method (35 to 86%) and moderate to excellent yields with the microwave-assisted method (46 to 98%). The reaction time also has been dramatically reduced, as using conventional heating the reaction is carried out in 12–24 h compared with 20–60 min heating in the microwave. Furthermore, the reaction yield has increased ranging between 3 to 20% with microwave assistance (Table 2). The synthesized compounds possess physical–chemical properties of fragments (M. Wt around 250) or lead-like (M. Wt around 350) that follow Lipinski's rules which is an excellent starting point for further development.34,35 Especially, sixteen derivatives (3b–3d, 3g, 3j, and 4a–4k) are new compounds.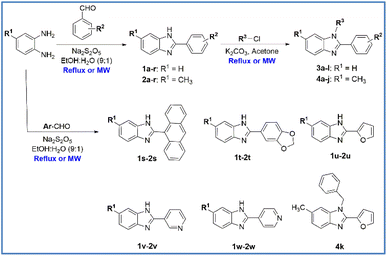 | ||
| Scheme 1 Synthesis of N,2,6-trisubstituted 1H-benzimidazole derivatives (MW: microwave irradiation, EtOH: ethanol). | ||
| Entry | R groups | Code | Physicochemical parameters | Yield | |||
|---|---|---|---|---|---|---|---|
| R1 | R2 | Re | MW | ||||
| a Re and MW – yields of conventional heating (or reflux) and microwave-assisted method (%), Re – reflux, MW – microwave, M. Wt – molecular weight, NHA – number of hydrogen bond acceptor, NHD – number of hydrogen bond donor, NRB – number rotatable bond, PSA – polar surface area (Angstroms squared). | |||||||
| 1 | 6-H | 2-Cl | 1a | M. Wt: 228.68 NHA: 1 NHD: 1 | NRB: 1, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 3.39 TPSA: 28.68 P: 3.39 TPSA: 28.68 |
81 | 94 |
| 2 | 6-H | 4-Cl | 1b | M. Wt: 228.68, NHA: 1, NHD: 1 | NRB: 1, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 3.48, TPSA: 28.68 P: 3.48, TPSA: 28.68 |
75 | 90 |
| 3 | 6-H | 2,4-Cl2 | 1c | M. Wt: 263.12, NHA: 1, NHD: 1 | NRB: 1, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 3.95, TPSA: 28.68 P: 3.95, TPSA: 28.68 |
80 | 92 |
| 4 | 6-H | 3,4-Cl2 | 1d | M. Wt: 263.12, NHA: 1, NHD: 1 | NRB: 1, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 3.99, TPSA: 28.68 P: 3.99, TPSA: 28.68 |
87 | 95 |
| 5 | 6-H | 2-Cl, 6-F | 1e | M. Wt: 246.67, NHA: 2, NHD: 1 | NRB: 1, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 3.75, TPSA: 28.68 P: 3.75, TPSA: 28.68 |
82 | 98 |
| 6 | 6-H | 3,4-(OCH3)2 | 1f | M. Wt: 254.28, NHA: 3, NHD: 1 | NRB: 3, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 2.88, TPSA: 47.14 P: 2.88, TPSA: 47.14 |
77 | 91 |
| 7 | 6-H | 4-OC2H5 | 1g | M. Wt: 238.28, NHA: 2, NHD: 1 | NRB: 3, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 3.23, TPSA: 37.91 P: 3.23, TPSA: 37.91 |
78 | 90 |
| 8 | 6-H | 3-OC2H5, 4-OH | 1h | M. Wt: 254.28, NHA: 3, NHD: 2 | NRB: 3, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 2.87, TPSA: 58.14 P: 2.87, TPSA: 58.14 |
83 | 92 |
| 9 | 6-H | 4-F | 1i | M. Wt: 212.22, NHA: 2, NHD: 1 | NRB: 1, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 3.25, TPSA: 28.68 P: 3.25, TPSA: 28.68 |
89 | 98 |
| 10 | 6-H | 2-OH | 1j | M. Wt: 210.23, NHA: 2, NHD: 2 | NRB: 1, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 2.60, TPSA: 48.91 P: 2.60, TPSA: 48.91 |
79 | 90 |
| 11 | 6-H | 2-OH, 5-Br | 1k | M. Wt: 289.13, NHA: 2, NHD: 2 | NRB: 1, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 3.16, TPSA: 48.91 P: 3.16, TPSA: 48.91 |
80 | 97 |
| 12 | 6-H | 3-OH | 1l | M. Wt: 210.23, NHA: 2, NHD: 2 | NRB: 1, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 2.53, TPSA: 48.91 P: 2.53, TPSA: 48.91 |
85 | 98 |
| 13 | 6-H | 3-OH, 4-OCH3 | 1m | M. Wt: 240.26, NHA: 3, NHD: 2 | NRB: 2, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 2.53, TPSA: 58.14 P: 2.53, TPSA: 58.14 |
87 | 98 |
| 14 | 6-H | 3-OCH3 | 1n | M. Wt: 224.26, NHA: 2, NHD: 1 | NRB: 2, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 2.94, TPSA: 37.91 P: 2.94, TPSA: 37.91 |
80 | 94 |
| 15 | 6-H | 4-SCH3 | 1o | M. Wt: 240.32, NHA: 1, NHD: 1 | NRB: 2, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 3.49, TPSA: 53.98 P: 3.49, TPSA: 53.98 |
76 | 91 |
| 16 | 6-H | 3-NO2 | 1p | M. Wt: 239.23, NHA: 3, NHD: 1 | NRB: 2, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 2.31, TPSA: 74.50 P: 2.31, TPSA: 74.50 |
84 | 94 |
| 17 | 6-H | 4-NO2 | 1q | M. Wt: 239.23, NHA: 3, NHD: 1 | NRB: 2, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 2.31, TPSA: 74.50 P: 2.31, TPSA: 74.50 |
93 | 99 |
| 18 | 6-H | 4-N(CH3)2 | 1r | M. Wt: 237.30, NHA: 1, NHD: 1 | NRB: 2, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 2.94, TPSA: 31.92 P: 2.94, TPSA: 31.92 |
80 | 90 |
| 19 | 6-H | 1s | M. Wt: 294.35, NHA: 1, NHD: 1 | NRB: 1, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 4.69, TPSA: 28.68 P: 4.69, TPSA: 28.68 |
86 | 97 | |
| 20 | 6-H | 1t | M. Wt: 238.24, NHA: 3, NHD: 1 | NRB: 1, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 2.76, TPSA: 47.14 P: 2.76, TPSA: 47.14 |
85 | 98 | |
| 21 | 6-H | 1u | M. Wt: 184.19, NHA: 2, NHD: 1 | NRB: 1, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 2.28, TPSA: 41.82 P: 2.28, TPSA: 41.82 |
81 | 96 | |
| 22 | 6-H | 1v | M. Wt: 195.22, NHA: 2, NHD: 1 | NRB: 1, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 2.20, TPSA: 41.57 P: 2.20, TPSA: 41.57 |
79 | 91 | |
| 23 | 6-H | 1w | M. Wt: 195.22, NHA: 2, NHD: 1 | NRB: 1, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 2.19, TPSA: 41.57 P: 2.19, TPSA: 41.57 |
77 | 90 | |
| 24 | 6-CH3 | 2-Cl | 2a | M. Wt: 242.70, NHA: 1, NHD: 1 | NRB: 1, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 3.77, TPSA: 28.68 P: 3.77, TPSA: 28.68 |
93 | 99 |
| 25 | 6-CH3 | 4-Cl | 2b | M. Wt: 242.70, NHA: 1, NHD: 1 | NRB: 1, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 3.82, TPSA: 28.68 P: 3.82, TPSA: 28.68 |
87 | 97 |
| 26 | 6-CH3 | 2,4-Cl2 | 2c | M. Wt: 277.15, NHA: 1, NHD: 1 | NRB: 1, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 4.27, TPSA: 28.68 P: 4.27, TPSA: 28.68 |
85 | 91 |
| 27 | 6-CH3 | 3,4-Cl2 | 2d | M. Wt: 277.15, NHA: 1, NHD: 1 | NRB: 1, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 4.31, TPSA: 28.68 P: 4.31, TPSA: 28.68 |
84 | 90 |
| 28 | 6-CH3 | 2-Cl, 6-F | 2e | M. Wt: 260.69, NHA: 2, NHD: 1 | NRB: 1, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 4.12, TPSA: 28.68 P: 4.12, TPSA: 28.68 |
83 | 92 |
| 29 | 6-CH3 | 3,4-(OCH3)2 | 2f | M. Wt: 268.31, NHA: 3, NHD: 1 | NRB: 3, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 3.21, TPSA: 47.14 P: 3.21, TPSA: 47.14 |
75 | 90 |
| 30 | 6-CH3 | 4-OC2H5 | 2g | M. Wt: 252.31, NHA: 2, NHD: 1 | NRB: 3, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 3.61, TPSA: 37.91 P: 3.61, TPSA: 37.91 |
83 | 90 |
| 31 | 6-CH3 | 3-OC2H5, 4-OH | 2h | M. Wt: 268.31, NHA: 3, NHD: 2 | NRB: 3, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 3.22, TPSA: 58.14 P: 3.22, TPSA: 58.14 |
78 | 91 |
| 32 | 6-CH3 | 4 F | 2i | M. Wt: 226.25, NHA: 2, NHD: 1 | NRB: 1, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 3.59, TPSA: 28.68 P: 3.59, TPSA: 28.68 |
82 | 93 |
| 33 | 6-CH3 | 2-OH | 2j | M. Wt: 224.26, NHA: 2, NHD: 2 | NRB: 1, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 2.93, TPSA: 48.91 P: 2.93, TPSA: 48.91 |
85 | 94 |
| 34 | 6-CH3 | 2-OH, 5-Br | 2k | M. Wt: 303.15, NHA: 2, NHD: 2 | NRB: 1, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 3.55, TPSA: 48.91 P: 3.55, TPSA: 48.91 |
86 | 95 |
| 35 | 6-CH3 | 3-OH | 2l | M. Wt: 224.26, NHA: 2, NHD: 2 | NRB: 1, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 2.87, TPSA: 48.91 P: 2.87, TPSA: 48.91 |
85 | 95 |
| 36 | 6-CH3 | 3-OH, 4-OCH3 | 2m | M. Wt: 254.28, NHA: 3, NHD: 2 | NRB: 2, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 2.84, TPSA: 58.14 P: 2.84, TPSA: 58.14 |
78 | 92 |
| 37 | 6-CH3 | 3-OCH3 | 2n | M. Wt: 238.28, NHA: 2, NHD: 1 | NRB: 2, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 3.27, TPSA: 37.91 P: 3.27, TPSA: 37.91 |
76 | 90 |
| 38 | 6-CH3 | 4-SCH3 | 2o | M. Wt: 254.35, NHA: 1, NHD: 1 | NRB: 2, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 3.80, TPSA: 53.98 P: 3.80, TPSA: 53.98 |
75 | 90 |
| 39 | 6-CH3 | 3-NO2 | 2p | M. Wt: 253.26, NHA: 3, NHD: 1 | NRB: 2, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 2.65, TPSA: 74.50 P: 2.65, TPSA: 74.50 |
88 | 94 |
| 40 | 6-CH3 | 4-NO2 | 2q | M. Wt: 253.26, NHA: 3, NHD: 1 | NRB: 2, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 2.65, TPSA: 74.50 P: 2.65, TPSA: 74.50 |
90 | 98 |
| 41 | 6-CH3 | 4-N(CH3)2 | 2r | M. Wt: 251.33, NHA: 1, NHD: 1 | NRB: 2, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 3.31, TPSA: 31.92 P: 3.31, TPSA: 31.92 |
77 | 91 |
| 42 | 6-CH3 | 2s | M. Wt: 308.38, NHA: 1, NHD: 1 | NRB: 1, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 5.01, TPSA: 28.68 P: 5.01, TPSA: 28.68 |
81 | 92 | |
| 43 | 6-CH3 | 2t | M. Wt: 252.27, NHA: 3, NHD: 1 | NRB: 1, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 3.09, TPSA: 47.14 P: 3.09, TPSA: 47.14 |
83 | 95 | |
| 44 | 6-CH3 | 2u | M. Wt: 198.22, NHA: 2, NHD: 1 | NRB: 1, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 2.62, TPSA: 41.82 P: 2.62, TPSA: 41.82 |
75 | 90 | |
| 45 | 6-CH3 | 2v | M. Wt: 209.25, NHA: 2, NHD: 1 | NRB: 1, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 2.50, TPSA: 41.57 P: 2.50, TPSA: 41.57 |
77 | 90 | |
| 46 | 6-CH3 | 2w | M. Wt: 209.25, NHA: 2, NHD: 1 | NRB: 1, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 2.52, TPSA: 41.57 P: 2.52, TPSA: 41.57 |
87 | 98 | |
| Entry | R groups | Code | Physicochemical parameters | Yield | ||||
|---|---|---|---|---|---|---|---|---|
| R1 | R2 | R3 | Re | MW | ||||
| a Re and MW – yields of conventional heating (or reflux) and microwave-assisted method (%), Re – reflux, MW – microwave, M. Wt – molecular weight, NHA – number of hydrogen bond acceptor, NHD – number of hydrogen bond donor, NRB – number rotatable bond, PSA – polar surface area (Angstroms squared). | ||||||||
| 1 | 6-H | 4-Cl | Allyl | 3a | M. Wt: 268.74 NHA: 1 NHD: 0 | NRB: 3, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 4.05 TPSA: 17.82 P: 4.05 TPSA: 17.82 |
82 | 98 |
| 2 | 6-H | 3,4-Cl2 | Allyl | 3b | M. Wt: 303.19, NHA: 1, NHD: 0 | NRB: 3, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 4.56, TPSA: 17.82 P: 4.56, TPSA: 17.82 |
76 | 94 |
| 3 | 6-H | 3,4-(OCH3)2 | Allyl | 3c | M. Wt: 294.35, NHA: 3, NHD: 0 | NRB: 5, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 3.48, TPSA: 36.28 P: 3.48, TPSA: 36.28 |
72 | 92 |
| 4 | 6-H | 4-NO2 | Allyl | 3d | M. Wt: 279.29, NHA: 3, NHD: 0 | NRB: 4, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 2.90, TPSA: 63.64 P: 2.90, TPSA: 63.64 |
86 | 98 |
| 5 | 6-H | 4-Cl | Benzyl | 3e | M. Wt: 318.80, NHA: 1, NHD: 0 | NRB: 3, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 4.73, TPSA: 17.82 P: 4.73, TPSA: 17.82 |
82 | 94 |
| 6 | 6-H | 3,4-Cl2 | Benzyl | 3f | M. Wt: 353.24, NHA: 1, NHD: 0 | NRB: 3, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 5.27, TPSA: 17.82 P: 5.27, TPSA: 17.82 |
74 | 94 |
| 7 | 6-H | 3,4-(OCH3)2 | Benzyl | 3g | M. Wt: 344.41, NHA: 3, NHD: 0 | NRB: 5, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 4.16, TPSA: 36.28 P: 4.16, TPSA: 36.28 |
76 | 96 |
| 8 | 6-H | 4-OC2H5 | Benzyl | 3h | M. Wt: 328.41, NHA: 2, NHD: 0 | NRB: 5, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 4.51, TPSA: 27.05 P: 4.51, TPSA: 27.05 |
70 | 88 |
| 9 | 6-H | 4-F | Benzyl | 3i | M. Wt: 302.34, NHA: 2, NHD: 0 | NRB: 3, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 4.51, TPSA: 17.82 P: 4.51, TPSA: 17.82 |
82 | 98 |
| 10 | 6-H | 3-OCH3 | Benzyl | 3j | M. Wt: 314.38, NHA: 2, NHD: 0 | NRB: 4, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 4.21, TPSA: 27.05 P: 4.21, TPSA: 27.05 |
78 | 96 |
| 11 | 6-H | 4-NO2 | Benzyl | 3k | M. Wt: 329.35, NHA: 3, NHD: 0 | NRB: 4, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 3.59, TPSA: 63.64 P: 3.59, TPSA: 63.64 |
84 | 96 |
| 12 | 6-H | 4-Cl | 4-Chlorobenzyl | 3l | M. Wt: 353.24, NHA: 1, NHD: 0 | NRB: 3, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 5.24, TPSA: 17.82 P: 5.24, TPSA: 17.82 |
80 | 98 |
| 13 | 6-CH3 | 4-Cl | Allyl | 4a | M. Wt: 282.77, NHA: 1, NHD: 0 | NRB: 3, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 4.39, TPSA: 17.82 P: 4.39, TPSA: 17.82 |
42 | 50 |
| 14 | 6-CH3 | 4-NO2 | Allyl | 4b | M. Wt: 293.32, NHA: 3, NHD: 0 | NRB: 4, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 3.21, TPSA: 63.64 P: 3.21, TPSA: 63.64 |
41 | 47 |
| 15 | 6-CH3 | 4-Cl | Benzyl | 4c | M. Wt: 332.83, NHA: 1, NHD: 0 | NRB: 3, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 5.08, TPSA: 17.82 P: 5.08, TPSA: 17.82 |
42 | 48 |
| 16 | 6-CH3 | 3,4-Cl2 | Benzyl | 4d | M. Wt: 367.27, NHA: 1, NHD: 0 | NRB: 3, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 5.58, TPSA: 17.82 P: 5.58, TPSA: 17.82 |
45 | 49 |
| 17 | 6-CH3 | 4-F | Benzyl | 4e | M. Wt: 316.37, NHA: 2, NHD: 0 | NRB: 3, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 4.84, TPSA: 17.82 P: 4.84, TPSA: 17.82 |
43 | 46 |
| 18 | 6-CH3 | 4-SCH3 | Benzyl | 4f | M. Wt: 344.47, NHA: 1, NHD: 0 | NRB: 4, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 5.05, TPSA: 43.12 P: 5.05, TPSA: 43.12 |
44 | 50 |
| 19 | 6-CH3 | 4-NO2 | Benzyl | 4g | M. Wt: 343.38, NHA: 3, NHD: 0 | NRB: 4, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 3.89, TPSA: 63.64 P: 3.89, TPSA: 63.64 |
42 | 50 |
| 20 | 6-CH3 | 4-Cl | 2-Chlorobenzyl | 4h | M. Wt: 367.27, NHA: 1, NHD: 0 | NRB: 3, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 5.55, TPSA: 17.82 P: 5.55, TPSA: 17.82 |
43 | 48 |
| 21 | 6-CH3 | 4-NO2 | 2-Chlorobenzyl | 4i | M. Wt: 377.82, NHA: 3, NHD: 0 | NRB: 4, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 4.39, TPSA: 63.64 P: 4.39, TPSA: 63.64 |
39 | 49 |
| 22 | 6-CH3 | 4-NO2 | 4-Chlorobenzyl | 4j | M. Wt: 377.82, NHA: 3, NHD: 0 | NRB: 4, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 4.42, TPSA: 63.64 P: 4.42, TPSA: 63.64 |
35 | 46 |
| 23 | 6-CH3 | Benzyl | 4k | M. Wt: 288.34, NHA: 2, NHD: 0 | NRB: 3, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 3.82, TPSA: 30.96 P: 3.82, TPSA: 30.96 |
36 | 47 | |
Structures of synthesized compounds were assigned using IR, 1H NMR, 13C NMR, and mass spectroscopies. In IR spectra, the medium absorbance band of the aromatic ring (ν 1520–1395 cm−1 region), as well as a strong absorbance band of imine (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) of imidazole nucleus of 1H-benzimidazole derivatives (ν 1650–1510 cm−1 region), were observed. In addition, in 1H NMR spectra of compounds 1 and 2 in DMSO characteristic chemical shifts of NH protons of 1H-benzimidazole (singlet in the δ 13.35–12.30 ppm region) and aromatic protons (in the δ 9.35–6.70 ppm region) were observed. On the other hand, 1H NMR spectra of compounds 3 and 4 revealed the appearance of a singlet in the 5.80–4.85 ppm region of the –CH2–CH
N) of imidazole nucleus of 1H-benzimidazole derivatives (ν 1650–1510 cm−1 region), were observed. In addition, in 1H NMR spectra of compounds 1 and 2 in DMSO characteristic chemical shifts of NH protons of 1H-benzimidazole (singlet in the δ 13.35–12.30 ppm region) and aromatic protons (in the δ 9.35–6.70 ppm region) were observed. On the other hand, 1H NMR spectra of compounds 3 and 4 revealed the appearance of a singlet in the 5.80–4.85 ppm region of the –CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 or –CH2–Ar group. Furthermore, the C
CH2 or –CH2–Ar group. Furthermore, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N group (δ 153.5–142.5 ppm) and the –CH2–CH
N group (δ 153.5–142.5 ppm) and the –CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 or –CH2–Ar group (δ 48.0–45.5 ppm) were identified in the 13C NMR spectrum of compounds 3 and 4. Finally, mass spectra showed the molecular ion peak M (m/z) of compounds 1–4 which helped to confirm the hypothesized structure.
CH2 or –CH2–Ar group (δ 48.0–45.5 ppm) were identified in the 13C NMR spectrum of compounds 3 and 4. Finally, mass spectra showed the molecular ion peak M (m/z) of compounds 1–4 which helped to confirm the hypothesized structure.
