Open Access Article
Open Access ArticleSynthetic aspects of 1,4- and 1,5-benzodiazepines using o-phenylenediamine: a study of past quinquennial
Sunita Teli
,
Pankaj Teli
,
Shivani Soni
,
Nusrat Sahiba
and
Shikha Agarwal
 *
*
Synthetic Organic Chemistry Laboratory, Department of Chemistry, MLSU, Udaipur-313001, Rajasthan, India. E-mail: shikhaagarwal@mlsu.ac.in
First published on 25th January 2023
Abstract
Benzodiazepines, seven-membered heterocyclic compounds having two nitrogen atoms at different positions, are ruling scaffolds in the area of pharmaceutical industry. They act as cardinal moieties in organic synthesis as well as in medicinal chemistry. Among the different benzodiazepines, 1,4- and 1,5-benzodiazepines play a far-reaching role in the field of biological activities such as anticonvulsion, anti-anxiety, sedation, and hypnotics. In the past few decades, researchers have conducted a lot of work on these moieties and developed broad, valuable, and significant approaches for their synthesis. In this review article, we recapitulate the systematic synthetic strategies of 1,4- and 1,5-benzodiazepines using o-phenylenediamine as a precursor over the past five years (2018–2022). This article will be helpful for scientists and researchers to examine and explore novel and efficient methods for the synthesis of these biologically active moieties.
1. Introduction
Heterocyclic moieties have gained tremendous interest due to their use in our daily life and their pivotal role in drug development and discovery. Among the heterocycles, benzodiazepines (BZDs) are one of the most significant and highly examined aromatic heterocycle, containing a benzene ring and a diazepine ring.In the past few decades, BZDs have played a remarkable role as heterocyclic moieties in the field of organic synthesis and medicine due to their wide range of applications in the pharmaceutical field, which include various types of activities such as antidepressant, anticonvulsant, muscle relaxant, anxiolytic, antiepileptic, hypnotic and sedative functions.1–9 The first commercially identified benzodiazepine drug is chlordiazepoxide/librium, which was discovered by Hoffmann-La Roche chemist Leo Sternbach and his colleague in 1955, but by 1960, it was marketed as Librium. In 1963, one more benzodiazepine drug, valium, was developed by Hoffmann-La Roche, which is a well-known drug named “diazepam”.
There are various types of BZDs, namely, 1,2-BZDs, 1,3-BZDs, 1,4-BZDs, 1,5-BZDs, 2,3-BZDs, and 2,4-BZDs, but widely used BZDs are 1,4- and 1,5-BZDs.10 In the nomenclature of BZDs, the nitrogen ring closest to the benzene ring is given priority,11 so the numbering of various BZDs in the simplest form is given in Fig. 1.
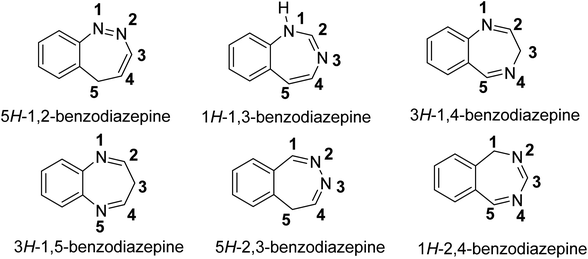 | ||
| Fig. 1 Structure of different BZDs.12 | ||
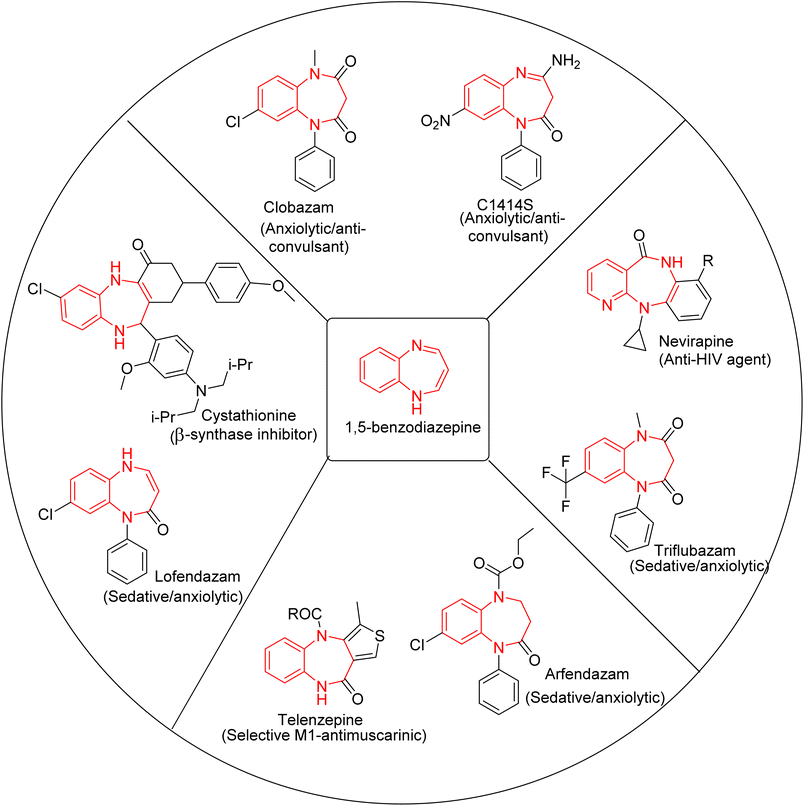
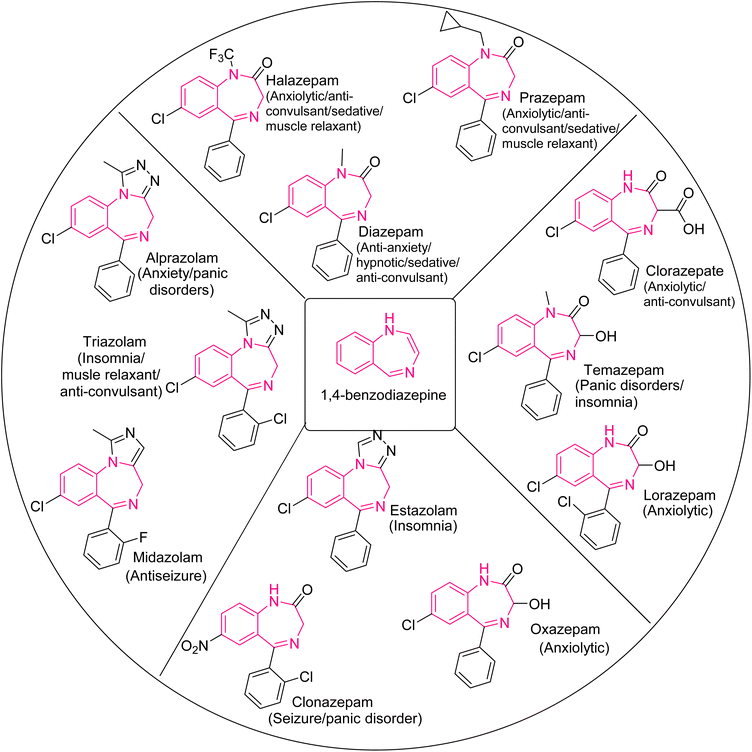
1,5-BZDs possess tremendous activities such as amnesia,13 anticonvulsant,14 hypnotics,15 analgesic,16 antimalarial,17 antifungal,18 antibacterial,19,20 anti-viral,21 anti-HIV,22 anti-inflammatory,23 antitumor,24 and phytotoxic functions.25 There are several commercially approved drugs based on 1,5-BZDs such as clobazam, lofendazam, arfendazam, triflubazam, CP-1414S, cystathionine, nevirapine, and telenzepine (Fig. 2). 1,4-BZDs also possess various activities in the biological and pharmacological field.21,26,27 Diazepam, halazepam, prazepam, alprazolam, triazolam, midazolam, clorazepate, temazepam, lorazepam, estazolam, oxazepam and clonazepam are clinically used drugs containing 1,4-benzodiazepine rings, illustrated in Fig. 3.28 The 1,4- and 1,5-BZD skeletons have been synthesized by various protocols such as metal-catalyzed tandem reactions, redox-neutral [5 + 2] annulation with 2-aminobenzaldehyde, isocyanide-based multicomponent reactions, cycloaddition reactions and cyclocondensation reactions.29–44
Among the diverse synthetic strategies, the most common synthesis of BZDs involves the condensation of o-phenylenediamine (OPD) with various carbonyl compounds in the presence of various catalysts or catalyst-free systems. OPD is an aromatic diamine, which is widely used as an important precursor to many heterocyclic compounds such as benzimidazoles,45,46 tetrahydro BZDs47 and some other scaffolds. Over the past few years, many improvements have been made in the synthesis of BZDs with respect to reaction efficiency, purity, eco-friendly process, ambient reaction conditions.48,49
Several reviews have been published28,50–52 on the synthesis of BZDs but they cannot encapsulate the latest research work done on this moiety. Singh et al.50 presented synthetic approaches for the synthesis of 1,5-BZDs from 2013 to 2018. The present review displays recent methodologies for the synthesis of 1,5-BZDs as well as 1,4-BZDs using OPD as a precursor from 2018 to 2022. The review comprises mainly two parts: synthesis of 1,5-BZDs and synthesis of 1,4-BZDs using OPD. Further, the synthesis of 1,5-BZDs is divided into three sections: a two-component reaction, a three-component reaction and a four-component reaction based on the type of reactants. The review will aid the scientific community toward developing systematic and rational approaches for the synthesis of 1,4- and 1,5-BZDs.
