Open Access Article
Open Access ArticleAxially chiral N-alkyl-N-cinnamoyl amide type P,olefin ligands for Pd-catalyzed reactions†
Takashi
Mino
 *abc,
Kaho
Takaya‡
a,
Kaito
Koki‡
a,
Natsume
Akimoto‡
a,
Yasushi
Yoshida
*abc,
Kaho
Takaya‡
a,
Kaito
Koki‡
a,
Natsume
Akimoto‡
a,
Yasushi
Yoshida
 abc,
Yoshio
Kasashima
d and
Masami
Sakamoto
abc,
Yoshio
Kasashima
d and
Masami
Sakamoto
 ab
ab
aGraduate School of Engineering, Chiba University, 1-33, Yayoi-cho, Inage-ku, Chiba 263-8522, Japan. E-mail: tmino@faculty.chiba-u.jp
bMolecular Chirality Research Center, Chiba University, 1-33, Yayoi-cho, Inage-ku, Chiba 263-8522, Japan
cSoft Molecular Activation Research Center, Chiba University, 1-33, Yayoi-cho, Inage-ku, Chiba 263-8522, Japan
dEducation Center, Chiba Institute of Technology, Shibazono 2-2-1, Narashino, Chiba 275-0023, Japan
First published on 11th March 2023
Abstract
We synthesized N-alkyl-N-cinnamoyl amide type phosphine–olefin compounds 1 and found axial chirality in a C(aryl)–N(amide) bond in compounds 1 by HPLC analysis using a chiral stationary phase column. We successfully obtained enantiomeric isomers of 1 and demonstrated the use of (–)-1 for chiral ligands in Pd-catalyzed asymmetric allylic substitution reactions of allylic esters with indoles (up to 97% ee).
Introduction
The development of chiral ligands is significant for transition metal catalyzed reactions because the enantioselectivity in asymmetric induction depends on chiral ligand selection. Recently, new chiral ligands with axial chirality for the Pd-catalyzed asymmetric allylic substitution reaction of allylic esters with indoles were reported by several groups.1 For example, the Zhou group reported a P,olefin type chiral ligand L1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 with C(aryl)–C(aryl) bond axial chirality for this reaction (Fig. 1). We also recently reported N-alkyl-N-cinnamyl type chiral ligands L2
2 with C(aryl)–C(aryl) bond axial chirality for this reaction (Fig. 1). We also recently reported N-alkyl-N-cinnamyl type chiral ligands L2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and L3
3 and L3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 with C(aryl)–N(amine) bond axial chirality and an N-cinnamoyl amide type chiral ligand L4
4 with C(aryl)–N(amine) bond axial chirality and an N-cinnamoyl amide type chiral ligand L4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 with central chirality and C(aryl)–N(amide) bond axial chirality.
5 with central chirality and C(aryl)–N(amide) bond axial chirality.
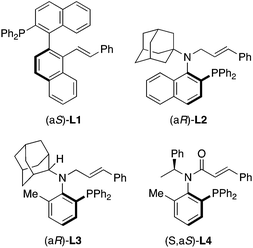 | ||
| Fig. 1 Recently reported chiral ligands with axial chirality for Pd-catalyzed asymmetric allylic substitution reaction. | ||
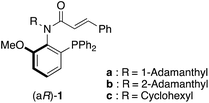
Related to investigations of ligands L2 and L3, we are interested in P,olefin chiral ligands with the cinnamoyl group instead of the cinnamyl group. Here, we describe the synthesis of N-alkyl-N-cinnamoyl amides 1 with only C(aryl)–N(amide) bond axial chirality without a chiral center (Fig. 2) and their applications to chiral ligands for the Pd-catalyzed asymmetric allylic substitution reaction of allylic esters with indoles.