2.2. In vitro antibacterial and antifungal activities
Antimicrobial activities (exhibited by MIC values) including antibacterial activities against two strains of Gram-negative (EC – Escherichia coli and PA – Pseudomonas aeruginosa) and three strains of Gram-positive (SF – Streptococcus faecalis, MSSA, MRSA) and antifungal activities (CA – Candida albicans and AN – Aspergillus niger) of all synthesized compounds are summarized in Tables 3 and 4. In antimicrobial evaluation, a series of 2,6-disubstituted 1H-benzimidazole derivatives were inactive against Gram-negative strain PA (MIC ≥ 1024 μg mL−1). Compounds 1a–1n, 1p–1w, 2a–2c, 2e–2n, and 2p–2w showed weak to moderate activities against 4 strains of bacteria (EC, SF, MSSA, and MRSA) and 2 strains of fungi (MIC ≥ 32 μg mL−1). Compound 1o (4-methylthio) showed good antibacterial activities against the Gram-positive strains MSSA and MRSA with MIC ranging between 16 to 32 μg mL−1 as compared to ciprofloxacin (Cipro, MIC = 8–16 μg mL−1), but showed moderate activities against the strains EC, SF, CA, and AN (MIC 64 μg mL−1). In addition, compounds 2d (3,4-dichloro) and 2o (4-methylthio) showed good antibacterial activities against the Gram-positive strains SF, MSSA, and MRSA with MIC of 16, 16, and 32 μg mL−1, respectively as compared to Cipro (MIC = 8–16 μg mL−1). However, these compounds showed moderate activities against the strains EC, CA, and AN (MIC 32–64 μg mL−1). The results suggested that the 4-methylthio group of the aromatic ring at position 2 of the 1H-benzimidazole scaffold enhanced antibacterial activities against MSSA and MRSA strains.| Entry | Code | Antibacterial | Antifungal | Anticancer | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EC | PA | SF | MSSA | MRSA | CA | AN | HepG2 | MDA-MB-231 | MCF7 | RMS | C26 | ||
| a MIC ≥ 1024 μg mL−1, ND – not determined, EC – Escherichia coli ATCC 25922, PA – Pseudomonas aeruginosa ATCC 27853, SF – Streptococcus faecalis ATCC 29212, MSSA – Methicillin-susceptible strains of Staphylococcus aureus ATCC 29213, MRSA – Methicillin-resistant strains of Staphylococcus aureus ATCC 43300, CA – Candida albicans ATCC 10321, AN – Aspergillus niger ATCC 16404, Cipro – ciprofloxacin, Flu – fluconazole, MIC (μg mL−1) ± 0.5 μg mL−1. PTX – paclitaxel, HepG2 – human hepatocyte carcinoma cell line, MDA-MB-231 – human breast adenocarcinoma cell line, MCF7 – human breast cancer cell line, RMS – human rhabdomyosarcoma cell line, C26 – colon carcinoma cell line. IC50 ± SEM (μM, SEM – standard error of the mean). The values in bold highlight the best compounds with the best MIC and IC50 values compared to positive controls. | |||||||||||||
| 1 | 1a | — | — | — | 64 | 128 | 512 | 512 | 31.50 ± 1.34 | 50.31 ± 2.52 | 69.02 ± 2.18 | 42.44 ± 1.98 | 35.81 ± 1.45 |
| 2 | 1b | — | — | — | 64 | 128 | 256 | 256 | 51.46 ± 6.27 | >100 | 67.12 ± 1.63 | >100 | 78.93 ± 2.86 |
| 3 | 1c | 128 | — | 64 | 128 | 256 | — | — | 37.50 ± 1.60 | 52.16 ± 3.02 | 55.76 ± 2.05 | 35.01 ± 2.47 | 28.90 ± 1.54 |
| 4 | 1d | 32 | — | 32 | 32 | 64 | 256 | 256 | 7.45 ± 1.72 | 9.83 ± 1.56 | 11.08 ± 1.44 | 10.41 ± 1.06 | 6.43 ± 1.35 |
| 5 | 1e | 64 | — | — | 64 | 128 | — | — | >100 | 68.95 ± 2.54 | >100 | >100 | 63.78 ± 2.67 |
| 6 | 1f | — | — | — | 128 | 256 | — | — | 44.06 ± 4.73 | 50.05 ± 2.81 | >100 | >100 | 43.59 ± 2.64 |
| 7 | 1g | — | — | — | 256 | 512 | — | — | 43.08 ± 2.97 | >100 | >100 | 25.07 ± 1.43 | >100 |
| 8 | 1h | — | — | — | 128 | 256 | 512 | 512 | 42.51 ± 2.24 | 48.26 ± 4.02 | 59.25 ± 2.65 | 29.38 ± 1.89 | 31.93 ± 2.77 |
| 9 | 1i | 256 | — | 256 | 256 | 512 | — | — | 35.82 ± 3.36 | >100 | 68.03 ± 3.14 | >100 | 26.07 ± 1.66 |
| 10 | 1j | — | — | — | 256 | 256 | — | — | 68.26 ± 3.01 | 35.94 ± 2.34 | >100 | 15.37 ± 0.97 | 50.53 ± 2.85 |
| 11 | 1k | 64 | — | 128 | 64 | 128 | 512 | 512 | 8.94 ± 1.66 | 12.83 ± 2.45 | 5.10 ± 1.43 | 7.25 ± 1.41 | 6.81 ± 1.23 |
| 12 | 1l | 64 | — | 64 | 64 | 128 | — | — | 83.02 ± 3.59 | >100 | >100 | 73.80 ± 2.55 | >100 |
| 13 | 1m | 256 | — | 64 | 128 | 256 | 256 | 512 | 64.22 ± 2.97 | 88.13 ± 2.45 | 29.67 ± 1.24 | 53.05 ± 2.07 | 66.38 ± 2.31 |
| 14 | 1n | 256 | — | — | 256 | 512 | — | — | 47.13 ± 4.69 | 25.47 ± 1.43 | 51.38 ± 2.55 | 79.05 ± 3.96 | 28.78 ± 1.95 |
| 15 | 1o | 64 | — | 64 | 16 | 32 | 64 | 64 | 40.05 ± 1.32 | 36.59 ± 1.14 | 33.18 ± 1.71 | 21.56 ± 1.19 | 37.44 ± 2.08 |
| 16 | 1p | — | — | — | 64 | 64 | 512 | 512 | 39.03 ± 3.28 | >100 | 24.41 ± 1.12 | 50.34 ± 3.81 | 42.66 ± 2.79 |
| 17 | 1q | 128 | — | 64 | 128 | 256 | 512 | 512 | 21.04 ± 2.87 | 26.89 ± 1.38 | 27.22 ± 2.35 | 23.45 ± 1.27 | 21.89 ± 2.42 |
| 18 | 1r | 64 | — | 64 | 64 | 128 | 256 | 512 | 37.49 ± 2.36 | 29.07 ± 1.66 | >100 | 50.51 ± 2.04 | 61.52 ± 3.29 |
| 19 | 1s | 64 | — | 64 | 32 | 64 | 256 | 256 | 9.79 ± 0.78 | 8.40 ± 1.13 | 13.20 ± 1.07 | 7.66 ± 1.05 | 8.15 ± 0.94 |
| 20 | 1t | — | — | — | 128 | 256 | — | — | >100 | >100 | >100 | >100 | >100 |
| 21 | 1u | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 22 | 1v | 64 | — | 64 | 128 | 256 | — | — | 70.61 ± 2.93 | >100 | >100 | 87.72 ± 3.71 | >100 |
| 23 | 1w | 128 | — | — | 128 | 256 | — | — | 87.72 ± 3.46 | >100 | >100 | 31.25 ± 2.09 | >100 |
| 24 | 2a | 64 | — | 256 | 64 | 256 | 512 | — | 95.58 ± 4.23 | 64.94 ± 2.80 | >100 | 46.49 ± 2.33 | 36.70 ± 2.11 |
| 25 | 2b | 256 | — | — | 256 | 512 | 256 | 256 | 28.91 ± 2.55 | 27.11 ± 1.48 | 25.62 ± 1.62 | 48.31 ± 2.41 | 18.47 ± 0.98 |
| 26 | 2c | 64 | — | — | 64 | 128 | — | — | 30.65 ± 1.59 | 27.47 ± 2.05 | 57.24 ± 2.13 | 29.94 ± 1.69 | 34.92 ± 1.66 |
| 27 | 2d | 32 | — | 16 | 16 | 32 | 32 | 32 | 56.74 ± 2.42 | 60.24 ± 2.70 | 50.81 ± 2.54 | 14.41 ± 1.02 | 39.36 ± 1.72 |
| 28 | 2e | 128 | — | 128 | 64 | 64 | — | — | >100 | 34.39 ± 1.53 | >100 | 45.05 ± 2.01 | >100 |
| 29 | 2f | 64 | — | 128 | 128 | 256 | — | — | 43.57 ± 1.98 | 52.57 ± 1.86 | >100 | >100 | 34.59 ± 1.75 |
| 30 | 2g | — | — | — | 128 | 256 | — | — | 27.40 ± 1.39 | 13.23 ± 0.94 | >100 | 76.22 ± 2.44 | 61.08 ± 2.94 |
| 31 | 2h | 64 | — | — | 32 | 64 | — | — | 77.11 ± 2.88 | >100 | 54.89 ± 3.60 | 51.62 ± 2.28 | 32.75 ± 1.55 |
| 32 | 2i | 64 | — | 64 | 64 | 128 | — | — | 80.35 ± 3.67 | >100 | 38.62 ± 2.25 | >100 | 19.43 ± 1.21 |
| 33 | 2j | 128 | — | 64 | 128 | 256 | 512 | 512 | 26.14 ± 1.78 | 31.85 ± 1.90 | 18.04 ± 1.63 | 6.76 ± 0.83 | 15.67 ± 2.20 |
| 34 | 2k | 64 | — | 64 | 64 | 128 | 256 | 256 | 8.93 ± 1.11 | 6.69 ± 1.67 | 4.37 ± 1.09 | 10.37 ± 1.04 | 9.75 ± 1.25 |
| 35 | 2l | 64 | — | 64 | 32 | 64 | 128 | 128 | 95.34 ± 4.16 | >100 | >100 | 19.31 ± 1.35 | 69.28 ± 2.50 |
| 36 | 2m | 64 | — | 64 | 64 | 128 | 512 | 512 | 59.76 ± 3.31 | 55.08 ± 2.44 | 23.70 ± 1.39 | 48.68 ± 2.61 | 52.47 ± 2.19 |
| 37 | 2n | 128 | — | 128 | 128 | 256 | 256 | 256 | 26.86 ± 2.73 | 15.58 ± 0.99 | 50.63 ± 2.50 | 22.34 ± 1.85 | 34.90 ± 1.89 |
| 38 | 2o | 32 | — | 16 | 16 | 32 | 64 | 64 | >100 | >100 | 45.01 ± 1.64 | 38.97 ± 1.69 | 33.21 ± 2.13 |
| 39 | 2p | 256 | — | 128 | 64 | 64 | 256 | 256 | 71.39 ± 3.18 | >100 | 19.20 ± 2.08 | 93.28 ± 2.58 | 47.94 ± 2.56 |
| 40 | 2q | 64 | — | 64 | 128 | 256 | 512 | 512 | 18.62 ± 2.29 | 17.59 ± 2.23 | 10.46 ± 1.44 | 26.22 ± 1.88 | 19.87 ± 1.15 |
| 41 | 2r | 64 | — | 64 | 64 | 128 | 256 | 256 | 35.07 ± 1.09 | 31.52 ± 2.55 | 47.69 ± 2.40 | 24.34 ± 1.65 | 58.33 ± 1.88 |
| 42 | 2s | 128 | — | 64 | 64 | 128 | — | — | 21.40 ± 1.49 | 36.78 ± 2.24 | 39.01 ± 2.31 | 26.97 ± 1.42 | 20.02 ± 1.53 |
| 43 | 2t | — | — | — | 256 | 512 | — | — | 78.95 ± 3.77 | >100 | >100 | >100 | >100 |
| 44 | 2u | 128 | — | 256 | 256 | 256 | 512 | 512 | >100 | >100 | >100 | >100 | >100 |
| 45 | 2v | 128 | — | 64 | 64 | 128 | — | — | 68.37 ± 3.47 | 89.01 ± 2.96 | 51.06 ± 4.12 | 83.64 ± 3.81 | 55.08 ± 2.78 |
| 46 | 2w | 64 | — | 64 | 64 | 128 | — | — | 52.63 ± 2.43 | 74.62 ± 2.53 | 54.65 ± 3.35 | 28.39 ± 2.17 | 47.05 ± 2.28 |
| 47 | Cipro | 16 | 16 | 8 | 8 | 16 | ND | ND | ND | ND | ND | ND | ND |
| 48 | Flu | ND | ND | ND | ND | ND | 4 | 128 | ND | ND | ND | ND | ND |
| 49 | PTX | ND | ND | ND | ND | ND | ND | ND | 4.75 ± 0.67 | 1.38 ± 0.42 | 2.35 ± 0.51 | 6.13 ± 0.83 | 3.32 ± 0.55 |
| Entry | Code | Antibacterial | Antifungal | Anticancer | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EC | PA | SF | MSSA | MRSA | CA | AN | HepG2 | MDA-MB-231 | MCF7 | RMS | C26 | ||
| a MIC ≥ 1024 μg mL−1, ND – not determined, EC – Escherichia coli ATCC 25922, PA – Pseudomonas aeruginosa ATCC 27853, SF – Streptococcus faecalis ATCC 29212, MSSA – Methicillin-susceptible strains of Staphylococcus aureus ATCC 29213, MRSA – Methicillin-resistant strains of Staphylococcus aureus ATCC 43300, CA – Candida albicans ATCC 10321, AN – Aspergillus niger ATCC 16404, Cipro – ciprofloxacin, Flu – fluconazole, MIC (μg mL−1) ± 0.5 μg mL−1. PTX – paclitaxel, HepG2 – human hepatocyte carcinoma cell line, MDA-MB-231 – human breast adenocarcinoma cell line, MCF7 – human breast cancer cell line, RMS – human rhabdomyosarcoma cell line, C26 – colon carcinoma cell line. IC50 ± SEM (μM, SEM – standard error of the mean). The values in bold highlight the best compounds with the best MIC and IC50 values compared to positive controls. | |||||||||||||
| 1 | 3a | 64 | — | 128 | 64 | 128 | 512 | 512 | 58.92 ± 3.59 | 73.14 ± 3.91 | 79.03 ± 3.30 | 67.39 ± 3.69 | 51.28 ± 3.11 |
| 2 | 3b | 64 | 512 | 64 | 64 | 64 | 256 | 256 | 66.07 ± 3.43 | 40.81 ± 2.87 | 50.03 ± 2.99 | 44.48 ± 3.03 | 47.16 ± 2.94 |
| 3 | 3c | 128 | — | 64 | 128 | 128 | 512 | 512 | 53.76 ± 3.65 | 31.24 ± 2.46 | 37.02 ± 1.95 | 50.38 ± 3.05 | 31.12 ± 2.21 |
| 4 | 3d | 64 | — | 64 | 32 | 64 | 256 | 256 | 36.84 ± 3.12 | 43.86 ± 2.82 | 34.09 ± 2.67 | 32.60 ± 2.44 | 29.13 ± 2.37 |
| 5 | 3e | 128 | — | — | 64 | 256 | — | — | 47.18 ± 4.65 | 35.01 ± 2.36 | 40.18 ± 2.70 | 31.65 ± 2.14 | 41.91 ± 2.50 |
| 6 | 3f | 64 | 256 | 128 | 8 | 16 | 64 | — | 49.91 ± 3.05 | 52.47 ± 2.09 | 62.35 ± 3.14 | 42.78 ± 2.55 | 36.94 ± 2.69 |
| 7 | 3g | 64 | 256 | 32 | 4 | 8 | — | — | 19.95 ± 3.08 | 27.08 ± 1.89 | 22.25 ± 2.01 | 18.37 ± 2.54 | 20.96 ± 2.42 |
| 8 | 3h | 64 | — | 64 | 64 | 64 | 128 | 256 | 33.76 ± 3.22 | 21.12 ± 1.61 | 40.15 ± 1.90 | 48.64 ± 2.38 | 29.03 ± 2.78 |
| 9 | 3i | — | 128 | 128 | — | — | — | — | 45.93 ± 3.19 | 34.57 ± 1.80 | 31.54 ± 2.35 | 27.96 ± 2.67 | 21.12 ± 1.74 |
| 10 | 3j | 128 | — | 64 | 256 | 256 | — | — | 40.72 ± 3.98 | 43.29 ± 2.63 | 24.76 ± 1.81 | 33.02 ± 1.99 | 28.89 ± 1.60 |
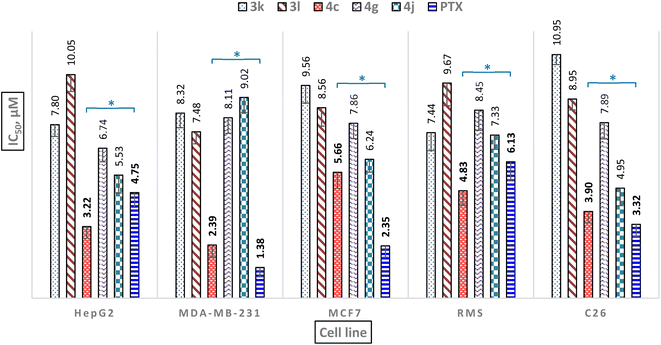
| 11 | 3k | 64 | 128 | 64 | 4 | 4 | 16 | 64 | 7.80 ± 0.53 | 8.32 ± 0.66 | 9.56 ± 0.79 | 7.44 ± 0.81 | 10.95 ± 0.45 |
| 12 | 3l | 16 | 256 | 16 | 8 | 16 | 32 | 32 | 10.05 ± 0.76 | 7.48 ± 0.54 | 8.56 ± 0.83 | 9.67 ± 1.02 | 8.95 ± 0.49 |
| 13 | 4a | 256 | — | 128 | 32 | 32 | — | — | 38.77 ± 2.35 | 30.11 ± 1.92 | 22.24 ± 2.60 | 48.81 ± 1.56 | 9.04 ± 0.88 |
| 14 | 4b | 64 | — | 64 | 32 | 64 | 512 | 512 | 63.45 ± 3.11 | >100 | 71.09 ± 2.84 | 85.97 ± 3.24 | 59.55 ± 2.74 |
| 15 | 4c | 16 | 64 | 16 | 4 | 8 | 16 | 32 | 3.22 ± 0.53 | 2.39 ± 0.54 | 5.66 ± 0.72 | 4.83 ± 0.64 | 3.90 ± 0.51 |
| 16 | 4d | 32 | — | 32 | 32 | 32 | 128 | 128 | >100 | 83.25 ± 4.43 | 80.50 ± 3.85 | 78.92 ± 2.34 | 64.45 ± 4.11 |
| 17 | 4e | 128 | — | 64 | 64 | 64 | 32 | 64 | 27.24 ± 1.48 | 28.54 ± 1.07 | 43.12 ± 2.36 | 35.05 ± 2.89 | 24.43 ± 1.78 |
| 18 | 4f | 128 | — | 128 | 128 | 256 | — | — | 17.74 ± 1.37 | 21.87 ± 2.02 | 29.01 ± 2.46 | 31.74 ± 2.33 | 19.65 ± 1.85 |
| 19 | 4g | 64 | 128 | 8 | 8 | 16 | 256 | 256 | 6.74 ± 0.61 | 8.11 ± 0.70 | 7.86 ± 0.69 | 8.45 ± 0.90 | 7.89 ± 0.73 |
| 20 | 4h | 64 | 512 | 64 | 64 | 64 | 64 | 64 | 47.88 ± 4.13 | 74.17 ± 3.63 | 59.78 ± 2.50 | 41.03 ± 2.15 | 38.49 ± 3.25 |
| 21 | 4i | 32 | 256 | 64 | 32 | 64 | 128 | 128 | 34.34 ± 3.22 | 28.46 ± 2.54 | 20.20 ± 1.93 | 17.61 ± 1.64 | 23.75 ± 2.15 |
| 22 | 4j | 64 | 256 | 64 | 8 | 8 | 32 | 64 | 5.53 ± 0.80 | 9.02 ± 0.68 | 6.24 ± 0.57 | 7.33 ± 0.64 | 4.95 ± 0.79 |
| 23 | 4k | 128 | — | 128 | 128 | 128 | 512 | 512 | 55.45 ± 3.32 | 60.51 ± 4.72 | 41.46 ± 4.04 | 39.18 ± 2.62 | 34.66 ± 2.01 |
| 24 | Cipro | 16 | 16 | 8 | 8 | 16 | ND | ND | ND | ND | ND | ND | ND |
| 25 | Flu | ND | ND | ND | ND | ND | 4 | 128 | ND | ND | ND | ND | ND |
| 26 | PTX | ND | ND | ND | ND | ND | ND | ND | 4.75 ± 0.67 | 1.38 ± 0.42 | 2.35 ± 0.51 | 6.13 ± 0.83 | 3.32 ± 0.55 |
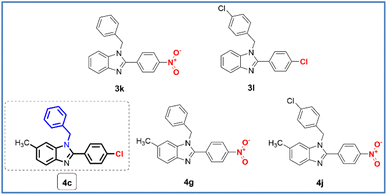
With antimicrobial activities of series of N,2,6-trisubstituted 1H-benzimidazole derivatives, compounds 3a–3e, 3h–3j, 4a, 4b, 4d–4f, 4h, 4i, and 4k showed weak to moderate activities against 5 strains of bacteria and 2 strains of fungi (MIC ≥ 32 μg mL−1). Compounds 3f (3,4-dichloro, N-benzyl), 3l (4-chloro, N-(4-chlorobenzyl)), and 4g (4-nitro, N-Benzyl) showed good antibacterial activities against the Gram-positive strains MSSA and MRSA with MIC of 8 and 16 μg mL−1, respectively. Compound 3f showed weak antimicrobial activities against strains EC, SF, CA, and AN with MIC ≥ 64 μg mL−1. Compound 3l showed good antimicrobial activities against strains EC, SF, CA, and AN with MIC ranging between 16 to 32 μg mL−1 and weak antibacterial activity against the Gram-negative strain PA with a MIC value of 256 μg mL−1. Compound 4g showed good antimicrobial activity against strain SF with MIC of 8 μg mL−1 as compared to Cipro (MIC = 8 μg mL−1) and weak antimicrobial activity against the strains EC, PA, CA, and AN with MIC ≥ 64 μg mL−1. Moreover, compounds 3g (3,4-dimethoxy, N-benzyl), 3k (4-nitro, N-benzyl), 4c (4-chloro, N-benzyl), and 4j (4-nitro, N-(4-chlorobenzyl)) exhibited the strongest activity among the synthesized compounds against the Gram-positive strains MSSA and MRSA with MIC ranging between 4 to 8 μg mL−1 as compared to Cipro. However, compounds 3g, 3k, and 4c showed weak to moderate activities against strains EC, PA, SF, CA, and AN. In contrast, compound 4c showed good antimicrobial activities against the bacteria strains EC, SF, and the fungi strain CA with the MIC value of 16 μg mL−1 as compared to ciprofloxacin and fluconazole (Flu, MIC of 4 μg mL−1), except for showed moderate antibacterial activity against Gram-negative strain PA. In particular, for antifungal activity, compound 4c also displayed promising activity against Aspergillus niger with the MIC value of 32 μg mL−1 as compared to Flu (MIC = 128 μg mL−1). From the structure–activity relationship (SAR), the presence of the N-benzyl group and the chloro/nitro group in the aromatic ring at position 2 of the 1H-benzimidazole scaffold is more desirable for enhanced antibacterial activity in 3f, 3l, 3k, 4c, and 4j, and antifungal activity in 3l and 4c (Fig. 3).
