Synthesis of N-isoindolinonyl peptides via Pd-catalyzed C(sp2)–H olefination–activation and their conformational studies†
Received
12th May 2023
, Accepted 22nd May 2023
First published on 23rd May 2023
Abstract
Isoindolinone is a constituent of several natural products that show a wide range of bioactivity, such as anticancer, antimicrobial, antiviral and anti-inflammatory properties. It would be interesting to explore the carbonyl group (H-bond acceptor) of isoindolinone and its structural and conformational changes. However, the synthesis of isoindolinone-comprising peptides in short steps is challenging. Herein, we have developed a synthetic methodology for introducing the isoindolinone residue to peptides via Pd-catalyzed C(sp2)–H activation/olefination, and demonstrated the conformational changes owing to the isoindolinone scaffold. Hence, isoindolinonyl peptides provide an avenue for the synthesis of novel foldamers and therapeutic agents.
Introduction
Isoindolinone is heterocyclic compound with a benzo fused γ-lactam scaffold that is a constituent of various natural products and synthetic bioactive molecules such as pagoclone, indoprofen, erinacerins, lennoxamine, lenalidomide, isohericenone, and pazinaclone (Fig. 1a).1–3 These molecules show antimicrobial, antioxidant, antifungal, antiviral, anxiolytic, anti-Parkinson's, anti-inflammatory, antihypertension, and anticancer activities.4 In the repertoire of isoindoline scaffolding into peptides, the incorporation of isoindolinone moiety into naturally occurring bioactive cyclic peptides enhances their bioactivity. Fenestin A and zygosporamide are marine-derived natural cyclic peptides possessing a wide range of bioactivity, including anticancer. Jin has synthesized Fenestin analogues containing isoindolinone derivatives that improved their apoptosis of tumor cells and led to cycle arrest in the G2/M phase.5 Zygosporamide cyclic depsipeptides isolated from marine-derived fungus and their analogues are also synthesized by incorporating the isoindolinone moiety, which significantly improved their bioactivity (Fig. 1b).6 Nevertheless, Chen has synthesized various stapled peptides by incorporating isoindolinone derivatives (Fig. 1c).7 Thus, the demand for isoindolinone-containing peptides has suddenly increased and become a lucrative research area for both chemists and biologists. In the literature, various heterocyclic scaffolds have been used for the structural and conformational changes of native peptides.8–10 The isoindolinone moiety of isoindolinonyl peptides can also participate in intramolecular hydrogen bonding with the other amide N–H of peptides and play a significant role in the conformational changes of peptides.
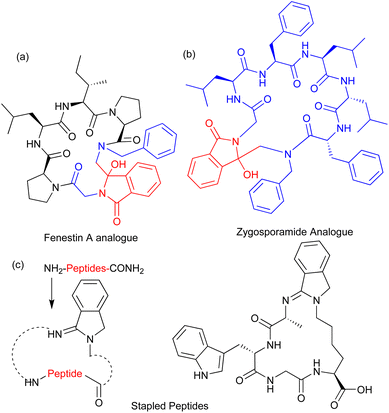 |
| | Fig. 1 Isoindolinone derivative: (a,b) Isoindolinone related natural peptides; (c) stapled peptides. | |
The classical synthesis of isoindolinone compounds is achieved through multiple challenging steps.11–13 In recent times, various heterocycle compounds are synthesized in one step from an economical reactant precursor through metal-catalyzed C–H activation, which is a modern synthetic methodology. However, the incorporation of isoindolinone residue in the native peptides is challenging through chemical synthesis. Recently, we reported the synthesis of isoindolinone-containing amino acid ester derivatives through Pd-catalyzed C–H activation/olefination from N-benzoyl amino acid esters and olefins without using an auxiliary directing group, which is a modern synthetic methodology by C–H activation.14 Transition metal-catalysed C–H activation and C–H olefination are emerging synthetic methodologies for the synthesis of various natural products and their precursors, including isoindolinone derivatives.15–18 The Pd-catalysed C(sp2)–H olefination methodologies have reached a new milestone owing to their easy handling and cost effectiveness.18–29 The Co-/Rh-catalyzed substrate-specific C(sp2)–H olefination is also known.30–32 The regioselective metal-catalyzed C–H activation also requires a directing group and ligand for metal complexation before inert C–H activation and functionalization. The directing groups could be auxiliary, transient or intrinsic. The directing group containing arylamides has also been used for synthesizing isoindolinone derivatives from non-activated olefins through C–(sp2)–H olefination.33 For example, the quinoline directing group containing arylamides gives N-quinolinyl substituted isoindolinones from acrylate in the presence of Pd(II)-catalyst. However, N-tosylated benzamide requires a ligand to prepare isoindolinone derivatives from olefin through Pd-catalyzed olefination.34,35 Amino acids and peptides are natural ligands of various transition metals and are being used as the directing group/ligand in the different transition metal-catalyzed C–H activation/C-olefination reactions.36 Yu has explored amino acids/peptides as the ligand or directing group in the metal-catalyzed C(sp3)–H functionalization of di-/tri/tetra-peptides at the N-terminus/site-specific C–H alkynylation/β-C–H arylation.37–39 Albericio has shown the synthesis of stapled peptides by the late-stage C(sp3)–H activation of peptides.40,41 Daugulis has used 2-thiomethylaniline as the directing group for C(sp3)–H functionalization of N-protected amino acids at the β-position with different aryl halides.42 The metal-catalyzed C–H olefinations of amino acid/peptide-related compounds have generated opportunities to prepare various synthetic and natural peptide analogues.43,44 Metal-catalyzed olefination of ligand enabled an aryl amide with olefin, which is an emerging synthetic methodology attractive for the synthesis of substituted aryl amide derivatives.24,45–51 Recently, Maiti has explored Pd-catalyzed 8-AQ-directed and template-based regioselective C–H olefinations with non-active aliphatic olefins.47–53 Wang has demonstrated a Pd-catalyzed ortho-olefination reaction and cyclization reaction at benzylsufonamide to yield benzosultam peptidomimetics.54 The synthesis of isoindolinone derivatives without comprising amino acids was accomplished from activated arylamides and non-reactive olefins through transition-metal-catalyzed C–H activation using metal ions Sc, Yb, Au, Rh, Ir, Ru, Co, Cu, Ni, Zn, In, and Pd (Fig. 2a and b).55 Herein, we have rationally designed peptides containing an isoindolinone olefin ester at the N-terminal to explore the role of isoindolinone's lactam carbonyl in the folding of peptide structure through noncovalent interactions (Fig. 2c). This report describes the post-synthesis isoindolinonyl peptides from N-arylamide peptides and olefins through Pd-catalysed C(sp2)–H olefination. Their conformational analysis was also studied by NMR and computational technique. To examine the biocompatibility and therapeutic value, cell cytotoxicity studies were also performed before exploring their therapeutic value.
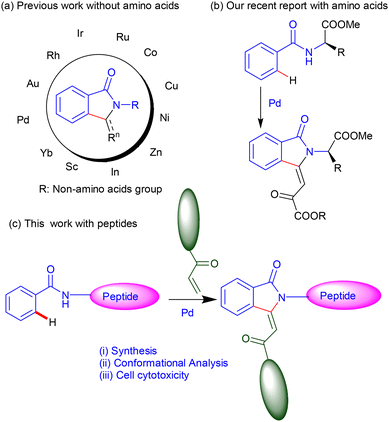 |
| | Fig. 2 (a) The previously reported C–H hydroarylation, (b) C–H functionalization and (c) (this report) post-synthesis of isoindolinyl peptides. | |
Results and discussion
The conjugation of isoindolinone scaffold with peptides could be achieved by two ways: (a) coupling of previously synthesized isoindolinonyl amino acid carboxylates at the free NH group of the designed peptides; (b) C(sp2)–H olefination/activation reaction at the N-benzamide of target peptides. However, we were unable to achieve the mono carboxylate derivative of isoindolinoyl amino acid ester through the base-catalyzed hydrolysis under mild condition (Scheme 1a). Thus, we attempted C(sp2)–H olefination/activation at the N-benzamide of peptides. We synthesized dipeptides (1–4), containing glycine, valine, phenyl alanine and phenyl glycine ester, and their N-benzamide peptides (6a–6d) with benzoic acid under peptide coupling conditions. These peptides were subjected to Pd-catalyzed C–H activation/olefination reactions with different olefins by using our previously reported method.56 Unfortunately, we could not obtain any olefinated/activated product. We noticed the formation of isoindolinonyl peptides (8a) from peptide 6a and olefin methyl acrylate (7a) under Cu(OAc)2/NaOAc at 100 °C in the presence of Pd catalyst.54 Importantly, additive Cu(OAc)2/NaOAc was found essential for mono C–H olefination/activation reaction in N-benzamide peptides (Scheme 1b). Similarly, we synthesized other isoindolinonyl peptides 8b–8d from peptide 6a and different olefins: ethyl acrylate (7b), n-butyl acrylate (7c), and t-butyl acrylate (7d). Next, peptide 6b was treated with various olefins (7a–7d) under similar optimized reaction conditions of mono C(sp2)–H olefination/activations that produced isoindolinonyl peptides 9a–9d (Scheme 1b). We performed similar reaction with N-benzamide of additional phenyl ring – containing dipeptides (6c–6d) and different olefins (7a–7d). Pleasantly, we isolated isoindolinonyl peptides 10a–10d/11a–11d from the respective peptides and olefins through chemoselective/regioselective C–H olefination/activation at only the ring. Herein, we could not get C(sp2)–H olefination reaction at the phenyl glycine residue of peptide 6c and phenyl alanine residue of peptide 6d though there is presence of a probable intrinsic amide-directing group. Thus, only benzamide ring is susceptible to the C–H olefination which led to the formation of isoindolinonyl peptides.
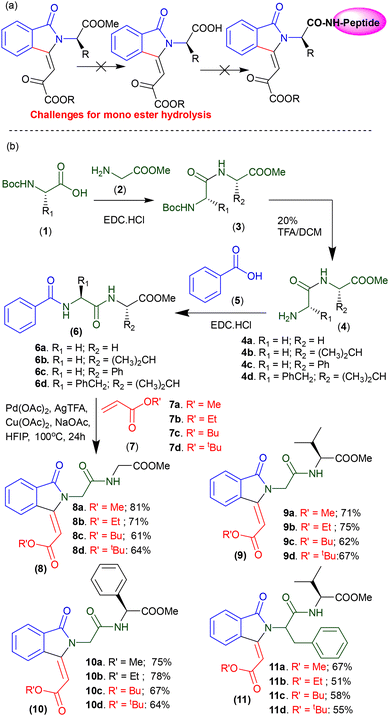 |
| | Scheme 1 Synthesis of isoindolinonyl peptide derivatives with different peptides and olefins. | |
To explore the diversity of arylamide substrates for Pd-catalyzed isoindolinonyl peptide synthesis, we synthesized arylamide dipeptides 13a/13b from 2-napthoic acid and dipeptides 4b/4c (Scheme 2). Similarly, we synthesized arylamide peptide substrates 16a/16b from 4-biphenyl carboxylic acid and peptides 4b/4c. These peptides were subjected to Pd-catalyzed olefination reaction under optimized conditions. Importantly, these peptides also produced chemoselective naphthyl isoindolinonyl peptides 14a–14d from the respective 2-napthoyl peptides 13a/13b and acrylate 7a/7b, as well as biphenyl isoindolinonyl peptides 17a–17d from the respective 4-biphenyl-containing peptides 16a/16b and acrylate 7a/7b. Here too, we could not achieve C(sp2)–H olefination reaction at the phenyl glycine residue of peptide 13b/16b though there is presence of probable intrinsic amide-directing group.
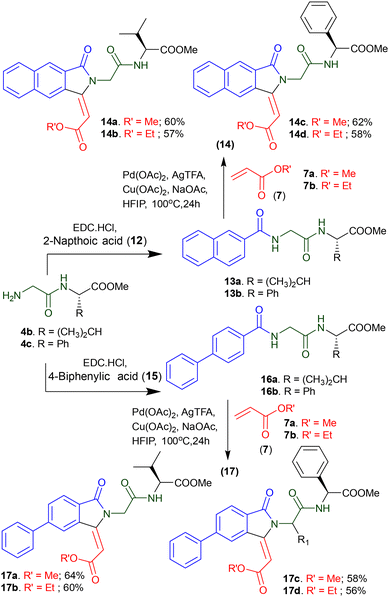 |
| | Scheme 2 Synthesis of isoindolinonyl peptides with different aromatic acids. | |
Further, we examined the C–H olefination/isoindolinonylation reaction with different N-benzamide dipeptides and olefines under Pd-catalysed optimized reaction conditions. We synthesized arylamide peptides 19a–19c containing different types of benzoyl residues (18), such as p-methyl benzoyl in 19a, p-tert-butylbenzoyl in peptide 19b, and m-nitrobenzoyl in peptide 19c (Scheme 3). These peptides were subjected to olefination with different acrylates (7) under Pd-catalyzed olefination reaction condition. We isolated isoindolinone derivatives 20a–20b from p-methylbenzoyl peptide and 21a–21d from p-tert-butyl benzoyl peptides though their yields are low. However, nitrobenzoyl peptide (19c) could not give any C–H olefinated/activated product. It seems an electron-withdrawing group possibly perturbs the metal (Pd-II) complexation of the phenyl ring of nitrobenzoyl peptide. Thus, unsubstituted aryl amides are more suitable substrates for Pd- catalysed C(sp2)–H olefination/activation reactions under the optimized conditions.
