Open Access Article
Open Access ArticleTuning water chemistry for the recovery of greener products: pragmatic and sustainable approaches
A. O. Adeeyo*af,
J. A. Oyetade b,
M. A. Alabi
b,
M. A. Alabi c,
R. O. Adeeyoa,
A. Samie
c,
R. O. Adeeyoa,
A. Samie d and
R. Makungoe
d and
R. Makungoe
aEcology and Resource Management Unit, Faculty of Science, Engineering and Agriculture, University of Venda, Thohoyandou 0950, South Africa. E-mail: firstrebby@gmail.com
bMaterial Science and Engineering, School of Materials, Water, Energy and Environmental Science, Nelson Mandela African Institution of Science and Technology, Arusha, Tanzania
cDepartment of Microbiology, School of Life Sciences, Federal University of Technology, Akure, Nigeria
dDepartment of Microbiology, Faculty of Science, Engineering and Agriculture, University of Venda, Thohoyandou 0950, South Africa
eDepartment of Earth Science, University of Venda, Thohoyandou 0950, South Africa
fAqua Plantae Research Group, University of Venda, Thohoyandou 0950, South Africa
First published on 28th February 2023
Abstract
The environmental impact and denaturing propensity of organic solvents in the extraction of plant bioactives pose great challenges in extraction systems. As a result, proactive consideration of procedures and evidence for tuning water properties for better recovery and positive influence on the green synthesis of products become pivotal. The conventional maceration approach takes a longer duration (1–72 h) for product recovery while percolation, distillation, and Soxhlet extractions take about 1 to 6 h. An intensified modern hydro-extraction process was identified for tuning water properties with an appreciable yield similar to organic solvents within 10–15 min. The percentage yield of tuned hydro-solvents achieved close to 90% recovery of active metabolites. The additional advantage of using tuned water over organic solvents is in the preservation of the bio-activities and forestalling the possibility of contamination of the bio-matrices during extractions with an organic solvent. This advantage is based on the fast extraction rate and selectivity of the tuned solvent when compared to the traditional approach. This review uniquely approaches the study of biometabolite recovery through insights from the chemistry of water under different extraction techniques for the very first time. Current challenges and prospects from the study are further presented.
1. Introduction
The early techniques for recovery of bioactive metabolites involve conventional cold or hot solvent extraction.1 The choice is a function of the nature of the bioactive compound of interest.2 The adverse effect of organic solvents (Table 1) which are mostly preferred extraction techniques has warranted the search for greener alternatives. One of the ways green extractions is described involves the isolation of medicinally active portions from a bio-material,3 with the simultaneous use of eco-friendly solvents and optimal use of energy.4–9 Prospecting for green solvents has brought water to the fore of extraction technology.10 Water is affirmatively described as the “greenest solvent” imaginable, with its availability at the required purity, it is cost-effective, readily recycled, non-toxic, non-flammable, and eco-friendly.10–13 Based on the green chemistry precept, water is considered a green chemical per excellence.14–16 Water is useful in the recovery of various phytochemicals including alcohols, sugars, proteins, and organic acids with natural water-soluble properties.12,16–21 However, water as a solvent has some physical and chemical property disadvantages when compared to organic solvent.21–23 The polar nature of water in its natural form reduces its efficacy and acceptability when compared with organic solvents for some kinds of extractions. Organic solvents are extensively desirable since they exhibit better recovery than water at ambient conditions.3 Further setbacks experienced when using conventional hydro-extraction include time and energy consumption, thermal decomposition of thermo-sensitive metabolites and low recovery of hydro-solvent in its natural form.| Solvents | Toxicological effects |
|---|---|
| Toluene | Appreciably fatal if it penetrates the airways or is swallowed. Has the potential of damaging the fetus. Prolonged exposure may warrant the damaging of organs |
| Dichloromethane (DCM) | Suspected of causing cancer |
| Chloroform | Suspected of causing cancer |
| Dimethylformamide (DMF), dimethylacetamide (DMA) and N-methyl pyrrolidinone (NMP) | May damage fertility or affect the unborn child |
| 1,2,3-Trichloropropane and trichloroethylene and 1,2-dichloroethane | May reduce fertility or affect the unborn child |
| 2-Methoxyethanol, 2-ethoxyethanol and 2-ethoxyethyl acetate | May damage fertility or affect the unborn child |
| Benzene | Low flash point (−11 °C), carcinogenic. It has a high toxicological impact on man and its immediate environment, hence, strongly regulated in the US (HAP) and the EU |
| n-Hexane(s) | Very low flash point (−23 °C), toxic, carcinogenic, pollutant |
| Pyridine | Has reprotoxic and carcinogenic effects on long exposure |
| n-Pentane | Classified as a hazardous airborne |
There exists the need to investigate water properties that can be improved to complement its natural advantage and eradicate its attendant limitations as a solvent for extraction.5,8,10,28,29 have indicated that improving traditional extraction must entail decreased energy input, sustainability and a non-toxic final product. Improving water to own variable chemistry will aid the extraction of a broad range of polar and non-polar biomolecules from sustainable natural products with non-toxic quality and eco-friendliness.10,21,29 This approach will prevent the use of organic solvents, fossil energy, chemical waste and risks of extraction. It is known that water existing in its tunable form satisfies the conditions of green solvents.11–13 Recently, the usage of water for extraction has been considered based on its negligible environmental impact.30,31 To date, processes developed imply extraction with water could proffer net benefits concerning eco-friendliness, reduced process time, improved selectivity, preservation of heat-sensitive compounds and reduced energy input.7
2. Methods
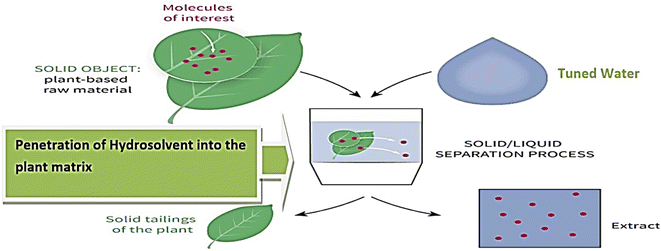
This review is centered on the tunable chemistry of water. The search methodology adopted was according to Feli et al.32 The study investigates the tunable properties of water with a positive influence on green synthesis. Data mining and processing of secondary information in literature engaged procedures as described in Fig. 1a and involve the review of 268 publications. About 125 articles reviewed comprehensively covered conventional and non-conventional techniques studied, 35 discussed the challenges of the green extraction techniques and 45 presented possibilities of tunable technology of water as an alternative extraction process.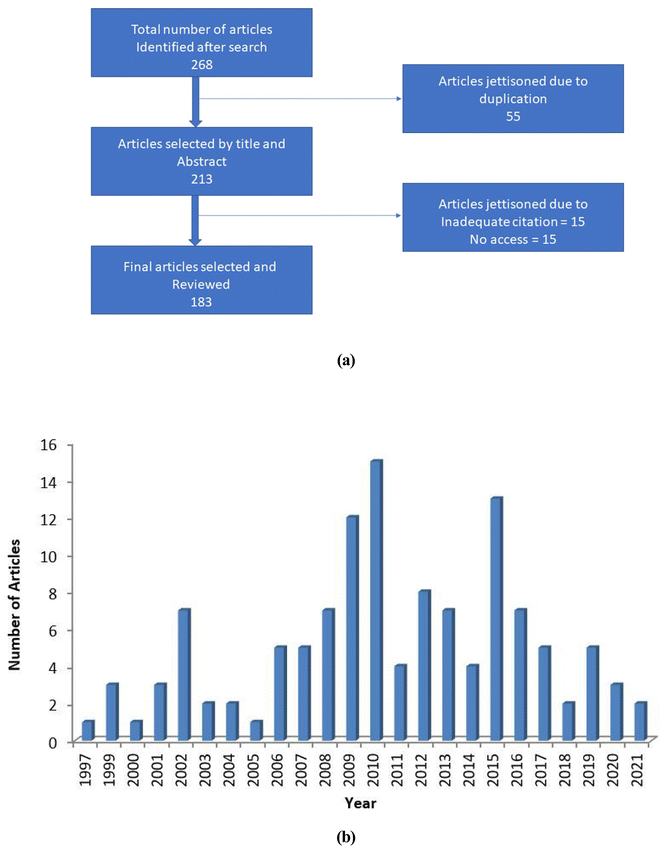 | ||
| Fig. 1 Flow chart for the article selection process (a) and yearly distribution of articles used for extracting data (b). | ||
Cumulatively, 183 articles were selected based on the extensive and quantifiable information and their systematic mode of data presentation on the alternative extraction processes. Various cogent keywords such as properties of water, tunable water, hydro extraction, merits of hydro solvent extraction and development of aqueous solvents and systems were typed and searched on notable databases such as Google scholar, science direct, pub med and web of science. Articles within the year of publication ranging from 1997 to 2021 were studied for review (Fig. 1b).
3. Hydro extraction and mechanism of tuned water
3.1 Water and extraction processes
The water molecule is very small with a hard-sphere diameter of 2.75 Å. The small size of the molecule is of vital importance in solute hydration. The dipole moment (1.85 D) of water molecules is a result of the two partial positive charges on the hydrogen [H+] atoms and the only single zone of negative charge on the oxygen [O−]. The contribution of the total electron density of the water molecule in the H–O–H plane makes it spherical one.33,34 Also, one of the most essential parameters use in categorizing the polarity of the medium and the control exerted over the ionic dissociation of salts is the macroscopic dielectric constant of a solvent (εr). The high polarity of water can be attributed to dipole orientations of the hydrogen-bond network which gives it a dielectric constant value of 78.3. At higher temperatures and pressures, the polarity of water is significantly reduced as the hydrogen bond network is disintegrated.35,36 Hence, using water as a solvent in extraction processes might be energy-demanding in those cases where water needs to be removed by evaporation. Furthermore, the energy demand to heat liquid water (25 °C to 250 °C, 5 MPa) is almost three times less than needed to vaporize the water to create steam (25 °C to 250 °C, 0.1 MPa).37The chemical and physical properties of water are largely affected by temperature variation.38 This possibility of change in the properties of water gives it variable characteristics which could be harnessed during the extraction of plant metabolites. One of the most dramatic changes for liquid water at saturation pressure is the static permittivity (εr), going from 78 at 25 °C to 14 at 350 °C.39 Studies show that the property of water at near-critical conditions dissolves hydrophobic organic compounds.38,40 At this point, inorganic solute such as NaCl becomes insoluble in water which is the attribute of organic solvents. The increasing temperature at a critical point diminishes electrostatic interactions within water molecules, as well as between water molecules and surrounding ions or molecules (i.e., both εr and π* (polarizability) decrease with increasing temperature). At higher temperatures, there is an observed increase in the movement/rotation of water molecules. Hence, the use of liquid water at higher temperature and pressure allows the dissolving of less polar bioactive compounds. Intermolecular interactions involving hydrogen bonding become less pronounced; thereby favoring dispersion forces (induced dipole-induced dipole forces). In other words, liquid water at elevated temperature (and pressure) becomes less polar of a solvent. Liquid water at temperatures of 200–275 °C and saturation pressure has εr similar to that of methanol and ethanol at ambient conditions.41 The notable properties of water include a strong hydrogen bond, which results in a very high specific heat capacity (Cp, m, 75.3 J mol−1 K−1, isobaric, molar, at 25 °C), high heat of vaporization (Hv, 40.7 kJ mol−1 at 100 °C) and extremely high relative static permittivity (εr, also referred to as the dielectric constant) of 76–80 at 20 °C. This implies that water creates electrostatic bonds with other molecules, thereby decreasing or eliminating intermolecular interaction between surrounding ions.42 Water is non-toxic and non-flammable, it is cheap and readily available. Thus, it provides opportunities for clean processing and pollution prevention.