2. Synthetic approaches of BZDs
OPD easily gets condensed with various carbonyl compounds, and is involved in various multicomponent green reactions for the synthesis of N-containing heterocycles.53 Condensation of OPD with various substrates such as β-haloketones, α,β-unsaturated carbonyl compounds or β-dicarbonyl compounds produces BZDs. This review aims to explore the various current approaches of 1,5- and 1,4-BZD synthesis.2.1. 1,5-BZDs
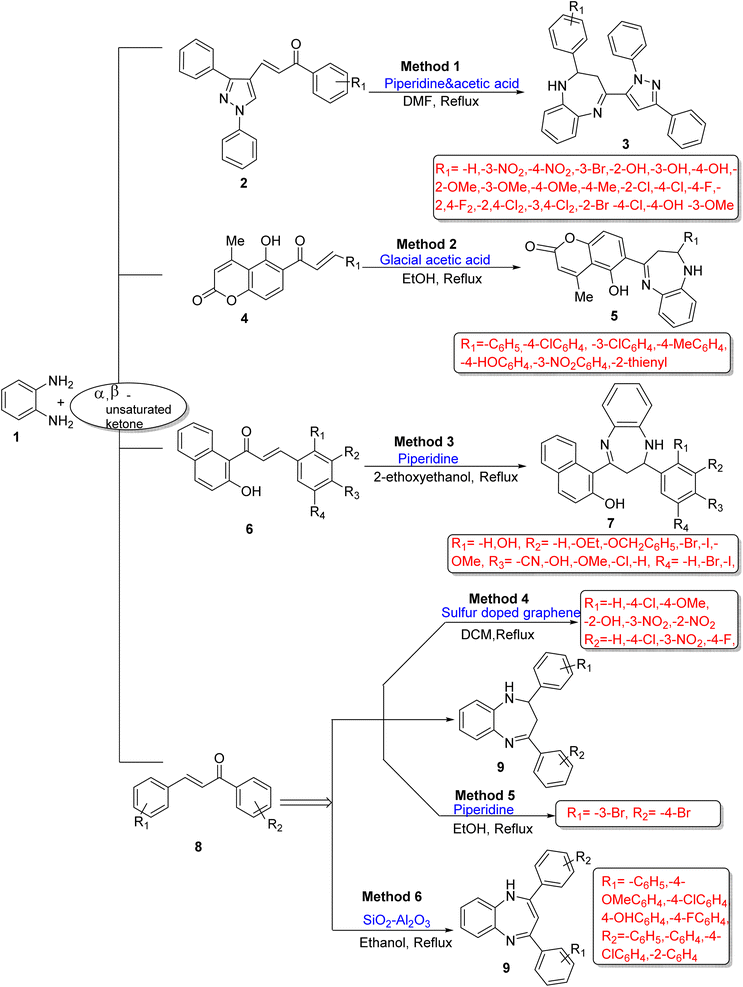
The growth of 1,5-BZDs in organic synthesis and industrial fields shows their importance and versatility. This literature categorized the synthetic strategies of 1,5-BZDs into three parts on the basis of the number of components taking part in the synthesis, namely, two-component, three-component and four-component reactions for the synthesis of BZDs.2.1.1.1. Using OPD(OPD) and α,β-unsaturated ketones. Desai et al.54 developed an efficient methodology for the synthesis of pyrazole-bearing BZDs (3) using OPD (1) and 3-(1,3-diphenyl-1H-pyrazol-4-yl)-1-phenylprop-2-en-1-ones (2) with piperidine and acetic acid as catalysts. The desired products were obtained in 8–10 h using a DMF solvent under reflux conditions. Twenty derivatives were synthesized in 56–79% yields. The designed pathway did not give satisfactory yields of products and consumed long reaction times (8–10 h), but has a wide substrate scope, and easily available solvent and catalyst were used (Scheme 1; Method 1).
Toan et al.55 synthesized some 1H-1,5-BZDs (5) containing a chromene ring using OPD (1) and α,β-unsaturated ketone (5-hydroxy-4-methyl-6-[(2E)-3′-(aryl)-prop-2′-enoyl]-2H-chromen-2-ones) (4) in the presence of glacial acetic acid as a catalyst and ethanol as a solvent. This ketone (4) was prepared from 6-acetyl-5-hydroxy-4-methylcoumarin and aldehyde with piperidine/TEA (triethylamine)/pyridine as a catalyst in ethanol. This protocol showed a range of product yields (39–67%) and reaction time variations with different substituted aldehydes (Scheme 1; Method 2).
Kottapalle and Shinde56 developed a method for the synthesis of BZDs (7) using chalcone (6) and OPD (1) with piperidine in 2-ethoxyethanol. The authors also examined some other organic solvents such as ethanol, DMF, DCM (dichloromethane), acetic acid and THF (tetrahydrofuran) in 55–71% yield, but 2-ethoxyethanol at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio gave 88% yield in 3 h reaction time (Scheme 1; Method 3).
2 molar ratio gave 88% yield in 3 h reaction time (Scheme 1; Method 3).
Chermahini et al.57 developed an efficient and robust carbocatalyst (sulfur-doped graphene) to catalyse the synthesis of 1,5-BZDs (9) using OPD (1) and chalcone (8). The reaction occurred in the DCM solvent using 8 mol% catalyst at a reflux temperature in 6 h with high 85% yield. The authors also examined other organic solvents such as CHCl3, THF, H2O, DMF, EtOH, and hexane, but DCM was found the best. The catalyst was reused up to ten times. The protocol has many advantages such as easy workup process, mild reaction conditions and low catalyst loading (Scheme 1; Method 4).
Pathade and Jagdale58 fabricated a significant, facile method for the synthesis of 2,3-dihydro-1H-1,5-BZDs (9) using OPD (1) and chalcone (8) in ethanol as reaction media and piperidine as a catalyst. The product 2-(3-bromophenyl)-4-(4-bromophenyl)-2,3-dihydro-1H-1,5-benzodiazepine (9) was obtained in a 6 h reaction time period at reflux temperature in 85% yield. The authors also found that the product has a high energy gap between HOMO and LUMO, which explains eventual charge transfer interaction within the molecule (Scheme 1; Method 5).
Tayde et al.59 developed an efficient method for the synthesis of 1,5-BZDs (9) using OPD (1) and chalcone (8) with SiO2–Al2O3 (silica-alumina) as a binary mixed metal oxide catalyst and ethanol as a solvent in 93% yield at 80 °C in 60 min. The authors also examined other solvents such as MeOH, CH2Cl2, CH3CN, 1,4-dioxane and solvent-free conditions but not obtained satisfactory yields. The catalyst (SiO2–Al2O3) was prepared by a hydrothermal method at 150 °C and reused for three times with significant loss in the catalytic activity (Scheme 1; Method 6).
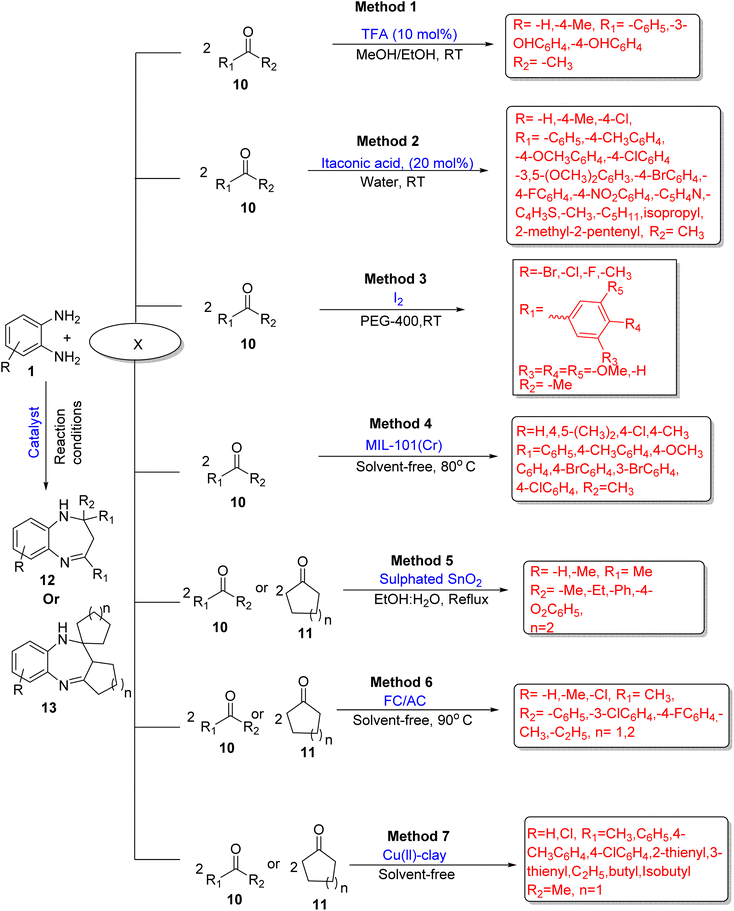
2.1.1.2. OPD and various ketones (cyclic and acyclic). Caiana and co-authors60 formulated a method for the synthesis of 1,5-BZDs (12) using OPD (1) and various acetophenones (10) as starting materials and TFA (trifluoroacetic acid) as a catalyst, and 91–95% yield was obtained using methanol and ethanol solvents at RT. The yields were not satisfactory from other examined solvents such as DMF (dimethylformamide), CH3CN (acetonitrile), and DMSO (dimethyl sulfoxide). The position of hydroxy group on acetophenone also affected the yield and reaction time. Meta-hydroxyacetophenone gave slightly high yields in a short reaction time period as compared to ortho- and para-hydroxyacetophenone (Scheme 2; Method 1).
 | ||
| Scheme 2 Two-component reactions of OPD with various ketones for the synthesis of 1,5-BZDs (‘X’ denotes the second component of the reactions). *n is the number of carbon atoms. | ||
Tamuli and co-workers61 developed an eco-friendly methodology for the synthesis of 1,5-BZDs (12) using itaconic acid as a green catalyst from OPD (1) and ketones (10) in water solvent at RT. The authors examined a large substrate scope and prepared 30 derivatives in good to excellent yields (61–95%). It was found that 20 mol% of catalyst gave 95% yields in half an hour at RT. Green solvent, easily available material, recyclable catalyst (five times), mild conditions, short reaction time, easy workup, excellent yield, wide substrate scope and gram scale synthesis are among the several advantages of this green protocol (Scheme 2; Method 2).
Peerzade et al.62 devised an efficient green method for the synthesis of 1,5-BZDs (12) using OPD (1) and substituted acetophenones (10) with I2 as a catalyst and PEG-400 (polyethylene glycol) as a solvent. The authors synthesized eight derivatives in 68–88% yields under 5–6 h reaction time at room temperature. The protocol has several benefits such as green solvent, green catalyst, short reaction time, low temperature and good to high yields (Scheme 2; Method 3).
Sarkar and co-workers63 formulated a new method for the synthesis of 1,5-BZDs (12) using OPD (1) and acetophenones (10). The reaction was promoted by a MOF (metal organic framework), MIL-101(Cr), which was prepared by a solvothermal method, using 1,4-benzene-dicarboxylic acid. The presence of electron-withdrawing and -donating groups on diamine or ketones did not affect the selectivity of product. The authors examined 26 derivatives in 84–96% yield at 80 °C in 30 min under SFRC (solvent-free reaction conditions). The main advantages of the protocol are low catalyst loading, recyclable catalyst, solvent-free reaction, high yields, short reaction time, and gram-scale synthesis (Scheme 2; Method 4).
