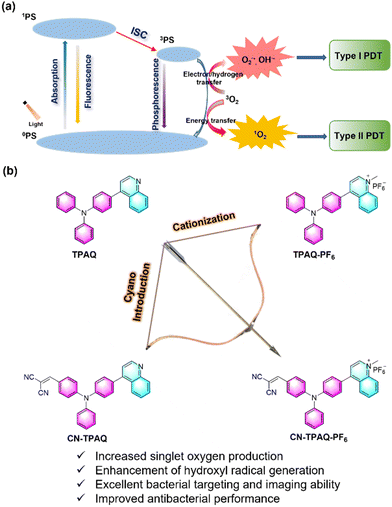
Cationic AIE-active photosensitizers for highly efficient photodynamic eradication of drug-resistant bacteria†
Yuewen
Yu‡
a,
Yubo
Liu‡
a,
Yitao
Chen
a,
Jinke
Chen
a,
Guangxue
Feng
 *a and
Ben Zhong
Tang
*b
*a and
Ben Zhong
Tang
*b
aState Key Laboratory of Luminescent Materials and Devices, Guangdong Provincial Key Laboratory of Luminescence from Molecular Aggregates, School of Materials Science and Engineering, AIE Institute, South China University of Technology, Guangzhou, 510640, China. E-mail: fenggx@scut.edu.cn
bShenzhen Institute of Aggregate Science and Technology, School of Science and Engineering, The Chinese University of Hong Kong, Shenzhen, 2001 Longxiang Boulevard, Longgang District, Shenzhen City, Guangdong 518172, China. E-mail: tangbenz@cuhk.edu.cn
First published on 21st November 2022
Abstract
Drug-resistant bacteria present a grave threat to human health. Photodynamic therapy (PDT) holds enormous potential as an innovative treatment in antimicrobial therapy. However, the generation of reactive oxygen species (ROS) for traditional photosensitizers in a hypoxic microenvironment or aggregated state is always restricted, limiting the antimicrobial effect. Herein, a cationization and cyano introduction molecular engineering strategy is reported to develop aggregation-induced emission active photosensitizers with enhanced type I ROS generation and bacteria binding ability for successful drug-resistant bacteria eradication. The introduction of a cyano group improves the light harvesting ability and ROS generation. This cationization can convert neutral molecules (TPAQ and CN-TPAQ) to their cationic counterparts (TPAQ-PF6 and CN-TPAQ-PF6), and enhance electron separation as well as transfer processes, which further promotes the ROS generation capacity, and in particular highly toxic hydroxyl radicals in aggregates that are 5.4-fold stronger than commercial crystal violet (CV) can be produced. As both the cationic charge and cyano group possess excellent bacterial binding affinity, the cationic CN-TPAQ-PF6 shows an excellent photodynamic killing efficiency of >99.999999% toward MRSA and >99.99999% toward S. aureus respectively at a very low concentration (2 μM) and under low intensity daylight exposure (40 mW cm−2), and the antibacterial performance is superior to that of clinical vancomycin antibiotics. Furthermore, CN-TPAQ-PF6 is also successfully applied in bacteria sterilization in natural lake water. This work provides a powerful guide for the appropriate design of novel and efficient type I AIE PSs to effectively conquer antibiotic resistance.
Introduction
Pathogenic bacteriological infections can result in serious illness and increased morbidity, posing a grave threat to worldwide public health.1–3 Since the development of penicillin for the management of bacteriological infections in 1928, antibiotics have given hope to humans for fighting bacteria.4 However, super bacteria or drug-resistant bacteria have emerged as a new threat due to the abuse of antibiotics, leading to the failure of many traditional antibacterial drugs.5,6 According to the Interagency Antimicrobial Resistance Coordination Group (IACG), drug-resistant infections cause over 700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 deaths each year in the world, and this will be increasing to 10 million per year as of 2050 if no action is taken.7 Therefore, the exploration of alternative therapeutic approaches and new bacteriostatic agents is of great significance for pharmacological and biomedical research.
000 deaths each year in the world, and this will be increasing to 10 million per year as of 2050 if no action is taken.7 Therefore, the exploration of alternative therapeutic approaches and new bacteriostatic agents is of great significance for pharmacological and biomedical research.
Photodynamic therapy (PDT) is considered an efficacious and alternative emerging antibacterial therapeutic approach due to its precise controllability, high spatial and temporal accuracy, noninvasiveness, and limited drug resistance.8–10 As the key component, photosensitizers (PSs) play a vitally important role in PDT, producing reactive oxygen species (ROS) in the presence of light, and then inactivating the bacteria.11–16 Generally, PSs are excited to their singlet excited state (S1) after light irradiation and subsequently proceed through intersystem crossing (ISC) to the triplet state (T1). The energetic long lifetime state (T1) of PSs could react with the surrounding substrates to generate type I ROS such as superoxide radicals (O2˙−), hydrogen peroxide (H2O2) and hydroxyl radicals (HO˙−) by an electron transfer mechanism, or type II ROS which is singlet oxygen (1O2) through an energy exchange process (Scheme 1a).17–19 Furthermore, type I PSs are more promising for antibacterial treatment with high therapeutic outcomes under a hypoxia microenvironment due to the reasons of less dependence on oxygen, and this is mainly attributed to the disproportionation involved in intracellular superoxide dismutase (SOD).20 To date, however, the great majority of reported PSs are related to the type II mechanism, and these PSs have many drawbacks, such as poor chemical stability, low photo-bleaching resistance, and small Stokes shifts.21 In addition, most PSs including these newly emerged type I PSs with planar structures suffer from a π–π stacking associated aggregation-caused quenching (ACQ) effect, resulting in unacceptable fluorescence quenching as well as low ROS efficiency in the aggregated state or high concentrations such as enriching in infected areas, which seriously hinders the clinical application of PDT antimicrobials.22,23
The concept of aggregation-induced emission (AIE) established in 2001 offers a novel solution to organic fluorophores and PSs.24 Quite different from these ACQ molecules, AIE-active fluorogens (AIEgens) show negligible fluorescence in the molecular state, but emit amplified fluorescence in the aggregated state because the nonradiative pathway is interrupted by the restriction of intramolecular motion (RIM), which also leads to promoted ISC processes as well.24–26 Thus, it makes AIEgens a promising candidate for ideal PSs for image-guided photodynamic inactivation of bacteria. It is critical to accelerate the ISC processes to enhance the generation capacity of ROS, which is normally realized by accelerating ISC processes such as through minimizing the energy gap (ΔEST) between S1 and T1 states. Separating the distribution of the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) was widely used to reduce ΔEST by constructing a donor–acceptor (D–A) backbone.27–29 However, this strategy mainly focuses on promoting the formation of the T1 state rather than emphasizing the improvement of electron separation and transfer processes to increase type I ROS generation. As a consequence, only a few AIEgens with type I ROS capabilities have been reported to date;30–36 nevertheless, we will mention their applications in the photodynamic inactivation of drug-resistant bacteria. In this regard, a general strategy to design type I AIE PSs with excellent bacteria targeting ability remains in high demand.
