Cobalt-catalyzed asymmetric phospha-Michael reaction of diarylphosphine oxides for the synthesis of chiral organophosphorus compounds†
Xu-Hui
Yu
a,
Liang-Qiu
Lu
 a,
Zhi-Han
Zhang
a,
De-Qing
Shi
a,
Zhi-Han
Zhang
a,
De-Qing
Shi
 *a and
Wen-Jing
Xiao
*a and
Wen-Jing
Xiao
 ab
ab
aKey Laboratory of Pesticide & Chemical Biology, Ministry of Education, College of Chemistry, Central China Normal University, 152 Luoyu Road, Wuhan, Hubei 430079, China. E-mail: chshidq@ccnu.edu.cn
bState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, 345 Lingling Road, Shanghai 200032, China
First published on 14th November 2022
Abstract
The asymmetric Michael addition of phosphorus nucleophiles to electron-deficient alkenes is one of the most direct and atom-economical methods to provide chiral organophosphorus compounds with high efficiency in recent years. Herein, we report a cobalt-catalyzed imidazolyl-directed asymmetric phospha-Michael-type reaction of diarylphosphine oxides with electron-deficient alkenes for synthesizing chiral organophosphorus compounds in moderate to good yields and good to excellent enantioselectivities (25 examples, up to 99% yield, and 99% ee). This protocol features broad substrate scope, good functional group tolerance, and mild conditions as well as avoids the release of massive metal wastes and the use of noble transition metal catalysts. The excellent enantioselectivity of the phospha-Michael reaction can be due to the adoption of a novel chiral N4-ligand. Furthermore, the DFT calculation indicates that the bulky 2,4,6-(i-Pr)3C6H2 group of the ligand induces large steric hindrance which blocks the nucleophilic attack from the Si-face.
Chiral organophosphorus compounds are not only used as versatile ligands, or organocatalysts in asymmetric catalytic transformation,1 but also frequently found in natural products, pharmaceuticals, agrochemicals or functional materials due to their unique properties.2 Therefore, numerous efforts have been made toward the catalytic asymmetric synthesis of organophosphorus compounds. Among them, the asymmetric phospha-Michael addition3 is one of the most direct and atom-economical methods to provide chiral organophosphorus compounds with high efficiency. In the past few decades, transition metal-catalyzed (including Pd, Cu, etc.),4 organocatalyzed (i.e., cinchona alkaloids, proline derivatives, N-heterocyclic carbenes, chiral phosphoric acids, etc.)5 and Lewis acid-catalyzed (i.e., Zn, Mg, etc.)6 asymmetric phospha-Michael addition of secondary phosphine oxides to electron deficient alkenes have been well developed. Recently, Liu and coworkers7 reported the first Cu(I)-catalyzed enantioselective phosphinocyanation of styrenes through a tandem radical relay strategy; in 2020, Fu et al.8 developed a nickel-catalyzed enantioconvergent reductive hydroalkylation of olefins with an α-phosphorus alkyl electrophile to construct chiral organophosphorus compounds; recently, Wang's group9 reported an enantioselective palladium-catalyzed hydrophosphinylation of allenes and a copper-catalyzed hydroamination for the synthesis of chiral organophosphorus compounds, respectively. Shortly afterwards, Xu and coworkers10 presented a Ni/photoredox-catalyzed enantioconvergent reductive cross-coupling between α-bromoalkylphosphates and aryl iodide for the synthesis of chiral α-aryl phosphorus compounds. Despite these remarkable advances, exploring a novel and environmentally friendly phospha-Michael-type reaction for the preparation of enantioenriched organophosphorous compounds under mild conditions is still highly desired.
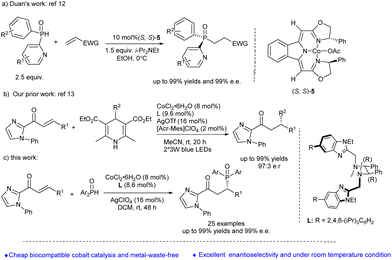
Cobalt, a cheap and low toxic element in comparison with other transition metals, was found to exhibit excellent activity in asymmetric catalysis and has received growing attention from the synthetic community in the last two decades.11 Recently, Duan's group12 reported a cobalt-catalysed asymmetric phospha-Michael addition for the synthesis of P-stereogenic compounds, in which a pyridyl moiety was adopted as the coordinating group in secondary phosphine oxides in order to obtain good enantioselectivity (Scheme 1a). In 2019, we reported a visible-light-induced cobalt-catalyzed enantioselective radical conjugate addition when the novel chiral Co-centered octahedral complex was used as the catalyst and an imidazolyl moiety as the coordinating group in the Michael acceptors (Scheme 1b).13 Based on our continuing interest in the synthesis of biologically active organophosphorus compounds,14 herein, we report a cobalt-catalyzed asymmetric phospha-Michael-type reaction of diarylphosphine oxides with electron-deficient alkenes by introducing an imidazolyl moiety to coordinate with the cobalt complex catalyst in a bidentate chelating form in order to obtain a high enantioselectivity control (Scheme 1c).
