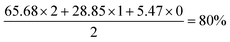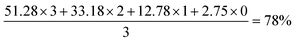Open Access Article
Open Access ArticleDeuterated squalene and sterols from modified Saccharomyces cerevisiae†
Carl
Recsei
 ,
Robert A.
Russell
,
Marina
Cagnes
and
Tamim
Darwish
*
,
Robert A.
Russell
,
Marina
Cagnes
and
Tamim
Darwish
*
Australian Nuclear Science and Technology Organisation, National Deuteration Facility, New Illawarra Rd, Lucas Heights, New South Wales 2234, Australia. E-mail: tde@ansto.gov.au
First published on 31st July 2023
Abstract
Uniformly deuterated sterols and biosynthetically related materials are important for neutron, NMR, tracing and bioanalysis studies as well as critical tools for the creation of improved lipid nanoparticle formulations. The production of sufficient quantities of materials relies not only on the engineering of microorganisms to selectively accumulate desired materials but also methods for the isolation, purification and characterisation of these materials to ensure their usefulness. Uniformly deuterated squalene, the universal precursor to sterols in biological systems, has been produced and characterised. Cholesterol has been produced with controlled levels of uniform deuteration, increased biosynthetic yield and a methodology developed for the extraction and purification of this material without HPLC. Two sterols, not previously produced in deuterated forms, have been prepared with uniform deuteration: 22,23-dihydrobrassicasterol and 24-methylenecholesterol. This report triples the number of sterols that have been produced with uniform deuteration, purified and characterised and provides a silylation/silver ion chromatography protocol for the separation of sterols which differ by the degree of unsaturation. The techniques for the 13C NMR analysis of deuterated sterols, site-specific deuteration levels and an analysis of key biosynthetic steps based on these data are reported.
1. Introduction
The production of deuterated biomolecules with specific levels of isotopic purity is of sustained interest as they enable characterisation of structures and complexes using mass spectrometry, NMR, FT-IR and neutron beam instruments that distinguish between protiated and deuterated analogues.1–5 Furthermore, there is interest in deuterated molecules that display kinetic differences in activity for new pharmaceuticals and functional materials.6–9 Although deuterated fatty acids and compounds derived therefrom are relatively freely available by metal-catalysed H/D exchange chemistry, the same is not true for deuterated sterols and related lipids. Even cholesterol, which is approximately 30% of the composition of cell membranes and is the biosynthetic precursor to other important steroids, as well as an important component in lipid nanoparticle (LNP) technologies including mRNA vaccines, is not available commercially in a uniformly deuterated form. Technologies such as LNPs rely on sterols to modulate the properties of lipid assemblies mimicking membranes, with closely related sterols showing strongly divergent properties with respect to mRNA delivery.10 It is desirable to have a range of deuterated cholesterol analogues for neutron studies to create the most promising LNP formulations, as well as for the elucidation of biosynthetic pathways and for NMR, tracing and bioanalysis studies.Previous reports in which isotopically labelled cholesterol was produced biosynthetically have relied on preparative HPLC to purify a mixture of sterols produced by engineered yeast.11–15 Although materials of satisfactory purity were isolated, dependence on HPLC is deleterious to the production of larger quantities of deuterated sterols.
Herein we report the production and characterisation of uniformly deuterated cholesterol, using a cholesterol-producing strain of Saccharomyces cerevisiae first reported by Riezman and co-workers.16 This bioengineered S. cerevisiae strain RH6829 produces a high proportion of cholesterol as the principal component (96%) of its total sterol production.16 We report the adaption and testing of this strain for the production of deuterium-labelled cholesterol, with NMR analysis that permits the verification of the uniformity of deuteration, the interrogation of site-specific deuteration and a means to examine biosynthetic mechanisms. We furthermore report a near tenfold increase in biosynthetic yield compared to a previous report using strain RH6829.13
The advantage of this approach is its applicability to other sterols. Using a 24-methylated sterol-producing strain (RH6827) from the Riezman laboratory we have additionally produced two other deuterated sterols, 22,23-dihydrobrassicasterol and 24-methylenecholesterol. We also report the production of deuterated squalene using S. cerevisiae strain Y2805 obtained from Choi and co-workers.17 The biosynthetic production of deuterated squalene was explored first to probe the uniformity and extent of deuterium labelling in the parent molecule of all naturally derived sterols.
2. Discussion
2.1 The reaction medium for the production of deuterated squalene by biosynthesis
A biosynthetic reaction involving only deuterated compounds in a deuterium oxide medium will produce fully deuterated biomolecules. The use of a culture with intermediate levels of isotopic purity will result in intermediate deuteration levels in the biosynthetic products when an exchange of hydrogen atoms occurs between the carbon source or biosynthetic intermediates and the medium. Not all hydrogen atoms in the medium are equally likely to be incorporated in deuterated biomolecules, since the construction of biomolecules such as squalene requires a carbon source, generally a sugar. Where the hydrogen atoms attached to the carbon atoms of the sugar are unaffected by metabolism into squalene, the hydrogen isotopic labelling of the sugar will be transferred to the biosynthetic intermediates of squalene (notably, mevalonate and dimethylallyl/isopentenyl pyrophosphate) and then incorporated into the squalene itself.18In practice, the biosynthesis of squalene via the mevalonate pathway occurs such that each carbon atom of the sugar feedstock is transformed into acidic intermediates (such as acetylcoenzyme A, acetylacetylcoenzyme A and pyruvate) before being assembled into the building blocks of squalene. This gives an opportunity for exchange of hydrogen ions with the solvent, potentially catalysed within the organism, and therefore an opportunity for deuterium incorporation if a deuterated medium is used. NADPH is employed as a reductant in multiple steps of the biosynthesis of sterols.18 The reducing proton in NADPH also arises from glucose but is also known to be exchanged with the solvent by the catalytic activity of flavin-dependent dehydrogenases.19–23
It is therefore practical to use deuterium oxide as a medium alongside protiated carbon sources as feedstocks and achieve above 80% levels of deuterium incorporation in biomolecules in a Saccharomyces-based biosynthesis of squalene and its sterol metabolites. Since deuterated sugars are several orders of magnitude more expensive than deuterium oxide per mole of deuterium atoms, this observation is key to the efficient biosynthetic production of deuterated squalene, deuterated cholesterol and related biomolecules and abrogates the disadvantageous preference of Saccharomyces for carbon sources that are expensive in deuterated forms (i.e. glucose) compared to Pichia, which grows robustly on glycerol.24 If the sterol is required at very high levels of deuteration, Pichia would likely provide a more economical platform due to the lower cost of the deuterated carbon source.
