Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Coupling agents with 2,4,6,8-tetramethylcyclotetrasiloxane core – synthesis and application in styrene–butadiene rubber production†
Tomasz
Sokolnicki
 ab,
Adrian
Franczyk
ab,
Adrian
Franczyk
 a,
Radosław
Kozak
c and
Jędrzej
Walkowiak
a,
Radosław
Kozak
c and
Jędrzej
Walkowiak
 *a
*a
aCenter for Advanced Technology, Adam Mickiewicz University, Uniwersytetu Poznańskiego 10, 61-614 Poznań, Poland. E-mail: jedrzejw@amu.edu.pl
bFaculty of Chemistry, Adam Mickiewicz University, Uniwersytetu Poznańskiego 8, 61-614 Poznań, Poland
cSynthos S.A., Chemików 1, 32-600 Oświęcim, Poland
First published on 27th July 2023
Abstract
A facile one-pot procedure for the functionalization of 2,4,6,8-tetramethylcyclotetrasiloxane DH4 to branched organosilicon compounds DSP3 or DS3P bearing various, but clearly defined, numbers of silyl groups with an affinity to silica (S) and organic groups (alkyl, alkenyl, perfluoroaryl, epoxy, ether and ester) capable of bonding with polymers (P) has been demonstrated. Sequential hydrosilylation of allyltrimethoxysilane in the first step and olefin or alkyne in the second (and reverse order of reagents) has been applied in the presence of Karstedt's catalyst, which is commercially available and commonly used in industry. The developed procedure permitted the synthesis of 13 new, branched organosilicon compounds DSP3 and DS3P with excellent yields and precise distribution of functional groups. All products were easily isolated by simple filtration followed by drying under reduced pressure and fully characterized by spectroscopic methods (1H, 13C, 29Si NMR, IR) and mass spectrometry (ESI or MALDI). The obtained compounds, because of the presence of two different functional groups with an affinity to inorganic filler and polymer matrix, are regarded as versatile silica modifiers, silane coupling agents for various applications, and starting materials in the preparation of polymer networks and vapor-deposited coatings. The vulcanizates prepared using cyclosiloxane-functionalized rubber showed enhanced rolling resistance and wet skid resistance, proving the compound's potential in producing novel “Green Tire” treads.
Introduction
Over the years, silica and other inorganic fillers have become important reinforcing agents in the synthesis of organic–inorganic hybrid materials.1,2 The main problem those siliceous fillers generate for industrial applications is related to their hydrophilic nature, which causes difficulties in processing with hydrophobic polymers. Silica particles easily adhere to each other through hydrogen bonding between silanol groups and create agglomerates. Therefore, modification of the silica surface is required to overcome filler–filler interactions and improve filler-polymer compatibility. For this purpose, silane coupling agents (SCAs), which are bifunctional organosilicon compounds, are used. They belong to the most frequently used silica modifiers.3 These compounds contain two functional end-groups capable of forming covalent bonds with both organic and inorganic substances. One of these groups is a silicon atom with easily hydrolyzable groups (typically alkoxy, acyloxy, or halogen atoms). The other constitutes an organic moiety with an affinity to polymers. Functionalization of the silica surface with SCAs greatly increases the hydrophobicity of the filler and facilitates its dispersion in polymers. Consequently, the introduction of a silica/silane system into polymer matrices improves the mechanical, dynamic, and heat resistance of filled resins and rubbers.4 On the other hand, SCAs are also commonly applied as adhesion promoters, which are used in the production of adhesives, coatings, dental fillings, films, paints, and inks. Other applications involve cross-linking action (polymer networks, wire insulations), catalyst immobilization, and binding of biomaterials. The global SCAs market is expected to grow from USD 1.2 billion in 2021 to USD 1.6 billion by 2026, indicating the huge applicability of these organosilanes in academia and various branches of industry.5 Accordingly, a constant demand for new molecules with interesting properties has been observed in recent years.6,7The catalytic hydrosilylation reaction, which refers to the addition of silicon hydrides across multiple bonds, is a fundamental transformation in the synthesis of organosilicon fine chemicals, silicones, and SCAs.8 Bifunctional organosilanes able to act as coupling agents can be obtained by the hydrosilylation of two different substrates with silane, which has more than one Si–H bond. Several methodologies for the synthesis of such reagents with various alkenes and alkynes using different Si–H donors, including 1,1,3,3-tetramethyldisiloxane (having two Si–H bonds)9 and silsesquioxanes (having eight Si–H bonds)10,11 have recently been described. Herein, we would like to expand the library of new bifunctional silanes obtained by the functionalization of 2,4,6,8-tetramethylcyclo-tetrasiloxane (DH4) (bearing four Si–H bonds) with two different unsaturated substrates: allyltrimethoxysilane and another olefin or alkyne. Such molecules have potential applications as silica modifiers, SCAs or substrates in the synthesis of polymer materials.
DH4 is an example of a cyclic siloxane. This class of compounds is described by the general formula (SiR2O)n. They might be regarded as silicon analogs of crown ethers, since they form different-sized rings of alternating silicon and oxygen atoms, with various side chains (R = hydrogen atoms, alkyl, or vinyl groups) attached to silicon.12 These compounds are characterized by flexibility, high strength of Si–O bonds, and high reactivity under specified conditions in miscellaneous organic reactions, i.e., hydrolysis,13 halogenation,14 dehydrogenative couplings,15,16 silylative coupling,17,18 alkylative cleavage,19 Piers–Rubinsztajn reaction,20 ring-opening polymerizations,21,22 and hydrosilylation.23–39 8-Membered compounds such as DH4, because of the possible modification of peripheral Si–H groups by a hydrosilylation reaction, have opened access to several functional materials. While cyclosiloxanes have been utilized as substrates in the synthesis of dendritic molecules,40 films41,42 and coatings,30,43 sensors,44,45 liquid crystallines,46,47 electrolytes,48 ceramics,49 and membranes,50 there are no reports of their use as precursors of silica modifiers or coupling agents. The addition reactions of DH4 to various olefins have been the subject of numerous reports. The most widely described group of products is DR4 derivatives, which have four identical groups pendent from the cyclosiloxane core and are obtained by hydrosilylation between DH4 and unsaturated compounds, including non-functional aliphatic olefins,38,51 perfluoroalkenes,23 cycloalkenes,32 alkenylbenzenes,24,27,39 allyl alcohol derivatives,28,34 allylamines,35,37 alkenyl acid esters,31,33 vinylsilanes,25,29,36 and alkynes.26 Hydrosilylation reactions focused on the modification of DH4 with two different substrates (olefin or alkyne) leading to products with different content of the groups DSP3, DS2P2 or DS3P are a more challenging task, definitely less frequently described in the scientific journals, and mainly disclosed in the patent literature.52–58 Nevertheless, it must be noted that the distribution of both groups coming from two starting materials in the structures of synthesized products is often random. Moreover, detailed characterization of these compounds by NMR methods has not been provided in the majority of these reports. Therefore, it is impossible to elucidate the precise structure of bifunctional products and the distribution of both groups attached to the cyclosiloxane core. As a result, there has been no reliable method of the synthesis of 2,4,6,8-tetramethylcyclotetrasiloxane derivative with DRR′3 structure reported in the literature.
