Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Catalytic synthesis and physical properties of CO2-based cross-linked poly(cyclohexene carbonate)s†
Chihiro
Maeda
 *,
Kenta
Kawabata
,
Kaito
Niki
,
Yuma
Sako
,
Takumi
Okihara
*,
Kenta
Kawabata
,
Kaito
Niki
,
Yuma
Sako
,
Takumi
Okihara
 * and
Tadashi
Ema
* and
Tadashi
Ema
 *
*
Division of Applied Chemistry, Graduate School of Natural Science and Technology, Okayama University, Tsushima, Okayama 700-8530, Japan. E-mail: cmaeda@okayama-u.ac.jp; okihara@cc.okayama-u.ac.jp; ema@cc.okayama-u.ac.jp
First published on 5th September 2023
Abstract
Bifunctional aluminum porphyrins (0.001 mol%) catalyzed the terpolymerization of cyclohexene oxide (CHO), bis(CHO), and CO2 to give cross-linked polycarbonates (CLPs) under solvent-free conditions. A small amount of bis(CHO) acted as a cross-linking agent, and the use of only 0.1 mol% bis(CHO) to CHO produced polymers of quite large sizes. The thermal and mechanical properties of CLPs could be altered by changing the structure and amount of bis(CHO), and the CLPs showed improved thermal stability and tensile strength as compared to linear poly(cyclohexene carbonate)s (PCHCs). The degradation of the CLPs was also investigated, and the selective cleavage of the cross-links was achieved by UV light irradiation to give linear PCHCs. The present study disclosed the potentials of cross-linking terpolymerization for the preparation of various CLPs with a constant CO2 content (31 wt%).
Introduction
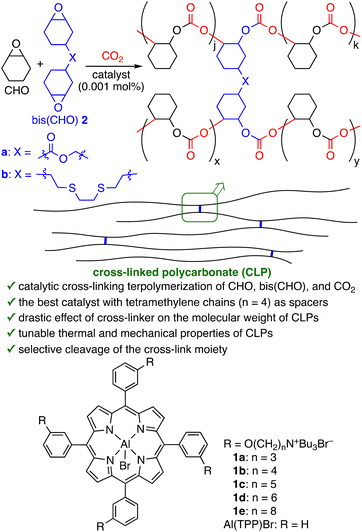
Conversions of carbon dioxide (CO2) into value-added materials are important molecular technologies.1 Synthesis of polycarbonates from epoxides and CO2 is one of the most important CO2 fixations with 100% atom economy, and a variety of catalysts have been reported since the discovery of this reaction by Inoue in 1969.2–4 In particular, poly(cyclohexene carbonate)s (PCHCs) have received considerable attention partly because they can be prepared directly via the copolymerization of cyclohexene oxide (CHO) and CO2 and because they exhibit good physical properties such as high glass transition temperature and tensile strength. Recently, the terpolymerization of CHO, CO2, and different comonomers has been extensively studied to control the properties of the PCHC-based polymers.5 Various comonomers such as different epoxides, cyclic acid anhydrides, lactones, and heteroallenes have been used.6–9 The physical properties of such terpolymers have significantly changed with an increase in the content of the comonomer. In most cases, however, such terpolymers have lower CO2 contents than PCHC.Synthesis of cross-linked polycarbonates (CLPs) via CO2 terpolymerization is a practical method to prepare large polymers without the loss of the CO2 content because the incorporation of a small amount of cross-linkers causes a drastic increase in the molar masses (Fig. 1). Several cross-linked poly(propylene carbonate)s (PPCs) have been synthesized via the terpolymerization of propylene oxide, CO2, and cross-linking agents such as diepoxides10 and cyclic acid anhydride oligomers,11 and the CLPs exhibited the enhanced thermal and mechanical properties as compared to linear PPCs. On the other hand, despite the potentiality of cross-linked PCHCs, there have been no reports on the terpolymerization of CHO, CO2, and diepoxides as far as we know.12 A highly active and robust catalyst is required to elongate a large network of branched polymers in a possible viscous reaction mixture even at high temperature.
Previously, we have reported highly active and robust bifunctional metalloporphyrin catalysts for the synthesis of cyclic carbonates and polycarbonates.13,14 Bifunctional aluminum porphyrin 1d (Fig. 1) was used for the synthesis of PCHC via the copolymerization of CHO and CO2.14 We envisioned that our highly active bifunctional catalysts might achieve the terpolymerization of CHO, CO2, and bis(CHO) such as 3,4-epoxycyclohexylmethyl 3′,4′-epoxycyclohexanecarboxylate (2a) and bis(3,4-epoxycyclohexylethylthio)ethane (2b) for the synthesis of cross-linked PCHCs (Fig. 1). Here we have found that newly synthesized bifunctional aluminum porphyrin 1b with tetramethylene chains is the best catalyst for the cross-linking terpolymerization, giving novel CLPs under solvent-free conditions. The structure and amount of the cross-linkers could modulate the thermal and mechanical properties of the CLPs. We have also found that UV photoirradiation can cleave the cross-link moieties (thioether bonds) of CLPs selectively to give linear PCHCs.
