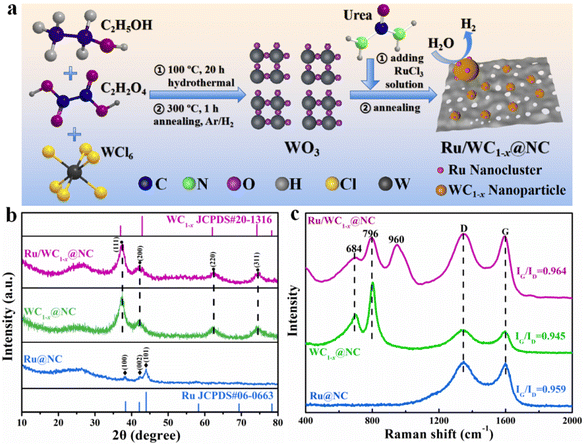
Synergistic effect of Ru nanoclusters on WC1−x anchored on N-doped carbon nanosheets to promote highly efficient alkaline hydrogen evolution†
Hong
Li
ab,
Lanxin
Dai
a,
Yinan
Zheng
a,
Hu
Yao
a,
Jiayu
Bai
a and
Xiaohui
Guo
 *a
*a
aKey Lab of Synthetic and Natural Functional Molecule Chemistry of Ministry of Education, and College of Chemistry and Materials Science, Northwest University, Xi'an, 710069, P. R. China. E-mail: guoxh2009@nwu.edu.cn; Tel: +86-2981535031
bCollege of Chemistry and Chemical Engineering, Yan'an University, Yan'an, 716000, P. R. China
First published on 5th November 2022
Abstract
Developing novel non-noble electrocatalysts with high efficiency and low cost for the alkaline hydrogen evolution reaction (HER) remains a great challenge. Although tungsten carbides with a Pt-like electronic structure have been considered as promising candidates to replace noble metal electrocatalysts, their alkaline HER performance is seriously restricted by the sluggish kinetics of water dissociation, the difficulty of hydrogen desorption and low conductivity. Herein, Ru nanocluster-anchored WC1−x nanoparticles supported on a porous N-doped carbon nanosheet (Ru/WC1−x@NC) hybrid was successfully prepared through a facile pyrolysis of a mixture of WO3, RuCl3 and molten urea. The Ru nanoclusters significantly promote the dissociation of water molecules, and the synergistic effect between the Ru nanoclusters and WC1−x nanoparticles and the strong coupling between the N-doped carbon substrate and WC1−x nanoparticles greatly facilitate the reaction kinetics for the alkaline HER. Therefore, the resulting Ru/WC1−x@NC hybrid displays outstanding catalytic activity with a very low overpotential of 24 mV at 10 mA cm−2 and a small Tafel slope of 45 mV dec−1 in 1 M KOH, and shows excellent long-term durability for the HER. This work sheds light on a feasible strategy by which to develop low-cost electrocatalysts with high efficiency and stability towards the alkaline HER via composition and nanostructure engineering modulation.
Introduction
The ever-increasing consumption of fossil fuels and the resulting environmental pollution problems have drawn attention to green and renewable hydrogen energy due to its zero carbon emissions and high energy density and utilization efficiency.1–3 The electrochemical hydrogen evolution reaction (HER) is considered to be one of the most effective and economical technologies by which to obtain industrially produced high-purity hydrogen.4–6 At present, highly efficient electrocatalysts are necessary to achieve hydrogen generation in a high yield from the HER because of the inevitable kinetics barriers that must be overcome during the water splitting process.7 Platinum noble metal electrocatalysts have been ranked as the most efficient electrocatalysts for the HER over the past several decades.8 However, the high cost and scarcity of noble metal catalysts hinder their large-scale industrial applications. Besides this, considering the overall process of electrochemical water splitting, the alkaline HER is more beneficial for practical applications as most state-of-the-art OER electrocatalysts exhibit poor stability in acid media, with alkaline media providing a more benign reaction environment for industrial water splitting devices.9,10 It is worth noting that the alkaline HER exhibits more sluggish reaction kinetics than those of the HER in acidic media, since protons need to be released first from the water dissociation process, which has a high activation barrier (Kw = 1 × 10−14, 298 K) in alkaline solution.11,12 Therefore, to promote the popularization and industrialization of hydrogen production through water electrolysis, it is highly desirable yet remains challenging to develop cost-effective electrocatalysts with high activity and stability for the alkaline HER.Recently, various non-noble electrocatalysts for the alkaline HER have been developed, such as transition-metal oxides, phosphides, nitrides, sulfides, carbides and hydroxides. Among them, earth-abundant tungsten-based compounds are regarded as promising low-cost non-noble metal catalysts for the HER in alkaline media, as they have favorable surface electronic properties with a W 5d orbital, which is similar to the d-band electronic structures of Pt group metals, and the W atoms at the surface can serve as Lewis acid sites, adsorbing H2O molecules.13,14 In particular, Tong et al.15 found that cubic WC1−x displays a much higher electrocatalytic HER activity with a low overpotential of 216 mV and small Tafel slope of 122 mV dec−1 in 1 M KOH solution than that of hexagonal WS2 (418 mV, 154.2 mV dec−1), WP (290 mV, 145.8 mV dec−1), W2N3 (379 mV, 193.4 mV dec−1), and WN (571 mV, 164 mV dec−1) under similar conditions. As a result, it was proved that WC1−x presents the best alkaline HER performance due to it has near-zero Gibbs free energy (∼0.23 eV), based on first-principles calculations. Moreover, Suetin et al.16 studied the electronic properties of tungsten mono- and semi-carbides through theoretical calculations, and discovered that the electronic density of states at the Fermi level in cubic WC1−x is six times higher than that of hexagonal WC and W2C. However, as far as we know, most reported studies on the HER performance of WC1−x electrocatalysts have been carried out in acidic media,17–19 so their hydrogen evolution activity and stability in alkaline solution still needs to be further explored.
In addition, the electrocatalytic HER activity of the reported WC1−x nanostructures in alkaline media is still far inferior to that of noble metals catalysts. There are three main reasons for this. (I) Compared with metal-based catalysts, the lower electrical conductivity of WC1−x impedes fast electron transfer during HER reactions.18,19 (II) The sluggish reaction kinetics of the water dissociation process with a high energy barrier in alkaline solutions seriously hinder the release of protons.20–22 (III) The strong W–H bonds on the surface of W atoms are not conducive to the timely desorption of the generated hydrogen (Heyrovsky step), which can occupy the catalytic active sites of H+ reduction (Volmer step), thus decaying the HER catalytic activity of tungsten carbides.23,24
Previous studies have demonstrated that combining transition-metal carbides with highly conductive carbon materials can significantly facilitate charge transfer during the HER reaction and increase the exposure of active sites.25–28 And, very recently, Deng et al.24 discovered that the synergistic effects between tungsten carbides and carbon support materials reduce the bonding strength of the W–H bonds on the surface of the catalyst towards the HER. In addition, loading a small amount of noble-metal nanoparticles on the surface of transition-metal compounds has been advocated as an effective strategy by which to dramatically improve the electrocatalytic activity for the alkaline HER.29–32 According to the reported experimental and theoretical results, Ru not only possesses the best water dissociation capability among almost all the noble metals, but also has a low hydrogen bond energy similar to that of Pt. However, its price is only about 4% that of Pt, which together with its favorable properties makes it a promising candidate as an alkaline HER electrocatalyst. Wang et al.31 prepared a Ru/CeO2 hybrid catalyst with 3 wt% Ru loading and found that the overpotential of this hybrid catalyst at 10 mA cm−2 was as low as 28.9 mV and the corresponding Tafel slope was only 53.2 mV dec−1. Inspired by all the above cases, we speculated that simultaneously loading a tiny amount of Ru nanoparticles and constructing composites with carbon materials may remarkably enhance the HER catalytic activity of WC1−x nanostructures in alkaline media.
Herein, we fabricated a Ru nanoclusters-anchored WC1−x hybrid on porous N-doped carbon nanosheets (Ru/WC1−x@NC) through a facile annealing treatment process. Porous N-doped carbon was used as a substrate to support the WC1−x nanoparticles, which not only suppresses their aggregation to enable the exposure of more catalytic active sites, but also ensures fast charge transport at high current densities in the HER process and favors the release of generated H2 on the surface of the catalyst, with the synergistic effect between the Ru nanoclusters and WC1−x nanoparticles facilitating the dissociation of the water and promoting the reduction of absorbed H*. The resulting Ru/WC1−x@NC catalysts with a low Ru loading of 2.5 wt% exhibited a low overpotential of 24 mV at 10 mA cm−2 and a small Tafel slope of 45 mV dec−1, which are much lower than those of Ru nanoclusters on N-doped carbon (Ru@NC) and WC1−x nanoparticles on N-doped carbon (WC1−x@NC), even superior to that of commercial 20 wt% Pt/C. This work further exploits the potential of WC1−x-based catalysts for the alkaline HER, and provides a facile approach by which to develop cheap transition-metal electrocatalysts for clean-energy utilization.
Experimental section
Synthesis of WO3 nanosheets
WO3 nanosheets were prepared via a one-step hydrothermal process followed by annealing treatment. Firstly, WCl6 (200 mg) and oxalic acid (2 g) were dissolved in ethanol (40 ml) under stirring for half an hour to obtain the precursor solution. Then, the precursor solution was transferred into a Teflon-lined autoclave and maintained at 100 °C for 24 h. After that, the product was repeatedly washed with deionized water and ethanol, and dried overnight in a vacuum oven at 60 °C. WO3 nanosheets were obtained by annealing of the dried product obtained after hydrothermal processing in a 10% H2/Ar atmosphere at 300 °C for 1 h.Synthesis of the Ru/WC1−x@NC hybrid
The Ru/WC1−x@NC hybrid was synthesized as follows. RuCl3 aqueous solution (1 mL) was mixed with urea (10 g) and then heated to obtain a molten mixture. Subsequently, WO3 nanosheets (0.1 g) were added into the molten mixture under magnetic stirring, and heated until they were evenly dispersed in the above-mentioned molten mixture. Then, the heating was stopped, and a solid powder mixture of WO3 nanosheets, RuCl3 and urea was obtained after cooling to room temperature. The resulting solid powder mixture was heated to 550 °C at a heating rate of 2 °C min−1 and held at temperature for 10 min, before being heated to 800 °C over 1 h and held for 2 h at temperature in a tube furnace under the protection of Ar gas. After that, the annealed product was further annealed at 300 °C for 30 min in a muffle furnace in air to obtain the Ru/WC1−x@NC hybrid as a black powder. In addition, as a comparison, WC1−x@NC and Ru@NC hybrids were prepared via a preparation process similar to that of Ru/WC1−x@NC without the addition of the aqueous solution of RuCl3 or WO3 nanosheets.Characterization
The phase compositions of the prepared Ru/WC1−x@NC samples were determined via powder X-ray diffraction (XRD) on a Bruker AXS D8 diffractometer operated at 40 kV and 40 mA using Cu Kα radiation (λ = 0.15418 nm). The sample morphologies were investigated using scanning electron microscopy (SEM) on a Hitachi SU-8010 field emission scanning electron microscope (FESEM). Transmission electron microscopy (TEM, Talos F200X) was performed at an accelerating voltage of 200 kV coupled with energy-dispersive spectroscopy (EDS). Raman spectra were obtained using a Raman spectrometer (Jobin Yvon Co., France, HR800) employing a 10 mW helium/neon laser at 532 nm. X-ray photoelectron spectroscopy (XPS) (PHI 5000 VersaProbe III XPS) was used to investigate the surface electron states of the prepared samples. The specific surface areas and pore structures of the materials were determined using the Brunauer–Emmett–Teller (BET) method on a N2 adsorption instrument (Micromeritics Tristar-3020). The loading amount of ruthenium in the catalysts was determined using inductively coupled plasma atomic emission spectrometry (ICP-AES, Shimadzu ICPS-8100).Electrochemical measurements
All the catalyst inks were prepared by ultrasonically dispersing 5 mg of the as-synthesized catalysts and 50 μL of Nafion® solution (Sigma, 5 wt%) in 950 μL of ethanol for at least 30 min. To prepare the electrode for catalytic tests, 5 μL of catalyst ink (5 mg mL−1) obtained as described above was loaded on a polished circular glassy carbon electrode (GCE, 3 mm in diameter) with an exposed area of 0.07 cm−2, leading to a catalyst loading mass of 0.36 mg cm−2.All electrochemical measurements were carried out on an electrochemical workstation (CHI 660E, Shanghai Chenhua, China) using a three-electrode electrochemical system. The GCE loaded with catalysts, carbon rod and Hg/HgO electrode were used as the working, counter and reference electrodes, respectively. All potentials measured are reported against the reversible hydrogen electrode (RHE) according to the following equation:
| ERHE = EHg/HgO + 0.059 × pH + 0.098 V | (1) |
All HER polarization curves were measured in 1.0 M KOH solution. The HER polarization tests were conducted via linear sweep voltammetry (LSV) measurements recorded at a scan rate of 5 mV s−1, and the LSV polarization curves were calculated according to the geometric area of the electrodes. The Tafel plots were obtained from LSV polarization curves using the equation:
η = b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) j + a j + a | (2) |
The turnover frequency (TOF) values of all the catalysts for the HER were calculated according to the following equation:
| TOF (s−1) = (j × A)/(2 × F × n) | (3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 C mol−1), and n (mol) is mole number of Ru contained in the catalysts loaded on the working electrode, which was obtained according to the results of ICP-MS analysis.
500 C mol−1), and n (mol) is mole number of Ru contained in the catalysts loaded on the working electrode, which was obtained according to the results of ICP-MS analysis.
Results and discussion
The preparation process of the Ru/WC1−x@NC hybrid is illustrated in Fig. 1a. First, WO3 nanosheets were synthesized via a hydrothermal reaction followed by a annealing reduction process under a 10% H2/Ar flow based on a method reported in the literature,33 as shown in Fig. S1 and S2.† Then, WO3 nanosheets and RuCl3 were dispersed in molten urea to obtain a precursor mixture. The resulting precursor was first held at 550 °C for 10 min and then pyrolyzed at 800 °C for 2 h in a tube furnace under the protection of Ar gas. During the pyrolysis process, urea was decomposed into C3N4 at a lower heating temperature and further converted to porous N-doped graphited carbon sheets at higher temperature, accompanied by the carbonization of the WO3 nanosheets by the released volatile carbonitride species and the reduction of Ru3+ under this reducing atmosphere as a result of the thermal decomposition of urea.34–36 Herein, urea not only serves as a raw material for the formation of porous N-doped carbon, but also provides a carbothermal reduction atmosphere to carbonize WO3 into WC1−x, while Ru3+ is reduced to Ru nanoclusters on the surface of WC1−x nanoparticles. The newly-generated porous N-doped carbon acts as a substrate to support WC1−x nanoparticles and prevent their agglomeration.The XRD pattern of the resulting Ru/WC1−x@NC hybrid is shown in Fig. 1b. The characteristic diffraction peaks at 36.9°, 42.8°, 62° and 74.2° can be assigned to the (111), (200), (220) and (311) planes of cubic WC1−x (JCPDS no. 20-1316) phase, respectively, which is consistent with the previously reported results.37 In the spectrum of the comparison compound WC1−x@NC, the broad peak at 20–30° corresponds to amorphous N-doped carbon,38 which can also be observed in the spectra of WC1−x@NC and Ru@NC. ICP-MS analysis results (Table S1†) demonstrated that the loading amount of Ru in the optical Ru/WC1−x@NC hybrid is only 2.5 wt%, which is much lower than those of most reported Ru-based catalysts for alkaline HER in recent years (Table S2†). Besides this, Raman spectroscopy was employed to further detect the structural information of the Ru/WC1−x@NC, WC1−x@NC and Ru@NC hybrids (Fig. 1c). It was found that the peaks located at 684 and 796 cm−1 can be ascribed to W–C stretching modes,17 implying the formation of a WC1−x phase. The special peak at around 960 cm−1 can be observed in the spectrum of Ru/WC1−x@NC, but not in the spectra of WC1−x@NC and Ru@NC, which is probably related to the generated crystalline disorder when Ru nanoclusters are anchored in the WC1−x nanoparticles,39 suggesting that Ru nanoclusters were successfully anchored on WC1−x nanoparticles in the Ru/WC1−x@NC hybrid. The peaks at 1350 and 1600 cm−1 corresponding to the disordered D and graphitized G bands of underlying carbon layers appear in the spectra of Ru/WC1−x@NC, WC1−x@NC and Ru@NC, but the value of IG/ID of Ru/WC1−x@NC is higher than that of WC1−x@NC and Ru@NC, revealing that the Ru/WC1−x@NC hybrid exhibits higher conductivity, which is beneficial to its HER catalytic performance.
The microstructure of the Ru/WC1−x@NC hybrid was detected, with the results shown in Fig. 2. It can be seen clearly from scanning electron microscopy (SEM, Fig. 2a) and transmission electron microscopy (TEM, Fig. 2b and c) images that the Ru/WC1−x@NC hybrid is composed of numerous porous N-doped carbon nanosheets loaded with nanoparticles, and that these nanoparticles with a small size of 4–14 nm are uniformly dispersed on the surface of these porous N-doped carbon nanosheets without aggregations. The high-resolution transmission electron microscopy (HRTEM) image (Fig. 2d) indicates that these small nanoparticles display regular lattice fringes with a spacing of 0.243 nm, which belong to the (111) crystal plane of face-centered cubic WC1−x. Moreover, some partial interplanar spacings of 0.206 nm that fit well to the (101) crystal plane of hexagonal metallic Ru can be observed among the lattice fringes of the WC1−x nanoparticles in the orange dashed line area, revealing that Ru nanoclusters are anchored on the surface of the WC1−x nanoparticles. Moreover, in the corresponding selected area electron diffraction (SAED) pattern, the white patterns can be indexed to the (220), (111), (311) and (331) planes of WC1−x and the orange patterns can be assigned to the (002) plane of Ru, demonstrating the coexistence of WC1−x nanoparticles and Ru nanoclusters. Energy-dispersive X-ray spectroscopy (EDX) elemental mappings (Fig. 2e) verified that the W, C, N and Ru elements are uniformly distributed in the Ru/WC1−x@NC hybrid (Fig. S3†), which is consistent with the ICP-MS results.
According to BET measurements (Fig. S4†), the Ru/WC1−x@NC hybrid has a high specific surface area of 146.8 m−2 g−1, which is almost 12 times higher than that of WC1−x@NC (12.1 m−2g−1), but, they have similar porous structures with a pore size distribution in the range of 20–90 nm. However, the introduction of Ru nanoclusters further promotes the uniform dispersion of WC1−x nanoparticles on the porous carbon surface, thereby increasing the specific surface area. Without the Ru nanoclusters, the partial agglomeration of WC1−x nanoparticles was observed in the WC1−x@NC hybrid (Fig. S5a and b and Fig. S6†); without the existence of WC1−x, the prepared Ru@NC hybrid displays an irregular bulk agglomeration morphology (Fig. S5c and d†). In the Ru/WC1−x@NC hybrid, the porous N-doped carbon layers as a substrate support the WC1−x nanoparticles and the Ru nanoclusters tightly attached to the WC1−x nanoparticles not only prevent the aggregation of WC1−x nanoparticles to expose more active sites, but also facilitate fast electrons transport during the HER process.
To further investigate the surface chemical state of the Ru/WC1−x@NC hybrid, XPS was carried out. From the XPS surveys shown in Fig. S7,† the coexistence of the W, Ru, N, and C elements in Ru/WC1−x@NC hybrid was confirmed. In the high-resolution C 1s and Ru 3d5/2 spectra, the peaks at higher binding energy of 284.5, 285.4 and 288.3 eV can be assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–N and oxidized carbon,40 respectively. The peak at lower binding energy of 283.2 eV observed in the spectra of the Ru/WC1−x@NC and WC1−x@NC hybrids was indexed to W–C bonding,17,19 verifying the existence of WC1−x. Compared with WC1−x@NC, the peak of Ru 3d5/2 at a binding energy of 280.6 eV was detected in the high-resolution C 1s and Ru 3d5/2 spectra of Ru/WC1−x@NC (Fig. 3a), which were also observed for Ru@NC, confirming that Ru was successfully introduced into the Ru/WC1−x@NC hybrid. In the high-resolution Ru 3p spectrum of Ru/WC1−x@NC (Fig. 3b), the two strong peaks at binding energies of around 462.6 and 484.9 eV correspond to the Ru 3p3/2 and Ru 3p1/2 of metallic Ru, respectively, and the other two weak peaks at binding energies of 464.9 and 487.4 eV can be assigned to oxidized Ru species,41,42 indicating that the ruthenium species in the hybrid is metallic Ru. The high-resolution W 4f spectra of Ru/WC1−x@NC (Fig. 3c) can be deconvoluted into four peaks at binding energies of 32.2, 34.3, 36 and 38.1 eV, in which the peaks at 32.2 and 34.3 eV can be attributed to the 4f7/2 and 4f5/2 orbitals of W4+ from W–C bonding, while the other bands can be indexed to W6+.43–45 The binding energy of W–C bonding in the spectrum of the Ru/WC1−x@NC hybrid displays a slight negative shift in comparison to that of WC1−x@NC, while the binding energy of metallic Ru exhibits a positive shift compared to those of Ru@NC, suggesting that the introduction of Ru leads to partial electron transport from the Ru nanoclusters to WC1−x nanoparticles due to the W atom exhibiting a higher electronegativity than the Ru atom, and thus resulting in the accumulation of electrons around the W atoms, which is beneficial to the generation of H2 on the surface of the WC1−x nanoparticles. The high-resolution N 1s spectrum of the Ru/WC1−x@NC hybrid (Fig. 3d) can be deconvoluted into three strong binding energy peaks of pyridinic-N (∼398.10 eV), pyrrolic-N (∼399.20 eV) and graphitic-N (∼400.60 eV), and a weak binding energy of oxidized-N (∼402.70 eV),26 revealing the successful doping of N into the porous carbon nanosheets.
C, C–N and oxidized carbon,40 respectively. The peak at lower binding energy of 283.2 eV observed in the spectra of the Ru/WC1−x@NC and WC1−x@NC hybrids was indexed to W–C bonding,17,19 verifying the existence of WC1−x. Compared with WC1−x@NC, the peak of Ru 3d5/2 at a binding energy of 280.6 eV was detected in the high-resolution C 1s and Ru 3d5/2 spectra of Ru/WC1−x@NC (Fig. 3a), which were also observed for Ru@NC, confirming that Ru was successfully introduced into the Ru/WC1−x@NC hybrid. In the high-resolution Ru 3p spectrum of Ru/WC1−x@NC (Fig. 3b), the two strong peaks at binding energies of around 462.6 and 484.9 eV correspond to the Ru 3p3/2 and Ru 3p1/2 of metallic Ru, respectively, and the other two weak peaks at binding energies of 464.9 and 487.4 eV can be assigned to oxidized Ru species,41,42 indicating that the ruthenium species in the hybrid is metallic Ru. The high-resolution W 4f spectra of Ru/WC1−x@NC (Fig. 3c) can be deconvoluted into four peaks at binding energies of 32.2, 34.3, 36 and 38.1 eV, in which the peaks at 32.2 and 34.3 eV can be attributed to the 4f7/2 and 4f5/2 orbitals of W4+ from W–C bonding, while the other bands can be indexed to W6+.43–45 The binding energy of W–C bonding in the spectrum of the Ru/WC1−x@NC hybrid displays a slight negative shift in comparison to that of WC1−x@NC, while the binding energy of metallic Ru exhibits a positive shift compared to those of Ru@NC, suggesting that the introduction of Ru leads to partial electron transport from the Ru nanoclusters to WC1−x nanoparticles due to the W atom exhibiting a higher electronegativity than the Ru atom, and thus resulting in the accumulation of electrons around the W atoms, which is beneficial to the generation of H2 on the surface of the WC1−x nanoparticles. The high-resolution N 1s spectrum of the Ru/WC1−x@NC hybrid (Fig. 3d) can be deconvoluted into three strong binding energy peaks of pyridinic-N (∼398.10 eV), pyrrolic-N (∼399.20 eV) and graphitic-N (∼400.60 eV), and a weak binding energy of oxidized-N (∼402.70 eV),26 revealing the successful doping of N into the porous carbon nanosheets.
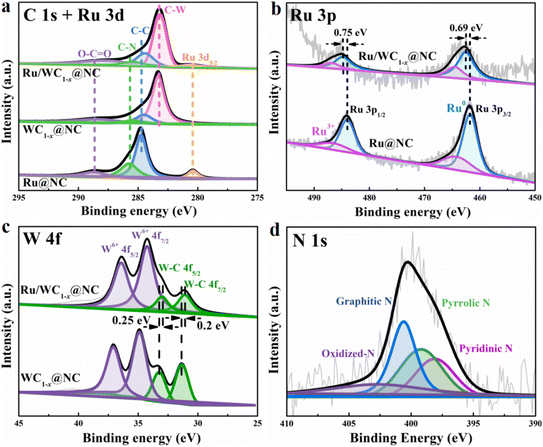 | ||
| Fig. 3 High-resolution XPS spectra of (a) C 1s, (b) Ru 3p, (c) W 4f and (d) N 1s in the resulting Ru/WC1−x@NC, WC1−x@NC and Ru@NC hybrids. | ||
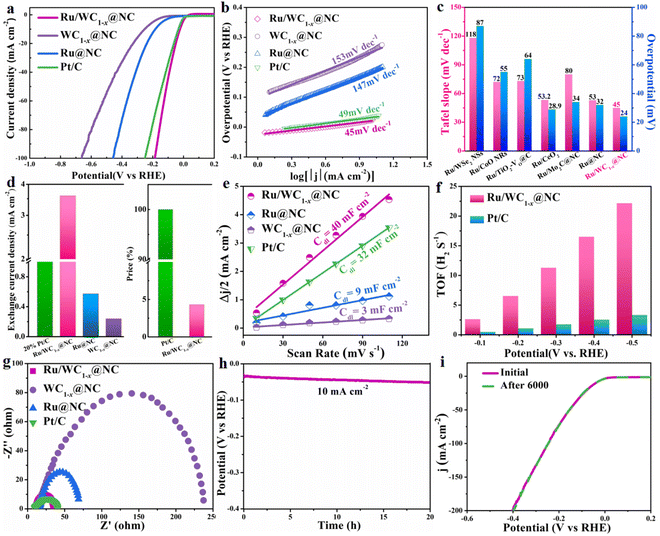
The electrocatalytic performance of the Ru/WC1−x@NC hybrid toward the HER was evaluated in 1 M KOH solution using a standard three-electrode system, alongside those of WC1−x@NC, Ru@NC and commercial Pt/C (20 wt%) for comparison. It was noted that the HER catalytic performance of the nitrogen-doped carbon (NC) is negligible (Fig. S8†). LSV curves were recorded at 5 mV s−1 to investigate the catalytic activity of these electrocatalysts. As shown in Fig. 4a, the Ru/WC1−x@NC hybrid exhibits much better alkaline HER activity with a very low overpotential of 24 mV at a current density of 10 mA cm−2 (η10 = 24 mV) compared with the WC1−x@NC (η10 = 253 mV) and Ru@NC (η10 = 183 mV) hybrids, which is comparable to that of commercial Pt/C (η10 = 30 mV), suggesting that the synergistic effect between the Ru nanoclusters and WC1−x nanoparticles plays an important role in promoting the high catalytic activity of Ru/WC1−x@NC for the alkaline HER. It can be calculated from Fig. 4b that the Tafel slope of the Ru/WC1−x@NC hybrid is as low as 45 mV dec−1, which is much lower than those of the WC1−x@NC (153 mV dec−1) and Ru@NC (147 mV dec−1) hybrids, and also lower than that of commercial Pt/C (49 mV dec−1), indicating that the combination of Ru nanoclusters and WC1−x nanoparticles remarkably improves the HER kinetics of the Ru/WC1−x@NC hybrid in alkaline electrolyte and that the alkaline HER process over Ru/WC1−x@NC catalyst probably follows a Volmer–Heyrovsky route. It is worth noting that the overpotential at 10 mA cm−2 and Tafel slope of the resulting Ru/WC1−x@NC hybrid in this work are also much lower than those of most reported Ru-based electrocatalysts for the alkaline HER (Fig. 4c)20,30,31,46,47 and most reported WC1−x-based HER catalysts,15,17–19 with the detailed comparisons displayed in Table S3,† further demonstrating the superior HER catalytic activity of the Ru/WC1−x@NC hybrid in alkaline electrolyte.
Moreover, the exchange current density can be used to directly evaluate the catalytic activity of a catalyst, i.e., the hydrogen evolution rate per surface area at the electrode potential.48 It can be observed from Fig. 4d that the exchange current density of the Ru/WC1−x@NC hybrid can reach 3.59 mA cm−2, which is around 6 and 15 times as high as those of the WC1−x@NC and Ru@NC hybrids, respectively, and even 1.5 times higher than that of commercial Pt/C, verifying the outstanding intrinsic electrocatalytic activity of the Ru/WC1−x@NC hybrid. When the activity was normalized by the mass of the contained noble metal, the mass activity of the Ru/WC1−x@NC (2.5 wt%) hybrid was almost 6 times higher than that of the commercial Pt/C catalyst (20 wt%) (Fig. S9†). In other words, employing Ru/WC1−x@NC as an electrocatalyst for the alkaline HER, the cost can be reduced by nearly 96% without any loss in activity in comparison with commercial Pt/C, leading to a competitive advantage for practical applications.
To gain further insight into the intrinsic mechanism for improved alkaline HER activity of the Ru/WC1−x@NC hybrid, the double-layer capacitance (Cdl) of the catalysts was determined through CV testing at different scan rates in the non-faradaic potential region (0.085–0.185 V vs. RHE) (Fig. S10†) to estimate the electrochemically active surface areas (ECSAs). It is considered that the value of Cdl of catalysts is linearly proportional to their ECSA, with a high Cdl value indicates a larger ECSA for the HER process.49 There are almost no electrochemical active sites on the surface of the NC support (CdI = 1.9 mF cm−2, as seen in Fig. S11†). As shown in Fig. 4e, the Cdl value of the Ru/WC1−x@NC hybrid (40 mF cm−2) is much higher than that of the commercial Pt/C (32 mF cm−2), WC1−x@NC (3 mF cm−2) and Ru@NC (9 mF cm−2) hybrids, revealing that there are many more available active sites exposed on the surface of the Ru/WC1−x@NC catalyst. Moreover, the specific activity (normalized by ECSA) of the Ru/WC1−x@NC hybrid is also greater than that of commercial Pt/C (Fig. S9†), suggesting the higher utilization efficiency of the noble metal. Furthermore, the TOF values (namely the number of H2 molecules evolved per second per active site) of the Ru/WC1−x@NC and commercial Pt/C catalysts were also calculated under the assumption that all noble metal atoms in the catalyst were active sites during the HER process, with the results shown in Fig. 4f. The TOF values of Ru/WC1−x@NC hybrid are 6.5 and 22.1 H2 s−1 at an overpotential of 200 and 500 mV, respectively, which are almost 6 and 10 times with the values of commercial Pt/C (1.1 and 2.3 H2 s−1), showing the high electrocatalytic efficiency of the Ru/WC1−x@NC hybrid for the alkaline HER. This may be ascribed to the abundant electrochemical active sites and the fast electron transfer dynamics benefiting from the special microstructure of the material conferred by the uniformly dispersed WC1−x nanoparticles with Ru nanoclusters anchored on the surface of the porous N-doped carbon nanosheets. In addition, the HER kinetics of the Ru/WC1−x@NC hybrid were examined by electrochemical impedance spectroscopy (EIS). The corresponding Nyquist plots (Fig. 4g) suggest that the charge transfer resistance of the Ru/WC1−x@NC hybrid (12.2 Ω) is much lower than that of the WC1−x@NC (26.5 Ω) and Ru@NC hybrids (112 Ω), which is comparable to that of commercial Pt/C (12.6 Ω) and close to that of the NC support (12 Ω) (Fig. S12†), confirming that the Ru/WC1−x@NC hybrid exhibits fast electron transfer capability toward the HER. The comparison of the experimental and theoretically calculated H2 evolution (Fig. S13†) over the Ru/WC1−x@NC hybrid also demonstrates that Ru/WC1−x@NC catalyst shows fast reactive kinetics for the HER in alkaline solution.
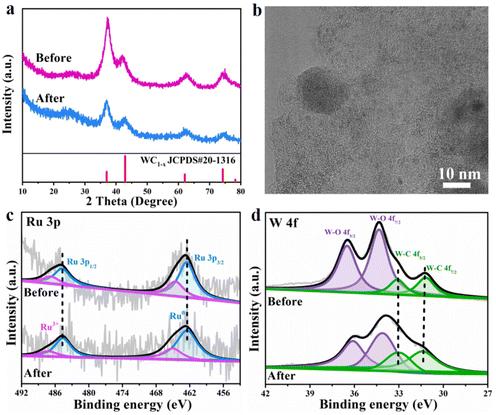
In addition, stability and durability are also very important for the practical application of an advanced catalyst, so the long-term chronopotentiometry curve at a current density of 10 mA cm−2 and the polarization curves before and after 6000 continuous CV cycles at a scan rate of 50 mV s−1 for the Ru/WC1−x@NC hybrid were recorded in 1 M KOH. It can be seen from Fig. 4h that the catalytic activity of the Ru/WC1−x@NC hybrid remains steady without significant activity attenuation after continuous operation for 20 h, demonstrating its excellent long-term stability toward the HER in alkaline solution. Further comparing the LSV curves of Ru/WC1−x@NC before and after 6000 CV cycles (Fig. 4i), almost no catalytic activity degradation occurred, verifying that the Ru/WC1−x@NC hybrid shows superior operation durability for the alkaline HER. The morphology and composition of the Ru/WC1−x@NC catalyst were characterized after 6000 CV cycles. A comparison of XRD patterns indicates that the phase composition of the Ru/WC1−x@NC hybrid shows no changes after cycling (Fig. 5a). It was observed from SEM (Fig. S14a†) and TEM (Fig. 5b) images that the Ru/WC1−x@NC hybrid still retains a well-defined porous nanostructure, also supported by the EDX results (Fig. S14b and S15†), confirming that the Ru nanoclusters are still uniformly distributed in the Ru/WC1−x@NC hybrid without agglomeration after cycling tests. In the Ru 3p and W 4f XPS spectra (Fig. 5c and d), the binding energies of the Ru metal and W–C bonding before and after cycling display a negligible shift, suggesting the good stability of the chemical states of the Ru/WC1−x@NC catalyst. Peaks with a lower binding energy are observed for Ru 3p after cycling, which may be due to the adsorption of trace electrolyte ions on the catalyst surface after HER testing. All these results demonstrates that the obtained Ru/WC1−x@NC hybrid has a robust structure and compositional stability, which enables the Ru/WC1−x@NC catalyst to exhibit excellent cycling stability and durability.
Based on the above results analysis, it can be inferred that the superior HER performance of the Ru/WC1−x@NC hybrid in alkaline solution originates from the following aspects: (1) the anchored Ru nanoclusters remarkably promote the dissociation of water molecules to provide sufficiently available hydrogen protons, which has been verified in previous reports,20,31,32,38,41,42,50 thus accelerating the generation of an adsorbed H* intermediate; (2) the special microstructure composed of Ru nanoclusters-anchored WC1−x nanoparticles uniformly dispersed on the porous N-doped carbon nanosheets contributes towards a large electrochemically active area and high conductivity, which accelerate charge transfer and improve activity and stability during the HER process; (3) the synergistic effect between Ru nanoclusters and WC1−x nanoparticles is beneficial to the adsorption of the H* intermediate on the exposed surface of WC1−x and the combination of the adsorbed H* and another H atom from water dissociation for H2 generation; (4) the strong electron coupling between the WC1−x nanoparticles and N-doped carbon facilitates the desorption of generated H2 from WC1−x and further improves catalytic durability.
Conclusion
In summary, an advanced Ru/WC1−x@NC hybrid catalyst for the HER was successfully prepared via a facile pyrolysis process. Benefiting from its unique composition and microstructure, as well as the synergistic effect between Ru nanoclusters and WC1−x nanoparticles, together with the strong coupling between WC1−x nanoparticles and N-doped carbon, the as-synthesized Ru/WC1−x@NC hybrid exhibits superior catalytic performance for the alkaline HER. Specifically, the Ru/WC1−x@NC hybrid delivers a very low overpotential of 24 mV and small Tafel slope of 45 mV dec−1 at a current density of 10 mA cm−2 in 1 M KOH, which is superior to most reported results and the commercial Pt/C catalyst. Moreover, the Ru/WC1−x@NC hybrid also displays excellent electrochemical stability and outstanding structural durability after long-term operation under alkaline conditions. These interesting findings provide a feasible strategy by which to design and fabricate other efficient and low-cost hybrid electrocatalysts for future clean energy utilization.Author contributions
Hong Li: Conceptualization, methodology, formal analysis, writing-original draft. Lanxin Dai: Investigation, methodology, formal analysis. Yinan Zheng: Investigation. Hu Yao: Methodology. Jiayu Bai: Methodology. Xiaohui Guo: Conceptualization, supervision, project administration, funding acquisition, writing review-editing.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported by the Key projects of intergovernmental international cooperation in key R & D programs of the Ministry of Science and Technology of China (No. 2021YFE0115800), the National Science Funding Committee of China (No. U20A20250), and the Science and Technology Committee of Shaanxi Province (Grant No. 2020JZ-42).References
- Z. W. Seh, J. Kibsgaard, C. F. Dickens, I. B. Chorkendorff, J. K. Norskov and T. F. Jaramillo, Combining theory and experiment in electrocatalysis: Insights into materials design, Science, 2017, 355, eaad4998 CrossRef.
- C. G. Morales-Guio, L.-A. Stern and X. L. Hu, Nanostructured hydrotreating catalysts for electrochemical hydrogen evolution, Chem. Soc. Rev., 2014, 43, 6555–6569 RSC.
- J. Wang, T. Liao, Z. Z. Wei, J. T. Sun, J. J. Guo and Z. Q. Sun, Heteroatom-doping of non-noble metal-based catalysts for electrocatalytic hydrogen evolution: An electronic structure tuning strategy, Small Methods, 2021, 5, 2000988 CrossRef CAS PubMed.
- J. Q. Zhang, Y. F. Zhao, X. Guo, C. Chen, C. L. Dong, R. S. Liu, C. P. Han, Y. D. Li, Y. Gogotsi and G. X. Wang, Single platinum atoms immobilized on an MXene as an efficient catalyst for the hydrogen evolution reaction, Nat. Catal., 2018, 1, 985–992 CrossRef CAS.
- J. Zhu, L. S. Hu, P. X. Zhao, L. Y. S. Lee and K. Y. Wong, Recent advances in electrocatalytic hydrogen evolution using nanoparticles, Chem. Rev., 2020, 120, 851–918 CrossRef CAS.
- Y. Gu, A. P. Wu, Y. Q. Jiao, H. R. Zheng, X. Q. Wang, Y. Xie, L. Wang, C. G. Tian and H. G. Fu, Two-dimensional porous molybdenum phosphide/nitride heterojunction nanosheets for pH-universal hydrogen evolution reaction, Angew. Chem., 2021, 133, 6747–6675 CrossRef.
- Y. Li, X. F. Wei, L. S. Chen and J. L. Shi, Electrocatalytic hydrogen production trilogy, Angew. Chem., Int. Ed., 2020, 60, 19550–19571 CrossRef.
- X. X. Zou and Y. Zhang, Noble metal-free hydrogen evolution catalysts for water splitting, Chem. Soc. Rev., 2015, 44, 5148–5180 RSC.
- Z. P. Zhao, H. T. Liu, W. P. Gao, W. Xue, Z. Y. Liu, J. Huang, X. Q. Pan and Y. Huang, Surface-engineered PtNi-O nanostructure with record-high performance for electrocatalytic hydrogen evolution reaction, J. Am. Chem. Soc., 2018, 140, 9046–9050 CrossRef CAS PubMed.
- Y. J. Li, W. Pei, J. T. He, K. Liu, W. H. Qi, X. H. Gao, S. Zhou, H. P. Xie, K. Yin, Y. L. Gao, J. He, J. J. Zhao, J. H. Hu, T. S. Chan, Z. Li, G. F. Zhang and M. Liu, Hybrids of PtRu nanoclusters and black phosphorus nanosheets for highly efficient alkaline hydrogen evolution reaction, ACS Catal., 2019, 9, 10870–10875 CrossRef CAS.
- T. Y. Kou, M. P. Chen, F. Wu, T. J. Smart, S. W. Wang, Y. S. Wu, Y. Zhang, S. T. Li, S. Lall, Z. H. Zhang, Y. S. Liu, J. H. Guo, G. M. Wang, Y. Ping and Y. Li, Carbon doping switching on the hydrogen adsorption activity of NiO for hydrogen evolution reaction, Nat. Commun., 2020, 11, 590 CrossRef CAS PubMed.
- Y. Zheng, Y. Jiao, A. Vasileff and S. Z. Qiao, The hydrogen evolution reaction in alkaline solution: from theory, single crystal models, to practical electrocatalysts, Angew. Chem., Int. Ed., 2018, 57, 7568–7579 CrossRef CAS.
- W.-F. Chen, J. T. Muckerman and E. Fujita, Recent developments in transition metal carbides and nitrides as hydrogen evolution electrocatalysts, Chem. Commun., 2013, 49, 8896–8909 RSC.
- E. Albanese, C. D. Valentin and G. Pacchioni, H2O adsorption on WO3 and WO3−X (001) surfaces, ACS Appl. Mater. Interfaces, 2017, 9, 23212–23221 CrossRef CAS PubMed.
- R. Tong, Y. J. Qu, Q. Zhu, X. N. Wang, Y. H. Lu, S. P. Wang and H. Pan, Combined experimental and theoretical assessment of WXy (X = C, N, S, P) for hydrogen evolution reaction, ACS Appl. Energy Mater., 2020, 3, 1082–1088 CrossRef CAS.
- D. V. Suetin, I. R. Shein and A. L. Ivanovskii, Structural, electronic properties and stability of tungsten mono- and semi-carbides: A first principles investigation, J. Phys. Chem. Solids, 2009, 70, 64–71 CrossRef CAS.
- I. Shanenkov, A. Ivashutenko, Y. Shanenkova, D. Nikitin, Y. K. Zhu, J. Z. Li, W. Han and A. Sivkov, Composite material WC1−-x@C as a noble-metal-economic material for hydrogen evolution reaction, J. Alloys Compd., 2020, 834, 155116 CrossRef CAS.
- I. Kim, S.-W. Park and D.-W. Kim, Carbon-encapsulated multi-phase nanocomposite of W2C@WC1−X as a highly active and stable electrocatalyst for hydrogen generation, Nanoscale, 2018, 10, 21123–21131 RSC.
- S. S. Xu, L. Q. Yang, Y.-Z. Liu, Y. Hua, X. M. Gao and A. Neville, Boosting hydrogen evolution performance by using a plasma-sputtered porous monolithic W2C@WC1−X/Mo film electrocatalyst, J. Mater. Chem. A, 2020, 8, 19473–19483 RSC.
- J. D. Chen, C. H. Chen, Y. Z. Chen, H. Y. Wang, S. J. Mao and Y. Wang, Improving alkaline hydrogen evolution reaction kinetics on molybdenum carbide: Introducing Ru dopant, J. Catal., 2020, 392, 313–321 CrossRef CAS.
- Y. Wen, J. Y. Qi, D. Q. Zhao, J. H. Liu, P. C. Wei, X. Kang and X. Li, O doping hierarchical NiCoP/Ni2P hybrid with modulated electron density for efficient alkaline hydrogen evolution reaction, Appl. Catal., B, 2021, 293, 120196 CrossRef CAS.
- Z. G. Chen, W. B. Gong, S. Cong, Z. Wang, G. Song, T. Pan, X. Q. Tang, J. Chen, W. B. Lu and Z. G. Zhao, Eutectoid-structured WC/W2C heterostructures: A new platform for long-term alkaline hydrogen evolution reaction at low overpotentials, Nano Energy, 2020, 68, 104335 CrossRef CAS.
- R. Michalsky, Y. J. Zhang and A. A. Peterson, Trends in the hydrogen evolution activity of metal carbide catalysts, ACS Catal., 2014, 4, 1274–1078 CrossRef CAS.
- X. C. Deng, H. Q. Chang and G. H. Zhang, N-doped graphene supported W2C/WC as efficient electrocatalyst for hydrogen evolution reaction, Int. J. Hydrogen Energy, 2022, 47, 902–916 CrossRef CAS.
- X. F. Lu, L. Yu, J. T. Zhang and X. W. Lou, Ultrafine dual-phased carbide nanocrystals confined in porous nitrogen-doped carbon dodecahedrons for efficient hydrogen evolution reaction, Adv. Mater., 2019, 31, 1900699 CrossRef.
- L. Lin, Z. M. Sun, M. W. Yuan, H. Yang, H. F. Li, C. Y. Nan, H. Y. Jiang, S. S. Ge and G. B. Sun, α-MoC1−X quantum dots encapsulated in nitrogen-doped carbon for hydrogen evolution reaction at all pH values, ACS Sustainable Chem. Eng., 2019, 7, 9637–9645 CrossRef CAS.
- Y. P. Liu, G. D. Li, L. Yuan, L. Ge, H. Ding, D. J. Wang and X. X. Zou, Carbon-protected bimetallic carbide nanoparticles for a highly efficient alkaline hydrogen evolution reaction, Nanoscale, 2015, 7, 3130–3136 RSC.
- Z. G. Chen, Y. F. Xu, D. Ding, G. Song, X. X. Gan, H. Li, W. Wei, J. Chen, Z. Y. Li, Z. M. Gong, X. M. Dong, C. F. Zhu, N. N. Yang, J. Y. Ma, R. Gao, D. Luo, S. Cong, L. Wang, Z. G. Zhao and Y. Cui, Thermal migration towards constructing W-W dual-sites for boosted alkaline hydrogen evolution reaction, Nat. Commun., 2022, 13, 763 CrossRef CAS.
- L. Wang, E. G. Mahoney, S. Zhao, B. L. Yang and J. G. Chen, Low loadings of platinum on transition metal carbides for hydrogen oxidation and evolution reactions in alkaline electrolytes, Chem. Commun., 2016, 52, 3697–3700 RSC.
- Y. M. Zhao, G. X. Mao, C. Z. Huang, P. Cai, G. Z. Cheng and W. Luo, Decorating WSe2 nanosheets with ultrafine Ru nanoparticles for boosting electrocatalytic hydrogen evolution in alkaline electrolytes, Inorg. Chem. Front., 2019, 6, 1382–1387 RSC.
- W. Wang, Y. R. Tao, X. C. Wu and L. J. Yang, Flower-like CeO2-supported small-sized Ru nanoparticle hybrids for highly efficient alkaline hydrogen evolution: Roles of interfacial effects, Appl. Surf. Sci., 2022, 581, 152256 CrossRef CAS.
- Z. Z. Wei, Z. J. Zhao, J. Wang, Q. Zhou, C. X. Zhao, Z. H. Yao and J. G. Wang, Oxygen-deficient TiO2 and carbon coupling synergistically boost the activity of Ru nanoparticles for the alkaline hydrogen evolution reaction, J. Mater. Chem. A, 2021, 9, 10160–10168 RSC.
- Z. Y. Sun, R. P. Huo, C. Choi, S. Hong, T.-S. Wu, J. S. Qiu, C. Yan, Z. S. Han, Y. C. Liu, Y.-L. Soo and Y. Jung, Oxygen vacancy enables electrochemical N2 fixation over WO3 with tailored structure, Nano Energy, 2019, 62, 869–875 CrossRef CAS.
- J. H. Liu, T. K. Zhang, Z. C. Wang, G. Dawson and W. Chen, Simple pyrolysis of urea into graphitic carbon nitride with recyclable adsorption and photocatalytic activity, J. Mater. Chem., 2011, 21, 14398–14401 RSC.
- R. Gao, Y. Zhou, X. F. Liu and J. C. Wang, N-doped defective carbon layer encapsulated W2C as a multifunctional cathode catalyst for high performance Li-O2 battery, Electrochim. Acta, 2017, 245, 430–437 CrossRef CAS.
- C. M. Zhao, Y. Wang, Z. J. Li, W. X. Chen, Q. Xu, D. S. He, D. S. Xi, Q. H. Zhang, T. W. Yuan, Y. T. Qu, J. Yang, F. Y. Zhou, Z. K. Yang, X. Q. Wang, J. Wang, J. Luo, Y. F. Li, H. H. Duan, Y. E. Wu and Y. D. Li, Solid-diffusion synthesis of single-atom catalysts directly from bulk metal for efficient CO2 reduction, Joule, 2019, 3, 584–594 CrossRef CAS.
- J. N. Cai, X. F. Zhang, M. X. Yang, Y. D. Shi, W. K. Liu and S. Lin, Constructing Co@WC1−X heterostructure on N-doped carbon nanotubes as an efficient bifunctional electrocatalyst for zinc-air batteries, J. Power Sources, 2021, 485, 229251 CrossRef CAS.
- J. W. Su, Y. Yang, G. L. Xia, J. T. Chen, P. Jiang and Q. W. Chen, Ruthenium-cobalt nanoalloys encapsulated in nitrogen-doped graphene as active electrocatalysts for producing hydrogen in alkaline media, Nat. Commun., 2017, 8, 14969 CrossRef.
- Y. Fujioka, J. Frantti, R. M. Nieminen and A. M. Asiri, Formation of ruthenium cluster on nanocrystalline tungsten trioxide, J. Phys. Chem. C, 2013, 117, 7506–7510 CrossRef CAS.
- G. Yan, C. X. Wu, H. Q. Tan, X. J. Feng, L. K. Yan, H. Y. Zang and Y. G. Li, N-Carbon, coated P-W2C composite as efficient electrocatalyst for hydrogen evolution reactions over the whole pH range, J. Mater. Chem. A, 2017, 5, 765–772 RSC.
- S. W. Sun, G. F. Wang, Y. Zhou, F. B. Wang and X. H. Xia, High-performance Ru@C4N electrocatalyst for hydrogen evolution reaction in both acidic and alkaline solutions, ACS Appl. Mater. Interfaces, 2019, 11, 19176–19182 CrossRef CAS.
- G. Meng, H. Tian, L. X. Peng, Z. H. Ma, Y. F. Chen, C. Chen, Z. W. Chang, X. Z. Cui and J. L. Shi, Ru to W electron donation for boosted HER from acidic to alkaline on Ru/WNO sponges, Nano Energy, 2021, 80, 105531 CrossRef CAS.
- S. C. Sun, H. Jiang, Z. Y. Chen, Q. Chen, M. Y. Ma, L. Zhen, B. Song and C. Y. Xu, Bifunctional WC-supported RuO2 nanoparticles for robust water splitting in acidic media, Angew. Chem., Int. Ed., 2022, e202202519 CAS.
- Z. K. Kou, T. T. Wang, Z. H. Pu, L. Wu, K. Xi and S. C. Mu, Realizing the extraction of carbon from WC for in situ formation of W/WC heterostructures with efficient photoelectrochemical hydrogen evolution, Nanoscale Horiz., 2019, 4, 196–201 RSC.
- O. Lori, S. Gonen, O. Kapon and L. Elbaz, Durable tungsten carbide support for Pt-based fuel cells cathodes, ACS Appl. Mater. Interfaces, 2021, 13, 8315–8323 CrossRef CAS.
- J.-X. Guo, D.-Y. Yan, K.-W. Qiu, C. Mu, D. Jiao, J. Mao, H. Wang and T. Ling, High electrocatalytic hydrogen evolution activity on a coupled Ru and CoO hybrid electrocatalyst, J. Energy Chem., 2019, 37, 143–147 CrossRef.
- J. Wang, Z. Z. Wei, S. J. Mao, H. R. Li and Y. Wang, Highly uniform Ru nanoparticles over N-doped carbon: pH and temperature-universal hydrogen release from water reduction, Energy Environ. Sci., 2018, 11, 800–806 RSC.
- H. B. Wu, B. Y. Xia, L. Yu, X. Y. Yu and X. W. Lou, Porous molybdenum carbide nano-octahedrons synthesized via confined carburization in metal-organic frameworks for efficient hydrogen production, Nat. Commun., 2015, 6, 6512 CrossRef CAS PubMed.
- R. Q. Ye, P. del Angel-Vicente, Y. Y. Liu, M. J. ArellanoJimenez, Z. W. Peng, T. Wang, Y. L. Li, B. I. Yakobson, S. H. Wei, M. J. Yacaman and J. M. Tour, High-performance hydrogen evolution from MoS2(1−X)P(X) solid solution, Adv. Mater., 2016, 28, 1427–1432 CrossRef CAS PubMed.
- S. H. Ye, F. Y. Luo, T. T. Xu, P. Y. Zhang, H. D. Shi, S. Q. Qin, J. P. Wu, C. X. He, X. P. Ouyang, Q. L. Zhang, J. H. Liu and X. L. Sun, Boosting the alkaline hydrogen evolution of Ru nanoclusters anchored on B/N-doped graphene by accelerating water dissociation, Nano Energy, 2020, 68, 104301 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2qi01923j |
| This journal is © the Partner Organisations 2023 |