3.2 Mechanisms of tuned water extraction
Generally, water can exist in three different states based on temperature change. These states include liquid water, solid water (ice) and gaseous water (steam or vapour).43 The physicochemical properties of water subjected to various tunable effects includes surface tension, dielectric constant and viscosity. These properties are tuned under external influences such as temperature, pressure, ultrasound, and the incorporation of some other bio-based solubilizing compounds and hydrotropes. Mottaleb and Sarker44 affirmed that at high temperature and pressure, the viscosity of solvent is lowered, surface tension reduces and the rate of the penetration of hydro-solvent into the pores of the sample matrix may increase (Fig. 2).44 In addition to this, at room temperature and atmospheric pressure, water is a highly polar solvent with a high dielectric constant based on the presence of hydrogen-bonded structure.42 Dielectric constant points to the strength of the polarity of water. When heat and pressure are applied to water, a drastic change is observed in the properties of the water as the hydrogen-bonded lattice is disrupted with increasing thermal motion and a fall in the value of the dielectric constant with the resultant lesser polarity of water.45,46 Hence, water begins to exhibit similar properties of organic solvents with no environmental concerns.47–49 The tuning effect also speeds up the rate of molecular diffusion and interaction of the liquid phase (hydro-solvents) with the material. The ease of diffusion and penetration of matrices observed in the tuned hydro-solvent is an appreciable attribute predominantly known in many extraction systems that use organic solvent.50Brignole,51 Smith,46 and Carr et al.41 in their studies revealed that under conditions of high temperature (250 °C) and sufficient pressure (25 bar) water remains in its liquid state. Though the dielectric constant of water is 80 at 25 °C; however, at elevated temperature, the dielectric constant drops to 25, which falls between those of methanol (ε = 33) and ethanol (ε = 24) at 25 °C. Under such conditions, water exhibits properties that mimic some organic solvents, dissolving compounds with low polarity.33,36,42,52–56 In addition, it has been reported that intensified techniques are faster when compared to the conventional extraction technique.57
4. Extraction processes
4.1 Conventional extraction processes
There are diverse traditional extraction methods such as described in Table 2. However, these methods are generally challenged with contamination of extracted bio-actives resulting from prolonged exposure to organic solvents and other disadvantages. Solvents may penetrate through the cell, move into the cytoplasm containing neutral lipids, and form a solvent–lipid interaction by van der Waals forces. During this process, lipids may be extracted from the cell matrix which may contaminate extracellular metabolites of interest and bioactivities (Fig. 3).| Methods | Process | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Squeezing | The techniques involve the application of pressure on moistened plant samples via pestle, mortars, mullers, presses, etc., on the plant samples to get the extrudate | Simple | Possibility of contamination of bio-actives | 59–61 |
| Little or no solvent required | ||||
| Requires no thermal degradation | ||||
| Low extraction efficiency | ||||
| Maceration | Powdered plant samples are added to the solvent already in a stoppered container with frequent agitation. The aqueous extracting solvent is then drained off followed by pressing and centrifugation to remove the remaining miscella from the plant material | Can be used for a large amount of sample | Long extraction duration | 39, 62 and 63 |
| A limited solvent is required | ||||
| Long extraction time | Only useful for soluble or thermolabile bio-actives | |||
| Low extraction efficiency | ||||
| Decoction | The plant matrix comes in contact with the aqueous solvent at boiling point for a maximum duration of 30 min. The liquid is then filtered at end of the extraction. Then the liquid is filtered, and the squeezed liquid of the extracted matrix impregnated with the aqueous solvent is added to it | Use predominantly for phenolics | Only useful for thermoresistant bioactive | 64–66 |
| Requires moderate heat | Limited validity | |||
| Long extraction time | Extracts have a short shelf life | |||
| Low extraction efficiency | ||||
| Infusion | Extraction in this regard involves soaking the solid plant powder in cold or boiling water for a short time | Long extraction time | Easily altered extract | 4 and 67 |
| Low extraction efficiency | Limited validity | |||
| Percolation | The method makes use of narrow shaped percolator which holds the moistened plant samples. The plant material is then rinsed with the solvent several times until the active ingredient is extracted | Easy to operate | Good grinding required | 68–70 |
| Very fast | Requires preliminary humidification | |||
| Not exhaustive |
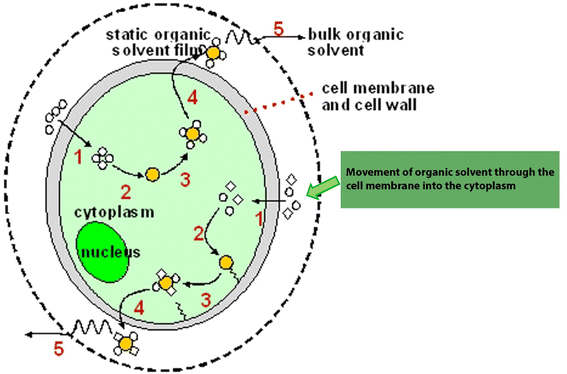 | ||
| Fig. 3 Schematic representation of extraction processes of bioactives in cell. Source: Halim et al.58 | ||
4.2 Intensified modern hydro-extraction processes
The intensified modern extraction processes include microwave-assisted, supercritical or pressurized liquid water, ultrasound-enhanced, water–oil-based solvents and enhanced hydrotropic extraction which is summarized in Table 3. These extraction systems make use of water as their solvent for extraction under critically controlled conditions and is commonly called “green solvent”.9,11,12,71 The extraction principles alongside the representations of conventional and intensified processes are presented in Table 3 and Fig. 4 respectively.| Techniques | Mechanism | Advantage (s) | Limitations | References |
|---|---|---|---|---|
| Enhanced hydro-accelerated extraction | Water is subjected to elevated temperatures above the boiling point and pressures while maintaining its liquid state (i.e., liquid water). The tuned aqueous solvent accelerates selective desorption of the analytes thus, giving room for selective extraction by solubilizing the analytes from plant samples | High solvent strength | Expensive | 73 and 74 |
| Fast extraction rate | Requires extraction clean up | |||
| No phase change of liquids | ||||
| Microwave-enhanced hydro-extraction/microwave-assisted hydro distillation | The dielectric of water is tuned under non-ionizing electromagnetic fields in the frequency range from 300 MHz to 300 GHz. The supplied electromagnetic energy is converted to heat following ionic conduction and dipole rotation mechanisms of the hydro-solvent. This leads to the separation of solutes from active sites of the sample matrix | High selectivity | Formation of free radicals | 75–78 |
| Lower extraction time | Possibility of thermal degradation | |||
| Enhanced bio-active recovery | ||||
| Pressurized or subcritical hydro-extraction | Water is tuned at critical pressure (1–22.1 MPa) and critical temperature (between 100–374 °C) while maintaining its liquid state. Under this condition, properties such as dielectric constant and viscosity are tuned which consequently decreases the surface tension of the water and increases its diffusivity | Possibility of dielectric variation over a wide range | Expensive | 57, 79–84 |
| Efficient mass transfer and diffusion. Fast extraction rate | ||||
| Aqueous ultrasonic hydro-extraction | The properties of water are tuned under ultrasound from 20 kHz to 2000 kHz. The ultrasound enhances the propagation of mechanical waves, formed compressions and rarefactions, respectively. Thus, in contact with a bio-matrix, the cell of the material is damaged by the principle of acoustic cavitation favouring the release of bioactive compounds | High extraction efficiency and faster extraction rate | — | 4, 77, 85–89 |
| Enhanced hydrotropic extraction | The solubility of water is enhanced by the incorporation of hydrotropes. These compounds consist of a hydrophilic part and a hydrophobic part in the similitude of surfactants. However, they act as coupling agents to tune the polar property of water and aid its extraction | Simplicity | 90–94 | |
| Cost-effective | ||||
| Eco-friendly nature | ||||
| High solubilization and selectivity capacity | ||||
| Water–oil biosurfactant extraction | This involves the dispersion of thermodynamically stable micelles in water. These micelles consist of the polar head which attracts the aqueous core (water) and the non-polar part of the hydrocarbon chain is attracted by the organic phase which points outside. The coupling is known as water–oil surfactant. During the extraction, water and hydrophilic plant metabolites are solubilized inside the cores | High selectivity | 95–100 | |
| Bioactivities protection | ||||
| Shorter phase separation time | ||||
| Thermodynamically stable, low costs, energy savings, ease of operation at a continuous steady-state |
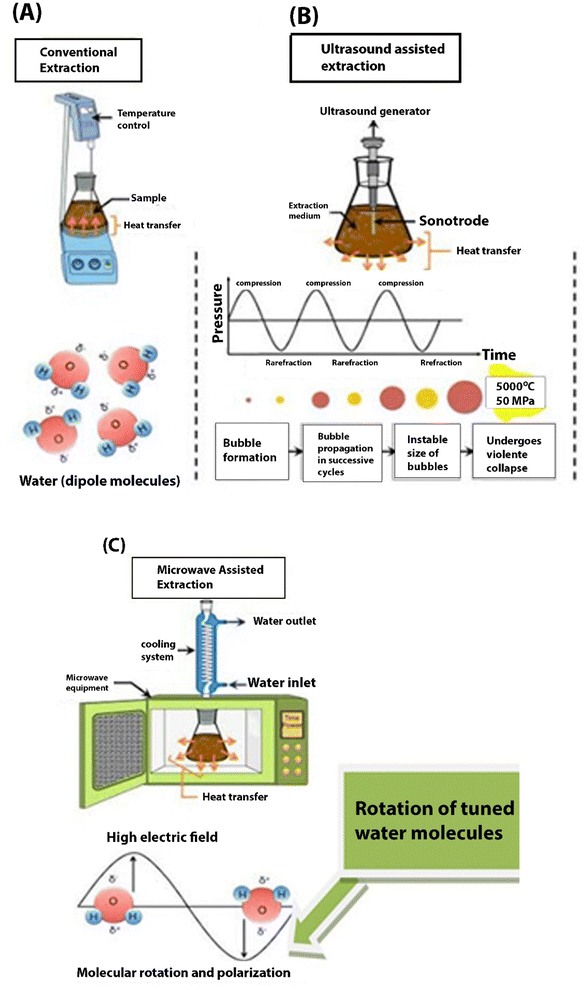 | ||
| Fig. 4 Comparative mechanism of conventional (A) and selected intensified methods (B and C).72 | ||
5. Results and discussion
5.1 Effect of extraction techniques on yield of biometabolites
From Tables 4 and 5, the extracted bio-actives can be categorized into phenolics and polyphenolics, essential oils, alkaloids and carotenoids, terpenes, carbohydrates, proteins pigments and vitamins.| Methods | Sample | Desired product | Experiment temp. (°C) | Optimum time (min) | Yields | Sources |
|---|---|---|---|---|---|---|
| Maceration | Brassica oleracea var. italica | Essential oil | 4 | 1440 | 82.2 mg GAE/100 g DW | 122 and 123 |
| Solanum scabrum leaves | Essential oil | — | 4320 | 34.2 g GAE/100 g | 124 | |
| Lepidium sativum | Essential oil | 50 | 1440 | 25 mg RuE/g DW | 125 | |
| Artocarpus heterophyllus wastes | 25 | 4320 | 871.4 mg QuE/g DW | 126 | ||
| Quercus robur L. | Phenolic compounds | 40 | — | 412 mg CE/g bark | 127 | |
| Percolation | Artocarpus heterophyllus wastes | Essential oil | 25 | 60 | 511.6 mg QuE/g DW | 128 |
| Infusion | Moroccan Acacia mollissima bark | Phenolic compounds | 20 | 120 | 258.4 mg GAE/g bark | 129 |
| Soxhlet | Artocarpus heterophyllus wastes | Essential oil | 10 | 300 | 381.4 mg QuE/g DW | 128 |
| Vernonia cinerea leaves | Essential oil | — | 120 | 26.22 mg QuE/g DW | 130 | |
| Pinus radiata bark | Essential oil | 82 | 60, 120, 180, and 360 | 622.40 mg GAE/g | 131 | |
| Morus nigra (dried) | Essential oil | 50 | 180 | 58.94% of flavonoid yield | 132 | |
| Steam distillation | Lavandula flowers | Essential oil | 100 | 90 | 8.75% | 133 |
| Hydro distillation | Thymus vulgaris | Essential oil | — | 240 | — | 134 |
| Pinus pinaster | Polyphenols | 100 | 180 | 14.3 mg GAE/g bark | 135 | |
| Pressurized hydro-extraction | Gossypium herbaceum seed | Oil | 180–280 | 270 | 30% | 136 |
| Solanum tuberosum peel | Glucose | 140–240 | 240 | 15% | 137 | |
| Pinus densiflora | Organic acid product | — | 270 | 10% | 123 | |
| M. chamomilla | Essential oils | 100–175 | 150 | 120% | 138 | |
| Zea mays stalk | Fermentable hexose | 180–392 | 280 | 27% | 139 | |
| Triticum aestivum L. straw | Fermentable hexose | — | 280 | 54% | 139 | |
| Cellulose | Oligosaccharides | — | 380 | 16% | 140 | |
| Triticum aestivum L. straw | Reducing sugars | 170–210 | 190 | 30% | 136 | |
| Fish proteins | Amino acid | 180–320 | 260 | 30% | 141 | |
| Thymus vulgaris | Essential oils | 100–175 | 150 | 150% | 138 | |
| Gramineae Saccharum officinarum L. waste | Reducing sugars | 200–240 | 240 | 2% | 142 | |
| Defatted rice bran | Sugars and proteins | 200–260 | 200 | 5% | 143 | |
| Simmondsia chinensis seed | Oil | 180–260 | 240 | 30% | 144 | |
| Rosmarinic acid | Terpenes | 60–100 | 25% | 145 | ||
| Microwave enhanced hydro solvent | Lavandula flowers | Essential oil | 100 | 10 | 8.86% | 133 |
| Thymus vulgaris | Essential oil | — | 75 | — | 134 | |
| Ultrasound-assisted hydro solvent | Artocarpus heterophyllus | Pectin | 90 | 10 | — | 146 |
| Camellia sinensis leaves | Polyphenols, amino acid and caffeine | 65 | 15 | — | 147 | |
| Solid–liquid extraction | Pinus nuts | Inositol | 60 | 120 | 3.7 mg g−1 | 148 |
| Analytes | Matrix and yield | Reference methods and conditions | Temp. (°C) | Pressure | Mode | Flow rate (ml min−1) | Extraction time (min) | References |
|---|---|---|---|---|---|---|---|---|
| Stevioside, rebaudioside A | Stevia rebaudiana, 91.8% | Reflux 60 °C, 1–1.5 h, 84.3% | 100 | 11–13 bar | Dynamic | 1.5 | 15 | 149 and 150 |
| Gastrodin, vanillyl alcohol | Gastrodia elata, 8.6% | Reflux with 95% methanol, 2 h and 70 °C and 0.075% | 100 | 8–10 bar | Dynamic | 1.5 | 20 | 151 |
| Phenolics and polyphenolic compounds | Momordica charantia, 29.8% | Soxhlet extraction, 180 °C, 30 ml methanol, 0.4% | 150–200 | 10 Mpa | Dynamic | 2.0 | 320 | 152 |
| Pinus radiata bark | Hydrodistillation ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water 3 water 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w, 0.55 g GAE/g extract 1 w/w, 0.55 g GAE/g extract |
120 | 1.01 bar | Nil | Nil | 153 | ||
| Quercus petraea | Heat reflux with water, 11.7 GAE/g extract | 100 | 1.01 bar | Nil | Nil | 120 | 154 | |
| Quercus robur L. and Quercus petraea | Hydrosolvent extraction with water and ethanol, 5.0–13.4 mg GAE/g DW | 1.01 bar | Nil | Nil | 360 | 154 | ||
| Phyllanthus amarus, 52.97 mg g−1 | Reflux, 17.67 mg g−1 | 192.4 | 110 bar | Static | 1.0 | 15 | 155 | |
| 85 | 30 | |||||||
| Essential oil | Fructus amomi, >12% | Steam distillation, 40 min, 12% | 150 | 60 bar | Dynamic | 1.0 | 5 | 156 |
| Borneol, terpinen-4-ol, carvacrol | Origanum Onites, 5.05% | Steam distillation, Soxhlet extraction, 50 ml n-hexane, 150 °C, 12 h, 3.16% | 100, 125, 150, 175 | 60 bar | 2.0 | 30 | 157 | |
| Volatile oil | Cuminum cyminum L., 16.2% | Hydro distillation, Soxhlet extraction, 175 °C, 3 h, 3.16% | 100–175 | 20 bar | Dynamic | 2.0, 4.0 | Nil | 113 |
| Catechins, proanthocyanidins | Grape seed, 95% | Extraction with 75% methanol, 4.6%, 200 ml n-hexane, 12 h | 50, 100, 150 | 1500 psi | Static | Nil | 30 | 112 |
| Capsaicin dihydrocapsaicin | Peppers | Nil | 50–200 | 100 atm | Static | Nil | Nil | 158 |
| Anthocyanins phenolics | Dried red grape skin, 45% | Nil | 100–160 | Nil | Static | Nil | 40 s | 159 and 160 |
| Rosmaric acid | Salvia rosmarinus | Reflux with water | 78 | 1.01 bar | Nil | Nil | 30 | 161 |
| Inositols | Pinus pinea L. | Pressurized hydrosolvent, 5.7 mg g−1 | 50 | 10 mpa | Dynamic | 18 | 148 | |
| Solid–liquid extraction, 3.7 mg g−1 | 60 | 120 | ||||||
| Anthocyanin and phenolic | P. cauliflora skin, 18.7% | Low-pressure solvent extraction (LPSE) with ethanol, 22–33 °C at 120 min 13.0% | 80 | 50 bar | Static | — | 9 | 162 |
| Rubus fruticosus, 12.10% and 14.27% | Soxhlet extraction with ethanol and mixture, at 300 min and 5.02% | 80 | — | — | 3.0–3.8 | 30 | 163 | |
| Polysaccharide | Grossularia, pressurized hydro-solvent extraction with water, 11.68% | — | 52 | 16 bar | — | — | 51 | 163 |
| Capsaicinoids | Capsicum annuum, pressurized hydro-solvent extraction with water, higher than Soxhlet extraction at 20 min | Soxhlet extraction, 80 °C with ethanol at 300 min and 5.243 mg g−1 | 120–240 | 200 bar | — | — | 20 | 164 |
| Protein | Prunus cerasus | Soxhlet extraction with n-hexane, 80.48% | — | — | — | — | 60 | 165 |
Generally, from Table 4, the extraction technique with the higher time frame is maceration, although at a lower temperature. However, when compared with extraction of essential oil via pressurized hydro extraction, pressurized technique operates at a lesser time frame for the extraction of oil from M. chamomilla and Thymus vulgaris with yield of 120% and 150% respectively. Similarly, in Table 5 the use of hydro-solvent via steam distillation compared to the pressurized technology has a faster oil extraction rate with a yield of 12% at 40 min and >12% at 5 min respectively.
Although the extraction process in traditional maceration took place at low temperature but disadvantaged with longer extraction time when compared to intensified methods of pressurized hydro-extraction, microwave-enhanced hydro solvent and ultrasound-assisted hydro extraction with lower recovery duration. The longer extraction durations in conventional extractions make extracted bio-products susceptible to deterioration and reduced pharmacological activity.104 Furthermore, it is imperative to add that extracts of organic solvent exhibit toxicological impact in the environment.105,106
Similarly, from Table 5, the extraction of capsaicinoids from Capsicum annuum using Soxhlet was reported to have 5.243 mg g−1 of bio-actives at 300 min while the green technology using pressurized hydro-solvent had improved performance compared to the conventional technology at 20 min. These appreciable attributes are connected to the mimicking ability of the tuned hydro-solvent and its ease of disrupting the cell wall of the plant leading to diffusion, transportation and effective mass transfer of the bio-actives at low time duration.111–114 Other bio-actives such as carbohydrates, proteins, vitamins and pigments exhibit similar extraction performance. Apart from the eco-friendliness, Cao et al.115 also showed that the use of pressurized extraction protocol results in significant reduction of extraction time while simultaneously increasing the yield to about four times when compared with the traditional method.116
The extraction capacity of tuned water does not only stand out with greater and greener potential, but it also influences vital properties of other green solvents such as ethanol and methanol.117 Although high selectivity of organic solvents is the reason behind their choice during extraction over water. For instance, water in its natural state, as studied by Carrero-Carralero et al.118 is less selective when compared to alcohol.119 However, the use of intensified technology can effectively alter the polarity, penetration and selectivity of water as solvent for the extraction of both hydrophilic and hydrophobic plant bio-actives.120 Water tuning via ultrasound technology at known frequencies and amplitude generates cavitation bubbles at a non-stable point resulting in the release of high temperature and pressure via imploding. This improves the penetration of the tune hydro-solvent beyond the plant cell wall into the plant matrix thereby releasing the targeted bioactives.117 One of the reported actions of this technology involves the hydrolysis of bio-active compounds such as tricaffeoylquinic acid due to the presence of OH radicals initiated by ultrasonic waves.121 The impact of this technology generates H2O2 which combines with the radical leading to hydrolysis reaction and improved selectivity of the water for extraction of phenolics in Phyllanthus amarus (Table 5) to a notable yield other than the conventional techniques. Also, the variation of the ultrasonic power also accounts for the variation in the yield and purity of carnosic acid and rosmarinic acid from Salvia rosmarinus. The proficient extraction performance of tuned water under microwave-enhanced technology is attributed to the high dielectric constant of the polar molecules. These molecules are characterized with the ability to absorb irradiated energy and to re-emit it for the heating of the extraction system as compared to the commonly used n-hexane with low dielectric constant.41
5.2 Effect of extraction conditions on yields of biometabolites
The data in Table 6 reveals conditions of two intensified methods namely aqueous ultrasonic hydro-extraction and microwave-enhanced hydro-extraction for their potencies. From the Table 6, extraction of polyphenolic from pomegranate peel using the pulse flow aqueous ultrasonic extraction gives a yield of 41.6% at a lesser extraction time of 10 min when compared to the continuous flow techniques in ultrasonic enhanced hydro-extraction with 30 min extraction time. Also, the mild extraction conditions of phenolics in grape and Withania somifera which are 60 °C, 24 Hz and 65 °C, 45 Hz respectively, helped to conserve the pharmacological activities. During the extraction, the penetration of the tuned solvent led to the disruption of cell walls by acoustical cavitation.74,104 Research reveals that at high temperatures and frequencies, the phenolics extracted from plants undergo degradation during extraction which makes the extracted metabolites commercially and industrially unacceptable.104| Samples | Desired products | Experimental conditions | Extraction time (min) | Yield | Hydro-solvent used | Sources |
|---|---|---|---|---|---|---|
| Centella asiatica | Triterpene | 45 °C; 600 W | 1.83 | 27.10% | Microwave enhanced hydro-solvent | 171 and 172 |
| Dioscorea hispida | Essential oil | 75 °C; 100 W | 20 | 85% | Microwave enhanced hydro-solvent | 173 |
| Chaerophyllum macropodum | Essential oil | — | 45 | 8.10% | Microwave enhanced hydro-solvent | 174 |
| Oliveira procumbens | Essential oil | — | 45 | 7.91% | Microwave enhanced hydro-solvent | 174 |
| Vitis vinifera | Phenolics | 60 °C; 24 kHz | 30 | 24–28% | Aqueous ultrasonic solvent | 175 |
| Cabernet franc grapes | Essential oil | 60 °C; 24 kHz | 15 | 7% | Aqueous ultrasonic solvent | 176 |
| Punica granatum peel | Polypenols | 105 W cm−2 (pulse mode) | 10 | 41.6% | Aqueous ultrasonic solvent | 177 |
| 105 W cm−2 (continuous mode) | 30 | 45.4% | ||||
| Withania somnifera | Phenolics | 65 °C; 45 kHz | 15 | 11.85% | Aqueous ultrasonic solvent | 178 |
| Fresh leaves of Vernonia amygdalina | Flavonoids | 100 °C | 7 | 87.05 mg | Microwave enhanced hydrosolvent | 130 |
| Uncaria sinensis | Flavonoids | 100 °C | 20 | 44 mg/100 g | Microwave enhanced hydrosolvent | 179 |
| Tea residues (oolong) | Flavonoids | 230 °C | 2 | 144.0 mg GAE g−1 | Microwave enhanced hydrosolvent | 180 |
| Genita scabra Bunge stem | Polysaccharides | — | 5.8 | 15.97% | Microwave enhanced hydrosolvent | 120 |
| Ipomoea batatas | Chlorogenic acid | 500 W, 25 KHz | 20 | — | Aqueous ultrasonic solvent | 121 |
| Phyllanthus amarus | Phenolic compounds | 30 W, 19 KHz | 7 | 27.23 mg g−1 | Aqueous ultrasonic solvent | 155 |
| Camellia sinensis | Polysaccharide | 127.5–750 W | 5 | 37.0% | Aqueous ultrasonic solvent | 181 |
| 25 °C, 100–300 W | 5 | 21.4–29.5% | Microwave enhanced hydrosolvent | |||
| Salvia rosmarinus | Carnosic acid and rosmarinic acid | 40 °C 150 W | 30 | 18.1% | Aqueous ultrasonic solvent | 161 |
| 70 °C, 1.2 kW | 20 | 25.2% | Aqueous ultrasonic solvent | |||
| A. melanocarpa | Polyphenolics | 70 °C, 144 W | 60 | 7.428 g/100 g | Aqueous ultrasonic solvent | 182 |
| Malus domestica | Gallic | 100 °C, 1500 W | 20 | 4.77 mg g−1 | Microwave enhanced hydrosolvent | 183 |
| Flavonoids | 100 °C, 1500 W | 20 | 17.1 mg g−1 | Microwave enhanced hydrosolvent | ||
| Ascorbic acid | 100 °C, 1500 W | 20 | 36.1 mg g−1 | Microwave enhanced hydrosolvent | ||
| Pinus radiata bark | Phenolic compounds | 900 W, 2450 MHz | 479 mg CE/g bark | Microwave enhanced hydrosolvent | 153 | |
| 35 kHz, 85 W | 388 mg CE/g bark | Aqueous ultrasonic solvent | ||||
| Moroccan Acacia mollissima bark | Phenolic compounds | 150 W | 279.7 mg GAE/g bark | Microwave enhanced hydrosolvent | 129 |
Furthermore, the extraction of essential oil at 75 °C and 100 W gives an excellent yield of 85% in 20 min using microwave-enhanced hydro-extraction when compared to 7% yield of essential oil from cabernet franc grapes in 15 h using ultrasonic enhanced hydro-extraction.
This is because tuned hydro-solvent under the influence of microwaves has a higher penetrating ability making the solvent to easily interact with the biomaterials for extraction purposes.4,77 The extraction of essential oils from plant samples mostly uses microwave-enhanced hydro-solvent than ultrasonic hydro-solvent based on its faster extraction rate.146
6. Current challenges and future prospects
The appreciable environmental advantage of using tunable hydro-solvent for extraction includes improved mass transfer, selectivity, extraction efficiency, higher yield and shortened extraction time. Future progressive research has been channeled on tackling challenges of large-scale operation and the design of industrial equipment that can use the hydro-solvent and extraction processes.80,157,158 The possibility of recovering and reusing spent hydro-solvents to facilitate cost-effectiveness, especially the water–oil-based solvent remains a great challenge. Furthermore, bio-surfactant may bind to proteins and other bio-active molecules to affect the stability or activity of the extracted products.159 In addition to these, the occasional but deleterious effect of ultrasound energy (more than 20 kHz) on the active constituents of medicinal plants exists. This is through the formation of free radicals and consequently undesirable changes in the active molecules.1607. Conclusion, key report findings and suggestions
The toxicological impact of various organic solvents and the contamination of the bioactive extract necessitate the study and the use of hydro-solvent (water) for extraction. From this review, it is possible to tune the properties of water to enhance its feature similar to organic solvents, increase extraction efficiency and create the possibility of the use of water in the extraction of a broad range of solutes which has been the limitation of hydro extraction. The intensified hydro-solvent system is faster and more selective for metabolite recovery than the traditional approach method. In the light this, the use of tuned solvents with intensified techniques should be employed to forestall the contamination of bio-actives extracted from plants and to enhance the rapid extraction process.Author contributions
AOA, JAO, ROA conceptualized and wrote the original draft of the study; MAA, RM, SA worked on the methodology, formal analysis and data curation; AOA, JAO, ROA, MAA, RM, SA reviewed and edited the final draft. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
A. O. A acknowledged the contributions of Prof J. O. Odiyo and the WRC and NRF for funding support.References
- J. Azmir, I. Zaidul, M. Rahman, K. Sharif, A. Mohamed, F. Sahena, M. Jahurul, K. Ghafoor, N. Norulaini and A. Omar, Techniques for extraction of bioactive compounds from plant materials: A review, J. Food Eng., 2013, 117(4), 426–436 CrossRef CAS.
- I. Naboulsi and A. Aboulmouhajir, Plants extracts and secondary metabolites, their extraction methods and use in agriculture for controlling crop stresses and improving productivity: A review, Acad. J. Med. Plants, 2018, 6(8), 223–240, DOI:10.15413/ajmp.2018.0139.
- N. Azwanida, A Review on the Extraction Methods Use in Medicinal Plants, Principle, Strength and Limitation, Med. Aromat. Plants, 2015, 4, 196 Search PubMed.
- S. Handa, S. Khanuja, G. Longo and D. Rakesh, Extraction Technologies for Medicinal and Aromatic Plants, United Nations Industrial Development Organization and the International Centre for Science and High Technology, 2015, vol. 18, pp. 214–243 Search PubMed.
- F. Chemat, M. Vian and G. Cravotto, Green extraction of natural products: concepts and principles, Int. J. Mol. Sci., 2021, 13(7), 8615–8627 CrossRef.
- N. Rombaut, A. S. Tixier, A. Bily and F. Chemat, Green extraction processes of natural products as tools for biorefinery, Biofuels, Bioprod. Biorefin., 2014, 8(4), 530–544 CrossRef CAS.
- F. Chemat, and J. Strube. Green extraction of natural products: theory and practice. Wiley-VCH Verlag GmbH & Co. KGaA, Boschstr. Weinheim, Germany. 2015 Search PubMed.
- F. Chemat, A. Fabiano-Tixier, M. Vian, T. Allaf and E. Vorobiev, Solvent-free extraction of food and natural products, Trends Anal. Chem., 2015, 71, 157–168 CrossRef CAS.
- M. Yiz, Review on the Extraction by Subcritical and Supercritical Water, Methanol, Ethanol and Their Mixtures, Institute of Chemistry, The Hebrew University of Jerusalem, 2018 Search PubMed.
- H. González and M. Muñoz. Water Extraction of Bioactive Compounds: From Plants to Drug Development. 2017, pp. 1–530 Search PubMed.
- K. Minami, M. Mizura, M. Suzuki, T. Aizawa and K. Aray, Determination of Kamlet-Taft solvent parameter p* of high pressure and supercritical water by the UV-vis absorption spectral shift of 4-nitroanisole, Phys. Chem. Chem. Phys., 2006, 8, 2257–2264 RSC.
- Y. Marcus. Supercritical Water: A green solvent, properties and uses. Wiley: New York, NY, USA, 2012 Search PubMed.
- S. Aritra and Y. Richa, Reverse micellar extraction: technological aspects, applications and recent developments. School of Biosciences and Technology, VIT University, Vellore, Tamil Nadu, India, J. Pharm. Res., 2015, 9(2), 145–156 Search PubMed.
- C. Anokwuru, G. Anyasor, O. Ajibaye, O. Fakoya and P. Okebugwu, Erect of extraction solvents on phenolic, flavonoid and antioxidant activities of three Nigerian medicinal plants, Nat. Sci., 2011, 9, 53–61 Search PubMed.
- M. Abraham and N. Nguyen, Green engineering: defining the principles- results from the Sandestin Conference, Environ. Prog., 2003, 22(4), 233–236 CrossRef CAS.
- R. Breslow. The principles of and reasons for using water as a solvent for green chemistry, in Handbook of Green Chemistry, Wiley-VCH Verlag GmbH & Co, 2010 Search PubMed.
- T. S. Tochikura, H. Nakashima, K. Hirose and N. Yamamoto, A biological response modifier, PSK, inhibits human immunodeficiency virus infection in vitro, Biochem. Biophys. Res. Commun., 1987, 148, 726–733 CrossRef CAS.
- S. T. Chang and J. A. Buswell, Mushroom nutraceutical, World J. Microbiol. Biotechnol., 1996, 12(5), 473–476 CrossRef CAS.
- C. Hobbs, Medicinal Value of Turkey Tail Fungus Trametes versicolor(L.:Fr.) Pilát (Aphyllophoromycetideae). A Literature Review, Int. J. Med. Mushrooms, 2004, 6(3), 1–24 Search PubMed.
- V. Vetvicka and J. Vetvickova, Immune enhancing effects of Maitake (Grifola frondosa) and Shiitake (Lentinula edodes) extracts, Ann. Transl. Med., 2014, 2(2), 1–6 Search PubMed.
- A. Filly, A. Fabiano-Tixier, C. Louis, X. Fernandez and F. Chemat, Water as a green solvent combined with different techniques for extraction of essential oil from lavender flowers, C. R. Chim., 2016, 19(6), 707–717 CrossRef CAS.
- A. Kimbaris, N. Siatis, D. Daferera, P. Tarantilis, C. Pappas and M. Polissiou, Comparison of distillation and ultrasound-assisted extraction methods for the isolation of sensitive aroma compounds from garlic (Allium sativum), Ultrason. Sonochem., 2006, 13, 54–60 CrossRef CAS PubMed.
- Y. Zhao, C. Liu, M. Feng, Z. Chen, S. Li, G. Tian, L. Wang, J. Hunag and S. Li, Solid phase extraction of uranium (VI) onto benzoylthiourea-anchored activated carbon, J. Hazard. Mater., 2010, 176, 119–124 CrossRef CAS PubMed.
- K. Alfonsi, Green chemistry tools to influence a medicinal chemistry and research chemistry based organisation, Green Chem., 2008, 10(1), 31–36 RSC.
- C. Capello, U. Fischer and K. Hungerbühler, What is a green solvent? A comprehensive framework for the environmental assessment of solvents, Green Chem., 2007, 9(9), 927–934 RSC.
- M. Tobiszewski, A solvent selection guide based on chemometrics and multicriteria decision analysis, Green Chem., 2015, 17(10), 4773–4785 RSC.
- D. R. Joshi and N. Adhikari, An Overview on Common Organic Solvents and Their Toxicity June 2019, J. Pharm. Res. Int., 2019, 28, 33–203 Search PubMed.
- R. Vardanega, D. T. Santos and M. A. A. Meireles, Intensification of bioactive compounds extraction from medicinal plants using ultrasonic irradiation, Pharmacogn. Rev., 2014, 8(16), 88–95 CrossRef.
- F. Chemat, N. Rombaut, A. Sicaire, A. Meullemiestre and M. Abert-vian, Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications, Ultrason. Sonochem., 2017, 34, 540–560 CrossRef CAS PubMed.
- Y. Hernández, M. Lobo and M. González, Factors affecting sample extraction in the liquid chromatographic determination of organic acids in papaya and pineapple, Food Chem., 2009, 114, 734–741 CrossRef.
- M. Plaza and C. Turner, Pressurized hot water extraction of bioactives, Trends Anal. Chem., 2015, 71, 39–54 CrossRef CAS.
- G. Feli, R. Pezeshki and M. Chizari, The effects of services of crop prospects’ advisers on the farmers in Tehran province, Iranian Agricultural Training and Promotional Science Journal, 2007, 3(1), 73–81 Search PubMed.
- A. Nieto, F. Borrull, R. Marcé and E. Pocurull, Pressurized liquid extraction of contaminants from environmental samples, Curr. Anal. Chem., 2008, 4, 157–167 CrossRef CAS.
- A. Zaibunnisa, S. Norashikin, S. Mamot and H. Osman, An experimental design approach for the extraction of volatile compounds from turmeric leaves (Curcuma domestica) using pressurised liquid extraction (PLE), LWT–Food Sci. Technol., 2009, 42, 233–238 CrossRef CAS.
- M. Hassas-Roudsari, P. Chang, R. Pegg and R. Tyler, Antioxidant capacity of bioactives extracted from canola meal by subcritical water, ethanolic and hot water extraction, Food Chem., 2009, 114, 717–726 CrossRef CAS.
- W. Kim, J. Kim, B. Veriansyah, J. Kim, Y. Lee, S. Oh and R. Tjandrawinata, Extraction of bioactive components from Centella asiatica using subcritical water, J. Supercrit. Fluids, 2009, 48, 211–216 CrossRef CAS.
- G. Brunner, Near critical and supercritical water. Part I. Hydrolytic and hydrothermal processes, J. Supercrit. Fluids, 2009, 47, 373–381 CrossRef CAS.
- A. Amid, R. Salim and M. Adenan, The Factors Affecting the Extraction Conditions for Neuroprotective Activity of Centella asiatica evaluated by Metal Chelating Activity Assay, J. Appl. Sci., 2010, 10, 837–842 CrossRef CAS.
- O. H. Yung, M. Y. Maskat and M. W. A. Wan, Effect of extraction on polyphenol content, antioxidant activity and pH in pegaga (Centella asiatica), Sains Malays., 2010, 39, 747–752 CAS.
- D. C. Willcox, B. J. Willcox, H. Todoriki and M. Suzuki, The Okinawan diet: health implications of a low-calorie, nutrient-dense, antioxidant-rich dietary pattern low in glycemic load, J. Am. Coll. Nutr., 2009, 28, 500S–516S CrossRef CAS.
- A. Carr and N. Mammucari, A review of subcritical water as a solvent and its utilization for the processing of hydrophobic organic compounds, Chem. Eng. J., 2011, 172, 1–17 CrossRef CAS.
- C. C. Teo, S. N. Tana, J. W. H. Yonga, E. S. Hew and E. S. Ong, Pressurised hot water extraction (PHWE), J. Chromatogr. A, 2010, 1217, 2484–2494 CrossRef CAS.
- J. Kronholm, K. Hartonen and M. Riekkola, Analytical extractions with water at elevated temperatures and pressures, TrAC, Trends Anal. Chem., 2011, 26, 396–412 CrossRef.
- M. Mottaleb and S. Sarker, Accelerated solvent extraction for natural products isolation, Methods Mol. Biol., 2012, 864, 75–87 CrossRef CAS PubMed.
- M. Co, C. Zettersten, L. Nyholm, P. J. R. Sjoberg and C. Turner, Degradation effect in the extraction of antioxidants from brich nark using water at elevated temperature and pressure, Anal. Chim. Acta, 2011, 716, 40–48 CrossRef PubMed.
- R. M. Smith, Extraction with superheated water, J. Chromatogr. A, 2002, 975, 31–46 CrossRef CAS PubMed.
- C. Chin, N. Swee, W. Jean, S. Choy and S. Eng, Pressurized hot water extraction (PHWE), J. Chromatogr. A, 2010, 1217, 2484–2494 CrossRef PubMed.
- N. Kapadiya, L. Singhvi, K. Mehta, G. Karwani and J. Dhrubo, Hydrotropy: A promising tool for solubility enhancement: A Review, Int. J. Drug Dev. Res., 2011, 3(2), 26–33 CAS.
- B. Mohsin and J. Abida, Ultrasonic assisted solvent extraction of bioactive compounds: A review, Int. J. Food Sci., 2019, 4(2), 102–106 Search PubMed.
- Z. Cai, Z. Qu, Y. Lan, S. Zhao, X. Ma, Q. Wan and P. Li, Conventional, ultrasound-assisted, and accelerated-solvent extractions of anthocyanins from purple sweet potatoes, Food Chem., 2016, 197, 266–272 CrossRef CAS PubMed.
- E. A. Brignole, Supercritical fluid extraction, Fluid Ph. Equilibria, 1986, 29(C), 133–144 CrossRef CAS.
- A. Kubatova, A. J. Lagadec, D. J. Miller and S. B. Hawthorne, Selective extraction of oxygenates from savory and peppermint using subcritical water, Flavour Fragrance J., 2001, 16, 64–73 CrossRef CAS.
- J. Kronholm, T. Kuosmanen, K. Hartonen and M. Riekkola, Destruction of PAHs from soil by using pressurized hot water extraction coupled with supercritical water oxidation, Waste Manage. Res., 2003, 23, 253–260 CrossRef CAS PubMed.
- L. Gamiz Gracia and M. D. Luque de Castro, Continuous subcritical water extraction of medicinal plant essential oil: comparison with conventional techniques, Talanta, 2000, 51, 1179–1185 CrossRef CAS PubMed.
- E. Ibañez, A. Kubatova, F. J. Senorans, S. Cavero, G. Reglero and S. B. Hawthorne, Subcritical water extraction of antioxidant compounds from rosemary plants, J. Agric. Food Chem., 2003, 51, 375–382 CrossRef PubMed.
- M. D. A. Saldaña and C. S. Valdivieso-Ramírez, Pressurized fluid systems: Phytochemical production from biomass, J. Supercrit. Fluids, 2015, 96, 228–244 CrossRef.
- X. L. Qi, T. T. Li, Z. F. Wei, N. Guo, M. Luo, W. Wang, Y. G. Zu, Y. J. Fu and X. Peng, Solvent-free microwave extraction of essential oil from pigeon pea leaves Cajanus cajan (L.) Millsp and evaluation of its antimicrobial activity, Ind. Crops Prod., 2014, 1(58), 322–328 CrossRef.
- R. Halim, M. K. Danquah and P. A. Webley, Extraction of oil from microalgae for biodiesel production: a review, Biotechnol. Adv., 2012, 30(2), 709–732 CrossRef CAS PubMed.
- E. Baldwin, J. Bai, A. Plotto, R. Cameron, G. Luzio, J. Narciso and B. Ford, Elect of extraction method on quality of orange juice: Hand-squeezed, commercial-fresh squeezed and processed, J. Sci. Food Agric., 2012, 92, 2029–2042 CrossRef CAS PubMed.
- S. Armenta, S. Garrigues and M. De la Guardia, The role of green extraction techniques in Green Analytical Chemistry, Trends Anal. Chem., 2015, 71, 2–8 CrossRef CAS.
- T. Vanek, A. Nepovium and P. Valice, Membrane- based separation scheme for processing sweeteners from Stevia leaves, Food Res. Int., 2001, 33, 617–620 Search PubMed.
- Y. Choon, A. Hanaa and S. Rabiha, Extraction and quantification of saponins: A review, Food Res. Int., 2014, 5, 16–40 Search PubMed.
- B. S. Rathi, S. L. Bodhankar and A. M. Baheti, Evaluation of aqueous leaves extract of Moringa oleifera Linn for wound healing in albino rats, Indian J. Exp. Biol., 2006, 44, 898–901 CAS.
- M. De Castro and F. Priego-Capote, Soxhlet extraction: Past and present panacea, J. Chromatogr. A, 2010, 1217, 2383–2389 CrossRef PubMed.
- S. Chanda and M. Kaneria, Optimization of conditions for the extraction of antioxidants from leaves of Syzygium cumini L. using different solvents, Food Anal. Methods, 2012, 5, 332–338 CrossRef.
- N. Manousi, I. Sarakatsianos and V. Samanidou, Extraction techniques of phenolic compounds and other bioactive compounds from medicinal and aromatic plants, in Engineering Tools in the Beverage Industry, Woodhead Publishing: Sawston, UK. 2019, pp. 283–314 Search PubMed.
- M. Bimakr, Comparison of different extraction methods for the extraction of major bioactive flavonoid compounds from spearmint (Mentha spicata L.) leaves, Food Bioprod. Process., 2010, 89(1), 67–72 CrossRef.
- M. Cowan, Plant products as antimicrobial agents, Clin. Microbiol. Rev., 1999, 12(4), 564–582 CrossRef CAS.
- M. Njila, E. Mahdi, D. Massoma Lembe, Z. Nde and D. Nyonseu, Review on Extraction and Isolation of Plant Secondary Metabolites, 7th Int'l Conference on Agricultural, Chemical, Biological and Environmental Sciences, 2017 Search PubMed.
- F. Ekezie, D. Sun and J. Cheng, Acceleration of microwave-assisted extraction processes of food components by integrating technologies and applying emerging solvents: A review of latest developments, Trends Food Sci. Technol., 2017, 67, 160–172 CrossRef CAS.
- F. B. Gerardo, Extraction and Analysis of Natural Product in Plant, Agronomy, 2011, 11, 415 Search PubMed.
- M. Chávez-González, L. Sepúlveda, D. Verma, H. Luna-García, L. Rodríguez-Durán, A. Ilina and C. Aguilar, Conventional and emerging extraction processes of flavonoids, Processes, 2020, 8(4), 434 CrossRef.
- D. Naviglio, P. Scarano, M. Ciaravolo and M. Gallo, Rapid Solid-Liquid Dynamic Extraction (RSLDE): A Powerful and Greener Alternative to the Latest Solid-Liquid Extraction Techniques, Foods, 2019, 8, 245 CrossRef CAS PubMed.
- O. Gusthinnadura, T. Achala, M. Malamige and A. Weroshana, Extraction methods, qualitative and quantitative techniques for screening of phytochemicals from plants, Am. J. Essent. Oil. Nat. Prod., 2017, 5(2), 29–32 Search PubMed.
- B. Nayak, F. Dahmoune, K. Moussi, H. Remini, S. Dairi, O. Aoun and M. Khodir, Comparison of microwave, ultrasound and accelerated-assisted solvent extraction for recovery of polyphenols from Citrus sinensis peels, Food Chem., 2015, 187, 507–516 CrossRef CAS.
- C. Rodríguez-Pérez, B. Gilbert-López, J. A. Mendiola, R. Quirantes-Piné, R. Segura-Carretero and A. Ibáñez, Optimization of microwave-assisted extraction and pressurized liquid extraction of phenolic compounds from Moringa oleifera leaves by multi-response surface methodology, Electrophoresis, 2017, 37, 1938–1946 CrossRef PubMed.
- B. Kaufmann and P. Christen, Recent extraction techniques for natural products: microwave-assisted extraction and pressurized solvent extraction, Phytochem. Anal., 2002, 13, 105–113 CrossRef CAS PubMed.
- T. Jain, Microwave assisted extraction for phytoconstituents – an overview, Asian J. Res. Chem., 2009, 2(1), 19–25 CAS.
- M. Numata, T. Yarita, Y. Aoyagi, Y. Tsuda, M. Yamazaki, A. Takatsu, K. Ishikawa, K. Chiba and K. Okamaoto, Sediment certified reference materials for the determination of polychlorinated biphenyls and organochlorine pesticides from the National Metrology Institute of Japan (NMIJ), Anal. Bioanal. Chem., 2007, 387, 2313–2323 CrossRef CAS PubMed.
- Z. Jixian, W. Chaoting, Z. Haihui, D. Yuqing and M. Haile, Recent advances in the extraction of bioactive compounds with subcritical water: A review, Trends Food Sci. Technol., 2020, 95, 183–195 CrossRef.
- J. Pol, E. Ostra, P. Karasek, M. Roth, K. Benesova, P. Kotlarikova and J. Caslavsky, Comparison of two different solvents employed for pressurised fluid extraction of stevioside from Stevia rebaudiana: methanol versus water, Anal. Bioanal. Chem., 2007, 388, 1847 CrossRef CAS.
- L. Ramos, E. Kristenson and U. T. Brinkman, Current use of pressurised liquid extraction and subcritical water extraction in environmental analysis, J. Chromatogr. A, 2002, 975, 3–29 CrossRef CAS.
- Z. Ju and L. Howard, Subcritical water and sulfured water extraction of anthocyanins and other phenolics from dried red grape skin, J. Food Sci., 2005, 70, S270–S276 CrossRef CAS.
- I. Sereewatthanawut, S. Prapintip, K. Watchiraruji, M. Goto, M. Sasaki and A. Shotipruk, Extraction of protein and amino acids from deoiled rice bran by subcritical water hydrolysis, Bioresour. Technol., 2008, 99(3), 555–561 CrossRef CAS PubMed.
- T. Awad, H. Moharram, O. Shaltout, D. Asker and M. Youssef, Applications of ultrasound in analysis, processing and quality control of food: A review, Food Res. Int., 2012, 48, 410–427 CrossRef CAS.
- G. Musielak, D. Mierzwa and J. Kroehnke, Food drying enhancement by ultrasound—A review, Trends Food Sci. Technol., 2016, 56, 126–141 CrossRef CAS.
- M. Vinatoru, An overview of the ultrasonically assisted extraction of bioactive principles from herbs, Ultrason. Sonochem., 2001, 8, 303–313 CrossRef CAS PubMed.
- M. Esclapez, J. Garcia-Perez, A. Mulet and J. Cárcel, Ultrasound-assisted extraction of natural products, Food Eng. Rev., 2011, 3, 108–120 CrossRef.
- B. K. Tiwari, A clean, green extraction technology, Trends Anal. Chem., 2015, 71, 100–109 CrossRef CAS.
- R. Maheshwari, M. Rajput and S. Sinha, New quantitative estimation of benzoic acid bulk sample using calcium disodium edetate as hydrotropic solubilizing agent, Asian J. Pharm. Clin. Res., 2010, 3(1), 43–45 CAS.
- N. Karanth, P. Deo and N. Veenanadig, Microbial production of biosurfactants and their importance, Curr. Sci., 1999, 77, 116–123 CAS.
- P. Jain, A. Goel, S. Sharma and M. Parmar, Solubility Enhancement Techniques with Special Emphasis on Hydrotrophy, Int. J. Pharm. Pharm. Res., 2010, 1(1), 34–45 Search PubMed.
- R. Maheshwari, S. Sharma, N. Rai and M. Rajput, Simple titrimetric method to estimate ketoprofen in bulk using mixed hydrotropy, J. Pharm. Res., 2010, 3(3), 442–443 CAS.
- G. Raman and V. Gaikar, Extraction of piperine from piper nigrum (Blac pepper) by hydrotropic solubilization, Ind. Eng. Chem. Res., 2002, 41, 2966–2976 CrossRef CAS.
- M. Malik, Y. Mohammad and A. Mohd, Microemulsion method: A novel route to synthesize organic and inorganic nanomaterials, Arabian J. Chem., 2010, 5(4), 397–417 CrossRef.
- M. Freda, G. Onori, A. Paciaroni and A. Santucci, Influence of hydration on dynamical properties of reverse micelles, J. Non-Cryst. Solids, 2002, 307, 874–877 CrossRef.
- T. Havre. Formation of calcium naphthenate in water/oil systems, naphthenic acid chemistry and emulsion stability, Doctoral diss., Norwegian University of Science and Technology, Trondheim, 2002.
- S. Debnath, D. Das and P. Das, Unsaturation at the surfactant head: influence on the activity of lipase and horseradish peroxidase in reverse micelles, Biochem. Biophys. Res. Commun., 2007, 356, 163–168 CrossRef CAS PubMed.
- G. Tingyue. Liquid-liquid Partitioning methods for bioseparations, handbook of Bioseparations, 2nd edn, Academic Press, New York, 2002 Search PubMed.
- S. Mohd-Setapara, S. Mohamad-Aziza, N. Haruna and C. Mohd-Azizia, Review on the Extraction of Biomolecules by Biosurfactant Reverse Micelles, APCBEE Proc., 2012, 3, 78–83 CrossRef.
- G. M. Priscilla, M. L. Andre, A. H. Francilene, F. J. Angela, C. V. P. Thereza, O. M. Perola, O. R. Carlota and P. J. Adalberto, Liquid-liquid extraction of biomolecules: an overview and update of the main techniques, J. Chem. Technol. Biotechnol., 2008, 83, 143–157 CrossRef.
- Z. Li, K. Smith and G. Stevens, The use of environmentally sustainable bio-derived solvents in solvent extraction applications—a review, Chin. J. Chem. Eng., 2015, 24, 215–220 CrossRef.
- N. Imane, A. Aziz, K. Lamfeddal, B. Faouzi and Y. Abdelaziz, Plants extracts and secondary metabolites, their extraction methods and use in agriculture for controlling crop stresses and improving productivity: A review, J. Med. Plants Res., 2018, 6(8), 223–240 Search PubMed.
- T. Reighard and S. Olesik, Bridging the Gap between Supercritical Fluid Extract ions and Liquid Extraction Techniques: Alternative Approaches of the Extract ion of Solid and Liquid Environmental Matrices, Crit. Rev. Anal. Chem., 2006, 26(2&3), 1–39 Search PubMed.
- S. Arora and P. Itankar, 2018. Extraction, isolation and identification of flavonoid from Chenopodium album aerial parts, J. Tradit. Chin. Med., 2018, 8, 476–482 Search PubMed.
- O. Alara, N. Abdurahman and O. A. Olalere, Optimization of microwave-assisted extraction of flavonoids and antioxidants from Vernonia amygdalina leaf using response surface methodology, Food Bioprod. Process., 2018, 107, 36–48 CrossRef CAS.
- M. Puspita, M. Deniel, I. Widowati, O. K. Radjasa, P. Douzenel, G. Bedoux and N. Bourgougnon, Antioxidant and antibacterial activity of solid-liquid and enzyme-assisted extraction of phenolic compound from three species of tropical Sargassum, IOP Conf. Ser.: Earth Environ. Sci., 2021, 55012057 Search PubMed.
- S. Armenta, S. Garrigues and M. De la Guardia, The role of green extraction techniques in Green Analytical Chemistry, Trends Anal. Chem., 2015, 71, 2–8 CrossRef CAS.
- L. S. Chua, F. I. Abdullah and M. A. F. Azlah, Phytochemical Profile of Andrographis paniculataExtract from Solvent Partition and Precipitation, J. Biol. Act. Prod. Nat., 2019, 9(3), 238–249 CAS.
- M. Aourach, A. González-de-Peredo, M. Vázquez-Espinosa, H. Essalmani, M. Palma and G. Barbero, Optimization and Comparison of Ultrasound and Microwave-Assisted Extraction of Phenolic Compounds from Cotton-Lavender (Santolina chamaecyparissus L.), Agronomy, 2011, 11, 84 CrossRef.
- H. Giergielewicz-Możajska, L. Dąbrowski and J. Namieśnik, Accelerated Solvent Extraction (ASE) in the Analysis of Environmental Solid Samples — Some Aspects of Theory and Practice, Crit. Rev. Anal. Chem., 2001, 31(3), 149–165 CrossRef.
- D. Vividha and M. Piyush, Advances in hydrotropic solutions: an updated review, St. Petersburg Polytechnical University Journal: Physics and Mathematics, 2016, 424–435 Search PubMed.
- B. Vongsak, P. Sithisarn, S. Mangmool, S. Thongpraditchote, Y. Wongkrajang and W. Gritsanapan, Maximizing total phenolics, total flavonoids contents and antioxidant activity of Moringa oleifera leaf extract by the appropriate extraction method, Ind. Crops Prod., 2013, 44, 566–571 CrossRef CAS.
- A. Dawidowicz, E. Rado, D. Wianowska, M. Mardarowicz and J. Gawdzik, Application of PLE for the determination of essential oil components from Thymus Vulgaris L, Talanta, 2008, 76, 878–884 CrossRef CAS PubMed.
- J. Cao, J. Han, H. Xiao, J. Qiao and M. Han, Effect of tea polyphenol compounds on anticancer drugs in terms of anti-tumor activity, toxicology, and pharmacokinetics, Nutrients, 2016, 8(12), 762 CrossRef PubMed.
- O. Benito-Román, E. Alonso and M. Cocero, Pressurized hot water extraction of β- glucans from waxy barley, J. Supercrit. Fluids, 2013, 73, 120–125 CrossRef.
- T. Lefebvre, E. Destandau and E. Lesellier, Selective extraction of bioactive compounds from plants using recent extraction techniques: A review, J. Chromatogr. A, 2021, 1635, 461770 CrossRef CAS PubMed.
- C. Carrero-Carralero, D. Mansukhani, A. I. Ruiz-Matute, I. Martínez-Castro, L. Ramos and M. L. Sanz, Extraction and characterization of low molecular weight 1396 bioactive carbohydrates from mung bean (Vigna radiata), Food Chem., 2018, 266 1397, 146–154 CrossRef PubMed.
- X. J. Qu, Y.-J. Fu, M. Luo, C.-J. Zhao, Y.-G. Zu, C.-Y. Li, W. Wang, J. Li and Z. F. Wei, Acidic pH based microwave-assisted aqueous extraction of seed oil from 1405 yellow horn (Xanthoceras sorbifolia Bunge.), Ind. Crops Prod., 2013, 43 1406, 420–426 CrossRef.
- Z. Cheng, H. Song, X. Cao, Q. Shen, D. Han, F. Zhong, H. Hu and Y. Yang, Simultaneous extraction and purification of polysaccharides from Gentiana scabra Bunge by microwave-assisted ethanol-salt aqueous two-phase system, Ind. Crops Prod., 2017, 102, 75–87 CrossRef CAS.
- E. G. Alves Filho, V. M. Sousa, S. Rodrigues, E. S. de Brito and F. A. N. Fernandes, Green ultrasound-assisted extraction of chlorogenic acids from sweet potato peels and sonochemical hydrolysis of caffeoylquinic acids derivatives, Ultrason. Sonochem., 2020, 63, 104911 CrossRef CAS PubMed.
- N. Cujic, K. Šavikin, T. Jankovic, D. Pljevljakušic and G. Zdunic, Ibric, Optimization of polyphenols extraction from dried chokeberry using maceration as traditional technique, Food Chem., 2016, 194, 135–142 CrossRef CAS PubMed.
- M. Daud, D. Fatanah, N. Abdullah and R. Ahmad, Evaluation of antioxidant potential of Artocarpus heterophyllus L. J33 variety fruit waste from different extraction methods and identification of phenolic constituents by LCMS, Food Chem., 2017, 232, 621–632 CrossRef CAS PubMed.
- N. N. Wang, J. A. Anuka, H. O. Kwanashie, S. Gyang and A. Auta, Anti-seizure activity of the aqueous leaf extract of Solanum nigrum linn (solanaceae) in experimental animals, Afr. Health Sci., 2008, 8(2), 74–79 Search PubMed.
- O. Ait-Yahia, F. Perreau, S. A. Bouzroura, Y. Benmalek, T. Dob and A. Belkebir, Chemical composition and biological activities of n-butanol extract of Lepidium sativum L (Brassicaceae) seed, Trop. J. Pharm. Res., 2018, 17(5), 891–896 CrossRef CAS.
- Y. Vázquez-González, J. A. Ragazzo-Sánchez and M. Calderón-Santoyo, Characterization and antifungal activity of jackfruit (Artocarpus heterophyllus Lam.) leaf extract obtained using conventional and emerging technologies, Food Chem., 2020, 330, 127211 CrossRef PubMed.
- M. Bouras, M. Chadni, F. J. Barba, N. Grimi, O. Bals and E. Vorobiev, Optimization of microwave-assisted extraction of polyphenols from Quercus bark, Ind. Crops Prod., 2015, 77, 590–601 CrossRef CAS.
- O. Alara, N. Abdurahman and L. Ukaegbu, Soxhlet extraction pf phenolic compounds from Vernonia cinereal leaves and its antioxidant activity, J. Appl. Res. Med. Aromat. Plants, 2018, 11, 12–17 Search PubMed.
- R. Naima, M. Oumam, H. Hannache, A. Sesbou, B. Charrier, A. Pizzi, E. Charrier and F. Bouhtoury, Comparison of the impact of different extraction methods on polyphenols yields and tannins extracted from Moroccan Acacia mollissima barks, Ind. Crops Prod., 2015, 70, 245–252 CrossRef CAS.
- E. Aspé and K. Fernández, The elect of different extraction techniques on extraction yield, total phenolic, and anti-radical capacity of extracts from Pinus radiata Bark, Ind. Crops Prod., 2011, 34, 838–844 CrossRef.
- S. L. Rodríguez De Luna, R. E. Ramírez-Garza and S. O. Serna Saldívar, Environmentally Friendly Methods for Flavonoid Extraction from Plant Material: Impact of Their Operating Conditions on Yield and Antioxidant Properties, Sci. World J., 2020, 6792069 Search PubMed.
- F. Chemat, M. Lucchesi, J. Smadja, L. Favretto, G. Colnaghi and F. Visinoni, Microwave accelerated steam distillation of essential oil from lavender: A rapid, clean and environmentally friendly approach, Anal. Chim. Acta, 2006, 555(1), 157–160 CrossRef CAS.
- J. Asghari, C. K. Touli, M. Mazaheritehrani and M. Aghdasi, Comparison of the microwave-assisted hydrodistillation with the traditional hydrodistillation method in the extraction of essential oils from Ferulagoangulata (Schelcht.), Eur. J. Med. Plants, 2012, 2(4), 324–334 CrossRef.
- K. A. Shams, N. S. Abdel-Azim, I. A. Saleh, M. E. F. Hegazy, M. M. El-Missiry and F. M. Hammouda, Green technology: Economically and environmentally innovative methods for extraction of medicinal & aromatic plants (MAP) in Egypt, J. Chem. Pharm. Res., 2015, 7(5), 1050–1074 CAS.
- L. Chupin, C. Motillon, F. Charrier-El Bouhtoury, A. Pizzi and B. Charrier, Characterisation of maritime pine (Pinus pinaster) bark tannins extracted under different conditions by spectroscopic methods, FTIR and HPLC, Ind. Crops Prod., 2013, 49, 897–903 CrossRef CAS.
- M. V. Alvarez, S. Cabred, C. L. Ramirez and M. A. Fanovich, Fluids valorization of an agroindustrial soybean residue by supercritical fluid extraction of phytochemical compounds, J. Supercrit. Fluids, 2019, 143, 90–96, DOI:10.1016/j.supflu.2018.07.012.
- M. Khajenoori, A. Haghighi Asl and H. Noori Bidgoli, Subcritical Water Extraction of Essential Oils from Matricaria Chamomilla L., Int. J. Eng., 2013, 26(5), 489–494 Search PubMed.
- L. Zhao, G. H. Zhao and M. Du, et al., Effect of selenium on increasing free radical scavenging activities of polysaccharide extracts from a Se-enriched mushroom species of the genus Ganoderma, Eur. Food Res. Technol., 2008, 226, 499–505 CrossRef CAS.
- X. Zhao, M. Gao, Q. Wang and X. Luan, Extraction, separation and structural characterization of polysaccharides from Crassostrea gigas, Chin. J. Mar. Drugs, 2014, 33, 8–14 Search PubMed.
- H. Cheng, X. Zhu, C. Zhu, J. Qian, N. Zhu, L. Zhao and J. Chen, Hydrolysis technology of biomass waste to produce amino acids in subcritical water, Bioresour. Technol., 2008, 99, 3337–3341 CrossRef CAS PubMed.
- Y. Chen, J. Tou and J. Jaczynski, Composition and recovery yield of protein and other components isolated from whole Antarctic krill (Euphausia superba) by isoelectric solubilization/precipitation, J. Food Sci., 2009, 74(2), H31–H39 CrossRef CAS PubMed.
- G. Zhu, Z. Xiao and X. Zhu, Reducing sugars production from sugarcane bagasse wastes by hydrolysis in sub-critical water. Clean Technologies and Environmental Policy, February, 2012, 15(1), 55–61 Search PubMed.
- S. Hata, J. Wiboonsirikul and A. Maeda, Extraction of defatted rice bran by subcritical water treatment, Biochem. Eng. J., 2008, 40(1), 44–53 CrossRef CAS.
- H. Yoshida, W. Abdelmoez, S. M. Nage and A. Bastawess, Ihab. Subcritical Water Technology for Wheat Straw Hydrolysis to Produce Value Added Products, J. Cleaner Prod., 2014, 70, 68–77 CrossRef.
- R. Rodríguez-herrera, C. Aguilar, D. Muñiz-márquez, G. Martínez-ávila, J. Wong-paz and R. Belmares-cerda, Ultrasound-assisted extraction of phenolic compounds from Laurus nobilis L. and their antioxidant activity, Ultrason. Sonochem., 2013, 20, 1149–1154 CrossRef PubMed.
- P. Koh, C. Leong and M. Noranizan, Microwave-assisted extraction of pectin from jackfruit rinds using different power levels, Int. Food Res. J., 2014, 21, 2091–2097 Search PubMed.
- T. Xia, S. Shi and X. Wan, Impact of ultrasonic-assisted extraction on the chemical and sensory quality of tea infusion, J. Food Eng., 2006, 74, 557–560 CrossRef CAS.
- L. Ruiz-Aceituno, S. Rodríguez-Sánchez, J. Sanz, M. L. Sanz and L. Ramos, Optimization of pressurized liquid extraction of inositols from pine nuts (Pinus pinea L.), Food Chem., 2014, 153, 450–456 CrossRef CAS PubMed.
- C. C. Teo, S. N. Tan, J. W. H. Yong, C. S. Hew and E. S. Ong, Validation of green-solvent extraction combined with chromatographic chemical fingerprint to evaluate quality of Stevia rebaudianaBertoni, J. Sep. Sci., 2009, 32, 613–622 CrossRef CAS PubMed.
- D. Vishwas and M. Pradeep, Advances in hydrotropic solutions: an updated review, St. Petersburg Polytechnical University Journal: Physics and Mathematics, 2015, 30(4), 119–138 Search PubMed.
- C. C. Teo, S. N. Tan, J. W. H. Yong, C. S. Hew and E. S. Ong, Evaluation of the extraction efficiency of thermally labile bioactive compounds in Gastrodiaelata Blume by pressurized hot water extraction and microwave-assisted extraction, J. Chromatogr. A, 2008, 1182(1), 34–40 CrossRef CAS PubMed.
- P. Budrat and A. Shotipurk, Extraction of phenolic compounds from fruits of bitter melon (Momordica charantia) with subcritical water extraction and antioxidant activities of these extracts, Chiang Mai J. Sci., 2008, 35(1), 123–130 CAS.
- C. Bocalandro, V. Sanhueza, A. M. Gómez-Caravaca, J. González-Álvarez, K. Fernández, M. Roeckel and M. T. Rodríguez-Estrada, Comparison of the composition of Pinus radiata bark extracts obtained at bench- and pilot-scales, Ind. Crops Prod., 2012, 38, 21–26 CrossRef CAS.
- M. Dedrie, N. Jacquet, P.-L. Bombeck, J. Hébert and A. Richel, Oak barks as raw materials for the extraction of polyphenols for the chemical and pharmaceutical sectors. A regional case study, Ind. Crops Prod., 2015, 70, 316–321 CrossRef CAS.
- A. D. Sousa, A. I. V. Maia, T. H. S. Rodrigues, K. M. Canuto, P. R. V. Ribeiro, R. de Cassia Alves Pereira, R. F. Vieira and E. S. de Brito, Ultrasound-assisted and pressurized liquid extraction of phenolic compounds from Phyllanthus amarus and its composition evaluation by UPLC-QTOF, Ind. Crops Prod., 2016, 79, 91–103 CrossRef CAS.
- C. Deng, N. Yao, B. Wang and X. Zhang, Development of microwave-assisted extraction followed by headspace single-drop microextraction for fast determination of paeonol in traditional chinese medicines, J. Chromatogr. A, 2006, 1103, 15–21, DOI:10.1016/j.chroma.2005.11.023.
- M. Z. Ozel and A. A. Clifford, Superheated water extraction of fragrance compounds from Rosa canina, Flavour Fragrance J., 2004, 19(4), 354–359 CrossRef.
- M. Aliaño-González, E. Espada-Bellido, M. Ferreiro-González, C. Carrera, M. Palma, J. Ayuso, J. Álvarez and G. Barbero, Extraction of Anthocyanins and Total Phenolic Compounds from Açai (Euterpe oleracea Mart.) Using an Experimental Design Methodology. Part 2: Ultrasound-Assisted Extraction, Agronomy, 2020, 10, 326 CrossRef.
- Z. Y. Ju and L. R. Howard, Subcritical water and sulfured water extraction of anthocyanins and other phenolics from dried red grape skin, J. Food Sci., 2005, 70, S270–S276 CrossRef CAS.
- K. Monrad, R. Howard, R. King, K. Srinivas and A. Mauromoustakos, Subcritical solvent extraction of anthocyanins from dried red grape pomace, J. Agric. Food Chem., 2010, 58(5), 2862–2868 CrossRef PubMed.
- M. Jacotet-Navarro, N. Rombaut, A.-S. Fabiano-Tixier, M. Danguien, A. Bily and F. Chemat, Ultrasound versus microwave as green processes for extraction of rosmarinic, carnosic and ursolic acids from rosemary, Ultrason. Sonochem., 2015, 27, 102–109 CrossRef CAS PubMed.
- M. Cvjetko Bubalo, N. Ćurko, M. Tomašević, K. Kovačević Ganić and I. Radojčić Redovniković, Green extraction of grape skin phenolics by using deep eutectic solvents, Food Chem., 2016, 200, 159–166 CrossRef CAS PubMed.
- F. Montañés, T. Fornari, P. J. Martín-Álvarez, N. Corzo, A. Olano and E. Ibáñez, Selective Recovery of Tagatose from Mixtures with Galactose by Direct Extraction with Supercritical CO2 and Different Cosolvents, J. Agric. Food Chem., 2006, 54, 8340–8345 CrossRef PubMed.
- A. Bogdanovic, V. Tadic, I. Arsic, S. Milovanovic, S. Petrovic and D. Skala, Supercritical and high pressure subcritical fluid extraction from Lemon balm (Melissa officinalis L., Lamiaceae), J. Supercrit. Fluids, 2016, 107, 234–242 CrossRef CAS.
- A. T. Serra, I. J. Seabra, M. E. M. Braga, M. R. Bronze, H. C. de Sousa and C. M. M. Duarte, Processing cherries (Prunus avium) using supercritical fluid technology. Part 1: Recovery of extract fractions rich in bioactive compounds, J. Supercrit. Fluids, 2010, 55, 184–191 CrossRef CAS.
- L. Wang and C. Waller, Recent advances in extract ion of nutraceuticals from plants, Trends Food Sci. Technol., 2006, 17, 300–312 CrossRef CAS.
- K. Michael and A. Danquah Paul, Extraction of oil from microalgae for biodiesel production: A review, Biotechnol Adv., 2012, 30, 709–732 CrossRef PubMed.
- B. Yingngam, M. Monschein and A. Brantner, Ultrasound-assisted extraction of phenolic compounds from Cratoxylum formosum ssp. Formosum leaves using central composite design and evaluation of its protective ability against H2O2-induced cell death, Asian Pac. J. Trop. Med., 2014, 7, S497–S505 CrossRef PubMed.
- M. Goto, T. Ono, F. Nakashio and T. Hatton, Design of surfactants suitable for protein extraction by reversed micelles, Biotechnol. Bioeng., 1997, 54(1), 26–32 CrossRef CAS PubMed.
- P. Komal, N. Panchal and P. Ingle, Techniques Adopted for Extraction of Natural Products Extraction Methods: Maceration, Percolation, Soxhlet Extraction, Turbo distillation, Supercritical Fluid Extraction, Int. J. Adv. Res. Chem. Sci., 2019, 6(Issue 4), 1–12 Search PubMed.
- P. Puttarak and P. Panichayupakaranant, A new method for preparing pentacyclic triterpene rich Centella asiatica extracts, Nat. Prod. Res., 2013, 27, 7 CrossRef PubMed.
- C. Kumoro and I. Hartati, Microwave Assisted Extraction of Dioscorin from Gadung (Dioscorea Hispida Dennst) Tuber Flour, Procedia Chem., 2015, 14, 47–55 CrossRef.
- O. Hartati, L. Kurniasari and Y. Anas, Mathematical Model of the Hydrotropic Microwave assisted Extraction of Anti-Malarial Agent from Andrographis paniculata, Procedia Chem., 2015, 14, 186–192 CrossRef.
- A. F. Qui, F. Xavier, M. Minuti, F. Visinoni, G. Cravotto and F. Chemat, Solvent-free microwave extraction of essential oil from aromatic herbs: From laboratory to pilot and industrial scale, Food Chem., 2014, 150, 193–198 CrossRef PubMed.
- Y. J. Cho, J. Y. Hong, H. S. Chun, S. K. Lee and H. Y. Min, Unltrasonication extraction of resveratrol from grapes, J. Food Eng., 2006, 77, 725–730 CrossRef CAS.
- N. El-Darra, N. Grimi-Eugene, V. Nicolas and L. R. Maroun, . Extraction of polyphenols from red grape pomace assisted by pulse ohmic heating, Food Bioprocess Technol., 2013, 6, 1281–1289 CrossRef.
- B. He, L. Zhang, X. Yue, J. Liang, J. Jiang, X. Gao and P. Yue, Optimization of Ultrasound-Assisted Extraction of phenolic compounds and anthocyanins from blueberry (Vaccinium ashei) wine pomace, Food Chem., 2016, 204, 70–76 CrossRef CAS PubMed.
- T. Dhanani, S. Shah, N. Gajbhiye and S. Kumar, Effect of extraction methods on yield, phytochemical constituents and antioxidant activity of Withaniasomnifera, Arabian J. Chem., 2013, 10(1), S1193–S1199 Search PubMed.
- S. N. Tan, J. H. Yong, C. C. Teo and C. S. Hew, Determination of metabolites in Uncaria sinensis by HPLC and GC-MS after green solvent microwave-assisted extraction, Talanta, 2011, 83(3), 891–898 CrossRef CAS PubMed.
- S. Tsubaki, M. Sakamoto and J. Azuma, Microwave-assisted extraction of phenolic compounds from tea residues under autohydrolytic conditions, Food Chem., 2010, 123, 1255–1258 CrossRef CAS.
- C. Zhu, X. Zhai, L. Li, X. Wu and B. Li, Response surface optimization of ultrasound-assisted polysaccharides extraction from pomegranate peel, Food Chem., 2015, 177, 139–146 CrossRef CAS PubMed.
- M. Ramić, S. Vidović, Z. Zeković, J. Vladić, A. Cvejin and B. Pavlić, Modeling and optimization of ultrasound-assisted extraction of polyphenolic compounds from Aronia melanocarpa by-products from filter-tea factory, Ultrason. Sonochem., 2015, 23, 360–368 CrossRef PubMed.
- M. M. Moreira, M. F. Barroso, A. Boeykens, H. Withouck, S. Morais and C. Delerue Matos, Valorization of apple tree wood residues by polyphenols extraction: Comparison between conventional and microwave-assisted extraction, Ind. Crops Prod., 2017, 104, 210–220 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |