Open Access Article
Open Access ArticleFrom AgTeO2F and Ag2(TeO2F2) to Ag3F3(TeF6)(TeO2)12: the first silver tellurite oxyfluorides with linear and nonlinear optical properties†
Bo
Zhang
abc,
Jia-Hang
Wu
ab,
Chun-Li
Hu
a,
Ya-Feng
Li
b,
Fang
Kong
 *ac and
Jiang-Gao
Mao
*ac and
Jiang-Gao
Mao
 *ac
*ac
aState Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou 350002, P. R. China. E-mail: kongfang@fjirsm.ac.cn; mjg@fjirsm.ac.cn
bCollege of Chemistry, Fuzhou University, Fuzhou 350108, P. R. China
cUniversity of Chinese Academy of Sciences, Beijing 100049, P. R. China
First published on 4th January 2023
Abstract
The first examples of silver fluorotellurites, namely, AgTeO2F and Ag2(TeO2F2), and the first silver fluoride tellurite, Ag3F3(TeF6)(TeO2)12, have been obtained successfully under mild hydrothermal conditions. AgTeO2F displays a new 2D structure composed of a 2D silver oxide layer strengthened by 1D [TeO2F]∞ chains. Ag2(TeO2F2) exhibits a new 3D construction consisting of a 3D silver oxyfluoride framework with 1D polyhedral ring channels occupied by the isolated TeO2F2 groups. Ag3F3(TeF6)(TeO2)12 features the first 3D neutral [TeO2]∞ open framework with 8- and 4-MPR channels along the a-, b- and c-axes. AgTeO2F and Ag2(TeO2F2) crystallize in the centrosymmetric space group while Ag3F3(TeF6)(TeO2)12 is in a non-centrosymmetric space group. Ag3F3(TeF6)(TeO2)12 shows a unique nonlinear optical property with an SHG intensity of about 70% that of commercial KH2PO4, while AgTeO2F and Ag2(TeO2F2) present an apparent linear optical property and their birefringence is calculated as 0.078 and 0.032@1064 nm, respectively. This work further confirms that fluorination in tellurium(IV) oxides can greatly enrich the structural chemistry and optical properties of metal tellurites.
Introduction
Second harmonic generation (SHG) materials have continued to be in great demand due to their capacity of laser frequency conversion, material micromachining, photolithography and so on.1–9 The first requirement for an SHG material is a non-centrosymmetric (NCS) structure since it is a physical quantity described by an odd-order tensor.10–14 The lone pair cation of Te(IV), with a 5s2 electronic configuration, can exhibit three different kinds of asymmetric coordination mode, namely, TeO3 trigonal pyramid, TeO4 seesaw-like group, and TeO5 tetragonal pyramid, when it coordinates with oxygen ligands due to the hybridization between the s- and p-orbitals of the cation and anions.15 These polar tellurite groups can stimulate the formation of NCS structures, making metal tellurites good candidates for SHG materials.16–20 Metal tellurites can also display plentiful structure types because the polar oxyanions can join together by condensation to generate 0D clusters,21–23 1D chains,24–27 2D layers28,29 and even 3D frameworks.30,31Fluorine, as the most electronegative element, could replace oxygen ligands due to their similar ionic radii.32–34 When fluorine atoms are introduced into oxide compounds, fluoride compounds often can display excellent comprehensive performance and abundant structure types,35–38 such as RbTeMo2O8F (27 × KDP, 3.63 eV),39 Ba(MoO2F)2(TeO3)2 (7.8 × KDP, 2.96 eV),40 and BaF2TeF2(OH)2 (3 × KDP, 5.9 eV)41 in metal tellurites. Fluorine-containing tellurite compounds (or tellurite oxyfluorides) can be classified into fluoride tellurites and fluorotellurites according to the connection mode of the fluorine element. In fluorotellurites, the fluorine atoms are connected with the lone pair cation of Te(IV); in other words, there are Te(IV)–F bonds in the structures, such as Bi3F(TeO3)(TeO2F2)342 and HgTeO2F(OH).43 BaF2TeF2(OH)2 is the first reported SHG material in metal fluorotellurites, which has proved to be a promising UV nonlinear optical material.41 The Te–F bonds in BaF2TeF2(OH)2 play an important role in the wide band gap. In fluoride tellurites, there are no Te(IV)–F bonds, and the fluorine atoms are linked with the other cations, such as d0 transition metals (TM), RbTeMo2O8F39 and Ba(MoO2F)2(TeO3)2,40 and alkali metals, Li7(TeO3)3F.44 RbTeMo2O8F containing a MoO5F octahedron features the strongest SHG material of metal tellurites at both the visible and near-infrared ranges, and the MoO5F octahedron contributed the most to the SHG efficiency of RbTeMo2O8F. Therefore, fluorination in tellurites not only broadens the band gap of metal tellurites, but can also increase the SHG intensity of crystals. However, compared with tellurite oxides, tellurite oxyfluorides, including fluoride tellurites and fluorotellurites, are still rare due to their synthetic difficulty. It is still very necessary to enrich the structural chemistry and the SHG crystal types in metal tellurite oxyfluorides.
To increase the success rate of NCS structures, the d10 TM cation Ag+ was chosen to balance the charge due to its large polar displacement.28,45–48 After an extensive survey, we found that no silver tellurites with the fluorine element have been reported yet. Our efforts in the silver–tellurite–fluorine system successfully result in the first examples of silver fluorotellurites and the first silver fluoride tellurite, namely, AgTeO2F and Ag2(TeO2F2), and Ag3F3(TeF6) (TeO2)12. Among these compounds, Ag3F3(TeF6) (TeO2)12 crystallized in an NCS space group and presented a moderate SHG response of 0.7 × KH2PO4 (KDP) at 1064 nm. Herein, we report their syntheses, crystal structures, and thermal and optical properties from theoretical and experimental aspects.
Experimental section
Reagents
Silver fluoride (AgF, 98+%, AR), tellurium dioxide (TeO2, 99.9%, AR) and hydrofluoric acid (HF, 40+%, AR) were obtained commercially and used as received. Caution! Hydrofluoric acid is toxic and corrosive! It must be handled with extreme caution and with the appropriate protective equipment and training.Syntheses
The three compounds were successfully synthesized via a mild hydrothermal method based on the following chemical proportions: AgF (0.127 g, 1.0 mmol), TeO2 (0.160 g, 1.0 mmol), hydrofluoric acid (0.25 mL) and 1 mL of deionized water for AgTeO2F; AgF (0.127 g, 1.0 mmol), TeO2 (0.080 g, 0.5 mmol), hydrofluoric acid (0.25 mL) and 1.5 mL of deionized water for Ag2(TeO2F2); AgF (0.102 g, 0.8 mmol), TeO2 (0.256 g, 1.6 mmol), hydrofluoric acid (0.25 mL) and 0.5 mL of deionized water for Ag3F3(TeF6)(TeO2)12. It is worth noting that the amount of deionized water is critical in these reactions besides the proportions of the raw materials. The mixtures were placed in a 23 mL Teflon liner equipped with a stainless-steel autoclave. These samples were heated to 220 °C, maintained for 48 h, and then cooled to 30 °C at 2.3 °C h−1. After washing with deionized water, the products were dried in air at room temperature. As shown in Fig. S1,† the three compounds exhibit three different morphologies: lamellar AgTeO2F, columnar Ag2(TeO2F2) and octahedral Ag3F3(TeF6)(TeO2)12, and the three kinds of crystals were obtained in yields of about 58%, 43% and 75% (based on Te), respectively. The existence and distribution of Ag, Te and F were demonstrated via the elemental distribution maps (Fig. 1). As shown in Fig. S2,† the experimental powder X-ray diffraction (PXRD) patterns are in good agreement with the calculated ones, so their purities are certified. | ||
| Fig. 1 SEM images of AgTeO2F (a), Ag2(TeO2F2) (b) and Ag3F3(TeF6)(TeO2)12 (c) and their elemental distribution maps. | ||
Single-crystal structure determination
Single-crystal X-ray diffraction data of AgTeO2F, Ag2(TeO2F2) and Ag3F3(TeF6) (TeO2)12 were collected on an Agilent Technologies SuperNova dual-wavelength CCD diffractometer with graphite-monochromated Mo Kα radiation (λ = 0.71073 Å) at room temperature. Cell refinement and data reduction were conducted with CrysAlisPro and absorption correction based on the multi-scan method was applied.49 The structures were determined by direct methods and refined by full-matrix least-squares fitting on F2 using the SHELXL-2017 software package.50 The atoms were refined with anisotropic thermal parameters. The detailed crystallographic data of the three structures are listed in Table 1, and some selected atomic coordinates, bond lengths and angles are listed in Tables S1–S3.† Detailed information on the crystal structure of the three compounds can be obtained from the Cambridge Crystallographic Data Center.| Molecular formula | AgTeO2F | Ag2(TeO2F2) | Ag3F3(TeF6)(TeO2)12 |
|---|---|---|---|
| a R 1 = ∑||Fo| − |Fc||/∑|Fo|, wR2 = {∑w[(Fo)2 − (Fc)2]2/∑w[(Fo)2]2}1/2. | |||
| Formula weight | 286.47 | 413.34 | 2575.07 |
| Crystal system | Monoclinic | Orthorhombic | Cubic |
| Space group | P21/c | Pbca |
P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3n 3n |
| Temperature (K) | 292.38(10) | 293.39(10) | 294.15 |
| F(000) | 728 | 2880 | 2245 |
| a/Å | 8.7523(14) | 15.6642(13) | 11.3998(5) |
| b/Å | 6.3759(9) | 6.8862(5) | 11.3998(5) |
| c/Å | 5.4505(8) | 16.5328(12) | 11.3998(5) |
| α (°) | 90 | 90 | 90 |
| β (°) | 91.396(14) | 90 | 90 |
| γ (°) | 90 | 90 | 90 |
| V/Å3 | 304.07(8) | 1783.3(2) | 1481.47(19) |
| Z | 4 | 16 | 2 |
| D c (g cm−3) | 6.258 | 6.158 | 5.773 |
| GOF on F2 | 1.008 | 1.073 | 1.138 |
| Flack factor | — | — | 0.48(9) |
| R 1, wR2 [I > 2σ(I)]a | 0.0223, 0.0439 | 0.0306, 0.0704 | 0.0200, 0.0434 |
| R 1, wR2 (all data)a | 0.0267, 0.0462 | 0.0377, 0.0750 | 0.0251, 0.0460 |
Powder X-ray diffraction
Powder X-ray diffraction (PXRD) data of the three crystals were recorded on a Rigaku Miniflex600 diffractometer equipped with graphite-monochromated Cu Kα radiation at room temperature. The 2θ range is set to 5–70°, and the scan step size is 0.02°.Energy-dispersive X-ray spectroscopy
Microprobe elemental analysis was conducted with the aid of a field-emission scanning electron microscope (JSM6700F) outfitted with an energy-dispersive X-ray spectroscope (Oxford INCA).Spectroscopic measurements
The IR spectrum was recorded using a Magna 750 FT-IR spectrometer under an air background, and the selected range is 4000–400 cm−1. The UV-vis-NIR diffuse reflection spectrum was measured using a PerkinElmer Lambda 950 UV-vis-NIR spectrophotometer with a BaSO4 powder board as a reference for 100% reflectance, and the recording range was 200–2000 nm. Absorption data were calculated from the diffuse reflection data by the Kubelka–Munk function: α/S = (1 − R)2/2R, in which α and S represent the absorption coefficient and the scattering coefficient, respectively. The band gap value can be given by extrapolating the absorption edge to the baseline in the α/S versus energy graph.Thermal analysis
A NETZCH STA 449F3 thermal analyzer was used to analyze the thermal stability of the three crystals. The three samples were heated in alumina crucibles from 20 °C to 1000 °C under nitrogen gas at a rate of 15 °C min−1.SHG measurements
Powder SHG measurements were carried out using a modified method of Kurtz and Perry. An irradiation laser beam (λ = 1.064 μm) is generated with a Nd:YAG solid-state laser equipped with a Kurtz and Perry setup.51 The SHG signal oscilloscope traces of the Ag3F3(TeF6)(TeO2)12 and KDP samples in a particle size range (150–210 μm) were both recorded.Results and discussion
Structure of AgTeO2F
Equivalent silver and tellurium sources resulted in the production of AgTeO2F. AgTeO2F crystallizes in the monoclinic space group P21/c. Its asymmetric unit contains 1 Ag, 1 Te, 2 O and 1 F atoms (Table S1†). The Ag(1) atom is 4-coordinated into a distorted tetrahedron AgO4 with the lengths of Ag–O bonds ranging from 2.319(4) to 2.478(5) Å. The Te(1) atom connects with 3 O and 1 F atoms, forming a polar TeO3F group in a seesaw-like configuration. The Te–O bond distances are in the range of 1.832(4)–2.162(4) Å and the Te–F bond length is 2.029(4) Å, which are consistent with the reported metal tellurites.43,52–57 The calculated total bond valences for Ag and Te are 0.825 and 4.031, indicating their oxidation states of +1 and +4 respectively (Table S2†).AgTeO2F presents a new 2D layered structure. Within the structure, the AgO4 tetrahedra were corner- and edge-shared into a silver oxide layer parallel to the bc plane (Fig. 2a). The TeO3F groups were corner-shared into a zigzag chain along the c-axis (Fig. 2b). The fluorotellurite 1D chains were linked on the both sides of the silver oxygen layers via Te–O–Ag bonds, forming the 2D layered structure of AgTeO2F (Fig. 2c). The lone pairs of tellurites were pointed to the space between layers. The interlayer distance was calculated as 8.75 Å.
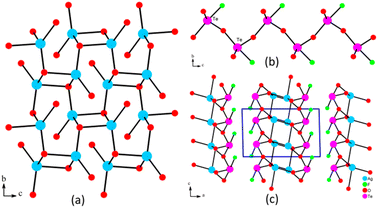 | ||
| Fig. 2 Silver oxide layer in the bc plane (a), the 1D chain of Te–O–F (b), and the layer structure of AgTeO2F (c). | ||
Structure of Ag2(TeO2F2)
When we increased the ratio of silver, a new compound of Ag2(TeO2F2) was achieved. Ag2(TeO2F2) presents a new 3D construction composed of a 3D silver oxygen framework strengthened by TeO2F2 units. The structure crystallized in the orthorhombic space group Pbca. Its asymmetric unit includes 4 Ag, 2 Te, 4 O and 2 F atoms. Both of the Te(IV) cations were four-coordinated in seesaw-like TeO2F2 groups with the Te–O and Te–F bonds in the ranges of 1.848–1.869 Å and 2.021–2.068 Å, respectively (Fig. S3a and S3b†). The four Ag+ cations were connected with both O and F atoms, forming Ag(1)O3F, Ag(2)O3F2, Ag(3)O4F, and Ag(4)O3F polyhedra, respectively. The Ag–O and Ag–F bond distances were in the ranges of 2.19(6)–2.663(8) and 2.458(6)–2.68(6) Å, respectively. The oxidation states of the Ag and Te atoms were proved to be +1 and +4, respectively. The calculated total bond valences for Ag(1), Ag(2), Ag(3), Ag(4), Te(1) and Te(2) are 0.769, 0.961, 0.711, 0.809, 4.007, and 4.066, respectively.The Ag(1)O3F polyhedra were corner-shared in a Ag(1) chain along the b-axis, so were the Ag(2) polyhedra (Fig. 3a and b). The Ag(3)O4F and Ag(4)O3F polyhedra were corner- and edge-shared into a 1D Ag(3)Ag(4) chain along the b-axis with four-member polyhedral rings (MPRs) (Fig. 3c). The Ag(3)Ag(4) chains were connected by the Ag(1) chain to form a 2D layer parallel with the ab plane (Fig. S3c†), which were further linked by the Ag(2) chains to a 3D silver oxyfluoride open framework with 1D 18-MPR channels along the b-axis (Fig. S3d†). The isolated Te(1)O2F2 and Te(2)O2F2 units were filled in the 18-MPR channels to strengthen the 3D structure of Ag2(TeO2F2) (Fig. 3d). Due to the blocking effect of TeO2F2 groups, the 18-MPR channels have been shrunk to 10-MPR channels with the lone pairs of tellurites pointed to the center of them.
 | ||
| Fig. 3 Ag(1)–O–F zigzag chain (a), Ag(2)–O–F wave chain (b), 1D Ag(3)–Ag(4)–O–F chains (c), and 3D structure of Ag2(TeO2F2) (d). | ||
Structure of Ag3F3(TeF6)(TeO2)12
A mixed valence tellurium compound of Ag3F3(TeVIF6)(TeIVO2)12 was obtained when we adjusted the reaction conditions further. Ag3F3(TeF6)(TeO2)12 is crystallized in the cubic space group P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3n. There are 1 Ag, 2 Te, 2 O and 2 F atoms in the asymmetric unit. Tetravalent Te(1) is coordinated with four O2− anions into a TeO4 quadrangular pyramid with the Te–O bond lengths ranging from 1.827(4) to 2.107(5) Å while the hexavalent Te(2) atom connects with 6 F atoms to form a TeF6 octahedron with a Te–F bond length of 1.906(17) Å (Fig. S4a†). The Ag+ cation is 8-coordinated into AgO8 polyhedra with the length of Ag–O bonds ranging from 2.589(7) to 2.559(7) Å (Fig. S4b†). The oxidation state of Ag, Te(1) and Te(2) atoms was proved to be +1, +4 and +6, respectively, based on the calculated total bond valences of 1.000, 4.078 and 6.180 for Ag, Te(1) and Te(2), respectively.
3n. There are 1 Ag, 2 Te, 2 O and 2 F atoms in the asymmetric unit. Tetravalent Te(1) is coordinated with four O2− anions into a TeO4 quadrangular pyramid with the Te–O bond lengths ranging from 1.827(4) to 2.107(5) Å while the hexavalent Te(2) atom connects with 6 F atoms to form a TeF6 octahedron with a Te–F bond length of 1.906(17) Å (Fig. S4a†). The Ag+ cation is 8-coordinated into AgO8 polyhedra with the length of Ag–O bonds ranging from 2.589(7) to 2.559(7) Å (Fig. S4b†). The oxidation state of Ag, Te(1) and Te(2) atoms was proved to be +1, +4 and +6, respectively, based on the calculated total bond valences of 1.000, 4.078 and 6.180 for Ag, Te(1) and Te(2), respectively.
Ag3F3(TeF6)(TeO2)12 features an interesting 3D structure formed by the 3D Ag3F3(TeO2)12 framework embedded with the isolated (TeF6) octahedra. The tetravalent Te(1)O4 units were interconnected into a 3D neutral [TeO2]∞ open framework by corner-sharing with 8- and 4-MPR channels along the a-, b- and c-axes (Fig. 4a). The Ag(1) cations were located in the two different 1D channels to form the 3D cationic framework of [Ag3(TeO2)12]3+ (Fig. S4c†). Interestingly, the silver cations were situated at the sites with symmetry of 222, namely, the twelve axis-center and the six face-center of the unit cell. The body center of the cubic structure was empty, and was filled by the isolated F(2) atoms and the (TeF6) octahedron to balance the charge and support the framework, respectively (Fig. 4b). So, the structure of Ag3F3(TeF6)(TeO2)12 can be described as a 3D construction composed of a 3D Ag3F3(TeO2)12 framework embedded with the neutral (TeF6) octahedra.
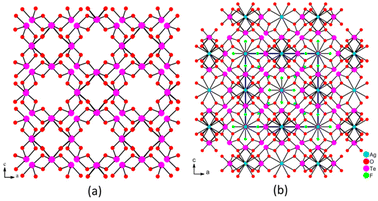 | ||
| Fig. 4 The 3D skeleton with two different 1D channels (a), and the structure chart of AgO8 and TeF6 residing in the centre of 8-MPRs and 4-MPRs (b). | ||
From the elucidation of the structures, we can find that the quantity and site of the fluorine element are totally different in the three tellurite oxyfluorides. In AgTeO2F, only one oxygen was replaced in the TeO3F groups while two oxygen atoms were substituted in TeO2F2 of Ag2(TeO2F2). Only the Te(VI) groups were fluoride in Ag3F3(TeF6)(TeO2)12. We think that the difference should be caused by the different synthesis conditions, especially from the molar ratio of AgF to TeO2. When the ratio of AgF/TeO2 was 1/1, AgTeO2F was obtained, of which the tellurite was a mono-substituted TeO3F group. If the ratio was increased to 2/1, Ag2(TeO2F2) was isolated and disubstituted TeO2F2 groups were found. When the ratio was decreased to 1/2 and the content of solvent water was halved, partial Te(IV) oxidized into Te(VI) and mixed valence Ag3F3(TeF6)(TeO2)12 was achieved. In the structure of Ag3F3(TeF6)(TeO2)12, only Te(VI) groups were fluoride, which can be explained by the Hard–Soft–Acid–Base (HSAB) theory. Compared with a Te(IV) ion, Te(VI) has higher charge and a smaller radius, so it has stronger acidity. The fluorine element with strong electronegativity can be regarded as a hard base, which is liable to coordinate with cations with strong acidity. This work revealed that the fluorination in tellurium(IV) oxides can enrich the structural chemistry of metal tellurites greatly.
Thermal analyses
Thermogravimetric analyses (TGA) were performed to explore the thermal behavior of the three compounds. From Fig. 5a we can find that there are slight weight losses of about 0.94% and 0.97% in the ranges of 294–494 °C and 271–509 °C for AgTeO2F and Ag2(TeO2F2), respectively, corresponding to the escape of partial F2 molecules. We checked the thermal stability of AgTeO2F and Ag2(TeO2F2) at 20, 300, 400 and 500 °C, respectively, by PXRD measurements. From the results shown in Fig. S5,† we can find that the PXRD patterns of the two samples at 300 °C could not match the patterns at room temperature, which indicates that the two crystals have been decomposed at 300 °C. The release of the TeO2 molecule occurred at about 662 °C and 689 °C for AgTeO2F and Ag2(TeO2F2), respectively. The thermogravimetric-mass spectrometry (TG-MS) measurement was performed for Ag3F3(TeF6)(TeO2)12 (Fig. 5b). The TG curve of Ag3F3(TeF6)(TeO2)12 involves two steps of weight-losses. The first one (exp. 6.6%) in the range of 328 to 524 °C is consistent with the release of 4.5 F2 molecules (cal. 6.7%). The second weight loss that occurred above 684 °C can be attributed to the evaporation of the TeO2 molecules, which did not complete even at 1000 °C, just like the situations in AgTeO2F and Ag2(TeO2F2). The ion current curves of Ag3F3(TeF6)(TeO2)12 indicate the existence of the fluorine element and the absence of the OH− group or the H2O molecule, which further proved the correctness of the formula.IR and UV-vis-NIR spectra
The IR spectra revealed that no obvious absorption was found in the region 4000–800 cm−1 for the three compounds, indicating no hydroxy bonds in the structures (Fig. S5†). Their vibration bands were focused over the range of 400–780 cm−1, which can be attributed to the Te–O and Te–F vibrations. These assignments correspond to the reported tellurites.52,58–63The UV-vis-NIR diffuse-reflectance spectra of the three compounds show that they have no obvious absorption in the range of 500–2000 nm (Fig. 6). The ultraviolet absorption cutoff edges of AgTeO2F, Ag2(TeO2F2) and Ag3F3(TeF6)(TeO2)12 were 283, 328 and 276 nm, respectively, and their optical band gaps were measured as 3.41, 3.22 and 3.69 eV, respectively.
 | ||
| Fig. 6 UV-vis-NIR diffuse reflectance spectra of AgTeO2F (a), Ag2(TeO2F2) (b) and Ag3F3(TeF6)(TeO2)12 (c). | ||
SHG measurements
For Ag3F3(TeF6)(TeO2)12, measurement of the powder frequency-doubling effect was carried out by the method of Kurtz and Perry, employing a Q-switched Nd:YAG laser under 1064 nm radiation. A sieved sample (70–100 mesh) was used to assess its second-order susceptibility coefficient. Powder SHG examination revealed that Ag3F3(TeF6)(TeO2)12 displayed a moderate frequency-doubling efficiency of about 0.7 × KDP (Fig. 7). | ||
| Fig. 7 The oscilloscope traces of the SHG signals for the samples (150–210 μm) of Ag3F3(TeF6)(TeO2)12 and KDP under laser irradiation at 1064 nm. | ||
Theoretical calculation
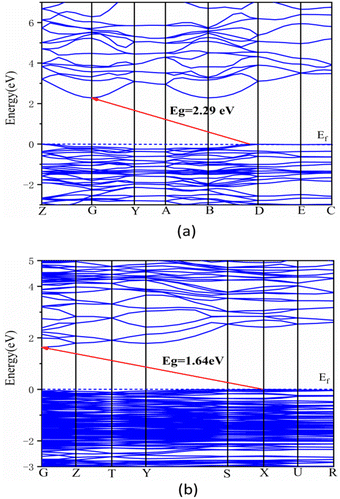
To access the birefringence of AgTeO2F and Ag2(TeO2F2), their linear optical properties have been studied using CASTEP based on DFT methods. Such work has not been performed for Ag3F3(TeF6)(TeO2)12 due to its isotropic nature.Fig. 8 presents the band structures along the high symmetry points of the first Brillouin zone for AgTeO2F and Ag2(TeO2F2). The state energies of the CBs and highest VBs of the two structures are shown in Table S4.† For AgTeO2F, the maximum of VBs is situated at the Z point and the minimum of CBs is placed at the G point with a band gap of 2.29 eV, indicating that it is an indirect band gap compound (Fig. 8a). For Ag2(TeO2F2), the top of the VBs is located at the X point while the bottom of CBs is placed at the G point with a band gap of 1.64 eV, which shows that it also is an indirect band gap material (Fig. 8b). The calculated band gaps are much smaller than the experimental results, which was caused by the discontinuity of the exchange–correlation functional.64 Therefore, scissor operators are 1.12 eV and 1.58 eV for AgTeO2F and Ag2(TeO2F2), respectively, which are used in the following optical property calculations.
The total and partial density of states are displayed in Fig. 9. The TDOS and PDOS of the two structures are very similar. In AgTeO2F and Ag2(TeO2F2), the VBs in the lower energy from −20 to −17 eV mostly originate from O 2s and Te 5s5p. The CBs in the higher energy between 3.0 and 10.0 eV are composed of Te 5s5p and O 2p states primarily. To assign the electronic bands explicitly, we have concentrated our attention on the top of VBs and the bottom of CBs close to the Fermi level, which are able to clarify the majority of the bonding character. In AgTeO2F and Ag2(TeO2F2), we can find that the O-2p states and F-2p states match well with the states of Te-5p, which indicates the strong Te–O and Te–F bonding interactions. For AgTeO2F and Ag2(TeO2F2), the maximum of valence bands are mainly from the O-2p and Ag-4d non-bonded states, while the minimum of valence bands come from the empty Te-5p and Ag-5s orbitals primarily (Fig. 9). Therefore, the band gaps of AgTeO2F and Ag2(TeO2F2) are determined by O, Ag and Te atoms.
The linear optical response properties were calculated by the complex dielectric function ε(ω) = ε1(ω) + iε2(ω). The dispersion curves of refractive indices based on the formula n2(ω) = ε(ω) displayed strong anisotropy. The frequency-dependent refractive indices for AgTeO2F and Ag2(TeO2F2) are shown in Fig. 10. For AgTeO2F, the refractive indices follow the order of n010 > n001 > n100. The birefringence of AgTeO2F is 0.078 at 1064 nm. For Ag2(TeO2F2), the refractive indices are in a sequence of n001 > n010 > n100 at 1064 nm. The birefringence of Ag2(TeO2F2) was calculated to be 0.032 at 1064 nm, which is much smaller than that of AgTeO2F. The large birefringence of AgTeO2F can be attributed to the regularly arranged TeO3F groups.
Conclusions
The first cases of silver tellurite oxyfluorides, namely, AgTeO2F, Ag2(TeO2F2) and Ag3F3(TeF6)(TeO2)12, have been successfully synthesized through a facile hydrothermal method. AgTeO2F and Ag2(TeO2F2) exhibit the first examples of silver fluorotellurites and the NCS Ag3F3(TeF6)(TeO2)12 is the first silver fluoride tellurite. The tellurite groups in these structures display three different dimensional structures, namely, 1D [TeO2F]∞ chains in AgTeO2F, 0D (TeO2F2) groups in Ag2(TeO2F2) and a 3D [TeO2]∞ architecture in Ag3F3(TeF6)(TeO2)12. The neutral [TeO2]∞ open framework with 8- and 4-MPR tunnels along three crystallographic axes is first reported here. In addition, the NCS Ag3F3(TeF6)(TeO2)12 can exhibit a moderate powder SHG effect of about 0.7 times that of commercial KDP. The birefringence of AgTeO2F was calculated to be 0.078@1064 nm, which is much larger than that of Ag2(TeO2F2) (0.032@1064). This work has enriched the syntheses, structures and optical properties of tellurite oxyfluorides further. Other related works about metal fluorotellurites and fluoride tellurites are underway.Author contributions
Bo Zhang: investigation, validation and writing – original draft; Jia-Hang Wu: validation; Chun-Li Hu: formal analysis; Ya-Feng Li: validation; Fang Kong: conceptualization, supervision and writing – review and editing; Jiang-Gao Mao: supervision and funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No.: 91963105, 22031009 and 21921001).References
- C. T. Chen, Y. B. Wang, B. C. Wu, K. C. Wu, W. L. Zeng and L. H. Yu, Design and Synthesis of an Ultraviolet-Transparent Nonlinear Optical Crystal Sr2Be2B2O7, Nature, 1995, 373, 322–324 CrossRef CAS.
- P. S. Halasyamani and K. R. Poeppelmeier, Noncentrosymmetric Oxides, Chem. Mater., 1998, 10, 2753–2769 CrossRef CAS.
- K. M. Ok, Toward the Rational Design of Novel Noncentrosymmetric Materials: Factors Influencing the Framework Structures, Acc. Chem. Res., 2016, 49, 2774–2785 CrossRef CAS PubMed.
- F. Kong, T. K. Jiang and J. G. Mao, Role of fluorine on the structure and second-harmonic-generation property of inorganic selenites and tellurites, Chem. Commun., 2021, 57, 12575–12586 RSC.
- H. H. Cheng, W. Q. Jin, Z. H. Yang and S. L. Pan, Performance of optical materials with the derivative of planar π-conjugated groups: Recent advances and future prospects, Inorg. Chem. Front., 2022, 9, 4554–4568 RSC.
- L. Huang and G. H. Zou, Recent Progresses of UV Nonlinear Optical Materials, Chin. J. Struct. Chem., 2020, 39, 1571–1577 Search PubMed.
- S. P. Guo, Y. Chi and H. G. Xue, SnI4·(S8)2: A Novel Adduct-Type Infrared Second-Order Nonlinear Optical Crystal, Angew. Chem., Int. Ed., 2018, 57, 11540–11543 CrossRef CAS PubMed.
- X. Huang, S. H. Yang, X. H. Li, W. Liu and S. P. Guo, Eu2P2S6: The First Rare-Earth Chalcogenophosphate Exhibiting Large Second-Harmonic Generation Response and High Laser-Induced Damage Threshold, Angew. Chem., Int. Ed., 2022, 61, e202206791 CAS.
- X. H. Li, Z. H. Shi, M. Yang, W. Liu and S. P. Guo, Sn7Br10S2: The First Ternary Halogen-Rich Chalcohalide Exhibiting a Chiral Structure and Pronounced Nonlinear Optical Properties, Angew. Chem., Int. Ed., 2022, 61, e202115871 CAS.
- Y. Y. Xu, C. S. Lin, D. Zhao, B. X. Li, L. L. Cao, N. Ye and M. Luo, Chemical substitution – oriented design of a new polar PbFIO3 achieving a balance between large second-harmonic generation response and wide band gap, Scr. Mater., 2022, 208, 114347 CrossRef CAS.
- W. Zeng, X. H. Dong, Y. Tian, L. Huang, H. M. Zeng, Z. E. Lin and G. H. Zou, Unprecedented boat-shaped [Mo2O5(IO3)4]2− polyanions induced a strong second harmonic generation response, Chem. Commun., 2022, 58, 3350–3353 RSC.
- H. S. Ra, K. M. Ok and P. S. Halasyamani, Combining Second-Order Jahn-Teller Distorted Cations to Create Highly Efficient SHG Materials: Synthesis, Characterization, and NLO Properties of BaTeM2O9 (M = Mo6+ or W6+), J. Am. Chem. Soc., 2003, 125, 7764–7765 CrossRef CAS PubMed.
- X. L. Du, X. J. Guo, Z. L. Gao, F. A. Liu, F. F. Guo, S. Y. Wang, H. Y. Wang, Y. X. Sun and X. T. Tao, Li2MTeO6 (M=Ti, Sn): Mid-Infrared Nonlinear Optical Crystal with Strong Second Harmonic Generation Response and Wide Transparency Range, Angew. Chem., Int. Ed., 2021, 60, 23320–23326 CrossRef CAS PubMed.
- H. Chen, M. Y. Ran, W. B. Wei, X. T. Wu, H. Lin and Q. L. Zhu, A comprehensive review on metal chalcogenides with three-dimensional frameworks for infrared nonlinear optical applications, Coord. Chem. Rev., 2022, 470, 214706 CrossRef CAS.
- P. F. Li, C. L. Hu, F. Kong, S. M. Ying and J. G. Mao, Y2(Te4O10)(SO4): a new sulfate tellurite with a unique Te4O10 polyanion and large birefringence, Inorg. Chem. Front., 2021, 8, 164–172 RSC.
- K. C. Chen, C. S. Lin, G. Peng, Y. Chen, H. Z. Huang, E. Z. Chen, Y. X. Min, T. Yan, M. Luo and N. Ye, LiNbTeO5: A High-Performance Multifunctional Crystal Material with a Very Large Second-Harmonic Generation Response and Piezoelectric Coefficient, Chem. Mater., 2021, 34, 399–404 CrossRef.
- S. G. Zhao, X. X. Jiang, R. He, S. Q. Zhang, Z. H. Sun, J. H. Luo, Z. S. Lin and M. C. Hong, A combination of multiple chromophores enhances second-harmonic generation in a nonpolar noncentrosymmetric oxide: CdTeMoO6, J. Mater. Chem. C, 2013, 1, 2906–2912 RSC.
- J. J. Zhang, Z. H. Zhang, Y. X. Sun, C. Q. Zhang, S. J. Zhang, Y. Liu and X. T. Tao, MgTeMoO6: A neutral layered material showing strong second-harmonic generation, J. Mater. Chem., 2012, 22, 9921–9927 RSC.
- A. Cammarata, W. G. Zhang, P. S. Halasyamani and J. M. Rondinelli, Microscopic Origins of Optical Second Harmonic Generation in Noncentrosymmetric–Nonpolar Materials, Chem. Mater., 2014, 26, 5773–5781 CrossRef CAS.
- Y. Q. Feng, H. T. Fan, Z. G. Zhong, H. W. Wang and D. F. Qiu, Cd3(MoO4)(TeO3)2: A Polar 3D Compound Containing d10-d0 SCALP-Effect Cations, Inorg. Chem., 2016, 55, 11987–11992 CrossRef CAS PubMed.
- Q. Wu, J. F. Zhou, X. M. Liu, X. X. Jiang, Q. X. Zhang, Z. S. Lin and M. J. Xia, Ca3(TeO3)2(MO4) (M = Mo, W): Mid-Infrared Nonlinear Optical Tellurates with Ultrawide Transparency Ranges and Superhigh Laser-Induced mage ThreDasholds, Inorg. Chem., 2021, 60, 18512–18520 CrossRef CAS PubMed.
- C. Bai, Y. Chu, J. Z. Zhou, L. N. Wang, L. Luo, S. L. Pan and J. J. Li, Two new tellurite halides with cationic layers: syntheses, structures, and characterizations of CdPb2Te3O8Cl2 and Cd13Pb8Te14O42Cl14, Inorg. Chem. Front., 2022, 9, 1023–1030 RSC.
- M. Weil and M. Shirkhanlou, Incorporation of Sulfate or Selenate Groups into Oxotellurates(IV): I. Calcium, Cadmium, and Strontium Compounds, Z. Anorg. Allg. Chem., 2017, 643, 330–339 CrossRef CAS.
- T. Wang, Y. G. Chen, Y. Guo, F. Wang, Q. Song, Y. J. Jia and X. M. Zhang, BaLiTe2O5X (X = Cl, Br): mixed alkali/alkaline-earth metal tellurite halides with [Te2O5] infinity chains, Dalton Trans., 2020, 49, 4914–4919 RSC.
- H. E. Lee, H. Jo, M. H. Lee and K. M. Ok, Unique synthesis, structure determination, and optical properties of seven new layered rare earth tellurite nitrates, RE(TeO3)(NO3) (RE = La, Nd, Eu, Gd, Dy, Er, and Y), J. Alloys Compd., 2021, 851, 156855 CrossRef CAS.
- K. K. Mishra, S. N. Achary, S. Chandra, T. R. Ravindran, K. K. Pandey, A. K. Tyagi and S. M. Sharma, Study of Phase Transformation in BaTe2O6 by in Situ High-Pressure X-ray Diffraction, Raman Spectroscopy, and First-Principles Calculations, Inorg. Chem., 2016, 55, 227–238 CrossRef CAS PubMed.
- J. Lin, J. Diwu, J. N. Cross, E. M. Villa and T. E. Albrecht-Schmitt, Cerium(IV) tellurite halides [Ce2Te7O17]X2 (X = Cl− or Br−): the first cerium-containing cationic frameworks, Inorg. Chem., 2012, 51, 10083–10085 CrossRef CAS PubMed.
- P. F. Li, C. L. Hu, Y. P. Gong, F. Kong and J. G. Mao, Hg3(Te3O8)(SO4): a new sulfate tellurite with a novel structure and large birefringence explored from d10 metal compounds, Chem. Commun., 2021, 57, 7039–7042 RSC.
- B. Stöger and M. Weil, The Barium Oxotellurate(IV) Bromides Ba6Te10O25Br2 and Ba3Te3O8Br2 with Channel Structures, Z. Anorg. Allg. Chem., 2012, 638, 2150–2157 CrossRef.
- W. Klein, J. Curda, E. M. Peters and M. Jansen, Neue Silber(I)-oxotellurate(IV/VI), Z. Anorg. Allg. Chem., 2005, 631, 2893–2899 CrossRef CAS.
- D. W. Lee, H. R. Noh, T. H. Kim, J. Lee, J. Y. Kim, K. T. Ko and S. H. Lim, Mixed-valent titanium tellurium oxides, Ti1−x TexTe3O8 + x (x = 0, 0.1, and 0.12): Hydrothermal synthesis, structure, and characterization, J. Solid State Chem., 2021, 297, 122024 CrossRef CAS.
- J. Chen, C. L. Hu, X. H. Zhang, B. X. Li, B. P. Yang and J. G. Mao, CsVO2F(IO3): An Excellent SHG Material Featuring an Unprecedented 3D [VO2F(IO3)]− Anionic Framework, Angew. Chem., Int. Ed., 2020, 59, 5381–5384 CrossRef CAS PubMed.
- G. S. Yang, G. Peng, N. Ye, J. Y. Wang, M. Luo, T. Yan and Y. Q. Zhou, Structural Modulation of Anionic Group Architectures by Cations to Optimize SHG Effects: A Facile Route to New NLO Materials in the ATCO3F (A = K, Rb; T = Zn, Cd) Series, Chem. Mater., 2015, 27, 7520–7530 CrossRef CAS.
- Q. T. Xu and S. P. Guo, Middle-infrared Second-order Nonlinear Optical Chalcogenides and Halides Containing Multiple Anions, Chin. J. Struct. Chem., 2020, 39, 1964–1570 Search PubMed.
- F. G. You, P. F. Gong, F. Liang, X. X. Jiang, H. Tu, Y. Zhao, Z. G. Hu and Z. S. Lin, M2(SeO3)F2 (M = Zn, Cd): understanding the structure directing effect of [SeO3]2− groups on constructing ordered oxyfluorides, CrystEngComm, 2019, 21, 2485–2489 RSC.
- M. L. Liang, C. L. Hu, F. Kong and J. G. Mao, BiFSeO3: An Excellent SHG Material Designed by Aliovalent Substitution, J. Am. Chem. Soc., 2016, 138, 9433–9436 CrossRef CAS PubMed.
- Y. J. Jia, X. Y. Zhang, Y. G. Chen, X. X. Jiang, J. N. Song, Z. S. Lin and X. M. Zhang, PbBi(SeO3)2F and Pb2Bi(SeO3)2Cl3: Coexistence of Three Kinds of Stereochemically Active Lone-Pair Cations Exhibiting Excellent Nonlinear Optical Properties, Inorg. Chem., 2022, 61, 15368–15376 CrossRef CAS PubMed.
- M. Mutailipu, M. Zhang, H. P. Wu, Z. H. Yang, Y. H. Shen, J. L. Sun and S. L. Pan, Ba3Mg3(BO3)3F3 polymorphs with reversible phase transition and high performances as ultraviolet nonlinear optical materials, Nat. Commun., 2018, 9, 3089 CrossRef PubMed.
- Y. L. Hu, C. Wu, X. X. Jiang, Z. J. Wang, Z. P. Huang, Z. S. Lin, X. F. Long, M. G. Humphrey and C. Zhang, Giant Second-Harmonic Generation Response and Large Band Gap in the Partially Fluorinated Mid-Infrared Oxide RbTeMo2O8F, J. Am. Chem. Soc., 2021, 143, 12455–12459 CrossRef CAS PubMed.
- M. L. Liang, Y. X. Ma, C. L. Hu, F. Kong and J. G. Mao, Ba(MoO2F)2(QO3)2 (Q = Se, Te): Partial Fluorination of MoO6 Octahedra Enabling Two Polar Solids with Strong and Phase Matchable SHG Response, Chem. Mater., 2020, 32, 9688–9695 CrossRef CAS.
- J. J. Zhou, H. P. Wu, H. W. Yu, S. T. Jiang, Z. G. Hu, J. Y. Wang, Y. C. Wu and P. S. Halasyamani, BaF2TeF2(OH)2: A UV Nonlinear Optical Fluorotellurite Material Designed by Band-Gap Engineering, J. Am. Chem. Soc., 2020, 142, 4616–4620 CrossRef CAS PubMed.
- J. Y. Chung, S. Yeon, H. Ryu, T. S. You, J. I. Jang and K. M. Ok, Nonlinear optical properties of a new polar bismuth tellurium oxide fluoride, Bi3F(TeO3)(TeO2F2)3, J. Alloys Compd., 2022, 895, 162603 CrossRef CAS.
- R. L. Tang, M. Yan, W. D. Yao, W. L. Liu and S. P. Guo, HgTeO2F(OH): A Nonlinear Optical Oxyfluoride Constructed of Active [TeO2F(OH)]2− Pyramids and V-Shaped [HgO2]2− Groups, Inorg. Chem., 2022, 61, 2333–2339 CrossRef CAS PubMed.
- J. H. Feng, C. L. Hu, H. P. Xia, F. Kong and J. G. Mao, Li7(TeO3)3F: A Lithium Fluoride Tellurite with Large Second Harmonic Generation Responses and a Short Ultraviolet Cutoff Edge, Inorg. Chem., 2017, 56, 14697–14705 CrossRef CAS PubMed.
- X. X. Wang, X. B. Li, C. L. Hu, F. Kong and J. G. Mao, Ag4Hg(SeO3)2(SeO4): a novel SHG material created in mixed valent selenium oxides by in situ synthesis, Sci. China Mater., 2019, 62, 1821–1830 CrossRef.
- T. N. Poe, F. D. White, V. Proust, E. M. Villa and M. J. Polinski, [Ag2M(Te2O5)2]SO4 (M = Ce(IV) or Th(IV)): A New Purely Inorganic d/f-Heterometallic Cationic Material, Inorg. Chem., 2018, 57, 4816–4819 CrossRef CAS PubMed.
- P. F. Li, C. L. Hu, F. Kong and J. G. Mao, Hg2(SeO3)(SO4): the first sulfate selenite with large birefringence explored from d10 transition metal compounds, Mater. Chem. Front., 2022, 6, 3567–3576 RSC.
- S. N. Yan, X. X. Wang, C. L. Hu, B. X. Li, F. Kong and J. G. Mao, Na3Ti3O3(SeO3)4F: A Phase-Matchable Nonlinear-Optical Crystal with Enlarged Second-Harmonic-Generation Intensity and Band Gap, Inorg. Chem., 2022, 61, 2686–2694 CrossRef CAS PubMed.
- R. H. Blessing, An Empirical Correction for Absorption Anisotropy, Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater., 1995, A51, 33–38 CrossRef CAS PubMed.
- G. M. Sheldrick, Crystal structure refinement with SHELXL, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3–8 Search PubMed.
- S. K. Kurtz and T. T. Perry, A Powder Technique for the Evaluation of Nonlinear Optical Materials, J. Appl. Phys., 1968, 39, 3798–3813 CrossRef CAS.
- Y. Y. Yang, Y. Guo, B. B. Zhang, T. Wang, Y. G. Chen, X. H. Hao, X. X. Yu and X. M. Zhang, Lead Tellurite Crystals BaPbTe2O6 and PbVTeO5F with Large Nonlinear-/Linear-Optical Responses due to Active Lone Pairs and Distorted Octahedra, Inorg. Chem., 2022, 61, 1538–1545 CrossRef CAS PubMed.
- M. Zhao, Y. Sun, Y. Wu, D. Mei, S. Wen and T. Doert, NaTePO5, SrTeP2O8 and Ba2TeP2O9: Three tellurite-phosphates with large birefringence, J. Alloys Compd., 2021, 854, 157243 CrossRef CAS.
- N. Jennene Boukharrata and J. P. Laval, Synthesis and structure determination of In3TeO3F7: An indium oxyfluorotellurate IV derived from W bronze structure with Te4+ in hexagonal tunnels, J. Alloys Compd., 2011, 509, 1517–1522 CrossRef CAS.
- D. D. Wang, P. F. Gong, X. Y. Zhang, Z. S. Lin, Z. G. Hu and Y. C. Wu, Centrosymmetric Rb[Te2O4(OH)5] and noncentrosymmetric K2[Te3O8(OH)4]: metal tellurates with corner and edge-sharing (Te4O18)12− anion groups, Inorg. Chem. Front., 2022, 9, 2628–2636 RSC.
- Y. P. Gong, Y. X. Ma, S. M. Ying, J. G. Mao and F. Kong, Two Indium Sulfate Tellurites: Centrosymmetric In2(SO4)(TeO3)(OH)2(H2O) and Non-centrosymmetric In3(SO4)(TeO3)2F3(H2O), Inorg. Chem., 2019, 58, 11155–11163 CrossRef CAS PubMed.
- J. L. Song and C. Qian, Syntheses, Crystal Structures, and Properties of Two Quaternary Selenite/Tellurite-Nitrates with Formula of Bi(SeO3)(NO3) and Bi3(μ3-OH)(TeO3)3(NO3)2, ChemistrySelect, 2017, 2, 1681–1685 CrossRef CAS.
- Y. G. Chen, N. Yang, X. N. Yao, C. B. Li, Y. Guo and X. M. Zhang, Synergetic Influence of Alkali-Metal and Lone-Pair Cations on Frameworks of Tellurites, Inorg. Chem., 2018, 57, 5406–5412 CrossRef CAS PubMed.
- S. Y. Song, D. W. Lee and K. M. Ok, Rich structural chemistry in scandium selenium/tellurium oxides: mixed-valent selenite-selenates, Sc2(SeO3)2(SeO4) and Sc2(TeO3)(SeO3)(SeO4), and ternary tellurite, Sc2(TeO3)3, Inorg. Chem., 2014, 53, 7040–7046 CrossRef CAS PubMed.
- P. F. Li, F. Kong and J. G. Mao, MII2M3IIIF3(Te6F2O16) (MII = Pb, Ba; MIII = Al, Ga): New mixed anionic tellurites with isolated Te6 coplanar rings, J. Solid State Chem., 2020, 286, 121288 CrossRef CAS.
- M. Zhao, W. M. Dong, Y. D. Wu, D. J. Mei, S. G. Wen and T. Doert, A2(TeO)P2O7 (A = K, Rb, Cs): Three new tellurite-pyrophosphates with large birefringence, J. Alloys Compd., 2021, 865, 158785 CrossRef CAS.
- Z. Li, S. Z. Zhang, W. L. Yin, Z. S. Lin, J. Y. Yao and Y. C. Wu, Na3Ca4(TeO3)(PO4)3: a new noncentrosymmetric tellurite phosphate with fascinating multimember-ring architectures and intriguing nonlinear optical performance, Dalton Trans., 2018, 47, 17198–17201 RSC.
- S. G. Zhao, Y. Yang, Y. G. Shen, B. Q. Zhao, L. N. Li, C. M. Ji, Z. Y. Wu, D. Q. Yuan, Z. S. Lin, M. C. Hong and J. H. Luo, Cooperation of Three Chromophores Generates the Water-Resistant Nitrate Nonlinear Optical Material Bi3TeO6OH(NO3)2, Angew. Chem., Int. Ed., 2017, 56, 540–544 CrossRef CAS PubMed.
- D. Vanderbilt, Soft self-consistent pseudopotentials in a generalized eigenvalue formalism, Phys. Rev. B: Condens. Matter Mater. Phys., 1990, 41, 7892–7895 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Crystallographic data, important bond distances and angles, simulated and measured PXRD patterns, infrared spectra, and computational method. CCDC 2214235–2214237. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2qi02272a |
| This journal is © the Partner Organisations 2023 |