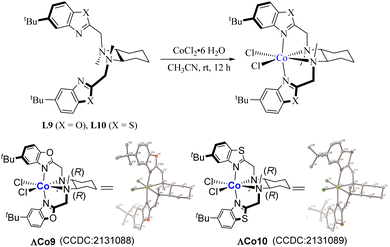
We commenced our investigation on the cobalt-catalyzed asymmetric phospha-Michael-type reaction of diarylphosphine oxides with electron-deficient alkenes for synthesizing chiral organophosphorus compounds using enone (1a) containing a phenylimidazolyl directing group and diphenylphosphine oxide (2a) as the model substrates, CoCl2·6H2O as the catalyst in the presence of various chiral N4 ligands in dichloromethane (DCM) at room temperature. To our delight, when cyclohexyldiamine-derived benzoimidazolyl N4 ligand (L1, 8.6 mol%) was used, the asymmetric phospha-Michael reaction indeed occurred, giving the product 3a in excellent yield with moderate enantioselectivity (Table 1, entry 1: 99% yield, 49% ee). Screening of N4 ligands showed that increasing the steric hindrance of the benzene ring of N4 ligands could improve the enantioselectivity of the phospha-Michael reaction (Table 1, entries 4 and 5, 86% ee and 91% ee, respectively); replacement of the chiral cyclohexane-1,2-diamine unit with the 1,2-diphenylethane-1,2-diamine skeleton could further increase the enantioselectivity of the phospha-Michael reaction slightly (Table 1, entry 6, 93% ee); however, when N4 ligands containing a benzoxazolyl or benzothiazolyl moiety were explored, both the reactivity and enantioselectivity of the phospha-Michael reaction decreased remarkably (Table 1, entries 7–10). In order to investigate how the steric environments of the cobalt complex catalyst affect the reaction activity and enantioselectivity of the phospha-Michael reaction, we obtained the chiral cobalt octahedral complexes ΛCo9 and ΛCo10 by stirring ligand L9 or L10 with CoCl2·6H2O in acetonitrile at room temperature, their structures were confirmed by X-ray crystallography analysis (Scheme 2).15 As shown in Scheme 2, the coordination of two nitrogen atoms of the chiral diamine moiety with the Co center, though preferably induces the chirality of the cobalt center, makes the sterically hindered tert-butyl groups move far away from the plane comprising Co and two Cl atoms or a Cl atom and an O atom of H2O (distance: SΛCo9 = 4.95 Å, SΛCo10 = 4.69 Å vs. SΛCo2 = 5.02 Å). Furthermore, by analyzing the bond angle of Cl–Co–Cl (or O) in different octahedral complexes (∠2 = 95.9° for ΛCo2, ∠2′ = 100.3° for ΛCo9, ∠2′′ = 99.9° for ΛCo10, vs. ∠2′′′ = 87.8° for Meggers’ catalyst ΛRh116), the potential coordination environments of ΛCo9, ΛCo10 and ΛCo2 were found to provide relatively unrestricted access to nucleophiles compared to Meggers’ catalyst ΛRh1. Therefore, these steric characteristics would be unfavorable for the stereoinduction capacity of the cobalt catalysts ΛCo2, ΛCo9 and ΛCo10 (Fig. 1) in contrast to Meggers’ catalyst ΛRh1. So, we concluded that these cobalt complexes (ΛCo2, ΛCo9 and ΛCo10) were not good candidate catalysts for the phospha-Michael reaction with good stereochemical outcomes. In addition, DFT calculation indicates that the bulky 2,4,6-(i-Pr)3C6H2 group of the ligand L6 induces a large steric hindrance which blocks the nucleophilic attack from the Si-face, so the cobalt complex ΛCo6 was a suitable catalyst for guaranteeing the phospha-Michael reaction with good enantioselectivities.
