Open Access Article
Open Access ArticleExtraction techniques for bioactive compounds of cannabis
Aitor
Sainz Martinez†
 a,
Olga
Lanaridi†
a,
Olga
Lanaridi†
 a,
Kristof
Stagel
a,
Heidi
Halbwirth
a,
Kristof
Stagel
a,
Heidi
Halbwirth
 b,
Michael
Schnürch
b,
Michael
Schnürch
 a and
Katharina
Bica-Schröder
a and
Katharina
Bica-Schröder
 *a
*a
aInstitute of Applied Synthetic Chemistry, TU Wien, Getreidemarkt 9/163, Vienna, Austria. E-mail: katharina.schroeder@tuwien.ac.at
bInstitute of Chemical, Environmental and Bioscience Engineering, TU Wien, Getreidemarkt 9/166, Vienna, Austria
First published on 10th January 2023
Abstract
Historically, cannabis has always constituted a component of the civilized world; archaeological discoveries indicate that it is one of the oldest crops, while, up until the 19th century, cannabis fibers were extensively used in a variety of applications, and its seeds comprised a part of human and livestock nutrition. Additional evidence supports its exploitation for medicinal purposes in the ancient world. The cultivation of cannabis gradually declined as hemp fibers gave way to synthetic fibers, while the intoxicating ability of THC eventually overshadowed the extensive potential of cannabis. Nevertheless, the proven value of certain non-intoxicating cannabinoids, such as CBD and CBN, has recently given rise to an entire market which promotes cannabis-based products. An increase in the research for recovery and exploitation of beneficial cannabinoids has also been observed, with more than 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 peer-reviewed research articles published annually. In the present review, a brief overview of the history of cannabis is given. A look into the classification approaches of cannabis plants/species as well as the associated nomenclature is provided, followed by a description of their chemical characteristics and their medically valuable components. The application areas could not be absent from the present review. Still, the main focus of the review is the discussion of work conducted in the field of extraction of valuable bioactive compounds from cannabis. We conclude with a summary of the current status and outlook on the topics that future research should address.
000 peer-reviewed research articles published annually. In the present review, a brief overview of the history of cannabis is given. A look into the classification approaches of cannabis plants/species as well as the associated nomenclature is provided, followed by a description of their chemical characteristics and their medically valuable components. The application areas could not be absent from the present review. Still, the main focus of the review is the discussion of work conducted in the field of extraction of valuable bioactive compounds from cannabis. We conclude with a summary of the current status and outlook on the topics that future research should address.
1. Introduction
1.1. History of cannabis
Cannabis sativa is an annual herbaceous flowering plant native to eastern Asia; nevertheless, its sophisticated uses and wide range of applications lead to its global distribution.1 Humans across different eras have cultivated it for a variety of applications, such as nutrition, recreation, generation of seed oil and fiber for industrial purposes, religious and spiritual practices and medicine.
Cannabis sativa existed prior to the development of agriculture, which began about 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 years ago, therefore, it is assumed to have been one of the most critical crops for the development of civilization.2 Hemp strands discovered in clay pots from tombs dating back to 10
000 years ago, therefore, it is assumed to have been one of the most critical crops for the development of civilization.2 Hemp strands discovered in clay pots from tombs dating back to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 BC constitute the earliest archaeological proof of human use of the plant, thus, indicating that Cannabis sativa is one of the oldest crops on earth.3,4 Outlining the ancient history of Cannabis sativa in its entirety is almost impossible since its utilization and cultivation dates before the time of the earliest discovered written texts.
000 BC constitute the earliest archaeological proof of human use of the plant, thus, indicating that Cannabis sativa is one of the oldest crops on earth.3,4 Outlining the ancient history of Cannabis sativa in its entirety is almost impossible since its utilization and cultivation dates before the time of the earliest discovered written texts.
Cannabis sativa was predominantly valuable as a fiber source for the most part of the documented history. It was considered to be one of the most important crops up until the 19th century, because of its useful properties, precisely its rot-resistance capabilities, strength and durability5 and it was utilized for several applications, such as production of coarse fabrics and paper and manufacturing of components used in the maritime sector. It is noteworthy that traditional planting, harvesting, and processing of hemp for fiber were exhausting processes, mostly performed by sentenced criminals and slaves. Principally, the abolition of slavery and, additionally, the industrial revolution, the manufacturing of synthetic fibers and its prohibition due to its intoxicating potency lead to the downfall of the hemp fiber. Eventually, hemp cultivation was forbidden in various parts of the world; however, in the majority of the Soviet Union, in most of Eastern Europe, and in Asia, specifically in China, it was authorized.6
In the 1990s, Western countries, specifically Europe and the British Commonwealth, started developing an increasing interest in the re-launch of the hemp sector driven by purely economic motives. Nowadays, around 36 countries produce remarkable amounts of hemp, while China still holds its place as the largest producer of hemp on a global scale.6
The discovery of cannabis seeds in Chinese tombs dates back to over 4500 years.7 They have been used for human consumption as nourishment and for livestock for at least three millennia.8 Even though there is not much evidence from ancient times in Europe, traditional European recipes, including hempseeds as ingredients, support the assumption that these seeds, at least to a lesser degree, were utilized in human nutrition for centuries9 and were generally consumed by the underprivileged. Hempseed oil was also used as lighting oil up until the commencement of the 19th century, due to its lower cost compared to whale oil.6
The 19th and the beginning of the 20th century were the golden ages of hemp cultivation; nevertheless, the economic importance of hemp seeds was minor for the major part of history and by the mid-20th century it became insignificant. During the second half of the 20th century, hemp seeds were usually used as animal foodstuff and only occasionally for human consumption.6
Regarding the intoxicating properties of cannabis, evidence has been found that it was used for shamanistic rituals in ancient China prior to the Han Dynasty, during which its medicinal properties were first documented in a written format.10–12 Cannabis remains (leaves and bracts, which have the highest THC content) along with utensils discovered in the 2500 years-old Yanghai tombs, located in China, suggest that the intoxicating effects of cannabis may have been known.13 In southern Asia, cannabis usage was established for medicinal purposes and religious events.14,15 In other major countries of the ancient world, such as Rome, the Islamic empire, Greece, India, Egypt and Assyria, cannabis was utilized as medicine.16–18 However, access to cannabis for medicinal purposes was extremely limited during the first millennium A.D. and it seems that in Europe the species were mainly cultivated for hemp fiber.
By the late 19th century, recreational marijuana use reached Mexico and in the southern US cannabis tinctures were first listed in the American pharmacopoeia in 1850.14,19 Between the mid-19th century and World War II, cannabis was largely employed in the West for its medical value, whereas its recreational use was strictly associated with low-class and underprivileged people of rural areas. In the second half of the 20th century, cannabis became highly popular as a recreational intoxicant among cultured people residing in urban areas. Eventually, the rise of hedonism and psychedelia in the late 60s and their globally perceived inextricable link with marijuana brought about an extensive global illegal market. Cannabis developed into the world's leading illicit entertainment drug during the last century.6
1.2. Cannabis classification and nomenclature
The naming of Cannabis sativa species follows the binomial nomenclature, which is a formal system of naming living organisms by assigning to them a two-part Latin name. The first part of the name establishes the genus and the second part identifies the species within the genus.20 Sativa comes from the Latin botany-related adjective sativa (f), sativus (m) and sativum (n), which means cultivated, and it is assigned to seed-grown domestic crops which are beneficial for human health.The species of Cannabis sativa L. belong to the family of Cannabaceae with which the former family of Celtidaceae was recently merged.21 The conventional botanical taxonomy of cannabis acknowledges two subspecies: Cannabis sativa subspecies sativa, and Cannabis sativa subspecies indica (Fig. 1),22 which are regarded as disparate species by some botanists.23,24 The terms “Sativa” and “Indica” are typically used by aficionados and medicinal users of cannabis and this vernacular taxonomy of drug-type cannabis has rapidly spread worldwide. However, we should draw attention to the fact that the terms Cannabis sativa and Cannabis indica (in italics) have no relation to the terms “Sativa” and “Indica” (in quotations marks). This difference has developed into a source of confusion in proper labelling.25
 | ||
| Fig. 1 Cannabis vernacular taxonomy.6 Public domain. The Biodiversity Heritage Library considers that this work is no longer under copyright protection.30 | ||
Cannabis species were initially classified in 1753, by the Swedish botanist Carl Linnaeus.26,27 The initial description of the appearance of the Cannabis sativa flower by Linnaeus is utilized for all the plants of the cannabis genus.27,28 The formal botanical name, Cannabis indica, was introduced by Lamarck and was assigned to the plants of Indian origin and to their South African and Southeast Asian descendants.29Cannabis indica has noticeable morphological differences from Cannabis sativa, in the flowers, leaflets, branching habitus and stalks. Additionally, Cannabis indica produces a strong distinctive scent which can induce intoxication when smoked.6
The vernacular categorization defines three terms: “Indica” as Cannabis afghanica, “Sativa” as Cannabis indica, and “Ruderalis” as Cannabis sativa, which is quite misleading (Table 1). The botanists McPartland and Guy suggested to align the formal botanical name with the vernacular nomenclature by matching the names Cannabis indica and Cannabis sativa with “Indica” and “Sativa”, respectively, for the sake of consistency.31,32 Nevertheless, only some researchers have taken this classification into consideration.32–34
| Vernacular taxonomy | Description | Formal botanical name |
|---|---|---|
| “Indica” | Broad leaflets, compact habits, early maturation | Cannabis afghanica |
| “Sativa” | Narrow leaflets, slender and tall habit, late growth | Cannabis indica |
| “Ruderalis” | Varied leaflets, shorter stature, small size and wild-looking | Cannabis sativa |
The lack of a universally accepted classification system for cannabis varieties has, unfortunately, enabled the development of the vernacular classification. Consequently, the classification used by consumers of commercial cannabis can be distinct from the one assigned by botanical taxonomy.35
1.3. Valuable compounds present in cannabis and their medicinal merit
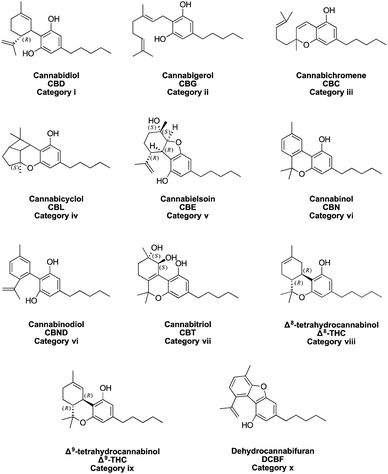
Cannabinoids in their neutral form have a skeleton comprising 21 carbon atoms. The structures of the principal cannabinoids are depicted in Fig. 2. They have been classified in 10 different sub-categories, namely, (i) cannabidiol (CBD), (ii) cannabigerol (CBG), (iii) cannabichromene (CBC), (iv) cannabicyclol (CBL), (v) cannabielsoin (CBE), (vi) cannabinol (CBN) and cannabinodiol (CBND), (vii) cannabitriol (CBT), (viii) Δ8-tetrahydrocannabinol (Δ8-THC), (ix) Δ9-tetrahydrocannabinol (Δ9-THC) and (x) miscellaneous.38
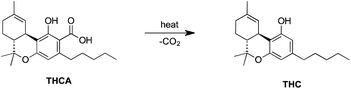
Cannabinoids are mainly encountered in their carboxylated form in the living plant. Decarboxylation naturally occurs in the plant (Fig. 3); however, the storage conditions after harvesting can accelerate this process (refer to section 1.6 for details). Their production primarily takes place in the glandular hairs.39
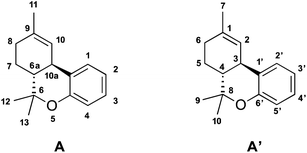
Cannabinoid nomenclature is based on 2 different numbering systems, meaning that the same compound can have 2 different names; numbering can start either at the dibenzopyran ring (used for pyran-type compounds, preferred in North America) or at the monoterpenoid ring/unit (refers to cannabinoids as substituted monoterpenoids, preferred in Europe). The classification according to the dibenzopyran nomenclature of Δ9-THC, indicates the existence of a carbon–carbon double bond between C-9 and C-10 carbon atoms40 and it is equivalent to Δ1-THC in the monoterpenoid nomenclature (Fig. 4A and A′, respectively).
Typically, drug-type plants (e.g., medicinal marihuana) contain THCA and THC as the most abundant cannabinoids, whereas fibre-type plants (e.g., hemp) have CBDA and CBGA, followed by their decarboxylated analogues, CBD and CBG. Cannabinoid acids are not regarded as intoxicants, however, when they are exposed to heat, their decarboxylation naturally occurs, generating the corresponding cannabinoids, namely THC and CBD. Like THC, CBD is also one of the most abundant constituents in the plant; however, it is non-intoxicating, and it has tremendous medical potential.41–44 THC was characterized and synthesized for the first time in the middle of the 1960s.36,45 It affects the central nervous and cardiovascular systems, and it has been reported that it produces pleasant effects, such as euphoria; however, it may produce hallucinations and tachycardia if large quantities are consumed.46,47 The intoxicant capability of Cannabis sativa can be mainly attributed to the decarboxylated form of THC. Although this intoxicating effect is modified by the presence of other cannabinoids and possibly terpenes, THC remains the chief intoxicating component. It also possesses properties that can be medically exploited; however, CBD, which is also present in considerable amounts, demonstrates higher medical potential than THC.6
CBD has received significant consideration in the course of recent years, because of its numerous beneficial properties, both medicinal and pharmacological.48–50 Due to its relatively low toxicity, it is suitable for medical applications and it can be used alone or combined with several cannabinoids. It has proven to have antiinflammatory properties and can be useful in the treatment of neuroinflammatory disorders, including various central nervous system and peripheral disorders, for instance, Alzheimer and it can function as an antimicrobial, anticonvulsive and antiepileptic,49,50 analgesic, antibacterial, antiemetic, antipsychotic, and antispasmodic.14,51 It has been proven that it can also reduce or prevent the anxiogenic effect of THC.47 CBD is the main cannabinoid encountered in the seeds, while no THC is present.52 Nonetheless, the seeds can be contaminated with THC either via contact with the plant resin or ineffective sieving. Depending on the country of origin, hemp seed products can contain THC in the range of 0.005 to 10 ppm.53
Overall, cannabinoids have exhibited outstanding therapeutic potential, as they can alleviate the effects of nausea and emesis in patients undergoing chemotherapy, can stimulate the appetite of HIV-positive patients, and can reduce spasticity in adults suffering with multiple sclerosis.51 They have also shown potential in controlling Tourette's syndrome and chronic pain.54,55 Moreover, they have demonstrated a beneficial effect as antitumor or anticancer agents and in the treatment of diseases, such as epilepsy, glaucoma and schizophrenia.56,57
Over the last decades, considerable steps have been made in the direction of interpreting the interaction of cannabis with the human physiology. Furthermore, new medicinal products and technologies are being developed, tested or, in certain cases, already accepted as beneficial and the volume of the available literature data has significantly increased; however, there is still no unanimous agreement on the merit of medical marijuana. In various jurisdictions, medical marijuana has become commercially available to a great extent owing to the spread of medical dispensaries.14
Currently, many different medicines are prepared using the bulk material of Cannabis sativa to treat chronic diseases, epilepsy, neuropathic pain and multiple sclerosis.58–60 As a matter of fact, the first phytocannabinoid medicine was authorized in the UK in 2011 to treat multiple sclerosis muscle spasms.61,62 In 2018, a CBD-containing anticonvulsant drug was approved in the USA.63
Many researchers use the term “medical marijuana” which particularly refers to the herbal material, while others take additionally into account the extracts and the natural and synthetic cannabinoids. Others suggest the term “medical cannabis” for non-herbal material, as it has less negative connotation.6
On the one hand, the plants that do not produce or produce limited amounts of THC, but generate high quantities of CBD, are designated for fiber and oilseed production, and are classified in Cannabis sativa subspecies sativa. On the other hand, plants that produce high content of the intoxicating THC are classified in Cannabis sativa subspecies indica. The inflorescence, typically the female one, contains the majority of THC and it is used for drug preparations.6
It becomes, thus, apparent, that appropriate labelling of cannabis products enables their proper categorization and allows end users/consumers to select the product that best suits their needs. The chemical content of each commercially available product should be provided. This is especially important for users of medicinal cannabis, who face the major challenge of obtaining a product that is appropriate for their respective treatments, since the chemical profile of the product must precisely fit their medical needs. A wide variety of legally accessible, high-quality, consistent, and safe cannabis products must be guaranteed.65 Medical consumers and their physicians could benefit from an overview of chemical content in commercially available cannabis to effectively shift from a beneficial illegally acquired product to a high-quality equivalent accessed through a legal route. Creating a new classification method based on chemical profiling (“chemovars”) might be complex, but the main benefit is that the chemical content description is detailed and the composition reproducible.65
Varieties of cannabis that contain 0.3% or less THC by dry weight are characterized as hemp, whereas varieties that contain more than 0.3% THC by dry weight are classified as marijuana.14 According to their cannabinoid content, cannabis varieties can be further classified as follows: type I – THC-abundant > 0.3% and CBD < 0.5%; type II – a mixture of THC and CBD with different moderate concentrations, which is virtually CBD dominant, and type III – CBD-dominant with low THC content.
Type I varieties can have intoxicating effects, with THC reaching >30%, and they are suitable for medicinal and therapeutic use. Type II CBD-rich varieties can help attenuate the intoxicating effect of THC, thereby increasing the chance of patients strictly adhering to their daily recommended dosage and, at the same time, increasing the therapeutic effect of CBD on the receptors. Type III refers to cannabis that contains mainly CBD in concentrations higher than THC, while the THC content is less than 0.3%. This range has been randomly assigned for legal hemp production. Type III cannabis is considered for fiber or drug-production, and if it is both rich in cannabinoids and terpenes, for industrial goods, such as cellulose plastics, paper, and fabric.66
Hemp seeds contain many components, which can have a beneficial effect on health, such as polyunsaturated fatty acids (PUFAs), digestible proteins and trace amounts of terpenes and cannabinoids.70 The fatty acid composition of the seeds determines the quality of the oil, specifically, the higher the amount of PUFAs the better the nutritional value. Hempseed oil has PUFAs in abundance (normally over 80%), among them, necessary fatty acids, such as γ-linolenic acid (GLA, 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, ω-6, 1–6%) and stearidonic acid (SDA, 18
3, ω-6, 1–6%) and stearidonic acid (SDA, 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, ω-3, 0–3%), and two essential fatty acids (EFAs), principally linoleic acid (50–60%) and α-linolenic acid (20–30%). Besides, hempseed oil also contains a monosaturated fatty acid (MUFA), namely oleic acid (18
4, ω-3, 0–3%), and two essential fatty acids (EFAs), principally linoleic acid (50–60%) and α-linolenic acid (20–30%). Besides, hempseed oil also contains a monosaturated fatty acid (MUFA), namely oleic acid (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 10–16%).14 The aforementioned PUFAs have a positive influence on the cell membrane functions, stimulate cell immunology and have demonstrated their potential in alleviating atopic dermatitis and psoriasis,71 while the omega fatty acids can promote cardiovascular health.72
1, 10–16%).14 The aforementioned PUFAs have a positive influence on the cell membrane functions, stimulate cell immunology and have demonstrated their potential in alleviating atopic dermatitis and psoriasis,71 while the omega fatty acids can promote cardiovascular health.72
Hempseed oil is rich in natural essential fatty acids and, additionally, it is the most balanced among commonly consumed plant oils for human nutrition; the ω-6 and ω-3 EFAs ratio (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–3
1–3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) is the most favorable for human digestion.73–75 The dietary EFAs, which are easily absorbed by skin tissues, contribute to the formation and regeneration of cell membranes and function as precursors in the synthetic route of several biochemicals that regulate the body's metabolic pathways.76,77
1) is the most favorable for human digestion.73–75 The dietary EFAs, which are easily absorbed by skin tissues, contribute to the formation and regeneration of cell membranes and function as precursors in the synthetic route of several biochemicals that regulate the body's metabolic pathways.76,77
Several tocopherols, such as α-, β-, γ- and δ-tocopherols, are present in low amounts (0.1%) in hemp seeds.75,78 Tocopherols, which have antioxidant properties, are part of the vitamin E group and are vital for human nourishment. In comparison to other available dietary oils, hempseed contains higher relative quantities of vitamin E, typically 100 to 150 mg g−1 of oil.79–81 Moreover, hempseed contains around 0.7% of phytosterols, in particular campesterol and β-sitosterol. They also provide many health-beneficial effects, such as reduction of the total blood cholesterol and low-density lipoprotein (LDL) cholesterol levels in human serum, thus, treating atherosclerosis. Hemp seeds, additionally, consist of 25–30% proteins, which include the eight essential amino acids.82,83
High content of phenols and polyphenols can be found in hempseeds. Positive health effects are attributed to phenolic compounds since they are considered to be effective antioxidants and frequently exhibit antiinflammatory and cardioprotective properties.84 Apart from that, hemp seeds also contain carbohydrates (20–30%), oil (25–35%), dietary fiber (10–15%) and minerals (4–6%), namely zinc, sulfur, potassium, phosphorus, magnesium, iron and calcium.14,73
Cannabis plants give out a characteristic scent, provided by the essential oil. Essential oils are a complex mixture of volatile secondary metabolites that consist of terpenes (monoterpenes, sesquiterpenes and other terpene-like compounds) and non-terpene hydrocarbons and their oxygenated derivatives, such as alcohols, ethers, ketone, aldehydes, esters, phenols, phenol ethers and lactones.85,86
Generally, terpenes are the major component of essential oils and many of them are responsible for the intense scent, since they can be detected by the sense of smell even at low concentrations. Currently, around 140 terpenes and terpenoids have been identified in Cannabis sativa and their medicinal properties are widely acknowledged (Table 2).86 In the case of cannabis plants, monoterpenes normally represent most of the essential oil and its particular aroma is produced by pinene and limonene, which comprise more than 75% of the volatile components.87–89 The essential oil composition varies significantly among different strains and cultivars of Cannabis sativa.90,91 It is worth mentioning, that the interaction between natural terpenes and cannabinoids may have a therapeutic effect.92,93
| Terpene/terpenoid | Properties |
|---|---|
| α-Pinene | Antiinflammatory, antibacterial, bronchodilatory |
| β-Caryophyllene | Antiinflammatory, protects lining of digestive tract, antimalarial |
| β-Myrcene | Analgactesic, antiinflammatory, sedative, muscle relaxant |
| Caryophyllene oxide | Antifungal, decreases platelet aggregation, treats nail infections |
| Limonene | Antidepressant, immunostimulant, antibacterial |
| Linalool | Antianxiety, sedative, local anesthetic, anticonvulsant |
| Nerolidol | Antimalarial, sedative |
| Phytol | Sedative, prevents certain congenital malformations |
An overview of the valuable compounds encountered in the cannabis plant along with their location in the respective plant parts is presented in Fig. 5.
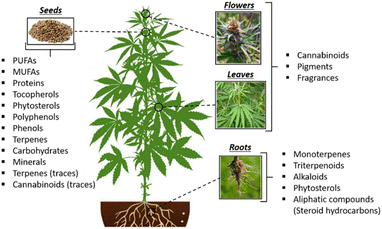 | ||
| Fig. 5 Valuable compounds present in the cannabis plant. PUFA: polyunsaturated fatty acids; MUFA: monosaturated fatty acid.94–96 Open access, CC BY 4.0, https://creativecommons.org/licenses/by/4.0/. | ||
1.4. Non-medical applications of cannabis
Cannabis sativa can be grouped into 2 main categories; the one is the cannabis cultivated for the production of financially significant materials, i.e., fiber, seed oil and psychotropic drugs, whereas, the other category comprises “wild” (weedy) plants which are not subject to the applied cultivation practices. Hemp fibers are used for the manufacturing of ropes and textiles, and seed oil is employed in the production of industrial oils and dietary supplements, while it can be additionally utilized as livestock feed, human food and occasionally as biofuel.Even though certain hemp applications, such as clothing and paper, are currently obsolete, a diversity of novel applications has emerged and revived the hemp industry, to name a few, horticultural planting media, biodegradable mulch, cordage products, pressed and molded fiber products, building construction products, animal bedding based on hurds, compressed cellulose plastics, plastic biocomposites and as insulation material in the automotive industry.14,99,100
Often the terms “hemp oil” and “hempseed oil” are erroneously used interchangeably; however, they do not refer to the same type of oil. “Hemp oil” may refer either to the vegetable oil or to the essential oil, thus, the oilseed industry has assigned the term “hempseed oil” to the edible vegetable oil to avoid ambiguity. The term “cannabis oil” is even more vague since it can be used to refer to either of the 3 oils derived from the cannabis plant.6
Recently, the seeds have proven to be an important source of edible oil and suitable for the production of pharmaceuticals, nutraceuticals and functional foods. Europe and Canada have recently cultivated varieties of cannabis that focus on oilseed production. It is foreseen that the oilseed crops of cannabis will create a more profitable market than the fiber crops, at least in industrially advanced countries. Importing hemp seed from China, which is currently the leading country in hempseed production, is not at the best interest of other countries due to various reasons; required sterilization of the seeds prior to import signifies delay in the import process, increased costs and a decrease in the quality of the seeds. Additionally, sterilized seeds go stale rather fast, which is an undesirable property of seeds intended for human consumption. Domestic-seed production is, therefore, preferable. The EU, which was heavily focused on fiber crops, has only recently realized the promising future of the oilseed. In contrast, Canada, which has always exploited the oilseed crops, has emerged as the global provider of hempseed products used in the manufacturing of natural foods, nutraceuticals and cosmetics. The USA is expected to follow suit ensuing the local legalization of industrial hemp.6
Nowadays, many food preparations, such as breads, yogurts, and salad dressings, contain hemp seeds and/or hemp seed oil. Additionally, hemp essential oil is used as a flavorant for alcoholic beverages. Hemp seeds have been typically used complementary to poultry feed.101 The seed cake, obtained from extracting the oil from the seeds, has proven to be an exceptional nutrition source for sheep, lambs and cattle.102,103 Since the late 20th century, hempseed oil has been used in a different array of cosmetic products, such as soaps, shampoos, and lip balms. It is also present in industrial products, such as printing inks, lubricants for machinery, sealants, varnishes and oil paints.104 In addition, it has been transformed into biodiesel and has been used as a green alternative to non-renewable fuel sources.105
The essential oil of Cannabis sativa is costly (approximately 1100 € per liter) since the obtained yields are quite low (around 1.3 liter per ton fresh weight – 10 liter per hectare).90,106
1.6. Cannabis storage and impact on composition
Ensuring the integrity of sample characteristics from the time of collection until the time of analysis implies that the transportation and storage conditions should be carefully monitored in order to minimize its degradation and/or contamination.A crucial factor that can affect the overall quality, medical efficacy and value of cannabis products is the humidity level upon storage of the harvested and cured flower. According to the American Society of Testing and Materials (ASTM) International, the optimal humidity level for cannabis storage is in the range of 55–65%. Exceeding the recommended values exposes the harvested flower to possible growth of mold and fungus, while failing to reach the minimum value can lead to extensive dryness, thus, substantial loss of critical components, such as cannabinoids and volatile constituents, such as terpenes.107
Sample processing prior to analysis or extraction is another important consideration that will largely determine the sample homogeneity and associated reliability of the analytical outcome. Despite the advantageous effects of sample grinding, i.e., increased homogeneity and surface area and decreased particle size, all of which benefit extraction efficiency, at the same time, grinding also impacts the sample in an undesirable manner; increased contamination risk, introduction of humidity, possible losses of volatile compounds and alteration of labile ones. Cryogenic grinding is an alternative that can largely circumvent the unwanted outcomes mentioned above.108
Studies have demonstrated that THC, both in herbal and resin cannabis preparations (marijuana and hashish), progressively decomposes over time to CBN,109 which is believed to be a mere chemical degradation product rather than a naturally/biochemically occurring component.110 Decomposition of Δ9-THC, when exposed to light and air, to CBN, proceeds through an oxidation reaction.
