Ru(II)-catalysed oxidative (4 + 2) annulation of chromene and coumarin carboxylic acids with alkynes/propargylic alcohols: isolation of Ru(0) complexes†
Mallepalli
Shankar
and
K. C.
Kumara Swamy
 *
*
School of Chemistry, University of Hyderabad, Hyderabad 500 046, Telangana, India. E-mail: kckssc@uohyd.ac.in; kckssc@yahoo.com; Tel: +91-40-23134856
First published on 9th December 2022
Abstract
Ru(II)-catalysed oxidative (4 + 2) annulation of chromene and coumarin-3-carboxylic acids with alkynes/propargylic alcohols via sp2 C–H bond activation is reported. While the reaction with alkynes affords highly substituted pyrano-chromones in good to excellent yields, the use of methyl-tethered propargylic alcohols in place of alkynes leads to novel Ru(0)–metal complexes in moderate to good yields; these complexes were stable in MeCO2H/O2. The generality of the reaction with alkynes is illustrated in the case of both symmetrical and unsymmetrical internal alkynes with excellent regioselectivity in the case of unsymmetrical alkynes.
Introduction
Transition metal-catalysed oxidative annulations via C–H bond activation involving the weakly coordinating carboxylic acid functionality are important methods for the construction of polycyclic heterocycles (e.g., pyrans and chromenes) and biologically active drug molecules (cf.Fig. 1).1,2 A wide range of chromenes/coumarins and their analogues possess pharmaceutically significant anti-HIV RT,3a–b anti-tuberculosis,3e diuretic, analgesic,3c and anti-cancer3d,f–g activities.A rapid expansion of methods to construct the above cyclic systems via transition metal, Ru,4 Rh,5 Pd,6 Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 and Ir
7 and Ir![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, catalysed cyclisation of carboxylic acids with C–C π-components has been observed in the last couple of decades. Ackermann and co-workers4b–d have recently reported a Ru(II)-catalysed oxidative annulation of alkynes with benzoic acids for the synthesis of isocoumarins via ortho-C–H bond activation. Jeganmohan and co-workers4a reported the same annulation reaction of alkynes with benzoic acid by using catalytic amounts of the Ru(II)-catalyst to provide highly substituted isocoumarins with excellent regioselectivity. In addition to the Ru(II)-catalyst, Miura and Satoh and co-workers5a–c,e,g,8a have carried out pioneering studies on rhodium and iridium catalysis for the annulation reactions of aromatic/heterocyclic carboxylic acids with alkynes for the regioselective synthesis of isocoumarins or related architectures. Ison and co-workers8b reported an iridium-catalysed oxidative annulation of benzoic acids with alkynes through mechanistic studies of the annulation process (Scheme 1a). Sundararaju and co-workers7a recently described a chelation-assisted selective cobalt-catalysed annulation of carboxylic acids/acrylic acids with alkynes with excellent regioselectivity overcoming the strong directing influence of the pyridyl nitrogen (Scheme 1b). In addition to these, the groups of Miura,5b,e Jiang6c and Gogoi4c have reported the synthesis of α-pyrones from the oxidative annulation of acrylic and cinnamic acids with alkynes (Scheme 1c). Nicewicz's9 group demonstrated the synthesis of novel γ-butyrolactones via an unusual (3 + 2) annulation of alkenes and unsaturated carboxylic acids by using a photoredox catalyst (Scheme 1d). Hernandez and co-workers10 described the solid-phase assisted intramolecular halocyclisation methodology for the synthesis of isocoumarins under transition metal-free conditions using a simple halogen (electrophile) source (Scheme 1e). There are also important reports from the groups of Tanaka,5g Wang,8c Bhanage,4e and Yi5d on the annulation of carboxylic acids with alkynes. In contrast to the general carboxylic acid annulations, Lee's group11 reported the synthesis of phospha-isocoumarins by using arylphosphonic acid monoesters and alkynes under rhodium catalysis (Scheme 1f). Allenes can also undergo cyclisation reactions with carboxylic acids under palladium-catalysed C–H activation (Scheme 1g).12 To the best of our knowledge, there are no reports on the annulation of chromene/coumarin carboxylic acids with alkynes/propargylic alcohols. Thus, in continuation of our studies on C–H activation/functionalisation as well as propargylic chemistry,12–15 we herein report a regioselective [Ru]-catalysed oxidative annulation involving the vinylic C(sp2)–H bond of chromene- and coumarin-3-carboxylic acids and alkynes to afford multisubstituted pyrano-chromones; the regioselectivity observed in the reactions using unsymmetrical internal alkynes is noteworthy (Scheme 1h). The reaction takes place at the vinylic group for which only limited examples are available.4c,g,i,6b Rather surprisingly, the reaction of methyl-tethered propargylic alcohols with chromene-3-carboxylic acids in a stoichiometric reaction with [RuCl2(p-cymene)]2 produced novel Ru(0)-complexes of pyrano-chromenones in moderate to good yields; to our knowledge, such a reaction has not been reported earlier.4d The ruthenium in these complexes was reluctant to move out by the use of MeCOOH/O2.
8, catalysed cyclisation of carboxylic acids with C–C π-components has been observed in the last couple of decades. Ackermann and co-workers4b–d have recently reported a Ru(II)-catalysed oxidative annulation of alkynes with benzoic acids for the synthesis of isocoumarins via ortho-C–H bond activation. Jeganmohan and co-workers4a reported the same annulation reaction of alkynes with benzoic acid by using catalytic amounts of the Ru(II)-catalyst to provide highly substituted isocoumarins with excellent regioselectivity. In addition to the Ru(II)-catalyst, Miura and Satoh and co-workers5a–c,e,g,8a have carried out pioneering studies on rhodium and iridium catalysis for the annulation reactions of aromatic/heterocyclic carboxylic acids with alkynes for the regioselective synthesis of isocoumarins or related architectures. Ison and co-workers8b reported an iridium-catalysed oxidative annulation of benzoic acids with alkynes through mechanistic studies of the annulation process (Scheme 1a). Sundararaju and co-workers7a recently described a chelation-assisted selective cobalt-catalysed annulation of carboxylic acids/acrylic acids with alkynes with excellent regioselectivity overcoming the strong directing influence of the pyridyl nitrogen (Scheme 1b). In addition to these, the groups of Miura,5b,e Jiang6c and Gogoi4c have reported the synthesis of α-pyrones from the oxidative annulation of acrylic and cinnamic acids with alkynes (Scheme 1c). Nicewicz's9 group demonstrated the synthesis of novel γ-butyrolactones via an unusual (3 + 2) annulation of alkenes and unsaturated carboxylic acids by using a photoredox catalyst (Scheme 1d). Hernandez and co-workers10 described the solid-phase assisted intramolecular halocyclisation methodology for the synthesis of isocoumarins under transition metal-free conditions using a simple halogen (electrophile) source (Scheme 1e). There are also important reports from the groups of Tanaka,5g Wang,8c Bhanage,4e and Yi5d on the annulation of carboxylic acids with alkynes. In contrast to the general carboxylic acid annulations, Lee's group11 reported the synthesis of phospha-isocoumarins by using arylphosphonic acid monoesters and alkynes under rhodium catalysis (Scheme 1f). Allenes can also undergo cyclisation reactions with carboxylic acids under palladium-catalysed C–H activation (Scheme 1g).12 To the best of our knowledge, there are no reports on the annulation of chromene/coumarin carboxylic acids with alkynes/propargylic alcohols. Thus, in continuation of our studies on C–H activation/functionalisation as well as propargylic chemistry,12–15 we herein report a regioselective [Ru]-catalysed oxidative annulation involving the vinylic C(sp2)–H bond of chromene- and coumarin-3-carboxylic acids and alkynes to afford multisubstituted pyrano-chromones; the regioselectivity observed in the reactions using unsymmetrical internal alkynes is noteworthy (Scheme 1h). The reaction takes place at the vinylic group for which only limited examples are available.4c,g,i,6b Rather surprisingly, the reaction of methyl-tethered propargylic alcohols with chromene-3-carboxylic acids in a stoichiometric reaction with [RuCl2(p-cymene)]2 produced novel Ru(0)-complexes of pyrano-chromenones in moderate to good yields; to our knowledge, such a reaction has not been reported earlier.4d The ruthenium in these complexes was reluctant to move out by the use of MeCOOH/O2.
Results and discussion
The precursors used in the present study are shown in Chart 1. They have been prepared by methods reported in the literature.3g,16,17To begin with, we treated 2H-chromene-3-carboxylic acid 1a (0.57 mmol) with diphenylacetylene 3a (0.57 mmol) in the presence of [RuCl2(p-cymene)]2 (2.5 mol%) as the catalyst, Cu(OAc)2·H2O (30 mol%) as the oxidant and AgSbF6 (10 mol%) as the additive in DCE solvent at 80 °C for 14 h. As expected, we obtained the desired annulated product 5aa in a moderate yield of 48% (Table 1, entry 1). Increasing the temperature to 100 °C increased the yield of 5aa to 56% (entry 2). We have also used other solvents like MeOH, t-AmOH, CH3CN, THF, xylene, PEG-400, and 1,4-dioxane (entries 3–9 at mentioned temperatures) for the reaction. A better yield of 76% was obtained when the solvent was 1,4-dioxane (entry 9); decreasing the temperature to 80 °C decreased the yield to 65% (entry 10). Increasing the reaction time to 20 h led to a very good yield of the final product 5aa (88%; entry 11). There was a marginal increase in the yield (92%) when the catalyst loading was increased to 5 mol% (entry 12). We tested the additives AgOAc, KPF6, AgOTf, AgNTf2 and Ag2CO3, among which AgNTf2 and Ag2CO3 gave yields of 74% and 53%, respectively (entries 13–17). In the absence of the catalyst or oxidant, the reaction failed to give the product, whereas without the additive, the reaction occurred and gave the final product in 43% yield (entries 18–20). We concluded that the conditions shown in entry 11 were the best for the reaction since we wanted to keep the catalyst loading lower than that shown in entry 12.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
| Entry | Additive | Solvent | Temp (°C) | Yieldb (%) |
|---|---|---|---|---|
| a Chromene-3-carboxylic acid 1a (0.57 mmol), diphenylacetylene 3a (0.57 mmol), [RuCl2(p-cymene)]2 (2.5 mol%), Cu(OAc)2·H2O (30 mol%), additive (10 mol%), solvent (2 mL), temp °C (oil bath temperature). Reaction time: 14 h for entries 1–10 and 20 h for entries 11–20. b Isolated yield. c 5 mol% of the catalyst was used. d In the absence of [RuCl2(p-cymene)]2. e In the absence of Cu(OAc)2·H2O. | ||||
| 1 | AgSbF6 | DCE | 80 | 48 |
| 2 | AgSbF6 | DCE | 100 | 56 |
| 3 | AgSbF6 | MeOH | 80 | Trace |
| 4 | AgSbF6 | t-AmOH | 100 | 25 |
| 5 | AgSbF6 | CH3CN | 80 | 51 |
| 6 | AgSbF6 | THF | 80 | Trace |
| 7 | AgSbF6 | Xylene | 100 | 12 |
| 8 | AgSbF6 | PEG-400 | 100 | n.r. |
| 9 | AgSbF6 | Dioxane | 100 | 76 |
| 10 | AgSbF6 | Dioxane | 80 | 65 |
| 11 | AgSbF 6 | Dioxane | 100 | 88 |
| 12 | AgSbF6 | Dioxane | 100 | 92c |
| 13 | AgOAc | Dioxane | 100 | 21 |
| 14 | KPF6 | Dioxane | 100 | 31 |
| 15 | AgOTf | Dioxane | 100 | 26 |
| 16 | AgNTf2 | Dioxane | 100 | 74 |
| 17 | Ag2CO3 | Dioxane | 100 | 53 |
| 18d | AgSbF6 | Dioxane | 100 | n.d. |
| 19e | AgSbF6 | Dioxane | 100 | n.d. |
| 20 | — | Dioxane | 100 | 43 |
As shown in Scheme 2, the reaction of 1a with 3a under the optimised conditions gave 5aa in 88% yield. The structure of 5aa was confirmed by X-ray crystallography. Various internal symmetrical (alkyl/alkyl or aryl/aryl; 3a–c and 3h–j) and unsymmetrical alkynes (alkyl/aryl or aryl/aryl; 3d–g and 3k) worked very well and delivered the final products 5aa–5cj in good to excellent yields. It is noteworthy that in the case of the unsymmetrical alkyne 3d, we observed excellent regioselectivity (cf. compound 5ad); the other isomer was present (<10%) but could not be isolated. Subtle steric factors associated with the [Ru]-intermediate and the alkyne are possibly the reason for this regioselectivity. The reaction of 1a with the electron-rich aryl alkynes 3a–c proceeded well under the optimised conditions and afforded the products 5aa–5ac in 70–88% yields. Unsymmetrical (alkyl/aryl) alkynes 3d–g and 3k also reacted well and delivered the final products 5ad–ag and 5ak in good yields with excellent regioselectivity. Dialkyl alkynes 3h–j were also amenable to this reaction and reacted with 1a and 1b, delivering the products in good yields. We also checked the reaction using dimethyl acetylene dicarboxylate (DMAD, 3l), but in this case, we obtained the simple addition product 5′al. Other internal alkynes like 2-butyn-1,4-diol 3-(4-nitrophenyl)prop-2-yn-1-ol, ether-tethered symmetrical alkyne 1,4-dimethoxy-2-butyne and bis(trimethylsilyl)acetylene were unreactive under the conditions employed here. The scope of this oxidative annulation reaction was also extended to substituted chromene-3-carboxylic acids. Thus 8-ethoxy-2H-chromene-3-carboxylic acid (1b) and 6-bromo-8-methoxy-2H-chromene-3-carboxylic acid (1c) upon treatment with alkyne partners afforded the final products 5ba–cj in good yields.
As an extension of the above reaction, we wanted to check the reactivity of chromene-3-carboxylic acid 1 with propargylic alcohols 4 in the presence of [RuCl2(p-cymene)]2, since a reaction similar to that discussed above can also take place. Thus, we employed an equimolar ratio of chromene-3-carboxylic acid 1a and propargylic alcohol 4a under the above-optimized reaction conditions but did not observe any product formation. Based on our earlier observations on the reactivity of the propargylic alcohols,15e we thought that use of Cu(OTf)2 (0.5 equiv.) would help, but no reaction was observed. Unexpectedly, by using Cu(OTf)2 (0.5 equiv.) along with K2CO3 (1.0 equiv.) in dioxane at 100 °C for 24 h, the reaction afforded the Ru(p-cymene) complex 6aa containing the (4 + 2) annulated product in a moderate yield (41% based on the [Ru]-precursor). In the absence of AgSbF6 also, the complex was obtained in 49% yield. However, as can be noticed from the formula, stoichiometrically, 0.5 mole of [RuCl2(p-cymene)]2 is required per each mole of 1a and 4a. Using this stoichiometry, we obtained 6aa in a yield of 61%. Similarly, by using chromene-3-carboxylic acids 1b and 1d, propargylic alcohols 4a–c and [RuCl2(p-cymene)]2, we obtained the ruthenium(0) complexes 6aa–ad in decent yields (cf.Scheme 3). The structure of complex 6aa was confirmed by single crystal X-ray analysis. Although complexes similar to 6aa have been reported by Ackermann,4d the fact that it may be a general reaction has not been explored. We tried to prepare the demetalated product from 6aa by using (i) Cu(OAc)2·H2O,4d (ii) AcOH/O2 or (iii) AgOAc, but did not observe any reaction and the Ru(0)–metal complex remained intact. It is likely that Cu(OTf)2 makes the alkynic part of the propargylic alcohol more reactive for the annulation process by coordinating with the alcoholic –OH group. The counter anion triflate (TfO−; a weak conjugate base) or the acetate anion may facilitate the exchange of ligands to give catalytically active [Ru]-species. We believe that in the reactions using alkynes also similar Ru(0) complexes are formed (vide infra), but under the conditions employed, they are not stable enough and release the [Ru]-moiety. These Ru(0)-complexes (e.g., 6aa) were not catalytically active under the conditions shown in Scheme 2; thus after treating 2a with 3a using 2.5 mol% of 6aa in place of [RuCl2(p-cymene)]2, we recovered the complex 6aa and the starting materials as such.
To extend the above [Ru]-catalysed cyclisation, we checked the reactivity of coumarin-3-carboxylic acid to know the effect of the additional carbonyl group on product formation. Hence we employed coumarin-3-carboxylic acid 2a along with alkyne 3a and as expected, the (4 + 2) annulation occurred to give the annulated product 7aa in 58% yield. Similarly, 3b gave the annulated product 7ab in 61% yield and 3c gave pyrano[3,4-c]chromene-4,5-dione 7ac in 64% yield. Unsymmetrical alkynes 3e (methyl and phenyl) and 3g (n-propyl and phenyl) also reacted well and afforded products 7ae and 7ag in 60% and 68% yields, respectively, with excellent regioselectivity. The dialkyl substituted alkynes 3i and 3j also provided the annulation products 7ai and 7aj in good yields. These results are shown in Scheme 4. The yields were a bit lower as compared to the reaction using chromene-3-carboxylic acid. This may be because of the coordination of the metal catalyst with the carbonyl functionality, making it less accessible for the annulation process via C–H activation. When we reacted the substituted coumarin-3-carboxylic acids 2b and 2c with diphenylacetylene 3a under the optimized reaction conditions, surprisingly, only the Ru(0)-complexes 7′ba and 7′ca, rather than simple (4 + 2) annulation products, were isolated. The structure of the annulated product 7ab was confirmed by single crystal X-ray diffraction studies.
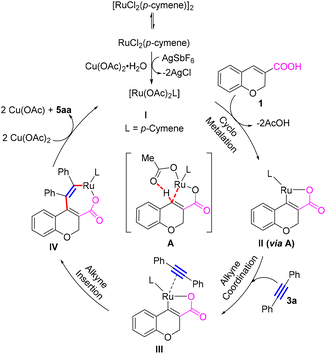
Plausible pathway for the annulation
Based on our experimental studies and previous literature reports on the annulation of carboxylic acids with alkynes,1c,2h,4a,d we propose the catalytic cycle shown in Scheme 5 for the annulation of the chromene/coumarin carboxylic acids (1 or 2) with alkynes 3. Here, [RuCl2(p-cymene)]2 first undergoes ligand exchange with Cu(OAc)2·H2O and AgSbF6 and generates the active intermediate I. Then the acid partner 1 undergoes cyclometalation with intermediate I to give the five-membered cyclic ruthenium intermediate II (viaA). Then alkyne coordinates with intermediate II to produce species III. This species undergoes alkyne insertion to give the seven-membered cyclic intermediate IV, which upon reductive elimination gives the annulated product 5aa. The catalyst may be regenerated by oxidation with Cu(OAc)2·H2O for the next cycle. However, in view of the isolated Ru-complexes 6aa–6da under similar conditions as discussed above, there is a possibility that the reaction could take place via a Ru(0) complex of this type in the penultimate step (IV to I).Based on our experimental studies and previous literature reports on the annulations of carboxylic acids with alkynes and Ru(0)–metal complex formation,1c,2b,4e,i–j we propose a possible pathway shown in Scheme 6 for the formation of the Ru(0)-complex 6aa. Here, [RuCl2(p-cymene)]2 first undergoes ligand exchange with Cu(OTf)2 to generate the active intermediate V. Then the acid partner 1 undergoes cyclometalation with intermediate V to give the ruthenium intermediate VI. Then propargylic alcohol 4a coordinates with intermediate VI to produce species VII. This species undergoes propargylic alcohol insertion to give intermediate VIII, which subsequently converts to intermediate IX by elimination of TfOH. This species upon dehydration in the presence of K2CO3 delivers the Ru(0)–metal complex. To check the other possibility of the reaction of in situ generated diene (by the elimination of water from the propargylic alcohol) with the intermediate Ru(p-cymene)(OTf)2, we treated propargylic alcohol 4a with Cu(OTf)2/(with/without) K2CO3. Although 4a reacted, we could not isolate the diene. Thus, we concluded that the water elimination takes place only at a later stage, as shown in Scheme 6.
