Aluminium-catalysed synthesis of aryl enol ethers from phenols and dimethyl ketals†
Kwihwan
Kobayashi
 *a,
Shingo
Komatsuzaki
a,
Shun-ya
Onozawa
a,
Koichiro
Masuda
*a,
Shingo
Komatsuzaki
a,
Shun-ya
Onozawa
a,
Koichiro
Masuda
 *a and
Shū
Kobayashi
*a and
Shū
Kobayashi
 *ab
*ab
aInterdisciplinary Research Center for Catalytic Chemistry, National Institute of Advanced Industrial Science and Technology Central 5, Higashi 1-1-1, Tsukuba, Ibaraki 305-8565, Japan. E-mail: kobayashi-kwihwan@aist.go.jp; koichiro-masuda@aist.go.jp; shu_kobayashi@chem.s.u-tokyo.ac.jp
bDepartment of Chemistry, School of Science, The University of Tokyo, Hongo, Bunkyo-ku, Tokyo 111-0033, Japan
First published on 5th September 2023
Abstract
We report an environmentally friendly, aluminium-catalysed, halide- and transition metal-free method for the synthesis of aryl enol ethers from phenols and dimethyl ketals that involves ketal exchange driven by the removal of methanol. The obtained aryl enol ethers were transformed into the corresponding diaryl ethers by Pd/C-catalysed dehydrogenation or DDQ oxidation.
Enol ethers have been widely used as building blocks in organic synthesis.1–3 In particular, silyl enol ethers and alkyl enol ethers have been widely explored in terms of synthesis and reactivity.4–7 However, compared to these ethers, the use of aryl enol ethers as intermediates in organic synthesis and polymer chemistry8–10 has been less explored because of their difficult synthesis. To the best of our knowledge, synthetic methods for aryl enol ethers from phenols have been limited to the Buchwald–Hartwig alkoxylation and Ullmann-type etherification with vinyl halides or vinyl triflates, which require halogenated substrates, transition metals, and equimolar amounts of bases.11–14 New methods with novel approaches are highly desirous for enhancing the use of aryl enol ethers.
Williamson ether synthesis of ketone enolates affords alkyl enol ethers by employing bases and alkyl halides.15 Non-Williamson-type reactions have been reported for the formation of alkyl cyclohexenyl ethers from cyclohexanones and aliphatic alcohols in the dehydrogenative synthesis of alkyl aryl ethers over Pd/C.16,17 However, the scope of these reactions is limited to alkyl aryl ethers because preparing the corresponding aryl enol ether intermediates from phenol derivatives is difficult due to their less nucleophilic nature than aliphatic alcohols. Regardless of the more stable nature of aryl enol ethers than alkyl enol ethers, to the best of our knowledge, there appear to be no reports on the direct preparation of an aryl enol ether from a phenol and a ketone derivative.
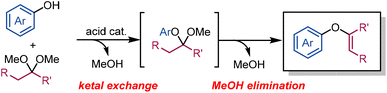
Ketals are well-known ketone-protecting groups in the synthetic organic chemistry field. A ketone and an alcohol form the corresponding ketal under acidic conditions and further treatment with another alcohol leads to a ketal-exchange equilibrium. Dimethyl ketals derived from the smallest alcohol are known to form methyl enol ethers under acidic conditions with the loss of one methanol molecule.18–20 Consequently, we envisaged the synthesis of aryl enol ethers by eliminating methanol from aryl methyl ketals, which are the products of phenol/dimethyl ketal exchange equilibria. Herein, we describe the development of a halogen- and transition metal-free method for the synthesis of aryl enol ethers from phenols and dimethyl ketals via an acid-catalysed ketal-exchange and methanol-elimination protocol (Scheme 1).
We first investigated the reactivity of the Lewis acid used to synthesise aryl enol ether 3aa from 2-naphthol (1a) and cyclohexanone dimethyl ketal (2a) (Table 1). Methanol was removed from the reaction environment with Dean–Stark apparatus and 4 Å molecular sieves (MS 4 Å). A Lewis acid was added to a solution of 1a in toluene in a flask fitted with a Dean–Stark trap. Compound 2a was added to the reaction solution using a syringe pump and the mixture was reacted for 16 h. Further details of the reaction apparatus and setup are provided in the ESI.† Aluminium ethoxide (Al(OEt)3) effectively promoted this transformation to afford 3aa in 72% yield (entry 1). In contrast, other metal alkoxides (entry 2) and aluminium compounds, such as aluminium chloride (AlCl3), aluminium acetate (Al2O(OAc)4·nH2O), aluminium nitrate (Al(NO3)3·9H2O), and aluminium acetylacetonate (Al(acac)3) (entries 3–6), catalysed the reaction poorly compared to Al(OEt)3. Trimethylaluminium (AlMe3) exhibited favourable performance to deliver 3aa in 61% yield (entry 7), while AlMe3 and 2 equiv. of 2a afforded 3aa in 71% yield (entry 8). A lower amount of AlMe3 (1 mol%) afforded a higher yield of 3aa because a small amount of 1a was consumed to form Al(ONap)3 (entry 9). The reaction proceeded efficiently to afford 3aa in 86% yield when 2a was added directly (without a syringe pump) (entry 10). A five-fold scale-up (50 mmol of 1a) of the reaction gave 3aa in 91% yield (entry 11). Further optimisation data are provided in the ESI.†
| Entry | Catalyst | Conv. (%) | Yield (%) |
|---|---|---|---|
| a Lewis acid (10 mol%) was added to a solution of 1a (10 mmol) and dodecane (10 mol%; internal standard) in toluene (40 mL), and the mixture was refluxed using the Dean–Stark apparatus filled with MS 4 Å. 2a (1 equiv.) was injected into the reaction mixture at 0.5 mL h−1 using a syringe pump and the reaction mixture was stirred for 16 h. Yields were determined by gas chromatography analysis. Further details of the reaction apparatus and setup are provided in the ESI.† b 2 equiv. of 2a were used. c 1 mol% AlMe3 was used. d 2a was added directly without a syringe pump. e 50 mmol of 1a, 200 mL of toluene, and 100 g of MS 4 Å were used. | |||
| 1 | Al(OEt)3 | 83 | 72 |
| 2 | Other metal alkoxides | 1–88 | 0–58 |
| 3 | AlCl3 | 69 | 51 |
| 4 | Al2O(OAc)4·nH2O | 33 | 29 |
| 5 | Al(NO3)3·9H2O | 23 | 3 |
| 6 | Al(acac)3 | 6 | 0 |
| 7 | AlMe3 | 74 | 61 |
| 8b | AlMe3 | 83 | 71 |
| 9b,c | AlMe3 | 92 | 74 |
| 10b,c,d | AlMe3 | 92 | 86 |
| 11b,c,d,e | AlMe3 | 93 | 91 |
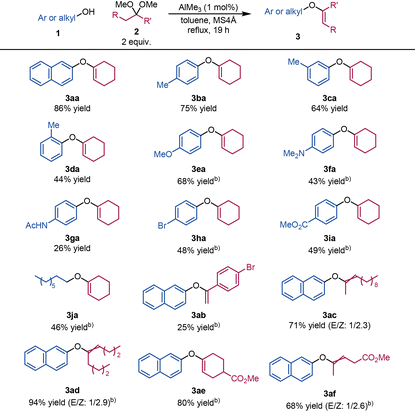
We next investigated the substrate scope for the synthesis of aryl enol ethers 3 from phenols 1 and dimethyl ketals 2 (Table 2). Compounds 3aa and 3ba were obtained in excellent yields from 2-naphthol (1a) and p-cresol (1b) under the optimised conditions (i.e., Table 1, entry 10). m-Cresol (1c) and o-cresol (1d) were less reactive toward cyclohexanone dimethyl ketal (2a) than 1b, which is ascribable to steric hindrance. 4-Methoxyphenol (1e), 4-(dimethylamino)phenol (1f), and p-acetamidophenol (1g), which contain electron-rich aromatic rings, were converted into the corresponding aryl enol ethers 3ea, 3fa, and 3ga in moderate yields. Phenols bearing electron-withdrawing groups, such as a bromine atom (1h) and a methyl ester (1i), were also converted to the corresponding aryl enol ethers 3ha and 3ia in moderate yields. Unstable aliphatic enol ether 3ja was obtained in 46% yield under the optimised conditions from aliphatic alcohol 1j. Enol ether possessing exo-methylene 3ab was obtained in 25% yield from 4′-bromoacetophenone dimethyl ketal (2b). Linear dimethyl ketals 2c and 2d and ester-bearing dimethyl ketals 2e and 2f afforded the corresponding aryl enol ethers 3ac, 3ad, 3ae, and 3af in good yields.
We next investigated the reaction mechanism using control experiments. The reaction of 1a and 1-methoxycyclohexene (2d) provided 3aa in 54% yield, which suggests that 2d is less reactive than cyclohexanone dimethyl ketal (2a) under the present reaction conditions (eqn (1)). Cyclohexanone ethylene ketal (2e) (instead of 2a) did not react and was completely recovered after the attempted reaction (eqn (2)), which reveals that the removal of methanol from the reaction environment using MS 4 Å is important for a successful reaction.
 | (1) |
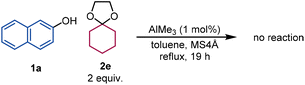 | (2) |
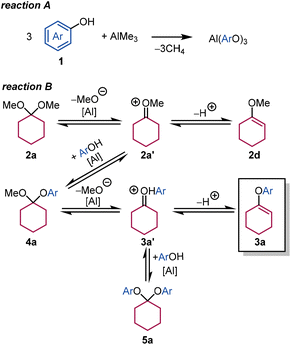
The reaction pathway shown in Scheme 2 is proposed based on our results. Al(OAr)3 is formed from 1 and AlMe3 accompanied by the release of methane (reaction A). AlMe3 is a pre-catalyst and easily transformed into aluminium alkoxide by a reaction with the corresponding alcohol.21–23 The proposed reaction pathway to aryl enol ether 3a is shown in reaction B. Elimination of methanol from 2a provides methyl enol ether 2dvia oxonium intermediate 2a′. Some amount of 2d is protonated to reform 2a′, with the nucleophilic addition of a phenol proceeding to give aryl methyl ketal 4a, which is detectable by GC-MS. 4a eliminates methanol to produce the desired aryl enol ether 3avia intermediate 3a′, and diaryl ketal 5a is obtained by the nucleophilic addition of phenol to 3a′. The reaction equilibrium is driven toward 3a because methanol is eliminated from 2a and 4a by the molecular sieves in the Dean–Stark apparatus.
We next investigated the synthesis of the corresponding diaryl ethers 6, which are useful substances,24–28 from the prepared aryl enol ethers 3 (Table 3). Alkyl enol ethers, which were in situ prepared from aliphatic alcohols and cyclohexanone, were dehydrogenated over Pd/C to give the corresponding aryl alkyl ethers.16,17 However, a method for dehydrogenating aryl enol ethers to the corresponding diaryl ethers was not established. Optimisation details for this dehydrogenation reaction are provided in the ESI.† Aryl enol ethers 3 were transformed under Condition A or B (Table 3), with the former involving Pd/C-catalysed dehydrogenation in the presence of styrene as the hydrogen acceptor. On the other hand, 3 is oxidised by DDQ29 under Condition B.
| Entry | Substrate | Condition: yield (%) | ||
|---|---|---|---|---|
| a Condition A: A suspension of 3 (1.0 mmol), 20% Pd/C (5 mol%), and styrene (2.0 equiv.) in xylene (4 mL) was refluxed for 19 h. Condition B: A suspension of 3 (0.2 mmol) and DDQ (2.0 equiv.) in toluene (2 mL) was refluxed for 17 h. Isolated yields are reported. | ||||
| 1 |

|
3aa | A: 64 | 6aa |
| B: 89 | ||||
| 2 |

|
3ba | A: 35 | 6ba |
| B: 68 | ||||
| 3 |

|
3ea | A: 54 | 6ea |
| B: 85 | ||||
| 4 |

|
3fa | A: 20 | 6fa |
| B: 90 | ||||
| 5 |
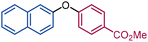
|
3ab | A: 0 | 6ab |
| B: 80 | ||||
While most aryl enol ethers 3 were dehydrogenated under Pd/C catalysis to afford the corresponding diaryl ethers 6aa, 6ba, 6ea, and 6fa in moderate yields (entries 1–4), 3ab did not react (entry 5). In contrast, DDQ-promoted oxidations proceeded efficiently, to afford the product in excellent yields under Condition B (entries 1–5). Hence, the desired diaryl ethers were obtained efficiently under catalytic (A) or equimolar (B) conditions.
One-pot target-molecule synthesis represents an important approach for shortening and simplifying a synthetic route.30–32 Accordingly, we investigated the one-pot synthesis of diaryl ether 6aa (Scheme 3). Aryl enol ether 3aa was first synthesised in xylene, after which Pd/C and 4-tert-butylstyrene were added, and 6aa was obtained in 54% yield through dehydrogenation of the in situ prepared 3aa. Interestingly, the dehydrogenation of 3aa was accompanied by hydrogenation when styrene was used as the hydrogen acceptor; the use of 4-tert-butylstyrene, which has a higher boiling point than styrene, prevented hydrogenation to afford 6aa in a good yield.
In summary, we have developed a method for the synthesis of aryl enol ethers from phenol derivatives and dimethyl ketals with AlMe3 as a pre-catalyst. The reaction proceeds in the absence of a halide or transition metal and involves ketal exchange driven by the elimination of methanol. The obtained aryl enol ethers were also transformed into diaryl ethers via Pd/C-catalysed dehydrogenation or DDQ-promoted oxidation. Moreover, a one-pot diaryl ether synthesis protocol was established involving aryl enol ether synthesis and subsequent Pd/C-catalysed dehydrogenation. Our method provides a promising approach for the efficient syntheses of aryl enols and diaryl ethers that are useful in a wide range of chemistry fields.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was partially supported by the New Energy and Industrial Technology Development Organization (JPNP19004).References
- S. A. Z. Ahmad, T. K. Jena and F. A. Khan, Chem. – Asian J., 2021, 16, 1685–1702 CrossRef CAS PubMed
.
- L. Lempenauer, G. Lemière and E. Duñach, Adv. Synth. Catal., 2019, 361, 5284–5304 CrossRef CAS
.
- Y. Nassar, F. Rodier, V. Ferey and J. Cossy, ACS Catal., 2021, 11, 5736–5761 CrossRef CAS
.
- S. B. J. Kan, K. K.-H. Ng and I. Paterson, Angew. Chem., Int. Ed., 2013, 52, 9097–9108 CrossRef CAS PubMed
.
- J. Matsuo and M. Murakami, Angew. Chem., Int. Ed., 2013, 52, 9109–9118 CrossRef CAS PubMed
.
- S. R. Polimera, A. Ilangovan and M. A. M. Subbaiah, Green Chem., 2023, 25, 2368–2377 RSC
.
- A. Dewanji, L. van Dalsen, J. A. Rossi-Ashton, E. Gasson, G. E. M. Crisenza and D. J. Procter, Nat. Chem., 2023, 15, 43–52 CrossRef CAS PubMed
.
- M. M. Diaz-Requejo, D. DiSalvo and M. Brookhart, J. Am. Chem. Soc., 2003, 125, 2038–2039 CrossRef CAS PubMed
.
- D. L. Boger, W. L. Corbett, T. T. Curran and A. M. Kasper, J. Am. Chem. Soc., 1991, 113, 1713–1729 CrossRef CAS
.
- K. Kojima, M. Sawamoto and T. Higashimura, Macromolecules, 1989, 22, 1552–1557 CrossRef CAS
.
- Z. Wan, C. D. Jones, T. M. Koenig, Y. J. Pu and D. Mitchell, Tetrahedron Lett., 2003, 44, 8257–8259 CrossRef CAS
.
- D. Winternheimer, R. Shade and C. Merlic, Synthesis, 2010, 2497–2511 CAS
.
- F. M. Moghaddam, A. Jarahiyan, M. H. Haris and A. Pourjavadi, Sci. Rep., 2021, 11, 11387 CrossRef CAS PubMed
.
- M. C. Willis, D. Taylora and A. T. Gillmore, Chem. Commun., 2002, 2222–2223 Search PubMed
.
- D. Seebach, Angew. Chem., Int. Ed. Engl., 1988, 27, 1624–1654 CrossRef
.
- M. Sutter, R. Lafon, Y. Raoul, E. Métay and M. Lemaire, Eur. J. Org. Chem., 2013, 5902–5916 CrossRef CAS
.
- I. Y. El-Deeb, M. Tian, T. Funakoshi, R. Matsubara and M. Hayashi, Eur. J. Org. Chem., 2017, 409–413 CrossRef CAS
.
- P. G. Gassman and S. J. Burns, J. Org. Chem., 1988, 53, 5574–5576 CrossRef CAS
.
- P. G. Gassman, S. J. Burns and K. B. Pfister, J. Org. Chem., 1993, 58, 1449–1457 CrossRef CAS
.
- M. Marsi and J. A. Gladysz, Tetrahedron Lett., 1982, 23, 631–634 CrossRef CAS
.
- K. Maruoka, T. Itoh, M. Sakurai, K. Nonoshita and H. Yamamoto, J. Am. Chem. Soc., 1988, 110, 3588–3597 CrossRef CAS
.
- K. Maruoka, S. Nagahara and H. Yamamoto, J. Am. Chem. Soc., 1990, 112, 6115–6117 CrossRef CAS
.
- H. Yamamoto, Tetrahedron, 2007, 63, 8377–8412 CrossRef CAS
.
- J. S. Sawyer, Tetrahedron, 2000, 56, 5045–5065 CrossRef
.
- F. Theil, Angew. Chem., Int. Ed., 1999, 38, 2345–2347 CrossRef CAS PubMed
.
- S. V. Ley and A. W. Thomas, Angew. Chem., Int. Ed., 2003, 42, 5400–5449 CrossRef CAS PubMed
.
- M. Chebaiki, E. Delfourne, R. Tamhaev, S. Danoun, F. Rodriguez, P. Hoffmann, E. Grosjean, F. Goncalves, J. Azéma-Despeyroux, A. Pál, J. Korduláková, N. Preuilh, S. Britton, P. Constant, H. Marrakchi, L. Maveyraud, L. Mourey and C. Lherbet, Eur. L. Chem. Med., 2023, 259, 115646 CrossRef CAS PubMed
.
- H. Bao, Y. Chen and X. Yang, Angew. Chem., Int. Ed., 2023, 62, e202300481 CrossRef CAS PubMed
.
- M. A. Alsharif, Q. A. Raja, N. A. Majeed, R. S. Jassas, A. A. Alsimaree, A. Sadiq, N. Naeem, E. U. Mughal, R. I. Alsantali, Z. Moussa and S. A. Ahmed, RSC Adv., 2021, 11, 29826–29858 RSC
.
- Y. Hayashi, Chem. Sci., 2016, 7, 866–880 RSC
.
- T. Newhouse, P. S. Baran and R. W. Hoffmann, Chem. Soc. Rev., 2009, 38, 3010 RSC
.
- P. T. Anastas and M. M. Kirchhoff, Acc. Chem. Res., 2002, 35, 686–694 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ob01266b |
| This journal is © The Royal Society of Chemistry 2023 |