Engineering fungal terpene biosynthesis
Zhiyong
Yin
and
Jeroen S.
Dickschat
 *
*
Kekulé-Institute for Organic Chemistry and Biochemistry, University of Bonn, Gerhard-Domagk-Straße 1, 53121 Bonn, Germany. E-mail: dickschat@uni-bonn.de
First published on 23rd May 2022
Abstract
Covering: 2015 to 2022
Fungal terpenoids are of large structural diversity and often exhibit interesting biological activities. Recent work has focused on two main aspects: (1) the discovery and understanding of unknown biosynthetic genes and pathways, and (2) the usage of already known biosynthetic genes in the construction of high yielding production strains. Both aspects will be covered in this review article that aims to summarise the most important work of the past few years.
1 Introduction
Fungi are an important source of bioactive natural products.1,2 Although the exact number of fungal species is unknown, it is estimated that the diversity of fungal species exceeds that of land plants by a ratio of approximately 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 They can be used not only as food resources, but also in medicinal applications for the treatment of diseases.3 In order to adapt to their ecological niches and environmental conditions, fungi have evolved extensive metabolic pathways towards natural products with antibacterial, antifungal or cytotoxic effects.4 Among these, terpenes constitute the largest class and often exhibit substantial biological activities.5,6 Their biosynthesis usually proceeds through four stages, initiated by formation of the monomers dimethylallyl diphosphate (DMAPP) and isopentenyl diphosphate (IPP), followed by their fusion to oligoprenyl diphosphates, terpene cyclisation and functionalisation e.g. through oxidations.7–9
1.1 They can be used not only as food resources, but also in medicinal applications for the treatment of diseases.3 In order to adapt to their ecological niches and environmental conditions, fungi have evolved extensive metabolic pathways towards natural products with antibacterial, antifungal or cytotoxic effects.4 Among these, terpenes constitute the largest class and often exhibit substantial biological activities.5,6 Their biosynthesis usually proceeds through four stages, initiated by formation of the monomers dimethylallyl diphosphate (DMAPP) and isopentenyl diphosphate (IPP), followed by their fusion to oligoprenyl diphosphates, terpene cyclisation and functionalisation e.g. through oxidations.7–9
Engineering of metabolic pathways towards terpenes in fungi may follow two main aims. First, increase of the metabolic flux towards a specific product may be addressed, for which targeting the first steps towards the oligoprenyl diphosphates is of special interest.10 Second, metabolic engineering has a large potential in the discovery of unknown pathways and the development of new drugs with tailored properties for which addressing the late biosynthetic stages are relevant.11,12 Widely applied methods in pathway engineering include heterologous gene expressions,13 overexpressions of silent or poorly expressed genes in their native hosts for pathway activation, gene deletions and knockdown strategies.14 The catalytic efficiency of single enzymes or their product spectra can be addressed by site-directed mutagenesis,15 domain swapping16 and similar approaches. Current gene transformation techniques focus on the incorporation of exogenous DNA fragments into the fungal chromosome for stable expression. Commonly used transformation methods include polyethylene glycol (PEG) transformation,17Agrobacterium tumefaciens-mediated transformation (ATMT)18 and electroporation.19 Gene overexpression in fungi is mainly achieved through promoter engineering,20 but also interference with global or epigenetic regulators can be applied. Homologous recombination strategies allow for gene deletions or substitutions. As an alternative, gene knockdown through RNA interference (RNAi) is widely applied.21 This technique leaves the target gene intact, but can inhibit protein biosynthesis and consequently biosynthetic transformations by promoting mRNA degradation. All these strategies are important for the analysis of gene functions in biosynthesis, but are also of interest for metabolic flux regulation to increase the yield of a specific natural product. This review summarises the recent examples of engineering fungal terpene biosynthesis, covering not only investigations on fungal terpenoids, but also heterologous expressions of terpenoid biosynthesis genes from other organisms such as bacteria and plants in fungal hosts. While another recent review mainly addressed the biology of engineering fungal pathways towards terpenes,14 this article will focus on the structural diversity of terpenoids obtained through metabolic engineering and the discovery of biosynthetic pathways leading to them.
2 Biosynthesis of terpenes
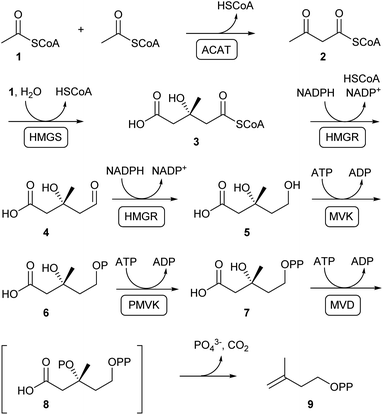
Terpenoids are assembled from DMAPP and IPP that are produced through the mevalonate (MVA) or the methylerythritol 4-phosphate (MEP) pathway, depending on the organism.22,23 In plants both pathways exist with the MVA pathway operating in the cytosol and the MEP pathway located in the plastids. Bacteria with some exceptions usually produce terpenes through the MEP pathway, while terpene biosynthesis in fungi strictly depends on the MVA pathway. Despite the fact that both pathways operate through completely different intermediates, the final products DMAPP and IPP are the same.The MVA pathway relevant to fungi (Scheme 1) starts with a Claisen condensation of two molecules of acetyl-coenzyme A (acetyl-CoA, 1) to generate acetoacetyl-CoA (2), followed by aldol addition of another unit of acetyl-CoA to obtain 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA, 3). The thioester in 3 is subsequently reduced through the aldehyde 4 to mevalonate (5), the eponymous pathway intermediate. Two sequential phosphorylations of the primary alcohol proceed via6 to mevalonate diphosphate (7). A subsequent third ATP-dependent reaction leads to the hypothetical intermediate 8 that collapses by elimination of phosphate and decarboxylation to IPP (9).
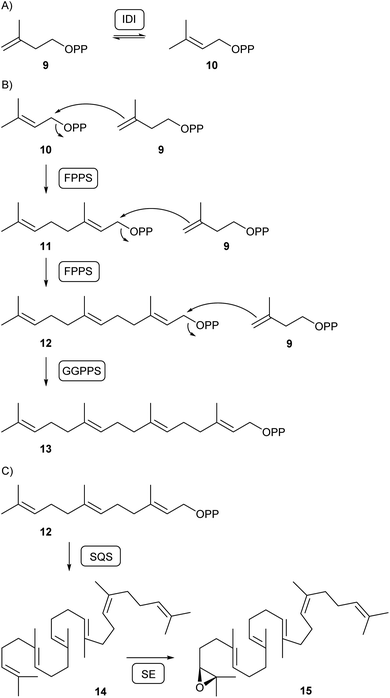
Isopentenyl diphosphate isomerase (IDI) is then responsible for the isomerisation of IPP and DMAPP (10, Scheme 2A). While DMAPP is a C1 electrophile, C4 of IPP can react as a nucleophile, allowing for their fusion to geranyl diphosphate (GPP, 11) and, with attack of another IPP to GPP, farnesyl diphosphate (FPP, 12) by FPP synthase (FPPS) that was first characterised in fungi from Neurospora crassa and Fusarium fujikuroi (Scheme 2B).24 In N. crassa further elongation to geranylgeranyl diphosphate (GGPP, 13) requires a second specialised prenyltransferase, the GGPP synthase (GGPPS).25 Subsequent conversion of FPP into squalene (14) by the sqalene synthase (SQS) and epoxidation to (S)-2,3-oxidosqualene (15) by squalene epoxidase (SE) is relevant for the biosynthesis of triterpenes in all fungi (Scheme 2C).
3 Heterologous expression in yeast
After the pioneering work at Amyris Inc. that gave access to artemisinic acid by heterologous gene expression in yeast,26 several recent studies have made use of this approach to construct high yielding yeast strains for the production of valuable terpenoids. Such work is of particular interest for highly bioactive compounds with potential medicinal applications, but also for flavours, food ingredients and biofuels. Good production levels could frequently be obtained through the overexpression of genes for precursor supply, with HMGR and FPPS being the bottlenecks in many cases. This section will summarise examples that made use of Saccharomyces cerevisiae and other yeast strains as heterologous hosts. Yeast strains have the advantage that they are fast growing, easy to cultivate on cheap carbon sources (saccharose), and are generally regarded as safe.3.1 Heterologous production of monoterpenes in yeast
Limonene (16) is a cyclic monoterpene of plant origin and is widely used in the perfume and flavour industry.27 Its classical biosynthesis pathway proceeds through the FPPS-dependent formation of GPP (11) and cyclisation by limonene synthase (LS) to 16 (Scheme 3). A FPPS variant (F96W,N127W; FPPSF96W,N127W) has been developed that selectively produces GPP without further elongation to FPP.28 Expression of the gene for FPPSF96W,N127W, in combination with nine plant LS genes with truncation of the N-terminal sequence for the transit peptide that targets the plastid membrane (tLS), in a yeast strain engineered for high isoprenoid production gave with 15.5 mg L−1 of 16 best results for Citrus limon tLS1 (CltLS1).29 Another pathway towards 16 is known from tomato that proceeds through coupling of DMAPP and IPP to nerolidyl diphosphate (NPP, (Z)-11), by Solanum lycopersicum nerolidyl diphosphate synthase (SlNDPS1) and cyclisation to 16.30 Expression of the genes for SlNDPS1 and Citrus limon tLS2 (CltLS2) in the same yeast strain gave a higher production of 16 than through the classical pathway (28.9 mg L−1). Extensive pathway engineering with placing the competing FPPS gene under the glucose-sensing promoter HXT1 allowed to raise production levels of 16 to more than 900 mg L−1.29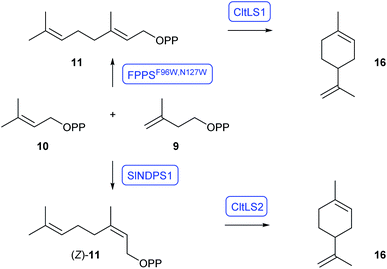 | ||
