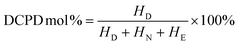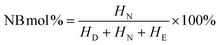Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Terpolymerization of ethylene, norbornene and dicyclopentadiene catalyzed by modified cyclopentadienyl scandium complexes†
Linfeng
Wang
ab,
Shuqi
Dong
ab,
Hui
Tian
ab,
Guangbi
Gong
c,
Baoli
Wang
 *ab,
Chunji
Wu
*a and
Dongmei
Cui
*ab,
Chunji
Wu
*a and
Dongmei
Cui
 ab
ab
aState Key Laboratory of Polymer Physics and Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, People's Republic of China. E-mail: wang@ciac.ac.cn; wuchunji@ciac.ac.cn
bSchool of Applied Chemistry and Engineering, University of Science and Technology of China, Hefei 230026, People's Republic of China
cLanzhou Chemical Research Center, PetroChina Company Limited, Lanzhou 730060, People's Republic of China
First published on 6th June 2023
Abstract
Cyclic olefin copolymers (COCs) with high glass-transition temperature (Tg) are of fundamental interest and practical importance. Herein we report the terpolymerization of ethylene (E), norbornene (NB) and dicyclopentadiene (DCPD) catalysed by fused-heterocyclic and pyridyl-modified cyclopentadienyl scandium complexes (1 and 2) in high activities (1.27 to 1.86 × 106 g molSc−1 h−1 bar−1) to produce E/NB/DCPD terpolymers with adjustable structures. The terpolymerization proceeded in a controlled fashion, forming E/NB/DCPD terpolymers with moderate number average molecular weight (Mn = 4.9 × 104–13.6 × 104 Da) and relatively narrow polymer dispersity index (PDI = 1.7–2.3). By controlling the concentration of NB and DCPD in-feed, a series of terpolymers with NB + DCPD contents from 47.4 mol% to 59.8 mol% (NB 17.7 mol% to 42.2 mol%, and DCPD 8.8 mol% to 38.6 mol%) was obtained. The highest total cycloolefin incorporation was more than 50 mol%, indicating the existence of continuous cycloolefin –E(NB)(DCPD)E– units as proved by 1H, 13C, 1H–1H COSY, 1H–13C HSQC and 1H–13C HMBC NMR spectroscopy. This phenomenon has not been observed in the binary copolymerization of E/NB and E/DCPD catalysed by rare earth catalyst systems in reported publications. The surprising finding may be ascribed to the use of two kinds of cycloolefins and novel efficient scandium catalysts. Hetero-cycloolefins promoted the cyclic olefin insertion of each other for rare earth catalysts. Tg values of the terpolymers range from 133.4 °C to 162.8 °C, which is in good agreement with NB and DCPD incorporation. The high DCPD content provides greater contribution to the high Tg value compared with NB in terpolymers. Further transformation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond in E/NB/DCPD terpolymers to the hydroxyl group was achieved via sequential epoxidation and hydroxylation reactions, resulting in a huge increase of Tg from 162.8 °C to 202.3 °C and a large decrease in the water contact angle from 84.1° to 33.4°.
C double bond in E/NB/DCPD terpolymers to the hydroxyl group was achieved via sequential epoxidation and hydroxylation reactions, resulting in a huge increase of Tg from 162.8 °C to 202.3 °C and a large decrease in the water contact angle from 84.1° to 33.4°.
Introduction
A cyclic olefin copolymer (COC) is an amorphous material prepared via the copolymerization of various cyclic olefins and ethylene (E). A COC has excellent properties, such as high transparency, good UV resistance, low dielectric constant, high refractive index and low birefringence due to its novel polymer chain structure composed of ethylidene and cyclic moieties.1–5 Norbornene (NB) and dicyclopentadiene (DCPD) are the most widely used commercial cyclic monomers, and the binary copolymerizations of E/NB6–28 and E/DCPD29–38 have attracted tremendous attention from both industry and academia. Waymouth et al. reported the copolymerization of ethylene with norbornene using ansa-cyclopentadienyl-amido group 4 metal complexes and the obtained copolymers with adjustable NB incorporation (11.7 to 46.3 mol%) possess moderate glass-transition temperatures (Tg = 34.8 to 127.1 °C).12 Hou et al. reported the binary copolymerizations of E/NB and E/DCPD using the half-sandwich scandium complex [(C5Me4SiMe3)Sc(CH2SiMe3)2THF] and the products were enriched alternating copolymers with Tg values up to 126 °C for the E/NB copolymer (41.2 mol% incorporation) and 125 °C for the E/DCPD copolymer (45.0 mol% incorporation).13,32 The Tg value of COCs is usually sensitive to the cyclic olefin incorporation ratio, which increases with a high cyclic olefin content;3 thus, the E/NB and E/DCPD copolymers show variable Tg values in a broad range from −28 °C to 220 °C.1–38 The cycloolefin content in polymers could be more than 50 mol% through the copolymerization of the cyclic olefin and ethylene catalyzed by titanium,16,28,30 zirconium10,18 and palladium complexes.19–21 However, the synthesis of cyclic olefin copolymers with high cycloolefin incorporation (>50 mol%) is still challenging for rare earth metal catalysts due to their strong Lewis acidity and relatively small ionic radii (particularly scandium, which is widely used in olefin polymerization),13,25,32,36 although they serve as efficient catalysts for the (co)polymerization of a wide range of olefins and dienes.39–47 On the other hand, the controllable terpolymerization of ethylene, norbornene and dicyclopentadiene was less successful because of the high complexity of terpolymerization and the lack of effective catalysts, although the binary copolymerizations of E/NB6–28 and E/DCPD29–38 have been studied extensively. Tritto et al. reported the polymerization of ethylene with norbornene and 5-norbornene-2-methanol catalysed by [(C5Me4SiMe3)Sc(CH2SiMe3)2THF] and [Ph3C][B(C6F5)4], using AliBu3 as the shielding agent for the hydroxyl group, and the Tg value of the resulting copolymer is 105 °C when the cycloolefin content is 42.7 mol%.48 Boggioni et al. reported primary results of the terpolymerization of E/NB/DCPD catalysed by ansa-metallocenes [Zr{(η5-2,5-Me2C5H2)2CHEt}Cl2] and methylaluminoxane (MAO) and the Tg value of the resulting copolymer is 152 °C when the total cycloolefin content is 57.3 mol%.49We have previously reported the binary copolymerizations of E/NB and E/DCPD using the N-heterocyclic carbene (NHC) modified fluorenyl scandium complex [Flu-CH2CH2(NHC)Sc(CH2SiMe3)2] and the fused-heterocyclic cyclopentadienyl scandium complex [(2,3,4,5,6-Me5-4H-cyclopenta[b]thiophenyl)Sc(CH2SiMe3)2THF].25,36 The resulting E/NB copolymer shows Tg = 117.3 °C when the NB incorporation is 46.3 mol%, while the Tg value of the E/DCPD copolymer increases to 166 °C with a DCPD incorporation as high as 46.1 mol%. These results encouraged us to examine whether scandium catalytic systems were efficient for the terpolymerization of ethylene, norbornene and dicyclopentadiene. Herein, we report our studies on the E/NB/DCPD terpolymerization using modified cyclopentadienyl scandium complexes. NB and DCPD contents were readily adjusted via the cyclic olefin concentration in-feed, which ranged from 17.7% to 42.2% for NB and from 8.8% to 38.6% for DCPD, respectively, to reach the highest total cycloolefin content of 59.8%. We found an interesting phenomenon that hetero-cycloolefins NB and DCPD mutually promoted the total cycloolefin insertion with high activities during E/NB/DCPD terpolymerization using rare earth catalysts. Thus, the E/NB/DCPD terpolymers show high glass-transition temperatures ranging from 133.4 °C to 162.8 °C. Further transformation of E/NB/DCPD terpolymers can be easily achieved via sequential epoxidation and hydroxylation reactions, furnishing the hydroxyl functional terpolymer with a higher Tg (202.3 °C).