Conclusions
In summary, we have developed an efficient and viable methodology for the construction of pyranochromene-4(5H)-ones and pyranochromene-4,5-diones with excellent regioselectivity, via ortho-C–H bond activation under ruthenium catalysis using simple starting materials. We were also successful in synthesizing novel Ru(0) metal complexes using chromene-3-carboxylic acids and methyl-tethered tert-propargylic alcohols using [Ru(p-cymene)Cl2]2. This methodology shows excellent regioselectivity and good functional group tolerance. It worked well for the reactions of a wide variety of symmetrical (alkyl/alkyl or aryl/aryl) and unsymmetrical (alkyl/aryl or aryl) alkynes with chromene and coumarin-3-carboxylic acids affording the products with very good regioselectivity.Experimental section
General information
All the reactions were carried out under air. All the required chemicals were procured from Aldrich or local manufacturers and used as such without further purification unless otherwise noted. Melting points were recorded on MR-visual melting range apparatus and are uncorrected. 1H and 13C{1H} NMR spectra were recorded using 5 mm tubes on 400 and 500 MHz NMR spectrometers [field strengths: for 13C{1H} NMR 400/100 or 500/125 MHz] in CDCl3 or DMSO-D6 solution (unless specified otherwise) with shifts referenced to TMS (1H, 13C: = 0). Data for 1H and 13C{1H} NMR are reported in δ (ppm). All J values are in Hz. Infrared spectra were recorded using the ATR technique on an FT-IR spectrophotometer. Mass spectra were recorded using HRMS (ESI-Thermo Scientific EXACTIVE ORBITRAP/TOF analyzer) equipment. Crystallographic data were collected using an X-ray diffractometer system using Mo-Kα radiation (λ = 0.71073 Å). Structures were solved and refined using standard methods. Thin-layer chromatography was performed on silica/alumina plates and components were visualized under iodine/UV light at 254 nm. Column chromatography was performed on silica gel (100–200 mesh); for the elution process, a hexane–EtOAc mixture was used as the eluent.Starting materials 2H-chromene-3-carboxylic acids 1a–c were synthesized by using standard literature procedures.3g,16 Among 2H-chromene-3-carboxylic acids 1a–c, 1a has already been reported, and 1b and 1c are new. Alkynes 3a–d were prepared by following literature procedures.17 Coumarin-3-carboxylic acid 2 and alkynes 3e–l are commercially available and were used as such. Analytical grade (AR grade) solvents were bought from local chemical vendors and were dried according to standard procedures.18 Crystallographic data were collected at 293 K on an X-ray diffractometer system using Mo-Kα radiation (λ = 0.71073 Å). Structures were solved and refined using standard methods.19
General procedure for the synthesis of 2H-chromene-3-carboxylic acids 1b and 1c
8-Ethoxy-2H-chromene-3-carboxylic acid (1b). Yield 5.5 g (84%) as a pale yellow solid; mp 218–220 °C; Rf = 0.36 (4
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1hexane/ethyl acetate); 1H NMR (400 MHz, DMSO-d6) δ 7.42 (s, 1H), 7.00–6.99 (m, 1H), 6.92–6.86 (m, 2H), 4.88 (s, 2H), 4.02 (q, J = 14.0 Hz, 2H), 1.32 (t, J = 7.0 Hz, 3H); 13C{1H} NMR (100 MHz, DMSO-d6) δ 166.0, 147.2, 144.1, 133.0, 123.9, 122.1, 121.9, 121.3, 166.7, 64.5, 15.2; IR (neat) 2973, 2923, 2875, 2516, 1663, 1635, 1469, 1436, 1308, 1340, 1263, 1111, 1084, 936, 778, 715 cm−1; HRMS (ESI-TOF): calcd for C12H12O4Na [M + Na]+: m/z 243.0633. Found: 243.0631.
1hexane/ethyl acetate); 1H NMR (400 MHz, DMSO-d6) δ 7.42 (s, 1H), 7.00–6.99 (m, 1H), 6.92–6.86 (m, 2H), 4.88 (s, 2H), 4.02 (q, J = 14.0 Hz, 2H), 1.32 (t, J = 7.0 Hz, 3H); 13C{1H} NMR (100 MHz, DMSO-d6) δ 166.0, 147.2, 144.1, 133.0, 123.9, 122.1, 121.9, 121.3, 166.7, 64.5, 15.2; IR (neat) 2973, 2923, 2875, 2516, 1663, 1635, 1469, 1436, 1308, 1340, 1263, 1111, 1084, 936, 778, 715 cm−1; HRMS (ESI-TOF): calcd for C12H12O4Na [M + Na]+: m/z 243.0633. Found: 243.0631.
6-Bromo-8-methoxy-2H-chromene-3-carboxylic acid (1c). Yield 4.9 g (81%) as a yellow solid; mp 235–237 °C; Rf = 0.39 (4
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1hexane/ethyl acetate); 1H NMR (400 MHz, DMSO-d6) δ 7.40 (s, 1H), 7.18–7.16 (m, 2H), 4.90 (s, 2H), 3.79 (s, 3H); 13C{1H} NMR (100 MHz, DMSO-d6) δ 165.7, 149.0, 143.3, 131.6, 125.1, 123.5, 123.2, 117.9, 113.0, 64.7, 56.6; IR (neat) 2841, 2580, 1679, 1637, 1567, 1476, 1433, 1343, 1247, 1211, 1124, 975, 901, 853, 822, 726 cm−1; HRMS (ESI-TOF): calcd For C11H9BrO4Na [M + Na]+, [M + Na + 2]+: m/z 306.9582, 308.9562. Found: 306.9581, 308.9561.
1hexane/ethyl acetate); 1H NMR (400 MHz, DMSO-d6) δ 7.40 (s, 1H), 7.18–7.16 (m, 2H), 4.90 (s, 2H), 3.79 (s, 3H); 13C{1H} NMR (100 MHz, DMSO-d6) δ 165.7, 149.0, 143.3, 131.6, 125.1, 123.5, 123.2, 117.9, 113.0, 64.7, 56.6; IR (neat) 2841, 2580, 1679, 1637, 1567, 1476, 1433, 1343, 1247, 1211, 1124, 975, 901, 853, 822, 726 cm−1; HRMS (ESI-TOF): calcd For C11H9BrO4Na [M + Na]+, [M + Na + 2]+: m/z 306.9582, 308.9562. Found: 306.9581, 308.9561.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture as the eluent to afford the corresponding annulated products 5aa–5ak, 5ba, 5bb, 5bg–5bj, 5ca–5cc, 5ce, 5ci–5cj and 5′al.
1) mixture as the eluent to afford the corresponding annulated products 5aa–5ak, 5ba, 5bb, 5bg–5bj, 5ca–5cc, 5ce, 5ci–5cj and 5′al.
1,2-Diphenyl-4H,5H-pyrano[3,4-c]chromen-4-one (5aa). Following the general procedure, the reaction of 1a (100 mg, 0.56 mmol) with 3a (101.2 mg, 0.56 mmol), [Ru] (8.7 mg, 2.5 mol%), AgSbF6 (19.5 mg, 10 mol%) and Cu(OAc)2·H2O (34.0 mg, 30 mol%) afforded 5aa as a white solid using ethyl acetate/hexane (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) as the eluent. Yield 176.0 mg (88%); mp 138–140 °C; Rf = 0.52 (9
9) as the eluent. Yield 176.0 mg (88%); mp 138–140 °C; Rf = 0.52 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/ethyl acetate);1H NMR (500 MHz, CDCl3) δ 7.37–7.34 (m, 2H), 7.32–7.30 (m, 2H), 7.29–7.26 (m, 3H), 7.21–7.20 (m, 2H), 7.12–7.10 (m, 2H), 7.04 (dd, J1 = 8.3 Hz, J2 = 1.5 Hz, 1H), 6.58–6.54 (m, 1H), 6.37 (dd, J1 = 8.0 Hz, J2 = 1.5 Hz, 1H), 5.07 (s, 2H); 13C{1H} NMR (125 MHz, CDCl3) δ 159.9, 158.0, 157.7, 143.9, 135.0, 132.8, 132.0, 131.3, 129.4, 129.3, 129.0, 128.8, 128.3, 127.8, 121.1, 120.1, 117.8, 116.3, 63.4; IR (neat) 2848, 2648, 1702, 1567, 1527, 1485, 1393, 1109, 998, 758, 696 cm−1; HRMS (ESI-TOF): calcd for C24H17O3 [M + H]+: m/z 353.1178. Found: 353.1177. X-ray structure has been determined for this compound.
1 hexane/ethyl acetate);1H NMR (500 MHz, CDCl3) δ 7.37–7.34 (m, 2H), 7.32–7.30 (m, 2H), 7.29–7.26 (m, 3H), 7.21–7.20 (m, 2H), 7.12–7.10 (m, 2H), 7.04 (dd, J1 = 8.3 Hz, J2 = 1.5 Hz, 1H), 6.58–6.54 (m, 1H), 6.37 (dd, J1 = 8.0 Hz, J2 = 1.5 Hz, 1H), 5.07 (s, 2H); 13C{1H} NMR (125 MHz, CDCl3) δ 159.9, 158.0, 157.7, 143.9, 135.0, 132.8, 132.0, 131.3, 129.4, 129.3, 129.0, 128.8, 128.3, 127.8, 121.1, 120.1, 117.8, 116.3, 63.4; IR (neat) 2848, 2648, 1702, 1567, 1527, 1485, 1393, 1109, 998, 758, 696 cm−1; HRMS (ESI-TOF): calcd for C24H17O3 [M + H]+: m/z 353.1178. Found: 353.1177. X-ray structure has been determined for this compound.
1,2-Di-p-tolyl-4H,5H-pyrano[3,4-c]chromen-4-one (5ab). Following the general procedure, the reaction of 1a (100 mg, 0.56 mmol) with 3b (117.1 mg, 0.56 mmol), [Ru] (8.7 mg, 2.5 mol%), AgSbF6 (19.5 mg, 10 mol%) and Cu(OAc)2·H2O (34.0 mg, 30 mol%) afforded 5ab as a pale-yellow solid using ethyl acetate/hexane (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) as the eluent. Yield 151.2 mg (70%); mp 218–220 °C; Rf = 0.56 (9
9) as the eluent. Yield 151.2 mg (70%); mp 218–220 °C; Rf = 0.56 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/ethyl acetate); 1H NMR (400 MHz, CDCl3) δ 7.24–7.19 (m, 1H), 7.13–7.11 (m, 4H), 7.04–6.98 (m, 5H), 6.60–6.56 (m, 1H), 6.42 (dd, J1 = 8.0 Hz, J2 = 1.6 Hz, 1H), 5.06 (s, 2H), 2.40 (s, 3H), 2.31 (s, 3H); 13C{1H} NMR (125 MHz, CDCl3) δ 160.1, 158.1, 157.7, 144.1, 139.4, 138.0, 132.0, 131.9, 131.1, 130.0, 129.7, 129.2, 128.9, 128.5, 121.0, 120.3, 117.7, 115.88, 115.85, 63.4, 21.4, 21.3; IR (neat) 2920, 2851, 1717, 1603, 1569, 1500, 1455, 1391, 1353, 1014, 820, 761, cm−1; HRMS (ESI-TOF): calcd for C26H21O3 [M + H]+: m/z 381.1491. Found: 381.1492.
1 hexane/ethyl acetate); 1H NMR (400 MHz, CDCl3) δ 7.24–7.19 (m, 1H), 7.13–7.11 (m, 4H), 7.04–6.98 (m, 5H), 6.60–6.56 (m, 1H), 6.42 (dd, J1 = 8.0 Hz, J2 = 1.6 Hz, 1H), 5.06 (s, 2H), 2.40 (s, 3H), 2.31 (s, 3H); 13C{1H} NMR (125 MHz, CDCl3) δ 160.1, 158.1, 157.7, 144.1, 139.4, 138.0, 132.0, 131.9, 131.1, 130.0, 129.7, 129.2, 128.9, 128.5, 121.0, 120.3, 117.7, 115.88, 115.85, 63.4, 21.4, 21.3; IR (neat) 2920, 2851, 1717, 1603, 1569, 1500, 1455, 1391, 1353, 1014, 820, 761, cm−1; HRMS (ESI-TOF): calcd for C26H21O3 [M + H]+: m/z 381.1491. Found: 381.1492.
1,2-Bis(4-methoxyphenyl)-4H,5H-pyrano[3,4-c]chromen-4-one (5ac). Following the general procedure, the reaction of 1a (100 mg, 0.56 mmol) with 3c (135.3 mg, 0.56 mmol), [Ru] (8.7 mg, 2.5 mol%), AgSbF6 (19.5 mg, 10 mol%) and Cu(OAc)2·H2O (34.0 mg, 30 mol%) afforded 5ac as a light green solid using ethyl acetate/hexane (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) as the eluent. Yield 189.6 mg (81%); mp 199–201 °C; Rf = 0.46 (9
9) as the eluent. Yield 189.6 mg (81%); mp 199–201 °C; Rf = 0.46 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/ethyl acetate); 1H NMR (500 MHz, CDCl3) δ 7.24–7.20 (m, 1H), 7.19–7.16 (m, 2H), 7.04–7.0 (m, 3H), 6.88–6.85 (m, 2H), 6.74–6.71 (m, 2H), 6.62–6.59 (m, 1H), 6.46 (dd, J1 = 8.2 Hz, J2 = 1.0 Hz, 1H), 5.05 (s, 2H), 3.86 (s, 3H), 3.79 (s, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ 160.18, 160.15, 159.4, 158.0, 157.7, 144.3, 132.4, 131.9, 130.9, 128.9, 127.3, 125.3, 121.1, 120.4, 117.7, 115.5, 115.1, 114.6, 113.3, 63.4, 55.3, 55.2; IR (neat) 2922, 2852, 1734, 1604, 1512, 1463, 1262, 1177, 1080, 1031, 970, 749 cm−1; HRMS (ESI-TOF): calcd for C26H20O5Na [M + Na]+: m/z 435.1209. Found: 435.1209.
1 hexane/ethyl acetate); 1H NMR (500 MHz, CDCl3) δ 7.24–7.20 (m, 1H), 7.19–7.16 (m, 2H), 7.04–7.0 (m, 3H), 6.88–6.85 (m, 2H), 6.74–6.71 (m, 2H), 6.62–6.59 (m, 1H), 6.46 (dd, J1 = 8.2 Hz, J2 = 1.0 Hz, 1H), 5.05 (s, 2H), 3.86 (s, 3H), 3.79 (s, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ 160.18, 160.15, 159.4, 158.0, 157.7, 144.3, 132.4, 131.9, 130.9, 128.9, 127.3, 125.3, 121.1, 120.4, 117.7, 115.5, 115.1, 114.6, 113.3, 63.4, 55.3, 55.2; IR (neat) 2922, 2852, 1734, 1604, 1512, 1463, 1262, 1177, 1080, 1031, 970, 749 cm−1; HRMS (ESI-TOF): calcd for C26H20O5Na [M + Na]+: m/z 435.1209. Found: 435.1209.
1-(4-Methoxyphenyl)-2-phenyl-4H,5H-pyrano[3,4-c]chromen-4-one (5ad). Following the general procedure, the reaction of 1a (100 mg, 0.56 mmol) with 3d (118.2 mg, 0.56 mmol), [Ru] (8.7 mg, 2.5 mol%), AgSbF6 (19.5 mg, 10 mol%) and Cu(OAc)2·H2O (34.0 mg, 30 mol%) afforded 5ad as a pale-yellow solid using ethyl acetate/hexane (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) as the eluent. Yield 169.3 mg (78%); mp 221–223 °C; Rf = 0.48 (9
9) as the eluent. Yield 169.3 mg (78%); mp 221–223 °C; Rf = 0.48 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/ethyl acetate); 1H NMR (500 MHz, CDCl3) δ 7.24–7.21 (m, 6H), 7.03 (dd, J1 = 8.3 Hz, J2 = 1.0 Hz, 1H), 7.01–6.98 (m, 2H), 6.85–6.82 (m, 2H), 6.63–6.60 (m, 1H), 6.47 (dd, J1 = 8.0 Hz, J2 = 1.5 Hz, 1H), 5.06 (s, 2H), 3.84 (s, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ 160.1, 159.5, 158.0, 157.7, 144.2, 132.9, 132.4, 132.0, 129.3, 129.2, 128.8, 127.9, 126.9, 121.2, 120.3, 117.8, 116.2, 115.9, 114.5, 63.4, 55.3; IR (neat) 3058, 2926, 2839, 1708, 1678, 1506, 1489, 1295, 1255, 1179, 1029, 836, 760, 699 cm−1; HRMS (ESI-TOF): calcd for C25H19O4 [M + H]+: m/z 383.1283. Found: 383.1283.
1 hexane/ethyl acetate); 1H NMR (500 MHz, CDCl3) δ 7.24–7.21 (m, 6H), 7.03 (dd, J1 = 8.3 Hz, J2 = 1.0 Hz, 1H), 7.01–6.98 (m, 2H), 6.85–6.82 (m, 2H), 6.63–6.60 (m, 1H), 6.47 (dd, J1 = 8.0 Hz, J2 = 1.5 Hz, 1H), 5.06 (s, 2H), 3.84 (s, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ 160.1, 159.5, 158.0, 157.7, 144.2, 132.9, 132.4, 132.0, 129.3, 129.2, 128.8, 127.9, 126.9, 121.2, 120.3, 117.8, 116.2, 115.9, 114.5, 63.4, 55.3; IR (neat) 3058, 2926, 2839, 1708, 1678, 1506, 1489, 1295, 1255, 1179, 1029, 836, 760, 699 cm−1; HRMS (ESI-TOF): calcd for C25H19O4 [M + H]+: m/z 383.1283. Found: 383.1283.
1-Methyl-2-phenyl-4H,5H-pyrano[3,4-c]chromen-4-one (5ae). Following the general procedure, the reaction of 1a (100 mg, 0.56 mmol) with 3e (65.9 mg, 0.56 mmol), [Ru] (8.7 mg, 2.5 mol%), AgSbF6 (19.5 mg, 10 mol%) and Cu(OAc)2·H2O (34.0 mg, 30 mol%) afforded 5ae as a pale-yellow solid using ethyl acetate/hexane (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) as the eluent. Yield 121.9 mg (74%); mp 160–162 °C; Rf = 0.60 (9
9) as the eluent. Yield 121.9 mg (74%); mp 160–162 °C; Rf = 0.60 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/ethyl acetate); 1H NMR (500 MHz, CDCl3) δ 7.76 (dd, J1 = 8.3 Hz, J2 = 1.5 Hz, 1H), 7.66–7.64 (m, 2H), 7.51–7.49 (m, 3H), 7.43–7.38 (m, 1H), 7.14–7.10 (m, 2H), 5.02 (s, 2H), 2.38 (s, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ 160.3, 158.3, 157.9, 145.7, 132.9, 132.3, 130.0, 129.4, 128.4, 121.7, 121.1, 118.1, 116.1, 109.2, 63.2, 18.2; IR (neat) 2922, 2852, 1762, 1702, 1604, 1571, 1534, 1492, 1450, 751, 699 cm−1; HRMS (ESI-TOF): calcd for C19H14O3Na [M + Na]+: m/z 313.0841. Found: 313.0842.
1 hexane/ethyl acetate); 1H NMR (500 MHz, CDCl3) δ 7.76 (dd, J1 = 8.3 Hz, J2 = 1.5 Hz, 1H), 7.66–7.64 (m, 2H), 7.51–7.49 (m, 3H), 7.43–7.38 (m, 1H), 7.14–7.10 (m, 2H), 5.02 (s, 2H), 2.38 (s, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ 160.3, 158.3, 157.9, 145.7, 132.9, 132.3, 130.0, 129.4, 128.4, 121.7, 121.1, 118.1, 116.1, 109.2, 63.2, 18.2; IR (neat) 2922, 2852, 1762, 1702, 1604, 1571, 1534, 1492, 1450, 751, 699 cm−1; HRMS (ESI-TOF): calcd for C19H14O3Na [M + Na]+: m/z 313.0841. Found: 313.0842.