| Scheme 3 Orthogonal strategies for the production of 16 in Saccharomyces cerevisiae. Enzymes for which genes were heterologously expressed are shown in blue. | ||
Nepetalactol (20) is an intermediate towards the large class of monoterpene indole alkaloids. The whole pathway towards strictosidin as it occurs in plants has been reconstructed in yeast by O'Connor and coworkers with an impressive number of genes transferred for heterologous expression.31 Despite this promising success, strictosidine production reached only levels of 0.5 mg L−1 (ref. 31) and further strain optimisation is desired. This problem was recently addressed by the Tang group through heterologous expression of the whole terpene pathway towards GPP, using the N144W variant of Gallus gallus FPPS (GgFPPSN144W) that is selective for GPP production,32 with targeting to the mitochondria to prevent cytosolic GPP elongation to FPP by native S. cerevisiae FPPS.33 Additional expression of Ocimum basilicum geraniol synthase (ObGES) and Catharanthus roseus geraniol 8-hydroxylase (CrG8H) in the mitochondria gave ca. 230 mg L−1 of 8-hydroxygeraniol (18) in fed-batch fermentations. The strain was further elaborated by cytosolic expression of C. roseus geraniol oxidoreductase (CrGOR) and iridoid synthase (CrISY) for the oxidation to 19 and reductive cyclisation to 20, yielding 6 mg L−1 of nepetalactol (Scheme 4).33
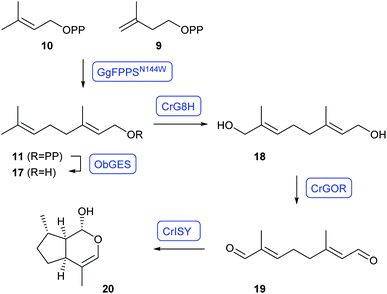 | ||
| Scheme 4 Reconstitution of nepetalactol (20) biosynthesis in S. cerevisiae. Enzymes for which genes were heterologously expressed are shown in blue. | ||
3.2 Heterologous production of sesquiterpenes in yeast
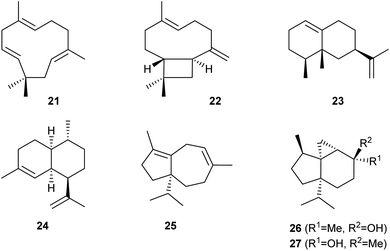
Heterologous expression in S. cerevisiae YZL141 was also used to investigate the functions of eleven candidate sesquiterpene synthase genes from Ocimum sanctum. In six cases, the production of sesquiterpenes was observed, including (E)-β-caryophyllene (21) by OsaTPS02, α-humulene (22) by OsaTPS12, and (−)-eremophilene (23) by OsaTPS07 (Scheme 5). A high yielding strain for the production of 23 was obtained by multiple insertions of the coding sequence for OsaTPS07 into the chromosome, resulting in a high gene copy number and a production of 700 mg L−1 of 23 in shaken flasks. Optimisation of the fermentation conditions in a 15 L steel tank gave excellent yields of 35 g L−1.34Amorpha-4,11-diene (24, Scheme 5) is the parent hydrocarbon of the antimalaria drug artemisinin. Pioneering work by Keasling and coworkers has made the biosynthetic intermediate artemisinic acid available from an engineered yeast strain,35 which can be converted through chemical synthesis into artemisinin.36 Recently, an engineered yeast strain has been constructed in which the enzymes for the whole biosynthetic pathway from acetyl-CoA to FPP and amorpha-4,11-diene synthase were expressed in the mitochondria.37 The use of mitochondria is considered a promising approach to terpene biosynthesis not only because of the abundant supply of acetyl-CoA and redox equivalents in mitochondria, but also because compartmentalisation of the pathway to terpenes allows for higher substrate and enzyme concentrations and will thus speed up the enzymatic reactions. Furthermore, competing pathways e.g. to ergosterol cannot interfere, because the mitochondrial membrane may serve as a barrier for the translocation of FPP to the cytosole. High yields of 24 (ca. 400 mg L−1) were reported.37
The sesquiterpene synthase gene FgJ03939 from Fusarium graminearum was studied in vitro by incubation with GPP, FPP and GGPP, showing terpene production with all three substrates. The most efficient conversion was observed with FPP, suggesting that this enzyme naturally functions as a sesquiterpene synthase. This was confirmed by heterologous expression in an FPP overproducing S. cerevisiae, allowing the isolation of the three new compounds fusariumdiene (25), fusagramineol (26) and epi-fusagramineol (27) by this metabolic engineering approach (Scheme 5).38
3.3 Heterologous production of diterpenes in yeast
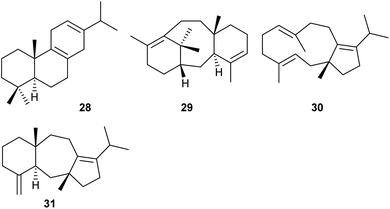
S. cerevisiae was also selected as a chassis for heterologous miltiradiene (28, Scheme 6) production. Miltiradiene is a precursor to many bioactive abietane diterpenes.39 A high-yielding strain for GGPP was obtained by overexpression of key genes for terpene precursor supply, including genes for HMGR, FPPS and GGPPS, down-regulation of genes for the competing metabolic pathway towards squalene, knockout of transcription factors that exert a repressive effect on the metabolic flow towards terpenes, and medium optimisation.39 In this background, different enzymes for the biosynthesis of 28 were expressed. Miltiradiene biosynthesis requires a type II and a type I terpene synthase for conversion of GGPP into copalyl diphosphate and its subsequent cyclisation to 28. The best titres (550 mg L−1 of 28) were obtained for an N-terminally truncated fusion protein of the copalyl diphosphate synthase CfTPS1 from Coleus forskohlii and the type I terpene synthase SmKSL1 from Salvia miltiorrhiza.39Taxadiene (29, Scheme 6) from Taxus brevifolia is the biosynthetic precursor of the anticancer drug taxol, requiring several cytochrome P450 mediated oxidations and other modifications.40 High titer production of 29 has been achieved in engineered Escherichia coli strains, yielding more than 1 g L−1 of 29 in fed-batch bioreactor cultivations.41 A drawback of this host may be that CYP450 oxidations are often difficult to achieve in E. coli. To overcome this problem, recently an engineered yeast strain was constructed that gave access to 29 with yields of ca. 130 mg L−1, using multi-copy chromosomal integration of taxadiene synthase and cultivation at 20 °C. Furthermore, the enzyme solubility was improved through removal of 60 amino acids at the N-terminus that cause formation of inclusion bodies and fusion to a protein solubility tag.42
The chimeric diterpene synthase from the endophytic fungus Colletotrichum gloeosporioides ES026 encoded by the Cgl13742 gene is composed of a prenyltransferase domain (PT) for GGPP biosynthesis and a terpene synthase domain (TS). The bifunctional PTTS enzyme was studied by overexpression of key enzymes for precursor supply in the native host, through in vitro incubation of the recombinant enzyme with oligoprenyl diphosphates, and through heterologous expression in S. cerevisiae ZW140 engineered for terpene precursor supply. Taken together, the experiments resulted in the isolation and identification of the products (R)-δ-araneosene (30) and (5R,12R,14S)-dolasta-1(15),8-diene (31, Scheme 6). Their absolute configurations were determined by chemical correlation through the enzymatic conversion of stereoselectively deuterated precursors, and the enzyme mechanism was studied in extensive isotopic labelling experiments.43
3.4 Heterologous production of sesterterpenes in yeast
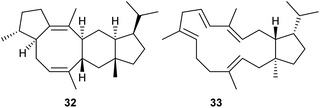
Heterologous expression of 74 selected fungal PTTS genes in yeast engineered for terpene precursor supply resulted in di- or sesterterpene production in 34 cases.44 Most of the investigated enzymes yielded known compounds, while the enzymes ZbSS from Zymoseptoria brevis and CoSS from Colletotrichum orbiculare yielded the new sesterterpenes sesterevisene (32) and sesterorbiculene (33) (Scheme 7).443.5 Heterologous production of triterpenes in yeast
Compound K (36) is a dammarane-type triterpenoid from the ginsenoside family isolated from Panax ginseng with antitumor activity in the low micromolar range.45,46 Its biosynthesis proceeds through cyclisation of 36 by the dammarenediol II synthase (DS) to dammarenediol II (34), followed by cytochrome P450 (CYP450, PPDS) mediated oxidation to protopanaxadiol (35, Scheme 8). Glycosylation by UGT1 leads to 36. Heterologous expression of the genes for DS, PPDS and UGT1 together with the gene for a CYP450 reductase from Arabidopsis thaliana (ATR1) in the yeast Yarrowia lipolytica yielded 36 (5.1 mg L−1). Overexpression of key genes of the MVA pathway together with fusion of PPDS to the CYP450 reductase and optimisation of fermentation conditions resulted in an increased production (160 mg L−1).47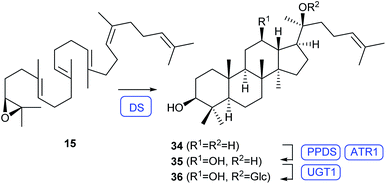 | ||
| Scheme 8 Biosynthesis of 36 in Y. lipolytica. Enzymes for which genes were heterologously expressed are shown in blue. | ||