Experimental
General procedure and materials
All experiments were performed under a dry and oxygen-free nitrogen atmosphere using standard high-vacuum Schlenk techniques or in a glove box filled with dinitrogen. All solvents were purified using an MBRAUN SPS system. Dicyclopentadiene and norbornene were stirred with sodium for 2 days at 50 °C followed by distillation and kept in a glove box. All chemicals except as mentioned, were purchased from the Energy Chemical Company in China. Complexes 1 and 2 (as shown in Fig. 1) were prepared according to the reported methods.36,50,511H, 13C, 1H–1H COSY,1H–13C HSQC and 1H–13C HMBC NMR spectra were recorded on Bruker AV500 and AV400 spectrometers. The molecular weight (Mn) and molecular weight distribution (PDI) were measured using a TOSOH HLC-8220 GPC at 40 °C using THF as solvent (the flow rate is 0.35 mL min−1) against polystyrene standards for the E/NB/DCPD terpolymer, and a PL GPC-200 GPC at 150 °C using 1,2,4-trichlorobenzene as solvent (the flow rate is 0.35 mL min−1) against polystyrene standards for the E/NB copolymer. The glass-transition temperature (Tg) was measured through differential scanning calorimetry (DSC) analysis, which was carried out using a METTLER TOPEM DSC instrument under a nitrogen atmosphere, at a heating rate of 10 °C min−1 in a range of 25 to 200 °C (25 to 250 °C for hydroxylated terpolymer). The FT-IR spectra were recorded using a Bruker Invenio-R Fourier transform infrared spectrometer. Static water contact angles on the surfaces of terpolymers were measured using a contact angle goniometer (KRUSS GMBH, Germany). The water contact angle values were recorded from 2 μL droplet depositions, and at least five measurements detected from different locations were averaged. | ||
| Fig. 1 Structures of modified cyclopentadienyl scandium complexes used for the copolymerization of ethylene, norbornene and dicyclopentadiene. | ||
Polymerization procedure
Terpolymerization was carried out under atmospheric pressure in toluene in a 75 mL glass reactor equipped with a mechanical stirrer. The total volume of the solution was 10 mL. The reactor was charged with the prescribed volume of toluene and comonomer under a nitrogen atmosphere, and then the ethylene gas feed was started, followed by the addition of half the amount of AliBu3 into the reactor. After equilibration at the desired terpolymerization temperature for 10 min, the terpolymerization was initiated by addition of the toluene solution of the catalyst systems composed of scandium complexes 1 (or 2), the residual amount of AliBu3 and [Ph3C][B(C6F5)4]. After the desired of period time, the reactor was vented. The resulting terpolymers were precipitated from hydrochloric acid/ethanol (2 vol%), filtered, washed three times with ethanol, marinated in trichloromethane for 12 h to remove the unreacted comonomer, and then dried in vacuo at 40 °C to a constant weight.According to Lee52 and Leone,53 the assignment of NMR spectra of ethylene/norbornene/dicyclopentadiene terpolymers and the cycloolefin incorporation into the copolymers was calculated from the intensities of relative protons using the following equations:
| HD = I3.0 ppm |
| 4HD + 2HN = I1.75–2.25 ppm |
| 4HD + 8HN + 4HE = I0.75 ppm–1.75 ppm |
where, D and DCPD refer to dicyclopentadiene, N and NB refer to norbornene, I3.0 ppm is the peak area of the proton at 3.0 ppm, and I1.75–2.25 ppm and I0.75–1.75 ppm were analogical.