Kagne et al.64 demonstrated the synthesis of 1,5-BZDs (12/13) using OPD (1) and various ketones (10/11) in the presence of sulphated tin oxide as a heterogenous solid super acid catalyst. The author examined various solvents in this method such as acetonitrile, DMF, toluene, chloroform, ethanol, methanol, and ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (1
water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in 72–88% yield. The catalyst showed high recyclability and easy separation, and was recycled for five times without losing its catalytic action and selectivity. High yield, mild reaction conditions and eco-friendly nature are among the several benefits of the method (Scheme 2; Method 5).
1) in 72–88% yield. The catalyst showed high recyclability and easy separation, and was recycled for five times without losing its catalytic action and selectivity. High yield, mild reaction conditions and eco-friendly nature are among the several benefits of the method (Scheme 2; Method 5).
Kusuma et al.65 reported an efficient and eco-friendly method for the synthesis of 1,5-BZDs (12/13) using OPD (1) and various ketones (10/11) in the presence of FC/AC (ferrocene-supported activated carbon) as heterogeneous acidic catalysts under solvent-free and mild conditions. The authors obtained 90% yield using 10 wt% catalyst at 90 °C in 8 h with 99% conversion of diamine and 91% selectivity of the desired product. The authors optimized various reaction conditions such as catalyst loading, solvent effects, temperature, effect of time period and found good to excellent yields with all parameters. The protocol has many benefits such as solvent-free conditions, easy availability of material, up to six times recyclability of catalyst, easy work-up, easy separation of catalyst and excellent yield (Scheme 2; Method 6).
Shaikh et al.66 developed an efficient, novel nanocatalyst Cu(II)-clay via a MW-assisted condensation reaction of OPD (1) and various ketones (10/11) for the synthesis of 1,5-BZDs (12/13). The catalyst was prepared using a Cu oligomer and clay. It showed specific activity without using any additive for the synthesis of 1,5-BZDs and reusable up to five cycles. The presence of electron-withdrawing and -donating groups has no effect on the yield. In total, 20 new compounds were prepared in 8–12 min in 90–98% yield under solvent-free conditions (Scheme 2; Method 7).
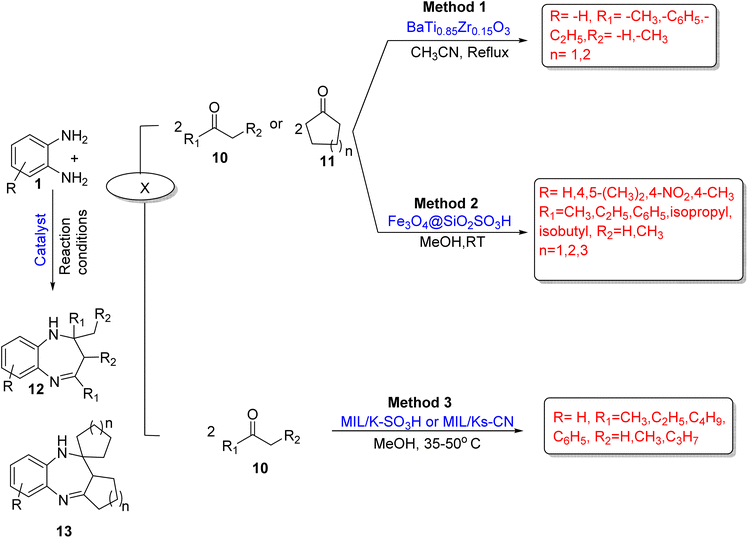
Amouhadi and co-workers67 fabricated a novel methodology for the synthesis of 1,5-BZDs (12/13) using diamine (1) and ketones (10/11) with BTZ (BaTi0.85Zr0.15O3) as an efficient and heterogenous catalyst under solvent-free conditions. The catalyst was prepared by a hydrothermal method using HAD (hexadecyl amine) as a surfactant, followed by solvothermal synthesis. The authors obtained 85–98% yield using 0.05 g catalyst in a CH3CN solvent in 10 min under reflux conditions. Reusable catalyst, easy workup, mild conditions, appropriate reaction time, and easy availability of materials are among the several advantages of the protocol (Scheme 3; Method 1).
 | ||
| Scheme 3 Two-component reactions of OPD with various ketones for the synthesis of 1,5-BZDs (‘X’ denotes the second component of the reactions). *n is the number of carbon atoms. | ||
Sathe and co-workers68 devised an efficient, facile method catalyzed by an iron nanocatalyst Fe3O4@SiO2SO3H for the synthesis of 1,5-BZDs (12/13). The protocol comprises OPD (1) and various ketones (10/11). The authors optimized various solvents such as EtOH, THF, DCM, iso-propanol, ethyl acetate, CH3CN, toluene, and MeOH but obtained excellent yields in MeOH at RT. In total, 16 new compounds (12/13) were synthesized in 3–6 h in 70–98% yield (Scheme 3; Method 2).
Isaeva and co-workers69 designed a cyclo-condensation reaction of OPD (1) with various ketones (10). It was functionalized with MIL/K–SO3H (para-sulfonatocalix[4]arene) and MIL/Ks-CN (para-tert-butylthiacalix[4]arene), and they have –SO3H(strong) and –CN(weak) acidic functions. The catalysts were synthesized via a MW-assisted reaction and they have MOFs (metal organic frameworks) with LAS (Lewis acid sites). The catalytic activity of MIL/K–SO3H was higher than that of MIL/Ks-CN due to the presence of the high acidic functional group –SO3H. The desired products (12) were obtained in methanol at 35–50 °C in good to excellent yields (Scheme 3; Method 3).
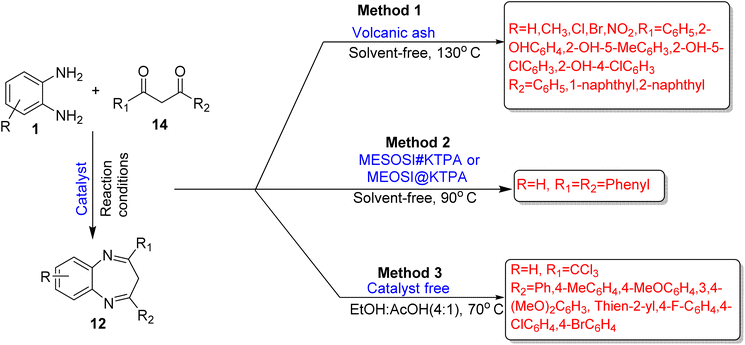
2.1.1.3. OPD and 1,3-dicarbonyls. Muñoz and co-workers70 developed a green method for the synthesis of 3H-1,5-BZDs (12), in which volcanic ash was used as a heterogenous acid catalyst. The catalyst was found from the Andes mountain range. It is a safe and recyclable catalyst, insoluble in organic solvents, which allows easy removal of products without affecting their catalytic activity. The catalyst did not show large variations in the yield even after five times of reusability. The product was obtained with 86% yield in 20 min, under solvent free conditions at 130 °C. The protocol followed eco-friendly conditions with excellent yields (Scheme 4; Method 1).
Morales et al.71 fabricated an efficient protocol for the synthesis of 3H-1,5-BZDs (12) from OPD (1) and 1,3-diphenyl-1,3-propanedione (14) using MESOSI#KTPA or MESOSI@KTPA (tungstophosphoric acid included in mesoporous silica) as heterogenous solid acid catalysts at 90 °C under solvent-free conditions. MESOSI#KTPA was prepared by impregnation of TPA (tungstophosphoric acid) in MESOSI (mesoporous silica) and MESOSI@KTPA was prepared by inclusion of TPA in MESOSI. MESOSI#KTPA showed a higher acidic strength than that of MESOSI@KTPA because MESOSI#KTPA displayed small crystals of H3PW12O40.6H2O in the XRD result. Solvent-free conditions, four times recyclability and easy separation of catalyst are the various advantages of the protocol but high yields were not obtained (Scheme 4; Method 2).
Myshkina et al.72 developed a novel pathway for the synthesis of substituted 3H-1,5-BZDs (12) from OPD (1) and 1,3-diketones (14) in an ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetic acid (4
acetic acid (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture in a catalyst-free system and the authors obtained the highest yield (77%) at 70 °C in 3 h reaction time. The author also examined other solvents like CHCl3 and the reaction proceeded at RT and 50 °C, but satisfactory yield was not obtained. In total, 7 compounds were prepared and they were screened for antimicrobial and antinociceptive activities (Scheme 4; Method 3).
1) mixture in a catalyst-free system and the authors obtained the highest yield (77%) at 70 °C in 3 h reaction time. The author also examined other solvents like CHCl3 and the reaction proceeded at RT and 50 °C, but satisfactory yield was not obtained. In total, 7 compounds were prepared and they were screened for antimicrobial and antinociceptive activities (Scheme 4; Method 3).
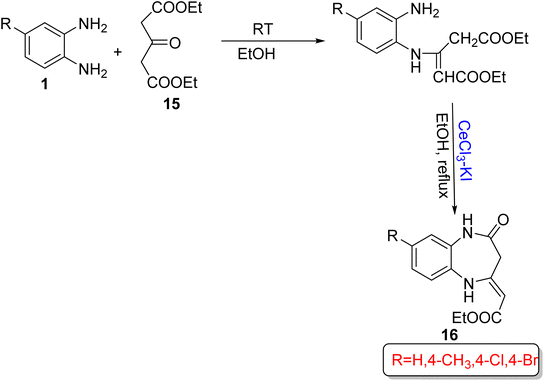
Wu and Wang73 devised a significant and eco-friendly method for the synthesis of 1,5-BZDs (16) using OPD (1) and 1,3-acetonedicarboxylate (15) with CeCl3-Kl or γ-Fe2O3@SiO2/CeCl3 as catalysts in ethanol. By using 4-substituted OPD (1), four examples were prepared in 35–45 min in 89–95% yield. The protocol involved the preparation of an enamine ester as an intermediate at RT, and then the final product was obtained at reflux temperature. The designed pathway has several beneficial effects such as mild reaction conditions, non-toxic solvents, simple operation, and excellent yield in a short reaction time (Scheme 5).