Bearing in mind the above-quoted facts about the application and synthesis of modified 2,4,6,8-tetramethylcyclo-tetrasiloxane, as well as the poor selectivity and unclear synthetic protocols for providing bifunctional compounds, here we present a one-pot synthetic protocol for the preparation of a wide gamut of functional organocyclotetrasiloxanes by platinum-catalyzed hydrosilylation of olefins and alkynes. The developed strategy involves the modification of DH4 in two steps: (i) reaction of DH4 with olefin possessing alkoxysilyl groups that allow for binding with silica (S), leading to a mono-decorated silyl derivative DH3R with three active Si–H bonds, which then in the second step (ii) react with alkenes or alkynes, which have groups capable of bonding with polymers (P). This pathway leads to derivatives with a DSP3 structure. Another procedure using both reagents in the reverse order produced coupling agents with a DS3P structure.
This work constitutes the first example of the synthesis of 2,4,6,8-tetramethylcyclotetrasiloxanes DSP3 or DS3P with a well-defined number of functional groups supported by comprehensive NMR, IR, and MS characterization of the synthesized compounds. Moreover, application tests of a selected compound in the production of “Green Tires” are presented.
Experimental
General procedure for the one-pot hydrosilylation of 2,4,6,8-tetramethylcyclotetrasiloxane DH4 with olefin 2a–c and allyltrimethoxysilane 2d – synthesis of silane coupling agents 5a–5c
The syntheses of compounds were conducted in two steps in a one-pot mode.Hydrosilylation of 2,4,6,8-tetramethylcyclotetrasiloxane DH4 with allyltrimethoxysilane 2d and second olefin 2a–c, 2e–h – synthesis of silane coupling agents 5d–5j
The syntheses were carried out using an analogous procedure to that described in the previous section, but allyltrimethoxy-silane 2d was applied in step I (leading to intermediate 4d), and another olefin 2a–c, 2e–h (3 equiv.) in step II.Hydrosilylation of 2,4,6,8-tetramethylcyclotetrasiloxane DH4 with allyltrimethoxysilane 2d and alkyne 2i–k – synthesis of silane coupling agents 5k–5m
The syntheses were carried out using an analogous procedure to that described in the previous section, but alkyne 2i–k (3 equiv.) was used instead of olefin in step II, and both steps were conducted at 50 °C.Results and discussion
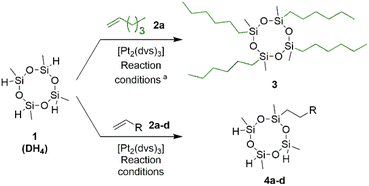
The selective functionalization of DH4 with two different substrates is challenging, due to the presence of four reactive Si–H bonds. Moreover, hydrosilylation of alkenes or alkynes with this reagent can lead to different constitutional isomers. Dehydrogenative silylation, olefin isomerization, or oligomerization are possible side reactions promoted by transition metal catalysts. Another problem constitutes the control of Si–H polyaddition to two different substrates, which may result in the formation of mixtures of products with different (very random) distributions of both functional groups (DR2R′2, DR3R′, DRR′3, and others with Si–H bonds that are not fully reacted). Taking into consideration the process's complexity, the proper selection of the catalytic system seems to be of major importance. Due to its excellent effectivity and high functional group tolerance, Pt-catalyzed hydrosilylation has been recognized as one of the most important reactions of homogeneous catalysis, allowing for the synthesis of a plethora of organosilanes and silicons. Therefore, based on reports in the literature and our previous experience59–65 in the synthesis of functionalized silanes via hydrosilylation reactions, we selected Karstedt's catalyst [Pt2(dvs)3, platinum(0)-1,3-divinyl-1,1,3,3-tetramethyldisiloxane] for our research.In the first stage of our studies, to prove the effectivity of Karstedt's catalyst, we carried out the reaction of DH4 with an excess of 1-hexene 2a (5 equiv.), leading to DR4 derivative 3. The reaction was conducted in anhydrous toluene in the presence of [Pt2(dvs)3] (1 × 10−3 mmol Pt/1 mmol of Si–H bond) at 100 °C for 24 hours (Table 1, entry 1). Tetrahexyl derivative (3) was isolated by filtration through a short plug of Cellite® and dried under reduced pressure, giving the product in excellent 97% yield. 1H and 13C NMR analyses of the pure product confirmed the exclusive formation of the anti-Markovnikov product. On the other hand, the multiplicity of signals around 20 ppm on 29Si NMR may imply that the product exists as a mixture of cis–trans isomers with different spatial arrangements of methyl and n-hexyl groups on silicon centers. Next, our efforts were focused on the introduction of only one aliphatic chain into the cyclosiloxane ring. Substrates with more than one Si–H bond suffer from the possible uncontrolled polyaddition of Si–H bonds to the unsaturated substrates, giving mixtures of products with a different number of hydrogens and alkyl groups attached to the silicon atoms. Therefore, the hydrosilylation of 1-hexene (2a) with an excess of 2,4,6,8-tetramethylcyclotetrasiloxane (DH4) was conducted to verify the feasibility of incorporation of only one n-hexyl group into the cyclotetrasiloxane ring and to check the stability of targeted compounds with reactive Si–H functionalities. Indeed, applying 6 equiv. excess of DH4 to 1-hexene (2a) resulted in the formation of the hydrosilylation product DH3(n-hexyl) decorated with one n-hexyl chain 4a in 94% yield (Table 1, entry 2). In this case, milder conditions compared to the synthesis of the tetra-n-hexyl product 3 (lower temperature of 50 °C and lower catalyst concentration of 2.5 × 10−4 mmol Pt/1 mmol of Si–H bond) were applied. Moreover, we observed that 6-fold excess of DH4 was required to prevent the formation of adducts with more than one Si–H bond reacted. Decreasing the volume of DH4 resulted in a higher content of DH2(n-hexyl)2 in the post-reaction mixture. The excess of DH4 was removed by fast and simple trap-to-trap distillation which allows its reuse in further tests. GC-MS and NMR techniques confirmed that distillate collected in a cold trap contained only DH4, and other low boiling cyclosiloxane derivatives or products of DH4 decomposition were not observed.