In published studies, 4-substituent 5,6-dichloro-1H-benzimidazole derivatives showed antibacterial activity against S. aureus with MIC 3.12 mg mL−1.6 Besides, the 4-nitro 1H-benzimidazole-5-carbohydrazide derivative exhibited good inhibitory activity against lanosterol 14α-demethylase (CYP51) with IC50 value at 0.19 μg mL−1 compared to fluconazole as reference IC50 value at 0.62 μg mL−1.36 In addition, the pyridin-3-yl-1H-benzimidazole-5-carboxylate derivative was found to be potent activity against Mycobacterium tuberculosis H37Rv and INH-resistant Mycobacterium tuberculosis with MIC value of 0.112 μM and 6.12 μM, respectively.37 Especially, the 6-fluoro-1H-benzimidazole derivative showed potent antibacterial activities against the Gram-positive strains MSSA (MIC of 4 μg mL−1) and MRSA (MIC of 2–8 μg mL−1).8 Two synthesized compounds 3k and 4c with 2-(4-nitro/chloro-phenyl) moiety also exhibited potent antibacterial activity with MICs of 4–8 μg mL−1 against MSSA and MSRA strains. This may be due to the structure of compound 3k with the presence of a 4-nitro group on the phenyl ring of the 1H-benzimidazole nucleus is similar to that of Morcoss et al. (2020) and the structure of compound 4c with the presence of 4-chloro group on the phenyl ring of the 1H-benzimidazole nucleus is similar to that of Tunçbilek et al. (2009) and Em et al. (2022).4,6,36 However, compounds 3k and 4c have different substituent patterns compared to our previous most potent compounds.4
2.3. Anticancer activity
Next, we assessed the anticancer activity of compounds 1a–1w, 2a–2w, 3a–3l, and 4a–4k on five cancer cell lines hepatocellular carcinoma cell line (HepG2), human breast cancer cell lines (MDA-MB-231 and MCF7), the aggressive and highly malignant rhabdomyosarcoma cell line (RMS), and colon carcinoma cell line (C26) using paclitaxel (PTX) as a non-selective positive control in MTT assay. The results are summarized in Tables 3 and 4In both series of 1H-benzimidazole derivatives, several compounds exhibited moderate (IC50 = 15.0–50.0 μM) or weak activity (IC50 > 50 μM) toward HepG2, MDA-MB-231, MCF7, RMS, and C26. Compounds 2d and 2j showed good anticancer activity with IC50 14.41 and 6.76 μM, respectively as compared to PTX (IC50 = 6.13 μM) at the RMS cell line. Compound 2g showed moderate anticancer activity against the MDA-MB-231 cell line with an IC50 value of 13.23 μM as compared to PTX (IC50 = 1.38 μM). On the other hand, compound 2q showed good anticancer activity against the MCF7 cell line with an IC50 value of 10.46 μM as compared to PTX (IC50 = 2.35 μM). Compound 4a showed good anticancer activity against the C26 cell line with an IC50 value of 9.04 μM as compared to PTX (IC50 = 3.32 μM). Particularly, nine compounds 1d (3,4-dichloro), 1k (5-bromo-2-hydroxy), 1s (Anthracen-9-yl), 2k (5-bromo-2-hydroxy), 3k (4-nitro, N-benzyl), 3l (4-chloro, N-(4-chlorobenzyl)), 4c (4-chloro, N-benzyl), 4g (4-nitro, N-benzyl), and 4j (4-nitro, N-(4-chlorobenzyl)) showed the strongest anticancer activity among the synthesized compounds against all tested cell lines with IC50 ranging between 2.39 to 13.20 μM comparable to PTX (IC50 = 1.38–6.13 μM). Moreover, compound 4c showed the strongest anticancer activity among all active compounds against HepG2, MDA-MB-231, MCF7, RMS, and C26 with IC50 of 3.22, 2.39, 5.66, 4.83, and 3.90 μM, respectively as compared to PTX. Compound 4c exhibited a weaker anticancer activity than PTX on MDA-MB-231, MCF7, and C26 cell lines, but exhibited better anticancer activity than PTX on HepG2 and RMS cell lines (Fig. 4), and especially also showed potent antimicrobial activities (Table 4). Target engagement with electron-withdrawing substituents 4-Cl and 4-NO2 on the phenyl ring, and N-phenyl and N-(4-chlorobenzyl) substituents of the 1H-benzimidazole scaffold may be responsible for its anticancer activity as compared to other compounds.
In published studies with similar structures, the 4-fluorophenyl benzoimidazolylquinazolinamine derivative showed potent activity against tyrosine-protein kinase Met (IC50 of 0.05 μM) and vascular endothelial growth factor receptor 2 (VEGFR-2, IC50 of 0.02 μM).38 Besides, the 3,5-difluorophenyl benzimidazole–oxindole conjugate derivative exhibited 43.7% and 64.8% apoptosis against MCF-7 at 1 and 2 μM, respectively.39 On the other hand, the 4-(N,N-dimethylamino)phenyl N,2,5-trisubstituted-1H-benzimidazole derivative exhibited Sirtuins inhibitory activity for SIRT1 and SIRT2 with IC50 value of 54.21 and 26.85 μM, respectively. In addition, the 3-hydroxyphenyl 6-benzoyl-1H-benzimidazole derivative exhibited good antitumor activity against human lung adenocarcinoma epithelial (A549, IC50 of 4.47 μM), human breast adenocarcinoma (MDA-MB-231, IC50 of 4.68 μM), and human prostate cancer (PC3, IC50 of 5.50 μM) cell lines.5 Cell proliferation assay demonstrated that this compound had pronounced anticancer activity against breast MDA-MB-468, colon HCT-116, and blood-leukemia CCRF-CEM cell lines.40 Moreover, the N-(3-phenylpropyl) N,2,5-trisubstituted-1H-benzimidazole derivative has been found to induce autophagy in MCF7 cells with IC50 value of 5.73 ± 0.95 μM by fluorescence microscope assays and western blot analysis.41 The 5-chloro-N-benzyl-1H-benzimidazole also exhibited to arrest MCF-7 cell growth at the G2/M and S phases with IC50 value of 7.01 ± 0.20 μM.42 Similar to reported potent compounds in literature, among our most active 2,6-disubstituted 1H-benzimidazole derivatives 3l, 4c, and 4j contain halogen substituents. This is similar to our previous most active compounds.4 Especially, compound 4c exhibited more potential antitumor activity against five different types of cancer cells when compared with the compounds of Yoon et al. (2014), Zhang et al. (2017), and Em et al. (2022).4,40,41 This may be due to the structure of 4c having the presence of a chlorine substituent (–Cl) at position 4 on the phenyl ring and the N-benzyl group on the 1H-benzimidazole scaffold.4
The development of compounds with multiple effects has been of increasing interest, especially with anticancer and antimicrobial activities. The dual-acting anticancer and antimicrobial chemotherapy agents have been published in many studies.4,43–46 Moreover, people with cancer may have a higher risk of infection due to changes in the immune system that controls their body's defenses.47 Therefore, our potential derivatives have shown to be promising agents in the development of dual therapeutic effects.
2.4. In silico ADMET profile
In this study, a computational study of all synthesized compounds was performed to determine the surface area and other physicochemical properties in the direction of Lipinski's rules (Tables 1 and 2).4,29 The five most active compounds 3k, 3l, 4c, 4g, and 4j follow all of Lipinski's rules. All the highest active derivatives have a number of hydrogen bonding acceptor groups ranging between 1 to 3, and nonhydrogen bonding donors. Also, molecular weights range between 329.35 to 377.82, and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values range between 3.59 to 5.24, and all these values agree with Lipinski's rules such as HB donor groups ≤ 5, HB acceptor groups ≤ 10, M. Wt < 500, and log
P values range between 3.59 to 5.24, and all these values agree with Lipinski's rules such as HB donor groups ≤ 5, HB acceptor groups ≤ 10, M. Wt < 500, and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P < 5.
P < 5.
Computational ADMET profiling of active compounds (Table S1†), showed that these derivatives have better intestinal absorption in humans than Cipro, Flu, and PTX. In fact, all compounds showed Caco-2 permeability higher than the control drugs while only compounds 3k and 4g showed MDCK permeability higher than the control drugs. This preference may be due to the superior lipophilic of the designed ligands, which would facilitate passage along different biological membranes.4,29 Accordingly, they may have remarkably good bioavailability after oral administration. All compounds are highly likely to be Pgp-inhibitor similar to the PTX reference drug. This is advantageous for overcoming multidrug resistance in cancer. In addition, all compounds showed high plasma protein binding. Moreover, compound 4c demonstrated a high potential to penetrate the blood–brain barrier (BBB), while Cipro and PTX are unable to do it. Therefore, compound 4c showed potential for the treatment of brain tumors compared with reference drugs.
The molecule is less skin permeant, the more negative the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kp (with Kp in cm s−1). Therefore, all active compounds (log
Kp (with Kp in cm s−1). Therefore, all active compounds (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kp in the range of −5.10 to −4.23) showed better skin permeation than Cipro (log
Kp in the range of −5.10 to −4.23) showed better skin permeation than Cipro (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kp of −9.09) and Flu (log
Kp of −9.09) and Flu (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kp of −7.92). The cytochrome enzymes could be weak to strongly inhibited under the effect of active compounds especially CYP1A2, CYP2C19, and CYP2C9, while Cipro and Flu couldn't. Compounds 3l and 4c also strongly inhibit CYP2D6, while PTX couldn't. However, all compounds did not show the effect of CYP3A4 inhibition compared with PTX.
Kp of −7.92). The cytochrome enzymes could be weak to strongly inhibited under the effect of active compounds especially CYP1A2, CYP2C19, and CYP2C9, while Cipro and Flu couldn't. Compounds 3l and 4c also strongly inhibit CYP2D6, while PTX couldn't. However, all compounds did not show the effect of CYP3A4 inhibition compared with PTX.
The CL (clearance) is a significant parameter in deciding dose intervals as a tool for the assessment of excretion. All active compounds (5.05–6.94 mL min−1 kg−1) and Flu (CL = 5.69 mL min−1 kg−1) was classified as a moderate clearance level ranging between 5 to 15 mL min−1 kg−1. In contrast, Cipro (3.21 mL min−1 kg−1) and PTX (3.42 mL min−1 kg−1) showed lower CL values and were classified as low clearance levels (CL < 5 mL min−1 kg−1).
Toxicity is the last parameter examined in the ADMET profile. As displayed in Table S1,† all the new ligands did not show H-HT (human hepatotoxicity), DILI (drug-induced liver injury), rat oral acute toxicity, and eye corrosion. In particular, the most potent compound 4c showed lower respiratory toxicity as well as the “Tox21 pathway” and “Toxicophore rules” properties better than the reference drugs.
2.5. In silico molecular docking studies
Following ADMET profiling, docking was used to assess the potential targets for the most active compounds. Based on the principle that similar compounds tend to bind to the same proteins as well as in vitro enzymes inhibition of the reported homologous benzimidazole structures, seven protein targets were chosen for docking study for the five most active compounds and reference compounds (Cipro – ciprofloxacin, Flu – fluconazole, and PTX – paclitaxel).4 Four different target proteins were selected for antimicrobial activity including dihydrofolate reductase (DHFR-F) and N-myristoyl transferase (NMT) from Candida albicans as fungal targets together with dihydrofolate reductase (DHFR-B) and gyrase B (GyrB) from Staphylococcus aureus as bacterial targets.29 Seven target proteins were selected for anticancer activity including DHFR-B, GyrB, DHFR-F, NMT, vascular endothelial growth factor receptor 2 (VEGFR-2), fibroblast growth factor receptor 1 (FGFR-1), and histone deacetylase 6 (HDAC6) whose dysregulation is linked to cancer cell proliferation. On the other hand, nine poses of each potent compound were obtained by the docking simulations with each receptor and the pose with the highest affinity (model 0) was chosen to validate the activity.Among all these seven proteins, two proteins (DHFR-B and NMT) as both antimicrobial and antitumor targets presented good binding affinity with a higher affinity than −9.5 kcal mol−1. On the other hand, two proteins (FGFR-1 and HDAC6) as antitumor targets presented good interactions with affinity in the range of −8.6 to −10.0 kcal mol−1, while VEGFR-2 showed weaker interactions with affinity in the range of −8.3 to −9.0 kcal mol−1 with active derivatives (Table 5). Here in our study, compound 4c being the most potent antimicrobial and antitumor agent displayed the highest negative affinity of −10.0 kcal mol−1 against DHFR-B, and the second negative affinity of −11.1 kcal mol−1 against NMT from S. aureus which is comparable to Cipro (DHFR-B), Flu (NMT) and PTX (DHFR-B and NMT) with the affinity of −9.1, −7.9 and (−10.0 and −11.4) kcal mol−1, respectively. Besides, this compound established one strong hydrogen bond with SER49 amino acid of DHFR-B with a bond length of 2.97 Å being similar to that of Cipro (2.20 Å) and PTX (1.87 Å). In addition, compound 4c also established one strong hydrogen bond with HIS227 amino acid of NMT with a bond length of 2.21 Å which is comparable to Flu (TYR225, 2.36 Å), and PTX (GLY213, 2.23 Å). Although no hydrogen bond was established, compound 4c showed a good affinity for FGFR1 of −9.6 kcal mol−1 compared with PTX (−10.5 kcal mol−1 which established three hydrogen bonds at ASN628, GLU486, and THR658 amino acids. Hence compound 4c is considered the best dock conformation in antimicrobial and antitumor targets.
| Compound | DHFR-B | GyrB | DHFR-F | NMT | VEGFR-2 | FGFR-1 | HDAC6 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a | b | a | b | a | b | a | b | a | b | a | b | a | b | |
| a The bacterial targets consist of DHFR-B – Dihydrofolate Reductase-Bacteria, GyrB – Gyrase B. The fungal targets consist of DHFR-F – Dihydrofolate Reductase-Fungi, NMT – N-myristoyl transferase, VEGFR-2 – vascular endothelial growth factor receptor 2, FGFR-1 – fibroblast growth factor receptor 1, HDAC6 – histone deacetylase 6. The cancer targets include all seven receptors. Cipro – Ciprofloxacin, Flu – Fluconazole, PTX – paclitaxel. a – affinity (kcal mol−1), b – hydrogen bond (number, position). | ||||||||||||||
| 3k | −9.6 | 2 ASN18, THR121 | −8.0 | 0 | −8.5 | 2 ALA11, TRP27 | −11.0 | 1 ASN392 | −8.3 | 2 ARG1027 | −9.7 | 0 | −9.4 | 2 HIS192, HIS193 |
| 3l | −9.5 | 1 SER49 | −7.9 | 0 | −8.0 | 1 GLY23 | −11.3 | 1 ASN392 | −8.6 | 0 | −9.3 | 0 | −9.1 | 1 LYS330 |
| 4c | −10.0 | 1 SER49 | −7.9 | 0 | −8.4 | 0 | −11.1 | 1 HIS227 | −8.7 | 0 | −9.6 | 0 | −8.6 | 1 HIS232 |
| 4g | −10.0 | 1 ASN18 | −8.1 | 0 | −8.8 | 1 ALA11 | −10.3 | 0 | −8.7 | 2 ARG1027 | −10.0 | 0 | −9.5 | 2 HIS192, HIS193 |
| 4j | −9.9 | 1 ASN18 | −8.0 | 0 | −8.5 | 0 | −10.6 | 0 | −9.0 | 2 ARG1027 | −9.5 | 1 ASP641 | −9.4 | 2 HIS192, HIS193 |
| Cipro | −9.1 | 1 SER49 | −7.3 | 2 ASP81, SER55 | — | — | — | — | — | — | — | — | — | — |
| Flu | — | — | — | — | −7.0 | 4 ALA115, GLU116, LYS57 | −7.9 | 1 TYR225 | — | — | — | — | — | — |
| PTX | −10.0 | 3 LEU20, SER49, THR121 | −7.8 | 5 ASN54, ARG84, GLY85, THR173 | −8.5 | 2 ARG28 | −11.4 | 1 GLY213 | −7.8 | 1 GLY1048 | −10.5 | 3 ASN628, GLU486, THR658 | −8.8 | 4 LYS330, SER150, VAL151 |
On the DHFR-B receptor, compound 3k established two hydrogen bonds (2.30–2.67 Å) with the affinity (−9.6 kcal mol−1) with ASN18, THR121 amino acids, but compounds 4g and 4j only established one hydrogen bond (2.35–2.58 Å) with the affinity (−9.9 to −10.0 kcal mol−1) with ASN18 amino acid when compared with the standard drug Cipro (−9.1 kcal mol−1) with one hydrogen bond (2.20 Å) with SER49 amino acid and PTX (−10.0 kcal mol−1) with three hydrogen bonds (1.87–3.01 Å) with LEU20, SER49, THR121 amino acids (Fig. 5). However, compound 3l (−9.5 kcal mol−1) established one hydrogen bond (2.89 Å) with SER49 amino acid similar to 4c, Cipro, and PTX. These results have demonstrated that compound 4c is the most potential in vitro antibacterial and antitumor activities.
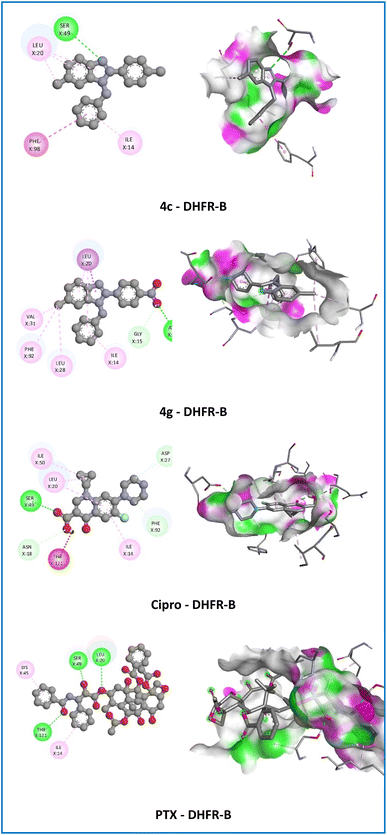 | ||
| Fig. 5 2D and 3D representation of the interaction of the active compounds (4c and 4g), ciprofloxacin (Cipro), and paclitaxel (PTX) with dihydrofolate reductase of bacteria (DHFR-B). | ||
On the GyrB receptor, all active compounds showed good interactions with affinity in the range of −7.9 to −8.1 kcal mol−1 compared with the standard drug Cipro (−7.3 kcal mol−1) and PTX (−7.8 kcal mol−1). Similarly, all active compounds also showed good interactions with affinity in the range of −8.0 to −8.8 kcal mol−1 compared with the standard drug Flu (−7.0 kcal mol−1) and PTX (−8.5 kcal mol−1) on DHFR-F receptor. However, these compounds established fewer hydrogen bonds than the standard drugs.
On the NMT receptor, compounds 3k and 3l established one hydrogen bond (2.61–2.71 Å) with good affinity (−11.0 to −11.3 kcal mol−1) with ASN392 amino acid compared with Flu (−7.9 kcal mol−1), PTX (−11.4 kcal mol−1), and 4c (−11.1 kcal mol−1) (Fig. 6). On the contrary, compounds 4g and 4j did not establish hydrogen bonds with affinity at −10.3 and −10.6 kcal mol−1, respectively.
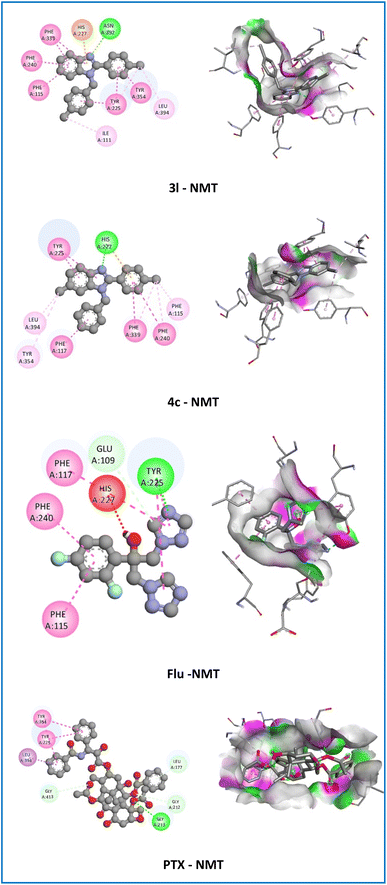 | ||
| Fig. 6 2D and 3D representation of the interaction of the active compounds (3l and 4c), fluconazole (Flu), and paclitaxel (PTX) with N-myristoyl transferase (NMT). | ||
On the VEGFR-2 receptor, all active compounds showed stronger interactions with the affinity between −8.3 and −9.0 kcal mol−1 compared with the reference drug PTX (−7.8 kcal mol−1). Compounds 3k, 4g, and 4j established one hydrogen bond (2.56–2.78 Å) with ARG1027 amino acid. Compounds 3l and 4c did not establish conventional hydrogen bonds but established carbon-hydrogen bonds with ASP1046 amino acid with bond lengths in the range of 3.13 to 3.56 Å.
On the FGFR-1 receptor, all active compounds did not establish a hydrogen bond except for 4j established one hydrogen bond (2.67 Å) with ASP641 amino acid. In addition, these compounds showed weaker interactions with the affinity between −9.3 and −10.0 kcal mol−1 compared with the reference drug PTX (−10.5 kcal mol−1). On the HDAC6 receptor, all active compounds showed stronger interactions with the affinity between −9.1 and −9.5 kcal mol−1 except for 4c when compared with reference drug PTX (−8.8 kcal mol−1). However, these compounds have formed fewer hydrogen bonds than PTX (Fig. 7). These results suggested that FGFR-1 and HDAC6 also are the most likely targets for the anticancer activity of these newly synthesized agents.
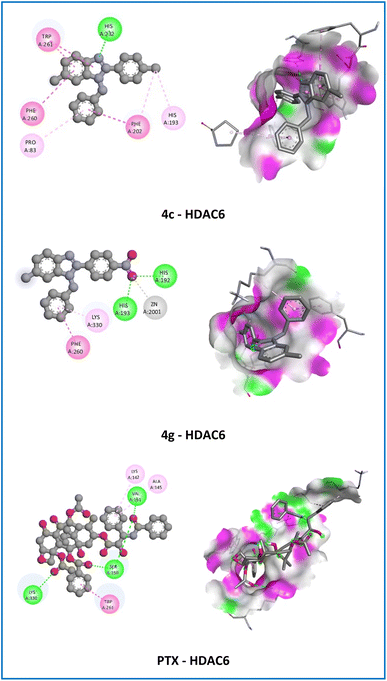 | ||
| Fig. 7 2D and 3D representation of the interaction of the active compounds (4c and 4g), and paclitaxel (PTX) with histone deacetylase 6 (HDAC6). | ||
Among all the derivatives, compound 4c showed hydrophobic interactions (π–π T-shaped, alkyl, π–alkyl) with PHE98, LEU20, and ILE14 with the crucial residue of the DHFR-B protein from S. aureus that resembles the co-crystallization ligand, Cipro, and PTX. As illustrated in Fig. 5, the 6-methyl (6-CH3) group and 1H-benzimidazole nucleus of compound 4c were engaged in the formation of alkyl and π–alkyl interactions with LEU20 amino acid with bond length in the range of 4.17–5.15 Å. Moreover, the N-benzyl group displayed π–π T-shaped interaction with the crucial residue PHE98 of the target protein with a bond length of 5.47 Å and π–alkyl interaction with ILE14 amino acid with a bond length of 4.80 Å. On the other hand, compound 4c also established electrostatic interaction (π–cation) and hydrophobic interactions (π–π stacked, π–π T-shaped, alkyl, π–alkyl) with the crucial residue of the NMT protein from Candida albicans that resembles the co-crystallization ligand, Flu, and PTX. The 6-methyl (6-CH3) group showed alkyl and π–alkyl interactions with LEU394 and TYR354 amino acids with bond lengths in the range of 4.09–5.31 Å. In addition, the substituted part of compound 4c moved inside the cavity where both the benzene ring of N-benzyl and 2-phenyl groups and the 1H-benzimidazole nucleus were observed to establish hydrophobic interactions (π–π stacked and π–π T-shaped) with TYR225, PHE240, PHE117, and PHE339 amino acids with a bond length of 3.82, 5.36, 5.03, and 5.04 Å, respectively. Besides, the 2-phenyl groups of the 1H-benzimidazole nucleus showed electrostatic interaction (π–cation) with HIS227 amino acid with a bond length of 4.24 Å. Especially, 4-chloro (4-Cl) of 2-phenyl ring displayed π–alkyl interaction with PHE115, PHE240, and PHE339 amino acids with a bond length of 3.55, 4.45, and 4.64 Å, respectively (Fig. 6). The resulting docking may therefore suggest that its potent antibacterial, antifungal, and antitumor activities are mediated via interaction with DHFR and NMT proteins.