Although this gradual conversion of THC to CBN is an inevitable process, storage conditions can significantly delay or accelerate it. Taking into consideration that there might be either a considerable time lapse between sample reception and analysis or the need for re-evaluation of an old sample or even both, it is crucial to be able to accurately determine the effect of time and storage conditions on the sample composition.
It has been known since 1969 that the THC content of marijuana stored at RT is decreasing at a rate of 3–5% per month.111 Three independent 4 year long stability studies, demonstrated that light and temperature have a dramatic effect on the decomposition of THC to CBN, while they mediate different aspects of the process; light impacts the stoichiometry of the conversion of THC to CBN, whereas temperature accelerates the conversion.109,112,113 In any case, samples stored at RT in direct contact with the atmospheric air, either in light or darkness, suffered the most pronounced losses in THC, ranging from 65% to almost 100%, depending on the sample origin and initial composition. Since many of the substances in cannabinoids can undergo oxidation, sealing the samples in plastic bags can reduce the losses to 25–42%. It should be mentioned that the increase in the total amount of CBN does not correspond with the total decrease of THC, an observation which implies that THC degrades to other products apart from CBN. Negligible impact on the initial concentrations of cannabinoids is observed when samples are stored in the freezer at −20 °C. In contrast, storage conditions have no significant impact on the CBD content.112
Short-term stability studies (15–30 days) of cannabinoids (THC, CBN, CBD) extracted in organic solvents (methanol, chloroform, light petroleum, methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chloroform 9
chloroform 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) indicate that the form of cannabinoids and storage temperature, rather than the nature of the extractant, are crucial parameters affecting their stability in solution. Specifically, neutral cannabinoids are stable for up to 15 (at 20 °C) to 30 days (at −18 °C and 4 °C) stored in the dark,114 while exposure to daylight leads to their dramatic decomposition.115 In the case of acidic cannabinoids, decomposition occurs both in dark and light with the rate increasing with temperature.114
1) indicate that the form of cannabinoids and storage temperature, rather than the nature of the extractant, are crucial parameters affecting their stability in solution. Specifically, neutral cannabinoids are stable for up to 15 (at 20 °C) to 30 days (at −18 °C and 4 °C) stored in the dark,114 while exposure to daylight leads to their dramatic decomposition.115 In the case of acidic cannabinoids, decomposition occurs both in dark and light with the rate increasing with temperature.114
In addition to the discussed parameters, the material of the containers in which cannabinoid extracts are stored can also have a significant effect on the cannabinoid content determined in solution. The loss of acidic THC on container walls is a function of both solvent type and container material since they both appear to compete to bind with it. Generally, the loss determined in organic diluents is significantly lower than in the case water is used as the cannabinoid diluent, owing to the higher solubility of cannabinoids in organic solvents. As far as material is concerned, the losses are far less pronounced in the case of polar materials (contact angle < 70°), such as glass and acrylic, than in non-polar materials (contact angle > 70°), such as HDPE. Additional losses are observed with increasing surface tension and decreasing pH and conductivity. Large volumes of stored solution are preferrable because losses attributable to both pipetting and exposed surface area are minimized.116
1.7. Inconsistencies and misconceptions in result reporting in literature
When one reviews the developed and reported extraction processes, it is quite apparent that an accurate and meaningful comparison of their performance is a rather challenging task. The inherent differences of the various cultivars extracted cannot be overcome; however, it is rather the inconsistent reporting that constitutes a major obstacle in the evaluation of the reported results.As far as the input sample is concerned, only a handful of manuscripts report the composition of the input sample material (either relying on Soxhlet as a reference method or on data provided by the raw-material supplier, while in some cases no specification is given),42,117–123 whereas most researchers only focus on the extracted amount of valuable compounds. Undoubtedly, the amount extracted is highly valuable information; nevertheless, the recovery of the target compounds, which is largely absent, is indicative of the performance of the developed method and, thus, a crucial factor for the evaluation of its potential economic and ecological merit in comparison to state-of-the-art approaches. Additionally, information regarding the input sample moisture78,121,122,124–130 or the particle size131–137 or both42,118,138–148 is often omitted, thereby rendering result interpretation further complicated; water content affects both the extraction outcome/extracted amount of the valuable compounds and the accuracy of the reported concentrations, when those are calculated with reference to the input sample.
With respect to extraction conditions with supercritical solvents, namely supercritical carbon dioxide (scCO2), variable pressure (bar, MPa) and CO2 flow rate units (g min−1, kg h−1, mL min−1) are reported,119,124,125,133,138,149 whereas, in certain cases, scCO2 density is provided in lieu of pressure150 or flow rate;117 furthermore, the value of the density is reported in different units (g mL−1,150 kg m−3,117) which sometimes can be attributed to differences in journal requirements. Lamentably, in certain cases, failure to report the overall extraction time of the process117,119,120,125,133 or even the process conditions altogether,118,126,132,151–154 as well as ambiguity in the reported conditions,78,130,144,147 does not allow fair and complete assessment of the presented results.
As we previously discussed, the storage conditions of the samples are crucial for the outcome of the quantification procedures. Review of the available literature reveals that the employed storage conditions can significantly vary between experiments conducted by different researchers. Milling and grinding prior to storage can have a considerable impact on the outcome, since these processes can not only contaminate the sample but also modify its composition, thus storage and analysis after milling/grinding117,124,155,156 means that information on the composition of the original plant material has been lost. Drying of the plant material prior to storage and analysis is often reported; however, when drying is performed at elevated temperature157–160 it leads to decarboxylation, thereby modifying the original composition of the material. It is not uncommon though that the drying is simply performed at RT.120,124,151 As a far as storage is concerned, storage conditions of RT,117,149,156 4 °C,124,126 <18 °C/refrigerator155,161 and even −20 °C157 have been reported. Even more unfortunate than the variation of storage conditions though is the unclear and/or incomplete storage and/or pre-treatment information of the sample material,118–120,125,131–133,151–154,158–160,162–164 while even in the cases that this information is complete the duration of the storage is not discussed at all. It is also quite important to point out that no information on the storage containers has been provided, which is rather important considering the impact the material of the containers can have, as we saw earlier.
Aside from the evidently inconsistent information provided on the input material and the process conditions, the reported results are a source of additional confusion. Unfortunately, % recovery data is rarely reported,149,153 even when the input-material composition has been determined with the aid of a reference extraction method. On the other hand, the yield from hemp flowers and residues (overall or per extracted valuable compound or both) is most commonly reported; however, the yield is not calculated consistently. It has been calculated with reference to the input sample material,42,78,123,124,127–129,133–135,139,143–148,153,165–167 to the recovered extract117–119,121,122,136,138,140–142,162,168,169 and to the overall cannabinoid extract process,117,125 while in some cases it is not clearly specified,122,131,163,170 thereby, causing confusion and only leading to assumptions and misunderstandings on the part of the reader. Providing the equation on which the calculations are based, as certain publications do,78,134,144,145,153,158,159,163,167 could effectively eliminate this problem. In contrast, the fatty acid yield from hemp seeds is invariably referenced to the extracted hempseed oil.126,131,149,151–153 Additionally, cannabinoid yields have been reported either as % (w/w),119,122,124,125,134,138,148 as μg g−1,141,143 as mg g−1,117,118,140,162 as mg mL−1,142,168 as percentage %136 and as cumulative yield,141 while the input sample amount is not always provided.117,118,124,139,147 Regarding the oil yield, it has been reported in % w/w,78,144–146,165,167 in g144 and in percentage %.127,130,147
Certain authors make a distinction between THC and total THC,125 however, in the majority of cases THC is reported without clarification as to whether this refers to pure THC or total THC (THC + THCA). Unfortunately, error values (procedural, measurement or both) of the reported results are frequently excluded;117,122–124,127,138,139,142,150,153,163,170 this information is quite significant, not only because in certain cases it can be considerable, which is not unexpected when working with naturally derived samples133 but also because its absence renders the comparison of different experimental outcomes problematic.
Concerning the statistical aspect of the reported procedures, it seems that performed extraction repetitions are not enough, in some cases, to confidently accept the reliability of the reported results. We consider that this might not be feasible in certain techniques, e.g., scCO2; however, at least triplicate extractions would be necessary to exclude/eliminate any erroneous conclusions. Nevertheless, the majority of the publications report triplicates,128–130,134–137,140,141,143–145,147,149,152,158,163,164,166–168,171 while some report duplicates.42,120,121,125,138,146,148,150,162,165 Unfortunately, sometimes, this crucial information is entirely omitted.117–119,123,124,126,127,131–133,139,140,142,151,153–155,159–161,163,166,170,172
It becomes thus apparent, that it is, currently, an impossible challenge to compare different publications on this topic, due to the lack of consensus between researchers on specifying in a standardized manner the experimental parameters and the obtained results. Arriving at an agreement of consistent data reporting would be an extremely valuable contribution to the field, which cannot be tackled by an individual researcher but would need to be an effort with broad support of the community.
With regard to misconceptions, a critical point that needs to be addressed, which appears not only in the referenced reviews (except Lazarjani et al.)173 but also in the majority of the publications reported herein, is the seemingly existing confusion between intoxicating and psychotropic/psychoactive properties. Intoxication is associated with a state of euphoria and mild cognitive impairment, i.e., in layman's terms “getting high”, and according to the Cambridge dictionary an intoxicating substance is “able to make you lose some control of your actions or behaviour”. However, a psychoactive substance does not simply “get one high” but rather affects mental processes, e.g., perception, consciousness, cognition or mood and emotions.174 Psychotropic substances have effects on psychological function.175 According to the Anatomical Therapeutic Chemical classification system the five classes of psychotropic drugs are antipsychotics, antidepressants, anxiolytics, hypnotics and mood stabilizers. Based on this nomenclature, it is clearly wrong to assign CBD as non-psychoactive/non-psychotropic;176 it clearly falls into the psychoactive/psychotropic classification by definition due to its anxiolytic capacity.
An apparent term misuse in the reported literature concerns the terms “terpenes” and “terpenoids”. They have been used in an interchangeable manner in several publications reported herein. Nevertheless, there is a distinct difference between the two; terpenes are hydrocarbons that arise from the head-to-tail joining of five-carbon isoprene units, while terpenoids are oxygenated forms of terpenes.177 Some authors collectively refer to terpenes and terpenoids as “terpenoids”118,134 while others collectively refer to terpenes and terpenoids as “terpenes”.120,121,148,164
1.8. Recent reviews on cannabis extraction
It should be mentioned here that a number of reviews on the topic of cannabinoid and bioactive compound extraction from cannabis has been published. Specifically, Lazarjani et al.,173 Pattnaik et al.,178 Al Ubeed et al.,179 and Qamar et al.180 have published most recently on this topic.There is, currently, no review that provides an exhaustive overview of all available extraction methods, conventional and more recently developed, from all different parts of the cannabis plant but there is rather a focus on certain compound groups (i.e., cannabinoids) or certain extraction methods (e.g., supercritical CO2). Additionally, in our review other groups of known value (e.g., polyphenols) have been addressed, in addition to cannabinoids. The review does not only focus on the medicinal value of the plant but also on the sometimes overlooked industrial (nutrition, cosmetics) value of the plant, while a historical overview allows a clear understanding of the long-standing value of the cannabis plant for the human civilization. Unlike other reviews, we have decided to address several inconsistencies that appear in experimental procedures and reported results which obscure their value and practicality.
2. Extraction techniques for cannabinoids and other valuable bioactive cannabis compounds
In the present section of the review, the techniques that have been employed for the extraction of valuable compounds from the cannabis plant will be discussed in detail. An overview of the employed techniques as well as the respective plant parts to which they have been applied along with the distribution of each technique is presented in Fig. 6 and 7.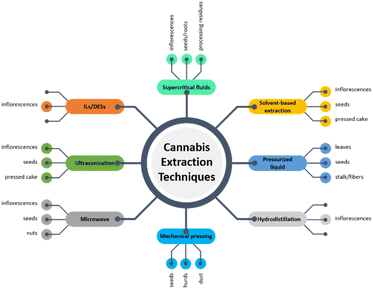 | ||
| Fig. 6 Reported techniques for the extraction of valuable compounds from various parts of the cannabis plant. | ||
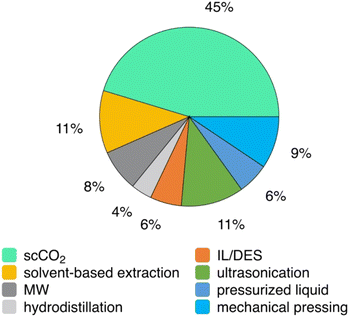 | ||
| Fig. 7 Distribution of the reported extraction techniques. scCO2: supercritical CO2, IL: ionic liquid; DES: deep eutectic solvent, MW: microwave. | ||
2.1. Supercritical fluid-based extraction
The contemporary quest towards sustainable processes has placed supercritical CO2 (scCO2) in the forefront of alternative solvents which are under investigation as promising candidates that can promote this goal. Supercritical CO2 possesses a range of unique properties that are superior to the commonly used organic solvents. Namely, its non-toxic and non-flammable nature, its tunability by simple variation of the process parameters, its recyclability, as well as its low price and easy separation from the extracted products, render it a highly versatile alternative solvent. Additionally, the supercritical state can be reached at relatively mild pressure (73.8 bar) and temperature (31.0 °C) conditions. Although the non-polar nature of scCO2 renders it ideal for the solvation of non-polar and weakly polar compounds, addition of polar co-solvents can expand its solvation range. It should be pointed out here that the properties of non-toxicity and the recovery of products without any solvent residues make the scCO2 extraction technology particularly attractive to the pharmaceutical and food industries.181,182| Entry no. | Input sample | Particle size (mm) | Conditions | Extracted compounds | Ref. no. |
|---|---|---|---|---|---|
a All concentrations of extracted compounds within the table are reported as %, regardless of the units in which they were originally reported by the authors, in order to facilitate their comparison.
b Reported as % of the cannabinoid extract.
c Reported as % of the total extract.
d Reported as % of the input sample.
e Pretreatment at 70 °C for 15 min with IL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O 1 H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. (n. r. = not reported), [C2mim][OAc] = 1-ethyl-3-methylimidazolium acetate, [Ch][OAc] = choline acetate, [C2mim][DMP] = 1-ethyl-3-methylimidazolium dimethyl phosphate. 3. (n. r. = not reported), [C2mim][OAc] = 1-ethyl-3-methylimidazolium acetate, [Ch][OAc] = choline acetate, [C2mim][DMP] = 1-ethyl-3-methylimidazolium dimethyl phosphate.
|
|||||
| 1 | Cannabis sativa L., strain: hash berry (Sativa 50%, Indica 50%), leaves & buds (ground), 500 g | 1.2 | 55 °C, 340 bar, 200 g min−1 (12 kg h−1) | THC: 25.78 ± 1.82, THCA: 49.75 ± 2.54, total THC: 69.41 ± 2.87, CBDA: 1.09 ± 0.93, extraction efficiency: 90 | 125 |
| 2 | Cannabis sativa L., strain: sour alien OG (Sativa 40%, Indica 60%), leaves & buds (ground), 500 g | 1.2 | 55 °C, 340 bar, 200 g min−1 (12 kg h−1) | THC: 20.55, THCA: 46.37, total THC: 61.21, CBDA: 1.66, extraction efficiency: 89 | 125 |
| 3 | Cannabis sativa L., strain: white widow (Sativa 60%, Indica 40%), leaves & buds (ground), 500 g | 1.2 | 55 °C, 340 bar, 200 g min−1 (12 kg h−1) | THC: 23.07, THCA: 39.68, total THC: 57.86, CBDA: 0.97, extraction efficiency: 90 | 125 |
| 4 | Cannabis sativa L., strain: abusive OG (indica), leaves & buds (ground), 500 g | 1.2 | 55 °C, 340 bar, 200 g min−1 (12 kg h−1) | THC: 27.69, THCA: 39.68, total THC: 56.06, CBDA: 0.01, extraction efficiency: 92 | 125 |
| 5 | Mixed plant material (dried, ground), 500 g | n.a. | 60 °C, 320 bar, 150 g min−1, 600 min | THC: 28, CBD: 17 | 138 |
| 6 | Ripe cannabis inflorescences (dried, milled) | <0.5 | 60 °C, 330 bar, 0.55 kg h−1, 240 min, 2% wt EtOH | THC: 6.06, total THC: 37.85, overall yield: 16 | 124 |
| 7 | Marijuana (air-dried, ground), 0.5 g | 0.063 < d < 0.125 | 40 °C, density 0.9 g mL−1, 1.5 mL min−1, 130 min | THC: complete removal | 150 |
| 8 | Girl scout cookies (hybrid: 60% Sativa, 40% Indica), flowers (milled), 6 g | 0.24 | 50 °C, 165 bar, 2.5 mL min−1, 6% (v/v) EtOH | THC: 87.91 ± 1.10, CBD: 5.08 ± 1.01, CBN: 0.53 ± 0.59, overall yield: 30, α-pinene: 4.79 ± 0.45, β-pinene: 2.20 ± 0.08, myrcene: 15.35 ± 2.38, (R)-limonene: 0.81 ± 0.13, caryophyllene: 31.67 ± 0.28, α-humulene: 11.09 ± 0.63, β-panasinsene: 12.62 ± 1.57, selina-3,7(11)-diene: 16.49 ± 2.10 | 119 |
| 9 | Durga mata II (10% Sativa, 90% Indica), flowers (milled), 6 g | 0.32 | 50 °C, 240 bar, 2.5 mL min−1, 6% (v/v) EtOH | THC: 27.96 ± 0.57, CBD: 33.81 ± 0.43, CBN: 0.23 ± 0.13, overall yield: 27, α-pinene: 17.99 ± 1.32, β-pinene: 6.08 ± 0.86, myrcene: 12.34 ± 0.50, (R)-limonene: 7.45 ± 0.44, caryophyllene: 20.97 ± 1.78, α-humulene: 8.75 ± 0.26, β-panasinsene: 9.15 ± 1.96, selina-3,7(11)-diene: 11.17 ± 2.15 | 119 |
| 10 | Cannabis sativa L., cultivar: a (undisclosed), flowers buds, leaves, trimmings (milled) | 2 | Near-critical propane | THC: 18.1, THCA: 1.18, CBD: 315, CBDA: 86.6, CBG: 5.76, CBGA:0.71, CBN: 0.36 | 117 |
| 11 | Cannabis sativa L., cultivar: B1 (Ferimon 12), flowers buds, leaves, trimmings (milled) | 2 | Pressurized dimethylether | THC: 18.0, THCA: 0.03, CBD: 229, CBDA: 5.03, CBG: 6.94, CBGA: 0.17, CBN: 0.82 | 117 |
| 12 | Cannabis sativa L., cultivar: B2 (Ferimon 12), flowers buds, leaves, trimmings (milled) | 2 | 40 °C, 100 bar, density 628.6 kg m−3 | CBD: 97.8 | 117 |
| 13 | Cannabis sativa L., cultivar Felina, inflorescences (dried), 150 g | 0.2–0.6 | 40 °C, 100 bar, density < 600 kg m−3 | n.r. | 120 |
| 14 | Cannabis sativa L., chemovar: cherry kush, flowers | n. r. | n. r. | THC: 7.75 ± 0.19, THCA: 69.4 ± 5.2, CBD: 0.53 ± 0.06, CBDA: 9.12 ± 0.21, CBG: not detected, CBN: 0.15 ± 0.01, α-pinene: 0.48 ± 0.11, β-pinene: 0.48 ± 0.08, β-myrcene: 0.13 ± 0.03, (R)-limonene: 1.19 ± 0.33, β-caryophyllene: 20.60 ± 2.40, α-humulene: 5.69 ± 0.55, linalool: 3.14 ± 0.66, fenchyl alcohol: 4.04 ± 0.47, α-terpineol: 5.06 ± 0.46, α-bisabolol: 4.53 ± 1.25 | 118 |
| 15 | Cannabis sativa L., chemovar: blackberry kush, flowers | n. r. | n. r. | THC: 10.6 ± 0.23, THCA: 74.9 ± 8.4, CBD: 0.09 ± 0.06, CBDA: 0.70 ± 0.05, CBG: 0.79 ± 0.06, CBN: 1.1 ± 0.1, α-pinene: 0.31 ± 0.05, β-pinene: 0.39 ± 0.08, β-myrcene: 0.14 ± 0.02, (R)-limonene: 1.09 ± 0.20, β-caryophyllene: 2.10 ± 0.65, α-humulene: 1.65 ± 1.43, linalool: 0.93 ± 0.17, fenchyl alcohol: 4.01 ± 0.45, α-terpineol: 4.65 ± 0.36, α-bisabolol: not detected | 118 |
| 16 | Cannabis sativa L., chemovar: pineapple kush, flowers | n. r. | n. r. | THC: 17.4 ± 0.38, THCA: 65.1 ± 4.5, CBD: 0.19 ± 0.05, CBDA: 0.33 ± 0.06, CBG: 0.11 ± 0.02, CBN: 0.1 ± 0.02, α-pinene: 0.44 ± 0.60, β-pinene: 0.32 ± 0.05, β-myrcene: 1.30 ± 0.21, (R)-limonene: 1.40 ± 0.19, β-caryophyllene: 10.38 ± 0.24, α-humulene: 3.23 ± 0.12, linalool: 4.00 ± 0.56, fenchyl alcohol: 2.68 ± 0.17, α-terpineol: 3.35 ± 0.18, α-bisabolol: 4.96 ± 0.54 | 118 |
| 17 | Cannabis sativa L., chemovar: purple sour diesel, flowers | n. r. | n. r. | THC: 0.998 ± 0.27, THCA: 67.5 ± 4.4, CBD: not detected, CBDA: 2.03 ± 0.60, CBG: 2.05 ± 0.09, CBN: 1.5 ± 0.007, α-pinene: 1.30 ± 0.14, β-pinene: 0.57 ± 0.01, β-myrcene: 5.46 ± 0.40, (R)-limonene: 1.06 ± 0.21, β-caryophyllene: 14.55 ± 0.46, α-humulene: 4.10 ± 0.09, linalool: 4.72 ± 0.28, fenchyl alcohol: 2.05 ± 0.33, α-terpineol: 2.60 ± 0.38, α-bisabolol: 2.05 ± 0.90 | 118 |
| 18 | Cannabis sativa L., chemovar: ripped Bubba, flowers | n. r. | n. r. | THC: 0.97 ± 0.21, THCA: 71.5 ± 5.3, CBD: not detected, CBDA: 0.31 ± 0.02, CBG: 1.14 ± 0.08, CBN: 0.16 ± 0.07, α-pinene: 0.17 ± 0.01, β-pinene: 0.210 ± 0.003, β-myrcene: 0.55 ± 0.05, (R)-limonene: 0.60 ± 0.01, β-caryophyllene: 7.59 ± 0.96, α-humulene: 2.06 ± 0.22, linalool: 3.64 ± 0.16, fenchyl alcohol: 2.84 ± 0.16, α-terpineol: 3.63 ± 5.3, α-bisabolol: not detected | 118 |
| 19 | Cannabis sativa L., chemovar: harlequin, flowers | n. r. | n. r. | THC: 5.5 ± 0.2, THCA: 33.3 ± 2.6, CBD: 1.74 ± 0.09, CBDA: 44.5 ± 0.37, CBG: not detected, CBN: not detected, α-pinene: 1.51 ± 0.26, β-pinene: 0.65 ± 0.26, β-myrcene: 1.78 ± 0.40, (R)-limonene: 1.27 ± 0.24, β-caryophyllene: 8.40 ± 0.96, α-humulene: 2.48 ± 0.27, linalool: 3.58 ± 0.71, fenchyl alcohol: 2.53 ± 0.28, α-terpineol: 3.94 ± 0.31, α-bisabolol: not detected | 118 |
| 20 | Cannabis sativa L., chemovar: Futura 75, flowers, leaves and stems | 2 | 70 °C, 200 bar, 5.0 mL min−1 [C2mim][OAc] | , THC: 0.542 ± 0.016 CBD: 15.6 ± 0.7 CBG: 0.335 ± 0.016 | 157 |
| 21 | Cannabis sativa L., chemovar: Futura 75, flowers, leaves and stems | 2 | 70 °C, 200 bar, 5.0 mL min−1 [Ch][OAc] | , THC: 0.535 ± 0.010 CBD: 15.4 ± 0.5 CBG: 0.401 ± 0.024 | 157 |
| 22 | Cannabis sativa L., chemovar: Futura 75, flowers, leaves and stems | 2 | 70 °C, 200 bar, 5.0 mL min−1 [C2mim][DMP] | , THC: 0.449 ± 0.025 CBD: 11.8 ± 0.9, CBG: 0.292 ± 0.028 | 157 |
Cannabinoids (THC, THCA, CBDA) from ground plant material (leaves, buds) were extracted with scCO2via one-step and multi-step approaches (Table 3, entries 1–4).125 While a three-step cascade extraction with sequential increase in applied pressure (sequential pressure increase 170 → 240 → 340 bar yields (82.99 ± 1.87) g cannabinoid extract) offers the possibility for selectivity tuning of extraction speed and overall yield at different steps, it is not as effective as a one-step extraction at higher pressures ((92.57 ± 2.14) g cannabinoid extract); high pressure implies increased solvating power, faster extraction rate and, consequently, lower solvent consumption (a solvent/feed ratio >70 is required at 240 bar to obtain the same extraction efficiency as with solvent/feed = 40 at 340 bar). Nevertheless, the extraction behavior seems to be dependent on the plant material used, specifically, the lower the cannabinoid concentration in the plant material the lower the extraction yield and the slower the extraction rate. Regardless of the selected scCO2 extraction approach, partial decarboxylation of THCA occurs during the process as a result of the exposure of the plant material to increased temperatures.