Epoxidation and hydroxylation of terpolymers
Epoxidation and hydroxylation were performed in a 100 mL flask. 200 mg of the terpolymer was dissolved in 50 mL of chloroform. The terpolymer was stirred for 30 min, and then m-chloroperbenzoic acid (0.032 g, 0.18 mmol) and Na2HPO4 (0.13 g, 0.37 mmol) were added. The reaction was stirred for 6 h at room temperature. After epoxidation, NaOH (0.6 g, 15 mmol) in 10 mL of water and 2.0 g of 30% H2O2 solution were added. The hydroxylation reaction was performed for 8 h at room temperature, followed by pouring into 100 mL of MeOH and then filtering, and drying in vacuo at 40 °C to a constant weight.Results and discussion
The binary copolymerization of ethylene and norbornene was first studied using the combination of 1–2/[Ph3C][B(C6F5)4]/AliBu3 (Table 1, entries 1–6). As the amount of NB in-feed was increased from 10 to 15 and 20 mmol under 1 bar of ethylene in toluene at 25 °C, the activity of the copolymerization of ethylene and norbornene catalysed by 1 increased from 1.22 × 106 to 1.88 × 106 and 2.16 × 106 g molSc−1 h−1 bar−1, and the NB incorporation into E/NB copolymers increased from 31.6 to 36.2 and 41.5 mol% (Table 1, entries 1–3). The number averaged molecular weight (Mn) ranged from 3.7 × 104 to 4.9 × 104 Da with narrow polymer dispersity index (PDI = 1.9). Glass-transition temperature (Tg) of the E/NB copolymer was related to NB content and ranged from 107.3 to 127.4 °C. When switching to complex 2, NB insertion into resultant E/NB copolymers was higher than that of copolymers produced by complex 1 under the same conditions, and ranged from 35.3 to 43.3 mol% (entries 4–6). Tg of the E/NB copolymer formed by 2 slightly increased to 112.2–129.1 °C because of higher NB incorporation. Similar results for the Tg of and NB incorporation into E/NB copolymers have been observed in the scandium-catalysed copolymerization of ethylene and norbornene.13,25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
| Entry | Sc | NB (mmol) | DCPD (mmol) | Activity (106 g molSc−1 h−1 bar−1) |
f
NB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (mol%) b (mol%) |
f
DCPD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (mol%) b (mol%) |
f NB+DCPD (mol%) |
M
n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c (104 g mol−1) c (104 g mol−1) |
PDIc |
T
g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d (°C) d (°C) |
|---|---|---|---|---|---|---|---|---|---|---|
a General conditions: scandium complex, 10 μmol; [Sc]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Ph3C][B(C6F5)4] [Ph3C][B(C6F5)4]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [AliBu3] = 1 [AliBu3] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (mole ratio); toluene, 10 mL; t = 10 min; T = 25 °C; ethylene 1 bar.
b Determined by 1H NMR for the E/NB/DCPD terpolymer, and 13C NMR for the E/NB copolymer.
c Determined by GPC in tetrahydrofuran at 40 °C against polystyrene standard for the E/NB/DCPD terpolymer, and in 1,2,4-trichlorobenzene at 150 °C against polystyrene standard for the E/NB copolymer.
d Determined by DSC. 5 (mole ratio); toluene, 10 mL; t = 10 min; T = 25 °C; ethylene 1 bar.
b Determined by 1H NMR for the E/NB/DCPD terpolymer, and 13C NMR for the E/NB copolymer.
c Determined by GPC in tetrahydrofuran at 40 °C against polystyrene standard for the E/NB/DCPD terpolymer, and in 1,2,4-trichlorobenzene at 150 °C against polystyrene standard for the E/NB copolymer.
d Determined by DSC.
|
||||||||||
| 1 | 1 | 10 | 0 | 1.22 | 31.6 | 0 | 31.6 | 3.7 | 1.9 | 107.3 |
| 2 | 1 | 15 | 0 | 1.88 | 36.2 | 0 | 36.2 | 4.4 | 1.9 | 115.9 |
| 3 | 1 | 20 | 0 | 2.16 | 41.5 | 0 | 41.5 | 4.9 | 1.9 | 127.4 |
| 4 | 2 | 10 | 0 | 1.51 | 35.3 | 0 | 35.3 | 7.8 | 1.3 | 112.2 |
| 5 | 2 | 15 | 0 | 1.86 | 38.8 | 0 | 38.8 | 8.1 | 1.4 | 122.7 |
| 6 | 2 | 20 | 0 | 2.18 | 43.3 | 0 | 43.3 | 8.9 | 1.4 | 129.1 |
| 7 | 1 | 10 | 10 | 1.45 | 29.4 | 20.6 | 50.0 | 6.4 | 2.0 | 140.7 |
| 8 | 2 | 10 | 10 | 1.30 | 25.5 | 22.9 | 48.4 | 5.0 | 2.3 | 143.9 |
| 9 | 1 | 20 | 20 | 1.54 | 33.2 | 20.4 | 53.6 | 5.2 | 2.3 | 149.4 |
| 10 | 2 | 20 | 20 | 1.76 | 32.1 | 18.5 | 50.6 | 4.9 | 1.8 | 148.4 |
| 11 | 1 | 5 | 15 | 1.32 | 18.4 | 30.4 | 48.8 | 10.8 | 1.9 | 149.0 |
| 12 | 1 | 5 | 20 | 1.41 | 17.8 | 31.2 | 49.0 | 13.6 | 1.9 | 151.2 |
| 13 | 1 | 15 | 5 | 1.35 | 37.9 | 9.5 | 47.4 | 7.2 | 1.7 | 137.4 |
| 14 | 2 | 5 | 15 | 1.71 | 17.7 | 31.8 | 49.5 | 5.7 | 2.0 | 149.6 |
| 15 | 2 | 5 | 20 | 1.86 | 20.1 | 34.3 | 54.4 | 6.8 | 1.8 | 154.7 |
| 16 | 2 | 15 | 5 | 1.27 | 42.2 | 8.8 | 51.0 | 5.5 | 1.7 | 133.4 |
| 17 | 1 | 5 | 40 | 1.46 | 21.2 | 38.6 | 59.8 | 9.0 | 1.9 | 162.8 |
Then, the terpolymerization of ethylene, norbornene and dicyclopentadiene was examined using catalyst systems of 1–2/[Ph3C][B(C6F5)4]/AliBu3 under mild conditions and the representative results are summarized in Table 1, entries 7–17. When the E/NB/DCPD terpolymerization was carried out at the same cycloolefin in-feed amount (10 mmol) for either NB or DCPD under 1 bar of ethylene pressure in toluene at 25 °C catalysed by catalyst system 1/[Ph3C][B(C6F5)4]/AliBu3, the activity of the terpolymerization was up to 1.45 × 106 g molSc−1 h−1 bar−1. The NB content in the resultant terpolymer was 29.4%, which is higher than that of DCPD (20.6%), indicating that the polymerization rate of NB is faster than that of DCPD (entry 7, Table 1). The number averaged molecular weight (Mn) of the terpolymer was 6.4 × 104 Da with a narrow polymer dispersity index (PDI = 2.0). It was noted that its Tg value (140.7 °C) is higher than those of both copolymers of E/NB (Tg 110 °C and NB incorporation 43.6%) and E/DCPD (Tg 125 °C, DCPD 45.0%) produced by [(C5Me4SiMe3)Sc(CH2SiMe3)2THF],13,32 but it is lower than that of the E/DCPD