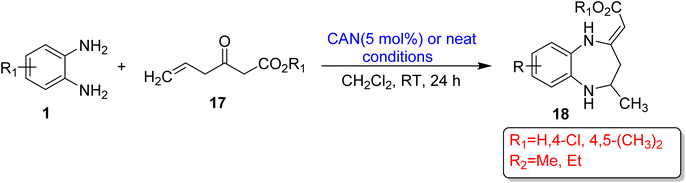
Maiti et al.74 developed a significant method for the synthesis of 1,5-BZDs (18) using OPD (1) and alkyl-3-oxo-5-hexenoates (Nazarov reagent) (17) in a solvent-free system at RT. 1,5-BZDs (18) were synthesized in a 24 h reaction time period in good to excellent yields using CAN (5 mol%) or neat conditions and DCM as a solvent. The mechanism of the protocol initially involved condensation of starting materials and produced an enaminoester as an intermediate and then formed the aza-Nazarov reagent by double bond isomerization, which gave desired products by intramolecular vinylogous-Michael addition (Scheme 6).
 | ||
| Scheme 6 Two-component reaction for the synthesis of 1,5-BZD derivatives using OPD and the Nazarov reagent. | ||
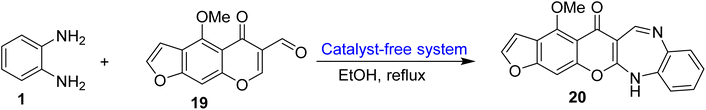
Ibrahim and co-workers75 developed 1,5-BZDs (20) via condensation of 6-formylvisnagin (19) (carboxaldehyde) with OPD in ethanol reaction media and using a catalyst-free system. The desired product (20) was obtained in 30 min at reflux temperature in 71% yield (Scheme 7).
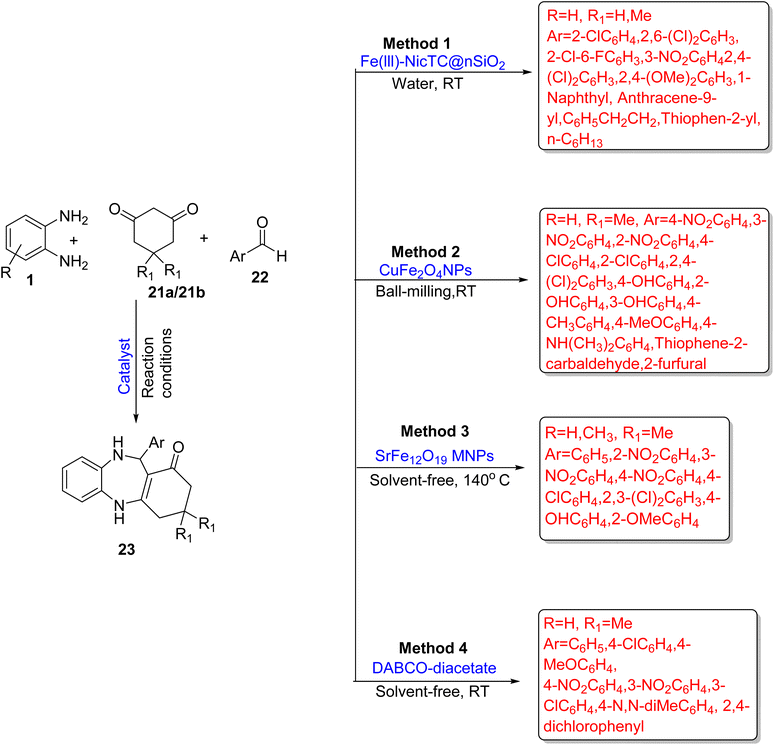
A series of 1,5-BZDs (23) was prepared by Nasr-Esfahani et al.76 using OPD (1), substituted 1,3-cyclohexanedione (21a/21b) and aldehyde (22). The reaction was promoted by a nicotine-based organocatalyst supported on silica (Fe(III)-NicTC@nSiO2). The desired product (23) was obtained in water at RT. This protocol is also applicable for the synthesis of mono and bis-1,5-benzodiazepine. The protocol has several beneficial effects such as a short reaction time, reusable catalysts, mild reaction conditions, easy separation of catalysts and simple operation. In total, 15 derivatives were prepared in a 5–40 min time period in 90–95% yield (Scheme 8; Method 1).
 | ||
| Scheme 8 Three-component reactions of OPD with substituted 1,3-cyclohexanedione and aldehydes for the synthesis of 1,5-BZDs. | ||
Maleki et al.77 reported a robust, facile magnetic nanocatalyst, CuFe2O4, for the eco-friendly synthesis of 1,5-BZDs (23) using OPD (1), dimedone (21b) and aldehyde (22). The catalyst was synthesized by thermal decomposition (80 °C) of Cu(II) and Fe(III) nitrate. It was easily separated using a magnet. By optimization of the substrate scope, 14 derivatives were obtained in 25–46 min in 68–98% yield at RT. High atom-economy, simple operation, reusability and easy handlings of catalyst are among the several cutting-edge benefits of this protocol (Scheme 8; Method 2).
Ahmadi et al.78 discovered a green and significant method for the synthesis of 1,5-BZDs (23) using OPDs (1), dimedone (21b) and aldehydes (22) with SrFe12O19 as a magnetic solid nanocatalyst under solvent-free conditions. The catalyst was prepared by a sol–gel auto combustion method using Fe(NO3)3·9H2O and Sr(NO3)3 powder and has many benefits such as reusable, easy recovery, high activity and easy handling. The designed pathway gave 75–96% yield in a very short time (140 s) at 140 °C (Scheme 8; Method 3).
Sarhandi et al.79 discovered an ultrasound irradiation methodology to obtain BZDs (23) using OPD (1), aldehydes (22) and dimedone (21b) with DABCO-diacetate (1,4-diazabicyclo[2.2.2]octanium diacetate) as an acidic bis ionic liquid catalyst. The authors examined various substrate scopes using 0.5 g catalyst and found 93–98% yield in 8–15 min at RT. Easy recovery, reusability, inexpensiveness, and non-toxicity are the characteristics of the catalyst. Several advantages such as green catalyst, mild reaction conditions, short reaction time, easy work up, and excellent yield make this protocol a green method (Scheme 8; Method 4).
Esfandiari et al.80 devised a novel and reusable catalyst CeO2/CuO@N-GQDs@NH2 (nitrogen-graphene quantum dots) as a nano-catalyst. The catalyst promoted the reaction of OPD (1) with dimedone (21b) and aromatic aldehydes (22) to synthesize 1,5-BZDs (23) and give 94% yield in 30 min using EtOH at RT. The protocol also involved the reaction of OPD (1) with Meldrum's acid (24) and isocyanide (25) to prepare BZD derivatives (26) in 91% yield in 50 min using a DCM solvent at RT. The authors optimized some other catalysts such as MgO NPs, triethylamine, Nano-CuO, Nano-CeO2, CeO2/CuO and CeO2/CuO@GQDs, but they did not give satisfactory results with respect to the reaction time and yield (Scheme 9; Method 1) and (Scheme 10; Method 1).
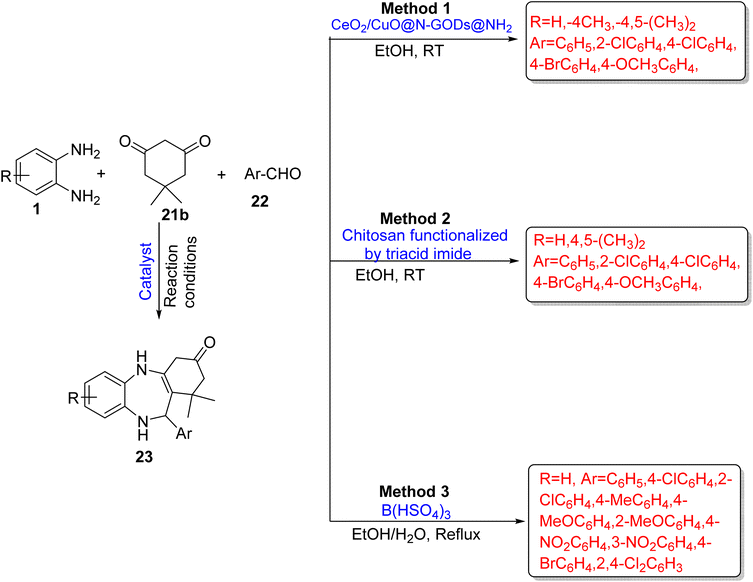 | ||
| Scheme 9 Three-component reactions of OPD with dimedone and aromatic aldehydes for the synthesis of 1,5-BZDs. | ||
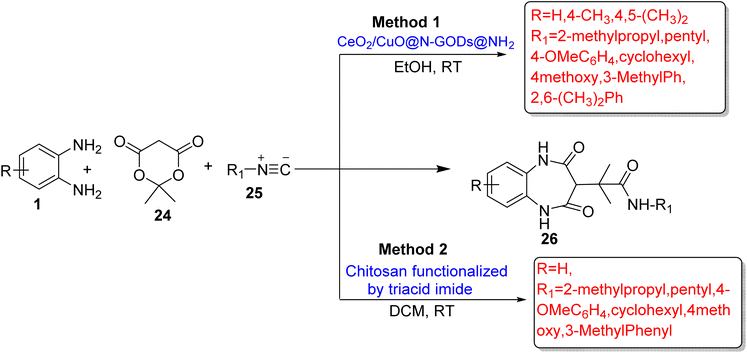 | ||
| Scheme 10 Three-component reactions of OPD with Meldrum's acid and isocyanide for the synthesis of 1,5-BZDs. | ||
Esfandiari et al.81 formulated a one-pot synthesis of 1,5-BZDs (23/26) using OPD (1), dimedone (21b), aryl aldehyde (22) or isocyanide (25) and Meldurm's acid (24) with chitosan operationalized by triacid imide as an efficient catalyst. The use of 7 mg catalyst gave high yields (93%) in 40 min. The designed pathway has some advantages such as low catalyst loading, easy workup and simple recovery process and recyclability of catalyst (Scheme 9; Method 2) and (Scheme 10; Method 2).
Karimi-Jaberi et al.82 devised a green synthesis strategy of 4-substituted-1,5-BZDs (23) using OPD (1), dimedone (21b), and aldehydes (22) in the presence of B(HSO4)3[tris(hydrogen sulfato)boron] acid catalyst. The reaction occurred under reflux conditions in ethanol and gave 85–93% yield in 20–40 min. A non-toxic and easily available catalytic system, excellent yields, short reaction times, avoidance of toxic and expensive solvents, and easy work-up are the advantages of this protocol (Scheme 9; Method 3).