| Entry | Product | R | Isolated yield of 3 or 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b [%] b [%] |
|---|---|---|---|
Reaction conditions: [DH4]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [2] [2]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Pt] = 6 [Pt] = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 × 10−4, 50 °C, 24 h.a Reaction conditions: [DH4] 2.5 × 10−4, 50 °C, 24 h.a Reaction conditions: [DH4]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [2] [2]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Pt] = 1 [Pt] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 × 10−3, 100 °C, 24 h, anhydrous toluene, total conversions of reagents were confirmed by 1H NMR; the selectivity was determined by GC-MS, and 1H, 13C, 29Si NMR.b The products exist as a mixture of stereoisomers.c Yields based on the amount of alkenes. 1 × 10−3, 100 °C, 24 h, anhydrous toluene, total conversions of reagents were confirmed by 1H NMR; the selectivity was determined by GC-MS, and 1H, 13C, 29Si NMR.b The products exist as a mixture of stereoisomers.c Yields based on the amount of alkenes. |
|||
| 1 | 3 |

|
97a |
| 2 | 4a |

|
94c |
| 3 | 4b |

|
92c |
| 4 | 4c |
|
90c |
| 5 | 4d |

|
93c |
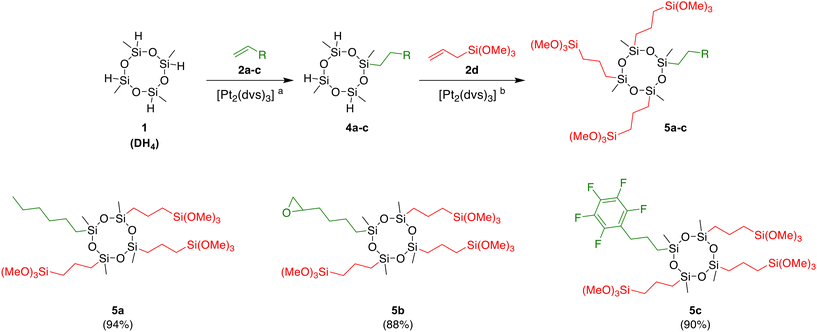
On the other hand, analyses of the isolated product 4a showed that only traces of by-product DH2(n-hexyl)2 with two aliphatic arms in its structure had been formed. An analogous procedure was followed for the reactions with 1,2-epoxy-5-hexene 2b, allylpentafluorobenzene 2c, and allyltrimethoxysilane 2d. Targeted products 4b–d were obtained in 92%, 90%, and 93% yields respectively. The compounds 4a–c were next subjected to hydrosilylation reactions with allyltrimethoxysilane 2d (3 equiv.), giving potential silica modifiers (5) equipped with one arm with affinity to polymer matrices and three pendent organosilicon moieties (DS3P modifiers) capable of hydrolysis and condensation with OH-rich materials, e.g., silica and other siliceous fillers (Scheme 1).
Products 5a–c were isolated with excellent yields (94%, 88%, and 90% respectively). Similar results were obtained when the syntheses were repeated in a one-pot procedure, without isolation of the intermediates 4a–c. Therefore, the next experiments were carried out according to this method (Scheme 2). After completion of the first step, volatiles were removed under reduced pressure, and then a new portion of the solvent, alkene 2a–c, 2e–h or alkyne 2i–k (3 equiv.) and the catalyst solution were added, followed by immediate placement of the reaction vessel in an oil bath heated to 50 °C. Using the described method, three new organocyclosiloxanes with one silylpropyl group and three alkyl 5a, alkyl epoxide 5b or perfluoroaryl 5c chains were synthesized. The desired products with the DS3P structure were obtained with an excellent yield of 88–94% after filtration through a pad of Cellite and drying under reduced pressure.
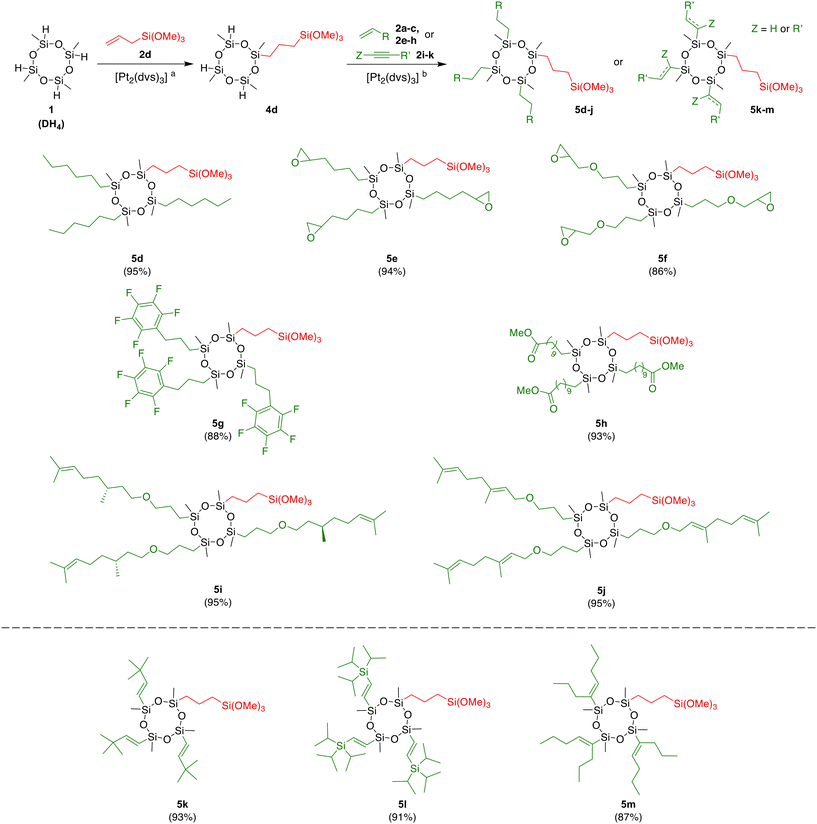
In the next step of our research, we focused on the synthesis of new silanes equipped with one organosilicon moiety, capable of modifying the surface of inorganic fillers, and three moieties reactive towards polymers (DSP3 modifiers). For this purpose, we applied the reverse order of reactions. First, the reaction of DH4 with allyltrimethoxysilane (2d) furnished the intermediate 4d, which after the evacuation of volatiles was used as the substrate in the second step (Table 1, entry 5). Based on our previous work on the hydrosilylation of various substrates with different silanes, we selected olefins 2a–c, 2e–h with groups with an affinity to polymers (alkenyl, epoxy, perfluoroaryl, ester), which might react with compound 4d in the presence of Karstedt's catalyst, giving access to the new molecules. Such compounds have potential as attractive additives or modifiers in materials chemistry and in various branches of industry. All hydrosilylation experiments between compound 4d and olefins 2a–c, 2e–h (3 equiv.) were carried out in anhydrous toluene at 100 °C for 24 h. The total conversion of reagents was confirmed by 1H NMR spectroscopy. Moreover, the equimolar ratio of reagents permitted simplification of the separation procedure. Products were isolated using the aforementioned procedure, giving viscous oils with excellent yields (Scheme 2). The hydrosilylation of 1-hexene 2a furnished compound 5d in an almost quantitative yield (95%). Although this compound does not possess a chemical group with an affinity to polymers, it may be considered a hydrophobing agent. Further reactions conducted with 1,2-epoxy-5-hexene 2b and allyl glycidyl ether 2e led to the cyclotetrasiloxanes 5e and 5f, equipped with a reactive epoxy group in 94% and 86% yields, respectively. Silanes functionalized with oxirane rings are essential components of modern resins, coatings, adhesives, and other epoxy-containing hybrid organic–inorganic materials.66,67 The hydrosilylation of allylpentafluorobenzene 2c with 4d resulted in the formation of derivative 5g with three perfluoroaryl moieties in 88% yield. Fluorosilanes are commonly known for their wide applicability in the preparation of superhydrophobic materials.68,69 The developed strategy was also compatible with compounds that have ester moieties in their structures. The hydrosilylation of methyl 10-undecenoate 2f furnished long-chain functionalized cyclotetrasiloxane 5h in 93% yield. We also investigated the hydrosilylation of unsymmetrical allyl ethers derived from citronellol 2g, and geraniol 2h, which have one and two trisubstituted C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds in their structures, respectively. The addition of Si–H bonds of intermediate 4d to these bio-based substrates proceeded chemoselectively on allyl endings, producing β-isomers exclusively. Competitive hydrosilylation of more substituted terpene multiple bonds was not observed. The products 5i and 5j, which have alkenyl functionalities, were obtained with excellent isolated yields (95%). The synthesis of new additives of high implementation potential from naturally occurring compounds rather than petrochemical resources complies with the Sustainable Goals highlighted by the United Nations. Coupling agents with alkenyl moieties have been reported as valuable fiber modifiers in thiol–ene click reactions,70 and were also used in the synthesis of nanocomposites by copolymerization.71
C bonds in their structures, respectively. The addition of Si–H bonds of intermediate 4d to these bio-based substrates proceeded chemoselectively on allyl endings, producing β-isomers exclusively. Competitive hydrosilylation of more substituted terpene multiple bonds was not observed. The products 5i and 5j, which have alkenyl functionalities, were obtained with excellent isolated yields (95%). The synthesis of new additives of high implementation potential from naturally occurring compounds rather than petrochemical resources complies with the Sustainable Goals highlighted by the United Nations. Coupling agents with alkenyl moieties have been reported as valuable fiber modifiers in thiol–ene click reactions,70 and were also used in the synthesis of nanocomposites by copolymerization.71
Another approach which also led to cyclosiloxane modifiers fitted with unsaturated moieties was the hydrosilylation of alkynes 2i–k with compound 4d. All reactions were performed using analogical reaction conditions, previously optimized for olefins; however, alkynes seemed to be more reactive and 50 °C was sufficient to achieve the total conversion of reagents (100 °C was applied for olefins). Very good yields were observed for the three starting materials tested. Hydrosilylation of 3,3-dimethyl-1-butyne 2i with 4d led to regio- and stereoselective addition of Si–H across carbon–carbon multiple bonds with the predominant formation of (E)-isomer 5k (3JH–H = 19.1 Hz) in 93% yield. On the other hand, the presence of a silicon atom in the alkyne structure impaired the selectivity of the reaction. Hydrosilylation of (triisopropylsilyl)acetylene 2j afforded product 5l bearing three alkenyl arms in 91% yield (mixture of geometric isomers). For internal alkenes, cis-hydrosilylation occurred, giving the product 5m in 87% yield, obtained by the reaction of compound 4d with 3 equiv. of 4-octyne 2k.
All organocyclosiloxanes obtained by the selective modification of 2,4,6,8-tetramethylcyclotetrasiloxane DH4 with two different substrates were synthesized with very good to excellent yields. They were fully characterized by spectroscopic methods (1H, 13C, 29Si NMR, IR) and mass spectrometry (ESI or MALDI). In particular, 29Si NMR analysis is of crucial importance in the identification of such systems, as it permits fast differentiation between the possible products (Fig. 1). 2,4,6,8-Tetramethylcyclotetrasiloxane DH4 displays one signal split into four resonance peaks around −32 ppm, while 2,4,6,8-tetrahexyl-2,4,6,8-tetramethylcyclotetrasiloxane 3 shows a signal shifted to ca. −20 ppm, which is also visible in the form of four peaks. On the other hand, cyclotetrasiloxane derivative decorated with one n-hexyl arm 4a is characterized by a signal at −16.6 ppm, representing the silicon atom substituted with two alkyl groups (Me and n-hex), and by a group of signals around −32 ppm, which correspond to three Si atoms with hydrogen and methyl groups bonded respectively (mixture of cis–trans stereoisomers). The 29Si NMR spectrum of tetramethylcyclotetrasiloxane decorated with one propyltrimethoxy-silyl moiety 4d shows the same chemical shifts as the monohexyl-decorated compound. Additionally, it has a signal around −42 ppm, which comes from the trimethoxysilyl group. Ultimately, the final products with two types of silicon atoms in their structures 5d can be distinguished by the presence of the two signals at ca. −20 and −42 ppm, coming from silicon atoms in the cyclosiloxane ring and the aliphatic external chain respectively.
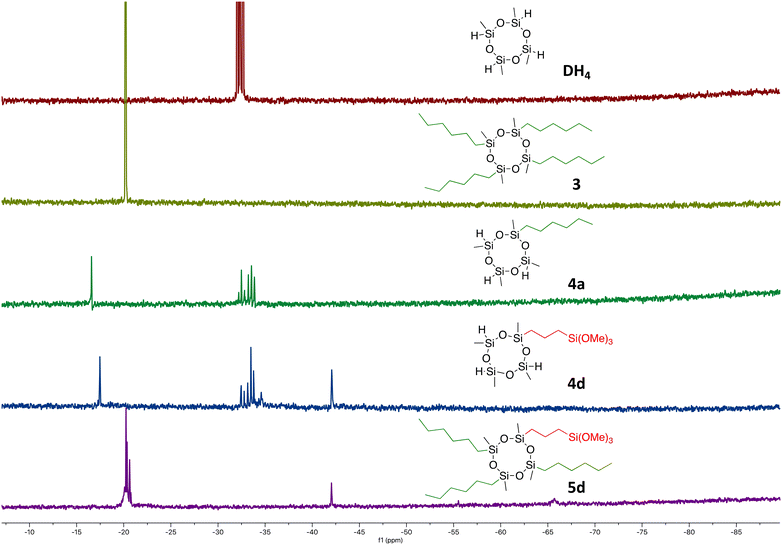 | ||
| Fig. 1 29Si NMR analysis of products obtained by the modification of 2,4,6,8-tetramethylcyclotetrasiloxane DH4via hydrosilylation reactions of 1-hexene 2a and allyltrimethoxysilane 2d. | ||
The application of Karstedt's catalyst, which is simple, commercially available and of high industrial importance, allowed for the introduction of structurally different groups such as alkyl, alkenyl, epoxy, perfluoroaryl, ester and alkoxysilyl into the cyclosiloxane core. A series of new compounds have been synthesized for the first time (three compounds with the structure DS3P, and 10 products with the structure DSP3). The simple isolation procedure, which involves filtration of the post-reaction mixture through a short Cellite® plug and drying under reduced pressure is advantageous and reduces time-consuming purification steps. Moreover, the DH4 used in excess in the first step of the protocol can easily be evaporated under vacuum, condensed in a cooling trap and reused in the next syntheses. The proper management of reagents is important for the possible application of the procedure on a larger scale. Due to the presence of two reactive groups, these newly synthesized silanes might be considered potential silica modifiers, SCAs for various applications, or additives in the synthesis of polymers or vapor-deposited coatings.