copolymer (166 °C, DCPD incorporation 46.1%) synthesized by the same complex 1.36 These results illustrated that the terpolymer generated by complex 1 maybe possessed certain stereoselectivity for cycloolefin and then showed higher Tg compared with those of samples synthesized by [(C5Me4SiMe3)Sc(CH2SiMe3)2THF] at closed cycloolefin content (43.6% to 46.1%) in polymers.13,36 Under similar conditions (amount of NB and DCPD was 10 mmol), using the pyridyl-modified cyclopentadienyl scandium complex 2 instead, the formed terpolymers still have higher NB content than DCPD (25.5% vs. 22.9%), which suggested that NB was also a favourite monomer for complex 2 compared to DCPD during the terpolymerization (entry 8), although the difference of cycloolefin insertions was not very obvious as compared to that in the terpolymer isolated from catalytic system 1 (NB 25.5% and DCPD 22.9% vs. NB 29.4% and DCPD 20.6%) due to the diverse structures of catalysts (entries 7 and 8). Four methyls on the cyclopentadienyl ring of 1 provided less space for monomer coordination, resulting in the large difference between NB insertion and DCPD insertion into the terpolymer, because DCPD is bulkier than NB. The Mn of the terpolymer yielded by 2 was lower compared with that from 1 (5.0 × 104vs. 6.4 × 104 Da) under the same conditions. For either 1 or 2, the NB polymerization rate was faster than that of DCPD. By increasing the amount of NB and DCPD in-feed to 20 mmol, the NB incorporation was raised to 33.2% while the DCPD content (20.4%) was almost unchanged when catalysed by complex 1 (entry 9). The Mn of the terpolymer slightly decreased to 5.2 × 104 Da and the PDI almost did not change. As the total content of NB and DCPD increased to 53.6% in the terpolymer, the Tg value increased to 149.4 °C, correspondingly. The NB incorporation into the terpolymer increased to 32.1% while the DCPD content slightly decreased to 18.5% when the NB and DCPD in-feed amount was 20 mmol using complex 2 as the catalyst (entry 10). The plausible reason was that more NB insertions hindered bulky DCPD insertions at high NB concentrations (entries 7–10). The catalytic activities and the total incorporations of cycloolefin somewhat increased with increasing monomer concentration. When the terpolymerization was carried out at different monomer in-feed amounts, with an NB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DCPD feed-ratio of 1
DCPD feed-ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (5 mmol:15 mmol), DCPD insertion (30.4%) was much higher than that of NB (18.4%) produced from complex 1 (entry 11). It is noteworthy that a significant increase was detected for DCPD incorporation into the resultant product and that the Mn increased to 10.8 × 104 Da (entry 11). Continuing to increase the DCPD/NB in-feed ratio to 4
3 (5 mmol:15 mmol), DCPD insertion (30.4%) was much higher than that of NB (18.4%) produced from complex 1 (entry 11). It is noteworthy that a significant increase was detected for DCPD incorporation into the resultant product and that the Mn increased to 10.8 × 104 Da (entry 11). Continuing to increase the DCPD/NB in-feed ratio to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (20 mmol
1 (20 mmol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 mmol) caused just a slight increase in DCPD incorporations (31.2% vs. 30.4%, entries 11 and 12) and a slight decline in NB content (17.8% vs. 18.4%). Switching the NB/DCPD feed ratio to 3
5 mmol) caused just a slight increase in DCPD incorporations (31.2% vs. 30.4%, entries 11 and 12) and a slight decline in NB content (17.8% vs. 18.4%). Switching the NB/DCPD feed ratio to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (15 mmol
1 (15 mmol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : 5 mmol), the NB content in the terpolymer (37.9%) was much higher than that of DCPD (9.5%) (entry 13). This was consistent with the observed phenomenon that NB showed faster polymerization rate than DCPD using either catalyst 1 or 2. However, the Tg value decreased to 137.4 °C (NB 37.9%, DCPD 9.5%, entry 13), which was lower than that of the terpolymer (Tg 151.2 °C, NB 17.8%, DCPD 31.2%, entry 12) although they had similar total content of cycloolefin (49.0% in entry 12, 47.4% in entry 13), which illustrated that the contribution of DCPD in terpolymers towards the Tg value is larger than that of NB. Compared with fused-heterocyclic cyclopentadienyl scandium complex 1, the pyridyl-modified cyclopentadienyl scandium complex 2 showed similar results with respect to NB and DCPD contents and Tg values (entries 11, 13, 14, 16) when the NB/DCPD in-feed ratio was 1
: 5 mmol), the NB content in the terpolymer (37.9%) was much higher than that of DCPD (9.5%) (entry 13). This was consistent with the observed phenomenon that NB showed faster polymerization rate than DCPD using either catalyst 1 or 2. However, the Tg value decreased to 137.4 °C (NB 37.9%, DCPD 9.5%, entry 13), which was lower than that of the terpolymer (Tg 151.2 °C, NB 17.8%, DCPD 31.2%, entry 12) although they had similar total content of cycloolefin (49.0% in entry 12, 47.4% in entry 13), which illustrated that the contribution of DCPD in terpolymers towards the Tg value is larger than that of NB. Compared with fused-heterocyclic cyclopentadienyl scandium complex 1, the pyridyl-modified cyclopentadienyl scandium complex 2 showed similar results with respect to NB and DCPD contents and Tg values (entries 11, 13, 14, 16) when the NB/DCPD in-feed ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (5 mmol
3 (5 mmol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 mmol) and 3
15 mmol) and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (15 mmol