Results and discussion
First, we screened catalysts 1a–e with different methylene chain lengths for the terpolymerization of CHO, bis(CHO) 2a, and CO2 (2 MPa) in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400
400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (1
000 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a
2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CHO) at 130 °C for 24 h under solvent-free conditions (Table 1). Interestingly, the catalytic activities highly depended on the methylene chain lengths, and 1b with tetramethylene chains showed the highest conversion (entries 1–5). It should be noted that the weight-average molar masses (Mw) significantly increased, and the dispersity (Đ) values were high. In addition, 1b and 1c produced insoluble polymers, which suggested the formation of the high-molar mass cross-linked PCHCs. In sharp contrast, a binary catalytic system composed of Al(TPP)Br and tetrabutylammonium bromide (TBAB) was inactive under otherwise identical reaction conditions (entry 6).15
CHO) at 130 °C for 24 h under solvent-free conditions (Table 1). Interestingly, the catalytic activities highly depended on the methylene chain lengths, and 1b with tetramethylene chains showed the highest conversion (entries 1–5). It should be noted that the weight-average molar masses (Mw) significantly increased, and the dispersity (Đ) values were high. In addition, 1b and 1c produced insoluble polymers, which suggested the formation of the high-molar mass cross-linked PCHCs. In sharp contrast, a binary catalytic system composed of Al(TPP)Br and tetrabutylammonium bromide (TBAB) was inactive under otherwise identical reaction conditions (entry 6).15
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
| Entry | Catalyst | Convb (%) | M n (kg mol−1) | M w (kg mol−1) | Đ |
|---|---|---|---|---|---|
a Reaction conditions: CHO (29 mmol), 2a (0.12 mmol), catalyst (0.29 μmol, 0.001 mol% based on CHO), CO2 (2.0 MPa), 130 °C, 24 h in an autoclave.
b Conversion based on CHO determined by 1H NMR spectroscopy using mesitylene as an internal standard.
c Determined by SEC analysis.
d Insoluble polymer products formed. The ratios of the soluble to insoluble polymers were 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 (entry 2) and 89 26 (entry 2) and 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 (entry 3).
e 1.2 μmol of TBAB was used as a cocatalyst. 11 (entry 3).
e 1.2 μmol of TBAB was used as a cocatalyst.
|
|||||
| 1 | 1a | 20 | 37 | 122 | 3.3 |
| 2d | 1b | 58 | 61 | 219 | 3.6 |
| 3d | 1c | 39 | 58 | 222 | 3.8 |
| 4 | 1d | 40 | 30 | 93 | 3.1 |
| 5 | 1e | 17 | 14 | 42 | 3.0 |
| 6e | Al(TPP)Br | <1 | — | — | — |
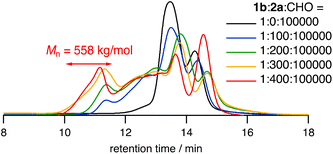
We further investigated the terpolymerization by changing the amount of bis(CHO) 2a using the best catalyst 1b (0.001 mol%), and the polymer products were analyzed by SEC (Table 2, entries 1–4 and Fig. 2). In contrast to the bimodal peaks observed for PCHC,14,16 surprisingly, the CLPs synthesized by using only 0.1 mol% of 2a to CHO showed a broad SEC profile, which indicated the formation of higher-molar mass polymers as compared with typical linear PCHC.17 The fraction at 11–12 min significantly increased with an increase of 2a, and the Mn reached 558 kg mol−1. A further increase of 2a to more than 0.4 mol% produced polymers showing poor solubility. These results suggest that the larger polymers consist of several PCHC chains cross-linked with the ester linkage of 2a. The number of the PCHC chains included in the CLPs with a Mn of 558 kg mol−1 was roughly calculated to be 8 by comparing this Mn value with that of linear PCHC. The intrinsic viscosity of the CHCl3 solution of the polymers increased with an increase in the content of 2a (Table 2). Electrospray ionization mass spectra were measured to confirm the incorporation of 2a into the polymers. CHO and 2a were heated in the presence of 1b under a CO2 pressure of 2 MPa at 130 °C for 5 min. Mass spectra of the mixture confirmed the ion peaks of the terpolymers as well as typical PCHC (Fig. S1 in ESI†); only one epoxide of 2a was ring-opened, and the other epoxide moiety remained intact because of the early reaction stage. This result together with the SEC analysis strongly suggests that cross-linking occurs at the later stage of the polymerization. It should also be noted that the copolymerization of 2a and CO2 (without CHO) did not proceed well probably because of the steric hindrance of 2a. We also performed the terpolymerization using bis(CHO) 2b with the thioether bonds (Table 2, entries 5–7). The successful synthesis of the corresponding CLPs was confirmed by the SEC analysis and the intrinsic viscosity measurements.