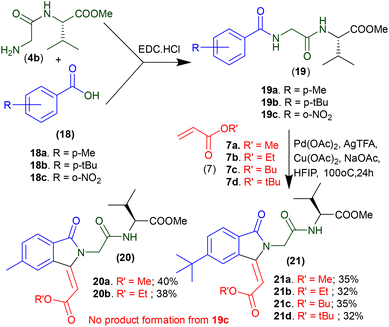 |
| | Scheme 3 Synthesis of aryl-substituted isoindolinonyl peptide derivatives. | |
Further, we attempted to apply this methodology for the synthesis of isoindolinone-containing tri-peptides. Thus, we synthesized the benzamide of tri-peptides (22) such as BzNHGly-Ala-Phe (22a) and BzNHVal-Leu-Phe-OMe (22b). These peptides were subjected to Pd-catalyzed olefination/activation reaction with olefin (7a) under the above optimized reaction conditions (Scheme 4). Importantly, benzamide peptides 22a–22b also produced the respective isoindolinone derivative peptides 23/24 though their yields are quite low (∼25–30%) as compared to dipeptides.
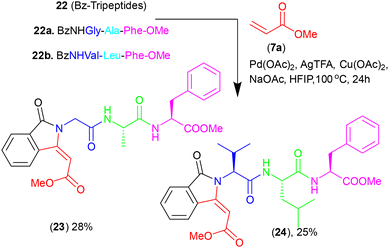 |
| | Scheme 4 Synthesis of substituted isoindolinonyl tri-peptide. | |
Herein, we propose the reaction mechanism of isoindolinone-peptide synthesis through Pd-catalyzed C(sp2)–H olefination/activation (Fig. 3). Additive Cu(OAc)2 facilitates the formation of a stable palladacycle with amide carbonyl through N,O-type chelation by preventing N,N covalent chelation. In the literature, the coordination of amide carbonyl with catalyst Pd(OAc)2 is known as well.57 The benzoyl amino acid residue of the peptide (reactant6a) acts as the intrinsic directing group for Pd complexation (5-membered palladacycle) and proceeds to C(sp2)–H activation at the ortho position of its phenyl ring (I-1). Olefin forms Pd complex I-2 by displacement of ligand OAc, and then proceeds to I-3 through 1,2-migration as the intermediate I-3. This intermediate (I-3) leads to olefinated product (6′) via β-hydride elimination reaction. Again, palladacycle forms with the benzoyl amino acid residue of olefinated peptide (6′) and proceeds to C(sp2)–H activation at olefin as the intermediate I-4, which leads to stable isoindolinonyl peptides (8a) via reductive elimination. The regeneration of catalyst occurs in situ through reoxidation.
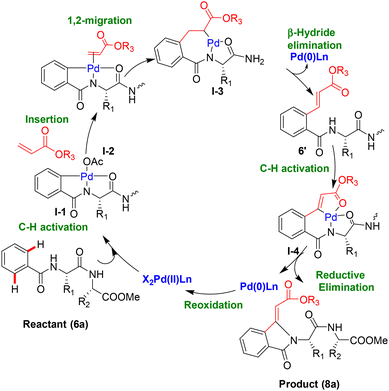 |
| | Fig. 3 The proposed mechanism for isoindolinone peptide synthesis. | |
After the successful synthesis of isoindolinonyl-conjugated peptides through C–H activation, we attempted to find the conformation of isoindolinyl tripeptides (23) in solution by NMR technique. We recorded their NMR spectra (1H/1H–1HCOSY/1H–1H NOESY) only in DMSO-d6 solvent because of poor solubility (or insolubility) in other solvents. Their spectra are provided in the ESI (Fig. S88†). The chemical shift of all protons were assigned from the COSY spectra, then the NOE cross peaks were used to find the interaction of non-vicinal protons. Our analyses helped to find the 1H–1H interactions which are in close proximity. In Fig. 4, the phenyl ring proton (d) of the isoindolinone residue interacts with the methyl protons of acrylate residue. Olefin protons of acrylate residue exhibit two cross peaks with glycinate protons (a) and alaninyl methyl protons. Thus, the orientation of isoindolinyl residue is in close proximity with alanine methyl (i + 2) to form a stable conformation in solution.
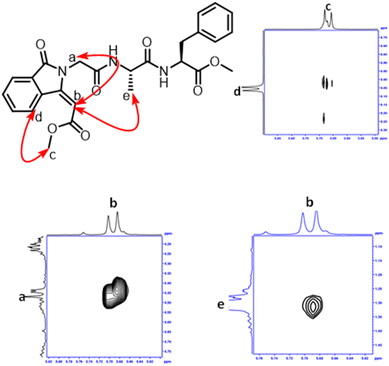 |
| | Fig. 4 NOESY spectra of isoindolinone derivative 23. | |
The DMSO-d6 titration NMR (1H-NMR) experiment is a well-established technique to find the intramolecular hydrogen bonding N–H in CDCl3.58 Herein, we performed the DMSO-d6 titration 1H-NMR experiment with representative isoindolinonyl peptides derived from methyl acrylates (8a–11a/14a/14c/17a/17c) and the control peptide BzNHGly-ValOMe (6b). Their titration profiles are depicted in Fig. 5, while their spectra are provided in ESI.† However, peptides 23 and 24 are insoluble in CDCl3 (soluble only in DMSO). In Fig. 5B, we have also extracted a bar diagram for peptides vs. Δδ(ppm), where Δδ(ppm) = (δfinal − δinitial). The control peptide (6b) has two amide N–H groups, whose titration profile shows that one N–H is unaffected while the other has a small downfield shift with DMSO-d6 titration owing to the strong and moderate intramolecular hydrogen bonding, respectively. The isoindolinonyl peptides (8a–11a/14a/14c/17a/17c) have one amide N–H that exhibits a small downfield shift (∼0.4–1.3 ppm) with DMSO-d6 titration, possibly due to intramolecular hydrogen bonding with amide N–H (Fig. 5A and B). The amide N–H groups of peptide 11a and 9a have small shifts as compared to other isoindolinonyl peptides (Fig. 5B). Thus, sequence-specific isoindolinone carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) has significant roles in the intramolecular hydrogen bonding with amide N–H, which could be useful for tuning the peptide folding.
O) has significant roles in the intramolecular hydrogen bonding with amide N–H, which could be useful for tuning the peptide folding.
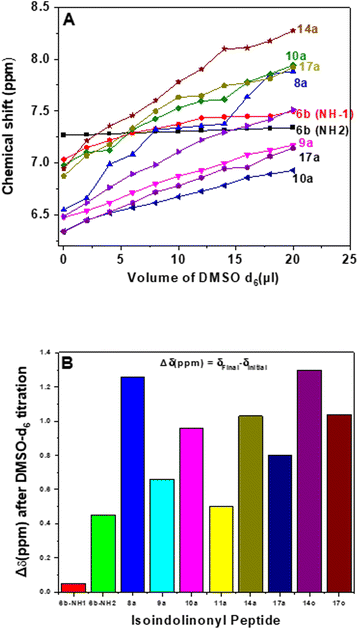 |
| | Fig. 5 DMSO-d6 titration NMR profile of isoindonyl derivatives (A) and Bar diagram of isoindolinyl peptides vs. Δδ(ppm), where Δδ(ppm) = (δfinal − δinitial) (B). | |
Next, we performed computational studies with representative isoindolinonyl peptides derived from methyl acrylates (8a–11a/14a/14c/17a/17c/23/24) and the control peptide BzNHGly-ValOMe (6b) using MMFF94 force field and GMMX. We visualized their conformations on PyMol (Fig. 6). The control peptide (6b), without containing isoindolinone scaffold, exhibits hydrogen bonding between the benzoyl carbonyl and the second amino acid residue valine N–H (i + 3) with bond distance of 1.9 Å (N–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) and bond angle ∠N–H–O ∼ 148° that nearly matched with the γ-turn. Isoindolinone-containing dipeptides (8a–11a/14a/14c/17a/17c) exhibit intramolecular hydrogen bonding between the isoindolinone carbonyl (C
C) and bond angle ∠N–H–O ∼ 148° that nearly matched with the γ-turn. Isoindolinone-containing dipeptides (8a–11a/14a/14c/17a/17c) exhibit intramolecular hydrogen bonding between the isoindolinone carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and N–H of the second amino acid residue, with bond distance (N–H⋯O) ∼2.0 and bond angle ∠N–H–O ∼150° with the respective substituents of the amino acid and nature of the aryl group of the isoindolinone residue. In the case of isoindolinonyl tri-peptides (23/24), they exhibit two intramolecular hydrogen bonds between isoindolinonyl carbonyl and the i + 3 amide (N–H⋯O
O) and N–H of the second amino acid residue, with bond distance (N–H⋯O) ∼2.0 and bond angle ∠N–H–O ∼150° with the respective substituents of the amino acid and nature of the aryl group of the isoindolinone residue. In the case of isoindolinonyl tri-peptides (23/24), they exhibit two intramolecular hydrogen bonds between isoindolinonyl carbonyl and the i + 3 amide (N–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C). The bond length and angle of the hydrogen bonds are almost same, ∼2.0 Å/∼150° for peptide 23, and possibly lead to the formation of a helix. In contrast, for 24, the bond length and angle of hydrogen bonds are different, at ∼2.0 Å/∼150° (strong) and ∼2.8 Å/136° (weak), presumably due to the leu residue at the 2nd position, and may lead to only γ-turn structure (Fig. 6). These data strongly support that isoindolinone-containing peptides are potential building blocks for designing unique foldamers.
C). The bond length and angle of the hydrogen bonds are almost same, ∼2.0 Å/∼150° for peptide 23, and possibly lead to the formation of a helix. In contrast, for 24, the bond length and angle of hydrogen bonds are different, at ∼2.0 Å/∼150° (strong) and ∼2.8 Å/136° (weak), presumably due to the leu residue at the 2nd position, and may lead to only γ-turn structure (Fig. 6). These data strongly support that isoindolinone-containing peptides are potential building blocks for designing unique foldamers.
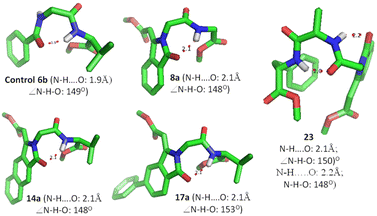 |
| | Fig. 6 Conformation of isoindolinone peptides (computationally optimized structure of isoindolinone peptides MMFF94 and GMMX). | |
Finally, we examined the cell cytotoxicity of isoindolinonyl peptides derived from methyl acrylates (8a–11a/14a/14c/17a/17c/23/24) with Hek293T cells using MTT assay. Their cytotoxicity data are provided in ESI (Fig. S89†), which indicate that isoindolinonyl peptides have negligible cytotoxicity as compared to the control (DMSO).
Conclusions
Isoindolinonyl-incorporated cyclopeptides are emerging therapeutic target drug molecules, and their synthesis involves multiple challenging steps. Herein, we have developed a novel methodology for the synthesis of isoindolinone scaffold comprising di/tri-peptides through Pd-catalyzed C(sp2)–H olefination/activation from various arylamide peptides and acrylate olefins. We have also studied the conformational changes of those peptides owing to the isoindolinone residue. Our conformational analyses strongly support the formation of γ-turn in dipeptide and helical formation in less stearic tripeptides. Finally, these peptides are biocompatible owing to their negligible cytotoxicity effect on Hek293T cells. Hence, isoindolinone-containing peptides are potential building blocks for novel foldamers with therapeutic value.
Experimental details
Material and instrumentation
All required materials were obtained from commercial suppliers and used without purification. Dry DMF was freshly prepared by distilling over calcium hydride. Reactions were monitored by thin-layer chromatography, visualized by UV and ninhydrin. Column chromatography was performed in 100–200 mesh silica. Mass spectra were obtained from Bruker Micro TOF-Q II Spectrometer. NMR spectra were recorded on Bruker AV-400 for 1H (400 MHz) and 13C (100.6 MHz). 1H and 13C NMR chemical shifts were recorded in ppm downfield from tetramethylsilane; splitting patterns are abbreviated as s, singlet; d, doublet; dd, doublet of doublet; t, triplet; q, quartet; dq, doublet of the quartet; m, multiplet.
General procedure for benzamide derivative peptide synthesis
Benzoic acid or its derivative was dissolved in DMF before triethylamine (TEA, 3.0 eq.), EDC.HCl (1.3 eq.) and HOAT (1.3 eq.) were added, followed by free N-terminal peptide methyl ester (1.2 eq.). Further, this mixture was heated to 60 °C for 8–12 h. The reaction was monitored by TLC. The crude reaction mixture was concentrated under reduced pressure before water was added. The aqueous layer was extracted with EtOAc. The organic layer was combined, dried over anhydrous Na2SO4, and concentrated under reduced pressure. The residue was purified with column chromatography by EtOAc/hexane solvent system to yield the corresponding substrates.