1-Ethyl-2-phenyl-4H,5H-pyrano[3,4-c]chromen-4-one (5af). Following the general procedure, the reaction of 1a (100 mg, 0.56 mmol) with 3f (73.9 mg, 0.56 mmol), [Ru] (8.7 mg, 2.5 mol%), AgSbF6 (19.5 mg, 10 mol%) and Cu(OAc)2·H2O (34.0 mg, 30 mol%) afforded 5af as a white solid using ethyl acetate/hexane (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) as the eluent. Yield 124.4 mg (72%); mp 122–124 °C; Rf = 0.62 (9
9) as the eluent. Yield 124.4 mg (72%); mp 122–124 °C; Rf = 0.62 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/ethyl acetate); 1H NMR (500 MHz, CDCl3) δ 7.83 (dd, J1 = 8.0 Hz, J2 = 1.5 Hz, 1H), 7.56–7.54 (m, 2H), 7.50–7.49 (m, 3H), 7.41–7.39 (m, 1H), 7.14–7.09 (m, 2H), 5.00 (s, 2H), 2.85 (q, J = 9.0 Hz, 2H), 0.94 (t, J = 9.0 Hz, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ 160.1, 158.6, 157.7, 144.4, 133.3, 132.2, 129.8, 129.2, 128.5, 127.1, 122.1, 121.2, 118.2, 117.6, 115.7, 63.2, 21.6, 14.5; IR (neat) 2924, 2852, 1705, 1604, 1571, 1533, 1491, 751, 701 cm−1; HRMS (ESI-TOF): calcd for C20H17O3 [M + H]+: m/z 305.1178. Found: 305.1117.
1 hexane/ethyl acetate); 1H NMR (500 MHz, CDCl3) δ 7.83 (dd, J1 = 8.0 Hz, J2 = 1.5 Hz, 1H), 7.56–7.54 (m, 2H), 7.50–7.49 (m, 3H), 7.41–7.39 (m, 1H), 7.14–7.09 (m, 2H), 5.00 (s, 2H), 2.85 (q, J = 9.0 Hz, 2H), 0.94 (t, J = 9.0 Hz, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ 160.1, 158.6, 157.7, 144.4, 133.3, 132.2, 129.8, 129.2, 128.5, 127.1, 122.1, 121.2, 118.2, 117.6, 115.7, 63.2, 21.6, 14.5; IR (neat) 2924, 2852, 1705, 1604, 1571, 1533, 1491, 751, 701 cm−1; HRMS (ESI-TOF): calcd for C20H17O3 [M + H]+: m/z 305.1178. Found: 305.1117.
2-Phenyl-1-propyl-4H,5H-pyrano[3,4-c]chromen-4-one (5ag). Following the general procedure, the reaction of 1a (100 mg, 0.56 mmol) with 3g (81.9 mg, 0.56 mmol), [Ru] (8.7 mg, 2.5 mol%), AgSbF6 (19.5 mg, 10 mol%) and Cu(OAc)2·H2O (34.0 mg, 30 mol%) afforded 5ag as a yellow solid using ethyl acetate/hexane (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) as the eluent. Yield 142.8 mg (79%); mp 118–120 °C; Rf = 0.63 (9
9) as the eluent. Yield 142.8 mg (79%); mp 118–120 °C; Rf = 0.63 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/ethyl acetate); 1H NMR (400 MHz, CDCl3) δ 7.80 (dd, J1 = 8.0 Hz, J2 = 1.6 Hz, 1H), 7.56–7.54 (m, 2H), 7.50–4.49 (m, 3H), 7.41–7.38 (m, 1H), 7.14–7.09 (m, 2H), 4.99 (s, 2H), 2.81–2.77 (m, 2H), 1.32–1.23 (m, 2H), 0.71 (t, J = 7.2 Hz, 3H); 13C{1H} NMR (125 MHz, CDCl3) δ 160.2, 158.8, 157.6, 144.6, 133.4, 132.3, 129.8, 129.4, 128.5, 126.9, 122.0, 121.4, 118.3, 117.6, 114.2, 63.2, 30.4, 22.8, 13.5; IR (neat) 2961, 2927, 2877, 1706, 1534, 1491, 1448, 1090, 750, 701 cm−1; HRMS (ESI-TOF): calcd for C21H19O3 [M + H]+: m/z 319.1334. Found: 319.1335.
1 hexane/ethyl acetate); 1H NMR (400 MHz, CDCl3) δ 7.80 (dd, J1 = 8.0 Hz, J2 = 1.6 Hz, 1H), 7.56–7.54 (m, 2H), 7.50–4.49 (m, 3H), 7.41–7.38 (m, 1H), 7.14–7.09 (m, 2H), 4.99 (s, 2H), 2.81–2.77 (m, 2H), 1.32–1.23 (m, 2H), 0.71 (t, J = 7.2 Hz, 3H); 13C{1H} NMR (125 MHz, CDCl3) δ 160.2, 158.8, 157.6, 144.6, 133.4, 132.3, 129.8, 129.4, 128.5, 126.9, 122.0, 121.4, 118.3, 117.6, 114.2, 63.2, 30.4, 22.8, 13.5; IR (neat) 2961, 2927, 2877, 1706, 1534, 1491, 1448, 1090, 750, 701 cm−1; HRMS (ESI-TOF): calcd for C21H19O3 [M + H]+: m/z 319.1334. Found: 319.1335.
1,2-Diethyl-4H,5H-pyrano[3,4-c]chromen-4-one (5ah). Following the general procedure, the reaction of 1a (100 mg, 0.56 mmol) with 3h (46.6 mg, 0.56 mmol), [Ru] (8.7 mg, 2.5 mol%), AgSbF6 (19.5 mg, 10 mol%) and Cu(OAc)2·H2O (34.0 mg, 30 mol%) afforded 5ah as a pale-yellow solid using ethyl acetate/hexane (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) as the eluent. Yield 106.2 mg (73%); mp 221–223 °C; Rf = 0.62 (9
9) as the eluent. Yield 106.2 mg (73%); mp 221–223 °C; Rf = 0.62 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/ethyl acetate); 1H NMR (400 MHz, CDCl3) δ 7.65 (dd, J1 = 8.2 Hz, J2 = 1.6 Hz, 1H), 7.38–7.34 (m, 1H), 7.10–7.06 (m, 2H), 4.90 (s, 2H), 2.69–2.62 (m, 4H), 1.31–1.26 (m, 6H); 13C{1H} NMR (100 MHz, CDCl3) δ 163.1, 160.7, 157.8, 144.9, 132.1, 127.3, 121.9, 120.9, 118.2, 115.5, 113.8, 63.2, 24.5, 20.4, 15.2, 12.3; IR (neat) 2972, 2936, 1719, 1684, 1605, 1571, 1532, 1454, 1202, 1038, 981, 759, 736 cm−1; HRMS (ESI-TOF): calcd for C16H16O3Na [M + Na]+: m/z 279.0997. Found: 279.0995.
1 hexane/ethyl acetate); 1H NMR (400 MHz, CDCl3) δ 7.65 (dd, J1 = 8.2 Hz, J2 = 1.6 Hz, 1H), 7.38–7.34 (m, 1H), 7.10–7.06 (m, 2H), 4.90 (s, 2H), 2.69–2.62 (m, 4H), 1.31–1.26 (m, 6H); 13C{1H} NMR (100 MHz, CDCl3) δ 163.1, 160.7, 157.8, 144.9, 132.1, 127.3, 121.9, 120.9, 118.2, 115.5, 113.8, 63.2, 24.5, 20.4, 15.2, 12.3; IR (neat) 2972, 2936, 1719, 1684, 1605, 1571, 1532, 1454, 1202, 1038, 981, 759, 736 cm−1; HRMS (ESI-TOF): calcd for C16H16O3Na [M + Na]+: m/z 279.0997. Found: 279.0995.
1,2-Dipropyl-4H,5H-pyrano[3,4-c]chromen-4-one (5ai). Following the general procedure, the reaction of 1a (100 mg, 0.56 mmol) with 3i (62.5 mg, 0.56 mmol), [Ru] (8.7 mg, 2.5 mol%), AgSbF6 (19.5 mg, 10 mol%) and Cu(OAc)2·H2O (34.0 mg, 30 mol%) afforded 5ai as a pale-yellow solid using ethyl acetate/hexane (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) as the eluent. Yield 124.3 mg (77%); mp 102–104 °C; Rf = 0.65 (9
9) as the eluent. Yield 124.3 mg (77%); mp 102–104 °C; Rf = 0.65 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/ethyl acetate); 1H NMR (400 MHz, CDCl3) δ 7.60 (dd, J1 = 8.4 Hz, J2 = 1.6 Hz, 1H), 7.40–7.35 (m, 1H), 7.11–7.07 (m, 2H), 4.90 (s, 2H), 2.62–2.56 (m, 4H), 1.82–1.72 (m, 2H), 1.65–1.56 (m, 2H), 1.03 (t, J = 7.4 Hz, 6H); 13C{1H} NMR (125 MHz, CDCl3) δ 162.0, 160.6, 157.9, 144.7, 132.1, 127.1, 121.8, 121.1, 118.2, 115.7, 113.1, 63.2, 33.2, 29.4, 23.8, 21.3, 13.90, 13.86; IR (neat) 2961, 2931, 2872, 1704, 1605, 1572, 1532, 1457, 1397, 1233, 758 cm−1; HRMS (ESI-TOF): calcd for C18H21O3 [M + H]+: m/z 285.1491. Found: 285.1490.
1 hexane/ethyl acetate); 1H NMR (400 MHz, CDCl3) δ 7.60 (dd, J1 = 8.4 Hz, J2 = 1.6 Hz, 1H), 7.40–7.35 (m, 1H), 7.11–7.07 (m, 2H), 4.90 (s, 2H), 2.62–2.56 (m, 4H), 1.82–1.72 (m, 2H), 1.65–1.56 (m, 2H), 1.03 (t, J = 7.4 Hz, 6H); 13C{1H} NMR (125 MHz, CDCl3) δ 162.0, 160.6, 157.9, 144.7, 132.1, 127.1, 121.8, 121.1, 118.2, 115.7, 113.1, 63.2, 33.2, 29.4, 23.8, 21.3, 13.90, 13.86; IR (neat) 2961, 2931, 2872, 1704, 1605, 1572, 1532, 1457, 1397, 1233, 758 cm−1; HRMS (ESI-TOF): calcd for C18H21O3 [M + H]+: m/z 285.1491. Found: 285.1490.
1,2-Dibutyl-4H,5H-pyrano[3,4-c]chromen-4-one (5aj). Following the general procedure, the reaction of 1a (100 mg, 0.56 mmol) with 3j (78.5 mg, 0.56 mmol), [Ru] (8.7 mg, 2.5 mol%), AgSbF6 (19.5 mg, 10 mol%) and Cu(OAc)2·H2O (34.0 mg, 30 mol%) afforded 5aj as a gummy liquid using ethyl acetate/hexane (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) as the eluent. Yield 134.8 mg (76%); Rf = 0.68 (9
9) as the eluent. Yield 134.8 mg (76%); Rf = 0.68 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/ethyl acetate); 1H NMR (400 MHz, CDCl3) δ 7.63–7.61 (m, 1H), 7.39–7.35 (m, 1H), 7.10–7.06 (m, 2H), 4.89 (s, 2H), 2.63–2.58 (m, 4H), 1.75–1.67 (m, 2H), 1.59–1.53 (m, 2H), 1.48–1.40 (m, 4H), 1.00–0.95 (m, 6H); 13C{1H} NMR (125 MHz, CDCl3) δ 162.2, 160.6, 157.9, 144.7, 132.1, 127.2, 121.8, 121.1, 118.2, 115.6, 113.0, 63.2, 32.6, 31.1, 30.0, 27.1, 22.6, 22.5, 13.8, 13.7; IR (neat) 2958, 2930, 2871, 1723, 1606, 1456, 1207, 1108, 1042, 979, 756 cm−1; HRMS (ESI-TOF): calcd for C20H24O3Na [M + Na]+: m/z 335.1623. Found: 335.1623.
1 hexane/ethyl acetate); 1H NMR (400 MHz, CDCl3) δ 7.63–7.61 (m, 1H), 7.39–7.35 (m, 1H), 7.10–7.06 (m, 2H), 4.89 (s, 2H), 2.63–2.58 (m, 4H), 1.75–1.67 (m, 2H), 1.59–1.53 (m, 2H), 1.48–1.40 (m, 4H), 1.00–0.95 (m, 6H); 13C{1H} NMR (125 MHz, CDCl3) δ 162.2, 160.6, 157.9, 144.7, 132.1, 127.2, 121.8, 121.1, 118.2, 115.6, 113.0, 63.2, 32.6, 31.1, 30.0, 27.1, 22.6, 22.5, 13.8, 13.7; IR (neat) 2958, 2930, 2871, 1723, 1606, 1456, 1207, 1108, 1042, 979, 756 cm−1; HRMS (ESI-TOF): calcd for C20H24O3Na [M + Na]+: m/z 335.1623. Found: 335.1623.
2-Butyl-1-ethyl-4H,5H-pyrano[3,4-c]chromen-4-one (5ak). Following the general procedure, the reaction of 1a (100 mg, 0.57 mmol) with 3k (62.5 mg, 0.57 mmol), [RuCl2(p-cymene)]2 (8.7 mg, 0.014 mmol), Cu(OAc)2·H2O (34.0 mg, 0.17 mmol) and AgSbF6 (19.5 mg, 0.057 mmol) afforded 5ak as a gummy liquid using ethyl acetate/hexane (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) as the eluent. Yield 114.6 mg (71%); Rf = 0.67 (9
9) as the eluent. Yield 114.6 mg (71%); Rf = 0.67 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/ethyl acetate); 1H NMR (500 MHz, CDCl3) δ 7.66 (d, J = 8.5, 1.5 Hz, 1H), 7.38–7.35 (m, 1H), 7.09–7.06 (m, 2H), 4.89 (s, 2H), 2.66 (q, J = 7.5 Hz, 2H), 5.22 (q, J = 7.5 Hz, 2H), 1.74–1.68 (m, 2H), 1.46–1.39 (m, 2H), 1.26 (t, J = 7.5 Hz, 3H), 0.96 (t, J = 7.5 Hz, 3H); 13C{1H} NMR (125 MHz, CDCl3) δ 162.3, 160.6, 157.9, 144.7, 132.1, 127.3, 121.9, 121.0, 118.2, 115.6, 114.2, 63.3, 31.0, 30.0, 22.6, 20.5, 15.2, 13.8; IR (neat) 2960, 2929, 1697, 1572, 1533, 1457, 904, 728, 649 cm−1; HRMS (ESI-TOF): calcd for C18H21O3 [M + H]+: m/z 285.1490. Found: 285.1487.
1 hexane/ethyl acetate); 1H NMR (500 MHz, CDCl3) δ 7.66 (d, J = 8.5, 1.5 Hz, 1H), 7.38–7.35 (m, 1H), 7.09–7.06 (m, 2H), 4.89 (s, 2H), 2.66 (q, J = 7.5 Hz, 2H), 5.22 (q, J = 7.5 Hz, 2H), 1.74–1.68 (m, 2H), 1.46–1.39 (m, 2H), 1.26 (t, J = 7.5 Hz, 3H), 0.96 (t, J = 7.5 Hz, 3H); 13C{1H} NMR (125 MHz, CDCl3) δ 162.3, 160.6, 157.9, 144.7, 132.1, 127.3, 121.9, 121.0, 118.2, 115.6, 114.2, 63.3, 31.0, 30.0, 22.6, 20.5, 15.2, 13.8; IR (neat) 2960, 2929, 1697, 1572, 1533, 1457, 904, 728, 649 cm−1; HRMS (ESI-TOF): calcd for C18H21O3 [M + H]+: m/z 285.1490. Found: 285.1487.
7-Ethoxy-1,2-diphenyl-4H,5H-pyrano[3,4-c]chromen-4-one (5ba). Following the general procedure, the reaction of 1b (100 mg, 0.45 mmol) with 3a (80.9 mg, 0.45 mmol), [Ru] (7.0 mg, 2.5 mol%), AgSbF6 (15.6 mg, 10 mol%) and Cu(OAc)2·H2O (27.2 mg, 30 mol%) afforded 5ba as a pale-yellow solid using ethyl acetate/hexane (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) as the eluent. Yield 133.2 mg (74%); mp 202–204 °C; Rf = 0.48 (9
9) as the eluent. Yield 133.2 mg (74%); mp 202–204 °C; Rf = 0.48 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/ethyl acetate); 1H NMR (500 MHz, CDCl3) δ 7.35–7.32 (m, 1H), 7.30 (br, 1H), 7.29–7.25 (m, 2H), 7.22–7.19 (m, 4H), 7.10–7.08 (m, 2H), 6.86–6.84 (m, 1H), 6.48 (t, J = 8.3 Hz, 1H), 6.01–5.99 (m, 1H), 5.12 (s, 2H), 4.13 (q, J = 7.0 Hz, 2H), 1.49 (t, J = 7.0 Hz, 3H); 13C{1H} NMR (125 MHz, CDCl3) δ 159.9, 158.0, 148.3, 147.3, 144.1, 135.0, 132.8, 131.3, 129.4, 129.3, 128.9, 128.2, 127.8, 121.1, 120.7, 120.4, 116.4, 155.7, 64.8, 63.6, 14.8; IR (neat) 3057, 2928, 2850, 1696, 1533, 1489, 1463, 1443, 1388, 1284, 1204, 1085, 779, 740, 696 cm−1; HRMS (ESI-TOF): calcd for C26H21O4 [M + H]+: m/z 397.1440. Found: 397.1442.
1 hexane/ethyl acetate); 1H NMR (500 MHz, CDCl3) δ 7.35–7.32 (m, 1H), 7.30 (br, 1H), 7.29–7.25 (m, 2H), 7.22–7.19 (m, 4H), 7.10–7.08 (m, 2H), 6.86–6.84 (m, 1H), 6.48 (t, J = 8.3 Hz, 1H), 6.01–5.99 (m, 1H), 5.12 (s, 2H), 4.13 (q, J = 7.0 Hz, 2H), 1.49 (t, J = 7.0 Hz, 3H); 13C{1H} NMR (125 MHz, CDCl3) δ 159.9, 158.0, 148.3, 147.3, 144.1, 135.0, 132.8, 131.3, 129.4, 129.3, 128.9, 128.2, 127.8, 121.1, 120.7, 120.4, 116.4, 155.7, 64.8, 63.6, 14.8; IR (neat) 3057, 2928, 2850, 1696, 1533, 1489, 1463, 1443, 1388, 1284, 1204, 1085, 779, 740, 696 cm−1; HRMS (ESI-TOF): calcd for C26H21O4 [M + H]+: m/z 397.1440. Found: 397.1442.
7-Ethoxy-1,2-di-p-tolyl-4H,5H-pyrano[3,4-c]chromen-4-one (5bb). Following the general procedure, the reaction of 1b (100 mg, 0.45 mmol) with 3b (93.7 mg, 0.45 mmol), [Ru] (7.0 mg, 2.5 mol%), AgSbF6 (15.6 mg, 10 mol%) and Cu(OAc)2·H2O (27.2 mg, 30 mol%) afforded 5bb as a pale-yellow solid using ethyl acetate/hexane (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) as the eluent. Yield 148.4 mg (77%); mp 220–222 °C; Rf = 0.52 (9
9) as the eluent. Yield 148.4 mg (77%); mp 220–222 °C; Rf = 0.52 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/ethyl acetate); 1H NMR (400 MHz, CDCl3) δ 7.12–7.09 (m, 4H), 7.01–6.99 (m, 2H), 6.97–6.95 (m, 2H), 6.84 (dd, J1 = 8.4 Hz, J2 = 1.2 Hz, 1H), 6.50 (t, J = 8.2 Hz, 1H), 6.04 (dd, J1 = 8.2 Hz, J2 = 1.6 Hz, 1H), 5.10 (s, 2H), 4.12 (q, J = 7.2 Hz, 2H), 2.39 (s, 3H), 2.30 (s, 3H), 1.49 (t, J = 7.2 Hz, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ 160.1, 158.0, 148.2, 147.3, 144.3, 139.4, 137.9, 132.0, 131.1, 130.0, 129.6, 129.3, 128.5, 121.3, 120.8, 120.3, 116.03, 115.99, 115.5, 64.8, 63.7, 21.4, 21.3, 14.8; IR (neat) 2980, 2923, 2858, 1696, 1615, 1535, 1502, 1461, 1387, 1355, 1278, 1200, 1085, 1048, 1017, 823, 762, 741 cm−1; HRMS (ESI-TOF): calcd for C28H25O4 [M + H]+: m/z 425.1753. Found: 425.1753.