As to the selectivity prediction, the binding affinity in the range of −9.4 to −10.6 kcal mol−1 of compounds 4g and 4j are essentially similar on the DHFR-B, NMT, FGFR-1, and HDAC6 receptors. Compounds 3k and 3l showed similar affinity (−9.1 to −9.7 kcal mol−1) on the DHFR-B, FGFR-1, and HDAC6 receptors. However, compounds 3k, 3l, and 4c are predicted to be selective on the NMT receptor as having high affinity in the range of −11.0 to −11.3 kcal mol−1. Moreover, compound 4c also exhibited higher selectivity on the DHFR receptor than other potential compounds due to the difference in the range of −1.3 to −2.1 kcal mol−1 compared with GyrB, DHFR-F, VEGFR-2, and HDAC6 receptors.
2.6. In vitro DHFR inhibitory activity
The results of in silico molecular docking studies have predicted that DHFR is a potential receptor to explain the mechanism of antimicrobial and anticancer activities for the active derivatives. So, these compounds were tested for their ability to inhibit human DHFR and their potencies (IC50 values) were measured in vitro. DHFR inhibition assay kit, involving the DHFR-mediated conversion of dihydrofolate to tetrahydrofolate in the presence of NADPH (reduced nicotinamide adenine dinucleotide phosphate) has been used to investigate the inhibition of DHFR of active compounds 3k, 3l, 4c, 4g, and 4j. It has been observed that compound 4c showed the best activity at a low μM concentration of 2.35 μM (Table 6). In addition, compounds 3k, 3l, 4g, and 4j showed good inhibitory activity towards DHFR enzyme immunoassay with IC50 in the range of 6.78–12.32 μM. On the other hand, benzimidazole derivatives, for example, quinazolinone-benzimidazole and triazine-benzimidazole hybrids have also been reported to strongly inhibit DHFR.30,48 Therefore, the results suggest that DHFR is a target for compound 4c's antimicrobial and anticancer activities as shown by both in silico and in vitro studies.| Compound | DHFR inhibitory activities (IC50, μM) |
|---|---|
| 3k | 12.32 |
| 3l | 10.64 |
| 4c | 2.35 |
| 4g | 6.78 |
| 4j | 8.01 |
| Methotrexate | 0.021 |
3. Conclusion
In summary, starting from 1,2phenylenediamine and 4-Me-1,2-phenylenediamine, forty-six 2,6-disubstituted 1H-benzimidazole and twenty-three N,2,6-trisubstituted 1H-benzimidazole derivatives including sixteen new compounds have been designed, synthesized, and evaluated for their antimicrobial and anticancer activities. The microwave-assisted method has contributed to a significant reduction in reaction time and a significant increase in product yield. In addition, the values of the MIC against microorganisms showed that some compounds have significant inhibitory effects, especially compounds 3k, 3l, 4c, 4g, and 4j are potent for antibacterial activity against Gram-positive and Gram-negative bacteria compared with standard drug Cipro while compounds 3k, 3l, and 4c are potent for antifungal activity compared with standard drug Flu. In particular, these compounds also exhibited potent anticancer activity with IC50 < 10 μM against all tested cell lines (HepG2, MDA-MB-231, MCF7, RMS, and C26) compared with the reference drug PTX. From the structure–activity relationship, the presence of the N-benzyl group and the 4-chloro/4-nitro group in the aromatic ring at position 2 of the 1H-benzimidazole scaffold is more desirable for enhanced antibacterial activity as well as antitumor activity in 3f, 3l, 3k, 4c, and 4j, and antifungal activity in 3l and 4c. Molecular docking predicted that DHFR (dihydrofolate reductase) protein from S. aureus and NMT (N-myristoyl transferase) protein from C. albicans are the most suitable targets for the antimicrobial and anticancer activities. Compound 4c being the most potent antimicrobial and anticancer displayed a good affinity of −11.1 kcal mol−1 with the NMT enzyme from C. albicans and showed a good affinity of −10.0 kcal mol−1 with the crucial residue of the DHFR-B protein from S. aureus as well as showed electrostatic and hydrophobic interactions that resemble the co-crystallization ligand and reference drugs. Moreover, compound 4c showed good activity at a low μM concentration of 2.35 μM. Computational ADMET profiling for the five most active compounds in comparison to ciprofloxacin, fluconazole, and paclitaxel as reference drugs suggests that our derivatives have good ADMET profiles. Moreover, all compounds show physical–chemical properties of fragment and lead-like compounds which are of great interest for further drug development. This work paved the way for the synthesis of more potent antimicrobial and anticancer benzimidazole derivatives.4. Experimental section
4.1. Materials
All chemicals and solvents were of analytical grade and obtained from Merck, Germany. The reactions were monitored by thin-layer chromatography (TLC, E-Merck Kieselgel 60 F254). The column chromatography was carried out with the indicated solvents using silica gel (particle size 0.040–0.063 mm) from Merck (Germany). The microwave-assisted reactions were performed by the microwave synthesizer (CEM Discover, USA) with continuous stirring and controlled temperature. Melting points (mp, °C) of all compounds were determined in an open capillary using a Gallenkamp melting point apparatus without any correction. The infrared (IR) spectra were recorded using a Shimadzu FT-IR (IRAffinity-1S) spectrometer. An Agilent Technology LC-mass spectrometer with ESI ionization (1100 series LC/MSD Trap) was used to record the mass spectra (MS). A Bruker Avance 500 (1H, 500 MHz; 13C, 125 MHz) NMR spectrometer was used to record the 1H NMR and 13C NMR spectra at ambient temperature using DMSO-d6 as solvent. Chemical shifts are reported in parts per million (ppm) relative to the residual solvent peak as follows: DMSO-d6 = 2.50 ppm (1H NMR) and DMSO-d6 = 40.00 ppm (13C NMR). The Multiskan microplate reader was used to measure optical density (OD) at 570 nm.4.2. Experimental procedures
4.2.1.1 Refluxing method. A mixture of benzene-1,2-diamine or 4-methylbenzene-1,2-diamine (5 mmol), the substituted aromatic aldehydes (5 mmol), and Na2S2O5 (20 mmol) in a mixture of EtOH
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (30 mL, 9
H2O (30 mL, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) was refluxed for 6–12 h at 80 °C. After cooling down, the mixture was poured into cooled water and filtered off in a Büchner funnel. The resulting solid was purified by silica gel column chromatography using hexane/ethyl acetate as eluent. Yields: 75–93%.
1, v/v) was refluxed for 6–12 h at 80 °C. After cooling down, the mixture was poured into cooled water and filtered off in a Büchner funnel. The resulting solid was purified by silica gel column chromatography using hexane/ethyl acetate as eluent. Yields: 75–93%.
4.2.1.2 Microwave-assisted method. A mixture of benzene-1,2-diamine or 4-methylbenzene-1,2-diamine (5 mmol), the substituted aromatic aldehydes (5 mmol), Na2S2O5 (20 mmol) in a mixture of EtOH
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (10 mL, 9
H2O (10 mL, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) was placed in a microwave oven and irradiated at a power of 300 W for 10–15 min at 80 °C. After cooling down, the mixture was poured into cooled water and filtered off in a Büchner funnel. The resulting solid was purified by silica gel column chromatography using hexane/ethyl acetate as eluent. Yields: 90–99%.
1, v/v) was placed in a microwave oven and irradiated at a power of 300 W for 10–15 min at 80 °C. After cooling down, the mixture was poured into cooled water and filtered off in a Büchner funnel. The resulting solid was purified by silica gel column chromatography using hexane/ethyl acetate as eluent. Yields: 90–99%.2-(2-Chlorophenyl)-1H-benzimidazole (1a): yellow solid, mp 228–229 °C. 1H NMR (500 MHz, DMSO-d6, δ ppm): 12.70 (1H, s, –NH–), 7.90 (1H, dd, J = 7.5, 2.0 Hz, HAr), 7.66–7.61 (3H, m, HAr), 7.56–7.50 (2H, m, HAr), 7.24 (2H, m, HAr). 13C NMR (125 MHz, DMSO-d6, δ ppm): 149.0, 132.0, 131.6, 131.1, 130.7, 130.3, 129.9, 127.4, 122.2. LC-MS (m/z) [M − H]− calcd for C13H8ClN2 227.0381, found 227.0399; [M + H]+ calcd for C13H10ClN2 229.0527, found 229.0462.
2-(4-Chlorophenyl)-1H-benzimidazole (1b): yellow solid, mp 290–291 °C. 1H NMR (500 MHz, DMSO-d6, δ ppm): 8.19 (2H, d, J = 8.5 Hz, HAr), 7.63–7.60 (4H, m, HAr), 7.22 (2H, d, J = 8.5 Hz, HAr). 13C NMR (125 MHz, DMSO-d6, δ ppm): 150.2, 134.6, 131.2, 130.8, 129.1, 129.0, 128.98, 128.8, 128.78, 128.2, 128.0, 122.4. LC-MS (m/z) [M − H]− calcd for C13H8ClN2 227.0381, found 227.0389; [M + H]+ calcd for C13H10ClN2 229.0527, found 229.0636.
2-(2,4-Dichlorophenyl)-1H-benzimidazole (1c): white solid, mp 232–233 °C. 1H NMR (500 MHz, DMSO-d6, δ ppm): 12.77 (1H, s, –NH–), 7.94 (1H, d, J = 8.5 Hz, HAr), 7.85 (1H, d, J = 2.0 Hz, HAr), 7.70 (1H, s, HAr), 7.63–7.58 (2H, m, HAr), 7.25 (2H, s, HAr). 13C NMR (125 MHz, DMSO-d6, δ ppm): 148.1, 143.1, 135.0, 134.6, 133.2, 132.6, 129.9, 128.9, 127.7, 122.9, 121.8, 119.2, 111.8. LC-MS (m/z) [M − H]− calcd for C13H7Cl2N2 260.9992, found 260.9952; [M + H]+ calcd for C13H9Cl2N2 263.0137, found 262.9776.
2-(3,4-Dichlorophenyl)-1H-benzimidazole (1d): white solid, mp 237–238 °C. 1H NMR (500 MHz, DMSO-d6, δ ppm): 13.07 (1H, s, –NH–), 8.39 (1H, d, J = 1.0 Hz, HAr), 8.15 (1H, dd, J = 8.0, 1.0 Hz, HAr), 7.83 (1H, d, J = 8.5 Hz, HAr), 7.67–7.57 (2H, m, HAr), 7.24 (2H, s, HAr). 13C NMR (125 MHz, DMSO-d6, δ ppm): 148.8, 132.2, 131.8, 131.3, 131.0, 130.9, 130.7, 127.9, 126.4, 123.1, 122.1, 119.0, 111.5. LC-MS (m/z) [M − H]− calcd for C13H7Cl2N2 260.9992, found 260.9905; [M + H]+ calcd for C13H9Cl2N2 263.0137, found 262.9993.
2-(2-Chloro-6-fluorophenyl)-1H-benzimidazole (1e): white solid, mp 225–226 °C. 1H NMR (500 MHz, DMSO-d6, δ ppm): 12.91 (1H, s, –NH–), 7.80–7.50 (4H, m, HAr), 7.46 (1H, d, J = 8.0 Hz, HAr), 7.26 (2H, s, HAr). 13C NMR (125 MHz, DMSO-d6, δ ppm): 161.7, 159.7, 143.5, 134.30, 134.27, 132.65, 132.57, 125.86, 125.84, 122.78, 121.6, 119.90, 119.74, 119.27, 115.0, 114.82, 111.6. LC-MS (m/z) [M − H]− calcd for C13H7ClFN2 245.0287, found 245.0257; [M + H]+ calcd for C13H9ClFN2 247.0433, found 247.0338.
2-(3,4-Dimethoxyphenyl)-1H-benzimidazole (1f): yellow solid, mp 232–233 °C. 1H NMR (500 MHz, DMSO-d6, δ ppm): 12.74 (1H, s, –NH–), 7.78 (1H, s, HAr), 7.75 (1H, d, J = 8.0 Hz, HAr), 7.62–7.50 (2H, m, HAr), 7.18–7.12 (3H, m, HAr), 3.88 (3H, s, –OCH3), 3.84 (3H, s, –OCH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 151.4, 150.3, 148.9, 143.9, 134.9, 122.7, 122.1, 121.5, 119.3, 118.5, 118.3, 111.9, 111.7, 111.0, 109.8, 55.6. LC-MS (m/z) [M − H]− calcd for C15H13N2O2 253.0983, found 254.1055; [M + H]+ calcd for C15H15N2O2 255.1128, found 255.0914.
2-(4-Ethoxyphenyl)-1H-benzimidazole (1g): white solid, mp 259–261 °C. 1H NMR (500 MHz, DMSO-d6, δ ppm): 12.71 (1H, s, –NH–), 8.10 (2H, d, J = 8.5 Hz, HAr), 7.56–7.54 (2H, m, HAr), 7.17–7.16 (2H, m, HAr), 7.09 (2H, d, J = 9.0 Hz, HAr), 4.11 (2H, q, J = 7.0 Hz, –CH2–), 1.36 (3H, t, J = 7.0 Hz, –CH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 159.8, 151.3, 128.0, 122.5, 121.7, 114.7, 63.2, 14.6. LC-MS (m/z) [M − H]− calcd for C15H13N2O 237.1033, found 237.0655; [M + H]+ calcd for C15H15N2O 239.1179, found 239.0670.
4-(1H-Benzimidazol-2-yl)-2-ethoxyphenol (1h): yellow solid, mp 193–194 °C. 1H NMR (500 MHz, DMSO-d6, δ ppm): 12.62 (1H, s, –NH–), 9.44 (1H, s, –OH), 7.73 (1H, d, J = 2.0 Hz, HAr), 7.61 (1H, dd, J = 8.5, 2.0 Hz, HAr), 7.53 (2H, s, HAr), 7.21–7.14 (2H, m, HAr), 6.93 (1H, d, J = 8.5 Hz, HAr), 4.14 (2H, q, J = 7.0 Hz, –CH2–), 1.40 (3H, t, J = 7.0 Hz, –CH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 151.8, 148.7, 147.0, 121.4, 119.7, 115.8, 111.6, 64.0, 14.7. LC-MS (m/z) [M − H]− calcd for C15H13N2O2 253.0983, found 253.1013; [M + H]+ calcd for C15H15N2O2 255.1128, found 255.1011.
2-(4-Fluorophenyl)-1H-benzimidazole (1i): yellow solid, mp 255–256 °C. 1H NMR (500 MHz, DMSO-d6, δ ppm): 12.89 (1H, s, –NH–), 8.23–8.20 (2H, m, HAr), 7.66 (1H, d, J = 7.5 Hz, HAr), 7.53 (1H, d, J = 7.5 Hz, HAr), 7.40 (2H, t, J = 9.0 Hz, HAr), 7.25–7.17 (2H, m, HAr). 13C NMR (125 MHz, DMSO-d6, δ ppm): 164.0, 162.1, 150.4, 143.7, 135.0, 128.72, 128.65, 126.78, 126.76, 122.5, 121.7, 118.8, 116.1, 115.9, 111.3. LC-MS (m/z) [M − H]− calcd for C13H8FN2 211.0677, found 211.0679; [M + H]+ calcd for C13H10FN2 213.0823, found 213.0708.
2-(1H-Benzimidazol-2-yl)phenol (1j): white solid, mp 246–248 °C. 1H NMR (500 MHz, DMSO-d6, δ ppm): 13.19 (1H, s, –NH–), 7.85 (1H, d, J = 7.0 Hz, HAr), 7.64 (1H, d, J = 7.5 Hz, HAr), 7.48 (1H, d, J = 7.5 Hz, HAr), 7.30–7.16 (4H, m, HAr), 7.02 (1H, d, J = 7.5 Hz, HAr). 13C NMR (125 MHz, DMSO-d6, δ ppm): 158.5, 151.7, 141.5, 133.6, 131.4, 129.6, 124.2, 119.2, 117.3, 111.5. LC-MS (m/z) [M − H]− calcd for C13H9N2O 209.0720, found 209.0822; [M + H]+ calcd for C13H11N2O 211.0866, found 211.0854.
2-(1H-Benzimidazol-2-yl)-4-bromophenol (1k): brown solid, mp 280–282 °C. 1H NMR (500 MHz, DMSO-d6, δ ppm): 13.27 (1H, s, –NH–), 8.29 (1H, d, J = 2.0 Hz, HAr), 7.70–7.66 (2H, m, HAr), 7.52 (1H, dd, J = 9.0, 2.0 Hz, HAr), 7.32–7.30 (2H, m, HAr), 7.02 (1H, d, J = 9.0 Hz, HAr). 13C NMR (125 MHz, DMSO-d6, δ ppm): 157.1, 150.2, 134.0, 128.4, 122.7, 119.4, 114.6, 111.7, 110.1. LC-MS (m/z) [M − H]− calcd for C13H8BrN2O 286.9825, found 287.0522; [M + H]+ calcd for C13H10BrN2O 288.9971, found 289.0718.
3-(1H-Benzimidazol-2-yl)phenol (1l): yellow solid, mp 261–263 °C. 1H NMR (500 MHz, DMSO-d6, δ ppm): 12.84 (1H, s, –NH–), 9.78 (1H, s, –OH), 7.66–7.51 (4H, m, HAr), 7.34 (1H, t, J = 8.0 Hz, HAr), 7.27–7.08 (2H, m, HAr), 6.91 (1H, d, J = 8.0 Hz, HAr). 13C NMR (125 MHz, DMSO-d6, δ ppm): 157.8, 151.4, 143.8, 135.0, 131.4, 130.1, 129.9, 122.5, 121.7, 118.9, 117.3, 117.0, 113.4, 111.3. LC-MS (m/z) [M − H]− calcd for C13H9N2O 209.0720, found 209.0724; [M + H]+ calcd for C13H11N2O 211.0866, found 211.0859.
5-(1H-Benzimidazol-2-yl)-2-methoxyphenol (1m): yellow solid, mp 238–240 °C. 1H NMR (500 MHz, DMSO-d6, δ ppm): 12.68 (1H, s, –NH–), 9.32 (1H, s, –OH), 7.67 (1H, s, HAr), 7.62 (1H, d, J = 8.5 Hz, HAr), 7.56–7.54 (2H, m, HAr), 7.17–7.15 (2H, m, HAr), 7.09 (1H, d, J = 8.5 Hz, HAr), 3.85 (3H, s, –OCH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 151.6, 149.4, 146.7, 123.0, 121.7, 118.0, 113.8, 112.2, 55.7. LC-MS (m/z) [M − H]− calcd for C14H11N2O2 239.0826, found 239.0592; [M + H]+ calcd for C14H13N2O2 241.0972, found 241.0712.
2-(3-Methoxyphenyl)-1H-benzimidazole (1n): yellow solid, mp 207–208 °C. 1H NMR (500 MHz, DMSO-d6, δ ppm): 12.87 (1H, s, –NH–), 7.76 (2H, d, J = 7.0 Hz, HAr), 7.67 (1H, d, J = 8.0 Hz, HAr), 7.53 (1H, d, J = 7.5 Hz, HAr), 7.46 (1H, t, J = 8.0 Hz, HAr), 7.24–7.17 (2H, m, HAr), 7.06 (1H, dd, J = 7.0, 2.5 Hz, HAr), 3.87 (3H, s, –OCH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 159.6, 151.0, 143.7, 134.9, 131.4, 130.0, 122.5, 121.6, 118.8, 118.7, 115.8, 111.4, 111.3, 55.3. LC-MS (m/z) [M − H]− calcd for C14H11N2O 223.0877, found 223.0852; [M + H]+ calcd for C14H13N2O 225.1022, found 225.0894.
2-(4-(Methylthio)phenyl)-1H-benzimidazole (1o): brown solid, mp 101–102 °C. 1H NMR (500 MHz, DMSO-d6, δ ppm): 12.81 (1H, s, –NH–), 8.05 (2H, d, J = 8.5 Hz, HAr), 7.66 (1H, d, J = 7.5 Hz, HAr), 7.50 (1H, d, J = 7.5 Hz, HAr), 7.43 (2H, d, J = 8.5 Hz, HAr), 7.23–7.15 (2H, m, HAr), 2.57 (3H, s, –SCH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 151.2, 141.5, 131.8, 127.4, 126.9, 126.0, 124.1, 14.9. LC-MS (m/z) [M − H]− calcd for C14H11N2S 239.0648, found 239.0601; [M + H]+ calcd for C14H13N2S 241.0794, found 241.0801.
2-(3-Nitrophenyl)-1H-benzimidazole (1p): yellow solid, mp 205–207 °C. 1H NMR (500 MHz, DMSO-d6, δ ppm): 13.26 (1H, s, –NH–), 8.99 (1H, s, HAr), 8.59 (1H, d, J = 8.0 Hz, HAr), 8.28 (1H, d, J = 8.0 Hz, HAr), 7.81 (1H, t, J = 8.0 Hz, HAr), 7.64 (2H, s, HAr), 7.25–7.23 (2H, m, HAr). 13C NMR (125 MHz, DMSO-d6, δ ppm): 149.0, 148.3, 132.4, 131.7, 130.6, 124.1, 122.6, 120.8. LC-MS (m/z) [M − H]− calcd for C13H8N3O2 238.0622, found 238.0592; [M + H]+ calcd for C13H10N3O2 240.0768, found 240.0730.
2-(4-Nitrophenyl)-1H-benzimidazole (1q): yellow solid, mp 319–320 °C. 1H NMR (500 MHz, DMSO-d6, δ ppm): 13.29 (1H, s, –NH–), 8.43–8.39 (4H, m, HAr), 7.70–7.63 (2H, m, HAr), 7.27 (2H, s, HAr). 13C NMR (125 MHz, DMSO-d6, δ ppm): 149.0, 147.8, 136.0, 127.4, 124.3, 123.5, 119.5, 111.8. LC-MS (m/z) [M − H]− calcd for C13H8N3O2 238.0622, found 238.0647; [M + H]+ calcd for C13H10N3O2 240.0768, found 240.0723.
4-(1H-Benzimidazol-2-yl)-N,N-dimethylaniline (1r): yellow solid, mp 287–289 °C. 1H NMR (500 MHz, DMSO-d6, δ ppm): 12.33 (1H, s, –NH–), 7.96 (2H, d, J = 8.5 Hz, HAr), 7.67 (1H, d, J = 7.5 Hz, HAr), 7.53 (1H, d, J = 7.5 Hz, HAr), 7.22–7.11 (2H, m, HAr), 6.81 (2H, d, J = 9.0 Hz, HAr), 2.98 (6H, s, –N(CH3)2). 13C NMR (125 MHz, DMSO-d6, δ ppm): 151.4, 127.5, 122.8, 117.7, 111.6, 40.2. LC-MS (m/z) [M + H]+ calcd for C15H16N3 238.1339, found 238.1368.
