Recyclable gold(i)-catalyzed heterocyclization of ynamides with benzyl or indolyl azides towards 2-aminoindoles or 3-amino-β-carbolines†
Abstract
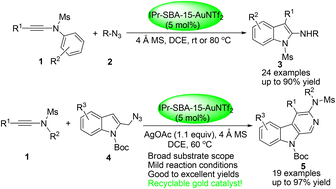
A highly efficient heterogeneous gold(I)-catalyzed heterocyclization of ynamides with benzyl or indolyl azides has been achieved in 1,2-dichloroethane under mild conditions via a heterogenized α-imino gold carbene intermediate using 5 mol% of SBA-15-anchored strongly hindered NHC–gold(I) complex [IPr-SBA-15-AuNTf2] as the catalyst, delivering a wide range of valuable 2-aminoindoles or 3-amino-β-carbolines in mostly good to excellent yields with high regioselectivity. Furthermore, the new heterogenized NHC–gold(I) complex displays the same catalytic activity as IPrAuNTf2 and is facile to recover by centrifugation of the reaction mixture and can be reused at least seven times without any appreciable drop in its catalytic activity.


 Please wait while we load your content...
Please wait while we load your content...