The use of Pichia in biosynthesis of lipids is generally beneficial in terms of yield and ease of culture.11 Although this work is centred around the use of Saccharomyces, this was largely a reflection of the strains available to us, and a unique property of RH6829, namely its aforementioned ability to produce cholesterol at 96% of total sterol production. For ergosterol, the principal native fungal sterol, production of a deuterated material in Pichia and its use in neutron studies have been reported, including a methodology for the isolation of the material in high purity by precipitation.25,26
2.2 Biosynthetic preparation of deuterated squalene
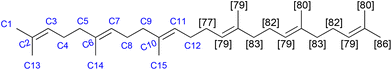
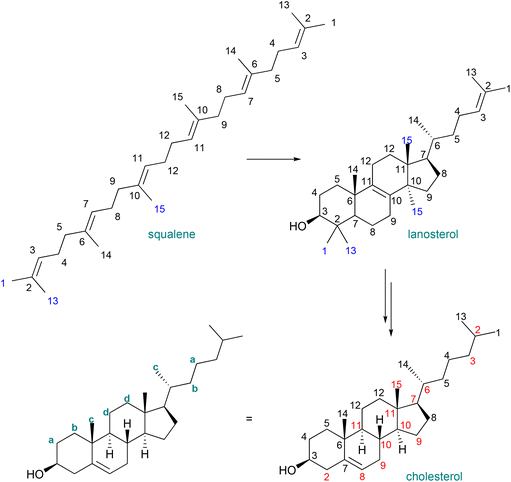
The biosynthesis of squalene begins with the production of dimethallyl/isopentenyl pyrophosphate, which is employed as the modular building block in the biosynthesis of farnesyl pyrophosphate, which is dimerised to form squalene.18Fig. 1 shows the C2-symmetric structure with the modular pattern of the molecule (left, with atom numbering). Squalene, like many similar biomolecules, is amenable not only for mass spectrometric measurements of overall deuteration, but also site-specific measurements of deuteration levels. Deuterated squalene was produced with protiated glucose in highly enriched deuterium oxide (99.8%-d), using the method of Choi and co-workers and their strain Y2805.17Fig. 1 shows the squalene numbering scheme (left) and the site-specific deuteration levels of the resulting material (right), measured by the integration of the 13C{1H,2H} NMR spectrum and with reference to the known extent of chemical shift perturbation arising from deuteration as well as known NMR properties of the protiated material.27–32
The overall deuteration level as measured by the integration of the 13C{1H,2H} NMR spectrum is 81% (standard error of the mean (SEM): 1%), corresponding to the value obtained by mass spectrometry (81 ± 2%). This level of deuteration is sufficient for neutron diffraction and reflectometry studies. The material was isolated in >98% purity (GC). To the best of our knowledge, this is the first report of the production of uniformly deuterated squalene.
Having established that the biosynthesis of squalene in a deuterium oxide produced a relatively uniform level of deuteration throughout the molecule and that this material could furthermore be isolated in pure form from the deuterium oxide culture with a biosynthetic yield of 800 mg L−1, we turned our attention to the production of deuterated sterols.
2.3 Biosynthetic preparation of deuterated cholesterol
We have found that with the use of deuterium oxide of high isotopic purity (99.8%-d) in conjunction with a protiated carbon source – glucose –cholesterol of 87–90% deuteration can reliably be obtained by growing strain RH6829 in highly enriched deuterium oxide. We additionally produced cholesterol of 79–81% isotopic purity, suitable for neutron applications, by recycling the deuterium oxide in the culture medium (by distillation from activated charcoal).33The isotopic purity of the recycled deuterium oxide is effectively depleted by the addition of further protiated glucose during the culture. This effect could potentially render the produced cholesterol unsuitable for neutron experiments by giving rise to an excessively broad spectrum of isotopologues with varying degrees of deuteration, as the isotopic purity of water is effectively depleted by addition of further protiated glucose, inducing a time-dependent variance in the mass distribution of the product. In the case of a hypothetical culture containing 12.5 g of glucose-h12 in 99.8%-d deuterium oxide the initial mole fraction of deuterium is 99.1% of the total hydrogen atoms. If a complete redistribution of hydrogen atoms in the system occurs during the metabolism of the glucose-h12 then another 12.5 g portion of glucose-h12 is added and the mole fraction is reduced to 98.3%, and then to 97.6% upon a third feeding. In practice the incomplete exchange of protium atoms with the deuterium atoms of the medium that is invariably observed means that the true decrease in the mole fraction of deuterium upon additional feeding events is lower than this theoretical maximum. A feeding study was performed in which one litre of a stationary phase 3.5 L culture was decanted after 5 days and the cholesterol was isolated and purified, while adding glucose-h12 to reinstate the initial concentration supplied to the culture. The partial decantation and extraction were repeated twice. The shape of the cholesterol mass distribution over three successive harvests did not change appreciably, nor did the deuteration level of the isolated sample (measuring 90%-d after one feeding, 90%-d after two feedings and 89%-d after three feedings). This result suggests the possibility of further yield increase in a multiple-feeding protocol.
Preparative HPLC, a technique widespread in biochemical laboratories, has been used to isolate isotopically-labelled cholesterol from a biosynthetically-derived mixture of sterols.11,14 We have found that strain RH6829 growing in deuterium oxide produces deuterated cholesterol which does not require HPLC separation from other sterols, and which may be purified by conventional flash column chromatography, such that no extraneous sterols were detected by NMR, nor by GCMS analysis of the trimethylsilyl derivative.11
The cholesterol was liberated from the RH6829 cells via base-mediated cell lysis – which destroys the cell membranes and cleaves cholesterol esters – following growth in a deuterated medium in a procedure similar to that in the original report.13 Heating of the mixture to reflux for several hours is also required for maximum recovery of cholesterol.‡ It was found that strict isolation of the reaction mixture from air during base-mediated cell lysis, with sparging of nitrogen, is advantageous for the production of pure cholesterol. Addition of a small amount of pyrogallol to the lysis medium following the sparging provides further assurance that any remaining dissolved oxygen is scavenged, including oxygen liberated from the lysed cells. The absence of oxygen reduces the loss of cholesterol by oxidation and formation of by-products which would otherwise occur under these harsh conditions. Although cholesterol may be obtained by less stringent measures to exclude air, we expect that this method will permit recovery of other, more oxidation-sensitive sterols. The dual benefits of improved cholesterol yield and the large reduction in closely eluting by-products greatly simplify the purification of the crude material. The extraction is made somewhat difficult by the slight solubility of cholesterol in methanol and the presence of other cell components in the lysis mixture which tend to promote emulsion formation with the organic solvent. The efficiency of extracting cholesterol from the reaction medium was improved in comparison with neat hexane by the addition of 10% v/v toluene to the extraction solvent. Deuterated cholesterol was isolated by flash column chromatography in >300 mg quantities from a single culture, with a biosynthetic yield up to 98 mg L−1.
A lower glucose concentration may help reduce the shock on the organism as it moves through the growth phase. In addition, a reduction in the glucose concentration reduces the amount of protium in the media. This is a consideration when a reasonably high deuteration level of the cholesterol product is desired. Thus, our preferred concentration is 12.5–20 mg L−1 of glucose, leading to typical yields of approximately 65 mg L−1 of deuterated cholesterol, which are up to 100 mg L−1 with higher feeding protocols.
A small amount of antibiotic may be employed in the culture to prevent the growth of bacteria. Our experience has shown that the greatest risk of bacterial contamination occurs in bioreactors where the run time is longer due to the slower growth (and extended culture time) on deuterium oxide compared to that with H2O. Addition of 20 mg L−1 of kanamycin and 20 mg L−1 of ampicillin was found to suppress the growth of bacteria in a bioreactor culture of 4 days’ duration. A shake flask culture containing antibiotics showed only a slight reduction in yeast growth (less than 10% lower optical density) compared to that of a control flask, over 3 days of incubation. Therefore, we believe that the yield is not seriously impacted by antibiotic use.
The resulting material, when the biosynthesis was conducted in recycled deuterium oxide, was found by mass spectrometry to have a deuteration level of 79 ± 2%. The uniformity of the labelling was probed by NMR.
2.4 Analysis of biosynthetically derived, deuterated cholesterol
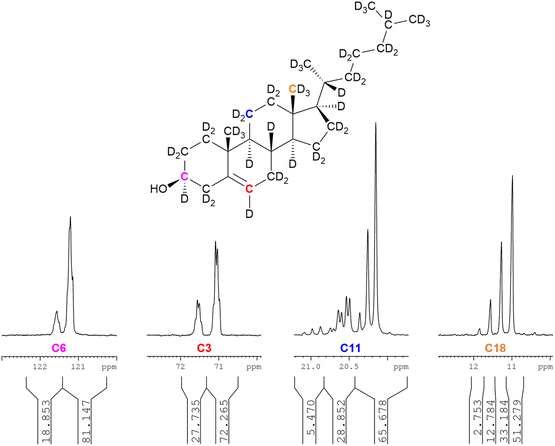
As for squalene, it is possible to calculate site-specific deuteration levels by the integration of the 13C{1H,2H} NMR spectrum, particularly the characteristic upfield shift induced by the replacement of a proton by a deuteron with diminishing magnitude at a greater distance from the relevant carbon atom.27,28,30,31 Consideration is necessary for adequate dwell times between NMR scans; it was found that a SEM of 1% when normalising integrals obtained by automatic integration of the 13C{1H,2H} NMR spectrum of cholesterol-d45 (79%-d) did not improve upon doubling of the dwell time (d1) from 30 seconds to 60 seconds. Conversely, squalene-d50 (81%-d) required d1 = 300 seconds for an SEM of 1% for normalised integrals of the 13C{1H,2H} NMR spectrum.Fig. 2 shows (left to right) the partially deuterated signals for C6 (vinyl), C3 (secondary, hydroxyl-bearing carbon), C11 (ring methylene) and C18 (methyl).
An analysis of the four signals in Fig. 2 suffices to demonstrate the practicality of 13C{1H,2H} NMR as an analytic tool in determining site-specific deuteration of complex biomolecules. The leftmost selected spectrum region is C6. The two broad peaks represent (left to right) CH and CD, with the integration of these signals indicating 81%-d for a vinylic methine with two vicinal hydrogen atoms.
The second to left selected spectrum region is the hydroxyl-bearing C3 – showing 72%-d for CD with four vicinal hydrogen atoms. The CD and CH peaks are split due to variable isotopic substitution at the vicinal positions.
The second to right selected spectrum region is C11, showing a pattern for CD2 with three vicinal hydrogens. Three sets of peaks are evident. The upfield set of three peaks represent CD2, with, moving downfield, three, two and one neighbouring deuterium atoms. The CD2 peak with three neighbouring protons is insufficiently intense to be observed. The middle set of peaks represents CHD, with two possible scenarios of one axial and one equatorial deuteron, a pattern known to induce different perturbations in the 13C chemical shift.30,31 The most downfield set of three peaks is CH2. The deuteration level is therefore given by:
Application of this method to the three upfield, CD2 peaks indicates a deuteration level of 84% for the group of three hydrogens vicinal to the C11 methylene (i.e. the two hydrogen atoms on C9 and one on C12), although this does not account for the contribution of the smallest peak (representing CD2 with three vicinal protons), which is coincident with the neighbouring signal and very low in relative intensity. It is assumed that this peak is, at most, at half the intensity of the second-smallest peak with two vicinal protons and at least a tenth of its intensity gives a deuteration level for the three vicinal hydrogens of 80–84%-d.
The rightmost selected spectrum region is C18, with 78%-d for CD3 with no vicinal hydrogens. Four peaks represent (from upfield to downfield) CD3, CHD2, CH2D and CH3. The deuteration level is therefore given by:
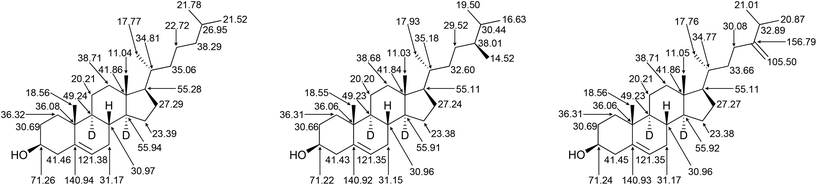
These selected measurements of site-specific deuterated cholesterol produced with recycled deuterium oxide and a protiated carbon source show a low degree of variation, congruent with the analysis of biosynthetically produced, deuterated squalene. Since squalene is the biosynthetic precursor to sterols, inspection of the biosynthetic pathway from squalene to cholesterol allows for the mapping of positions unaffected by intermediate transformations (Fig. 3), and thus determination of site specific deuteration at positions that may not be directly measurable due to signal overlap.34 A previous report identified the possibility of atom mapping for the biosynthesis of deuterated cholesterol, but, for the carbon atoms shown in Fig. 3, that report suggested that only C18 could possess a significant level of protiation.12
Recalling that the deuteration levels of C11 (d, Fig. 3, leftmost carbon of the pair) could be measured directly from the carbon NMR spectrum, C12, the rightmost carbon of the pair, should also have a deuteration level of 80%. This value is congruent with the estimate for the deuteration level of the three hydrogen atoms vicinal to C11 obtained by recursive application of the integral method of determining deuteration from the 13C{1H,2H} NMR spectrum (80–84%). This measurement implies that the remaining methine hydrogen at C9 is deuterated at a level of 80–89%. Indeed, this carbon atom gives a signal at δC = 49.2 without the overlap of other signals, from which a site-specific deuteration level of 87% is measured. Fig. 4 shows selected deuteration levels obtained from the 13C{1H,2H} NMR spectrum, with additional consideration of paired carbons arising from the biosynthesis of cholesterol.
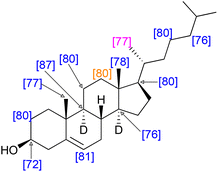 | ||
| Fig. 4 Selected, measured and calculated deuteration levels of cholesterol with an overall deuteration of 79%. Blue: deuteration measured directly by the integration of the 13C{1H,2H} NMR spectrum. Orange: deuteration measured directly by the integration of the 13C{1H,2H} NMR spectrum and also calculated from the neighbouring carbon atom. Purple: deuteration assigned with reference to the biosynthetic map in Fig. 3. Note that deuteration was in some cases calculated from partially overlapping spectra where it was possible to infer the value of the integral of, for example, CH where the value of the integral CD was measured and the integrals assumed to sum to unity. Note also that it was possible to measure both integrals of the biosynthetic pair for C3 and C24 as well as C11 and C12 (Fig. 3). In both cases the equal integrals accorded with the biosynthetic mapping. Deuterium atoms omitted for clarity except when used for stereochemical designation. | ||
The use of site-specific NMR calculations and atom-mapping from the biosynthesis of cholesterol shows that the relatively even deuteration levels seen in squalene are observed also in its biosynthetic daughter molecule, cholesterol. The positions indicated in Fig. 4 have a mean deuteration of 79% (SEM: 1%), indicative of the overall 79% deuteration level.
We have also produced cholesterol-d45 (98%-d) where glucose-d12 was employed. The biosynthetic yield (15 mg L−1) in this case is determined by the amount of labelled sugar made available rather than any attempt to optimise the production by attempting to favour continued growth of the bioreactor culture, for instance by repeated feeding. The NMR analysis of residual protiation of cholesterol-d45 (98%-d) was judged unlikely to be reliably informative as it would rely on the integration of very small (2%-h) peaks in the 13C{1H,2H} NMR spectrum for which a low signal to noise ratio prevents accurate determination of deuteration levels.
2.5 Biosynthetic preparation of deuterated 22,23-dihydrobrassicasterol and 24-methylenecholesterol
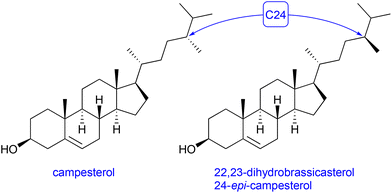
Another S. cerevisiae strain – RH6827 – was reported to overexpress a 24-methylated sterol, campesterol.16 Analysis of the 1H NMR of the crude, lysed extract of shake-flask cultures of strain RH6827 appeared initially to show co-production of campesterol and variable quantities of 24-methylenecholesterol. The close biosynthetic relationship between these compounds has been extensively described.35 Indeed, some commercial standards of campesterol contain a considerable fraction of 24-methylenecholesterol. Furthermore, the engineering of Saccharomyces cerevisiae to produce 24-methylenecholesterol has been reported, in which it is co-produced with campesterol.36Closer inspection of the 13C NMR spectra of the sterol fraction of a protiated RH6827 culture and the use of the definitive spectroscopic data collated by McCarthy and co-workers in their synthesis of both protiated isomers suggested that the major product of RH6827 in our hands was not campesterol but rather 24-epi-campesterol (22,23-dihydrobrassicasterol, Fig. 5).37 We anticipated that the isolation of a pure sample of the 24-methylated sterol would be necessary to definitively identify which isomer had been produced.
Regardless of which isomer was present, the mixture of sterols produced by RH6827 would be of limited use in elucidating, for instance, the lipid-bilayer modulating properties of one of the produced sterols or for NMR studies. The purification of this mixture was therefore required. The reported methods may allow for selective oxidation of 24-methylenecholesterol to permit its removal as a more polar impurity.38,39 These methods would also eliminate the possibility of isolating this potentially useful material and would be restricted to the removal of this particular material, with its sterically unencumbered methylene apt to react preferentially with the trisubstituted Δ5 alkene. Thus, we sought a more generally applicable, preparative separation of sterols bearing degrees of unsaturation surplus to the main sterol being sought biosynthetically.
Since the only difference between 24-methylenecholesterol and 22,23-dihydrobrassicasterol or campesterol is a single methylene versus a methyl group at C24 and remote from the C3 hydroxyl moiety (see: Fig. 5) it was evident that a plausible chromatographic separation mechanism would need to suppress the binding of the alcohol to a stationary phase while allowing for separation based on the affinity of the stationary phase for the C24 unsaturated methylene moiety versus the saturated C24 methyl. For this purpose, silver-ion chromatography was employed after having protected the C3 alcohols of the mixture of deuterated sterols as the tert-butyldimethylsilyl ether using tert-butyldimethylsilyl chloride and the standard imidazole catalyst. The reaction was found to be sluggish in dichloromethane and thus DMF was employed as the solvent (requiring 40 hours reaction time at 30 °C).