2-(Anthracen-9-yl)-1H-benzimidazole (1s): yellow solid, mp 313–314 °C. 1H NMR (500 MHz, DMSO-d6, δ ppm): 13.01 (1H, s, –NH–), 8.85 (1H, s, HAr), 8.22 (2H, d, J = 8.5 Hz, HAr), 7.60 (4H, d, J = 8.5 Hz, HAr), 7.60–7.50 (4H, m, HAr), 7.32 (2H, m, HAr). 13C NMR (125 MHz, DMSO-d6, δ ppm): 149.5, 130.6, 130.5, 128.8, 128.4, 126.8, 125.8, 125.6, 125.5, 122.0. LC-MS (m/z) [M − H]− calcd for C21H13N2 293.1084, found 293.1032; [M + H]+ calcd for C21H15N2 295.1230, found 295.1241.
2-(Benzo[d][1,3]dioxol-5-yl)-1H-benzimidazole (1t): yellow solid, mp 251–252 °C. 1H NMR (500 MHz, DMSO-d6, δ ppm): 12.72 (1H, s, –NH–), 7.72 (1H, dd, J = 8.0, 2.0 Hz, HAr), 7.68 (1H, d, J = 1.5 Hz, HAr), 7.62 (1H, d, J = 7.5 Hz, HAr), 7.49 (1H, d, J = 7.0 Hz, HAr), 7.18 (2H, t, J = 7.5 Hz, HAr), 7.09 (1H, d, J = 8.0 Hz, HAr), 6.12 (2H, s, –CH2–). 13C NMR (125 MHz, DMSO-d6, δ ppm): 151.1, 148.7, 147.9, 143.7, 134.9, 124.2, 122.2, 121.5, 120.9, 118.6, 111.1, 108.7, 106.5, 101.6. LC-MS (m/z) [M − H]− calcd for C14H9N2O2 237.0670, found 237.0655; [M + H]+ calcd for C14H11N2O2 239.0815, found 239.0670.
2-(Furan-2-yl)-1H-benzimidazole (1u): brown solid, mp 280–282 °C. 1H NMR (500 MHz, DMSO-d6, δ ppm): 12.74 (1H, s, –NH–), 7.92 (1H, d, J = 4.0 Hz, HAr), 7.69 (1H, d, J = 7.5 Hz, HAr), 7.55 (1H, d, J = 7.5 Hz, HAr), 7.23–7.19 (2H, m, HAr), 7.14 (1H, d, J = 4.0 Hz, HAr), 6.72 (1H, dd, J = 4.0, 2.0 Hz, HAr). 13C NMR (125 MHz, DMSO-d6, δ ppm): 146.8, 144.7, 143.5, 142.3, 132.8, 123.6, 118.5, 112.8, 110.4. LC-MS (m/z) [M − H]− calcd for C11H7N2O 183.0564, found 183.0571; [M + H]+ calcd for C11H9N2O 185.0709, found 185.0802.
2-(Pyridin-3-yl)-1H-benzimidazole (1v): yellow solid, mp 240–241 °C. 1H NMR (500 MHz, DMSO-d6, δ ppm): 12.95 (1H, s, –NH–), 9.33 (1H, d, J = 1.5 Hz, HAr), 8.69 (1H, d, J = 3.5 Hz, HAr), 8.51 (1H, d, J = 8.0 Hz, HAr), 7.71 (1H, d, J = 7.5 Hz, HAr), 7.60 (1H, d, J = 7.5 Hz, HAr), 7.37 (1H, s, HAr), 7.25–7.20 (2H, m, HAr). 13C NMR (125 MHz, DMSO-d6, δ ppm): 150.5, 148.6, 147.5, 133.5, 126.2, 124.3, 123.8, 118.9, 111.2. LC-MS (m/z) [M − H]− calcd for C12H8N3 194.0724, found 194.0732.
2-(Pyridin-4-yl)-1H-benzimidazole (1w): yellow solid, mp 216–217 °C. 1H NMR (500 MHz, DMSO-d6, δ ppm): 13.26 (1H, s, –NH–), 8.76 (2H, dd, J = 4.5, 1.5 Hz, HAr), 8.10 (2H, dd, J = 4.5, 1.5 Hz, HAr), 7.74 (1H, d, J = 8.0 Hz, HAr), 7.60 (1H, d, J = 8.0 Hz, HAr), 7.31–7.23 (2H, m, HAr). 13C NMR (125 MHz, DMSO-d6, δ ppm): 150.5, 148.8, 143.6, 137.1, 135.0, 123.6, 122.3, 120.3, 119.5, 111.8. LC-MS (m/z) [M − H]− calcd for C12H8N3 194.0724, found 194.0728.
2-(2-Chlorophenyl)-6-methyl-1H-benzimidazole (2a): brown solid, mp 140–141 °C. 1H NMR (500 MHz, DMSO-d6, δ ppm): 12.55 (1H, s, –NH–), 7.89 (1H, d, J = 5.5 Hz, HAr), 7.65 (1H, dd, J = 9.0, 1.5 Hz, HAr), 7.58–7.45 (3H, m, HAr), 7.35 (1H, s, HAr), 7.10–7.04 (1H, m, HAr), 2.45 (3H, s, –CH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 141.4, 134.9, 132.0, 131.6, 131.1, 130.3, 130.0, 127.4, 124.2, 123.3, 118.74, 118.65, 111.4, 111.2, 21.3. LC-MS (m/z) [M − H]− calcd for C14H10ClN2 241.0538, found 241.0005; [M + H]+ calcd for C14H12ClN2 243.0684, found 243.0598.
2-(4-Chlorophenyl)-6-methyl-1H-benzimidazole (2b): brown solid, mp 216–217 °C. 1H NMR (500 MHz, DMSO-d6, δ ppm): 12.81 (1H, s, –NH–), 8.15 (2H, d, J = 8.5 Hz, HAr), 7.62 (2H, d, J = 8.5 Hz, HAr), 7.48–7.32 (2H, m, HAr), 7.03 (1H, d, J = 8.0 Hz, HAr), 2.50 (3H, s, –CH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 134.2, 129.2, 129.0, 128.0, 21.3. LC-MS (m/z) [M + H]+ calcd for C14H12ClN2 243.0684, found 243.0676.
2-(2,4-Dichlorophenyl)-6-methyl-1H-benzimidazole (2c): white solid, mp 142–144 °C. 1H NMR (500 MHz, DMSO-d6, δ ppm): 12.78 (1H, s, –NH–), 7.93 (1H, d, J = 8.5 Hz, HAr), 7.82 (1H, d, J = 2.0 Hz, HAr), 7.60 (1H, dd, J = 8.5, 2.0 Hz, HAr), 7.52 (1H, d, J = 8.0 Hz, HAr), 7.42 (1H, s, HAr), 7.08 (1H, d, J = 8.0 Hz, HAr), 2.44 (3H, s, –CH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 147.6, 134.9, 133.2, 132.5, 131.7, 129.8, 128.8, 127.7, 123.9, 21.3. LC-MS (m/z) [M − H]− calcd for C14H9Cl2N2 275.0148, found 275.0288; [M + H]+ calcd for C14H11Cl2N2 277.0294, found 277.1055.
2-(3,4-Dichlorophenyl)-6-methyl-1H-benzimidazole (2d): white solid, mp 134–136 °C. 1H NMR (500 MHz, DMSO-d6, δ ppm): 12.76 (1H, s, –NH–), 8.36 (1H, d, J = 1.5 Hz, HAr), 8.11 (1H, dd, J = 8.5, 1.5 Hz, HAr), 7.79 (1H, d, J = 8.5 Hz, HAr), 7.49 (1H, d, J = 8.5 Hz, HAr), 7.39 (1H, s, HAr), 7.05 (1H, d, J = 8.0 Hz, HAr), 2.42 (3H, s, –CH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 148.5, 132.1, 132.0, 131.8, 131.2, 131.0, 130.9, 130.8, 129.3, 127.8, 126.3, 124.1, 21.3. LC-MS (m/z) [M + H]+ calcd for C14H11Cl2N2 277.0294, found 277.0366.
2-(2-Chloro-6-fluorophenyl)-6-methyl-1H-benzimidazole (2e): white solid, mp 193–195 °C. 1H NMR (500 MHz, DMSO-d6, δ ppm): 12.74 (1H, s, –NH–), 8.19 (1H, d, J = 8.5 Hz, HAr), 8.18 (1H, d, J = 8.5 Hz, HAr), 7.50–7.33 (3H, m, HAr), 7.02 (1H, d, J = 7.5 Hz, HAr), 2.42 (3H, s, –CH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 163.9, 162.0, 128.6, 128.5, 127.1, 126.9, 118.3, 116.0, 115.8, 111.1, 21.3. LC-MS (m/z) [M − H]− calcd for C14H9ClFN2 259.0444, found 259.0794; [M + H]+ calcd for C14H11ClFN2 261.0589, found 261.0896.
2-(3,4-Dimethoxyphenyl)-6-methyl-1H-benzimidazole (2f): white solid, mp 228–230 °C. 1H NMR (500 MHz, DMSO-d6, δ ppm): 12.61 (1H, s, –NH–), 7.76 (1H, d, J = 2.0 Hz, HAr), 7.73 (1H, dd, J = 8.0, 2.0 Hz, HAr), 7.45 (1H, d, J = 8.0 Hz, HAr), 7.35 (1H, s, HAr), 7.12 (1H, d, J = 8.5 Hz, HAr), 7.00 (1H, dd, J = 8.5, 1.5 Hz, HAr), 3.88 (3H, s, –OCH3), 3.84 (3H, s, –OCH3), 2.43 (3H, s, –CH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 151.6, 150.6, 149.4, 123.7, 123.4, 119.6, 112.3, 110.1, 56.1, 56.0, 21.8. LC-MS (m/z) [M − H]− calcd for C16H15N2O2 267.1139, found 267.1076; [M + H]+ calcd for C16H17N2O2 269.1285, found 269.1175.
2-(4-Ethoxyphenyl)-6-methyl-1H-benzimidazole (2g): yellow solid, mp 258–260 °C. 1H NMR (500 MHz, DMSO-d6, δ ppm): 12.57 (1H, s, –NH–), 8.06 (2H, d, J = 8.0 Hz, HAr), 7.48 (1H, d, J = 8.0 Hz, HAr), 7.36 (1H, d, J = 8.5 Hz, HAr), 7.27 (1H, s, HAr), 7.07 (2H, d, J = 8.5 Hz, HAr), 4.11 (2H, q, J = 7.0 Hz, –CH2–), 2.42 (3H, s, –CH3), 1.36 (3H, t, J = 7.0 Hz, –CH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 159.8, 151.3, 150.8, 142.0, 135.2, 133.0, 131.3, 130.3, 127.9, 127.8, 123.4, 122.9, 122.7, 118.3, 118.0, 114.7, 110.8, 110.5, 63.3, 21.3, 14.6. LC-MS (m/z) [M − H]− calcd for C16H15N2O 251.1190, found 251.0255; [M + H]+ calcd for C16H17N2O 253.1335, found 253.0133.
δ2-Ethoxy-4-(6-methyl-1H-benzimidazol-2-yl)phenol (2h): brown solid, mp 223–225 °C. 1H NMR (500 MHz, DMSO-d6, δ ppm): 12.49 (1H, s, –NH–), 9.40 (1H, s, –OH), 7.70 (1H, s, HAr), 7.58 (1H, d, J = 8.0 Hz, HAr), 7.47 (1H, d, J = 8.0 Hz, HAr), 7.35 (1H, d, J = 8.0 Hz, HAr), 7.26 (1H, s, HAr), 6.91 (1H, d, J = 8.5 Hz, HAr), 4.14 (2H, q, J = 7.0 Hz, –CH2–), 2.42 (3H, s, –CH3), 1.40 (3H, t, J = 7.0 Hz, –CH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 151.3, 148.5, 146.9, 144.2, 142.0, 135.2, 131.2, 130.2, 123.3, 122.9, 121.6, 121.2, 119.6, 119.5, 118.1, 117.9, 115.8, 111.5, 110.7, 110.4, 64.0, 63.8, 21.4, 14.8, 14.6. LC-MS (m/z) [M − H]− calcd for C16H15N2O2 267.1139, found 267.0427; [M + H]+ calcd for C16H17N2O2 269.1285, found 269.0582.
2-(4-Fluorophenyl)-6-methyl-1H-benzimidazole (2i): brown solid, mp 217–219 °C. 1H NMR (500 MHz, DMSO-d6, δ ppm): 12.74 (1H, s, –NH–), 7.63 (2H, d, J = 8.5 Hz, HAr), 7.58 (1H, d, J = 8.0 Hz, HAr), 7.53 (2H, d, J = 8.0 Hz, HAr), 7.50 (1H, s, HAr), 7.44 (1H, t, J = 8.5 Hz, HAr), 7.34 (1H, s, HAr), 7.10 (1H, d, J = 8.0 Hz, HAr), 7.07 (1H, d, J = 8.0 Hz, HAr), 2.44 (3H, s, –CH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 161.7, 159.7, 142.9, 141.4, 134.5, 134.3, 132.5, 132.2, 130.6, 125.81, 125.78, 124.3, 123.2, 120.0, 119.8, 118.9, 118.8, 114.9, 114.8, 111.2, 111.1, 21.3. LC-MS (m/z) [M − H]− calcd for C14H10FN2 225.0834, found 225.0014; [M + H]+ calcd for C14H12FN2 227.0979, found 227.1081.
2-(6-Methyl-1H-benzimidazol-2-yl)phenol (2j): white solid, mp 250–252 °C. 1H NMR (500 MHz, DMSO-d6, δ ppm): 13.21 (1H, s, –NH–), 9.71 (1H, s, –OH), 8.04 (1H, d, J = 7.0 Hz, HAr), 7.60–7.37 (2H, m, HAr), 7.35 (1H, s, HAr), 7.10–7.07 (1H, m, HAr), 7.04 (1H, d, J = 8.5 Hz, HAr), 7.00 (1H, d, J = 7.5 Hz, HAr), 2.45 (3H, s, –CH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 158.0, 151.5, 151.3, 141.2, 139.0, 133.4, 131.5, 131.3, 129.8, 129.7, 126.0, 124.7, 124.1, 124.0, 119.0, 117.1, 112.7, 111.2, 21.4, 21.1. LC-MS (m/z) [M − H]− calcd for C14H11N2O 223.0877, found 223.0852; [M + H]+ calcd for C14H13N2O 225.1022, found 225.0894.
4-Bromo-2-(6-methyl-1H-benzimidazol-2-yl)phenol (2k): white solid, mp 277–278 °C. 1H NMR (500 MHz, DMSO-d6, δ ppm): 13.31 (1H, s, –NH–), 9.72 (1H, s, –OH), 8.26 (1H, d, J = 2.0 Hz, HAr), 7.60–7.57 (1H, s, HAr), 7.50 (1H, dd, J = 9.0, 2.5 Hz, HAr), 7.40 (1H, s, HAr), 7.15–7.06 (1H, m, HAr), 7.00 (1H, d, J = 8.5 Hz, HAr), 2.45 (3H, s, –CH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 157.0, 133.8, 128.2, 124.2, 119.4, 117.7, 114.7, 111.3, 110.1, 21.3. LC-MS (m/z) [M + H]+ calcd for C14H12BrN2O 303.0128, found 302.9765.
3-(6-Methyl-1H-benzimidazol-2-yl)phenol (2l): yellow solid, mp 294–296 °C. 1H NMR (500 MHz, DMSO-d6, δ ppm): 12.67 (1H, s, –NH–), 9.70 (1H, s, –OH), 7.57 (1H, d, J = 8.5 Hz, HAr), 7.52 (1H, d, J = 8.0 Hz, HAr), 7.43 (1H, s, HAr), 7.33 (1H, t, J = 8.0 Hz, HAr), 7.29 (1H, s, HAr), 7.02 (1H, d, J = 8.0 Hz, HAr), 6.88 (1H, dd, J = 8.0, 2.0 Hz, HAr), 2.43 (3H, s, –CH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 157.7, 131.5, 129.9, 118.4, 117.1, 116.8, 113.2, 110.0, 21.3. LC-MS (m/z) [M − H]− calcd for C14H11N2O 223.0877, found 223.0852; [M + H]+ calcd for C14H13N2O 225.1022, found 225.0894.
2-Methoxy-5-(6-methyl-1H-benzimidazol-2-yl)phenol (2m): yellow solid, mp 248–249 °C. 1H NMR (500 MHz, DMSO-d6, δ ppm): 12.69 (1H, s, –NH–), 9.25 (1H, s, –OH), 7.60 (1H, s, HAr), 7.56 (1H, dd, J = 8.5, 1.5 Hz, HAr), 7.41 (1H, d, J = 8.0 Hz, HAr), 7.31 (1H, s, HAr), 7.06 (1H, d, J = 8.5 Hz, HAr), 6.98 (1H, d, J = 8.5 Hz, HAr), 3.84 (3H, s, –OCH3), 2.41 (3H, s, –CH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 151.2, 149.2, 146.6, 130.8, 123.1, 117.8, 113.7, 112.1, 55.7, 21.3. LC-MS (m/z) [M − H]− calcd for C15H13N2O2 253.0983, found 253.1013; [M + H]+ calcd for C15H15N2O2 255.1128, found 255.1011.
2-(3-Methoxyphenyl)-6-methyl-1H-benzimidazole (2n): white solid, mp 202–204 °C. 1H NMR (500 MHz, DMSO-d6, δ ppm): 12.79 (1H, s, –NH–), 7.74 (1H, d, J = 8.0 Hz, HAr), 7.73 (1H, d, J = 1.5 Hz, HAr), 7.48 (1H, d, J = 6.5 Hz, HAr), 7.44 (1H, t, J = 8.0 Hz, HAr), 7.38 (1H, s, HAr), 7.05–7.02 (2H, m, HAr), 3.85 (3H, s, –OCH3), 2.42 (3H, s, –CH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 159.8, 150.9, 131.6, 130.2, 123.8, 118.8, 115.8, 111.4, 55.4, 21.4. LC-MS (m/z) [M − H]− calcd for C15H13N2O 237.1033, found 237.1105; [M + H]+ calcd for C15H15N2O 239.1179, found 239.0899.
6-Methyl-2-(4-(methylthio)phenyl)-1H-benzimidazole (2o): brown solid, mp 94–95 °C. 1H NMR (500 MHz, DMSO-d6, δ ppm): 12.65 (1H, s, –NH–), 8.09 (2H, d, J = 8.5 Hz, HAr), 7.46 (1H, d, J = 8.0 Hz, HAr), 7.41 (2H, d, J = 8.5 Hz, HAr), 7.36 (1H, s, HAr), 7.02 (1H, d, J = 8.0 Hz, HAr), 2.55 (3H, s, –SCH3), 2.43 (3H, s, –CH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 151.1, 140.9, 131.7, 127.2, 127.1, 126.2, 124.0, 21.2, 14.8. LC-MS (m/z) [M − H]− calcd for C15H13N2S 253.0805, found 253.0834; [M + H]+ calcd for C15H15N2S 255.0950, found 255.0866.
6-Methyl-2-(3-nitrophenyl)-1H-benzimidazole (2p): yellow solid, mp 200–201 °C. 1H NMR (500 MHz, DMSO-d6, δ ppm): 13.12 (1H, s, –NH–), 8.99 (1H, s, HAr), 8.59 (1H, d, J = 8.0 Hz, HAr), 8.32 (1H, dd, J = 8.0, 1.0 Hz, HAr), 7.84 (1H, t, J = 8.0 Hz, HAr), 7.55–7.39 (2H, m, HAr), 7.08 (1H, d, J = 7.5 Hz, HAr), 2.50 (3H, s, –CH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 148.4, 132.3, 131.9, 130.7, 124.0, 120.7, 21.3. LC-MS (m/z) [M − H]− calcd for C14H10N3O2 252.0779, found 252.0872; [M + H]+ calcd for C14H12N3O2 254.0924, found 254.0882.
6-Methyl-2-(4-nitrophenyl)-1H-benzimidazole (2q): yellow solid, mp 240–242 °C. 1H NMR (500 MHz, DMSO-d6, δ ppm): 13.14 (1H, s, –NH–), 8.60–8.30 (4H, m, HAr), 7.60–7.36 (2H, m, HAr), 7.16–7.02 (1H, m, HAr), 2.44 (3H, s, –CH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 147.7, 136.2, 127.2, 124.3, 124.0, 119.0, 111.4, 21.3. LC-MS (m/z) [M + H]+ calcd for C14H12N3O2 254.0924, found 254.0874.
N,N-Dimethyl-4-(6-methyl-1H-benzimidazol-2-yl)aniline (2r): yellow solid, mp 246–248 °C. 1H NMR (500 MHz, DMSO-d6, δ ppm): 12.36 (1H, s, –NH–), 7.97 (2H, d, J = 8.5 Hz, HAr), 7.35–7.28 (2H, m, HAr), 6.95 (1H, d, J = 8.0 Hz, HAr), 6.83 (2H, d, J = 9.0 Hz, HAr), 2.99 (6H, s, –N(CH3)2), 2.50 (3H, s, –CH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 151.1, 127.4, 122.7, 117.6, 111.8, 39.84, 21.3. LC-MS (m/z) [M + H]+ calcd for C16H18N3 252.1495, found 252.1590.
2-(Anthracen-9-yl)-6-methyl-1H-benzimidazole (2s): yellow solid, mp 323–324 °C. 1H NMR (500 MHz, DMSO-d6, δ ppm): 13.02 (1H, s, –NH–), 8.84 (1H, s, HAr), 8.25 (2H, d, J = 8.5 Hz, HAr), 7.62 (4H, d, J = 8.5 Hz, HAr), 7.61–7.48 (3H, m, HAr), 7.33 (2H, q, J = 3.0 Hz, HAr), 2.43 (3H, s, –CH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 150.2, 132.4, 130.6, 128.7, 128.2, 126.5, 125.8, 125.7, 125.3, 122.1, 118.5, 111.2, 21.9. LC-MS (m/z) [M − H]− calcd for C22H15N2 307.1241, found 307.1253; [M + H]+ calcd for C22H17N2 309.1386, found 308.1327.
2-(Benzo[d][1,3]dioxol-5-yl)-6-methyl-1H-benzimidazole (2t): yellow solid, mp 258–259 °C. 1H NMR (500 MHz, DMSO-d6, δ ppm): 12.81 (1H, s, –NH–), 7.76 (1H, dd, J = 8.0, 2.0 Hz, HAr), 7.67 (1H, d, J = 1.5 Hz, HAr), 7.60 (1H, d, J = 7.5 Hz, HAr), 7.51 (1H, d, J = 7.0 Hz, HAr), 7.17 (1H, t, J = 7.5 Hz, HAr), 7.05 (1H, d, J = 8.0 Hz, HAr), 6.14 (2H, s, –CH2–), 2.41 (3H, s, –CH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 151.2, 148.6, 147.8, 143.9, 134.6, 124.5, 122.0, 121.6, 120.3, 118.5, 111.5, 108.6, 106.4, 101.5, 21.8. LC-MS (m/z) [M − H]− calcd for C15H11N2O2 251.0826, found 251.0843; [M + H]+ calcd for C15H13N2O2 253.0972, found 253.0986.