 | ||
| Fig. 1 Comparison of the stereochemical parameters of ΛCo9 and ΛCo10 with ΛCo2 (CCDC 1870638).13 | ||
| Entry | Ligand | Solvent | Yieldb (%) | ee ratioc (%) |
|---|---|---|---|---|
| a Unless noted otherwise, reactions were performed with 1a (0.10 mmol), 2a (0.15 mmol, 1.5 equiv.), CoCl2·6H2O (8 mol%), L (8.6 mol%), AgOTf (16 mol%) in DCM (1.0 mL) at room temperature for 48 h. b Determined by 1H NMR using triphenylmethane as an internal standard. c Determined by chiral HPLC analysis. d 2.0 mL of DCM was used. e 1.0 equiv. of 2a was used. f 2.0 equiv. of 2a was used. g Replaced AgOTf with AgClO4 (16 mol%). h Isolated yield in parenthesis. | ||||
| 1 | L1 | DCM | 99 | 49 |
| 2 | L2 | DCM | 99 | 53 |
| 3 | L3 | DCM | 99 | 48 |
| 4 | L4 | DCM | 99 | 86 |
| 5 | L5 | DCM | 88 | 91 |
| 6 | L6 | DCM | 98 | 93 |
| 7 | L7 | DCM | 42 | 3 |
| 8 | L8 | DCM | 59 | 43 |
| 9 | L9 | DCM | 36 | 24 |
| 10 | L10 | DCM | 40 | 47 |
| 11 | L6 | Acetonitrile | 75 | 48 |
| 12 | L6 | Acetyl acetate | 93 | 6 |
| 13 | L6 | CHCl3 | 92 | 84 |
| 14 | L6 | THF | 99 | 18 |
| 15 | L6 | Et2O | 80 | 2 |
| 16d | L6 | DCM | 96 | 86 |
| 17e | L6 | DCM | 94 | 93 |
| 18f | L6 | DCM | 95 | 85 |
| 19 | L6 | DCM | 99 (92) | 96 |
Encouraged by this result, we continued to evaluate the effect of solvents and found that, highly polar solvents such as acetonitrile or medium polar ethyl acetate and ether solvents such as THF or ethyl ether are not effective at improving the yields of products and enantioselectivity; however, halogenated solvents such as DCM or chloroform are suitable one, and DCM is the best solvent for improving the outcome of the phospha-Michael reaction (Table 1, entries 6 and 11–15). When the amount of DCM used was increased to 2 mL, the enantioselectivity of the phospha-Michael reaction decreased slightly (Table 1, entry 16). Subsequently, increasing the molecular ratio of substrates 1a and 2a to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 or decreasing the ratio to 1
2 or decreasing the ratio to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, could not improve the reaction efficiency and stereoselectivity (Table 1, entries 17 and 18). In addition, the addition of bases is not beneficial for the phospha-Michael reaction (please see the ESI†). Furthermore, when the additive AgClO4 was used instead of AgOTf, the ratio of excess enantioselectivity was further increased to 96% (Table 1, entry 19). Finally, the results of the control experiments showed that the cobalt source, N4 ligand and the additive AgClO4 were crucial for the asymmetric phospha-Michael addition (please see the ESI†).
1, could not improve the reaction efficiency and stereoselectivity (Table 1, entries 17 and 18). In addition, the addition of bases is not beneficial for the phospha-Michael reaction (please see the ESI†). Furthermore, when the additive AgClO4 was used instead of AgOTf, the ratio of excess enantioselectivity was further increased to 96% (Table 1, entry 19). Finally, the results of the control experiments showed that the cobalt source, N4 ligand and the additive AgClO4 were crucial for the asymmetric phospha-Michael addition (please see the ESI†).
After the optimal conditions were established, the scope of β-aryl substituted α,β-unsaturated enones containing phenylimidazolyl directing group 1 was then investigated. As shown in Table 2, substrates 1 bearing either an electron-donating group (i.e., methyl-, methoxy-, tert-butyl, N,N-dimethylamino) or an electron-withdrawing one (i.e., fluoro-, chloro-, bromo-, iodo-, trifluoromethyl, methoxycarbonyl) on the benzene ring, in either ortho-, meta- or para-substituted pattern, were found to be compatible for the cobalt-catalyzed asymmetric phospha-Michael reactions, generating the corresponding products in moderate to excellent yields and good to excellent enantioselectivities (3a–3p, 68%–99% yield, 87%–99% ee). It's worth mentioning that furyl and thienyl substituted enones were tolerated well in this reaction and furyl substituted enone gave the target compound (3q) with the highest yield and stereochemistry. Furthermore, β-alkyl substituted α,β-unsaturated enone was also found to be suitable for the reaction, and the corresponding product 3s was obtained with excellent enantioselectivity (>99% ee), albeit in moderate yield (50% yield). Next, we turned to investigate whether other diaryl phosphine oxides or dialkyl phosphites could act as effective phosphorus nucleophiles when 1a was used (Table 2). To our delight, a series of diaryl phosphine oxides were all good candidates in this reaction. As highlighted in Table 2, diaryl phosphine oxides 2 bearing either diphenyl, naphthyl substituted or different halogen (fluoro or chloro) substituted or the halogen atom in different positions on the benzene ring all worked well and the corresponding products (3u–3y) were obtained in excellent yields and enantioselectivities (92–98% yields, 93–99% ee). However, dialkyl phosphites (for example, dimethyl or diethyl phosphite) were not active in the cobalt-catalyzed asymmetric phospha-Michael-type reaction, which might probably be due to the poor nucleophilicity of the dialkyl phosphites. In order to determine the absolute configuration of products 3, a single crystal of 3a was cultured and unambiguously confirmed by X-ray single-crystal diffraction analysis.15 The result showed that 3a was presented in S-configuration.