1 hexane/ethyl acetate); 1H NMR (400 MHz, CDCl3) δ 7.12–7.09 (m, 4H), 7.01–6.99 (m, 2H), 6.97–6.95 (m, 2H), 6.84 (dd, J1 = 8.4 Hz, J2 = 1.2 Hz, 1H), 6.50 (t, J = 8.2 Hz, 1H), 6.04 (dd, J1 = 8.2 Hz, J2 = 1.6 Hz, 1H), 5.10 (s, 2H), 4.12 (q, J = 7.2 Hz, 2H), 2.39 (s, 3H), 2.30 (s, 3H), 1.49 (t, J = 7.2 Hz, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ 160.1, 158.0, 148.2, 147.3, 144.3, 139.4, 137.9, 132.0, 131.1, 130.0, 129.6, 129.3, 128.5, 121.3, 120.8, 120.3, 116.03, 115.99, 115.5, 64.8, 63.7, 21.4, 21.3, 14.8; IR (neat) 2980, 2923, 2858, 1696, 1615, 1535, 1502, 1461, 1387, 1355, 1278, 1200, 1085, 1048, 1017, 823, 762, 741 cm−1; HRMS (ESI-TOF): calcd for C28H25O4 [M + H]+: m/z 425.1753. Found: 425.1753.
7-Ethoxy-2-phenyl-1-propyl-4H,5H-pyrano[3,4-c]chromen-4-one (5bg). Following the general procedure, the reaction of 1b (100 mg, 0.45 mmol) with 3g (65.5 mg, 0.45 mmol), [Ru] (7.0 mg, 2.5 mol%), AgSbF6 (15.6 mg, 10 mol%) and Cu(OAc)2·H2O (27.2 mg, 30 mol%) afforded 5bg as a pale-yellow solid using ethyl acetate/hexane (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) as the eluent. Yield 131.7 mg (80%); mp 128–130 °C; Rf = 0.60 (9
9) as the eluent. Yield 131.7 mg (80%); mp 128–130 °C; Rf = 0.60 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/ethyl acetate); 1H NMR (500 MHz, CDCl3) δ 7.56–7.53 (m, 2H), 7.50–7.48 (m, 3H), 7.41–7.38 (m, 1H), 7.03–7.02 (m, 2H), 5.03 (s, 2H), 4.18 (q, J = 9.5 Hz, 2H), 2.78 (t, J = 9.5 Hz, 2H), 1.52 (t, J = 9.0 Hz, 3H), 1.27–1.19 (m, 2H), 0.68 (t, J = 9.5 Hz, 3H); 13C{1H} NMR (125 MHz, CDCl3) δ 160.1, 158.7, 148.8, 147.3, 144.9, 133.4, 129.8, 129.4, 128.4, 122.4, 121.4, 118.6, 117.8, 115.8, 114.3, 64.8, 63.5, 30.4, 22.7, 14.8, 13.5; IR (neat) 3059, 2973, 2927, 1708, 1538, 1465, 1360, 1266, 1221, 1088, 1021, 732, 701 cm−1; HRMS (ESI-TOF): calcd for C23H23O4 [M + H]+: m/z 363.1596. Found: 363.1597.
1 hexane/ethyl acetate); 1H NMR (500 MHz, CDCl3) δ 7.56–7.53 (m, 2H), 7.50–7.48 (m, 3H), 7.41–7.38 (m, 1H), 7.03–7.02 (m, 2H), 5.03 (s, 2H), 4.18 (q, J = 9.5 Hz, 2H), 2.78 (t, J = 9.5 Hz, 2H), 1.52 (t, J = 9.0 Hz, 3H), 1.27–1.19 (m, 2H), 0.68 (t, J = 9.5 Hz, 3H); 13C{1H} NMR (125 MHz, CDCl3) δ 160.1, 158.7, 148.8, 147.3, 144.9, 133.4, 129.8, 129.4, 128.4, 122.4, 121.4, 118.6, 117.8, 115.8, 114.3, 64.8, 63.5, 30.4, 22.7, 14.8, 13.5; IR (neat) 3059, 2973, 2927, 1708, 1538, 1465, 1360, 1266, 1221, 1088, 1021, 732, 701 cm−1; HRMS (ESI-TOF): calcd for C23H23O4 [M + H]+: m/z 363.1596. Found: 363.1597.
7-Ethoxy-1,2-diethyl-4H,5H-pyrano[3,4-c]chromen-4-one (5bh). Following the general procedure, the reaction of 1b (100 mg, 0.45 mmol) with 3h (37.3 mg, 0.45 mmol), [Ru] (7.0 mg, 2.5 mol%), AgSbF6 (15.6 mg, 10 mol%) and Cu(OAc)2·H2O (27.2 mg, 30 mol%) afforded 5bh as a yellow solid using ethyl acetate/hexane (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) as the eluent. Yield 102.3 mg (75%); mp 104–106 °C; Rf = 0.60 (9
9) as the eluent. Yield 102.3 mg (75%); mp 104–106 °C; Rf = 0.60 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/ethyl acetate); 1H NMR (400 MHz, CDCl3) δ 7.29–7.25 (m, 1H), 7.02–7.00 (m, 2H), 4.95 (s, 2H), 4.15 (q, J = 7.0 Hz, 2H), 2.69–2.63 (m, 4H), 1.49 (t, J = 7.0 Hz, 3H), 1.30 (t, J = 7.5 Hz, 3H), 1.26 (t, J = 7.5 Hz, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ 163.0, 160.7, 148.8, 147.6, 145.1, 122.0, 121.3, 119.0, 115.8, 115.7, 113.9, 64.8, 63.6, 24.6, 20.4, 15.2, 14.8, 12.3; IR (neat) 2976, 2930, 2877, 1702, 1577, 1534, 1464, 1391, 1270, 1092, 1045, 874, 787, 738 cm−1; HRMS (ESI-TOF): calcd for C18H21O4 [M + H]+: m/z 301.1440. Found: 301.1441.
1 hexane/ethyl acetate); 1H NMR (400 MHz, CDCl3) δ 7.29–7.25 (m, 1H), 7.02–7.00 (m, 2H), 4.95 (s, 2H), 4.15 (q, J = 7.0 Hz, 2H), 2.69–2.63 (m, 4H), 1.49 (t, J = 7.0 Hz, 3H), 1.30 (t, J = 7.5 Hz, 3H), 1.26 (t, J = 7.5 Hz, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ 163.0, 160.7, 148.8, 147.6, 145.1, 122.0, 121.3, 119.0, 115.8, 115.7, 113.9, 64.8, 63.6, 24.6, 20.4, 15.2, 14.8, 12.3; IR (neat) 2976, 2930, 2877, 1702, 1577, 1534, 1464, 1391, 1270, 1092, 1045, 874, 787, 738 cm−1; HRMS (ESI-TOF): calcd for C18H21O4 [M + H]+: m/z 301.1440. Found: 301.1441.
7-Ethoxy-1,2-dipropyl-4H,5H-pyrano[3,4-c]chromen-4-one (5bi). Following the general procedure, the reaction of 1b (100 mg, 0.45 mmol) with 3i (50.0 mg, 0.45 mmol), [Ru] (7.0 mg, 2.5 mol%), AgSbF6 (15.6 mg, 10 mol%) and Cu(OAc)2·H2O (27.2 mg, 30 mol%) afforded 5bi as a pale-yellow solid using ethyl acetate/hexane (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) as the eluent. Yield 120.8 mg (81%); mp 116–118 °C; Rf = 0.62 (9
9) as the eluent. Yield 120.8 mg (81%); mp 116–118 °C; Rf = 0.62 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/ethyl acetate); 1H NMR (500 MHz, CDCl3) δ 7.21–7.18 (m, 1H), 7.02–6.98 (m, 2H), 4.93 (s, 2H), 4.14 (q, J = 7.0 Hz, 2H), 2.60–2.55 (m, 4H), 1.79–1.72 (m, 2H), 1.60–1.53 (m, 2H), 1.48 (t, J = 7.0 Hz, 3H), 1.01 (t, J = 7.5 Hz, 3H), 0.99 (t, J = 7.0 Hz, 3H); 13C{1H} NMR (125 MHz, CDCl3) δ 162.0, 160.6, 148.8, 147.7, 145.0, 122.1, 121.2, 118.9, 115.8, 113.2, 64.8, 63.5, 33.2, 29.4, 23.8, 21.3, 14.8, 13.88, 13.85; IR (neat) 2959, 2871, 1697, 1538, 1466, 1388, 1269, 1146, 1050, 1020, 759 cm−1; HRMS (ESI-TOF): calcd for C20H25O4 [M + H]+: m/z 329.1753. Found: 329.1753.
1 hexane/ethyl acetate); 1H NMR (500 MHz, CDCl3) δ 7.21–7.18 (m, 1H), 7.02–6.98 (m, 2H), 4.93 (s, 2H), 4.14 (q, J = 7.0 Hz, 2H), 2.60–2.55 (m, 4H), 1.79–1.72 (m, 2H), 1.60–1.53 (m, 2H), 1.48 (t, J = 7.0 Hz, 3H), 1.01 (t, J = 7.5 Hz, 3H), 0.99 (t, J = 7.0 Hz, 3H); 13C{1H} NMR (125 MHz, CDCl3) δ 162.0, 160.6, 148.8, 147.7, 145.0, 122.1, 121.2, 118.9, 115.8, 113.2, 64.8, 63.5, 33.2, 29.4, 23.8, 21.3, 14.8, 13.88, 13.85; IR (neat) 2959, 2871, 1697, 1538, 1466, 1388, 1269, 1146, 1050, 1020, 759 cm−1; HRMS (ESI-TOF): calcd for C20H25O4 [M + H]+: m/z 329.1753. Found: 329.1753.
1,2-Dibutyl-7-ethoxy-4H,5H-pyrano[3,4-c]chromen-4-one (5bj). Following the general procedure, the reaction of 1b (100 mg, 0.45 mmol) with 3j (62.8 mg, 0.45 mmol), [Ru] (7.0 mg, 2.5 mol%), AgSbF6 (15.6 mg, 10 mol%) and Cu(OAc)2·H2O (27.2 mg, 30 mol%) afforded 5bj as a white solid using ethyl acetate/hexane (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) as the eluent. Yield 118.2 mg (73%); mp 102–104 °C; Rf = 0.65 (9
9) as the eluent. Yield 118.2 mg (73%); mp 102–104 °C; Rf = 0.65 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/ethyl acetate); 1H NMR (400 MHz, CDCl3) δ 7.25–7.20 (m, 1H), 7.03–6.98 (m, 2H), 4.95 (s, 2H), 4.15 (q, J = 7.2 Hz, 2H), 2.63–2.58 (m, 4H), 1.75–1.67 (m, 4H), 1.58–1.47 (m, 3H), 1.46–1.37 (m, 4H), 0.99–0.95 (m, 6H); 13C{1H} NMR (100 MHz, CDCl3) δ 162.2, 160.6, 148.8, 147.6, 145.1, 122.1, 121.1, 119.0, 115.74, 115.70, 113.1, 64.8, 63.6, 32.6, 31.1, 30.0, 27.1, 22.6, 14.8, 13.8, 13.7; IR (neat) 2957, 2927, 2870, 1706, 1616, 1535, 1465, 1392, 1271, 1182, 1145, 1114, 789, 740 cm−1; HRMS (ESI-TOF): calcd for C22H29O4 [M + H]+: m/z 357.2066. Found: 357.2065.
1 hexane/ethyl acetate); 1H NMR (400 MHz, CDCl3) δ 7.25–7.20 (m, 1H), 7.03–6.98 (m, 2H), 4.95 (s, 2H), 4.15 (q, J = 7.2 Hz, 2H), 2.63–2.58 (m, 4H), 1.75–1.67 (m, 4H), 1.58–1.47 (m, 3H), 1.46–1.37 (m, 4H), 0.99–0.95 (m, 6H); 13C{1H} NMR (100 MHz, CDCl3) δ 162.2, 160.6, 148.8, 147.6, 145.1, 122.1, 121.1, 119.0, 115.74, 115.70, 113.1, 64.8, 63.6, 32.6, 31.1, 30.0, 27.1, 22.6, 14.8, 13.8, 13.7; IR (neat) 2957, 2927, 2870, 1706, 1616, 1535, 1465, 1392, 1271, 1182, 1145, 1114, 789, 740 cm−1; HRMS (ESI-TOF): calcd for C22H29O4 [M + H]+: m/z 357.2066. Found: 357.2065.
9-Bromo-7-methoxy-1,2-diphenyl-4H,5H-pyrano[3,4-c]chromen-4-one (5ca). Following the general procedure, the reaction of 1c (100 mg, 0.35 mmol) with 3a (62.5 mg, 0.35 mmol), [Ru] (5.4 mg, 2.5 mol%), AgSbF6 (12.0 mg, 10 mol%) and Cu(OAc)2·H2O (21.0 mg, 30 mol%) afforded 5ca as a pale-yellow solid using ethyl acetate/hexane (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) as the eluent. Yield 131.0 mg (81%); mp 246–248 °C; Rf = 0.44 (9
9) as the eluent. Yield 131.0 mg (81%); mp 246–248 °C; Rf = 0.44 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/ethyl acetate); 1H NMR (400 MHz, CDCl3) δ 7.42–7.33 (m, 3H), 7.30–7.18 (m, 5H), 7.10–7.08 (m, 2H), 6.95 (d, J = 2.0 Hz, 1H), 6.02 (d, J = 2.4 Hz, 1H), 5.12 (s, 2H), 3.90 (s, 3H); 13C{1H} NMR (125 MHz, CDCl3) δ 159.7, 158.1, 149.6, 146.1, 142.9, 134.3, 132.5, 131.2, 129.5, 129.4, 129.1, 128.6, 127.9, 123.4, 122.0, 117.2, 116.5, 116.0, 112.8, 63.8, 56.4; IR (neat) 3017, 2934, 2840, 2360, 1980, 1709, 1614, 1525, 1385, 1355, 1278, 1202, 1105, 875, 843, 712, 583 cm−1; HRMS (ESI-TOF): calcd for C25H18BrO4 [M + H]+, [M + H + 2]+: m/z 461.0388, 463.0368. Found: 461.0386, 463.0369.
1 hexane/ethyl acetate); 1H NMR (400 MHz, CDCl3) δ 7.42–7.33 (m, 3H), 7.30–7.18 (m, 5H), 7.10–7.08 (m, 2H), 6.95 (d, J = 2.0 Hz, 1H), 6.02 (d, J = 2.4 Hz, 1H), 5.12 (s, 2H), 3.90 (s, 3H); 13C{1H} NMR (125 MHz, CDCl3) δ 159.7, 158.1, 149.6, 146.1, 142.9, 134.3, 132.5, 131.2, 129.5, 129.4, 129.1, 128.6, 127.9, 123.4, 122.0, 117.2, 116.5, 116.0, 112.8, 63.8, 56.4; IR (neat) 3017, 2934, 2840, 2360, 1980, 1709, 1614, 1525, 1385, 1355, 1278, 1202, 1105, 875, 843, 712, 583 cm−1; HRMS (ESI-TOF): calcd for C25H18BrO4 [M + H]+, [M + H + 2]+: m/z 461.0388, 463.0368. Found: 461.0386, 463.0369.
9-Bromo-7-methoxy-1,2-di-p-tolyl-4H,5H-pyrano[3,4-c]chromen-4-one (5cb). Following the general procedure, the reaction of 1c (100 mg, 0.35 mmol) with 3b (72.3 mg, 0.35 mmol), [Ru] (5.4 mg, 2.5 mol%), AgSbF6 (12.0 mg, 10 mol%) and Cu(OAc)2·H2O (21.0 mg, 30 mol%) afforded 5cb as a yellow solid using ethyl acetate/hexane (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) as the eluent. Yield 135.6 mg (79%); mp 226–228 °C; Rf = 0.48 (9
9) as the eluent. Yield 135.6 mg (79%); mp 226–228 °C; Rf = 0.48 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/ethyl acetate); 1H NMR (500 MHz, CDCl3) δ 7.18–7.16 (m, 4H), 7.02 (d, J = 8.0 Hz, 2H), 6.97–6.93 (m, 3H), 5.98 (d, J = 2.0 Hz, 1H), 5.09 (s, 2H), 3.88 (s, 3H), 2.41 (s, 3H), 2.30 (s, 3H); 13C{1H} NMR (125 MHz, CDCl3) δ 159.8, 158.1, 149.6, 146.2, 143.2, 139.7, 138.5, 131.5, 131.0, 129.82, 129.78, 129.3, 128.6, 123.6, 122.2, 117.1, 116.0, 115.6, 112.8, 63.8, 56.4, 21.30, 21.25; IR (neat) 3108, 2921, 1712, 1616, 1562, 1531, 1500, 1277, 1100, 1012, 973, 904, 819, 771, 731 cm−1; HRMS (ESI-TOF): calcd for C27H22BrO4 [M + H]+, [M + H + 2]+: m/z 489.0701, 491.0681. Found: 489.0703, 491.0690.
1 hexane/ethyl acetate); 1H NMR (500 MHz, CDCl3) δ 7.18–7.16 (m, 4H), 7.02 (d, J = 8.0 Hz, 2H), 6.97–6.93 (m, 3H), 5.98 (d, J = 2.0 Hz, 1H), 5.09 (s, 2H), 3.88 (s, 3H), 2.41 (s, 3H), 2.30 (s, 3H); 13C{1H} NMR (125 MHz, CDCl3) δ 159.8, 158.1, 149.6, 146.2, 143.2, 139.7, 138.5, 131.5, 131.0, 129.82, 129.78, 129.3, 128.6, 123.6, 122.2, 117.1, 116.0, 115.6, 112.8, 63.8, 56.4, 21.30, 21.25; IR (neat) 3108, 2921, 1712, 1616, 1562, 1531, 1500, 1277, 1100, 1012, 973, 904, 819, 771, 731 cm−1; HRMS (ESI-TOF): calcd for C27H22BrO4 [M + H]+, [M + H + 2]+: m/z 489.0701, 491.0681. Found: 489.0703, 491.0690.
9-Bromo-7-methoxy-1,2-bis(4-methoxyphenyl)-4H,5H-pyrano[3,4-c]chromen-4-one (5cc). Following the general procedure, the reaction of 1c (100 mg, 0.35 mmol) with 3c (83.6 mg, 0.35 mmol), [Ru] (5.4 mg, 2.5 mol%), AgSbF6 (12.0 mg, 10 mol%) and Cu(OAc)2·H2O (21.0 mg, 30 mol%) afforded 5cc as a yellow solid using ethyl acetate/hexane (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) as the eluent. Yield 153.6 mg (84%); mp 228–230 °C; Rf = 0.40 (9
9) as the eluent. Yield 153.6 mg (84%); mp 228–230 °C; Rf = 0.40 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/ethyl acetate); 1H NMR (400 MHz, CDCl3) δ 7.23 (d, J = 8.8 Hz, 2H), 7.01–6.98 (m, 2H), 6.95–6.91 (m, 3H), 6.74 (d, J = 8.8 Hz, 2H), 6.06 (d, J = 2.0 Hz, 1H), 5.09 (s, 2H), 3.89 (s, 3H), 3.88 (s, 3H), 3.80 (s, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ 160.3, 159.9, 158.0, 149.6, 146.2, 143.4, 132.3, 131.0, 126.7, 125.0, 123.6, 122.3, 116.9, 115.6, 114.84, 114.80, 113.4, 112.8, 63.8, 56.4, 55.5, 55.3; IR (neat) 2935, 2836, 1711, 1601, 1499, 1459, 1414, 1246, 1174, 1101, 1024, 973, 832, 771, 732 cm−1; HRMS (ESI-TOF): calcd for C27H22BrO6 [M + H]+, [M + H + 2]+: m/z 521.0600, 523.058. Found: 521.0600, 523.0615.
1 hexane/ethyl acetate); 1H NMR (400 MHz, CDCl3) δ 7.23 (d, J = 8.8 Hz, 2H), 7.01–6.98 (m, 2H), 6.95–6.91 (m, 3H), 6.74 (d, J = 8.8 Hz, 2H), 6.06 (d, J = 2.0 Hz, 1H), 5.09 (s, 2H), 3.89 (s, 3H), 3.88 (s, 3H), 3.80 (s, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ 160.3, 159.9, 158.0, 149.6, 146.2, 143.4, 132.3, 131.0, 126.7, 125.0, 123.6, 122.3, 116.9, 115.6, 114.84, 114.80, 113.4, 112.8, 63.8, 56.4, 55.5, 55.3; IR (neat) 2935, 2836, 1711, 1601, 1499, 1459, 1414, 1246, 1174, 1101, 1024, 973, 832, 771, 732 cm−1; HRMS (ESI-TOF): calcd for C27H22BrO6 [M + H]+, [M + H + 2]+: m/z 521.0600, 523.058. Found: 521.0600, 523.0615.