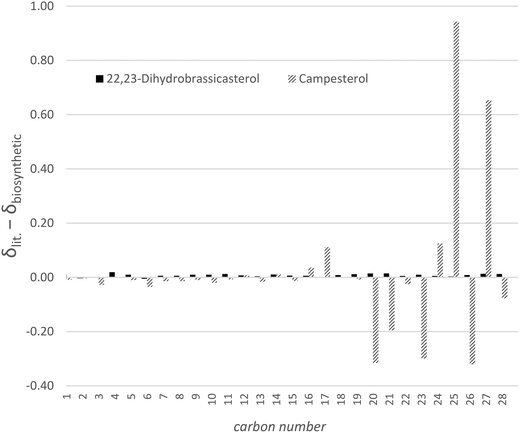
Silver-ion chromatography employs the affinity of bound silver ions for alkene moieties to separate molecules based on the degree of unsaturation. In the case of 24-methylenecholesterol and 22,23-dihydrobrassicasterol/campesterol the Δ5 unsaturation is presumably barely accessible to the solid phase for steric reasons and O-tert-butyldimethylsilyl-22,23-dihydrobrassicasterol displayed no greater affinity for the argentated silica than for conventional chromatographic silica, whereas O-tert-butyldimethylsilyl-24-methylenecholesterol was retained and required polar solvents to induce elution. We expect that this technique could be applied to separate sterols with varying positions and degrees of tail unsaturation. Once separation had been accomplished on the protiated sterols produced by RH6827 in a shake flask culture it became apparent that the material that had been produced was not campesterol but indeed 22,23-dihydrobrassicasterol. Fig. 6 shows the difference between the chemical shifts of the protiated material produced by us and the reported chemical shifts for the two 24-methylated sterols. This graph confirms the deviation from expected chemical shifts to be centred around the part of the sterol tail remote from the tetracyclic core where the stereocentre of interest lies and the identity of the major product of RH6827 as 22,23-dihydrobrassicasterol.
From a 115 mg mixed sterol fraction containing principally deuterated 22,23-dihydrobrassicasterol and 24-methylenecholesterol along with minor impurities, 61 mg of 22,23-dihydrobrassicasterol-d47 (87%-d) and 12 mg of 24-methylenecholesterol-d45 (87%-d) were isolated after silylation, separation and desilylation – an overall recovery of >60% of these two materials present in the original mixture. Desilylation was accomplished according to the method of Guerrero and co-workers, using wetted alumina.40
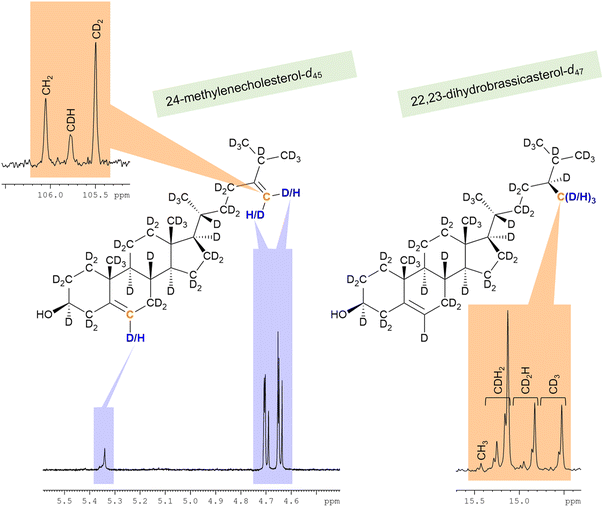
The C28 positions of deuterated 22,23-dihydrobrassicasterol and 24-methylenecholesterol from strain RH6827 were found to bear anomalously low levels of deuteration. The anomalously low level of deuteration at the C28 position of 24-methylenecholesterol was most dramatically revealed in the 1H NMR spectrum (Fig. 7), where the residual proton methylene signals at 4.6–4.7 ppm appeared much larger than other vinylic signals present. Indeed, an NMR spectrum of the crude sterol extract of strain RH6827 could mislead if this methylene signal is taken as indicative of the relative concentration of this sterol in the mixture produced by growing this strain in a deuterium-rich medium with a protiated carbon source. The methylation of C24 that produces 22,23-dihydrobrassicasterol and 24-methylenecholesterol presumably occurs from S-adenosylmethionine (SAM). This pathway likely has reduced capacity for exchange of hydrogen atoms with the medium with respect to the mevalonate pathway, leading to lower deuteration levels.41
The carbon NMR spectra were also indicative of low levels of deuteration (Fig. 7). In the 13C{1H,2H} and 13C{1H} spectra of 24-methylenecholesterol-d45 (87%-d), a strong peak for CH2 is present at 106.1 ppm. The 13C{1H,2H} and 13C{1H} spectra of 22,23-dihydrobrassicasterol-d47 (87%-d) showed an approximate 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of CDH2, CD2H, and CD3 signals observed in a set of signals between 14.5 and 15.5 ppm. The CH3 signal was present at a very low intensity of <1%. These observations show that the reduction of the 24-methylene unit in RH6827 is consistent with hydride delivery to C24 with C28 acquiring a hydrogen atom from the deuterium oxide solvent (a one-step process). These observations are also consistent with isomerisation to the Δ24(25) analogue 24-methyldesmosterol and subsequent reduction (a two-step process) if full solvent exchange of the hydridic hydrogen atom in the FADH2 cofactor associated with enzymatic 24-methylene reduction occurs, in contrast to partial exchange for the NADPH cofactor associated with the earlier stages of the biosynthesis of the sterol framework.18,21 Since even among plants both the one-step and two-step mechanisms have been shown to operate depending on the species, the near complete delivery of deuterium over protium to C28 upon reduction in a deuterated medium is consistent with known biosynthetic pathways.21,35,42 The synthesis of 22,23-dihydrobrassicasterol by enzymatic reduction of the 24-methylenecholesterol-d45 (87%-d) produced in this work in a fully protiated system would allow the resolution of this question for specific enzyme origins as the 13C{1H,2H} NMR spectrum of the 22,23-dihydrobrassicasterol product would show retention or loss of deuteration at C25, depending on the pathway.
1 mixture of CDH2, CD2H, and CD3 signals observed in a set of signals between 14.5 and 15.5 ppm. The CH3 signal was present at a very low intensity of <1%. These observations show that the reduction of the 24-methylene unit in RH6827 is consistent with hydride delivery to C24 with C28 acquiring a hydrogen atom from the deuterium oxide solvent (a one-step process). These observations are also consistent with isomerisation to the Δ24(25) analogue 24-methyldesmosterol and subsequent reduction (a two-step process) if full solvent exchange of the hydridic hydrogen atom in the FADH2 cofactor associated with enzymatic 24-methylene reduction occurs, in contrast to partial exchange for the NADPH cofactor associated with the earlier stages of the biosynthesis of the sterol framework.18,21 Since even among plants both the one-step and two-step mechanisms have been shown to operate depending on the species, the near complete delivery of deuterium over protium to C28 upon reduction in a deuterated medium is consistent with known biosynthetic pathways.21,35,42 The synthesis of 22,23-dihydrobrassicasterol by enzymatic reduction of the 24-methylenecholesterol-d45 (87%-d) produced in this work in a fully protiated system would allow the resolution of this question for specific enzyme origins as the 13C{1H,2H} NMR spectrum of the 22,23-dihydrobrassicasterol product would show retention or loss of deuteration at C25, depending on the pathway.
2.6 The production of the unexpected epimer
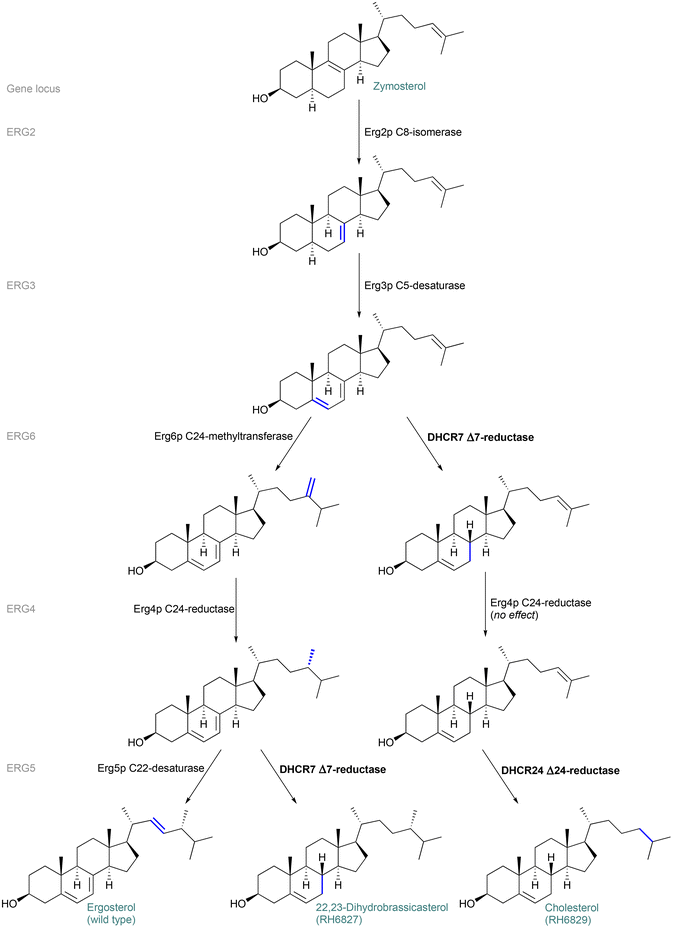
Although we had been able to identify the major biosynthetic product of the strain RH6827 as the 24-epimer of the originally reported major product – campesterol – we found that reliable distinguishing of these two materials is hampered by some available information. Examples include database entries in which these diastereomeric materials are considered synonyms43 and the fact that when prompted to draw ‘campesterol’, the chemical drawing software ChemDraw (20.0.0.41) provides its 24-epimer as the drawn product.The above considerations led us to map the enzymatic steps and sterol intermediates occurring in the yeast strains RH6827 and RH6829. We followed the stereochemistry through these routes to understand how the final sterol structures are achieved and to confirm their identities to ensure that our conclusion about which sterol is produced by RH6827 is consistent with the known genetic architecture of the strain. RH6827 and RH6829 were created through gene disruption of a wild-type (ergosterol-producing) strain. Fig. 8 shows the biosynthesis of the sterols (starting from the intermediate zymosterol), and the genes (and encoded enzymes) involved in each position-specific reaction. The enzymes Erg2p to Erg6p can act on a range of similar sterol substrates.44 There is no particular order to the steps along the natural pathway (apart from the installation of the 24-methylene group by Erg6p occurring before reduction by Erg4p). The order shown is to demonstrate the divergence of the sterol products between strains. The non-native sterols 22,23-dihydrobrassicasterol and cholesterol result from mutations of specific genes as described by Riezman and co-workers.16 Specifically, 22,23-dihydrobrassicasterol synthesis by RH6827 is analogous to ergosterol biosynthesis apart from a change on the ERG5 locus resulting in the occurrence of DHCR7 Δ7-reductase instead of Erg5p C22-desaturase. This excludes the installation of the double bond at C22–23 while effecting reduction of the C7–8 double bond. Production of cholesterol results from changes on the ERG6 and ERG5 loci which replace Erg6p C24-methyltransferase and Erg5p C22-desaturase with DHCR7 and DHCR24 reductases, respectively.
The suggested origin of the sense of symmetry at the 24-position in the major product of RH6827 concurred with the opinion of the lead author in the work describing the creation of the strain (personal communication).45
2.7 Further techniques for 13C NMR analysis of deuterated sterols
The observation of lower deuteration levels associated with C28 in 22,23-dihydrobrassicasterol and 24-methylenecholesterol is indicative of the ambiguity that may arise during the production of sterols of intermediate (i.e. 80–90%-d) deuteration levels for neutron studies. Since intermediate deuteration induces shifts in retention times in GCMS, turns single peaks in mass spectrometry into distributions and results in complex and/or broad 1H and 2H NMR spectra it is necessary to complete 13C NMR assignments to be certain of the identity of biosynthetically derived, deuterated sterols, a postulate that closely mirrors the conclusion of McCarthy and co-workers in their synthesis of 22,23-dihydrobrassicasterol and campesterol.37 It would be impossible to distinguish the relative quantities of 22,23-dihydrobrassicasterol and 24-methylenecholesterol at 80% deuteration by GCMS, for instance. These compounds generally co-elute on GCMS (the reason they are found mixed in commercial campesterol standards) and their mass distributions would overlap at intermediate deuteration levels.The complex spectra typical of moderately deuterated materials can often be assigned with the aid of the observation that deuteration typically induces a characteristic upfield 13C chemical shift commensurate with stronger shielding by the deuteron with respect to the proton.31Fig. 9 shows that sp3-hybridised carbon atoms in cholesterol-d45 (98%-d) show a typical shift when perdeuterated of approximately −1 ppm in the 13C NMR spectrum. The partially deuterated signals lie between the perdeuterated and perprotiated shifts, often at highly regular intervals (Fig. 9). The effect of deuteration is, as expected, less profound on quaternary carbon atoms not bearing hydrogen. This analysis also allows deuterated 22,23-dihydrobrassicasterol and campesterol to be differentiated, as the apparent change in the chemical shift for the C24 methine upon deuteration would appear to be an anomalously large −2 ppm from the literature C24 chemical shift of protiated campesterol.
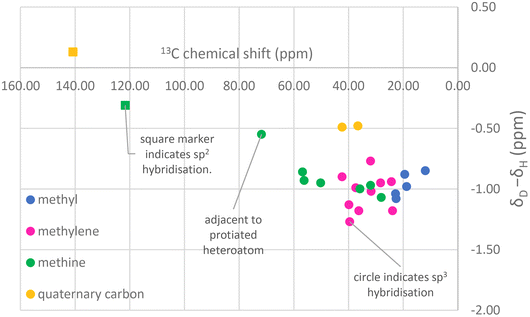 | ||
| Fig. 9 Characteristic chemical shift changes that occur in cholesterol upon deuteration (using data for 98%-d cholesterol). | ||
Using the characteristic chemical shift changes that occur upon deuteration, with the 13C NMR spectrum of cholesterol-d45 (98%-d) as the reference, the chemical shift changes associated with O-silylation and the pattern of partially deuterated signals in the 13C{1H,2H} NMR and 13C NMR spectra of the deuterated sterols produced in this work were assigned (Fig. 10).
The NMR assignments in Fig. 10 have not previously been reported, with only partially deuterated sterols being previously characterised completely in the literature. Although the NMR methods described herein (particularly the collection of 13C{1H,2H} NMR) are emerging techniques in biochemical laboratories, the inability to differentiate closely related deuterated sterols by conventional methods may necessitate their use. Cultures performed in protiated media and analysed by NMR, particularly 13C{1H} NMR, alongside methods such as GCMS, are likely to be the best means for unambiguous determination of the identity of biosynthetic sterols in such laboratories.
3. Conclusion
Production of deuterated squalene, the universal parent molecule in the biosynthesis of sterols, using strain Y2805 provided by Dr Eui-Sung Choi is reported, along with the finding of a highly uniform pattern of deuteration. We have also shown that the use of S. cerevisiae strains from the Riezman laboratory that selectively accumulate sterols are a practical biosynthetic tool for the synthesis of sterols at defined, uniformly distributed deuteration levels of 80–98% at 100 milligram scales. The identity of the sterols was confirmed by 13C NMR and allowed us to postulate a biosynthetic mechanism for the production of 22,23-dihydrobrassicasterol by the yeast strain RH6827.NMR data suitable for verifying the identity and purity of biosynthetically produced deuterated sterols have been reported. These data showed an even distribution of deuterium atoms within the sterol framework but lower deuteration upon further methylation, with the C28 deuteration patterns evident in 24-methylenecholesterol-d45 (87%-d) and 22,23-dihydrobrassicasterol-d47 (87%-d) found to be consistent with the known biosynthetic relationship between these compounds.
We anticipate that this work will facilitate further characterisation studies by making two new sterols, 22,23-dihydrobrassicasterol and 24-methylenecholesterol, available in uniformly deuterated forms and by providing tools to isolate and characterise further useful sterols from existing and emerging strains of unicellular organisms engineered to produce them selectively.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The National Deuteration Facility is partly supported by the National Collaborative Research Infrastructure Strategy – an initiative of the Australian Government. The authors gratefully acknowledge the provision of S. cerevisiae strains Y2805 by Dr Eui-Sung Choi and RH6827 and RH6829 by Emeritus Professor Howard Riezman.References
- A. Okuda, R. Inoue, K. Morishima, T. Saio, Y. Yunoki, M. Yagi-Utsumi, H. Yagi, M. Shimizu, N. Sato, R. Urade, K. Kato and M. Sugiyama, Biophys. Physicobiol., 2021, 18, 16–27 CrossRef CAS PubMed.
- R. A. Russell, T. A. Darwish, L. Puskar, D. E. Martin, P. J. Holden and L. J. R. Foster, Biomacromolecules, 2014, 15, 644–649 CrossRef CAS PubMed.
- A. Duff, M. Cagnes, T. Darwish, A. Krause-Heuer, M. Moir, C. Recsei, A. Rekas, R. Russell, K. Wilde and N. Yepuri, in Methods in Enzymology, ed. J. A. Tainer, Academic Press, 2022, vol. 677, pp. 85–126 Search PubMed.
- S. Nebl, W. Alwan, M. Williams, G. Sharma, A. Taylor, B. Doak, K. Wilde, R. McMahon, M. Halili, B. Capuano, R. Fenwick, B. Mohanty and M. Scanlon, J. Biomol. NMR, 2020, 74, 595–611 CrossRef CAS PubMed.
- M. Haertlein, M. Moulin, J. M. Devos, V. Laux, O. Dunne and V. Trevor Forsyth, in Methods in Enzymology, ed. Z. Kelman, Academic Press, 2016, vol. 566, pp. 113–157 Search PubMed.
- S. L. Harbeson and R. D. Tung, in Annual Reports in Medicinal Chemistry, ed. J. E. Macor, Academic Press, 2011, vol. 46, pp. 403–417 Search PubMed.
- A. Danos, R. MacQueen, Y. Y. Cheng, M. Dvořák, T. Darwish, D. McCamey and T. Schmidt, J. Phys. Chem. Lett., 2015, 6, 3061–3066 CrossRef CAS PubMed.
- C. C. Tong and K. C. Hwang, J. Phys. Chem. C, 2007, 111, 3490–3494 CrossRef CAS.
- J. H. Z. Lee, M. N. Podgorski, M. Moir, A. R. Gee and S. G. Bell, Chem. – Eur. J., 2022, 28, e202201366 CAS.
- S. Patel, N. Ashwanikumar, E. Robinson, Y. Xia, C. Mihai, J. P. Griffith, S. Hou, A. A. Esposito, T. Ketova, K. Welsher, J. L. Joyal, Ö. Almarsson and G. Sahay, Nat. Commun., 2020, 11, 983 CrossRef CAS PubMed.
- M. Moulin, G. A. Strohmeier, M. Hirz, K. C. Thompson, A. R. Rennie, R. A. Campbell, H. Pichler, S. Maric, V. T. Forsyth and M. Haertlein, Chem. Phys. Lipids, 2018, 212, 80–87 CrossRef CAS PubMed.
- S. Waldie, M. Moulin, L. Porcar, H. Pichler, G. A. Strohmeier, M. Skoda, V. T. Forsyth, M. Haertlein, S. Maric and M. Cárdenas, Sci. Rep., 2019, 9, 5118 CrossRef PubMed.
- A. A. García, S. G. Pfisterer, H. Riezman, E. Ikonen and E. O. Potma, J. Biomed. Opt., 2015, 21, 061003 CrossRef PubMed.
- R. Shivapurkar, C. M. Souza, D. Jeannerat and H. Riezman, J. Lipid Res., 2011, 52, 1062–1065 CrossRef CAS PubMed.
- C. G. Borcik, I. R. Eason, B. Vanderloop and B. J. Wylie, ACS Omega, 2022, 7, 17151–17160 CrossRef CAS PubMed.
- C. M. Souza, T. M. E. Schwabe, H. Pichler, B. Ploier, E. Leitner, X. L. Guan, M. R. Wenk, I. Riezman and H. Riezman, Metab. Eng., 2011, 13, 555–569 CrossRef CAS PubMed.
- J. Y. Han, S. H. Seo, J. M. Song, H. Lee and E.-S. Choi, J. Ind. Microbiol. Biotechnol., 2018, 45, 239–251 CrossRef CAS PubMed.
- W. D. Nes, Chem. Rev., 2011, 111, 6423–6451 CrossRef CAS PubMed.
- C. A. Lewis, S. J. Parker, B. P. Fiske, D. McCloskey, D. Y. Gui, C. R. Green, N. I. Vokes, A. M. Feist, M. G. Vander Heiden and C. M. Metallo, Mol. Cell, 2014, 55, 253–263 CrossRef CAS PubMed.
- J. S. Rowbotham, M. A. Ramirez, O. Lenz, H. A. Reeve and K. A. Vincent, Nat. Commun., 2020, 11, 1454 CrossRef CAS PubMed.
- E. N. Smith, J. S. O. McCullagh, R. G. Ratcliffe and N. J. Kruger, Metabolites, 2019, 9(10), 205 CrossRef CAS PubMed.
- A. Yahashiri, A. Sen and A. Kohen, J. Labelled Compd. Radiopharm., 2009, 52, 463–466 CrossRef CAS PubMed.
- Z. Zhang, L. Chen, L. Liu, X. Su and J. D. Rabinowitz, J. Am. Chem. Soc., 2017, 139, 14368–14371 CrossRef CAS PubMed.
- M. Tomàs-Gamisans, A. S. R. Ødum, M. Workman, P. Ferrer and J. Albiol, New Biotechnol., 2019, 50, 52–59 CrossRef PubMed.
- R. Delhom, A. Nelson, V. Laux, M. Haertlein, W. Knecht, G. Fragneto and H. P. Wacklin-Knecht, Nanomaterials, 2020, 10, 2439 CrossRef CAS PubMed.
- A. de Ghellinck, H. Schaller, V. Laux, M. Haertlein, M. Sferrazza, E. Maréchal, H. Wacklin, J. Jouhet and G. Fragneto, PLoS One, 2014, 9, e92999 CrossRef PubMed.
- J. E. Baldwin, D. J. Kiemle and A. P. Kostikov, J. Org. Chem., 2009, 74, 3866–3874 CrossRef CAS PubMed.
- J. E. Baldwin, D. J. Kiemle and A. P. Kostikov, J. Org. Chem., 2009, 74, 7866–7872 CrossRef CAS PubMed.
- T. A. Darwish, N. R. Yepuri, P. J. Holden and M. James, Anal. Chim. Acta, 2016, 927, 89–98 CrossRef CAS PubMed.
- R. Aydin and H. Günther, Angew. Chem., Int. Ed. Engl., 1981, 20, 985–986 CrossRef.
- R. Aydin, J. R. Wesener, H. Guenther, R. L. Santillan, M. E. Garibay and P. Joseph-Nathan, J. Org. Chem., 1984, 49, 3845–3847 CrossRef CAS.
- A.-M. Nam, A. Bighelli, F. Tomi, J. Casanova and M. Paoli, Magnetochemistry, 2017, 3, 34 CrossRef.
- Y. Correa, R. Del Giudice, S. Waldie, M. Thépaut, S. Micciula, Y. Gerelli, M. Moulin, C. Delaunay, F. Fieschi, H. Pichler, M. Haertlein, V. T. Forsyth, A. Le Brun, M. Moir, R. A. Russell, T. Darwish, J. Brinck, T. Wodaje, M. Jansen, C. Martín, F. Roosen-Runge and M. Cárdenas, J. Colloid Interface Sci., 2023, 645, 627–638 CrossRef CAS PubMed.
- H. Schaller, in Comprehensive Natural Products II, ed. H.-W. Liu and L. Mander, Elsevier, Oxford, 2010, pp. 755–787, DOI:10.1016/B978-008045382-8.00008-3.
- R. Eggers, A. Jammer, S. Jha, B. Kerschbaumer, M. Lahham, E. Strandback, M. Toplak, S. Wallner, A. Winkler and P. Macheroux, Phytochemistry, 2021, 189, 112822 CrossRef CAS PubMed.
- J. Yang, C. Li and Y. Zhang, Biomolecules, 2021, 11(11), 1710 CrossRef CAS PubMed.
- N. M. O'Connell, Y. C. O'Callaghan, N. M. O'Brien, A. R. Maguire and F. O. McCarthy, Tetrahedron, 2012, 68, 4995–5004 CrossRef.
- M.-A. Bazin, P. M. Loiseau, C. Bories, Y. Letourneux, S. Rault and L. El Kihel, Eur. J. Med. Chem., 2006, 41, 1109–1116 CrossRef CAS PubMed.
- X. Wei, A. D. Rodríguez, Y. Wang and S. G. Franzblau, Bioorg. Med. Chem. Lett., 2008, 18, 5448–5450 CrossRef CAS PubMed.
- J. Feixas, A. Capdevila and A. Guerrero, Tetrahedron, 1994, 50, 8539–8550 CrossRef CAS.
- K. I. Minard and L. McAlister-Henn, J. Biol. Chem., 2005, 280, 39890–39896 CrossRef CAS PubMed.
- Y. Tsukagoshi, H. Suzuki, H. Seki, T. Muranaka, K. Ohyama and Y. Fujimoto, J. Biol. Chem., 2016, 291, 8189–8198 CrossRef CAS PubMed.
- n.d., Dihydrobrassicasterol, https://commonchemistry.cas.org/detail?cas_rn=4651-51-8 (accessed 2023-03-26, 2023).
- E. J. Johnston, T. Moses and S. J. Rosser, Yeast, 2020, 37, 27–44 CrossRef CAS PubMed.
- H. Riezman , personal communication.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ob00754e |
| ‡ A strictly aqueous alkaline lysis protocol was not successful upon attempted scale-up, nor a biphasic alkaline water/toluene protocol. Physical disruption of the cells by bead-beating was found to have little effect on cholesterol recovery. |
| This journal is © The Royal Society of Chemistry 2023 |