| a Unless noted otherwise, all reactions were performed with 1 (0.20 mmol), 2 (0.30 mmol, 1.5 equiv.), CoCl2·6H2O (0.016 mmol, 8 mol%), L6 (0.018 mmol, 8.6 mol%), AgClO4 (0.032 mmol, 16 mol%) in dichloromethane (2.0 mL) at room temperature for 48 h. b Isolated yield. c Determined by chiral HPLC analysis using Chiral OD-H or AS-H column, and hexane/i-PrOH as the eluent. d Reaction time of 7 h. e Reaction time of 72 h. |
|---|

|
In order to evaluate the synthetic utility of the cobalt-catalyzed asymmetric phospha-Michael-type reaction, a gram-scale reaction of 1a (3.5 mmol) and 2a (5.25 mmol, 1.5 equiv.) was performed under standard conditions, the target product 3a (1.60 g) was obtained in 91% isolated yield with 96% ee (Scheme 3).
Furthermore, the target compounds could be conveniently transformed into functionalized chiral organophosphorus compounds. 3a could easily transform into chiral organophosphorus compound bearing an ester group 4a in 93% yield with 81% ee in a simple one-step procedure; additional, chiral ketone compound containing phosphorus 5a was obtained in moderate yield with excellent stereoselectivity (54% yield, 90% ee) by the addition of 3a with Grignard reagent (Scheme 3).
On the basis of some related literature reports12,13 and our control experiment results, a plausible mechanism is suggested in Fig. 2. Initially, CoCl2 combined with N4 ligand L6 to form a cobalt complex, which acted as a Lewis acid to activate enones 1 by coordinating with the N atom of phenylimidazole and O atom of enone 1 in a bidentate chelating form (Int-1). Subsequently, diphenylphosphine oxide 2a could tautomerize to 2a′ and undergo conjugate addition with activated enone (Int-1) to form Int-2. Product 3 was generated through a proton transfer process and subsequently the liberation of the cobalt complex catalyst.
In addition, in order to gain insight into the origin of stereocontrol of the cobalt complex ΛCo6 that catalyzed the phospha-Michael addition reactions, the DFT calculations were carried out and a stereoinduction model was also proposed to rationalize the stereochemical outcome. As shown in Fig. 2, the Re-face addition of the phosphorus nucleophile to the β-position of enones, is preferential because of the reduced steric impulsion from the N4 ligand.
In conclusion, we developed a cobalt-catalyzed imidazolyl-directed asymmetric phospha-Michael-type reaction of diarylphosphine oxides with electron-deficient alkenes for synthesizing chiral organophosphorus compounds. This protocol provides an efficient access to chiral organophosphorus compounds in moderate to excellent yields with good to excellent enantioselectivities under mild reaction conditions, meanwhile avoiding the release of massive metal wastes and the use of noble transition metal catalysts. The excellent enantioselectivity of the phospha-Michael reaction can be due to the adoption of a novel chiral N4-ligand. In addition, the phospha-Michael adducts 3 can be conveniently transformed into functionalized chiral organophosphorus compounds.
Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
We are grateful to the National Natural Science Foundation of China (No. 51573066) and the Region Joint Funds of the National Natural Science Foundation of China (No. U21A20384) for support of this research.References
- (a) W. Tang and X. Zhang, New chiral phosphorus ligands for enantioselective hydrogenation, Chem. Rev., 2003, 103, 3029–3070 CrossRef CAS; (b) T. Wang, X. Han, F. Zhong, W. Yao and Y. Lu, Amino acid-derived bifunctional phosphines for enantioselective transformations, Acc. Chem. Res., 2016, 49, 1369–1378 CrossRef CAS; (c) H. Ni, W.-L. Chan and Y. Lu, Phosphine-catalyzed asymmetric organic reactions, Chem. Rev., 2018, 118, 9344–9411 CrossRef CAS PubMed; (d) W. Li and J. Zhang, Recent developments in the synthesis and utilization of chiral β-amino-phosphine derivatives as catalysts or ligands, Chem. Soc. Rev., 2016, 45, 1657–1677 RSC; (e) Y. Wei and M. Shi, Applications of Chiral phosphine-based organocatalysts in catalytic asymmetric reactions, Chem. – Asian J., 2014, 9, 2720–2734 CrossRef CAS; (f) A. Börner, Phosphorus ligands in asymmetric catalysis: synthesis and applications, Wiley-VCH, Weinheim, 2008, Vol. 1–3 Search PubMed; (g) H. Fernández-Pérez, P. Etayo, A. Panossian and A. Vidal-Ferran, Phosphine–phosphinite and phosphine–phosphite ligands: preparation and applications in asymmetric catalysis, Chem. Rev., 2011, 111, 2119–2176 CrossRef PubMed; (h) H. Guo, Y. C. Fan, Z. Sun, Y. Wu and O. Kwon, Phosphine organocatalysis, Chem. Rev., 2018, 118, 10049–10293 CrossRef CAS; (i) A. L. Clevenger, R. M. Stolley, J. Aderibigbe and J. Louie, Trends in the usage of bidentate phosphines as ligands in nickel catalysis, Chem. Rev., 2020, 120, 6124–6196 CrossRef CAS; (j) Q.-L. Zhou, Privileged chiral ligands and catalysts, Wiley-VCH, Weinheim, 2011, Vol. 6, pp. 55–91 CrossRef; (k) C. A. Busacca and C. H. Senanayake, The use of new phosphines as powerful tools in asymmetric synthesis of biologically active compounds, comprehensive chirality, ed. E. M. Carreira and H. Yamamoto, Elsevier, Amsterdam, 2012, Vol. 1 Search PubMed; (l) P. C. J. Kamer and P. W. N. M. van Leeuwen, Phosphorus(III) ligands in homogeneous catalysis: design and synthesis, Wiley, Chichester, 2012 CrossRef.
- (a) F. H. Westheimer, Why nature chose phosphates, Science, 1987, 235, 1173–1178 CrossRef CAS PubMed; (b) C. S. Demmer, N. Krogsgaard-Larsen and L. Bunch, Review on modern advances of chemical methods for the introduction of a phosphonic acid group, Chem. Rev., 2011, 111, 7981–8006 CrossRef CAS; (c) D. E. C. Corbridge, Phosphorus: chemistry, biochemistry and technology, CRC Press, 6th edn, 2013 Search PubMed; (d) H. Seto and T. Kuzuyama, Bioactive natural products with carbon–phosphorus bonds and their biosynthesis, Nat. Prod. Rep., 1999, 16, 589–596 RSC; (e) K. Yoshino, T. Kohno, T. Morita and G. Tsukamoto, Organic phosphorus compounds. 2. Synthesis and coronary vasodilator activity of (benzothiazolylbenzyl)phosphonate derivatives, J. Med. Chem., 1989, 32, 1528–1532 CrossRef CAS; (f) W.-Q. Jiang, G. Allan, X. Chen, J. J. Fiordeliso, O. Linton, P. Tannenbaum, J. Xu, P.-F. Zhu, J. Gunnet, K. Demarest, S. Lundeen and Z.-H. Sui, Novel phosphorus-containing 17 β-side chain mifepristone analogues as progesterone receptor antagonists, Steroids, 2006, 71, 949–954 CrossRef CAS PubMed; (g) P. Tatti, M. Pahor, R. P. Byington, P. D. Mauro, R. Guarisco, G. Strollo and F. Strollo, Outcome results of the fosinopril versus amlodipine cardiovascular events randomized trial (FACET) in patients with hypertension and NIDDM, Diabetes Care, 1998, 21, 597–603 CrossRef CAS; (h) L.-B. Han, Y. Ono, Q. Xu and S. Shimada, Highly selective Markovnikov-type addition of hypervalent H-spirophosphoranes to alkynes mediated by palladium acetate: generality and mechanism, Bull. Chem. Soc. Jpn., 2010, 83, 1086–1099 CrossRef CAS; (i) L. G. Costa, Organophosphorus compounds at 80: Some old and new issues, Toxicol. Sci., 2018, 162, 24–35 CrossRef CAS; (j) S. Monge and G. David, Phosphorus-based polymers from synthesis to applications, The Royal Society of Chemistry, Cambridge, 2014 RSC.
- (a) A. Yu. Rulev, Recent advances in Michael addition of H-phosphonates, RSC Adv., 2014, 4, 26002–26012 RSC; (b) Z. Li and W. Duan, Recent advances in the asymmetric conjugate addition reactions of phosphorus nucleophiles to electron-deficient alkenes, Chin. J. Org. Chem., 2016, 36, 1805–1813 CrossRef CAS; (c) D. S. Glueck, Catalytic asymmetric synthesis of chiral phosphanes, Chem. – Eur. J., 2008, 14, 7108–7117 CrossRef CAS; (d) D. Zhao and R. Wang, Recent developments in metal catalyzed asymmetric addition of phosphorus nucleophiles, Chem. Soc. Rev., 2012, 41, 2095–2108 RSC; (e) D. Enders, A. Saint-Dizier, M.-I. Lannou and A. Lenzen, The Phospha-Michael addition in organic synthesis, Eur. J. Org. Chem., 2006, 29–49 CrossRef CAS; (f) D. Zhao and R. Wang, Recent developments in metal catalyzed asymmetric addition of phosphorus nucleophiles, Chem. Soc. Rev., 2012, 41, 2095–2018 RSC.