9-Bromo-7-methoxy-1-methyl-2-phenyl-4H,5H-pyrano[3,4-c]chromen-4-one (5ce). Following the general procedure, the reaction of 1c (100 mg, 0.35 mmol) with 3e (40.7 mg, 0.35 mmol), [Ru] (5.4 mg, 2.5 mol%), AgSbF6 (12.0 mg, 10 mol%) and Cu(OAc)2·H2O (21.0 mg, 30 mol%) afforded 5ce as a pale-yellow solid using ethyl acetate/hexane (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) as the eluent. Yield 106.4 mg (76%); mp 182–184 °C; Rf = 0.46 (9
9) as the eluent. Yield 106.4 mg (76%); mp 182–184 °C; Rf = 0.46 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/ethyl acetate); 1H NMR (400 MHz, CDCl3) δ 7.65–7.62 (m, 2H), 7.51–7.48 (m, 4H), 7.13 (d, J = 2.0 Hz, 1H), 5.06 (s, 2H), 3.94 (s, 3H), 2.35 (s, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ 159.9, 158.6, 150.1, 146.3, 144.7, 132.7, 130.2, 129.4, 128.4, 123.1, 122.5, 117.5, 116.6, 113.5, 108.8, 63.6, 56.5, 18.1; IR (neat) 2923, 2850, 2359, 2117, 1707, 1621, 1537, 1386, 1277, 1227, 1153, 1106, 1010, 883, 764, 700 cm−1; HRMS (ESI-TOF): calcd For C20H16BrO4 [M + H]+, [M + H + 2]+: m/z 399.0232, 401.0212. Found: 399.0231, 401.0217.
1 hexane/ethyl acetate); 1H NMR (400 MHz, CDCl3) δ 7.65–7.62 (m, 2H), 7.51–7.48 (m, 4H), 7.13 (d, J = 2.0 Hz, 1H), 5.06 (s, 2H), 3.94 (s, 3H), 2.35 (s, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ 159.9, 158.6, 150.1, 146.3, 144.7, 132.7, 130.2, 129.4, 128.4, 123.1, 122.5, 117.5, 116.6, 113.5, 108.8, 63.6, 56.5, 18.1; IR (neat) 2923, 2850, 2359, 2117, 1707, 1621, 1537, 1386, 1277, 1227, 1153, 1106, 1010, 883, 764, 700 cm−1; HRMS (ESI-TOF): calcd For C20H16BrO4 [M + H]+, [M + H + 2]+: m/z 399.0232, 401.0212. Found: 399.0231, 401.0217.
9-Bromo-7-methoxy-1,2-dipropyl-4H,5H-pyrano[3,4-c]chromen-4-one (5ci). Following the general procedure, the reaction of 1c (100 mg, 0.35 mmol) with 3i (38.6 mg, 0.35 mmol), [Ru] (5.4 mg, 2.5 mol%), AgSbF6 (12.0 mg, 10 mol%) and Cu(OAc)2·H2O (21.0 mg, 30 mol%) afforded 5ci as a pale-yellow solid using ethyl acetate/hexane (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) as the eluent. Yield 109.0 mg (79%); mp 150–152 °C; Rf = 0.48 (9
9) as the eluent. Yield 109.0 mg (79%); mp 150–152 °C; Rf = 0.48 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/ethyl acetate); 1H NMR (500 MHz, CDCl3) δ 7.34 (d, J = 2.0 Hz, 1H), 7.10 (d, J = 2.0 Hz, 1H), 4.95 (s, 2H), 3.92 (s, 3H), 2.59 (t, J = 7.8 Hz, 2H), 2.55–2.52 (m, 2H), 1.80–1.73 (m, 2H), 1.64–1.56 (m, 2H), 1.04 (t, J = 7.3 Hz, 3H), 1.02 (t, J = 7.3 Hz, 3H); 13C{1H} NMR (125 MHz, CDCl3) δ 162.4, 160.2, 150.2, 146.5, 143.7, 123.1, 121.6, 117.3, 116.0, 113.6, 112.8, 63.7, 56.5, 33.2, 29.3, 23.8, 21.2, 13.8, 13.7; IR (neat) 2953, 2867, 1709, 1622, 1537, 1467, 1385, 1268, 1154, 1071, 933, 855, 831, 767 cm−1; HRMS (ESI-TOF): calcd for C19H22BrO4 [M + H]+, [M + H + 2]+: m/z 393.0701, 395.0681. Found: 393.0702, 395.0686.
1 hexane/ethyl acetate); 1H NMR (500 MHz, CDCl3) δ 7.34 (d, J = 2.0 Hz, 1H), 7.10 (d, J = 2.0 Hz, 1H), 4.95 (s, 2H), 3.92 (s, 3H), 2.59 (t, J = 7.8 Hz, 2H), 2.55–2.52 (m, 2H), 1.80–1.73 (m, 2H), 1.64–1.56 (m, 2H), 1.04 (t, J = 7.3 Hz, 3H), 1.02 (t, J = 7.3 Hz, 3H); 13C{1H} NMR (125 MHz, CDCl3) δ 162.4, 160.2, 150.2, 146.5, 143.7, 123.1, 121.6, 117.3, 116.0, 113.6, 112.8, 63.7, 56.5, 33.2, 29.3, 23.8, 21.2, 13.8, 13.7; IR (neat) 2953, 2867, 1709, 1622, 1537, 1467, 1385, 1268, 1154, 1071, 933, 855, 831, 767 cm−1; HRMS (ESI-TOF): calcd for C19H22BrO4 [M + H]+, [M + H + 2]+: m/z 393.0701, 395.0681. Found: 393.0702, 395.0686.
9-Bromo-1,2-dibutyl-7-methoxy-4H,5H-pyrano[3,4-c]chromen-4-one (5cj). Following the general procedure, the reaction of 1c (100 mg, 0.35 mmol) with 3j (48.5 mg, 0.35 mmol), [Ru] (5.4 mg, 2.5 mol%), AgSbF6 (12.0 mg, 10 mol%) and Cu(OAc)2·H2O (21.0 mg, 30 mol%) afforded 5cj as a yellow solid using ethyl acetate/hexane (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) as the eluent. Yield 112.3 mg (76%); mp 100–102 °C; Rf = 0.50 (9
9) as the eluent. Yield 112.3 mg (76%); mp 100–102 °C; Rf = 0.50 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/ethyl acetate); 1H NMR (400 MHz, CDCl3) δ 7.36 (d, J = 2.0 Hz, 1H), 7.09 (d, J = 2.0 Hz, 1H), 4.93 (s, 2H), 3.90 (s, 3H), 2.61–2.53 (m, 4H), 1.73–1.65 (m, 2H), 1.58–1.37 (m, 6H), 0.99 (t, J = 7.2 Hz, 3H), 0.96 (t, J = 7.2 Hz, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ 162.6, 160.3, 150.1, 146.4, 143.8, 123.0, 121.5, 117.2, 115.9, 113.6, 112.7, 63.7, 56.4, 32.6, 31.1, 30.0, 27.1, 22.54, 22.51, 13.8, 13.7; IR (neat) 2957, 2931, 2869, 1699, 1614, 1529, 1459, 1414, 1383, 1267, 1212, 1154, 1011, 853, 838, 768, 686, 578 cm−1; HRMS (ESI-TOF): calcd for C21H26BrO4 [M + H]+, [M + H + 2]+: m/z 421.1014, 423.0994. Found: 421.1014, 423.0996.
1 hexane/ethyl acetate); 1H NMR (400 MHz, CDCl3) δ 7.36 (d, J = 2.0 Hz, 1H), 7.09 (d, J = 2.0 Hz, 1H), 4.93 (s, 2H), 3.90 (s, 3H), 2.61–2.53 (m, 4H), 1.73–1.65 (m, 2H), 1.58–1.37 (m, 6H), 0.99 (t, J = 7.2 Hz, 3H), 0.96 (t, J = 7.2 Hz, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ 162.6, 160.3, 150.1, 146.4, 143.8, 123.0, 121.5, 117.2, 115.9, 113.6, 112.7, 63.7, 56.4, 32.6, 31.1, 30.0, 27.1, 22.54, 22.51, 13.8, 13.7; IR (neat) 2957, 2931, 2869, 1699, 1614, 1529, 1459, 1414, 1383, 1267, 1212, 1154, 1011, 853, 838, 768, 686, 578 cm−1; HRMS (ESI-TOF): calcd for C21H26BrO4 [M + H]+, [M + H + 2]+: m/z 421.1014, 423.0994. Found: 421.1014, 423.0996.
Dimethyl 2-((2H-chromene-3-carbonyl)oxy)maleate (5′al). Following the general procedure, the reaction of 1a (100 mg, 0.57 mmol) with 3l (80.7 mg, 0.57 mmol), [RuCl2(p-cymene)]2 (8.7 mg, 0.014 mmol), Cu(OAc)2·H2O (34.0 mg, 0.17 mmol) and AgSbF6 (19.5 mg, 0.057 mmol) afforded 5al as a gummy liquid using ethyl acetate/hexane (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) as the eluent. Yield 142.7 mg (79%); Rf = 0.61 (9
9) as the eluent. Yield 142.7 mg (79%); Rf = 0.61 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/ethyl acetate); 1H NMR (500 MHz, CDCl3) δ 7.62 (s, 1H), 7.31–7.28 (m, 1H), 7.19 (dd, J = 7.5, 1.5 Hz, 1H), 6.98–6.95 (m, 1H), 6.88 (d, J = 8.5 Hz, 1H), 6.19 (s, 1H), 5.0 (d, J = 1.0 Hz, 2H), 3.87 (s, 3H), 3.81 (s, 3H); 13C{1H} NMR (125 MHz, CDCl3) δ 164.2, 161.6, 161.3, 155.6, 147.1, 137.4, 133.1, 129.5, 122.1, 120.4, 119.6, 116.4, 115.3, 63.9, 53.0, 52.3; IR (neat) 2953, 2842, 1719, 1632, 1605, 1570, 1434, 1342, 1283, 1185, 1111, 1058, 1018, 911, 819, 759 cm−1; HRMS (ESI-TOF): calcd for C16H15O7 [M + H]+: m/z 319.0818. Found: 319.0814.
1 hexane/ethyl acetate); 1H NMR (500 MHz, CDCl3) δ 7.62 (s, 1H), 7.31–7.28 (m, 1H), 7.19 (dd, J = 7.5, 1.5 Hz, 1H), 6.98–6.95 (m, 1H), 6.88 (d, J = 8.5 Hz, 1H), 6.19 (s, 1H), 5.0 (d, J = 1.0 Hz, 2H), 3.87 (s, 3H), 3.81 (s, 3H); 13C{1H} NMR (125 MHz, CDCl3) δ 164.2, 161.6, 161.3, 155.6, 147.1, 137.4, 133.1, 129.5, 122.1, 120.4, 119.6, 116.4, 115.3, 63.9, 53.0, 52.3; IR (neat) 2953, 2842, 1719, 1632, 1605, 1570, 1434, 1342, 1283, 1185, 1111, 1058, 1018, 911, 819, 759 cm−1; HRMS (ESI-TOF): calcd for C16H15O7 [M + H]+: m/z 319.0818. Found: 319.0814.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluent to afford the corresponding final product 6aa; the same experimental procedure was followed to synthesize compounds 6ab–6bc and 6da.
1) as the eluent to afford the corresponding final product 6aa; the same experimental procedure was followed to synthesize compounds 6ab–6bc and 6da.
Ru-complex (6aa). Following the general procedure, the reaction of 1a (100 mg, 0.57 mmol) with 4a (126.2 mg, 0.57 mmol), [RuCl2(p-cymene)]2 (173.8 mg, 0.28 mmol), Cu(OTf)2 (205.3 mg, 0.57 mmol) and K2CO3 (78.4 mg, 0.57 mmol) afforded 6aa as a yellow solid using ethyl acetate/hexane (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) as the eluent. Yield 160.5 mg (61%); mp 142–144 °C; Rf = 0.40 (4
4) as the eluent. Yield 160.5 mg (61%); mp 142–144 °C; Rf = 0.40 (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/ethyl acetate); 1H NMR (500 MHz, CDCl3) δ 7.71 (dd, J = 7.5, 1.0 Hz, 1H), 7.52–7.47 (m, 4H), 7.23–7.20 (m, 2H), 7.17–7.13 (m, 2H), 7.08–7.05 (m, 2H), 6.97–6.95 (m, 1H), 6.91–6.89 (m, 1H), 6.81–6.78 (m, 1H), 6.20 (d, J = 1.5 Hz 1H), 5.85 (d, J = 1.0 Hz, 1H), 5.81 (d, J = 6.0 Hz, 1H), 5.65 (d, J = 5.5 Hz, 1H), 4.90 (d, J = 6.0 Hz, 1H), 4.79 (d, J = 6.0 Hz, 1H), 4.59 (d, J = 12.5 Hz, 1H), 4.49 (d, J = 13.0 Hz, 1H), 2.08–2.00 (m, 1H), 1.63 (s, 3H), 1.04 (d, J = 7.0 Hz, 3H), 0.90 (d, J = 6.5 Hz, 3H); 13C{1H} NMR (125 MHz, CDCl3) δ 174.9, 155.8, 143.4, 142.5, 138.8, 128.7, 128.4, 128.0, 127.1, 126.9, 126.6, 125.0, 124.8, 124.1, 121.6, 121.1, 117.5, 112.0, 101.9, 89.6, 89.1, 88.4, 87.9, 87.6, 84.3, 84.1, 69.9, 58.0, 30.7, 25.1, 21.0, 17.6; IR (neat) 3057, 2959, 2924, 2852, 1706, 1600, 1492, 1462, 1380, 1256, 1217, 1126, 1096, 1034, 1006, 978, 907, 757, 699 cm−1; HRMS (ESI-TOF): calcd for C36H33O3Ru [M + H]+: m/z 615.1473. Found: 615.1477.
1 hexane/ethyl acetate); 1H NMR (500 MHz, CDCl3) δ 7.71 (dd, J = 7.5, 1.0 Hz, 1H), 7.52–7.47 (m, 4H), 7.23–7.20 (m, 2H), 7.17–7.13 (m, 2H), 7.08–7.05 (m, 2H), 6.97–6.95 (m, 1H), 6.91–6.89 (m, 1H), 6.81–6.78 (m, 1H), 6.20 (d, J = 1.5 Hz 1H), 5.85 (d, J = 1.0 Hz, 1H), 5.81 (d, J = 6.0 Hz, 1H), 5.65 (d, J = 5.5 Hz, 1H), 4.90 (d, J = 6.0 Hz, 1H), 4.79 (d, J = 6.0 Hz, 1H), 4.59 (d, J = 12.5 Hz, 1H), 4.49 (d, J = 13.0 Hz, 1H), 2.08–2.00 (m, 1H), 1.63 (s, 3H), 1.04 (d, J = 7.0 Hz, 3H), 0.90 (d, J = 6.5 Hz, 3H); 13C{1H} NMR (125 MHz, CDCl3) δ 174.9, 155.8, 143.4, 142.5, 138.8, 128.7, 128.4, 128.0, 127.1, 126.9, 126.6, 125.0, 124.8, 124.1, 121.6, 121.1, 117.5, 112.0, 101.9, 89.6, 89.1, 88.4, 87.9, 87.6, 84.3, 84.1, 69.9, 58.0, 30.7, 25.1, 21.0, 17.6; IR (neat) 3057, 2959, 2924, 2852, 1706, 1600, 1492, 1462, 1380, 1256, 1217, 1126, 1096, 1034, 1006, 978, 907, 757, 699 cm−1; HRMS (ESI-TOF): calcd for C36H33O3Ru [M + H]+: m/z 615.1473. Found: 615.1477.
Ru-complex (6ab). Following the general procedure, the reaction of 1a (100 mg, 0.57 mmol) with 4b (145.7 mg, 0.57 mmol), [RuCl2(p-cymene)]2 (173.8 mg, 0.28 mmol), Cu(OTf)2 (205.3 mg, 0.57 mmol) and K2CO3 (78.4 mg, 0.57 mmol) afforded 6ab as a yellow solid using ethyl acetate/hexane (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) as the eluent. Yield 130.6 mg (71%); mp 206–208 °C; Rf = 0.42 (4
4) as the eluent. Yield 130.6 mg (71%); mp 206–208 °C; Rf = 0.42 (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/ethyl acetate); 1H NMR (major isomer, >95%; 500 MHz, CDCl3) δ 7.61 (d, J = 7.0 Hz, 1H), 7.44–7.40 (m, 4H), 7.15–7.11 (m, 3H), 7.06–7.03 (m, 2H), 6.97–6.94 (m, 1H), 6.88 (d, J = 8.0 Hz, 1H), 6.79–6.76 (m, 1H), 6.17 (s, 1H), 5.85 (s, 1H), 5.79 (d, J = 5.5 Hz, 1H), 5.61 (d, J = 5.5 Hz, 1H), 4.88 (d, J = 5.5 Hz, 1H), 4.77 (d, J = 5.5 Hz, 1H), 4.56 (d, J = 12.5 Hz, 1H), 4.46 (d, J = 12.5 Hz, 1H), 2.06–1.98 (m, 1H), 1.61 (s, 3H), 1.01 (d, J = 7.0 Hz, 3H), 0.88 (d, J = 6.5 Hz, 3H); 13C{1H} NMR (125 MHz, CDCl3) δ 174.8, 155.8, 142.5, 142.3, 137.3, 133.9, 128.9, 128.6, 127.8, 127.1, 126.8, 125.0, 124.9, 123.9, 121.61, 121.57, 117.6, 112.2, 102.0, 89.4, 89.2, 88.5, 88.0, 87.6, 84.4, 83.6, 69.8, 58.0, 30.7, 25.1, 21.0, 17.6; IR (neat) 2964, 1706, 1600, 1490, 1461, 1377, 1247, 1210, 1122, 1093, 1002, 975, 906, 837, 753, 692 cm−1; HRMS (ESI-TOF): calcd for C36H32O3ClRu [M + H]+: m/z 649.1083. Found: 649.1086.
1 hexane/ethyl acetate); 1H NMR (major isomer, >95%; 500 MHz, CDCl3) δ 7.61 (d, J = 7.0 Hz, 1H), 7.44–7.40 (m, 4H), 7.15–7.11 (m, 3H), 7.06–7.03 (m, 2H), 6.97–6.94 (m, 1H), 6.88 (d, J = 8.0 Hz, 1H), 6.79–6.76 (m, 1H), 6.17 (s, 1H), 5.85 (s, 1H), 5.79 (d, J = 5.5 Hz, 1H), 5.61 (d, J = 5.5 Hz, 1H), 4.88 (d, J = 5.5 Hz, 1H), 4.77 (d, J = 5.5 Hz, 1H), 4.56 (d, J = 12.5 Hz, 1H), 4.46 (d, J = 12.5 Hz, 1H), 2.06–1.98 (m, 1H), 1.61 (s, 3H), 1.01 (d, J = 7.0 Hz, 3H), 0.88 (d, J = 6.5 Hz, 3H); 13C{1H} NMR (125 MHz, CDCl3) δ 174.8, 155.8, 142.5, 142.3, 137.3, 133.9, 128.9, 128.6, 127.8, 127.1, 126.8, 125.0, 124.9, 123.9, 121.61, 121.57, 117.6, 112.2, 102.0, 89.4, 89.2, 88.5, 88.0, 87.6, 84.4, 83.6, 69.8, 58.0, 30.7, 25.1, 21.0, 17.6; IR (neat) 2964, 1706, 1600, 1490, 1461, 1377, 1247, 1210, 1122, 1093, 1002, 975, 906, 837, 753, 692 cm−1; HRMS (ESI-TOF): calcd for C36H32O3ClRu [M + H]+: m/z 649.1083. Found: 649.1086.
Ru-complex (6ac). Following the general procedure, the reaction of 1a (100 mg, 0.57 mmol) with 4c (171.0 mg, 0.57 mmol), [RuCl2(p-cymene)]2 (173.8 mg, 0.28 mmol), Cu(OTf)2 (205.3 mg, 0.57 mmol) and K2CO3 (78.4 mg, 0.57 mmol) afforded 6ac as a yellow solid using ethyl acetate/hexane (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) as the eluent. Yield 147.4 mg (75%); mp 218–220 °C; Rf = 0.43 (4
4) as the eluent. Yield 147.4 mg (75%); mp 218–220 °C; Rf = 0.43 (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/ethyl acetate); 1H NMR (major isomer, >95%, 500 MHz, CDCl3) δ 7.60 (dd, J = 7.5, 1.0 Hz, 1H), 7.43 (d, J = 8.0 Hz 2H), 7.35–7.34 (m, 2H), 7.29–7.27 (m, 2H), 7.14–7.11 (m, 1H), 7.06–7.03 (m, 2H), 6.97–6.94 (m, 1H), 6.88 (d, J = 7.5 Hz, 1H), 6.78–6.75 (m, 1H), 6.19 (s, 1H), 5.86 (s, 1H), 5.79 (d, J = 6.0 Hz, 1H), 5.61 (d, J = 6.0 Hz, 1H), 4.88 (d, J = 6.0 Hz, 1H), 4.77 (d, J = 6.0 Hz, 1H), 4.56 (d, J = 12.5 Hz, 1H), 4.46 (d, J = 12.5 Hz, 1H), 2.06–1.98 (m, 1H), 1.60 (s, 3H), 1.01 (d, J = 7.0 Hz, 3H), 0.88 (d, J = 6.5 Hz, 3H); 13C{1H} NMR (125 MHz, CDCl3) δ 174.7, 155.7, 142.4, 142.2, 137.7, 131.5, 128.8, 128.0, 127.0, 126.7, 125.0, 124.8, 123.9, 122.1, 121.7, 121.6, 117.5, 112.2, 102.0, 89.3, 89.2, 88.5, 88.0, 87.5, 84.3, 83.4, 69.7, 57.9, 30.6, 25.0, 20.9, 17.5; IR (neat) 2963, 1705, 1599, 1488, 1461, 1385, 1247, 1209, 1122, 1091, 1003, 975, 906, 835, 754, 693 cm−1; HRMS (ESI-TOF): calcd for C36H32O3BrRu [M + H]+: m/z 693.0578. Found: 693.0577.
1 hexane/ethyl acetate); 1H NMR (major isomer, >95%, 500 MHz, CDCl3) δ 7.60 (dd, J = 7.5, 1.0 Hz, 1H), 7.43 (d, J = 8.0 Hz 2H), 7.35–7.34 (m, 2H), 7.29–7.27 (m, 2H), 7.14–7.11 (m, 1H), 7.06–7.03 (m, 2H), 6.97–6.94 (m, 1H), 6.88 (d, J = 7.5 Hz, 1H), 6.78–6.75 (m, 1H), 6.19 (s, 1H), 5.86 (s, 1H), 5.79 (d, J = 6.0 Hz, 1H), 5.61 (d, J = 6.0 Hz, 1H), 4.88 (d, J = 6.0 Hz, 1H), 4.77 (d, J = 6.0 Hz, 1H), 4.56 (d, J = 12.5 Hz, 1H), 4.46 (d, J = 12.5 Hz, 1H), 2.06–1.98 (m, 1H), 1.60 (s, 3H), 1.01 (d, J = 7.0 Hz, 3H), 0.88 (d, J = 6.5 Hz, 3H); 13C{1H} NMR (125 MHz, CDCl3) δ 174.7, 155.7, 142.4, 142.2, 137.7, 131.5, 128.8, 128.0, 127.0, 126.7, 125.0, 124.8, 123.9, 122.1, 121.7, 121.6, 117.5, 112.2, 102.0, 89.3, 89.2, 88.5, 88.0, 87.5, 84.3, 83.4, 69.7, 57.9, 30.6, 25.0, 20.9, 17.5; IR (neat) 2963, 1705, 1599, 1488, 1461, 1385, 1247, 1209, 1122, 1091, 1003, 975, 906, 835, 754, 693 cm−1; HRMS (ESI-TOF): calcd for C36H32O3BrRu [M + H]+: m/z 693.0578. Found: 693.0577.
Ru-complex (6ba). Following the general procedure, the reaction of 1b (100 mg, 0.45 mmol) with 4a (101.0 mg, 0.45 mmol), [RuCl2(p-cymene)]2 (139.0 mg, 0.23 mmol), Cu(OTf)2 (164.2 mg, 0.45 mmol) and K2CO3 (62.8 mg, 0.45 mmol) afforded 6ba as a yellow solid using ethyl acetate/hexane (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) as the eluent. Yield 94.3 mg (63%); mp 128–130 °C; Rf = 0.39 (4
4) as the eluent. Yield 94.3 mg (63%); mp 128–130 °C; Rf = 0.39 (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/ethyl acetate); 1H NMR (major isomer, >95%, 500 MHz, CDCl3) δ 7.51–7.47 (m, 4H), 7.31 (dd, J = 8.0, 1.5 Hz, 1H), 7.22–7.19 (m, 2H), 7.16–7.13 (m, 1H), 7.07–7.04 (m, 2H), 6.97–6.94 (m, 1H), 6.81 (dd, J = 8.0, 1.0 Hz, 1H), 6.74–6.71 (m, 1H), 6.19 (d, J = 1.5 Hz, 1H), 5.84 (d, J = 1.5 Hz, 1H), 5.80 (d, J = 6.0 Hz, 1H), 5.64 (d, J = 6.0 Hz, 1H), 4.95 (d, J = 5.5 Hz, 1H), 4.84 (d, J = 5.5 Hz, 1H), 4.72 (d, J = 12.5 Hz, 1H), 4.47 (d, J = 13.0 Hz, 1H), 4.16 (q, J = 14.0, 7.0 Hz, 2H), 2.09–2.01 (m, 1H), 1.65 (s, 3H), 1.45 (t, J = 7.0 Hz, 3H), 1.03 (d, J = 7.0 Hz, 3H), 0.91 (d, J = 6.5 Hz, 3H); 13C{1H} NMR (125 MHz, CDCl3) δ 174.9, 147.9, 145.4, 143.4, 142.5, 138.9, 127.9, 126.9, 126.6, 125.2, 125.0, 124.8, 121.3, 121.1, 119.4, 113.1, 112.1, 101.9, 89.0, 88.5, 88.1, 87.7, 84.3, 84.1, 70.3, 64.6, 58.1, 30.6, 25.1, 21.0, 17.5, 14.8; IR (neat) 2961, 2923, 2852, 1710, 1597, 1446, 1395, 1258, 1095, 1014, 863, 793, 695 cm−1; HRMS (ESI-TOF): calcd for C38H37O4Ru [M + H]+: m/z 659.1735. Found: 659.1732.
1 hexane/ethyl acetate); 1H NMR (major isomer, >95%, 500 MHz, CDCl3) δ 7.51–7.47 (m, 4H), 7.31 (dd, J = 8.0, 1.5 Hz, 1H), 7.22–7.19 (m, 2H), 7.16–7.13 (m, 1H), 7.07–7.04 (m, 2H), 6.97–6.94 (m, 1H), 6.81 (dd, J = 8.0, 1.0 Hz, 1H), 6.74–6.71 (m, 1H), 6.19 (d, J = 1.5 Hz, 1H), 5.84 (d, J = 1.5 Hz, 1H), 5.80 (d, J = 6.0 Hz, 1H), 5.64 (d, J = 6.0 Hz, 1H), 4.95 (d, J = 5.5 Hz, 1H), 4.84 (d, J = 5.5 Hz, 1H), 4.72 (d, J = 12.5 Hz, 1H), 4.47 (d, J = 13.0 Hz, 1H), 4.16 (q, J = 14.0, 7.0 Hz, 2H), 2.09–2.01 (m, 1H), 1.65 (s, 3H), 1.45 (t, J = 7.0 Hz, 3H), 1.03 (d, J = 7.0 Hz, 3H), 0.91 (d, J = 6.5 Hz, 3H); 13C{1H} NMR (125 MHz, CDCl3) δ 174.9, 147.9, 145.4, 143.4, 142.5, 138.9, 127.9, 126.9, 126.6, 125.2, 125.0, 124.8, 121.3, 121.1, 119.4, 113.1, 112.1, 101.9, 89.0, 88.5, 88.1, 87.7, 84.3, 84.1, 70.3, 64.6, 58.1, 30.6, 25.1, 21.0, 17.5, 14.8; IR (neat) 2961, 2923, 2852, 1710, 1597, 1446, 1395, 1258, 1095, 1014, 863, 793, 695 cm−1; HRMS (ESI-TOF): calcd for C38H37O4Ru [M + H]+: m/z 659.1735. Found: 659.1732.
Ru-complex (6bb). Following the general procedure, the reaction of 1b (100 mg, 0.45 mmol) with 4b (116.6 mg, 0.45 mmol), [RuCl2(p-cymene)]2 (139.0 mg, 0.23 mmol), Cu(OTf)2 (164.2 mg, 0.45 mmol) and K2CO3 (62.8 mg, 0.45 mmol) afforded 6bb as a yellow solid using ethyl acetate/hexane (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) as the eluent. Yield 116.3 mg (74%); mp 185–187 °C; Rf = 0.40 (4
4) as the eluent. Yield 116.3 mg (74%); mp 185–187 °C; Rf = 0.40 (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/ethyl acetate); 1H NMR (500 MHz, CDCl3) δ 7.46–7.42 (m, 4H), 7.22 (dd, J = 8.0, 1.5 Hz, 1H), 7.16 (d, J = 8.5 Hz, 2H), 7.08–7.05 (m, 2H), 7.0–6.96 (m, 1H), 6.81 (dd, J = 8.0, 1.0 Hz, 1H), 6.74–6.71 (m, 1H), 6.18 (d, J = 1.0 Hz, 1H), 5.86 (d, J = 1.0 Hz, 1H), 5.80 (d, J = 6.0 Hz, 1H), 5.64 (d, J = 6.0 Hz, 1H), 4.96 (d, J = 6.0 Hz, 1H), 4.84 (d, J = 5.5 Hz, 1H), 4.71 (d, J = 12.5 Hz, 1H), 4.46 (d, J = 12.5 Hz, 1H), 4.16 (q, J = 14.0, 7.0 Hz, 2H), 2.08–2.00 (m, 1H), 1.65 (s, 3H), 1.45 (t, J = 7.0 Hz, 3H), 1.03 (d, J = 7.0 Hz, 3H), 0.90 (d, J = 7.0 Hz, 3H); 13C{1H} NMR (125 MHz, CDCl3) δ 174.8, 148.0, 145.5,142.5, 142.3, 137.3, 133.8, 129.8, 128.6, 127.8, 127.0, 125.0, 124.9, 121.6, 121.1, 119.1, 113.2, 112.2, 101.9, 89.4, 89.1, 88.5, 88.1, 87.7, 84.3, 83.6, 70.2, 64.6, 58.2, 30.6, 25.0, 21.0, 17.5, 14.8; IR (neat) 2968, 1700, 1576, 1475, 1382, 1222, 1124, 1092, 1057, 970, 928, 833, 752, 734, 699 cm−1; HRMS (ESI-TOF): calcd for C38H36O4ClRu [M + H]+: m/z 693.1345. Found: 693.1349.
1 hexane/ethyl acetate); 1H NMR (500 MHz, CDCl3) δ 7.46–7.42 (m, 4H), 7.22 (dd, J = 8.0, 1.5 Hz, 1H), 7.16 (d, J = 8.5 Hz, 2H), 7.08–7.05 (m, 2H), 7.0–6.96 (m, 1H), 6.81 (dd, J = 8.0, 1.0 Hz, 1H), 6.74–6.71 (m, 1H), 6.18 (d, J = 1.0 Hz, 1H), 5.86 (d, J = 1.0 Hz, 1H), 5.80 (d, J = 6.0 Hz, 1H), 5.64 (d, J = 6.0 Hz, 1H), 4.96 (d, J = 6.0 Hz, 1H), 4.84 (d, J = 5.5 Hz, 1H), 4.71 (d, J = 12.5 Hz, 1H), 4.46 (d, J = 12.5 Hz, 1H), 4.16 (q, J = 14.0, 7.0 Hz, 2H), 2.08–2.00 (m, 1H), 1.65 (s, 3H), 1.45 (t, J = 7.0 Hz, 3H), 1.03 (d, J = 7.0 Hz, 3H), 0.90 (d, J = 7.0 Hz, 3H); 13C{1H} NMR (125 MHz, CDCl3) δ 174.8, 148.0, 145.5,142.5, 142.3, 137.3, 133.8, 129.8, 128.6, 127.8, 127.0, 125.0, 124.9, 121.6, 121.1, 119.1, 113.2, 112.2, 101.9, 89.4, 89.1, 88.5, 88.1, 87.7, 84.3, 83.6, 70.2, 64.6, 58.2, 30.6, 25.0, 21.0, 17.5, 14.8; IR (neat) 2968, 1700, 1576, 1475, 1382, 1222, 1124, 1092, 1057, 970, 928, 833, 752, 734, 699 cm−1; HRMS (ESI-TOF): calcd for C38H36O4ClRu [M + H]+: m/z 693.1345. Found: 693.1349.
Ru-complex (6bc). Following the general procedure, the reaction of 1b (100 mg, 0.45 mmol) with 4c (136.8 mg, 0.45 mmol), [RuCl2(p-cymene)]2 (139.0 mg, 0.23 mmol), Cu(OTf)2 (164.2 mg, 0.45 mmol) and K2CO3 (62.8 mg, 0.45 mmol) afforded 6bc as a yellow solid using ethyl acetate/hexane (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) as the eluent. Yield 128.8 mg (77%); mp 172–174 °C; Rf = 0.41 (4
4) as the eluent. Yield 128.8 mg (77%); mp 172–174 °C; Rf = 0.41 (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/ethyl acetate); 1H NMR (major isomer, >95%; 500 MHz, CDCl3) δ 7.45 (d, J = 8.0 Hz, 2H), 7.37–7.28 (m, 4H), 7.21 (d, J = 7.0 Hz, 1H), 7.08–7.05 (m, 2H), 6.99–6.96 (m, 1H), 6.81 (d, J = 8.0 Hz, 1H), 6.74–6.71 (m, 1H), 6.19 (s, 1H), 5.86 (s, 1H), 5.80 (d, J = 5.5 Hz, 1H), 5.64 (d, J = 5.5 Hz, 1H), 4.96 (d, J = 6.0 Hz, 1H), 4.83 (d, J = 5.5 Hz, 1H), 4.71 (d, J = 13.0 Hz, 1H), 4.46 (d, J = 13.0 Hz, 1H), 4.15 (q, J = 14.0, 7.0 Hz, 2H), 2.08–2.00 (m, 1H), 1.65 (s, 3H), 1.45 (t, J = 7.0 Hz, 3H), 1.03 (d, J = 7.0 Hz, 3H), 0.90 (d, J = 6.5 Hz, 3H); 13C{1H} NMR (125 MHz, CDCl3) δ 174.7, 148.0, 145.5, 142.6, 142.3, 137.7, 131.6, 128.1, 127.0, 125.1, 125.0, 124.9, 122.1, 121.7, 121.1, 119.1, 113.2, 112.3, 101.9, 89.4, 89.1, 88.6, 88.1, 87.7, 84.4, 83.5, 70.2, 64.6, 58.2, 30.6, 25.0, 21.0, 17.5, 14.8; IR (neat) 2962, 1699, 1576, 1476, 1386, 1252, 1204, 1125, 1090, 967, 928, 857, 831, 749, 733, 697 cm−1; HRMS (ESI-TOF): calcd for C38H36O4BrRu [M + H]+: m/z 737.0840. Found: 737.0839.
1 hexane/ethyl acetate); 1H NMR (major isomer, >95%; 500 MHz, CDCl3) δ 7.45 (d, J = 8.0 Hz, 2H), 7.37–7.28 (m, 4H), 7.21 (d, J = 7.0 Hz, 1H), 7.08–7.05 (m, 2H), 6.99–6.96 (m, 1H), 6.81 (d, J = 8.0 Hz, 1H), 6.74–6.71 (m, 1H), 6.19 (s, 1H), 5.86 (s, 1H), 5.80 (d, J = 5.5 Hz, 1H), 5.64 (d, J = 5.5 Hz, 1H), 4.96 (d, J = 6.0 Hz, 1H), 4.83 (d, J = 5.5 Hz, 1H), 4.71 (d, J = 13.0 Hz, 1H), 4.46 (d, J = 13.0 Hz, 1H), 4.15 (q, J = 14.0, 7.0 Hz, 2H), 2.08–2.00 (m, 1H), 1.65 (s, 3H), 1.45 (t, J = 7.0 Hz, 3H), 1.03 (d, J = 7.0 Hz, 3H), 0.90 (d, J = 6.5 Hz, 3H); 13C{1H} NMR (125 MHz, CDCl3) δ 174.7, 148.0, 145.5, 142.6, 142.3, 137.7, 131.6, 128.1, 127.0, 125.1, 125.0, 124.9, 122.1, 121.7, 121.1, 119.1, 113.2, 112.3, 101.9, 89.4, 89.1, 88.6, 88.1, 87.7, 84.4, 83.5, 70.2, 64.6, 58.2, 30.6, 25.0, 21.0, 17.5, 14.8; IR (neat) 2962, 1699, 1576, 1476, 1386, 1252, 1204, 1125, 1090, 967, 928, 857, 831, 749, 733, 697 cm−1; HRMS (ESI-TOF): calcd for C38H36O4BrRu [M + H]+: m/z 737.0840. Found: 737.0839.
Ru-complex (6da). Following the general procedure, the reaction of 1d (100 mg, 0.48 mmol) with 4a (107.8 mg, 0.48 mmol), [RuCl2(p-cymene)]2 (148.5 mg, 0.24 mmol), Cu(OTf)2 (175.4 mg, 0.48 mmol) and K2CO3 (67.0 mg, 0.48 mmol) afforded 6da as a yellow solid using ethyl acetate/hexane (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) as the eluent. Yield 118.6 mg (76%); mp 96–98 °C; Rf = 0.37 (4
4) as the eluent. Yield 118.6 mg (76%); mp 96–98 °C; Rf = 0.37 (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/ethyl acetate); 1H NMR (500 MHz, CDCl3) δ 7.63 (d, J = 9.0 Hz, 1H), 7.50–7.46 (m, 4H), 7.22–7.19 (m, 2H), 7.17–7.14 (m, 1H), 7.07–7.04 (m, 2H), 6.97–6.94 (m, 1H), 6.48 (d, J = 2.5 Hz, 1H), 6.38 (dd, J = 9.5, 2.5 Hz, 1H), 6.21 (d, J = 1.0 Hz, 1H), 5.87 (d, J = 1.0 Hz, 1H), 5.80 (d, J = 6.0 Hz, 1H), 5.62 (d, J = 5.5 Hz, 1H), 4.89 (d, J = 6.0 Hz, 1H), 4.75 (d, J = 5.5 Hz, 1H), 4.55 (q, J = 12.5 Hz, 2H), 3.77 (s, 3H), 2.09–2.02 (m, 1H), 1.67 (s, 3H), 1.05 (d, J = 7.0 Hz, 3H), 0.95 (d, J = 6.5 Hz, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ 175.1, 160.3, 157.1, 143.6, 142.7, 138.8, 128.4, 127.9, 127.8, 126.9, 126.6, 125.0, 124.8, 121.1, 116.1, 111.8, 107.7, 103.1, 101.7, 89.6, 88.9, 88.4, 88.1, 87.7, 84.0, 70.0, 56.8, 55.3, 30.8, 25.0, 21.2, 17.8; IR (neat) 2959, 1698, 1611, 1572, 1493, 1444, 1233, 1193, 1158, 1085, 1030, 974, 907, 850, 727, 696 cm−1; HRMS (ESI-TOF): calcd for C37H35O4Ru [M + H]+: m/z 645.1579. Found: 645.1580.
1 hexane/ethyl acetate); 1H NMR (500 MHz, CDCl3) δ 7.63 (d, J = 9.0 Hz, 1H), 7.50–7.46 (m, 4H), 7.22–7.19 (m, 2H), 7.17–7.14 (m, 1H), 7.07–7.04 (m, 2H), 6.97–6.94 (m, 1H), 6.48 (d, J = 2.5 Hz, 1H), 6.38 (dd, J = 9.5, 2.5 Hz, 1H), 6.21 (d, J = 1.0 Hz, 1H), 5.87 (d, J = 1.0 Hz, 1H), 5.80 (d, J = 6.0 Hz, 1H), 5.62 (d, J = 5.5 Hz, 1H), 4.89 (d, J = 6.0 Hz, 1H), 4.75 (d, J = 5.5 Hz, 1H), 4.55 (q, J = 12.5 Hz, 2H), 3.77 (s, 3H), 2.09–2.02 (m, 1H), 1.67 (s, 3H), 1.05 (d, J = 7.0 Hz, 3H), 0.95 (d, J = 6.5 Hz, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ 175.1, 160.3, 157.1, 143.6, 142.7, 138.8, 128.4, 127.9, 127.8, 126.9, 126.6, 125.0, 124.8, 121.1, 116.1, 111.8, 107.7, 103.1, 101.7, 89.6, 88.9, 88.4, 88.1, 87.7, 84.0, 70.0, 56.8, 55.3, 30.8, 25.0, 21.2, 17.8; IR (neat) 2959, 1698, 1611, 1572, 1493, 1444, 1233, 1193, 1158, 1085, 1030, 974, 907, 850, 727, 696 cm−1; HRMS (ESI-TOF): calcd for C37H35O4Ru [M + H]+: m/z 645.1579. Found: 645.1580.
1,2-Diphenyl-4H,5H-pyrano[3,4-c]chromene-4,5-dione (7aa). Following the general procedure, the reaction of 2a (100 mg, 0.53 mmol) with 3a (93.7 mg, 0.53 mmol), [Ru] (8.0 mg, 2.5 mol%), AgSbF6 (18.0 mg, 10 mol%) and Cu(OAc)2·H2O (31.5 mg, 30 mol%) afforded 7aa as a yellow solid using ethyl acetate/hexane (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) as the eluent. Yield 111.8 mg (58%); mp 244–246 °C; Rf = 0.48 (9
9) as the eluent. Yield 111.8 mg (58%); mp 244–246 °C; Rf = 0.48 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/ethyl acetate); 1H NMR (500 MHz, CDCl3) δ 7.54–7.50 (m, 1H), 7.49–7.45 (m, 1H), 7.43–7.40 (m, 2H), 7.37–7.32 (m, 2H), 7.24–7.22 (m, 6H), 6.84–6.80 (m, 1H), 6.78–6.76 (m, 1H); 13C{1H} NMR (100 MHz, CDCl3) δ 164.1, 156.5, 156.3, 155.0, 154.3, 134.9, 134.4, 132.0, 131.5, 130.5, 129.7, 129.5, 129.2, 129.0, 128.0, 123.5, 118.0, 115.8, 115.7, 104.3, 164.2, 156.8, 156.5, 154.9, 154.5, 141.1, 139.2, 134.3, 131.9, 131.2, 130.5, 129.5, 129.2, 129.1, 128.7, 123.4, 117.9, 115.9, 115.4, 103.8, 21.5, 21.4; IR (neat) 3059, 1770, 1603, 1563, 1485, 1443, 1381, 1047, 766, 727 cm−1; HRMS (ESI-TOF): calcd for C24H14O4Na [M + Na]+: m/z 389.0790. Found: 389.0799.
1 hexane/ethyl acetate); 1H NMR (500 MHz, CDCl3) δ 7.54–7.50 (m, 1H), 7.49–7.45 (m, 1H), 7.43–7.40 (m, 2H), 7.37–7.32 (m, 2H), 7.24–7.22 (m, 6H), 6.84–6.80 (m, 1H), 6.78–6.76 (m, 1H); 13C{1H} NMR (100 MHz, CDCl3) δ 164.1, 156.5, 156.3, 155.0, 154.3, 134.9, 134.4, 132.0, 131.5, 130.5, 129.7, 129.5, 129.2, 129.0, 128.0, 123.5, 118.0, 115.8, 115.7, 104.3, 164.2, 156.8, 156.5, 154.9, 154.5, 141.1, 139.2, 134.3, 131.9, 131.2, 130.5, 129.5, 129.2, 129.1, 128.7, 123.4, 117.9, 115.9, 115.4, 103.8, 21.5, 21.4; IR (neat) 3059, 1770, 1603, 1563, 1485, 1443, 1381, 1047, 766, 727 cm−1; HRMS (ESI-TOF): calcd for C24H14O4Na [M + Na]+: m/z 389.0790. Found: 389.0799.
1,2-Di-p-tolyl-4H,5H-pyrano[3,4-c]chromene-4,5-dione (7ab). Following the general procedure, the reaction of 2a (100 mg, 0.53 mmol) with 3b (108.5 mg, 0.53 mmol), [Ru] (8.0 mg, 2.5 mol%), AgSbF6 (18.0 mg, 10 mol%) and Cu(OAc)2·H2O (31.5 mg, 30 mol%) afforded 7ab as a yellow solid using ethyl acetate/hexane (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) as the eluent. Yield 126.5 mg (61%); mp 238–240 °C; Rf = 0.50 (9
9) as the eluent. Yield 126.5 mg (61%); mp 238–240 °C; Rf = 0.50 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/ethyl acetate); 1H NMR (400 MHz, CDCl3) δ 7.53–7.48 (m, 1H), 7.34–7.32 (m, 1H), 7.23–7.21 (m, 2H), 7.15–7.09 (m, 4H), 7.04–7.02 (m, 2H), 6.83–6.82 (m, 2H), 2.45 (s, 3H), 2.32 (s, 3H); 13C{1H} NMR (125 MHz, CDCl3) δ 164.2, 156.8, 156.5, 154.9, 154.5, 141.1, 139.2, 134.3, 131.9, 131.2, 130.5, 129.5, 129.2, 129.1, 128.7, 123.4, 117.9, 115.9, 115.4, 103.8, 21.5, 21.4; IR (neat) 3059, 3031, 2920, 1754, 1701, 1598, 1488, 1437, 1248, 1184, 1130, 826, 768, 753 cm−1; HRMS (ESI-TOF): calcd for C26H18O4Na [M + Na]+: m/z 417.1103. Found: 417.1103.
1 hexane/ethyl acetate); 1H NMR (400 MHz, CDCl3) δ 7.53–7.48 (m, 1H), 7.34–7.32 (m, 1H), 7.23–7.21 (m, 2H), 7.15–7.09 (m, 4H), 7.04–7.02 (m, 2H), 6.83–6.82 (m, 2H), 2.45 (s, 3H), 2.32 (s, 3H); 13C{1H} NMR (125 MHz, CDCl3) δ 164.2, 156.8, 156.5, 154.9, 154.5, 141.1, 139.2, 134.3, 131.9, 131.2, 130.5, 129.5, 129.2, 129.1, 128.7, 123.4, 117.9, 115.9, 115.4, 103.8, 21.5, 21.4; IR (neat) 3059, 3031, 2920, 1754, 1701, 1598, 1488, 1437, 1248, 1184, 1130, 826, 768, 753 cm−1; HRMS (ESI-TOF): calcd for C26H18O4Na [M + Na]+: m/z 417.1103. Found: 417.1103.
1,2-Bis(4-methoxyphenyl)-4H,5H-pyrano[3,4-c]chromene-4,5-dione (7ac). Following the general procedure, the reaction of 2a (100 mg, 0.53 mmol) with 3c (125.3 mg, 0.53 mmol), [Ru] (8.0 mg, 2.5 mol%), AgSbF6 (18.0 mg, 10 mol%) and Cu(OAc)2·H2O (31.5 mg, 30 mol%) afforded 7ac as a pale-yellow solid using ethyl acetate/hexane (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) as the eluent. Yield 143.5 mg (64%); mp 148–150 °C; Rf = 0.46 (9
9) as the eluent. Yield 143.5 mg (64%); mp 148–150 °C; Rf = 0.46 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/ethyl acetate); 1H NMR (500 MHz, CDCl3) δ 8.03 (d, J = 8.5 Hz, 2H), 7.69–7.65 (m, 1H), 7.60 (d, J = 8.0 Hz, 1H), 7.42 (d, J = 8.5 Hz, 1H), 7.30–7.25 (m, 3H), 6.93–6.90 (m, 4H), 3.88 (s, 3H), 3.81 (s, 3H); 13C{1H} NMR (125 MHz, CDCl3) δ 170.6, 164.8, 164.6, 160.9, 156.5, 154.5, 135.3, 134.0, 129.4, 127.9, 126.6, 126.0, 124.7, 117.5, 115.5, 114.9, 113.9, 109.9, 92.6, 55.6, 55.4; IR (neat) 2932, 2842, 2360, 1791, 1745, 1674, 1597, 1511, 1397, 1253, 1168, 1024, 983, 838, 733, 701, 614 cm−1; HRMS (ESI-TOF): calcd for C26H19O6 [M + H]+: m/z 427.1182. Found: 427.1181.
1 hexane/ethyl acetate); 1H NMR (500 MHz, CDCl3) δ 8.03 (d, J = 8.5 Hz, 2H), 7.69–7.65 (m, 1H), 7.60 (d, J = 8.0 Hz, 1H), 7.42 (d, J = 8.5 Hz, 1H), 7.30–7.25 (m, 3H), 6.93–6.90 (m, 4H), 3.88 (s, 3H), 3.81 (s, 3H); 13C{1H} NMR (125 MHz, CDCl3) δ 170.6, 164.8, 164.6, 160.9, 156.5, 154.5, 135.3, 134.0, 129.4, 127.9, 126.6, 126.0, 124.7, 117.5, 115.5, 114.9, 113.9, 109.9, 92.6, 55.6, 55.4; IR (neat) 2932, 2842, 2360, 1791, 1745, 1674, 1597, 1511, 1397, 1253, 1168, 1024, 983, 838, 733, 701, 614 cm−1; HRMS (ESI-TOF): calcd for C26H19O6 [M + H]+: m/z 427.1182. Found: 427.1181.
1-Methyl-2-phenyl-4H,5H-pyrano[3,4-c]chromene-4,5-dione (7ae). Following the general procedure, the reaction of 2a (100 mg, 0.53 mmol) with 3e (61.1 mg, 0.53 mmol), [Ru] (8.0 mg, 2.5 mol%), AgSbF6 (18.0 mg, 10 mol%) and Cu(OAc)2·H2O (31.5 mg, 30 mol%) afforded 7ae as a pale-yellow solid using ethyl acetate/hexane (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) as the eluent. Yield 96.0 mg (60%); mp 137–139 °C; Rf = 0.48 (9
9) as the eluent. Yield 96.0 mg (60%); mp 137–139 °C; Rf = 0.48 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/ethyl acetate); 1H NMR (500 MHz, CDCl3) δ 8.41 (dd, J1 = 7.5 Hz, J2 = 1.0 Hz, 1H), 7.85–7.82 (m, 1H), 7.67 (d, J = 8.0 Hz, 1H), 7.63–7.61 (m, 2H), 7.60–7.56 (m, 1H), 7.51–7.49 (m, 1H), 7.48–7.44 (m, 2H), 2.35 (s, 3H); 13C{1H} NMR (125 MHz, CDCl3) δ 162.6, 151.2, 138.8, 134.8, 133.3, 129.8, 129.5, 129.4, 128.5, 128.3, 128.0, 123.4, 120.8, 109.1, 100.0, 13.6; IR (neat) 3068, 2968, 1730, 1672, 1594, 1460, 1445, 1376, 1321, 1286, 1229, 1205, 1028, 976, 956, 928, 902, 791, 749 cm−1; HRMS (ESI-TOF): calcd for C19H13O4 [M + H]+: m/z 305.0814. Found: 305.0814.
1 hexane/ethyl acetate); 1H NMR (500 MHz, CDCl3) δ 8.41 (dd, J1 = 7.5 Hz, J2 = 1.0 Hz, 1H), 7.85–7.82 (m, 1H), 7.67 (d, J = 8.0 Hz, 1H), 7.63–7.61 (m, 2H), 7.60–7.56 (m, 1H), 7.51–7.49 (m, 1H), 7.48–7.44 (m, 2H), 2.35 (s, 3H); 13C{1H} NMR (125 MHz, CDCl3) δ 162.6, 151.2, 138.8, 134.8, 133.3, 129.8, 129.5, 129.4, 128.5, 128.3, 128.0, 123.4, 120.8, 109.1, 100.0, 13.6; IR (neat) 3068, 2968, 1730, 1672, 1594, 1460, 1445, 1376, 1321, 1286, 1229, 1205, 1028, 976, 956, 928, 902, 791, 749 cm−1; HRMS (ESI-TOF): calcd for C19H13O4 [M + H]+: m/z 305.0814. Found: 305.0814.
2-Phenyl-1-propyl-4H,5H-pyrano[3,4-c]chromene-4,5-dione (7ag). Following the general procedure, the reaction of 2a (100 mg, 0.53 mmol) with 3g (75.8 mg, 0.53 mmol), [Ru] (8.0 mg, 2.5 mol%), AgSbF6 (18.0 mg, 10 mol%) and Cu(OAc)2·H2O (31.5 mg, 30 mol%) afforded 7ag as a pale-yellow solid using ethyl acetate/hexane (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) as the eluent. Yield 118.9 mg (68%); mp 180–182 °C; Rf = 0.51 (9
9) as the eluent. Yield 118.9 mg (68%); mp 180–182 °C; Rf = 0.51 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/ethyl acetate); 1H NMR (500 MHz, CDCl3) δ 8.25 (dd, J1 = 8.5 Hz, J2 = 1.0 Hz, 1H), 7.73–7.69 (m, 1H), 7.64–7.63 (m, 2H), 7.59–7.52 (m, 3H), 7.45 (dd, J = 8.3 Hz, J2 = 1.5 Hz, 1H), 7.41–7.38 (m, 1H), 3.04 (t, J = 7.8 Hz, 2H), 1.47–1.40 (m, 2H), 0.78 (t, J = 7.3 Hz, 3H); 13C{1H} NMR (125 MHz, CDCl3) δ 164.7, 156.7, 156.2, 155.7, 154.6, 134.7, 132.5, 131.1, 129.5, 128.7, 127.7, 124.4, 118.5, 116.5, 113.5, 105.9, 32.4, 22.6, 13.6; IR (neat) 2962, 1763, 1604, 1567, 1480, 1380, 1257, 1071, 998, 860, 764, 698 cm−1; HRMS (ESI-TOF): calcd for C21H17O4 [M + H]+: m/z 333.1127. Found: 333.1124.
1 hexane/ethyl acetate); 1H NMR (500 MHz, CDCl3) δ 8.25 (dd, J1 = 8.5 Hz, J2 = 1.0 Hz, 1H), 7.73–7.69 (m, 1H), 7.64–7.63 (m, 2H), 7.59–7.52 (m, 3H), 7.45 (dd, J = 8.3 Hz, J2 = 1.5 Hz, 1H), 7.41–7.38 (m, 1H), 3.04 (t, J = 7.8 Hz, 2H), 1.47–1.40 (m, 2H), 0.78 (t, J = 7.3 Hz, 3H); 13C{1H} NMR (125 MHz, CDCl3) δ 164.7, 156.7, 156.2, 155.7, 154.6, 134.7, 132.5, 131.1, 129.5, 128.7, 127.7, 124.4, 118.5, 116.5, 113.5, 105.9, 32.4, 22.6, 13.6; IR (neat) 2962, 1763, 1604, 1567, 1480, 1380, 1257, 1071, 998, 860, 764, 698 cm−1; HRMS (ESI-TOF): calcd for C21H17O4 [M + H]+: m/z 333.1127. Found: 333.1124.
1,2-Dipropyl-4H,5H-pyrano[3,4-c]chromene-4,5-dione (7ai). Following the general procedure, the reaction of 2a (100 mg, 0.53 mmol) with 3i (58.0 mg, 0.53 mmol), [Ru] (8.0 mg, 2.5 mol%), AgSbF6 (18.0 mg, 10 mol%) and Cu(OAc)2·H2O (31.5 mg, 30 mol%) afforded 7ai as a gummy liquid using ethyl acetate/hexane (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) as the eluent. Yield 105.1 mg (67%); Rf = 0.54 (9
9) as the eluent. Yield 105.1 mg (67%); Rf = 0.54 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/ethyl acetate); 1H NMR (400 MHz, CDCl3) δ 8.05 (dd, J1 = 8.4 Hz, J2 = 1.2 Hz, 1H), 7.70–7.66 (m, 1H), 7.42–7.35 (m, 2H), 2.83–2.79 (m, 2H), 2.72–2.69 (m, 2H), 1.88–1.79 (m, 2H), 1.77–1.66 (m, 2H), 1.12 (t, J = 7.4 Hz, 3H), 1.07 (t, J = 7.4 Hz, 3H); 13C{1H} NMR (125 MHz, CDCl3) δ 160.8, 154.1, 143.4, 131.9, 131.8, 130.0, 127.9, 126.2, 124.4, 118.9, 116.9, 116.6, 31.9, 29.7, 29.4, 22.7, 14.4, 14.1; IR (neat) 2923, 2853, 1723, 1606, 1452, 1176, 1119, 998, 829, 753cm−1; HRMS (ESI-TOF): calcd for C18H18O4Na [M + Na]+: m/z 321.1103. Found: 321.1103.
1 hexane/ethyl acetate); 1H NMR (400 MHz, CDCl3) δ 8.05 (dd, J1 = 8.4 Hz, J2 = 1.2 Hz, 1H), 7.70–7.66 (m, 1H), 7.42–7.35 (m, 2H), 2.83–2.79 (m, 2H), 2.72–2.69 (m, 2H), 1.88–1.79 (m, 2H), 1.77–1.66 (m, 2H), 1.12 (t, J = 7.4 Hz, 3H), 1.07 (t, J = 7.4 Hz, 3H); 13C{1H} NMR (125 MHz, CDCl3) δ 160.8, 154.1, 143.4, 131.9, 131.8, 130.0, 127.9, 126.2, 124.4, 118.9, 116.9, 116.6, 31.9, 29.7, 29.4, 22.7, 14.4, 14.1; IR (neat) 2923, 2853, 1723, 1606, 1452, 1176, 1119, 998, 829, 753cm−1; HRMS (ESI-TOF): calcd for C18H18O4Na [M + Na]+: m/z 321.1103. Found: 321.1103.
1,2-Dibutyl-4H,5H-pyrano[3,4-c]chromene-4,5-dione (7aj). Following the general procedure, the reaction of 2a (100 mg, 0.53 mmol) with 3j (72.7 mg, 0.53 mmol), [Ru] (8.0 mg, 2.5 mol%), AgSbF6 (18.0 mg, 10 mol%) and Cu(OAc)2·H2O (31.5 mg, 30 mol%) afforded 7aj as a gummy liquid using ethyl acetate/hexane (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) as the eluent. Yield 111.6 mg (65%); Rf = 0.58 (9
9) as the eluent. Yield 111.6 mg (65%); Rf = 0.58 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/ethyl acetate); 1H NMR (400 MHz, CDCl3) δ 8.08 (dd, J1 = 8.4 Hz, J2 = 1.2 Hz, 1H), 7.70–7.66 (m, 1H), 7.41 (dd, J = 8.4 Hz, J2 = 1.2 Hz, 1H), 7.38–7.34 (m, 1H), 2.86–2.82 (m, 2H), 2.74–2.70 (m, 2H), 1.81–1.75 (m, 2H), 1.73–1.68 (m, 2H), 1.58–1.51 (m, 2H), 1.50–1.43 (m, 2H), 1.05 (t, J = 7.4 Hz, 3H), 1.00 (t, J = 7.4 Hz, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ 169.5, 157.0, 156.3, 155.3, 154.6, 134.4, 127.7, 124.1, 118.5, 116.1, 112.7, 104.6, 32.2, 32.0, 29.7, 28.5, 22.63, 22.57, 13.7, 13.6; IR (neat) 2924, 2853, 1729, 1606, 1563, 1453, 1178, 1102, 929, 828, 752 cm−1; HRMS (ESI-TOF): calcd for C20H22O4Na [M + Na]+: m/z 349.1416. Found: 349.1412.
1 hexane/ethyl acetate); 1H NMR (400 MHz, CDCl3) δ 8.08 (dd, J1 = 8.4 Hz, J2 = 1.2 Hz, 1H), 7.70–7.66 (m, 1H), 7.41 (dd, J = 8.4 Hz, J2 = 1.2 Hz, 1H), 7.38–7.34 (m, 1H), 2.86–2.82 (m, 2H), 2.74–2.70 (m, 2H), 1.81–1.75 (m, 2H), 1.73–1.68 (m, 2H), 1.58–1.51 (m, 2H), 1.50–1.43 (m, 2H), 1.05 (t, J = 7.4 Hz, 3H), 1.00 (t, J = 7.4 Hz, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ 169.5, 157.0, 156.3, 155.3, 154.6, 134.4, 127.7, 124.1, 118.5, 116.1, 112.7, 104.6, 32.2, 32.0, 29.7, 28.5, 22.63, 22.57, 13.7, 13.6; IR (neat) 2924, 2853, 1729, 1606, 1563, 1453, 1178, 1102, 929, 828, 752 cm−1; HRMS (ESI-TOF): calcd for C20H22O4Na [M + Na]+: m/z 349.1416. Found: 349.1412.
Ru-complex (7′ba). Following the general procedure, the reaction of 2b (100 mg, 0.45 mmol) with 3a (81.0 mg, 0.45 mmol), [RuCl2(p-cymene)]2 (6.95 mg, 0.01 mmol), Cu(OAc)2·H2O (27.2 mg, 0.13 mmol) and AgSbF6 (15.6 mg, 0.045 mmol) afforded 7′ba as a yellow solid using ethyl acetate/hexane (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) as the eluent. Yield 5.8 mg (81%); mp 246–248 °C; Rf = 0.32 (4
4) as the eluent. Yield 5.8 mg (81%); mp 246–248 °C; Rf = 0.32 (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/ethyl acetate); 1H NMR (500 MHz, CDCl3) δ 7.61 (d, J = 7.0 Hz, 1H), 7.45–7.39 (m, 2H), 7.33–7.29 (m, 2H), 7.07–7.01 (m, 5H), 6.86 (dd, J = 8.0, 1.0 Hz, 1H), 6.76–6.73 (m, 1H), 6.38 (dd, J = 8.5, 1.5 Hz, 1H), 5.80 (d, J = 5.5 Hz, 1H), 5.66 (d, J = 5.5 Hz, 1H), 4.63 (d, J = 5.5 Hz, 1H), 4.59 (d, J = 5.0 Hz, 1H), 3.89 (s, 3H), 2.00–1.92 (m, 1H), 1.63 (s, 3H), 0.99 (d, J = 6.5 Hz, 3H), 0.95 (d, J = 7.0 Hz, 3H); 13C{1H} NMR (125 MHz, CDCl3) δ 169.4, 165.1, 147.7, 141.1, 140.4, 134.4, 133.3, 131.8, 129.3, 128.6, 127.2, 125.7, 123.1, 121.2, 118.3, 113.9, 112.2, 103.6, 91.0, 90.1, 89.9, 87.8, 87.2, 85.4, 84.4, 56.4, 46.7, 30.5, 24.7, 21.0, 17.3; IR (neat) 2973, 2928, 1756, 1712, 1599, 1479, 1444, 1377, 1274, 1202, 1178, 1131, 908, 781, 730 cm−1; HRMS (ESI-TOF): calcd for C35H31RuO5 [M + H]+: m/z 633.1215. Found: 633.1206.
1 hexane/ethyl acetate); 1H NMR (500 MHz, CDCl3) δ 7.61 (d, J = 7.0 Hz, 1H), 7.45–7.39 (m, 2H), 7.33–7.29 (m, 2H), 7.07–7.01 (m, 5H), 6.86 (dd, J = 8.0, 1.0 Hz, 1H), 6.76–6.73 (m, 1H), 6.38 (dd, J = 8.5, 1.5 Hz, 1H), 5.80 (d, J = 5.5 Hz, 1H), 5.66 (d, J = 5.5 Hz, 1H), 4.63 (d, J = 5.5 Hz, 1H), 4.59 (d, J = 5.0 Hz, 1H), 3.89 (s, 3H), 2.00–1.92 (m, 1H), 1.63 (s, 3H), 0.99 (d, J = 6.5 Hz, 3H), 0.95 (d, J = 7.0 Hz, 3H); 13C{1H} NMR (125 MHz, CDCl3) δ 169.4, 165.1, 147.7, 141.1, 140.4, 134.4, 133.3, 131.8, 129.3, 128.6, 127.2, 125.7, 123.1, 121.2, 118.3, 113.9, 112.2, 103.6, 91.0, 90.1, 89.9, 87.8, 87.2, 85.4, 84.4, 56.4, 46.7, 30.5, 24.7, 21.0, 17.3; IR (neat) 2973, 2928, 1756, 1712, 1599, 1479, 1444, 1377, 1274, 1202, 1178, 1131, 908, 781, 730 cm−1; HRMS (ESI-TOF): calcd for C35H31RuO5 [M + H]+: m/z 633.1215. Found: 633.1206.
Ru-complex (7′ca). Following the general procedure, the reaction of 2c (100 mg, 0.38 mmol) with 3a (68.8 mg, 0.38 mmol), [RuCl2(p-cymene)]2 (5.91 mg, 0.0096 mmol), Cu(OAc)2·H2O (23.1 mg, 0.11 mmol) and AgSbF6 (13.3 mg, 0.03 mmol) afforded 7′ca as a yellow solid using ethyl acetate/hexane (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) as the eluent. Yield 5.6 mg (87%); mp >250 °C; Rf = 0.43 (4
4) as the eluent. Yield 5.6 mg (87%); mp >250 °C; Rf = 0.43 (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/ethyl acetate); 1H NMR (500 MHz, CDCl3) δ 7.57–7.56 (m, 1H), 7.53–7.48 (m, 2H), 7.40–7.36 (m, 3H), 7.11–7.05 (m, 5H), 6.59 (d, J = 2.5 Hz, 1H), 5.86 (d, J = 6.0 Hz, 1H), 5.77 (d, J = 5.5 Hz, 1H), 4.68 (d, J = 6.0 Hz, 2H), 1.98–1.89 (m, 1H), 1.66 (s, 3H), 1.03 (d, J = 7.0 Hz, 3H), 0.99 (d, J = 7.0 Hz, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ 168.8, 164.0, 146.0, 139.6, 133.4, 132.7, 131.7, 129.8, 129.3, 129.1, 128.6, 127.3, 125.8, 124.8, 123.7, 122.8, 114.7, 104.4, 91.2, 90.7, 90.4, 88.2, 85.5, 84.8, 84.4, 45.9, 30.4, 24.6, 21.0, 17.3; IR (neat) 3062, 2963, 2925, 1765, 1717, 1445, 1368, 1240, 1169, 1120, 1007, 966, 764, 734 cm−1; HRMS (ESI-TOF): calcd for C34H27Cl2RuO4 [M + H]+: m/z 671.0330. Found: 671.0327.
1 hexane/ethyl acetate); 1H NMR (500 MHz, CDCl3) δ 7.57–7.56 (m, 1H), 7.53–7.48 (m, 2H), 7.40–7.36 (m, 3H), 7.11–7.05 (m, 5H), 6.59 (d, J = 2.5 Hz, 1H), 5.86 (d, J = 6.0 Hz, 1H), 5.77 (d, J = 5.5 Hz, 1H), 4.68 (d, J = 6.0 Hz, 2H), 1.98–1.89 (m, 1H), 1.66 (s, 3H), 1.03 (d, J = 7.0 Hz, 3H), 0.99 (d, J = 7.0 Hz, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ 168.8, 164.0, 146.0, 139.6, 133.4, 132.7, 131.7, 129.8, 129.3, 129.1, 128.6, 127.3, 125.8, 124.8, 123.7, 122.8, 114.7, 104.4, 91.2, 90.7, 90.4, 88.2, 85.5, 84.8, 84.4, 45.9, 30.4, 24.6, 21.0, 17.3; IR (neat) 3062, 2963, 2925, 1765, 1717, 1445, 1368, 1240, 1169, 1120, 1007, 966, 764, 734 cm−1; HRMS (ESI-TOF): calcd for C34H27Cl2RuO4 [M + H]+: m/z 671.0330. Found: 671.0327.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the Department of Science & Technology (DST, New Delhi; IRHPA and FIST grants) for the single crystal X-ray diffractometer and HRMS facility. We also thank UGC (New Delhi) for the NRC program. Financial support from SERB (KCKS; JBR/2020/000038), UGC-BSR (MS; F.25-1/2014-15(BSR)/5-129/2007(BSR)) is gratefully acknowledged.References
- For selected reviews on C–H activation, see: (a) A. E. Shilov and G. B. Shul'pin, Chem. Rev., 1997, 97, 2879 Search PubMed; (b) V. Ritleng, C. Sirlin and M. Pfeffer, Chem. Rev., 2002, 102, 1731 CrossRef CAS PubMed; (c) L. Ackermann, Chem. Rev., 2011, 111, 1315 CrossRef CAS PubMed; (d) P. B. Arockiam, C. Bruneau and P. H. Dixneuf, Chem. Rev., 2012, 112, 5879 CrossRef CAS PubMed; (e) F. Chen, T. Wang and N. Jiao, Chem. Rev., 2014, 114, 8613 CrossRef CAS PubMed; (f) R. Chinchilla and C. Nájera, Chem. Rev., 2014, 114, 1783 CrossRef CAS PubMed; (g) L. Ackermann, Acc. Chem. Res., 2014, 47, 281 CrossRef CAS PubMed; (h) Z. Dong, Z. Ren, S. J. Thompson, Y. Xu and G. Dong, Chem. Rev., 2017, 117, 9333 CrossRef CAS PubMed; (i) R. H. Crabtree and A. Lei, Chem. Rev., 2017, 117, 8481 CrossRef CAS PubMed.
- Selected reviews: (a) K. Nozava, M. Yamada, Y. Tsuda, K.-I. Kawai and S. Nakajima, Chem. Pharm. Bull., 1981, 29, 2689 CrossRef PubMed; (b) L. Pochet, R. Frederick and B. Masereel, Curr. Pharm. Des., 2004, 10, 3781 Search PubMed; (c) M. A. Musa, J. S. Cooperwood and M. O. F. Khan, Curr. Med. Chem., 2008, 15, 2664 CrossRef CAS PubMed; (d) D. Balcells, E. Clot and O. Eisenstein, Chem. Rev., 2010, 110, 749 Search PubMed; (e) R. Manikandan and M. Jeganmohan, Org. Biomol. Chem., 2015, 13, 10420 Search PubMed; (f) B. Su, Z.-C. Cao and Z.-J. Shi, Acc. Chem. Res., 2015, 48, 886 CrossRef CAS PubMed; (g) J. S. Lee, Mar. Drugs, 2015, 13, 1581 Search PubMed; (h) J.-P. Wan, L. Gan and Y. Liu, Org. Biomol. Chem., 2017, 15, 9031 RSC; (i) S.-L. Zheng and L. Chen, Org. Biomol. Chem., 2021, 19, 10530 Search PubMed; (j) D. Xia, H. Liu, X. Cheng, M. Maraswami, Y. Chen and X. Lv, Curr. Top. Med. Chem., 2022, 22, 269 CrossRef CAS PubMed.
- (a) Y. Kashman, K. R. Gustafson, R. W. Fuller, J. H. Cardellina-II, J. B. McMahon, M. J. Currens, R. W. Buckheit-Jr, S. H. Hughes, G. M. Cragg and M. R. Boyd, J. Med. Chem., 1992, 35, 2735 CrossRef CAS PubMed; (b) A. D. Patil, A. J. Freyer, D. S. Eggleston, R. C. Haltiwanger, M. F. Bean, P. B. Taylor, M. J. Caranfa, A. L. Breen, H. R. Bartus, R. K. Johnson, R. P. Hertzberg and J. W. Westley, J. Med. Chem., 1993, 36, 4131 CrossRef CAS PubMed; (c) L. Bonsigore, G. Loy, D. Secci and A. Calignano, Eur. J. Med. Chem., 1993, 28, 517 CrossRef; (d) E. Budzisz, E. Brzezinska, U. Krajewska and M. Rozalski, Eur. J. Med. Chem., 2003, 38, 597 CrossRef CAS PubMed; (e) D. C. Mungra, M. P. Patel, D. P. Rajani and R. G. Patel, Eur. J. Med. Chem., 2011, 46, 4192 CrossRef CAS PubMed; (f) N. R. Emmadi, K. Atmakur, G. K. Chityal, S. Pombala and J. B. Nanubolu, Bioorg. Med. Chem. Lett., 2012, 22, 7261 CrossRef CAS PubMed; (g) G. Gudipudi, S. R. Sagurthi, S. Perugu, G. Achaiah and G. L. D. Krupadanam, RSC Adv., 2014, 4, 56489 RSC.
- (a) R. K. Chinnagolla and M. Jeganmohan, Chem. Commun., 2012, 48, 2030 RSC; (b) L. Ackermann, J. Pospech, K. Graczyk and R. Karsten, Org. Lett., 2012, 14, 930 Search PubMed; (c) R. Prakash, K. Shekarrao and S. Gogoi, Org. Lett., 2015, 17, 5264 CrossRef CAS PubMed; (d) S. Warratz, C. Kornhaaβ, A. Cajaraville, B. Niepötter, D. Stalke and L. Ackermann, Angew. Chem., Int. Ed., 2015, 54, 5513 Search PubMed; (e) S. L. Yedage and B. M. Bhanage, Green Chem., 2016, 18, 5635 RSC; (f) K. S. Singh, S. G. Sawant and P. H. Dixneuf, ChemCatChem, 2016, 8, 1046 CrossRef CAS; (g) Y. Qiu, C. Tian, L. Massignan, T. Rogge and L. Ackermann, Angew. Chem., Int. Ed., 2018, 57, 5818 CrossRef CAS PubMed; (h) A. Mandal, B. Garai, S. Dana, R. Bera and M. Baidya, Chem. – Asian J., 2020, 15, 4009 CrossRef CAS PubMed; (i) J. Wu, B. Qian, Y. Liu and Y. Shang, ChemistrySelect, 2020, 5, 10269 Search PubMed; (j) I. Choi, A. M. Messinis, X. Hou and L. Ackermann, Angew. Chem., Int. Ed., 2021, 60, 27005 Search PubMed.
- (a) K. Ueura, T. Satoh and M. Miura, Org. Lett., 2007, 9, 1407 CrossRef CAS PubMed; (b) S. Mochida, K. Hirano, T. Satoh and M. Miura, J. Org. Chem., 2009, 74, 6295 CrossRef CAS PubMed; (c) M. Shimizu, K. Hirano, T. Satoh and M. Miura, J. Org. Chem., 2009, 74, 3478 CrossRef CAS PubMed; (d) Q. Li, Y. Yan, X. Wang, B. Gong, X. Tang, J. J. Shi, H. E. Xu and W. Yi, RSC Adv., 2013, 3, 23402 Search PubMed; (e) M. Itoh, M. Shimizu, K. Hirano, T. Satoh and M. Miura, J. Org. Chem., 2013, 78, 11427 CrossRef CAS PubMed; (f) Y. Liu, Y. Yang, Y. Shi, X. Wang, L. Zhang, Y. Cheng and J. You, Organometallics, 2016, 35, 1350 CrossRef CAS; (g) E. Kudo, Y. Shibata, M. Yamazaki, K. Masutomi, Y. Miyauchi, M. Fukui, H. Sugiyama, H. Uekusa, T. Satoh, M. Miura and K. Tanaka, Chem. – Eur. J., 2016, 22, 14190 CrossRef CAS PubMed; (h) Y.-T. Li, Y. Zhu, G.-L. Tu, J.-Y. Zhang and Y.-S. Zhao, Chem. – Asian J., 2018, 13, 3281 CrossRef CAS PubMed; (i) A. P. Molotkov, M. A. Arsenov, D. A. Kapustin, D. V. Muratov, N. E. Shepel’, Y. V. Fedorov, A. F. Smol'yakov, E. I. Knyazeva, D. A. Lypenko, A. V. Dmitriev, A. E. Aleksandrov, E. I. Maltsev and D. A. Loginov, ChemPlusChem, 2020, 85, 334 CrossRef CAS PubMed; (j) C. Maeng, J.-Y. Son, S. C. Lee, Y. Baek, K. Um, S. H. Han, G. H. Ko, G. U. Han, K. Lee and P. H. Lee, J. Org. Chem., 2020, 85, 3824 CrossRef CAS PubMed.
- (a) K. Yonehara, Y. Miyoshi, A. Tsukajima, T. Akatsuka and M. Saito, Adv. Synth. Catal., 2011, 353, 1071 CrossRef CAS; (b) D. Nandi, D. Ghosh, S.-J. Chen, B.-C. Kuo, N. M. Wang and H. M. Lee, J. Org. Chem., 2013, 78, 3445 Search PubMed; (c) Y. Yu, L. Huang, W. Wu and H. Jiang, Org. Lett., 2014, 16, 2146 CrossRef CAS PubMed.
- (a) R. Mandal and B. Sundararaju, Org. Lett., 2017, 19, 2544 Search PubMed; (b) T. T. Nguyen, L. Grigorjeva and O. Daugulis, Angew. Chem., Int. Ed., 2018, 57, 1688 CrossRef CAS PubMed.
- (a) K. Ueura, T. Stoh and M. Miura, J. Org. Chem., 2007, 72, 5362 CrossRef CAS PubMed; (b) D. A. Frasco, C. P. Lilly, P. D. Boyle and E. A. Ison, ACS Catal., 2013, 3, 2421 Search PubMed; (c) X.-G. Liu, H. Gao, H.-H. Zhang, Q. Li and H. Wang, ACS Catal., 2017, 7, 5078 CrossRef CAS.
- M. A. Zeller, M. Riener and D. A. Nicewicz, Org. Lett., 2014, 16, 4810 Search PubMed.
- M. Peuchmaur, V. Lisowski, C. Gandreuil, L. T. Maillard, J. Martinez and J.-F. Hernandez, J. Org. Chem., 2009, 74, 4158 CrossRef CAS PubMed.
- J. Seo, Y. Park, I. Jeon, T. Ryu, S. Park and P. H. Lee, Org. Lett., 2013, 15, 3358 CrossRef CAS PubMed.
- R. Rama Suresh and K. C. Kumara Swamy, J. Org. Chem., 2012, 77, 6959 CrossRef PubMed.
- R. N. P. Tulichala, M. Shankar and K. C. Kumara Swamy, J. Org. Chem., 2017, 82, 5068 Search PubMed.
- S. Allu and K. C. Kumara Swamy, Adv. Synth. Catal., 2015, 357, 2665 CrossRef CAS.
- (a) S. Allu and K. C. Kumara Swamy, RSC Adv., 2015, 5, 92045 RSC; (b) R. N. P. Tulichala and K. C. Kumara Swamy, Chem. Commun., 2015, 51, 12008 Search PubMed; (c) S. Allu, M. Ravi and K. C. Kumara Swamy, Eur. J. Org. Chem., 2016, 5697 Search PubMed; (d) R. N. P. Tulichala and K. C. Kumara Swamy, Org. Biomol. Chem., 2016, 14, 4519 RSC; (e) M. Shankar, U. Anasuyamma and K. C. Kumara Swamy, Adv. Synth. Catal., 2022, 364, 643 CrossRef CAS.
- (a) E. J. Corey and L. I. Wu, J. Am. Chem. Soc., 1993, 115, 9327 CrossRef CAS; (b) A. A. Ravichandran, Synth. Commun., 2001, 31, 1233 CrossRef; (c) A. Elangovan, Y.-H. Wang and T.-I. Ho, Org. Lett., 2003, 5, 1841 CrossRef CAS PubMed.
- (a) Y. Liang, Y.-X. Xie and J.-H. Li, J. Org. Chem., 2006, 71, 379 Search PubMed; (b) J. Moon, M. Jeong, H. Nam, J. Ju, J. H. Moon, H. M. Jung and S. Lee, Org. Lett., 2008, 10, 945 CrossRef CAS PubMed.
- D. D. Perrin, W. L. F. Armarego and D. R. Perrin, Purification of laboratory Chemicals, Pergamon, Oxford, UK, 1986 Search PubMed.
- (a) G. M. Sheldrick, SADABS, Siemens Area Detector Absorption Correction, University of Gottingen, Germany, 1996 Search PubMed; (b) G. M. Sheldrick, SHELX-97-A program for crystal structure solution and refinement, University of Gottingen, 1997 Search PubMed; (c) G. M. Sheldrick, SHELXTL NT Crystal Structure Analysis Package, version 5.10, Bruker AXS, Analytical X-ray System, WI, USA, 1999 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental data, crystal data and copies of 1H/13C{1H} NMR spectra. CCDC 2213160–2213164. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2ob01890j |
| This journal is © The Royal Society of Chemistry 2023 |