The effect of co-solvent on the one-step scCO2 extraction was evaluated with two different modes of addition; ethanol was supplied either at a constant flow rate or in a pulse mode (Fig. 8). As expected, the addition of a polar co-solvent increases the solvating power of scCO2 towards polar molecules, thus, favors the overall extraction yield of cannabinoids and the extraction rate. The pulse-mode experiments were designed so that the same amount of ethanol as in the constant-flow experiments was supplied, i.e., higher % EtOH was supplied at three shorter intervals. Although the overall extraction yield between the two approaches was comparable, the pulse mode delivered the maximum extraction yield already after the first pulse was applied, which means that a lower amount of ethanol than expected was consumed to achieve the same extraction yield as in the constant-flow experiments at significantly shorter time.125 It would have been very interesting if the authors had also considered running the extraction experiments with a higher EtOH concentration but for a shorter duration to assess whether the outcome would be the same.
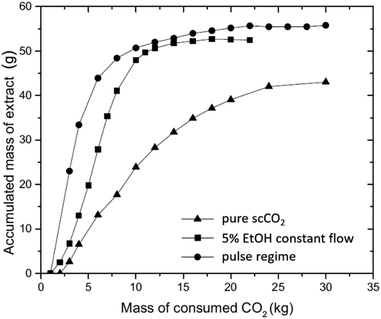 | ||
| Fig. 8 Comparison of pure scCO2, constant EtOH flow rate and pulse regime on the extraction (white widow).125 Reprinted with permission from Elsevier. | ||
Highly efficient extraction of medicinally valuable cannabinoids, namely CBD and THC, from cannabis buds was performed with scCO2 without the aid of a co-solvent (Table 3, entry 5).138 The impact of critical input parameters, i.e., CO2 flow rate, time and pressure, with a fixed temperature of 60 °C, on the obtained yields was established; extraction of CBD is clearly favored with increasing flow rate, while both higher flow rate and longer time positively impact THC yield. Interestingly, the ratio of the output CBD to THC can be tuned by adjusting the flow rate; however, increasing the ratio to recover higher amounts of the desirable CBD is detrimental to the overall extraction yield. Complete cannabinoid recoveries and overall extraction yield were obtained at increased flow rates, high pressure (320 bar) and long extraction time (60 °C, 320 bar, 150 g min−1: 101.1% CBD, 98.6% THC, yield 7.1% vs. 60 °C, 320 bar, 40 g min−1: 78.1% CBD, 73.6% THC, yield 5.6%).138
THC of high purity (90.1%) could be obtained from dried cannabis plant material (inflorescences) with the aid of supercritical fluid extraction (SFE) combined with solid phase extraction (SPE) (Table 3, entry 6).124 Models were constructed to determine the optimum SFE parameters for the enrichment of the extracts in THC. While an increase in pressure, temperature and amount of co-solvent (EtOH) increases the overall extraction yield, at the same time, it decreases the amount of extracted THC, which can be attributed to the variation of the solvent selectivity with the modification of these extraction parameters (40 °C, 150 bar: yield 4.83%, THC 32.25%; 80 °C, 150 bar, yield 6.32%, THC 31.08%; 80 °C, 330 bar: yield 10.41%, THC 24.73%; 80 °C, 330 bar, 5% wt. EtOH: yield 26.36%, THC 15.52%). Addition of ethanol as a co-solvent has proven to be necessary to boost the extraction yields and THC amounts since it has the capacity to extract highly polar compounds; however, it should be kept within a certain range to maximize its extracting ability. Subsequent isolation and recovery of the extracted THC was achieved via SPE, yielding a highly pure product, as NMR and GC-FID analysis demonstrated. For the SPE, the extract was dissolved (0.05% trifluoroacetic acid in water) and injected into an SPE column packed with octadecyl-modified silica gel. A linear solvent gradient (0.05% trifluoracetic acid in acetonitrile) from 0% to 100%, at a constant flow rate of 1.0 mL min−1, was employed.124
The particle size of ground marijuana plants has a noticeable effect on the extracted amount of THC via scCO2 (Table 3, entry 7).150 Various particle sizes in the range of 0.800 mm < d < 0.063 mm (where d is the particle diameter) were evaluated. Even though complete removal of THC was observed in all samples, the highest amount of THC was recovered from the fractions with particle sizes of 0.063 mm < d < 0.125 mm, since the THC concentration varies with particle size (Fig. 9). Microscopic observation elucidated the observed differences; smaller particle sizes contain resinous parts rich in THC, whereas bigger particle sizes mainly consist of leaves and plant parts poor in THC.150
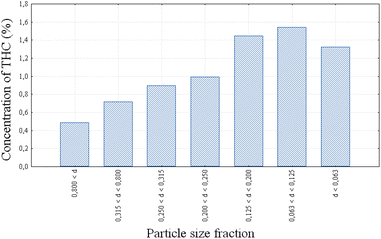 | ||
| Fig. 9 THC concentration of different sieve fractions of marijuana.150 Reprinted with permission. | ||
The effect of decarboxylation prior to scCO2 extraction and consequent winterization on the enrichment of cannabinoids extracted from cannabis flowers via scCO2, has been reported (Table 3, entries 8 and 9).119 Decarboxylation serves the conversion of the acids of THC and CBD present in the flowers to their respective neutral compounds and is simply performed by exposing the flowers to increased temperature for a set amount of time. Winterization involves the suspension of the scCO2 extracts to an organic solvent for the removal, via decanting, of the extracted flower waxes. Two varieties of cannabis with different cannabinoid composition were evaluated. Highest extraction yields were obtained with conventional organic solvent-based extraction (girl scout cookie: 37%, durga mata II: 31%), followed by scCO2 extraction with the aid of a co-solvent (girl scout cookie: 32%, durga mata II: 26%) and, lastly, extraction with pure scCO2 on samples decarboxylated prior to the extraction (girl scout cookie: 18%, durga mata II: 17%). In any case, scCO2-based extractions exhibit higher selectivity towards the target cannabinoids compared to conventional extraction. Additionally, decarboxylation enriches the extracts in THC and CBD (28% and 34% with decarboxylation vs. 9% and 6% without, respectively, for durga mata II), while winterization only has a negligible impact on the cannabinoid content of the recovered extracts. Concerning the essential oils extracted by scCO2, it is observed that higher pressures favor their recovery.119
Efficient cannabinoid extraction from hemp material (buds, leaves, trimmings) can be obtained with the aid of scCO2 and near-critical propane (Table 3, entries 10–12).117 Although the solubility of cannabinoids follows an upward trend with increasing extraction pressure, the overall yield achieved in the case of raw (non-decarboxylated) material is higher than in the decarboxylated one (approx. 4% difference), which can be probably attributed to (i) the water present in the raw material, which acts as a polar co-solvent that favors the extraction of the more polar sample components and (ii) the presence of volatile compounds in the raw material that are extractable by scCO2. Despite the lower overall yield obtained from decarboxylated samples, the selectivity towards cannabinoids as well as their respective recovery is higher (THC: 23 mg g−1, CBD: 342 mg g−1vs. THC: 5.49 mg g−1, CBD: 24.7 mg g−1) due to the much lower amount of the cannabinoid acidic forms present in the decarboxylated samples. An increase in pressure positively affects the extraction of cannabinoids, while addition of ethanol as co-solvent has a positive impact mainly on the acidic cannabinoids, specifically CBDA and THCA (for non-decarboxylated sample; with EtOH-CBDA: 442 mg g−1, THCA: 24.0 mg g−1vs. without EtOH-CBDA: 278 mg g−1, THCA: 11.4 mg g−1). Propane seems to be more efficient and faster than CO2 in the recovery of the acidic cannabinoids from raw material and the recovered extracts are significantly richer (approx. 20%) in the desirable CBD.117
Tuning of the scCO2 parameters for extraction of volatile compounds (monoterpenes, sesquiterpenes) from inflorescences of Cannabis sativa L. allows the recovery of fractions of different chemical profiles (Table 3, entry 13).120 Additionally, the low temperatures employed, as opposed to conventional hydrodistillation processes, result in an aromatic extract of high quality since thermo labile components are spared degradation, while less energy is consumed in the process. The results are verified by their comparison and overlap with the output values of head space solid-phase microextraction.120
A study performed on six cannabis chemovars demonstrated that the chemical profile of extracts obtained via scCO2 is not representative of the original flower (Table 3, entries 14–19).118 Specifically, the scCO2-derived concentrates are characterized by higher THC and CBD potency than the flower (THC: 75%, CBD: 41% vs. THC: 28%, CBD: 11%), while the observed decrease of monoterpene content and the increase in terpene alcohols and sesquiterpenes fail to reproduce the organoleptic characteristics (flavor, fragrance) of the flower (Fig. 10).118 In this interesting study, the authors failed to point out that differences in terpene and terpenoid concentration between flowers and extracts are to be expected, if we consider that the volatility of these compounds differs in these two cases.
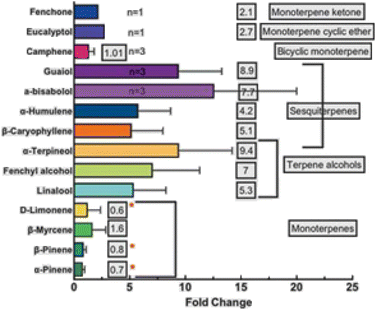 | ||
| Fig. 10 Changes in the extraction efficiency of 14 terpenes/terpenoids across 6 cannabis chemotypes. Bars are the average fold change between flower and concentrate across all samples and error bars are the standard deviations (n is labelled when <6 samples contained this terpene or terpenoid).118 Reprinted with permission, ©Georg Thieme Verlag KG. | ||
Recently, the combination of scCO2 with ionic liquids (ILs) for the extraction of cannabinoids from industrial hemp has been reported (Table 3, entries 20–22).157 The ability of ILs for partial dissolution of the hemp material prior to scCO2 extraction was exploited; a relatively short time (15 min) at 70 °C, is enough to maximize the cannabinoid yield (THC, CBD, CBG) during the IL-based pretreatment step. Subsequent dilution before the scCO2 extraction with H2O is deemed necessary to reduce viscosity and optimize the mass transport, thereby allowing better scCO2 penetration and higher extraction yields. Yields obtained with pure IL verify this hypothesis while results obtained with pretreatment based on pure H2O indicate that ILs have the capacity to preserve the neutral forms of the target cannabinoids. The authors further demonstrated that the extraction yield and the IL cation are inextricably connected with acetate-based ILs exhibiting higher extraction efficiency than phosphate-based ones. Although the scCO2-based approach had only slightly higher yield than the reference solvent-based approach, scCO2 has the major advantage of enabling recovery of the target compounds in pure (non-contaminated with solvents) form, while the ratio of carboxylated to decarboxylated cannabinoids in the extract can be adjusted by simple tuning of the extraction parameters. Additionally, the IL used in the process can be recovered and reused for subsequent extraction cycles. An overview of the process developed by the authors is presented in Fig. 11.157
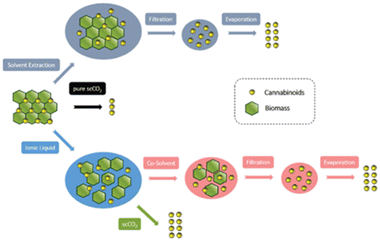 | ||
| Fig. 11 Conceptualization for the comparison of work up steps and yields of cannabinoid extraction techniques.157 Used with permission of the Royal Society of Chemistry, permission conveyed through Copyright Clearance Center, Inc. | ||
| Entry no. | Input sample | Particle size (mm) | scCO2 conditions | Oil characteristics | Ref. no. |
|---|---|---|---|---|---|
| a All concentrations of extracted compounds within the table are reported as %, regardless of the units in which they were originally reported by the authors, in order to facilitate their comparison. b Ultrasonication prior to scCO2 extraction (20 KHz, 10 min). c Reported in g oil per 100 g seeds. d Reported in mM eq. Vit E. e Reported in eq. a-tocopherol per mL oil. f Reported in % of the extracted hempseed oil. g Reported in meq. O2 per kg oil. h Reported in mg of gallic acid eq. per kg of oil. i Reported in mg KOH per mg oil. j Reported in mmol O2 per kg oil. (n. r. = not reported). | |||||
| 23 | Cannabis sativa L., cultivar: Felina, seeds (ground), 300 g | 1.50 | 80 °C, 300 bar, 10 kg h−1, 30 kg CO2 per kg feed | Yield: 16.8 ± 0.7, doxidation stability: 2.18 ± 0.04 | 151 |
| 24 | Cannabis sativa L., cultivar: Felina, seeds (ground), 15 g | 0.71 | 40 °C, 300 bar, 8 × 10−5 kg s−1 | Yield: 21.5, eoxidation stability: 1.87 ± 5.6, fpalmitic acid: 5.85 ± 0.06, stearic acid: 1.45 ± 0.04, oleic acid: 10.67 ± 0.14, linoleic acid: 59.21 ± 0.70, γ-linolenic acid: 3.40 ± 0.09, α-linolenic acid: 18.47 ± 0.63, eicosenoic acid: 0.12 ± 0.06, behenic acid: 0.84 ± 0.01 | 126 |
| 25 | Cannabis sativa L., cultivar: Felina, seeds (ground),b 15 g | 0.83 ± 0.05 | 40 °C, 300 bar, 8 × 10−5 kg s−1, 4 h | Yield: 24.50 ± 0.06, eoxidation stability: 0.80 ± 0.4, fpalmitic acid: 5.70 ± 0.21, stearic acid: 1.91 ± 0.09, oleic acid: 11.10 ± 0.21, linoleic acid: 58.86 ± 0.01, γ-linolenic acid: 3.32 ± 0.02, α-linolenic acid: 18.14 ± 0.15, eicosenoic acid: 0.17 ± 0.04, behenic acid: 0.79 ± 0.01 | 152 |
| 26 | Cannabis sativa L., seeds (dried, ground), 4 g | 0.70 | 80 °C, 400 bar, 3 mL min−1 | Yield: 0.442 | 153 |
| 27 | Cannabis sativa L., genotype: Fedora 17, seeds (ground), 100 g | 0.38 ± 0.15 | 40 °C, 400 bar, 1.94 kg h−1 | Oil content: 33.34 ± 0.23, fpalmitic acid: 6.36 ± 0.05, oleic acid: 12.67 ± 0.03, linoleic acid: 57.04 ± 0.02, γ-linolenic acid: 2.99 ± 0.03, α-linolenic acid: 15.68 ± 0.04, stearic acid: 2.68 ± 0.02, lignoceric acid: 0.19 ± 0.02, behenic acid: 0.44 ± 0.02, arachidic acid: 0.98 ± 0.01, β-citosterol: 85.83 ± 0.54, campesterol: 9.69 ± 0.08, stigmasterol: 4.00 ± 0.06 | 154 |
| 28 | Cannabis sativa L., seeds (ground), 50 g | 0.43 | 76 °C, 300 bar, 13 g min−1, EtOH 8% of scCO2 flow rate, 4 h | n. r. | 155 |
| 29 | Cannabis sativa L., cultivar: USO31, seeds (milled), 18 g | d ≤ 1 | 40 °C, 300 bar, 10 mL min−1, 195 min | Yield: 30.98 ± 1.02, gperoxide value: 5.50 ± 1.0, fpalmitic acid: 6.36 ± 0.05, oleic acid: 12.67 ± 0.03, linoleic acid: 57.04 ± 0.02, γ-linolenic acid: 2.99 ± 0.03, α-linolenic acid: 15.68 ± 0.04, stearic acid: 2.68 ± 0.02, lignoceric acid: 0.19 ± 0.02, behenic acid: 0.44 ± 0.02, arachidic acid: 0.98 ± 0.01, β-citosterol: 85.83 ± 0.54, campesterol: 9.69 ± 0.08, stigmasterol: 4.00 ± 0.06, chlorophyll α: (107.23 ± 2.80) × 10−4, chlorophyll β: (23.29 ± 3.99) × 10−4, carotenoids: (61.00 ± 1.04) × 10−4, α-tocopherol: (39.57 ± 0.72) × 10−4, γ-tocopherol: (770.08 ± 10.75) × 10−4, hpolyphenols: 51.42 ± 0.31, fhexanal: (39.57 ± 0.71) × 10−4, octadienal: (10.29 ± 3.18) × 10−4, heptadienal: (9.38 ± 1.41) × 10−4, nonanal: (8.34 ± 1.28) × 10−4, nonenal: (8.77 ± 1.27) × 10−4, hexanol: (30.66 ± 0.95) × 10−4 | 149 |
| 30 | Cannabis sativa L., dehulled seeds (milled), 4 g | n. r. | 40 °C, 400 bar, 1.15 mL min−1 scCO2, 4 h | Acidity index: 5.21 ± 0.08, fpalmitic acid: 6.14 ± 0.08, oleic acid: 12.64 ± 0.03, linoleic acid: 57.97 ± 0.10, α-linolenic acid: 18.43 ± 0.04, stearic acid: 2.61 ± 0.03, eicosenoic acid: 0.31 ± 0.01, palmitoleic acid: 0.09 ± 0.00, erucic acid: 0.02 ± 0.01 | 131 |
| 31 | Cannabis sativa L., dehulled seeds (milled), 4 g | n. r. | 40 °C, 60 bar, 2.45 mL min−1n-propane, 30 min | acidity index: 4.95 ± 0.01, fpalmitic acid: 6.09 ± 0.01, oleic acid: 13.16 ± 0.05, linoleic acid: 57.11 ± 0.05, α-linolenic acid: 18.53 ± 0.03, stearic acid: 2.83 ± 0.03, eicosenoic acid: 0.35 ± 0.02, palmitoleic acid: 0.11 ± 0.02, erucic acid: 0.21 ± 0.01 | 131 |
| 32 | Cannabis sativa L., pressed-seed cake (ground) | 0.38 ± 0.15 | 45 °C, 250 bar, 1.2 kg h−1, 3.5 h | Oil content: 9.97, jperoxide value: 2.98, fchlorophyll α: 19.35 × 10−3, chlorophyll β: 3.54 × 10−3, carotene: 12.54 × 10−3 | 163 |
| 33 | Cannabis sativa L., genotype: Fedora 17, pressed-seed cake (ground) | n. r. | 60 °C, 300 bar, 2.80 kg h−1, 1.75 h | Yield: 14.97, fchlorophyll α: 27.27 × 10−3, chlorophyll β: 9.79 × 10−3, carotene: 9.18 × 10−3 | 132 |
The quality characteristics of oil extracted from ground hemp seeds with scCO2 under varying conditions of temperature and pressure were investigated by Da Porto et al. (Table 4, entry 23).151 With constant scCO2 flow rate and hemp seed particle size, it was determined that there was no significant difference in the yield of fatty acids extracted with various scCO2 conditions of temperature (40, 60, 80 °C) and pressure (300, 400 bar) and Soxhlet extraction performed with n-hexane. The highest total oil recovery was obtained at two different sets of scCO2 conditions (40 °C, 300 bar: 72.2% and 80 °C, 400 bar: 72.1%); nevertheless, it was still lower than the 100% yield obtained via Soxhlet extraction. On the other hand, the high selectivity of scCO2 towards tocopherols results in a tremendously increased oxidation stability of the oil (2.18 vs. 0.17 mM eq. Vit E) compared to Soxhlet extraction, however, at the expense of total oil recovery.151
After experimentally setting the preliminary scCO2 extraction parameters, the research group focused on determining, via response surface methodology (RSM), the effect of temperature, pressure and particle size on the total oil yield and its respective quality (expressed as oxidation stability) (Table 4, entry 24).126 At a fixed flow rate and temperature of 50 °C, an increase in pressure (250 to 350 bar) significantly impacts the total oil yield in a positive manner, while a decrease in particle size further assists the observed positive impact. An increase in pressure implies increased scCO2 density, thus, higher oil solubility, while smaller particle size, which results from seed grinding, means release of the oil from the broken seed cells as well as increased contact surface between solvent and seed. When the particle size is kept constant, maximum total oil yield is obtained at 40 and 60 °C, provided the pressure is over 300 bar, whereas, at values below 300 bar increasing temperature has a negative impact on the total yield. This behavior clearly implies the dominant effect of increased oil vapor pressure on the extraction at high pressures, while at lower pressures the density drop of scCO2 is the dominant extraction factor. As far as oxidative stability is concerned, pressure and temperature are the factors with the highest influence, when a constant particle size is used; highest stability is favored at low pressures, regardless of the employed temperature, while higher pressures need to be combined with high temperatures to maximize the oxidative stability. When the temperature is kept constant at 50 °C, maximum yield stability is observed at the lowest pressure combined with the largest particle size. At constant pressure of 300 bar, highest temperature applied at the smallest particle sizes maximizes the oxidative stability of the extracted oil. The oil derived by scCO2 is of higher quality than the oil extracted via solvent extraction with Soxhlet (1.87 ± 5.6 vs. 0.84 ± 1.2 Eq α-toc/mL oil), however, the overall oil yield is lower (30% vs. 21.5%). It could be argued, considering the significantly large error margin in the oxidation stability of the oil, that the argument for higher quality of scCO2-extracted oil based on this parameter alone can be easily questioned. However, the major factor which contributes to oil quality derived by scCO2 is its higher selectivity; lack of selectivity in Soxhlet extraction implies removal of pigments and waxes, along with the oil, which are contaminated with solvent residues. In this case, refining of the oil is required which decreases its health value. As far as fatty acids are concerned, there is no significant difference in the obtained yields between scCO2 and Soxhlet approach. Optimized scCO2 conditions that would simultaneously maximize the total oil yield and the oxidative stability of the oil could not be determined.126
The research group further investigated the effect of ultrasonication on the scCO2 extraction capacity of hempseed oil and fatty acids from intact hemp seeds (Table 4, entry 25).152 Ultrasonication of the hemp seeds, in the absence of solvent, for 10 min, exerted a minor positive effect on the extraction yield (3.3% increase), compared to scCO2 extraction without prior ultrasonication, most probably due to the enhanced mass transfer brought about by the augmented penetration rate of scCO2. An increase, however, in the ultrasonication time had a reverse effect on the extraction yield, which can be attributed to ensuing degradation reactions occurring following the temperature increase generated by the effect of sonic cavitation during the ultrasonication process. Differences in fatty acid extraction yields obtained by n-hexane, scCO2 and scCO2 with prior ultrasound pre-treatment were only negligible (30%, 21% and 25%, respectively). Slightly higher primary oxidation of the oil was observed when ultrasonication was employed prior to scCO2 extraction; however, it was still approximately half of the oxidation levels obtained by Soxhlet extraction. Concerning antiradical capacity (expressed in equivalents of tocopherol per mL of oil), the highest levels were obtained via scCO2 due to its high selectivity towards tocopherols, while a significant decrease was observed via ultrasonication and Soxhlet extraction (1.87 vs. 0.80, 0.84).152
The impact of varying the parameters of temperature (40–80 °C) and pressure (200–400 bar) on the scCO2-based recovery of oil from hemp seeds has been evaluated by Tomita et al. (Table 4, entry 26).153 At low pressure, a reverse significant dependency of the yield on the extraction temperature was established (at 200 bar; 40 °C: 39%, 60 °C: 18%, 80 °C: 6%). In contrast, when high pressure was employed the correlation of the extraction efficiency on the employed temperature was negligible (at 400 bar; 40 °C: 39%, 60 °C: 41%, 80 °C: 44%), while the obtained yield was comparable to that achieved by Soxhlet extraction with n-hexane (42%). This is in contrast to the observation that Da Porto et al. made,151 where scCO2 could not reach a total extraction yield comparable to Soxhlet, however, in that case a lower flow rate was employed. As temperature rises, the positive impact of increasing temperature on the overall oil recovery becomes quite notable. In any case, concurrently employing high temperatures and pressures favors the overall extraction yield. It should be noted that even though the nutritionally desirable ratio of ω-6 to ω-3 fatty acids (∼3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) is similar when scCO2, Soxhlet or cold-press extractions are employed, unsaturated fatty acid, linoleic acid and linolenic acid are selectively extracted by scCO2.153
1) is similar when scCO2, Soxhlet or cold-press extractions are employed, unsaturated fatty acid, linoleic acid and linolenic acid are selectively extracted by scCO2.153
The effect of variable scCO2 conditions on the extraction of tocopherols, fatty acids and pigments from hempseed oil has been studied and compared to traditional extraction approaches, namely, Soxhlet and cold pressing (Table 4, entry 27).154 Temperature, pressure and time were varied, while hemp particle size and solvent flow rate were kept constant throughout the experiments. Fatty acid extraction (α- and γ-linolenic, oleic and linoleic acid) showed no dependency on the process temperature, in contrast to tocopherols (α- and γ-) which were reduced to almost half by an increase in temperature by 20 °C (40 to 60 °C). In a similar fashion, no effect of pressure was exhibited on fatty acid extraction efficiency, while an increase in pressure by 100 bar (300 to 400 bar) exerted a dramatically negative impact on tocopherol extraction, which was more pronounced for the α-form (α-form: 110 to 31 mg g−1, γ-form: 182 to 164 mg g−1). These observations regarding the dependency of fatty acid yield on the employed extraction conditions so far agree with what has been previously reported by Tomita et al.153
Pigment extraction can widely vary depending on the scCO2 extraction parameters; however, extraction time appears to have the most marked impact (Table 4, entry 27). With regard to overall hempseed oil yield, it was affected to a negligible degree by a 20 °C increase in temperature and it was favored by higher pressure. In terms of the amount of accessed tocopherols, scCO2 extraction had superior performance to the other approaches, while all 3 approaches performed equally in fatty acid extraction. The lowest total pigment recovery is obtained with scCO2, whereas the mechanical force applied in cold pressing results in the highest pigment recovery.154
A statistical model (central composite design of response surface methodology) was developed and experimentally verified to obtain the optimum scCO2-based extraction of ω-6 linoleic and ω-3 α-linolenic fatty acids from hemp seed oil (Table 4, entry 28).155 The output response of several input parameters, namely temperature, pressure, CO2 flow rate, hemp seed particle size and co-solvent flow rate, was evaluated for the process optimization, while all input parameters were found to be statistically significant. Experimental results demonstrate that, in practice, variations in temperature do not affect the extraction profile of the fatty acids; although mass transfer is favored by an increase in temperature, at the same time, the impact it has on solvent density, i.e., by reducing it, implies reduced solubility of the target compound, therefore, these 2 concurrent phenomena balance out each other's effect on extraction efficiency. Reducing the particle size of the hemp seed only has a positive effect on the extraction efficiency (27% to 34%). Pressure also has a positive effect, with a 150 bar increase (200 bar to 350 bar) leading to an approx. 10% higher overall extraction efficiency (24% to 36%), which can be explained by the pressure-induced increase of the scCO2 density, thus, increased solubility in the solvent. Higher flow rates, both for scCO2 and co-solvent, have a considerable impact on the overall extraction (scCO2: 5 g min−1-22%, 10 g min−1-34% and co-solvent: 0% of CO2: 20%, 10% of CO2: 35%), due to increased solute–solvent interaction and hydrogen bonding formation between –OH groups of the co-solvent and the charged polar groups of the fatty acids, respectively.155
The advantageous properties of CO2, either in super- or sub-critical state, in contrast to conventional liquid extraction of hemp seed oils has been demonstrated (Table 4, entry 29).149 Aside of its environmental compatibility, CO2 has the ability to enhance either the yield or selectivity towards valuable compounds.