- (a) J.-J. Feng, X.-F. Chen, M. Shi and W.-L. Duan, Palladium-catalyzed asymmetric addition of diarylphosphines to enones toward the synthesis of chiral phosphines, J. Am. Chem. Soc., 2010, 132, 5562–5563 CrossRef CAS PubMed; (b) J. Lu, J. Ye and W.-L. Duan, Palladium-catalyzed asymmetric 1,6-addition of diarylphosphines to a,b,c,d-unsaturated sulfonic esters: controlling regioselectivity by rational selection of electron-withdrawing groups, Chem. Commun., 2014, 50, 698–700 RSC; (c) J. Lu, J. Ye and W.-L. Duan, Palladium-catalyzed asymmetric addition of diarylphosphines to α,β-unsaturated sulfonic esters for the synthesis of chiral phosphine sulfonate compounds, Org. Lett., 2013, 15, 5016–5019 CrossRef CAS PubMed; (d) C. Li, Q.-L. Bian, S. Xu and W.-L. Duan, Palladium-catalyzed 1,4-addition of secondary alkylphenylphosphines to α,β-unsaturated carbonyl compounds for the synthesis of phosphorus- and carbon-stereogenic compounds, Org. Chem. Front., 2014, 1, 541–545 RSC; (e) W.-J. Yue, J.-Z. Xiao, S. Zhang and L. Yin, Rapid synthesis of chiral 1,2-bisphosphine derivatives through copper(I)-catalyzed asymmetric conjugate hydrophosphination, Angew. Chem., Int. Ed., 2020, 59, 7057–7062 CrossRef CAS; (f) Y.-R. Chen, J.-J. Feng and W.-L. Duan, NHC–copper-catalyzed asymmetric 1,4-addition of diarylphosphines to α, β-unsaturated ketones, Tetrahedron Lett., 2014, 55, 595–597 CrossRef CAS.
- (a) R. Maiti, J.-L. Yan, X. Yang, B. Mondal, J. Xu, H. Chai, Z. Jin and Y. R. Chi, Carbene-catalyzed enantioselective hydrophosphination of bromoenals to prepare phosphine-containing chiral molecules, Angew. Chem., Int. Ed., 2021, 60, 26616–26621 CrossRef CAS; (b) Y. Chen, Z. Yu, Z. Jiang, J.-P. Tan, J.-H. Wu, Y. Lan, X. Ren and T. Wang, Asymmetric construction of tertiary/secondary carbon−phosphorus bonds via bifunctional phosphonium salt catalyzed 1,6-addition, ACS Catal., 2021, 11, 14168–14180 CrossRef CAS; (c) L. Hou, J. Kikuchi, H. Ye, M. Bao and M. Terada, Chiral phosphoric acid-catalyzed enantioselective phospha-Michael-type addition reaction of diarylphosphine oxides with alkenyl benzimidazoles, J. Org. Chem., 2020, 85, 14802–14809 CrossRef CAS; (d) M. Terada, T. Ikehara and H. Ube, Enantioselective 1,4-addition reactions of diphenyl phosphite to nitroalkenes catalyzed by an axially chiral guanidine, J. Am. Chem. Soc., 2007, 129, 14112–14113 CrossRef CAS; (e) A. Carlone, G. Bartoli, M. Bosco, L. Sambri and P. Melchiorre, Organocatalytic asymmetric hydrophosphination of α, β-unsaturated aldehydes, Angew. Chem., Int. Ed., 2007, 46, 4504–4506 CrossRef CAS PubMed; (f) I. Ibrahem, R. Rios, J. Vesely, P. Hammar, L. Eriksson, F. Himo and A. Cordova, Enantioselective organocatalytic hydrophosphination of α, β-unsaturated aldehydes, Angew. Chem., Int. Ed., 2007, 46, 4507–4510 CrossRef CAS; (g) Y. Zhu, J. P. Malerich and V. H. Rawal, Squaramide-catalyzed enantioselective Michael addition of diphenyl phosphite to nitroalkenes, Angew. Chem., Int. Ed., 2010, 49, 153–156 CrossRef CAS; (h) Z. Gu, J. Zhou, G.-F. Jiang and Y.-G. Zhou, Synthesis of chiral γ-aminophosphonates through the organocatalytic hydrophosphonylation of azadienes with phosphites, Org. Chem. Front., 2018, 5, 1148–1151 RSC.
- (a) M. Hatano, T. Horibe and K. Ishihara, Chiral magnesium(II) binaphtholates as cooperative Brønsted/Lewis acid−base catalysts for the highly enantioselective addition of phosphorus nucleophiles to α, β-unsaturated esters and ketones, Angew. Chem., Int. Ed., 2013, 52, 4549–4553 CrossRef CAS PubMed; (b) N. Shao, Y.-Y. Luo, H.-J. Lu, Y.-Z. Hua and M.-C. Wang, Enantioselective phospha-Michael reaction of diethyl phosphonate with exocyclic α, β-unsaturated benzocyclic ketones catalyzed by a dinuclear zinc-AzePhenol catalyst, Tetrahedron, 2018, 74, 2130–2142 CrossRef CAS; (c) D. Zhao, L. Mao, D. Yang and R. Wang, Zinc-mediated asymmetric additions of dialkylphosphine oxides to α, β-unsaturated ketones and N-sulfinylimines, J. Org. Chem., 2010, 75, 6756–6763 CrossRef CAS PubMed.
- G. Zhang, L. Fu, P. Chen, J. Zou and G. Liu, Proton-coupled electron transfer enables tandem radical relay for asymmetric copper-catalyzed phosphinoylcyanation of styrenes, Org. Lett., 2019, 21, 5015–5020 CrossRef CAS.
- S.-J. He, J.-W. Wang, Y. Li, Z.-Y. Xu, X.-X. Wang, X. Lu and Y. Fu, Nickel-catalyzed enantioconvergent reductive hydroalkylation of olefins with α-heteroatom phosphorus or sulfur alkyl electrophiles, J. Am. Chem. Soc., 2020, 142, 214–221 CrossRef CAS PubMed.
- (a) Z. Yang and J. Wang, Enantioselective palladium-catalyzed hydrophosphinylation of allenes with phosphine oxides: access to chiral allylic phosphine oxides, Angew. Chem., Int. Ed., 2021, 60, 27288–27292 CrossRef CAS; (b) Z. Yang, Q. Du, Y. Jiang and J. Wang, Asymmetric access of γ-amino acids and γ-amino phosphonic acid derivatives via copper-catalyzed enantioselective and regioselective hydroamination, CCS Chem., 2022, 4, 1901–1911 CrossRef CAS.
- H. Wang, P. Zheng, X. Wu, Y. Li and T. Xu, Modular and facile access to chiral α-aryl phosphates via dual nickel- and photoredox-catalyzed reductive cross-coupling, J. Am. Chem. Soc., 2022, 144, 3989–3997 CrossRef CAS.
- (a) H. Pellissier and H. Clavier, Enantioselective cobalt-catalyzed transformations, Chem. Rev., 2014, 114, 2775–2823 CrossRef CAS; (b) H. Pellissier, Recent developments in enantioselective cobalt-catalyzed transformations, Coord. Chem. Rev., 2018, 360, 122–168 CrossRef CAS; (c) L.-M. Jin, X. Xu, H. Lu, X. Cui, L. Wojtas and X. P. Zhang, Effective synthesis of chiral N-fluoroaryl aziridines through enantioselective aziridination of alkenes with fluoroaryl azides, Angew. Chem., Int. Ed., 2013, 52, 5309–5313 CrossRef CAS PubMed; (d) X. Cui, X. Xu, L.-M. Jin, L. Wojtas and X. P. Zhang, Stereoselective radical C–H alkylation with acceptor/acceptor-substituted diazo reagents via Co(II)-based metalloradical catalysis, Chem. Sci., 2015, 6, 1219–1224 RSC; (e) Y. N. Belokon, V. I. Maleev, D. A. Kataev, T. F. Saveleva, T. V. Skrupskaya, Y. V. Nelyubina and M. North, Chiral ion pairs in catalysis: lithium salts of chiral metallocomplex anions as catalysts for asymmetric C–C bond formation, Tetrahedron: Asymmetry, 2009, 20, 1746–1752 CrossRef CAS; (f) V. I. Maleev, T. V. Skrupskaya, L. V. Yashkina, A. F. Mkrtchyan, A. S. Saghyan, M. M. Il'in and D. A. Chusov, Aza-Diels–Alder reaction catalyzed by novel chiral metalocomplex Brønsted acids, Tetrahedron: Asymmetry, 2013, 24, 178–183 CrossRef CAS; (g) H. Jiang, K. Lang, H. Lu, L. Wojtas and X. P. Zhang, Asymmetric radical bicyclization of allyl azidoformates via cobalt(II)-based metalloradical catalysis, J. Am. Chem. Soc., 2017, 139, 9164–9167 CrossRef CAS PubMed; (h) Y. Wang, X. Wen, X. Cui, L. Wojtas and X. P. Zhang, Asymmetric radical cyclopropanation of alkenes with in situ- generated donor-substituted diazo reagents via Co(II)-based metalloradical catalysis, J. Am. Chem. Soc., 2017, 139, 1049–1052 CrossRef CAS PubMed; (i) X. Xu, S. Zhu, X. Cui, L. Wojtas and X. P. Zhang, Cobalt(II)-catalyzed asymmetric olefin cyclopropanation with α-ketodiazoacetates, Angew. Chem., Int. Ed., 2013, 52, 11857–11861 CrossRef CAS PubMed; (j) X. Xu, H. Lu, J. V. Ruppel, X. Cui, S. L. de Mesa, L. Wojtas and X. P. Zhang, Highly asymmetric intramolecular cyclopropanation of acceptor-substituted diazoacetates by Co(II)-based metalloradical catalysis: iterative approach for development of new-generation catalysts, J. Am. Chem. Soc., 2011, 133, 15292–15295 CrossRef CAS; (k) S. Zhu, X. Xu, J. A. Perman and X. P. Zhang, A general and efficient cobalt(II)-based catalytic system for highly stereoselective cyclopropanation of alkenes with α-cyanodiazoacetates, J. Am. Chem. Soc., 2010, 132, 12796–12799 CrossRef CAS; (l) X. Cui, X. Xu, H. Lu, S. Zhu, L. Wojtas and X. P. Zhang, Enantioselective cyclopropenation of alkynes with acceptor/acceptor-substituted diazo reagents via Co(II)-based metalloradical catalysis, J. Am. Chem. Soc., 2011, 133, 3304–3307 CrossRef CAS PubMed; (m) S. Zhu, J. V. Ruppel, H. Lu, L. Wojtas and X. P. Zhang, Cobalt-catalyzed asymmetric cyclopropanation with diazosulfones: rigidification and polarization of ligand chiral environment via hydrogen bonding and cyclization, J. Am. Chem. Soc., 2008, 130, 5042–5043 CrossRef CAS PubMed; (n) Y. Chen, J. V. Ruppel and X. P. Zhang, Cobalt-catalyzed asymmetric cyclopropanation of electron-deficient olefins, J. Am. Chem. Soc., 2007, 129, 12074–12075 CrossRef CAS.
- Z.-H. Wu, A.-Q. Cheng, M. Yuan, Y.-X. Zhao, H.-L. Yang, L.-H. Wei, H.-Y. Wang, T. Wang, Z. Zhang and W.-L. Duan, Cobalt-catalysed asymmetric addition and alkylation of secondary phosphine oxides for the synthesis of P-stereogenic compounds, Angew. Chem., Int. Ed., 2021, 60, 27241–27246 CrossRef CAS.
- K. Zhang, L.-Q. Lu, Y. Jia, Y. Wang, F.-D. Lu, F. Pan and W.-J. Xiao, Exploration of chiral cobalt catalyst for Visible-Light-Induced enantioselective radical conjugate addition, Angew. Chem., Int. Ed., 2019, 58, 13375–13379 CrossRef CAS.
- (a) H.-M. Guo, Q.-Q. Zhou, X. Jiang, D.-Q. Shi and W.-J. Xiao, Catalyst- and oxidant-free desulfonative CP couplings for the synthesis of phosphine oxides and phosphonates, Adv. Synth. Catal., 2017, 359, 4141–4146 CrossRef CAS; (b) M.-M. Qiao, Y.-Y. Liu, S. Yao, T.-C. Ma, Z.-L. Tang, D.-Q. Shi and W.-J. Xiao, Photoredox/cobalt-catalyzed phosphinyloxy radical addition/cyclization cascade: Synthesis of phosphaisocoumarins, J. Org. Chem., 2019, 84, 6798–6806 CrossRef CAS; (c) J.-L. Chen, W. Tang, J.-Y. Che, K. Chen, G. Yan, Y.-C. Gu and D.-Q. Shi, Synthesis and biological activity evaluation of novel α-amino phosphonate derivatives containing a pyrimidinyl moiety as potential herbicidal agents, J. Agric. Food Chem., 2015, 63, 7219–7722 CrossRef CAS.
- The structures of chiral Co(II)-complex ΔCo9, ΔCo10 and chiral phospha-Michael product 3a were established through the X-ray diffraction analysis. The crystallographic data (CCDC 2131088, 2131089 and 2131091†).
- (a) L. Zhang and E. Meggers, Steering asymmetric Lewis acid catalysis exclusively with octahedral metal-centered chirality, Acc. Chem. Res., 2017, 50, 320–330 CrossRef CAS PubMed; (b) X. Huang and E. Meggers, Asymmetric photocatalysis with bis-cyclometalated rhodium complexes, Acc. Chem. Res., 2019, 52, 833–847 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC: 2131088, 2131089 and 2131091. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2qo01483a |
| This journal is © the Partner Organisations 2023 |