2-(Furan-2-yl)-6-methyl-1H-benzimidazole (2u): brown solid, mp 191–193 °C. 1H NMR (500 MHz, DMSO-d6, δ ppm): 12.77 (1H, s, –NH–), 7.92 (1H, d, J = 4.0 Hz, HAr), 7.49–7.29 (2H, m, HAr), 7.16 (1H, d, J = 4.0 Hz, HAr), 7.02 (1H, d, J = 8.0 Hz, HAr), 6.72 (1H, dd, J = 4.0, 2.0 Hz, HAr), 2.42 (3H, s, –CH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 146.2, 144.9, 143.7, 142.2, 132.5, 123.9, 118.8, 112.7, 111.5, 110.6, 21.8. LC-MS (m/z) [M − H]− calcd for C12H9N2O 197.0720, found 197.0773; [M + H]+ calcd for C12H11N2O 199.0866, found 199.0822.
6-Methyl-2-(pyridin-3-yl)-1H-benzimidazole (2v): yellow solid, mp 246–248 °C. 1H NMR (500 MHz, DMSO-d6, δ ppm): 12.92 (1H, s, –NH–), 9.32 (1H, d, J = 1.5 Hz, HAr), 8.66 (1H, d, J = 3.5 Hz, HAr), 8.47 (1H, d, J = 8.0 Hz, HAr), 7.58–7.56 (1H, m, HAr), 7.48–7.41 (1H, m, HAr), 7.35 (1H, s, HAr), 7.06–7.04 (1H, m, HAr), 2.44 (3H, s, –CH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 150.3, 148.4, 147.4, 133.6, 126.3, 124.0, 123.6, 118.6, 111.1, 99.4, 89.2, 21.3. LC-MS (m/z) [M + H]+ calcd for C13H12N3 210.1026, found 210.0951.
6-Methyl-2-(pyridin-4-yl)-1H-benzimidazole (2w): brown solid, mp 149–150 °C. 1H NMR (500 MHz, DMSO-d6, δ ppm): 13.09 (1H, s, –NH–), 8.74 (2H, d, J = 5.5 Hz, HAr), 8.06 (2H, d, J = 5.5 Hz, HAr), 7.60–7.40 (2H, m, HAr), 7.09 (1H, d, J = 6.0 Hz, HAr), 2.44 (3H, s, –CH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 150.4, 137.2, 133.1, 120.2, 119.1, 111.3, 21.3. LC-MS (m/z) [M − H]− calcd for C13H10N3 208.0880, found 208.1029; [M + H]+ calcd for C13H12N3 210.1026, found 210.0911.
4.2.2.1 Refluxing method. The mixture of 2,6-disubstituted 1H-benzimidazole derivatives 1–2 (1 mmol), potassium carbonate (1 mmol), and substituted halides (1.2 mmol) in acetonitrile (10 mL) was heated at 80 °C for 12–24 h and monitored by TLC. After cooling down, the mixture was poured into cooled water and filtered off in a Büchner funnel. The resulting solid was purified by silica gel column chromatography using hexane/ethyl acetate as eluent. Yields: 35–86%.
4.2.2.2 Microwave-assisted method. The mixture of 2,6-disubstituted 1H-benzimidazole derivatives 1–2 (1 mmol), potassium carbonate (1 mmol), and substituted halides (1.2 mmol) in acetonitrile (10 mL) was irradiated at a power of 300 W for 20–60 min at 80 °C. After cooling down, the mixture was poured into cooled water and filtered off in a Büchner funnel. The resulting solid was purified by silica gel column chromatography using hexane/ethyl acetate as eluent. Yields: 46–98%.
1-Allyl-2-(4-chlorophenyl)-1H-benzimidazole (3a): yellow solid, mp 99–101 °C. IR (ν, cm−1): 1601 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1454 (C
N), 1454 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 842 (C–Cl). 1H NMR (500 MHz, DMSO-d6, δ ppm): 7.80 (2H, d, J = 8.5 Hz, HAr), 7.71 (1H, d, J = 7.0 Hz, HAr), 7.63 (2H, d, J = 8.5 Hz, HAr), 7.54 (1H, d, J = 7.5 Hz, HAr), 7.31–7.25 (2H, m, HAr), 6.10–6.03 (1H, m, –CH
C), 842 (C–Cl). 1H NMR (500 MHz, DMSO-d6, δ ppm): 7.80 (2H, d, J = 8.5 Hz, HAr), 7.71 (1H, d, J = 7.0 Hz, HAr), 7.63 (2H, d, J = 8.5 Hz, HAr), 7.54 (1H, d, J = 7.5 Hz, HAr), 7.31–7.25 (2H, m, HAr), 6.10–6.03 (1H, m, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 5.20 (1H, d, J = 10.5 Hz,
), 5.20 (1H, d, J = 10.5 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 4.93 (2H, s, –CH2–), 4.88 (1H, d, J = 17.5 Hz,
CH2), 4.93 (2H, s, –CH2–), 4.88 (1H, d, J = 17.5 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2). 13C NMR (125 MHz, DMSO-d6, δ ppm): 151.8, 142.5, 135.8, 134.7, 133.3, 130.7, 128.9, 128.8, 122.7, 122.2, 119.2, 116.6, 111.0, 46.6. LC-MS (m/z) [M − H]− calcd for C16H12ClN2 267.0694, found 267.0917; [M + H]+ calcd for C16H14ClN2 269.0840, found 269.0883.
CH2). 13C NMR (125 MHz, DMSO-d6, δ ppm): 151.8, 142.5, 135.8, 134.7, 133.3, 130.7, 128.9, 128.8, 122.7, 122.2, 119.2, 116.6, 111.0, 46.6. LC-MS (m/z) [M − H]− calcd for C16H12ClN2 267.0694, found 267.0917; [M + H]+ calcd for C16H14ClN2 269.0840, found 269.0883.
1-Allyl-2-(3,4-dichlorophenyl)-1H-benzimidazole (3b): yellow solid, mp 97–99 °C. IR (ν, cm−1): 1607 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1450 (C
N), 1450 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 731 (C–Cl). 1H NMR (500 MHz, DMSO-d6, δ ppm): 8.02 (1H, d, J = 1.0 Hz, HAr), 7.84 (1H, d, J = 8.0 Hz, HAr), 7.76 (1H, dd, J = 8.0, 1.0 Hz, HAr), 7.72 (1H, d, J = 7.5 Hz, HAr), 7.56 (1H, d, J = 7.5 Hz, HAr), 7.33–7.26 (2H, m, HAr), 6.11–6.04 (1H, m, –CH
C), 731 (C–Cl). 1H NMR (500 MHz, DMSO-d6, δ ppm): 8.02 (1H, d, J = 1.0 Hz, HAr), 7.84 (1H, d, J = 8.0 Hz, HAr), 7.76 (1H, dd, J = 8.0, 1.0 Hz, HAr), 7.72 (1H, d, J = 7.5 Hz, HAr), 7.56 (1H, d, J = 7.5 Hz, HAr), 7.33–7.26 (2H, m, HAr), 6.11–6.04 (1H, m, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 5.21 (1H, d, J = 10.5 Hz,
), 5.21 (1H, d, J = 10.5 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 4.96 (2H, s, –CH2–), 4.89 (1H, d, J = 17.0 Hz,
CH2), 4.96 (2H, s, –CH2–), 4.89 (1H, d, J = 17.0 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2). 13C NMR (125 MHz, DMSO-d6, δ ppm): 150.5, 142.4, 135.9, 133.3, 132.7, 131.5, 131.0, 130.62, 130.59, 128.9, 123.0, 122.4, 119.4, 116.7, 111.1, 46.6. LC-MS (m/z) [M + H]+ calcd for C16H13Cl2N2 303.0450, found 303.1268.
CH2). 13C NMR (125 MHz, DMSO-d6, δ ppm): 150.5, 142.4, 135.9, 133.3, 132.7, 131.5, 131.0, 130.62, 130.59, 128.9, 123.0, 122.4, 119.4, 116.7, 111.1, 46.6. LC-MS (m/z) [M + H]+ calcd for C16H13Cl2N2 303.0450, found 303.1268.
1-Allyl-2-(3,4-dimethoxyphenyl)-1H-benzimidazole (3c): yellow solid, mp 198–200 °C. IR (ν, cm−1): 1586 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1468 (C
N), 1468 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1253 (C–O). 1H NMR (500 MHz, DMSO-d6, δ ppm): 7.68 (1H, dd, J = 8.5, 2.0 Hz, HAr), 7.50 (1H, dd, J = 8.5, 1.5 Hz, HAr), 7.34 (1H, s, HAr), 7.31 (1H, dd, J = 8.0, 2.0 Hz, HAr), 7.27–7.22 (2H, m, HAr), 7.14 (1H, d, J = 8.5 Hz, HAr), 6.16–6.09 (1H, m, –CH
C), 1253 (C–O). 1H NMR (500 MHz, DMSO-d6, δ ppm): 7.68 (1H, dd, J = 8.5, 2.0 Hz, HAr), 7.50 (1H, dd, J = 8.5, 1.5 Hz, HAr), 7.34 (1H, s, HAr), 7.31 (1H, dd, J = 8.0, 2.0 Hz, HAr), 7.27–7.22 (2H, m, HAr), 7.14 (1H, d, J = 8.5 Hz, HAr), 6.16–6.09 (1H, m, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 5.24 (1H, d, J = 10.0 Hz,
), 5.24 (1H, d, J = 10.0 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 4.94 (2H, s, –CH2–), 4.93 (1H, d, J = 15.5 Hz,
CH2), 4.94 (2H, s, –CH2–), 4.93 (1H, d, J = 15.5 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 3.85 (3H, s, –OCH3), 3.82 (3H, s, –OCH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 153.0, 150.1, 148.6, 142.5, 135.9, 133.6, 122.3, 122.2, 121.9, 121.4, 118.9, 116.4, 112.4, 111.6, 110.7, 55.6, 55.5, 46.6. LC-MS (m/z) [M − H]− calcd for C18H17N2O2 293.1296, found 293.1032; [M + H]+ calcd for C18H19N2O2 295.1441, found 295.1241.
CH2), 3.85 (3H, s, –OCH3), 3.82 (3H, s, –OCH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 153.0, 150.1, 148.6, 142.5, 135.9, 133.6, 122.3, 122.2, 121.9, 121.4, 118.9, 116.4, 112.4, 111.6, 110.7, 55.6, 55.5, 46.6. LC-MS (m/z) [M − H]− calcd for C18H17N2O2 293.1296, found 293.1032; [M + H]+ calcd for C18H19N2O2 295.1441, found 295.1241.
1-Allyl-2-(4-nitrophenyl)-1H-benzimidazole (3d): yellow solid, mp 127–129 °C. IR (ν, cm−1): 1599 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1516 (C
N), 1516 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1344 (N
C), 1344 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR (500 MHz, DMSO-d6, δ ppm): 8.41 (2H, d, J = 8.5 Hz, HAr), 8.08 (2H, d, J = 8.5 Hz, HAr), 7.76 (1H, d, J = 7.5 Hz, HAr), 7.60 (1H, d, J = 8.0 Hz, HAr), 7.36–7.29 (2H, m, HAr), 6.11–6.06 (1H, m, –CH
O). 1H NMR (500 MHz, DMSO-d6, δ ppm): 8.41 (2H, d, J = 8.5 Hz, HAr), 8.08 (2H, d, J = 8.5 Hz, HAr), 7.76 (1H, d, J = 7.5 Hz, HAr), 7.60 (1H, d, J = 8.0 Hz, HAr), 7.36–7.29 (2H, m, HAr), 6.11–6.06 (1H, m, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 5.22 (1H, d, J = 10.0 Hz,
), 5.22 (1H, d, J = 10.0 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.01 (1H, s, –CH2–), 5.00 (1H, s, –CH2–), 4.90 (1H, d, J = 17.0 Hz,
CH2), 5.01 (1H, s, –CH2–), 5.00 (1H, s, –CH2–), 4.90 (1H, d, J = 17.0 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2). 13C NMR (125 MHz, DMSO-d6, δ ppm): 150.7, 148.0, 142.5, 136.2, 136.1, 133.2, 130.2, 123.9, 123.3, 122.6, 119.6, 116.8, 111.2, 46.7. LC-MS (m/z) [M + H]+ calcd for C16H14N3O2 280.1081, found 280.2779.
CH2). 13C NMR (125 MHz, DMSO-d6, δ ppm): 150.7, 148.0, 142.5, 136.2, 136.1, 133.2, 130.2, 123.9, 123.3, 122.6, 119.6, 116.8, 111.2, 46.7. LC-MS (m/z) [M + H]+ calcd for C16H14N3O2 280.1081, found 280.2779.
1-Benzyl-2-(4-chlorophenyl)-1H-benzimidazole (3e): yellow solid, mp 148–149 °C. IR (ν, cm−1): 1514 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1425 (C
N), 1425 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 754 (C–Cl). 1H NMR (500 MHz, DMSO-d6, δ ppm): 7.76–7.73 (3H, m, HAr), 7.59 (2H, d, J = 8.5 Hz, HAr), 7.48 (1H, d, J = 8.5 Hz, HAr), 7.29–7.23 (5H, m, HAr), 6.99 (2H, d, J = 7.5 Hz, HAr), 5.59 (2H, s, –CH2–). 13C NMR (125 MHz, DMSO-d6, δ ppm): 152.1, 142.6, 136.8, 136.0, 134.7, 130.8, 130.0, 128.9, 128.8, 127.5, 126.1, 122.9, 122.3, 119.3, 111.1, 47.4. LC-MS (m/z) [M + H]+ calcd for C20H16ClN2 319.0997, found 319.0913.
C), 754 (C–Cl). 1H NMR (500 MHz, DMSO-d6, δ ppm): 7.76–7.73 (3H, m, HAr), 7.59 (2H, d, J = 8.5 Hz, HAr), 7.48 (1H, d, J = 8.5 Hz, HAr), 7.29–7.23 (5H, m, HAr), 6.99 (2H, d, J = 7.5 Hz, HAr), 5.59 (2H, s, –CH2–). 13C NMR (125 MHz, DMSO-d6, δ ppm): 152.1, 142.6, 136.8, 136.0, 134.7, 130.8, 130.0, 128.9, 128.8, 127.5, 126.1, 122.9, 122.3, 119.3, 111.1, 47.4. LC-MS (m/z) [M + H]+ calcd for C20H16ClN2 319.0997, found 319.0913.
1-Benzyl-2-(3,4-dichlorophenyl)-1H-benzimidazole (3f): yellow solid, mp 113–114 °C. IR (ν, cm−1): 1545 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1409 (C
N), 1409 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 743 (C–Cl). 1H NMR (500 MHz, DMSO-d6, δ ppm): 7.94 (1H, d, J = 1.5 Hz, HAr), 7.78 (1H, d, J = 8.0 Hz, HAr), 7.75 (1H, dd, J = 9.0, 1.5 Hz, HAr), 7.70 (1H, dd, J = 8.5, 2.0 Hz, HAr), 7.53 (1H, dd, J = 9.0, 2.0 Hz, HAr), 7.30–7.25 (4H, m, HAr), 7.23 (1H, d, J = 7.0 Hz, HAr), 7.00 (2H, d, J = 7.0 Hz, HAr), 5.62 (2H, s, –CH2–). 13C NMR (125 MHz, DMSO-d6, δ ppm): 150.7, 142.5, 136.7, 136.1, 132.7, 131.6, 131.0, 130.8, 130.7, 129.0, 128.8, 127.6, 126.1, 123.2, 122.5, 119.5, 111.2, 47.5. LC-MS (m/z) [M + H]+ calcd for C20H15Cl2N2 353.0607, found 353.0698.
C), 743 (C–Cl). 1H NMR (500 MHz, DMSO-d6, δ ppm): 7.94 (1H, d, J = 1.5 Hz, HAr), 7.78 (1H, d, J = 8.0 Hz, HAr), 7.75 (1H, dd, J = 9.0, 1.5 Hz, HAr), 7.70 (1H, dd, J = 8.5, 2.0 Hz, HAr), 7.53 (1H, dd, J = 9.0, 2.0 Hz, HAr), 7.30–7.25 (4H, m, HAr), 7.23 (1H, d, J = 7.0 Hz, HAr), 7.00 (2H, d, J = 7.0 Hz, HAr), 5.62 (2H, s, –CH2–). 13C NMR (125 MHz, DMSO-d6, δ ppm): 150.7, 142.5, 136.7, 136.1, 132.7, 131.6, 131.0, 130.8, 130.7, 129.0, 128.8, 127.6, 126.1, 123.2, 122.5, 119.5, 111.2, 47.5. LC-MS (m/z) [M + H]+ calcd for C20H15Cl2N2 353.0607, found 353.0698.
1-Benzyl-2-(3,4-dimethoxyphenyl)-1H-benzimidazole (3g): yellow solid, mp 140–141 °C. IR (ν, cm−1): 1601 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1461 (C
N), 1461 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C). 1H NMR (500 MHz, DMSO-d6, δ ppm): 7.71 (1H, d, J = 7.0 Hz, HAr), 7.44 (1H, d, J = 7.5 Hz, HAr), 7.32 (2H, t, J = 7.5 Hz, HAr), 7.27–7.20 (5H, m, HAr), 7.08 (1H, d, J = 8.5 Hz, HAr), 7.04 (2H, d, J = 7.5 Hz, HAr), 5.59 (2H, s, –CH2–), 3.81 (3H, s, –OCH3), 3.66 (3H, s, –OCH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 153.3, 150.1, 148.6, 142.6, 137.2, 136.1, 128.8, 127.4, 125.9, 122.5, 122.3, 122.1, 121.6, 119.0, 112.3, 111.7, 110.8, 55.6, 55.3, 47.5. LC-MS (m/z) [M + H]+ calcd for C22H21N2O2 345.1598, found 345.1474.
C). 1H NMR (500 MHz, DMSO-d6, δ ppm): 7.71 (1H, d, J = 7.0 Hz, HAr), 7.44 (1H, d, J = 7.5 Hz, HAr), 7.32 (2H, t, J = 7.5 Hz, HAr), 7.27–7.20 (5H, m, HAr), 7.08 (1H, d, J = 8.5 Hz, HAr), 7.04 (2H, d, J = 7.5 Hz, HAr), 5.59 (2H, s, –CH2–), 3.81 (3H, s, –OCH3), 3.66 (3H, s, –OCH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 153.3, 150.1, 148.6, 142.6, 137.2, 136.1, 128.8, 127.4, 125.9, 122.5, 122.3, 122.1, 121.6, 119.0, 112.3, 111.7, 110.8, 55.6, 55.3, 47.5. LC-MS (m/z) [M + H]+ calcd for C22H21N2O2 345.1598, found 345.1474.
1-Benzyl-2-(4-ethoxyphenyl)-1H-benzimidazole (3h): yellow solid, mp 227–229 °C. IR (ν, cm−1): 1608 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1457 (C
N), 1457 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1257 (C–O). 1H NMR (500 MHz, DMSO-d6, δ ppm): 7.69 (1H, d, J = 7.5 Hz, HAr), 7.65 (2H, d, J = 8.0 Hz, HAr), 7.42 (1H, d, J = 7.5 Hz, HAr), 7.29 (2H, d, J = 7.0 Hz, HAr), 7.25–7.19 (3H, m, HAr), 7.05 (2H, d, J = 8.0 Hz, HAr), 7.01 (2H, d, J = 7.0 Hz, HAr), 5.37 (2H, s, –CH2–), 4.09 (2H, q, J = 6.5 Hz, –CH
C), 1257 (C–O). 1H NMR (500 MHz, DMSO-d6, δ ppm): 7.69 (1H, d, J = 7.5 Hz, HAr), 7.65 (2H, d, J = 8.0 Hz, HAr), 7.42 (1H, d, J = 7.5 Hz, HAr), 7.29 (2H, d, J = 7.0 Hz, HAr), 7.25–7.19 (3H, m, HAr), 7.05 (2H, d, J = 8.0 Hz, HAr), 7.01 (2H, d, J = 7.0 Hz, HAr), 5.37 (2H, s, –CH2–), 4.09 (2H, q, J = 6.5 Hz, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 1.34 (3H, t, J = 6.5 Hz, –CH
), 1.34 (3H, t, J = 6.5 Hz, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ). 13C NMR (125 MHz, DMSO-d6, δ ppm): 159.6, 153.2, 142.7, 137.0, 135.9, 130.4, 128.7, 127.4, 126.0, 122.3, 122.1, 122.0, 119.0, 114.6, 110.8, 63.2, 47.4, 14.5. LC-MS (m/z) [M − H]− calcd for C22H19N2O 327.1503, found 327.1003; [M + H]+ calcd for C22H21N2O 329.1648, found 329.1559.
). 13C NMR (125 MHz, DMSO-d6, δ ppm): 159.6, 153.2, 142.7, 137.0, 135.9, 130.4, 128.7, 127.4, 126.0, 122.3, 122.1, 122.0, 119.0, 114.6, 110.8, 63.2, 47.4, 14.5. LC-MS (m/z) [M − H]− calcd for C22H19N2O 327.1503, found 327.1003; [M + H]+ calcd for C22H21N2O 329.1648, found 329.1559.
1-Benzyl-2-(4-fluorophenyl)-1H-benzimidazole (3i): yellow solid, mp 129–130 °C. IR (ν, cm−1): 1572 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1397 (C
N), 1397 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1220 (C–F). 1H NMR (500 MHz, DMSO-d6, δ ppm): 7.77 (1H, d, J = 8.5 Hz, HAr), 7.67 (1H, d, J = 8.5 Hz, HAr), 7.72 (1H, dd, J = 8.0, 1.0 Hz, HAr), 7.48 (1H, dd, J = 8.0, 1.5 Hz, HAr), 7.34 (2H, t, J = 8.5 Hz, HAr), 7.30–7.23 (5H, m, HAr), 6.99 (2H, d, J = 7.5 Hz, HAr), 5.58 (2H, s, –CH2–). 13C NMR (125 MHz, DMSO-d6, δ ppm): 163.9, 161.9, 152.3, 142.5, 136.8, 135.9, 131.42, 131.36, 128.8, 127.5, 126.64, 126.61, 126.1, 122.8, 122.3, 119.2, 116.0, 115.8, 111.1, 47.4. LC-MS (m/z) [M + H]+ calcd for C20H16FN2 303.1292, found 303.1268.
C), 1220 (C–F). 1H NMR (500 MHz, DMSO-d6, δ ppm): 7.77 (1H, d, J = 8.5 Hz, HAr), 7.67 (1H, d, J = 8.5 Hz, HAr), 7.72 (1H, dd, J = 8.0, 1.0 Hz, HAr), 7.48 (1H, dd, J = 8.0, 1.5 Hz, HAr), 7.34 (2H, t, J = 8.5 Hz, HAr), 7.30–7.23 (5H, m, HAr), 6.99 (2H, d, J = 7.5 Hz, HAr), 5.58 (2H, s, –CH2–). 13C NMR (125 MHz, DMSO-d6, δ ppm): 163.9, 161.9, 152.3, 142.5, 136.8, 135.9, 131.42, 131.36, 128.8, 127.5, 126.64, 126.61, 126.1, 122.8, 122.3, 119.2, 116.0, 115.8, 111.1, 47.4. LC-MS (m/z) [M + H]+ calcd for C20H16FN2 303.1292, found 303.1268.