In this work, we report an economic cationization and cyano introduction molecular strategy that simultaneously amplifies type II (1O2) and type I (HO˙−) ROS generation and enhances the bacteria binding affinity for highly efficient PDT of drug-resistant bacteria. Triphenylamine (TPA) with a propeller configuration is selected as the electron donor, as its twisted geometry also helps to reduce the tight π–π stacking in the aggregate and to bring about the AIE feature. The quinolinium hexafluorophosphate (PF6−) group and cyano group with a strong electron withdrawing ability were conjugated with TPA to afford the asymmetric acceptor–donor–acceptor (A–D–A′) typed AIEgen CN-TPAQ-PF6 (Scheme 1b). The introduction of a cyano group improves the light harvesting ability and ROS generation. Benefitting from the electron-rich environment contributed by hexafluorophosphate after cationization,31CN-TPAQ-PF6 exhibits further enhanced ROS generation capability, especially the generation of 1O2 and HO˙−, and it is preferred over these widely used commercial PSs, including Chlorin e6 (Ce6) and crystal violet (CV). In addition, both the cyano group and the cationic hexafluorophosphate moiety also bestow CN-TPAQ-PF6 with an excellent bacteria recognition and targeting ability, providing outstanding in vitro fluorescence image guided PDT killing efficiency even for methicillin-resistant Staphylococcus aureus (MRSA) super bacteria. We anticipate that this proposed design approach can be adapted to develop additional type I PSs with AIE activity to fight against drug-resistant bacteria and super bacterial infections.
Results and discussion
Synthesis and characterization of cationic asymmetric AIE PSs
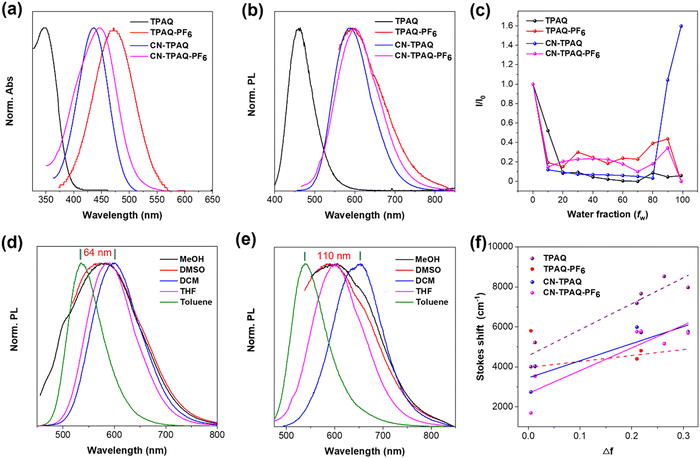
TPA was selected as an AIE active electron donor (D) group due to its nonplanar structure and strong electron donating ability. A quinoline fragment and a cyano group were employed as two different electron acceptors (A, A′). Suzuki coupling and condensation reactions and Vilsmeier reaction were used to obtain the neutral precursors of D–A type TPAQ and A–D–A′ type CN-TPAQ. The alkylation of quinoline in TPAQ and CN-TPAQ using iodomethane was followed by ion exchange in saturated KPF6 solution to obtain the cationic TPAQ-PF6 and CN-TPAQ-PF6 with yields of 80% and 83%, respectively. The detailed synthetic routes of these four PSs are displayed in the ESI† (Fig. S1), and furthermore, 1H NMR, 13C NMR, and high-resolution mass spectrometry (HRMS) were employed to confirm their chemical structures (Fig. S2–S15, ESI†).First, UV-vis and photoluminescence (PL) spectroscopy were engaged to evaluate the optical properties of these four PSs in THF solution. As displayed in Fig. 1a, the maximal absorption wavelengths of TPAQ, TPAQ-PF6, CN-TPAQ and CN-TPAQ-PF6 were located at 350, 472, 437 and 448 nm, respectively (Table S1, ESI†). In addition, the introduction of a cyano group not only changes the electron distribution of these molecules but also greatly increases the light harvesting ability of CN-TPAQ and CN-TPAQ-PF6 that is beneficial for ROS generation, and their molar absorption coefficients were 4.69 × 104 (at 437 nm) and 4.61 × 104 M−1 cm−1 (at 448 nm), respectively, much higher than those of their neutral precursors (Fig. S16, ESI†). TPAQ and CN-TPAQ had emission peaks at 464 and 590 nm, respectively, whereas the emission peaks of cationic TPAQ-PF6 and CN-TPAQ-PF6 with PF6− as the counter anion were bathochromic shifted to 595 and 602 nm, respectively (Fig. 1b and Table S1, ESI†). The bathochromic shifted absorption and emission peaks of 122 and 131 nm upon cationizing TPAQ to TPAQ-PF6 clearly indicate that cationization could greatly reinforce the electron withdrawing ability of the quinoline acceptor to narrow the HOMO–LUMO bandgap to achieve long absorption and emission wavelengths. In addition, the introduction of a cyano group leads to an increased hypochromatic shift of the absorption peak but a bathochromic shift of the emission peak for CN-TPAQ-PF6 as compared to TPAQ-PF6, indicating a more twisted excited state geometry for CN-TPAQ-PF6, which is beneficial for lowering the S1 state for better ISC processes and ROS generation. Instead, the absolute fluorescence quantum yields (η) of TPAQ, TPAQ-PF6, CN-TPAQ and CN-TPAQ-PF6 in THF solution were determined to be 85.3%, 0.97%, 11% and 2.4%, respectively (Table S1, ESI†). The η of TPAQ, TPAQ-PF6, CN-TPAQ and CN-TPAQ-PF6 in the solid state were measured to be 4.8%, 18.1%, 64.3% and 10.8%, respectively (Table S1, ESI†). The higher solid state η values of TPAQ-PF6, CN-TPAQ and CN-TPAQ-PF6 as compared to those in THF solution clearly indicate their AIE feature. The AIE properties of these four PSs were further studied by measuring their PL spectra in THF/H2O mixtures with increased water fraction (fw) to induce aggregate formation. As shown in Fig. 1c and Fig. S17 (ESI†), CN-TPAQ has negligible PL intensities in solution when the fw was below 80%. When increasing fw to 90%, the PL intensities of CN-TPAQ amplified dramatically and have a maximum value at fw = 99%, indicating its typical AIE feature. As for other PSs, their fluorescence gradually decreased when increasing fw. The main reason for this descended fluorescence intensity at high fw was attributed to the competition between the twisted intramolecular charge transfer (TICT) and AIE effect, where the polar aqueous environment overpasses the AIE feature. In addition, taking CN-TPAQ-PF6 as an example, the particle size distribution at different fw was determined by dynamic light scattering (DLS). As indicated in Fig. S18 (ESI†), CN-TPAQ-PF6 showed a very small hydrodynamic size of ∼2.0 nm when fw was below 50%, indicating it failed to form nanoparticles in this case. Further increasing fw leads to the formation of nanoaggregates but this aggregation formation could not compete with the ICT effect in highly polar aqueous solutions. To further evaluate the AIE feature of these PSs, their fluorescence in the solid state and solution state was evaluated under a 365 nm UV lamp. Remarkable bright fluorescence from these powders was observed, suggesting that these four PSs possess excellent AIE tendency and the strong π–π stacking is greatly reduced by the twisted molecular geometry in the solid state (Fig. S19, ESI†).