Mozafari and ghadermazi83 developed a significant method for the synthesis of 1,5-BZDs (23) using CoFe2O4@GO-K22·Ni as a reusable magnetic nanocatalyst. It involved the reaction between OPD (1), dimedone (21b), and aromatic aldehyde (22) in water under stirring at 60 °C. The authors optimized various substrate scopes and prepared 14 derivatives in 8–18 min reaction time and obtained excellent yields (87–96%). The protocol has many remarkable benefits such as inexpensive starting material, water as a solvent, excellent yields in a very short reaction time and reusability of catalysts for up to 6 times (Scheme 11; Method 1).
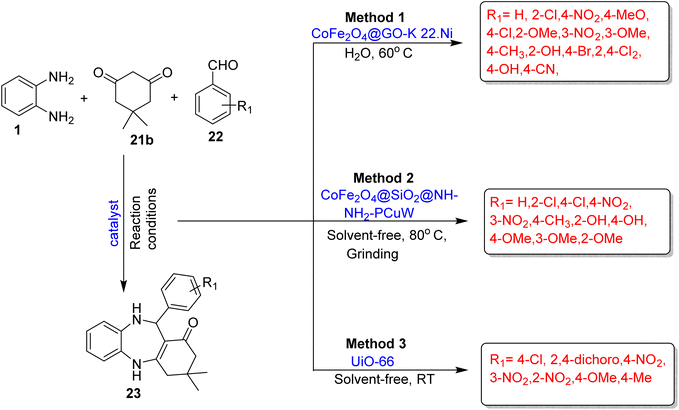 | ||
| Scheme 11 Three-component reactions for the synthesis of 1,5-BZDs using OPD, dimedone and aromatic aldehyde. | ||
A novel and eco-friendly one-pot synthetic methodology of 1,5-BZDs (23) using CoFe2O4@SiO2@NH–NH2–PCuW as a magnetic nanocatalyst was developed by Savari and co-workers.84 The catalyst has acidic properties and the presence of Cu increased the acidity by enhancing the electron deficiency of polyoxometalate. The protocol comprised the use of OPD (1), dimedone (21b) and aromatic aldehyde (22) under a solvent-free system at 80 °C and gave 96% yield in 15 min. A solvent-free system, reuse of catalysts up to six times, easy operation, short reaction times and excellent yields are the various cutting-edge benefits of the protocol (Scheme 11; Method 2).
Mirhosseini-Eshkevari et al.85 fabricated an efficient methodology for the synthesis of the pharmaceutically interesting moiety 1,5-BZD (23) and its derivatives using OPD (1), dimedone (21b) and aromatic aldehyde (22) in a solvent-free system. The protocol was catalyzed by UiO-66 and gave excellent yields in less than one hour reaction time at RT. Short reaction times, solvent-free systems, simple operation, low catalyst loadings, and excellent yields are among the several remarkable advantages of the protocol (Scheme 11; Method 3).
A series of 1,5-BZDs (23) was prepared by Kumar and co-workers86 in a catalyst-free system. The protocol involved condensation of OPD (1) with dimedone (21b) and substituted aldehyde (22) using toluene as a reaction medium at reflux temperature. The reaction mechanism also involved an intermediate formation. The authors optimized various substitutions on aldehyde and prepared 18 derivatives in low to good yields. The desired products possessed significant anti-TB activity (Scheme 12).
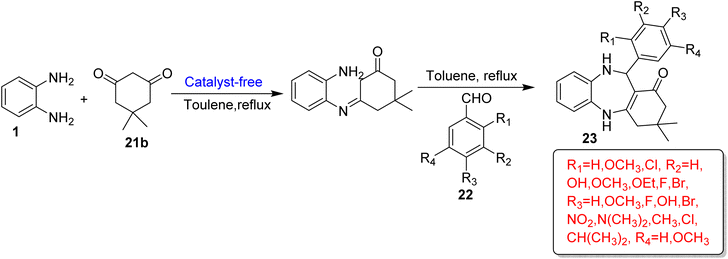 | ||
| Scheme 12 Three-component reaction for the synthesis of 1,5-BZD derivatives using OPD, dimedone and aromatic aldehyde. | ||
A library of BZDs (23/28) was developed by Sun and Wang87 using OPD (1), β-cyclopentanedione (21c) or β-cyclohexanedione (21a) and various aldehydes (22) or ketones (27) in the presence of γ-Fe2O3@SiO2/CeCl3 as a catalyst in EtOH reaction media. In total, 40 compounds were synthesized in 82–97% yield at RT in a short reaction time (21–60 min). Simple process, wide substrate scope, non-toxic solvent, easily available materials, mild reaction conditions, and excellent yields are the various cutting-edge benefits of the protocol (Scheme 13; Method 1 and 2).
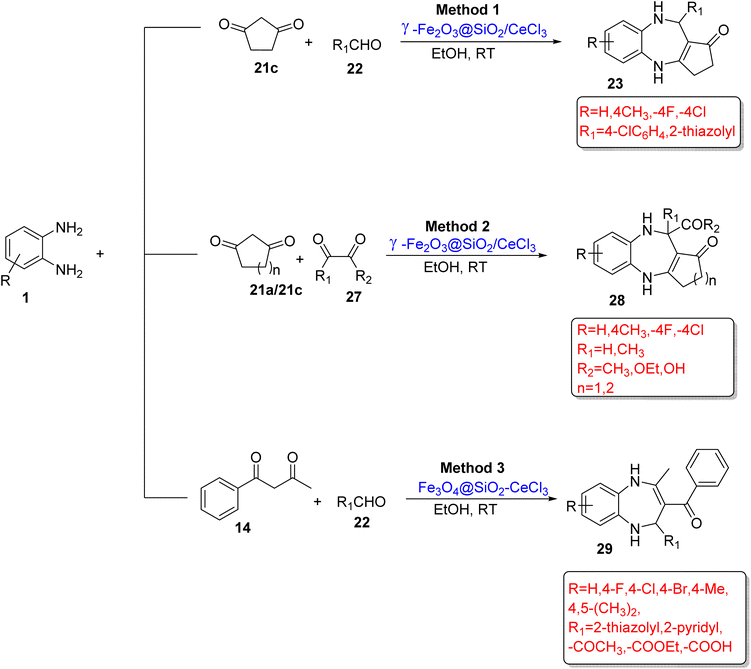 | ||
| Scheme 13 Three-component reactions of OPD with 1,3-diketones and aldehydes for the synthesis of 1,5-BZDs. *n denotes the number of carbon atoms. | ||
Zhou et al.88 designed intramolecular H+ transfer and cyclization of OPD (1), 1,3-diketones (14) and aldehyde/ethylglyoxylate/glyoxylic acid (22) to synthesize 1,5-BZDs (29) using Fe3O4@SiO2–CeCl3 as a reusable magnetic nanocatalyst in ethanol at RT. The authors optimized various substrate scopes and prepared 30 derivatives (29) in 71–93% yield in 0.5–2.6 h. The protocol is compatible with a broad substrate scope and gram-scale synthesis (Scheme 13; Method 3).
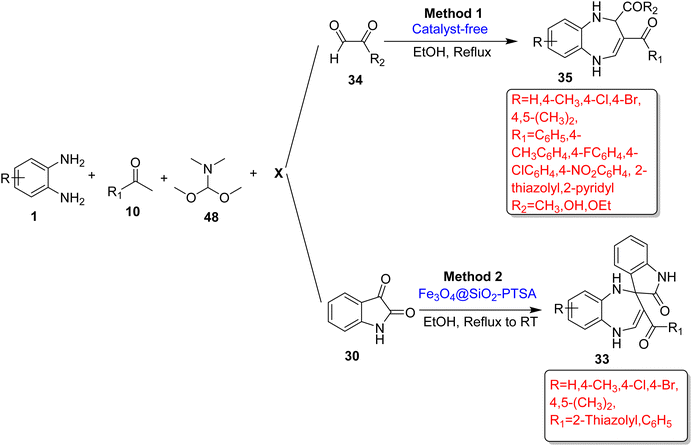
Zhang et al.89 reported three different three-component reactions for the synthesis of 1,5-BZDs (33) containing a indole ring introduced by Fe3O4@SiO2-PTSA as a magnetic nanocatalyst in EtOH. All reactions involved two common components, namely, OPD (1) and isatin (30), but the third component was ethyl acetoacetate (31) or 3-butyne-2-one (32) or benzoylacetone (14) and gave product (33). In total, 15 new compounds were synthesized in 80–94% yields in a 5.5–11 h reaction period (Scheme 14).
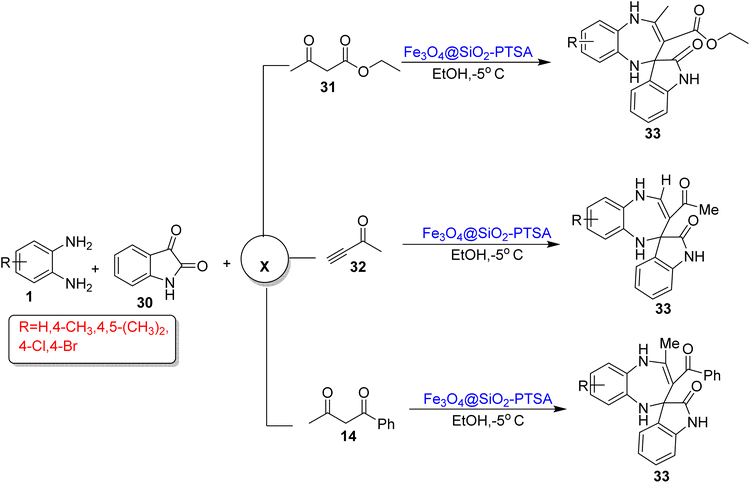 | ||
| Scheme 14 Three-component reactions of OPD with isatin and various carbonyl compounds for the synthesis of 1,5-BZDs (‘X’ denotes the third component of the reactions). | ||
An and co-workers90 reported a cyclization reaction of OPD (1), β-carbonyl ester (31) and ethyl glyoxylate (34a) or ethyl pyruvate (34b) using EtOH as the reaction medium at RT and gave 1,5-benzodiazepine-2,3-dicarboxylates (35). The reaction was promoted by γ-Fe2O3@SiO2/Ce(OTf)3 as a reusable nanocatalyst, and totally, 32 derivatives were synthesized in 63–92% yield. The reaction involved 2 steps; in the first step, an intermediate enamine ester was obtained in 30–60 min using OPD and β-carbonyl ester, and a final desired product was obtained in 1.5–5 h using ethyl glyoxylate or ethyl pyruvate (Scheme 15).