1 (15 mmol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 mmol). The total cycloolefin content (NB + DCPD) was around 50%. To our surprise, the total cycloolefin content (NB + DCPD) of terpolymers was 54.4% (20.1% for NB and 34.3% for DCPD) at high DCPD/NB in-feed ratio 4
5 mmol). The total cycloolefin content (NB + DCPD) was around 50%. To our surprise, the total cycloolefin content (NB + DCPD) of terpolymers was 54.4% (20.1% for NB and 34.3% for DCPD) at high DCPD/NB in-feed ratio 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (20 mmol:5 mmol) when catalysed by complex 2, indicating the presence of continuous cycloolefin units (the structural analysis is discussed below), and the Tg value was 154.7 °C (entry 15). This was in sharp contrast to binary copolymerization of E/NB and E/DCPD catalysed by scandium complexes [(C5Me4SiMe3)Sc(CH2SiMe3)2THF], [Flu-CH2CH2(NHC)Sc(CH2SiMe3)2] and complex 1, in which the cycloolefin unit (either NB or DCPD) was isolated in polymer chains with the absence of continuous cycloolefin units, and cyclic olefin content was lower than 50%.13,25,32,36 The total cycloolefin incorporation for the terpolymer reached 59.8% (21.2% for NB and 38.6% for DCPD) and Tg increased to 162.8 °C when increasing the DCPD/NB in-feed ratio to 8
1 (20 mmol:5 mmol) when catalysed by complex 2, indicating the presence of continuous cycloolefin units (the structural analysis is discussed below), and the Tg value was 154.7 °C (entry 15). This was in sharp contrast to binary copolymerization of E/NB and E/DCPD catalysed by scandium complexes [(C5Me4SiMe3)Sc(CH2SiMe3)2THF], [Flu-CH2CH2(NHC)Sc(CH2SiMe3)2] and complex 1, in which the cycloolefin unit (either NB or DCPD) was isolated in polymer chains with the absence of continuous cycloolefin units, and cyclic olefin content was lower than 50%.13,25,32,36 The total cycloolefin incorporation for the terpolymer reached 59.8% (21.2% for NB and 38.6% for DCPD) and Tg increased to 162.8 °C when increasing the DCPD/NB in-feed ratio to 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (40 mmol
1 (40 mmol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : 5 mmol) (entry 17), indicating that two kinds of cyclic olefins (NB and DCPD) promoted insertion for each other during E/NB/DCPD terpolymerization. All terpolymerization activity reached 106 g molSc−1 h−1 bar−1 and the PDI was around 2.0 (entries 7–17).
: 5 mmol) (entry 17), indicating that two kinds of cyclic olefins (NB and DCPD) promoted insertion for each other during E/NB/DCPD terpolymerization. All terpolymerization activity reached 106 g molSc−1 h−1 bar−1 and the PDI was around 2.0 (entries 7–17).
The terpolymers showed good solubility in THF and CHCl3 at room temperature. The 13C spectra of terpolymers with various cycloolefin contents of 48.4% (entry 8, NB 25.5%, DCPD 22.9%), 54.4% (entry 15, NB 20.1%, DCPD 34.3%) and 59.8% (entry 17, NB 21.2%, DCPD 38.6%) are shown in Fig. 2. The structure of the terpolymer (entry 15) is discussed in detail below. For DCPD units in this terpolymer (entry 15), two singlets appearing at 130.8 and 132.8 ppm belong to C1 and C2 of the CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH moiety of the DCPD unit in the 13C NMR spectrum (Fig. 2) while the corresponding protons are at around 5.5–5.7 ppm in the 1H NMR spectrum (Fig. S53†), respectively, revealing that the terpolymer contains unreacted cyclopentene units arising from DCPD.36 The signals appearing at 53.3 (C9), 45.8 (C6), 44.0 (C7), 42.5 (C4), 40.4 (C8), 37.3 (C5), 36.1 (C10) and 32.5 (C3) in the 13C spectrum were assigned to the isolated DCPD units –EE(DCPD)EE–, while the signals at 46.5 (C6), 45.1 (C7), 41.5 (C8) and 38.2 (C5) ppm are characteristic of –E(DCPD)E(DCPD)E– alternating sequences according to an earlier study.32 Continuous DCPD units –(DCPD)(DCPD)– were not observed based on the 13C NMR spectrum compared with reported results.32,36 E/DCPD alternating units –E(DCPD)E(DCPD)E– were dominant at the initial period of polymerization, and DCPD isolated parts –EE(DCPD)EE– began to form with the consumption of DCPD. For NB units (entry 15 in Fig. 2), the peaks in the region of 46.6–47.4 ppm are assigned to C12 and C13 on isolated NB units –EE(NB)EE– and E/NB alternating sequences –E(NB)E(NB)E–, and these were comparable with E/NB binary copolymers produced by [(C5Me4SiMe3)Sc(CH2SiMe3)2THF] and [Flu-CH2CH2(NHC)Sc(CH2SiMe3)2].13,25 The peaks in the region of 40.8–41.6 ppm are ascribed to C11 and C14 on isolated NB units –EE(NB)EE– and alternating sequences –E(NB)E(NB)E–. Additionally, the broad peak at around 30.1 ppm is attributed to C15, C16 and successive ethylene segments.11 The peaks at 30.5 ppm and in the region of 28.4–29.6 ppm are assigned to the carbons derived from the alternating E/NB units –E(NB)E(NB)E– and the alternating E/DCPD units –E(DCPD)E(DCPD)E–.10,32 The signal of carbon C17 was a singlet and not split indicating the absence of continuous NB units.13,25,26 Based on the above NMR spectroscopy analysis, it can be concluded that the obtained terpolymers predominantly consist of alternating –E(NB)E(NB)E– and –E(DCPD)E(DCPD)E– segments.27,37 Furthermore, it is found that the signals belonging to C8, C11 and C14 for these three samples at around 39.5–42.0 ppm in their 13C NMR spectra were different (Fig. 2), indicating that the obvious connection unit for a continuous hetero-cycloolefin is –E(NB)(DCPD)E– (for detailed analysis, see below). The plausible reason was that the catalysts possessed stereoselectivity to a certain extent for bulky monomers NB and DCPD, and favoured E or hetero-cyclic olefin insertion kinetically or thermodynamically after one-cycloolefin insertion into the Sc-carbon bond.
CH moiety of the DCPD unit in the 13C NMR spectrum (Fig. 2) while the corresponding protons are at around 5.5–5.7 ppm in the 1H NMR spectrum (Fig. S53†), respectively, revealing that the terpolymer contains unreacted cyclopentene units arising from DCPD.36 The signals appearing at 53.3 (C9), 45.8 (C6), 44.0 (C7), 42.5 (C4), 40.4 (C8), 37.3 (C5), 36.1 (C10) and 32.5 (C3) in the 13C spectrum were assigned to the isolated DCPD units –EE(DCPD)EE–, while the signals at 46.5 (C6), 45.1 (C7), 41.5 (C8) and 38.2 (C5) ppm are characteristic of –E(DCPD)E(DCPD)E– alternating sequences according to an earlier study.32 Continuous DCPD units –(DCPD)(DCPD)– were not observed based on the 13C NMR spectrum compared with reported results.32,36 E/DCPD alternating units –E(DCPD)E(DCPD)E– were dominant at the initial period of polymerization, and DCPD isolated parts –EE(DCPD)EE– began to form with the consumption of DCPD. For NB units (entry 15 in Fig. 2), the peaks in the region of 46.6–47.4 ppm are assigned to C12 and C13 on isolated NB units –EE(NB)EE– and E/NB alternating sequences –E(NB)E(NB)E–, and these were comparable with E/NB binary copolymers produced by [(C5Me4SiMe3)Sc(CH2SiMe3)2THF] and [Flu-CH2CH2(NHC)Sc(CH2SiMe3)2].13,25 The peaks in the region of 40.8–41.6 ppm are ascribed to C11 and C14 on isolated NB units –EE(NB)EE– and alternating sequences –E(NB)E(NB)E–. Additionally, the broad peak at around 30.1 ppm is attributed to C15, C16 and successive ethylene segments.11 The peaks at 30.5 ppm and in the region of 28.4–29.6 ppm are assigned to the carbons derived from the alternating E/NB units –E(NB)E(NB)E– and the alternating E/DCPD units –E(DCPD)E(DCPD)E–.10,32 The signal of carbon C17 was a singlet and not split indicating the absence of continuous NB units.13,25,26 Based on the above NMR spectroscopy analysis, it can be concluded that the obtained terpolymers predominantly consist of alternating –E(NB)E(NB)E– and –E(DCPD)E(DCPD)E– segments.27,37 Furthermore, it is found that the signals belonging to C8, C11 and C14 for these three samples at around 39.5–42.0 ppm in their 13C NMR spectra were different (Fig. 2), indicating that the obvious connection unit for a continuous hetero-cycloolefin is –E(NB)(DCPD)E– (for detailed analysis, see below). The plausible reason was that the catalysts possessed stereoselectivity to a certain extent for bulky monomers NB and DCPD, and favoured E or hetero-cyclic olefin insertion kinetically or thermodynamically after one-cycloolefin insertion into the Sc-carbon bond.
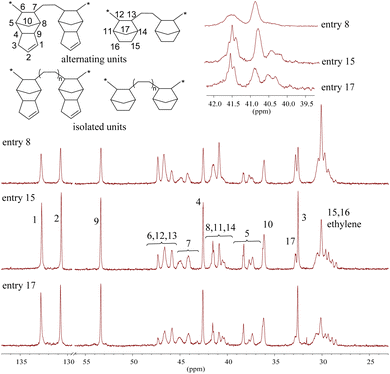 | ||
| Fig. 2 13C NMR spectra of ethylene/norbornene/dicyclopentadiene terpolymers in CDCl3 at 25 °C (Table 1, entries 8, 15 and 17). | ||
The total cycloolefin content (NB% + DCPD%) of selected terpolymers was more than 50% (53.6% for entry 9, 54.4% for entry 15, 59.8% for entry 17), which indicated the existence of continuous connection segments of cycloolefins. This information was in sharp contrast to the results for E/NB and E/DCPD binary copolymers from various scandium complexes, whose cyclic olefin incorporation was less than 50%.13,25,32,36 For instance, the reported highest NB content of E/NB copolymers was 48.1% yielded by [(C5Me4SiMe3)Sc(CH2SiMe3)2THF]13 and 46.3% from an N-heterocyclic carbene-modified fluorenyl scandium complex,25 while the highest DCPD content of E/DCPD copolymers was 46.1% from fused-heterocyclic cyclopentadienyl scandium complex 1.36 To further confirm the structure of terpolymers, we selected the sample in entry 15 (NB 20.1%, DCPD 34.3%) for further analysis using 1H–1H COSY (Fig. S54†), 1H–13C HSQC (Fig. S55†) and 1H–13C HMBC (Fig. 3 and S56†) NMR spectroscopy.
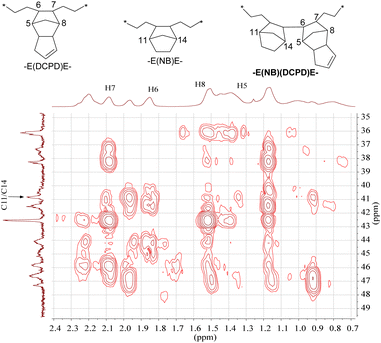 | ||
| Fig. 3 The 1H–13C HMBC NMR spectrum of an ethylene/norbornene/dicyclopentadiene terpolymer in CDCl3 at 25 °C (Table 1, entry 15). | ||
In the 1H–13C HMBC spectrum, a correlation between the C11/C14 carbons on NB with H5, H6, H7 and H8 on DCPD is shown to exist (Fig. 3), which suggests the presence of a structure having continuous –E(NB)(DCPD)E– units according to the analysis of E/NB and E/DCPD copolymers in the literature.13,32
All the terpolymer products are amorphous without the observation of melting temperature (Tm). The Tg values of the terpolymers depended on the content of either DCPD or NB, which could be controlled by the change of DCPD and NB monomer concentrations. The Tg value increased with increasing the content of total cycloolefin, and DCPD played more important roles in the increase of Tg. For instance, the Tg value of terpolymers with greater DCPD content (Tg 149.0 °C, NB 18.4%, DCPD 30.4%, entry 11) was higher than that of terpolymers with greater NB content (Tg 143.9 °C, NB 25.5% DCPD 22.9%, entry 8) at similar total NB + DCPD incorporations. The highest Tg of 162.8 °C was observed for the sample with 38.6 mol% DCPD content and 21.2 mol% NB content (entry 17). All of the GPC curves are unimodal with relatively narrow molecular weight distributions (PDI = 1.7–2.3) and consistent with the predominance of a single homogeneous catalytic species during copolymerization when compared with reported results.25,36
The obtained E/NB/DCPD terpolymers contained pendant reactive C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds, which can be readily transformed into various functional groups. For example, the epoxidation of the terpolymers can be easily achieved using m-chloroperbenzoic acid and Na2HPO4. 1H NMR analyses indicated that the complete epoxidation of the olefinic groups was achieved based on the fact that the disappearing signals belonged to CH
C double bonds, which can be readily transformed into various functional groups. For example, the epoxidation of the terpolymers can be easily achieved using m-chloroperbenzoic acid and Na2HPO4. 1H NMR analyses indicated that the complete epoxidation of the olefinic groups was achieved based on the fact that the disappearing signals belonged to CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH in the 1H NMR spectrum (as shown in Fig. S57†). Hydroxylation of the resulting products could be further achieved using NaOH and H2O2. FT-IR and 1H NMR spectra further demonstrated that the hydroxylated terpolymers were successfully obtained (as shown in Fig. S58 and S59†).38 Hydroxylated terpolymers have higher glass transition temperatures up to 202.3 °C (as shown in Fig. S60†), and their water-contact angle drastically decreased from 84.1° to 33.4° (for entry 17) as shown in Fig. S61,† which may further expand the application of these materials.