To further investigate the influence of a polar microenvironment on the optical behavior of these four PSs, a variety of solvents with different polarities were employed to measure their absorption and emission spectra. The emission peaks showed an obvious red-shift along with increased solvent polarities for all four PSs, while minimal changes were observed for their absorption spectra (Fig. 1d and Fig. S20, ESI†), especially for the neutral ones. The apparently increased Stokes’ shifts demonstrate the typical intramolecular charge transfer (ICT) features of these PSs (Fig. 1e and Fig. S21, ESI†). Taking CN-TPAQ for instance, its emission peak was red-shifted by 64 nm (from 536 to 600 nm) from toluene (low-polar solvent) to dichloromethane (high-polar solvent). Intriguingly, CN-TPAQ-PF6 showed an approximately 110 nm (from 540 to 652 nm) red-shift in emission peak under the same conditions. These experimental results suggest that cationization could enhance the ICT characteristics. Furthermore, the Lippert–Mataga model was carried out to further quantify the ICT level of these molecules by plotting the Stokes shift against the solvent orientation polarizability (Δf). As shown in Fig. 1e, the cationic CN-TPAQ-PF6 showed a larger slope as compared to its neutral precursor CN-TPAQ, noticeably suggesting that cationization can promote the ICT effect. However, it is difficult to acquire an accurate comparison between TPAQ and TPAQ-PF6 due to the PL intensity of TPAQ-PF6 in various solvents being extremely low (Fig. S21b, ESI†). Furthermore, the inhibited molecular motions of AIEgens in the aggregate could suppress the heat dissipation process and promote the radiative decay and ISC process, which is promising for higher fluorescence and ROS generation.37 A stronger ICT effect helps to reduce the excited S1 state energy level to reduce the ΔEST, as well as to increase the electron separation and transfer process for free radical generation.30,34,35 In this regard, AIE-active CN-TPAQ-PF6 with better light harvesting properties and a stronger ICT effect is anticipated to be the highly efficient AIE-active type I PS.
ROS generation of AIE PSs
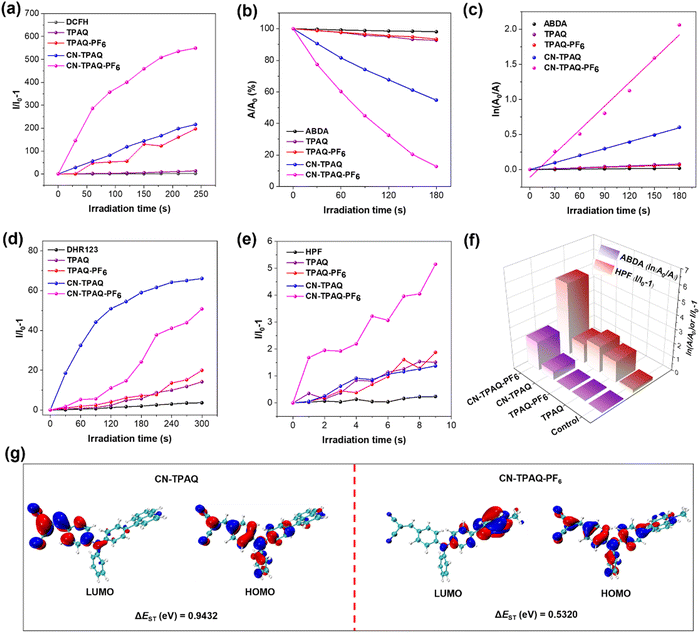
To verify our hypothesis, the ROS production capacities of the four AIE PSs were subsequently investigated. 2′,7′-Dichlorodihydrofluorescein (DCFH) was employed as a total ROS indicator.38 As illustrated in Fig. 2a and Fig. S22 (ESI†), the green fluorescence of DCFH is greatly amplified in the presence of TPAQ-PF6, CN-TPAQ and CN-TPAQ-PF6 within a short period of light irradiation (20 mW cm−2, 3 min), with fluorescence enhancement factors of 196.8, 216.0, and 549.3, respectively (Table S1, ESI†). However, this phenomenon was negatively observed for DCFH alone and in the presence of TPAQ, where an increase in fluorescence intensity was hardly detected under light irradiation. TPAQ-PF6 and CN-TPAQ-PF6 showed much higher ROS generation abilities than their neutral counterparts, which are nearly 15.0- and 2.5-fold higher than TPAQ and CN-TPAQ, respectively, as revealed by the DCFH fluorescence enhancement factors, clearly indicating that cationization is a progressive strategy to promote the ROS generation efficiency of PSs. Remarkably, cationization could convert the TPAQ with minimal ROS generation to TPAQ-PF6 with promising ROS generation ability, further reinforcing the effectiveness of our design strategy. In addition, CN-TPAQ-PF6 also showed a better ROS generation over TPAQ-PF6, suggesting that the cyano group introduction to afford this kind of A–D–A′ configuration is also beneficial for improving ROS production.The types of ROS generated by these AIE PSs were subsequently investigated. Primarily, 9,10-anthracenediyl-bis(methylene) dimalonic acid (ABDA),39 a famed indicator was employed to detect 1O2 generated by these four PSs. As shown in Fig. 2b and Fig. S23 (ESI†), the absorption of ABDA slightly decreased about 7.0% in the existence of TPAQ or TPAQ-PF6 after light irradiation for 3 minutes, indicating their insufficient 1O2 generation efficiency. Differently, the A–D–A′ type CN-TPAQ and CN-TPAQ-PF6 showed promising 1O2, where ABDA absorbance was reduced by 45.3% for CN-TPAQ and 87.3% for cationic CN-TPAQ-PF6, respectively (Table S1, ESI†), indicating that introducing a cyano group to redistribute the electron distribution helps to promote type II ROS generation. The decomposition rate result of the ABDA showed that the 1O2 generation efficiency of CN-TPAQ-PF6 is 3.4-fold higher than that of CN-TPAQ (Fig. 2c). It is worth noting that TPAQ-PF6 demonstrates promising ROS generation although it is not a type II PS, hinting that a type I ROS generation mechanism is involved in cationic AIE PSs (Fig. 2a–c).