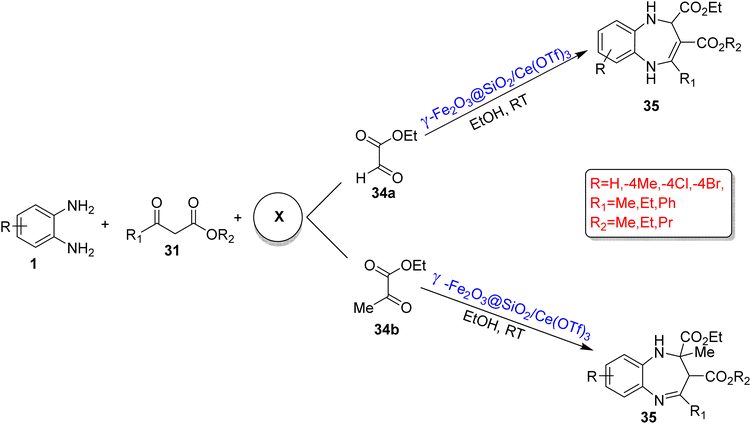 | ||
| Scheme 15 Three-component reactions of OPD with β-carbonyl ester and ethyl glyoxylate or ethyl pyruvate for the synthesis of 1,5-BZDs (‘X’ denotes the third component of the reactions). | ||
Yuan et al.91 developed a novel method for benzodiazepine-fused isoindolinones (37) as pseudo natural products, which is a biologically relevant molecule. The reaction occurred by using OPD (1), 2-formyl benzoic acid (36) and acetophenone (10). It was promoted by MSNPs (mesoporous silica nanoparticles) as reusable and green catalysts. The catalyst gave 88% yield in 24 h at 120 °C using AcOH solvent and 78% yield in gram-scale synthesis. In total, 21 derivatives (37) were prepared in 55–91% yield at 120 °C (Scheme 16; Method 1).
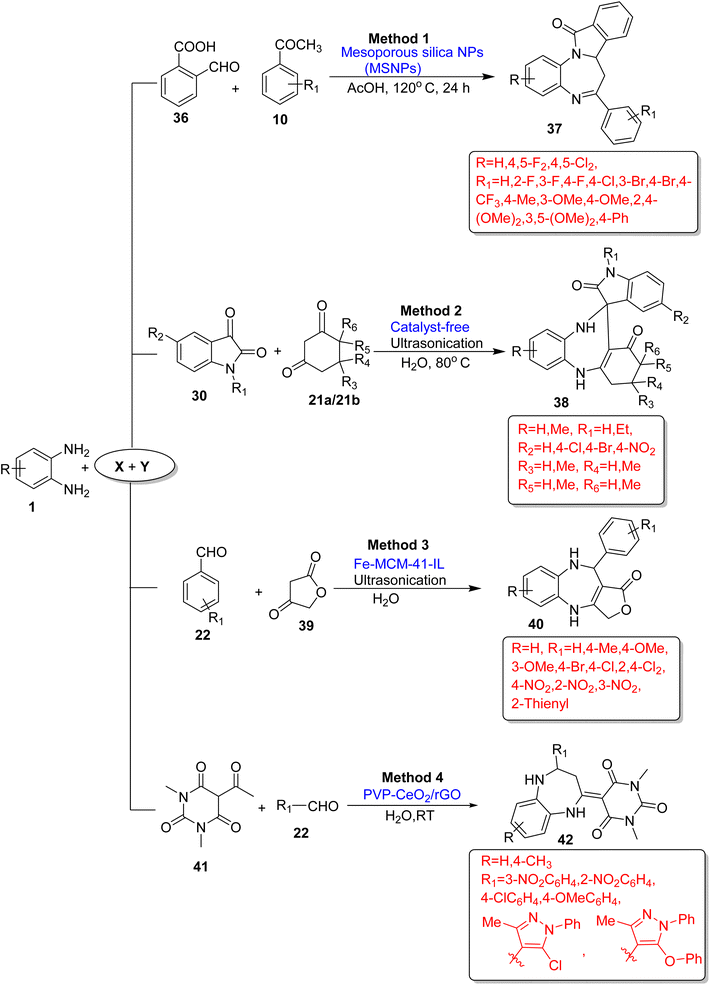 | ||
| Scheme 16 Three-component reactions of OPD with various substrates for the synthesis of 1,5-BZDs (‘X’ and ‘Y’ denote the second and third components of the reactions). | ||
Maury et al.92 devised a green synthesis strategy of the benzodiazepine ring via ultrasonication of OPD (1), isatin (30) and 1,3-diketone (21) at 80 °C for 10 min using water as a solvent and a catalyst-free system in 95% yield. The authors also examined both polar and non-polar solvents to identify solvent effects and the desired product (38) not obtained in non-polar solvents, while the product yield was good with some polar solvents such as ethanol, methanol, and acetonitrile. The protocol was validated for gram-scale synthesis, and it has many benefits such as green solvents, catalyst-free conditions, short reaction times, low temperatures, simple workup processes and excellent yields (Scheme 16; Method 2).
Nejadshafiee et al.93 fabricated an efficient method for the synthesis of BZDs (40) using OPD (1), tetronic acid (39) and aldehydes (22) with 15 g Fe-MCM-41-IL (ionic liquid) as a nano-catalyst under ultrasonication in 93% yield in 5 min. The authors examined various solvents such as EtOH, CH3CN, and THF, but H2O gave excellent yields. The authors also examined various substrate scopes and prepared 11 derivatives in 89–97% yield in 5–10 min. 4-Nitrobenzaldehyde gave the highest yield (97%) in 5 min (Scheme 16; Method 3).
Siddiqui et al.94 fabricated an efficient methodology for the synthesis of BZDs (42) using 5-acetyl-1,3-dimethylbarbituric acid (41), substituted benzaldehyde (22) and OPD (1) with PVP (polyvinylpyrrolidone)-CeO2/rGO(reduced graphene oxide) as a catalyst and water as a solvent in 98% yield in 7 min at RT. The amount of catalyst increased the yield but beyond 30 mg, the increase in the catalyst amount did not affect the yield of product. The catalyst was recycled up to sixth run, after which it lost minor catalytic activities. The recyclability of catalysts, very short reaction times, excellent yields, water as a solvent, etc., are the advantages of the protocol (Scheme 16; Method 4).
Wu and Wang73 devised a novel and eco-friendly approach for the synthesis of 1,5-BZDs (43) using OPD (1), 1,3-acetonedicarboxylate (15) and various aldehydes (22) in EtOH and the reaction was promoted by CeCl3-Kl or γ-Fe2O3@SiO2/CeCl3. In total, 44 derivatives (43) were prepared by optimizing the substrate scope in 37 min–7 h in 69–96% yield at RT. The protocol has several benefits such as mild reaction conditions, non-toxic solvents, simple operation, a broad substrate scope, and good to excellent yields (Scheme 17; Method 1).
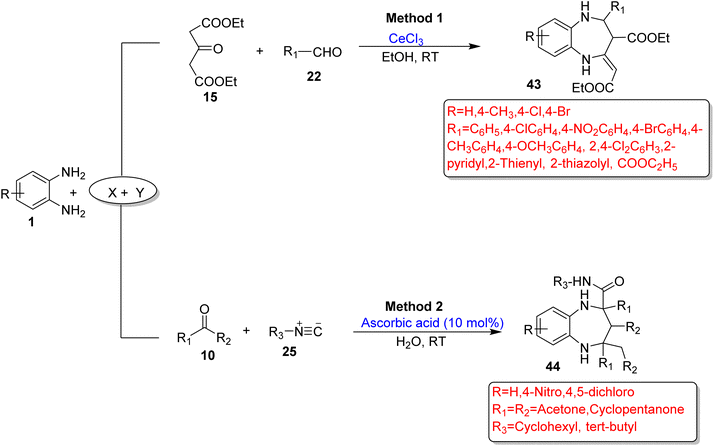 | ||
| Scheme 17 Three-component reactions of OPD with X and Y substrates for the synthesis of 1,5-BZDs (‘X’ and ‘Y’ denote the second and third components of the reactions). | ||
Shaabani et al.95 devised a green methodology for the synthesis of 1,5-BZDs (44) using OPD (1), ketones (10) and isocyanides (25) with vitamin C (L-ascorbic acid) as a catalyst in water as a solvent. The authors obtained excellent yields in a 5 h reaction time at RT. The protocol has some benefits such as use of water as a solvent, easily available materials, mild reaction conditions and high yields (Scheme 17; Method 2).
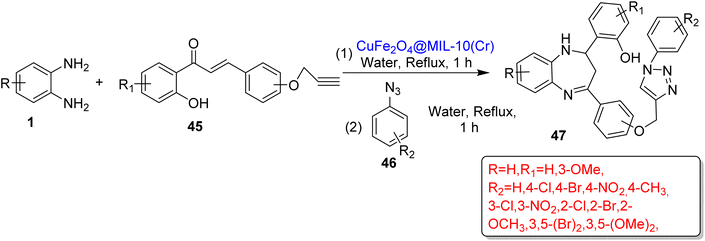
Gupta et al.96 developed a magnetically separable and metal–organic framework containing a nano-catalyst, CuFe2O4@MIL-10. The catalyst was used to catalyze the synthesis of benzodiazepine triazole derivatives (47) using OPD (1), chalcone (45) and azides (46) in water in 2 h under reflux conditions. In total, 21 derivatives (47) were obtained in 87–96% yield by examining the broad substrate scope (Scheme 18).
 | ||
| Scheme 19 Four-component reactions for the synthesis of 1,5-BZDs (‘X’ denotes the third component of the reactions). | ||
Zhang and colleges89 developed an efficient method for the synthesis of BZDs (33) using substituted OPD (1), N,N-dimethylformamide dimethylacetal (44), aromatic acetone (10) and isatin (30) in ethanol at reflux to RT. The authors obtained 10 new compounds (33) in 5–12 h in 79–90% yield using various aromatic ketones and substituted OPD. The protocol involved three steps to obtain the final product (33), in which high yields and easy operation of initial two steps are considerable for the product (33) yield in the final step (Scheme 19; Method 2).