CH in the 1H NMR spectrum (as shown in Fig. S57†). Hydroxylation of the resulting products could be further achieved using NaOH and H2O2. FT-IR and 1H NMR spectra further demonstrated that the hydroxylated terpolymers were successfully obtained (as shown in Fig. S58 and S59†).38 Hydroxylated terpolymers have higher glass transition temperatures up to 202.3 °C (as shown in Fig. S60†), and their water-contact angle drastically decreased from 84.1° to 33.4° (for entry 17) as shown in Fig. S61,† which may further expand the application of these materials.
Conclusions
The terpolymerization of ethylene, norbornene and dicyclopentadiene has been achieved using catalyst systems composed of fused-heterocyclic and pyridyl-modified cyclopentadienyl scandium complexes (1 and 2), [Ph3C][B(C6F5)4] and AliBu3. The terpolymerization led to the formation of ethylene/norbornene/dicyclopentadiene terpolymers with high total cycloolefin content (>50 mol%), which has not been observed in the binary copolymerization of E/NB and E/DCPD catalysed by various rare earth metal complexes. The existence of –E(NB)(DCPD)E– segments was proved by 1H, 13C and 1H–13C HMBC NMR spectroscopy. Hetero-cycloolefins (NB and DCPD) could promote insertion for each other and afford total cycloolefin content of more than 50%. The incorporation of norbornene and dicyclopentadiene was adjustable via the amount of cycloolefin in-feed. Dicyclopentadiene content provided a large contribution to the Tg value of terpolymers compared with the content of norbornene. Further transformation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond in the terpolymer to the hydroxyl group was achieved via sequential epoxidation and hydroxylation reactions to further increase the Tg value to 202.3 °C from 162.8 °C, and decrease the water-contact angle from 84.1° to 33.4°. We expect that our findings will guide the design and synthesis of various cyclic olefin copolymers.
C double bond in the terpolymer to the hydroxyl group was achieved via sequential epoxidation and hydroxylation reactions to further increase the Tg value to 202.3 °C from 162.8 °C, and decrease the water-contact angle from 84.1° to 33.4°. We expect that our findings will guide the design and synthesis of various cyclic olefin copolymers.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is partially supported by the Natural Science Foundation of China for projects No. 52273017, 52273016 and U21A20279. We are grateful to the Scientific Research Foundation supported by the Changchun Institute of Applied Chemistry, CAS.References
- W. Kaminsky, A. Bark and M. Arndt, Makromol. Chem. Macromol. Symp., 1991, 47, 83–93 CrossRef CAS.
- X. Li and Z. Hou, Coord. Chem. Rev., 2008, 252, 1842–1869 CrossRef CAS.
- L. Boggioni and I. Tritto, Adv. Polym. Sci., 2013, 258, 117–141 CrossRef CAS.
- L. Boggioni and I. Tritto, MRS Bull., 2013, 38, 245–251 CrossRef CAS.
- I. Tritto, L. Boggioni and D. Ferro, Coord. Chem. Rev., 2006, 250, 212–241 CrossRef CAS.
- H. Terao, A. Iwashita, S. Ishii, H. Tanaka, Y. Yoshida, M. Mitani and T. Fujita, Macromolecules, 2009, 42, 4359–4361 CrossRef CAS.
- I. Tritto, C. Marestin, L. Boggioni, L. Zetta, A. Provasoli and D. Ferro, Macromolecules, 2000, 33, 8931–8944 CrossRef CAS.
- K. Nomura, M. Tsubota and M. Fujiki, Macromolecules, 2003, 36, 3797–3799 CrossRef CAS.
- J. Kiesewetter and W. Kaminsky, Chem. – Eur. J., 2003, 9, 1750–1758 CrossRef CAS PubMed.
- Y. Li, J. Yang, B. Wang and Y. Li, RSC Adv., 2016, 6, 59590–59599 RSC.
- Y. Yoshida, J. Mohri, S. Ishii, M. Mitani, J. Saito, S. Matsui, H. Makio, T. Nakano, H. Tanaka, M. Onda, Y. Yamamoto, A. Mizuno and T. Fujita, J. Am. Chem. Soc., 2004, 126, 12023–12032 CrossRef CAS PubMed.
- A. McKnight and R. Waymouth, Macromolecules, 1999, 32, 2816–2825 CrossRef CAS.
- X. Li, J. Baldamus and Z. Hou, Angew. Chem., Int. Ed., 2005, 44, 962–965 CrossRef CAS PubMed.
- K. Thorshaug, R. Mendichi, L. Boggioni, I. Tritto, S. Trinkle, C. Friedrich and R. Mülhaupt, Macromolecules, 2002, 35, 2903–2911 CrossRef CAS.
- A. Ravasio, C. Zampa, L. Boggioni, I. Tritto, J. Hitzbleck and J. Okuda, Macromolecules, 2008, 41, 9565–9569 CrossRef CAS.
