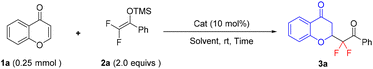A highly efficient metal-free selective 1,4-addition of difluoroenoxysilanes to chromones†
Xi-Yu
Wang
a,
Min
Yang
a,
Ying
Zhou
*a,
Jian
Zhou
 *ba and
Yong-Jia
Hao
*ba and
Yong-Jia
Hao
 *a
*a
aSchool of Pharmacy, Guizhou University of Traditional Chinese Medicine, Guiyang 550025, China. E-mail: haoyongjia026@gzy.edu.cn; yingzhou71@126.com
bShanghai Engineering Research Center of Molecular Therapeutics and New Drug Development, and Shanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai 200062, China. E-mail: jzhou@chem.ecnu.edu.cn
First published on 29th December 2022
Abstract
A highly efficient metal-free selective 1,4-addition reaction of difluoroenoxysilanes to chromones was developed using the low-cost and readily available HOTf as the catalyst, which is a facile and straightforward method to access valuable C2-difluoroalkylated chroman-4-one derivatives. Interestingly, the products could be readily converted to the difluorinated bioisostere of the natural product (S)-2,6-dimethylchroman-4-one and a difluorinated benzo-seven-membered heterocycle via the Schmidt rearrangement reaction. In addition, the in vitro anti-proliferative activities of these synthesized derivatives against human colon carcinoma cells (HCT116) revealed that compound 3g exhibited potent inhibitory effect on HCT116 cancer cells with an IC50 value of 6.37 μM, representing a novel lead compound for further structural optimization and biological evaluation.
The selective introduction of fluorine at appropriate positions of organic and biologically active molecules has strong beneficial effects on their physical, chemical, and biological properties and has emerged as a widely used strategy for structural modification in drug research and development.1 In particular, due to its unique physicochemical properties, for example its H-bond donor ability,2 the introduction of a gem-difluoroalkyl (CF2) moiety in therapeutic drugs and bioactive molecules was realized to enhance their metabolic stabilities, biological activities and drug properties,3 such as the selective type-2 chloride channel activator lubiprostone,4 the anti-hepatitis C drugs glecaprevir and voxilaprevir,5 the PDE-4 inhibitor roflumilast,6 and a range of natural analogues.7 Consequently, the development of new reagents and new synthetic methods toward structurally diverse difluoroalkyl-containing derivatives has become an appealing area of research.
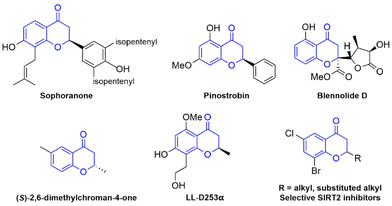
On the other hand, the synthesis of natural product hybrid systems with varied biological and pharmacological properties has received increasing attention in the past decades.8 Chroman-4-ones, especially 2-substituted chroman-4-ones, are the core structural scaffolds of various natural compounds and bioactive molecules. For instance, the natural products sophoranone,9 pinostrobin,10 blennolide D,11 (S)-2,6-dimethylchroman-4-one,12 and LL-D253α,13 all contain such privileged units and have shown a wide variety of biological activities, including antioxidant, anti-inflammatory, antimicrobial, and antitumor activities (Fig. 1). Besides, the substituents at the 2-position of chroman-4-ones usually play a crucial role in their biological activities and pharmacokinetic properties, for example, the potent and selective SIRT2 inhibitors14 (Fig. 1). Therefore, the introduction of a difluoro substituent into a chroman-4-one structural motif to assemble the difluoroalkylated chroman-4-one skeleton can not only enrich the diversity of chroman-4-ones but also offer novel bioactivities for drug discovery campaigns.