Although the recoveries obtained with CO2-based approaches were lower (93% for supercritical, 68% for subcritical) compared with the liquid extraction relying on n-hexane (100%), the oxidation of the recovered hemp seed oil was lower. The effect of fluctuating temperature, air and light in the opened-system conventional extraction is partly accountable for the observed difference. An additional factor, which explains the significantly lower oxidation in the case of subcritical CO2, is the substantially smaller amount of extracted chlorophyll (27 mg kg−1 oil vs. 143 mg kg−1 oil), which acts as a photosensitizer that accelerates the oil oxidation.
Total polyphenol extraction between CO2 and n-hexane does not have a statistically significant difference, however CO2 is marked by higher selectivity towards these compounds. Even though higher tocopherol yields are obtained with the conventional extraction, subsequent refinement leads to substantial content reduction, thus, the yield of the food grade product obtained with all 3 approaches is ultimately comparable.
Non-intoxicating cannabinoids, cannabidiol (CBD) and cannabinol (CBN), are selectively assessed by subcritical CO2 which also enhances the obtained yields by approx. a factor of 2 compared to n-hexane and scCO2-based approaches (subcritical CO2; CBD: 72 mg kg−1 oil, CBN: 114 mg kg−1 oil vs. n-hexane; CBD: 41 mg kg−1, CBN: 47 mg kg−1 and scCO2; CBD: 69 mg kg−1, CBN: 77 mg kg−1, respectively). The extraction method had no significant effect on the yields of fatty acids, triglycerides and phytosterols. Volatile organic compounds, namely aldehydes (hexanal, octadienal, heptadienal, nonanal, nonenal), characterized by pleasant odor are dominantly extracted with CO2.149
The merit of pressurized n-propane as an alternative to scCO2 for the extraction of oil from dehulled hemp seeds has been reported (Table 4, entries 30 and 31).131 Reduction in operational costs can be achieved due to the ability of propane to access the same amount of oil as scCO2 at lower pressures (approx. 37–40% at 42.5 bar instead of 73.8 bar for scCO2) and with significantly lower solvent consumption (flow rates: 1.15 mL min−1 for propane, 2.45 mL min−1 for scCO2). Additionally, it was demonstrated that the use of dehulled hemp seeds favored the extraction yield with the same scCO2 conditions described in the literature. Product of high nutritional value, as this is expressed in ω-3 to ω-6 fatty acid ratio (2.5–5.0), is obtained by employing a propane-based extraction, as well as of lower acidity and humidity values. Furthermore, the total tocopherol yield is higher and, unlike scCO2 where higher temperatures favor tocopherol extraction, independent of the employed extraction temperature, thus, yielding an oil of superior antioxidant value with lower energy consumption.131
Aladić et al.163 demonstrated the residual oil from hemp pressed cake can be fully removed with the aid of scCO2 (Table 4, entry 32). The residual oil obtained with this approach is richer in pigments (chlorophyll α & β, carotene) than the cold press-based extracted oil (chlorophyll α: 194 μg g−1 oil, chlorophyll β: 35 μg g−1 oil & carotene: 125 μg g−1 oil vs. chlorophyll α: 59 μg g−1, chlorophyll β: 39 μg g−1 & carotene: 31 μg g−1, respectively).163
In a subsequent study, the same research group performed additional studies on the recovery of residual oil from hemp pressed cake with the aid of scCO2 (Table 4, entry 33).132 An RSM experimental design was utilized to optimize the extraction parameters; the effect of the variation in pressure, solvent flow rate and temperature on the output responses of extraction yield, pigment content (chlorophyll α & β, carotene) in the recovered oil and extraction time was evaluated. An increase in pressure and solvent flow rate shortens the duration of the extraction process (from 7.5 h to 1.5 h), while temperature does not appear to have any statistically significant effect. The oil solubility is favored at higher pressures, thus, the overall yield is favored at high pressure, whereas concurrent increase in temperature enhances the positive impact. The most decisive factor in the extraction of pigments is pressure; an increase has a dramatically positive effect on the extraction yield, which is statistically independent from the variations in temperature and solvent flow rate.
Extraction via scCO2 was successful in the complete removal of residual oil from pressed hemp cake, which has been in the prior step subjected to cold-pressing for oil recovery. Following the cold-pressing process, the defatted hemp cake is enriched in proteins and fibers suitable for other food-related applications, meaning that a zero-waste process has been developed.132
| Entry no. | Input sample | Particle size (mm) | scCO2 conditions | Extracted compounds | Ref. no. |
|---|---|---|---|---|---|
| a All concentrations of extracted compounds within the table are reported as %, regardless of the units in which they were originally reported by the authors, in order to facilitate their comparison. b n. r. c Reported in μg g−1 of hemp dust. d Reported as % of the total extract. e Reported as % of the input sample. (n. r. = not reported). | |||||
| 34 | Cannabis sativa L., variety: Santhica, hemp dust residues | n. r. | 50 °C, 350 bar, 35 g min−1, 4 h | CBD: 0.12 ± 0.04, cfatty acids: 2000 ± 100, n-policosanols: 790 ± 20, fatty aldehydes: 990 ± 10, n-alkanes: 1250 ± 20, wax esters: 1350 ± 30, sterols: 1600 ± 10 | 160 |
| 35 | Cannabis sativa L., cultivar: Beniko, threshing residues (dried, ground), 10 g | 0.2 | 70 °C, 465 bar, 2–3 standard L min−1, density 0.0018 g mL−1, 2 h | CBD: 2.47 ± 0.03, CBDA: 26.1 ± 0.22 | 162 |
| 36 | Cannabis sativa L., cultivar: Felina 32, threshing residues, 500 g | n. r. | 45 °C, 450 bar, 7 kg h−1 | THC: 5.7, d8-THC: 1.6, THCA: 0.9, CBD: 509, CBDA: 109, CBC: 27.3, CBG: 9.1, CBN: 6.1 | 133 |
| 37 | Cannabis sativa L., cultivar: Kompolti (2014), threshing residues, 500 g | n. r. | 45 °C, 450 bar, 7 kg h−1 | THC: 18.6, d8-THC: 3.6, THCA: 0.5, CBD: 791, CBDA: 118, CBC: 36.4, CBG: 18.2, CBN: 7.9 | 133 |
| 38 | Cannabis sativa L., cultivar: Kompolti (2016), threshing residues, 500 g | n. r. | 45 °C, 350 bar, 7 kg h−1 | THC: (10 ± 4.3) × 10−3, d8-THC: (2.6 ± 0.4) × 10−3, THCA: (46.1 ± 3) × 10−3, CBD: (160.7 ± 71.4) × 10−3, CBDA: (1660.7 ± 53.6) × 10−3, CBC: (17.9 ± 1.8) × 10−3, CBG: not detected, CBN: (1.3 ± 0.3) × 10−3 | 133 |
| 39 | Cannabis sativa L., cultivar: Kompolti, threshing residues (ground), 500 g | 0.44 ± 0.01 | 45 °C, 450 bar, 7 kg h−1, 10% (w/w) EtOH | n. r. | 161 |
The feasibility of recovery and exploitation of valuable compounds from waste dust generated during processing of hemp for fiber extraction was demonstrated, while two extraction approaches relying on Soxhlet and scCO2 were evaluated and compared (Table 5, entry 34).160 The chemical composition of fractions collected from different steps of the fiber extraction process indicates that the machinery used in each step leads to the generation of residues with specific profiles; specifically, fractionation of the lipophilic components (unsaturated fatty acids, long-chain alcohols, fatty aldehydes and long-chain hydrocarbons, wax esters) was observed. It should be noted, that the highly desirable CBD was accumulated in one step of the mechanical process. Although the overall extraction yield of crude wax with scCO2 is favored at high temperatures and pressures (65 °C, 400 bar), the majority of the lipophilic compounds, with the exception of long-chain fatty acids, reaches the highest extraction yields at milder conditions (50 °C, 350 bar). Contrary to conventional extraction approaches, scCO2 offers the possibility of simple separation of CBD from intoxicating and lipophilic compounds, by simple tuning of the temperature; CBD exhibits higher solubility at lower temperatures.160
Quantitative removal (90–99%) of the bioactive components present in Cannabis sativa threshing residues was successfully obtained with a combination of three extraction processes performed in a consecutive order; scCO2, pressurized liquid (PLE) and enzyme-assisted extractions (EAE) (Table 5, entry 35).162 The majority of the lipophilic cannabinoid fraction, which contains the bioactive CBD and CBDA, was recovered by applying the optimized scCO2 parameters, which were generated via an RSM approach. Although all 3 parameters considered during RSM (pressure, temperature and extraction time) are significant in terms of the final yield, an increase in pressure seems to have the most dramatic impact on increasing the extraction of cannabinoids. It was observed, however, that during the extraction process decarboxylation of CBDA most probably took place, as demonstrated by the changing ratios of CBD and CBDA in the extract compared with their ratios in the threshing residue. For the recovery of the polar constituents with antioxidant properties, the residue of the scCO2 extraction was subjected to PLE, which serves as a faster alternative to conventional extraction approaches, at elevated temperature and pressure. A two-step PLE with the aid of acetone and subsequently a mixture of EtOH/H2O results in the removal of the polar phenolic and flavonoid constituents. Additionally, in the final step, hydrophilic mono- and disaccharide fractions (glucuronic, malic, citric and succinic acid) can be accessed with EAE, resulting in a 94% recovery of sugars.162
The feasibility of a pilot plant scale scCO2 set-up for the recovery of cannabinoids from different industrial hemp threshing residues was demonstrated (Table 5, entries 36–38).133 The solvent flow rate and extraction temperature were kept constant, while the effect of pressure and fractionation on the cannabinoid yield were evaluated. Although an increase in pressure generally increases the solubility of solutes in scCO2, thus, favoring the yield, it was concluded, in contrast to other reported studies, that mild pressures are sufficient to maximize the yield and any further increase in pressure does not exert any additional impact (Fig. 12). Fractionation of the extracted compounds was obtained by using two separators set at different pressure levels; the cannabinoids were recovered in the separator set at higher pressure and in the other fraction the fragrant compounds of the hemp residues were collected. Additionally, the dependency of the extracted cannabinoid amount on the quality of the threshing residue was demonstrated as well as the dependency of the nature of the extracted cannabinoids on storage time; freshly collected residues had higher amounts of the acidic form of the cannabinoids compared to older samples, in which cannabinoid decarboxylation naturally occurs over time.133
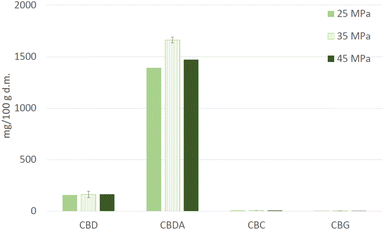 | ||
| Fig. 12 Effect of extraction pressure on the non-intoxicating cannabinoids (d. m. = dry matter).133 Open access, CC BY 3.0, https://creativecommons.org/licenses/by/3.0/. | ||
The research group subsequently employed the pilot plant to investigate the effect of super- and sub-critical CO2 on the recovery of valuable cannabinoids from six different industrial hemp threshing residues (Table 5, entry 39).161 Comparison of the CO2-based extractions with Soxhlet extraction revealed that conventional extraction leads to higher overall yield (1.8–2.3% vs. 21% with Soxhlet employing EtOH); however, higher selectivity towards the target cannabinoids (THC, CBD, CBN, CBG, CBC) can be achieved with CO2 extractions. Low pressure and moderate temperature favor the cannabinoid extraction, while neutral cannabinoids are extracted at the beginning of the process followed by their acidic equivalents. Fractionation of the cannabinoids was also performed by using two separators set at different pressures (Fig. 13). The majority of the cannabinoids was recovered in the separator operating at lower pressure, while their efficient separation from the hemp fragrant compounds was obtained. Addition of a co-solvent in the scCO2 extraction significantly increases both the overall and cannabinoid yield (by 30% each). The subcritical CO2 extraction operates at milder conditions and requires lower solvent consumption resulting in highly concentrated extracts.161
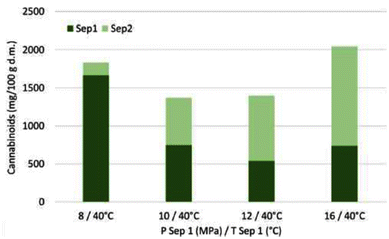 | ||
| Fig. 13 Effect of separator pressure on the yield of cannabinoids in the extract from 1st and 2nd separator by scCO2 extraction.161 Open access, CC BY-NC-ND 4.0, https://creativecommons.org/licenses/by-nc-nd/4.0/. | ||
| Entry no. | Input sample | Particle size (mm) | scCO2 conditions | Extracted compounds | Ref. no. |
|---|---|---|---|---|---|
| a All concentrations of extracted compounds within the table are reported as %, regardless of the units in which they were originally reported by the authors, in order to facilitate their comparison. b Reported as % in dried hemp root. | |||||
| 40 | Cannabis sativa L., cultivar: Futura 75 (dried, milled), Jul. 2019, 0.5 g | 1 | 60 °C, 200 bar, 3 mL min−1, 10% (v/v) EtOH, 2 h (1 h static/1 h dynamic) | Friedelin: 0.0373 ± 0.0012 | 156 |
| 41 | Cannabis sativa L., cultivar: Futura 75 (dried, milled), Aug. 2019, 0.5 g | 1 | 60 °C, 200 bar, 3 mL min−1, 10% (v/v) EtOH, 2 h (1 h static/1 h dynamic) | Friedelin: 0.0434 ± 0.0038 | 156 |
| 42 | Cannabis sativa L., cultivar: Futura 75 (dried at 45 °C for 30 h, milled), Oct. 2019, 0.5 g | 1 | 60 °C, 200 bar, 3 mL min−1, 10% (v/v) EtOH, 2 h (1 h static/1 h dynamic) | Friedelin: 0.0100 ± 0.0005 | 156 |
The impact of a scCO2-based extraction on a largely neglected part of the hemp plant, namely its roots, has been recently reported (Table 6, entries 40–42).156 From a chemical perspective, the profile of the roots differs significantly from the rest of the plant, since it is characterized by an abundance in triterpenes and phytosterols. As the authors demonstrated, both harvesting period and sample drying conditions seem to have an impact on the amount of the accessed triterpenoids, with harvesting after the summer months and increased drying temperatures exerting a negative impact. Comparison of the scCO2 approach with conventional solvent-based extraction proves that conventional extraction has the advantage of higher extraction efficiencies with lower solvent consumption (Fig. 14); for the conventional extraction (n-hexane or EtOH) 8 mL of solvent are required, while for the scCO2 approach, where EtOH is used as a modifier, 180 mL of CO2 are consumed and 18 mL EtOH (3 mL min−1 CO2, 10% EtOH, 60 min). Nevertheless, this is one of the first studies where extraction of triterpenoids, namely epifriedelinol and friedelin, with scCO2 has been reported in the literature. Additionally, the study identified some previously unidentified metabolites, i.e., two triterpenoids, four phytosterols and one aliphatic compound.156
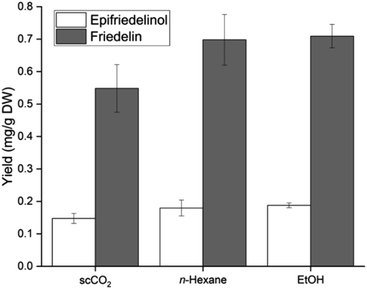 | ||
| Fig. 14 Yield of epifriedelinol and friedelin in mg g−1 dry weight by different extraction approaches.156 Open access, CC BY 4.0, https://creativecommons.org/licenses/by/4.0/. | ||
It is evident thus far that employing scCO2 in the recovery of valuable compounds from various cannabis sources (flowers, seeds, processing residues) is quite promising; apart from having certain ecological advances, it offers the additional advantages of easy product separation from the extraction solvent by simple depressurization, which is a very attractive feature for food and pharmaceutical applications since there is no contamination of the product by solvent residues, increased extraction speed and selectivity, tunability of the extract composition by variation in pressure and temperature conditions, as well as fractionation of the recovered constituents.
Despite the extensive commercialization of scCO2 in the cannabis industry, on a research level it is still at a nascent stage. There is still room to further understand and subsequently exploit the possibilities that scCO2 has to offer; however, the basis that will eventually lead to this desirable direction has already been set.
2.2. Ionic liquids and deep eutectic solvents
Ionic liquids (ILs) are liquids that consist exclusively of ions and have melting points below 100 °C.183 The distinctive and unique properties that characterize ILs, such as negligible vapor pressure, wide solubility range, non-flammability and tunable nature, by simple variation of their building anions and cations, has rendered them suitable for a wide range of extractions and separations. They are considered to be viable candidates for more sustainable extraction approaches, since they can replace the usually toxic and flammable organic solvents, reduce chemical waste and improve the safety of chemical processes and products.183,184Ionic liquids are of particular interest for biomass extraction; the strong hydrogen bonding between the lignocellulose polymers poses a challenge for conventional solvents, while ILs have the ability to break these bonds, thus, allowing higher access to the active target compounds (Fig. 15). Apart from the higher extraction yield for active ingredients that can be obtained by employing ILs, they also significantly shorten the extraction time since they can be combined with microwave or ultrasound irradiation techniques.185 The selection of the IL anion clearly has an impact on the extraction efficiency of the IL with an increase in anion basicity being in line with extraction capacity. There does not seem to be a trend regarding the cation; however, the aromatic-based pyridinium and imidazolium ILs exhibit better extraction capacity than their phosphonium and ammonium counterparts.186
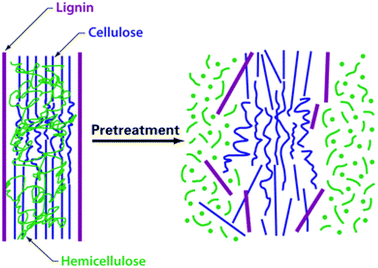 | ||
| Fig. 15 Schematic of pretreatment on lignocellulosic material.187 Reprinted with permission from Elsevier. | ||
Deep eutectic solvents (DESs) are eutectic mixtures formed by the combination of two or more components; specifically, Lewis or Brønsted acids and bases comprising a variety of anionic and/or cationic species. They are considered to be IL analogues,188 and are ideal candidates to replace commonly employed organic solvents due to their low toxicity and low vapor pressure, while, at the same time, they offer the advantage of tunability of their properties by simple variation of the mixing components and/or their respective ratios. Additionally, their comparably low price and biodegradable nature make them both financially desirable and environmentally compatible.189
The extraction conditions with ionic liquids/deep eutectic solvents and the respective results that are discussed in this section are summarized in Table 7.
| Entry no. | Input sample | Particle size (mm) | Conditions | Extracted compounds | Ref. no. |
|---|---|---|---|---|---|
| a All concentrations of extracted compounds within the table are reported as %, regardless of the units in which they were originally reported by the authors, in order to facilitate their comparison. b Reported as % of the input sample. (n. r. = not reported). | |||||
| 43 | Cannabis sativa L., leaves (dried, powdered), 0.2 g | 0.25 | 68% DES (choline chloride/(+)-diethyl-L-tartrate) in water, solid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) liquid 1 liquid 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24, 48 °C, 55 min (with the aid of ultrasonication) 24, 48 °C, 55 min (with the aid of ultrasonication) |
CBD: 1.2 | 158 |
| 44 | Cannabis sativa L., inflorescences (ground), 0.02 g | n. r. | 0.8 mL DES (L-menthol/acetic acid), 30 °C, 10 min (with the aid of ultrasonication) | THC: 6.32 ± 0.72, THCA: 7.92 ± 1.44, CBD: 0.40 ± 0.08, CBDA: 1.68 ± 0.32 | 164 |
| 45 | Cannabis sativa L., leaves (dried, powdered) | 0.25 | Pure IL [C6mim][NTf2], solid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) liquid 1 liquid 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25, 60 °C, 50 min 25, 60 °C, 50 min |
CBD: 0.72 | 159 |
Deep eutectic solvents were investigated for the extraction of CBD from powdered leaves of industrial hemp as a low cost and environmentally friendly approach with possibility for upscaling (Table 7, entry 43).158 The evaluated DESs were based on choline chloride and betaine combined with various hydrogen bond donors. A preliminary screening at set conditions (DES concentration, solid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) liquid ratio, temperature, time) revealed choline chloride/(+)-diethyl L-tartrate as the most efficient extractant for CBD with comparable performance to the less environmentally friendly methanol, which was used as a control sample. The highest polarity (experimentally determined) which is exhibited by this DES, compared to its counterparts, is a key indicator of its superior dissolving capacity towards the polar CBD. Nevertheless, calculations of the hydrogen bond ability of the evaluated DESs indicate that polarity alone is not the decisive factor of the DES's extracting capacity.
liquid ratio, temperature, time) revealed choline chloride/(+)-diethyl L-tartrate as the most efficient extractant for CBD with comparable performance to the less environmentally friendly methanol, which was used as a control sample. The highest polarity (experimentally determined) which is exhibited by this DES, compared to its counterparts, is a key indicator of its superior dissolving capacity towards the polar CBD. Nevertheless, calculations of the hydrogen bond ability of the evaluated DESs indicate that polarity alone is not the decisive factor of the DES's extracting capacity.
The impact of the individual extraction parameters on the process performance were individually evaluated. In order to maximize the hydrogen bond capacity of the DES, while maintaining the viscosity at manageable levels, 70% of DES in water is deemed the optimum option; too much water (exceeding 50%) eliminates the desirable hydrogen bond interaction between the DES and the solute, whereas too little water (less than 30%) complicates the separation due to the high viscosity of the mixture. Similarly, driven by viscosity considerations, the solid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) liquid ratio for the separation was set at 1
liquid ratio for the separation was set at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25. An increase in temperature favors the extraction (no CBD is detected at samples in RT) by enabling the mass transfer and diffusion processes, while it has the beneficial effect of reducing the system viscosity. However, it should be carefully adjusted to avoid the degradation of heat sensitive natural compounds. Prolonging the extraction process benefits the extraction up to a certain time point beyond which no further effect is observed. Mildly acidic pH, in which the extraction system itself results, is necessary to maximize the CBD yield (Fig. 16). An RSM approach, verified that the selected conditions were indeed the optimum to maximize CBD extraction.