1-Benzyl-2-(3-methoxyphenyl)-1H-benzimidazole (3j): yellow solid, mp 107–108 °C. IR (ν, cm−1): 1568 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1453 (C
N), 1453 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C). 1H NMR (500 MHz, DMSO-d6, δ ppm): 7.74 (1H, dd, J = 7.0, 1.0 Hz, HAr), 7.47–7.42 (2H, m, HAr), 7.31–7.28 (3H, m, HAr), 7.26–7.23 (4H, m, HAr), 7.09 (1H, dd, J = 8.0, 2.0 Hz, HAr), 7.02 (2H, d, J = 7.5 Hz, HAr), 5.59 (2H, s, –CH2–), 3.72 (3H, s, –OCH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 159.2, 153.0, 142.6, 137.0, 135.9, 131.3, 129.9, 128.8, 127.4, 126.0, 122.7, 122.2, 121.2, 119.3, 115.8, 114.2, 111.0, 55.1, 47.5. LC-MS (m/z) [M + H]+ calcd for C21H19N2O 315.1492, found 315.1444.
C). 1H NMR (500 MHz, DMSO-d6, δ ppm): 7.74 (1H, dd, J = 7.0, 1.0 Hz, HAr), 7.47–7.42 (2H, m, HAr), 7.31–7.28 (3H, m, HAr), 7.26–7.23 (4H, m, HAr), 7.09 (1H, dd, J = 8.0, 2.0 Hz, HAr), 7.02 (2H, d, J = 7.5 Hz, HAr), 5.59 (2H, s, –CH2–), 3.72 (3H, s, –OCH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 159.2, 153.0, 142.6, 137.0, 135.9, 131.3, 129.9, 128.8, 127.4, 126.0, 122.7, 122.2, 121.2, 119.3, 115.8, 114.2, 111.0, 55.1, 47.5. LC-MS (m/z) [M + H]+ calcd for C21H19N2O 315.1492, found 315.1444.
1-Benzyl-2-(4-nitrophenyl)-1H-benzimidazole (3k): yellow solid, mp 191–192 °C. IR (ν, cm−1): 1602 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1498 (C
N), 1498 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1343 (N
C), 1343 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR (500 MHz, DMSO-d6, δ ppm): 8.35 (2H, d, J = 9.0 Hz, HAr), 8.04 (2H, d, J = 8.5 Hz, HAr), 7.78 (1H, d, J = 9.0 Hz, HAr), 7.56 (1H, d, J = 9.0 Hz, HAr), 7.31–7.21 (5H, m, HAr), 6.99 (2H, d, J = 7.5 Hz, HAr), 5.67 (2H, s, –CH2–). 13C NMR (125 MHz, DMSO-d6, δ ppm): 151.0, 148.0, 142.6, 136.6, 136.3, 136.2, 130.3, 128.8, 127.6, 126.1, 123.9, 123.5, 122.7, 119.7, 111.4, 47.6. LC-MS (m/z) [M + H]+ calcd for C20H16N3O2 330.1237, found 330.1215.
O). 1H NMR (500 MHz, DMSO-d6, δ ppm): 8.35 (2H, d, J = 9.0 Hz, HAr), 8.04 (2H, d, J = 8.5 Hz, HAr), 7.78 (1H, d, J = 9.0 Hz, HAr), 7.56 (1H, d, J = 9.0 Hz, HAr), 7.31–7.21 (5H, m, HAr), 6.99 (2H, d, J = 7.5 Hz, HAr), 5.67 (2H, s, –CH2–). 13C NMR (125 MHz, DMSO-d6, δ ppm): 151.0, 148.0, 142.6, 136.6, 136.3, 136.2, 130.3, 128.8, 127.6, 126.1, 123.9, 123.5, 122.7, 119.7, 111.4, 47.6. LC-MS (m/z) [M + H]+ calcd for C20H16N3O2 330.1237, found 330.1215.
1-(4-Chlorobenzyl)-2-(4-chlorophenyl)-1H-benzimidazole (3l): white solid, mp 147–148 °C. IR (ν, cm−1): 1557 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1445 (C
N), 1445 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 744 (C–Cl). 1H NMR (500 MHz, DMSO-d6, δ ppm): 7.76 (1H, d, J = 8.0 Hz, HAr), 7.68 (2H, d, J = 8.5 Hz, HAr), 7.58 (2H, d, J = 8.5 Hz, HAr), 7.50 (1H, d, J = 7.5 Hz, HAr), 7.42 (1H, d, J = 7.5 Hz, HAr), 7.33–7.20 (4H, m, HAr), 6.62 (1H, d, J = 8.0 Hz, HAr), 5.61 (2H, s, –CH2–). 13C NMR (125 MHz, DMSO-d6, δ ppm): 142.5, 135.9, 134.8, 133.8, 131.3, 130.6, 129.7, 129.4, 128.9, 128.8, 127.7, 127.3, 123.0, 122.5, 119.4, 110.9, 45.8. LC-MS (m/z) [M + H]+ calcd for C20H15Cl2N2 353.0607, found 353.0698.
C), 744 (C–Cl). 1H NMR (500 MHz, DMSO-d6, δ ppm): 7.76 (1H, d, J = 8.0 Hz, HAr), 7.68 (2H, d, J = 8.5 Hz, HAr), 7.58 (2H, d, J = 8.5 Hz, HAr), 7.50 (1H, d, J = 7.5 Hz, HAr), 7.42 (1H, d, J = 7.5 Hz, HAr), 7.33–7.20 (4H, m, HAr), 6.62 (1H, d, J = 8.0 Hz, HAr), 5.61 (2H, s, –CH2–). 13C NMR (125 MHz, DMSO-d6, δ ppm): 142.5, 135.9, 134.8, 133.8, 131.3, 130.6, 129.7, 129.4, 128.9, 128.8, 127.7, 127.3, 123.0, 122.5, 119.4, 110.9, 45.8. LC-MS (m/z) [M + H]+ calcd for C20H15Cl2N2 353.0607, found 353.0698.
1-Allyl-2-(4-chlorophenyl)-6-methyl-1H-benzimidazole (4a): white solid, mp 138–140 °C. IR (ν, cm−1): 1608 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1460 (C
N), 1460 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 803 (C–Cl). 1H NMR (500 MHz, DMSO-d6, δ ppm): 7.78 (2H, d, J = 8.0 Hz, HAr), 7.63 (2H, d, J = 8.0 Hz, HAr), 7.58 (1H, d, J = 8.0 Hz, HAr), 7.32 (1H, s, HAr), 7.09 (1H, d, J = 8.5 Hz, HAr), 6.09–6.04 (1H, m, –CH
C), 803 (C–Cl). 1H NMR (500 MHz, DMSO-d6, δ ppm): 7.78 (2H, d, J = 8.0 Hz, HAr), 7.63 (2H, d, J = 8.0 Hz, HAr), 7.58 (1H, d, J = 8.0 Hz, HAr), 7.32 (1H, s, HAr), 7.09 (1H, d, J = 8.5 Hz, HAr), 6.09–6.04 (1H, m, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 5.20 (1H, d, J = 10.0 Hz,
), 5.20 (1H, d, J = 10.0 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 4.89 (2H, s, –CH2–), 4.84 (1H, d, J = 20.5 Hz,
CH2), 4.89 (2H, s, –CH2–), 4.84 (1H, d, J = 20.5 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 2.45 (3H, s, –CH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 151.3, 140.6, 136.1, 134.5, 133.4, 132.2, 130.6, 129.0, 124.2, 123.8, 118.9, 116.5, 110.6, 46.6, 21.4. LC-MS (m/z) [M − H]− calcd for C17H14ClN2 281.0851, found 281.0440; [M + H]+ calcd for C17H16ClN2 283.0997, found 283.0922.
CH2), 2.45 (3H, s, –CH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 151.3, 140.6, 136.1, 134.5, 133.4, 132.2, 130.6, 129.0, 124.2, 123.8, 118.9, 116.5, 110.6, 46.6, 21.4. LC-MS (m/z) [M − H]− calcd for C17H14ClN2 281.0851, found 281.0440; [M + H]+ calcd for C17H16ClN2 283.0997, found 283.0922.
1-Allyl-6-methyl-2-(4-nitrophenyl)-1H-benzimidazole (4b): orange solid, mp 100–102 °C. IR (ν, cm−1): 1601 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1515 (C
N), 1515 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1340 (N
C), 1340 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR (500 MHz, DMSO-d6, δ ppm): 8.38 (2H, d, J = 8.5 Hz, HAr), 8.05 (2H, d, J = 8.0 Hz, HAr), 7.46 (1H, d, J = 8.5 Hz, HAr), 7.38 (1H, s, HAr), 7.15 (1H, d, J = 9.0 Hz, HAr), 6.11–6.04 (1H, m, –CH
O). 1H NMR (500 MHz, DMSO-d6, δ ppm): 8.38 (2H, d, J = 8.5 Hz, HAr), 8.05 (2H, d, J = 8.0 Hz, HAr), 7.46 (1H, d, J = 8.5 Hz, HAr), 7.38 (1H, s, HAr), 7.15 (1H, d, J = 9.0 Hz, HAr), 6.11–6.04 (1H, m, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 5.21 (1H, d, J = 9.5 Hz,
), 5.21 (1H, d, J = 9.5 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 4.96 (2H, s, –CH2–), 4.87 (1H, d, J = 17.5 Hz,
CH2), 4.96 (2H, s, –CH2–), 4.87 (1H, d, J = 17.5 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 2.46 (3H, s, –CH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 150.2, 147.8, 142.9, 136.4, 134.3, 133.2, 131.7, 130.1, 124.9, 123.8, 119.2, 116.7, 110.8, 46.7, 21.5. LC-MS (m/z) [M − H]− calcd for C17H14N3O2 292.1092, found 292.0119; [M + H]+ calcd for C17H16N3O2 294.1237, found 294.0211.
CH2), 2.46 (3H, s, –CH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 150.2, 147.8, 142.9, 136.4, 134.3, 133.2, 131.7, 130.1, 124.9, 123.8, 119.2, 116.7, 110.8, 46.7, 21.5. LC-MS (m/z) [M − H]− calcd for C17H14N3O2 292.1092, found 292.0119; [M + H]+ calcd for C17H16N3O2 294.1237, found 294.0211.
1-Benzyl-2-(4-chlorophenyl)-6-methyl-1H-benzimidazole (4c): yellow solid, mp 123–125 °C. IR (ν, cm−1): 1563 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1500 (C
N), 1500 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 750 (C–Cl). 1H NMR (500 MHz, DMSO-d6, δ ppm): 7.74–7.71 (2H, m, HAr), 7.62–7.56 (3H, m, HAr), 7.31–7.24 (4H, m, HAr), 7.10 (1H, d, J = 8.5 Hz, HAr), 6.98 (2H, d, J = 7.0 Hz, HAr), 5.55 (2H, s, –CH2–), 2.42 (3H, s, –CH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 151.6, 140.8, 136.9, 136.3, 134.5, 132.4, 130.7, 129.1, 128.9, 128.8, 127.5, 126.0, 123.9, 119.0, 110.7, 47.3, 21.4. LC-MS (m/z) [M + H]+ calcd for C21H18ClN2 333.1153, found 333.1102.
C), 750 (C–Cl). 1H NMR (500 MHz, DMSO-d6, δ ppm): 7.74–7.71 (2H, m, HAr), 7.62–7.56 (3H, m, HAr), 7.31–7.24 (4H, m, HAr), 7.10 (1H, d, J = 8.5 Hz, HAr), 6.98 (2H, d, J = 7.0 Hz, HAr), 5.55 (2H, s, –CH2–), 2.42 (3H, s, –CH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 151.6, 140.8, 136.9, 136.3, 134.5, 132.4, 130.7, 129.1, 128.9, 128.8, 127.5, 126.0, 123.9, 119.0, 110.7, 47.3, 21.4. LC-MS (m/z) [M + H]+ calcd for C21H18ClN2 333.1153, found 333.1102.
1-Benzyl-2-(3,4-dichlorophenyl)-6-methyl-1H-benzimidazole (4d): brown solid, mp 138–140 °C. IR (ν, cm−1): 1613 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1459 (C
N), 1459 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 714 (C–Cl). 1H NMR (500 MHz, DMSO-d6, δ ppm): 7.93 (1H, dd, J = 8.0, 2.0 Hz, HAr), 7.71 (1H, d, J = 2.0 Hz, HAr), 7.63 (1H, d, J = 8.5 Hz, HAr), 7.54 (1H, s, HAr), 7.41 (1H, d, J = 8.5 Hz, HAr), 7.35–7.23 (3H, m, HAr), 7.11 (1H, d, J = 9.0 Hz, HAr), 7.00 (2H, d, J = 8.5 Hz, HAr), 5.60 (2H, s, –CH2–), 2.43 (3H, s, –CH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 151.1, 143.3, 141.1, 137.4, 136.9, 134.8, 133.2, 132.2, 131.5, 131.2, 129.4, 128.0, 126.5, 125.2, 124.6, 119.6, 111.3, 48.0, 22.0. LC-MS (m/z) [M + H]+ calcd for C21H17Cl2N2 367.0763, found 367.0701.
C), 714 (C–Cl). 1H NMR (500 MHz, DMSO-d6, δ ppm): 7.93 (1H, dd, J = 8.0, 2.0 Hz, HAr), 7.71 (1H, d, J = 2.0 Hz, HAr), 7.63 (1H, d, J = 8.5 Hz, HAr), 7.54 (1H, s, HAr), 7.41 (1H, d, J = 8.5 Hz, HAr), 7.35–7.23 (3H, m, HAr), 7.11 (1H, d, J = 9.0 Hz, HAr), 7.00 (2H, d, J = 8.5 Hz, HAr), 5.60 (2H, s, –CH2–), 2.43 (3H, s, –CH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 151.1, 143.3, 141.1, 137.4, 136.9, 134.8, 133.2, 132.2, 131.5, 131.2, 129.4, 128.0, 126.5, 125.2, 124.6, 119.6, 111.3, 48.0, 22.0. LC-MS (m/z) [M + H]+ calcd for C21H17Cl2N2 367.0763, found 367.0701.
1-Benzyl-2-(4-fluorophenyl)-6-methyl-1H-benzimidazole (4e): brown solid, mp 111–112 °C. IR (ν, cm−1): 1607 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1479 (C
N), 1479 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1219 (C–F). 1H NMR (500 MHz, DMSO-d6, δ ppm): 7.75 (2H, d, J = 8.5 Hz, HAr), 7.61 (1H, d, J = 8.0 Hz, HAr), 7.52 (1H, s, HAr), 7.39–7.23 (5H, m, HAr), 7.08 (1H, d, J = 9.0 Hz, HAr), 6.99 (2H, d, J = 8.5 Hz, HAr), 5.55 (2H, s, –CH2–), 2.43 (3H, s, –CH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 164.5, 152.7, 143.3, 141.2, 137.4, 134.5, 132.7, 131.8, 129.3, 127.9, 126.5, 124.7, 119.3, 116.4, 111.2, 47.9, 21.9. LC-MS (m/z) [M + H]+ calcd for C21H18FN2 317.1449, found 317.1362.
C), 1219 (C–F). 1H NMR (500 MHz, DMSO-d6, δ ppm): 7.75 (2H, d, J = 8.5 Hz, HAr), 7.61 (1H, d, J = 8.0 Hz, HAr), 7.52 (1H, s, HAr), 7.39–7.23 (5H, m, HAr), 7.08 (1H, d, J = 9.0 Hz, HAr), 6.99 (2H, d, J = 8.5 Hz, HAr), 5.55 (2H, s, –CH2–), 2.43 (3H, s, –CH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 164.5, 152.7, 143.3, 141.2, 137.4, 134.5, 132.7, 131.8, 129.3, 127.9, 126.5, 124.7, 119.3, 116.4, 111.2, 47.9, 21.9. LC-MS (m/z) [M + H]+ calcd for C21H18FN2 317.1449, found 317.1362.
1-Benzyl-6-methyl-2-(4-(methylthio)phenyl)-1H-benzimidazole (4f): brown solid, mp 123–124 °C. IR (ν, cm−1): 1599 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1496 (C
N), 1496 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 588 (C–S). 1H NMR (500 MHz, DMSO-d6, δ ppm): 7.65 (2H, d, J = 8.5 Hz, HAr), 7.59 (1H, d, J = 8.0 Hz, HAr), 7.39–7.35 (3H, m, HAr), 7.33–7.23 (3H, m, HAr), 7.08 (1H, d, J = 9.0 Hz, HAr), 7.00 (2H, d, J = 8.5 Hz, HAr), 5.56 (2H, s, –CH2–), 2.52 (3H, s, –SCH3), 2.42 (3H, s, –CH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 153.8, 150.8, 141.4, 136.6, 136.5, 129.6, 128.4, 127.2, 126.1, 125.7, 121.7, 119.5, 116.3, 111.4, 110.1, 47.5, 21.8, 14.9. LC-MS (m/z) [M + H]+ calcd for C22H21N2S 345.1420, found 345.1344.
C), 588 (C–S). 1H NMR (500 MHz, DMSO-d6, δ ppm): 7.65 (2H, d, J = 8.5 Hz, HAr), 7.59 (1H, d, J = 8.0 Hz, HAr), 7.39–7.35 (3H, m, HAr), 7.33–7.23 (3H, m, HAr), 7.08 (1H, d, J = 9.0 Hz, HAr), 7.00 (2H, d, J = 8.5 Hz, HAr), 5.56 (2H, s, –CH2–), 2.52 (3H, s, –SCH3), 2.42 (3H, s, –CH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 153.8, 150.8, 141.4, 136.6, 136.5, 129.6, 128.4, 127.2, 126.1, 125.7, 121.7, 119.5, 116.3, 111.4, 110.1, 47.5, 21.8, 14.9. LC-MS (m/z) [M + H]+ calcd for C22H21N2S 345.1420, found 345.1344.
1-Benzyl-6-methyl-2-(4-nitrophenyl)-1H-benzimidazole (4g): yellow solid, mp 165–167 °C. IR (ν, cm−1): 1560 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1513 (C
N), 1513 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1341 (N
C), 1341 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR (500 MHz, DMSO-d6, δ ppm): 8.34 (2H, d, J = 8.5 Hz, HAr), 8.02 (2H, d, J = 8.5 Hz, HAr), 7.66 (1H, d, J = 8.0 Hz, HAr), 7.36 (1H, s, HAr), 7.30–7.21 (3H, m, HAr), 7.12 (1H, d, J = 7.5 Hz, HAr), 6.98 (2H, d, J = 7.0 Hz, HAr), 5.64 (2H, s, –CH2–), 2.44 (3H, s, –CH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 150.9, 147.9, 143.0, 140.8, 136.7, 134.4, 133.1, 131.9, 130.3, 128.9, 127.6, 126.1, 124.4, 119.3, 111.0, 47.6, 21.5. LC-MS (m/z) [M + H]+ calcd for C21H18N3O2 344.1394, found 344.1229.
O). 1H NMR (500 MHz, DMSO-d6, δ ppm): 8.34 (2H, d, J = 8.5 Hz, HAr), 8.02 (2H, d, J = 8.5 Hz, HAr), 7.66 (1H, d, J = 8.0 Hz, HAr), 7.36 (1H, s, HAr), 7.30–7.21 (3H, m, HAr), 7.12 (1H, d, J = 7.5 Hz, HAr), 6.98 (2H, d, J = 7.0 Hz, HAr), 5.64 (2H, s, –CH2–), 2.44 (3H, s, –CH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 150.9, 147.9, 143.0, 140.8, 136.7, 134.4, 133.1, 131.9, 130.3, 128.9, 127.6, 126.1, 124.4, 119.3, 111.0, 47.6, 21.5. LC-MS (m/z) [M + H]+ calcd for C21H18N3O2 344.1394, found 344.1229.
1-(2-Chlorobenzyl)-2-(4-chlorophenyl)-6-methyl-1H-benzimidazole (4h): yellow solid, mp 137–138 °C. IR (ν, cm−1): 1634 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1468 (C
N), 1468 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 754 (C–Cl). 1H NMR (500 MHz, DMSO-d6, δ ppm): 7.65 (2H, d, J = 8.5 Hz, HAr), 7.56 (2H, d, J = 8.5 Hz, HAr), 7.51 (1H, d, J = 9.0 Hz, HAr), 7.33–7.20 (3H, m, HAr), 7.20 (1H, d, J = 8.5 Hz, HAr), 7.10 (1H, d, J = 8.0 Hz, HAr), 6.58 (1H, t, J = 8.5 Hz, HAr), 5.56 (2H, s, –CH2–), 2.43 (3H, s, –CH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 152.1, 142.9, 136.2, 134.6, 134.0, 132.6, 131.6, 131.3, 130.5, 129.6, 128.8, 127.7, 127.2, 124.5, 124.1, 119.1, 110.5, 45.8, 21.4. LC-MS (m/z) [M − H]− calcd for C13H10N3 365.0618, found 364.9981; [M + H]+ calcd for C21H17Cl2N2 367.0763, found 367.0769.
C), 754 (C–Cl). 1H NMR (500 MHz, DMSO-d6, δ ppm): 7.65 (2H, d, J = 8.5 Hz, HAr), 7.56 (2H, d, J = 8.5 Hz, HAr), 7.51 (1H, d, J = 9.0 Hz, HAr), 7.33–7.20 (3H, m, HAr), 7.20 (1H, d, J = 8.5 Hz, HAr), 7.10 (1H, d, J = 8.0 Hz, HAr), 6.58 (1H, t, J = 8.5 Hz, HAr), 5.56 (2H, s, –CH2–), 2.43 (3H, s, –CH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 152.1, 142.9, 136.2, 134.6, 134.0, 132.6, 131.6, 131.3, 130.5, 129.6, 128.8, 127.7, 127.2, 124.5, 124.1, 119.1, 110.5, 45.8, 21.4. LC-MS (m/z) [M − H]− calcd for C13H10N3 365.0618, found 364.9981; [M + H]+ calcd for C21H17Cl2N2 367.0763, found 367.0769.
1-(2-Chlorobenzyl)-6-methyl-2-(4-nitrophenyl)-1H-benzimidazole (4i): yellow solid, mp 215–217 °C. IR (ν, cm−1): 1600 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1518 (C
N), 1518 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1345 (N
C), 1345 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR (500 MHz, DMSO-d6, δ ppm): 8.33 (2H, d, J = 8.5 Hz, HAr), 7.96 (2H, d, J = 9.0 Hz, HAr), 7.69 (1H, d, J = 8.5 Hz, HAr), 7.52 (1H, s, HAr), 7.35–7.29 (2H, m, HAr), 7.21 (1H, d, J = 8.5 Hz, HAr), 7.15 (1H, d, J = 8.0 Hz, HAr), 6.64 (1H, d, J = 8.0 Hz, HAr), 5.65 (2H, s, –CH2–), 2.44 (3H, s, –CH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 151.1, 147.9, 140.8, 136.4, 134.3, 133.7, 132.0, 131.4, 130.1, 129.7, 127.8, 127.5, 125.1, 124.5, 123.9, 119.4, 110.8, 46.0, 21.4. LC-MS (m/z) [M + H]+ calcd for C21H17ClN3O2 378.1004, found 378.0929.
O). 1H NMR (500 MHz, DMSO-d6, δ ppm): 8.33 (2H, d, J = 8.5 Hz, HAr), 7.96 (2H, d, J = 9.0 Hz, HAr), 7.69 (1H, d, J = 8.5 Hz, HAr), 7.52 (1H, s, HAr), 7.35–7.29 (2H, m, HAr), 7.21 (1H, d, J = 8.5 Hz, HAr), 7.15 (1H, d, J = 8.0 Hz, HAr), 6.64 (1H, d, J = 8.0 Hz, HAr), 5.65 (2H, s, –CH2–), 2.44 (3H, s, –CH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 151.1, 147.9, 140.8, 136.4, 134.3, 133.7, 132.0, 131.4, 130.1, 129.7, 127.8, 127.5, 125.1, 124.5, 123.9, 119.4, 110.8, 46.0, 21.4. LC-MS (m/z) [M + H]+ calcd for C21H17ClN3O2 378.1004, found 378.0929.
1-(4-Chlorobenzyl)-6-methyl-2-(4-nitrophenyl)-1H-benzimidazole (4j): yellow solid, mp 178–180 °C. IR (ν, cm−1): 1601 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1518 (C
N), 1518 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1342 (N
C), 1342 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR (500 MHz, DMSO-d6, δ ppm): 8.35 (2H, d, J = 8.5 Hz, HAr), 8.00 (2H, d, J = 8.0 Hz, HAr), 7.66 (1H, d, J = 8.0 Hz, HAr), 7.57 (1H, s, HAr), 7.34 (2H, t, J = 8.0 Hz, HAr), 7.14 (1H, d, J = 8.0 Hz, HAr), 6.99 (2H, d, J = 8.0 Hz, HAr), 5.63 (2H, s, –CH2–), 2.44 (3H, s, –CH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 150.9, 147.9, 143.0, 140.8, 136.4, 134.3, 133.3, 132.2, 130.3, 128.9, 128.1, 125.1, 124.5, 119.4, 110.9, 46.9, 21.5. LC-MS (m/z) [M + H]+ calcd for C21H17ClN3O2 378.1004, found 378.0932.
O). 1H NMR (500 MHz, DMSO-d6, δ ppm): 8.35 (2H, d, J = 8.5 Hz, HAr), 8.00 (2H, d, J = 8.0 Hz, HAr), 7.66 (1H, d, J = 8.0 Hz, HAr), 7.57 (1H, s, HAr), 7.34 (2H, t, J = 8.0 Hz, HAr), 7.14 (1H, d, J = 8.0 Hz, HAr), 6.99 (2H, d, J = 8.0 Hz, HAr), 5.63 (2H, s, –CH2–), 2.44 (3H, s, –CH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 150.9, 147.9, 143.0, 140.8, 136.4, 134.3, 133.3, 132.2, 130.3, 128.9, 128.1, 125.1, 124.5, 119.4, 110.9, 46.9, 21.5. LC-MS (m/z) [M + H]+ calcd for C21H17ClN3O2 378.1004, found 378.0932.
1-Benzyl-2-(furan-2-yl)-6-methyl-1H-benzimidazole (4k): brown solid, mp 140–142 °C. IR (ν, cm−1): 1515 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1495 (C
N), 1495 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C). 1H NMR (500 MHz, DMSO-d6, δ ppm): 7.92 (1H, d, J = 5.0 Hz, HAr), 7.57 (1H, d, J = 8.5 Hz, HAr), 7.39 (1H, s, HAr), 7.31–7.23 (3H, m, HAr), 7.12–7.07 (4H, m, HAr), 6.71 (1H, dd, J = 4.0, 1.5 Hz, HAr), 5.77 (2H, s, –CH2–), 2.42 (3H, s, –CH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 145.3, 144.1, 143.4, 137.7, 136.3, 134.3, 133.0, 132.1, 129.2, 127.9, 126.7, 124.9, 119.2, 112.8, 110.8, 47.8, 21.9. LC-MS (m/z) [M + H]+ calcd for C19H17N2O 289.1335, found 289.1230.
C). 1H NMR (500 MHz, DMSO-d6, δ ppm): 7.92 (1H, d, J = 5.0 Hz, HAr), 7.57 (1H, d, J = 8.5 Hz, HAr), 7.39 (1H, s, HAr), 7.31–7.23 (3H, m, HAr), 7.12–7.07 (4H, m, HAr), 6.71 (1H, dd, J = 4.0, 1.5 Hz, HAr), 5.77 (2H, s, –CH2–), 2.42 (3H, s, –CH3). 13C NMR (125 MHz, DMSO-d6, δ ppm): 145.3, 144.1, 143.4, 137.7, 136.3, 134.3, 133.0, 132.1, 129.2, 127.9, 126.7, 124.9, 119.2, 112.8, 110.8, 47.8, 21.9. LC-MS (m/z) [M + H]+ calcd for C19H17N2O 289.1335, found 289.1230.
4.3. In vitro antibacterial and antifungal activities
The minimum inhibitory concentration (MIC) was determined by the microtitre broth dilution method with positive controls (ciprofloxacin for antibacterial activity and fluconazole for antifungal activity).4,29 All bacterial strains were maintained at ±37 °C for 24–48 h on a nutrient agar medium. Meanwhile, all fungal strains were maintained at ±25 °C for 48 h on potato dextrose agar. The different concentration gradients (2, 4, 8, 16, 32, 64, 128, 256, 512, and 1024 μg mL−1) of tested compounds and positive controls were prepared in the media. The inoculum was prepared by dilution in broth media of each bacteria and fungi to give a final concentration of 5 × 105 CFU mL−1. The trays were covered and placed in plastic bags to prevent evaporation and are then incubated at 35 °C for 18–20 h with the bacteria, and at 25 °C for 72 h with fungi. The MIC was determined to be the lowest concentration that completely inhibits the growth of the organism. All MIC determinations were done in triplicates in independent experiments.4.4. In vitro anticancer activity
The cytotoxic activity of the synthesized compounds was evaluated using the methyl thiazolyl tetrazolium (MTT) method. Paclitaxel as anticancer drug was used as the positive control. The MTT assay detects the reduction of yellow tetrazolium by metabolically active cells to be purple formazan measured using spectrophotometry.2,50 The cells lines were seeded into 96-well plates at a density of 5 × 103 cells per well and replenished with growth media including Eagle's Minimum Essential Medium (EMEM), 10% Fetal Calf Serum (FCS), 2 mM L-glutamine, 100 IU per mL penicillin, and 100 μg per mL streptomycin. The cells were incubated at 37 °C for 24 h in 5% CO2. A series of concentrations (0.5, 1, 5, 10, 25, 50, 80, and 100 μM) of the tested compounds and paclitaxel in DMSO was then added to each well of the 96-well plate and incubated for 48 h using the control DMSO at the same concentration. Next, the plate was incubated at 37 °C for 4 h in a CO2 incubator after 10 μL fresh solution of MTT reagent was added to each well. Finally, after the purple precipitate was obtained, the cells were dissolved in ethanol and their optical density was recorded at 570 nm using a microplate reader. The experiment was conducted on 6 wells for each concentration of the test sample. The percent of proliferation inhibition was calculated using the following formula:where ODt is the optical density of test compound, ODb is the optical density of blank, ODc is the optical density of control.
The 50% inhibitory concentration (IC50) of each compound was calculated using the correlation plot between percent of proliferation inhibition and corresponding concentration via Graphpad Prism version 8.30.
4.5. ADMET predictions
The physicochemical properties of all compounds were calculated using the SwissADME web tool and ADMETlab 2.0 descriptors algorithm protocol. In silico prediction of the ADME (absorption, distribution, metabolism, and excretion) properties and the toxicity (T) risks was performed using ADMETlab 2.0 descriptors algorithm protocol.494.6. In silico molecular docking studies
The structure of ligands were drawn in ChemBioDraw Ultra 19. The energy of these ligands was minimized using ChemBio3D Ultra 19. Protein molecules of dihydrofolate reductase (PDB ID: 4HOF and 3FYV), N-myristoyl transferase (PDB ID: 1IYL), gyrase B (PDB ID: 4URM), vascular endothelial growth factor receptor 2 (PDB ID: 5EW3), fibroblast growth factor receptor 1 (PDB ID: 5A46), and histone deacetylase 6 (PDB ID: 5EEF) were retrieved from the protein data bank (https://rcsb.org). After all the water molecules have been removed, the receptors were added to only polar hydrogen and Kollman charges. The grid box for docking simulations was set by AutoDock tools. Next, the ligand molecules with minimized energy were inputted and carried out in the docking simulation using AutoDock Vina.51All the minimizations were performed by AutoDock Vina docking simulation protocol with AMBER force field and the partial charges were automatically calculated. The electrostatic potential was shown for the interaction of two oppositely-charged atoms with a full atomic charge. The search algorithm of AutoDock Vina is a Monte-Carlo iterated search combined with the BFGS17 gradient-based optimizer, which comprises iterations of sampling, scoring, and optimization. AutoDock Vina actually uses a united-atom scoring function (one that involves only the heavy atoms) with combines knowledge-based and empiric scoring function features as well as supports the AutoDock4.2 scoring function.52 Besides, AutoDock Vina was compiled and run under Windows 10.0 Professional operating system. Discovery Studio 2021 was used to deduce the pictorial representation of the interaction between the ligands and the target protein.
4.7. In vitro dihydrofolate reductase inhibition assay
The dihydrofolate reductase (DHFR) inhibition assay was performed as per the manual of the CS0340 DHFR assay kit (Sigma, USA). 10 mM stock solutions of dihydrofolic acid and NADPH (reduced nicotinamide adenine dinucleotide phosphate) were prepared in assay buffer with a pH value of 7.5. The five different concentrations (10−8, 10−7, 10−6, 10−5, and 10−4 M) of the test compounds and methotrexate (as a positive control) in DMSO solvent were added to the respective wells of the 96-well plate containing assay buffer so the final concentration of DMSO was 0.4% in each experiment. The changes in absorbance were monitored at 340 nm wavelength as a function of time using the test samples. After nullifying the effects (such as NADPH, folate, and solvent), the percentage inhibition of enzymatic activity was calculated. The 50% inhibitory concentration (IC50) of each compound was calculated by plotting a graph between percentage inhibition and the corresponding concentration of the compound using Graphpad Prism version 8.504.8. Statistical analysis
All values are expressed in mean ± SEM (Standard Error of Mean). The difference in IC50 value between tested compounds and positive control was analyzed by one-way ANOVA (analysis of variance) with Tukey HSD (Tukey's honestly significant difference) post hoc test using Minitab version 19.0 software. The results were considered statistically significant if the p-value < 0.05. The chart is drawn using Microsoft Excel 2021 software.Author contributions
Em Canh Pham: conceptualization, methodology, investigation, data curation, supervision, writing-original draft preparation, writing – review & editing. Tuong Vi Thi Le: investigation, software. Huong Ha Hong Ly: investigation. Bich Ngoc Thi Vo: investigation. Long Binh Vong: supervision, investigation. Thao Thanh Vu: investigation. Duy Duc Vo: writing – review & editing. Ngoc Vi Tran Nguyen: investigation. Khanh N. B. Le: supervision, investigation. Tuyen Ngoc Truong: data curation, supervision, writing-original draft preparation, writing – review & editing.Conflicts of interest
The authors have stated that there is no conflict of interest associated with the publication and no financial support, which could have influenced the outcome.References
- B. T. Buu Hue, H. T. Kim Quy, W. K. Oh, V. D. Duy, C. N. Tram Yen, T. T. Kim Cuc, P. C. Em, T. P. Thao, T. T. Loan and M. V. Hieu, Tetrahedron Lett., 2016, 57, 887–891 CrossRef.
- P. C. Em, T. N. Tuyen, D. H. Nguyen, V. D. Duy and D. T. Hong Tuoi, Med. Chem., 2022, 18, 558–573 CrossRef PubMed.
- S. Yadav, B. Narasimhan and H. Kaur, Anticancer Agents Med. Chem., 2016, 16, 1403–1425 CrossRef CAS PubMed.
- P. C. Em, L. T. Tuong Vi and N. T. Tuyen, RSC Adv., 2022, 12, 21621–21646 RSC.
- T. H. Kim Chi, T. N. Hong An, T. N. Cam Thu and T. H. Kim Dung, RSC Adv., 2020, 10, 20543 RSC.
- M. Tunçbilek, T. Kiper and N. Altanlar, Eur. J. Med. Chem., 2009, 44, 1024–1033 CrossRef PubMed.
- H. Z. Zhang, G. L. Damu, G. X. Cai and C. H. Zhou, Eur. J. Med. Chem., 2013, 64, 329–344 CrossRef CAS PubMed.
- S. Malasala, M. N. Ahmad, R. Akunuri, M. Shukla, G. Kaul, A. Dasgupta, Y. V. Madhavi, S. Chopra and S. Nanduri, Eur. J. Med. Chem., 2021, 212, 112996 CrossRef CAS PubMed.
- K. C. Achar, K. M. Hosamani and H. R. Seetharamareddy, Eur. J. Med. Chem., 2010, 45, 2048–2054 CrossRef CAS PubMed.
- N. Pribut, A. E. Basson, W. A. L. van Otterlo, D. C. Liotta and S. C. Pelly, ACS Med. Chem. Lett., 2019, 10, 196–202 CrossRef CAS PubMed.
- M. C. Sharma, D. V. Kohli and S. Sharma, Int. J. Drug Deliv., 2010, 2, 228–237 CrossRef CAS.
- D. C. Cole, J. L. Gross, T. A. Comery, S. Aschmies, W. D. Hirst, C. Kelley, J. I. Kim, K. Kubek, X. Ning, B. J. Platt, A. J. Robichaud, W. R. Solvibile, J. R. Stock, G. Tawa, M. J. Williams and J. W. Ellingboe, Bioorg. Med. Chem. Lett., 2010, 20, 1237–1240 CrossRef CAS PubMed.
- G. Surineni, Y. Gao, M. Hussain, Z. Liu, Z. Lu, C. Chhotaray, M. M. Islam, H. M. A. Hameed and T. Zhang, Medchemcomm, 2019, 10, 49–60 RSC.
- D. S. Rudra, U. Pal, N. Chowdhury, N. C. Maiti, A. Bagchi and S. Swarnakar, Free Radic. Biol. Med., 2022, 181, 221–234 CrossRef CAS PubMed.
- M. Gaba, S. Singh and C. Mohan, Eur. J. Med. Chem., 2014, 76, 494–505 CrossRef CAS PubMed.
- M. Ouattara, D. Sissouma, M. W. Koné, H. E. Menan, S. A. Touré and L. Ouattara, Trop. J. Pharm. Res., 2011, 10, 767–775 CAS.
- D. Valdez-Padilla, S. Rodriguez-Morales, A. Hernandez-Campos, F. Hernandez-Luis, L. Yepez-Mulia, A. Tapia-Contreras and R. Castillo, Bioorg. Med. Chem., 2009, 17, 1724–1730 CrossRef CAS PubMed.
- N. Bharti, M. T. Shailendra, G. Garza, D. E. Cruz-Vega, J. Catro-Garza, K. Saleem, F. Naqvi, M. R. Maurya and A. Azam, Bioorg. Med. Chem. Lett., 2002, 12, 869–871 CrossRef CAS PubMed.
- B. Bhrigu, N. Siddiqui, D. Pathak, M. S. Alam, R. Ali and B. Azad, Acta Pol. Pharm. Drug Res., 2012, 69, 53–62 CAS.
- A. A. Farahat, M. A. Ismail, A. Kumar, T. Wenzler, R. Brun, A. Paul, W. D. Wilson and D. W. Boykin, Eur. J. Med. Chem., 2018, 143, 1590–1596 CrossRef CAS PubMed.
- S. Tahlan, K. Ramasamy, S. M. Lim, S. A. A. Shah, V. Mani and B. Narasimhan, BMC Chem., 2019, 13, 12 CrossRef PubMed.
- B. G. Tuna, P. B. Atalay, G. Kuku, E. E. Acar, H. K. Kara, M. D. Yilmaz and V. C. Ozalp, RSC Adv., 2019, 9, 36005–36010 RSC.
- H. Joensuu, J. Y. Blay, A. Comandone, J. Martin-Broto, E. Fumagalli, G. Grignani, X. G. Del Muro, A. Adenis, C. Valverde, A. L. Pousa, O. Bouché, A. Italiano, S. Bauer, C. Barone, C. Weiss, S. Crippa, M. Camozzi, R. Castellana and A. Le Cesne, Br. J. Cancer, 2017, 117, 1278–1285 CrossRef CAS PubMed.
- C. Y. Hsieh, P. W. Ko, Y. J. Chang, M. Kapoor, Y. C. Liang, H. H. Lin, J. C. Horng and M. H. Hsu, Molecules, 2019, 24, 3259 CrossRef PubMed.
- G. Mariappan, R. Hazarika, F. Alam, R. Karki, U. Patangia and S. Nath, Arab. J. Chem., 2015, 8, 715–719 CrossRef CAS.
- P. Boggu, E. Venkateswararao, M. Manickam, D. Kwak, Y. Kim and S. H. Jung, Bioorg. Med. Chem., 2016, 24, 1872–1878 CrossRef CAS PubMed.
- D. R. Gund, A. P. Tripathi and S. D. Vaidya, Eur. J. Chem., 2017, 8, 149–154 CrossRef CAS.
- E. M. E. Dokla, N. S. Abutaleb, S. N. Milik, D. Li, K. El-Baz, M. W. Shalaby, R. Al-Karaki, M. Nasr, C. D. Klein, K. A. M. Abouzid and M. N. Seleem, Eur. J. Med. Chem., 2020, 186, 111850 CrossRef CAS PubMed.
- P. C. Em, L. T. Tuong Vi, T. P. Long, T. N. Huong-Giang, N. B. L. Khanh and N. T. Tuyen, Arab. J. Chem., 2022, 15, 103682 CrossRef.
- P. Singla, V. Luxami and K. Paul, Bioorg. Med. Chem., 2015, 23, 1691–1700 CrossRef CAS PubMed.
- S. B. Nabuurs, M. Wagener and J. de Vlieg, J. Med. Chem., 2007, 50, 6507–6518 CrossRef CAS PubMed.
- S. M. Rizvi, S. Shakil and M. Haneef, EXCLI J., 2013, 12, 831–857 Search PubMed.
- N. El-Hachem, B. Haibe-Kains, A. Khalil, F. H. Kobeissy and G. Nemer, Methods Mol. Biol., 2017, 1598, 391–403 CrossRef CAS PubMed.
- M. Congreve, R. Carr, C. Murray and H. Jhoti, Drug Discov. Today, 2003, 8, 876–877 CrossRef PubMed.
- C. A. Lipinski, Drug Discov. Today Technol., 2004, 1, 337–341 CrossRef CAS PubMed.
- M. M. Morcoss, E. S. M. N. Abdelhafez, R. A. Ibrahem, H. M. Abdel-Rahman, M. Abdel-Aziz and D. A. Abou El-Ella, Bioorg. Chem., 2020, 101, 103956 CrossRef CAS PubMed.
- Y. Keng Yoon, M. Ashraf Ali, T. S. Choon, R. Ismail, A. Chee Wei, R. Suresh Kumar, H. Osman and F. Beevi, BioMed Res. Int., 2013, 2013, 926309 Search PubMed.
- L. Shi, T. T. Wu, Z. Wang, J. Y. Xue and Y. G. Xu, Bioorg. Med. Chem., 2014, 22, 4735–4744 CrossRef CAS PubMed.
- L. V. Nayak, B. Nagaseshadri, M. V. P. S. Vishnuvardhan and A. Kamal, Bioorg. Med. Chem. Lett., 2016, 26, 3313–3317 CrossRef PubMed.
- Y. K. Yoon, M. A. Ali, A. C. Wei, A. N. Shirazi, K. Parang and T. S. Choon, Eur. J. Med. Chem., 2014, 83, 448–454 CrossRef CAS PubMed.
- J. Zhang, D. Yao, Y. Jiang, J. Huang, S. Yang and J. Wang, Bioorg. Chem., 2017, 72, 168–181 CrossRef CAS PubMed.
- N. S. Goud, S. M. Ghouse, J. Vishnu, D. Komal, V. Talla, R. Alvala, J. Pranay, J. Kumar, I. A. Qureshi and M. Alvala, Bioorg. Chem., 2019, 89, 103016 CrossRef CAS PubMed.
- S. A. Al-Trawneh, J. A. Zahra, M. R. Kamal, M. M. El-Abadelah, F. Zani, M. Incerti, A. Cavazzoni, R. R. Alfieri, P. G. Petronini and P. Vicini, Bioorg. Med. Chem., 2010, 18, 5873–5884 CrossRef CAS PubMed.
- M. A. Divakar, S. Shanmugam, T. Muneeswaran and M. Ramakritinan, ChemistrySelect, 2016, 1, 6151–6155 CrossRef CAS.
- X. Deng and M. Song, J. Enzyme Inhib. Med. Chem., 2020, 35, 354–364 CrossRef CAS PubMed.
- K. Gholivand, M. H. H. Koupaei, F. Mohammadpanah, R. Roohzadeh, N. Fallah, M. Pooyan, M. Satari and F. Pirastehfar, J. Mol. Struct., 2022, 1263, 133024 CrossRef CAS.
- Z. Tatiana and S. Alexis, Infectious Diseases in Cancer Patients: An Overview, Springer International Publishing, Switzerland, 2015, pp. 295–311 Search PubMed.
- T. Lam, M. T. Hilgers, M. L. Cunningham, B. P. Kwan, K. J. Nelson, V. Brown-Driver, V. Ong, M. Trzoss, G. Hough, K. J. Shaw and J. Finn, J. Med. Chem., 2014, 57, 651–668 CrossRef CAS PubMed.
- G. Xiong, Z. Wu, J. Yi, L. Fu, Z. Yang, C. Hsieh, M. Yin, X. Zeng, C. Wu, A. Lu, X. Chen, T. Hou and D. Cao, Nucleic Acids Res., 2021, 49, W5–W14 CrossRef CAS PubMed.
- P. C. Em and N. T. Tuyen, ACS Omega, 2022, 7, 33614–33628 CrossRef PubMed.
- P. C. Em, V. V. Lenh, V. N. Cuong, N. D. Ngoc Thoi, L. T. Tuong Vi and N. T. Tuyen, Biomed. Pharmacother., 2022, 146, 112611 CrossRef PubMed.
- T. Gaillard, J. Chem. Inf. Model., 2018, 58, 1697–1706 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ra06667j |
| This journal is © The Royal Society of Chemistry 2023 |