| Entry | Bis(CHO) |
1b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CHO CHO |
Convb (%) | M n (kg mol−1) | Đ | Intrinsic viscosityd (mL g−1) | T 50 (°C) | T g (°C) | E , (MPa) | σ , (MPa) | ε , (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
a Reaction conditions: CHO (29 mmol), 2 (0–87 μmol), 1b (0.29 μmol, 0.001 mol% based on CHO), CO2 (2.0 MPa), 130 °C, 24 h in an autoclave.
b Conversion based on CHO determined by 1H NMR spectroscopy using mesitylene as an internal standard.
c Determined by SEC analysis.
d Measured by an Ostwald viscometer in CHCl3 at 30 °C.
e Temperature at 50% decomposition determined by TGA.
f Glass transition temperature determined by DSC.
g Dog-bone-shaped specimens with the thickness of 0.2–0.3 mm were tested with a strain rate of 5 mm min−1. Average values of three measurements are shown. Values in parenthesis are standard deviations.
h Young's modulus.
i Tensile strength.
j Elongation at break.
k Insoluble polymers formed. The ratio of the soluble to insoluble polymers was 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. 4.
|
|||||||||||
| 1 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
58 | 49 | 1.3 | 37 | 312 | 125 | 2780 (±90) | 34 (±1) | 1.4 (±0.02) |
| 2 | 2a | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
66 | 57 | 1.8 | 44 | 318 | 127 | 2940 (±160) | 36 (±2) | 1.5 (±0.1) |
| 3 | 2a | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
52 | 50 | 2.5 | 51 | 314 | 127 | 3100 (±200) | 41 (±2) | 1.7 (±0.1) |
| 4k | 2a | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
52 | 57 | 3.6 | 68 | 314 | 127 | 2900 (±280) | 33 (±1) | 1.3 (±0.1) |
| 5 | 2b | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
44 | 53 | 1.6 | 47 | 321 | 124 | 2870 (±140) | 35 (±2) | 1.4 (±0.1) |
| 6 | 2b | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
55 | 57 | 2.0 | 50 | 323 | 124 | 2950 (±150) | 36 (±2) | 1.5 (±0.2) |
| 7 | 2b | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
48 | 50 | 2.1 | 53 | 321 | 125 | 2990 (±90) | 35 (±2) | 1.4 (±0.02) |
Polymer products were purified by reprecipitation with CH2Cl2/MeOH to remove the remaining monomers and a small amount of cyclic carbonates (<1%). The thermal properties of the purified polymers were characterized by means of thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) measurements (Table 2). TGA indicated that all the samples of cross-linked and linear PCHCs showed a large weight loss at ca. 320 °C, which suggests the cleavage of the carbonate linkages. When 50% weight loss temperatures (T50) were compared, importantly, the CLPs showed higher T50 than linear PCHC, which suggests that the cross-linkers especially containing the thioether bonds improved the thermal stability. DSC analysis revealed slight changes in the glass transition temperature (Tg): 127 °C for CLPs with the ester cross-linkers (entries 2–4) and 124–125 °C for those with the flexible thioether cross-linkers (entries 5–7). The rigidity of the cross-linkers affects the thermal properties of the polymers.
When we further increased the amount of 2a, CLPs obtained with 3 mol% of 2a showed a T50 of 320 °C and a broad DSC curve at 127 °C (Fig. 3). We expected the improvement of the thermal properties by the incorporation of a larger amount of cross-linkers. Therefore, CLPs containing more cross-linkers were prepared by the terpolymerization using 10–40 mol% of 2a under 4 MPa CO2 pressure for 48 h, which gave insoluble polymers. The products were ground with a mill and washed with MeOH. IR spectra of the resulting powder samples were measured by the attenuated total reflection (ATR) method (Fig. S2 in ESI†). The IR spectra of CLPs showed C–H stretching vibrations at 2942 and 2868 cm−1 and a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration at 1730 cm−1, which are quite similar to those of PCHC. Careful observations allowed us to find that the C
O stretching vibration at 1730 cm−1, which are quite similar to those of PCHC. Careful observations allowed us to find that the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of the CLPs was slightly broadened. Since the CLPs contain the ester group, the broadened peak was assigned as the C
O stretching vibration of the CLPs was slightly broadened. Since the CLPs contain the ester group, the broadened peak was assigned as the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations of both the carbonate and ester groups. In addition, we observed a new peak at 1196 cm−1, which increased with an increase in the content of 2a and therefore could be assigned as the C–O stretching vibration of the ester bond. These insoluble CLPs prepared from 10–40 mol% of 2a were investigated by TGA and DSC. The decomposition temperature of the CLPs increased with an increase in the content of 2a, and T50 reached 332 °C. In addition, the DSC peaks at 127 °C disappeared and broadened up to 160 °C. Clearly, the thermal properties could be altered by the amount of the cross-linker. Although there are reports on the improved thermal stability of cross-linked PPCs as compared to linear PPC,10,11 such high T50 and Tg values observed for the cross-linked PCHCs in this work have never been reported.