25. An increase in temperature favors the extraction (no CBD is detected at samples in RT) by enabling the mass transfer and diffusion processes, while it has the beneficial effect of reducing the system viscosity. However, it should be carefully adjusted to avoid the degradation of heat sensitive natural compounds. Prolonging the extraction process benefits the extraction up to a certain time point beyond which no further effect is observed. Mildly acidic pH, in which the extraction system itself results, is necessary to maximize the CBD yield (Fig. 16). An RSM approach, verified that the selected conditions were indeed the optimum to maximize CBD extraction.
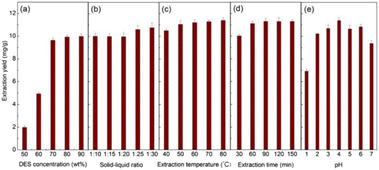 | ||
Fig. 16 Single factor effect on the extraction of CBD using choline chloride/(+)-diethyl L-tartrate; (a) solid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) liquid 1 liquid 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, 40 °C, 60 min, pH not adjusted, (b) 70% DES, 40 °C, 60 min, pH not adjusted, (c) 70% DES, solid 20, 40 °C, 60 min, pH not adjusted, (b) 70% DES, 40 °C, 60 min, pH not adjusted, (c) 70% DES, solid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) liquid 1 liquid 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25, 60 min, pH not adjusted, (d) 70% DES, solid 25, 60 min, pH not adjusted, (d) 70% DES, solid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) liquid 1 liquid 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25, 50 °C, pH not adjusted, (e) 70% DES, solid 25, 50 °C, pH not adjusted, (e) 70% DES, solid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) liquid 1 liquid 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25, 50 °C, 60 min.158 Reprinted with permission from Elsevier. 25, 50 °C, 60 min.158 Reprinted with permission from Elsevier. | ||
Recovery of the CBD from the crude extract is feasible with the aid of macroporous resins. The highest recovery is reported with the microporous absorbent resin DM-130 (81%), resulting in a dried sample of 29% purity.158
An environmentally benign approach relying on hydrophobic DESs was developed for the recovery of cannabinoids (THC, CBD, THCA, CBDA) from ground cannabis inflorescences (Table 7, entry 44).164 Menthol and carboxylic acids were the evaluated hydrogen-bond acceptor and hydrogen-bond donor, respectively. The extraction process was performed near RT (30 °C) with the aid of ultrasonication. The highest yields for all target cannabinoids were obtained with the DES comprising menthol and acetic acid, except for THC for which menthol combined with formic acid generated slightly higher yields. Additionally, replacing menthol with other terpenes, established menthol as the optimum hydrogen-bond donor to maximize extraction yields. Comparison of the developed DES extractant with commonly used organic solvents demonstrated the superior performance, in terms of extraction, of the DES (Fig. 17).164
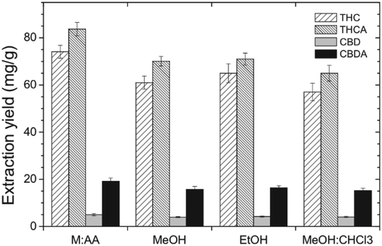 | ||
Fig. 17 Comparison of the extraction yields (mg g−1) of target cannabinoids obtained with different organic solvents and the menthol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetic acid (M acetic acid (M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AA) hydrophobic DES.164 Reprinted with permission from Elsevier. AA) hydrophobic DES.164 Reprinted with permission from Elsevier. | ||
Imidazolium-based ILs were evaluated as alternative solvents for CBD extraction from powdered industrial hemp leaves (Table 7, entry 45).159 Decarboxylation of CBDA provides the desirable CBD. The rate of this conversion in the evaluated imidazolium IL extractants is much higher than in methanol due to the lower polarity of these ILs compared to methanol. The solvation effect provided by ILs lowers the energy barrier required for the conversion reaction. Additionally, ILs enhance the stability of the extracted CBD via formation of hydrogen bonding with the photooxidation site of CBD. Increasing of the IL lipophilicity enhances the extraction of the lipophilic CBD; the IL with the longest cation chain and a hydrophobic anion, [C6mim][NTf2], was the optimum extractant with yields exceeding 80%, under optimized conditions of temperature, time and solid-to-liquid ratio. The majority of the extracted CBD can be recovered by back-extraction with a mixture of organic solvents or an AgNO3 solution (>90% recovered in both cases), thus, enabling the recycling of the IL.159
2.3. Solvent-based extraction
The solvent-based extraction conditions and the respective results that are discussed in this section are summarized in Table 8.| Entry no. | Input sample | Solvent | Conditions | Extracted compounds | Ref. no. |
|---|---|---|---|---|---|
| a All concentrations of extracted compounds within the table are reported as %, regardless of the units in which they were originally reported by the authors, in order to facilitate their comparison. b Reported as % of the total extracted THC + THCA content. c Reported as % of total monoterpene content. d Reported as % of total sesquiterpene content. e Reported as % extracted oil from hemp seed. f Reported as % in olive oil extracts. g Reported as mg of gallic acid equivalent per g dry weight hemp. h Reported as mg of catechin equivalent per g dry weight hemp. i Reported as mg gallic acid equivalent per 100 g extract. | |||||
| 46 | Cannabis, variety: Bedrocan, female flower tops (air dried, ground), 5 g | Naphtha, petroleum ether, ethanol, olive oil –water, olive oil | 98 °C, 20–120 min, 4–20 mL solvent per g cannabis | THC: 5–33, THCA: 67–95, cβ-pinene: 7.5, myrcene: 23.1, β-phellandrene: 8.4, cis-ocimene: 12.1, terpinolene: 43.8, terpineol: 5.2, dβ-caryophyllene: 25.8, humulene: 14.8, δ-guaiene: 14.4, γ-cadinene: 12.4, eudesma-3,7-(11)-diene: 13.1, elemene: 19.5 | 121 |
| 47 | Cannabis sativa L., seeds (ground), 5 g | n-hexane | 20–70 °C, 5–15 min, 3–10 mL solvent per g cannabis | Hempseed oil: 26.6–30.4 | 165 |
| 48 | Cannabis sativa L., seeds (ground), 5 g | n-hexane | 20–70 °C, 10 min, 3–10 mL solvent per g cannabis | Hempseed oil: 25.6–30.0 | 146 |
| 49 | Cannabis sativa L., female inflorescences (ground), 5 g | Olive oil | 35–145 °C, 40–120 min, 10 mL solvent per g cannabis | THC: 0.3–15, THCA: 0.01–15.5 | 122 |
| 50 | Cannabis sativa L., aerial parts of young and mature hemp (ground), 5 g | H2O, EtOH (30–90%) | RT, 24 h, 20 mL solvent per g cannabis | Phenols: 6.2–17.1, hflavonoids: 1.8–11.2 | 127 |
| 51 | Cannabis sativa L., hemp press cake | 200 mM NaCl, pH 12 | RT, 30 min, pH adjusted with NaOH or HCl, 10% (w/w) press cake suspension, RT, 12 h (after pH adjustment) | Total phenolic content: 81 | 172 |
Romano et al.121 evaluated different organic solvents for cannabis oil extraction with the aim of obtaining an extract rich in health beneficial compounds (cannabinoids, terpenes) (Table 8, entry 46). Although sample pre-treatment via heating (oven or water bath) lead to the decarboxylation of the target cannabinoids, at the same time, it significantly reduced the terpene content, thus, sample heating prior to extraction was disregarded. Although all the solvents tested (naptha, petroleum ether, ethanol, olive oil) extracted only small amounts of THC, due to the relatively low extraction temperatures, olive oil exhibited the capacity for maximum terpene extraction.121
The n-hexane extraction of hempseed oil has been optimized by adjusting the parameters of extraction temperature, solvent-to-seed ratio and extraction time (Table 8, entry 47).165 Outcomes of RSM indicate that increasing solvent-to-seed ratio (up to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), at fixed temperature (20 °C), favors the hempseed oil yield regardless of the extraction time, an effect which is more noticeable at high solvent-to-seed ratios. The impact of time is dependent on the solvent-to-seed ratio; although negligible at low ratios, it becomes more pronounced as the ratio increases by increasing the oil yield. At high temperatures (45 °C; 70 °C), the hempseed oil yield increases up to a maximum point (30.4%) beyond which it decreases when increasing both extraction time and solvent-to-seed ratio. At the highest extraction temperature (70 °C), the influence of solvent-to-seed ratio and extraction time are less appreciable since temperature becomes the main influencing factor. Increased temperatures promote the oil solubility and diffusion rate, thus, an increase in the oil yield is observed. Additionally, a multivariate data analysis approach was used to determine the optimum extraction parameters; although the parameters do not differ considerably from the RSM ones, the multivariate data analysis approach was found to be a more accurate prediction model than RSM, since the relative standard deviation between theoretical and experimentally obtained values was smaller.165
1), at fixed temperature (20 °C), favors the hempseed oil yield regardless of the extraction time, an effect which is more noticeable at high solvent-to-seed ratios. The impact of time is dependent on the solvent-to-seed ratio; although negligible at low ratios, it becomes more pronounced as the ratio increases by increasing the oil yield. At high temperatures (45 °C; 70 °C), the hempseed oil yield increases up to a maximum point (30.4%) beyond which it decreases when increasing both extraction time and solvent-to-seed ratio. At the highest extraction temperature (70 °C), the influence of solvent-to-seed ratio and extraction time are less appreciable since temperature becomes the main influencing factor. Increased temperatures promote the oil solubility and diffusion rate, thus, an increase in the oil yield is observed. Additionally, a multivariate data analysis approach was used to determine the optimum extraction parameters; although the parameters do not differ considerably from the RSM ones, the multivariate data analysis approach was found to be a more accurate prediction model than RSM, since the relative standard deviation between theoretical and experimentally obtained values was smaller.165
Kostić et al.146 published an investigation on the kinetic and thermodynamic behavior of hempseed oil extraction via n-hexane (Table 8, entry 48). Elevated temperatures (45 °C; 70 °C) favor the oil yield, due to its increased solubility, while, from a kinetic perspective, the yield initially increases dramatically and progressively decelerates. The calculated temperature extraction coefficient indicates an increase factor of approx. 1 for every increase of 10 °C. Additionally, lower seed-to-solvent ratios (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 6.5
1; 6.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) promote oil extraction, since increasing amounts of solvent improve its dissolution capacity. Thermodynamic data support the endothermic nature of the reaction.146
1) promote oil extraction, since increasing amounts of solvent improve its dissolution capacity. Thermodynamic data support the endothermic nature of the reaction.146
The growing demand for oil extracts of cannabis for medicinal purposes prompted Casiraghi et al. (Table 8, entry 49).122 to investigate the extraction of valuable constituents with the aid of olive oil. Prior to extraction, the impact of varying temperatures (70–110 °C) on cannabinoid decarboxylation was evaluated. A standardized medicinal sample comprising cannabis inflorescences was used for the experiments. Pre-heating promotes the conversion of THCA to THC, thus, the corresponding oil extracts are rich in THC, which is the pharmacologically valuable constituent. In contrast, no heat pre-treatment of the sample allows the recovery of oil-based extracts with higher THCA content than the other approaches. Careful selection of pre-treatment temperature can afford high quality and quantity THC extracts. The extraction of THCA along with THC observed at temperatures up to 100 °C can be almost completely eliminated by further increase of the temperature. Combination of high temperature (130 °C) and prolonged heating time (40 min) elevates the THC amounts (up to 15%); however, prolonged time leads to formation of degradation products. Therefore, a compromise in the combination of temperature and time can lead to the recovery of THC-rich extracts of high quality.
Stability experiments indicated that the oil preparation is stable for at least 3 weeks, if stored in the fridge.122
The potential for recovery of valuable compounds from aerial parts of young and mature hemp via solvent extraction was investigated by Drinić et al. (Table 8, entry 50).127 The study focused on the extraction yield, total phenolic (TP) content, total flavonoid (TF) content, antioxidant activity, and reductive capacity (Fig. 18). Solvent polarity has a positive impact on the overall extraction efficiency; therefore, water was more effective than aqueous ethanolic solutions of variable concentrations (30–90%), since it can solubilize more efficiently the target compounds. The obtained yields (yield ranges) of young and mature hemp were comparable. Nevertheless, TP and TF values for young hemp were twice those of the mature one. Additionally, an aqueous ethanolic solution (50%) afforded the highest TP (17.1 mg g−1) and TF (11.2 mg g−1) contents, as well as the highest antioxidant capacity.127
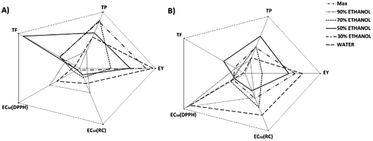 | ||
| Fig. 18 Radar chart for all investigated parameters in obtained extracts of aerial parts of (A) young hemp and (B) mature hemp; EY – extraction yield, TP – total phenols content, TF – total flavonoids content, EC50 (DPPH) – effective concentration obtained by DPPH assay and EC50 (RC) effective concentration obtained by reducing power assay.127 Open access, CC BY 4.0, https://creativecommons.org/licenses/by/4.0/. | ||
The effect of varying a number of parameters on the extraction efficiency of proteins from undelipidated hemp press-cakes was evaluated (Table 8, entry 51).172 Hemp proteins are recovered as aqueous extracts by simple suspension of the cake in distilled water and concurrent stirring, followed by continuous pH adjustment (to the desired level) and centrifugation. A decisive factor in the protein extraction efficiency is the pH level; a significant increase in the yield is observed as the pH exceeds 9 (8.4% at pH 8, 67.1% at pH 12), which is in line with the previously reported increase in the yield of the major hemp protein fraction, the globulins, at alkaline pH values.83,190 In contrast, the minor hemp protein fraction, the albumins, are preferentially extracted at pH below 6. Concerning the appearance of the extractants, moving towards alkaline conditions leads to increasing brown coloration and turbidity, which can be attributed to progressive solubilization and/or modification of phenolic compounds. In principle, the modification of the ionic strength of the solution should impact the protein extraction yield (either by salting-in effect at low concentrations or salting-out effect at high concentrations), however, this effect is very much dependent on the pH and the surface properties of the proteins. Adjustment of the ionic strength by NaCl addition at the studied pH range of 9–12 did not have any significant impact on the protein extraction yield, while low ratios of press-cake to water (5–10 w/w %) are favorable. Even though alkaline pH maximizes the protein extraction efficiency, at the same time, this happens to the detriment of the nutritional and organoleptic characteristics of the extracts due to the oxidation of their phenolic compounds at these pH levels.172
2.4. Ultrasonication-assisted extraction
Ultrasound is regarded as mechanical waves of higher frequency than the human-audible frequency (>20 kHz). It is used to enhance the extraction of compounds of interest from plant matter. Ultrasonication-assisted extraction (UAE) consumes small amount of energy and it has a low investment cost; however, large solvent volumes might be required, a filtration step is necessary and it might deteriorate target compounds. The ultrasonic waves spread due to rarefaction and compression and as a result negative pressure is generated. Consequently, when the tensile strength of the solvent is smaller than the generated pressure, vapor bubbles are created that implode (cavitation), generating macroturbulences and perturbation. This cavitation near to the liquid–solid interface leads a fast-moving stream of liquid through the cavity at the surface. The impact of these microjets on the surface, generate erosion, particle breakdown and surface peeling, promoting the liberation of compounds of interest from the plant matter, hence, higher extraction efficiencies are obtained owing to the augmentation of mass transfer. Therefore, cell disruption and effective mass transfer are cited as major factors enhancing extraction with ultrasonic power. Ultrasound permits changes in processing conditions, as compared to other techniques lower pressures and temperatures are employed, allowing the extraction of thermolabile compounds.191Regarding the most important parameters of ultrasound, frequency, wavelength, amplitude, power and the shape of the reactor play an important role in the extraction efficiency.192
The ultrasonication-assisted extraction conditions and the respective results that are discussed in this section are summarized in Table 9.
| Entry no. | Input sample | Solvent | Conditions | Extracted compounds | Ref. no. |
|---|---|---|---|---|---|
| a All concentrations of extracted compounds within the table are reported as %, regardless of the units in which they were originally reported by the authors, in order to facilitate their comparison. b Reported as percentage of the amount extracted by the Soxhlet method. c Reported as % extracted oil from hemp seed. d Reported in mg of gallic acid equivalent per 100 g fresh hemp. e Reported in mg of luteolin equivalent per 100 g fresh hemp. f Reported as mg of catechin equivalents per g dry matter, extracted compounds: N-trans-caffeoyltryamine, cannabisin B (or isomers) and 2 unknown compounds. g Reported in mg of gallic acid equivalent per g of dry weight. h Reported in quercetin equivalents per g of dry weight. i Reported in ascorbic acid equivalents per g of dry weight. j Reported as % of plant dry weight. | |||||
| 52 | Cannabis sativa L., seeds (ground), 5 g | n-Hexane | 25 °C, 100–300 W, 15–40 min, 1–15 mL solvent per g cannabis | Hempseed oil: 21.4–26.4 | 130 |
| 53 | Cannabis sativa L., seeds (ground), 10 g | n-Hexane | 25 °C, 20–100 W, 10–30 min | Hempseed oil: 31.9–36.1 | 78 |
| 54 | Cannabis sativa L., seed cakes (ground), 5 g | Methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetone acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (7 water (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 v/v/v) 6 v/v/v) |
20–70 °C, 200 W, 20–40 min, 8–20 mL solvent per g cannabis | Flavonoids: 5.6–38.3, ephenols: 475.4–2563.5 | 128 |
| 55 | Cannabis sativa L., seed cake (ground), 5 g | Methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetone acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (7 water (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 v/v/v) 6 v/v/v) |
70 °C, 200 W, 20 min | Flavonoids: 6.4–19.2, ephenols: 467.5–1328.9 | 129 |
| 56 | Cannabis sativa L., cold-pressed seed cake (ground), 1 g | Methanol (70%), acetone (80%), methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetone (1 acetone (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) 1 v/v) |
1 min, 3 × 9 mL solvent per g cannabis | 4 main phenols: 2.2–2.8 | 135 |
| 57 | Cannabis sativa L., flowers, leaves and husks (dried) | 80% methanol | 130 W, 15 min | Total phenolic content: 312.452, htotal flavonoids: 28.173, iferric reducing antioxidant power: 18.79, jyield: 11 | 166 |
Lin et al.130 first reported the effect of UAE on hempseed oil yield (Table 9, entry 52). The influence of the extraction time, the solid-to-solvent ratio, the acting on–off ratio and the ultrasonic power were evaluated using single-factor experiments (Fig. 19).
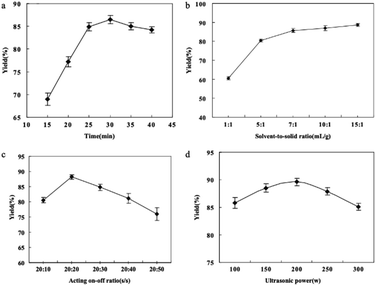 | ||
| Fig. 19 Relationship between yield and various parameters: (a) extraction time, (b) solvent-to-solid ratio, (c) acting on-off ratio and (d) ultrasonic power.130 Used with permission, John Wiley and Sons, ©2011 Wiley Periodicals, Inc. | ||
Extraction duration of 30 min was sufficient to obtain the maximum yield (86.5%), whereas any further increase in time is detrimental to the yield, considering that longer extraction times may promote the oxidation of unsaturated fatty acids (UFAs) and their decomposition. An increase of solvent-to-solid ratio initially leads to a significant increase in the extraction yield, but eventually the increase gets progressively less significant as the ratio further increases. With regard to the effect of acting on–off, a ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 s s−1 afforded the highest yield (88.20%). Either increase or decrease of the acting off ratio generates lower yields; an increase implies inadequate use of ultrasonic waves, while a decrease leads to degradation of UFAs as a result of hot spots generated by the cavitation bubbles. The positive influence of ultrasonic power reaches a maximum value at 200 W and any further increase leads to progressive decrease of the yield. The optimum experimental conditions that maximize the obtained yield were determined using a three-variable, four-level orthogonal design (L9(34)).193 In terms of yield, the UAE outperformed the solvent-based extraction, however, no significant difference in the quality of the obtained oil was observed, which implies that the UAE has no influence on the fatty-acid content. Additionally, the antioxidant activity of the UAE-recovered oil was much higher.130
20 s s−1 afforded the highest yield (88.20%). Either increase or decrease of the acting off ratio generates lower yields; an increase implies inadequate use of ultrasonic waves, while a decrease leads to degradation of UFAs as a result of hot spots generated by the cavitation bubbles. The positive influence of ultrasonic power reaches a maximum value at 200 W and any further increase leads to progressive decrease of the yield. The optimum experimental conditions that maximize the obtained yield were determined using a three-variable, four-level orthogonal design (L9(34)).193 In terms of yield, the UAE outperformed the solvent-based extraction, however, no significant difference in the quality of the obtained oil was observed, which implies that the UAE has no influence on the fatty-acid content. Additionally, the antioxidant activity of the UAE-recovered oil was much higher.130
The impact of UAE on the recovered hempseed oil quality and its antioxidant activity has also been reported, focusing on the extraction of PUFAs and tocopherols, which are representative of the oil quality and its antioxidant activity, respectively, with the aid of n-hexane (Table 9, entry 53).78 The effect of different variables, namely, extraction time (10–30 min) and ultrasound power level (20–100 W), was evaluated, while the parameters of duty cycle (percentage of the total process time during which the ultrasound signal is “on”) and temperature were maintained constant. Increasing the ultrasound power and time favors the oil extraction efficiency, due to the affinity of hempseed oil for hexane and the advantageous effect of cavitation on its diffusion. Nevertheless, the combined effect of high ultrasound power, high temperature and long extraction times, lead to a reduction in the oxidation stability of the oil, since, under these conditions, thermal decomposition of long chain fatty acids occurs. With respect to antioxidant scavenging activity, longer extraction times and high ultrasound power, lead to higher quantities of tocopherol and phenolic compounds. By rising the ultrasound power from 20 W to 100 W the extraction of antioxidants was incremented. A compromise between ultrasound power and time needs to be found, so that energy is reserved and oil quality is maintained; this objective is achieved at the optimized conditions of 91 W and 10 min. Although Soxhlet-based extraction with n-hexane slightly favors the extraction yield (approx. by 4%), higher quality oil, characterized by stability and antioxidant activity (higher tocopherol content), can be derived via UAE.78
Ultrasonic assisted extraction (UAE) was applied for the extraction of TP and TF compounds from defatted hemp seed cake and other plant sources (Table 9, entry 54).128 The impact of solvent volume, ultrasonication time (20–40 min) and temperature (20–70 °C) was evaluated. The TP and TF yields obtained from seed cakes with ultrasonication are dramatically higher than the respective conventional extraction-based yields. Additionally, UAE is more favorable than conventional solvent-based extraction in terms of antioxidant capacity of the recovered oil. Interestingly, the double amount of solvent (100 mL) than the one used for maximum TP extraction (50 mL) is required in order to obtain the maximum TF value. The ideal UAE time proved to be 20 min; exceeding this time leads to reduced recovery of polyphenols and lowers the antioxidant activity of the oil, which could be attributed to the degradation of polyphenols occurring at longer ultrasonication times. Increased polyphenol yield is in line with rising temperature; highest TP and antioxidant activity are achieved at 70 °C, while lower temperatures are ideal to acquire the maximum TF yield.128
The effect of different pre-treatment approaches, namely, microwave (MW) and pulsed electric field (PEF), on the ultrasonic-based extraction of polyphenolic compounds from defatted hemp seed cake has been reported (Table 9, entry 55).129 The optimization of the MW and PEF processes was performed with the aid of RSM. In the MW pre-treatment, the influence of MW power (440–1100 W), time (1–5 min) and liquid-to-solid ratio (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–6
1–6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) on the total phenolic and total flavonoid extraction was investigated. An increase of the liquid-to-solid ratio and the processing time favors the extraction yield for both phenolics and flavonoids, whereas the positive impact of increased MW power reaches a maximum value beyond which it has a detrimental effect on the yields, possibly due to the thermal degradation of the target compounds. In the PEF pre-treatment, the influence of frequency, voltage, ethanol concentration and electroporation time on the extraction yields was investigated. Short processing times and low voltage values maximize the extraction of total flavonoids, while additional voltage increase further promotes the extraction of total phenolics. The low frequency and the use of ethanol as a solvent are advantageous for the yield, however, voltage is the factor that exerts the most decisive influence. The positive impact of ethanol can be attributed to its polarity, which (i) enhances cell permeability, thus, accelerating the extraction of polyphenols and (ii) implies increased conductivity of the solvent, thereby accelerating heat transfer to the cells.129
1) on the total phenolic and total flavonoid extraction was investigated. An increase of the liquid-to-solid ratio and the processing time favors the extraction yield for both phenolics and flavonoids, whereas the positive impact of increased MW power reaches a maximum value beyond which it has a detrimental effect on the yields, possibly due to the thermal degradation of the target compounds. In the PEF pre-treatment, the influence of frequency, voltage, ethanol concentration and electroporation time on the extraction yields was investigated. Short processing times and low voltage values maximize the extraction of total flavonoids, while additional voltage increase further promotes the extraction of total phenolics. The low frequency and the use of ethanol as a solvent are advantageous for the yield, however, voltage is the factor that exerts the most decisive influence. The positive impact of ethanol can be attributed to its polarity, which (i) enhances cell permeability, thus, accelerating the extraction of polyphenols and (ii) implies increased conductivity of the solvent, thereby accelerating heat transfer to the cells.129
Liang et al.135 reported a study on the UAE of phenolic compounds from cold-pressed hemp seed cake, particularly focusing on the effect of solvent, preheating temperature, and ultrasonication time (Table 9, entry 56). The developed process was divided into two steps; preheating treatment followed by UAE. Comparison of preheated hemp seed cakes with an unheated control sample demonstrated the positive effect of preheating on the TP yield in all the aqueous organic solvents that were evaluated. Regardless of the employed preheating conditions (temperature and exposure time), the highest TP (4.29 mg of gallic acid equivalent per g of dry matter) yield was obtained with an aqueous (80%) solution of acetone, which was higher by 30% and 40% compared to aqueous methanol and the mix of the 2 solvents, respectively. While the TP yield is significantly affected by the employed solvent and the preheating temperature, the preheating duration only has a negligible effect.135
Ultrasonication-based extraction for the recovery of bioactive compounds from cannabis inflorescence proved to be an approach of considerable merit, compared to conventional extraction, in terms of associated time, energy and costs (Table 9, entry 57).166 Response surface methodology was used to evaluate the effect of the input extraction parameters, namely, time, input power and methanol concentration, on the output responses, i.e., TP, TF, ferric reducing ability of plasma (FRAP) and total yield. The optimum parameters were experimentally verified and the respective outputs were compared to a control extraction process, which relied on mixing of the sample with 50% MeOH while elevated temperature and constant stirring were applied.
