Organocatalytic aldol approach for the protecting group-free asymmetric synthesis of (7R′)-parabenzlactone, (−)-hinokinin, (−)-yatein, (−)-bursehernin, (−)-pluviatolide, (+)-isostegane and allied lignans†
Abstract
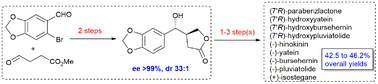
A short and efficient catalytic asymmetric protection-free synthesis of dibenzylbutyrolactone lignans, such as (−)-hinokinin, (−)-yatein, (−)-bursehernin, (−)-pluviatolide, and their 7′-hydroxylignans – (7′R)-parabenzlactone, (7′R)-hydroxyyatein, (7′R)-hydroxybursehernin, and (7′R)-hydroxy pluviatolide, respectively, is described. The syntheses of (+)-isostegane and the formal synthesis of (−)-podophyllotoxin and bicubebins are also described. Organocatalytic aldol-reduction-lactonization and Pd/C-catalyzed hydrogenative debromination are two-pot sequential reactions for the enantioselective synthesis of hydroxybutyrolactone 13b with excellent diastereo- and enantioselectivity (dr 33 : 1 and >99% ee). The protecting group-free chemoselective α-alkylation of 13b directly led to 7′-hydroxydibenzylbutyrolactone lignans, followed by hydrogenative dehydroxylation, which led to their (deoxy) dibenzylbutyrolactone lignans, and the syntheses were completed in three to five steps from 6-bromopiperonal.


 Please wait while we load your content...
Please wait while we load your content...