Subsequently, the evaluation of type I ROS generated from these PSs was conducted. Dihydrorhodamine 123 (DHR 123) was utilized to assess the generation of O2˙−.40 As displayed in Fig. 2d and Fig. S24 (ESI†), TPAQ, TPAQ-PF6, CN-TPAQ and CN-TPAQ-PF6 adequately enhanced the green fluorescence signal of DHR 123 nearly by 14.2-, 20.0-, 66.1- and 50.8-fold, respectively, and the O2˙− production capacity followed the sequence of CN-TPAQ > CN-TPAQ-PF6 > TPAQ-PF6 > TPAQ (Table S1, ESI†). In addition, the production of OH˙− was further evaluated with hydroxyphenyl fluorescein (HPF).41 As shown, CN-TPAQ-PF6 caused a significant enhancement of HPF fluorescence signal, and the enhancement factors of HPF intensity upon adding CN-TPAQ and CN-TPAQ-PF6 with light irradiation were 1.4 and 5.2, respectively. This result indicated that cationization could increase the generation of highly toxic HO˙−. The enhancement factors of HPF intensity of TPAQ and TPAQ-PF6 were 1.5 and 1.9, respectively (Fig. 2e, Fig. S25 and Table S1, ESI†). Furthermore, two popular commercially available PSs, Ce6 (a type II PS)42 and CV (a type I PS)43 are employed as the benchmark to evaluate the ROS generation performance of our PSs. As shown in Fig. S26 (ESI†), CN-TPAQ-PF6 showed a superior ability to generate different ROS in contrast to the two commercial PSs. Additionally, both CN-TPAQ and CN-TPAQ-PF6 showed excellent photostability in water and PBS buffer solution, and a virtually minimal absorbance change was observed after white light irradiation (20 mW cm−2) for 5 min (Fig. S27 and S28, ESI†). Fig. 2f summarizes the production capability of type I and type II ROS for these four AIE PSs. It reveals that cationization can effectively enhance type I ROS (HO˙−) generation (TPAQversusTPAQ-PF6), while cyano group introduction to form an asymmetric A–D–A′ molecular structure could promote type II ROS (1O2) generation (TPAQ-PF6versusCN-TPAQ-PF6). The cationic A–D–A′ type CN-TPAQ-PF6 showed the best performance in producing both HO˙− and 1O2, promising for photodynamic eradication of drug-resistant bacteria.
To more profoundly understand why the cationization strategy could improve ROS generation, especially for type I ROS, time-dependent density functional theory (TD-DFT) calculations (B3LYP/6-31G (d, p)) were performed. Taking into account the more pronounced ROS enhancement capabilities, CN-TPAQ and CN-TPAQ-PF6 were selected for further mechanism analysis. As displayed in Fig. 2g, the HOMO and LUMO of CN-TPAQ are mainly located on the TPA and cyano moiety with an inferior separation. Cationization transforms quinoline segments from a moderate electron acceptor to a much stronger electron acceptor. Therefore, the LUMO of CN-TPAQ-PF6 is mainly located on the quinoline cation moiety, resulting in a significant separation of the HOMO–LUMO distribution. The larger separation of HOMO–LUMO would result in a smaller ΔEST, thus facilitating the ISC process and enhancing the ROS generation capacity of PSs.30 Furthermore, the ΔEST band gap of CN-TPAQ-PF6 (0.5320 eV) is quite small compared with CN-TPAQ (0.9530 eV), thus CN-TPAQ-PF6 shows a better ROS production efficiency than its neutral CN-TPAQ counterpart.
Bacteria imaging and light-enhanced PDT antibacterial study
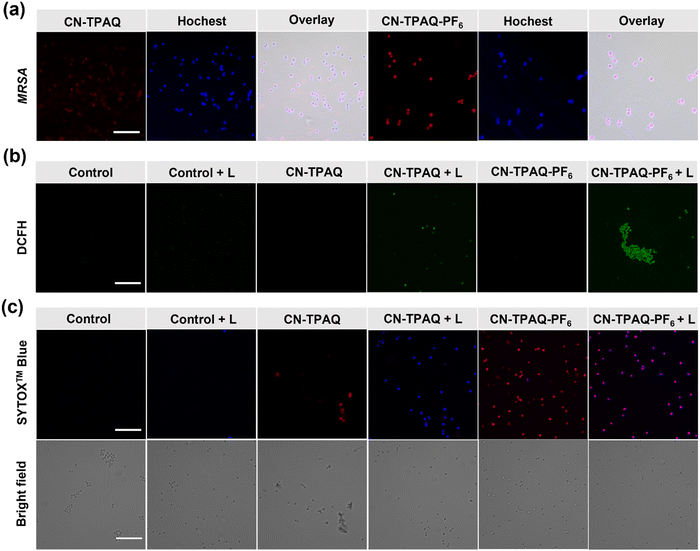
Pathogens are usually negatively charged, thus cationization also possibly provided these AIE PSs with the binding affinity.44–46 Furthermore, nitrile-containing molecules could have a high binding affinity to the phospholipid membrane of the bacterial cell wall by hydrogen bonding interaction.40 Considering the best ROS production with optimal HO˙− generation and the dual effect of bacterial binding ability of CN-TPAQ-PF6, CN-TPAQ and CN-TPAQ-PF6 were selected for further bacterial imaging and PDT antibacterial research. Initially, Staphylococcus aureus (S. aureus) and MRSA were employed for bacterial imaging respectively. Confocal laser scanning microscopy (CLSM) displayed similar bright red fluorescence from S. aureus after incubating with CN-TPAQ or CN-TPAQ-PF6 (2 μM) for 30 min (Fig. S29, ESI†). This phenomenon is likely due to the binding which could limit the intramolecular motions of AIEgens and hence light up their fluorescence signal. However, this situation is quite different in MRSA. MRSA incubated with CN-TPAQ-PF6 showed obviously stronger fluorescence than those incubated with CN-TPAQ (although CN-TPAQ possessed a much higher fluorescence brightness over CN-TPAQ-PF6) (Fig. 3a). Furthermore, CN-TPAQ-PF6 treated Escherichia coli (E. coli) also showed stronger fluorescence than CN-TPAQ treated E. coli (Fig. S30, ESI†), further indicating the stronger bacterial binding ability of cationic AIE PS. These results indicate that the cationic quinoline group also contributes to the improved bacterial binding of CN-TPAQ-PF6 apart from the nitrile segments. As a consequence, CN-TPAQ-PF6 has a higher binding affinity toward peptidoglycan on the MRSA cell membrane, making it more suitable for imaging and inactivating MRSA.Before estimating the PDT antibacterial efficacy of CN-TPAQ and CN-TPAQ-PF6, their ROS generation ability on a bacteria cell was firstly determined. CN-TPAQ and CN-TPAQ-PF6 pretreated MRSA were incubated with 2′,7′-dichlorodihydro fluorescein diacetate (DCFH-DA), a well-known ROS probe, followed by white light irradiation (20 mW cm−2, 5 min), subsequently recorded with a confocal laser scanning microscope (CLSM). As shown, the green fluorescence signal of DCFH-DA displays a more significant improvement upon white light exposure (20 mW cm−2, 5 min) for CN-TPAQ-PF6 than CN-TPAQ treated MRSA, while a nonobvious fluorescence signal was observed in the other groups (Fig. 3b and Fig. S31, ESI†), indicating that the cationic AIE PSs can effectively produce ROS inside bacteria upon light irradiation. The ability of CN-TPAQ and CN-TPAQ-PF6 to kill MRSA under light exposure was further visualized by the dead staining method with SYTOXTM Blue. As shown in Fig. 3c and Fig. S32 (ESI†), both CN-TPAQ and CN-TPAQ-PF6 treated MRSA with white light exposure displayed large ratios of blue fluorescence, while there is no obvious blue fluorescence signal in the control and the dark groups, suggesting the existence of AIE PSs and light could fairly easily and effectively eradicate MRSA.
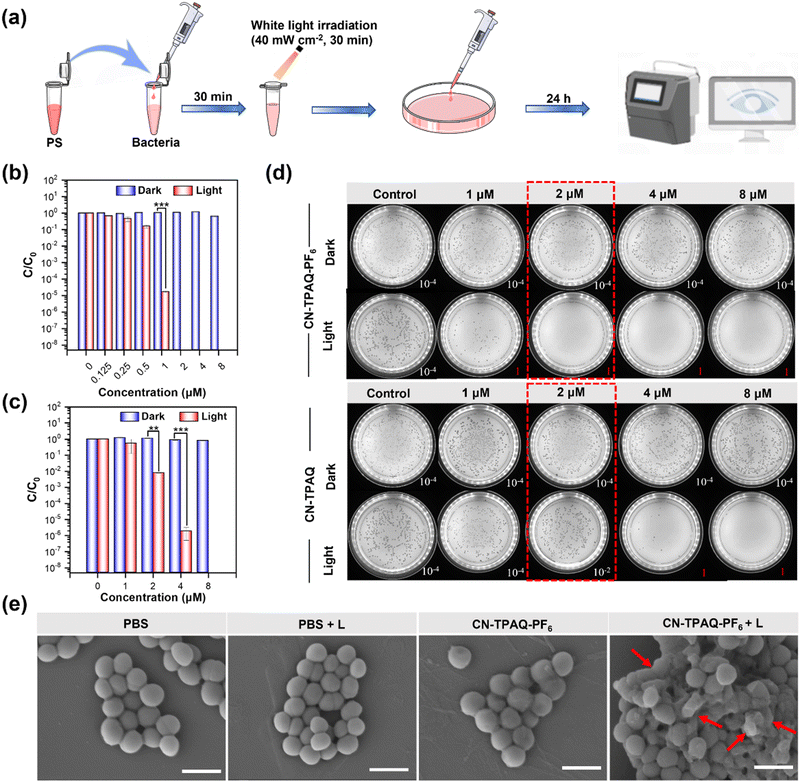
Encouraged by the excellent imaging capacity and type I ROS (OH˙−) generation efficiency of CN-TPAQ and CN-TPAQ-PF6, their antibacterial activity against MRSA was investigated through the colony formatting unit (CFU) plate count method (Fig. 4a–d and Fig. S33–S36, ESI†). Initially, the minimum inhibitory concentration (MIC) for MRSA and S. aureus of CN-TPAQ and CN-TPAQ-PF6 was studied. The MIC of CN-TPAQ-PF6 was 8 μM for both MRSA and S. aureus under dark conditions. However, CN-TPAQ showed a non-significant bacterial inhibitory effect on MRSA and S. aureus under dark conditions, even when the concentration increases up to 32 μM (Fig. S33 and S34, ESI†). Moreover, as shown in Fig. 4b–d and Fig. S35a (ESI†), the colony counting experiment results show that both CN-TPAQ and CN-TPAQ-PF6 in the absence of light irradiation hardly deliver an antibacterial effect towards MRSA and S. aureus. At an AIE PS concentration of 1 μM, CN-TPAQ-PF6 showed a sterilization efficiency of 99.998% toward MRSA under light irradiation, while the value for CN-TPAQ is only 44.63%. It is worth noting that CN-TPAQ-PF6 with a concentration of 2 μM demonstrates a sterilization efficiency of >99.999999% toward MRSA and >99.99999% toward S. aureus within 30 min of light exposure (40 mW cm−2), despite the bactericidal experiments being conducted in 0.9% (w/v) saline with MRSA and S. aureus cell densities of 108 CFU mL−1, indicating cationic CN-TPAQ-PF6 possess an excellent PDT antibacterial effect. Whereas 4 μM of CN-TPAQ could demonstrate an improved antimicrobial effect toward MRSA and S. aureus (Fig. S36, ESI†), it is still inferior to CN-TPAQ-PF6, as the sterilization efficiency of CN-TPAQ-PF6 (2 μM) toward MRSA is 6 orders of magnitude higher than that of CN-TPAQ (2 μM), and the sterilization efficiency of CN-TPAQ (8 μM) toward S. aureus is nearly 3 orders of magnitude lower than that of CN-TPAQ-PF6 (8 μM). Vancomycin (Van) is a common medical antibiotic, frequently used for a variety of drug-resistant bacterial infectious diseases. Thus, Van was also selected for comparison with CN-TPAQ-PF6, but Van failed to exert an observable antibacterial effect toward MRSA under similar conditions (incubated at 2 μM for 30 min) (Fig. S35b, ESI†), further indicating that CN-TPAQ-PF6 possesses a high bacterium killing efficiency.
In order to deeply understand the structure-function relationships of cationization AIE PSs, scanning electron microscopy (SEM) was utilized to investigate the morphological changes of the bacteria incubated with PBS (as control) and CN-TPAQ-PF6 under darkness or under light exposure, and MRSA was selected as the representative bacteria. As shown in Fig. 4e, the control group with PBS treatment did not impact the integrity of the MRSA cell walls, where a smooth bacterial lamina with well-defined and acute edges was visualized. However, the cell walls of MRSA significantly swelled, split and collapsed after the photodynamic treatment with CN-TPAQ-PF6. In brief, this cationization strategy could possess a huge potential to develop excellent antibacterial agents.
In view of the excellent PDT antimicrobial capability exhibited by CN-TPAQ-PF6, a sterilization experiment was further conducted with natural lake water. A certain volume of natural water was collected from the West Lake of South China University of Technology (SCUT) to test the sterilization efficacy of CN-TPAQ-PF6. As demonstrated in Fig. S37 (ESI†), CN-TPAQ-PF6 can efficiently kill bacteria in lake water upon light irradiation, further indicating that CN-TPAQ-PF6 has excellent practical application potential.
Conclusion
In summary, an asymmetrically cationic AIE PS with enhanced type I and type II ROS generation as well as enhanced bacterial binding affinity was reported for photodynamic inactivation of drug-resistant bacteria. A cationization molecular engineering strategy is developed to afford such PSs for antibacterial PDT, which preferably enhances the type I ROS generation capacity, especially the highly toxic OH˙− and improves the binding affinity capability towards bacteria. Specifically, cationization can significantly improve the electron-withdrawing capacity of the quinoline moiety, resulting in more superior separation of HOMO–LUMO to decrease ΔEST and further facilitate the ISC process as well as stronger electron transfer processes for generating free radicals. In addition, the introduction of a cyano group also renders the asymmetric CN-TPAQ-PF6 with greatly improved light harvesting ability, enhanced ROS generation and increased bacteria binding affinity. With this design, CN-TPAQ-PF6 could preferably bind to bacteria, even for MRSA. As such, the cationic CN-TPAQ-PF6 can be used at very low concentrations (2 μM) for photodynamic inactivation of bacteria, and it shows a sterilization efficiency of >99.999999% toward MRSA and >99.99999% toward S. aureus under low white light irradiation (40 mW cm−2), superior to clinical vancomycin antibiotics. With the excellent antibacterial performances, CN-TPAQ-PF6 is also successfully applied in natural lake water sterilization. We believe that eradication of drug-resistant bacteria by type I PDT is an effective approach, and our strategy of cationization as well as cyano introduction could simultaneously boost type I ROS generation and enhancing bacteria binding affinity could be a general method for the design of type I PSs as the next-generation of exclusive antimicrobial agents.Author contributions
Y. Y. and G. F. conceived the research; G. F. and B. Z. T. designed the research approach and supervised the study; Y. Y. performed the experiments and analyzed the results with the help of Y. L., Y. C. and J. C.; Y. Y. wrote the manuscript with input from all the authors; G. F. and B. Z. T. reviewed and revised the manuscript; Y. Y. and Y. L. contributed equally to this work.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
We are grateful for the support from Guangzhou Municipal Science and Technology Bureau (202102021224) and Guangdong Provincial Key Laboratory of Luminescence from Molecular Aggregates (2019B030301003).References
- A. Zlitni, G. Gowrishankar, I. Steinberg, T. Haywood and S. S. Gambhir, Maltotriose-based probes for fluorescence and photoacoustic imaging of bacterial infections, Nat. Commun., 2020, 11, 1250 CrossRef CAS PubMed.
- J. M. A. Blair, M. A. Webber, A. J. Baylay, D. O. Ogbolu and L. J. V. Piddock, Molecular mechanisms of antib-iotic resistance, Nat. Rev. Microbiol., 2015, 13, 42–51 CrossRef CAS PubMed.
- C. E. Moore, Changes in antibiotic resistance in animals, Science, 2019, 365, 1251–1252 CrossRef CAS PubMed.
- S. Nolivos, J. Cayron, A. Dedieu, A. Page, F. Delolme and C. Lesterlin, Role of AcrAB-TolC multidrug efflux pump in drug-resistance acquisition by plasmid transfer, Science, 2019, 364, 778–782 CrossRef CAS PubMed.
- A. J. Alanis, Resistance to Antibiotics: Are We in the Post-Antibiotic Era?, Arch. Med. Res., 2005, 36, 697–705 CrossRef PubMed.
- S. C. Davies, T. Fowler, J. Watson, D. M. Livermore and D. Walker, Annual Report of the Chief Medical Officer: infection and the rise of antimicrobial resistance, Lancet, 2013, 381, 1606–1609 CrossRef PubMed.
- WHO, No Time to Wait: Securing the Future from Drug-Resistant Infections, World Health Organization, Geneva, 2019, https://www.who.int/publications/i/item/no-time-to-wait-securing-the-future-from-drug-resistant-infections Search PubMed.
- A. P. Castano, P. Mroz and M. R. Hamblin, Photodynamic therapy and anti-tumour tmmunity, Nat. Rev. Cancer, 2006, 6, 535–545 CrossRef CAS PubMed.
- B. Wang, M. Wang, A. Mikhailovsky, S. Wang and G. C. Bazan, A Membrane-Intercalating Conjugated Oligoelectrolyte with High-Efficiency Photodynamic Antimicrobial Activity, Angew. Chem., Int. Ed., 2017, 56, 5031–5034 CrossRef CAS PubMed.
- Q. Jia, Q. Song, P. Li and W. Huang, Rejuvenated Photodynamic Therapy for Bacterial Infections. Rejuvenated Photodynamic Therapy for Bacterial Infections, Adv. Healthcare Mater., 2019, 8, 1900608 CrossRef PubMed.
- J. Qi, H. Ou, Q. Liu and D. Ding, Gathering brings strength: how organic aggregates boost disease phototheranostics, Aggregate, 2021, 2, 95–113 CrossRef.
- G. B. Kharkwal, S. K. Sharma, Y. Huang, T. Dai and M. R. Hamblin, Photodynamic therapy for infections: Clinical applications, Lasers Surg. Med., 2011, 43, 755–767 CrossRef PubMed.
- B. Yang, F. Gao, Z. Li, M. Li, L. Chen, Y. Guan, G. Liu and L. Yang, Selective Entropy Gain-Driven Adsorption of Nanospheres onto Spherical Bacteria Endows Photodynamic Treatment with Narrow-Spectrum Activity, J. Phys. Chem. Lett., 2020, 11, 2788–2796 CrossRef CAS PubMed.
- X. Shi, S. H. P. Sung, J. H. C. Chau, Y. Li, Z. Liu, R. T. K. Kwok, J. Liu, P. Xiao, J. Zhang, B. Liu, J. W. Y. Lam and B. Z. Tang, Killing G (+) or G (−) Bacteria? The Important Role of Molecular Charge in AIE-Active Photo-Sensitizers, Small Methods, 2020, 4, 2000046 CrossRef CAS.
- T. Dai, F. Ye, P. Hu, X. Pan, J. Chen, Y. Huang, H. Wang, L. Zhang, M. Huang, J. Liu, J. Su, X. Chen and Z. Chen, A strategy for enhanced tumor targeting of photodynamic therapy based on escherichia coli-driven drug delivery system, Sci. China Mater., 2021, 64, 232–240 CrossRef CAS.
- V.-N. Nguyen, Z. Zhao, B. Z. Tang and J. Yoon, Organic photosensitizers for antimicrobial phototherapy, Chem. Soc. Rev., 2022, 51, 3324–3340 RSC.
- S. Pervaiz and M. Olivo, Art and Science of Photodynamic Therapy, Clin. Exp. Pharmacol. Physiol., 2006, 33, 551–556 CrossRef CAS PubMed.
- K. Plaetzer, B. Krammer, J. Berlanda, F. Berr and T. Kiesslich, Photophysics and Photochemistry of Photodynamic Therapy: Fundamental Aspects, Lasers Med. Sci., 2009, 24, 259–268 CrossRef CAS PubMed.
- D. Chen, Z. Wang, H. Dai, X. Lv, Q. Ma, D.-P. Yang, J. Shao, Z. Xu and X. Dong, Boosting O2˙− Photogeneration via Promoting Intersystem-Crossing and Electron-Donating Efficiency of Aza-BODIPY-Based Nanoplatforms for Hypoxic-Tumor Photodynamic Therapy, Small Methods, 2020, 4, 2000013 CrossRef CAS.
- J. Yao, Y. Cheng, M. Zhou, S. Zhao, S. Lin, X. Wang, J. Wu, S. Li and H. Wei, ROS scavenging Mn3O4 nanozymes for: in vivo anti-inflammation, Chem. Sci., 2018, 9, 2927–2933 RSC.
- F. Hu, S. Xu and B. Liu, Photosensitizers with Aggregation-Induced Emission: Materials and Biomedical Applications, Adv. Mater., 2018, 30, 1801350 CrossRef PubMed.
- L. E. Bennett, K. P. Ghiggino and R. W. Henderson, Singlet oxygen formation in monomeric and aggregated porphyrin c, J. Photochem. Photobiol., B, 1989, 3, 81 CrossRef CAS PubMed.
- Y. Hu, S.-Y. Yin, W. Liu, Z. Li, Y. Chen and J. Li, Rationally designed monoamine oxidase a-activatable AIE molecular photosensitizer for the specific imaging and cellular therapy of tumors, Aggregate, 2022, e256 Search PubMed.
- J. Luo, Z. Xie, J. W. Y. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu, D. Zhu and B. Z. Tang, Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole, Chem. Commun., 2001, 1740–1741 RSC.
- J. Mei, Y. Hong, J. W. Lam, A. Qin, Y. Tang and B. Z. Tang, Aggregation-Induced Emission: The Whole Is More Brilliant Than the Parts, Adv. Mater., 2014, 26, 5429–5479 CrossRef CAS PubMed.
- J. Li, H. Gao, R. Liu, C. Chen, S. Zeng, Q. Liu and D. Ding, Endoplasmic reticulum targeted AIE bioprobe as a highly efficient inducer of immunogenic cell death, Sci. China: Chem., 2020, 63, 1428–1434 CAS.
- S. Xu, J. Li, P. Cai, X. Liu, B. Liu and X. Wang, Self-Improving Photosensitizer Discovery System via Bayesian Search with First-Principle Simulations, J. Am. Chem. Soc., 2021, 143, 19769–19777 CrossRef CAS PubMed.
- S. Liu, G. Feng, B. Z. Tang and B. Liu, Recent advances of AIE light-up probes for photodynamic therapy, Chem. Sci., 2021, 12, 6488–6506 RSC.
- P. Zhang, H. Kuang, Y. Xu, L. Shi, W. Cao, K. Zhu, L. Xu and J. Ma, Rational Design of a High-Performance Quinoxalinone-Based AIE Photosensitizer for Image-Guided Photodynamic Therapy, ACS Appl. Mater. Interfaces, 2020, 12, 42551–42557 CrossRef CAS PubMed.
- Q. Wan, R. Zhang, Z. Zhuang, Y. Li, Y. Huang, Z. Wang, W. Zhang, J. Hou and B. Z. Tang, Molecular Engineering to Boost AIE-Active Free Radical Photogenerators and Enable High-Performance Photodynamic Therapy under Hypoxia, Adv. Funct. Mater., 2020, 30, 2002057 CrossRef CAS.
- Z. Zhuang, J. Dai, M. Yu, J. Li, P. Shen, R. Hu, X. Lou, Z. Zhao and B. Z. Tang, Type I photosensitizers based on phosphindole oxide for photodynamic therapy: apoptosis and autophagy induced by endoplasmic reticulum stress, Chem. Sci., 2020, 11, 3405–3417 RSC.
- H. Huang, W. Xie, Q. Wan, L. Mao, D. Hu, H. Sun, X. Zhang and Y. Wei, A Self-Degradable Conjugated Polymer for Photodynamic Therapy with Reliable Postoperative Safety, Adv. Sci., 2022, 9, 2104101 CrossRef CAS PubMed.
- Y. Wang, Y. Li, Z. Zhang, L. Wang, D. Wang and B. Z. Tang, Triple-Jump Photodynamic Theranostics: MnO2 Combined Upconversion Nanoplatforms Involving a Type-I Photosensitizer with Aggregation-Induced Emission Characteristics for Potent Cancer Treatment, Adv. Mater., 2021, 33, 2103748 CrossRef CAS PubMed.
- Y. Yu, S. Wu, L. Zhang, S. Xu, C. Dai, S. Gan, G. Xie, G. Feng and B. Z. Tang, Cationization to boost both type I and type II ROS generation for photodynamic therapy, Biomaterials, 2022, 280, 121255 CrossRef CAS PubMed.
- X. Zhao, Y. Dai, F. Ma, S. Misal, K. Hasrat, H. Zhu and Z. Qi, Molecular engineering to accelerate cancer cell discrimination and boost AIE-active type I photosensitizer for photodynamic therapy under hypoxia, Chem. Eng. J., 2021, 410, 128133 CrossRef CAS.
- H. Li, M. Yang, J. Kim, J. Ha, J. Han, H. Kim, Y. Cho, J. Wang, K. Nam and J. Yoon, Structure-oriented design strategy to construct NIR AIEgens to selectively combat gram (+) multidrug-resistant bacteria in vivo, Biomaterials, 2022, 286, 121580 CrossRef CAS PubMed.
- L. Yang, X. Wang, G. Zhang, X. Chen, G. Zhang and J. Jiang, Aggregation-induced intersystem crossing: a novel strategy for efficient molecular phosphorescence, Nanoscale, 2016, 8, 17422–17426 RSC.
- K. Setsukinai, Y. Urano, K. Kakinuma, H. J. Majima and T. Nagano, Development of novel fluorescence probes that can reliably detect reactive oxygen species and distinguish specific species, J. Biol. Chem., 2003, 278, 3170–3175 CrossRef CAS PubMed.
- Y. Bu, T. Xu, X. Zhu, J. Zhang, L. Wang, Z. Yu, J. Yu, A. Wang, Y. Tian, H. Zhou and Y. Xie, A NIR-I light-responsive superoxide radical generator with cancer cell membrane targeting ability for enhanced imaging-guided photodynamic therapy, Chem. Sci., 2020, 11, 10279–10286 RSC.
- J. S. Nam, M.-G. Kang, J. Kang, S.-Y. Park, S. J. C. Lee, H.-T. Kim, J. K. Seo, O.-H. Kwon, M. H. Lim, H.-W. Rhee and T.-H. Kwon, Endoplasmic Reticulum-Localized Iridium (III) Complexes as Efficient Photodynamic Therapy Agents via Protein Modifications, J. Am. Chem. Soc., 2016, 138, 10968–10977 CrossRef CAS PubMed.
- J. Ni, T. Min, Y. Li, M. Zha, P. Zhang, C. Ho and K. Li, Planar AIEgens with Enhanced Solid-State Luminescence and ROS Generation for Multidrug-Resistant Bacteria Treatment, Angew. Chem., Int. Ed., 2020, 59, 10179–10185 CrossRef CAS PubMed.
- M. Li, J. Xia, R. Tian, J. Wang, J. Fan, J. Du, S. Long, X. Song, J. W. Foley and X. Peng, Near-Infrared Light-Initiated Molecular Superoxide Radical Generator: Rejuvenating Photodynamic Therapy against Hypoxic Tumors, J. Am. Chem. Soc., 2018, 140, 14851–14859 CrossRef CAS PubMed.
- K. Reszka, F. S. Cruz and R. Docampo, Photosensitization by the trypanocidal agent crystal violet. Type I versus type II reactions, Chem.-Biol. Interact., 1986, 58, 161–172 CrossRef CAS PubMed.
- W. Liu, L. Miao, X. Li and Z. Xu, Development of fluorescent probes targeting the cell wall of pathogenic bacteria, Coord. Chem. Rev., 2021, 429, 213646 CrossRef CAS.
- X. Wu, M. Yang, J. Kim, R. Wang, G. Kim, J. Ha, H. Kim, Y. Cho, K. Nam and J. Yoon, Reactivity Differences Enable ROS for Selective Ablation of Bacteria, Angew. Chem., Int. Ed., 2022, 61, e202200808 CAS.
- Y. Wang, Y. Du and N. Huang, A survey of the role of nitrile groups in protein-ligand interactions, Future Med. Chem., 2018, 10, 2713–2728 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2qm01043g |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2023 |