The biosynthesis of glycyrrhetinic acid (41) in liquorice plants requires the cyclisation of 15 to β-amyrin (37) by β-amyrin synthase (βAS), its oxidation to 11-oxo-β-amyrin (38) by the cytochrome P450 monooxygenase CYP88D6, and downstream oxidations to 41 by CYP72A154 (Scheme 9).48,49 The functionality of this pathway was demonstrated by its genetic reconstitution in yeast that resulted in the production of 41.48–50 This work also identified CYP72A63 from Medicago truncatula, an enyzme with high sequence identity to CYP72A154 that can functionally substitute in the C30 oxidation of 38 in yeast.49 Recently a high yielding production strain for 38 (>100 mg L−1) was reported that made use of high copy number expressions of Glycyrrhiza genes for βAS, the CYP450 Uni25647 showing 97% amino acid similarity to CYP88D6, and the CYP450 reductase GuCPR1 in S. cerevisiae.51 Additional expression of CYP72A63 from M. truncatula resulted in the formation of four oxidation products, including glycyrrhetol (39), glycyrrhetaldehyde (40), 41 and 29-hydroxy-11-oxo-β-amyrin (42).51,52 Based on a structural homology model with docked substrates key enzyme residues of CYP72A63 were identified. Site-directed mutagenesis and heterologous expression in yeast selectively yielded the oxidation products 39 (91% of total oxidation products, from the L509I variant), 40 (82%, from T338S in conjunction with the CYP450 reductase GuCPR2), 41 (94%, from T338S), and 42 as a single product from the L398I variant.52
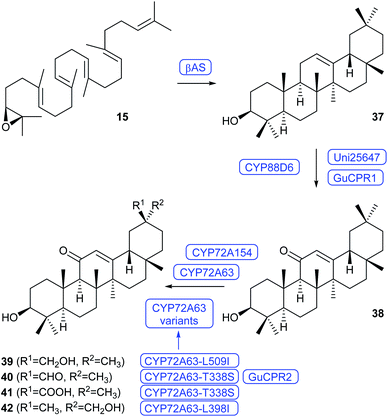 | ||
| Scheme 9 Biosynthesis of the liquorice root compounds 39–42 in S. cerevisiae. Enzymes for which genes were heterologously expressed are shown in blue. | ||
3.6 Heterologous production of carotenoids in yeast
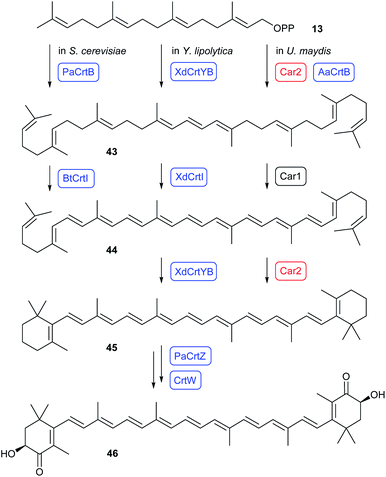
Lycopene is a compound with a variety of biological activities including antioxidant and anti-cancer activity.53 The production of lycopene (44, Scheme 10, left) in S. cerevisiae was optimised by systematically investigating all possible combinations of genes for a GGPPS (CrtE) from five organisms (Pantoea agglomerans, Sulfolobus acidocaldarius, Archaeglobus fulgidus, Blakeslea trispora and Taxus media), the phytoene (43) synthase CrtB from two organisms (Agrobacterium aurantiacum and P. agglomerans), and the lycopene synthase CrtI from three organisms (Agrobacterium aurantiacum, P. agglomerans and B. trispora), placed under galactose inducible promoters in all cases.Further strain engineering gave a lycopene production of ca. 55 mg g−1 cell dry weight (cdw).54 Based on this work, an upregulation of HMGR was conducted to improve metabolic flux through the MVA pathway.55 Subsequent heterologous expression of the established optimal combination of lycopene pathway genes for a GGPPS (CrtE from Taxus media), the phytoene synthase CrtB from P. agglomerans (PaCrtB) and the lycopene synthase CrtI from B. trispora (BtCrtI)54 resulted in a lycopene production of 4.9 mg g−1 cdw.55 Additionally, pathway engineering was used to direct the carbon flux from ethanol to acetyl-CoA, to improve NADPH cofactor availability, and to optimise triacylglycerol biosynthesis and lipid droplet formation for efficient accumulation of 44 in lipid droplets. This work resulted in a lycopene production of ca. 70 mg g−1 cdw.55
Astaxanthin (46) is a carotenoid and lipid-soluble pigment that is widely used in the food industry.56 The full pathway to 46 was reconstituted in Y. lipolytica by heterologous expression of the bifunctional phytoene synthase/lycopene cyclase CrtYB and the phytoene desaturase CrtI from the red yeast Xanthophyllomyces dendrorhous (Scheme 10, middle). Together with pathway engineering for increased precursor supply including downregulation of competing genes this resulted in a good β-carotene (45) production (800 mg L−1). Additional heterologous expression of the hydroxylase CrtZ from Pantoea ananatis and of the β-carotene ketolase CrtW from Paracoccus sp. yielded >50 mg L−146, besides various oxidised on-pathway intermediates along the transformations from 45 to 46.57
4 Heterologous expression in basidiomycetes
Besides yeast strains, basidiomycetes have also been established as heterologous expression hosts. Ustilago maydis produces β-carotene (45) from GGPP (13) through the bifunctional enzyme Car2 that couples two GGPP units to phytoene (43, Scheme 10, right). The lycopene synthase Car1 then carries out the oxidation to lycopene 44, followed by cyclisation to 45 by the second domain of Car2. Deletion of the car2 locus and heterologous expression of the phytoene synthase AaCrtB from Agrobacterium aurantium under the constitutive promoter Prpl40 with simultaneous overexpression of mevalonate pathway genes in U. maydis resulted in the production of 44 (0.7 mg L−1).58In the same background of overexpressing the MVA pathway genes the heterologous expression of the gene for Callitropsis nootkatensis (+)-valencene synthase (CnVS)59 gave (+)-valencene (47, Scheme 11) with a yield of 5.5 mg L−1.58 While this work established U. maydis as a new host for heterologous terpene production, the production was much lower than reported before for the expression of the CnVS gene in an optimised Rhodobacter sphaeroides (352 mg L−1)59 or Saccharomyces cerevisiae (540 mg L−1).60 However, the heterologous expression of the coding sequence for the α-cuprenene synthase from Coprinus cinereus (Cop6)61 gave the satisfying result of 0.1 g L−1 of α-cuprenene (48).58
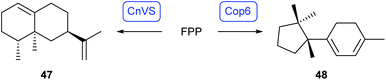 | ||
| Scheme 11 Biosynthesis of (+)-valencene (47) and of α-cuprenene (48) in U. maydis. Enzymes for which genes were heterologously expressed are shown in blue. | ||
5 Heterologous expression in Aspergillus
In 1987 Gomi et al. reported on the transformation of Aspergillus oryzae with a plasmid carrying the argB gene from Aspergillus nidulans and showed integration of the plasmid into the chromosome by non-homologous recombination.62 Traditionally, A. oryzae is used in Japanese sake brewing, and to avoid unpleasant flavours of toxic compounds strains have been selected over the centuries that do not produce secondary metabolites. Therefore, A. oryzae is a host that is ideal for the heterologous expression of genes for biosynthetic pathways to secondary metabolites, because compound isolations will be comparably easy. This strategy is today widely used and recent examples will be discussed in this section.5.1 Heterologous expression of sesquiterpenoid pathways in Aspergillus
The biosynthetic gene cluster for brasilane glycosides from the fungus Annulohypoxylon truncatum encoding the five genes braA–braE was identified and studied by heterologous expression in A. oryzae.63 The pathway proceeds by cyclisation of FPP by BraA likely to trichobrasilenol (49), the same compound as reported previously from a sesquiterpene synthase from Trichoderma,64 followed by glycosylation with N-acetylglycosamine by BraB to brasilane A (50) and sequential oxidations by the CYP450 BraC to brasilanes D (51) and E (52, Scheme 12).63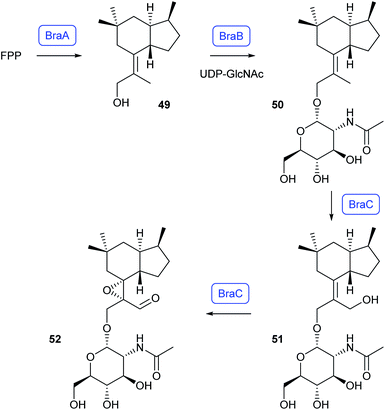 | ||
| Scheme 12 Biosynthesis of brasilane glycosides. Enzymes for which genes were heterologously expressed are shown in blue. | ||
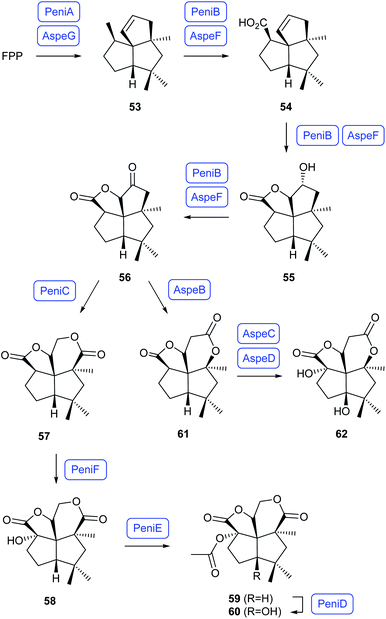
The biosyntheses of the dioxafenestrane sesquiterpenes penifulvenes and asperaculins have been investigated in the terrestrial plant symbiont Penicillium griseofulvum and the marine fungus Aspergillus aculeatus. Heterologous expression of the genes for the terpene synthase PeniA, the CYP450 PeniB and the flavin dependent Baeyer–Villiger monooxygenase (BVMO) in Aspergillus nidulans in conjunction with gene knockout experiments and in vitro incubations with recombinant PeniA revealed the early steps of the pathway (Scheme 13). FPP is first cyclised to silphinene (53) by PeniA, followed by sequential oxidations to 54, 55 and 56 by PeniB and Baeyer–Villiger oxidation to 57 by PeniC.65 A similar gene cluster was discovered from A. aculeatus, and heterologous expression of the genes for the terpene synthase AspeG and the CYP450 AspeF in A. nidulans confirmed these enzymes have the same function as PeniA and PeniB in the biosynthesis of 56.66 Additional expression of the BMVO AspeB that exhibits a low sequence identity to PeniC in A. nidulans resulted in the formation of the lactone 61, demonstrating that the BMVOs PeniC and AspeB serve as a branching point in the two pathways.66 Heterologous expression of further genes from the biosynthetic gene clusters and in vitro incubations with purified enzymes revealed a strict sequence of steps in the Penicillium pathway with first hydroxylation to 58 by the α-ketoglutarate-dependent dioxygenase (αKGD) PeniF, acetylation by PeniE to 59, and another hydroxylation by the αKGD PeniD to 60, while the sequence of steps for two corresponding hydroxylations from 61 to 62 by AspeC and AspeD in the Aspergillus pathway is less strict.66
 | ||
| Scheme 13 Biosynthesis of penifulvenes and asperaculins. Enzymes for which genes were heterologously expressed are shown in blue. | ||
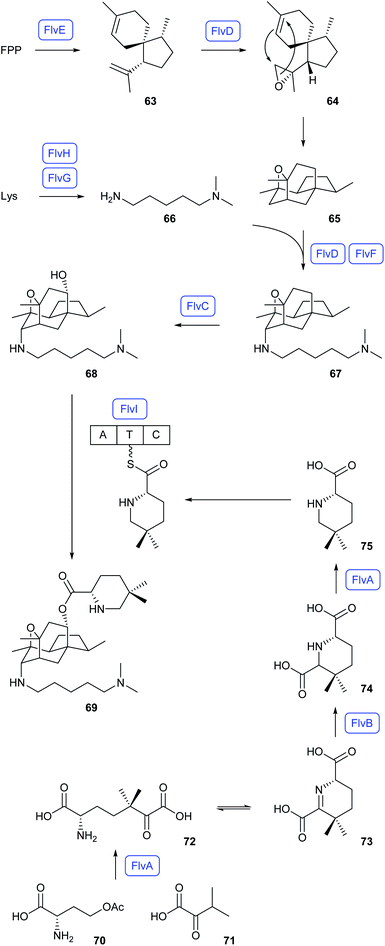