To check the application potential of the synthesized products, in the final stage of our research, a representative example (chosen with the Synthos S.A. company specialized in the production of rubbers), compound 5e, equipped with one organosilicon moiety (capable of forming stable bonds with silica) and three epoxy groups with an affinity to polymer was applied as an SCA in the synthesis of silica-filled styrene–butadiene rubber vulcanizates with the potential to produce “Green Tire” treads. Such tires are characterized by lower rolling resistance and enhanced wet grip compared to tires that only contain carbon black as a filler. Tires with low rolling resistance need less energy to keep rolling, which corresponds to decreased fuel consumption by mechanical vehicles and therefore contributes to the reduction of global emissions of CO2 into the atmosphere. This is actually of a great importance due to the need to reduce the emission of greenhouse gases. On the other hand, the improvement in the dry handling and wet grip properties of car tire treads has a profound influence on driving comfort and safety. Tread parameters are highly dependent on the tire components and the coupling agents used in their production.
In this work, we decided to investigate the influence of the functionalization of Syntion 2150® rubber with cyclosiloxane SCA 5e on the properties of the final composites and compare them with unmodified commercially available rubber. Modified rubber was prepared via anionic polymerization using n-butyl-lithium as an initiator (the detailed procedure is described in ESI, section 4.1†). Rubber compounds were prepared based on a model recipe for a passenger car tire tread using Syntion 2150® and Synteca 44® rubbers, silica as the main filler, and various processing additives.72 Compounding of the rubbers was conducted in a Banbury-type internal mixer, while the final pass was completed on a lab-sized two-roll mill. Next, rubber compounds and vulcanizates were subjected to several tests to determine their dynamic and mechanical properties (the detailed rubber characterization, compound composition, mixing procedure, and analytical methods used to characterize the compounds and vulcanizates are presented in the ESI, sections 4.2.–4.4. and Tables S1–S5†).
After preparing the rubber compounds, the Payne effect was measured using a rotorless shear rheometer (please see ESI, section 4.5., Tables S6, S7 and Fig. S58†). The Payne effect is an important parameter which can be considered a measure of filler micro-dispersion, as it is attributed to strain-induced breakage and recovery of weak physical bonding between filler clusters. The results show that the Payne effect for rubber functionalized with 5e is lower than for unmodified rubber. The lower the value of the Payne effect, the better the micro-dispersion of the filler in the rubber compound, which usually corresponds to lower energy losses (lower values of the rolling resistance predictor). Next, rubber compounds were vulcanized at 170 °C (curing characteristics are presented in ESI, Table S8 and Fig. S59†) and mechanical and dynamic characterization of vulcanizates was carried out. Dynamic mechanical analysis (DMA) was performed with the aid of DMA 450 + Metravib with the attached 450 N load cell in shear. The loss factor (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ) and storage modulus (G′) for vulcanizates as a function of temperature are listed in ESI in Table S9† and depicted in Fig. S60.† The tire predictors were determined as follows: rolling resistance measured as tan
δ) and storage modulus (G′) for vulcanizates as a function of temperature are listed in ESI in Table S9† and depicted in Fig. S60.† The tire predictors were determined as follows: rolling resistance measured as tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ at 60 °C, wet traction measured as tan
δ at 60 °C, wet traction measured as tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ at 0 °C, ice traction measured as tan
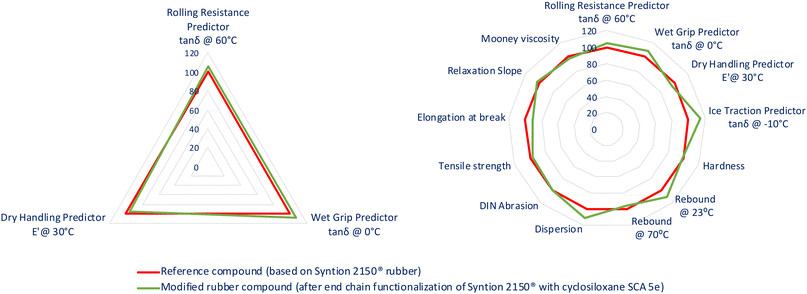
δ at 0 °C, ice traction measured as tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ at −10 °C and dry handling as G′ at 30 °C. Crucial parameters for the development of new “Green Tire” rubbers prepared with the use of the synthesized cyclosiloxane coupling agent have been depicted in the form of a triangle chart, including rolling resistance, dry handling, and wet grip predictors, while all measured parameters are presented as a radar chart in Fig. 2 (please see also ESI, Table S10†).
δ at −10 °C and dry handling as G′ at 30 °C. Crucial parameters for the development of new “Green Tire” rubbers prepared with the use of the synthesized cyclosiloxane coupling agent have been depicted in the form of a triangle chart, including rolling resistance, dry handling, and wet grip predictors, while all measured parameters are presented as a radar chart in Fig. 2 (please see also ESI, Table S10†).
It was observed that the rolling resistance of vulcanizates functionalized with 5e was improved by 6% compared to that of unmodified vulcanizates, showing the compound's potential in the preparation of fuel-saving tire treads. Moreover, the wet grip and ice traction predictors for the modified rubber turned out to be enhanced by 7% and 15% respectively, which would have a positive impact on driving safety. Vulcanizates modified with 5e were also characterized by improved filler dispersion and rebound at room temperature (by 11% for both parameters). Furthermore, the rubber's mechanical properties, i.e., hardness, tensile strength, and abrasion resistance, were comparable to those of the unmodified rubber.
The improvement of rolling resistance and wet skid properties (wet grip and ice traction predictors) of vulcanizates prepared with the rubbers functionalized with a newly synthesized cyclosiloxane coupling agent confirmed the possibility of its application in the production of “Green Tire” treads. Reduced rolling resistance causes lower fuel consumption since less energy is being used as the tires roll along the road and therefore has a positive impact on the environment by reducing a vehicle's carbon footprint. On the other hand, wet grip properties are crucial parameters which influence road traction, directional control, and deceleration on wet and icy surfaces. Enhanced wet grip predictors indicate a shorter braking distance on slippery roads, which would significantly improve driving safety.
Currently, we are working on the synthesis of new compounds intended to improve the wear resistance of rubbers. The results of this investigation will be the subject of another article in a more specialized journal.
Conclusions
In conclusion, we have developed a one-pot procedure for the synthesis of new organosilanes by selective modification of 2,4,6,8-tetramethylcyclotetrasiloxane via the subsequent hydrosilylation reaction of two different substrates: allyltrimethoxysilane and another olefin or alkyne. The application of Karstedt's catalyst, which is highly effective and commercially available, permitted the incorporation of a wide spectrum of functional groups, including alkenyl, perfluoroaryl, ether, ester, and epoxy moieties, into the cyclotetrasiloxane core, leading to 13 new products in very high, almost quantitative yields. All compounds were isolated by simple filtration through a thin pad of Cellite® and drying under reduced pressure, which makes the target products easily accessible. Hydrosilylation of alkenes proceeded in a regioselective manner, giving anti-Markovnikov isomers as major products. The addition of DH3R intermediates to sterically hindered terminal alkyne led to the predominant formation of trans isomer, while hydrosilylation of silylacetylene gave a mixture of geometric isomers. All products were characterized by spectroscopic methods (1H, 13C, 29Si NMR, IR) and by mass spectrometry (ESI or MALDI).Due to the presence of two groups of different chemical reactivity – readily hydrolyzable organosilicon moiety and organic functions with an affinity to polymers – the synthesized compounds might be considered novel, versatile silica modifiers, coupling agents, or substrates in the synthesis of polymer networks or vapor-deposited coatings, potentially leading to materials with improved physicochemical properties.
To prove its application potential, cyclosiloxane coupling agent 5e was applied in the functionalization of styrene–butadiene rubber (Syntion 2150®) via anionic polymerization. The obtained modified rubber was applied in the preparation of silica-reinforced vulcanizates intended for the production of “Green Tires”. The final composites exhibited improved rolling resistance and wet grip properties compared to unmodified rubber, showing the potential of this compound in the manufacture of novel tire treads for the car industry that could reduce fuel consumption and CO2 emissions.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financed by the National Science Centre in Poland (Grants No. UMO-2019/35/N/ST4/02594, UMO-2018/31/G/ST4/04012) and by the National Centre for Research and Development in Poland, Lider Programme No. LIDER/6/0017/L-9/17/NCBR/2018, and No. POWR.03.02.00-00-I026/16, co-financed by the EU through the European Social Fund under the Operational Program Knowledge Education Development. The authors acknowledge financial support from Synthos S.A.References
- F. Hussain, M. Hojjati, M. Okamoto and R. E. Gorga, Polymer-matrix nanocomposites, processing, manufacturing, and application: an overview, J. Compos. Mater., 2006, 40, 1511–1575 CrossRef CAS.
- R. Scotti, M. D'Arienzo, B. Di Credico, L. Giannini and F. Morazzoni, in Hybrid organic–inorganic interfaces: towards advanced functional materials, ed. M.-H. Delville and A. Taubert, Wiley–VCH Verlag GmbH & Co. KGaA, 2018, pp. 151–198 Search PubMed.
- K. L. Mittal, Silanes and other coupling agents, CRC Press, London, 2007 Search PubMed.
- M.-J. Wang, Effect of polymer-filler and filler-filler Interactions on dynamic properties of filled vulcanizates, Rubber Chem. Technol., 1998, 71, 520–589 CrossRef CAS.
- Web page, https://www.marketsandmarkets.com/Market-Reports/silane-coupling-agent-market-152751887.html.
- T. Aziz, A. Ullah, H. Fan, M. I. Jamil, F. U. Khan, R. Ullah, M. Iqbal, A. Ali and B. Ullah, Recent progress in silane coupling agent with its emerging applications, J. Polym. Environ., 2021, 29, 3427–3443 CrossRef CAS.
- S. Shokoohi, A. Arefazar and R. Khosrokhavar, Silane coupling agents in polymer-based reinforced composites: a review, J. Reinf. Plast. Compos., 2008, 27, 473–485 CrossRef CAS.
- B. Marciniec, H. Maciejewski, C. Pietraszuk and P. Pawluć, in Applied homogeneous catalysis with organometallic compounds, ed. B. Cornils, W. A. Herrmann, M. Beller and R. Paciello, Wiley–VCH Verlag GmbH & Co. KGaA, 2017, pp. 569–620, DOI:10.1002/9783527651733.ch8.
- J. Szyling, R. Januszewski, K. Jankowska, J. Walkowiak, I. Kownacki and A. Franczyk, Synthesis of bifunctional disiloxanes via subsequent hydrosilylation of alkenes and alkynes, Chem. Commun., 2021, 57, 4504–4507 RSC.
- M. Walczak, R. Januszewski, M. Dutkiewicz, A. Franczyk and B. Marciniec, A facile approach for the synthesis of novel silsesquioxanes with mixed functional groups, New J. Chem., 2019, 43, 18141–18145 RSC.
- M. Walczak, A. Franczyk, M. Dutkiewicz and B. Marciniec, Synthesis of bifunctional silsesquioxanes (RSiMe2O)∼4(R′SiMe2O)∼4Si8O12 via hydrosilylation of alkenes, Organometallics, 2019, 38, 3018–3024 CrossRef CAS.
- F. Dankert and C. von Hänisch, Siloxane coordination revisited: Si−O bond character, reactivity and magnificent molecular shapes, Eur. J. Inorg. Chem., 2021, 2021, 2907–2927 CrossRef CAS.
- A. S. Lee, S.-S. Choi, S. Y. Oh, H. S. Lee, B. Kim, S. S. Hwang and K.-Y. Baek, Incompletely condensed POSS-based spin-on-glass networks for impeccable ultra low-k Integration, J. Mater. Chem. C, 2015, 3, 11605–11611 RSC.
- S. Varaprath and D. H. Stutts, Utility of trichloroisocyanuric acid in the efficient chlorination of silicon hydrides, J. Organomet. Chem., 2007, 692, 1892–1897 CrossRef CAS.
- B. P. S. Chauhan and P. Boudjouk, Dehydrogenative condensation of SiH and SH bonds. A metal-catalyzed protocol to stable thiopolysiloxanes, Tetrahedron Lett., 2000, 41, 1127–1130 CrossRef CAS.
- H. Seki, T. Kajiwara, Y. Abe and T. Gunji, Synthesis and structure of ladder polymethylsilsesquioxanes from sila-functionalized cyclotetrasiloxanes, J. Organomet. Chem., 2010, 695, 1363–1369 CrossRef CAS.
- Y. Itami, B. Marciniec and M. Kubicki, Functionalization of vinyl-substituted cyclosiloxane and cyclosilazane via ruthenium-catalyzed silylative coupling reaction, Organometallics, 2003, 22, 3717–3722 CrossRef CAS.
- B. Dudziec and B. Marciniec, A new catalytic route to monoalkynyl-functionalized di- and trivinyl-substituted cyclosiloxanes and divinylcyclosilazanes, Organometallics, 2008, 27, 5598–5604 CrossRef CAS.
- A. Mori, H. Sato, K. Mizuno, T. Hiyama, K. Shintani and Y. Kawakami, A facile preparation and polymerization of 1,1-difunctionalized disiloxanes, Chem. Lett., 1996, 25, 517–518 CrossRef.
- A. Y. Khalimon, B. K. Shaw, A. J. Marwitz, W. E. Piers, J. M. Blackwell and M. Parvez, Photo lewis acid generators: photorelease of B(C6F5)3 and applications to catalysis, Dalton Trans., 2015, 44, 18196–18206 RSC.
- V. R. Ziatdinov, G. Cai and W. P. Weber, Anionic ring-opening polymerization of trimethylsiloxy-substituted 1-oxa-2, 5-disilacyclopentanes: synthesis of trimethylsiloxy-substituted poly[1-oxa-2, 5-disila-1, 5-pentanylene]s, Macromolecules, 2002, 35, 2892–2897 CrossRef CAS.
- F. Ganachaud, S. Boileau, in Handbook of ring–opening polymerization, Wiley–VCH Verlag GmbH & Co. KGaA, 2009, pp. 65–95, DOI:10.1002/9783527628407.ch3.
- B. Buotevin and B. Youssef, Synthese de polysiloxanes fluores. Partie 7 copolycondensation de 1,4 bis(hydroxydimethylsilyl) benzene avec les dichlorosilanes fluores, J. Fluor. Chem., 1989, 45, 355–376 CrossRef.
- K. Matyjaszewski, P. J. Miller, J. Pyun, G. Kickelbick and S. Diamanti, Synthesis and characterization of star polymers with varying arm number, length, and composition from organic and hybrid inorganic/organic multifunctional initiators, Macromolecules, 1999, 32, 6526–6535 CrossRef CAS.
- B. Alonso, B. González, B. García, E. Ramírez-Oliva, M. Zamora, C. M. Casado and I. Cuadrado, Functionalization via hydrosilylation of linear and cyclic siloxanes with appendent first generation dendrons containing electronically communicated ferrocenyl units, J. Organomet. Chem., 2001, 637–639, 642–652 CrossRef CAS.
- M. Suguro, Y. Yamamura, T. Koike and A. Mori, Silicone as an organosilicon reagent for the palladium-catalyzed cross-coupling reaction, React. Funct. Polym., 2007, 67, 1264–1276 CrossRef CAS.
- A. El Malki, A. Hannioui, E. M. Rakib, N. Knouzi and M. Vaultier, Regioselective platinum catalyzed β-hydrosilylation of allylic benzene derivatives with cyclic siloxane D4H, Lett. Org. Chem., 2011, 8, 361–363 CrossRef CAS.
- B. Gierczyk, G. Schroeder and M. Cegłowski, New polymeric metal ion scavengers with polyamine podand moieties, React. Funct. Polym., 2011, 71, 463–479 CrossRef CAS.
- S. Bruña, D. Nieto, A. M. González-Vadillo, J. Perles and I. Cuadrado, Cubic octasilsesquioxanes, cyclotetrasiloxanes, and disiloxanes maximally functionalized with silicon-bridged interacting triferrocenyl units, Organometallics, 2012, 31, 3248–3258 CrossRef.
- L. Cui, A. N. Ranade, M. A. Matos, L. S. Pingree, T. J. Frot, G. Dubois and R. H. Dauskardt, Atmospheric plasma deposited dense silica coatings on plastics, ACS Appl. Mater. Interfaces, 2012, 4, 6587–6598 CrossRef CAS PubMed.
- M. Igarashi, T. Matsumoto, T. Kobayashi, K. Sato, W. Ando, S. Shimada, M. Hara and H. Uchida, Hydrosilylation of allyl derivatives with T, D and M type hydrosilanes, J. Organomet. Chem., 2014, 749, 421–427 CrossRef CAS.
- R. Srivastava and D. M. Dukes, High refractive index siloxanes, WO2014/152686A2, 2014.
- M. Frampton, T. Jones and P. Zelisko, Cyclotetrasiloxane frameworks for the chemoenzymatic synthesis of oligoesters, RSC Adv., 2015, 5, 1999–2008 RSC.
- B. Gierczyk, M. Cegłowski and M. Zalas, New gel-like polymers as selective weak-base anion exchangers, PLoS One, 2015, 10, e0122891 CrossRef PubMed.
- B. Wagner, Merocyanine derivatives, WO2008/080645A1, 2015.
- M. Huang, R. W. Cruse, B. Falk, E. Pohl, S.-C. Su, J. Nesheiwat and P. Ramdatt, Moisture curable silylated polymer compositions containing reactive modifiers, US9714315B2, 2017.
- R. J. Perry, Absorbent compositions including amino-siloxanes, WO2018/190815A1, 2018.
- L. Chen, C. Jariwala and J. S. Rathore, Cyclic siloxanes, compositions, methods, and articles, WO2019/058229A1, 2019.
- P. Zhang, T. Yao, K. Xue, X. Meng, J. Zhang and L. Liu, Low–dielectric constant and viscosity tetrafunctional bio–based epoxy resin containing cyclic siloxane blocks, J. Appl. Polym. Sci., 2022, 139, 52176 CrossRef CAS.
- C. M. Casado, I. Cuadrado, M. Morán, B. Alonso, M. Barranco and J. Losada, Cyclic siloxanes and silsesquioxanes as cores and frameworks for the construction of ferrocenyl dendrimers and polymers, Appl. Organomet. Chem., 1999, 13, 245–259 CrossRef CAS.
- R. Pinho, E. Radovanovic, I. Torriani and I. Yoshida, Hybrid materials derived from divinylbenzene and cyclic siloxane, Eur. Polym. J., 2004, 40, 615–622 CrossRef CAS.
- A. Demirci, S. Yamamoto, J. Matsui, T. Miyashita and M. Mitsuishi, Facile synthesis of cyclosiloxane-based polymers for hybrid film formation, Polym. Chem., 2015, 6, 2695–2706 RSC.
- J. Thomas, S.-B. Choi, R. Fjeldheim and P. Boudjouk, Silicones containing pendant biocides for antifouling coatings, Biofouling, 2004, 20, 227–236 CrossRef CAS PubMed.
- J. Losada, M. G. Armada, I. Cuadrado, B. Alonso, B. González, C. Casado and J. Zhang, Ferrocenyl and permethylferrocenyl cyclic and polyhedral siloxane polymers as mediators in amperometric biosensors, J. Organomet. Chem., 2004, 689, 2799–2807 CrossRef CAS.
- R. Sun, S. Feng, B. Zhou, Z. Chen, D. Wang and H. Liu, Flexible cyclosiloxane-linked fluorescent porous polymers for multifunctional chemical sensors, ACS Macro Lett., 2020, 9, 43–48 CrossRef CAS PubMed.
- J. T. Sołtysiak, E. Białecka-Florjańczyk and J. Przedmojski, Liquid crystalline cyclic oligosiloxanes with polar end groups, Eur. Polym. J., 2006, 42, 1662–1669 CrossRef.
- Q. Y. Li, J. Xu, W. Z. Zhang and P. Li, Preparation and characterization of chiral cyclosiloxane-based liquid-crystalline elastomers bearing menthyl groups, Appl. Mech. Mater., 2012, 466–467, 445–448 CAS.
- S. H. Lee, D. W. Seo, Y. D. Lim, Y. T. Jeon, H. H. Joo, S. Y. Lee, J. S. Lim and W. G. Kim, Novel cyclotetrasiloxane tetraimidazolium salts as high performance quasi-solid state electrolyte for dye-sensitized solar cells, Macromol. Res., 2013, 21, 732–737 CrossRef CAS.
- M. A. Schiavon, N. A. Armelin and I. V. P. Yoshida, Novel poly(borosiloxane) precursors to amorphous SiBCO ceramics, Mater. Chem. Phys., 2008, 112, 1047–1054 CrossRef CAS.
- S. Granados-Focil, J. Conway, Y. Meng and L. Smith, Triazole functionalized sol-gel membranes, effect of crosslink density and heterocycle content on water free proton conduction and membrane mechanical properties, J. Macromol. Sci., Part A: Pure Appl.Chem., 2010, 47, 1197–1202 CrossRef CAS.
- X. Cui, K. Junge, X. Dai, C. Kreyenschulte, M.-M. Pohl, S. Wohlrab, F. Shi, A. Brückner and M. Beller, Synthesis of single atom based heterogeneous platinum catalysts: high selectivity and activity for hydrosilylation reactions, ACS Cent. Sci., 2017, 3, 580–585 CrossRef CAS PubMed.
- H. Enami and S. Akamatsu, Organocyclosiloxane and method for its preparation, EP475437A1, 1992.
- F. G. Mizori, Adhesive compositions containing cyclic siloxanes and methods for use thereof, US7777064B2, 2010.
- O. Mukbaniani, J. Aneli, T. Tatrishvili, E. Markarashvili, M. Chigvinadze and M. J. M. Abadie, Synthesis of cross-linked comb-type polysiloxane for polymer electrolyte membranes, e-Polymers, 2012, 12, 89 Search PubMed.
- N. Gao, W. Liu, Z. Yan and Z. Wang, Synthesis and properties of transparent cycloaliphatic epoxy–silicone resins for opto-electronic devices packaging, Opt. Mater., 2013, 35, 567–575 CrossRef CAS.
- N. Miyagawa, M. Tateno and N. Taniguchi, Carboxylic anhydride-containing cyclic siloxane compounds, epoxy resin compositions and cured materials therewith, JP2016079129A, 2016.
- T. Yamada and Y. Yoshikawa, Epoxy group-containing cyclic organosiloxane, WO2016/170850A1, 2016.
- M. Kawakami and A. Kiyomori, Bissilylamino group-containing organic polysilazane compound, method for producing same, and composition containing same and cured product, US11203670B2, 2021.
- K. Stefanowska, A. Franczyk, J. Szyling, K. Salamon, B. Marciniec and J. Walkowiak, An effective hydrosilylation of alkynes in supercritical CO2−a green approach to alkenyl silanes, J. Catal., 2017, 356, 206–213 CrossRef CAS.
- K. Stefanowska, A. Franczyk, J. Szyling, M. Pyziak, P. Pawluć and J. Walkowiak, Selective hydrosilylation of alkynes with octaspherosilicate (HSiMe2O)8Si8O12, Chem. – Asian J., 2018, 13, 2101–2108 CrossRef CAS PubMed.
- J. Walkowiak, K. Salamon, A. Franczyk, K. Stefanowska, J. Szyling and I. Kownacki, Pt-catalyzed hydrosilylation of 1, 3-diynes with triorganosilanes: regio-and stereoselective synthesis of mono-or bis-silylated adducts, J. Org. Chem., 2019, 84, 2358–2365 CrossRef CAS PubMed.
- K. Stefanowska, A. Franczyk, J. Szyling and J. Walkowiak, Synthesis of functional 3−buten–1−ynes and 1, 3−butadienes with silsesquioxane moiety via hydrosilylation of 1, 3−diynes, ChemCatChem, 2019, 11, 4848–4853 CrossRef CAS.
- T. Sokolnicki, A. Franczyk, B. Janowski and J. Walkowiak, Synthesis of bio–based silane coupling agents by the modification of eugenol, Adv. Synth. Catal., 2021, 363, 5493–5500 CrossRef CAS.
- K. Stefanowska, J. Szyling, J. Walkowiak and A. Franczyk, Alkenyl-functionalized open-cage silsesquioxanes (RSiMe2O)3R′7Si7O9: a novel class of building nanoblocks, Inorg. Chem., 2021, 60, 11006–11013 CrossRef CAS PubMed.
- K. Stefanowska, T. Sokolnicki, J. Walkowiak, A. Czapik and A. Franczyk, Directed cis-hydrosilylation of borylalkynes to borylsilylalkenes, Chem. Commun., 2022, 58, 12046–12049 RSC.
- S. Choi, S. Maul, A. Stewart, H. R. Hamilton and E. P. Douglas, Effect of silane coupling agent on the durability of epoxy adhesion for structural strengthening applications, Polym. Eng. Sci., 2013, 53, 283–294 CrossRef CAS.
- J. Zheng, X. Zhang, J. Cao, R. Chen, T. Aziz, H. Fan and C. Bittencourt, Behavior of epoxy resin filled with nano-SiO2 treated with a eugenol epoxy silane, J. Appl. Polym. Sci., 2021, 138, e50138 CrossRef.
- J. Hu, Z. Fang, Y. Huang and J. Lu, Fabrication of superhydrophobic surfaces based on fluorosilane and TiO2/SiO2 nanocomposites, Surf. Eng., 2021, 37, 271–277 CrossRef CAS.
- H. Zhou, H. Wang, H. Niu, A. Gestos, X. Wang and T. Lin, Fluoroalkyl silane modified silicone rubber/nanoparticle composite: a super durable, robust superhydrophobic fabric coating, Adv. Mater., 2012, 24, 2409–2412 CrossRef CAS PubMed.
- D. Gao, J. Chen, W. Qian, Y. He, P. Song and R. Wang, Improving wettability of feather fiber by surface modification, Waste Biomass Valorization, 2020, 11, 6993–7003 CrossRef CAS.
- N. Bravaya, S. Saratovskikh, A. Panin, E. Faingol'd, I. Zharkov, O. Babkina, S. Vasil'ev, M. Bubnova, V. Volkov and M. Lobanov, Influence of silane coupling agent on the synthesis and properties of nanocomposites obtained via in situ catalytic copolymerization of ethylene and propylene in the presence of modified Nafen™ Al2O3 nanofibers, Polymer, 2019, 174, 114–122 CrossRef CAS.
- R. Rauline, Rubber compound and tires based on such a compound, EP0501227B1, 1995.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3qi00619k |
| This journal is © the Partner Organisations 2023 |