2.2. Synthesis of 1,4-BZDs
BZDs, in particular 1,4-BZDs, are found in various natural alkaloids,98–100 but the first 1,4-benzodiazepine framework was discovered in the 1960s.101 From the past few decades, the synthesis of 1,4-BZDs gained tremendous attention due to their multiple use in the medical field.102 In this literature review, several current synthetic approaches of 1,4-BZD derivatives using OPD as one of the reactants are given with their advantages and drawbacks.Rajput and co-workers103 reported a novel microwave-assisted process to synthesize 1,4-BZDs derivatives (49) using OPD (1) and N-propargylated-2-aminobenzaldehyde (22) with a copper(II) catalyst (10 mol%), K2CO3 (3 equiv.) and a DMF solvent. The authors prepared 12 (49) compounds at 100 °C in a 15 min reaction period with 73–88% yield. The authors also compared the reaction with the conventional heating process, but the obtained yields (68–82% at 110 °C) were lower than those for MW-assisted conditions. The conventional process also consumed a long time period (6 h). The protocol has many benefits such as one-pot and operationally simple processes, high atom economy, short reaction periods and high yields (Scheme 20).
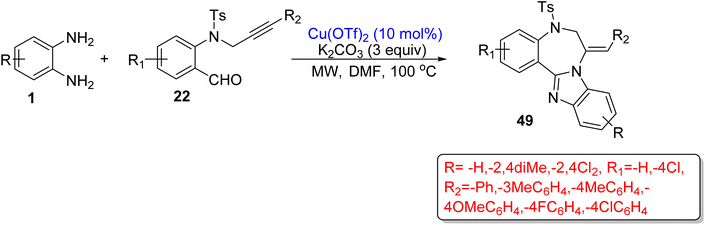 | ||
| Scheme 20 Two-component reaction of OPD with substituted 2-aminobenzaldehyde for the synthesis of 1,4-BZDs. | ||
3. Conclusion
In the past few decades, 1,4- and 1,5-BZDs have displayed considerable evolution due to their wide applicability in the field of medicinal, organic synthesis and industrial field. The present review article implements the current progress in the synthetic approaches of 1,4 and 1,5-BZDs using OPD as a common substrate with a variety of other substrates such as aldehydes, ketones, and isocyanides under miscellaneous reaction conditions. This research helps to dispatch the multicomponent synthetic strategies, namely, two-, three- and four-component reactions to design benzodiazepine scaffolds under eco-friendly conditions and techniques such as MW-assisted, ultrasonication, and ball-milling with their benefits and drawbacks. We hope that this review article will guide researchers, chemists and scientists to obtain the systematic knowledge of synthetic strategies of 1,4- and 1,5-BZDs and will inspire for further development in this area.Conflicts of interest
The authors confirmed that this article has no conflict of interest.Acknowledgements
The authors are grateful to Department of Chemistry, M.L.S.U. Udaipur, Rajasthan, India, for providing necessary library facilities.References
- J. B. Hester Jr, A. D. Rudzik and B. V. Kamdar, J. Med. Chem., 1971, 14, 1078–1081 CrossRef CAS PubMed.
- T. Kaneko, H. Wong, T. Doyle, W. Rose and W. Bradner, J. Med. Chem., 1985, 28, 388–392 CrossRef CAS PubMed.
- R. M. Keenan, J. F. Callahan, J. M. Samanen, W. E. Bondinell, R. R. Calvo, L. Chen, C. DeBrosse, D. S. Eggleston, R. C. Haltiwanger and S. M. Hwang, J. Med. Chem., 1999, 42, 545–559 CrossRef CAS PubMed.
- G. Grossi, M. Di Braccio, G. Roma, V. Ballabeni, M. Tognolini, F. Calcina and E. Barocelli, Eur. J. Med. Chem., 2002, 37, 933–944 CrossRef CAS PubMed.
- R. A. Kusanur, M. Ghate and M. V. Kulkarni, Chem. Sci. J., 2004, 116, 265–270 CrossRef CAS.
- S. J. R. Rajarao, B. Platt, S. J. Sukoff, Q. Lin, C. N. Bender, B. W. Nieuwenhuijsen, R. H. Ring, L. E. Schechter, S. Rosenzweig-Lipson and C. E. Beyer, Neuropeptides, 2007, 41, 307–320 CrossRef CAS PubMed.
- S. K. Ha, D. Shobha, E. Moon, M. A. Chari, K. Mukkanti, S.-H. Kim, K.-H. Ahn and S. Y. Kim, Bioorg. Med. Chem. Lett., 2010, 20, 3969–3971 CrossRef CAS PubMed.
- R. Kumar and Y. Joshi, J. Serb. Chem. Soc., 2008, 73, 937–943 CrossRef CAS.
- A. V. da Silva, S. M. Meneghetti and M. R. Meneghetti, Molecules, 2021, 26, 2796 CrossRef CAS PubMed.
- R. I. Fryer, Bicyclic Diazepines: Diazepines with an Additional Ring, John Wiley & Sons, 2009, vol. 50 Search PubMed.
- D. J. Twardy, Benzodiazepine coordination chemistry and nitrogen heterocyclic compounds from reactions of carbonyl alkynes with o-phenylenediamines, PhD thesis, Oakland University, 2022.
- O. O. Tolu-Bolaji, S. O. Sojinu, A. P. Okedere and O. O. Ajani, Arab J. Basic Appl. Sci., 2022, 29, 287–306 CrossRef.
- H. Naeimi and H. Foroughi, Chin. J. Catal., 2015, 36, 734–741 CrossRef CAS.
- N. Kausar, P. Mukherjee and A. R. Das, RSC Adv., 2016, 6, 88904–88910 RSC.
- V. Nejadshafiee and H. Naeimi, Curr. Org. Synth., 2019, 16, 136–144 CrossRef CAS PubMed.
- J. V. Pergolizzi and J. A. LeQuang, Postgrad. Med., 2020, 132, 10–12 CrossRef PubMed.
- S. M. Anil, R. Shobith, K. R. Kiran, T. R. Swaroop, N. Mallesha and M. P. Sadashiva, New J. Chem., 2019, 43, 182–187 RSC.
- R. A. Mane and D. B. Ingle, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 1982, 21B(10), 973–974 CAS.
- A. Misra, J. Dwivedi, S. Shukla, D. Kishore and S. Sharma, J. Heterocycl. Chem., 2020, 57, 1545–1558 CrossRef CAS.
- S. D. Tupare and R. Pawar, Int. J. Appl. Chem., 2017, 13, 369–376 Search PubMed.
- F. Guo, S. Wu, J. Julander, J. Ma, X. Zhang, J. Kulp, A. Cuconati, T. M. Block, Y. Du and J.-T. Guo, J. Virol., 2016, 90, 10774–10788 CrossRef CAS PubMed.
- S. Chander, C.-R. Tang, H. M. Al-Maqtari, J. Jamalis, A. Penta, T. B. Hadda, H. M. Sirat, Y.-T. Zheng and M. Sankaranarayanan, Bioorg. Chem., 2017, 72, 74–79 CrossRef CAS PubMed.
- P. Theodosis-Nobelos, G. Papagiouvannis, P. N. Kourounakis and E. A. Rekka, Molecules, 2019, 24, 3277 CrossRef CAS PubMed.
- N. S. Chowdari, Y. Zhang, I. McDonald, W. Johnson, D. R. Langley, P. Sivaprakasam, R. Mate, T. Huynh, S. Kotapati and M. Deshpande, J. Med. Chem., 2020, 63, 13913–13950 CrossRef CAS PubMed.
- A. A. Zakharova, S. S. Efimova, V. N. Yuskovets, I. P. Yakovlev, Z. M. Sarkisyan and O. S. Ostroumova, Front. Cell Dev. Biol., 2020, 8, 535 CrossRef PubMed.
- C.-Y. Chen, P.-H. Lee, Y.-Y. Lin, W.-T. Yu, W.-P. Hu, C.-C. Hsu, Y.-T. Lin, L.-S. Chang, C.-T. Hsiao and J.-J. Wang, Bioorg. Med. Chem. Lett., 2013, 23, 6854–6859 CrossRef CAS PubMed.
- M. G. G. Di Braccio, J. Med. Chem., 2001, 36, 01283 Search PubMed.
- H. Farhid, V. Khodkari, M. T. Nazeri, S. Javanbakht and A. Shaabani, Org. Biomol. Chem., 2021, 19, 3318–3358 RSC.
- P. Kundu, A. Mondal, B. Das and C. Chowdhury, Adv. Synth. Catal., 2015, 357, 3737–3752 CrossRef CAS.
- V. Murugesh, B. Harish, M. Adiseshu, J. Babu Nanubolu and S. Suresh, Adv. Synth. Catal., 2016, 358, 1309–1321 CrossRef CAS.
- J. D. Neukom, A. S. Aquino and J. P. Wolfe, Org. Lett., 2011, 13, 2196–2199 CrossRef CAS PubMed.
- L. P. Tardibono Jr and M. J. Miller, Org. Lett., 2009, 11, 1575–1578 CrossRef PubMed.
- S. Wang, Y.-B. Shen, L.-F. Li, B. Qiu, L. Yu, Q. Liu and J. Xiao, Org. Lett., 2019, 21, 8904–8908 CrossRef CAS PubMed.
- H. Xie, J.-C. Liu and M.-W. Ding, Synthesis, 2016, 48, 4541–4547 CrossRef CAS.
- Y. Wang, M. Chen and M.-W. Ding, Tetrahedron, 2013, 69, 9056–9062 CrossRef CAS.
- K. G. Guggenheim, H. Toru and M. J. Kurth, Org. Lett., 2012, 14, 3732–3735 CrossRef CAS PubMed.
- C. S. Chambers, N. Patel and K. Hemming, Tetrahedron Lett., 2010, 51, 4859–4861 CrossRef CAS.
- K. Majumdar and S. Ganai, Tetrahedron Lett., 2013, 54, 6192–6195 CrossRef CAS.
- J. Shin, J. Lee, D. Ko, N. De and E. J. Yoo, Org. Lett., 2017, 19, 2901–2904 CrossRef CAS PubMed.
- J. Feng, M. Zhou, X. Lin, A. Lu, X. Zhang and M. Zhao, Org. Lett., 2019, 21, 6245–6248 CrossRef CAS PubMed.
- X.-Q. Pan, J.-P. Zou, Z.-H. Huang and W. Zhang, Tetrahedron Lett., 2008, 49, 5302–5308 CrossRef CAS.
- S. Liu, T. Zhao, J. Qu and B. Wang, Adv. Synth. Catal., 2018, 360, 4094–4098 CrossRef CAS.
- R. S. Borisov, A. I. Polyakov, L. A. Medvedeva, V. N. Khrustalev, N. I. Guranova and L. G. Voskressensky, Org. Lett., 2010, 12, 3894–3897 CrossRef CAS PubMed.