- J. Ni, C. Lu, Y. Zhang, Z. Liu and Y. Mu, Polymer, 2008, 49, 211–216 CrossRef CAS.
- E. Cho, U. Joung, B. Lee, H. Lee, Y. Park, C. Lee and D. Shin, Organometallics, 2004, 23, 4693–4699 CrossRef CAS.
- B. Lee, Y. Kim, Y. Won, J. Han, W. Suh, I. Lee, Y. Chung and K. Song, Organometallics, 2002, 21, 1500–1503 CrossRef CAS.
- J. Kiesewetter, B. Arikan and W. Kaminsky, Polymer, 2006, 47, 3302–3314 CrossRef CAS.
- J. Kiesewetter and W. Kaminsky, Chem. – Eur. J., 2003, 9, 1750–1758 CrossRef CAS PubMed.
- H. Wu, Y. Liu, S. Peng and S. Liu, Eur. J. Inorg. Chem., 2003, 2003, 3152–3159 CrossRef.
- H. Gao, Y. Liu, G. Li, Z. Xiao, G. Liang and Q. Wu, Polym. Chem., 2014, 5, 6012–6018 RSC.
- K. Nomura, W. Wang, M. Fujiki and J. Liu, Chem. Commun., 2006, 25, 2659–2661 RSC.
- D. Ruchatz and G. Fink, Macromolecules, 1998, 31, 4681–4683 CrossRef CAS PubMed.
- B. Wang, T. Tang, Y. Li and D. Cui, Dalton Trans., 2009, 41, 8963–8969 RSC.
- H. Gao, H. Hu and Q. Wu, Sci. China: Chem., 2010, 53, 1634–1640 CrossRef CAS.
- I. Tritto, C. Marestin, L. Boggioni, M. Sacchi, H. Brintzinger and D. Ferro, Macromolecules, 2011, 34, 5770–5777 CrossRef.
- T. Hasan, T. Ikeda and T. Shiono, Macromolecules, 2004, 37, 8503–8509 CrossRef CAS.
- V. Dougnac, R. Quijada, H. Palza and G. Galland, Eur. Polym. J., 2009, 45, 102–106 CrossRef CAS.
- K. Nishii, S. Hayano, Y. Tsunogae, Z. Cai, Y. Nakayama and T. Shiono, Chem. Lett., 2008, 37, 590–591 CrossRef CAS.
- A. Simanke, R. Mauler and G. Galland, J. Polym. Sci., Part A: Polym. Chem., 2002, 40, 471–485 CrossRef CAS.
- X. Li and Z. Hou, Macromolecules, 2005, 38, 6767–6769 CrossRef CAS.
- M. Gao, X. Sun, Y. Gu, X. Yao, C. Li, J. Bai, C. Wang, Z. Ma, Y. Tang, Z. Xie, S. Bu and C. Qian, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 2807–2819 CrossRef CAS.
- M. Hong, L. Pan, W. Ye, D. Song and Y. Li, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 1764–1772 CrossRef CAS.
- W. Wang, M. Chen, W. Pang, Y. Li, C. Zou and C. Chen, Macromolecules, 2021, 54, 3197–3203 CrossRef CAS.
- R. Chen, C. Yao, M. Wang, H. Xie, C. Wu and D. Cui, Organometallics, 2015, 34, 455–461 CrossRef CAS.
- J. Suzuki, Y. Kino, T. Uozumi, T. Sano, T. Teranishi, J. Jin, K. Soga and T. Shiono, J. Appl. Polym. Sci., 1999, 72, 103–108 CrossRef CAS.
- L. Pan, M. Hong, J. Liu, W. Ye and Y. Li, Macromolecules, 2009, 42, 4391–4393 CrossRef CAS.
- Y. Jiang, X. Kang, Z. Zhang, S. Li and D. Cui, ACS Catal., 2020, 10, 5223–5229 CrossRef CAS.
- B. Liu, K. Qiao, J. Fang, T. Wang, Z. Wang, D. Liu, Z. Xie, L. Maron and D. Cui, Angew. Chem., Int. Ed., 2018, 57, 14896–14901 CrossRef CAS PubMed.
- X. Yan, S. Zhang, D. Peng, P. Zhang, J. Zhi, X. Wu, L. Wang, Y. Dong and X. Li, Polym. Chem., 2018, 9, 984–993 RSC.
- X. Shi, M. Nishiura and Z. Hou, J. Am. Chem. Soc., 2016, 138, 6147–6150 CrossRef CAS PubMed.
- X. Yu, Q. You, X. Zhou and L. Zhang, ACS Catal., 2018, 8, 4465–4472 CrossRef CAS.
- M. Nishiura, F. Guo and Z. Hou, Acc. Chem. Res., 2015, 48, 2209–2220 CrossRef CAS PubMed.
- M. Nishiura and Z. Hou, Nat. Chem., 2010, 2, 257–268 CrossRef CAS PubMed.
- C. Li, L. Wang, M. Wang, B. Liu, X. Liu and D. Cui, Angew. Chem., Int. Ed., 2019, 58, 11434–11438 CrossRef CAS PubMed.
- D. Liu, M. Wang, Y. Chai, X. Wan and D. Cui, ACS Catal., 2019, 9, 2618–2625 CrossRef CAS.
- I. Tritto, A. Ravasio, L. Boggioni, F. Bertini, J. Hitzbleck and J. Okuda, Macromol. Chem. Phys., 2010, 211, 897–904 CrossRef CAS.
- L. Boggioni, N. Galotto, F. Bertini and I. Tritto, Polymers, 2016, 8, 60–78 CrossRef PubMed.
- Y. Pan, W. Rong, Z. Jian and D. Cui, Macromolecules, 2012, 45, 1248–1253 CrossRef CAS.
- L. Huang, Z. Liu, Y. Zhang, C. Wu and D. Cui, ACS Catal., 2022, 12, 953–962 CrossRef CAS.
- S. Na, C. Wu, J. Yoo, B. Kim and B. Lee, Macromolecules, 2008, 41, 4055–4057 CrossRef CAS.
- G. Zanchin, L. Vendier, I. Pierro, F. Bertini, G. Ricci, C. Lorber and G. Leone, Organometallics, 2018, 37, 3181–3195 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3py00383c |
| This journal is © The Royal Society of Chemistry 2023 |