General procedure for Pd-catalysed reactions
Typically, amide substrates were placed in a 15 ml sealed reaction tube under the indicated reaction conditions. The mixture was stirred at 100 °C for 12–24 h, cooled to room temperature, and then diluted with EtOAc. The resulting solution was filtered through a Celite pad, concentrated under reduced pressure, and the product was further purified by column chromatography by the EtOAc/hexane solvent system and was typically obtained as a white solid.
Methyl benzoylglycylglycinate (6a).
1H NMR (400 MHz, CDCl3) δ 7.85 (t, J = 8.0 Hz, 2H), 7.62 (d, J = 4.0 Hz, 1H), 7.50 (t, J = 8.0 Hz, 1H), 7.45 (s, 1H), 7.41 (d, J = 8.0 Hz, 2H), 4.21 (d, J = 4.0 Hz, 2H), 4.05 (d, J = 4.0 Hz, 2H), 3.72 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 170.28 (s), 169.88 (s), 168.04 (s), 133.35 (s), 131.91 (s), 128.59 (s), 127.23 (s), 52.41 (s), 43.57 (s), 41.20 (s). White solid (68% yield). ESI-HRMS m/z [M + H]+ calcd for C12H14N2O4 251.1032, found 251.1040.
Methyl (Z)-2-(2-(2-((2-methoxy-2-oxoethyl)amino)-2-oxoethyl)-3-oxoisoindolin-1-ylidene)acetate (8a).
1H NMR (400 MHz, CDCl3) δ 9.07 (d, J = 8.0 Hz, 1H), 7.88 (d, J = 8.0 Hz, 1H), 7.70 (d, J = 8.0 Hz, 1H), 7.61 (d, J = 8.0 Hz, 1H), 6.54 (s, 1H), 5.79 (s, 1H), 4.52 (s, 2H), 4.04 (d, J = 4.0 Hz, 2H), 3.81 (s, 3H), 3.72 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 169.77 (s), 167.26 (s), 166.82 (s), 166.08 (s), 147.65 (s), 133.88 (s), 133.79 (s), 131.55 (s), 129.32 (s), 128.27 (s), 123.56 (s), 99.68 (s), 52.49 (s), 51.82 (s), 43.39 (s), 41.23 (s). White solid (81% yield). ESI-HRMS m/z [M + Na]+ calcd for C12H14N2O4 355.0906, found 355.1041.
Ethyl (Z)-2-(2-(2-((2-methoxy-2-oxoethyl)amino)-2-oxoethyl)-3-oxoisoindolin-1-ylidene)acetate (8b).
1H NMR (400 MHz, CDCl3) δ 9.09 (d, J = 7.9 Hz, 1H), 7.90 (d, J = 7.4 Hz, 1H), 7.71 (t, J = 8.3 Hz, 1H), 7.62 (t, J = 7.9 Hz, 1H), 6.49 (d, J = 4.0 Hz, 1H), 5.80 (s, 1H), 4.54 (s, 2H), 4.28 (q, J = 8.0 Hz, 2H), 4.06 (d, J = 5.4 Hz, 2H), 3.74 (s, 3H), 1.36 (t, J = 8.0 Hz, 3H). 13C NMR (176 MHz, CDCl3) δ 169.70 (s), 167.27 (s), 166.85 (s), 165.65 (s), 147.37 (s), 133.87 (s), 133.76 (s), 131.31 (s), 129.32 (s), 128.32 (s), 123.81 (s), 100.29 (s), 60.77 (s), 52.42 (s), 43.47 (s), 41.24 (s), 14.24 (s). White solid (71% yield). ESI-HRMS m/z [M + H]+ calcd for C17H18N2O6 347.1243, found 347.1243.
Butyl (Z)-2-(2-(2-((2-methoxy-2-oxoethyl)amino)-2-oxoethyl)-3-oxoisoindolin-1-ylidene)acetate (8c).
1H NMR (400 MHz, CDCl3) δ 9.10 (d, J = 8.0 Hz, 1H), 7.90 (d, J = 8.0 Hz, 1H), 7.71 (t, J = 8.0 Hz, 1H), 7.62 (t, J = 8.0 Hz, 1H), 6.47 (s, 1H), 5.81 (s, 1H), 4.54 (s, 2H), 4.23 (t, J = 8.0 Hz, 2H), 4.06 (d, J = 8.0 Hz, 2H), 3.74 (s, 3H), 1.72–1.67 (m, 2H), 1.49–1.41 (m, 2H), 0.98 (t, J = 8.0 Hz, 3H). 13C NMR (176 MHz, CDCl3) δ 169.68 (s), 167.26 (s), 166.87 (s), 165.77 (s), 147.35 (s), 133.89 (s), 133.75 (s), 131.30 (s), 129.31 (s), 128.33 (s), 123.80 (s), 100.30 (s), 64.70 (s), 52.40 (s), 43.48 (s), 41.24 (s), 30.68 (s), 19.17 (s), 13.73 (s). White solid (61% yield). ESI-HRMS m/z [M + H]+ calcd for C19H22N2O6 375.1558, found 375.1553.
tert-Butyl (Z)-2-(2-(2-((2-methoxy-2-oxoethyl)amino)-2-oxoethyl)-3-oxoisoindolin-1-ylidene)acetate (8d).
1H NMR (400 MHz, CDCl3) δ 9.02 (d, J = 8.0 Hz, 1H), 7.86 (d, J = 8.0 Hz, 1H), 7.68 (t, J = 8.0 Hz, 1H), 7.58 (t, J = 8.0 Hz, 1H), 6.57 (s, 1H), 5.74 (s, 1H), 4.50 (s, 2H), 4.04 (d, J = 4.0 Hz, 2H), 3.71 (s, 3H), 1.55 (s, J = 8.0 Hz, 9H). 13C NMR (101 MHz, CDCl3) δ 169.71 (s), 167.28 (s), 167.09 (s), 165.03 (s), 146.31 (s), 133.97 (s), 133.61 (s), 131.21 (s), 129.33 (s), 128.21 (s), 123.41 (s), 102.55 (s), 81.19 (s), 52.41 (s), 43.44 (s), 41.22 (s), 28.23 (s). White solid (64% yield). ESI-HRMS m/z [M + H]+ calcd for C19H22N2O6 375.1556, found 375.1553.
Methyl benzoylglycyl-l-valinate (6b).
1H NMR (400 MHz, CDCl3) δ 7.85 (d, J = 8.0 Hz, 2H), 7.56–7.38 (m, 4H), 7.27 (s, 1H), 4.55 (d, J = 4.0 Hz, 1H), 4.26 (d, J = 4.0 Hz, 2H), 3.73 (s, 3H), 2.25–2.13 (m, 1H), 0.94 (dd, J = 8.0 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 172.21 (s), 169.34 (s), 167.93 (s), 133.53 (s), 131.86 (s), 128.59 (s), 127.21 (s), 57.52 (s), 52.25 (s), 43.78 (s), 31.07 (s), 19.05 (s), 17.79 (s). White solid (62% yield). ESI-HRMS m/z [M + H]+ calcd for C15H20N2O4 293.1501, found 293.1498.
Methyl (Z)-(2-(1-(2-methoxy-2-oxoethylidene)-3-oxoisoindolin-2-yl)acetyl)-L-valinate (9a).
1H NMR (400 MHz, CDCl3) δ 9.14–9.05 (m, 1H), 7.90 (d, 1H), 7.71 (t, J = 8.0 Hz, 1H), 7.62 (t, J = 8.0 Hz, 1H), 6.47 (d, J = 8.0 Hz, 1H), 5.78 (s, 1H), 4.61–4.49 (m, 3H), 3.81 (s, 3H), 3.70 (s, 3H), 2.16 (d, J = 8.0 Hz, 1H), 0.88 (dd, J = 8.0 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 171.91 (s), 167.26 (s), 166.45 (s), 166.08 (s), 147.77 (s), 133.82 (s), 133.77 (s), 131.55 (s), 129.33 (s), 128.29 (s), 123.60 (s), 99.53 (s), 57.27 (s), 52.32 (s), 51.80 (s), 43.57 (s), 31.22 (s), 18.93 (s), 17.73 (s). White solid (71% yield). ESI-HRMS m/z [M + H]+ calcd for C19H22N2O6 375.1556, found 375.1553.
Methyl (Z)-(2-(1-(2-ethoxy-2-oxoethylidene)-3-oxoisoindolin-2-yl)acetyl)-L-valinate (9b).
1H NMR (400 MHz, CDCl3) δ 9.09 (d, J = 4.0 Hz, 1H), 7.89 (d, J = 4.0 Hz, 1H), 7.69 (t, J = 8.0 Hz, 1H), 7.62 (t, 1H), 6.47 (d, J = 8.0 Hz, 1H), 5.77 (d, J = 4.0 Hz, 1H), 4.60–4.49 (m, 3H), 4.32–4.18 (m, 2H), 3.70 (s, 3H), 2.21–2.09 (m, 1H), 1.37–1.27 (m, 3H), 0.88 (dd, J = 8.0 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 171.90 (s), 167.26 (s), 166.50 (s), 165.63 (s), 133.82 (s), 133.73 (s), 131.48 (s), 129.33 (s), 128.32 (s), 123.58 (s), 100.13 (s), 60.67 (s), 57.26 (s), 52.31 (s), 43.59 (s), 31.23 (s), 18.95 (s), 17.72 (s), 14.28 (s). White solid (75% yield). ESI-HRMS m/z [M + H]+ calcd for C20H24N2O6 389.1713, found 389.1714.
Methyl (Z)-(2-(1-(2-butoxy-2-oxoethylidene)-3-oxoisoindolin-2-yl)acetyl)-L-valinate (9c).
1H NMR (400 MHz, CDCl3) δ 9.10 (d, J = 8.0 Hz, 1H), 7.91 (d, J = 8.0 Hz, 1H), 7.71 (d, J = 8.0 Hz, 1H), 7.62 (t, J = 8.0 Hz, 1H), 6.36 (d, J = 8.0 Hz, 1H), 5.77 (s, 1H), 4.59–4.49 (m, 3H), 4.21 (d, J = 8.0 Hz, 2H), 3.70 (s, 3H), 2.20–2.11 (m, 1H), 1.67 (d, J = 7.9 Hz, 2H), 1.45–1.39 (m, 2H), 0.96 (d, J = 4.0 Hz, 3H), 0.93–0.83 (m, 6H). 13C NMR (101 MHz, CDCl3) δ 171.81 (s), 167.23 (s), 166.46 (s), 165.72 (s), 147.47 (s), 133.87 (s), 133.73 (s), 131.47 (s), 129.34 (s), 128.36 (s), 123.59 (s), 100.13 (s), 64.60 (s), 57.25 (s), 52.26 (s), 43.68 (s), 31.24 (s), 30.70 (s), 19.16 (s), 18.93 (s), 17.69 (s), 13.71 (s). White solid (62% yield). ESI-HRMS m/z [M + H]+ calcd for C22H28N2O6 417.2026, found 417.2060.
Methyl (Z)-(2-(1-(2-(tert-butoxy)-2-oxoethylidene)-3-oxoisoindolin-2-yl)acetyl)-L-valinate (9d).
1H NMR (400 MHz, CDCl3) δ 9.04 (d, J = 8.0 Hz, 1H), 7.89 (d, J = 8.0 Hz, 1H), 7.70 (d, J = 8.0 Hz, 1H), 7.60 (d, J = 8.0 Hz, 1H), 6.41 (d, J = 8.7 Hz, 1H), 5.71 (s, 1H), 4.60–4.46 (m, 3H), 3.69 (s, 3H), 2.20–2.11 (m, 1H), 1.53 (s, 9H), 1.02–0.75 (m, 6H). 13C NMR (176 MHz, CDCl3) δ 171.83 (s), 167.23 (s), 166.62 (s), 164.93 (s), 146.44 (s), 133.94 (s), 133.62 (s), 131.26 (s), 129.37 (s), 128.28 (s), 123.50 (s), 102.42 (s), 57.24 (s), 52.26 (s), 43.66 (s), 31.25 (s), 28.21 (s), 18.98 (s), 17.68 (s). White solid (67% yield). ESI-HRMS m/z [M + H]+ calcd for C22H28N2O6 417.2026, found 417.2019.
Methyl (S)-2-(2-benzamidoacetamido)-2-phenylacetate (6c).
1H NMR (400 MHz, CDCl3) δ 7.76 (d, J = 8.0 Hz, 2H), 7.59 (d, J = 6.7 Hz, 1H), 7.52–7.45 (m, 1H), 7.43–7.30 (m, 7H), 7.19 (s, 1H), 5.57 (d, J = 8.0 Hz, 1H), 4.32–4.13 (m, 2H), 3.70 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 171.00 (s), 168.57 (s), 167.76 (s), 135.93 (s), 133.51 (s), 131.83 (s), 129.05 (s), 128.71 (s), 128.64 (d, J = 12.4 Hz), 128.58 (s), 127.36 (s), 127.18 (s), 56.77 (s), 52.89 (s), 43.57 (s). White solid (65% yield). ESI-HRMS m/z [M + H]+ calcd for C18H18N2O4 327.1345, found 327.1369.
Methyl-(S,Z)-2-(2-(1-(2-methoxy-2-oxoethylidene)-3-oxoisoindolin-2-yl)acetamido)-2-phenylacetate (10a).
1H NMR (400 MHz, CDCl3) δ 9.10 (d, J = 8.0 Hz, 1H), 7.91 (d, J = 8.0 Hz, 1H), 7.72 (d, J = 8.0 Hz, 1H), 7.63 (d, J = 8.0 Hz, 1H), 7.35–7.32 (m, 5H), 6.99 (d, J = 8.0 Hz, 1H), 5.75 (s, 1H), 5.57 (d, J = 8.0 Hz, 1H), 4.53 (s, 2H), 3.81 (s, 3H), 3.71 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 170.77 (s), 167.21 (s), 166.03 (s), 165.90 (s), 147.74 (s), 135.85 (s), 133.82 (s), 133.75 (s), 131.51 (s), 129.36 (s), 129.02 (s), 128.67 (s), 128.28 (s), 127.23 (s), 123.62 (s), 99.57 (s), 56.58 (s), 52.94 (s), 51.74 (s), 43.43 (s). White solid (75% yield). ESI-HRMS m/z [M + H]+ calcd for C22H20N2O6 409.1400, found 409.1439.
Methyl (S,Z)-2-(2-(1-(2-ethoxy-2-oxoethylidene)-3-oxoisoindolin-2-yl)acetamido)-2-phenylacetate (10b).
1H NMR (400 MHz, CDCl3) δ 9.08 (d, J = 8.0 Hz, 1H), 7.89 (d, J = 8.0 Hz, 1H), 7.69 (d, J = 8.0 Hz, 1H), 7.61 (t, J = 8.0 Hz, 1H), 7.37–7.28 (m, 5H), 6.95 (d, J = 8.0 Hz, 1H), 5.73 (s, 1H), 5.55 (d, J = 7.0 Hz, 1H), 4.51 (d, J = 4.0 Hz, 2H), 4.26 (d, J = 8.0 Hz, 2H), 3.69 (s, 3H), 1.34 (d, J = 8.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 170.80 (s), 167.24 (s), 166.03 (s), 165.64 (s), 147.55 (s), 133.85 (s), 133.69 (s), 131.44 (s), 129.37 (s), 129.00 (s), 128.85–128.75 (m), 128.66 (s), 128.30 (s), 127.24 (s), 123.57 (s), 100.13 (s), 60.67 (s), 56.57 (s), 52.95 (s), 43.36 (s), 14.31 (s). White solid (78% yield). ESI-HRMS m/z [M + H]+ calcd for C25H26N2O6 423.1556, found 423.1570.
Methyl (S,Z)-2-(2-(1-(2-butoxy-2-oxoethylidene)-3-oxoisoindolin-2-yl)acetamido)-2-phenylacetate (10c).
1H NMR (400 MHz, CDCl3) δ 9.09 (d, J = 8.0 Hz, 1H), 7.89 (d, J = 8.0 Hz, 1H), 7.70 (t, J = 8.0 Hz, 1H), 7.61 (t, J = 8.0 Hz, 1H), 7.40–7.29 (m, 5H), 7.09–6.98 (m, 1H), 5.74 (s, 1H), 5.57 (d, J = 8.0 Hz, 1H), 4.53 (d, J = 4.0 Hz, 2H), 4.21 (t, J = 8.0 Hz, 2H), 3.69 (s, 3H), 1.74–1.64 (m, 2H), 1.48–1.39 (m, 2H), 0.98 (t, J = 8.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 170.83 (s), 167.31 (s), 166.08 (s), 165.81 (s), 147.56 (s), 135.93 (s), 135.93 (s), 133.96 (s), 133.76 (s), 131.49 (s), 129.43 (s), 129.07 (s), 128.91 (s), 128.72 (s), 128.39 (s), 127.29 (s), 100.21 (s), 64.68 (s), 56.64 (s), 52.98 (s), 43.51 (s), 30.79 (s), 19.25 (s), 13.81 (s). White solid (67% yield). ESI-HRMS m/z [M + H]+ calcd for C25H26N2O6 451.1869, found 451.1873.
Methyl (S,Z)-2-(2-(1-(2-(tert-butoxy)-2-oxoethylidene)-3-oxoisoindolin-2-yl)acetamido)-2-phenylacetate (10d).
1H NMR (400 MHz, CDCl3) δ 9.03 (d, J = 8.0 Hz, 1H), 7.89 (d, J = 8.0 Hz, 1H), 7.69 (t, J = 8.0 Hz, 1H), 7.59 (t, J = 8.0 Hz, 1H), 7.36–7.28 (m, 5H), 6.95 (d, J = 8.0 Hz, 1H), 5.69 (s, 1H), 5.55 (d, J = 8.0 Hz, 1H), 4.50 (m, 2H), 3.69 (s, 3H), 1.54 (s, 9H). 13C NMR (101 MHz, CDCl3) δ 170.76 (s), 167.31 (s), 166.16 (s), 165.00 (s), 146.47 (s), 135.92 (s), 134.03 (s), 133.66 (s), 131.27 (s), 129.39 (s), 129.06 (s), 128.90 (s), 128.70 (s), 128.30 (s), 127.22 (s), 123.56 (s), 102.48 (s), 81.14 (s), 56.58 (s), 52.96 (s), 43.60 (s), 28.28 (s). White solid (64% yield). ESI-HRMS m/z [M + H]+ calcd for C25H26N2O6 451.1869, found 451.1873.
Methyl benzoyl-L-phenylalanyl-L-valinate (6d).
1H NMR (400 MHz, CDCl3) δ 7.72 (d, J = 8.0 Hz, 2H), 7.50 (d, J = 8.0 Hz, 1H), 7.40 (d, J = 4.0 Hz, 2H), 7.32–7.21 (m, 5H), 6.95 (d, J = 8.0 Hz, 1H), 6.50 (d, J = 8.0 Hz, 1H), 4.94 (q, J = 8.0 Hz, 1H), 4.44 (dd, J = 4.0 Hz, 1H), 3.71 (s, 3H), 3.29–3.06 (m, 2H), 2.19–1.93 (m, 1H), 0.83 (d, J = 8.0 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 171.66 (s), 170.93 (s), 167.30 (s), 136.52 (s), 133.80 (s), 131.82 (s), 129.41 (s), 128.71 (s), 128.61 (s), 127.06 (s), 57.52 (s), 54.83 (s), 52.14 (s), 38.25 (s), 31.08 (s), 18.85 (s), 17.74 (s). White solid (60% yield). ESI-HRMS m/z [M + H]+ calcd for C22H26N2O4 383.1971, found 383.1970.
Methyl ((S)-2-((Z)-1-(2-methoxy-2-oxoethylidene)-3-oxoisoindolin-2-yl)-3-phenylpropanoyl)-L-valinate (11a).
1H NMR (400 MHz, CDCl3) δ 9.05 (d, J = 8.0 Hz, 1H), 7.79 (d, J = 8.0 Hz, 1H), 7.69 (t, J = 8.0 Hz, 1H), 7.59 (t, J = 8.0 Hz, 1H), 7.22–7.09 (m, 5H), 6.38 (s, 1H), 5.98 (s, 1H), 5.43 (s, 1H), 4.59 (dd, J = 4.0 Hz, 1H), 3.84 (s, 3H), 3.71 (s, 3H), 3.67–3.60 (m, 1H), 3.44–3.34 (m, 1H), 2.21–2.07 (m, 1H), 0.83 (dd, J = 8.0 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 171.78 (s), 168.59 (s), 167.36 (s), 166.04 (s), 136.70 (s), 133.69 (s), 133.55 (s), 131.43 (s), 128.97 (s), 128.72 (s), 128.55 (s), 128.03 (s), 126.91 (s), 101.06 (s), 57.53 (s), 52.24 (s), 51.80 (s), 34.05 (s), 31.14 (s), 29.21 (s), 18.88 (s), 17.76 (s). White solid (67% yield). ESI-HRMS m/z [M + H]+ calcd for C26H28N2O6 464.1947, found 464.1995.
Methyl ((S)-2-((Z)-1-(2-ethoxy-2-oxoethylidene)-3-oxoisoindolin-2-yl)-3-phenylpropanoyl)-L-valinate (11b).
1H NMR (400 MHz, CDCl3) δ 9.05 (d, J = 8.0 Hz, 1H), 7.79 (d, J = 8.0 Hz, 1H), 7.68 (t, J = 8.0 Hz, 1H), 7.58 (t, J = 8.0 Hz, 1H), 7.23–7.08 (m, 4H), 6.40 (s, 1H), 5.94 (s, 1H), 5.45 (s, 1H), 4.60 (d, J = 4.0 Hz, 1H), 4.38–4.20 (m, 2H), 3.71 (s, 2H), 3.68–3.58 (m, 1H), 3.45–3.32 (m, 1H), 2.20–2.10 (m, 1H), 1.36 (t, J = 8.0 Hz, 3H), 0.87 (dd, J = 8.0 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 171.78 (s), 168.59 (s), 167.36 (s), 166.04 (s), 136.70 (s), 133.69 (s), 133.55 (s), 131.43 (s), 128.97 (s), 128.72 (s), 128.55 (s), 128.03 (s), 126.91 (s), 101.06 (s), 57.53 (s), 52.24 (s), 51.80 (s), 34.05 (s), 31.14 (s), 21.96 (s), 18.88 (s), 17.76 (s). White solid (51% yield). ESI-HRMS m/z [M + H]+ calcd for C27H30N2O6 479.2182, found 479.2165.
Methyl ((S)-2-((Z)-1-(2-butoxy-2-oxoethylidene)-3-oxoisoindolin-2-yl)-3-phenylpropanoyl)-L-valinate (11c).
1H NMR (400 MHz, CDCl3) δ 9.01 (d, J = 8.0 Hz, 1H), 7.74 (d, J = 8.0 Hz, 1H), 7.67–7.61 (t, J = 8.0 Hz, 1H), 7.55–7.51 (t, J = 8.0 Hz, 1H), 7.16–7.06 (m, 5H), 6.49–6.30 (s, 1H), 5.89 (s, 1H), 5.42 (s, 1H), 4.57 (d, J = 8.0 Hz, 1H), 4.30–4.10 (m, 2H), 3.64 (s, 3H), 3.62–3.59 (m, 1H), 3.35 (m, 1H), 2.16–2.06 (m, 1H), 1.71–1.63 (m, 2H), 1.46–1.37 (m, 2H), 0.96 (t, J = 8.0 Hz, 3H), 0.80 (d, J = 8.0 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 171.86 (s), 169.10–168.50 (m), 167.40 (s), 165.77 (s), 136.84 (s), 133.68 (s), 131.41 (s), 129.06 (s), 128.83 (s), 128.56 (s), 128.13 (s), 126.93 (s), 123.55 (s), 101.64 (s), 64.72 (s), 57.57 (s), 52.27 (s), 34.09 (s), 31.29 (s), 30.78 (s), 29.76 (s), 19.24 (s), 19.04 (s), 17.82 (s), 13.79 (s). White solid (58% yield). ESI-HRMS m/z [M + H]+ calcd for C29H34N2O6 507.2498, found 507.2518.
Methyl ((S)-2-((Z)-1-(2-(tert-butoxy)-2-oxoethylidene)-3-oxoisoindolin-2-yl)-3-phenylpropanoyl)-L-valinate (11d).
1H NMR (400 MHz, CDCl3) δ 8.97 (d, J = 8.0 Hz, 1H), 7.76 (d, J = 8.0 Hz, 1H), 7.66 (t, J = 8.0 Hz 1H), 7.55 (t, J = 8.0 Hz, 1H), 7.17–7.09 (m, 5H), 6.37 (s, 1H), 5.81 (s, 1H), 5.41 (s, 1H), 4.60 (dd, J = 8.0 Hz, 1H), 3.69 (s, 3H), 3.68–3.60 (m, 1H), 3.40–3.30 (m, 1H), 2.21–2.05 (m, 1H), 1.55 (s, 9H), 0.98–0.67 (dd, J = 8.0 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 170.76 (s), 167.25 (s), 165.95 (s), 165.62 (s), 147.49 (s), 135.85 (s), 133.87 (s), 133.74 (s), 131.47 (s), 129.34 (s), 129.03 (s), 128.87 (s), 128.69 (s), 128.34 (s), 127.22 (s), 123.62 (s), 100.19 (s), 60.68 (s), 56.57 (s), 52.97 (s), 45.71 (s), 43.50 (s), 29.71 (s), 14.30 (s), 14.17 (s). White solid (55% yield). ESI-HRMS m/z [M + H]+ calcd for C29H34N2O6 507.2498, found 507.2518.
Methyl (2-naphthoyl)glycyl-L-valinate (13a).
1H NMR (400 MHz, CDCl3) δ 8.37 (s, 1H), 8.07 (s, 1H), 7.89 (d, J = 4.0 Hz, 1H), 7.79 (t, J = 8.0 Hz, 1H), 7.71 (d, J = 8.0 Hz, 1H), 7.55–7.41 (m, 1H), 4.60–4.54 (m, 1H), 4.43–4.28 (m, 2H), 3.70 (s, 3H), 2.32–2.08 (m, 1H), 0.95 (dd, J = 8.0 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 172.39 (s), 170.15 (s), 168.16 (s), 134.76 (s), 132.48 (s), 130.62 (s), 129.02 (s), 128.21 (s), 128.08 (s), 127.66 (s), 127.60 (s), 126.56 (s), 123.72 (s), 57.73 (s), 52.14 (s), 43.86 (s), 30.98 (s), 19.08 (s), 17.92 (s). Yellow solid (66% yield). ESI-HRMS m/z [M + H]+ calcd for C19H22N2O4 343.1658, found 343.1665.
Methyl (Z)-(2-(1-(2-methoxy-2-oxoethylidene)-3-oxo-1,3-dihydro-2H-benzo[f]isoindol-2-yl)acetyl)-L-valinate (14a).
1H NMR (400 MHz, CDCl3) δ 9.70 (s, 1H), 8.40 (s, 1H), 8.12–8.06 (m, 1H), 8.06–7.98 (m, 1H), 7.78–7.49 (m, 2H), 6.46 (d, J = 8.0 Hz, 1H), 5.78 (s, 1H), 4.68–4.45 (m, 3H), 3.84 (s, 3H), 3.69 (s, 4H), 2.23–2.09 (m, 1H), 0.88 (dd, J = 8.0 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 171.83 (s), 167.17 (s), 166.50 (s), 166.34 (s), 148.26 (s), 136.02 (s), 133.87 (s), 130.52 (s), 129.96 (s), 129.65 (s), 128.64 (s), 128.54 (s), 126.11 (s), 125.20 (s), 124.73 (s), 97.97 (s), 57.32 (s), 52.26 (s), 51.69 (s), 43.93 (s), 31.20 (s), 18.91 (s), 17.72 (s). Yellow solid (60% yield). ESI-HRMS m/z [M + H]+ calcd for C23H24N2O6 425.1713, found 425.1697.
Methyl (Z)-(2-(1-(2-ethoxy-2-oxoethylidene)-3-oxo-1,3-dihydro-2H-benzo[f]isoindol-2-yl)acetyl)-L-valinate (14b).
1H NMR (400 MHz, CDCl3) δ 9.71 (s, 1H), 8.41 (s, 1H), 8.12–8.06 (m, 1H), 8.05–7.99 (m, 1H), 7.71–7.57 (m, 2H), 6.47 (d, J = 8.7 Hz, 1H), 5.77 (s, 1H), 4.61–4.52 (m, 2H), 4.30 (q, J = 8.0 Hz, 2H), 3.70 (s, 2H), 2.23–2.12 (m, 1H), 1.37 (t, J = 8.0 Hz, 3H), 0.88 (dd, J = 8.0 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 171.82 (s), 167.19 (s), 166.38 (s), 166.05 (s), 147.98 (s), 136.03 (s), 133.86 (s), 130.52 (s), 129.98 (s), 129.65 (s), 128.69 (s), 128.62 (s), 128.51 (s), 126.15 (s), 124.72 (s), 98.61 (s), 60.53 (s), 57.31 (s), 52.27 (s), 43.98 (s), 31.22 (s), 18.94 (s), 17.71 (s), 14.35 (s). Yellow solid (57% yield). ESI-HRMS m/z [M + H]+ calcd for C23H24N2O6 439.1869, found 439.1867.
Methyl ([1,1′-biphenyl]-4-carbonyl)glycyl-L-valinate (16a).
1H NMR (400 MHz, CDCl3) δ 8.07–8.01 (m, 1H), 7.93 (d, J = 8.0 Hz, 2H), 7.73 (d, J = 8.0 Hz, 1H), 7.55 (dd, J = 8.0 Hz, 4H), 7.44–7.31 (m, 3H), 4.57 (dd, J = 8.0 Hz, 1H), 4.31 (d, J = 4.0 Hz, 2H), 3.70 (s, 3H), 2.31–2.09 (m, 1H), 0.95 (dd, J = 6.0 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 172.33 (s), 170.04 (s), 167.85 (s), 144.40 (s), 139.92 (s), 132.18 (s), 128.89 (s), 127.99 (s), 127.89 (s), 127.15 (s), 127.08 (s), 57.69 (s), 52.15 (s), 43.83 (s), 31.03 (s), 19.08 (s), 17.91 (s). Yellow solid (62% yield). ESI-HRMS m/z [M + H]+ calcd for C23H24N2O6 369.1814, found 369.1799.
Methyl (Z)-(2-(3-(2-methoxy-2-oxoethylidene)-1-oxo-5-phenylisoindolin-2-yl)acetyl)-L-valinate (17a).
1H NMR (400 MHz, CDCl3) δ 9.41 (s, 1H), 7.97 (d, J = 8.0 Hz, 1H), 7.85 (d, J = 4.0 Hz, 1H), 7.71 (d, J = 4.0 Hz, 1H), 7.51 (d, J = 8.0 Hz, 1H), 7.43 (d, J = 8.0 Hz, 1H), 6.36 (d, J = 1.0 Hz, 1H), 5.81 (s, 1H), 4.60–4.44 (m, 3H), 3.81 (s, 3H), 3.71 (s, 3H), 2.24–2.08 (m, 1H), 0.89 (dd, J = 6.0 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 171.93 (s), 167.20 (s), 166.49 (s), 166.12 (s), 147.82 (s), 147.14 (s), 140.09 (s), 134.59 (s), 130.46 (s), 129.17 (s), 128.47 (s), 127.97 (s), 127.65 (s), 127.25 (s), 124.04 (s), 99.77 (s), 57.34 (s), 52.41 (s), 51.96 (s), 43.81 (s), 31.31 (s), 19.01 (s), 17.81 (s). Yellow solid (64% yield). ESI-HRMS m/z [M + H]+ calcd for C25H26N2O6 451.1869, found 451.1879.
Methyl (Z)-(2-(3-(2-ethoxy-2-oxoethylidene)-1-oxo-5-phenylisoindolin-2-yl)acetyl)-L-valinate (17b).
1H NMR (400 MHz, CDCl3) δ 9.43 (d, J = 4.0 Hz, 1H), 7.99 (d, J = 4.0 Hz, 1H), 7.87 (d, J = 4.0 Hz, 1H), 7.73 (d, J = 4.0 Hz, 2H), 7.53 (t, J = 8.0 Hz, 2H), 7.45 (t, J = 8.0 Hz, 1H), 6.37 (d, J = 8.0 Hz, 1H), 5.83 (s, 1H), 4.63–4.52 (m, 3H), 4.29 (d, J = 8.0 Hz, 2H), 3.73 (s, 3H), 2.25–2.12 (m, 1H), 1.35 (t, J = 8.0 Hz, 3H), 0.91 (dd, J = 8.0 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 171.91 (s), 167.20 (s), 166.54 (s), 165.66 (s), 147.52 (s), 147.12 (s), 140.12 (s), 134.63 (s), 130.41 (s), 129.16 (s), 128.45 (s), 127.99 (s), 127.66 (s), 127.14 (s), 124.02 (s), 100.40 (s), 60.79 (s), 57.33 (s), 52.40 (s), 43.84 (s), 31.33 (s), 19.03 (s), 17.80 (s), 14.38 (s). Yellow solid (60% yield). ESI-HRMS m/z [M + H]+ calcd for C26H28N2O6 465.2026 found 465.2043.
Methyl (S)-2-(2-(2-naphthamido)acetamido)-2-phenylacetate (13b).
1H NMR (400 MHz, CDCl3) δ 8.33 (s, 1H), 7.87–7.78 (m, 5H), 7.58–7.47 (m, 3H), 7.42–7.37 (m, 2H), 7.35–7.28 (m, 3H), 5.61 (d, J = 8.0 Hz, 1H), 4.46–4.22 (m, 2H), 3.70 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 171.14 (s), 168.90 (s), 167.93 (s), 136.01 (s), 134.94 (s), 132.62 (s), 130.72 (s), 129.13 (s), 128.78 (s), 128.50 (s), 128.00 (s), 127.87 (s), 127.79 (s), 127.47 (s), 126.81 (s), 123.74 (s), 56.91 (s), 52.99 (s), 43.78 (s). Yellow solid (60% yield). ESI-HRMS m/z [M + H]+ calcd for C22H20N2O4 377.1501, found 377.1418.
Methyl-(S,Z)-2-(2-(1-(2-methoxy-2-oxoethylidene)-3-oxo-1,3-dihydro-2H-benzo[f]isoindol-2-yl)acetamido)-2-phenylacetate (14c).
1H NMR (400 MHz, CDCl3) δ 9.70 (s, 1H), 8.39 (s, 1H), 8.12–8.07 (m, 1H), 8.04–7.98 (m, 1H), 7.67–7.61 (m, 2H), 7.37–7.29 (m, 5H), 7.00 (d, J = 8.0 Hz, 1H), 5.73 (s, 1H), 5.63–5.50 (m, 1H), 4.57 (s, 2H), 3.83 (s, 3H), 3.68 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 170.76 (s), 167.15 (s), 166.47 (s), 165.80 (s), 148.27 (s), 136.02 (s), 135.86 (s), 133.87 (s), 130.53 (s), 129.96 (s), 129.66 (s), 129.02 (s), 128.88 (s), 128.67 (s), 128.64 (s), 128.54 (s), 127.24 (s), 126.12 (s), 124.77 (s), 98.02 (s), 56.60 (s), 52.95 (s), 51.67 (s), 43.75 (s). Yellow solid (62% yield). ESI-HRMS m/z [M + H]+ calcd for C26H22N2O6 459.1556, found 459.1564.
Methyl(S,Z)-2-(2-(1-(2-ethoxy-2-oxoethylidene)-3-oxo-1,3-dihydro-2H-benzo[f]isoindol-2-yl)acetamido)-2-phenylacetate (14d).
1H NMR (400 MHz, CDCl3) δ 9.75 (s, 1H), 8.44 (s, 1H), 8.17–8.11 (m, 1H), 8.07–8.02 (m, 1H), 7.71–7.64 (m, 2H), 7.42–7.30 (m, 7H), 6.97 (d, J = 6.8 Hz, 1H), 5.76 (s, 1H), 5.60 (d, J = 8.0 Hz, 1H), 4.60 (d, J = 2.7 Hz, 2H), 4.32 (d, J = 8.0 Hz, 2H), 3.71 (s, 2H), 1.38 (t, J = 8.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 170.75 (s), 167.21 (s), 166.07 (s), 165.83 (s), 148.01 (s), 136.05 (s), 135.86 (s), 133.87 (s), 130.54 (s), 130.02 (s), 129.67 (s), 129.21 (s), 129.04 (s), 128.89–128.87 (m), 128.69 (s), 128.65 (s), 127.22 (s), 126.14 (s), 124.79 (s), 98.66 (s), 60.57 (s), 56.58 (s), 52.98 (s), 43.83 (s), 14.38 (s). Yellow solid (58% yield). ESI-HRMS m/z [M + H]+ calcd for C27H24N2O6 473.1713, found 473.1757.
Methyl (S)-2-(2-([1,1′-biphenyl]-4-carboxamido)acetamido)-2-phenylacetate (16b).
1H NMR (400 MHz, CDCl3) δ 7.88 (d, J = 8.0 Hz, 2H), 7.70–7.58 (m, 5H), 7.52–7.45 (m, 2H), 7.45–7.33 (m, 6H), 7.31 (s, 1H), 5.62 (d, J = 8.0 Hz, 1H), 4.39–4.14 (m, 2H), 3.74 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 171.12 (s), 168.75 (s), 167.55 (s), 144.66 (s), 140.01 (s), 135.98 (s), 132.16 (s), 129.16 (s), 129.01 (s), 128.81 (s), 128.13 (s), 127.81 (s), 127.47 (s), 127.30 (s), 56.87 (s), 53.02 (s), 43.67 (s). Yellow solid (55% yield). ESI-HRMS m/z [M + H]+ calcd for C24H22N2O4 403.1658, found 403.1653.
Methyl (S,Z)-2-(2-(3-(2-methoxy-2-oxoethylidene)-1-oxo-5-phenylisoindolin-2-yl)acetamido)-2-phenylacetate (17c).
1H NMR (400 MHz, CDCl3) δ 9.39 (d, J = 0.8 Hz, 1H), 7.94 (d, J = 8.0 Hz, 1H), 7.86–7.80 (m, J = 7.9, 1.4 Hz, 1H), 7.73–7.66 (m, 2H), 7.50 (t, J = 8.0 Hz, 2H), 7.43 (d, J = 8.0 Hz, 1H), 7.35–7.29 (m, 5H), 6.96 (d, J = 6.9 Hz, 1H), 5.76 (s, 1H), 5.56 (d, J = 8.0 Hz, 1H), 4.53 (d, J = 4.0 Hz, 2H), 3.80 (s, 3H), 3.69 (s, 3H).13C NMR (101 MHz, CDCl3) δ 170.78 (s), 167.08 (s), 166.02 (s), 165.92 (s), 147.75 (s), 147.05 (s), 140.04 (s), 135.86 (s), 134.55 (s), 130.34 (s), 129.09 (s), 129.03 (s), 128.69 (s), 128.38 (s), 127.94 (s), 127.57 (s), 127.24 (s), 127.15 (s), 123.96 (s), 99.72 (s), 56.58 (s), 52.96 (s), 51.82 (s), 43.55 (s). Yellow solid (58% yield). ESI-HRMS m/z [M + H]+ calcd for C28H24N2O6 485.1713, found 485.1740.
Methyl (S,Z)-2-(2-(3-(2-ethoxy-2-oxoethylidene)-1-oxo-5-phenylisoindolin-2-yl)acetamido)-2-phenylacetate (17d).
1H NMR (400 MHz, CDCl3) δ 9.39 (d, J = 0.9 Hz, 1H), 7.95 (d, J = 8.0 Hz, 1H), 7.86–7.80 (m, 1H), 7.72–7.66 (m, 2H), 7.53–7.46 (m, 2H), 7.46–7.39 (m, 1H), 7.38–7.27 (m, 5H), 6.96 (d, J = 6.9 Hz, 1H), 5.76 (s, 1H), 5.56 (d, J = 8.0 Hz, 1H), 4.59–4.44 (m, 2H), 4.26 (d, J = 8.0 Hz, 2H), 3.70 (s, 3H), 1.33 (d, J = 8.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 170.77 (s), 167.11 (s), 165.99 (s), 165.59 (s), 147.47 (s), 147.03 (s), 140.08 (s), 135.88 (s), 134.60 (s), 130.30 (s), 129.08 (s), 129.03 (s), 128.69 (s), 128.36 (s), 127.95 (s), 127.58 (s), 127.23 (s), 127.18 (s), 123.94 (s), 100.33 (s), 60.70 (s), 56.58 (s), 52.96 (s), 43.58 (s), 14.32 (s). Yellow solid (56% yield). ESI-HRMS m/z [M + H]+ calcd for C29H26N2O6 498.1869, found 498.1845.
Methyl (4-methylbenzoyl)-L-phenylalanylvalinate (19a).
1H NMR (400 MHz, CDCl3) δ 7.62 (d, J = 8.1 Hz, 2H), 7.32–7.18 (m, 7H), 6.87 (d, J = 7.1 Hz, 1H), 6.53 (d, J = 7.9 Hz, 1H), 5.00–4.85 (m, 1H), 4.43 (d, J = 8.0 Hz, 1H), 3.71 (s, 3H), 3.28–3.11 (m, J = 8.0 Hz, 2H), 2.38 (s, 3H), 2.12–2.04 (m, 1H), 0.98–0.71 (m, J = 8.0 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 171.68 (s), 170.99 (s), 167.26 (s), 142.31 (s), 136.59 (s), 130.95 (s), 129.41 (s), 129.27 (s), 128.69 (s), 127.06 (s), 127.03 (s), 57.51 (s), 54.74 (s), 52.12 (s), 38.19 (s), 31.07 (s), 21.46 (s), 18.85 (s), 17.74 (s). White solid (40% yield). ESI-HRMS m/z [M + H]+ calcd for C23H28N2O4 397.2127, found 397.2145.
Methyl ((S)-2-((Z)-3-(2-methoxy-2-oxoethylidene)-5-methyl-1-oxoisoindolin-2-yl)-3-phenylpropanoyl)-L-valinate (20a).
1H NMR (400 MHz, CDCl3) δ 8.85 (s, 1H), 7.64 (d, J = 8.0 Hz, 1H), 7.36 (d, J = 8.0 Hz, 1H), 7.19–7.02 (m, 5H), 6.40 (s, 1H), 5.92 (s, 1H), 5.41 (s, 1H), 4.56 (dd, J = 8.0 Hz, 1H), 3.81 (s, 3H), 3.67 (s, 3H), 3.64–3.56 (m, 1H), 3.42–3.30 (m, 1H), 2.50 (s, 3H), 2.16–1.98 (m, 1H), 0.82 (dd, J = 8.0 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 171.87 (s), 168.81 (s), 167.41 (s), 166.20 (s), 144.34 (s), 136.86 (s), 133.93 (s), 132.34 (s), 129.04 (s), 128.56 (d, J = 3.0 Hz), 126.91 (s), 126.31 (s), 123.42 (s), 100.40 (s), 57.59 (s), 52.27 (s), 51.83 (s), 34.03 (s), 31.19 (s), 29.72 (s), 22.42 (s), 18.96 (s), 17.84 (s). White solid (40% yield). ESI-HRMS m/z [M + H]+ calcd for C27H30N2O6 479.2182, found 479.2209.
Methyl ((S)-2-((Z)-3-(2-ethoxy-2-oxoethylidene)-5-methyl-1-oxoisoindolin-2-yl)-3-phenylpropanoyl)-L-valinate (20b).
1H NMR (400 MHz,) δ 8.83 (s, 1H), 7.62 (d, J = 8.0 Hz, 1H), 7.34 (d, J = 8.0 Hz, 1H), 7.18–7.05 (m, 5H), 6.40 (s, 1H), 5.86 (s, 1H), 5.39 (s, 1H), 4.56 (dd, J = 8.0 Hz, 1H), 4.29–4.21 (m, 2H), 3.65 (s, 3H), 3.64–3.57 (m, 1H), 3.41–3.26 (m, 1H), 2.49 (s,3H), 2.17–2.03 (m,1H), 1.33 (t, J = 8.0 Hz, 3H), 0.80 (dd, J = 8.0 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 171.86 (s), 168.88 (s), 167.40 (s), 165.74 (s), 144.73 (s), 136.96 (s), 133.99 (s), 132.29 (s), 129.06 (s), 128.55 (s), 126.90 (s), 126.38 (s), 123.41 (s), 101.26 (s), 60.72 (s), 57.58 (s), 52.21 (s), 34.03 (s), 31.24 (s), 29.81 (s), 22.39 (s), 19.04 (s), 17.83 (s), 14.38 (s). White solid (40% yield). ESI-HRMS m/z [M + H]+ calcd for C28H32N2O6 492.2339, found 492.2367.
Methyl (4-(tert-butyl)benzoyl)-L-phenylalanyl-L-valinate (19b).
1H NMR (400 MHz, CDCl3) δ 7.67 (d, J = 8.0 Hz, 2H), 7.43–7.39 (m, 2H), 7.30–7.19 (m, 6H), 6.98 (d, J = 8.0 Hz, 1H), 6.66 (d, J = 8.1 Hz, 1H), 4.96 (q, J = 8.0 Hz, 1H), 4.45 (dd, J = 8.0 Hz, 1H), 3.71 (s, 3H), 3.28–3.13 (m, 2H), 2.14–2.00 (m, 1H), 1.32 (s, 9H), 0.83 (dd, J = 8.0 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 171.70 (s), 171.12 (s), 167.28 (s), 155.35 (s), 136.62 (s), 130.88 (s), 129.41 (s), 128.67 (s), 126.97 (d, J = 4.6 Hz), 125.53 (s), 125.22 (s), 57.50 (s), 54.73 (s), 52.11 (s), 38.13 (s), 34.94 (s), 31.14 (s), 31.08 (s), 18.86 (s), 17.75 (s). White solid (55% yield). ESI-HRMS m/z [M + H]+ calcd for C26H34N2O4 439.2597, found 439.2552.
Methyl ((S)-2-((Z)-5-(tert-butyl)-3-(2-methoxy-2-oxoethylidene)-1-oxoisoindolin-2-yl)-3-phenylpropanoyl)-L-valinate (21a).
1H NMR (400 MHz, CDCl3) δ 9.18 (d, J = 4.0 Hz, 1H), 7.70 (d, J = 8.0 Hz, 1H), 7.61 (d, J = 8.0 Hz, 1H), 7.20–7.05 (m, 5H), 6.34 (s, 1H), 5.91 (s, 1H), 5.38 (s, 1H), 4.60–4.52 (m, 1H), 3.82 (s, 3H), 3.69 (s, 3H), 3.66–3.59 (m, 1H), 3.45–3.28 (m, 1H), 2.16–2.00 (m, 1H), 1.41 (s, 9H), 0.82 (d, J = 8.0 Hz, 7H). 13C NMR (101 MHz, CDCl3) δ 171.79 (s), 168.72 (s), 166.08 (s), 157.92 (s), 146.94 (s), 136.89 (s), 133.75 (s), 129.34 (s), 128.99 (s), 128.75 (s), 128.54 (s), 126.84 (s), 126.12 (s), 125.31 (s), 123.13 (s), 100.68 (s), 57.55 (s), 52.22 (s), 51.80 (s), 35.87 (s), 34.09 (s), 31.34 (s), 31.19 (s), 29.70 (s), 18.89 (s), 17.84 (s). White solid (35% yield). ESI-HRMS m/z [M + H]+ calcd for C30H36N2O6 521.2651, found 521.2672.
Methyl ((S)-2-((Z)-5-(tert-butyl)-3-(2-ethoxy-2-oxoethylidene)-1-oxoisoindolin-2-yl)-3-phenylpropanoyl)-L-valinate (21b).
1H NMR (400 MHz, CDCl3) δ 9.17 (d, J = 1.1 Hz, 1H), 7.69 (d, J = 8.0 Hz, 1H), 7.60 (dd, J = 8.0, 1.6 Hz, 1H), 7.21–7.08 (m, 1H), 6.37 (s, 1H), 5.88 (s, 1H), 5.40 (s, 1H), 4.58 (dd, J = 8.6, 5.0 Hz, 1H), 4.35–4.20 (m, 1H), 3.68 (s, 1H), 3.67–3.61 (m, 1H), 3.42–3.30 (m, 1H), 2.20–2.07 (m, 1H), 1.40 (s, 9H), 1.34 (t, J = 8.0 Hz, 3H), 0.84 (d, J = 8.0 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 171.80 (s), 168.80 (s), 165.62 (s), 157.86 (s), 136.94 (s), 133.79 (s), 129.00 (s), 128.96 (s), 128.69 (s), 128.51 (s), 126.82 (s), 126.14 (s), 125.31 (s), 123.10 (s), 101.15 (s), 60.62 (s), 57.56 (s), 52.22 (s), 35.86 (s), 34.09 (s), 31.34 (s), 31.24 (s), 29.70 (s), 18.97 (s), 17.86 (s), 14.34 (s). White solid (32% yield). ESI-HRMS m/z [M + H]+ calcd for C31H38N2O6 535.2730, found 535.2773.
Methyl ((S)-2-((Z)-3-(2-butoxy-2-oxoethylidene)-5-(tert-butyl)-1-oxoisoindolin-2-yl)-3-phenylpropanoyl)-L-valinate (21c).
1H NMR (400 MHz, CDCl3) δ 9.19 (s, 1H), 7.71 (d, J = 8.0 Hz, 1H), 7.62 (d, J = 9.4 Hz, 1H), 7.21–7.10 (m, 3H), 6.41 (s, 1H), 5.89 (s, 1H), 5.43 (s, 1H), 4.60 (dd, J = 8.6, 4.9 Hz, 1H), 4.31–4.16 (m, 2H), 3.69 (s, 2H), 3.67–3.62 (m, 1H), 3.44–3.30 (m, 1H), 2.19–2.10 (m, 1H), 1.74–1.65 (m, 2H), 1.49–1.44 (m, 2H), 1.42 (s, 9H), 0.99 (t, J = 8.0 Hz, 3H), 0.86 (dd, J = 8.0 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 171.79 (s), 168.81 (s), 165.74 (s), 157.85 (s), 150.88 (s), 136.95 (s), 133.81 (s), 129.00 (s), 128.68 (s), 128.50 (s), 128.25 (s), 126.81 (s), 126.14 (s), 125.31 (s), 123.09 (s), 101.17 (s), 64.58 (s), 57.55 (s), 52.21 (s), 35.86 (s), 34.07 (s), 31.34 (s), 31.27 (s), 30.75 (s), 29.70 (s), 19.18 (s), 18.97 (s), 17.85 (s), 13.75 (s). White solid (30% yield). ESI-HRMS m/z [M + H]+ calcd for C33H42N2O6 563.3121, found 563.3135.
Methyl ((S)-2-((Z)-3-(2-(tert-butoxy)-2-oxoethylidene)-5-(tert-butyl)-1-oxoisoindolin-2-yl)-3-phenylpropanoyl)-L-valinate (21d).
1H NMR (400 MHz, CDCl3) δ 9.03 (s, 1H), 7.67 (d, J = 8.0 Hz, 1H), 7.62–7.55 (m, 1H), 7.21–7.08 (m, 5H), 6.40 (s, 1H), 5.78 (s, 1H), 5.39 (s, 1H), 4.63–4.55 (m, 1H), 3.68 (s, 3H), 3.66–3.62 (m, 1H), 3.42–3.26 (m, 1H), 2.13 (m, 1H), 1.55 (s, 9H), 1.39 (s, 9H), 0.86 (dd, J = 8.0 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 171.78 (s), 168.95 (s), 165.01 (s), 157.60 (s), 137.08 (s), 133.91 (s), 129.03 (s), 128.95 (s), 128.47 (s), 128.28 (s), 126.77 (s), 126.17 (s), 124.83 (s), 123.01 (s), 103.33 (s), 81.00 (s), 57.57 (s), 52.21 (s), 35.81 (s), 34.08 (s), 31.30 (s), 31.24 (s), 29.70 (s), 28.21 (s), 19.10 (s), 17.87 (s). White solid (32% yield). ESI-HRMS m/z [M + H]+ calcd for C33H42N2O6 562.3043, found 562.3095.
Methyl (3-nitrobenzoyl)-L-phenylalanyl-L-valinate (19c).
1H NMR (400 MHz, CDCl3) δ 8.59 (s, 1H), 8.35 (d, J = 8.0 Hz, 1H), 8.08 (d, J = 8.0 Hz, 1H), 7.61 (t, J = 8.0 Hz, 1H), 7.41 (d, J = 8.0 Hz, 1H), 7.36–7.25 (m, 5H), 6.46 (d, J = 8.0 Hz, 1H), 4.97 (q, J = 8.0 Hz, 1H), 4.48 (dd, J = 8.0 Hz, 1H), 3.75 (s, 3H), 3.23 (m, 1H), 2.21–2.09 (m, 1H), 0.88 (dd, J = 8.0 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 171.61 (s), 170.89 (s), 164.79 (s), 148.27 (s), 136.21 (s), 135.38 (s), 132.92 (s), 129.73 (s), 129.37 (s), 128.78 (s), 127.25 (s), 126.27 (s), 122.36 (s), 57.64 (s), 55.23 (s), 52.22 (s), 38.47 (s), 31.08 (s), 18.87 (s), 17.77 (s). White solid (53% yield). ESI-HRMS m/z [M + H]+ calcd for C22H25N2O4 428.1822, found 428.1808.
Methyl benzoylglycyl-L-alanyl-L-phenylalaninate (22a).
1H NMR (400 MHz, CDCl3) δ 7.83 (d, J = 7.3 Hz, 1H), 7.51 (t, J = 7.3 Hz, 1H), 7.45–7.38 (m, 1H), 7.32 (d, J = 7.5 Hz, 1H), 7.24–7.15 (m, 1H), 7.10–7.02 (m, 1H), 4.88–4.79 (m, 1H), 4.58 (t, J = 8.0 Hz, 1H), 4.12–4.07 (m, 2H), 3.68 (s, 3H), 3.13 (m, 1H), 3.04 (m, 1H), 1.34 (d, J = 8.0 Hz, 2H).13C NMR (101 MHz, CDCl3) δ 171.82 (s), 168.98 (s), 167.88 (s), 166.79 (s), 135.82 (s), 133.50 (s), 131.89 (s), 129.23 (s), 128.58 (s), 128.51 (s), 127.28 (s), 127.09 (s), 53.31 (s), 52.39 (s), 48.98 (s), 43.47 (s), 37.80 (s), 18.18 (s). White solid (50% yield). ESI-HRMS m/z [M + H]+ calcd for C22H26N3O5 412.1873, found 412.1873.
Methyl (2-((Z)-1-(2-methoxy-2-oxoethylidene)-3-oxoisoindolin-2-yl)acetyl)-L-alanyl-L-phenylalaninate (23).
1H NMR (400 MHz, CDCl3) δ 9.05 (d, J = 7.9 Hz, 1H), 7.85 (d, J = 7.4 Hz, 1H), 7.71–7.63 (m, J = 9.6, 5.7 Hz, 1H), 7.61–7.54 (m, J = 13.2, 5.9 Hz, 1H), 7.29–7.17 (m, 1H), 7.07 (d, J = 6.8 Hz, 1H), 6.98 (t, J = 8.8 Hz, 1H), 6.74 (t, J = 7.9 Hz, 1H), 5.68 (s, 1H), 4.78 (m, 1H), 4.59–4.49 (m, 1H), 4.44 (s, 2H), 3.75 (s, 3H), 3.68 (s, 3H), 3.16–2.97 (m, 2H), 1.32 (d, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 171.62 (s), 171.53 (s), 167.20 (s), 166.33 (s), 166.04 (s), 147.96 (s), 135.60 (s), 133.78 (s), 133.66 (s), 131.48 (s), 129.42 (s), 129.22 (s), 128.60 (s), 128.25 (s), 127.19 (s), 123.51 (s), 99.13 (s), 53.43 (s), 52.43 (s), 51.73 (s), 48.91 (s), 43.06 (s), 37.74 (s), 18.37 (s). White solid (28% yield). ESI-HRMS m/z [M + H]+ calcd for C26H27N3O7 493.1849, found 493.4983.
Methyl benzoyl-L-valyl-L-leucyl-L-phenylalaninate (22b).
1H NMR (400 MHz, DMSO) δ 8.17 (d, J = 8.1 Hz, 1H), 7.94 (d, J = 8.2 Hz, 1H), 7.90–7.82 (m, 1H), 7.55–7.48 (m, 1H), 7.44 (dd, J = 9.2, 4.1 Hz, 1H), 7.23 (t, J = 7.1 Hz, 1H), 7.17 (d, J = 5.8 Hz, 1H), 4.53 (d, J = 8.0 Hz, 1H), 4.41 (q, J = 8.0 Hz, 1H), 4.32 (t, J = 8.0 Hz, 1H), 3.58 (s, 3H), 2.99 (m, 2H), 2.13 (m, 1H), 1.59 (m, 1H), 1.43 (t, J = 8.0 Hz, 2H), 0.94–0.79 (m, 12H). 13C NMR (101 MHz, DMSO) δ 172.32 (s), 171.91 (s), 171.14 (s), 167.07 (s), 137.21 (s), 134.86 (s), 131.48 (s), 129.30 (s), 128.50 (s), 128.44 (s), 127.82 (s), 126.82 (s), 59.56 (s), 53.62 (s), 52.05 (s), 51.67–51.39 (m), 41.45 (s), 37.16 (s), 30.59 (s), 24.49 (s), 23.28 (s), 22.11 (s), 19.76 (s), 19.06 (s). White solid (42% yield). ESI-HRMS m/z [M + H]+ calcd for C28H38N3O5 496.2812, found 496.3018.
Methyl ((S)-2-((Z)-1-(2-methoxy-2-oxoethylidene)-3-oxoisoindolin-2-yl)-3-methylbutanoyl)-L-leucyl-L-phenylalaninate (24).
1H NMR (400 MHz, CDCl3) δ 9.06 (d, J = 7.9 Hz, 1H), 7.95 (d, J = 15.8 Hz, 1H), 7.86 (d, J = 7.4 Hz, 1H), 7.69 (td, J = 7.8, 1.1 Hz, 1H), 7.61 (t, J = 7.2 Hz, 1H), 7.29 (dd, J = 16.1, 6.9 Hz, 1H), 7.15 (d, J = 7.4 Hz, 1H), 6.75 (d, J = 7.7 Hz, 1H), 6.41 (d, J = 15.7 Hz, 1H), 6.26 (s, 1H), 4.78 (d, J = 8.0 Hz, 1H), 4.46–4.38 (m, 1H), 3.80 (s, 3H), 3.78 (s, 3H), 3.65 (m, 2H), 3.34–3.16 (m, 2H), 1.62–1.53 (m, 1H), 1.48–1.38 (m, 2H), 1.13–0.67 (m, 12H). 13C NMR (101 MHz, CDCl3) δ 171.29 (s), 171.25 (s), 168.93 (s), 167.58 (s), 166.27 (s), 141.77 (s), 135.84 (s), 133.73 (s), 133.52 (s), 131.34 (s), 131.14 (s), 130.22 (s), 128.83 (s), 128.02 (s), 127.72 (s), 126.74 (s), 123.46 (s), 119.69 (s), 101.51 (s), 53.14 (s), 52.45 (s), 51.83 (s), 51.73 (s), 39.94 (s), 34.87 (s), 31.92 (s), 29.70 (s), 24.69 (s), 22.80 (s), 21.40 (s), 20.84 (s), 14.13 (s). White solid (25% yield). ESI-HRMS m/z [M + Na]+ calcd for C32H39N3O7 601.2686, found 601.4069.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
MKG thanks UGC for JRF/SRF. NKS thanks SERB-New Delhi Core Research Grant with Grant No. CRG/2020/001028.
References
- B. Şener, B. Gözler, R. D. Minard and M. Shamma, Alkaloids of Fumaria vaillantii, Phytochemistry, 1983, 22(9), 2073–2075 CrossRef.
- G. Blaskó, D. J. Gula and M. Shamma, The phthalideisoquinoline alkaloids, J. Nat. Prod., 1982, 45(2), 105–122 CrossRef.
- S. P. Upadhyay, P. Thapa, R. Sharma and M. Sharma, 1-Isoindolinone scaffold-based natural products with a promising diverse bioactivity, Fitoterapia, 2020, 104722 CrossRef CAS PubMed.
- A. F. B. Räder, M. Weinmüller, F. Reichart, A. Schumacher-Klinger, S. Merzbach, C. Gilon, A. Hoffman and H. Kessler, Orally Active Peptides: Is There a Magic Bullet?, Angew. Chem., Int. Ed., 2018, 57(44), 14414–14438 CrossRef PubMed.
- H. Zhang, J. Wu, J. Wang, S. Xiao, L. Zhao, R. Yan, X. Wu, Z. Wang, L. Fan and Y. Jin, Novel Isoindolinone-Based Analogs of the Natural Cyclic Peptide Fenestin A: Synthesis and Antitumor Activity, ACS Med. Chem. Lett., 2022, 13(7), 1118–1124 CrossRef CAS PubMed.
- U. C. Yoon, Y. X. Jin, S. W. Oh, C. H. Park, J. H. Park, C. F. Campana, X. Cai, E. N. Duesler and P. S. Mariano, A synthetic strategy for the preparation of cyclic peptide mimetics based on SET-promoted photocyclization processes, J. Am. Chem. Soc., 2003, 125(35), 10664–10671 CrossRef CAS PubMed.
- B. Li, L. Wang, X. Chen, X. Chu, H. Tang, J. Zhang, G. He, L. Li and G. Chen, Extendable stapling of unprotected peptides by crosslinking two amines with o-phthalaldehyde, Nat. Commun., 2022, 13(1), 311 CrossRef CAS PubMed.
- W. S. Horne, C. D. Stout and M. R. Ghadiri, A heterocyclic peptide nanotube, J. Am. Chem. Soc., 2003, 125(31), 9372–9376 CrossRef CAS PubMed.
- A. Boto, C. C. González, D. Hernández, I. Romero-Estudillo and C. J. Saavedra, Site-selective modification of peptide backbones, Org. Chem. Front., 2021, 8(23), 6720–6759 RSC.
- H. Yang, Y. Du, S. Wan, G. D. Trahan, Y. Jin and W. Zhang, Mesoporous 2D covalent organic frameworks based on shape-persistent arylene-ethynylene macrocycles, Chem. Sci., 2015, 6(7), 4049–4053 RSC.
- P. Thapa, E. Corral, S. Sardar, B. S. Pierce and F. W. Foss Jr., Isoindolinone Synthesis: Selective Dioxane-Mediated Aerobic Oxidation of Isoindolines, J. Org. Chem., 2018, 84(2), 1025–1034 CrossRef PubMed.
- O. Anamimoghadam, S. Mumtaz, A. Nietsch, G. Saya, C. A. Motti, J. Wang, P. C. Junk, A. M. Qureshi and M. Oelgemöller, The photodecarboxylative addition of carboxylates to phthalimides as a key-step in the synthesis of biologically active 3-arylmethylene-2, 3-dihydro-1H-isoindolin-1-ones, Beilstein J. Org. Chem., 2017, 13(1), 2833–2841 CAS.
- A. Abell, M. Oldham and J. Taylor, Synthesis of Cyclic Acylated Enamino Esters from Enol Lactones, 4-Keto Amides, and 5-Hydroxy Lactams, J. Org. Chem., 1995, 60(5), 1214–1220 CrossRef CAS.
- M. K. Gupta, C. K. Jena and N. K. Sharma, Pd-Catalyzed C (sp 2)–H olefination: synthesis of N-alkylated isoindolinone scaffolds from aryl amides of amino acid esters, Org. Biomol. Chem., 2021, 19(46), 10097–10104 RSC.
- J. M. Chalker, G. J. Bernardes and B. G. Davis, A “tag-and-modify” approach to site-selective protein modification, Acc. Chem. Res., 2011, 44(9), 730–741 CrossRef CAS PubMed.
- W. Ali, G. Prakash and D. Maiti, Recent development in transition metal-catalysed
C–H olefination, Chem. Sci., 2021, 12(8), 2735–2759 RSC.
- M. C. Reddy and M. Jeganmohan, Total synthesis of aristolactam alkaloids via synergistic C–H bond activation and dehydro-Diels–Alder reactions, Chem. Sci., 2017, 8(5), 4130–4135 RSC.
- A. Deb, A. Hazra, Q. Peng, R. S. Paton and D. Maiti, Detailed mechanistic studies on palladium-catalyzed selective C–H olefination with aliphatic alkenes: a significant influence of proton shuttling, J. Am. Chem. Soc., 2017, 139(2), 763–775 CrossRef CAS PubMed.
- S. Bag, T. Patra, A. Modak, A. Deb, S. Maity, U. Dutta, A. Dey, R. Kancherla, A. Maji and A. Hazra, Remote para-C–H functionalization of arenes by a D-shaped biphenyl template-based assembly, J. Am. Chem. Soc., 2015, 137(37), 11888–11891 CrossRef CAS PubMed.
- A. Deb, S. Bag, R. Kancherla and D. Maiti, Palladium-catalyzed aryl C–H olefination with unactivated, aliphatic alkenes, J. Am. Chem. Soc., 2014, 136(39), 13602–13605 CrossRef CAS PubMed.
- K. Seth, M. Bera, M. Brochetta, S. Agasti, A. Das, A. Gandini, A. Porta, G. Zanoni and D. Maiti, Incorporating unbiased, unactivated aliphatic alkenes in Pd(II)-catalyzed olefination of benzyl phosphonamide, ACS Catal., 2017, 7(11), 7732–7736 CrossRef CAS.
- N. Thrimurtulu, A. Dey, A. Singh, K. Pal, D. Maiti and C. M. Volla, Palladium Catalyzed Regioselective C4−Arylation and Olefination of Indoles and Azaindoles, Adv. Synth. Catal., 2019, 361(6), 1441–1446 CrossRef CAS.
- S. Maity, E. Hoque, U. Dhawa and D. Maiti, Palladium catalyzed selective distal C–H olefination of biaryl systems, Chem. Commun., 2016, 52(97), 14003–14006 RSC.
- R. Manoharan, G. Sivakumar and M. Jeganmohan, Cobalt-catalyzed C–H olefination of aromatics with unactivated alkenes, Chem. Commun., 2016, 52(69), 10533–10536 RSC.
- T. Patra, R. Watile, S. Agasti, T. Naveen and D. Maiti, Sequential meta-C–H olefination of synthetically versatile benzyl silanes: effective synthesis of meta-olefinated toluene, benzaldehyde and benzyl alcohols, Chem. Commun., 2016, 52(10), 2027–2030 RSC.
- T. K. Achar, K. Ramakrishna, T. Pal, S. Porey, P. Dolui, J. P. Biswas and D. Maiti, Regiocontrolled remote C− H olefination of small heterocycles, Chem. – Eur. J., 2018, 24(68), 17906–17910 CrossRef CAS PubMed.
- T. K. Achar, S. Maiti, S. Jana and D. Maiti, Transition metal catalyzed enantioselective C (sp2)–H bond functionalization, ACS Catal., 2020, 10(23), 13748–13793 CrossRef CAS.
- U. Dutta, S. Maiti, T. Bhattacharya and D. Maiti, Arene diversification through distal C (sp2)− H functionalization, Science, 2021, 372(6543), eabd5992 CrossRef CAS PubMed.
- A. Saha, S. Guin, W. Ali, T. Bhattacharya, S. Sasmal, N. Goswami, G. Prakash, S. K. Sinha, H. B. Chandrashekar and S. Panda, Photoinduced regioselective olefination of arenes at proximal and distal sites, J. Am. Chem. Soc., 2022, 144(4), 1929–1940 CrossRef CAS PubMed.
- U. Dutta, S. Maiti, S. Pimparkar, S. Maiti, L. R. Gahan, E. H. Krenske, D. W. Lupton and D. Maiti, Rhodium catalyzed template-assisted distal para-C–H olefination, Chem. Sci., 2019, 10(31), 7426–7432 RSC.
- S. Maity, P. Dolui, R. Kancherla and D. Maiti, Introducing unactivated acyclic internal aliphatic olefins into a cobalt catalyzed allylic selective dehydrogenative Heck reaction, Chem. Sci., 2017, 8(7), 5181–5185 RSC.
- A. Baccalini, S. Vergura, P. Dolui, S. Maiti, S. Dutta, S. Maity, F. F. Khan, G. K. Lahiri, G. Zanoni and D. Maiti, Cobalt-Catalyzed C (sp2)–H Allylation of Biphenyl Amines with Unbiased Terminal Olefins, Org. Lett., 2019, 21(21), 8842–8846 CrossRef CAS PubMed.
- C. Xia, A. J. White and K. K. M. Hii, Synthesis of isoindolinones by Pd-catalyzed coupling between N-methoxybenzamide and styrene derivatives, J. Org. Chem., 2016, 81(17), 7931–7938 CrossRef CAS PubMed.
- C. Zhu and J. Falck, N-acylsulfonamide assisted tandem C–H olefination/annulation: synthesis of isoindolinones, Org. Lett., 2011, 13(5), 1214–1217 CrossRef CAS PubMed.
- S. W. Youn, T. Y. Ko, Y. H. Kim and Y. A. Kim, Pd(II)/Cu(II)-Catalyzed regio-and stereoselective synthesis of (E)-3-arylmethyleneisoindolin-1-ones using air as the terminal oxidant, Org. Lett., 2018, 20(24), 7869–7874 CrossRef CAS PubMed.
- A. F. Noisier and M. A. Brimble, C–H functionalization in the synthesis of amino acids and peptides, Chem. Rev., 2014, 114(18), 8775–8806 CrossRef CAS PubMed.
- W. Gong, G. Zhang, T. Liu, R. Giri and J.-Q. Yu, Site-selective C (sp3)–H functionalization of di-, tri-, and tetrapeptides at the N-terminus, J. Am. Chem. Soc., 2014, 136(48), 16940–16946 CrossRef CAS PubMed.
- G. Chen, Z. Zhuang, G. C. Li, T. G. Saint-Denis, Y. Hsiao, C. L. Joe and J. Q. Yu, Ligand–Enabled β–C–H Arylation of α–Amino Acids Without Installing Exogenous Directing Groups, Angew. Chem., Int. Ed., 2017, 56(6), 1506–1509 CrossRef CAS PubMed.
- T. Liu, J. X. Qiao, M. A. Poss and J. Q. Yu, Palladium(II)–Catalyzed Site–Selective C (sp3)− H Alkynylation of Oligopeptides: A Linchpin Approach for Oligopeptide–Drug Conjugation, Angew. Chem., Int. Ed., 2017, 56(36), 10924–10927 CrossRef CAS PubMed.
- L. Mendive-Tapia, S. Preciado, J. García, R. Ramón, N. Kielland, F. Albericio and R. Lavilla, New peptide architectures through C–H activation stapling between tryptophan–phenylalanine/tyrosine residues, Nat. Commun., 2015, 6(1), 1–9 Search PubMed.
- A. F. Noisier, J. García, I. A. Ionuţ and F. Albericio, Stapled Peptides by Late–Stage C (sp3)− H Activation, Angew. Chem., 2017, 129(1), 320–324 CrossRef.
- L. D. Tran and O. Daugulis, Nonnatural amino acid synthesis by using carbon-hydrogen bond functionalization methodology, Angew. Chem., Int. Ed., 2012, 51(21), 5188–5191 CrossRef CAS PubMed.
- Y. Lu, D.-H. Wang, K. M. Engle and J.-Q. Yu, Pd(II)-catalyzed hydroxyl-directed C− H olefination enabled by monoprotected amino acid ligands, J. Am. Chem. Soc., 2010, 132(16), 5916–5921 CrossRef CAS PubMed.
- J. He, S. Li, Y. Deng, H. Fu, B. N. Laforteza, J. E. Spangler, A. Homs and J.-Q. Yu, Ligand-controlled C (sp3)–H arylation and olefination in synthesis of unnatural chiral α–amino acids, Science, 2014, 343(6176), 1216–1220 CrossRef CAS PubMed.
- C. S. Sevov and J. F. Hartwig, Iridium-catalyzed oxidative olefination of furans with unactivated alkenes, J. Am. Chem. Soc., 2014, 136(30), 10625–10631 CrossRef CAS PubMed.
- M.-Z. Lu, X.-R. Chen, H. Xu, H.-X. Dai and J.-Q. Yu, Ligand-enabled ortho-C–H olefination of phenylacetic amides with unactivated alkenes, Chem. Sci., 2018, 9(5), 1311–1316 RSC.
- M. Bera, A. Modak, T. Patra, A. Maji and D. Maiti, Meta-selective arene C–H bond olefination of arylacetic acid using a nitrile-based directing group, Org. Lett., 2014, 16(21), 5760–5763 CrossRef CAS PubMed.
- S. Agasti, B. Mondal, T. K. Achar, S. K. Sinha, A. S. Suseelan, K. J. Szabo, F. Schoenebeck and D. Maiti, Orthogonal selectivity in C–H olefination: synthesis of branched vinylarene with unactivated aliphatic substitution, ACS Catal., 2019, 9(10), 9606–9613 CrossRef CAS.
- M. Bera, S. K. Sahoo and D. Maiti, Room-temperature meta-functionalization: Pd(II)-catalyzed synthesis of 1, 3, 5-trialkenyl arene and meta-hydroxylated olefin, ACS Catal., 2016, 6(6), 3575–3579 CrossRef CAS.
- T. K. Achar, X. Zhang, R. Mondal, M. Shanavas, S. Maiti, S. Maity, N. Pal, R. S. Paton and D. Maiti, Palladium–catalyzed directed meta–selective C− H allylation of arenes: Unactivated internal olefins as allyl surrogates, Angew. Chem., 2019, 131(30), 10461–10468 CrossRef.
- T. K. Achar, J. P. Biswas, S. Porey, T. Pal, K. Ramakrishna, S. Maiti and D. Maiti, Palladium-catalyzed template directed C-5 selective olefination of thiazoles, J. Org. Chem., 2019, 84(12), 8315–8321 CrossRef CAS PubMed.
- S. Bag, S. Jana, S. Pradhan, S. Bhowmick, N. Goswami, S. K. Sinha and D. Maiti, Imine as a linchpin approach for meta-C–H functionalization, Nat. Commun., 2021, 12(1), 1–8 CrossRef PubMed.
- S. Bag and D. Maiti, Palladium-catalyzed olefination of aryl C–H bonds by using directing scaffolds, Synthesis, 2016,(06), 804–815 CAS.
- J. Tang, H. Chen, Y. He, W. Sheng, Q. Bai and H. Wang, Peptide-guided functionalization and macrocyclization of bioactive peptidosulfonamides by Pd(II)-catalyzed late-stage C–H activation, Nat. Commun., 2018, 9(1), 1–8 CrossRef PubMed.
- R. Savela and C. Méndez-Gálvez, Isoindolinone Synthesis via One–Pot Type Transition Metal Catalyzed C− C Bond Forming Reactions, Chemistry, 2021, 27(17), 5344 CrossRef CAS PubMed.
- M. K. Gupta and N. K. Sharma, A new amino acid, hybrid peptides and BODIPY analogs: synthesis and evaluation of 2-aminotroponyl-l-alanine (ATA) derivatives, Org. Biomol. Chem., 2022, 20(47), 9397–9407 RSC.
- J. Tang, H. Chen, Y. He, W. Sheng, Q. Bai and H. Wang, Peptide-guided functionalization and macrocyclization of bioactive peptidosulfonamides by Pd(II)-catalyzed late-stage C–H activation, Nat. Commun., 2018, 9(1), 3383 CrossRef PubMed.
- B. B. Palai and N. K. Sharma, N-Arylated, peptide: troponyl residue influences the structure and conformation of N-troponylated-(di/tri)-peptides, CrystEngComm, 2021, 23(1), 131–139 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: NMR and mass data of all new compounds are provided. 2D NMR of compound 19a and DMSO-titration spectra are also provided. Computational study outcomes (energy vs. conformation profiles are also provided. See DOI: https://doi.org/10.1039/d3ob00742a |
| ‡ These authors are equally contributed. |
|
| This journal is © The Royal Society of Chemistry 2023 |
Click here to see how this site uses Cookies. View our privacy policy here.  *ab
*ab


![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) has significant roles in the intramolecular hydrogen bonding with amide N–H, which could be useful for tuning the peptide folding.
O) has significant roles in the intramolecular hydrogen bonding with amide N–H, which could be useful for tuning the peptide folding.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) and bond angle ∠N–H–O ∼ 148° that nearly matched with the γ-turn. Isoindolinone-containing dipeptides (8a–11a/14a/14c/17a/17c) exhibit intramolecular hydrogen bonding between the isoindolinone carbonyl (C
C) and bond angle ∠N–H–O ∼ 148° that nearly matched with the γ-turn. Isoindolinone-containing dipeptides (8a–11a/14a/14c/17a/17c) exhibit intramolecular hydrogen bonding between the isoindolinone carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and N–H of the second amino acid residue, with bond distance (N–H⋯O) ∼2.0 and bond angle ∠N–H–O ∼150° with the respective substituents of the amino acid and nature of the aryl group of the isoindolinone residue. In the case of isoindolinonyl tri-peptides (23/24), they exhibit two intramolecular hydrogen bonds between isoindolinonyl carbonyl and the i + 3 amide (N–H⋯O
O) and N–H of the second amino acid residue, with bond distance (N–H⋯O) ∼2.0 and bond angle ∠N–H–O ∼150° with the respective substituents of the amino acid and nature of the aryl group of the isoindolinone residue. In the case of isoindolinonyl tri-peptides (23/24), they exhibit two intramolecular hydrogen bonds between isoindolinonyl carbonyl and the i + 3 amide (N–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C). The bond length and angle of the hydrogen bonds are almost same, ∼2.0 Å/∼150° for peptide 23, and possibly lead to the formation of a helix. In contrast, for 24, the bond length and angle of hydrogen bonds are different, at ∼2.0 Å/∼150° (strong) and ∼2.8 Å/136° (weak), presumably due to the leu residue at the 2nd position, and may lead to only γ-turn structure (Fig. 6). These data strongly support that isoindolinone-containing peptides are potential building blocks for designing unique foldamers.
C). The bond length and angle of the hydrogen bonds are almost same, ∼2.0 Å/∼150° for peptide 23, and possibly lead to the formation of a helix. In contrast, for 24, the bond length and angle of hydrogen bonds are different, at ∼2.0 Å/∼150° (strong) and ∼2.8 Å/136° (weak), presumably due to the leu residue at the 2nd position, and may lead to only γ-turn structure (Fig. 6). These data strongly support that isoindolinone-containing peptides are potential building blocks for designing unique foldamers.