Heterologous expressions in A. nidulans also revealed the flavunoidine biosynthetic pathway in Aspergillus flavus (Scheme 14).67 Herein, FPP is converted into (1R,4R,5S)-acoradiene (63) by the terpene synthase FlvE, followed by CYP450 epoxidation (FlvD) to 64 and spontaneous reaction to 65. Its coupling with N,N-dimethylcadaverine (66), generated from lysine through the N-methyltransferase FlvH and the decarboxylase FlvG, requires the CYP450 FlvD and FlvF, an enzyme that shows homology to terpene synthases. The product 67 is hydroxylated to 68 by FlvC, followed by reaction with the NRPS (FlvI) bound substrate 75 to flavunoidine (69). Compound 75 is itself made from O-acetylhomoserine (70) and α-ketoisovalerate (71) that are fused to 72 by the pyridoxalphosphate (PLP) dependent enzyme FlvA. Cyclisation to 73, FlvB-mediated reduction to 74 and decarboxylation by FlvA result in 75.67
 | ||
| Scheme 14 Biosynthesis of flavunoidine. Enzymes for which genes were heterologously expressed are shown in blue. | ||
5.2 Heterologous expression of diterpenoid pathways in Aspergillus
The biosynthesis of erinacine Q (82) from the basidiomycete Hericium erinaceus was studied by heterologous expression of genes from the biosynthetic gene cluster in A. oryzae (Scheme 15).68 Along the pathway, GGPP is converted by the UbiA-related diterpene synthase EriG into cyatha-3,12-diene (76).69 Subsequent hydroxylations by the CYP450s EriI and EriC leads to erinacol (77) and cyathadiol (78).68 A third hydroxylation to cyathatriol (79) by the CYP450 EriA requires coexpression of the gene for either the short chain dehydrogenase/reductase EriH or the cognate cytochrome P450 reductase (CPR) from H. erinaceus. Acetylation by EriL results in 11-O-acetylcyathatriol (80) that is, in the absence of the glycosyltransferase EriJ oxidised by EriH to yield 11-O-acetylcyathin A3 (81). In vitro competition experiments confirmed a faster glycosylation of 80 by EriJ in comparison to oxidation by EriH. Expression of all ten genes of the biosynthetic gene cluster in A. oryzae did not yield the expected pathway product erinacine Q (82) with a xylose moiety, but resulted in erinacine Q2 (83) with a glycose moiety, which was explained by a possibly too low supply of the glycosyl donor UDP-xylose in A. oryzae. In vitro reactions of 80 with EriJ showed indeed a faster conversion with UDP-xylose to 82 than with UDP-glucose to 83. Also coexpression of the biosynthetic genes for AtUGD1 and AtUXS3 from Arabidopsis thaliana involved in the biosynthesis of UDP-xylose in A. oryzae resulted in the formation of 82.69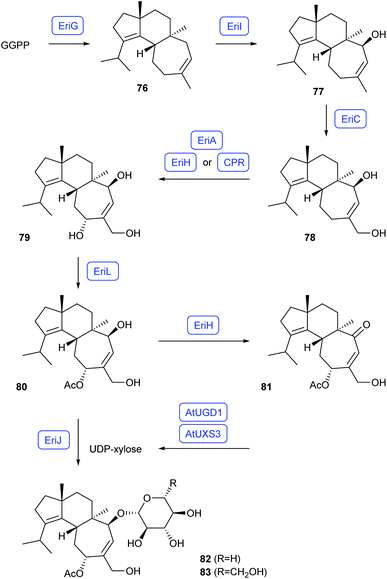 | ||
| Scheme 15 Biosynthesis of erinacine Q (82). Enzymes for which genes were heterologously expressed are shown in blue. | ||
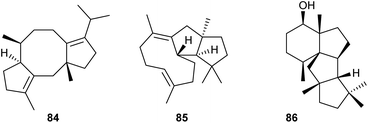
After the discovery of the first bifunctional chimeric diterpene synthase for fusicoccadiene (84) from Phomopsis amygdali (PaFS, Scheme 16),70 the research field has dramatically expanded and benefitted from the establishment of heterologous expression systems, particularly from the use of A. oryzae as a host. Subsequent discoveries of diterpene synthases that have made use of this approach include the enzymes from Emericella variecolor (EvVS) for variediene (85)71 and from Penicillium chrysogenum (PcCS) for the cyclopiane type diterpene (86).72 Interestingly, domain swapping of the PT domain from a domain with GGPPS activity to a domain with geranylfarnesyl diphosphate synthase (GFPPS) activity in EvVS allowed to turn this bifunctional enzyme into a sesterterpene synthase.71
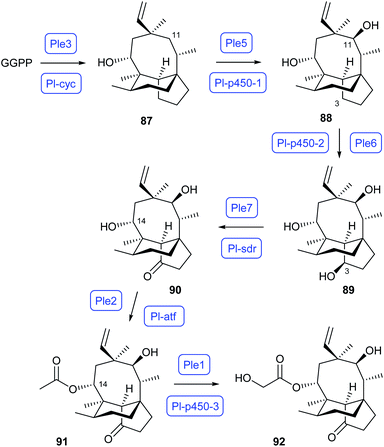
The biosynthetic pathway to pleuromutilin in Clitopilus passeckerianus (Scheme 17) has also been reconstituted in A. oryzae. Independent work by Oikawa and by Foster has demonstrated that the pathway operates through GGPP cyclisation to premutilin (87) by the bifunctional (type II/type I) terpene synthase Ple3/Pl-cyc, followed by two oxidation steps to 88 and 89 by two CYP450s (Ple5/Pl-p450-1 and Ple6/Pl-p450-2). Further oxidation to mutilin (90) is achieved by the short chain dehydrogenase/reductase Ple7/Pl-sdr, which is followed by acetylation to 14-O-acetylmutilin (91) through Ple2/Pl-atf and another CYP450 oxidation to pleuromutilin (92) by PleI/Pl-p450-3.73,74
5.3 Heterologous expression of sesterterpenoid pathways in Aspergillus
Also several bifunctional fungal sesterterpene synthases with a PT domain (GFPPS) and a TS domain for its cyclisation to sesterterpenes have been investigated during the past years through heterologous gene expression in A. oryzae. Eventually, genetically clustered CYP450s were characterised by coexpression of their genes (Scheme 18). This work resulted in the identification of the sesterfisherol synthase (NfSS) from Neosartorya fischeri. Sesterfisherol (93) contains a characteristic 5–6–8–5 tetracyclic system and is modified to sesterfisheric acid (94) by the CYP450 NfP450.75 Similarly, in Emericella variecolor the sesterterpene synthase Stl-SS generates stellata-2,6,19-triene (95) that becomes oxidised to stellatic acid (96) by Stl-P450.76 Heterologous gene expression in A. oryzae has demonstrated the formation of quiannulatene (97) by the sesterterpene synthase EvQS and oxidation to quiannulatic acid (98) by the genetically clustered Qnn-P450.77 The astellifadiene synthase EvAS from the same organism produces the sesterterpene hydrocarbon astellifadiene (99) with a 6–8–6–5 ring system,78 and PbSS from Penicillium brasilianum catalyses the formation of sesterbrasiliatriene (100) with a 5–12–5 membered skeleton.79 For these two compounds no downstream oxidative transformations are known.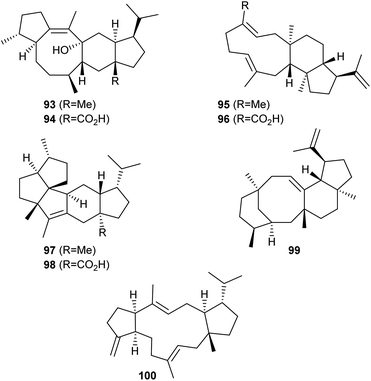 | ||
| Scheme 18 Sesterterpenoids obtained from bifunctional fungal PTTS enzymes and genetically clustered CYP450s. | ||
Genome analysis of Penicillium verruculosum revealed a new sesterterpene synthase (PvPS) whose product was identified as preasperterpenoid A (101).79 This hydrocarbon is the biosynthetic precursor to asperterpenoids A (102) and C (103) for which the CYP450s AstB and AstA were identified by Hu and coworkers through heterologous expression in A. oryzae (Scheme 19A).80 For ophiobolin F (104) sesterterpene synthases have been identified from Aspergillus clavatus (AcOS)81 and from Aspergillus calidoustus (AcldOS),82 in both cases by gene expression in A. oryzae (Scheme 19B). Hydrocarbon 104 is subsequently transformed by the CYP450 OblB into ophiobolin C (105) and then by the flavin-dependent oxygenase OblC to ophiobolin A (106).83
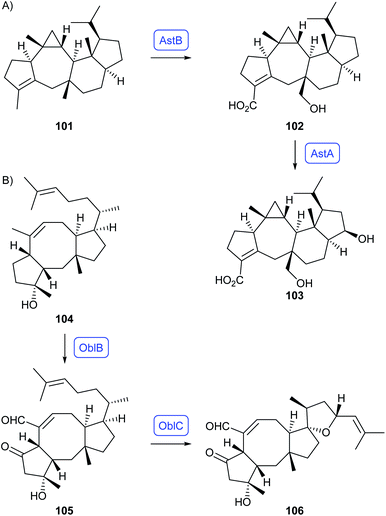 | ||
| Scheme 19 Sesterterpenoids obtained from bifunctional fungal PTTS enzymes and genetically clustered CYP450s. Enzymes for which genes were heterologously expressed are shown in blue. | ||
Heterologous expression of two homologous gene clusters from the plant endophytes Phoma betae and Colletotrichum orbiculare in A. oryzae resulted in the biosynthesis of betaestacin I (107) by the bifuncational terpene synthases BtcAPb and BtcACo in both cases (Scheme 20). The gene clusters furthermore encode two pairs of homologous CYP450s, but their functions differ. Along the P. betae pathway, BtcBPb catalyses oxidation to betaestacin II (108), but no further oxidation by BtcCPb is observed. In contrast, BtcBCo catalyses oxidation to betaestacin III (109) and then betaestacin IV (110), followed by downstream oxidations to the four betaestacins 111–114.84 These findings suggest that structural diversity in sesterterpenes may originate from closely homologous enzymes that have evolved different functions.84
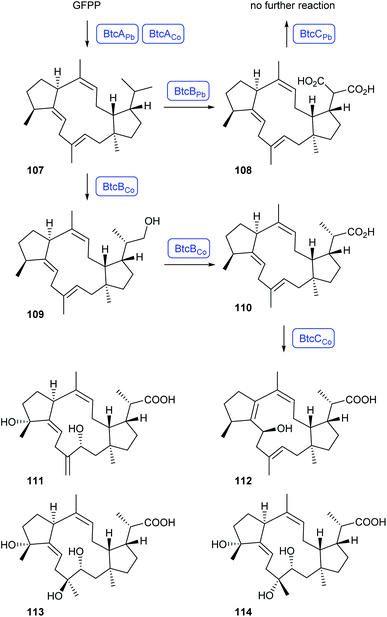 | ||
| Scheme 20 Biosynthesis of betaestacins. Enzymes for which genes were heterologously expressed are shown in blue. GFPP = geranylfarnesyl diphosphate. | ||
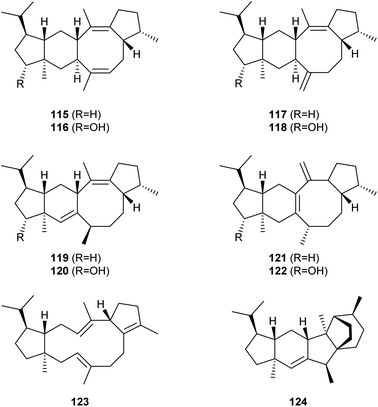
Based on a genome mining of Aspergillus ustus the Kong group identified the bifunctional enzyme AuAS (Scheme 21). Heterologous expression of its coding gene and of the upstream gene for the CYP450 AuAP450 in Aspergillus oryzae allowed the isolation of five sesterterpene hydrocarbons, including aspergildienes A–D (115, 117, 119, 121) and aspergiltriene (123). In addition, the CYP450 oxidation products aspergilols A–D (116, 118, 120, 122) were obtained.85In vitro incubations with the homolog AcAS from Aspergillus calidoustus resulted in the identification of rearranged calidoustene (124) for which the cyclisation mechanism was studied in isotopic labelling experiments and by DFT calculations.86
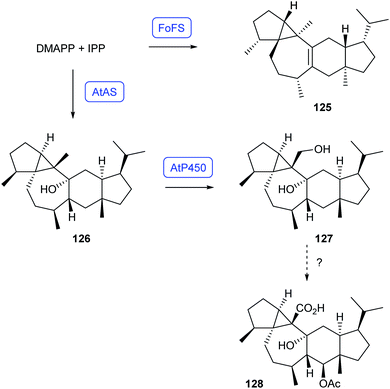
Recently, heterologous expression in A. oryzae has resulted in the identification of two bifunctional sesterterpene synthases that produce pseudoenantiomeric compounds (Scheme 22). The enzyme FoFS from Fusarium oxysporum converts DMAPP and IPP into fusoxypene A (125), while AtAS from Aspergillus terreus catalyses the formation of preaspterpenacid I (126). The genetically clustered AtP450 converts this alcohol into the diol preaspterpenacid II (127),87 but further oxidation to known aspterpenacid A (128) has so far not been reconstituted.