 | ||
| Fig. 1 Representative examples of bioactive compounds containing the 2-substituted chroman-4-one moiety. | ||
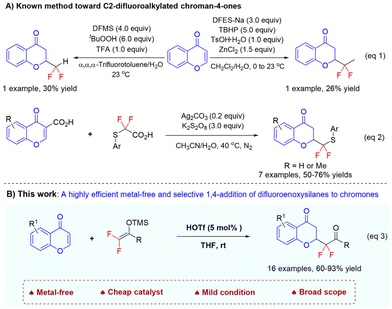
In this context, while some attention has been paid to develop methods to merge a gem-difluoroalkyl group into the chromone framework,15 until recently,16,17 only three protocols, with limited success, have been reported for the preparation of C2-difluorinated chroman-4-ones, as shown in Scheme 1A. For example, during the examination of the generality of difluoroalkylation using zinc difluoromethanesulfinate (DFMS)16a and heteroarylether bioisoster synthesis using sodium difluoroethylsulfinate (DFES-Na),16b Baran and co-workers also tested both reagents for conjugate addition reactions with chromone. However, although 4 and 3 equivalents of DFMS and DFES-Na were used, as well as stoichiometric amounts of oxidants and acids, the desired difluoroalkylated chroman-4-ones were obtained in only 30% and 26% yields, respectively (eqn (1)).16 Very recently, Chen et al. accomplished a Ag-mediated decarboxylative C-2 difluoromethylation of chromone-3-carboxylic acids and arylthio-difluoroacetic acids in the presence of 3 equivalents of K2S2O8 (eqn (2)).17 In their report, improved yields, 50–76%, were realized, but the use of the more active but expensive chromone-3-carboxylic acids was required; therefore, only seven examples were shown. Furthermore, the synthetic utility of the products was not shown. Therefore, the development of new and efficient synthetic methods for the selective incorporation of the gem-difluoroalkyl group at the C2 position of chroman-4-one scaffolds is highly desirable.
As part of our continuous efforts toward selective difluoralkylation18a using difluoroenoxysilanes 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ,19 which have been identified as one among the powerful synthons for the introduction of the gem-difluoroalkyl ketone functionality in the past decades,18,20–26 we recently conducted a program focusing on the synthesis of difluorinated heterocyclic systems based on natural product skeletons.27 For instance, by using Fe(OTf)3 as the catalyst, we established a highly efficient nucleophilic substitution of 3-hydroxyoxindoles with difluoroenoxysilanes 227a and a 1,6-conjugate addition of para-quinone methides with 2.27b On the basis of these results, we envisioned the possibility of developing a selective 1,4-addition reaction of chromone using difluoroenoxysilanes, thus providing a facile method for constructing C2-difluoroalkylated chroman-4-ones with structural diversity. Herein, we utilized the cheap HOTf as an acid catalyst to realize the selective 1,4-addition reaction of difluoroenoxysilanes with chromones, which is a straightforward and practical protocol toward C2-difluoroalkylated chroman-4-ones that are inaccessible via other methods (Scheme 1B, eqn (3)).
,19 which have been identified as one among the powerful synthons for the introduction of the gem-difluoroalkyl ketone functionality in the past decades,18,20–26 we recently conducted a program focusing on the synthesis of difluorinated heterocyclic systems based on natural product skeletons.27 For instance, by using Fe(OTf)3 as the catalyst, we established a highly efficient nucleophilic substitution of 3-hydroxyoxindoles with difluoroenoxysilanes 227a and a 1,6-conjugate addition of para-quinone methides with 2.27b On the basis of these results, we envisioned the possibility of developing a selective 1,4-addition reaction of chromone using difluoroenoxysilanes, thus providing a facile method for constructing C2-difluoroalkylated chroman-4-ones with structural diversity. Herein, we utilized the cheap HOTf as an acid catalyst to realize the selective 1,4-addition reaction of difluoroenoxysilanes with chromones, which is a straightforward and practical protocol toward C2-difluoroalkylated chroman-4-ones that are inaccessible via other methods (Scheme 1B, eqn (3)).
Initially, we began this study by using difluoroenoxysilane 2a and chromone 1a as model substrates for the optimization of 1,4-addition reaction conditions (Table 1). Based on our previous experience of metal Lewis acid catalyzed difluoroalkylation with 2, we first examined the performance of metal triflates. We were pleased to find that the use of 10 mol% of Fe(OTf)3, Al(OTf)3, Bi(OTf)3, Cu(OTf)2, or trimethylsilyl trifluoromethanesulfonate (TMSOTf) allowed the model reaction to take place and afforded the desired 1,4-addition product 3a in moderate yields (entries 1–5), while almost no reaction occurred in the presence of Y(OTf)3, Sc(OTf)3, Ga(OTf)3, and Zn(OTf)2. Interestingly, when changing the Lewis acid catalyst to the Brønsted acid catalyst CF3SO3H (HOTf), the reaction yield of 3a could be greatly improved to 78% within 19 h (entry 10). Other Brønsted acids, such as p-TsOH, MeSO3H or Tf2NH, did not deliver better results (entries 11–13). Among all the tested catalysts, HOTf was identified to be the best.
| Entry | Cat. (10 mol%) | Solvent | Time (h) | Yieldb (%) |
|---|---|---|---|---|
| a 2a (1.5 equiv.). b NMR yield. c Isolated yield. | ||||
| 1 | Fe(OTf)3 | CH2Cl2 | 27 | 67 |
| 2 | Al(OTf)3 | CH2Cl2 | 27 | 31 |
| 3 | Bi(OTf)3 | CH2Cl2 | 27 | 52 |
| 4 | Cu(OTf)2 | CH2Cl2 | 27 | 47 |
| 5 | TMSOTf | CH2Cl2 | 19 | 72 |
| 6 | Y(OTf)3 | CH2Cl2 | 27 | Trace |
| 7 | Sc(OTf)3 | CH2Cl2 | 27 | Trace |
| 8 | Ga(OTf)3 | CH2Cl2 | 27 | Trace |
| 9 | Zn(OTf)2 | CH2Cl2 | 27 | Trace |
| 10 | HOTf | CH2Cl2 | 19 | 78 |
| 11 | p-TsOH | CH2Cl2 | 8 | 41 |
| 12 | MeSO3H | CH2Cl2 | 19 | 42 |
| 13 | Tf2NH | CH2Cl2 | 19 | 70 |
| 14 | HOTf | Hexane | 28 | 85 |
| 15 | HOTf | Toluene | 28 | 81 |
| 16 | HOTf | THF | 9 | 84 |
| 17 | HOTf | CH3CN | 28 | 63 |
| 18 | HOTf | EtOAc | 9 | 67 |
| 19 | HOTf (5 mol%) | THF | 9 | 76c |
| 20a | HOTf (5 mol%) | THF | 9 | 76c |
Subsequently, the investigation of solvent effects using HOTf as the catalyst revealed that both polar and non-polar solvents could afford acceptable reaction yields, and tetrahydrofuran (THF) turned out to be the optimal one, affording the target product 3a in 84% yield within 9 h (entry 16). Additionally, decreasing the amount of HOTf from 10 to 5 mol% or that of 2a from 2.0 to 1.5 equiv. had no remarkable influence on the yield of 3a (entry 19 vs. 16, entry 20 vs. 16, respectively). Therefore, the optimal reaction conditions were identified as follows: 5 mol% of HOTf as the catalyst and THF as the solvent at room temperature (entry 20).
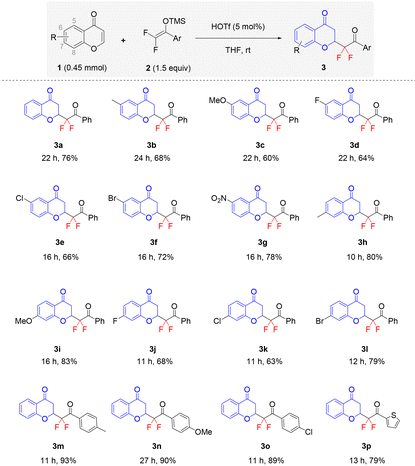
With the set of the optimized reaction conditions in hand, we investigated the scope and generality of this HOTf-catalyzed 1,4-addition reaction to access structurally diverse C2-difluoroalkylated chroman-4-ones. As shown in Table 2, chromone substrates bearing electron-donating groups at the C-6 position were first subjected to the reaction with 2a, and it turned out that all of them afforded the corresponding products 3b and 3c in good yields. Furthermore, substituted chromones with electron-deficient groups (F, Cl, Br, and NO2) at the C-6 position also gave the corresponding products in 64–78% yields (3d–3g). Notably, the C7-substituted chromone substrates with electron-donating and electron-withdrawing substituents were also found to be suitable for this 1,4-addition. It was found that these chromones bearing electron-donating groups afforded the desired products (3h and 3i) in a higher yield than the substrates bearing electron-withdrawing groups (3j–3l). The efficient formation of 3a–3l illustrated that the substituents at different positions and with different electronegativities were well tolerated under the optimized conditions, indicating the potential for further synthetic elaboration. Subsequently, the scope of phenyl substituted difluoroenoxysilanes 2 was evaluated. It is noteworthy that substrates 2 featuring electron-donating or electron-deficient substituents at the phenyl ring converted well to generate the target products 3m–3o in relatively high yields (89–93% yields). In addition, the heterocyclic substituted substrate 2, for example the 2-thienyl-derived difluoroenoxysilane, could also undergo the selective 1,4-addition reaction with 1a, affording the desired product 3p in 79% yield.
| a Isolated yields are given. |
|---|
|
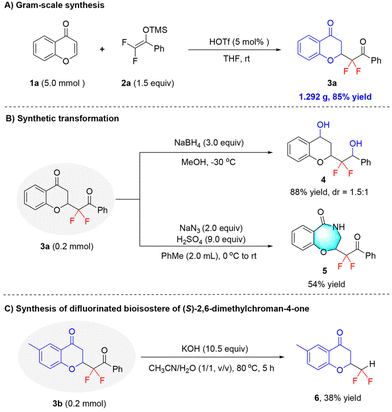
To demonstrate the synthetic utility of the current method, a gram-scale reaction between 1a (5.0 mmol) and 2a was conducted under the standard conditions. As shown in Scheme 2A, 1.292 g of adduct 3a could be obtained in 85% yield. In addition, the carbonyl moieties in product 3a could be readily reduced to give diol 4 in a high yield by using NaBH4. Moreover, the subsequent Schmidt rearrangement reaction of 3a enabled the facile construction of benzo-seven-membered heterocycles, as exemplified by the synthesis of compound 5 in a good yield (Scheme 2B). Finally, to further explore the reaction, we also tried to extend this difluoroalkylation to pharmaceutical molecules or natural products. For instance, the difluorinated bioisostere (target compound 6) of the plant product (S)-2,6-dimethylchroman-4-one was obtained under basic conditions from product 3b (Scheme 2C), providing a simple, feasible, and reliable means for medicinal chemists to synthesize difluoroalkylated mimics of natural product scaffolds. Thus, this useful product elaboration not only leads to novel molecular diversity in heterocyclic frameworks, but also further highlights the practicality of this methodology.
Next, to preliminarily demonstrate the potential anticancer effects of these difluorinated derivatives, we tested their in vitro anti-proliferative activity against human colon carcinoma cells (HCT116) by using the CCK-8 assay, and their inhibition rates at 20 μM are given in Table 3. The results demonstrated that compounds 3d, 3g, 3h, 3k, 3l, and 3o showed greater than 50% inhibition rate toward the HCT116 cell line, especially 3g, which potently inhibited the growth of HCT116 cancer cells with 99.5% inhibition rate. Furthermore, the IC50 values were determined when the inhibition rate was higher than 70% using cisplatin as the positive control (Table 3). Compounds 3h, 3l, and 3o displayed weak antiproliferative effects toward the HCT116 cell line (IC50 > 10 μM). As expected, 3g exhibited potent inhibitory activity with an IC50 value of 6.37 μM, and even showed a better inhibition activity relative to the positive control drug, cisplatin. The results also indicated that the synthesized difluorinated new chemical entities (NCEs) may be useful as promising lead compounds for further structural optimization and biological screening.
| Entry | Inhibition rate (%) | IC50 (μM) | Entry | Inhibition rate (%) | IC50 (μM) |
|---|---|---|---|---|---|
| a The given values are mean values of three experiments. b Not determined. | |||||
| 3a | 2.2 | NDb | 3k | 62.2 | ND |
| 3b | 16.7 | ND | 3l | 73.3 | >10 |
| 3c | 4.3 | ND | 3m | 7.1 | ND |
| 3d | 55.8 | ND | 3n | 25.6 | ND |
| 3e | 12.4 | ND | 3o | 90.9 | >10 |
| 3f | 48.8 | ND | 4 | 0 | ND |
| 3g | 99.5 | 6.37 ± 0.81 | 5 | 0 | ND |
| 3h | 90.0 | >10 | 6 | 0 | ND |
| 3i | 0 | ND | Cisplatin | ND | 9.47 ± 0.68 |
| 3j | 6.8 | ND | |||
Conclusions
In summary, a highly efficient selective 1,4-addition of chromones and difluoroenoxysilanes catalyzed by HOTf has been developed, which is highlighted by the following salient features: metal-free conditions, low-cost, simple operation and broad substrate scope. This method provides a facile means for the synthesis of a wide range of structurally novel C-2 difluorinated chroman-4-ones from readily available chromones under mild conditions. Notably, this methodology can be used to prepare novel difluoroalkylated mimics of drug molecules and natural products, providing a powerful strategy for structural modification and drug discovery. Moreover, in vitro anticancer evaluations of the synthesized difluorinated derivatives were also conducted, and a potential lead compound was obtained for further development and research on new drugs. We believe that the HOTf-catalyzed synthetic strategy will bring new possibilities to efficiently prepare structurally diverse and bioactive fluorinated oxygen-containing heterocycles. Further studies on the asymmetric version as well as on the evaluation of the biological activities of these difluorinated chroman-4-ones are underway in our lab.Author contributions
Conceptualization: Y. Z., J. Z., and Y. H.; investigation: X. W.; supervision: J. Z. and Y. H.; data curation: X. W., M. Y., and Y. H.; and writing: M. Y., Y. Z., J. Z., and Y. H.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The financial support from the National Natural Science Foundation of China (No. 81660576), Projects of Guizhou Province (Qian Ke He Ji Chu-ZK [2021] Yi Ban 561, Qian Jiao He KY Zi [2021]018), and the Projects of Guizhou University of Traditional Chinese Medicine (2018YFC170810207, 2018-27) is highly appreciated.References
- (a) J. Wang and H. Liu, Chin. J. Org. Chem., 2011, 31, 1785 CAS; (b) J. Wang, M. Sánchez-Roselló, J. L. Aceña, C. del Pozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok and H. Liu, Chem. Rev., 2014, 114, 2432 CrossRef CAS PubMed; (c) E. P. Gillis, K. J. Eastman, M. D. Hill, D. J. Donnelly and N. A. Meanwell, J. Med. Chem., 2015, 58, 8315 CrossRef CAS PubMed.
- (a) N. A. Meanwell, J. Med. Chem., 2011, 54, 2529 CrossRef CAS; (b) M. D. Martíne, L. Luna, A. Y. Tesio, G. E. Feresin, F. J. Durán and G. Burton, J. Pharm. Pharmacol., 2016, 68, 233 CrossRef.
- (a) K. Ando, F. Koike, F. Kondo and H. Takayama, Chem. Pharm. Bull., 1995, 43, 189 CrossRef CAS; (b) Y. Zhou, J. Wang, Z. Gu, S. Wang, W. Zhu, J. L. Aceña, V. A. Soloshonok, K. Izawa and H. Liu, Chem. Rev., 2016, 116, 422 CrossRef; (c) Y. Zafrani, D. Yeffet, G. Sod-Moriah, A. Berliner, D. Amir, D. Marciano, E. Gershonov and S. Saphier, J. Med. Chem., 2017, 60, 797 CrossRef PubMed; (d) Y. Zafrani, G. Sod-Moriah, D. Yeffet, A. Berliner, D. Amir, D. Marciano, S. Elias, S. Katalan, N. Ashkenazi, M. Madmon, E. Gershonov and S. Saphier, J. Med. Chem., 2019, 62, 5628 CrossRef PubMed.
- K. McKeage, G. L. Plosker and M. A. A. Siddiqui, Drugs, 2006, 66, 873 CrossRef.
- Y. Zhang, Acta Pharm. Sin. B, 2021, 56, 2182 Search PubMed.
- D. P. Tashkin, Expert Opin. Pharmacother., 2014, 15, 85 CrossRef.
- (a) K. Uoto, S. Ohsuki, H. Takenoshita, T. Ishiyama, S. Iimura, Y. Hirota, I. Mitsui, H. Terasawa and T. Soga, Chem. Pharm. Bull., 1997, 45, 1793 CrossRef; (b) K. Nakayama, H. C. Kawato, H. Inagaki, R. Nakajima, A. Kitamura, K. Someya and T. Ohta, Org. Lett., 2000, 2, 977 CrossRef.
- (a) K. C. Nicolaou, J. A. Pfefferkorn, A. J. Roecker, G.-Q. Cao, S. Barluenga and H. J. Mitchell, J. Am. Chem. Soc., 2000, 122, 9939 CrossRef; (b) H.-B. Zhang, Synlett, 2014, 1953 CrossRef; (c) E. N. Hancock, J. M. Wahl and M. K. Brown, Nat. Prod. Rep., 2019, 36, 1383 RSC; (d) S.-M. Fu and B. Liu, Org. Chem. Front., 2020, 7, 1903 RSC.
- W. Zhang, X. Wei, L. Zhang, X. Hu, Y.-Q. Zhou and Y. Zhou, Chem. Nat. Compd., 2020, 56, 1140 CrossRef.
- H. Häberlein and K. P. Tschiersch, Biochem. Syst. Ecol., 1998, 26, 97 CrossRef.
- I. N. Siddiqui, A. Zahoor, H. Hussain, I. Ahmed, V. U. Ahmad, D. Padula, S. Draeger, B. Schulz, K. Meier, M. Steinert, T. Kurtán, U. Flörke, G. Pescitelli and K. Krohn, J. Nat. Prod., 2011, 74, 365 CrossRef.
- N. Comey, I. Hook, H. Sheridan and J. Walsh, J. Nat. Prod., 1997, 60, 148 CrossRef.
- I. M. Chandler, C. R. Mclntyre and T. J. Simpson, J. Chem. Soc., Perkin Trans. 1, 1992, 2271 RSC.
- T. Seifert, M. Malo, T. Kokkola, K. Engen, M. Fridén-Saxin, E. A. A. Wallén, M. Lahtela-Kakkonen, E. M. Jarho and K. Luthman, J. Med. Chem., 2014, 57, 9870 CrossRef.
- (a) K. Li, J. Chen, C. Yang, K. Zhang, C. Pan and B. Fan, Org. Lett., 2020, 22, 4261 CrossRef; (b) C. Li, Y.-X. Cao, R. Wang, Y.-N. Wang, Q. Lan and X.-S. Wang, Nat. Commun., 2018, 9, 4951 CrossRef.
- (a) Y. Fujiwara, J. A. Dixon, R. A. Rodriguez, R. D. Baxter, D. D. Dixon, M. R. Collins, D. G. Blackmond and P. S. Baran, J. Am. Chem. Soc., 2012, 134, 1494 CrossRef PubMed; (b) Q.-H. Zhou, A. Ruffoni, R. Gianatassio, Y. Fujiwara, E. Sella, D. Shabat and P. S. Baran, Angew. Chem., Int. Ed., 2013, 52, 3949 CrossRef PubMed.
- Z. Chen, J. Sun, Z. Ke, X. Huang and Z. Li, Org. Chem. Front., 2022, 9, 757 RSC.
- For reviews, see: (a) X.-S. Hu, J.-S. Yu and J. Zhou, Chem. Commun., 2019, 55, 13638 RSC; (b) M. Decostanzi, J. M. Campagne and E. Leclerc, Org. Biomol. Chem., 2015, 13, 7351 RSC; (c) Y. Gong, J.-S. Yu, Y.-J. Hao, Y. Zhou and J. Zhou, Asian J. Org. Chem., 2019, 8, 610 CrossRef; (d) Y.-J. Hao, J.-S. Yu, Y. Zhou, X. Wang and J. Zhou, Acta Chim. Sin., 2018, 76, 925 CrossRef.
- For the synthesis of difluoroenoxysilanes 2, see: (a) H. Amii, T. Kobayashi, Y. Hatamoto and K. Uneyama, Chem. Commun., 1999, 1323 RSC; (b) G. K. S. Prakash, J. Hu and G. A. Olah, J. Fluorine Chem., 2001, 112, 355 CrossRef.
- For aldol-type reactions using 2, see: (a) S. Sasaki, T. Suzuki, T. Uchiya, S. Toyota, A. Hirano, M. Tanemura, H. Teramoto, T. Yamauchi and K. Higashiyama, J. Fluorine Chem., 2016, 192, 78 CrossRef; (b) J. Yang, S. Liu, P. Hong, J. Li, Z. Wang and J. Ren, J. Org. Chem., 2022, 87, 1144 CrossRef PubMed. For our contributions, see: (c) Y.-L. Liu, X.-P. Zeng and J. Zhou, Acta Chim. Sin., 2012, 70, 1451 CrossRef; (d) Y.-L. Liu and J. Zhou, Chem. Commun., 2012, 48, 1919 RSC; (e) J.-S. Yu, Y.-L. Liu, J. Tang, X. Wang and J. Zhou, Angew. Chem., Int. Ed., 2014, 53, 9512 CrossRef; (f) F.-M. Liao, Y.-L. Liu, J.-S. Yu, F. Zhou and J. Zhou, Org. Biomol. Chem., 2015, 13, 8906 RSC; (g) F.-M. Liao, X.-T. Gao, X.-S. Hu, S.-L. Xie and J. Zhou, Sci. Bull., 2017, 62, 1504 CrossRef CAS; (h) Y.-P. Tian, Y. Gong, X.-S. Hu, J.-S. Yu, Y. Zhou and J. Zhou, Org. Biomol. Chem., 2019, 17, 9430 RSC; (i) J.-X. He, Z.-H. Zhang, B.-S. Mu, X.-Y. Cui, J. Zhou and J.-S. Yu, J. Org. Chem., 2021, 86, 9206 CrossRef CAS PubMed.
- For selected examples, see: (a) L. Chu, X. Zhang and F.-L. Qing, Org. Lett., 2009, 11, 2197 CrossRef CAS; (b) Z. Yuan, Y. Wei and M. Shi, Chin. J. Chem., 2010, 28, 1709 CrossRef CAS; (c) Z. Yuan, L. Mei, Y. Wei, M. Shi, P. V. Kattamuri, P. McDowell and G. Li, Org. Biomol. Chem., 2012, 10, 2509 RSC; (d) J.-S. Yu and J. Zhou, Org. Biomol. Chem., 2015, 13, 10968 RSC; (e) J.-S. Yu and J. Zhou, Org. Chem. Front., 2016, 3, 298 RSC; (f) J.-S. Li, Y.-J. Liu, G.-W. Zhang and J.-A. Ma, Org. Lett., 2017, 19, 6364 CrossRef CAS; (g) X.-S. Hu, Y. Du, J.-S. Yu, F.-M. Liao, P.-G. Ding and J. Zhou, Synlett, 2017, 2194 CAS; (h) Y.-B. Wu, L. Wan, G.-P. Lu and C. Cai, Eur. J. Org. Chem., 2017, 3438 CrossRef CAS; (i) X.-S. Hu, J.-S. Yu, Y. Gong and J. Zhou, J. Fluorine Chem., 2019, 219, 106 CrossRef CAS; (j) X.-S. Hu, P.-G. Ding, J.-S. Yu and J. Zhou, Org. Chem. Front., 2019, 6, 2500 RSC; (k) M.-Y. Rong, J.-S. Li, Y. Zhou, F.-G. Zhang and J.-A. Ma, Org. Lett., 2020, 22, 9010 CrossRef CAS; (l) L. Wang, J. Zhang and X. Lin, Synlett, 2021, 417 CAS.
- (a) T. Chen and C. Cai, New J. Chem., 2017, 41, 2519 RSC; (b) J. Li, S. Liu, R. Zhong, Y. Yang, J. Xu, J. Yang, H. Ding and Z. Wang, Org. Lett., 2021, 23, 9526 CrossRef CAS; (c) J. Li, W. Xi, S. Liu, Y. Yang, J. Yang, H. Ding and Z. Wang, Chin. Chem. Lett., 2022, 33, 3007 CrossRef; (d) S. Liu, Y. Li, F. Wang, C. Ma, G. Yang, J. Yang and J. Ren, Synthesis, 2022, 161 Search PubMed; (e) H. Wu, P. Hong, W. Xi and J. Li, Org. Lett., 2022, 24, 2488 CrossRef PubMed.
- (a) J.-S. Yu, F.-M. Liao, W.-M. Gao, K. Liao, R.-L. Zuo and J. Zhou, Angew. Chem., Int. Ed., 2015, 54, 7381 CrossRef PubMed; (b) M. Mamone, E. Morvan, T. Milcent, S. Ongeri and B. Crousse, J. Org. Chem., 2015, 80, 1964 CrossRef PubMed; (c) X.-S. Hu, J.-X. He, S.-Z. Dong, Q.-H. Zhao, J.-S. Yu and J. Zhou, Nat. Commun., 2020, 11, 5500 CrossRef PubMed; (d) Y. Shi, B.-W. Pan, J.-X. He, Y. Zhou, J. Zhou and J.-S. Yu, J. Org. Chem., 2021, 86, 7797 CrossRef CAS PubMed; (e) J. Li, S. Liu, R. Zhong, Y. Yang, Y. He, J. Yang, Y. Ma and Z. Wang, Org. Lett., 2021, 23, 5859 CrossRef CAS PubMed; (f) G. Chen and Y. Liu, Chin. J. Org. Chem., 2021, 41, 869 CrossRef.
- (a) F.-M. Liao, Z.-Y. Cao, J.-S. Yu and J. Zhou, Angew. Chem., Int. Ed., 2017, 56, 2459 CrossRef CAS; (b) J. Li, W. Xi, S. Liu, C. Ruan, X. Zheng, J. Yang, L. Wang and Z. Wang, Org. Lett., 2021, 23, 7264 CrossRef CAS PubMed.
- X. Gao, R. Cheng, Y.-L. Xiao, X.-L. Wan and X.-G. Zhang, Chem, 2019, 5, 2987 CAS.
- (a) J. Li, Y. Chen, R. Zhong, Y. Zhang, J. Yang, H. Ding and Z. Wang, Org. Lett., 2020, 22, 1164 CrossRef CAS PubMed; (b) H. Song, R. Chen, Q.-Q. Min and X. Zhang, Org. Lett., 2020, 22, 7747 CrossRef CAS; (c) F.-S. He, Y. Yao, W. Xie and J. Wu, Chem. Commun., 2020, 56, 9469 RSC; (d) B.-W. Pan, Y. Shi, Y.-P. Tian, Y. Zhou, J. Zhou and J.-S. Yu, Chem. – Asian J., 2020, 15, 4028 CrossRef CAS; (e) J. Li, W. Xi, R. Zhong, J. Yang, L. Wang, H. Ding and Z. Wang, Chem. Commun., 2021, 57, 1050 RSC; (f) J.-Y. Zou, Y.-Z. Wang, W.-H. Sun, W.-J. Lin and X.-Y. Liu, Org. Biomol. Chem., 2021, 19, 8696 RSC; (g) Q.-P. Huang, Y. Huang, A.-J. Wang, L. Zhao, J. Jia, Y.-B. Yu, J. Tong, J.-W. Gu and C.-Y. He, Org. Chem. Front., 2021, 8, 4438 RSC.
- (a) Y.-J. Hao, Y. Gong, Y. Zhou, J. Zhou and J.-S. Yu, Org. Lett., 2020, 22, 8516 CrossRef CAS; (b) Y.-J. Hao, X.-S. Hu, J.-S. Yu, F. Zhou, Y. Zhou and J. Zhou, Tetrahedron, 2018, 74, 7395 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ob02152h |
| This journal is © The Royal Society of Chemistry 2023 |