Concerning TP extraction, the individual and combined effects of time and solvent composition have a pronounced positive effect; long sonication times favor the penetration of the solvent in the material, while higher content of methanol allows an increase in the solvent permeability. On the other hand, sonication power, either alone or in combination with solvent composition, has a negligible effect on TP extraction.
The most pronounced effect on TF extraction is brought about the solvent; in contrast to THC extraction, lower methanol content favors TF extraction. This observation can be attributed to the dual effect of water on the extraction; (i) expansion of the matrix resulting in an increase in the contact area of solvent and matrix and (ii) generation of hydroxyl radicals that further assist the extraction. Power and time, either separate or combined, exert a moderately positive effect on the TF extraction.
The ferric reducing ability of plasma seemed to be independent of the input experimental parameters. The overall yield is favored by lowering the methanol content due to the higher diffusivity of water as opposed to methanol, while it increases as a function of time. High power, however, can lead to degradation of heat sensitive compounds, thus, resulting in lower overall extraction, while its combined effect with either solvent or time is dependent on the nature of the compounds present in the sample.
Overall, the optimized output value of all evaluated responses was significantly higher than that of the control extraction (Fig. 20), thus, proving the undeniable value of the ultrasonication approach.166
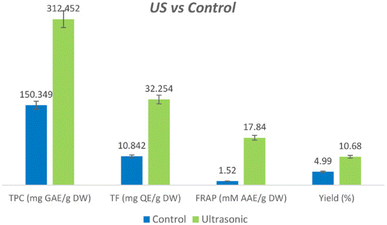 | ||
| Fig. 20 Extraction yields obtained through ultrasonication (green) and control (blue) extractions (TPC = total phenolic content, GAE = gallic acid equivalents, DW = dry weight, TF = total flavonoids, QE = quercetin equivalents, FRAP = ferric reducing antioxidant power, AAE = ascorbic acid equivalents). The error bars indicate percentage error.166 Used with permission, John Wiley and Sons, ©2018 Institute of Food Technologists®. | ||
2.5. Microwave-assisted extraction
Microwave-assisted extraction (MAE) is related to the generation of a non-ionizing electromagnetic radiation with a frequency of 300 MHz to 300 GHz. The microwave effect relies on the conversion of electromagnetic energy into heat by the direct influence of dipole polarization and ionic conduction. The molecules that possess a dipole moment align with the electric field, generating molecular friction, collisions and the release of heat energy into the medium, causing fast dielectric heating. The ionic conduction is also an important interaction that takes place. The ionic conduction generates higher temperatures and faster heating in solutions consisting of ions than the ones without. The influence of the electromagnetic waves can be determined by the dielectric properties of the solvent. The spreading of the heat all over the sample/matrix is promoted by solvents that have high dielectric constant and high dissipation factor, leading to higher extraction yields, however, non-polar solvents remain unaffected by the microwaves.194Microwave-assisted extraction is characterized by shorter extraction time, higher extraction yield, higher selectivity and better quality of the extracts. On the negative side, chemical reactions could be enhanced and, depending on the extraction conditions, chemical structures could be modified, negatively affecting the yield. In addition, this technique is ineffective when non-polar compounds are the target and when non-polar solvents are employed. Moreover, it is not suitable for thermally unstable compounds. Regarding this technique, several factors play a main role, such as power and frequency of microwave, duration of the microwave irradiation, extraction temperature and pressure, moisture content and particle size of the plant material, the nature and concentration of solvent and ratio of solid to liquid.195
The microwave-assisted extraction conditions and the respective results that are discussed in this section are summarized in Table 10.
| Entry no. | Input sample | Solvent | Conditions | Extracted compounds | Ref. No |
|---|---|---|---|---|---|
| a All concentrations of extracted compounds within the table are reported as %, regardless of the units in which they were originally reported by the authors, in order to facilitate their comparison. b Reported as % in hemp nut. c Reported as % in dried inflorescences. d Reported as % in the hemp essential oil. e Reported in mg gallic acid equivalent/mL extract. f Reported in mg catechin equivalent/mL extract. g Reported in mg mL−1 extract. h Reported as % in hemp seeds. | |||||
| 58 | Cannabis sativa L., nuts, 1 g | Methanol, ethanol, acetonitrile, isopropanol, ethyl acetate | 40–160 °C, 100–1300 W, 5–35 min, 12 mL solvent per g cannabis | THC: (1.4–2.7) × 10−4, CBD: (1.5–2.6) × 10−4, CBN: (0.8–1) × 10−4 | 143 |
| 59 | Cannabis sativa L., inflorescences (crushed), 500 g | Distilled water | 400–600 W, 46–100 min | Essential oil: 0.15, dCBD: 9.3 | 134 |
| 60 | Cannabis sativa L., cultivar: helena, leaves, blossoms, inflorescences and bracts (ground) | Ethanol | 10–30 min, 0.07–0.2 mL, solvent per g cannabis | Phenols: 0.9–2.7, fflavonoids: 0.5–1.4, gTHC: 0.03–0.06, gCBD: 0.2–1.8 | 168 |
| 61 | Cannabis sativa L., seeds (ground), 15 g | n-Hexane | 300–600 W, 5–15 min, 10 mL solvent per g cannabis | Hempseed oil: 25.7–36.0 | 167 |
A MAE process for the efficient recovery of cannabinoids from hemp nuts was developed by Chang et al. (Table 10, entry 58).143 and subsequently compared to other available extraction methods, namely, heat reflux extraction (HRE), Soxhlet extraction (SE), SFE and UAE. An RSM was employed in order to obtain the optimum MAE conditions, using as input parameters the solvent (methanol, ethanol, acetonitrile, isopropanol, ethyl acetate), the microwave power, the temperature and the extraction time (Fig. 21).
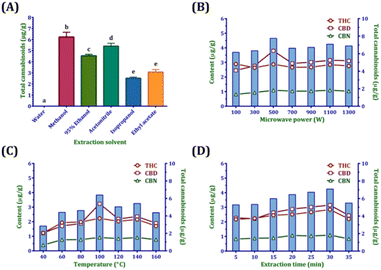 | ||
| Fig. 21 Effect of (A) extraction solvent, (B) microwave power, (C) temperature and (D) extraction time on the yields of cannabinoids. Different letters (a, b, c, d, e) indicate a significant difference (one-way ANOVA, p < 0.05).143 Open access, CC BY 4.0, https://creativecommons.org/licenses/by/4.0/. | ||
Of all the organic solvents tested, methanol afforded the highest yield. In contrast, water performed quite poorly due to its low solvating power towards cannabinoids and its large dielectric constant which leads to sample damage after exposure to high-temperature. Therefore, methanol was selected for further RSM optimization of the MAE process.
An increase in temperature favors the overall yield, however, temperatures beyond 100 °C are not considered since they lead to the degradation of cannabinoids. Specifically, THCA and CBDA progressively decarboxylate into THC and CBD, while THC can further convert to CBN, thus, affecting the yields of the carboxylated analogues by increasing the content of THC, CBD and CBN. Additionally, terpenes are sensitive to high temperatures.
Microwave power exerts a minor effect on the cannabinoid yield. While prolonging the extraction time (10–30 min) has a positive impact on the extraction yield, exceeding the 30 min mark seems to negatively affect it.
The optimized RSM output parameters were subsequently experimentally verified and used in the ensuing comparative study. Microwave-assisted extraction demonstrated superior performance to the other methods in terms of obtained yield (up to 0.00027% THC), extraction time, solvent consumption, and simplicity of operation.143
An RSM-based approach for the optimization of the MAE of valuable compounds from cannabis inflorescences was evaluated (Table 10, entry 59).134 The MAE approach was compared with hydrodistillation (HD), in terms of quality of the obtained essential oil. Concerning the MW experiments, microwave irradiation power (400–600 W), extraction time (46–100 min) and water added after moistening were evaluated. The RSM output values indicated that higher microwave power and longer extraction times augmented the essential oil yield and the CBD content. However, an increase in water content, decreased both. These theoretical predictions were experimentally verified. Both MAE and HD extracted similar amounts of oil, which were also similar with respect to their chemical make-up. Higher amounts of CBD were obtained with MAE (9%), while longer extraction time (almost double) was necessary in HD.
Overall, MAE is advantageous over HD due to its higher selectivity, shorter extraction time and reduced consumption of water and energy.134
A microwave-assisted process was reported as a simple, fast and environmentally benign approach for the extraction of polyphenols and cannabinoids from ground Cannabis sativa L. (leaves, blossoms, inflorescence, bracts) (Table 10, entry 60).168 Evaluation of the influence of different extraction parameters, namely ethanol concentration, extraction time and solid-to-liquid ratio, on several output variables (extraction yield, total phenol and flavonoid content, antioxidant activity IC50, reductive capacity EC50, CBD and THC content) was based on a statistical model. The constructed response surfaces provide a comprehensive overview of the effect that variations in the combined extraction parameters exert on the individual output variables.
An increase in ethanol concentration has a negative impact on total yield and total phenol content, it positively influences total flavonoid content, while it maximizes CBD extraction up to a certain point (1.8 mg mL−1) beyond which it negatively impacts it. Overall, increased solid-to-liquid ratios favor most parameters, whereas total yield and CBD are positively influenced after a certain point.168
Rezvankhah et al.167 published a study on the recovery of hempseed oil via MAE (Table 10, entry 61). The fatty acid composition, antioxidant activity, as well as the physiochemical and thermal properties of the oil extracted from Cannabis sativa were evaluated. An RSM approach was employed for the optimization of the MAE extraction parameters. The MAE study focused on the quantity and quality of the extracted oil; higher MW power (450–600 W) and higher extraction time (15 min) promotes the oil yield (up to 36%). However, time seems to have a more significant impact than MW power; increasing time beyond a certain point, regardless of the employed MW power, can lead to thermal decomposition of the oil. Higher oil yield characterized by lower oxidation stability is obtained with SE; thus, MAE affords oil of higher quality.
Similar amounts of PUFAs and MUFAs were obtained, regardless of the employed extraction method, however, MAE is the more efficient approach since shorter extraction times are required. Additionally, MAE accesses a higher amount of total tocopherols (930 vs. 833 mg kg−1).167
2.6. Pressurized-liquid extraction
Pressurized-liquid extraction (PLE) or accelerated-solvent extraction is based on the use of high temperature (50–200 °C) and pressure (35–200 bar) for the extraction of targeted chemicals in solid or semi-solid samples. On the one hand, high pressure positively influences mass transfer by promoting cell permeability. On the other hand, high temperatures enhance diffusion of the solvent into the sample, as the viscosity of the solvent is reduced. Moreover, it also increments the extraction rate by augmenting the solubility of the targeted compound and mass transfer. This technique is simple, provides a safe and fast extraction, has low solvent consumption, shorter extraction time, high reproducibility and accuracy. In addition, parameters can be modified and automatization is possible. However, it is expensive, high temperatures may result in the degradation of thermally labile compounds, the selectivity is governed by the solvent, meaning exhaustive and nonselective extractions and periodical cleaning of the equipment is necessary. With regard to the extraction yields and rate, the main key parameters are the temperature, pressure, type of solvent and number of cycles.191The pressurized-liquid extraction conditions and the respective results that are discussed in this section are summarized in Table 11.
| Entry no. | Input sample | Solvent | Conditions | Extracted compounds | Ref. no. |
|---|---|---|---|---|---|
| a All concentrations of extracted compounds within the table are reported as %, regardless of the units in which they were originally reported by the authors, in order to facilitate their comparison. b Reported as % relative GC-peak area. c Reported as % in total extract. d Reported as mg g−1 of dry sample. | |||||
| 62 | Cannabis sativa L., seeds (crushed), 5 g | Water | 50–200 °C, 5–60 min | THC: 0.02–3.2, CBD: 0.1–9.9, CBN: 2 × 10−3 to 0.7, CBG: 0.03–4.5, CBC: 0.03–6.7 | 136 |
| 63 | Cannabis sativa L., buds, leaves and stems, 0.2 g | Isopropanol | 500 mL solvent per g cannabis | THC: 1.6–9.5 (buds), 0.087–0.72% (leaves, stems), CBD: 0.015–0.024 (buds), <7.6 × 10−3 (leaves, stems), CBN: 0.43–2.1% (buds), 0.09–0.97% (leaves, stems) | 140 |
| 64 | Cannabis sativa KC Virtus, flowers, leaves, stems 7.5–43.2 g | Ethanol | 25–100 °C, 1–150 bar, 10–1000 min, 0.045–0.1 g hemp per g solvent | THC: 4.9–10.7, CBD: 0.1–0.37 | 196 |
| 65 | Cannabis sativa Finola, flowers, leaves, stems 7.5–43.2 g | Ethanol | 25–100 °C, 1–150 bar, 10–1000 min, 0.045–0.1 g hemp per g solvent | THC: 8.2–19.8, CBD: 1.5–2.6 | 196 |
The recovery of valuable compounds from hemp with the aid of pressurized hot water extraction (PHWE) has been reported.139 Specifically, the chemical make-up of untreated samples and steam-treated at different temperatures (100–160 °C) was evaluated. Fresh hemp stalk yielded the highest cellulose content (49%), while decorticated fibers attained the highest lignin content (27%). Similar amounts of proteins are contained in all plant parts. Higher amounts of cellulose and lignin were detected in dried and fresh hemp stalk and decorticated fibers, however, similar hemicellulose content was obtained regardless of the extracted plant part. By adjusting the temperature of PHWE, different compounds can be accessed; higher temperatures are ideal for hemicellulose extraction, while glucose and pectins can be recovered at lower temperatures. The phenolic compounds and fatty acids commonly found in industrial hemp bast fibers, were detected in the majority of the extracts.139
Nuapia et al.136 published a study on the selective extraction of cannabinoids from Cannabis sativa L. seeds via PHWE, aiming for higher extraction of CBD and lower extraction of THC and CBN (Table 11, entry 62).136 The study focused on 5 cannabinoids: THC, CBN, CBD, CBC and CBG. Response surface methodology (RSM) was used in order to get insight into the influence of the evaluated parameters, precisely, extraction time, extraction temperature and collector vessel temperature. The results showed that all 3 parameters have an impact on the THC and CBN extraction. Specifically, an increase in both temperatures favors solubility, thus, the yields of CBD, CBC and CBG increase. On the other hand, yields of THC and CBN diminish, due to their probable vaporization at elevated temperatures, as proven by their capture in methanol in the trapping system. Consequently, the final product has a high content of CBD, CBC and CBG and lower amounts of THC and CBN.136
A cannabinoid extraction approach relying on a hard-cap espresso machine, using 2-propanol, was developed as a rapid, simple and cheap alternative for their recovery from Cannabis sativa leaves, buds and stems (Table 11, entry 63).140 Following the verification of cannabinoid stability under extraction conditions with the aid of standards, it was demonstrated that the developed approach could quantitatively extract all target cannabinoids (THC, CBD, CBN) within 40 s. The addition of a filter on the sample-containing capsule allows the simultaneous extraction and filtration of the cannabinoids from the plant sample, thus, circumventing additional filtration or centrifugation steps normally required for the recovery of the cannabinoid extract from the solid plant material.140
In 2020, a PLE-based extraction method was reported for the extraction of CBD and THC from two different varieties of C. sativa (KC Virtus and Finola), using ethanol as solvent (Table 11, entries 64 and 65).196 The research examined the influence of different parameters, such as temperature, extraction time, and pressure on the extraction efficiency. Their results showed that relatively low pressures and 100 °C were sufficient to extract the cannabinoids with good yields. Approximately, 19.8 mg of CBD per gram of dry hemp has been extracted from the leaves of variety Finola.
2.7. Hydrodistillation
Hydrodistillation is a commonly used method for the extraction of bioactive compounds from plants. The key difference between steam distillation and hydrodistillation is that steam distillation uses steam, whereas hydrodistillation uses water, steam or the combination of both for the extraction. Since no organic solvents are involved in this process, this can be considered as an environmentally benign extraction method. On the other hand, because of the relatively high temperature needed in this method, volatile and thermo-labile compounds may be lost during the process.197 The hydrodistillation conditions and the respective results that are discussed in this section are summarized in Table 12.| Entry no. | Input sample | Solvent | Conditions | Extracted compounds | Ref. no. |
|---|---|---|---|---|---|
| a All concentrations of extracted compounds within the table are reported as %, regardless of the units in which they were originally reported by the authors, in order to facilitate their comparison. b Reported as % in the extracted hemp essential oil. c Reported as % CBD in the resulting volatile fraction, based on GC-MS relative areas. | |||||
| 66 | Cannabis sativa L., inflorescences, (i. ground, ii. fresh matter, iii. powdered), 100 g | Water | 240 min, 35 mL water per g cannabis | CBD: 4.6–9.1, α-pinene: 0.5–8.1, myrcene: 1–11.5, terpinolene: 0.5–3.2, (E)-caryophyllene: 25–52, α-humulene: 3.5–6.6, caryophyllene oxide: 10–22.5 | 148 |
| 67 | Cannabis sativa L., cultivar: Monoica, inflorescences, leaves, stalks | Water | 240 min, 1–5 kg plant per L solvent | CBD: 23.83, α-pinene: 10.78, β-ocimene: 7.02, β-myrcene: 6.74, α-terpinolene: 2.55, δ-3-carene: 3.55, limonene: 1.82, camphene: 1.65 | 198 |
Fiorini et al.148 reported a strategy for the enrichment of hempseed oil, derived by distillation of industrial fresh and dry hemp inflorescences, in cannabidiol (Table 12, entry 66). In order to maximize the enrichment efficiency, 5 different sample pre-treatment methods were evaluated; (i) no prior pre-treatment of fresh sample, (ii) drying for 1 month, (iii) grinding, (iv) chopping and heating at 120 °C and (v) cutting into small pieces and MW at 450 W (1 min) and 900 W (3 min).
Drying negatively impacts the extraction yield, since higher yield was obtained from fresh inflorescences; specifically, drying promotes the loss of monoterpenes.
While there was no significant difference in the overall oil yield between grinding and grinding combined with conventional or MW heating, the maximum extraction of CBD (9.1% in the extracted oil) was obtained with the aid of MW pre-treatment. Concerning the terpene content of the extracted oil, all pre-treatment approaches negatively impacted the monoterpene extraction, however, favored the extraction of sesquiterpenes.148
It has been demonstrated that microwave-assisted hydrodistillation has a considerable impact on the speed and yield of the extraction process (half time, triple yield) (Table 12, entry 67). Application of microwaves has the added advantage of inducing extensive carboxylation of the cannabinoids, which mostly remain in the residual biomass, thereby, providing more active forms of these compounds, while the reduced extraction time means that loss of volatile compounds and secondary metabolite degradation can be circumvented. The terpenoid fraction of MW-assisted hydrodistillation has a richer profile in terms of variety. Prolongation of the extraction time enriches the monoterpene content but, at the same time, the sesquiterpene content is decreased.198
2.8. Mechanical pressing
Mechanical pressing is regarded as a solid–liquid separation process utilized for the extraction of oils from oilseeds. It is preferentially used when the oil content is below 20%. This technique uses mechanical pressure and there are 2 classifications: whenever high temperature is applied (over 49 °C) it is defined as hot-pressing and if temperature is equal or below 49 °C it is called cold-pressing. The pressing machine contains one inlet for seeds and two outlets, one for the attained oil (also known as oil-cake) and the other for the non-oiled cake. Expellers, expanders and twin-cold systems fall into the category of cold presses. Mechanical pressing is a simple, automatic, low cost, environmentally friendly process, as no solvents are used, and can be applied for a wide variety of applications. However, the main drawbacks are its low yield and low reproducibility of the obtained-product quality. Concerning the oil yield, the most influential factors are the parameters related to the process, to name a few, nozzle size, screw rotational speed and temperature. In addition, the pretreatment of the seeds is also a key parameter, including peeling, drying, solvent or enzymatic treatment.199The mechanical pressing conditions and the respective results that are discussed in this section are summarized in Table 13.
| Entry no. | Input sample | Conditions | Extracted compounds | Ref. no. |
|---|---|---|---|---|
| a All concentrations of extracted compounds within the table are reported as %, regardless of the units in which they were originally reported by the authors, in order to facilitate their comparison. b Reported as % in hemp seeds. c Reported in mg kg−1 of raw organic material. d Reported in g of gallic acid eq. per kg of raw organic material. | ||||
| 68 | Cannabis sativa L., seeds | Press nozzle diameter: 4–10 mm | Hempseed oil: 24–30 | 123 |
| 69 | Cannabis sativa L., seeds, 1000 g | 40–70 °C, press nozzle diameter: 8–12 mm | Hempseed oil: 26, relative content; phenols: 10–50, non-phenolic compounds: 10–78, flavonoids: 5–75 | 169 |
| 70 | Cannabis sativa L., seeds, 1000 g | 50–70 °C, press nozzle diameter: 8 mm, screw speed: 22–32 rpm | Hempseed oil: 17–23 | 137 |
| 71 | Cannabis sativa L., seeds (ground) | 20 min, 294–410 bar | Hempseed oil: 28–33 | 147 |
| 72 | Cannabis sativa L., hurds (dried) | 50 °C, 60% EtOH, 13% NaOH, feed rate: 2.2–2.5 kg h−1, screw speed: 200 rpm | Ferulic acid: 99, p-coumaric acid: 1814, dpolyphenols: 5.8 | 171 |
| 73 | Cannabis sativa L., dust (dried) | 50 °C, 60% EtOH, 21% NaOH, feed rate: 2.2–2.5 kg h−1, screw speed: 200 rpm | Ferulic acid: 95, p-coumaric acid: 1150, dpolyphenols: 9.0 | 171 |
The impact of cold pressing on the quality and extraction efficiency of hempseed oil has been reported (Table 13, entry 68).123 Specifically, the pressing rate efficiency appears to be influenced not only by the diameter of the press nozzle but the hemp seed characteristics as well. Smaller nozzle diameters (4 mm) yield higher oil quantities, while hemp seeds that contain lower volatile matter promote the rate efficiency. Based on standard of quality, it was determined, by connecting the acidity and peroxide value, that exclusively the oil processed from the seeds with lower total volatile content meets the quality requirements for alimentary and therapeutic purposes.123
Apart from the cold pressing parameters, the fertilization techniques also appear to have an effect on the phenolic and polyphenolic content of hempseed oil extracted with cold-pressing (Table 13, entry 68).169 The impact of various parameters, such as plant density, soil fertilization, foliar fertilization, extraction temperature (40–70 °C) and nozzle diameter (8–12 mm), was evaluated by Faugno et al.169 It was demonstrated that foliar fertilization increased the extraction efficiency of phenolic compounds.
Extracts derived from non-fertilized plants of different densities, clearly show the impact of density on extraction efficiency (Table 13, entry 69); higher abundance of metabolites with a flavonoid skeleton were present in the higher density plants. High temperatures diminish the extraction of these metabolites, however, when larger nozzle sizes are employed, the effect of the temperature is less significant. Soil fertilization positively impacts the quality of the recovered hempseed oil which is abundant in phenolic and polyphenolic constituents. An overview of the mechanical screw press set-up used in the experiments is depicted in Fig. 22.169
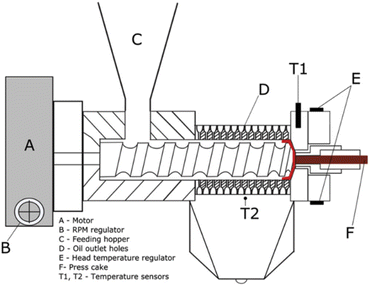 | ||
| Fig. 22 Mechanical screw press for tobacco oil extraction used by Faugno et al. and Crimaldi and co-workers.137,169 Reprinted with permission from Elsevier. | ||
The effect of several parameters of mechanical screw pressing on the yield of hempseed oil has also been reported (Table 13, entry 70).137 The nozzle size was not considered (kept constant), however, the press temperature, the screw speed and the sample pre-treatment (heating vs. no heating) were varied. The most influential parameters on the oil yield were the screw rotational speed, followed by the extraction temperature and seed preheating. Regarding the extraction temperature, high temperatures lead to higher yields (up to 23%). In preheated and non-preheated samples, high rotational screw speed had a significant negative influence on the yield. Higher extraction of oil was obtained by combining lowest screw rotational speed, with preheated seeds at high temperatures.137
An RSM approach was used by Aladić et al.163 in order to determine the input parameter values of cold pressing that maximize the extraction of high-quality oil; the input parameters of nozzle size, temperature and frequency were employed. Additional parameters, such as cold-press oil volume, oil after centrifugation, oil temperature, free fatty acid (FFA), insoluble impurities (II) and amount of residual oil in the press cake, were also considered.
Larger nozzle sizes lead to a notable decrease of the amount of obtained oil. The FFA amount is an indicator of oil quality; lower amounts are correlated with high quality and long shelf life. Low temperatures, high frequencies and bigger nozzle sizes help maintain the FFA content at the desirable low levels. A satisfactory quality can be attributed to the recovered oil, due to the low co-extraction of impurities (0.33–0.49%).
With regard to the optimization of screw pressing of hemp seed oil, several parameters were studied using RSM with the aim to maximize the oil quality properties and the oil recovery.
Among all the natural antioxidants tested, oregano provides the oil with the highest resistance towards oxidative degradation.163
An alternative cold-pressing approach, i.e., enzyme-assisted cold-pressing extraction, for recovery of hempseed oil has been reported (Table 13, entry 71).147 Enzyme-treated hemp seeds deliver significantly higher oil yields than untreated ones. Additionally, it was demonstrated that different enzymes favor the extraction yields of different fatty acids and tocopherol forms. In any case, the addition of an enzyme adjuvant is essential to maximize the yield of γ-tocopherol. The enzyme treated hempseed oil contains higher amounts of pigments, whereas its oxidation stability does not differ from the control sample.147
A twin-screw extrusion was evaluated for its potential to recover pharmaceutically valuable compounds from hemp by-products. Specifically, ferulic acid (FA) and p-coumaric acid (p-CA) in hemp hurds and dust were targeted (Table 13, entries 72 and 73).171 Hurds proved to be a richer source of the target compounds since they contain 3 times the amount found in dust.
The extrusion process comprises the steps of (i) mixing the solid sample with the liquid solvent, (ii) extracting and (iii) separating the solid and liquid fractions, which are performed in a continuous mode. An aqueous ethanolic solvent is used for the solubilization of the target compounds, while the addition of NaOH in the solvent favors their extraction yields. Both target compounds are not primarily encountered in their free form, therefore, addition of a mildly basic NaOH solution is essential to access the etherified FA and the both etherified and esterified p-CA. Under these conditions, the polyphenol yield is also significantly increased, since NaOH breaks the intermolecular hydrogen bonds, thereby releasing the phenolic compounds. An additional advantage of NaOH is its ability to reduce the viscosity of the mixture inside the extruder. Increasing the temperature (from 50 °C to 100 °C) does not seem to have an effect on the extraction yield, therefore, mild temperatures are selected.
A fraction of the extracted compounds is retained in the solid residue; however, they can be recovered by a second extraction step with a polar solvent. While a single pass from the extruder (15 min) can recover half of the amount of polyphenols that can be recovered by alkaline batch extraction in 24 h, the respective yields for FA and p-CA are 2–4 times lower. Nevertheless, this trade-off is more than counterbalanced by the significantly lower amounts of NaOH (45 times less) used in the extrusion process and the low solid-to-liquid ratio, both of which signify economic and environmentally advantageous solvent consumption. Additionally, compared to conventional approaches, twin-screw extrusion is less time consuming and less energy demanding.171
2.9. Comparative studies
A comparative study on essential oil extraction from Cannabis sativa leaves using three different techniques, precisely, supercritical fluid extraction (SFE), steam distillation (SD) and hydrodistillation (HD), was reported by Naz et al.144 The effect of temperature on extraction efficiencies was evaluated, while in SFE pressure was additionally considered. In the case of HD, increased temperature implies elevated vapor pressure which results in increased transfer rates, thus, higher extraction yields. On the other hand, in the case of SD, extraction is favored at lower temperatures which limit the negative impact of hydro diffusion, hydrolysis and thermal degradation. Between the two, higher oil yields were obtained with SD; lower hydrolysis rate, resulting from smaller water amounts used, as opposed to HD, minimizes thermal decomposition, thus, providing higher essential oil yields.Regarding SFE, increasing pressure has a positive impact on the extraction efficiency of the oil components; scCO2 density increases, thus, its dissolving ability is favored at elevated pressures. The combination of high pressure and low temperature is ideal to obtain high essential oil yield, which is also the highest among the 3 techniques that were compared. The natural aroma of the oil was preserved better when scCO2 was employed, due to the higher percentage of extracted sesquiterpenes. Supercritical fluid extraction proved to be superior with regard to extraction yields, isolation and recovery of target compounds and energy consumption.144
In a subsequent study, the research group further expanded their study to include Cannabis indica. Elevated temperatures in HD negatively impact the extraction yield of the essential oil due to possible thermal degradation occurring at higher temperature.145 However, this observation is contradictory to what the authors previously reported; that high temperatures in HD lead to higher extraction yields.144 In the case of SD, best yield was obtained at 130 °C, however, compared to the HD the yield was much lower.
This observed difference can be attributed to the lower penetration capacity of the steam in the plant material in the case of SD. While in HD the plant material is constantly submerged in boiling water, in SD the steam needs to vaporize and condense the water present in the plant material in order to access the target compounds; thus, longer extraction times are required, which can lead to thermal degradation of the target constituents.
In the case of scCO2 extraction, an increase in temperature up to a certain point increases the yield, however, any additional increase beyond this point has no impact on the obtained yield. This can be attributed to the decrease of the scCO2 density beyond this temperature point, since scCO2 controls the increase of the solute vapor pressure at the corresponding pressure.
Regardless of the cannabis species, SFE afforded the highest oil yield of all 3 techniques compared. In any case, higher oil yield was obtained from Cannabis sativa than Cannabis indica, which is a result of different factors regarding the biotype and chemotype of the plant, geographical cultivation, climatic conditions and extraction process. An overview of the yields obtained by the compared methods is presented in Fig. 23.145
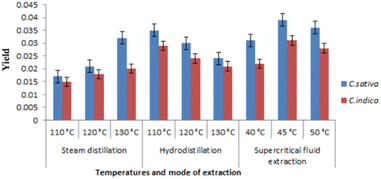 | ||
| Fig. 23 Extraction yields (%) obtained by various methods.145 Reprinted by permission of Taylor & Francis Ltd. | ||
A comparative study of focused ultrasound extraction (FUSE) and SFE on the recovery of cannabinoids and sesquiterpenes from cannabis leaves and buds has been reported.
The sonication time, amplitude, sonication cycles and the extracting solvent composition were varied during the optimization of the FUSE. As factorial fractionated design indicates, the number of cycles has no significant effect, hence the value was set to the minimum. The highest value of amplitude is achieved at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 solvent mixture, which had a positive impact on the extraction of sesquiterpenes and cannabinoids. For both, the sonication time did not have a significant impact, therefore, it was set to the minimum (5 min).
1 solvent mixture, which had a positive impact on the extraction of sesquiterpenes and cannabinoids. For both, the sonication time did not have a significant impact, therefore, it was set to the minimum (5 min).
The SFE was optimized with central composite design (CCD). Addition of co-solvent (EtOH) was deemed necessary to maximize the cannabinoid extraction, whereas lack of co-solvent and low temperature extractions are more favorable for terpene extraction. Cannabinoid extraction is not affected by changes in temperature. Supercritical fluid extraction allowed the fractionation in 2 different extracts; one rich in terpenes and the other in cannabinoids.
While higher extraction yields are accessible with FUSE, SFE offers the advantage of fractionation, which enables the separation of cannabinoids and terpenes.141
De Vita et al.142 published a comparative investigation of different methods, i.e., UAE, MAE and an emulsion-based extraction for the recovery of main cannabinoids (THC, THCA, CBD, CBDA, and CBN) from commercially available hemp varieties and medical cannabis samples.
Regarding the UAE, the influence of solvent and extraction time was studied. Use of ethanol, combined with shorter extraction times, favors the cannabinoid extraction. Overall, for the commercially available hemp, conventional ethanol-assisted extraction was more favorable for CBDA, THCA and total CBD, while the optimized UAE extraction was more efficient for the other cannabinoids. In the case of the medical samples, ethanol-based extraction was more efficient than the optimized UAE for all target cannabinoids.
With regard to MAE, the effect of extraction time, extraction temperature, ramp time and solvent was evaluated. Prolonged extraction time and elevated temperature extracts an amount of CBD that is 4 times higher than the conventional extraction, while employing ethanol and short ramp time favors this outcome. Conversely, by increasing the time and temperature of extraction, a significant decrease in the CBDA yield is observed, which may be attributed to the transformation of CBDA into its decarboxylated analogue, i.e., CBD, during the MAE process. Contrary to CBDA, an increase in temperature promotes THCA extraction. Overall, MAE obtained a higher amount of THC and THCA compared to the conventional extraction. Between MAE and UAE, higher extraction efficiency for CBD and THC is obtained with UAE.
Regarding the emulsion-aided extraction (Tween 20 in H2O), the influence of the solvent, the extraction time and the extraction temperature were evaluated. Both THC and CBD yields were lower compared to the ones obtained with conventional extraction. Apart from that, total THC was comparable in all the emulsion-based extractions, regardless of the employed extraction parameters. Interestingly, higher CBD than CBDA quantities were present in the tween-based extract, even though in the conventional ethanolic extract CBDA was 5 times higher than CBD.142
Brighenti et al.42 compared the efficiency of solvent extraction (SE), dynamic maceration (DM), ultrasound-assisted extraction (UAE), microwave-assisted extraction (MAE) and supercritical fluid extraction (SFE) on the recovery of cannabinoids from inflorescences.
Concerning the optimization of the extraction conditions, methanol and ethanol were the most appropriate solvents for the extraction of cannabinoids, followed by acetone, MeOH/CHCl3 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and hexane. Each of the evaluated techniques were optimized in terms of extraction time and temperature. UAE and SFE had the poorest performance of all compared methods in terms of extracted amounts of CBDA, CBD and CBGA. While similar amounts of CBGA were obtained with both DM and MAE, CBD yields were favored by MAE and CBDA by DM. Considering all the aforementioned, DM was selected as the best extraction method for non-intoxicating cannabinoids.42
1) and hexane. Each of the evaluated techniques were optimized in terms of extraction time and temperature. UAE and SFE had the poorest performance of all compared methods in terms of extracted amounts of CBDA, CBD and CBGA. While similar amounts of CBGA were obtained with both DM and MAE, CBD yields were favored by MAE and CBDA by DM. Considering all the aforementioned, DM was selected as the best extraction method for non-intoxicating cannabinoids.42
A comparative study of different extraction approaches for hemp seed oil and their industrially-relevant financial assessment was performed. The following extraction techniques were evaluated; Soxhlet (SOX), percolation (PER), supercritical fluid extraction (SFE), ultrasonication (ULT), pyrolysis (PYR), ULT treated SOX (UTS) and SOX treated ULT (STU).
Maximum extraction efficiency is obtained via UTS (Fig. 24), however, SFE provides product of the highest purity. Aside from efficiency, the profile of the obtained fatty acids is significantly affected by the extraction method of choice. SFE and SOX are not only the most efficient in terms of extraction but they also provide the ideal ratio (3.22 and 2.40, respectively), from a dietary perspective, of ω-6 to ω-3 acids. In contrast, the high ratio obtained via UTS (9.24) renders it unsuitable for the extraction of fatty acids intended for nutritional purposes.
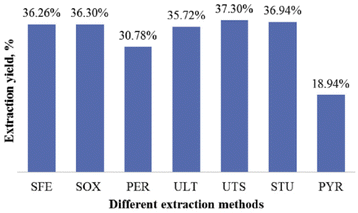 | ||
| Fig. 24 Extraction yields obtained through different extraction processes.170 Reprinted with permission from Elsevier. | ||
Concerning industrial upscaling, all the evaluated techniques are considered to be financially viable. Despite the high cost of SFE, the expected profit (high market value due to the high purity of the product), which exceeds 10-fold the profit of the other processes, more than counterbalances the high cost. The use of ultrasonication is the main reason that ULT and UTS exhibit the highest power consumption.170
3. Conclusion
In the last few years, there has been an increasing interest worldwide in cannabis-derived phytochemicals, mainly due to their medical potential, as well as their merit/role in the production of industrially valuable products (Fig. 25). Despite this dramatic boost, the extraction of high value-added products is still in its early stages, as new bioactive compounds are constantly discovered, and their identification and isolation remain challenging tasks. In this review, the extraction of phytochemicals present in cannabis plant with the aid of conventional techniques and more sophisticated ones has been discussed. | ||
| Fig. 25 Cannabinoids related publications based on the database of SciFindern (search term: cannabinoids, 10.05.2022). | ||
Evidently, the extraction of phytochemicals is a complex process and, up till now, progress in extraction of target compounds has been achieved with various levels of performance/effectiveness. It has been demonstrated that by substituting the conventional solvent extraction techniques with modern strategies has led to reduction of extraction time, decrease of solvent consumption, high extraction yields, increased selectivity, fractionation and stability of targeted compounds.
Nevertheless, there are still numerous questions that need to be addressed. The conducted research has mainly focused on valuable compounds, such as cannabinoids, essential oils and pigments, however, complete characterization of the extracts is notably absent. While high recovery of desirable compounds is the main objective, it is equally important to include data on the possible presence of contaminants, such as degradation products and/or residual pesticides and growth regulators, which apart from lowering the quality of the derived extracts, could also have a harmful effect if used in products intended for human consumption. Additionally, a concise interpretation of the obtained results could be useful; the impact of the extracted amount of each valuable compound on the extract quality, organoleptic features and stability should be clearly stated.
Regarding sample storage and preparation prior to extraction, both can drastically influence the chemical profile of the starting material and the yield and composition of the collected extract, as demonstrated in the research discussed in this review. Therefore, a reconciliation in the inconsistencies regarding storage conditions (fridge, freezer, RT, dark, light) and sample preparation (particle size, moisture content) is necessary in order to accurately evaluate the obtained results and compare the experimental outcomes of different research efforts. Furthermore, the merit of the developed extraction approaches could benefit from their further application to various cannabis sources (fibers, flowers, seeds, processing residues) and different cannabis varieties.
Reliable quantification of the extracts is of paramount importance and the development of a unified and validated analytical approach could mitigate quantification errors and deviations, which is especially important for extracts intended for medicinal applications. Additionally, a unified approach should be introduced regarding the input material characterization and the reported quantification results; we believe that this would significantly facilitate future research work and effectively simplify the comparison between different extraction approaches.
We should point out, that there is no universal extraction technique, as each extraction technique has advantages and disadvantages (Table 14). Moreover, it is dependent on the part of the plant used and the nature of the target compound. Considerable attention needs to be paid to the aim of the investigation at hand and the target compounds when deciding on the pretreatment approach of the input material and the extraction technique. Scientists, motivated by the increased demand of extracting bioactive compounds present in plants, are in continuous search for suitable and more sophisticated extraction techniques. However, a combination of processes may be the beautiful and rewarding future of the extraction of cannabis-related bioactive compounds.
| Extraction method | Principle | Advantages | Disadvantages |
|---|---|---|---|
| Supercritical fluid | Improved extraction efficiency due to low viscosity & high diffusivity | • Non-toxic & non-flammable | • Limited to non-polar and weakly polar compounds |
| • No solvent residues | • Energy-demanding | ||
| • Easy separation from product | • Special equipment required | ||
| Ionic liquids & deep eutectic solvents | Liquids with low vapour pressure, that consist of ions | • Tuneable properties | • High cost |
| • High extraction yields | • Viscosity issues | ||
| • Improved safety | |||
| Solvent-based Soxhlett extraction | Organic solvents | • Low investment costs | • Large solvent consumption |
| • High reproducibility | • Use of persistent/flammable chemicals | ||
| Ultrasound-assisted | Local hotspots generated via cavitation | • Low energy consumption | • Large solvent consumption |
| • Low investment costs | Use of persistent/flammable chemicals | ||
| Microwave-assisted | Direct conversion of electromagnetic energy to heat | • Short extraction time | • Ineffective for non-polar target compounds |
| • High extraction yields | • Not suitable for thermally instable compounds | ||
| Pressurized liquid | High temperature (enhances solvent diffusion due to reduced viscosity) and pressure (promotes cell permeability) is applied | • Fast | • High cost |
| • Low solvent consumption | • Degradation of thermo-labile compounds may occur | ||
| • High reproducibility | |||
| Hydrodistillation | Water and/or steam is applied for the extraction | • No organic solvent | • Volatile and thermo-labile compounds may be lost |
| • Energy-intense | |||
| Mechanical pressing | Mechanical pressure | • Simple | • Low yields and reproducibility |
| • Low cost | |||
| • No solvent-free |
4. Abbreviations
| AD | anno Domini |
| AgNO3 | Silver nitrate |
| ANOVA | Analysis of variance |
| BC | Before Christ |
| Cannabis sativa L. | Cannabis sativa Linnaeus |
| CBC | Cannabichromene |
| CBCA | Cannabichromenic acid |
| CBD | Cannabidiol |
| CBDA | Cannabidiolic acid |
| CBE | Cannabielsoin |
| CBG | Cannabigerol |
| CBGA | Cannabigerolic acid |
| CBL | Cannabicyclol |
| CBN | Cannabinodiol |
| CBT | Cannabitriol |
| CCD | Central composite design |
| CHCl3 | Chloroform |
| [C6mim][NTf2] | 1-Hexyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide |
| DES | Deep eutectic solvent |
| DM | Dynamic maceration |
| EAE | Enzyme-assisted extraction |
| EFA | Essential fatty acids |
| EtOH | Ethanol |
| EU | European Union |
| FA | Ferulic acid |
| FFA | Free fatty acid |
| FRAP | Ferric reducing ability of plasma |
| FUSE | Focused ultrasound extraction |
| g | Grams |
| GC-FID | Gas chromatography-flame ionization detector |
| GLA | Gamma-linolenic acid |
| HD | Hydro distillation |
| HDPE | High-density polyethylene |
| H2O | Water |
| II | Insoluble impurities |
| IL | Ionic liquid |
| LDL | Low-density lipoprotein |
| MAE | Microwave assisted extraction |
| MeOH | Methanol |
| mg | Milligrams |
| MUFA | Monounsaturated fatty acids |
| MW | Microwave |
| NMR | Nuclear magnetic resonance |
| RSC | Response surface methodology |
| p-CA | p-Coumaric acid |
| PCA | Principal component analysis |
| PEF | Pulsed electric field |
| PER | Percolation |
| PLE | Pressurized-liquid extraction |
| ppm | Part per millions |
| PUFA | Polyunsaturated fatty acid |
| PYR | Pyrolysis |
| RSM | Response surface methodology |
| scCO2 | Super critical carbon dioxide |
| SD | Steam distillation |
| SDA | Stearidonic acid |
| SE | Solvent extraction |
| SFE | Supercritical fluid extraction |
| SOX | Soxhlet |
| SPE | Solid phase extraction |
| STU | Soxhlet treated ultrasonication |
| TF | total flavonoids |
| THC | Δ9-Tetrahydrocannabinol |
| THCA | Δ9-Tetrahydrocannabinolic acid |
| TP | Total phenolics |
| Tween 20 | polysorbate 20 |
| UAE | Ultrasound assisted extraction |
| UFA | Unsaturated fatty acid |
| UK | United Kingdom |
| ULT | Ultrasonication |
| UN | United Nations |
| US(A) | United States of America |
| UTS | Ultrasonication treated Soxhlet |
5. Author contributions
A. S. M. & O. L. conceived the review, analysed the data; wrote the original draft, O. L. edited and reviewed the draft. K. S. reviewed, edited and contributed to the manuscript content. K. B.-S.& H. H. conceived and supervised, edited and reviewed the draft. M. S. reviewed the draft.6. Conflicts of interest
There are no conflicts of interest to declare.7. Acknowledgements
The authors acknowledge TU Wien for the support during this project.8. References
- E. Small and A. Cronquist, Taxon, 1976, 25, 405–435 CrossRef.
- J. F. Hancock, Plant evolution and the origin of crop species, CABI, 2012 Search PubMed.
- C. T. Kung, Archeology in China, University of Toronto Press, Toronto, 1959, vol. 1, p. 131 Search PubMed.
- K.-C. Chang, Science, 1968, 162, 519–526 CrossRef PubMed.
- I. Bocsa and M. Karus, The cultivation of hemp: botany, varieties, cultivation and harvesting, Hemptech, 1998 Search PubMed.
- S. Chandra, H. Lata and M. A. ElSohly, Cannabis sativa L.-botany and biotechnology, Springer, 2017 Search PubMed.
- H.-E. Jiang, X. Li, Y.-X. Zhao, D. K. Ferguson, F. Hueber, S. Bera, Y.-F. Wang, L.-C. Zhao, C.-J. Liu and C.-S. Li, J. Ethnopharmacol., 2006, 108, 414–422 CrossRef PubMed.
- R. E. Schultes, Nat. Hist., 1973, 82, 59 Search PubMed.
- G. Leson, in Hemp: industrial production and uses, ed. P. Bouloc, S. Allegret and L. Arnaud, CABI, 2013, ch. 16, pp. 229–238 Search PubMed.
- M. Touw, J. Psychoact. Drugs, 1981, 13, 23–34 CrossRef.
- H.-L. Li, Econ. Bot., 1974, 28, 437–448 CrossRef.
- H.-L. Li, J. Psychedelic Drugs, 1978, 10, 17–26 CrossRef.
- A. Mukherjee, S. C. Roy, S. De Bera, H.-E. Jiang, X. Li, C.-S. Li and S. Bera, Genet. Resour. Crop Evol., 2008, 55, 481–485 CrossRef.
- E. Small, Cannabis: a complete guide, CRC Press, 2016 Search PubMed.
- M. R. Aldrich, J. Psychedelic Drugs, 1977, 9, 227–233 CrossRef.
- R. Mechoulam and L. A. Parker, Annu. Rev. Psychol., 2013, 64, 21–47 CrossRef PubMed.
- E. L. Abel, Marihuana: the first twelve thousand years, Springer Science & Business Media, 2013 Search PubMed.
- S. Wills, in Cannabis: the genus cannabis, CRC Press, 1st edn, 1998, pp. 1–27 Search PubMed.
- M. B. Bridgeman and D. T. Abazia, Pharmacy and Therapeutics, 2017, vol. 42, p. 180 Search PubMed.
- USDA, Classification for Kingdom Plantae Down to Species Cannabis sativa L.,https://plants.usda.gov/java/ClassificationServlet?source=display%26classid=CASA3, accessed 27.10. 2020 Search PubMed.
- J. M. McPartland, Cannabis Cannabinoid Res., 2018, 3, 203–212 CrossRef.
- E. Small, P. Y. Jui and L. P. Lefkovitch, Syst. Bot., 1976, 1, 67–84 CrossRef.
- K. W. Hillig and P. G. Mahlberg, Am. J. Bot., 2004, 91, 966–975 CrossRef.
- R. C. Clarke and M. D. Merlin, Cannabis: evolution and ethnobotany, Univ of California Press, 2013 Search PubMed.
- J. M. McPartland and G. W. Guy, Bot. Rev., 2017, 83, 327–381 CrossRef.
- G. Green, The Cannabis Breeder's Bible: The Definitive Guide to Marijuana Genetics, Cannabis Botany and Creating Strains for the Seed Market, Green Candy Press, New York, 2005 Search PubMed.
- C. Linnaeus, Species Plantarum, Laurentius Salvius, Stockholm, 1st edn, 1753 Search PubMed.
- C. Linnaeus, Genera plantarum, Laurentius Salvius, Stockholm, 5 edn, 1754 Search PubMed.
- J. Lamarck, Encyclopédique méthodique, Botanique I, Paris, 1785 Search PubMed.
- L. C. Anderson, Leaf Variation among Cannabis Species from a Controlled Garden, https://www.biodiversitylibrary.org/part/168641, accessed November 2022 Search PubMed.
- J. M. McPartland and G. W. Guy, The evolution of Cannabis and coevolution with the cannabinoid receptor - a hypothesis, Royal Society of Pharmacists, London, 2004 Search PubMed.
- J. McPartland, O’Shaughnessy’s (J Calif Cannabis Res Med Group), Autumn, 2014, 1.
- P. Henry, PeerJ PrePrints, 2015, 3, e1553 Search PubMed.
- R. Clarke and M. Merlin, HerbalGram, 2016, 110, 44–49 Search PubMed.
- T. J. Sheehan, H. J. Hamnett, R. Beasley and P. S. Fitzmaurice, Forensic Sci. Prog., 2019, 4, 168–178 Search PubMed.
- R. Mechoulam, P. Braun and Y. Gaoni, J. Am. Chem. Soc., 1967, 89, 4552–4554 CrossRef PubMed.
- M. M. Radwan, S. Chandra, S. Gul and M. A. ElSohly, Molecules, 2021, 26, 2774 CrossRef PubMed.
- R. Brenneisen, in Marijuana and the Cannabinoids, Humana Press, New Jersey, 2007, ch. 2, pp. 17–49 Search PubMed.
- I. J. Flores-Sanchez and R. Verpoorte, Phytochem. Rev., 2008, 7, 615–639 CrossRef.
- D. M. Lambert and C. J. Fowler, J. Med. Chem., 2005, 48, 5059–5087 CrossRef.
- G. Appendino, G. Chianese and O. Taglialatela-Scafati, Curr. Med. Chem., 2011, 18, 1085–1099 CrossRef.
- V. Brighenti, F. Pellati, M. Steinbach, D. Maran and S. Benvenuti, J. Pharm. Biomed. Anal., 2017, 143, 228–236 CrossRef PubMed.
- F. Pellati, V. Brighenti, J. Sperlea, L. Marchetti, D. Bertelli and S. Benvenuti, Molecules, 2018, 23, 2639 CrossRef PubMed.
- F. Pellati, V. Borgonetti, V. Brighenti, M. Biagi, S. Benvenuti and L. Corsi, BioMed Res. Int., 2018, 2018, 1691428 Search PubMed.
- Y. Gaoni and R. Mechoulam, J. Am. Chem. Soc., 1964, 86, 1646–1647 CrossRef.
- N. M. Kogan and R. Mechoulam, Dialogues Clin. Neurosci., 2007, 9, 413 CrossRef PubMed.
- S. Ameur, B. Haddou, Z. Derriche, J.-P. Canselier and C. Gourdon, Anal. Bioanal. Chem., 2013, 405, 3117–3123 CrossRef CAS PubMed.
- R. Mechoulam, L. A. Parker and R. Gallily, J. Clin. Pharmacol., 2002, 42, 11S–19S CrossRef CAS.
- J. Fernández-Ruiz, O. Sagredo, M. R. Pazos, C. García, R. Pertwee, R. Mechoulam and J. Martínez-Orgado, Br. J. Clin. Pharmacol., 2013, 75, 323–333 CrossRef.
- R. Mechoulam, M. Peters, E. Murillo-Rodriguez and L. O. Hanuš, Chem. Biodiversity, 2007, 4, 1678–1692 CrossRef CAS.
- C. M. Andre, J.-F. Hausman and G. Guerriero, Front. Plant Sci., 2016, 7, 19 Search PubMed.
- H. Mölleken and R. R. Theimer, J. Ind. Hemp, 1997, 4, 13–17 Search PubMed.
- S. A. Ross, Z. Mehmedic, T. P. Murphy and M. A. ElSohly, J. Anal. Toxicol., 2000, 24, 715–717 CrossRef CAS.
- M. B. Amar, J. Ethnopharmacol., 2006, 105, 1–25 CrossRef.
- D. Baker, G. Pryce, G. Giovannoni and A. J. Thompson, Lancet Neurol, 2003, 2, 291–298 CrossRef CAS.
- S. P. Alexander, Prog. Neuro-Psychopharmacol. Biol. Psychiatry, 2016, 64, 157–166 CrossRef CAS PubMed.
- M. Guzman, Nat. Rev. Cancer, 2003, 3, 745–755 CrossRef CAS.
- M. P. Barnes, Expert Opin. Pharmacother., 2006, 7, 607–615 CrossRef CAS.
- O. Devinsky, C. Verducci, E. A. Thiele, L. C. Laux, A. D. Patel, F. Filloux, J. P. Szaflarski, A. Wilfong, G. D. Clark and Y. D. Park, Epilepsy Behav.Epilepsy Behav., 2018, 86, 131–137 CrossRef.
- B. Palmieri, C. Laurino and M. Vadalà, Int. J. Pharm. Pract., 2019, 27, 264–270 CrossRef.
- J. Sastre-Garriga, C. Vila, S. Clissold and X. Montalban, Expert Rev. Neurother., 2011, 11, 627–637 CrossRef PubMed.
- S. Giacoppo, P. Bramanti and E. Mazzon, Mult. Scler. Relat. Disord., 2017, 17, 22–31 CrossRef.
- US-Food-and-Drug-Administration, FDA approves first drug comprised of an active ingredient derived from marijuana to treat rare, severe forms of epilepsy, US Food and Drug Administration, 2018 Search PubMed.
- S. Elzinga, J. Fischedick, R. Podkolinski and J. C. Raber, Nat. Prod. Chem. Res., 2015, 3, 1–9 Search PubMed.
- A. Hazekamp, K. Tejkalová and S. Papadimitriou, Cannabis Cannabinoid Res., 2016, 1, 202–215 CrossRef.
- E. Small and H. D. Beckstead, Nature, 1973, 245, 147–148 CrossRef PubMed.
- J. C. Callaway, Euphytica, 2004, 140, 65–72 CrossRef.
- H. Wang and Y. Wei, Med. Plant, 2012, 3, 11–14 Search PubMed.
- C.-W. Cheng, Z.-X. Bian, L.-X. Zhu, J. C. Wu and J. J. Sung, Am. J. Gastroenterol., 2011, 106, 120–129 CrossRef.
- C. Leizer, D. Ribnicky, A. Poulev, S. Dushenkov and I. Raskin, J. Diet. Suppl., 2000, 2, 35–53 Search PubMed.
- C. R. Vogl, H. Mölleken, G. Lissek-Wolf, A. Surböck and J. Kobert, J. Ind. Hemp, 2004, 9, 51–68 CrossRef CAS.
- R. Wall, R. P. Ross, G. F. Fitzgerald and C. Stanton, Nutr. Rev., 2010, 68, 280–289 CrossRef.
- J.-L. Deferne and D. W. Pate, J. Ind. Hemp, 1996, 3, 1–7 Search PubMed.
- A. P. Simopoulos, Biomed. Pharmacother., 2002, 56, 365–379 CrossRef CAS.
- B. D. Oomah, M. Busson, D. V. Godfrey and J. C. Drover, Food Chem., 2002, 76, 33–43 CrossRef CAS.
- D. Spielmann, U. Bracco, H. Traitler, G. Crozier, R. Holman, M. Ward and R. Cotter, J. Parenter. Enteral Nutr., 1988, 12, 111S–123S CrossRef CAS PubMed.
- D. Wirtshafter, in Bioresource Hemp: proceedings of the symposium, Hemptech, Frankfurt Am Main, Germany, 1995, pp. 546–555 Search PubMed.
- A. Rezvankhah, Z. Emam-Djomeh, M. Safari, G. Askari and M. Salami, J. Food Process. Preserv., 2018, 42, e13766 CrossRef.
- A. Kamal-Eldin and L. Å. Appelqvist, Lipids, 1996, 31, 671–701 CrossRef CAS PubMed.
- S. Sapino, M. E. Carlotti, E. Peira and M. Gallarate, Int. J. Cosmet. Sci., 2005, 27, 355 CrossRef.
- L. L. Yu, K. K. Zhou and J. Parry, Food Chem., 2005, 91, 723–729 CrossRef CAS.
- C.-H. Tang, Z. Ten, X.-S. Wang and X.-Q. Yang, J. Agric. Food Chem., 2006, 54, 8945–8950 CrossRef CAS PubMed.
- S.-K. Park, J.-B. Seo and M.-Y. Lee, Biochim. Biophys. Acta, Proteins Proteomics, 2012, 1824, 374–382 CrossRef.
- E. Vonapartis, M.-P. Aubin, P. Seguin, A. F. Mustafa and J.-B. Charron, J. Food Compos. Anal., 2015, 39, 8–12 CrossRef.
- E. Guenther, Essent. Oils, 1972, 1, 87–226 Search PubMed.
- M. W. Giese, M. A. Lewis, L. Giese and K. M. Smith, J. AOAC Int., 2019, 98, 1503–1522 CrossRef PubMed.
- H. Hendriks, T. M. Malingré, S. Batterman and R. Bos, Phytochemistry, 1975, 14, 814–815 CrossRef.
- E. Lemberkovics, P. Veszki, G. Verzar-Petri and A. Trka, Sci. Pharmacol., 1981, 49, 401–408 Search PubMed.
- L. Hood, M. Dames and G. Barry, Nature, 1973, 242, 402–403 CrossRef PubMed.
- V. Mediavilla and S. Steinemann, J. Ind. Hemp, 1997, 4, 80–82 Search PubMed.
- J. Novak and C. Franz, J. Essent. Oil Res., 2003, 15, 158–160 CrossRef.
- E. B. Russo, Br. J. Pharmacol., 2011, 163, 1344–1364 CrossRef PubMed.
- J. M. McPartland and E. B. Russo, J. Cannabis Ther., 2001, 1, 103–132 CrossRef.
- M. Hesami, A. Baiton, M. Alizadeh, M. Pepe, D. Torkamaneh and A. M. P. Jones, Int. J. Mol. Sci., 2021, 22, 5671 CrossRef.
- B. Farinon, R. Molinari, L. Costantini and N. Merendino, Nutrients, 2020, 12, 1935 CrossRef PubMed.
- M. Kuddus, I. A. Ginawi and A. Al-Hazimi, Emir. J. Food Agric., 2013, 736–745 CrossRef.
- E. Small and D. Marcus, in Trends in New Crops and New Uses, Alexandria, VA, 2002, pp. 284–326 Search PubMed.
- E. Small, in Natural fibers and composites, Studium Press, Houston, 2014, pp. 29–64 Search PubMed.
- E. Kreuger, T. Prade, F. Escobar, S.-E. Svensson, J.-E. Englund and L. Björnsson, Biomass Bioenergy, 2011, 35, 893–900 CrossRef.
- L. Nissen, A. Zatta, I. Stefanini, S. Grandi, B. Sgorbati, B. Biavati and A. Monti, Fitoterapia, 2010, 81, 413–419 CrossRef.
- D. Gibb, M. Shah, P. Mir and T. McAllister, Can. J. Anim. Sci., 2005, 85, 223–230 CrossRef.
- A. Mustafa, J. McKinnon and D. Christensen, Can. J. Anim. Sci., 1999, 79, 91–95 CrossRef.
- A. Hessle, M. Eriksson, E. Nadeau, T. Turner and B. Johansson, Acta Agric. Scand., Sect. A, 2008, 58, 136–145 Search PubMed.
- J. W. Roulac, Hemp horizons: the comeback of the world's most promising plant, Chelsea Green Publishing Company, Vermount, United States, 1st edn, 1997 Search PubMed.
- S.-Y. Li, J. D. Stuart, Y. Li and R. S. Parnas, Bioresour. Technol., 2010, 101, 8457–8460 CrossRef PubMed.
- R. Weightman and D. Kindred, Review and analysis of breeding and regulation of hemp and flax varieties available for growing in the UK, ADAS Centre for Sustainable Crop Management, Boxworth, Cambridge, 2005 Search PubMed.
- The science of cannabis and hemp storage, https://bovedainc.com/the-science-of-cannabis-and-hemp-storage/, accessed October, 2020 Search PubMed.
- P. L. Atkins, J. AOAC Int., 2019, 102, 427–433 CrossRef PubMed.
- L. Zamengo, C. Bettin, D. Badocco, V. Di Marco, G. Miolo and G. Frison, Forensic Sci. Int., 2019, 298, 131–137 CrossRef.
- D. J. Harvey, J. Ethnopharmacol., 1990, 28, 117–128 CrossRef PubMed.
- P. Lerner, The precise determination of tetrahydrocannabinol in marihuana and hashish, United Nations Office on Drugs and Crime, Vienna, Bull. Narc., 1969, 21, 39–42 Search PubMed.
- K. Grafström, K. Andersson, N. Pettersson, J. Dalgaard and S. J. Dunne, Forensic Sci. Int., 2019, 301, 331–340 CrossRef PubMed.
- C. Lindholst, Aust. J. Forensic Sci., 2010, 42, 181–190 CrossRef.
- R. N. Smith and C. G. Vaughan, J. Pharm. Pharmacol., 1977, 29, 286–290 CrossRef PubMed.
- J. W. Fairbairn, J. A. Liebmann and M. G. Rowan, J. Pharm. Pharmacol., 1976, 28, 1–7 CrossRef.
- K. D. Roth, N. A. Siegel, R. W. Johnson Jr, L. Litauszki, L. Salvati Jr, C. A. Harrington and L. K. Wray, J. Anal. Toxicol., 1996, 20, 291–300 CrossRef PubMed.
- T. Moreno, F. Montanes, S. J. Tallon, T. Fenton and J. W. King, J. Supercrit. Fluids, 2020, 161, 104850 CrossRef.
- M. Sexton, K. Shelton, P. Haley and M. West, Planta Med., 2018, 84, 234–241 CrossRef.
- D. R. Grijó, I. A. V. Osorio and L. Cardozo-Filho, J. CO2 Util., 2018, 28, 174–180 CrossRef.
- C. Da Porto, D. Decorti and A. Natolino, Ind. Crops Prod., 2014, 58, 99–103 CrossRef.
- L. L. Romano and A. Hazekamp, Cannabinoids, 2013, 1, 1–11 Search PubMed.
- A. Casiraghi, G. Roda, E. Casagni, C. Cristina, U. M. Musazzi, S. Franzè, P. Rocco, C. Giuliani, G. Fico and P. Minghetti, Planta Med., 2018, 84, 242–249 CrossRef.
- M.-V. Morar, K. Dragan, C. Bele, C. Matea, I. Tarta, R. Suharovschi and C. Semeniuc, Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca, Agric., 2010, 67, 2 Search PubMed.
- A. C. Gallo-Molina, H. I. Castro-Vargas, W. F. Garzón-Méndez, J. A. M. Ramírez, Z. J. R. Monroy, J. W. King and F. Parada-Alfonso, J. Supercrit. Fluids, 2019, 146, 208–216 CrossRef.
- L. J. Rovetto and N. V. Aieta, J. Supercrit. Fluids, 2017, 129, 16–27 CrossRef.
- C. Da Porto, D. Voinovich, D. Decorti and A. Natolino, J. Supercrit. Fluids, 2012, 68, 45–51 CrossRef.
- Z. Drinić, S. Vidović, J. Vladić, A. Koren, B. Kiprovski and V. Sikora, Lek. Sirovine, 2018, 17–21 CrossRef.
- S.-S. Teh and E. J. Birch, Ultrason. Sonochem., 2014, 21, 346–353 CrossRef CAS PubMed.
- S.-S. Teh, B. E. Niven, A. E.-D. A. Bekhit, A. Carne and E. J. Birch, Food Bioprocess Technol., 2014, 7, 3064–3076 CrossRef CAS.
- J. Y. Lin, Q. X. Zeng, Q. An, Q. Z. Zeng, L. X. Jian and Z. W. Zhu, J. Food Process Eng., 2012, 35, 76–90 CrossRef CAS.
- D. R. Grijó, G. K. Piva, I. V. Osorio and L. Cardozo-Filho, J. Supercrit. Fluids, 2019, 143, 268–274 CrossRef.
- K. Aladić, S. Vidović, J. Vladić, D. Balić, H. Jukić and S. Jokić, Int. J. Food Sci. Technol., 2016, 51, 885–893 CrossRef.
- E. Vági, M. Balázs, A. Komóczi, I. Kiss, M. Mihalovits and E. Székely, Period. Polytech., Chem. Eng., 2019, 63, 357–363 CrossRef.
- D. Fiorini, S. Scortichini, G. Bonacucina, N. G. Greco, E. Mazzara, R. Petrelli, J. Torresi, F. Maggi and M. Cespi, Ind. Crops Prod., 2020, 154, 112688 CrossRef CAS.
- J. Liang, E. Zago, R. Nandasiri, R. Khattab, N. M. Eskin, P. Eck and U. Thiyam-Holländer, J. Am. Oil Chem. Soc., 2018, 95, 1319–1327 CrossRef CAS.
- Y. Nuapia, H. Tutu, L. Chimuka and E. Cukrowska, Molecules, 2020, 25, 1335 CrossRef CAS PubMed.
- M. Crimaldi, S. Faugno, M. Sannino and L. Ardito, Chem. Eng. Trans., 2017, 58, 373–378 Search PubMed.
- S. Rochfort, A. Isbel, V. Ezernieks, A. Elkins, D. Vincent, M. A. Deseo and G. C. Spangenberg, Sci. Rep., 2020, 10, 1–7 CrossRef.
- T. Väisänen, P. Kilpeläinen, V. Kitunen, R. Lappalainen and L. Tomppo, Ind. Crops Prod., 2019, 131, 224–233 CrossRef.
- K. Leiman, L. Colomo, S. Armenta, M. de la Guardia and F. A. Esteve-Turrillas, Talanta, 2018, 190, 321–326 CrossRef CAS PubMed.
- J. Omar, M. Olivares, M. Alzaga and N. Etxebarria, J. Sep. Sci., 2013, 36, 1397–1404 CrossRef CAS PubMed.
- D. De Vita, V. N. Madia, V. Tudino, F. Saccoliti, A. De Leo, A. Messore, P. Roscilli, A. Botto, I. Pindinello and G. Santilli, Nat. Prod. Res., 2020, 34, 2952–2958 CrossRef CAS PubMed.
- C.-W. Chang, C.-C. Yen, M.-T. Wu, M.-C. Hsu and Y.-T. Wu, Molecules, 2017, 22, 1894 CrossRef.
- S. Naz, M. A. Hanif, T. M. Ansari and J. N. Al-Sabahi, Spectrosc. Spectral Anal., 2017, 37, 306–311 Search PubMed.
- S. Naz, M. A. Hanif, H. N. Bhatti and T. M. Ansari, J. Essent. Oil-Bear. Plants, 2017, 20, 175–184 CrossRef.
- M. D. Kostić, N. M. Joković, O. S. Stamenković, K. M. Rajković, P. S. Milić and V. B. Veljković, Ind. Crops Prod., 2014, 52, 679–686 CrossRef.
- S. Latif and F. Anwar, Eur. J. Lipid Sci. Technol., 2009, 111, 1042–1048 CrossRef.
- D. Fiorini, A. Molle, M. Nabissi, G. Santini, G. Benelli and F. Maggi, Ind. Crops Prod., 2019, 128, 581–589 CrossRef.
- A. Aiello, F. Pizzolongo, G. Scognamiglio, A. Romano, P. Masi and R. Romano, Int. J. Food Sci. Technol., 2020, 55, 2472–2480 CrossRef.
- L. Eöry, B. Dános and T. Veress, Z Zagadnien Nauk Sadowych, 2001, 47, 322–327 Search PubMed.
- C. Da Porto, D. Decorti and F. Tubaro, Ind. Crops Prod., 2012, 36, 401–404 CrossRef CAS.
- C. Da Porto, A. Natolino and D. Decorti, J. Food Sci. Technol., 2015, 52, 1748–1753 CrossRef CAS.
- K. Tomita, S. Machmudah, A. T. Quitain, M. Sasaki, R. Fukuzato and M. Goto, J. Supercrit. Fluids, 2013, 79, 109–113 CrossRef CAS.
- K. Aladić, K. Jarni, T. Barbir, S. Vidović, J. Vladić, M. Bilić and S. Jokić, Ind. Crops Prod., 2015, 76, 472–478 CrossRef.
- V. Devi and S. Khanam, J. Environ. Chem. Eng., 2019, 7, 102818 CrossRef CAS.
- C. Kornpointner, A. Sainz Martinez, S. Marinovic, C. Haselmair-Gosch, P. Jamnik, K. Schröder, C. Löfke and H. Halbwirth, Ind. Crops Prod., 2021, 165, 113422 CrossRef CAS.
- C. Kornpointner, A. Sainz Martinez, M. Schnürch, H. Halbwirth and K. Bica-Schröder, Green Chem., 2021, 23, 10079–10089 RSC.
- C. Cai, W. Yu, C. Wang, L. Liu, F. Li and Z. Tan, J. Mol. Liq., 2019, 287, 110957 CrossRef CAS.
- C. Cai, Y. Wang, Y. Yi, F. Li and Z. Tan, Ind. Crops Prod., 2020, 155, 112796 CrossRef CAS.
- T. M. Attard, C. Bainier, M. Reinaud, A. Lanot, S. J. McQueen-Mason and A. J. Hunt, Ind. Crops Prod., 2018, 112, 38–46 CrossRef CAS.
- E. Vági, M. Balázs, A. Komoczi, M. Mihalovits and E. Székely, J. Supercrit. Fluids, 2020, 164, 104898 CrossRef.
- V. Kitrytė, D. Bagdonaitė and P. R. Venskutonis, Food Chem., 2018, 267, 420–429 CrossRef.
- K. Aladić, S. Jokić, T. Moslavac, S. Tomas, S. Vidović, J. Vladić and D. Šubarić, Chem. Biochem. Eng. Q., 2014, 28, 481–490 CrossRef.
- T. Křížek, M. Bursová, R. Horsley, M. Kuchař, P. Tůma, R. Čabala and T. Hložek, J. Cleaner Prod., 2018, 193, 391–396 CrossRef.
- M. D. Kostić, N. M. Joković, O. S. Stamenković, K. M. Rajković, P. S. Milić and V. B. Veljković, Ind. Crops Prod., 2013, 48, 133–143 CrossRef.
- C. Agarwal, K. Máthé, T. Hofmann and L. Csóka, J. Food Sci., 2018, 83, 700–710 CrossRef.
- A. Rezvankhah, Z. Emam-Djomeh, M. Safari, G. Askari and M. Salami, J. Food Sci. Technol., 2019, 56, 4198–4210 CrossRef.
- Z. Drinić, J. Vladić, A. Koren, T. Zeremski, N. Stojanov, B. Kiprovski and S. Vidović, J. Chem. Technol. Biotechnol., 2020, 95, 831–839 CrossRef.
- S. Faugno, S. Piccolella, M. Sannino, L. Principio, G. Crescente, G. M. Baldi, N. Fiorentino and S. Pacifico, Ind. Crops Prod., 2019, 130, 511–519 CrossRef.
- V. Devi and S. Khanam, J. Cleaner Prod., 2019, 207, 645–657 CrossRef.
- L. Candy, S. Bassil, L. Rigal, V. Simon and C. Raynaud, Ind. Crops Prod., 2017, 109, 335–345 CrossRef CAS.
- F. Potin, S. Lubbers, F. Husson and R. Saurel, J. Food Sci., 2019, 84, 3682–3690 CrossRef CAS PubMed.
- M. P. Lazarjani, O. Young, L. Kebede and A. Seyfoddin, J. Cannabis Res, 2021, 3, 32 CrossRef PubMed.
- World-Health-Organization, Drugs (psychoactive), https://www.who.int/health-topics/drugs-psychoactive#tab=tab_3, accessed October 2022 Search PubMed.
- NHS, Psychotropic Medication, https://www.datadictionary.nhs.uk/nhs_business_definitions/psychotropic_medication.html, accessed October 2022.
- F. Caraci, S. J. Enna, J. Zohar, G. Racagni, G. Zalsman, W. van den Brink, S. Kasper, G. F. Koob, C. M. Pariante, P. V. Piazza, K. Yamada, M. Spedding and F. Drago, Br. J. Clin. Pharmacol., 2017, 83, 1614–1616 CrossRef.
- J. McMurry, Organic Chemistry, Thomson-Brooks/Cole, 2004 Search PubMed.
- F. Pattnaik, S. Nanda, S. Mohanty, A. K. Dalai, V. Kumar, S. K. Ponnusamy and S. Naik, Chemosphere, 2022, 289, 133012 CrossRef CAS PubMed.
- H. M. S. AL Ubeed, D. J. Bhuyan, M. A. Alsherbiny, A. Basu and Q. V. Vuong, Molecules, 2022, 27, 604 CrossRef CAS.
- S. Qamar, Y. J. M. Torres, H. S. Parekh and J. Robert Falconer, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2021, 1167, 122581 CrossRef CAS.
- J. Peach and J. Eastoe, Beilstein J. Org. Chem., 2014, 10, 1878–1895 CrossRef.
- E. L. N. Escobar, T. A. Da Silva, C. L. Pirich, M. L. Corazza and L. P. Ramos, Front. Bioeng. Biotechnol., 2020, 8, 252 CrossRef PubMed.
- M. Freemantle, An introduction to ionic liquids, RSC Publishing, Cambridge, UK, 2009 Search PubMed.
- M. J. Earle and K. R. Seddon, Pure Appl. Chem., 2000, 72, 1391–1398 CrossRef CAS.
- A. K. Ressmann and K. Bica, in Ionic Liquids for Better Separation Processes, Springer, 2016, pp. 135–165 Search PubMed.
- H. Wang, G. Gurau and R. D. Rogers, Chem. Soc. Rev., 2012, 41, 1519–1537 RSC.
- N. Mosier, C. Wyman, B. Dale, R. Elander, Y. Y. Lee, M. Holtzapple and M. Ladisch, Bioresour. Technol., 2005, 96, 673–686 CrossRef CAS PubMed.
- E. L. Smith, A. P. Abbott and K. S. Ryder, Chem. Rev., 2014, 114, 11060–11082 CrossRef CAS.
- D. Rengstl, V. Fischer and W. Kunz, Phys. Chem. Chem. Phys., 2014, 16, 22815–22822 RSC.
- S. A. Malomo and R. E. Aluko, Food Hydrocolloids, 2015, 43, 743–752 CrossRef CAS.
- J. M. Danlami, A. Arsad, M. A. A. Zaini and H. Sulaiman, Rev. Chem. Eng., 2014, 30, 605–626 CAS.
- F. Chemat, N. Rombaut, A.-G. Sicaire, A. Meullemiestre, A.-S. Fabiano-Tixier and M. Abert-Vian, Ultrason. Sonochem., 2017, 34, 540–560 CrossRef CAS.
- B. Maazinejad, O. Mohammadnia, G. A. M. Ali, A. S. H. Makhlouf, M. N. Nadagouda, M. Sillanpää, A. M. Asiri, S. Agarwal, V. K. Gupta and H. Sadegh, J. Mol. Liq., 2020, 298, 112001 CrossRef.
- N. Flórez, E. Conde and H. Domínguez, J. Chem. Technol. Biotechnol., 2015, 90, 590–607 CrossRef.
- H.-F. Zhang, X.-H. Yang and Y. Wang, Trends Food Sci. Technol., 2011, 22, 672–688 CrossRef.
- S. Serna-Loaiza, J. Adamcyk, S. Beisl, C. Kornpointner, H. Halbwirth and A. Friedl, Processes, 2020, 8, 1334 CrossRef.
- A. Oreopoulou, D. Tsimogiannis and V. Oreopoulou, in Polyphenols in Plants, ed. R. R. Watson, 2nd edn, Academic Press, 2019, ch. 15, pp. 243–259 Search PubMed.
- V. Gunjević, G. Grillo, D. Carnaroglio, A. Binello, A. Barge and G. Cravotto, Ind. Crops Prod., 2021, 162, 113247 CrossRef.
- B. Cakaloglu, V. H. Ozyurt and S. Otles, Ukr. Food J., 2018, 7, 640–654 CrossRef.
Footnote |
| † Both authors have contributed equally to the manuscript. |
| This journal is © The Royal Society of Chemistry 2023 |