Results and discussion
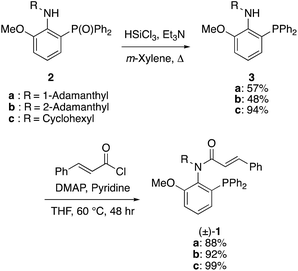
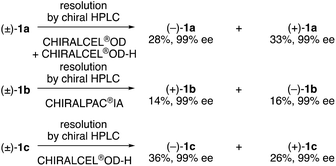
N-Alkyl-N-cinnamoyl amides 1 were prepared from phosphine oxide 2via reduction using trichlorosilane with triethylamine and N-acylation with cinnamoyl chloride in two steps (Scheme 1).We analyzed amide compounds 1 by HPLC analysis using a chiral stationary phase column with a CD detector. Although we previously failed to find the axial chirality in the cinnamyl type N-cyclohexylaniline compound,4 we found that C(aryl)–N(amide) bond axial chirality exists in amide compounds 1 including N-cyclohexyl type compound 1c. Next, we attempted the optical resolution of racemic compounds (±)-1a–c and obtained (+)-1a–c and (–)-1a–c using semi-preparative chiral HPLC on the 10 milligram scale (Scheme 2).
After the recrystallization of the optically active compound, we successfully performed the single-crystal X-ray analysis using (aS)-(+)-1c to determine the absolute configurations of 1 (Fig. 3).6
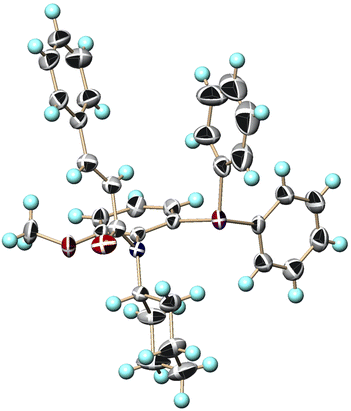 | ||
| Fig. 3 X-ray structure of (aS)-(+)-1c (CCDC 2227386†). Ellipsoids are shown at the 50% probability level. | ||
We also determined the racemization barriers in the axial chirality of 1a–c in a dodecane solution. For compound 1a, no racemization occurred in dodecane at 130 °C. On the other hand, we found that the racemization of 1b and 1c in dodecane occurred. We repeated this experiment at different temperatures and determined the rate constants (krac) at each temperature (see the ESI†). The rotational barrier (ΔG‡rac) of 1b in dodecane was 27.3 kcal mol−1 at 25 °C (Table 1), based on Arrhenius and Eyring equations.7 Although the half-life of N-cinnamyl type compound L3 is approximately only 3 days,4 the half-life of 1b is about 64 days in dodecane at 25 °C. We also found that the half-life of 1c, which has a less hindered alkyl group on a nitrogen atom than 1b, is about 3 days at 25 °C.
| Thermodynamic parameters in dodecane at 25 °C | 1b | 1c |
|---|---|---|
| Half-life (days) | 64.3 | 3.72 |
| K rac | 6.24 × 10−2 | 1.08 |
| ΔH (kcal mol−1) | 16.8 | 24.0 |
| ΔS (cal mol−1 K) | –35.2 | –5.40 |
| ΔG ‡rac (kcal mol−1) | 27.3 | 25.6 |
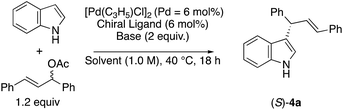
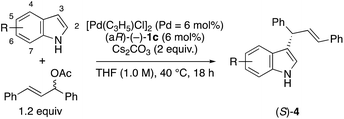