O stretching vibrations of both the carbonate and ester groups. In addition, we observed a new peak at 1196 cm−1, which increased with an increase in the content of 2a and therefore could be assigned as the C–O stretching vibration of the ester bond. These insoluble CLPs prepared from 10–40 mol% of 2a were investigated by TGA and DSC. The decomposition temperature of the CLPs increased with an increase in the content of 2a, and T50 reached 332 °C. In addition, the DSC peaks at 127 °C disappeared and broadened up to 160 °C. Clearly, the thermal properties could be altered by the amount of the cross-linker. Although there are reports on the improved thermal stability of cross-linked PPCs as compared to linear PPC,10,11 such high T50 and Tg values observed for the cross-linked PCHCs in this work have never been reported.
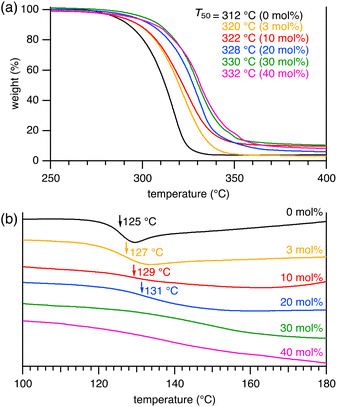 | ||
Fig. 3 (a) TGA and (b) DSC profiles of polymers. Polymer samples were prepared from 1b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a 2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CHO = 1 CHO = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (x = 0, 3000, 10 000 (x = 0, 3000, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 20 000, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 30 000, 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, and 40 000, and 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000). 000). | ||
The mechanical properties of CLPs prepared with 0.1–0.3 mol% of 2 to CHO and linear PCHC were investigated in the film state. Purified polymer powder was hot-pressed at 150 °C to give solvent-free films, and the dog-bone-shaped specimens were prepared by die cutting. Tensile tests of the specimens were conducted on a universal testing machine (Table 2). The PCHC film showed a Young's modulus (E) of 2780 MPa and a tensile strength (σ) of 34 MPa (entry 1), which were comparable to the values reported by Rieger.8d Interestingly, CLPs with 0.1–0.2 mol% of cross-linkers showed higher values for Young's modulus, tensile strength, and elongation at break (entries 2, 3, 5, and 6), which suggested that the cross-linkers increased the strength of the films. On the other hand, those with 0.3 mol% cross-linkers showed somewhat lower values (entries 4 and 7), probably because the cross-links hampered intermolecular forces between the polymer chains by restricting their mobility. Considering the solubility and processibility of the polymers and the tensile strength of the films, the use of 0.2 mol% cross-linker 2 (entries 3 and 6) is the most suitable for making robust films of the CLPs.
Degradation of polymers is of importance in view of sustainability, and the selective chemical recycling of polycarbonates has been studied.18 Here, we investigated the selective cleavage of the cross-linkers of the CLPs to give linear polycarbonates (Fig. 4a). We initially attempted the selective hydrolysis of the ester linkers of CLPs prepared from 2a, which resulted in failure. For example, the base-promoted hydrolysis of the carbonate linkages gave monomers such as CHO and 1,2-cyclohexanediol (Fig. S11a in ESI†). Gratifyingly, we found that photoirradiation selectively cleaved the thioether-containing linkers of CLPs prepared from 2b. A THF solution of the CLP (Table 2, entry 6) was irradiated by a high-pressure mercury lamp, and the resulting solution was analyzed by SEC. The faster fractions of the multimodal peaks disappeared after photoirradiation for 24 h, and the slower two fractions (bimodal peaks) corresponding to linear PCHC increased (Fig. 4b). In contrast, no changes were observed for the SEC chart of PCHC or the CLPs prepared from 2a upon irradiation (Fig. S11c and d in ESI†). Clearly, the selective cleavage of the thioether bonds occurred.19 It should be noted that photoirradiation enabled the highly selective cleavage of the thioether bonds without damaging the other framework of the polymers. Although several examples of the degradation of the backbone of polymers have been reported, the selective cleavage of specific positions of CO2-based polymers has been quite rare as far as we know.
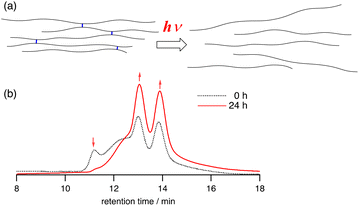 | ||
| Fig. 4 (a) Schematic representation of the selective cleavage of cross-linkers of CLPs. (b) SEC charts of the CLPs (Table 2, entry 6) before and after photoirradiation. | ||
Conclusions
In summary, we have prepared cross-linked PCHCs for the first time via the terpolymerization of CHO, bis(CHO) 2, and CO2 using bifunctional aluminum porphyrins 1 (0.001 mol%) as catalysts under solvent-free conditions. The catalytic activities of 1 were dependent on the methylene chain lengths, and the best catalyst was found to be 1b. The highly active and robust catalyst 1b achieved the cross-linking polymerization giving high-molar mass CLPs. Thermal and mechanical properties of the polymers could be altered by the cross-linkers, and enhanced thermal stability and tensile strength were observed. Importantly, the cross-linking terpolymerization reported herein enabled the preparation of polycarbonates of large molecular sizes without the loss of the high CO2 content (31 wt%), which contrasts with the previously reported terpolymerization methods giving linear terpolymers;7–9 for example, when comonomers such as lactones and cyclic acid anhydrides are incorporated, the corresponding PCHC-based terpolymers have lower CO2 contents than PCHC. Photoirradiation selectively cleaved the thioether linkages of the CLPs, giving linear PCHCs. Further investigation on the development of novel polycarbonates using bifunctional porphyrin catalysts is currently investigated in our laboratory.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was partly supported by Takahashi Industrial and Economic Research Foundation.References
- For reviews on CO2 fixation, see: (a) M. Cokoja, C. Bruckmeier, B. Rieger, W. A. Herrmann and F. E. Kühn, Angew. Chem., Int. Ed., 2011, 50, 8510 CrossRef CAS PubMed; (b) Y. Tsuji and T. Fujihara, Chem. Commun., 2012, 48, 9956 RSC; (c) N. Kielland, C. J. Whiteoak and A. W. Kleij, Adv. Synth. Catal., 2013, 355, 2115 CrossRef CAS; (d) C. Maeda, Y. Miyazaki and T. Ema, Catal. Sci. Technol., 2014, 4, 1482 RSC; (e) B. Yu and L.-N. He, ChemSusChem, 2015, 8, 52 CrossRef CAS PubMed; (f) A. Tlili, E. Blondiaux, X. Frogneux and T. Cantat, Green Chem., 2015, 17, 157 RSC; (g) Q. Liu, L. Wu, R. Jackstell and M. Beller, Nat. Commun., 2015, 6, 5933 CrossRef PubMed; (h) K. Sekine and T. Yamada, Chem. Soc. Rev., 2016, 45, 4524 RSC; (i) S.-S. Yan, Q. Fu, L.-L. Liao, G.-Q. Sun, J.-H. Ye, L. Gong, Y.-Z. Bo-Xue and D.-G. Yu, Coord. Chem. Rev., 2018, 374, 439 CrossRef CAS; (j) A. Tortajada, F. Juliá-Hernández, M. Börjesson, T. Moragas and R. Martin, Angew. Chem., Int. Ed., 2018, 57, 15948 CrossRef CAS PubMed; (k) L. Zhang, Z. Li, M. Takimoto and Z. Hou, Chem. Rec., 2020, 20, 494 CrossRef CAS PubMed; (l) P. Sreejyothi and S. K. Mandal, Chem. Sci., 2020, 11, 10571 RSC.
- For reviews on the synthesis of polycarbonates from epoxides and CO2, see: (a) S. Klaus, M. W. Lehenmeier, C. E. Anderson and B. Rieger, Coord. Chem. Rev., 2011, 255, 1460 CrossRef CAS; (b) X.-B. Lu, W.-M. Ren and G.-P. Wu, Acc. Chem. Res., 2012, 45, 1721 CrossRef CAS PubMed; (c) N. Ikpo, J. C. Flogeras and F. M. Kerton, Dalton Trans., 2013, 42, 8998 RSC; (d) Y. Zhu, C. Romain and C. K. Williams, Nature, 2016, 540, 354 CrossRef CAS PubMed; (e) S. J. Poland and D. J. Darensbourg, Green Chem., 2017, 19, 4990 RSC; (f) C. M. Kozak, K. Ambrose and T. S. Anderson, Coord. Chem. Rev., 2018, 376, 565 CrossRef CAS; (g) Y. Wang and D. J. Darensbourg, Coord. Chem. Rev., 2018, 372, 85 CrossRef CAS; (h) A. J. Plajer and C. K. Williams, Angew. Chem., Int. Ed., 2022, 61, e202104495 CrossRef CAS PubMed.
- (a) S. Inoue, H. Koinuma and T. Tsuruta, J. Polym. Sci., Part B: Polym. Lett., 1969, 7, 287 CrossRef CAS; (b) S. I. Vagin, R. Reichardt, S. Klaus and B. Rieger, J. Am. Chem. Soc., 2010, 132, 14367 CrossRef CAS PubMed; (c) K. Nakano, K. Kobayashi, T. Ohkawara, H. Imoto and K. Nozaki, J. Am. Chem. Soc., 2013, 135, 8456 CrossRef CAS PubMed; (d) F. Auriemma, C. De Rosa, M. R. Di Caprio, R. Di Girolamo, W. C. Ellis and G. W. Coates, Angew. Chem., Int. Ed., 2015, 54, 1215 CrossRef CAS PubMed; (e) S. Kissling, M. W. Lehenmeier, P. T. Altenbuchner, A. Kronast, M. Reiter, P. Deglmann, U. B. Seemann and B. Rieger, Chem. Commun., 2015, 51, 4579 RSC; (f) M. Schütze, S. Dechert and F. Meyer, Chem. – Eur. J., 2017, 23, 16472 CrossRef PubMed; (g) H. Nagae, R. Aoki, S. Akutagawa, J. Kleemann, R. Tagawa, T. Schindler, G. Choi, T. P. Spaniol, H. Tsurugi, J. Okuda and K. Mashima, Angew. Chem., Int. Ed., 2018, 57, 2492 CrossRef CAS PubMed; (h) G. Trott, J. A. Garden and C. K. Williams, Chem. Sci., 2019, 10, 4618 RSC; (i) A. C. Deacy, A. F. R. Kilpatrick, A. Regoutz and C. K. Williams, Nat. Chem., 2020, 12, 372 CrossRef CAS PubMed; (j) Y.-C. Su, C.-H. Tsui, C.-Y. Tsai and B.-T. Ko, Polym. Chem., 2020, 11, 3225 RSC; (k) F. de la Cruz-Martínez, M. Martínez de Sarasa Buchaca, J. Fernández-Baeza, L. F. Sánchez-Barba, A. M. Rodríguez, C. Alonso-Moreno, J. A. Castro-Osma and A. Lara-Sánchez, Organometallics, 2021, 40, 1503 CrossRef.
- (a) T. Aida, M. Ishikawa and S. Inoue, Macromolecules, 1986, 19, 8 CrossRef CAS; (b) S. Mang, A. I. Cooper, M. E. Colclough, N. Chauhan and A. B. Holmes, Macromolecules, 2000, 33, 303 CrossRef CAS; (c) H. Sugimoto and K. Kuroda, Macromolecules, 2008, 41, 312 CrossRef CAS; (d) C. E. Anderson, S. I. Vagin, W. Xia, H. Jin and B. Rieger, Macromolecules, 2012, 45, 6840 CrossRef CAS; (e) C. Chatterjee and M. H. Chisholm, Inorg. Chem., 2012, 51, 12041 CrossRef CAS PubMed; (f) W. Wu, X. Sheng, Y. Qin, L. Qiao, Y. Miao, X. Wang and F. Wang, J. Polym. Sci., Part A: Polym. Chem., 2014, 52, 2346 CrossRef CAS; (g) W. Xia, S. I. Vagin and B. Rieger, Chem. – Eur. J., 2014, 20, 15499 CrossRef CAS PubMed; (h) X. Sheng, Y. Wang, Y. Qin, X. Wang and F. Wang, RSC Adv., 2014, 4, 54043 RSC; (i) X. Sheng, W. Wu, Y. Qin, X. Wang and F. Wang, Polym. Chem., 2015, 6, 4719 RSC; (j) R. M. B. Carrilho, L. D. Dias, R. Rivas, M. M. Pereira, C. Claver and A. M. Masdeu-Bultó, Catalysts, 2017, 7, 210 CrossRef; (k) H. Cao, Y. Qin, C. Zhuo, X. Wang and F. Wang, ACS Catal., 2019, 9, 8669 CrossRef CAS.
- (a) A. C. Deacy, G. L. Gregory, G. S. Sulley, T. T. D. Chen and C. K. Williams, J. Am. Chem. Soc., 2021, 143, 10021 CrossRef CAS PubMed; (b) C. A. L. Lidston, S. M. Severson, B. A. Abel and G. W. Coates, ACS Catal., 2022, 12, 11037 CrossRef CAS.
- For terpolymerization with two different epoxides, see: (a) B. Li, R. Zhang and X.-B. Lu, Macromolecules, 2007, 40, 2303 CrossRef CAS; (b) W.-M. Ren, X. Zhang, Y. Liu, J.-F. Li, H. Wang and X.-B. Lu, Macromolecules, 2010, 43, 1396 CrossRef CAS; (c) G.-P. Wu, W.-M. Ren, Y. Luo, B. Li, W.-Z. Zhang and X.-B. Lu, J. Am. Chem. Soc., 2012, 134, 5682 CrossRef CAS PubMed; (d) M. Reiter, S. Vagin, A. Kronast, C. Jandl and B. Rieger, Chem. Sci., 2017, 8, 1876 RSC; (e) W. Mo, C. Zhuo, H. Cao, S. Liu, X. Wang and F. Wang, Macromol. Chem. Phys., 2022, 223, 2100403 CrossRef CAS.
- For terpolymerization with cyclic acid anhydrides, see: (a) S. Huijser, E. HosseiniNejad, R. Sablong, C. de Jong, C. E. Koning and R. Duchateau, Macromolecules, 2011, 44, 1132 CrossRef CAS; (b) J. Zhang, L. Wang, S. Liu, X. Kang and Z. Li, Macromolecules, 2021, 54, 763 CrossRef CAS; (c) Z. Wang and Y. Mu, Polym. Chem., 2021, 12, 1776 RSC; (d) V. K. Chidara, S. K. Boopathi, N. Hadjichristidis, Y. Gnanou and X. Feng, Macromolecules, 2021, 54, 2711 CrossRef CAS; (e) A. J. Plajer and C. K. Williams, Angew. Chem., Int. Ed., 2021, 60, 13372 CrossRef CAS PubMed.
- For terpolymerization with lactones, see: (a) M. Kröger, C. Folli, O. Walter and M. Döring, Adv. Synth. Catal., 2006, 348, 1908 CrossRef; (b) C. Romain and C. K. Williams, Angew. Chem., Int. Ed., 2014, 53, 1607 CrossRef CAS PubMed; (c) C. Romain, Y. Zhu, P. Dingwall, S. Paul, H. S. Rzepa, A. Buchard and C. K. Williams, J. Am. Chem. Soc., 2016, 138, 4120 CrossRef CAS PubMed; (d) S. Kernbichl, M. Reiter, F. Adams, S. Vagin and B. Rieger, J. Am. Chem. Soc., 2017, 139, 6787 CrossRef CAS PubMed; (e) S. Kernbichl, M. Reiter, J. Mock and B. Rieger, Macromolecules, 2019, 52, 8476 CrossRef CAS; (f) G. S. Sulley, G. L. Gregory, T. T. D. Chen, L. Peña Carrodeguas, G. Trott, A. Santmarti, K.-Y. Lee, N. J. Terrill and C. K. Williams, J. Am. Chem. Soc., 2020, 142, 4367 CrossRef CAS PubMed; (g) Z. Yang, C. Hu, F. Cui, X. Pang, Y. Huang, Y. Zhou and X. Chen, Angew. Chem., Int. Ed., 2022, 61, e202117533 CrossRef CAS PubMed.
- For terpolymerization with heteroallenes, see: (a) Y. Zhi, Y. Miao, W. Zhao, J. Wang, Y. Zheng, H. Su, Q. Jia and S. Shan, Polymer, 2019, 165, 11 CrossRef CAS; (b) T.-J. Yue, B.-H. Ren, W.-J. Zhang, X.-B. Lu, W.-M. Ren and D. J. Darensbourg, Angew. Chem., Int. Ed., 2021, 60, 4315 CrossRef CAS PubMed.
- (a) A. Okada, S. Kikuchi and T. Yamada, Chem. Lett., 2011, 40, 209 CrossRef CAS; (b) A. Cyriac, S. H. Lee and B. Y. Lee, Polym. Chem., 2011, 2, 950 RSC.
- (a) L. Gao and J. Feng, J. Mater. Chem. A, 2013, 1, 3556 RSC; (b) X. Chen, L. Wang, J. Feng, X. Huang, X. Guo, J. Chen, Z. Xiao, X. Liang and L. Gao, Polymers, 2018, 10, 552 CrossRef PubMed; (c) L. Gao, M. Huang, Q. Wu, X. Wan, X. Chen, X. Wei, W. Yang, R. Deng, L. Wang and J. Feng, Polymers, 2019, 11, 1467 CrossRef CAS PubMed; (d) M. Huang, L. Gao, J. Feng, X. Huang, Z. Li, Z. Huang and L. Wang, ACS Omega, 2020, 5, 17808 CrossRef CAS PubMed; (e) W.-J. Wang, S.-X. Ye, J.-X. Liang, C.-X. Fan, Y.-L. Zhu, S.-J. Wang, M. Xiao and Y.-Z. Meng, Chin. J. Polym. Sci., 2022, 40, 462 CrossRef CAS.
- Two-step access to CLPs via cross-metathesis or thiol–ene click reactions of vinyl-appended polycarbonates, see: (a) A. E. Cherian, F. C. Sun, S. S. Sheiko and G. W. Coates, J. Am. Chem. Soc., 2007, 129, 11350 CrossRef CAS PubMed; (b) M. Taherimehr, J. P. Cardoso Costa Sertã, A. W. Kleij, C. J. Whiteoak and P. P. Pescarmona, ChemSusChem, 2015, 8, 1034 CrossRef CAS PubMed; (c) Y. Wang, J. Fan and D. J. Darensbourg, Angew. Chem., Int. Ed., 2015, 54, 10206 CrossRef CAS PubMed; (d) C. Martín and A. W. Kleij, Macromolecules, 2016, 49, 6285 CrossRef; (e) K. A. Andrea and F. M. Kerton, ACS Catal., 2019, 9, 1799 CrossRef CAS.
- (a) T. Ema, Y. Miyazaki, S. Koyama, Y. Yano and T. Sakai, Chem. Commun., 2012, 48, 4489 RSC; (b) T. Ema, Y. Miyazaki, J. Shimonishi, C. Maeda and J. Hasegawa, J. Am. Chem. Soc., 2014, 136, 15270 CrossRef CAS PubMed; (c) C. Maeda, T. Taniguchi, K. Ogawa and T. Ema, Angew. Chem., Int. Ed., 2015, 54, 134 CrossRef CAS PubMed; (d) C. Maeda, J. Shimonishi, R. Miyazaki, J. Hasegawa and T. Ema, Chem. – Eur. J., 2016, 22, 6556 CrossRef CAS PubMed; (e) C. Maeda, S. Sasaki and T. Ema, ChemCatChem, 2017, 9, 946 CrossRef CAS; (f) C. Maeda, H. Inoue, A. Ichiki, T. Okihara and T. Ema, ACS Catal., 2022, 12, 13042 CrossRef CAS.
- J. Deng, M. Ratanasak, Y. Sako, H. Tokuda, C. Maeda, J. Hasegawa, K. Nozaki and T. Ema, Chem. Sci., 2020, 11, 5669 RSC.
- In the case of the bifunctional Al porphyrin catalysts, the linear carbonate anion dissociates from the Al center to form an ion pair with the tethered ammonium cation, which facilitates the coordination of epoxide and ring-opening of the coordinated epoxide. In addition, the high Lewis acidity of the Al porphyrin catalyst contributes to the superiority as compared to the Zn or Mg porphyrin catalyst.14.
- (a) H. Sugimoto, H. Ohtsuka and S. Inoue, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 4172 CrossRef CAS; (b) K. Nakano, T. Kamada and K. Nozaki, Angew. Chem., Int. Ed., 2006, 45, 7274 CrossRef CAS PubMed; (c) M. Jia, N. Hadjichristidis, Y. Gnanou and X. Feng, ACS Macro Lett., 2019, 8, 1594 CrossRef CAS PubMed.
- For the Mn, Mw, and Đ values of each of the multimodal peaks, see ESI.†.
- (a) D. J. Darensbourg, S.-H. Wei, A. D. Yeung and W. C. Ellis, Macromolecules, 2013, 46, 5850 CrossRef CAS; (b) F. N. Singer, A. C. Deacy, T. M. McGuire, C. K. Williams and A. Buchard, Angew. Chem., Int. Ed., 2022, 61, e202201785 CrossRef CAS PubMed; (c) Y. Yu, B. Gao, Y. Liu and X.-B. Lu, Angew. Chem., Int. Ed., 2022, 61, e202204492 CrossRef CAS PubMed; (d) T. M. McGuire, A. C. Deacy, A. Buchard and C. K. Williams, J. Am. Chem. Soc., 2022, 144, 18444 CrossRef CAS PubMed.
- When a model compound containing the thioether bond was subjected to UV irradiation, alcohol and thiol compounds that seemed to be formed via the homolytic cleavage of the C–S bond were detected by mass measurements (Fig. S12†).
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3py00870c |
| This journal is © The Royal Society of Chemistry 2023 |