5.4 Heterologous expression of triterpenoid pathways in Aspergillus
A combination of gene deletions in the native host Aspergillus fumigatus and pathway reconstitution in A. nidulans with the afum gene cluster uncovered the biosynthetic pathway to fumihopaside A (131, Scheme 23). Starting from squalene epoxide (15), the squalene-hopene cyclase AfumA catalyses the cyclisation to 129, followed by CYP450 oxidation by AfumB to 130 and glycosylation by AfumC to 131.88 Bioactivity testings revealed that fumihopaside A plays a role in protecting A. fumigatus from heat or UV stress.88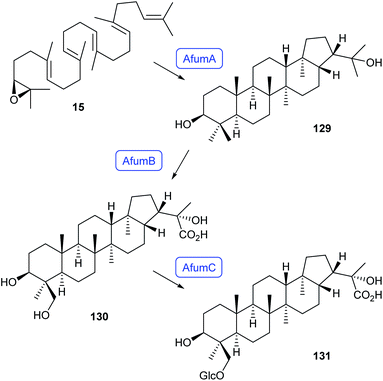 | ||
| Scheme 23 Biosynthesis of fumihopaside A (131) in A. fumigatus. Enzymes for which genes were heterologously expressed are shown in blue. | ||
5.5 Heterologous expression of meroterpenoid pathways in Aspergillus
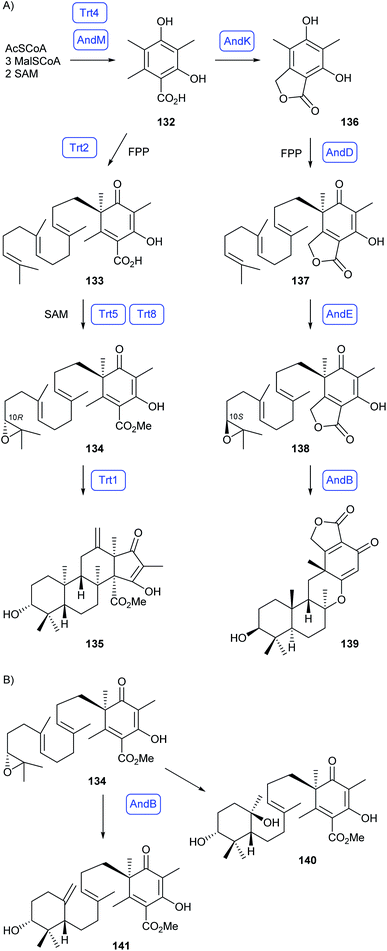
Meroterpenoids are a class of natural products of mixed biosynthetic origin whose skeletons are partly derived from terpene biosynthesis.89 The definition of this compound class is not very sharp and there are borderline cases. In this section only compounds will be discussed that contain two or more moieties, at least one of which is of terpenoid origin, that are fused by carbon–carbon bonds. Acylations, glycosylations and all other modifications with formation of carbon–heteroatom bonds are not considered as meroterpenoids and thus not included in this section.About one decade ago, Abe and coworkers reported about the reconstitution of the biosynthetic pathways towards the fungal meroterpenoids anditomin, andrastin A, terretonin and austinol by heterologous expression of biosynthesis genes in A. oryzae.90–92 The pathway to terretonin proceeds through 3,5-dimethylorsellinic acid (132) made by the polyketide synthase Trt4, followed by farnesylation to 133 by the prenyltransferase Trt2, methylation (Trt5) and epoxidation (Trt8) to 134 with 10R configuration, and terpene cyclisation to preterretonin A (135) by Trt1 (Scheme 24A).90 Reactions towards anditomin also proceed through 132 (AndM), followed by oxidation and lactonisation to 136 (AndK), farnesylation by the prenyltransferase AndD to 137, epoxidation by AndE to 138 with 10S configuration, and cyclisation to preandiloid A (139) by AndB.92 In a combinatorial biosynthesis approach biosynthetic genes for the biosynthesis of 134 and for the terpene synthase AndB were heterologously expressed in A. oryzae, resulting in the new cyclisation products 140 and 141 (Scheme 24B).93
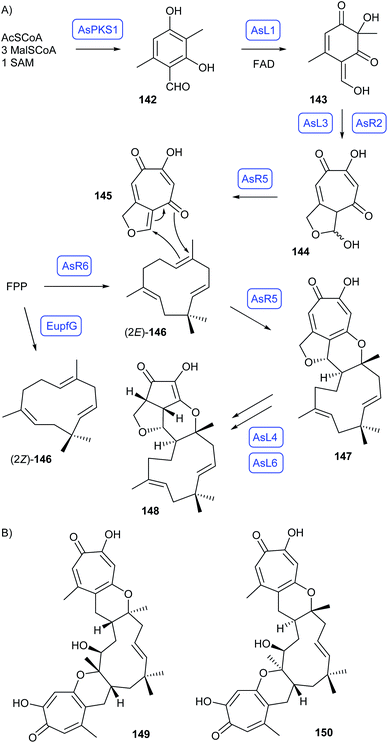
The biosynthesis of the fungal meroterpenoid xenovulene A (148) in Acremonium strictum has been studied by Cox and coworkers through heterologous expression in A. oryzae (Scheme 25A).94 The pathway involves formation of methylorcinaldehyde (142) by the polyketide synthase AsPKS1, followed by FAD-dependent oxidation to 143 by AsL1. Oxidative ring expansion by AsL3 and oxidation with hemiacetal formation by the CYP450 AsR2 leads to 144. The next enzyme AsR5 is bifunctional and catalyses elimination of water to 145 that reacts with humulene (146), the product of the unusual terpene synthase AsR6, in a hetero-Diels–Alder reaction to 147. Further oxidation steps with ring contractions by AsL4 and AsL6 result in 148.94 Similar biosynthetic gene clusters for eupenifeldin (149) and pycnidione (150) have been identified and partially studied (Scheme 25B).95–97 The incorporation of one tropolone (as in 148) or two tropolone units (as in 149 and 150) was shown to depend on the hetero-Diels-Alderase AsR5 versus PycR1,97 while the cis or trans ring junction depends on the double bond configuration in the humulene precursor. While AsR6 yields (2E)-146 from FPP in vitro through a well established stereochemical course,94,98 expression of EupfG in A. nidulans results in (2Z)-146.95 These different enzyme functions allowed for a combinatorial biosynthesis approach for the generation of non-natural tropolone sesquiterpenoids.97
As shown by Watanabe and coworkers who investigated the early steps towards subglutinols in Metarhizium robertsii through heterologous gene expression in A. nidulans, the biosynthesis of decalin-containing diterpenoid pyrones (DDP) in fungi starts with the formation of 151 by the polyketide synthase SubA and of GGPP by the GGPPS SubD (Scheme 26A).99 Both building blocks are then fused by a prenyltransferase SubC to 152, followed by SubE mediated epoxidation to 153 and terpene cyclisation to 154 by SubB.99 A genome mining study revealed the presence of DDP biosynthetic gene clusters in four additional fungal genera,100 showing that the genes for homologs of SubABCDE are conserved in all clusters.99,100 Additional genes occurred in different combinations, including genes for two short chain dehydrogenases (SDR1, SDR2) that can invert the stereochemistry at C8 by processing the alcohol through the ketone and reduction,100 a flavine monooxygenase for oxidation at C12,99 a CYP450 for oxidation of Me26 and Me27, and two O-methyltransferases (O-MT1 and O-MT2) for methylations in the polyketide portion.100 A combinatorial biosynthesis approach with heterologous expression of genes in different combinations in A. oryzae gave access to several known compounds, establishing their biosynthesis, but also a large variety of new derivatives (Scheme 26B).100
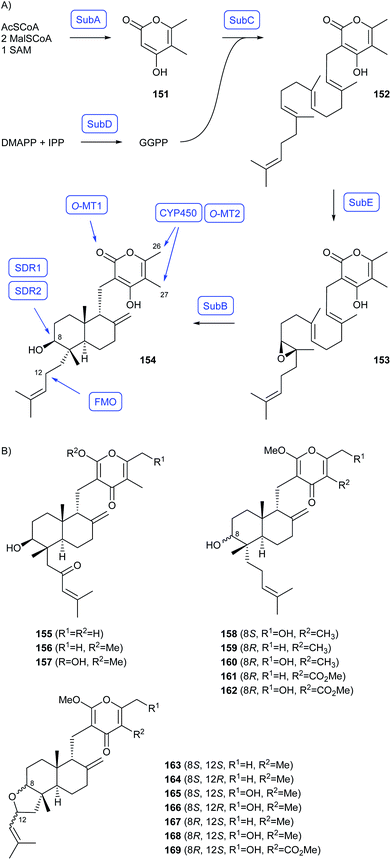 | ||
| Scheme 26 Biosynthesis of decalin-containing diterpenoid pyrones (DDP). (A) Early steps reported by Watanabe and coworkers,99 (B) new compounds obtained by combinatorial biosynthesis.100 Enzymes for which genes were heterologously expressed are shown in blue. | ||
6 Heterologous expression in Escherichia coli
Heterologous expressions of fungal biosynthetic genes towards terpenes have also occasionally been performed in Escherichia coli. The coding sequences of the TS domain of the bifunctional terpene synthase FgJ07578 and of the monodomain terpene synthase FgJ07623 from the endophyte Fusarium graminearum were fused to different nucleotide sequences for PT domains known to be specific for the biosynthesis of FPP (E. coli IspA), GGPP (from Taxus canadensis) or GFPP (from Geobacillus stearothermophilus). Expression of these artificial constructs in an engineered E. coli strain developed by Liu and coworkers for efficient supply of terpene precursors resulted in the production of terpenes for all six PTTS combinations, including the new compounds GJ1012A–E (170–174) from the FgJ07623-GGPPS fusion, and mangicdiene (175) and variecoltetraene (176) from the FgJ07578-GFPPS construct (Scheme 27).101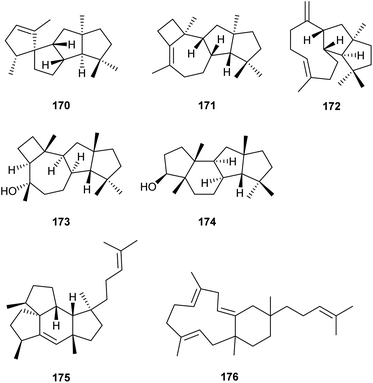 | ||
| Scheme 27 Terpenes obtained by expression of artificial PTTS fusions in an engineered E. coli for high titre precursor supply. | ||
7 Pathway activation in the original host
Pathway activations in the native host follow different strategies to upregulate gene transcription. Classical approaches make use of different culture conditions102 or co-cultivation of microorganisms,103 but pathway activation can also be achieved genetically, e.g. by placing the target genes under a strong constitutive or inducible promoter, targeting pathway specific transcription factors such as zinc finger proteins, global or epigenetic regulators. Details of these methods have recently been reviewed,104 and here only a few examples for applications in fungal terpene biosynthesis will be covered.7.1 Specific pathway activation
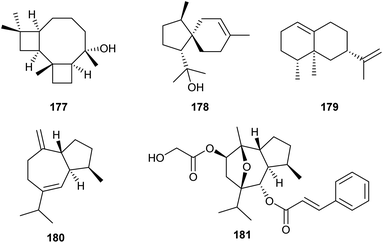
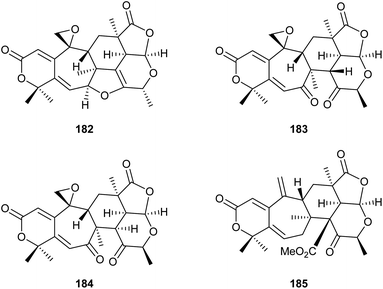
Analysis of the genome from Fusarium fujikuroi revealed the presence of at least six genes potentially coding for sesquiterpene cyclases (STC1–STC6). Deletions of the genes for STC4 and STC6 revealed that these enzymes are active in the wildtype. Overexpression of these genes was achieved using the gpd promoter derived from the glycolysis enzyme glyceraldehyde-3-phosphate dehydrogenase that is inducible by glucose. Simultaneously, the gene for the other active terpene synthase was deleted, and both manipulations were performed in the background of the mutant strain SG139 that is deficient in diterpene production. This allowed to easily isolate and characterise the products from STC4 as koraiol (177) and from STC6 as α-acorenol (178, Scheme 28).105 In contrast, the two sesquiterpene cyclases STC5 and STC3 are inactive in wildtype F. fujikuroi under laboratory culture conditions. Overexpression mutants for these terpene synthases under the gpd promoter were constructed that did not lead to the production of new compounds, but allowed to obtain the cDNA by quantitative reverse transcription (RT)-PCR. Gene cloning and protein expression in E. coli, followed by in vitro incubations with GPP, FPP and GGPP revealed the conversion of FPP into (+)-eremophilene (179) by STC3, while STC5 was efficiently expressed, but inactive due to a naturally occurring mutation at a key site. Sequence correction by site-directed mutagenesis gave an active enzyme that converted FPP into (−)-guaia-6,10(14)-diene (180).106 Based on this finding, a high yielding S. cerevisiae strain for the production of 180 was engineered, providing up to 0.8 g L−1 of this hydrocarbon. The material was used by Christmann and coworkers for a short seven step synthesis of englerin (181),107 a highly potent compound against renal cancer cells.108Also gene cluster activation by targeting of pathway-specific regulators has been used. The ber gene cluster in Neosartorya glabra is under control of the positive regulator BerA. Transformation of N. glabra with a plasmid containing the berA gene under control of the gpd promoter resulted in a strongly upregulated expression of the ber gene cluster and consequently the production of two new compounds, berkeleyacetal D (182) and 11-epi-berkeleyacetal C (183), besides the known compounds berkeleyacetal C (184) and purpurogenolide C (185, Scheme 29).109
7.2 Targeting of global regulators
Global regulators simultaneously control the expression of many genes in one organism. This is possible, because they interact with specific, but short and frequently occurring DNA sequence motifs, e.g. GATA type transcription factors can bind to the nucleotide sequence GATA and thereby control the expression of nearby genes. Knockout of the GATA-type transcription factor CSM1 in F. fujikuroi resulted not only in morphological changes and a depleted production of the PKS-derived red pigments bikaverin and fusarubins, but also in an activated production of (−)-germacrene D (186) by STC1 (Scheme 30).110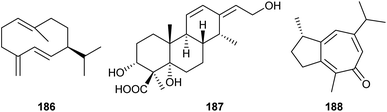 | ||
| Scheme 30 Structures of compounds for which the biosynthesis was activated by targeting global and epigenetic regulators. | ||
7.3 Epigenetic manipulation
Histones are proteins for packing of chromosomal DNA. Their charge and thus interaction with negatively charged DNA can be modified by phosphorylation of serine, acetylation of lysine, or methylation of lysine or arginine. Interference with histone modifying enzymes can thus have consequences on DNA packing and gene transcription.Genome sequencing of Calcarisporium arbuscula demonstrated the presence of 68 core genes for the biosynthesis of secondary metabolites. Deletion of the gene for the histone H3 Lys14 deacetylase (H3K14Ac, HDAC) in C. arbuscula activated many biosynthetic pathways to natural products, including the new labdane-related diterpenoid arbusculic acid A (187, Scheme 30).111 In a subsequent study on the same mutant twelve additional new diterpenoids were isolated that showed cytotoxic activity towards cancer cells.112
Instead of gene knockouts also small molecules that specifically inhibit the histone modifying enzymes can be used. Epigenetic manipulation of the deep-sea sediment-derived Spiromastix sp. by the addition of the HDAC inhibitor suberoylanilide hydroxamic acid (SAHA) was found to activate a terpenoid biosynthetic gene cluster. This strategy allowed the isolation of nine new sesquiterpenes with antineuroinflammatory effects, spiromaterpenes A–I, including the main compound 188.113
Besides the approach discussed in Section 7.1 through placing the gene for STC5 in F. fujikuroi under the gdp promoter, also an epigenetic approach has been used to awake this and other silent biosynthetic genes. In this fungus, the methyltransferase KMT6 catalyses triple methylation of Lys27 in histone H3 (H3K27me3). A gene knockout for the likely essential gene coding for KMT6 was not possible, but its downregulation by RNAi resulted in the activation of several secondary metabolite pathways, including activation of gene expression for STC5 and consequently in the production of 180 (Scheme 28).114
8 Conclusions
As summarised in this review article, metabolic engineering approaches towards fungal terpenoids have been applied very successfully in many recent cases. For the discovery and understanding of unknown biosynthetic pathways their heterologous reconstitution is of enormous potential. For this purpose, Aspergillus oryzae and A. nidulans are the most frequently used hosts, and the one-by-one introduction of biosynthetic genes together with isolation and structure elucidation of newly formed metabolites can give detailed insights into enzyme functions and pathway intermediates towards the final compound. On the other hand, pathways to known compounds are often reconstructed in heterologous hosts in which primary metabolism (especially the MVA pathway and oligoprenyl diphosphate biosynthesis) has been engineered for a high titre precursor supply. This work usually aims at high yielding production strains to get a biotechnological access to valuable terpenoids. In most cases, Saccharomyces cerevisiae or Escherichia coli are used for this purpose, because these organisms are easy to manipulate and to cultivate in large bioreactors.These two approaches, of course, do not strictly stand in parallel to each other with no conceptual crosstalk, but can also fruitfully stimulate each other, e.g. if unknown terpene biosynthesis genes are introduced into high yielding engineered strains, so that unknown pathway intermediates are obtained in large quantities for comparably easy compound isolation and structure elucidation.
The research of the past years has offered important tools for metabolic engineering and deep knowledge of biosynthetic pathways towards many valuable and bioactive terpenoids. Based on this work, it will be of enourmous interest to investigate, if artificial combinations of biosynthetic genes from different pathways can lead to new compounds. As demonstrated here and in another recent review,115 researchers have already begun to tap this promising source, but the number of possible gene combinations seems nearly unlimited and future research in this area can be expected to open the doors to a chemical terra incognita of great potential. Hopefully, this approach will also lead to new compounds with interesting biological activities that can help to cure some of the most severe plagues for mankind.
9 Conflicts of interest
There are no conflicts to declare.10 Acknowledgements
This work was funded by the Deutsche Forschungsgemeinschaft DFG (TRR 261, project ID 398967434).11 References
- M. B. Quin, C. M. Flynn and C. Schmidt-Dannert, Nat. Prod. Rep., 2014, 31, 1449–1473 RSC.
- S. E. Helaly, B. Thongbai and M. Stadler, Nat. Prod. Rep., 2018, 35, 992–1014 RSC.
- N. P. Keller, G. Turner and J. W. Bennett, Nat. Rev. Microbiol., 2005, 3, 937–947 CrossRef CAS PubMed.
- A. Evidente, A. Kornienko, A. Cimmino, A. Andolfi, F. Lefranc, V. Mathieu and R. Kiss, Nat. Prod. Rep., 2014, 31, 617–627 RSC.
- J. Gershenzon and N. Dudareva, Nat. Chem. Biol., 2007, 3, 408–414 CrossRef CAS PubMed.
- M. Avalos, P. Garbeva, L. Vader, G. P. van Wezel, J. S. Dickschat and D. Ulanova, Nat. Prod. Rep., 2022, 39, 249–272 RSC.
- J. S. Dickschat, Nat. Prod. Rep., 2011, 28, 1917–1936 RSC.
- D. W. Christianson, Chem. Rev., 2017, 117, 11570–11648 CrossRef CAS.
- A. Minami, T. Ozaki, C. Liu and H. Oikawa, Nat. Prod. Rep., 2018, 35, 1330–1346 RSC.
- V. J. J. Martin, D. J. Pitera, S. T. Withers, J. D. Newman and J. D. Keasling, Nat. Biotechnol., 2003, 21, 796–802 CrossRef CAS PubMed.
- C. Liu, A. Minami, T. Ozaki, J. Wu, H. Kawagishi, J. Maruyama and H. Oikawa, J. Am. Chem. Soc., 2019, 141, 15519–15523 CrossRef CAS.
- D. A. Yee, T. B. Kakule, W. Cheng, M. Chen, C. T. Y. Chong, Y. Hai, L. F. Hang, Y.-S. Hung, N. Liu, M. Ohashi, I. C. Okorafor, Y. Song, M. Tang, Z. Zhang and Y. Tang, J. Am. Chem. Soc., 2020, 142, 710–714 CrossRef CAS.
- C. Ma, K. Zhang, X. Zhang, G. Liu, T. Zhu, Q. Che, D. Li and G. Zhang, Curr. Opin. Biotechnol., 2021, 69, 281–289 CrossRef CAS PubMed.
- H. Xiao and J. J. Zhong, Trends Biotechnol., 2016, 34, 242–255 CrossRef CAS.
- H. Xu and J. S. Dickschat, Synthesis, 2022, 54, 1551–1565 CrossRef CAS.
- B. Qin, Y. Matsuda, T. Mori, M. Okada, Z. Quan, T. Mitsuhasi, T. Wakimoto and I. Abe, Angew. Chem., Int. Ed., 2016, 55, 1658–1661 CrossRef CAS.
- G. Li, R. Li, Q. Liu, Q. Wang, M. Chen and B. Li, FEMS Microbiol. Lett., 2006, 256, 203–208 CrossRef CAS.
- M. J. de Groot, P. Bundock, P. J. Hooykaas and A. G. Beijersbergen, Nat. Biotechnol., 1998, 16, 839–842 CrossRef CAS PubMed.
- C.-Y. Kuo and C.-T. Huang, J. Microbiol. Methods, 2008, 72, 111–115 CrossRef CAS.
- H. Alper, C. Fischer, E. Nevoigt and G. Stephanopoulos, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 12678–12683 CrossRef CAS.
- J. Kurreck, Angew. Chem., Int. Ed., 2009, 48, 1378–1398 CrossRef CAS.
- M. Rohmer, Nat. Prod. Rep., 1999, 16, 565–574 RSC.
- M. Rohmer, M. Knani, P. Simonin, B. Sutter and H. Sahm, Biochem. J., 1993, 295, 517–524 CrossRef CAS.
- V. Homann, K. Mende, C. Arntz, V. Ilardi, G. Macino, G. Morelli, G. Böse and B. Tudzynski, Curr. Genet., 1996, 30, 232–239 CrossRef CAS.
- A. Carattoli, N. Romano, P. Ballario, G. Morelli and G. Macino, J. Biol. Chem., 1991, 266, 5854–5859 CrossRef CAS.
- C. J. Paddon, P. J. Westfall, D. J. Pitera, K. Benjamin, K. Fisher, D. McPhee, M. D. Leavell, A. Tai, A. Main, D. Eng, D. R. Polichuk, K. H. Teoh, D. W. Reed, T. Treynor, J. Lenihan, M. Fleck, S. Bajad, G. Dang, D. Dengrove, D. Diola, G. Dorin, K. W. Ellens, S. Fickes, J. Galazzo, S. P. Gaucher, T. Geistlinger, R. Henry, M. Hepp, T. Horning, T. Iqbal, H. Jiang, L. Kizer, B. Lieu, D. Melis, N. Moss, R. Regentin, S. Secrest, H. Tsuruta, R. Vazquez, L. F. Westblade, L. Xu, M. Yu, Y. Zhang, L. Zhao, J. Lievense, P. S. Covello, J. D. Keasling, K. K. Reiling, N. S. Renninger and J. D. Newman, Nature, 2013, 496, 528–532 CrossRef CAS.
- E. Jongedijk, K. Cankar, M. Buchhaupt, J. Schrader, H. Bouwmeester and J. Beekwilder, Appl. Microbiol. Biotechnol., 2016, 100, 2927–2938 CrossRef CAS.
- C. Ignea, M. Pontini, M. E. Maffei, A. M. Makris and S. C. Kampranis, ACS Synth. Biol., 2014, 3, 298–306 CrossRef CAS PubMed.
- S. Cheng, X. Liu, G. Jiang, J. Wu, J. Zhang, D. Lei, Y. Yuan, J. Qiao and G. Zhao, ACS Synth. Biol., 2019, 8, 968–975 CrossRef CAS.
- A. L. Schilmiller, I. Schauvinhold, M. Larson, R. Xu, A. L. Charbonneau, A. Schmidt, C. Wilkerson, R. L. Last and E. Pichersky, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 10865–10870 CrossRef CAS PubMed.
- S. Brown, M. Clastre, V. Gourdavoult and S. E. O'Connor, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 3205–3210 CrossRef CAS PubMed.
- S. M. Stanley Fernandez, B. A. Kellogg and C. D. Poulter, Biochemistry, 2000, 39, 15316–15321 CrossRef CAS.
- D. A. Yee, A. B. DeNicola, J. M. Billingsley, J. G. Creso, V. Subrahmanyam and Y. Tang, Metab. Eng., 2019, 55, 76–84 CrossRef CAS PubMed.
- X. Deng, B. Shi, Z. Ye, M. Huang, R. Chen, Y. Cai, Z. Kuang, X. Sun, G. Bian and T. Liu, Metab. Eng., 2022, 69, 122–133 CrossRef CAS PubMed.
- D.-K. Ro, E. M. Paradise, M. Ouellet, K. J. Fisher, K. L. Newman, J. M. Ndungu, K. A. Ho, R. A. Eachus, T. S. Ham, J. Kirby, M. C. Y. Chang, S. T. Withers, Y. Shiba, R. Sarpong and J. D. Keasling, Nature, 2006, 440, 940–943 CrossRef CAS PubMed.
- F. Lévesque and P. H. Seeberger, Angew. Chem., Int. Ed., 2012, 51, 1706–1709 CrossRef.
- J. Yuan and C. B. Ching, Metab. Eng., 2016, 38, 303–309 CrossRef CAS.
- G. Bian, A. Hou, Y. Yuan, B. Hu, S. Cheng, Z. Ye, Y. Di, Z. Deng and T. Liu, Org. Lett., 2018, 20, 1626–1629 CrossRef CAS.
- T. Hu, J. Zhou, Y. Tong, P. Su, X. Li, Y. Liu, N. Liu, X. Wu, Y. Zhang, J. Wang, L. Gao, L. Tu, Y. Lu, Z. Jiang, Y. J. Zhou, W. Gao and L. Huang, Metab. Eng., 2020, 60, 87–96 CrossRef CAS PubMed.
- J. S. Dickschat, Biosynthesis of Diterpenoid Natural Products, in Comprehensive Natural Products III: Chemistry and Biology, ed. T. P. Begley, H. W. Liu and I. Abe, Elsevier, 2020, vol. 1, pp. 506–552 Search PubMed.
- P. K. Ajikumar, W.-H. Xiao, K. E. J. Tyo, Y. Wang, F. Simeon, E. Leonhard, O. Mucha, T. H. Phon, B. Pfeifer and G. Stephanopoulos, Science, 2010, 330, 70–74 CrossRef CAS.
- B. Nowrouzi, R. A. Li, L. E. Walls, L. d'Espaux, K. Malci, L. Liang, N. Jonguitud-Borrego, A. I. Lerma-Escalera, J. R. Morones-Ramirez, J. D. Keasling and L. Rios-Solis, Microb. Cell Fact., 2020, 19, 200 CrossRef CAS PubMed.
- G. Bian, J. Rinkel, Z. Wang, L. Lauterbach, A. Hou, Y. Yuan, Z. Deng, T. Liu and J. S. Dickschat, Angew. Chem., Int. Ed., 2018, 57, 15887–15890 CrossRef CAS.
- R. Chen, Q. Jia, X. Mu, B. Hu, X. Sun, Z. Deng, F. Chen, G. Bian and T. Liu, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2023247118 CrossRef CAS PubMed.
- I. Yosioka, T. Sugawara, K. Imai and I. Kitagawa, Chem. Pharm. Bull., 1972, 20, 2418–2421 CrossRef CAS.
- L. N. Atopkina, G. V. Malinovskaya, G. B. Elyakov, N. I. Uvarova, H. J. Woerdenbarg, A. Koulman, N. Pras and P. Potier, Planta Med., 1999, 65, 30–34 CrossRef CAS PubMed.
- D. Li, Y. Wu, C. Zhang, J. Sun, Z. Zhou and W. Lu, J. Agric. Food Chem., 2019, 67, 2581–2588 CrossRef CAS.
- H. Seki, K. Ohyama, S. Sawai, M. Mizutani, T. Ohnishi, H. Sudo, T. Akashi, T. Aoki, K. Saito and T. Muranaka, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 14204–14209 CrossRef CAS.
- H. Seki, S. Sawai, K. Ohyama, M. Mizutani, T. Ohnishi, H. Sudo, E. O. Fukushima, T. Akashi, T. Aoki, K. Saito and T. Muranaka, Plant Cell, 2011, 23, 4112–4123 CrossRef CAS.
- J. Kirby, D. W. Romanini, E. M. Paradise and J. D. Keasling, FEBS J., 2008, 275, 1852–1859 CrossRef CAS.
- M. Zhu, C. Wang, W. Sun, A. Zhou, Y. Wang, G. Zhang, X. Zhou, Y. Hou and C. Li, Metab. Eng., 2018, 45, 43–50 CrossRef CAS PubMed.
- W. Sun, H. Xue, H. Liu, B. Lv, Y. Yu, Y. Wang, M. Huang and C. Li, ACS Catal., 2020, 10, 4253–4260 CrossRef CAS.
- J. R. Mein, F. Lian and X. D. Wang, Nutr. Rev., 2008, 66, 667–683 CrossRef PubMed.
- Y. Chen, W. Xiao, Y. Wang, H. Liu, X. Li and Y. Yuan, Microb. Cell Fact., 2016, 15, 113 CrossRef PubMed.
- T. Ma, B. Shi, Z. Ye, X. Li, M. Liu, Y. Chen, J. Xia, J. Nielsen, Z. Deng and T. Liu, Metab. Eng., 2019, 52, 134–142 CrossRef CAS PubMed.
- M. Guerin, M. E. Huntley and M. Olaizola, Trends Biotechnol., 2003, 21, 210–216 CrossRef CAS.
- K. R. Kildegaard, B. Adiego-Pérez, D. D. Belda, D. D. Belda, J. K. Khangura, C. Holkenbrink and I. Borodina, Synth. Syst. Biotechnol., 2017, 2, 287–294 CrossRef.
- J. Lee, F. Hilgers, A. Loeschke, K. E. Jaeger and M. Feldbrügge, Front. Microbiol., 2020, 11, 1655 CrossRef.
- J. Beekwilder, A. van Houwelingen, K. Cankar, A. D. J. van Dijk, R. M. de Jong, G. Stoopen, H. Bouwmeester, J. Achkar, T. Sonke and D. Bosch, Plant Biotechnol. J., 2014, 12, 174–182 CrossRef CAS PubMed.
- H. Chen, C. Zhu, M. Zhu, J. Xiong, H. Ma, M. Zhuo and S. Li, Microb. Cell Fact., 2019, 18, 195 CrossRef PubMed.
- S. Agger, F. Lopez-Gallego and C. Schmidt-Dannert, Mol. Microbiol., 2009, 72, 1181–1195 CrossRef CAS PubMed.
- K. Gomi, Y. Iimura and S. Hara, Agric. Biol. Chem., 1987, 51, 2549–2555 CAS.
- J. Feng, F. Surup, M. Hauser, A. Miller, J. P. Wennrich, M. Stadler, R. J. Cox and E. Kuhnert, Chem. Commun., 2020, 56, 12419–12422 RSC.
- K. Murai, L. Lauterbach, K. Teramoto, Z. Quan, L. Barra, T. Yamamoto, K. Nonaka, K. Shiomi, M. Nishiyama, T. Kuzuyama and J. S. Dickschat, Angew. Chem., Int. Ed., 2019, 58, 15046–15050 CrossRef PubMed.
- H. Zeng, G. Yin, Q. Wei, D. Li, Y. Wang, Y. Hu, C. Hu and Y. Zou, Angew. Chem., Int. Ed., 2019, 58, 6569–6573 CrossRef CAS PubMed.
- Q. Wei, H. C. Zeng and Y. Zou, ACS Catal., 2021, 11, 948–957 CrossRef CAS.
- D. A. Yee, T. B. Kakule, W. Cheng, M. Chen, C. T. Y. Chong, Y. Hai, L. F. Hang, Y.-S. Hung, N. Liu, M. Ohashi, I. C. Okorafor, Y. Song, M. Tang, Z. Zhang and Y. Tang, J. Am. Chem. Soc., 2020, 142, 710–714 CrossRef CAS PubMed.
- C. Liu, A. Minami, T. Ozaki, J. Wu, H. Kawagishi, J. Maruyama and H. Oikawa, J. Am. Chem. Soc., 2019, 141, 15519–15523 CrossRef CAS PubMed.
- Y.-I. Yang, S. Zhang, K. Ma, Y. Xu, Q. Tao, Y. Chen, J. Chen, S. Guo, J. Ren, W. Wang, Y. Tao, W.-B. Yin and H. Liu, Angew. Chem., Int. Ed., 2017, 56, 4749–4752 CrossRef CAS PubMed.
- T. Toyomasu, M. Tsukahara, A. Kaneko, R. Niida, W. Mitsuhashi, T. Dairi, N. Kato and T. Sassa, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 3084–3088 CrossRef CAS.
- B. Qin, Y. Matsuda, T. Mori, M. Okada, Z. Quan, T. Mitsuhashi, T. Wakimoto and I. Abe, Angew. Chem., Int. Ed., 2016, 55, 1658–1661 CrossRef CAS.
- T. Mitsuhashi, T. Kikuchi, S. Hoshino, M. Ozeki, T. Awakawa, S. Shi, M. Fujita and I. Abe, Org. Lett., 2018, 20, 5606–5609 CrossRef CAS.
- M. Yamane, A. Minami, C. Liu, T. Ozaki, I. Takeuchi, T. Tsukagoshi, T. Tokiwano, K. Gomi and H. Oikawa, ChemBioChem, 2017, 18, 2317–2322 CrossRef CAS.
- F. Alberti, K. Khairudin, E. Rodriguez Venegas, J. A. Davies, P. M. Hayes, C. L. Willis, A. M. Bailey and G. D. Foster, Nat. Commun., 2017, 8, 1831 CrossRef.
- Y. Ye, A. Minami, A. Mandi, C. Liu, T. Taniguchi, T. Kuzuyama, K. Monde, K. Gomi and H. Oikawa, J. Am. Chem. Soc., 2015, 137, 11846–11853 CrossRef CAS PubMed.
- Y. Matsuda, T. Mitsuhashi, Z. Quan and I. Abe, Org. Lett., 2015, 17, 4644–4647 CrossRef CAS.
- M. Okada, Y. Matsuda, T. Mitsuhashi, S. Hoshino, T. Mori, K. Nakagawa, Z. Quan, B. Qin, H. Zhang, F. Hayashi, H. Kawaide and I. Abe, J. Am. Chem. Soc., 2016, 138, 10011–10018 CrossRef CAS PubMed.
- Y. Matsuda, T. Mitsuhashi, S. Lee, M. Hoshino, T. Mori, M. Okada, H. Zhang, F. Hayashi, M. Fujita and I. Abe, Angew. Chem., Int. Ed., 2016, 55, 5785–5788 CrossRef CAS PubMed.
- T. Mitsuhashi, J. Rinkel, M. Okada, I. Abe and J. S. Dickschat, Chem.–Eur. J., 2017, 23, 10053–10057 CrossRef CAS PubMed.
- J. Huang, J. Lv, Q. Wang, J. Zou, Y. Lu, Q. Wang, D. Chen, X. Yao, H. Gao and D. Hu, Org. Biomol. Chem., 2019, 17, 248–251 RSC.
- R. Chiba, A. Minami, K. Gomi and H. Oikawa, Org. Lett., 2013, 15, 594–597 CrossRef CAS.
- Z. Quan and J. S. Dickschat, Org. Biomol. Chem., 2020, 18, 6072–6076 RSC.
- K. Narita, R. Chiba, A. Minami, M. Kodama, I. Fujii, K. Gomi and H. Oikawa, Org. Lett., 2016, 18, 1980–1983 CrossRef CAS.
- L. Gao, K. Narita, T. Ozaki, N. Kumakura, P. Gan, A. Minami, C. Liu, X. Lei, K. Shirasu and H. Oikawa, Tetrahedron Lett., 2018, 59, 1136–1139 CrossRef CAS.
- J. Guo, Y. S. Cai, F. Cheng, C. Yang, W. Zhang, W. Yu, J. Yan, Z. Deng and K. Hong, Org. Lett., 2021, 23, 1525–1529 CrossRef CAS.
- Z. Quan, A. Hou, B. Goldfuss and J. S. Dickschat, Angew. Chem., Int. Ed., 2022, 61, e202117273 CAS.
- L. Jiang, X. Zhang, Y. Sato, G. Zhu, A. Minami, W. Zhang, T. Ozaki, B. Zhu, Z. Wang, X. Wang, K. Lv, J. Zhang, Y. Wang, S. Gao, C. Liu, T. Hsiang, L. Zhang, H. Oikawa and X. Liu, Org. Lett., 2021, 23, 4645–4650 CrossRef CAS PubMed.
- K. Ma, P. Zhang, Q. Tao, N. P. Keller, Y. Yang, W. B. Yin and H. Liu, Org. Lett., 2019, 21, 3252–3256 CrossRef CAS PubMed.
- L. Barra and I. Abe, Nat. Prod. Rep., 2021, 38, 566–585 RSC.
- Y. Matsuda, T. Awakawa, T. Itoh, T. Wakimoto, T. Kushiro, I. Fujii, Y. Ebizuka and I. Abe, ChemBioChem, 2012, 13, 1738–1741 CrossRef CAS.
- Y. Matsuda, T. Awakawa and I. Abe, Tetrahedron, 2013, 69, 8199–8204 CrossRef CAS.
- Y. Matsuda, T. Wakimoto, T. Mori, T. Awakawa and I. Abe, J. Am. Chem. Soc., 2014, 136, 15326–15336 CrossRef CAS PubMed.
- J. Jiang, X. Li, T. Mori, T. Awakawa and I. Abe, Chem. Pharm. Bull., 2021, 69, 444–446 CrossRef CAS.
- R. Schor, C. Schotte, D. Wibberg, J. Kalinowski and R. J. Cox, Nat. Commun., 2018, 9, 1963 CrossRef.
- Q. Chen, J. Gao, C. Jamieson, J. Liu, M. Ohashi, J. Bai, D. Yan, B. Liu, Y. Che, Y. Wang, K. N. Houk and Y. Hu, J. Am. Chem. Soc., 2019, 141, 14052–14056 CrossRef CAS.
- Y. Zhai, Y. Li, J. Zhang, Y. Zhang, F. Ren, X. Zhang, G. Liu, X. Liu and Y. Che, Fungal Genet. Biol., 2019, 129, 7–15 CrossRef CAS.
- C. Schotte, L. Li, D. Wibberg, J. Kalinowski and R. J. Cox, Angew. Chem., Int. Ed., 2020, 59, 23870–23878 CrossRef CAS.
- H. Xu, C. Schotte, R. J. Cox and J. S. Dickschat, Org. Biomol. Chem., 2021, 19, 8482–8486 RSC.
- H. Kato, Y. Tsunematsu, T. Yamamoto, T. Namiki, S. Kishimoto, H. Noguchi and K. Watanabe, J. Antibiot., 2016, 69, 561–566 CrossRef CAS.
- K. Tsukada, S. Shinki, A. Kaneko, K. Murakami, K. Irie, M. Murai, H. Miyoshi, S. Dan, K. Kawaji, H. Hayashi, E. N. Kodama, A. Hori, E. Salim, T. Kuraishi, N. Hirata, Y. Kanda and T. Asai, Nat. Commun., 2020, 11, 1–12 CrossRef.
- G. Bian, Y. Han, A. Hou, Y. Yuan, X. Liu, Z. Deng and T. Liu, Metab. Eng., 2017, 42, 1–8 CrossRef CAS.
- H. B. Bode, B. Bethe, R. Höfs and A. Zeeck, ChemBioChem, 2002, 3, 619–627 CrossRef CAS.
- H. Onaka, Y. Mori, Y. Igarashi and T. Furumai, Appl. Environ. Microbiol., 2011, 77, 400–406 CrossRef CAS PubMed.
- H. N. Lyu, H. W. Liu, N. P. Keller and W. B. Yin, Nat. Prod. Rep., 2020, 37, 6–16 RSC.
- N. L. Brock, K. Huss, B. Tudzynski and J. S. Dickschat, ChemBioChem, 2013, 14, 311–315 CrossRef CAS.
- I. Burkhardt, T. Siemon, M. Henrot, L. Studt, S. Rösler, B. Tudzynski, M. Christmann and J. S. Dickschat, Angew. Chem., Int. Ed., 2016, 55, 8748–8751 CrossRef CAS.
- T. Siemon, Z. Wang, G. Bian, T. Seitz, Z. Ye, Y. Lu, S. Cheng, Y. Ding, Y. Huang, Z. Deng, T. Liu and M. Christmann, J. Am. Chem. Soc., 2020, 142, 2760–2765 CrossRef CAS PubMed.
- Y. Akbulut, H. J. Gaunt, K. Muraki, M. J. Ludlow, M. S. Amer, A. Bruns, N. S. Vasudev, L. Radtke, M. Willot, S. Hahn, T. Seitz, S. Ziegler, M. Christmann, D. J. Beech and H. Waldmann, Angew. Chem., Int. Ed., 2015, 127, 3858–3862 CrossRef.
- T. Zhang, J. Wan, Z. Zhan, J. Bai, B. Liu and Y. Hu, Acta Pharm. Sin. B, 2018, 8, 478–487 CrossRef PubMed.
- E.-M. Niehaus, J. Schumacher, I. Burkhardt, P. Rabe, M. Münsterkötter, U. Güldener, J. S. Dickschat and B. Tudzynski, Front. Microbiol., 2017, 8, 1175 CrossRef PubMed.
- X. M. Mao, W. Xu, D. Li, W. B. Yin, Y. H. Chooi, Y. Q. Li, Y. Tang and Y. Hu, Angew. Chem., Int. Ed., 2015, 127, 7702–7706 CrossRef.
- J. Bai, R. Mu, M. Dou, D. Yan, B. Liu, Q. Wie, J. Wan, Y. Tang and Y. Hu, Acta Pharm. Sin. B, 2018, 8, 687–697 CrossRef.
- X. Guo, Q. Meng, S. Niu, J. Liu, X. Guo, Z. Sun, D. Liu, Y. Gu, J. Huang, A. Fan and W. Lin, J. Nat. Prod., 2021, 84, 1993–2003 CrossRef CAS PubMed.
- L. Studt, S. M. Rösler, I. Burkhardt, B. Arndt, M. Freitag, H.-U. Humpf, J. S. Dickschat and B. Tudzynski, Environ. Microbiol., 2016, 18, 4037–4054 CrossRef CAS PubMed.
- L. Kahlert, C. Schotte and R. J. Cox, Synthesis, 2021, 53, 2381–2394 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |