Open Access Article
Open Access ArticleAdsorptive removal of antibiotic pollutants from wastewater using biomass/biochar-based adsorbents
Oluwaseyi Aderemi AJALA a,
Solomon Oluwaseun AKINNAWO†
a,
Solomon Oluwaseun AKINNAWO†
 bc,
Abayomi BAMISAYE†
bc,
Abayomi BAMISAYE† d,
Demilade Tunrayo ADEDIPE†
d,
Demilade Tunrayo ADEDIPE† e,
Morenike Oluwabunmi ADESINA†d,
Omolabake Abiodun OKON-AKAN†bf,
Tosin Adewumi ADEBUSUYI†
e,
Morenike Oluwabunmi ADESINA†d,
Omolabake Abiodun OKON-AKAN†bf,
Tosin Adewumi ADEBUSUYI† g,
Adedamola Titi OJEDOKUN†b,
Kayode Adesina ADEGOKE
g,
Adedamola Titi OJEDOKUN†b,
Kayode Adesina ADEGOKE *b and
Olugbenga Solomon BELLO
*b and
Olugbenga Solomon BELLO
 *b
*b
aDepartment of Applied Chemistry, Graduate School of Advanced Science and Engineering, Hiroshima University, 1-4-1, Kagamiyama, Higashi-Hiroshima, 739-8527, Japan
bDepartment of Pure and Applied Chemistry, Ladoke Akintola University of Technology, P. M. B. 4000, Ogbomoso, Oyo State, Nigeria
cDepartment of Chemical Sciences, Olusegun Agagu University of Science and Technology, P. M. B. 353, Okitipupa, Ondo State, Nigeria
dDepartment of Chemical Sciences, Faculty of Natural and Applied Sciences, Lead City University, Ibadan, Oyo State, Nigeria
eState Key Laboratory of Marine Pollution and Department of Chemistry, City University of Hong Kong, Tat Chee Avenue, Kowloon, Hong Kong SAR, China
fWood and Paper Technology Department, Federal College of Forestry Jericho, Ibadan, Nigeria
gDepartment of Chemical Sciences, Augustine University, Ilara-Epe, Lagos State, Nigeria. E-mail: kwharyourday@gmail.com; osbello@lautech.edu.ng
First published on 3rd February 2023
Abstract
This study explores adsorptive removal measures to shed light on current water treatment innovations for kinetic/isotherm models and their applications to antibiotic pollutants using a broad range of biomass-based adsorbents. The structure, classifications, sources, distribution, and different techniques for the remediation of antibiotics are discussed. Unlike previous studies, a wide range of adsorbents are covered and adsorption of comprehensive classes of antibiotics onto biomass/biochar-based adsorbents are categorized as β-lactam, fluoroquinolone, sulfonamide, tetracycline, macrolides, chloramphenicol, antiseptic additives, glycosamides, reductase inhibitors, and multiple antibiotic systems. This allows for an assessment of their performance and an understanding of current research breakthroughs in applying various adsorbent materials for antibiotic removal. Distinct from other studies in the field, the theoretical basis of different isotherm and kinetics models and the corresponding experimental insights into their applications to antibiotics are discussed extensively, thereby identifying the associated strengths, limitations, and efficacy of kinetics and isotherms for describing the performances of the adsorbents. In addition, we explore the regeneration of adsorbents and the potential applications of the adsorbents in engineering. Lastly, scholars will be able to grasp the present resources employed and the future necessities for antibiotic wastewater remediation.
1 Introduction
Through the years, lives have been saved thanks to the discovery of antibiotics and their application in treating diseases, notably bacterial infections.1 Researchers are searching for more antibacterial chemical compounds, but they are constrained by the possibility of negative side effects. Due to an increase in the world population, there is a rise in the use of antibiotics, necessitating the development of better medications, apart from their use for non-medical purposes, which is a major contribution to their consumption.2 Since antibiotics are not completely digested after consumption, they can be discovered in sewage systems.3 These non-digested antibiotics are frequently not biodegradable and photolysis is useless against them. According to a recent study, India has been the world's most substantial end-user since 2015, posing a serious threat to their resistance.4–8 Antibiotics can be detected in water samples due to ineffective conventional methods of elimination from wastewater.9,10 However, some metabolized antibiotics are eliminated through defecation while the active non-biodegradable residues accumulate greatly, developing bacteria with antibiotic-resistance.11 As a result, antibiotic-resistant germs kill thousands of people yearly worldwide.12 Antimicrobial resistance reported in detectable concentrations in drinkable water is lower than that found in numerous effluents, such as hospital discharges, pharmaceutical industry emissions, and agricultural water.5,10,13 Antibiotic levels in the environment are also exacerbated by pharmaceutical firms and ineffective industrial wastewater treatment. According to the current literature, tetracyclines, sulfonamides, quinolones, ionophores, and lactams are quickly adsorbed but difficult to disintegrate.14 Conventional treatment plants only treated 48–77%,15 despite the fact that the presence of antibiotics in drinking water precedes the increase in antibiotic-resistant bacteria and transmission to both human and animal pathogens, posing serious hygienic and quantifiable clinical risks. Although existing treatment technologies cannot totally eliminate the problem, addressing the problem at its source can help prevent additional contamination of ecosystems. Because of their great variety and complete classification, separating antibiotics from wastewater necessitates the use of special procedures.Wide-ranging experimental reports on antibiotic adsorption and numerous studies on the kinetic and isotherm models have been reported. However, a review of these models is absent, thereby necessitating an extensive study in these areas, focusing on the adsorption technique. In addition, the previous literature did not cover the extensive utilization of biomass/biochar-based adsorbents for the removal of comprehensive classes of antibiotics. Additionally, the potential applications of adsorbents in engineering included in this study were not mentioned in past studies. As a result, our research is limited to the adsorptive removal of antibiotics utilizing various adsorbents. Unlike the previous literature, broad ranges of adsorbents are covered. They are categorized into the adsorption of different classes of antibiotics, including β-lactam, fluoroquinolone, sulfonamide, tetracycline, macrolides, chloramphenicol, antiseptic additives, glycosamides, reductase inhibitors, and multiple antibiotics systems onto agricultural biomass/biochar-based adsorbents. This provides an opportunity to assess their performances and understand current research breakthroughs in applying various adsorbent materials for the removal of antibiotics, thereby identifying the associated strengths and limitations and experimental insights into the efficacy of different kinetics and isotherms for describing the performances of the adsorbents. Regeneration of adsorbents and the potential applications of the adsorbents in engineering are also explored. Lastly, this study highlights significant challenges and knowledge gaps for directing specific research for large and industrial-scale applications.
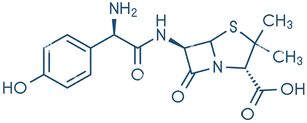
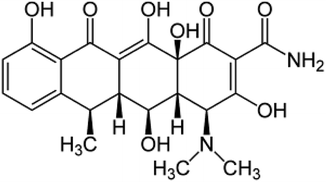
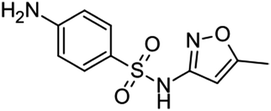
2 Antibiotic: structure, classifications, sources, and distribution
Antibiotics are divided into two major groups according to their mode of operation: bacteriostatic (restrains bacterial progression) and bactericidal (kills bacteria). The classification of antibiotics based on their structural unit is presented in (Table 1), along with some frequent sources (Fig. 1). Antibiotic concentrations can be detected from many sources to compile data for further research into antibiotic remediation worldwide. Ciprofloxacin (CPX) was abundantly prevalent in wastewater, followed by sulfamethoxazole.16 Quinolones are categorized as non-decomposable.16 Liquid chromatography combined with mass spectrometry (LC-MS/MS) and solid-phase extraction (SPE) are commonly used to extract antibiotics.17,18 Antibiotics in groundwater have been identified in surface water and effluents from hospitals, such as erythromycin, amoxicillin, norfloxacin, ciprofloxacin, levofloxacin, ofloxacin, trimethoprim, sulfamethoxazole, and tetracycline, as well as other medications in the g L−1 range. Sources of antibiotics have a direct impact on bacterial resistance. Hospital and untreated household discharge, which flows straight into the sewage system, are substantial contributors (Fig. 1).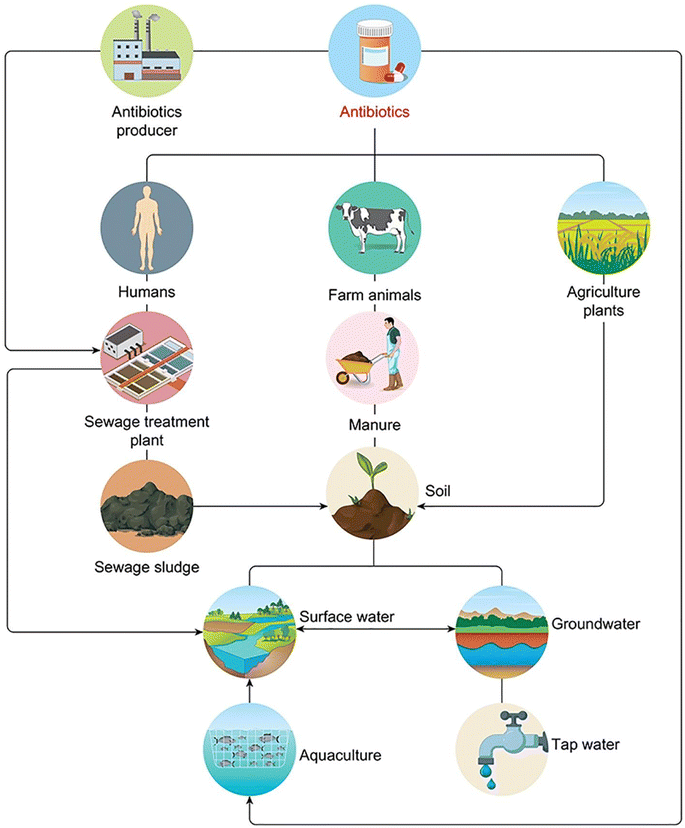 | ||
| Fig. 1 Common sources and distributions of antibiotics in the environment.30–33 | ||
Antibiotics and associated genes have recently been discovered in water samples all around the world due to their limited biodegradability, which can have a significant influence on human and animal health. Non-target infections are affected by antibiotics in aqueous media, and the algal structure is altered.19 The existence of antibiotics in groundwater, surface water, and wastewater, even at minimal amounts, is a serious problem, and numerous strategies for antibiotic removal from effluents have been implemented. Countless traditional procedures, such as distillation, reverse osmosis, sedimentation, and lime softening, are used at different phases of wastewater treatment; however, despite their low cost and harmless nature, these technologies are ineffective in entirely eliminating them from wastewater.20–22
Regardless of the use of foremost and derived wastewater treatments, external particles such as medicines, heavy metal ions and dyes continue to be released, which contain dangerous residues of hazardous pollutants. As a result, advanced treatment is required to eradicate dangerous contaminants.14 Effluent treatment using a technique other than traditional methods was investigated by De Andrade and his co-authors, including flotation, lime softening and coagulation, and innovative types of expertise such as advanced oxidation and ozonation, membrane separation, and electrodialysis because these processes require fewer chemicals.23 Nevertheless, when interacting with Cl2 or O3, some of these procedures produce poisonous by-products that are considerably more dangerous than the initial pollutants. As a result, these technologies are not in use because of their high energy requirements and inability to remediate wastewater on a larger scale attributed to their expensive cost. Reverse osmosis and nanofiltration have recently been focused on based on their high energy and material consumption.7,24 Conversely, these technologies exhibit several drawbacks, such as the waste of too much water in reverse osmosis/nanofiltration. In addition, the method is time-consuming and expensive to implement on a large scale.25
Furthermore, innovative hybrid-designed biological systems for wastewater treatment that combine various redox conditions and biomass conformations may successfully remove various antibiotic chemicals. However, further in-depth research is needed to assess their efficacy in full-scale wastewater treatment plants with a variety of operating characteristics. Currently, adsorption has various advantages over alternative remediation approaches, including simplicity, reliability, minimal energy use, and quick adsorbent recovery.26 The batch process and the continuous process are the major adsorptive processes for the removal of antibiotics from wastewater, both of which can be used with a variety of adsorbents, such as biochars, clay materials, ion-exchange resins, activated carbons (ACs), zeolites and carbon nanotubes (CNT). Because this technique has various improvements compared to other procedures, and because it is simple to combine with other technologies, incorporating a unified system into previously existing treatment plants for total elimination could be helpful. Several hybrid systems previously created by combining membrane technologies with other efficient technologies include ozonolysis, photocatalysis, chlorination, sedimentation, and filtration.27–29 However, these pairings possess some drawbacks, e.g., the aerobic digestion process frequently generates more damaging derivatives than the organic molecules themselves. Table 2 shows numerous treatment techniques for removing various antibiotics discovered thus far. Adsorbents such as CNTs, clay materials, ACs (PAC/GAC), and biochar have also been comprehensively researched for antibiotic elimination.25
| Method | Advantages | Disadvantages | Examples of antibiotics removed |
|---|---|---|---|
| Adsorption | It is easy to operate and has low cost | Product recovery usually requires expensive distillation. The capacity of the adsorbent also progressively deteriorates as you increase the number of cycles | Chlortetracycline,51 sulfamethazine52 |
| Photolysis and photocatalysis | It does not lead to the formation of bromated products. It can be applied to the full-scale drinking water treatment system | The penetration of light can be inhibited by turbidity. The process is also energy- and cost-intensive | Tetracycline,53 ciprofloxacin54 |
| Ozonation | It enhances the effective destruction of organic matter | It requires high cost | Roxithromycin55 |
| Biodegradation | It is a low-cost technology and plays a critical role in pollutant degradation | The rate of microbial metabolism may reduce (due to antibiotic wastewater exposure), thereby affecting the performance of biological treatment of the sewage | Cephalexin,41 sulfanilamide56 |
| Electrochemical oxidation | It can be integrated with other technologies | It is not widely used due to the expensive cost of electrode materials. It is also associated with low current efficiency and high energy consumption | Levofloxacin57 |
| Anaerobic treatment | It is effective in removing high-strength organic compounds and breaking down refractory materials | They are effective in removing non-organic pollutants from wastewater (such as nutrients and pathogens) | Tylosin,58 penicillin59 |
| Aerobic treatment | It is associated with reduced odor and improved nutrient removal efficiency | It requires high capital cost, high operating cost, and high maintenance requirements | Tetracycline,60 Sulfamerazine46 |
| Biofilm technology | It offers operational flexibility, reduced hydraulic retention time, low space requirements, high active biomass concentration, and increased biomass residence time | Operators need to be skilled in the area of biological water treatment. It also requires annual monitoring to ensure that the bacteria on the carriers are still thriving | Ibuprofen,61 terramycin62 |
| Coagulation/flocculation/sedimentation | Decrease in detention time of the wastewater treatment process. They can also target different contaminants via a single system | Since coagulation is an additive process, the addition of chemicals can produce a large volume of sludge that must be treated and disposed of after the treatment | Sulfamerazine, sulfadimethoxine48 |
3 Different techniques for antibiotics remediation
Antibiotics and adsorption are closely related.34 Adsorption and activated carbon (AC) have a close association, showing that AC is the most studied adsorbent. This is due to activated carbon's low cost and its well-developed pore structure and abundance of surface functional groups, which allow for both physical and chemical adsorption of antibiotics. Adsorption efficiency is affected by the kind of activated carbon, operating parameters (pH, adsorbent dosage, and ionic strength), activation methods, precursors, and adsorbates, used in the process.35 For instance, the size of granular activated carbon particles has a major impact on removing certain pollutants, with the smallest sizes exhibiting maximum removal effectiveness, which is likely to be due to greater mass transfer.36,37 Activated carbon can be modified in a variety of ways to boost its adsorption capacity by modifying the pore structure and surface chemical properties.After adsorption, photolysis/photocatalysis is the most researched therapeutic method. Despite the fact that both photolysis and photocatalysis involve light, their methods are somewhat different. Self-sensitization, and indirect and direct photolysis are the three photolysis routes. After photon adsorption, chemicals undergo pyrolysis, heterolysis, and photoionization. Indirect photolysis is induced by compounds found in the environment which absorb light energy and destroy organic matter by producing highly reactive molecules, including alkyl peroxy radicals (OOR), hydroxyl radicals (·HO), and singlet oxygen (1O2). Organics transition to an excited state by collecting light energy, transferring it to ground-state 3O2 or H2O, and releasing reactive oxygen species like O2 and ·OH, which destroy the organics' ground-state molecules.38
Photocatalysis has been getting a lot of attention lately. Its high mineralization efficiency for refractory organic contaminants makes it one of the most sophisticated oxidation processes. However, TiO2 only reacts to UV light. Additionally, g-C3N4 (and other newly developed visible-light-response catalysts) have a low quantum yield. As a result, there is still a long way to go in terms of the large-scale deployment of these photocatalysts.
Ozonation, like photocatalysis, is an advanced oxidation process with direct ozone oxidation and indirect ozone oxidation by ·OH as mechanisms. Due to the increase in reactive radicals (·OH) and the presence of indirect reactions, alkaline ozonation results in high chemical oxygen demand and total organic carbon removal rates. In contrast, the reaction is dominated by direct oxidation under acidic conditions and has a limited ability to remove pollutants.39
Apart from being an important route for breaking antibiotics down in the environment, biodegradation is also one of the most widely employed techniques for treating sewage in antibiotic wastewater. Biodegradation research hotspots are the conditions, consequences, and mechanisms.40 The most often utilized method is activated sludge. The efficiency of this method is largely determined by the adsorption and biodegradation capabilities of the sludge. Adsorption process is used to remove antibiotics like norfloxacin, ciprofloxacin, ofloxacin, ampicillin, β-lactams, quinolones, and tetracyclines, whereas biodegradation is used to remove antibiotics like cephalexin and β-lactams.41
Conventional activated sludge treatment technologies are unable to extract sulfonamide antibiotics fully. However, because of the exposure to antibiotic wastewater, the rate of microbial metabolism may reduce, thereby affecting the performance of biological treatment of the sewage. The use of technologies such as ozonation to pre-treat antibiotic effluent is an excellent technique to lessen the biological unit's disposal difficulties.
Electrochemical oxidation has grown in popularity as a therapeutic method. To a large extent, the electrochemical oxidation ability and efficiency are determined by the electrode materials' catalytic activity and stability, particularly the anode electrode.42 Electrode materials widely employed in electrochemical oxidation include platinum electrodes, dimensionally stable anodes, and boron-doped diamond electrodes. However, due to the expensive cost of electrode materials, electrochemical oxidation has not been widely utilized. Furthermore, when the conductivity of wastewater is low, mass transfer in the electrochemical oxidation reactor is slow, leading to a low current efficiency and high energy consumption. As a result, low-cost anode materials that have high stability and catalytic activity are needed. Additionally, new reactors, like the three-dimensional electrode reactor, should be studied to reduce mass transfer resistance and enhance current efficiency for electrochemical oxidation technology to be widely used.
Hydrolysis, acidogenesis, and methanogenesis are all steps in the anaerobic treatment process. Extracellular enzymes degrade solid cellulose, lignins, lipids, proteins, and complex organics into soluble organic fatty acids, carbon dioxide, ammonia, and alcohol in the hydrolysis phase, while microorganisms convert the products of the first stage into acetic acid, hydrogen, carbon dioxide, propionic acid, and other low molecular weight organic acids in the acidogenesis phase. Two groups of methane-forming bacteria work simultaneously in the final stage, the methanogenesis phase, to convert carbon dioxide and hydrogen to methane, while converting acetate to bicarbonate and methane.
For aerobic treatment, organic substances can be entirely degraded to CO2 in an aerobic process.43,44 Anaerobic processes have been used more frequently for antimicrobial wastewater treatment than aerobic processes. Nonetheless, certain antibiotics can be entirely metabolized to carbon dioxide and water under aerobic circumstances. The aerobic procedure would not be practicable for high-strength effluent from antibiotics manufacturers; hence, before aerobic treatment, dilution of the wastewater is required. As a result, the micro-aerobic or integrated anaerobic–aerobic process is capable of treating high-strength antibiotic manufacturing wastewater.45,46 COD has been employed to indicate process performance in most of the research mentioned above. However, it should be noted that high efficiency in removing COD does not imply high efficiency in removing antibiotics. As a result, direct detection of antibiotic concentration rather than using COD is strongly advised in future research.
Microorganisms used in biological processes typically come in two forms: biofilms and suspended activated sludge. Biofilms are microorganism aggregates that grow on a solid packaging substance. They have previously been identified as highly complex, diverse, and uncontrolled formations. The thickness and form of biofilm seen are a product of the operating parameters of the biofilm system.47 Biofilms are clusters with a mushroom-like appearance and cavities. Voids are open passages that connect the bulk fluid to the interior of biofilms. The liquid may flow through the spaces but is always stationary in the cell clusters. As a result, both convection and diffusion in voids may contribute to mass transfer, whereas only diffusion determines transport in cell clusters. Knowledge of the biofilms used in the treatment of wastewater polluted with antibiotics is currently limited.
Coagulation/flocculation/sedimentation is a method of removing soluble species in which chemicals are applied to water to promote colloidal particle instability, permitting aggregation through flocculation and then sedimentation. In this method, antibiotics would be brought into contact with clays and other natural colloidal matter for long periods in a natural system, affording the chance for antibiotic adsorption on the colloidal matter. If antibiotics are adsorbed on colloids, they could be co-removed in the coagulation, flocculation, and sedimentation process. On the other hand, Adams et al.48 found no substantial remediation of the antibiotics examined using ferric salt or aluminium as a coagulant. This method of removing antibiotics is not yet proven.
Despite the advantages of some available methods (Table 2), they have disadvantages, including poor removal efficiency, high energy requirements, production of poisonous by-products, which may pose health risks, and the production of large quantities of secondary sludge, that are harmful to both aquatic and human health.7 The adsorption technique using activated carbon (AC), has gained increasing attention due to its lower cost, simplicity of operation, large surface area, and efficacy.7 By comparison, adsorption is still the most prevalent method of treating wastewater for the two types of antibiotics because of the advantages of ease of operation and low cost. Carbon materials, like graphene oxide, biochar, activated carbon, and nanocarbon materials, like nanoporous carbon and nanotubes, can be used as adsorbents for antibiotics removal;49 other materials include chitosan beads, and bentonite.50
4 Agricultural biomass-based adsorbents for antibiotics removal
In recent years, the search for a simple, green, effective, inexpensive and sustainable material to control antibiotics has led the scientific community to develop a significant interest in a non-toxic and readily available agricultural biomass material for the adsorptive removal of antibiotic contaminants in water and wastewater.63 Agricultural biomass is a group of residues obtained from either forestry or agricultural undertakings (Table 3). Biomass-based adsorbents obtained from agricultural residues have been suggested to retain functional groups that include hydroxyl and carboxyl groups that expedite the removal of emerging contaminants that include pharmaceuticals such as antibiotics. Biochar and activated carbon obtained from agricultural biomass have been widely explored for the removal of pollutants in the water phase.| Material | Antibiotics | Pyrolysis condition/activating agents | Experimental conditions | Adsorption capacity/removal efficiency | Kinetics | Isotherm | Mechanisms | Ref. |
|---|---|---|---|---|---|---|---|---|
| a F = Freundlich, L = Langmuir, T = Temkin, IC = initial concentration, t = time, T = temperature, PSOM = pseudo-second-order model, PFOM = pseudo-first-order model. | ||||||||
| Red pine | Sulfamethoxazole SPY | 400 °C | t = 72 h, pH = 6, T = 25 °C, dose = 10–15 mg | 2.0 | — | F | π–π electron-donor–acceptor (EDA) interaction | 116 |
| 500 °C | 1.5 | |||||||
| Alfalfa Medicago sativa L. | Tetracycline | 500 °C | pH = 5, t = 5 d, dose = 0.1 g L−1 conc. = 10–100 mg L−1, T = 25 °C | 372.31 | PSOM | F | Surface complexation, hydrogen bonding and electrostatic interactions | 68 |
| Sugar cane bagasse | Chlortetracycline | 500 °C | T = 25 °C, t = 24 h, pH = 5, conc. = 200 mg L−1, dose = 10 g L−1 | 16.96 | PSOM | F | — | 117 |
| Wood | Tetracycline | 800 °C | T = 298 K, t = 48 h, pH = 3.5–10.0, dose = 10 mg, conc. = 6.0–48 mg L−1 | 163, 261 | — | L | — | 118 |
| Rise husk | Tetracycline | H2SO4, KOH, 500–550 °C | T = 30 °C, dose = 5 g L−1, conc. = 0.5–1 g L−1 | 23.26, 58.82 | PSOM | L | π–π interactions and hydrogen bonding | 119 |
| Treatment plant sludge | Tetracycline | NaOH, 700 °C | T = 25 °C, t = 8 d | 124.8 | — | L | — | 120 |
| Pinus taeda | Tetracycline | NaOH, 800 °C | T = 20 °C, t = 5 d, dose = 0.1g L−1, conc. = 10–100 mg L−1 | 434.36 | Elovich | F | Hydrogen bonding and π–π interaction | 73 |
| Graphene-oxide bamboo sawdust composite | SMT | Graphene oxide, 600 °C | t = 24 h, pH = 3–9, conc. = 10 g L−1, T = 25 °C | 6.5 | PSOM | F | p–p electron–donor–acceptor interaction | 121 |
| Cornhusk | Tetracycline, levofloxacin | FeCl3·H2O, 300 °C | t = 24 h, T = 30 °C, dose = 0.8 g L−1, C = 266 mg L−1 (TC) 200 mg L−1 (LEV) | 102 | — | — | Hydrogen bonding and electrostatic attraction | 122 |
| Municipal solid waste sludge | Ciprofloxacin | Bentonite clay support, 450 °C | pH = 3–9, dose = 1.0 g L−1, t = 12 h, conc. = 25 mg L−1 | 286.6 | PSOM, Elovich | Hill | Electrostatic interactions | 91 |
| Vinasse | Pefloxacin, ciprofloxacin | Manganese ferrite, 800 °C | pH = 3–10, T = 298 K, conc. = 1000 mg L−1, dose = 0.1–2 g L−1 | 256, 357 | PSOM, Bangham fitting | L | Pore filling effect, π–π stacking interaction, π–π EDA, hydrogen bonding and hydrophobicity | 123 |
| Pomelo peel | Tetracycline, oxytetracycline, chlortetracycline | KOH, 400, 600 °C | Dose = 10 mg, conc. = 5–50 mg L−1, t = 75 h, pH = 7, T = 294.15 K | 476.19, 407.5, 555.56 | PSOM | L | Pore filling, electrostatic interaction and π–π interactions | 124 |
| Fe/Zn-sawdust | Tetracycline | FeCl3·6H2O, ZnCl2, 600 °C | pH = 2–9, conc. = 40, 100, 150 mg L−1 | — | PSOM, Elovich | L | π–π EDA, hydrogen bonding | 63 |
| Chinese herb residue | Tetracycline, chlortetracycline, oxytetracycline | ZnCl2, 500 °C | T = 303 K, dose = 0.02 g, conc. = 30–110 mg L−1, pH = 3–10 | 188.7, 200.0, 129.9 | PSOM | L | Hydrogen bonding and electrostatic interaction | 72 |
| Self-functionalized corncob biochar | Amoxicillin | Ultrasonic, 700 °C | t = 60 min, dose = 0.2 g | 121.53 | Elovich | F | π–π attractions | 71 |
| Tetracycline | 161.58 | |||||||
| Levofloxacin | 164.42 | |||||||
| Pomegranate wood | IC: 500 mg L−1, dose: 0.04 g, pH: 6, T: 35 °C | 79 | ||||||
| (i) Activated carbon | Amoxicillin | 234.6 mg g−1 | PSOM | Electrostatic attraction | ||||
| (ii) Activated carbon | Amoxicillin | 437 mg g−1 | PSOM | L | Electrostatic attraction | |||
| NH4Cl-induced activated carbon | ||||||||
| Hazelnut-shell-derived activated carbon | Tetracycline | IC: 100 mg L−1, dose: 0.1 g, pH: 5, T: 293 K | 312.59 mg g−1 | PSOM | L | Hydrogen bonding and π–π interaction | 94 | |
| Oxytetracycline | 322.60 mg g−1 | PSOM | L | |||||
| Chlortetracycline | 333.30 mg g−1 | PSOM | L | |||||
| Cow manure-based activated carbon | Sulfamethazine | Dose: 04 mg, pH: 3, T: 25 °C | 3.62 mg g−1 | PSOM | F | — | 125 | |
| Pine tree AC | Sulfamethoxazole | IC: 1.0 mg L−1, dose: 0.003 g, pH: 3, T: 25 °C | 131 mg g−1 | PSOM | L | — | 88 | |
| KOH-modified pomegranate peel wastes | Ciprofloxacin | IC: 50–300 mg L−1, dose: 0.025–0.2 g, pH: 2–12, t: 15–150 min, T: 25 °C | 2.353 mg g−1 | — | F | Hydrogen bonding and π–π interaction | 86 | |
| Activated carbon from | Ciprofloxacin | IC: 20 mg L−1, dose: 0.05 g, pH: 8, time: 30 min | — | — | π–π interactions, hydrogen bonding and electron–donor–acceptor interaction | 85 | ||
| (i) Banana peel | 31.67% | |||||||
| (ii) Straw | 93.34% | |||||||
| (iii) Avocado peel | 39.49% | |||||||
| (iv) Limonia acidissima shell | 23.43% | |||||||
| (v) Tea waste | 31.67% | |||||||
| Pumpkin-seed-derived activated carbon derived | Ciprofloxacin | IC: 500 mg L−1, dose: 0.04 g, pH: 6, T: 35 °C | 234.6 mg g−1 | PSOM | L | Electrostatic attraction | 79 | |
| 437 mg g−1 | PSOM | L | Electrostatic attraction | |||||
| Pomegranate wood | IC: 100 mg L−1, dose: 0.1 g, pH: 5, T: 293 K | Hydrogen bonding and π–π interaction | 94 | |||||
| (i) Activated carbon | Amoxicillin | 312.59 mg g−1 | PSOM | L | ||||
| (ii) Activated carbon | Amoxicillin | 322.60 mg g−1 | PSOM | L | ||||
| NH4Cl-induced activated carbon | 333.30 mg g−1 | PSOM | L | |||||
| (i) Eucalyptus leaves | Cefuroxime | IC: 2.5–50 μmol L−1, dose: 0.5 g, T: 48 h | 862.05 μmol kg−1 | NA | L–F | — | 75 | |
| (ii) Wood ash | 817.67 | |||||||
| (iii) Pine bark | 700.09 | |||||||
| (iv) Pine needles | 1223.47 | |||||||
| Lemna minor biomass | Ciprofloxacin | IC: 10 to 5 0 mg L−1, time: 90 min, dose: 0.04 g, pH: 6, T: 273 to 323 K | 19.62 mg g−1 | PSOM | NA | Physical adsorption | 81 | |
| Sawdust | Ciprofloxacin hydrochloride | IC: 5–25 mg L−1, dose: 1.0–5.0 g L−1, pH: 3–12, T: 30 °C, t: 15–120 min | 11.18 mg g−1 | PSOM | NA | Intra-particle diffusion | 82 | |
| Modified sawdust Fe-coated sawdust | Tetracycline | IC: 10–20 mg L−1, dose: 50 mg, pH: 2–10, T: 30 °C, t: 0–60 min | 5.14 mg g−1 | PSOM | F | Electrostatic interaction hydrogen bonding | 95 | |
| ZnO-modified pistachio shells | Amoxicillin | IC: 30–70 mg L−1, dose: 0.1 g per 100 mL, pH: 3–9, T: 25–35 °C, t: 0–120 min | 132.240 mg g−1 | PSOM | L | Chemisorption | 114 | |
| Ciprofloxacin | 92.450 mg g−1 | PSOM | F | Intra-particle diffusion | ||||
| Tetracycline | 98.717 mg g−1 | PSOM | F | |||||
| Palm bark biomass | Amoxicillin | IC: 10–100 mg L−1, dose: 0.5–5 g L−1, pH: 6.5, T: 298 K, t: 10–180 min | 35.92 mg g−1 | — | L | — | 77 | |
| Magnetic chitosan graphene oxide composite | Ciprofloxacin | IC: 2–100 mg L−1, dose: 10 mg, pH: 4–9, t: 0.08–8 h | 282.9 mg g−1 | PSOM | L–F | Electrostatic attractions, π–π electron interaction | 83 | |
| Modified indian almond biomass | Dicloxacillin | IC: 20–500 mg L−1, dose: 0.025–3.0 g L−1, pH: 2–8, t: 24 h, T: 273.15–323.15 K | 71.94 mg g−1 | PSOM | L | Hydrogen bonding, van der Waals forces | 80 | |
| Moringa oleífera | Oxytetracycline | IC: 0.2–1.0 mg L−1, dose: 1–2 g L−1, t: 5–120 min, T: 20–40 °C | 50.3% | PSOM | F | Electrostatic attractions intra-particle diffusion | 92 | |
| Spent mushroom substrates | (i) Sulfamethyldiazine | IC: 0.5–10 mg L−1, dose: 1–2 g L−1, pH: 1–11 | 2.1072 mg g−1 | PSOM | L | — | 87 | |
| (ii) Sulfamethazine | 1.8103 mg g−1 | |||||||
| (iii) Sulfathiazole | 2.2991 mg g−1 | |||||||
| (iv) Sulfamethoxazole | 2.2133 | |||||||
| Magnetic functionalization of sugar cane bagasse | Tetracycline | IC: 0–200 mg L−1, dose: 0.05 g, t: 0–25 h, T: 30 °C, pH: 6.8 | 48.35 mg g−1 | PSOM | F | Hydrogen bonding and π–π interaction | 126 | |
| Grape slurry (activated carbon from grape slurry) | Chloramphenicol | 650 °C | 23 ± 2 °C, 150 rpm, dose = 0.10 g | PSOM | L and F | 104 | ||
| Corn stalk biomass fiber & Fe3O4 embedded chitosan | Chloramphenicol | 35 °C, reaction time = 3 h, dose = 30 mg | 58.75 mg g−1 | PSOM | L | 102 | ||
| Porous carbon from waste lignin | Chloramphenicol | 800 °C | Dose = 120 mg L−1 | 534.0 mg g−1 at 303 K | PSOM | L | 103 | |
| Biochar from peanut shell | Chloramphenicol (CHLR) | 105 °C for 12 h | Natural pH, 25 °C, 180 rpm, conc. = 100, 300, 600 mg L−1, dose = 1.0 g L−1 | 423.7 mg g−1 and 90% | PSOM | L | Hydrogen bonding interaction π–π interaction | 105 |
| Date palm fiber | Tyrosine | IC: 10 to 5 0 mg L−1, time: 90 min, dose: 0.04 g, pH: 6, T: 273 to 323 K | 147 | PSOM | L | Electrostatic, H-bonding, π–π EDA interaction | 97 | |
| Corchorus capsularis | Tyrosine | IC: 10 to 5 0 mg L−1, time: 90 min, dose: 0.04 g, pH: 4–7, T: 273 to 323 K | 25 | PSOM | L | Hydrophobic, electrostatic, H-bonding, π–π EDA interaction | 97 | |
| Banana peel graphene | Erythromycin | 286 | PSOM | L | N/A | 97 | ||
| Cotton gin waste and guayule bagasse biochar | Erythromycin | 17.12 | PSOM | L | Hydrophobic partitioning, H-bonding and π–π interactions | 97 | ||
| Cuttlefish bone powder | Clarithromycin | 34.5 | PSOM | F | Electrostatic interaction | |||
| Rice husk | Azithromycin | IC: 100 mg L−1, time: 0–250 min, dose: 0–20 g, pH: 4–7, T: 300 K | 612.22 | PFOM | L | N/A | 99 | |
| Erythromycin | 599.72 | L | N/A | |||||
| Corn cobs | Tyrosine | IC: 20–100 mg L−1, time: 5–540 min, dose: 0–20 g, pH: 6, T: 298, 308, 318 K | 14.0 | PSOM | L | N/A | 100 | |
| Azolla filiculoides – based activated porous carbon | Azithromycin | IC: 25–200 mg L−1, time: 0–180 min, dose: 0.1–1.5 g, T: 273–325 K | 374 | PSOM | L | Physical adsorption | 101 | |
| Feather-derived charcoal | TMC | 600 °C | pH: 2–12, T: 293, 303 and 313 K, t: 0–60 h, dose: 0.2 g, IC: 1000 mL | 164 mg g−1 | Intraparticle diffusion, PS1 and PS2 | L, F and DR | Exothermic and electrostatic interaction | 112 |
| Modified peanut husk with betaine (MPN-Bet) | TMC | Co-precipitation, 60 °C | pH: 2–11, dose: 0.005 to 0.05 g, t: 0–350 min, T: 293, 303 and 313 K, IC: 0–0.1 mol L−1 | 31.2 ± 3.2 mg g−1 | Intraparticle diffusion, PS1 and PS2 | L, F and T | Chemisorption | 111 |
| Waste citrus peel | TMC | NA | IC: 50–150 mg L−1, pH: 4–10, T: 293–323, dose: 0.05–0.15 g | 144.9 mg g−1 | PS1 and PS2 | L and F | Chemisorption | 113 |
| Red pine | SMX | 400 °C | t = 72 h, pH = 6, T = 25 °C, dose = 10–15 mg | 2.0 | — | F | 116 | |
| SPY | 500 °C | 1.5 | ||||||
| Alfalfa Medicago sativa L. | TC | 500 °C | pH = 5, t = 5 d, dose = 0.1 g L−1, conc. = 10–100 mg L−1, T = 25 °C | 372.31 | PSOM | F | 68 | |
| Waste rice straw | TCS | 300 °C | t = 30 min, dose = 50 mg L−1, conc. = 10–50 mg L−1, T = 25 °C | 714 | PSOM | L | 106 | |
| Kenaf biochar | TCS | 750 °C | pH = 5, t = 24 h, dose = 0.03 g L−1, conc. = 30 mg L−1, T = 25 °C | 77.4 mg g−1 | PSOM | L | 107 | |
| Magnetic carbon composites | TCS | 27 °C | t = 10 min, dose = 50 mg L−1, conc. = 10–50 mg L−1, T = 30 °C | 892.9 mg g−1 | PSOM | L | 127 | |
| Microwave-activated biochar | Lincomycin | 105 °C | pH = 3 to 10, conc. = 50 mg L−1, dose = 1 g L−1, temp = 25 °C | 190 mg g−1 | Pseudo-second-order | Redlich–Peterson | Electrostatic interaction | 109 |
Biochar is a carbon-rich product of biomass pyrolysis in an inert atmosphere (Table 3). The growing interest in biochar over the years for the adsorption of aqueous pollutants is owing to its intrinsic properties, such as its high specific surface areas and porous structure, surface functionality, and vast possibilities for modification.64 Its properties are mainly dependent on heating temperature, biomass type, carrier gases and catalysts.33
Due to advances in research, diverse methods have been devised to influence the properties of biochar depending on the method of preparation and modification so that they unveil a better adsorption capacity.33,65 Bare biochars can be obtained via physical methods (ball milling and microwave pyrolysis) or chemical methods using oxidants such as HCl, H2SO4, H3PO4, or reductants NaOH, KOH during pre- or post-synthesis. Some pristine or bare biochars are usually associated with a lack of affinity for antibiotic pollutants, which is influenced by the absence of sufficient surface functionality or their low porosity as an adsorbent, so they are subjected to modifications. Modified biochars using the aforementioned activating agents are referred to as activated carbon (AC).
AC is carbon produced from carbonaceous source materials mainly from biomass, e.g. coconut husk or wood, purportedly exhibiting large pore volumes, a well-built high specific area, small pore diameter, and high adsorption capacity and have been projected as one of the most effectual adsorbents (Table 3). Nevertheless, the characteristics of the prepared activated carbon depend on the raw precursors utilized and the preparation procedure, as depicted in Fig. 2.66,67 They have been found to have relatively comparable adsorption properties to commercial activated carbon. Additionally, they are more cost effective than commercial activated carbon. Furthermore, apart from modifying bare biochar using various activating agents, combining them with other components, such as clay minerals, graphene, metal oxides or hydroxides, graphene oxides, carbon nanotubes and polymers to form composites, has been employed to enhance their antibiotic removal efficiency. Biochar can be incorporated with these components either before pyrolysis or after pyrolysis.
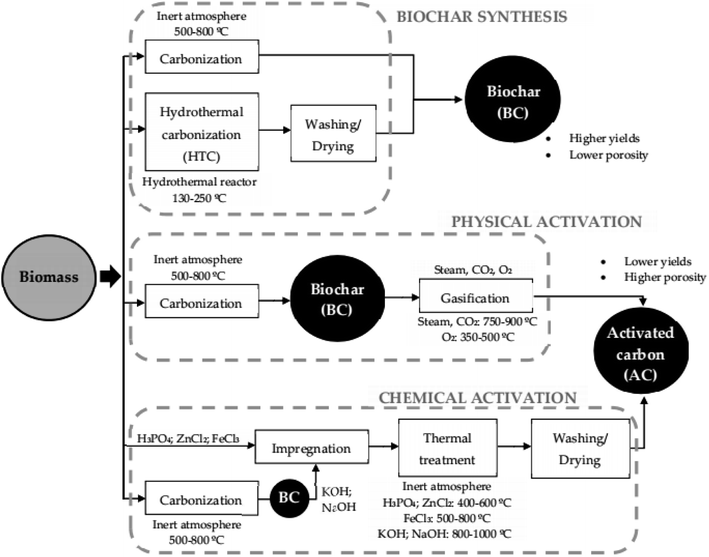 | ||
| Fig. 2 Synthetic route for preparing biochar and activated carbon from biomass.66 | ||
However, it is vital to select adsorbents with higher selectivity for targeted antibiotics because it is often challenging to control the type of contaminant occurring in a matrix or adsorption variables such as pH and temperature.63,68 The process of adsorbing contaminant molecules onto biomass-based adsorbents has been found to follow the principal steps of adsorption, which include: the movement of adsorbate molecules from the matrix, diffusion of the adsorbate through the liquid film surrounding the adsorbent molecule, movement of adsorbate molecules to the active sites of the adsorbent and adsorbent–adsorbent interactions, while the mechanism of adsorption depends on the latter step.33,69
Different interaction types have been postulated to be responsible for antibiotics adhering to the surface of biochar or (AC): for example, electrostatic, hydrophobic, π–π electron-donor–acceptor, and charge–dipole interactions.70,71 Other factors such as pore structure, functionality, nature of contaminants, and process conditions, such as temperature, pH, and competing ions can influence the mechanism and adsorption capacity of the adsorbents.72 It is, therefore, paramount to choose an adsorbent that is highly selective to the target antibiotics.73,74 A detailed scheme of the mechanism involved in the sorption of antibiotic molecules on a biochar material is simplified in the two pictorial illustrations in Fig. 3.
 | ||
| Fig. 3 A detailed scheme of the mechanism involved in the sorption of antibiotic molecules on a biomass-based material.25,33 | ||
4.1 Removal of selected classes of antibiotics onto different biomass-based adsorbents
Another biomass, palm bark, was utilized under adsorption conditions of 0.5 to 5 g L−1 (adsorbent dosage), 10 to 100 mg L−1 (initial concentration), and 10 to 180 min (contact time) for amoxicillin removal.77 Furthermore, the palm bark biomass was shown to yield 98.1% removal of amoxicillin at 90 min contact time, 3 g L−1 adsorbent dose and 10 mg L−1 of amoxicillin at a solution temperature of 25 °C. Additionally, the adsorption isotherm for palm bark biomass in removing amoxicillin from an aqueous solution was inferred to be best interpreted using the Langmuir model, compared to the Freundlich and Temkin isotherm models.77 Moreover, it was suggested from the experimental findings that there was an increase in the percentage removal of amoxicillin when the adsorbent dose was increased from 0.5 to 3.0 g because of the availability of binding sites positioned on the adsorbent surface. In contrast, there was no significant upsurge in the percentage removal of amoxicillin when there was a further increase in the adsorbent dosage as a result of the diminution of the amoxicillin concentration. In addition, there was a rapid removal of amoxicillin at the initial phase of contact period, but it subsequently became slower when approaching equilibrium.77 The rate of amoxicillin adsorption between the initial phase of contact time and equilibrium time was reported to be virtually constant as a result of the large number of binding sites located on the adsorbent surface, compared to the reduced number of binding sites after a lapse of time. The decrease in the number of binding sites obtained on the adsorbent surface resulted in repulsive forces between the amoxicillin ions and a resultant decline in the percentage removal of amoxicillin using palm bark biomass. Similarly, an increase in the initial amoxicillin concentration was inferred to result in a decrease in the percentage removal of amoxicillin, consequent to the saturation of the binding sites of the palm bark biomass at a fixed dose.77
Activated carbons derived from lignocellulosic precursors obtained from olive stones have been crucially utilized in removing amoxicillin from wastewater.78 The speedy adsorption kinetics were supposedly controlled by a pseudo-second-order model and were ascribed to the presence of an immense network of mesopores on the activated carbons. The rapid adsorption kinetics is an indication that there is no constraint on the accessibility of pores on the activated carbon surface. This outcome agreed closely with the molecular dimension of amoxicillin (ca. 1.24 × 0.56 × 0.46 nm), as computed from 3D optimization for the lowest energy configuration using Chem Sketch software.78 Similarly, investigation of the employment of activated carbon derived from NH4Cl-modified pomegranate wood in removing amoxicillin has been reported.79 The adsorptive capacities of unmodified and NH4Cl-modified activated carbons were suggested to be 262 and 437 mg g−1, and the effective adsorption process was attributed to the high surface area, functional groups, and interaction between the modified adsorbent and amoxicillin, which was pH-dependent.79 Moreover, the experimental findings established the existence of electrostatic interaction between the positively charged functional groups on the cationic surfactant of the adsorbent and carboxyl anions in the structure of amoxicillin antibiotic molecules as the governing mechanism of the adsorption process. Comparatively, the unmodified and NH4Cl-modified activated carbon respectively displayed 55 and 99% amoxicillin removal at optimum adsorption conditions with Langmuir and pseudo-second-order giving the best interpretation for the adsorption isotherm and kinetics, respectively.79
In another study, the modification of indian almond biomass with the use of concentrated acid and sodium bicarbonate solution has been investigated in preparing tannin to act as a suitable adsorbent for removing dicloxacillin from water.80 The impact of pH on utilizing tannin for removing dicloxacillin was studied as the percentage removal of dicloxacillin increases with an increase in pH from 2 to 6. This outcome was linked to the increase in the degree of ionization of phenolic hydroxyl ions of tannin that increases with pH, thereby resulting in higher removal of dicloxacillin.80 Contrariwise, there was a decrease in the percentage removal of dicloxacillin at pH values that exceeded 6. Furthermore, it was reported that there was a quick adsorption removal of dicloxacillin on tannin during the initial phase of contact time, but equilibrium was attained at 24 h of contact time.
Rapid removal of ciprofloxacin of about 80% was noted within the first 5 min of the adsorption process. Conversely, beyond 5 min, the removal of ciprofloxacin was noted to be slow and, lastly, equilibrium was reached within 40 to 60 min of contact time. The result from pH studies signified that the removal of ciprofloxacin increased with an increase in pH until an equilibrium capacity of 11.6 mg g−1 was attained at pH 5.8.82 Beyond this, there was a steady decrease in the removal of ciprofloxacin with a respective increase in the solution pH of the adsorption process. Furthermore, the effect of the sawdust/ciprofloxacin ratio was reported to impact the percentage removal of ciprofloxacin. An increase in percentage ciprofloxacin removal was suggested to occur with a corresponding increase in the sawdust/ciprofloxacin ratio until equilibrium was reached at a ratio of 2.0, beyond which there was no further increase in the removal of ciprofloxacin.82 This finding was ascribed to occupation by ciprofloxacin on the active sites of the sawdust adsorbents, thereby preventing further removal of ciprofloxacin from the solution. Additionally, the kinetics and the mechanism for ciprofloxacin removal using sawdust were supposedly controlled by a pseudo-second-order model and intra-particle diffusion process, respectively. At the same time, regeneration studies revealed 85% efficiency compared to the initial adsorption capacity in removing ciprofloxacin.82
Chitosan is a linear cationic amino-polysaccharide composed of α-D-glucosamine, which can be obtained from chitin naturally occurring in relative abundance. Reports on the modification of chitosan have been conveyed as obtaining a magnetic chitosan graphene oxide composite as a viable adsorbent for removing ciprofloxacin from wastewater.83 By reason of the magnitude of solution pH on the surface charge of adsorbents as well as speciation of adsorbate ions in circulation, the effect of pH on ciprofloxacin elimination has been reportedly regulated by electrostatic and π–π attraction. It was reported that the removal of ciprofloxacin decreases with pH values above 5, which was reported as the optimum pH for removing ciprofloxacin with modified chitosan.83 Similarly, a decrease in ciprofloxacin removal was reported when the solution pH decreased from 5 to 4. This observation can best be explained based on the existence of ciprofloxacin in the mostly cationic form at pH <6.1 and the anionic form of ciprofloxacin existing at pH >8.7, while in the pH range of 6.1 to 8.7, ciprofloxacin exists as zwitterions. The adsorptive removal of ciprofloxacin on modified chitosan was reported to be swayed by the existence of metal ions following the 40.9 and 37.5% declines in ciprofloxacin removal under the influence of calcium as well as sodium ions,83 consequently confirming the influence of electrostatic attraction on the adsorption removal of ciprofloxacin. In addition, the utilization of modified chitosan on removing ciprofloxacin was best interpreted with the Langmuir isotherm model with a maximum adsorption capacity of 282.9 mg g−1, but kinetically controlled by the pseudo-second-order model. Lastly, 72% of the preliminary adsorption capacity in removing ciprofloxacin was reported after four cycles of reuse from regeneration studies.83
Correspondingly, activated carbon derived from pumpkin seed has been employed in removing ciprofloxacin antibiotics from wastewater.84 The experimental outcome shows that pumpkin-seed-based activated carbon displayed 99% removal of ciprofloxacin antibiotics under optimum experimental conditions. The adsorption isotherm was best interpreted using the Langmuir model with a physical adsorption path, while the findings from thermodynamics confirmed an endothermic and spontaneous adsorption process.84 Similarly, the utilization of KOH has been explored as an activating agent for preparing activated carbons derived from agricultural wastes that include banana peel, straw, avocado peel, Limonia acidissima shell, and tea waste to remove ciprofloxacin.85 The experimental results revealed that the straw-based-activated carbon presented the highest adsorption removal of ciprofloxacin at 93.34%, whereas the Limonia acidissima shell-based activated carbon displayed the least adsorption removal of ciprofloxacin at 23.43%.85 Further insight into the adsorption process disclosed that the presence of hydroxyl, carboxyl, and carbonyl ester groups on the activated carbons plays a dynamic part in the binding of activated carbon to the ciprofloxacin molecules. This promotes the adsorption of ciprofloxacin on the activated carbon via π–π interactions and hydrogen bonding as well as an electron-donor–acceptor mechanism.85
Another activated carbon derived from KOH-modified pomegranate peel wastes for ciprofloxacin removal from aqueous system has been reported.86 The experimental findings revealed an optimum pH of 8, and recounted a high level of protonation on the adsorbent surface at lower pH that expedited electrostatic attraction between ciprofloxacin and the prepared activated carbon. Similarly, an optimum dose of 0.05 g was suggested from the experimental findings to yield the highest adsorption capacity, whereas there was a major decline in adsorption removal of ciprofloxacin at a higher adsorbent dose.86 Moreover, rapid adsorption removal of ciprofloxacin was attained within a contact period of 30 min, after which there was no significant increase in the adsorption removal of ciprofloxacin at a higher contact time. This outcome is consequent on the high initial concentration gradient occurring between the adsorbates and the number of unoccupied active sites on the adsorbents. In addition, the adsorption isotherm for removing ciprofloxacin was best interpreted with the Freundlich model, compared to the Langmuir model which produced a maximum adsorption capacity of 2.353 mg g−1.86
Investigation on the adsorption of ciprofloxacin (CIP) onto composite derived from solid waste supported on bentonite clay was carried out by Ashiq et al.91 A 40% increase in removal efficiency of CIP was reported compared with bare BC, which was attributed to the intercalation of CIP in the clay–BC layer. In addition, the improved active pores and existence of electrostatic attraction between the functional groups of CIP molecules and the BC composites favored increased removal efficiency. Experimental data best fitted the Hill isotherm alongside pseudo-second-order and Elovich kinetic models. The optimum adsorption capacity of 190 mg g−1 was attained at pH 6.
Sulfamethoxazole (SMZ) sorption from an aqueous solution was achieved using Azolla filiculoides (AF) as an adsorbent.89 Adsorption came into play as well. Thermodynamics, isotherms, and kinetics were investigated. During the experiments, the contact time, agitation speed, initial SMZ concentration, and temperature were varied. The Langmuir, Freundlich, and Temkin adsorption isotherms were all investigated. The Langmuir models were the best fit for describing SMZ sorption in aqueous solutions (because of their high R2 values). Pseudo-first-order, pseudo-second-order, and intraparticle diffusion models were all used to fit the experimental data. The pseudo-second-order kinetic model was more accurate than other kinetic models in describing the adsorption process. Standard free energy changes (G), standard enthalpy change (H), and standard entropy change (S) were all determined as thermodynamic parameters. The adsorption of SMZ on AF biomass was found to be practicable, spontaneous, and endothermic based on these criteria. This research discovered that AF biomass is an effective adsorbent for removing sulfamethoxazole antibiotics from an aqueous solution.
Kurup in 2012 (ref. 90) reported the adsorption removal of sulfamethoxazole using an alkali (NaOH)-treated agricultural-waste-based adsorbent obtained from deoiled soya. Compared to unmodified deoiled soya, the display of the hydroxyl group and the accessibility to a higher surface area were demonstrated by the alkali-modified deoiled soya. Besides, the detected decline in the porosity of the alkali-modified deoiled soya was ascribed to the occurrence of sodium ions. Nonetheless, the alkaline modification ensued in an increase from 10 to 20% of sulfamethoxazole exclusion and the adsorption mechanism was well defined by dint of electrostatic force and ion exchange.90
Similarly, Huang et al.121 investigated a GO-modified bamboo sawdust biochar composite for sulfamethazine (SMT) adsorption. The FTIR spectra showed more oxygen functional groups on the composite surface, which was also reflected in the SBET due to GO addition. The sorption of SMT onto GO–BC was best described by the Freundlich isotherm with R2 = 0.969, indicating the existence of electrostatic interactions in the core of the heterogenous active sites in the composite. Other mechanisms such as hydrogen bonding and cation exchange were suggested to support the adsorption. This established the potential of GO–BC nano-composites for antibiotic removal.121
Activated carbon derived from hazelnut shell for the elimination of tetracycline, oxytetracycline and chlortetracycline yielded 312.59, 322.60 and 333.30 mg g−1 adsorption capacities controlled by hydrogen bonding as well as a π–π interaction adsorption mechanism with an endothermic adsorption process. Furthermore, the maximum adsorption capacities of hazelnut-shell-derived activated carbon was obtained under optimum conditions of 5 (pH), 293 K (temperature) and 0.1 g (adsorbent dose) with the adsorption isotherm and kinetics best described using Langmuir and pseudo-second-order models, respectively.67,93,94 In 2020, Wang et al. synthesized a zinc–chloride–activated biochar derived from Flueggea suffruticosa to adsorb oxytetracycline (OTC), tetracycline (TC) and chlortetracycline (CTC) from aqueous solution. The surface area was found to be 2556 m2 g−1, and the isotherm data fitted well to the Langmuir model, which assumed monolayer adsorption occurred while kinetics were correlated best to pseudo-second-order. At 30 °C the maximum adsorption capacities of Zn–BC were 200, 188.7 and 129.9 mg g−1 for TC, CTC and OTC, respectively. From the thermodynamic studies, the entropy value was found to be positive while ΔG0 was negative, which is an indication of spontaneity. In addition, it also revealed that the adsorption of TC and CTC was an endothermic process, whereas that of OTC was exothermic from the negative and positive ΔH0 values obtained. In summary, at a wider pH and ionic strength range, Zn–BC had a larger adsorption capacity for TCs. Thus, Zn–BC is a prospective material for removing pollutants in an environmental manner.72
Alidadi95 demonstrated an improvement in adsorption capacity for removing tetracycline by using modified sawdust in the sequence order of FeCl3 > HCl > CaCl2 > NaHCO3 modifying agents. Furthermore, the utilization of FeCl3 for modifying sawdust yielded accessible, functional groups that include carboxylic, ferric, carboxylate and hydroxyl by means of the existence of oxygen, carbon and iron atoms on the surface of the sawdust. The demonstration of a pH of 4.15 by the modified sawdust denotes a positively and negatively charged surface of modified sawdust at pH values below and above 4.15, respectively, and a modified sawdust surface with a neutral charge at pH 4.15.95 A comparable trend in pH studies was reported when sulfonation-modified sawdust was experimented with in removing tetracycline with maximum adsorption capacity obtained at a neutral pH.96 A sulfonation modifying agent has been noted to generate a sulfur deposit on the surface of sawdust. It yielded sulfonic acid groups (–SO3H) that support the adsorption of tetracycline on sulfonation-modified sawdust via the mechanisms of electronic interaction, π–π interaction, and hydrogen bonding.96
Furthermore, the adsorbents derived from banana peel graphene, cotton gin waste and guayule bagasse biochar have been reported to be effective for the removal of 286 and 17.12 mg g−1 of erythromycin, respectively. In addition the adsorption kinetics and isotherm were best described using the pseudo-second-order and Langmuir models.97,98 The adsorption removal of clarithromycin from pharmaceutical effluent by employing an adsorbent derived from cuttlefish bone powder has been posited to yield a maximum adsorption capacity of 34.5 mg g−1 with an electrostatic mechanism. However, the adsorption kinetics and isotherm were best interpreted using pseudo-second-order and the Freundlich model.97
The employment of a low-cost adsorbent obtained as a biochar derived from rice husk at a temperature range of 450–600 °C has been effective for removing more than 95% of azithromycin and erythromycin from pharmaceutical effluents.99 Maximum adsorption capacities of 612.22 and 599.72 mg g−1 for the respective removal of azithromycin and erythromycin were reported using rice husk biochar derived at temperatures of 500 and 600 °C, respectively.99 The use of adsorbent derived from agricultural waste corn cobs has been successfully reported to yield a low adsorption capacity of 14.4 mg g−1 for removing tyrosine antibiotics using the Langmuir model which best described the adsorption process. However, the adsorption kinetics for the removal of tyrosine was best described using a pseudo-second-order model.100
The utilization of Azolla filiculoides-based activated porous carbon has been recommended to be effective in removing 87 and 98% of azithromycin after respective adsorption contact times of 75 min, at 303 and 333 K.101 Furthermore, the adsorption removal of azithromycin was endothermic and spontaneous with the adsorption isotherm best described using the Langmuir model with an adsorption capacity of 374 mg g−1, while the adsorption kinetics was best described using the pseudo-second-order model.101
Another study investigated the removal of chloramphenicol from water using porous carbon materials extracted from waste lignin.103 The highest adsorption capacity of this adsorbent at a starting concentration of chloramphenicol of 120 mg L−1 was 534.0 mg g−1 at 303 K. The adsorption capacity did not change significantly under a pH of 4.86, so the initial pH was chosen as the ideal condition for the future tests. In this study, the adsorption process of chloramphenicol was endothermic and spontaneous, according to a thermodynamic analysis of the adsorption isotherm. The high adsorption capability of the synthetic adsorbents was maintained in a complex aquatic environment, which was remarkable. It was concluded from the study that the porous carbon as adsorbent was a cheap and productive biomass-based adsorbent with a wide range of potential applications and it is also easy to use.103
A study on the removal of chloramphenicol from contaminated water using adsorbents made from grape slurry waste has been reported.104 A batch experiment was carried out using simulated antibiotic-contaminated water. According to the study, waste grape slurry could be a useful starting point for creating efficient adsorbents for treating wastewater polluted with antibiotics. Temperature variations appeared to have an impact on the affinity of antibiotics for the adsorbent surface which demonstrated that when the temperature of the solution increases, the adsorption capacity of the adsorbents increases. Thermodynamic analyses also revealed that the sorption of CHLR was an exothermic reaction that was conceivable but not spontaneous as the temperature rose.104
The adsorptive removal of antibiotic contaminants from wastewater using biochar from peanut shells was studied.105 In this study, chloramphenicol served as the reference antibiotic. Waste biomass was pyrolyzed using small amounts of ammonium polyphosphate to create porous biochar that can bind with chloramphenicol. The nitrogen and phosphorus of ammonium polyphosphate additionally encouraged the chemical activity of biochar surface. The Langmuir model and the pseudo-second-order model of chloramphenicol adsorption were the best fits. The study presented alternative routes to biochar preparation and also additional applications of biochar in antibiotic removal.
Liu et al.106 considered discarded rice straw which is hydrothermally liquefied (HTL) to create hydrochar. However, because of its small porosity and surface area, hydrochar material could not be used directly in the environmental field. The hydrochar produced from rice straw was therefore activated and magnetized to create magnetic activated carbon in order to increase the porosity and adsorption capability. The detrimental impact of the magnetic medium led researchers to first explore the activation requirement for hydrochar. The results showed that the magnetically activated carbon has a large surface area (about 674 m2 g−1), a high adsorption capacity, and a rapid adsorption rate for the removal of triclosan (TCS). An external magnetic field can also be used to recover magnetically activated carbon quickly from aqueous solutions. Overall, the hydrochar made from discarded rice straw may be converted into a very effective magnetic adsorbent for TCS elimination.
Recently, Cho et al.107 examined how triclosan can be removed from an aqueous solution using biochar made from kenaf. Physical and chemical analyses were used to investigate the triclosan adsorption process of biochars that were pyrolyzed at different temperatures (300, 400, 600, and 750 °C) (FE-SEM, EDS, EA, XRF, pHpzc, N2 adsorption–desorption, SAXS, ATRFTIR, and XPS). As the pyrolysis temperature climbed, triclosan adsorption by the kenaf biochar improved, with the exception of 450 °C, which showed the least sorption capacity. The maximum sorption capability was demonstrated by kenaf biochar produced at 750 °C (KNF-750), which had a high aromatic moiety and a sizable specific surface area. The pseudo-second-order model accurately described the kinetic adsorption of KNF-750, with equilibrium being reached in 3 hours. The maximum triclosan adsorption capacity for KNF-750 of the Langmuir model, which had a strong correlation coefficient, was 77.4 mg g−1. Because triclosan dissociated at a final solution pH higher than 9, triclosan adsorption drastically decreased at a pH of 5 for the starting solution. With 4 g L−1 of KNF-750, triclosan was removed 90% of the time. Triclosan was adsorbed endothermically, with a 32.8 kJ mol−1 enthalpy change. By demonstrating the disappearance of inorganic Cl and the appearance of organic Cl, XPS examination demonstrated that triclosan was adsorbed on the surface of biochar.
Yu et al.108 investigated how to effectively eliminate TCS with activated carbon (AC) made from nylon 6,6 nanofiber and waste coconut (Cocos nucifera) pulp. The effects of physico–chemical parameters and features for both nanofiber and AC were investigated. The AC was made by carbonizing discarded coconut pulp under a nitrogen flow for an hour at 300 °C after treatment with zinc chloride. Utilizing an electrospinning apparatus with a high voltage of 26 kV, an injection rate of 0.4 mL h−1, a tip-to-collector distance of 15 cm, and a rotational speed of 1000 rpm, the nylon 6,6 nanofiber [14 wt%] was created. Variables such as pH, adsorbent dosage, contact time, agitation speed, temperature, and initial TCS concentration were investigated. Additionally, a device for testing flat-sheet membranes was used to perform a filtration test at a pressure of 1.0 bar. Three techniques—Fourier transform infrared spectroscopy (FTIR), Brunauer–Emmett–Teller, and field emission scanning electron microscopy (FESEM)—were used to examine the properties of AC and nylon 6,6 nanofiber (BET). According to the research, while the adsorption method using AC can remove 83.3% TCS in 20 minutes, the filtering method using nylon 6,6 can remove 90.2% TCS in 5 minutes. After combining the adsorption and filtering techniques using AC and nylon 6,6 nanofibers, TCS elimination increased to 100% removal in less than 5 minutes. The Freundlich isotherm is used to research isotherms, while the Langmuir isotherm is used for nylon 6,6 nanofiber. While nylon 6,6 and AC both use the pseudo-second-order model for kinetics studies. This research demonstrated that the use of AC combined with nylon 6,6 nanofiber can enhance the elimination of TCS from water.
A study was carried out by Cheng et al.,112 where feather charcoal was utilized for the adsorption of TMP-polluted waste water. The characterization of feather-derived charcoal showed a well-organized microporous adsorbent with a surface area of 805.4 m2 g−1. Furthermore, 1.36 and 1.76 mmol g−1 were the observed acidic and basic functional groups, respectively, on the surface of the charcoal with a recorded pHpzc value of 7.52. The following adsorption parameters were studied: initial concentration, dosage, time, temperature, pH and ionic strength. The findings of the study show that adsorption kinetics is favored by pseudo-second-order kinetics with an R2 value of 0.9880. The TMP-feather-derived adsorption isotherms for the biosorbent were better explained by the Freundlich isotherm at lower temperature (293 K) with a qm value of 125 mg g−1 and R2 = 0.9913, while the adsorption process was well fitted to the Langmuir isotherm at higher temperature (313 K) with a qm value of 164 mg g−1 at R2 = 0.9984. The adsorption mechanism was observed to be a combination of hydrophobic interactions and ion exchange as well as electrostatic interaction. This study thereby confirmed the suitability of the feather-derived charcoal as a biosorbent for TMP-polluted wastewater.
Furthermore, a novel low-cost magnetic peanut-based adsorbent (MPN-Bet) was synthesized through a copolymerization technique with Fe3O4 and betaine.111 The modification of the peanut husk alters the available MPN-bet adsorption sites, thereby altering the physicochemical properties and thus enhancing the adsorption potential of the MPN-Bet composite. The finding of the study show that MPN-Bet has potential for the sequestration of TMP from its solution. The maximum adsorption capacity of 31.2 ± 3.2 mg g−1 with R2 = 0.978 at 293 K show that the uptake of TMP from its solution using MPN-Bet is exothermic in nature. Both physisorption and chemisorption as shown by the kinetic models employed in this study, suggesting that pseudo-first and pseudo-second-order kinetics play active roles in the adsorption process. However, chemisorption was observed to be the dominant adsorption mechanism. An evaluation of the biopotency of MPN-Bet against E. coli and S. aureus showed that it effectively inhibited the growth of these microorganisms. These properties promote MPN-Bet as a suitable sorbent for wastewater remediation.
Lee and Kam investigated the adsorption characteristics of TMP onto an activated carbon prepared from waste citrus peel.113 Response surface methodology was adopted to evaluate the influence of adsorption parameters on TMP adsorption. Batch experiments were carried out according to a four-factor Box–Behnken experimental design, which included concentration, amount of adsorbent, temperature, and pH. The experimental data was observed to be best fitted to the Langmuir isotherm with a recorded qm value of 144.9 mg g−1 at 293 K. The reaction kinetics were best described by the pseudo-second-order reaction kinetic model.
Another study assessed the adsorption capability of three biochars derived from spent coffee grounds, cattle manure, and biosolids on antibiotics, namely, tetracycline (TET), erythromycin (ERY), clarithromycin (CLA), trimethoprim (TMP) and ampicillin (AMP). Biochar dosage of 1 g L−1 or 10 g L−1 was applied to a spiked mixture of antibiotic mixtures of 100 μg L−1 of an aqueous solution in a batch adsorption process. The results showed that at low dosage, more than 70% of the antibiotics were removed by all biochars applied, while at high dosage, rapid adsorption within 5 min of incubation was observed, resulting in complete removal of TET, CLA, ERY, and CLA as well as >85% of AMP and TMP. From the study, it was revealed that the process is pH-dependent. The experimental data fitted well to the Freundlich isotherm model and the suggested mechanisms responsible for the adsorption were π–π electron-donor–acceptor and hydrogen-bonding. The overall result emphasized the possible utilization of the biochar for the decontamination of antibiotics in the water phase.69
In another recent study, self-functionalized biochar from corncob was designed using an ultrasonic-assistant fore-modified method to obtain enhanced adsorption of three targets traditional antibiotics, namely: amoxicillin (AMX), tetracycline (TC), and levofloxacin (LE). The adsorbent characterization revealed an ultra-large surface area of 2368 m2 g−1 and greater functionality, which played a vital role in the adsorbent–adsorbate interface interaction. The experimental data obtained from the batch process fitted well with the Freundlich isotherm model (R2 = 0.99), which indicated chemisorption. This correlated with the kinetics data, which fitted best to Elovich at a temperature ranging from 20–40 °C. An outstanding adsorptive capability of >497 mg g−1 was also observed. The thermodynamics parameters of enthalpy (ΔH) and entropy (ΔS) obtained from the studies were positive, while the Gibb's free energy change (ΔG) decreased with increasing temperature. This revealed that the sorption affinity became intense at a higher temperature. The removal efficiencies reached 97.98%, 72.26%, and 96.59% for LE, AMX and TC respectively. It can be inferred from the process that the ultrasonic-assisted method has the potential to develop a more efficient modified BC for pollutant removal in the water layer.71
The modification of powdered pistachio shells with ZnO nanoparticles to prepare a viable adsorbent has been exploited to get rid of tetracycline, amoxicillin, and ciprofloxacin antibiotics from aqueous solution.114 The adsorption isotherm for the removal of tetracycline and ciprofloxacin was best fitted with the Freundlich model, while the Langmuir model best interpreted the removal of amoxicillin. Moreover, the maximum adsorption capacities of 92.450, 98.717 and 132.24 mg g−1 were attained for tetracycline, amoxicillin and ciprofloxacin, as deduced from the Langmuir isotherm model. Additionally, the correlation of the pseudo-second-order kinetic data with the removal of the antibiotics implies a chemical adsorption controlled process in a spontaneous and exothermic approach pinpointed in the thermodynamic studies.114
The utilization of chemical agents, for instance, HNO3, NaOH, ZnCl2, KOH, and NaCl, for the modification of vine wood to generate carbon nanoparticles for removing selected antibiotics that include tetracycline, cephalexin, penicillin G and amoxicillin has been reported.115 From the experimental outcomes, it was pointed out that NaOH-activated adsorbents exhibited the highest removal rate compared to the adsorbents derived from other activating agents at the optimum condition of pH (2), 20 mg L−1 (antibiotic concentration), 8 h of contact time and 0.4 g L−1 adsorbent dose at 45 °C. Correspondingly, the kinetics studies revealed a pseudo-second-order controlled adsorption process.115
5 Adsorption kinetics models: theoretical basis
In past years, different models and mathematical formulas have been used to describe the kinetics128,129 and mechanisms of adsorption processes,130 specifically, the equilibrium adsorption proficiency of various solutes. The adsorption mechanism is also affected by the adsorbents' physical properties and chemical attributes.130 These models include but are not limited to the following models.5.1 Lagergren pseudo-first-order model
The pseudo-first-order kinetic model is commonly used to explain the adsorption process in relation to boundary diffusion. It is the most frequently used adsorption model.131 Lagergren presented a first-order rate equation to describe the kinetic process of liquid–solid phase adsorption. The pseudo-first-order kinetic model is often used for solute adsorption from liquid.132 It is said to be the first model established on adsorption capacity.133 It is used to explain the adsorption kinetics of various species. For lower solute concentrations, the pseudo-first-order kinetic model by Lagergren is particularly appropriate.130 This model has been frequently used in recent years to describe the adsorption of contaminants/pollutants from wastewater in several fields. It has been popularly used in recent years to explain contaminant adsorption from wastewater in a variety of disciplines.133 It can be expressed as in eqn (1) & (2) in Table 4.| Type | Expression | Equation | Ref. |
|---|---|---|---|
| a Notation: Qe = adsorption capacity at equilibrium (mg g−1), Qt = adsorption capacity at time t (mg g−1), k1 = pseudo-first-order adsorption equilibrium rate constant of (1/min), t = time of contact (min), k2 = equilibrium rate constant of pseudo-second-order adsorption (g mg−1 min−1), ki = intra-particle diffusion rate constant (mg g−1 min−0.5), I = constant that gives the information regarding the thickness of the boundary layer (mg g−1), t1/2 = half-life, Qav = Avrami theoretical value of the amount of the adsorption (mg g−1), Kav = Avrami constant rate, nav = Avrami order model, Qt = the amount of adsorbate in the adsorbent at time t (mg.g−1), Ct = solution concentration at time t, Ci = adsorbate initial concentration (mg L−1), Qt = the amount of adsorbate in the adsorbent at time t (mg g−1), m = the mass of the adsorbent in a litre of adsorbate (g L−1), kB = rate constant for Bangham's model, Bt = Boyd constant, β = the number of sites available for adsorption, α = the initial adsorption rate (mg g−1 min). | |||
| Lagergren pseudo-first-order |  |
(1) | 130, 135 and 143 |
ln(Qe − Qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Qe − k1t Qe − k1t |
(2) | 144 | |
| Pseudo-second-order |  |
(3) | 144 and 145 |
| Intra-particle diffusion model |  |
(4) | 146–149 |
| Avrami |  |
(5) | 131 and 144 |
| Bangham | Qt = Qe − (Qe − Q0)exp(−kBtμ) | (6) | 136 |
 |
(7) | 139 | |
| Boyd |  |
(8) | 139 |
| Elovich |  |
(9) | 150 |
5.2 Lagergren pseudo-second-order model
The Lagergren pseudo-second-order-model approach can be used to determine the rate constants, equilibrium adsorption capacity, and adsorption mechanism of adsorption processes.129 It can be expressed as eqn (3) in Table 4.5.3 Intra-particle diffusion model
The intra-particle diffusion model was developed to see whether intra-particle diffusion is the rate-limiting stage in an adsorption process.134 The intra-particle diffusion model implies that intra-particle diffusion is the only phase affecting the rate135 and that the membrane diffusion can be ignored.136 Doğan et al. reported in 2009 that the intra-particle diffusion step is most often the rate-limiting step in adsorption processes.132 It is written as eqn (4) in Table 4.5.4 Avrami kinetic model
In Avrami's model, adsorption kinetics is an exponential function of adsorption time. The fractional-order kinetic model of Avrami was created to explain phase transition and to evaluate crystal formation in materials.137 The mole fraction of the gas phase and sorption temperature are relevant considerations when the Avrami model is applied.131 The Avrami model depends on the overall rate of adsorption and can be used where there is multiple adsorption mechanism. It is written as eqn (5) in Table 4.5.5 Bangham kinetic model
The Bangham kinetic model has also been used to determine the rate-controlling step of an adsorption process.138 According to Wang et al.,136 the Bangham adsorption equation can also be written as in eqn (6) and (7).1395.6 Boyd kinetic model
This Boyd kinetic model differentiates the extra particle and intra-particle diffusion.139 It can be expressed mathematically as represented in eqn (8).5.7 Elovich kinetic model
Recently, the Elovich equation has been frequently utilized to characterize the kinetics of gas adsorption on solids140,141 as well as the adsorption of contaminants from aqueous solutions.139 The Elovich model assumes that the surfaces of solids are actively heterogeneous.142 It is mathematically expressed as eqn (9) in Table 4.6 Adsorption isotherm models: theoretical basis
The adsorption capacities of different adsorbents and their relationships with various adsorbates are often expressed using different types of adsorption isotherms. Since a single isotherm model cannot be used to describe an adsorption process in general, we have put together the mathematical expressions for frequently used isotherms in Table 5. Additionally, we summarize the unique features and characteristics of these isotherms in order to provide the theoretical background to which they could be further applied.| Isotherm | Nonlinear form | Equation | Linear form | Equation | Plot | Ref. |
|---|---|---|---|---|---|---|
| a Notation: QL is the maximum monolayer adsorption (mg g−1); KL is the Langmuir isotherm constant (L mg−1); Ce is the equilibrium concentration (mg L−1); Qe is the amount of adsorbate in the adsorbent at equilibrium (mg g−1); KF is the Freundlich isotherm constant (mg g−1) (L g−1)n related to adsorption capacity; n is the adsorption intensity; KBA is the Bohart–Adams rate constant; Z is the total bed depth; CBET is the BET adsorption isotherm relating to the energy of surface interaction (L mg−1); Cs is the adsorbate monolayer saturation concentration (mg L−1); Qs is the theoretical isotherm saturation capacity (mg g−1); KDR is the Dubinin–Radushkevich isotherm constant (mol2 kJ−2); QDR is the theoretical isotherm saturation capacity (mg g−1); Ci is the adsorbate initial concentration (mg L−1); KFH is the Flory–Huggins isotherm equilibrium constant (L g−1); nFH is the Flory–Huggins isotherm model exponent; θ is the degree of surface coverage; d is the interlayer spacing (m); R is the universal gas constant (8.314 J mol−1 K−1); r is the inverse power of the distance from the surface; T is the temperature (K); α is the Frenkel–Halsey–Hill isotherm constant (J mr per mole); ak is the Khan isotherm model exponent; bk is the Khan isotherm model constant; A is the Koble–Corrigan isotherm constant (Ln mg1−n g−1); B is the Koble–Corrigan isotherm constant (L mg−1)n; n is the adsorption intensity; aRp is the Radke–Prausnitz isotherm model constant; rR is the Radke–Prausnitz isotherm model constant; βR is the Radke–Prausnitz isotherm model exponent. aR is the Redlich–Peterson isotherm constant (L mg−1); g is the Redlich–Peterson isotherm exponent; KR is the Redlich–Peterson isotherm constant (L g−1); as is the Sips isotherm model constant (L mg−1); Ks is the Sips isotherm model constant (L g−1); βs is the Sips isotherm model exponent; AT is the Temkin isotherm equilibrium binding constant (L g−1); bT is the Temkin isotherm constant; aT is the Toth isotherm constant (L mg−1); KT is the Toth isotherm constant (mg g−1); t is the time; Ct is the solution concentration at the fixed bed outlet at time t; N0 is the maximum volumetric sorption capacity; U is the linear flow rate; Z is the total bed depth; β is the kinetic coefficient of the external mass transfer; KYN is the Yoon–Nelson rate constant; τ is the time required to reach 50% adsorbate breakthrough (min); AHJ is the Harkin–Jura isotherm constant (slope); BHJ is the Harkin–Jura isotherm constant (intercept); KH is the Halsey constant (slope); nH is the Halsey constant (intercept); KE is the Elovich–Larionov isotherm constant; QE is the Elovich–Larionov maximum adsorption capacity (mg g−1). | ||||||
| Langmuir |  |
(10) | 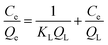 |
(11) |  |
154 |
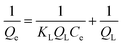 |
(12) |  |
||||
 |
(13) |  |
||||
 |
(14) |  |
||||
| Freundlich | Qe = KFC1/ne | (15) |  |
(16) | Ln(Qe) vs. Ln(Ce) | 155 |
| Bohart–Adams |  |
(17) |  |
(18) | — | 156 |
| Brunauer–Emmett–Teller (BET) |  |
(19) | 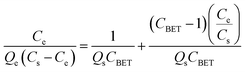 |
(20) |  |
157 |
| Dubinin–Radushkevich |  |
(21) |  |
(22) | Ln(Qe) vs. ε2 | 158 |
| Flory–Huggins |  |
(23) |  |
(24) | 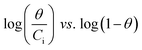 |
159 |
| Frenkel–Halsey–Hill |  |
(25) | — | — | 160 | |
| Khan |  |
(26) | — | — | 161 | |
| Koble–Corrigan |  |
(27) |  |
(28) | — | 162 |
| MacMillan–Teller | 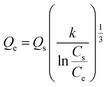 |
(29) | — | — | 163 | |
| Radke–Prausnitz |  |
(30) | — | — | 164 | |
| Redlich–Peterson | 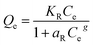 |
(31) |  |
(32) | — | 165 |
| Sips |  |
(33) |  |
(34) | 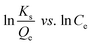 |
166 |
| Temkin |  |
(35) |  |
(36) | Qe vs. ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce Ce |
167 |
| Toth |  |
(37) | 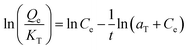 |
(38) |  |
168 |
| Wolborska |  |
(39) | — | — | 169 | |
| Yoon–Nelson |  |
(40) | — | — | 170 | |
| Harkin–Jura | — | 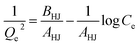 |
(41) |  |
171 | |
| Halsey | — |  |
(42) | Ln(Qe) vs. Ln(Ce) | Naz et al. 2021 | |
| Elovich–Larionov | — |  |
(43) | 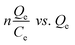 |
172 | |
The Langmuir isotherm model is one of the most common and simple to use isotherms due to its effectiveness in low concentrations, flexibility with computer simulations, and easy handling.151 This model works on the principle of homogeneous adsorption and monolayer formation with no interaction between the adsorbed species. It is expressed as eqn (10) and eqn (11)–(14) in non-linear and linear models. Together with the Langmuir isotherm model, the Freundlich isotherm is also commonly used. This model works on the principle of heterogeneity and multilayer adsorption and depends on the concentration of pollutants. The Freundlich model is frequently used because its capacity to describe nonlinear adsorption even in the smallest amount of the adsorbate coped with its functionality in heterogeneous systems, which are common for adsorption. Mathematically, the Freundlich isotherm is expressed as non-linear (eqn (15)) and linear (eqn (16)). Many studies have compared the Bohart–Adams isotherm model with that of the Thomas and Yoon–Nelson models, even though most of these studies present contrary ideas.152,153 The uniqueness of the Bohart–Adams isotherm is based on its assumption that equilibrium is not instantaneous and the dependence on surface reaction theory. The performance of the Bohart–Adams model can be evaluated using nonlinear (eqn (17)) and linear (eqn (18)) equations.
The Langmuir isotherm model had some barriers and the BET isotherm was developed years later to address these flaws. The BET theory extends the Langmuir theory to multilayer adsorption with additional assumptions that the principle of Langmuir theory can be applied to each layer, that a dynamic equilibrium exists between successive layers, and last that the enthalpy of adsorption of the first layer is constant. This model is expressed as non-linear (eqn (19)) and linear (eqn (20)). Since the Langmuir isotherm is limited to homogeneous surfaces, the Dubinin–Radushkevich isotherm was considered more advanced because it accounts for the effect of the porous structure of the adsorbents.173 The Dubinin–Radushkevich isotherm assumes that adsorption depends on the filling of the micropore volume, contrary to known layer-by-layer models.174,175 Since this model has been applied in aqueous solutions, its accuracy depends on the ε values.176 It can be expressed as non-linear (eqn (21)) and linear (eqn (22)). The Flory–Huggins isotherm believes that the chain elements arrange themselves randomly on a three-dimensional structure. Based on this assumption, the Flory–Huggins isotherm is used to describe the coverage characteristics of the adsorbate. This isotherm is expressed as nonlinear (eqn (23)) and linear forms (eqn (24)).
The Frenkel–Halsey–Hill isotherm basically works on the principle of pair potential that adsorbate particles interact with other particles such as the substrate during adsorption. It assumes that the sum of individual adsorbate–substrate or adsorbate–adsorbate interactions present the total interaction. Mathematically, this isotherm is expressed in a nonlinear (eqn (25)) form. The Khan isotherm is a summed-up model recommended for a pure mixture, which can address the two limits of the Langmuir and Freundlich types. It was created for both multicomponent and single-part adsorption frameworks. Mathematically, this isotherm is expressed in a nonlinear (eqn (26)) form. As a three-parameter equation, the Koble–Corrigan isotherm works on the Langmuir and Freundlich isotherm principle for assessing the equilibrium adsorption of various systems. When the concentration of adsorbate is high, the Koble–Corrigan isotherm conditions itself to the principle of the Freundlich isotherm.159 Mathematically, this model can be represented as a non-linear equation (eqn (27)) and a linear equation (eqn (28)). The MacMillan–Teller isotherm is an adsorption model deciphered from incorporating surface chemistry in the BET isotherm. This isotherm can be expressed as a non-linear model (eqn (29)).
The Radke–Prausnitz isotherm accepts that an adsorbent should be thermodynamically idle; for instance, its properties (for example, interior energy) do not influence the adsorption process. Mathematically, it is expressed as a non-linear equation (eqn (30)). The Redlich–Peterson isotherm is an experimental model with a consolidated component of both Langmuir and Freundlich isotherms, containing vague three boundaries joining its three conditions. This model can be applied for homogeneous and heterogeneous surfaces, and it does not follow ideal monolayer adsorption; as a result, this model gives the best understanding of trial information. Mathematically, this isotherm can be shown as a non-linear equation (eqn (31)) and linear equation (eqn (32)). The Sips isotherm is a joined type of Langmuir and Freundlich model derived for anticipating the adsorption in heterogeneous frameworks and evading the restriction of the rising adsorbate focus related to the Freundlich isotherm. At low adsorbate fixations, it reduces to the Freundlich isotherm, while at high focus, it predicts monolayer adsorption, like the Langmuir isotherm. Along these lines, the Sips isotherm will be utilized to portray just monolayer adsorption frameworks. It is expressed as a non-linear equation (eqn (33)) and a linear equation (eqn (34)). The Temkin isotherm model assumes that the adsorption heat of all molecules decreases linearly with the increase in coverage of the adsorbent surface and that adsorption is characterized by a uniform distribution of binding energies up to maximum binding energy. It is expressed as a non-linear equation (eqn (35)) and a linear equation (eqn (36)).
The Toth isotherm model is another empirical equation developed to improve the Langmuir isotherm model. It is used to describe heterogeneous adsorption systems in both low and high concentrations. It is expressed as a non-linear equation (eqn (37)) and a linear equation (eqn (38)). The Wolborska model depicts the adsorption elements utilizing the mass exchange conditions related to the diffusion means at low levels.169 It is expressed as a non-linear equation (eqn (39)). The Yoon–Nelson isotherm model described that the pace of decline in the adsorption indicates that the adsorbate particle is relative to the forward leap on the adsorbent.170 It is expressed as a non-linear equation (eqn (40)). The Harkin–Jura isotherm model assumes the possibility of multilayer adsorption on the adsorbent surface with a heterogeneous pore distribution.171 Mathematically, this model can be expressed as a linear equation (eqn (41)). The Halsey isotherm is largely applied for multilayer adsorption.177 It is expressed as a linear equation (eqn (42)). The Elovich–Larionov isotherm portrays the adsorption of non-electrolytes from a solution on a solid surface. It is expressed as a linear equation (eqn (43)).
7 Experimental insight into the application of kinetics and isotherm to antibiotics
7.1 Adsorption kinetics
The adsorption process is an established method extensively researched for how water and wastewater can be remediated. The major methods that are utilized in carrying out adsorption experiments could be either a continuous or a batch process. The latter has been observed to be adopted to a major degree and reported in adsorption experiments due to its lower complexity and the better reliability of its results.7 This phenomenon (batch or continuous operations) is based on the interaction between the adsorbate and the adsorbent surface through either physical or chemical bonding processes.178 There are basically four steps that are involved in adsorption kinetics: bulk transport, film transport, intra-particle transport, and adsorption on the adsorbent. The film transport has been reported to be the determinant of the interboundary rate of molecular diffusion and as such regarded as the rate-controlling step of the adsorption process.67,179However, a series of kinetic modeling experiments is carried out to evaluate the rate-controlling steps during the remediation process of an antibiotic or dye polluted wastewater solution by adsorbents (of either chemical or biological source, or a hybrid of both chemical and biological material). The models are categorized into different differential equations, which are basically solved by integral analysis methods.25 They include zero-order, first-order/pseudo-first-order, second-order/pseudo-second-order, and third-order, which are adopted to provide insight into adsorption kinetics. These kinetic models and their respective parameters, including rate constants, equilibrium adsorption capacities, and related correlation coefficients, are presented in Table 6 to utilize a series of biomass-based sorbents to remove antibiotics from their solution.
| Materials | Antibiotic | Experimental conditions | Adsorption capacity/removal efficiency | Kinetics | Isotherms | Mechanism | Ref. |
|---|---|---|---|---|---|---|---|
| a IC: initial concentration, AD: adsorbent dose, T: time and temp.: temperature (°C/K). | |||||||
| Waste tea residue | Hydralazine hydrochloride | IC: 100 mg L−1, AD: 10 mg, T: 60 min, pH: 6–8, temp.: nil | 131.63 mg g−1 | — | Langmuir and Freundlich isotherms, Brunauer–Emmett–Teller (BET) | — | 180 |
| Activated carbons from urban wastes (post-consumer plastics), and agro-industrial residues (cork powder and peach stones) | Acetaminophen | IC: 120 mg dm−3, AD: 10 mg, T: 5 min–24 h, pH: nil, temp.: 30 °C | 267 mg g−1 | Pseudo-second-order kinetics | Langmuir and Freundlich isotherms | 181 | |
| Cocoa shell biomass-based adsorbents | Ibuprofen | IC: 0.5 g L−1, AD: 10 mg, T: 5 min–24 h, pH: 2, temp.: 22–50 °C | 39 mg g−1 | IBP adsorption isotherms | 182 | ||
| Garlic peel | Quinolone | IC: 10 mg L−1, AD: nil, T: nil, pH: nil, temp.: 298 K | 9.8912 mg g−1 | Pseudo-first-order, pseudo-second-order and intraparticle diffusion kinetic models | Langmuir, Freundlich, Temkin and Dubinin–Radushkevich (D-R) models | Hydrogen-bonding | 183 |
| Sulfonated sawdust | Tetracycline, sulfamethoxazole and bisphenol A | IC: 20 mL., AD: 10 mg, T: nil, pH: 4, temp.: 25 °C, 45 °C and 65 °C | 270.53 mg g−1, 295.06 mg g−1, and 263.75 mg g−1 | Pseudo-first-order, pseudo-second-order and intra-particle diffusion kinetic models | Langmuir, Freundlich, Temkin and Dubinin–Radushkevich (D–R) isotherm | Physiosorption | 96 |
| Arundo donax Linn. | Amoxicillin | IC: 50–450 mg L−1, AD: 0.5 g L−1, T: 24 h, pH: 7.0, temp.: 323 K | 345.4 mg g−1 | Pseudo-first-order, pseudo-second-order and intra-particle diffusion kinetic models | Langmuir, Freundlich and Sips isotherms | Endothermic and physisorption | 184 |
| Aminated graphitic carbon derived from corn stover biomass | Tetracycline | pH: 7.4, AD: 0.98 g L−1, IC: 50–200 mg L−1 | 132.9 mg g−1 | Pseudo-first-order, pseudo-second-order and intra-particle diffusion kinetic models | Langmuir, Freundlich, Temkin and Dubinin–Radushkevich (D–R) isotherm | 185 | |
| Steam-activated biochars of Sicyos angulatus L.) | Sulfamethazine | IC: 2.5–50 mg L−1, AD: 1 g L−1, T: 72 h, pH: 3, 5, 7 and 9, temp.: 25 °C | 37.7 mg g−1 | Langmuir, Freundlich, Temkin, and Dubinin–Radushkevich (D–R) | Chemisorption and electrostatic interactions | 186 | |
| NaOH-activated macadamia nut shells | Tetracycline | IC: 500.0, 550.0 and 600.0 mg L−1, AD: 25 mg, T: 2.5–180 min, pH: 3–10, temp.: nil | 455.33 mg g−1 | Pseudo-first-order, pseudo-second-order, Elovich, and Avrami | Langmuir, Freundlich, and Temkin | Intraparticle diffusion and film diffusion | 187 |
| High surface area-activated carbons based on olive biomass | Amoxicillin | IC: 100–1400 mg L−1, AD: 30 mg, T: 1 to 360 min, pH: 7, temp.: 25 °C | 237.02 mg g−1 | Pseudo-first-order, pseudo-second order, and Avrami fractional-order | Langmuir, Freundlich and Liu | π–π stacking (π bonds of the drug molecules with π bonds of adsorbent | 188 |
| Non-living Chlorella sp. | Cephalexin | IC: 482.92 mg L−1, AD: 50 mg, T: 15–600 min, pH: , temp.: 27 °C | 129.87 mg g−1 | Freundlich and Langmuir isotherms | 189 | ||
| Pseudomonas putida | Ceftriaxone | IC: 15, 10, 20, and 50 mg L−1, AD: 0.1 g, T: 6, 12, 24, 48, and 72 h, pH: 7, temp.: 25, 35, and 42 °C | 109.5 mg g−1 | Freundlich and Langmuir isotherms | 190 | ||
| Rhizopus oryzae biomass | Tetracycline | IC: 10–200 mg L−1, AD: 0.25–5 g L−1, T: 5–200 min, pH: 2–11, temp.: 25–50 °C | 67.93 mg g−1 | Pseudo-first-order, pseudo-second-order, and Elovich | Langmuir, Freundlich and Dubinin–Radushkevich (D–R) | π–π or cation–π interaction | 191 |
| Bentonite, activated carbon, zeolite, and pumice | Ciprofloxacin | IC: 20, 25, 30, and 40 mg L−1, AD: 0.0125 g, T: 5, 10, 20, 30, 40, 50, and 60 min, pH: nil, temp.: 22 °C | 91, 87, and 51% for bentonite, activated carbon, and zeolite, respectively | Pseudo-first, pseudo-second, Elovich equations, and intraparticle diffusion | π–π interaction | 192 | |
| Super-magnetization of pectin from orange-peel biomass | Sulfamethoxazole | IC: 200 ppm, AD: 1 g, T: 24 h, pH: 3–8, temp.: 15, 25, 35 and 45 °C | 120 mg L−1 | Pseudo-first, pseudo-second-order | Langmuir, Freundlich, and Redlich–Peterson | Electrostatic-interactions | 193 |
| Vine wood | Amoxicillin, cephalexin, tetracycline and penicillin G | IC: 20, 30, 50, 80, 100, 150 and 200 mg L−1, AD: 0.05, 0.1, 0.2, 0.3, 0.4, 0.5 and 0.6 g L−1, T: 120, 240, 360, 480, 600, 720 and 840 min, pH: 1–12, temp.: 35, 45 and 55 °C | Amoxicillin (2.69 mg g−1), cephalexin (7.08 mg g−1), tetracycline (1.98 mg g−1), penicillin G (8.41 mg g−1) | Pseudo-first, pseudo-second and intraparticle diffusion | Langmuir and Freundlich isotherms | Protonation, hydrogen bonding or van der Waals forces | 194 |
| Lignin-derived biomass | Tetracycline | IC: 1–300 mg L−1, AD: 15 mg, T: 1–120 min, pH: nil, temp.: 298, 308, and 318 K | 173.9 mg g−1 | Pseudo-first-order, pseudo-second-order, intra-particle diffusion, Elovich | Langmuir, Freundlich, and Dubinin–Radushkevich isotherms | Chemisorption and intraparticle diffusion | 195 |
| Amino-functionalized biomass-derived porous carbons | Sulfonamide | IC: 1–20 mg L−1, AD: 5.0 mg, T: 720 min, pH: nil, temp.: 25 °C | 124.6 mg g−1 | Pseudo-second-order | Langmuir and Freundlich isotherms | Electron donor–acceptor interaction | 196 |
| Tea waste biochar | Sulfamethazine | IC: 0–50 mg L−1, AD: 1 g L−1, T: 72 h, pH: 3, 7, 9 and 10, temp.: 25 °C | 33.81 mg | Freundlich and Langmuir isotherms | π–π electron donor–acceptor interaction, cation–π interaction and cation exchange | 186 | |
| Grape stalk | Ofloxacin and chrysoidine | IC: 3.1 mM or 7.0 mM, AD: 0.5 g, T: 120 min, pH: 4, 7 and 9 | 137.3 mg g−1 | Pseudo-second-order | Langmuir isotherm | π–π interaction | 197 |
7.2 Adsorption isotherm
Adsorption isotherms represent the relationship between the adsorbate concentration in the adsorbent phase and determine the amount of dissolved concentration at equilibrium.67 This simply shows that isotherms are used to predict the adsorption capacity of an adsorbent effectively, that is, the amounts of solutes the sorbent can adsorb on its surface per time.7 Furthermore, the isotherms have the tendency to show the distribution ratio of the antibiotics on the adsorbent's surface at various equilibrium concentrations. There are several adsorption isotherm models; including the Langmuir, Freundlich, Brunauer–Emmett–Teller (BET), Temkin, Frumkin, Harkins–Jura, Smith, and Dubinin–Radushkevich (D–R) isotherms. The Langmuir model shows that once the available adsorptive site is filled, which is termed monolayer covering via homogeneous energy distribution, the adsorption process is terminated. The Freundlich isotherm is suitable for the heterogeneous surface adsorption process; there is a tendency for more adsorption to occur even when the monolayer surface is covered.7,37 While the D–R isotherm model is a combination of both Freundlich and D–R isotherms, it accommodates the adsorption of antibiotics at low concentrations onto both homogeneous and heterogeneous surfaces. It helps determine the mechanism of the adsorption processes: either physisorption or chemisorption.67 The earlier studies have reported different adsorption isotherm and antibiotics kinetics, as presented in Table 6.8 Regeneration of adsorbents
The process of regenerating biomass-based adsorbents is termed the inverse of the adsorption process. Two principles are involved in the regeneration of adsorbents: adsorbate decomposition and adsorbate desorption.198 A high-quality adsorbent should have good recycling and reusable abilities for industrial purposes and very good regenerative ability, which reduces adsorbent costs.199,200 Magnetic biochar adsorbent regeneration prepared with the eucalyptus leaf residue used in the tetracycline adsorption revealed no significant band shifts in Fourier transform infrared spectra. Furthermore, there was no significant difference in the functional groups present on the regenerated adsorbent while the ash content slightly increased. Thermal and solvent regeneration techniques can be adapted to regenerate biomass used in the adsorption of antibiotic pollutants.8.1 Thermal regeneration
One effective means of achieving desorption is through thermal regeneration. It involves the decomposition of carbonized adsorbate at high temperature, thus making its molecules smaller than the pore size of biochar adsorbent, thus making desorption occur.201 It has been established that regeneration efficiency increases with increasing pyrolysis temperature. Regenerated biochar made from Enteromorpha prolifera at 80 °C, 150 °C, and 200 °C retained pyrene uptake of 35.00%, 45.00%, and 48.00% while the rates of regeneration of benzo[a]pyrene were 31.00%, 41.00%, and 40.00%, respectively.202 Removal of dissolved organic carbon can be effectively carried out using thermal treatment.2038.2 Solvent regeneration
Regeneration of solvent uses an equilibrium relationship among biomass, adsorbate, and solvent to break the equilibrium set up in the adsorption process by altering the pH value and temperature of the solvent in order to desorb the adsorbate from activated carbon.204 Solvent regeneration is a good technique for organic matter adsorbents with low boiling points and high concentrations.205 Solvent regeneration can be achieved by using inorganic chemicals such as inorganic acids like hydrochloric acid or soluble bases such as sodium hydroxide and other reagents for adsorbate removal, termed the acid–base regeneration technique or through the use of organic solvent. Organic solvent regeneration involves the extraction of adsorbed adsorbents on activated carbon from organic solvents such as methanol, benzene, or acetone. The adsorption involves the principle of integration and similarity.2069 Potential application of the adsorbents in engineering
The use of a biomass-based adsorbent for the construction of fixed beds helps remove organic substances in water and is usually employed to treat contaminated water.207 The most recent adsorber in a fixed bed for water treatment is constructed such that it can either be an open gravity filter or a closed pressure filter. Concrete or steel that is resistant to corrosion is often employed as a filter material. The most common arrangement of a fixed bed is to fill the inlet with a certain amount of biomass-based adsorbent with very high adsorption capacity, and the outlet also has a certain amount of adsorbent to reduce bed resistance and improve the bed resistance efficiency of mass transfer.208 A small sand bed is placed between the bottom end of the fixed bed and the activated carbon to get rid of the toner, as shown in Fig. 4. Using a fixed bed, the wastewater treatment plant process can be made continuous by placing multiple adsorbers in series, thus minimizing capacity loss. Two possible ways of connecting multiple adsorbers to a single fixed bed exist: series and parallel. The process involves dividing the total mass of the adsorbent into four parts, with three being used for treatment while the fourth adsorbent is used for regeneration. The time it takes for the first adsorber to be inactive and the mass transfer zone (MTZ) between the third and fourth adsorbers is termed t1. After a while, the adsorbent present in the second adsorber becomes completely saturated at equilibrium and the MTZ therefore leaves the second adsorber. The time at which this process occur is denoted t2. The processes continue to obtain t3 and t4. Ideally, the adsorbent bed continues to operate until the equilibrium load is attained and this involves continuous operation of the four adsorbers. Different adsorbers are used at various starting times. | ||
| Fig. 4 Typical water treatment fixed-bed adsorbents: (a) corrosion-resistant pressure GAC filter made of steel and (b) rectangular gravity concrete filter.209 | ||
The advantage of parallel connections in fixed beds is that the total cross-sectional area increases with an increasing number of adsorbers. Therefore, this means that the multi-adsorber system can be adjusted based on the requirements of the water to be treated. A large amount of water to be treated can be suitably handled by this fixed bed. Factors such as humidity, gas velocity, temperature, side stream effect, pressure drop, and other factors affect the efficiency of the fixed bed in water treatment.
10 Concluding remarks and future perspectives
The global production of a high volume of agricultural wastes without conversion to sustainable products has been attributed to the cause of rural environmental pollution and to a certain degree, increasing the burden of rural agricultural production. By extension, the increase in sustainable products obtained from agricultural wastes can be used to effectively decrease the level of air pollution resulting from the incineration of agricultural wastes. Constructively, the production of biomass-based adsorbents has been postulated to play essential roles in water treatment operations, climate change, and environmental protection in general. Furthermore, global reports on the modification of biomass-based adsorbents for enhancing their adsorption capacities have received tremendous attention and encouragement. This has resulted in the drive for modifying agents that can further increase the adsorption capacity of biomass-based adsorbents. Conversely, attention has recently been drawn to the environmental problems connected with some chemical modifying agents as well as the cost implications and intricacy involved in the modification process. The deployment of agricultural wastes for bio-carbon is posited as the core universal competitive approach of green and sustainable technology. The spotlight is presently on carbon sequestration, resource cycling, energy saving, environmental waste management, emission reduction, and tackling the issue of climate change. Consequently, the future of biomass-based adsorbents should be organized according to the following perspective:(1) Technology for agricultural waste carbonization should be explored and advanced in order to promote the industrial development of biomass-based adsorbents.
(2) Highly efficient green modifying agents and modification processes should be developed that will be employed in the biological sorption process.
(3) Prospective research should be extended to the exploitation of biomass-based adsorbents in dealing with the engineering glitches of pollution scaling.
(4) Due to their merits allied with biomass-based adsorbents, agricultural wastes can be used in substituting for pricey commercial activated carbon for application in environmental protection.
(5) Unfortunately, there is little discussion on handling used adsorbents/biosorbents and sequestered antibiotics. As a result, it is unclear what happened to the residual solutions and the utilized sorbents (and where they were discharged) at the end of the experiments. This would also assist in determining whether or not such successful experiments and inquiries also constitute a risk to the environment.
(6) The majority of the literature reviews were conducted in a synthetic aqueous environment, which might restrict the potential of sorption processes owing to matrix effect(s) that can interfere with sorption in real-world practical systems. As a result, real-world applications and thorough assessments of potential impact interference on the sorption processes are necessary.
(7) To date, there has been only a little research on the use of machine learning to predict antibiotic adsorption onto various adsorbents/biosorbents. As a result, we propose using machine learning to estimate sorption capabilities/efficiencies in water/wastewater.
(8) We also propose employing the partition coefficient (PC) approach to conduct the ideal sorbent assessments, as this will aid in assessing the adsorbents' performance metrics in real applications. In a solid–liquid system, PC is defined as the ratio of qmax to equilibrium concentrations. Some biosorbents/adsorbents that have been found to be effective, based on qmax studies, may have decreased adsorption capacity in an ambient setting. As a result, performance metrics utilizing the PC approach are able to provide better assessments of the sorption capacities of different sorbents. This could also be used for comparing the effectiveness of these biosorbents/adsorbents.
Data availability
All data and materials used in this study are available within this article.Author contributions
All authors contributed equally.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors acknowledge their respective Universities for the platform to carry out this study. O. S. Bello acknowledges the support obtained from LAUTECH 2016 TET Fund Institution Based Research Intervention (TETFUND/DESS/UNI/OGBOMOSO/RP/VOL. IX).References
- D. Mangla, A. S. Annu and S. Ikram, J. Hazard. Mater., 2022, 425, 127946 CrossRef CAS PubMed.
- Z. Fang, J. Chen, X. Qiu, X. Qiu, W. Cheng and L. Zhu, Desalination, 2011, 268, 60–67 CrossRef CAS.
- S. I. Polianciuc, A. E. Gurzău, B. Kiss, M. G. Stefan and F. Loghin, Med. Pharm. Rep., 2020, 93, 231–240 Search PubMed.
- L. Karthik, G. Kumar, T. Keswani, A. Bhattacharyya, S. Sarath Chandar and K. V. Bhaskara Rao, PLoS One, 2014, 9(3), 1–13 CrossRef PubMed.
- A. Gulkowska, H. W. Leung, M. K. So, S. Taniyasu, N. Yamashita, L. W. Y. Yeung, B. J. Richardson, A. P. Lei, J. P. Giesy and P. K. S. Lam, Water Res., 2008, 42, 395–403 CrossRef CAS PubMed.
- K. A. Adegoke, O. O. Adesina, O. A. Okon-Akan, O. R. Adegoke, A. B. Olabintan, O. A. Ajala, H. Olagoke, N. W. Maxakato and O. S. Bello, Curr. Res. Green Sustainable Chem., 2022, 5, 100274 CrossRef CAS.
- K. A. Adegoke, A. O. Olagunju, T. C. Alagbada, O. C. Alao, M. O. Adesina, I. C. Afolabi, R. O. Adegoke and O. S. Bello, Water, Air, Soil Pollut., 2022, 233, 38 CrossRef CAS.
- A. O. Ibrahim, K. A. Adegoke, R. O. Adegoke, Y. A. AbdulWahab, V. B. Oyelami and M. O. Adesina, J. Mol. Liq., 2021, 333, 115593 CrossRef CAS.
- A. R. Mahmood, H. H. Al-Haideri and F. M. Hassan, Adv. Public Health., 2019, 1–10 CAS.
- T. Schwartz, W. Kohnen, B. Jansen and U. Obst, FEMS Microbiol. Ecol., 2003, 43, 325–335 CrossRef CAS PubMed.
- S. B. Levy and M. Bonnie, Nat. Med., 2004, 10, S122–S129 CrossRef CAS PubMed.
- A. Cassini, L. D. Högberg, D. Plachouras, A. Quattrocchi, A. Hoxha, G. S. Simonsen, M. Colomb-Cotinat, M. E. Kretzschmar, B. Devleesschauwer, M. Cecchini, D. A. Ouakrim, T. C. Oliveira, M. J. Struelens, C. Suetens, D. L. Monnet, R. Strauss, K. Mertens, T. Struyf, B. Catry, K. Latour, I. N. Ivanov, E. G. Dobreva, A. Tambic Andraševic, S. Soprek, A. Budimir, N. Paphitou, H. Žemlicková, S. Schytte Olsen, U. Wolff Sönksen, P. Märtin, M. Ivanova, O. Lyytikäinen, J. Jalava, B. Coignard, T. Eckmanns, M. Abu Sin, S. Haller, G. L. Daikos, A. Gikas, S. Tsiodras, F. Kontopidou, Á. Tóth, Á. Hajdu, Ó. Guólaugsson, K. G. Kristinsson, S. Murchan, K. Burns, P. Pezzotti, C. Gagliotti, U. Dumpis, A. Liuimiene, M. Perrin, M. A. Borg, S. C. de Greeff, J. C. Monen, M. B. Koek, P. Elstrøm, D. Zabicka, A. Deptula, W. Hryniewicz, M. Caniça, P. J. Nogueira, P. A. Fernandes, V. Manageiro, G. A. Popescu, R. I. Serban, E. Schréterová, S. Litvová, M. Štefkovicová, J. Kolman, I. Klavs, A. Korošec, B. Aracil, A. Asensio, M. Pérez-Vázquez, H. Billström, S. Larsson, J. S. Reilly, A. Johnson and S. Hopkins, Lancet Infect. Dis., 2019, 19, 56–66 CrossRef PubMed.
- C. Bouki, D. Venieri and E. Diamadopoulos, Ecotoxicol. Environ. Saf., 2013, 91, 1–9 CrossRef CAS PubMed.
- B. Li and T. Zhang, Environ. Sci. Technol., 2010, 44, 3468–3473 CrossRef CAS PubMed.
- A. S. Oberoi, Y. Jia, H. Zhang, S. K. Khanal and H. Lu, Environ. Sci. Technol., 2019, 53, 7234–7264 CrossRef CAS PubMed.
- M. Kumar, S. Jaiswal, K. K. Sodhi, P. Shree, D. K. Singh, P. K. Agrawal and P. Shukla, Environ. Int., 2019, 124, 448–461 CrossRef CAS PubMed.
- K. J. Choi, S. G. Kim and S. H. Kim, J. Hazard. Mater., 2008, 151, 38–43 CrossRef CAS PubMed.
- V. Diwan, A. J. Tamhankar, M. Aggarwal, S. Sen, R. K. Khandal and C. S. Lundborg, Curr. Sci., 2009, 97, 1752–1755 CAS.
- M. Malakootian, A. Nasiri and H. Mahdizadeh, Water Sci. Technol., 2018, 78, 2158–2170 CrossRef CAS PubMed.
- K. D. Burch, B. Han, J. Pichtel and T. Zubkov, Environ. Sci. Pollut. Res., 2019, 26, 6301–6310 CrossRef CAS PubMed.
- S. Sharma and A. Bhattacharya, Appl. Water Sci., 2017, 7, 1043–1067 CrossRef CAS.
- S. M. Ebrahimi, R. D. Reyhani, M. Asghari-JafarAbadi and Z. Fathifar, Environ. Evid., 2020, 9, 1–9 CrossRef.
- J. R. De Andrade, M. F. Oliveira, M. G. C. Da Silva and M. G. A. Vieira, Ind. Eng. Chem. Res., 2018, 57, 3103–3127 CrossRef CAS.
- D. Dolar, T. I. Zokić, K. Košutić, D. Ašperger and D. M. Pavlović, Environ. Sci. Pollut. Res., 2012, 19, 1033–1042 CrossRef CAS PubMed.
- M. B. Ahmed, J. L. Zhou, H. H. Ngo and W. Guo, Sci. Total Environ., 2015, 532, 112–126 CrossRef CAS PubMed.
- A. Choudhry, A. Sharma, T. A. Khan and S. A. Chaudhry, J. Water Process. Eng., 2021, 42, 102150 CrossRef.
- S. Purnell, J. Ebdon, A. Buck, M. Tupper and H. Taylor, Water Res., 2015, 73, 109–117 CrossRef CAS PubMed.
- C. A. Quist-Jensen, F. Macedonio and E. Drioli, Desalination, 2015, 364, 17–32 CrossRef CAS.
- H. Rondon, W. El-Cheikh, I. A. R. Boluarte, C. Y. Chang, S. Bagshaw, L. Farago, V. Jegatheesan and L. Shu, Bioresour. Technol., 2015, 183, 78–85 CrossRef CAS PubMed.
- Y. Ben, C. Fu, M. Hu, L. Liu, M. H. Wong and C. Zheng, Environ. Res., 2019, 169, 483–493 CrossRef CAS PubMed.
- K. Kümmerer, J. Antimicrob. Chemother., 2003, 52, 5–7 CrossRef PubMed.
- A. J. Ebele, M. Abou-Elwafa Abdallah and S. Harrad, Emerging Contam., 2017, 3, 1–16 CrossRef.
- P. Krasucka, B. Pan, Y. Sik Ok, D. Mohan, B. Sarkar and P. Oleszczuk, Chem. Eng. J., 2021, 405, 126926 CrossRef CAS.
- H. Shi, J. Ni, T. Zheng, X. Wang, C. Wu and Q. Wang, Environ. Chem. Lett., 2020, 18, 345–360 CrossRef CAS.
- F. Mansour, M. Al-Hindi, R. Yahfoufi, G. M. Ayoub and M. N. Ahmad, Rev. Environ. Sci. Biotechnol., 2018, 17, 109–145 CrossRef CAS.
- M. Östman, B. Björlenius, J. Fick and M. Tysklind, Sci. Total Environ., 2019, 649, 1117–1123 CrossRef PubMed.
- K. A. Adegoke and O. S. Bello, Water Resour. Ind., 2015, 12, 8–24 CrossRef.
- X. Xie, Z. Zhang, Y. Hu and H. Cheng, Chem. Eng. J., 2018, 334, 1242–1251 CrossRef CAS.
- I. Arslan-Alaton and S. Dogruel, J. Hazard. Mater., 2004, 112, 105–113 CrossRef CAS PubMed.
- J. Peng, X. Wang, F. Yin and G. Xu, Sci. Total Environ., 2019, 650, 2437–2445 CrossRef CAS PubMed.
- S. F. Yang, C. F. Lin, A. Yu-Chen Lin and P. K. Andy Hong, Water Res., 2011, 45, 3389–3397 CrossRef CAS PubMed.
- Y. Feng, L. Yang, J. Liu and B. E. Logan, Environ. Sci.: Water Res. Technol., 2016, 2, 800–831 RSC.
- M. Eddy, Wastewater Engineering: Treatment and Reuse, 4th edn, 2004 Search PubMed.
- A. R. Mareddy, Impacts on water environment, 2017 Search PubMed.
- P. Zhou, C. Su, B. Li and Y. Qian, J. Environ. Eng., 2006, 132, 129–136 CrossRef CAS.
- D. T. Sponza and P. Demirden, Sep. Purif. Technol., 2007, 56, 108–117 CrossRef CAS.
- M. C. M. van Loosdrecht, D. Eikelboom, A. Gjaltema, A. Mulder, L. Tijhuis and J. J. Heijnen, Water Sci. Technol., 1995, 32, 35–43 CrossRef CAS.
- C. Adams, Y. Wang, K. Loftin and M. Meyer, J. Environ. Eng., 2002, 128, 253–260 CrossRef CAS.
- H. Chen, B. Gao and H. Li, J. Hazard. Mater., 2015, 282, 201–207 CrossRef CAS PubMed.
- A. O. Ifebajo, A. A. Oladipo and M. Gazi, Environ. Chem. Lett., 2019, 17, 487–494 CrossRef CAS.
- A. Alahabadi, A. Hosseini-Bandegharaei, G. Moussavi, B. Amin, A. Rastegar, H. Karimi-Sani, M. Fattahi and M. Miri, J. Mol. Liq., 2017, 232, 367–381 CrossRef CAS.
- Y. Liu, X. Liu, W. Dong, L. Zhang, Q. Kong and W. Wang, Sci. Rep., 2017, 7(1), 12437 CrossRef PubMed.
- F. Chen, Q. Yang, J. Sun, F. Yao, S. Wang, Y. Wang, X. Wang, X. Li, C. Niu, D. Wang and G. Zeng, ACS Appl. Mater. Interfaces, 2016, 8, 32887–32900 CrossRef CAS PubMed.
- J. Di, J. Xia, M. Ji, B. Wang, S. Yin, Q. Zhang, Z. Chen and H. Li, Appl. Catal., B, 2016, 183, 254–262 CrossRef CAS.
- M. M. Huber, S. Canonica, G. Y. Park and U. Von Gunten, Environ. Sci. Technol., 2003, 37, 1016–1024 CrossRef CAS PubMed.
- E. Adamek, W. Baran and A. Sobczak, J. Hazard. Mater., 2016, 313, 147–158 CrossRef CAS PubMed.
- Y. Xia and Q. Dai, Chemosphere, 2018, 205, 215–222 CrossRef CAS PubMed.
- S. Chelliapan, T. Wilby and P. J. Sallis, Water Res., 2006, 40, 507–516 CrossRef CAS PubMed.
- I. Deǧirmentaş and N. Deveci, J. Biochem., 2004, 136, 177–182 CrossRef PubMed.
- S. Kim, P. Eichhorn, J. N. Jensen, A. S. Weber and D. S. Aga, Environ. Sci. Technol., 2005, 39, 5816–5823 CrossRef CAS PubMed.
- C. Zwiener and F. H. Frimmel, Sci. Total Environ., 2003, 309, 201–211 CrossRef CAS PubMed.
- W. L. Ma, R. Qi, Y. Zhang, J. Wang, C. Z. Liang and M. Yang, Biochem. Eng. J., 2009, 45, 30–34 CrossRef CAS.
- Y. Zhou, X. Liu, Y. Xiang, P. Wang, J. Zhang, F. Zhang, J. Wei, L. Luo, M. Lei and L. Tang, Bioresour. Technol., 2017, 245, 266–273 CrossRef CAS PubMed.
- Y. Xiang, Z. Xu, Y. Wei, Y. Zhou, X. Yang, Y. Yang, J. Yang, J. Zhang, L. Luo and Z. Zhou, J. Environ. Manage., 2019, 237, 128–138 CrossRef CAS PubMed.
- Z. W. Zeng, X. F. Tan, Y. G. Liu, S. R. Tian, G. M. Zeng, L. H. Jiang, S. B. Liu, J. Li, N. Liu and Z. H. Yin, Front. Chem., 2018, 6, 1–11 CrossRef PubMed.
- J. Bedia, M. Peñas-Garzón, A. Gómez-Avilés, J. Rodriguez and C. Belver, Chem. Eng. J., 2018, 333, 58–65 CrossRef CAS.
- J. O. Eniola, R. Kumar and M. A. Barakat, Environ. Sci. Pollut. Res., 2019, 26, 34775–34788 CrossRef CAS PubMed.
- H. M. Jang and E. Kan, Bioresour. Technol., 2019, 274, 162–172 CrossRef CAS PubMed.
- M. Stylianou, A. Christou, C. Michael, A. Agapiou, P. Papanastasiou and D. Fatta-Kassinos, J. Environ. Chem. Eng., 2021, 9, 105868 CrossRef CAS.
- X. R. Jing, Y. Y. Wang, W. J. Liu, Y. K. Wang and H. Jiang, Chem. Eng. J., 2014, 248, 168–174 CrossRef CAS.
- H. Li, J. Hu, L. Yao, Q. Shen, L. An and X. Wang, J. Hazard. Mater., 2020, 390, 122127 CrossRef CAS PubMed.
- H. Wang, X. Lou, Q. Hu and T. Sun, J. Mol. Liq., 2021, 325, 2–10 Search PubMed.
- H. M. Jang, S. Yoo, Y. K. Choi, S. Park and E. Kan, Bioresour. Technol., 2018, 259, 24–31 CrossRef CAS PubMed.
- L. ling Yu, W. Cao, S. chuan Wu, C. Yang and J. hua Cheng, Ecotoxicol. Environ. Saf., 2018, 164, 289–296 CrossRef PubMed.
- R. Cela-Dablanca, C. Nebot, L. R. López, D. Fernández-Calviño, M. Arias-Estévez, A. Núñez-Delgado, M. J. Fernández-Sanjurjo and E. Álvarez-Rodríguez, Processes, 2021, 9, 1–12 CrossRef.
- D. Naghipour, A. Amouei, K. T. Ghasemi and K. Taghavi, Desalin. Water Treat., 2020, 201, 219–227 CrossRef CAS.
- D. Balarak, A. Joghatayi, F. K. Mostafapour and H. Azarpira, Int. J. Life Sci. Pharma Res., 2017, 7, 1–8 Search PubMed.
- H. Mansouri, R. J. Carmona, A. Gomis-Berenguer, S. Souissi-Najar, A. Ouederni and C. O. Ania, J. Colloid Interface Sci., 2015, 449, 252–260 CrossRef CAS PubMed.
- G. Moussavi, A. Alahabadi, K. Yaghmaeian and M. Eskandari, Chem. Eng. J., 2013, 217, 119–128 CrossRef CAS.
- N. Sunsandee, P. Ramakul, S. Phatanasri and U. Pancharoen, Biotechnol. Rep., 2020, 27, e00488 CrossRef PubMed.
- D. Balarak and K. Chandrika, Int. J. Pharm. Invest., 2019, 9, 117–121 CrossRef CAS.
- S. K. Bajpai, M. Bajpai and N. Rai, Water SA, 2012, 38, 673–682 CrossRef CAS.
- F. Wang, B. Yang, H. Wang, Q. Song, F. Tan and Y. Cao, J. Mol. Liq., 2016, 222, 188–194 CrossRef CAS.
- İ. Alacabey, Molecules, 2022, 27, 1380 CrossRef PubMed.
- T. U. T. Dao, H. T. T. Nguyen, D. T. C. Nguyen, H. T. N. Le, H. T. T. Nguyen, S. T. Do, H. H. Loc, T. T. Nguyen, T. D. Nguyen and L. G. Bach, Cellul. Chem. Technol., 2020, 54, 811–819 CrossRef CAS.
- M. Elhag Elhussien, Adv. Biochem., 2017, 5, 89 CrossRef.
- A. Zhou, Y. Zhang, R. Li, X. Su and L. Zhang, Desalin. Water Treat., 2016, 57, 388–397 CAS.
- M. C. Tonucci, L. V. A. Gurgel and S. F. de Aquino, Ind. Crops Prod., 2015, 74, 111–121 CrossRef CAS.
- D. Balarak and F. Mostafapour, Br. J. Pharm. Res., 2016, 13, 1–14 CAS.
- L. Kurup, Water Sci. Technol., 2012, 65, 1531–1539 CrossRef CAS PubMed.
- A. Ashiq, N. M. Adassooriya, B. Sarkar, A. U. Rajapaksha, Y. S. Ok and M. Vithanage, J. Environ. Manage., 2019, 236, 428–435 CrossRef CAS PubMed.
- A. R. Olivera, Biosorption of pharmaceuticals from wastewater using Moringa oleífera as biosorbent, Master of double diploma with the National University of Missions, Engenharia Química, ESTiG – Dissertações de Mestrado Alunos, 2020.
- Y. Dai, Q. Sun, W. Wang, L. Lu, M. Liu, J. Li, S. Yang, Y. Sun, K. Zhang, J. Xu, W. Zheng, Z. Hu, Y. Yang, Y. Gao, Y. Chen, X. Zhang, F. Gao and Y. Zhang, Chemosphere, 2018, 211, 235–253 CrossRef CAS PubMed.
- H. T. Fan, L. Q. Shi, H. Shen, X. Chen and K. P. Xie, RSC Adv., 2016, 6, 109983–109991 RSC.
- H. Alidadi, M. Dolatabadi, M. Davoudi, F. Barjasteh-Askari, F. Jamali-Behnam and A. Hosseinzadeh, Process Saf. Environ. Prot., 2018, 117, 51–60 CrossRef CAS.
- M. A. Ahsan, M. T. Islam, C. Hernandez, E. Castro, S. K. Katla, H. Kim, Y. Lin, M. L. Curry, J. Gardea-Torresdey and J. C. Noveron, J. Environ. Chem. Eng., 2018, 6, 4329–4338 CrossRef CAS.
- W. Stuart, L. Shearer, S. Pap and S. W. Gibb, J. Environ. Chem. Eng., 2022, 10, 108106 CrossRef.
- M. C. Ndoun, H. A. Elliott, H. E. Preisendanz, C. F. Williams, A. Knopf and J. E. Watson, Biochar, 2021, 3, 89–104 CrossRef CAS.
- K. Herrera, L. F. Morales, N. A. Tarazona, R. Aguado and J. F. Saldarriaga, ACS Omega, 2022, 7, 7625–7637 CrossRef CAS PubMed.
- C. C. O. Alves, A. S. Franca and L. S. Oliveira, BioMed Res. Int., 2013, 2013, 978256 CrossRef PubMed.
- D. Balarak, A. H. Mahvi, S. Shahbaksh, M. A. Wahab and A. Abdala, Nanomaterials, 2021, 11, 3281 CrossRef CAS PubMed.
- W. Xing, Q. Liu, J. Wang, S. Xia, L. Ma, R. Lu, Y. Zhang, Y. Huang and G. Wu, Nanomaterials, 2021, 11, 2950 CrossRef CAS PubMed.
- A. Chen, J. Pang, X. Wei, B. Chen and Y. Xie, Environ. Sci. Pollut. Res., 2021, 28, 27398–27410 CrossRef CAS PubMed.
- R. Chitongo, B. O. Opeolu and O. S. Olatunji, Clean: Soil, Air, Water, 2018, 47, 1800077 Search PubMed.
- J. Yang, G. Ji, Y. Gao, W. Fu, M. Irfan, L. Mu, Y. Zhang and A. Li, J. Cleaner Prod., 2020, 264, 121516 CrossRef CAS.
- J. C. Yuchen Liu, X. Zhu, F. Qian and S. Zhang, RSC Adv., 2014, 4, 63620–63626 RSC.
- E. Cho, J. Kang, J. Moon, B. Um, C. Lee, S. Jeong and S. Park, J. Environ. Chem. Eng., 2021, 9, 106343 CrossRef CAS.
- Y. Yu, Q. Huang, J. Cui, K. Zhang, C. Tang and X. Peng, Springer, 2011, vol. 399, pp. 891–902.
- K. Zoroufchi Benis, S. Minaei, J. Soltan and K. N. McPhedran, Chem. Eng. Res. Des., 2022, 187, 140–150 CrossRef CAS.
- F. M. Mpatani, A. A. Aryee, A. N. Kani, R. Han, Z. Li, E. Dovi and L. Qu, J. Cleaner Prod., 2021, 308, 127359 CrossRef CAS.
- A. A. Aryee, F. M. Mpatani, E. Dovi, Q. Li, J. Wang, R. Han, Z. Li and L. Qu, J. Cleaner Prod., 2021, 329, 129722 CrossRef CAS.
- C. Cheng, J. Zhang, C. Zhang, H. Liu and W. Liu, Desalin. Water Treat., 2014, 52, 5401–5412 CrossRef CAS.
- M. G. Lee and S. K. Kam, Korean Chem. Eng. Res., 2018, 56, 568–576 CAS.
- A. A. Mohammed, T. J. Al-Musawi, S. L. Kareem, M. Zarrabi and A. M. Al-Ma’abreh, Arabian J. Chem., 2019, 13, 4629–4643 CrossRef.
- H. R. Pouretedal and N. Sadegh, J. Water Process. Eng., 2014, 1, 64–73 CrossRef.
- M. Xie, W. Chen, Z. Xu, S. Zheng and D. Zhu, Environ. Pollut., 2014, 186, 187–194 CrossRef CAS PubMed.
- L. Zhang, L. Tong, P. Zhu, P. Huang, Z. Tan, F. Qin, W. Shi, M. Wang, H. Nie, G. Yan and H. Huang, Water Sci. Technol., 2018, 78, 1336–1347 CrossRef CAS PubMed.
- C. Li, X. Zhu, H. He, Y. Fang, H. Dong, J. Lü, J. Li and Y. Li, J. Mol. Liq., 2019, 274, 353–361 CrossRef.
- P. Liu, W. J. Liu, H. Jiang, J. J. Chen, W. W. Li and H. Q. Yu, Bioresour. Technol., 2012, 121, 235–240 CrossRef CAS PubMed.
- C. V. Gómez-Pacheco, J. Rivera-Utrilla, M. Sánchez-Polo and J. J. López-Peñalver, J. Hazard. Mater., 2012, 217–218, 76–84 CrossRef PubMed.
- D. Huang, X. Wang, C. Zhang, G. Zeng, Z. Peng, J. Zhou, M. Cheng, R. Wang, Z. Hu and X. Qin, Chemosphere, 2017, 186, 414–421 CrossRef CAS PubMed.
- Y. Chen, J. Shi, Q. Du, H. Zhang and Y. Cui, RSC Adv., 2019, 9, 14143–14153 RSC.
- Y. Xiang, X. Yang, Z. Xu, W. Hu, Y. Zhou, Z. Wan, Y. Yang, Y. Wei, J. Yang and D. C. W. Tsang, Sci. Total Environ., 2020, 709, 136079 CrossRef CAS PubMed.
- D. Cheng, H. H. Ngo, W. Guo, S. W. Chang, D. D. Nguyen, X. Zhang, S. Varjani and Y. Liu, Sci. Total Environ., 2020, 720, 137662 CrossRef CAS PubMed.
- T. W. Tzeng, Y. T. Liu, Y. Deng, Y. C. Hsieh, C. C. Tan, S. L. Wang, S. T. Huang and Y. M. Tzou, Int. J. Environ. Sci. Technol., 2016, 13, 973–984 CrossRef CAS.
- N. Rattanachueskul, A. Saning, S. Kaowphong, N. Chumha and L. Chuenchom, Bioresour. Technol., 2017, 226, 164–172 CrossRef CAS PubMed.
- X. Zhu, Y. Liu, G. Luo, F. Qian, S. Zhang and J. Chen, Environ. Sci. Technol., 2014, 48, 5840–5848 CrossRef CAS PubMed.
- J. P. Simonin, Chem. Eng. J., 2016, 300, 254–263 CrossRef CAS.
- P. Saha, S. Chowdhury, S. Gupta and I. Kumar, Chem. Eng. J., 2010, 165, 874–882 CrossRef CAS.
- K. A. Guimarães Gusmão, L. V. Alves Gurgel, T. M. Sacramento Melo and L. F. Gil, Dyes Pigm., 2012, 92, 967–974 CrossRef.
- B. Ohs, M. Krödel and M. Wessling, Sep. Purif. Technol., 2018, 204, 13–20 CrossRef CAS.
- M. Doǧan, H. Abak and M. Alkan, J. Hazard. Mater., 2009, 164, 172–181 CrossRef PubMed.
- H. Qiu, L. Lv, B. C. Pan, Q. J. Zhang, W. M. Zhang and Q. X. Zhang, J. Zhejiang Univ., Sci., A, 2009, 10, 716–724 CrossRef CAS.
- J. Chen, X. Shi, Y. Zhan, X. Qiu, Y. Du and H. Deng, Appl. Surf. Sci., 2017, 397, 133–143 CrossRef CAS.
- D. H. K. Reddy, Y. Harinath, K. Seshaiah and A. V. R. Reddy, Chem. Eng. J., 2010, 162, 626–634 CrossRef CAS.
- K. Wang, H. Ren, Z. Wang, S. Ma, J. Wei, W. Ke and Y. Guo, Nat. Resour. Res., 2021, 30, 4597–4620 CrossRef CAS.
- R. Serna-Guerrero and A. Sayari, Chem. Eng. J., 2010, 161, 182–190 CrossRef CAS.
- M. A. Malana, R. B. Qureshi and M. N. Ashiq, Chem. Eng. J., 2011, 172, 721–727 CrossRef CAS.
- M. Benjelloun, Y. Miyah, G. Akdemir Evrendilek, F. Zerrouq and S. Lairini, Arabian J. Chem., 2021, 14, 103031 CrossRef CAS.
- J. A. Heimberg, K. J. Wahl, I. L. Singer and A. Erdemir, Appl. Phys. Lett., 2001, 78, 2449–2451 CrossRef CAS.
- W. Rudzinski and T. Panczyk, J. Phys. Chem. B, 2000, 104, 9149–9162 CrossRef CAS.
- N. Y. Mezenner and A. Bensmaili, Chem. Eng. J., 2009, 147, 87–96 CrossRef CAS.
- S. S. Baral, N. Das, G. Roy Chaudhury and S. N. Das, J. Hazard. Mater., 2009, 171, 358–369 CrossRef CAS PubMed.
- S. M. Ahmed, M. R. Taha and O. M. E. Taha, Nanotechnol. Environ. Eng., 2018, 3(1), 4 CrossRef.
- Y. dong Huang, Appl. Surf. Sci., 2019, 469, 564–565 CrossRef.
- S. Afroze and T. K. Sen, Water, Air, Soil Pollut., 2018, 229, 225 CrossRef.
- S. Chowdhury and P. Saha, Chem. Eng. J., 2010, 164, 168–177 CrossRef CAS.
- Z. Y. Yao, J. H. Qi and L. H. Wang, J. Hazard. Mater., 2010, 174, 137–143 CrossRef CAS PubMed.
- W. J. Weber and J. C. Morris, J. Sanit. Eng. Div., 1963, 89, 31–59 CrossRef.
- C. Aharoni and M. Ungarish, J. Chem. Soc., Faraday Trans. 1, 1977, 73, 456–464 RSC.
- I. I. Laskar and Z. Hashisho, Sep. Purif. Technol., 2020, 241, 241 CrossRef.
- K. L. Tan and B. H. Hameed, J. Taiwan Inst. Chem. Eng., 2017, 74, 25–48 CrossRef CAS.
- K. H. Chu, Chem. Eng. J., 2020, 398, 122513 CrossRef.
- I. Langmuir, J. Am. Chem. Soc., 1917, 39, 1848–1906 CrossRef CAS.
- H. M. F. Freundlich, J. Phys. Chem., 1906, 57, 385–471 CAS.
- G. S. Bohart and E. Q. Adams, J. Am. Chem. Soc., 1920, 42, 523–544 CrossRef.
- S. Brunauer, P. H. Emmett and E. Teller, J. Am. Chem. Soc., 1938, 60, 309–319 CrossRef CAS.
- M. M. Dubinin, Dokl. Akad. Nauk SSSR, 1947, 55, 327–329 CAS.
- R. Saadi, Z. Saadi, R. Fazaeli and N. E. Fard, Korean J. Chem. Eng., 2015, 32, 787–799 CrossRef CAS.
- T. L. Hill, Adv. Catal., 1952, 4, 211–258 CAS.
- A. R. Khan, R. Ataullah and A. Al-Haddad, J. Colloid Interface Sci., 1997, 194, 154–165 CrossRef CAS PubMed.
- R. A. Koble and T. E. Corrigan, Ind. Eng. Chem., 1952, 44, 383–387 CrossRef CAS.
- W. G. McMILLAN and E. TELLER, J. Phys. Colloid Chem., 1951, 55, 17–20 CrossRef CAS PubMed.
- A. L. Myers and J. M. Prausnitz, AIChE J., 1965, 11, 121–127 CrossRef CAS.
- O. Redlich and D. L. Peterson, J. Phys. Chem., 1959, 63, 1024 CrossRef CAS.
- R. Sips, J. Chem. Phys., 1948, 16, 490–495 CrossRef CAS.
- V. Tempkin and M. I. Pyzhev, Acta Physicochim. URSS, 1940, 12, 327–356 Search PubMed.
- J. Toth, Acta Chim. Acad. Sci. Hung., 1971, 69, 311–317 CAS.
- S. Das, A. Barui and A. Adak, J. Water Process. Eng., 2020, 37, 101497 CrossRef.
- M. J. Ahmed and B. H. Hameed, Ecotoxicol. Environ. Saf., 2018, 149, 257–266 CrossRef CAS PubMed.
- M. Wahab, M. Zahoor, S. Muhammad Salman, A. W. Kamran, S. Naz, J. Burlakovs, A. Kallistova, N. Pimenov and I. Zekker, Water, 2021, 13, 1969 CrossRef CAS.
- S. Y. Elovich and O. G. Larionov, Bull. Acad. Sci. USSR Div. Chem. Sci., 1962, 11, 198–203 CrossRef.
- S. Kaur, S. Rani, R. K. Mahajan, M. Asif and V. K. Gupta, J. Ind. Eng. Chem., 2015, 22, 19–27 CrossRef CAS.
- V. J. Inglezakis, Microporous Mesoporous Mater., 2007, 103, 72–81 CrossRef CAS.
- G. Alberti, V. Amendola, M. Pesavento and R. Biesuz, Coord. Chem. Rev., 2012, 256, 28–45 CrossRef CAS.
- Q. Hu and Z. Zhang, J. Mol. Liq., 2019, 277, 646–648 CrossRef CAS.
- T. Shahnaz, V. Vishnu Priyan, S. Pandian and S. Narayanasamy, Environ. Pollut., 2021, 268, 115494 CrossRef CAS PubMed.
- O. S. Bello, K. A. Adegoke, A. A. Inyinbor and A. O. Dada, Water Environ. Res., 2021, 93, 2308–2328 CrossRef CAS PubMed.
- L. Largitte and R. Pasquier, Chem. Eng. Res. Des., 2016, 109, 495–504 CrossRef CAS.
- C. S. Patil, D. B. Gunjal, V. M. Naik, N. S. Harale, S. D. Jagadale, A. N. Kadam, P. S. Patil, G. B. Kolekar and A. H. Gore, J. Cleaner Prod., 2019, 206, 407–418 CrossRef CAS.
- I. Cabrita, B. Ruiz, A. S. Mestre, I. M. Fonseca, A. P. Carvalho and C. O. Ania, Chem. Eng. J., 2010, 163, 249–255 CrossRef CAS.
- H. A. Al-Yousef, B. M. Alotaibi, F. Aouaini, L. Sellaoui and A. Bonilla-Petriciolet, J. Mol. Liq., 2021, 331, 115697 CrossRef CAS.
- Y. Zhao, W. Li, J. Liu, K. Huang, C. Wu, H. Shao, H. Chen and X. Liu, Chem. Eng. J., 2017, 326, 745–755 CrossRef CAS.
- M. A. Chayid and M. J. Ahmed, J. Environ. Chem. Eng., 2015, 3, 1592–1601 CrossRef CAS.
- G. A. Haghighat, M. H. Saghi, I. Anastopoulos, A. Javid, A. Roudbari, S. S. Talebi, S. K. Ghadiri, D. A. Giannakoudakis and M. Shams, J. Mol. Liq., 2020, 313, 113523 CrossRef CAS.
- A. U. Rajapaksha, M. Vithanage, M. Ahmad, D. C. Seo, J. S. Cho, S. E. Lee, S. S. Lee and Y. S. Ok, J. Hazard. Mater., 2015, 290, 43–50 CrossRef CAS PubMed.
- A. C. Martins, O. Pezoti, A. L. Cazetta, K. C. Bedin, D. A. S. Yamazaki, G. F. G. Bandoch, T. Asefa, J. V. Visentainer and V. C. Almeida, Chem. Eng. J., 2015, 260, 291–299 CrossRef CAS.
- D. L. C. Rodrigues, F. M. Machado, A. G. Osório, C. F. de Azevedo, E. C. Lima, R. S. da Silva, D. R. Lima and F. M. Gonçalves, Environ. Sci. Pollut. Res., 2020, 27, 41394–41404 CrossRef CAS PubMed.
- E. Angulo, L. Bula, I. Mercado, A. Montaño and N. Cubillán, Bioresour. Technol., 2018, 257, 17–22 CrossRef CAS PubMed.
- S. bozorginia, J. Jaafari, K. Taghavi, S. D. Ashraf, E. Roohbakhsh and D. Naghipour, Int. J. Environ. Anal. Chem., 2021, 1–15 CrossRef.
- E. Azamateslamtalab, M. Madani, B. Ramavandi and R. Mohammadi, Environ. Sci. Pollut. Res., 2020, 27, 35792–35801 CrossRef CAS PubMed.
- N. Genç and E. C. Dogan, Desalin. Water Treat., 2015, 53, 785–793 CrossRef.
- A. A. Kadam, B. Sharma, G. D. Saratale, R. G. Saratale, G. S. Ghodake, B. M. Mistry, S. K. Shinde, S. C. Jee and J. S. Sung, Cellulose, 2020, 27, 3301–3318 CrossRef CAS.
- H. R. Pouretedal and N. Sadegh, J. Water Process. Eng., 2014, 1, 64–73 CrossRef.
- M. Yuan, C. Li, B. Zhang, J. Wang, J. Zhu, J. Ji and Y. Ma, Chemosphere, 2021, 280, 130877 CrossRef CAS PubMed.
- Y. Wang, W. Bin Jiao, J. T. Wang, G. F. Liu, H. L. Cao and J. Lü, Bioresour. Technol., 2019, 277, 128–135 CrossRef CAS PubMed.
- V. M. Nurchi, M. Crespo-Alonso, M. I. Pilo, N. Spano, G. Sanna and R. Toniolo, Arabian J. Chem., 2019, 12, 1141–1147 CrossRef CAS.
- Y. Jia, Q. Zong, M. Zhang, Z. M. Wang and L. H. Zhang, Bull. Chin. Ceram. Soc., 2016, 35, 815–818 Search PubMed.
- J. Ifthikar, X. Jiao, A. Ngambia, T. Wang, A. Khan, A. Jawad, Q. Xue, L. Liu and Z. Chen, Bioresour. Technol., 2018, 262, 22–31 CrossRef CAS PubMed.
- S. ye Wang, Y. kui Tang, C. Chen, J. tao Wu, Z. Huang, Y. yuan Mo, K. xuan Zhang and J. bo Chen, Bioresour. Technol., 2015, 186, 360–364 CrossRef PubMed.
- G. Prasannamedha, P. S. Kumar, R. Mehala, T. J. Sharumitha and D. Surendhar, J. Hazard. Mater., 2021, 407, 124825 CrossRef CAS PubMed.
- K. Qiao, W. Tian, J. Bai, J. Dong, J. Zhao, X. Gong and S. Liu, Ecotoxicol. Environ. Saf., 2018, 149, 80–87 CrossRef CAS PubMed.
- X. He, M. Elkouz, M. Inyang, E. Dickenson and E. C. Wert, J. Hazard. Mater., 2017, 326, 101–109 CrossRef CAS PubMed.
- Y. Dai, N. Zhang, C. Xing, Q. Cui and Q. Sun, Chemosphere, 2019, 223, 12–27 CrossRef CAS PubMed.
- E. Gagliano, M. Sgroi, P. P. Falciglia, F. G. A. Vagliasindi and P. Roccaro, Water Res., 2020, 171, 115381 CrossRef CAS PubMed.
- L. Cseri, M. Razali, P. Pogany and G. Szekely, Green Chemistry: An Inclusive Approach, Elsevier, 2018, pp. 513–553 Search PubMed.
- P. N. Omo-Okoro, A. P. Daso and J. O. Okonkwo, Environ. Technol. Innovation, 2018, 9, 100–114 CrossRef.
- Y. Shao, H. Zhang and Y. Yan, Chem. Eng. J., 2013, 225, 481–488 CrossRef CAS.
- E. Worch, Adsorption Technology in Water Treatment: Fundamentals, Processes, and Modeling, De Gruyter, 2021 Search PubMed.
Footnote |
| † Share the same second authorship. |
| This journal is © The Royal Society of Chemistry 2023 |