We next investigated the ability of the optically active cinnamoyl amides 1 to function as chiral ligands for the Pd-catalyzed asymmetric allylic substitution reactions of allylic acetates, such as 1,3-diphenyl-2-propenyl acetate, with indoles.1 The reaction with indole was performed using 3 mol% of [Pd(C3H5)Cl]2 and 6 mol% of ligands 1 as a model reaction (Table 1). The reaction using (–)-1a as a ligand and 2 equiv. of K2CO3 in MeCN at 40 °C for 18 h gave the corresponding product (S)-4a in 70% yield with 88% ee (entry 1). The reaction using (–)-1b and (aR)-(–)-1c, respectively, gave an (S)-product with 82% ee and 91% ee (entries 2 and 3). We found that (aR)-(–)-1c was the best ligand among chiral ligands 1a–c. Next, we changed the base and used other bases instead of K2CO3 in MeCN (entry 3 vs. entries 4–7). Using KOAc as a base, the enantioselectivity of (S)-4a decreased to 52% ee (entry 4). When the reaction was carried out with Cs2CO3, the corresponding product (S)-4a was obtained with good enantioselectivity (92% ee) and 73% yield (entry 7). When the reaction was carried out with Cs2CO3 using Pd2(dba)3·CHCl3 instead of [Pd(C3H5)Cl]2, the enantioselectivity of (S)-4a decreased to 87% ee (entry 8). We next investigated the effect of solvents using (aR)-(–)-1c with Cs2CO3 (entries 7, 9–12). Using EtOAc, DCM (dichloromethane), or PhCF3 (benzotrifluoride) as a solvent instead of acetonitrile, we obtained the product (S)-4a with a similar level of enantioselectivities (89–91% ee) (entries 10–12). When the reaction was carried out using THF as a solvent, the corresponding product (S)-4a was obtained with the highest enantioselectivity (97% ee) and a good yield (69%) (entry 13).
With the optimized reaction conditions in hand (Table 2, entry 13), we examined the scope of the allylic substitution reaction of 1,3-diphenyl-2-propenyl acetate with various substituted indoles using (aR)-(–)-1c in THF with Cs2CO3 at 40 °C (Table 3). The reactions with 6-halogenated indoles gave the corresponding products (S)-4b–d in high enantioselectivities (90–93% ee) (entries 2–4). Although the reactions using 6-nitroindole and 6-methylindole gave the products (S)-4g and (S)-4h in low yields (entries 7 and 8), the reaction with 6-methoxyindole and 6-benzyloxyindole, respectively, gave the corresponding products (S)-4e and 4f in good yields with moderate to good enantioselectivities such as 72% ee and 87% ee (entries 5 and 6). The reaction of 7-bromoindole gave the corresponding product (S)-4i in good yield with 83% ee (entry 9). The reaction with 2-, 4-, or 5-substituted indoles also gave the corresponding products (S)-4i–m with high enantioselectivities (entries 10–13).
| Entry | Chiral ligand | Base | Solv. | Yieldb (%) | eec |
|---|---|---|---|---|---|
| a The reactions were carried out on a 0.2 mmol scale of indole in various solvents (0.2 mL) at 40 °C with 1.2 equiv. of 1,3-diphenyl-2-propenyl acetate and 2 equiv. of base, in the presence of a chiral ligand (6 mol%) and [Pd(C3H5)Cl]2 (3 mol%; Pd = 6 mol%). b Isolated yield. c Determined by HPLC analysis using a chiral column. d This reaction was carried out using Pd2(dba)3·CHCl3 instead of [Pd(C3H5)Cl]2. | |||||
| 1 | (–)-1a | K2CO3 | MeCN | 70 | 88 |
| 2 | (–)-1b | K2CO3 | MeCN | 67 | 82 |
| 3 | (aR)-(–)-1c | K2CO3 | MeCN | 74 | 91 |
| 4 | (aR)-(–)-1c | KOAc | MeCN | 80 | 52 |
| 5 | (aR)-(–)-1c | K3PO4 | MeCN | 69 | 88 |
| 6 | (aR)-(–)-1c | Na2CO3 | MeCN | 76 | 74 |
| 7 | (aR)-(–)-1c | Cs2CO3 | MeCN | 73 | 92 |
| 8d | (aR)-(–)-1c | Cs2CO3 | MeCN | 69 | 87 |
| 9 | (aR)-(–)-1c | Cs2CO3 | PhMe | 87 | 86 |
| 10 | (aR)-(–)-1c | Cs2CO3 | EtOAc | 60 | 89 |
| 11 | (aR)-(–)-1c | Cs2CO3 | DCM | 74 | 91 |
| 12 | (aR)-(–)-1c | Cs2CO3 | PhCF3 | 66 | 90 |
| 13 | (aR)-(–)- 1c | Cs 2 CO 3 | THF | 69 | 97 |
| Entry | R | Yieldb (%) | eec (%) |
|---|---|---|---|
| a The reactions were carried out on a 0.2 mmol scale of indole derivative in THF (0.2 mL) at 40 °C with 1.2 equiv. of 1,3-diphenyl-2-propenyl acetate and 2 equiv. of Cs2CO3, in the presence of (aR)-(–)-1c (6 mol%) and [Pd(C3H5)Cl]2 (3 mol%; Pd = 6 mol%). b Isolated yield. c Determined by HPLC analysis using a chiral column. | |||
| 1 | H | 69 (4a) | 97 |
| 2 | 6-Br | 70 (4b) | 90 |
| 3 | 6-Cl | 70 (4c) | 93 |
| 4 | 6-F | 72 (4d) | 93 |
| 5 | 6-MeO | 71 (4e) | 72 |
| 6 | 6-BnO | 77 (4f) | 87 |
| 7 | 6-NO2 | 26 (4g) | 76 |
| 8 | 6-Me | 34 (4h) | 87 |
| 9 | 7-Br | 64 (4i) | 83 |
| 10 | 5-Br | 60 (4j) | 91 |
| 11 | 4-Br | 68 (4k) | 95 |
| 12 | 5-MeO | 78 (4l) | 91 |
| 13 | 2-Ph | 88 (4m) | 92 |
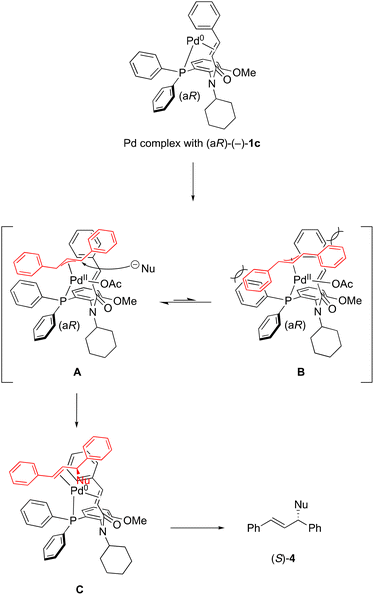
According to the observed stereochemical outcome and our previous reports,4,5 we propose a possible stereochemical pathway for the formation of (S)-4 as a product. As shown in Scheme 3, PdII complexes such as W-type A and M-type B intermediates were formed from ligand (aR)-(–)-1c. PdII complex A was favored more than complex B due to a steric effect. The indole anion as a nucleophile would preferentially attack the carbon at the trans position to the phosphorus of (aR)-(–)-1c from outside.8 Finally, the (S)-product 4 was obtained from Pd0 complex C.
Conclusions
We synthesized racemic N-alkyl-N-cinnamoyl amides (±)-1a–c from phosphine oxide 2 in two steps and successfully obtained enantiomeric isomers of 1a–c with C(aryl)–N(amide) bond axial chirality by optical resolution using semi-preparative chiral HPLC. We also found that the optically active compound 1c is an effective chiral ligand for the Pd-catalyzed asymmetric allylic substitution reaction of 1,3-diphenyl-2-propenyl acetate with indoles in high enantioselectivities (up to 97% ee).Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by a Grant-in-Aid for Scientific Research (C) (No. 18K05117 and 22K05107) from the Japan Society for the Promotion of Science (JSPS).References
- For examples, see (a) B. Feng, P.-W. Fang, G.-M. Lan, L.-Y. Peng, L.-F. Liang and G.-Y. You, Org. Biomol. Chem., 2022, 20, 7415–7418 RSC; (b) L. Zhang, S.-H. Xiang, J. Wang, J. Xiao, J.-Q. Wang and B. Tan, Nat. Commun., 2019, 10, 566 CrossRef PubMed; (c) T. Mino, D. Yamaguchi, M. Kumada, J. Youda, H. Saito, J. Tanaka, Y. Yoshida and M. Sakamoto, Synlett, 2021, 532–538 CAS; (d) Z. Qiu, R. Sun, K. Yang and D. Teng, Molecules, 2019, 24, 1575 CrossRef PubMed; (e) K. Yamamoto, T. Shimizu, K. Igawa, K. Tomooka, G. Hirai, H. Suemune and K. Usui, Sci. Rep., 2016, 6, 36211–36219 CrossRef PubMed; (f) B. Feng, X.-Y. Pu, Z.-C. Liu, W.-J. Xiao and J.-R. Chen, Org. Chem. Front., 2016, 3, 1246–1249 RSC; (g) J.-X. Xu, F. Ye, X.-F. Bai, J. Zhang, Z. Xu, Z.-J. Zheng and L.-W. Xu, RSC Adv., 2016, 6, 45495–45502 RSC; (h) T. Mino, M. Ishikawa, K. Nishikawa, K. Wakui and M. Sakamoto, Tetrahedron: Asymmetry, 2013, 24, 499–504 CrossRef CAS; (i) T. Hoshi, K. Sasaki, S. Sato, Y. Ishii, T. Suzuki and H. Hagiwara, Org. Lett., 2011, 13, 932–935 CrossRef CAS PubMed; (j) H. Y. Cheung, W.-Y. Yu, F. L. Lam, T. T.-L. Au-Yeung, Z. Zhou, T. H. Chan and A. S. C. Chan, Org. Lett., 2007, 9, 4295–4298 CrossRef CAS PubMed.
- Z.-S. Liu, Y. Hua, Q. Gao, Y. Ma, H. Tang, Y. Shang, H.-G. Cheng and Q. Zhou, Nat. Catal., 2020, 3, 727–733 CrossRef CAS.
- (a) T. Mino, J. Youda, T. Ebisawa, Y. Shima, K. Nishikawa, Y. Yoshida and M. Sakamoto, J. Oleo Sci., 2018, 67, 1189–1199 CrossRef CAS PubMed; (b) T. Mino, K. Nishikawa, M. Asano, Y. Shima, T. Ebisawa, Y. Yoshida and M. Sakamoto, Org. Biomol. Chem., 2016, 14, 7509–7519 RSC.
- T. Mino, D. Yamaguchi, C. Masuda, J. Youda, T. Ebisawa, Y. Yoshida and M. Sakamoto, Org. Biomol. Chem., 2019, 17, 1455–1465 RSC.
- T. Mino, Y. Fujisawa, S. Yoshida, M. Hirama, T. Akiyama, R. Saito, Y. Yoshida, Y. Kasashima and M. Sakamoto, Org. Biomol. Chem., 2021, 19, 10385–10389 RSC.
- CCDC 2227386† contains the supplementary crystallographic data for this paper.
- (a) A. S. Cooke and M. M. Harris, J. Chem. Soc. C, 1967, 988–992 RSC; (b) F. W. Cagle Jr. and H. Eyring, J. Am. Chem. Soc., 1951, 73, 5628–5630 CrossRef.
- For examples, see (a) J. M. Brown, D. I. Hulmes and P. J. Guiry, Tetrahedron, 1994, 50, 4493–4506 CrossRef CAS; (b) J. Sprinz, M. Kiefer, G. Helmchen, M. Reggelin, G. Huttner, O. Walter and L. Zsolnai, Tetrahedron Lett., 1994, 35, 1523–1523 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2227386. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3ob00224a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2023 |