- Y. Huang, K. Khoury, T. Chanas and A. Dömling, Org. Lett., 2012, 14, 5916–5919 CrossRef CAS PubMed.
- M. Zhuang, L. Tu, Y. Wu, Y. Jian, Y. Wang, W. Zhang, H. Sun and Z. Gao, Mol. Catal., 2022, 524, 112181 CrossRef CAS.
- R. A. Smiley, Ullmann's Encycl. Ind. Chem., 2000, 10, a19_405 Search PubMed.
- A. Shaabani, A. Maleki, F. Hajishaabanha, H. Mofakham, M. Seyyedhamzeh, M. Mahyari and S. W. Ng, J. Comb. Chem., 2010, 12, 186–190 CrossRef CAS PubMed.
- B. V. Kendre, M. G. Landge and S. R. Bhusare, Arabian J. Chem., 2019, 12, 2091–2097 CrossRef CAS.
- A. Shaabani, S. E. Hooshmand, M. T. Nazeri, R. Afshari and S. Ghasemi, Tetrahedron Lett., 2016, 57, 3727–3730 CrossRef CAS.
- R. K. Singh, S. Sharda, S. Sharma, S. Kumar and D. N. Prasad, Mini-Rev. Org. Chem., 2020, 17, 465–484 CrossRef CAS.
- R. Mishra, A. K. Sharma, R. Kumar, V. Baweja, P. Mothsra, M. K. Singh and S. B. Yadav, Synth. Commun., 2022, 52, 481–503 CrossRef CAS.
- N. Arora, P. Dhiman, S. Kumar, G. Singh and V. Monga, Bioorg. Chem., 2020, 97, 103668 CrossRef CAS PubMed.
- M. Verma, R. Sharma, R. Bharti and A. Tangri, Mater. Today: Proc., 2021, 37, 2321–2328 CAS.
- N. C. Desai, S. B. Joshi and V. M. Khedkar, Anal. Chem. Lett., 2020, 10, 307–320 CrossRef CAS.
- D. N. Toan, N. D. Thanh, M. X. Truong and N. M. Thao, Curr. Org. Synth., 2020, 17, 404–410 CrossRef CAS PubMed.
- G. Kottapalle and A. Shinde, Chem. Data Collect., 2021, 33, 100690 CrossRef CAS.
- M. T. Jafari-Chermahini, H. Tavakol and W. Salvenmoser, ChemistrySelect, 2020, 5, 968–978 CrossRef CAS.
- S. S. Pathade and B. S. Jagdale, J. Adv. Sci. Res., 2020, 11, 87–94 CAS.
- D. Tayde and M. Lande, Chem. Rev. Lett., 2021, 4, 30–36 CAS.
- E. C. Caiana, B. O. d. Veras, A. L. d. Souza and N. Queiroz, J. Braz. Chem. Soc., 2021, 32, 626–637 CAS.
- K. J. Tamuli and M. Bordoloi, ChemistrySelect, 2020, 5, 1353–1358 CrossRef CAS.
- N. A. Peerzade, S. Y. Jadhav, B. D. Varpe, A. A. Kulkarni and R. B. Bhosale, Polycycl. Aromat. Compd., 2021, 1–14 Search PubMed.
- F. K. Sarkar, A. Gupta, R. Jamatia, J. M. H. Anal and A. K. Pal, New J. Chem., 2021, 45, 19553–19564 RSC.
- R. Kagne, V. Kalalawe, S. Niwadange, R. Gutte and D. Munde, Int. J. Res. Anal. Rev., 2019, 6, 492–497 Search PubMed.
- S. Kusuma, K. N. Patil, P. M. Srinivasappa, N. Chaudhari, A. Soni, W. Nabgan and A. H. Jadhav, RSC Adv., 2022, 12, 14740–14756 RSC.
- I. N. Shaikh, M. Baseer, D. Ahmed, S. F. Adil, M. Khan and A. Alwarthan, J. King Saud Univ., Sci., 2020, 32, 979–985 CrossRef.
- E. Amouhadi, R. Fazaeli and H. Aliyan, J. Nanostruct., 2022, 12, 71–82 CAS.
- B. Sathe, P. Phatak, V. Dalve, A. Rote, R. Tigote and K. Haval, Int. Res. J. Sci. Eng. A, 2018, 5, 92–93 Search PubMed.
- V. I. Isaeva, M. N. Timofeeva, V. N. Panchenko, I. A. Lukoyanov, V. V. Chernyshev, G. I. Kapustin, N. A. Davshan and L. M. Kustov, J. Catal., 2019, 369, 60–71 CrossRef CAS.
- M. Muñoz, G. Pasquale, A. G. Sathicq, G. P. Romanelli, C. I. Cabello and D. Gazzoli, Green Process. Synth., 2019, 8, 600–610 Search PubMed.
- M. D. Morales, A. Infantes-Molina, J. M. Lázaro-Martínez, G. P. Romanelli, L. R. Pizzio and E. Rodríguez-Castellón, Mol. Catal., 2020, 485, 110842 CrossRef CAS.
- O. Myshkina, S. Y. Balandina, R. Makhmudov, M. Dmitriev and N. Y. Lisovenko, Russ. Chem. Bull., 2021, 70, 1408–1414 CrossRef CAS.
- H.-t. Wu and L.-z. Wang, New J. Chem., 2020, 44, 10428–10440 RSC.
- S. Maiti, M. Leonardi, Á. Cores, G. Tenti, M. T. Ramos, M. Villacampa and J. C. Menéndez, J. Org. Chem., 2020, 85, 11924–11933 CrossRef CAS PubMed.
- M. A. Ibrahim, S. A. Halim, N. Roushdy, A. Farag and N. M. El-Gohary, Optik, 2018, 166, 294–306 CrossRef CAS.
- M. Nasr-Esfahani, I. Mohammadpoor-Baltork, M. Moghadam, S. Tangestaninejad and V. Mirkhani, Catal. Lett., 2019, 149, 1057–1066 CrossRef.
- A. Maleki, R. Firouzi-Haji and P. Farahani, Org. Chem. Res., 2018, 4, 86–94 Search PubMed.
- T. Ahmadi, G. Mohammadi Ziarani, S. M. Masoumian Hoseini, A. Badiei and M. M. Ranjbar, J. Iran. Chem. Soc., 2021, 18, 2047–2056 CrossRef CAS.
- L. Z. Fekri, S. Sarhandi and E. Vessally, Acta Chim. Slov., 2018, 65, 246–252 CrossRef.
- M. Esfandiari, A. Kareem Abbas, H. Shahbazi-Alavi and J. Safaei-Ghomi, Polycycl. Aromat. Compd., 2022, 42, 1235–1248 CrossRef CAS.
- M. Esfandiari, A. K. Abbas, M. R. Vakili, H. Shahbazi-Alavi and J. Safaei-Ghomi, Res. Chem. Intermed., 2021, 47, 483–496 CrossRef CAS.
- Z. Karimi-Jaberi and A. Hooshmandpour, Polycycl. Aromat. Compd., 2020, 40, 432–436 CrossRef CAS.
- R. Mozafari and M. Ghadermazi, RSC Adv., 2020, 10, 15052–15064 RSC.
- A. Savari, F. Heidarizadeh and N. Pourreza, Polyhedron, 2019, 166, 233–247 CrossRef CAS.
- B. Mirhosseini-Eshkevari, F. Zamani and M. A. Ghasemzadeh, ChemistrySelect, 2020, 5, 14554–14558 CrossRef CAS.
- M. M. K. Kumar, T. Mohan, G. Sangeeta and K. P. Nagasree, J. Young Pharm., 2018, 10, 267 CrossRef CAS.
- Y.-W. Sun and L.-Z. Wang, New J. Chem., 2018, 42, 20032–20040 RSC.
- L. Zhou, M. Wang, K. Wang, T. Wen and L. Wang, Appl. Organomet. Chem., 2020, 34, e5707 CAS.
- K. Zhang, J. Li, K. Wang, X. An and L. Wang, ChemistrySelect, 2020, 5, 14056–14061 CrossRef CAS.
- X. An, L. Gao, M. Wang, H. Wu and L. Wang, Chem. Heterocycl. Compd., 2021, 57, 806–816 CrossRef CAS.
- S. Yuan, Y.-L. Yue, D.-Q. Zhang, J.-Y. Zhang, B. Yu and H.-M. Liu, Chem. Comm., 2020, 56, 11461–11464 RSC.
- S. K. Maury, D. Kumar, A. Kamal, H. K. Singh, S. Kumari and S. Singh, Mol. Diversity, 2021, 25, 131–142 CrossRef CAS PubMed.
- V. Nejadshafiee, H. Naeimi and M. R. Islami, Appl. Organomet. Chem., 2019, 33, e5072 Search PubMed.
- S. Siddiqui and Z. N. Siddiqui, Nanoscale Adv., 2020, 2, 4639–4651 RSC.
- A. Shaabani, V. Khodkari, M. T. Nazeri, S. Ghasemi, R. Mohammadian and S. Shaabani, J. Iran. Chem. Soc., 2019, 16, 1793–1800 CrossRef CAS.
- A. Gupta, F. K. Sarkar, R. Sarkar, R. Jamatia, C. Y. Lee, G. Gupta and A. K. Pal, Appl. Organomet. Chem., 2020, 34, e5782 CrossRef CAS.
- Y.-W. Sun, Y.-M. Bei and L.-Z. Wang, Org. Biomol. Chem., 2019, 17, 930–938 RSC.
- A. Witt and J. Bergman, J. Org. Chem., 2001, 66, 2784–2788 CrossRef CAS PubMed.
- R. Ookura, K. Kito, T. Ooi, M. Namikoshi and T. Kusumi, J. Org. Chem., 2008, 73, 4245–4247 CrossRef CAS PubMed.
- C. M. Cui, X. M. Li, C. S. Li, H. F. Sun, S. S. Gao and B. G. Wang, Helv. Chim. Acta, 2009, 92, 1366–1370 CrossRef CAS.
- J. Wick, Consult. Pharm., 2013, 28, 538–548 CrossRef PubMed.
- G. G. Mandawad, B. M. Shaikh, S. S. Chobe and S. G. Konda, Eur. J. Chem., 2020, 11, 276–279 CrossRef CAS.
- D. Rajput, A. Kumar, T. Jandial, M. Karuppasamy, N. Bhuvanesh, R. S. Kumar, A. I. Almansour and V. Sridharan, J. Org. Chem., 2022, 87, 8956–8969 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |