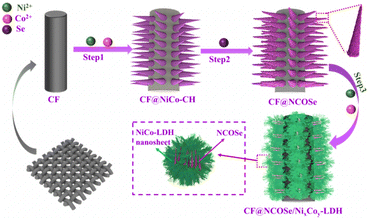
Novel core–shell nanoclusters composed of multiple nickel–cobalt-oxyselenide nanowires wrapped with NiCo-LDH nanosheets for high energy density supercapacitors†
Runmei
Luo
,
Qingjun
Yang
,
Yu
Liu
,
Lin
Sun
,
Changhong
Wang
,
Min
Chen
 * and
Weidong
Shi
* and
Weidong
Shi
 *
*
School of Chemistry and Chemical Engineering, Jiangsu University, Zhenjiang 212013, P. R. China. E-mail: swd1978@ujs.edu.cn; Tel: +86-511-88791800
First published on 4th November 2022
Abstract
Transition metal selenides are widely used in supercapacitors because of their high theoretical capacity but their poor electron or ion accessibility in electrochemical tests leads to low conductivity and weak energy density. Herein, a novel nanocluster made up of a nickel–cobalt-oxyselenide (NiCoO4Se3, denoted as NCOSe) nanowire core and a NixCoy-LDH nanosheet shell on carbon fiber (CF@NCOSe/NixCoy-LDH) is synthesized for the first time. Nanosheets encapsulated with multiple active nanowires have a multitude of active sites and high electrical conductivity simultaneously. This core–shell structure can enhance material stability and also further stimulate the redox reaction activity of the internal core nanowires. Due to the synergistic effect of NCOSe and Ni2Co1-LDH, the nanocluster CF@NCOSe/Ni2Co1-LDH exhibits an outstanding capacity of 3270 F g−1 (454.17 mA h g−1) at 1 A g−1. Significantly, the prepared hybrid supercapacitor (HSC) with CF@NCOSe/Ni2Co1-LDH//AC shows a particularly high energy density of 89.7 W h kg−1 at a power density of 800 W kg−1. In addition, the HSC exhibits an outstanding cycling performance of 95.6% capacitance retention after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge/discharge cycles. Therefore, constructing core–shell nanoclusters with an effective electrolyte buffer space can obtain high capacitance electrode materials, which is an effective strategy to build high energy density HSCs.
000 charge/discharge cycles. Therefore, constructing core–shell nanoclusters with an effective electrolyte buffer space can obtain high capacitance electrode materials, which is an effective strategy to build high energy density HSCs.
1. Introduction
In recent years, transition metal selenides (TMSes) have been widely used in supercapacitors due to their excellent redox activity and cycling stability.1,2 However, in electrochemical performance tests, the active Se utilization of TMSe is low and it eventually turns into the corresponding hydroxide, leading to a decrease in electrode conductivity.3 Conductivity is one of the important parameters determining electrochemical performance, and a lower conductivity leads to the rapid decay of performance at high current densities, i.e., poor multiplicative performance. In addition, unfavorable ionic or electronic conductivity can limit the energy density and electrochemical performance.4–6 Therefore, how to increase the electrical conductivity to improve the electrochemical performance is currently the bottleneck facing TMSe in supercapacitor applications.Many effective strategies, such as introducing defect engineering,7 designing composite structures,8 and non-metal doping,9 can enhance the conductivity of TMSes. Based on the synergistic effect of the composite structures, the redox reactions of multiple components can promote effective charge transfer and conductivity enhancement.10,11 For example, Ameri et al. synthesized NiCoSe2@NiMn-LDH layered core–shell materials by using synergistic effects.12 Both components undergo redox reactions with enhanced conductivity, and more importantly, the composite 2D-LDH nanosheets with highly interconnected morphology and high specific surface area can effectively contact the electrolyte, shorten the ion transport path, and improve conductivity.13 The core–shell material exhibits outstanding multiplicative performance and excellent energy density. Furthermore, the use of quasi-metallic doping can generate more active sites and improve intrinsic conductivity.14–16 For example, Zong et al. constructed P-(Ni, Co)Se2 nanomaterials using a nonmetallic doping strategy.17 P doping transformed the Prussian blue analog (PBA) from a nanocube into a hollow cube, and the PBA hollow cube, nanowires, and nanosheets formed a continuous conductive network with significantly enhanced conductivity, and the nanomaterials showed excellent area-specific capacity. Especially, methods have been developed for polyanion-doped bimetallic compounds (e.g. NiCoOS) with higher conductivity and theoretical capacity than single anion bimetallic compounds (NiCoS) because the polyanion strategy can adjust the composition of the metal compound and thus the electronic structure to improve the conductivity and electrochemical properties.18,19 Given this, two means of rational design of composite structures and the introduction of nonmetallic doping are feasible for improving the electrical conductivity of electrode materials. However, no systematic studies have been reported on the advantages of the simultaneous application of both approaches, i.e., composite 2D-LDH nanosheets and polyanion doping, to improve the electrical conductivity and to obtain excellent electrochemical properties of bimetallic compound electrode materials.
Herein, we first synthesized NCOSe nanowire cores and then wrapped NixCoy-LDH nanosheets around them to form nanocluster composites, in which NCOSe active nanowires can promote electron transport along the axial direction and improve energy storage kinetics, while the modulation of NixCoy-LDH nano component helps to form a more stable three-phase system. Finally, the nanocluster structure can provide sufficient space to effectively mitigate the volume changes generated during the continuous charge/discharge process and enhance cycling stability. Based on the synergistic effect of NCOSe and NixCoy-LDH, both nanowire cores and nanosheets can have great advantages in redox, resulting in a core–shell electrode with ultra-high energy density and cycling stability. The NCOSe/Ni2Co1-LDH electrode outperforms other electrodes in terms of capacity (an electrochemical capacity of 454.17 mA h g−1 at 1 A g−1). In addition, the hybrid supercapacitor constructed using the core–shell NCOSe/Ni2Co1-LDH electrode exhibits an excellent energy density of 89.7 W h kg−1 at a power density of 800 W kg−1 and a capacity retention of 95.6% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge/discharge cycles. Therefore, the construction of core–shell electrodes has a promising future in applications.
000 charge/discharge cycles. Therefore, the construction of core–shell electrodes has a promising future in applications.
2. Experimental section
For details of physical characterization and the corresponding electrochemical tests, please refer to the ESI.†2.1 Chemicals
NiCl2·6H2O, CoCl2·6H2O, NiNO3·6H2O, CoNO3·6H2O, urea, Se powder, carbon black (10%), and polyethylene-difluoride (10%), and N-methylpyrrolidone (NMP) were obtained by Zhenjiang Dewei Chemical Co. SCNs were bought from China's XFNAN. Carbon fiber fabrics (CF, 1 × 2 cm2) were washed for 30 min each with acetone (CH3COCH3), ethanol (C2H6O) and DI water.2.2 Preparation of NiCo-CH
First, 95 mg of NiCl2·6H2O and CoCl2·6H2O and 200 mg of urea were weighed and added to 30 mL of distilled water, stirred evenly, and subjected to ultrasound for 10 min. Then, a piece of CF (1 × 2 cm2) was placed into the solution prepared for the mixture and reacted at 120 °C for 10 hours. After 10 hours of reaction, the product is obtained by exchanging it several times with DI water and ethanol and baking in an oven at 60 °C for 12 hours.2.3 Preparation of NCOSe
NCOSe was obtained by selenization based on NiCo-CH nanowires. 0.114 g of Se powder and 0.285 g of NaOH were weighed, dissolved in 25 mL of deionized water, sonicated for 1 h, put into the oven at 140 °C, sealed for 4 h, 6 h, 8 h, and 10 h, and then removed, rinsed with a few exchanges of deionized water and ethanol, and dried in the oven at 60 °C for 12 h. According to the selenization time, it is named NCOSe-4, NCOSe-6, NCOSe-8(NCOSe), and NCOSe-10. For comparison, (Ni,Co)Se2 formed by heating at 180 °C for 8 h under the same reaction conditions was also prepared.2.4 Preparation of NCOSe/NixCoy-LDH
NCOSe/NixCoy-LDH was prepared by electrodeposition. First, 2.475 mmol of NiNO3·6H2O and CoNO3·6H2O were dissolved in 50 mL of deionized water containing and sonicated for 10 min to obtain the electrolyte solution. The prepared NCOSe, Pt plate (area of 4 cm2), and saturated AgCl2 electrode were put into the electrolyte solution as the working, counting, and comparison electrodes and the electrodeposition potential was controlled at −1.0 V for 300 s to obtain NCOSe/Ni1Co1-LDH. Then, NCOSe/Ni2Co1-LDH and NCOSe/Ni1Co2-LDH, NCOSe/Ni3Co1-LDH, and CF@Ni2Co1-LDH were obtained by varying the ratio of Ni/Co concentration in the electrolyte solution and designing Ni2Co1, Ni1Co2, Ni3Co1, and Ni2Co1-LDH by electrodeposition directly on carbon cloth at constant potential and time.2.5 Preparation of negative electrode materials
First, the nickel foam was cleaned with hydrochloric acid and the powder from the mixed active electrode material (80%), carbon black (10%), and polyethylene diene difluoride (PVDF 10%) was ground into a fine powder. The fine powder was placed in the center of the mortar, 1–2 drops of NMP were added, continued to be ground, and then the slurry was evenly coated on the nickel foam. The electrode was dried in an oven at 60 °C for 12 h and the mass loading of the electrode was about 4 mg cm−2.3. Results and discussion
To obtain high purity core–shell nanocluster structures of the NCOSe/NixCoy-LDH electrode, the synthesis strategy shown in Fig. 1 was followed. First, dense and smooth nanowires NiCo-CH [Fig. S1, ESI†] were synthesized on carbon fabrics by a solvothermal method, and these nanowires could be used as scaffolds or supports. Then, after a high-temperature selenization process, the NiCo-CH precursors were further converted into NiCo-oxyselenium (i.e., NCOSe) via an anion-exchange reaction and this active selenized nanowire slowed down the volume expansion in electrochemical cycles and provided an efficient transport pathway for ion and electron mobilization.20 Finally, to expose more active sites of NCOSe, we wrapped 2D-NixCoy-LDH nanosheets on the surface of multiple selenide nanowires by electrodeposition to obtain a core–shell nanocluster structure of NCOSe/NixCoy-LDH, which has a large specific surface area and can effectively promote charge transfer efficiency and also promote further core NCOSe redox reactions to improve the conductivity and electrochemical performance of the electrode.Regarding the X-ray diffraction of NiCo-CH, NCOSe-6, NCOSe-8(NCOSe), and NCOSe-10 (Fig. 2a), the XRD spectra show that although the selenization times are different, all of them show diffraction peaks typical of both mixed phases of CoSeO4 and NiSe2, indicating that the oxygen atoms in NiCo-CH are partially replaced by selenium atoms. The two diffraction peaks are shifted, probably due to the nature of the selenization. The 2θ values of the 19°, 27.3°, 32.9°, and 61.4° diffraction peaks are from the (110), (002), (200), and (151) planar CoSeO4 (JCPDS card no. 17-0844), while the four diffraction peaks at 36.8°, 52.2°, 58.8°, 74.3° correspond well to the (200), (031), (310), and (240) planes of NiSe2 (JCPDS card no. 18-0886). When selenized for 10 h, two different crystalline surfaces appeared, one containing (111) NiSe2 and one containing CoSeO4, respectively. The amount of selenium doped increased with the increase in selenization time, and when the selenization time was 10 h, it led to nanostructure agglomeration due to excessive selenization, which was verified by SEM. Fig. 2b shows the XRD data of NCOSe-/NixCoy-LDH formed by the electrodeposition of NixCoy-LDH. The crystallinity of NixCoy-LDH is relatively high and a series of diffraction planes can be indexed to the (003), (006), (100), (015), (102), and (110) of NixCoy-LDH planes.21,22 All diffraction peaks are broad and weak, indicating low crystallinity and the generation of lattice defects during the reaction process, which is attributed to interphase multiphase diffusion.23–25 This is in line with the results of the TEM and SEM.
To further determine the atomic valence and elemental composition of the composite, X-ray photoelectron spectroscopy (XPS) was carried out for NCOSe-8 and NCOSe/Ni2Co1-LDH. The XPS spectra of NCOSe/Ni2Co1-LDH are shown in Fig. S2.†Fig. 2(c–f) present the characteristic peaks of the Ni, Co, O, and Se elements. For NCOSe-8, the Ni 2p3/2 spectra of 856.8, 855.6, and 853.5 eV correspond to Ni3+, Ni-O-Se, and Ni2+, respectively,26–28 and similarly, the Co 2p spectra can be fitted to spectra with Co2+, Co-O-Se, Co3+, and associated recombination satellites (Fig. 2(c and d)). The peaks of each element in NCOSe/Ni2Co1-LDH spectra are shifted towards the high binding energy indicating that the molecular transfer to Ni, Co and O atoms in LDH increases the density of Ni, Co and O. Ni has two spin orbitals with peaks located at 876.1 eV and 856.6 eV attributed to the presence of Ni2+; the other two peaks at 881.2 eV and 858.1 eV belong to Ni3+. In the spectrum of Co 2p in Fig. 2d, the energy of binding 2p1/2 and 2p3/2 are 796.8 eV, 793.6 eV, 781.1 eV, and 778.6 eV indexed to the +2 and +3 states of the Co element, respectively.29 The XPS pattern of the seized O shows many oxygen defects and little surface adsorbed oxygen, while the electrodeposited O does not show oxygen vacancies, probably because the LDH nanosheets attached to the nanowire surface are too thick and the maximum depth that XPS can penetrate is 10 nm. Therefore, XPS did not penetrate the whole core–shell structure and only the O in LDH can be measured (Fig. 2e). In Fig. 2f, the fit peak at 59.1 eV is attributed to selenium oxide.30
Fig. 3(a–c) show the scanning electron microscopy (SEM) spectra after selenization for 6 h, 8 h, and 10 h. The homogeneous and monodisperse active nanowires can be seen after selenization until 8 h. However, after selenization until 10 h, the selenium oxide starts to show agglomeration, indicating that its shuttle effect in the redox reaction is severe and the nanowires are not enough to provide enough space to accommodate the volume expansion, leading to a decrease in performance.3Fig. 3(d–f) show the NCOSe/NixCoy-LDH morphology of the core–shell nanoclusters. It can be seen that the active nanowires can provide support for the deposition of nanosheets when the optimal Ni/Co ion ratio is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 3d). When the nanocluster structure is almost complete, it provides the largest specific surface area, which facilitates electron transport and ion diffusion in the inner layer, and the structure exhibits stable kinetic performance properties (Fig. S3(a and b)†). However, when Ni/Co ions are deposited in other ratios, the nanosheets thickly cover the active nanowires, hindering the electrolyte from reaching the nanowire cores and thus reducing the utilization of active sites (Fig. 3(d, f)). In addition, the effect of different ratios of Ni and Co on the growth of selenide was investigated. Among different NixCoy-CH arrangements, the most regular arrangement of nanowires was found to be NiCo-CH. Upon hydrothermal selenization of NixCoy-CH, it was found that different ratios of Ni and Co affected not only the growth of selenides but also the electrochemical properties (Fig. S4, S8†). The different morphologies of NCOSe-x were also found to affect the growth of LDH by plating LDH on the basis of different selenization times (Fig. S5†).
1 (Fig. 3d). When the nanocluster structure is almost complete, it provides the largest specific surface area, which facilitates electron transport and ion diffusion in the inner layer, and the structure exhibits stable kinetic performance properties (Fig. S3(a and b)†). However, when Ni/Co ions are deposited in other ratios, the nanosheets thickly cover the active nanowires, hindering the electrolyte from reaching the nanowire cores and thus reducing the utilization of active sites (Fig. 3(d, f)). In addition, the effect of different ratios of Ni and Co on the growth of selenide was investigated. Among different NixCoy-CH arrangements, the most regular arrangement of nanowires was found to be NiCo-CH. Upon hydrothermal selenization of NixCoy-CH, it was found that different ratios of Ni and Co affected not only the growth of selenides but also the electrochemical properties (Fig. S4, S8†). The different morphologies of NCOSe-x were also found to affect the growth of LDH by plating LDH on the basis of different selenization times (Fig. S5†).
 | ||
| Fig. 3 SEM images of (a) NCOSe-6 NAs; (b) NCOSe-8 NAs; (c) NCOSe-10 NAs; (d) NCOSe/Ni2Co1-LDH; (e) NCOSe/Ni1Co1-LDH; and (f) NCOSe/Ni1Co2-LDH. | ||
Transmission electron microscopy (TEM) further confirmed the structural features of the active nanowire NCOSe-8 and nanoclusters NCOSe/Ni2Co1-LDH in Fig. 4(a–c). Fig. 4a shows small nanosheets grown at the edges of the nanowires after high-temperature selenization, constituting the active nanowires, which have a larger specific surface area than normal nanowires and provide more active sites, as can be seen in the SEM. Fig. 4(b and c) show the Ni2Co1-LDH nanosheets anchor wrapped around the outside of multiple active selenium nanowires, forming a nanocluster structure. From Fig. S6,† it can be confirmed that the size of NCOSe/Ni2Co1-LDH nanoclusters is about 245 nm. Furthermore, high magnification transmission electron microscopy (HRTEM) shows the NCOSe/Ni2Co1-LDH visible lattice stripes with a spacing of 3.37, 2.44, and 2.31 nm pointing to the CoSeO4 (002) plane, the (200) plane of NiSe2, and the (015) plane of NiCo-LDH (Fig. 4(d–g)). The corresponding STEM-EDS mapping images show that Ni, Co, Se, and O atoms are equally distributed in all samples (Fig. 4h).
 | ||
| Fig. 4 TEM images of (a) NCOSe-8 NAs and (b and c) NCOSe/Ni2Co1-LDH. (d–g) HRTEM images of NCOSe/Ni2Co1-LDH and (h) HAADF-STEM and respective EDS mapping of images of NCOSe/Ni2Co1-LDH. | ||
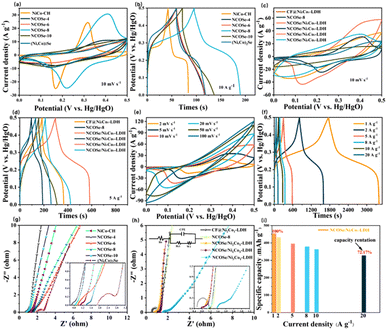
The electrochemical performance of the electrodes was analysed in an electrolyte of 6 M (KOH) and a three-electrode set up, and a series of cyclic voltammetric (CV) and galvanostatic charge/discharge (GCD) curves were obtained to evaluate the capacity performance of the electrode materials. As shown in Fig. 5a, according to the CV plots for different selenization times at 10 mV s−1, it can be demonstrated that NCOSe-8 has the largest integrated area and the largest redox peak, which may be due to the partial substitution of O in NiCo-CH by Se to generate active NCOSe-8 nanowires. This provides more redox active sites than complete selenization producing (Ni, Co)2Se and other selenization times. While after 10 h of selenization, the material structure agglomerates and blocks the ion transport path and thus leads to a decrease in performance. The corresponding GCD also confirms the excellent electrochemical properties of NCOSe-8 (Fig. 5b). The relative CV and GCD curves of NCOSe-4, NCOSe-6, NCOSe-8, NCOSe-10, (Ni, Co)2Se are shown in Fig. S7.† In addition, the effect of different NiCo ratios on selenization was also investigated, and Fig. S8† shows that NiCo = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 has the largest integrated area, the strongest redox peak, and the smallest corresponding internal resistance.
1 has the largest integrated area, the strongest redox peak, and the smallest corresponding internal resistance.
The CV curves of NCOSe-/NixCoy-LDH and CF@Ni2Co1-LDH are shown in Fig. 5c, where the CV closure curve area of NCOSe/Ni2Co1-LDH at 10 mV s−1 is larger than those of NCOSe/Ni1Co1-LDH, NCOSe/Ni1Co2-LDH, NCOSe/Ni3Co1-LDH, CF@Ni2Co1-LDH, and the corresponding selenide NCOSe-8. Meanwhile, NCOSe/Ni2Co1-LDH is shown to have the longest discharge time at 5A g−1, displaying superior specific capacity (Fig. 5d). Because when the molar mass ratio of Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co = 1
Co = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 1
1 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 or 3
2 or 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, as well as CF@Ni2Co1-LDH, the stacking of nanosheets is not favorable for electron transfer due to electrolyte penetration, resulting in less pronounced redox peaks and lower electrochemical capacity performance. Typical CV curves of NCOSe/Ni2Co1-LDH at scan rates ranging from 2 to 100 mV s−1 are shown in Fig. 5e. There were distinct cathodic and anodic peaks at different scan rates indicating reversible redox reactions, and the GCD curves of NCOSe/Ni2Co1-LDH exhibited a relatively symmetrical shape indicating good coulometric efficiency. The electrode exhibited electrochemical capacities of 454.17, 433.33, 395.83, 377.78, and 363.89 mA h g−1 at current densities of 1, 2, 5, 8, and 10 A g−1 (Fig. 5f). Combined, it can be concluded that the redox rate increased due to the incomplete substitution of selenium. Together with the effective modulation of the nanocomponent, the active sites of the active nanowires also increase. In addition, the electrodeposition accelerates the rapid adsorption of nickel and cobalt ions in the electrolyte, thus modulating the electronic structure and increasing the electrical conductivity. The resulting nanocluster structure with a significant increase in the specific surface area shows excellent electrochemical properties.31 The possible Faraday reaction associated with this is described by the following equation:3,32–35
1, as well as CF@Ni2Co1-LDH, the stacking of nanosheets is not favorable for electron transfer due to electrolyte penetration, resulting in less pronounced redox peaks and lower electrochemical capacity performance. Typical CV curves of NCOSe/Ni2Co1-LDH at scan rates ranging from 2 to 100 mV s−1 are shown in Fig. 5e. There were distinct cathodic and anodic peaks at different scan rates indicating reversible redox reactions, and the GCD curves of NCOSe/Ni2Co1-LDH exhibited a relatively symmetrical shape indicating good coulometric efficiency. The electrode exhibited electrochemical capacities of 454.17, 433.33, 395.83, 377.78, and 363.89 mA h g−1 at current densities of 1, 2, 5, 8, and 10 A g−1 (Fig. 5f). Combined, it can be concluded that the redox rate increased due to the incomplete substitution of selenium. Together with the effective modulation of the nanocomponent, the active sites of the active nanowires also increase. In addition, the electrodeposition accelerates the rapid adsorption of nickel and cobalt ions in the electrolyte, thus modulating the electronic structure and increasing the electrical conductivity. The resulting nanocluster structure with a significant increase in the specific surface area shows excellent electrochemical properties.31 The possible Faraday reaction associated with this is described by the following equation:3,32–35
| NiSe2 + OH− ↔ NiSe2OH + e− | (1) |
| NiSe2 + H2O + OH− ↔ NiSe2OH− + e− | (2) |
| Ni(OH)2 + OH− ↔ NiOOH + e− | (3) |
| Co(OH)2 + OH− ↔ CoOOH + e− | (4) |
| CoOOH + OH− ↔ CoO2 + H2O + e− | (5) |
The CV and GCD curves for NCOSe/Ni1Co1-LDH, NCOSe/Ni1Co2-LDH and NCOSe/Ni3Co1-LDH CF@Ni2Co1-LDH are shown in Fig. S9.†
In addition, we compare the different selenization times and the different Ni/Co molar mass ratios as well as CF@Ni2Co1-LDH in the Nyquist plot Fig. 5(g and h). The Nyquist plot consists of a diagonal line in the low-frequency region, reflecting the diffusion resistance, and a quasi-semicircle in the high-frequency region, indicating the charge transfer kinetic properties.36,37 Apparently, NCOSe/Ni2Co1-LDH has a smaller semicircle in the high frequency region, indicating a smaller charge equivalent series resistance (Rs) and charge transfer resistance (Rct). The fit values of Rs and Rct for the relevant samples (Table S1†) show that NCOSe/Ni2Co1-LDH has the smallest values of Rs (0.75 Ω) and Rct (0.12 Ω), indicating that Ni2Co1-LDH can modulate the electronic structure and conductivity of the electrode material. The lower frequency region is steeper and exhibits lower ion diffusion resistance, which may be due to the deficiency of selenium atoms, lower content of oxygen atoms in NCOSe-8, enhanced electronegativity, increased conductivity, and a thin layer of LDH on the nanowire surface adhering to the Seide surface, alleviating layer buildup.38,39 Then, Z versus frequency is shown in Fig. S10,† from which it can be seen that the resistance of NCOSe/NixCoy-LDH is smaller than that of NCOSe-8, where NCOSe/Ni2Co1-LDH has the lowest resistance and the best conductivity, which indicates that the synergistic effect can improve the conductivity of TMSe. Finally, the specific capacity Qs (mA h g−1) was calculated from the GCD curve:
| Qs = i × Δt/3.6Δm+ | (6) |
The equations are as follows where i, Δt, and Δm+ denote the discharge current (A) and discharge time (s), and load capacity. The specific capacities are 454.17 mA h g−1 and 327.78 mA h g−1 when the current density is 1 A g−1 and 20 A g−1. When the current density was increased from 1 to 20 A g−1, there was excellent capacitance retention of 72.17% in Fig. 5i. Taken together, the synergistic effect of NCOSe-8 and NiCo-LDH has an excellent role in electrochemical energy storage.
According to the CV curves at different scan rates, the current density (i) obeys a post-exponential relationship:4,40
| i = avb | (7) |
log(i) = log(a) + b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log(v) log(v) | (8) |
In light of the above results, the excellent performance of NCOSe/Ni2Co1-LDH is mainly attributed to three reasons. First, the active nanowire NCOSe component not only serves as a nano-substrate for NiCo-LDH but also plays an important role in long-range electron transfer. Second, the NiCo-LDH shell layer can improve the stability of the electrode material and can provide buffer space for the electrolyte to further stimulate the redox reaction activity of the internal nanowires.43 Third, since the NCOSe/Ni2Co1-LDH core–shell structure offers a large specific surface area, the permeable stacked nanosheets can not only increase the active sites but also provide ionization channels for the electrolyte ions to rapidly penetrate into the electrode material, and both the core–shell and the core can undergo redox reactions under continuous charge and discharge and the electrode material shows excellent multiplicative performance and electric capacity.
To further assess the energy storage principle and the electrochemical properties of the synthesized core–shell nanocluster NCOSe/Ni2Co1-LDH hybrid cathode, the hybrid supercapacitor was assembled with NCOSe/Ni2Co1-LDH as the anode and AC as the cathode. The assembly schematic of this HSC device was shown in Fig. 7a. To ascertain the voltage range of the HSC, the CV curves of AC from −1.0 V–0 V and NCOSe/Ni2Co1-LDH from 0–0.5 V were tested in Fig. 7b, respectively. The voltage values are determined based on the CV and GCD curves of the HSC at different voltage windows. Fig. S11(a)† showed the stable operating voltage of the HSC device for CF@NCOSe/Ni2Co1-LDH//AC at different potential windows extending up to 1.8 V. When the voltage window was increased to 1.8 V, the CV curve showed a typical rectangular shape which was well maintained without any distortion. Similarly, Fig. S11(b)† showed that the stable operating voltage under the GCD curve was also 1.8 V. Therefore, the voltage window for the CV curve and the GCD curve of the HSC was determined to be 0–1.6 V. As Fig. 7c shows, the different CV plots for a voltage of 1.6 V and a scan rate from 5 mV s−1–100 mV s−1. There were no obvious changes in the CV curves even at high scan rates, indicating that this HSC exhibited good rate performance. The GCD curves had symmetrical triangular characteristics, demonstrating that the hybrid supercapacitor had high Coulomb efficiency in Fig. 7d. The HSC achieves excellent capacitance capacities of 252.4, 238, 205.6, 171.0 and 158.1 F g−1 at 1, 2, 5, 8 and 10 A g−1, respectively, and energy densities up to 89.7 W h kg−1 when the power density is 800 W kg−1. Due to the incomplete substitution during selenization, the percentage of O atoms in NiCo-CH decreases. The excellent energy density is demonstrated by the enhanced electronegativity and increased conductivity, as well as the 2D-LDH nanosheets that promote ion transport and enhanced electronic conductivity. This high energy density HSC has great potential for application in electronic devices such as energy storage.
A cycling stability test of NCOSe/Ni2Co1-LDH//AC cells was done at a 10 A g−1 current density. The capacity was maintained at 106.7% at 5000 cycles and 95.6% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles (Fig. 8a). With the penetration of the electrolyte, on the one hand, the conductive selenide containing the main element of the surface electrode material with good spark discharge properties increases the stability of the electrode in an alkaline electrolyte by allowing the effective diffusion of electrolyte ions. On the other hand, the core–shell nanoclusters have a huge surface area and hierarchical structure, which in turn promote the diffusion of hydrogen ions and suppress the hydrogen ions providing sufficient space for swelling during both the charging and discharging processes.44,45 As shown in Fig. 8b, HSC has an outstanding specific capacity retention of 65.2% at a 10 A g−1 current density. To investigate the energy density and power density of this HSC, Ragone plots are plotted and are shown in Fig. 8c. When the power density is 800 W kg−1, the energy density of the HSC reaches 89.7 W h kg−1 and when the power density is 1600 W kg−1, the energy density is 84.6 W h kg−1. The energy and power density values of this HSC device are better than the previously reported HSC values, such as (Ni,Co)Se2/NiCo-LDH//PC (39 W h kg−1 at 1650 W kg−1),7 CNTs@NiCo-LDH//ZIF-8 (37.38 W h kg−1 at 800 W kg−1),29 ZnO/C@(Ni,Co)Se2//AC (65.67 W h kg−1 at 800 W kg−1),46 (Ni,Co)Se2@rGO//AC (52.6 W h kg−1 at 803.4 W kg−1),47 P-(Ni,Co)Se2 NAs//ZC (45 W h kg−1 at 446.3 W kg−1),48 CC@NiCo-LDH/Co9S8 (38 W h kg−1 at 800 W kg−1),49 Co(OH)2/CoSe2//CNTs (68.7 W h kg−1 at 189 W kg−1),9 CC/NiCoP @ NiCo-LDH//AC (57 W h kg−1 at 850 W kg−1),50 and PANI@NiSe2//AC (38.3 W h kg−1 at 308 W kg−1).51 To demonstrate the application potential of the device, we used solid-state HSCs in series. As seen in Fig. 8d, all the LEDs were lit, which provides a huge advantage for energy storage in electronic devices and as a potential power source (Fig. 8d).
000 cycles (Fig. 8a). With the penetration of the electrolyte, on the one hand, the conductive selenide containing the main element of the surface electrode material with good spark discharge properties increases the stability of the electrode in an alkaline electrolyte by allowing the effective diffusion of electrolyte ions. On the other hand, the core–shell nanoclusters have a huge surface area and hierarchical structure, which in turn promote the diffusion of hydrogen ions and suppress the hydrogen ions providing sufficient space for swelling during both the charging and discharging processes.44,45 As shown in Fig. 8b, HSC has an outstanding specific capacity retention of 65.2% at a 10 A g−1 current density. To investigate the energy density and power density of this HSC, Ragone plots are plotted and are shown in Fig. 8c. When the power density is 800 W kg−1, the energy density of the HSC reaches 89.7 W h kg−1 and when the power density is 1600 W kg−1, the energy density is 84.6 W h kg−1. The energy and power density values of this HSC device are better than the previously reported HSC values, such as (Ni,Co)Se2/NiCo-LDH//PC (39 W h kg−1 at 1650 W kg−1),7 CNTs@NiCo-LDH//ZIF-8 (37.38 W h kg−1 at 800 W kg−1),29 ZnO/C@(Ni,Co)Se2//AC (65.67 W h kg−1 at 800 W kg−1),46 (Ni,Co)Se2@rGO//AC (52.6 W h kg−1 at 803.4 W kg−1),47 P-(Ni,Co)Se2 NAs//ZC (45 W h kg−1 at 446.3 W kg−1),48 CC@NiCo-LDH/Co9S8 (38 W h kg−1 at 800 W kg−1),49 Co(OH)2/CoSe2//CNTs (68.7 W h kg−1 at 189 W kg−1),9 CC/NiCoP @ NiCo-LDH//AC (57 W h kg−1 at 850 W kg−1),50 and PANI@NiSe2//AC (38.3 W h kg−1 at 308 W kg−1).51 To demonstrate the application potential of the device, we used solid-state HSCs in series. As seen in Fig. 8d, all the LEDs were lit, which provides a huge advantage for energy storage in electronic devices and as a potential power source (Fig. 8d).
4. Conclusions
In summary, we successfully synthesized core–shell nanocluster NCOSe/Ni2Co1-LDH structures on carbon fibers. The double anionic bonding of multivalent cations can improve the reaction kinetics and accelerate the redox reaction, while the active nanowires can promote the transport of core electrons along the edge of the core shell and increase the intrinsic conductivity of selenide. On the other hand, the use of electrodeposition promotes the diffusion of Ni and Co ions in the electrolyte, which accelerates the rapid attachment of NiCo-LDH nanosheets to the nanowire surface, thus exposing more active sites. The above factors enable NCOSe/Ni2Co1-LDH to exhibit ultra-high capacity (454.17 mA h g−1 at 1 A g−1) and excellent multiplicative performance. CF@NCOSe/Ni2Co1-LDH//AC demonstrates high energy density and good cycling stability when used as a supercapacitor. Therefore, the construction of such high-energy density nanocluster-like electrode materials based on synergistic effects has good energy storage advantages and application prospects.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This research was supported by the National Natural Science Foundation of China (22108107, 22075111, and 22225808), the China Postdoctoral Science Foundation (2021M691302), and the China Postdoctoral Science Special Fund (2021TQ0131).References
- J. Wang, B. Wang, H. Sun, G. Wang, J. Bai and H. Wang, Heterogeneous interface containing selenium vacancies space-confined in double carbon to induce superior electronic/ionic transport dynamics for sodium/potassium-ion half/full batteries, Energy Storage Mater., 2022, 46, 394–405 CrossRef.
- X. Gao, Y. Zhang, S. Yin, Y. Mao, J. Gui, J. Li, Y. Zhao, C. Sun and S. Guo, Dual Redox Active Sites N-C@ Ni2P/NiSe2 Heterostructure Supercapacitor Integrated with Triboelectric Nanogenerator toward Efficient Energy Harvesting and Storage, Adv. Funct. Mater., 2022, 2204833 CrossRef CAS.
- X. Li, H. Wu, C. Guan, A. M. Elshahawy, Y. Dong, S. J. Pennycook and J. Wang, (Ni, Co) Se2/NiCo-LDH core/shell structural electrode with the cactus-like (Ni, Co) Se2 core for asymmetric supercapacitors, Small, 2019, 15, 1803895 Search PubMed.
- J. Chen, X. Wang, J. Wang and P. S. Lee, Sulfidation of NiMn-layered double hydroxides/graphene oxide composites toward supercapacitor electrodes with enhanced performance, Adv. Energy Mater., 2016, 6, 1501745 CrossRef.
- A. M. Sakita, R. Della Noce, P. L. Gastelois, W. A. Macedo and R. L. Lavall, Binder-free graphitic films with high conductivity for supercapacitor devices, Chem. Eng. J., 2022, 427, 131731 CrossRef CAS.
- G. H. Yu, L. B. Hu, M. Vosgueritchian, H. L. Wang, X. Xie, J. R. McDonough, X. Cui, Y. Cui and Z. N. Bao, Nanostructured conductive polypyrrole hydrogels as high-performance, flexible supercapacitor electrodes, J. Mater. Chem. A, 2011, 11, 2905 CAS.
- J. Zhao, H. Cheng, Z. Zhang, Y. Liu, J. Song, T. Liu, Y. He, A. Meng, C. Sun and M. Hu, The Semicoherent Interface and Vacancy Engineering for Constructing Ni (Co) Se2@ Co (Ni) Se2 Heterojunction as Ultrahigh-Rate Battery-Type Supercapacitor Cathode, Adv. Funct. Mater., 2022, 2202063 CrossRef CAS.
- L. Sun, Y. Liu, M. Yan, Q. Yang, X. Liu and W. Shi, Lewis acid etched NixCo1-xSe2 derived from ZIF-L on CoO nanowires for hybrid-supercapacitors, Chem. Eng. J., 2022, 431, 133472 CrossRef CAS.
- J. Kim, R. Tabassian, V. H. Nguyen, S. Umrao and I.-K. Oh, Interfaces, Crumpled quaternary Nanoarchitecture of Sulfur-doped Nickel Cobalt Selenide directly grown on Carbon cloth for making stronger Ionic Soft Actuators, ACS Appl. Mater. Interfaces, 2019, 11, 40451–40460 CrossRef CAS.
- L. Ye, Y. Zhou, Z. Bao, Y. Zhao, Y. Zou, L. Zhao and Q. Jiang, Sheet-membrane Mn-doped nickel hydroxide encapsulated via heterogeneous Ni3 S2 nanoparticles for efficient alkaline battery–supercapacitor hybrid devices, J. Mater. Chem. A, 2018, 6, 19020–19029 RSC.
- X. Wu, Z. Han, X. Zheng, S. Yao, X. Yang and T. Zhai, Core-shell structured Co3O4@ NiCo2O4 electrodes grown on flexible carbon fibers with superior electrochemical properties, Nano Energy, 2017, 33, 410–417 CrossRef.
- B. Ameri, A. M. Zardkhoshoui and S. Davarani, Fuels, Engineering of hierarchical NiCoSe 2@ NiMn-LDH core-shell nanostructures as a high-performance positive electrode material for hybrid supercapacitors, Sustainable Energy Fuels, 2020, 4, 5144–5155 RSC.
- S. Parvin, A. Kumar, A. Ghosh and S. Bhattacharyya, An earth-abundant bimetallic catalyst coated metallic nanowire grown electrode with platinum-like pH-universal hydrogen evolution activity at high current density, Chem. Sci., 2020, 11, 3893–3902 RSC.
- T. Wang, H. C. Chen, F. Yu, X. Zhao and H. Wang, Boosting the cycling stability of transition metal compounds-based supercapacitors, Energy Storage Mater., 2019, 16, 545–573 CrossRef.
- M. Sakthivel, S. Ramaraj, S.-M. Chen and K.-C. Ho, Bimetallic vanadium cobalt diselenide nanosheets with additional active sites for excellent asymmetric pseudocapacitive performance: comparing the electrochemical performances with M–CoSe2 (M = Zn, Mn, and Cu), J. Mater. Chem. A, 2019, 7, 12565–12581 RSC.
- C. Guan, W. Xiao, H. Wu, X. Liu, W. Zang, H. Zhang, J. Ding, Y. P. Feng, S. J. Pennycook and J. Wang, Hollow Mo-doped CoP nanoarrays for efficient overall water splitting, Nano Energy, 2018, 48, 73–80 CrossRef.
- Q. Zong, Y. Zhu, Q. Wang, H. Yang, Q. Zhang, J. Zhan and W. Du, Prussian blue analogues anchored P-(Ni, Co) Se2 nanoarrays for high performance all-solid-state supercapacitor, Chem. Eng. J., 2020, 392, 123664 CrossRef CAS.
- H. Kageyama, K. Hayashi, K. Maeda, J. P. Attfield, Z. Hiroi, J. M. Rondinelli and K. R. Poeppelmeier, Expanding frontiers in materials chemistry and physics with multiple anions, Nat. Commun., 2018, 9, 1–15 CrossRef CAS PubMed.
- Q. Guo, Y. Ma, T. Chen, Q. Xia, M. Yang, H. Xia and Y. Yu, Cobalt sulfide quantum dot embedded N/S-doped carbon nanosheets with superior reversibility and rate capability for sodium-ion batteries, ACS Nano, 2017, 11, 12658–12667 CrossRef CAS.
- S. Wang, H. Zhao, S. Lv, H. Jiang, Y. Shao, Y. Wu, X. Hao and Y. Lei, Insight into Nickel-Cobalt Oxysulfide Nanowires as Advanced Anode for Sodium-Ion Capacitors, Adv. Energy Mater., 2021, 11, 2100408 CrossRef CAS.
- C. Qiao, Y. Zhang, Y. Zhu, C. Cao, X. Bao and J. Xu, One-step synthesis of zinc–cobalt layered double hydroxide (Zn–Co-LDH) nanosheets for high-efficiency oxygen evolution reaction, J. Mater. Chem. A, 2015, 3, 6878–6883 RSC.
- H. Liang, J. Lin, H. Jia, S. Chen, J. Qi, J. Cao, T. Lin, W. Fei and J. Feng, Hierarchical NiCo-LDH@NiOOH core-shell heterostructure on carbon fiber cloth as battery-like electrode for supercapacitor, J. Power Sources, 2018, 378, 248–254 CrossRef CAS.
- S. Parvin, A. Kumar, A. Ghosh and S. Bhattacharyya, An earth-abundant bimetallic catalyst coated metallic nanowire grown electrode with platinum-like pH-universal hydrogen evolution activity at high current density, Chem. Sci., 2020, 11, 3893–3902 RSC.
- M. Dinari, H. Allami and M. M. Momeni, Construction of Ce-Doped NiCo-LDH@CNT Nanocomposite Electrodes for High-Performance Supercapacitor Application, Energy Fuels, 2020, 35, 1831–1841 CrossRef.
- F. Hao, A. Verma and P. P. Mukherjee, Electrodeposition stability of metal electrodes, Energy Storage Mater., 2019, 20, 1–6 CrossRef.
- J. Zhu, S.-X. Zhao, X. Wu, Y.-F. Wang, L. Yu and C.-W. Nan, Wrapping RGO/MoO2/carbon textile as supercapacitor electrode with enhanced flexibility and areal capacitance, Electrochim. Acta, 2018, 282, 784–791 CrossRef CAS.
- S. Wang, L. Li, Y. Shao, L. Zhang, Y. Li, Y. Wu and X. Hao, Transition-Metal Oxynitride: A Facile Strategy for Improving Electrochemical Capacitor Storage, Adv. Mater., 2019, 31, 1806088 CrossRef.
- Y. Liu, N. Xin, Q. Yang and W. Shi, 3D CNTs/graphene network conductive substrate supported MOFs-derived CoZnNiS nanosheet arrays for ultra-high volumetric/gravimetric energy density hybrid supercapacitor, J. Colloid Interface Sci., 2021, 583, 288–298 CrossRef CAS.
- F. Zhu, W. Liu, Y. Liu and W. Shi, Construction of porous interface on CNTs@NiCo-LDH core-shell nanotube arrays for supercapacitor applications, Chem. Eng. J., 2020, 383, 123150 CrossRef CAS.
- C. Xia, Q. Jiang, C. Zhao, M. N. Hedhili and H. N. Alshareef, Selenide-based electrocatalysts and scaffolds for water oxidation applications, Adv. Mater., 2016, 28, 77–85 CrossRef CAS PubMed.
- Y. Wang, L. Zhao, Y. Zhao, W. Y. Wang, Y. Liu, C. Gu, J. Li, G. Zhang, T. J. Huang and S. Yang, Electrocarving during electrodeposition growth, Adv. Mater., 2018, 30, 1805686 CrossRef.
- A. M. Zardkhoshoui and S. S. H. Davarani, Construction of complex copper-cobalt selenide hollow structures as an attractive battery-type electrode material for hybrid supercapacitors, Chem. Eng. J., 2020, 402, 126241 CrossRef CAS.
- D. Jia, D. Jiang, Y. Zheng, H. Tan, X. Cao, F. Liu, L. Yue, Y. Sun and J. Liu, Electrochemical synthesis of NiCo layered double hydroxide nanosheets decorated on moderately oxidized graphene films for energy storage, Nanoscale, 2019, 11, 2812–2822 RSC.
- D. Zha, Y. Fu, L. Zhang, J. Zhu and X. Wang, Design and fabrication of highly open nickel cobalt sulfide nanosheets on Ni foam for asymmetric supercapacitors with high energy density and long cycle-life, J. Power Sources, 2018, 378, 31–39 CrossRef CAS.
- H. Liang, J. Lin, H. Jia, S. Chen, J. Qi, J. Cao, T. Lin, W. Fei and J. Feng, Hierarchical NiCo-LDH@ NiOOH core-shell heterostructure on carbon fiber cloth as battery-like electrode for supercapacitor, J. Power Sources, 2018, 378, 248–254 CrossRef CAS.
- L. Wan, Z. Zhao, X. Chen, P.-F. Liu, P. Wang, Z. Xu, Y. Lin and B. Wang, Controlled Synthesis of Bifunctional NiCo2O4@FeNi LDH Core–Shell Nanoarray Air Electrodes for Rechargeable Zinc–Air Batteries, ACS Sustainable Chem. Eng., 2020, 8, 11079–11087 CrossRef CAS.
- H. Niu, Y. Liu, B. Mao, N. Xin, H. Jia and W. Shi, In situ embedding MOFs-derived copper sulfide polyhedrons in carbon nanotube networks for hybrid supercapacitor with superior energy density, Electrochim. Acta, 2020, 329, 135130 CrossRef CAS.
- H. Tong, S. Yue, L. Lu, F. Jin, Q. Han, X. Zhang and J. Liu, A binder-free NiCo2O4 nanosheet/3D elastic N-doped hollow carbon nanotube sponge electrode with high volumetric and gravimetric capacitances for asymmetric supercapacitors, Nanoscale, 2017, 9, 16826–16835 RSC.
- D. S. Hall, D. J. Lockwood, C. Bock and B. R. MacDougall, Nickel hydroxides and related materials: a review of their structures, synthesis and properties, Phys. Eng. Sci. Med., 2015, 471, 20140792 Search PubMed.
- B. Long, M. S. Balogun, L. Luo, W. Qiu, Y. Luo, S. Song and Y. Tong, Phase boundary derived pseudocapacitance enhanced nickel-based composites for electrochemical energy storage devices, Adv. Energy Mater., 2018, 8, 1701681 CrossRef.
- J. B. Goodenough, High-rate electrochemical energy storage through Li+ intercalation pseudocapacitance, Nat. Mater., 2013, 13, 518 Search PubMed.
- A. Vlad, N. Singh, J. Rolland, S. Melinte, P. Ajayan and J.-F. Gohy, Hybrid supercapacitor-battery materials for fast electrochemical charge storage, Sci. Rep., 2014, 4, 1–7 Search PubMed.
- H. Luo, B. Wang, T. Liu, F. Jin, R. Liu, C. Xu, C. Wang, K. Ji, Y. Zhou, D. Wang and S. Dou, Hierarchical design of hollow Co-Ni LDH nanocages strung by MnO2 nanowire with enhanced pseudocapacitive properties, Energy Storage Mater., 2019, 19, 370–378 CrossRef.
- W. Song, X. Teng, Y. Liu, J. Wang, Y. Niu, X. He, C. Zhang and Z. Chen, Rational construction of self-supported triangle-like MOF-derived hollow (Ni,Co)Se2 arrays for electrocatalysis and supercapacitors, Nanoscale, 2019, 11, 6401–6409 RSC.
- D. Tian, N. Song, M. Zhong, X. Lu and C. Wang, Bimetallic MOF Nanosheets Decorated on Electrospun Nanofibers for High-Performance Asymmetric Supercapacitors, ACS Appl. Mater. Interfaces, 2020, 12, 1280–1291 CrossRef CAS.
- W. Liu, F. Zhu, B. Ge, L. Sun, Y. Liu and W. Shi, MOF derived ZnO/C@(Ni,Co)Se2 core–shell nanostructure on carbon cloth for high-performance supercapacitors, Chem. Eng. J., 2022, 427, 130788 CrossRef CAS.
- X. Liu, S. Ding, L. Ye, Y. Du, L. Zhao and Y. Zhu, Optimizing the supercapacitive performance via encasing MOF-derived hollow (Ni,Co)Se2 nanocubes into reduced graphene oxide, Chem. Eng. J., 2020, 399, 125789 CrossRef CAS.
- Q. Zong, Y. Zhu, Q. Wang, H. Yang, Q. Zhang, J. Zhan and W. Du, Prussian blue analogues anchored P-(Ni,Co)Se2 nanoarrays for high performance all-solid-state supercapacitor, Chem. Eng. J., 2020, 392, 123664 CrossRef CAS.
- Q. Yang, Y. Liu, L. Xiao, M. Yan, H. Bai, F. Zhu, Y. Lei and W. Shi, Self-templated transformation of MOFs into layered double hydroxide nanoarrays with selectively formed Co9S8 for high-performance asymmetric supercapacitors, Chem. Eng. J., 2018, 354, 716–726 CrossRef CAS.
- X. Gao, Y. Zhao, K. Dai, J. Wang, B. Zhang and X. Shen, NiCoP nanowire@NiCo-layered double hydroxides nanosheet heterostructure for flexible asymmetric supercapacitors, Chem. Eng. J., 2020, 384, 123373 CrossRef CAS.
- T. Hao, Y. Liu, G. Liu, C. Peng, B. Chen, Y. Feng, J. Ru and J. Yang, Insight into faradaic mechanism of polyaniline@NiSe2 core-shell nanotubes in high-performance supercapacitors, Energy Storage Mater., 2019, 23, 225–232 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2qi01739c |
| This journal is © the Partner Organisations 2023 |