Surface double modification and photocatalytic performance of graphite carbon nitride
Jing
Luo
,
Yanxiu
Liu
 *,
Jinqi
Li
and
Hua
Song
*,
Jinqi
Li
and
Hua
Song

Provincial Key Laboratory of Oil & Gas Chemical Technology, College of Chemistry & Chemical Engineering, Northeast Petroleum University, Daqing, 163318, Heilongjiang, China. E-mail: liuyanxiu1126@126.com; Tel: 0459-6503035
First published on 15th November 2022
Abstract
Graphite carbon nitride (g-C3N4) has attracted much attention in the field of photocatalysis because of its visible light response and stable properties. However, its photocatalytic activity is not ideal because of its limited visible light response range, easy recombination of photogenerated electron holes, and poor dispersion. In this study, the visible light catalytic performance of g-C3N4 was significantly improved by the double modification method of surface hydroxyl and coupling agent grafting. Firstly, g-C3N4 was prepared by thermal polymerization with melamine as the raw material, and then the surface hydroxyl group was introduced by hydrogen peroxide. Finally, the organic groups were grafted on the surface of g-C3N4 by using silane coupling agent YDH171 as the grafting agent. The catalysts were characterized by FT-IR, XPS, XRD, contact angle measurements, UV-vis DRS, PL, electrochemical methods and particle size distribution. Taking simulated oily wastewater as the target material, the reaction rate of graphite carbon nitride was increased by 2.7 times after surface double modification. Moreover, a radical trap experiment was carried out, which indicated that the hole acted as the main reactive species for the photocatalytic degradation process. The catalytic mechanism of the material was investigated. The results showed that the synergistic effect of surface hydroxyl group grafting and coupling agent grafting increased the separation efficiency of photogenerated electron holes and improves their dispersion in water, thus promoting the photocatalytic performance of the catalyst.
1. Introduction
With the development of social economy, the energy crisis and environmental pollution have become the focus of researchers all over the world. Photocatalysis technology can convert solar energy into chemical energy and degrade most organic pollutants into small molecular inorganic substances. It shows great potential in the field of wastewater and waste gas pollution treatment because of its mild reaction conditions, safety, energy saving and high degradation efficiency.1–3In order to avoid the secondary pollution caused by the use of metal containing catalysts, the study of metal free catalysts is very important. As a metal-free photocatalyst, graphite phase carbon nitride (g-C3N4) has a special energy band structure and electronic structure. Wang et al.4 reported that the band gap of g-C3N4 is about 2.7 eV (the conduction band is about −1.1 eV, and the valence band is about 1.6 eV); this shows that g-C3N4 has corresponding absorption in the visible light range. In addition, g-C3N4 has strong thermal and chemical stability and does not easily decompose and react; so it has attracted much attention in the field of photocatalysis.5–7 However, it shows a relatively narrow response range (<460 nm) to visible light,8–10 and a narrow band gap and low bottom of the conduction band make the photo-generated carriers recombine easily.11 The aromatic ring and two-dimensional planar structure of g-C3N4 also lead to stacking and poor dispersion in the reaction process, which make the catalyst unable to absorb light and contact with the reactant thoroughly.12 Based on the above reasons, the photocatalytic activity of g-C3N4 is low.
In order to improve the photocatalytic performance of g-C3N4, researchers have taken a variety of methods to modify it in recent years, such as construction of semiconductor heterostructures,13–18 metal doping,19–22 nonmetallic element doping,23–26 multiple modification strategies combining doping and heterojunction,27,28 donor–acceptor modulation,29,30 regulation of morphology and structure31–33 and so on. In most methods, metal elements are inevitably introduced and some preparation processes are usually complicated.
In recent years, the strategy of controlling photocatalysts by grafting organic functional groups has attracted wide attention. This method has greater flexibility and can introduce specific functional groups onto the surface of the photocatalyst to modify its physicochemical properties. It can achieve goals such as enhanced light absorption, promoting the separation of electron hole pairs and accelerating charge transfer dynamics, and also helps to adsorb/activate reactant molecules, which provides a variety of strategies for improving the photocatalytic activity.34–36 Silane coupling agent, as a modifier, has been used to modify nano-TiO2. Through the formation of stable chemical bonds between the functional groups on the modifier and the hydroxyl groups on the surface of TiO2, while improving the dispersion, it is expected to improve other properties.37 However, some studies showed that the grafting of the silane coupling agent on the TiO2 surface leads to the decline of photocatalytic activity.38 This may be due to formation of excessive coating on the catalyst surface, resulting in the reduction of the light absorption capacity of the catalyst. And there are few reports on silane coupling agent used for surface modification of g-C3N4. The reason may be that the amount of surface hydroxyl of g-C3N4 is very small and it cannot be effectively grafted with the coupling agent. On the other hand, coupling agents may also undergo self-polycondensation and cross-linking, forming excessive coating on the surface of the catalyst, hindering light absorption, and leading to a decline in catalytic activity.
In this paper, a double modification route with surface hydroxyl and coupling agent grafting was designed to modify carbon nitride. Firstly, g-C3N4 was modified by hydrogen peroxide, and hydroxyls were introduced on its surface. Then the principle of condensation reaction with the surface hydroxyl group was used to connect the coupling agent group to the catalyst surface. In order to inhibit excessive coating, YDH-171 was selected as the coupling agent. Because of the steric hindrance effect of its vinyl double bond, its self-polycondensation became difficult, so it is difficult to form excessive coating on the catalyst surface, which would hinder the light absorption and affect its photocatalytic performance. The modification process was simple and no metal elements were introduced. The prepared catalysts were characterized by FT-IR, XPS, XRD, contact angle measurements, UV-vis DRS, PL, electrochemical methods and particle size distribution. The photocatalytic degradation performance for oily wastewater and mechanism of catalyst performance improvement were further explored.
2. Materials and methods
2.1. Reagents and materials
Melamine (C3N4H6N6, Shandong United Chemical Co., Ltd), hydrogen peroxide (H2O2, Heilongjiang Heihua Group Co., Ltd), cyclohexane (C6H12, Damao Reagent Factory), and vinyl trimethoxysilane (C5H12N2O3Si, YDH171) reagents are analytically pure.2.2. Preparation and surface modification of g-C3N4
Preparation of g-C3N4: 5.00 g of melamine was heated to 550 °C in a muffle furnace for 3 h. After cooling down to room temperature, the resulting product was ground and g-C3N4 was obtained.Surface hydroxyl modification of g-C3N4: typically, 0.50 g of g-C3N4 was dispersed into 50 mL of 30% hydrogen peroxide and further stirred at room temperature for 120 minutes. Afterwards, the resulting product was collected by filtration, washed with deionized water several times, then subjected to vacuum drying at 70 °C for 12 h. Surface hydroxyl modification of g-C3N4 was finished and the finally obtained product was named C3N4–OH.
Double surface modification of g-C3N4: typically, 0.2 mL of YDH171 was dissolved in 10 mL of cyclohexane to form a solution. 0.20 g of C3N4–OH was dispersed into the solution, then the obtained suspension was stirred at 80 °C for 8 hours. The resulting product was collected by filtration, washed with cyclohexane, ethyl alcohol and deionized water several times, then subjected to vacuum drying at 70 °C for 12 h. Double surface modification of g-C3N4 was done and the finally obtained product was named C3N4–OH/YDH171. For comparison, carbon nitride without hydroxyl modification was grafted with the coupling agent, named C3N4/YDH171.
2.3. Characterization
The Fourier-transform infrared (FT-IR) spectra were measured on a spectrometer (Tensor 27, Bruker) in the frequency range from 500 to 2000 cm−1. The XPS spectra were recorded on a Thermo SCIENTIFIC ESCALAB 250Xi spectrometer using Al Ka at 72 W at an angle of 45. Binding energies were registered with an accuracy of 0.4 eV and the analysis of the spectra was performed using a commercial curve fitting software. The contact angles were recorded using a JGW360A contact angle tester. Powder X-ray diffraction (XRD) patterns were recorded on an automated powder X-ray diffractometer (40 KV, 40 mA, Bruker-D8). UV-Visible diffuse reflectance spectra (DRS) were collected on a UV-2550 UV-visible spectrophotometer. The photoluminescence (PL) spectrum of the sample was determined using a PerkinElemer LS55 fluorescence spectrophotometer. The electrochemical impedance spectroscopy (EIS) and photocurrent responses (PR) measurements were performed using a CHI760E electrochemical workstation with a standard three-electrode cell at room temperature. The dispersion of samples in water was recorded on a Malvin2000 particle size analyzer. The oil concentration after degradation was detected using a UV1900/UV1901 spectrophotometer.2.4. Photocatalytic activity tests
The simulated diesel wastewater was prepared according to the concentration of 50 mg L−1 oil content, diesel oil and SDS with a mass percentage of 1% diesel were added to deionized water and stirred at high speed to obtain the required oily wastewater. Photocatalytic activity tests were conducted with a 300 W Xe lamp (with a 420 nm cutoff filter) as the light source and a 0.1 L quartz tube reactor. A circulating condensed water system kept the reactor system at 25 °C. In a typical experiment, 10 mg of catalyst was added into 50 mL of simulated diesel wastewater, then the suspension was stirred for 30 min in the dark before turning on the light. After the reaction, the catalyst was removed by centrifugation, and the liquid was extracted with 10 mL of petroleum ether twice; the absorbance value of the organic phase at the wavelength of 227 nm was measured by ultraviolet spectrophotometry, and the residual concentration was calculated by the standard curve, and the degradation ratio was calculated.393. Results and discussion
3.1. Modification mechanism
We hypothesize that the surface modification mechanism is carried through in the mode given in Fig. 1. Firstly, g-C3N4 was prepared by thermal polymerization with melamine as a raw material. Then H2O2 was chosen as the hydroxyl provider, decomposition of H2O2 generated hydroxyl groups, which were introduced on the C atoms40 of g-C3N4. Finally, the Me O–Si group in YDH171 was condensed with the hydroxyl group on the surface of g-C3N4 in cyclohexane solvent;41 the organic groups are grafted on the surface of g-C3N4, and thus the double modification of g-C3N4 was completed.3.2. Characterization
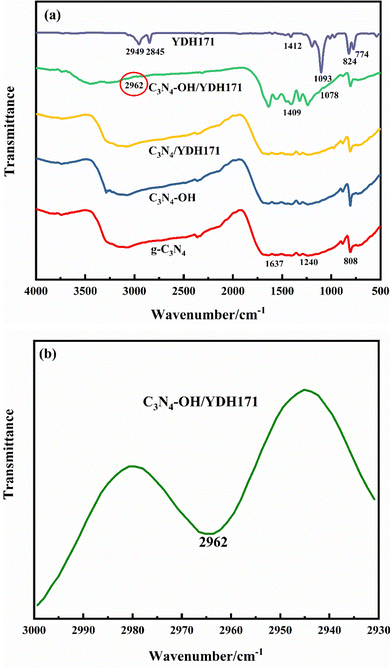
FT-IR spectra of g-C3N4, C3N4–OH, C3N4/YDH171 and C3N4–OH/YDH171 are shown in Fig. 2(a). It can be seen from Fig. 2(a) that the sharp absorption peak at 808 cm−1 is attributed to the characteristic bending vibration of the structural unit of g-C3N4. Multiple absorption peaks in the range of 1240–1637 cm−1 correspond to C–N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching vibration peaks of carbon nitrogen aromatic ring structure, and the wide absorption peaks of N–H or O–H symmetric and antisymmetric stretching vibration in the range of 3000–3400 cm−1.42–44 Compared with g-C3N4, the wide absorption band signal in the range of 3000–3400 cm−1 of C3N4–OH is enhanced, and splits into several small absorption peaks, indicating that more hydroxyl groups generated on the surface of the catalyst.45 YDH171 has peaks for CH2 stretching at 2800–3000 cm−1, which corresponds to the weak peak at 2962 cm−1 for C3N4–OH/YDH17146 (Fig. 2(b)). The peak at 1412 cm−1 belongs to –OCH3.47 There is a weak peak at 1078 cm−1, which corresponds to the Si–O symmetric stretching vibrations.46 In addition, hydroxyl groups peak of C3N4–OH/YDH171 in the range of 3000–3400 cm−1 became weak, which is due to the condensation reaction between some surface hydroxyl groups. These clearly suggest that the silane coupling agent YDH171 is successfully grafted on the surface of g-C3N4. It was also observed that C3N4/YDH171 still retains the characteristic carbon nitrogen heterocyclic structure of g-C3N4, because the surface hydroxyl content of g-C3N4 unmodified by hydrogen peroxide is very small, the grafted coupling agent group is very small too, and there is no obvious characteristic peak of the coupling agent group on its IR spectra. This shows that YDH171 is connected to the catalyst surface by condensation reaction with the surface hydroxyl of the sample, which is consistent with the speculated mechanism of the catalyst modification process.
N stretching vibration peaks of carbon nitrogen aromatic ring structure, and the wide absorption peaks of N–H or O–H symmetric and antisymmetric stretching vibration in the range of 3000–3400 cm−1.42–44 Compared with g-C3N4, the wide absorption band signal in the range of 3000–3400 cm−1 of C3N4–OH is enhanced, and splits into several small absorption peaks, indicating that more hydroxyl groups generated on the surface of the catalyst.45 YDH171 has peaks for CH2 stretching at 2800–3000 cm−1, which corresponds to the weak peak at 2962 cm−1 for C3N4–OH/YDH17146 (Fig. 2(b)). The peak at 1412 cm−1 belongs to –OCH3.47 There is a weak peak at 1078 cm−1, which corresponds to the Si–O symmetric stretching vibrations.46 In addition, hydroxyl groups peak of C3N4–OH/YDH171 in the range of 3000–3400 cm−1 became weak, which is due to the condensation reaction between some surface hydroxyl groups. These clearly suggest that the silane coupling agent YDH171 is successfully grafted on the surface of g-C3N4. It was also observed that C3N4/YDH171 still retains the characteristic carbon nitrogen heterocyclic structure of g-C3N4, because the surface hydroxyl content of g-C3N4 unmodified by hydrogen peroxide is very small, the grafted coupling agent group is very small too, and there is no obvious characteristic peak of the coupling agent group on its IR spectra. This shows that YDH171 is connected to the catalyst surface by condensation reaction with the surface hydroxyl of the sample, which is consistent with the speculated mechanism of the catalyst modification process.
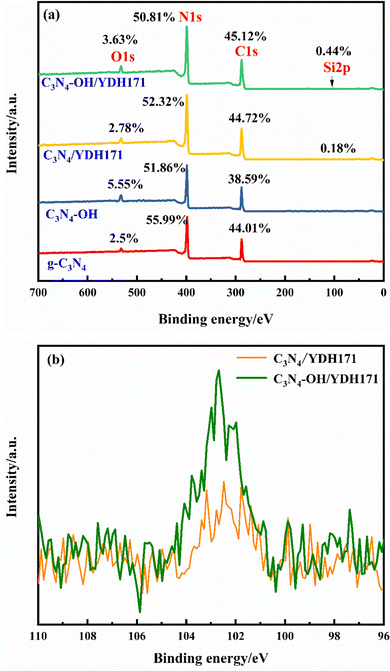
The XPS spectra of g-C3N4, C3N4–OH, C3N4/YDH171 and C3N4–OH/YDH171 are shown in Fig. 3(a). Compared with g-C3N4, the O content of C3N4–OH increased significantly, which corresponds to the introduction of a surface hydroxyl. After interacting with the coupling agent YDH171, the presence of Si was detected and the content of C increased, which verified the successful connection of C3N4–OH and silane coupling agent. In addition, as shown in Fig. 3(b), the presence of Si was also detected in C3N4/YDH171. This is because the original g-C3N4 surface also contains a small amount of surface hydroxyl, which has cross-linked with YDH171, but the content is very small, and there is little influence on the content of surface elements before and after modification.
The change of contact angle can also verify whether YDH171 is successfully grafted on the catalyst surface. If coupling groups are introduced to the catalyst surface, the hydrophobicity will inevitably increase. The contact angles of g-C3N4, C3N4–OH, C3N4/YDH171 and C3N4–OH/YDH171 are shown in Fig. 4. When measuring the contact angle of g-C3N4 and C3N4–OH, the rate of water droplet entering the material surface were extremely fast, the contact angles both were zero, indicating that g-C3N4 and C3N4–OH are super hydrophilic materials. The contact angles of C3N4/YDH171 and C3N4–OH/YDH171 are 21.72° and 37.19°, respectively. The decrease of hydrophilicity showed that YDH171 had been successfully grafted onto the surface of g-C3N4 and C3N4–OH. Compared with g-C3N4, C3N4–OH has more surface hydroxyl groups, so more organic groups are grafted on its surface, and the contact angle of C3N4–OH/YDH171 is the largest.
Fig. 5 displays the XRD patterns of g-C3N4, C3N4–OH, C3N4/YDH171 and C3N4–OH/YDH171. The XRD patterns of all the catalysts showed two prominent peaks at approximately 13.2° and 27.4°, which were assigned to the (100) and (002) crystal planes of carbon nitride, respectively. The low diffraction angle of 13.2° corresponds to the in-plane repeated arrangement of the trimethyltriazine ring structure, and the high diffraction angle of 27.4° corresponds to the interlayer periodic arrangement of the trimethyltriazine ring structure and the interlayer stacking reflection structure of aromatic organic compounds.48,49 Compared to g-C3N4, the XRD peak intensity of C3N4–OH is stronger, this is consistent with the literature.40,50 The characteristic peak intensity of the C3N4–OH sample at high diffraction angle increased obviously. It is speculated that the 2D crystal planes of g-C3N4 stacked more orderly after the treatment with H2O2. However, after C3N4–OH interacted with the coupling agent YDH171, the peak intensity decreased, which was due to the shielding effect of surface coating with YDH171 on X-rays.51
The SEM images of g-C3N4, C3N4–OH, C3N4/YDH171 and C3N4–OH/YDH171 are shown in Fig. 6(a–d). It can be seen that the surface of C3N4 before and after modification present a two-dimensional layered stacking structure, with little difference in the overall morphology. However, it can be seen that compared with the original g-C3N4, the modified samples present a more orderly layered stacking structure, which is consistent with the XRD characterization results.
3.3. Photocatalytic activity evaluation
The photocatalytic performance of g-C3N4, C3N4–OH, C3N4/YDH171 and C3N4–OH/YDH171 was investigated with visible light irradiation (λ > 420 nm). The results are shown in Fig. 7. The blank test showed that the oil contaminants are stable under visible light irradiation, which indicated that the contribution of the photolysis could be neglected.In the initial stage of the visible light catalytic reaction, the residual oil concentration decreased obviously. The residual concentration using g-C3N4, C3N4–OH, C3N4/YDH171 and C3N4–OH/YDH171 was 0.642, 0.413, 0.253 and 0.092 at 30 min, respectively. It showed that both surface hydroxyl modification and coupling agent grafting modification of g-C3N4 could improve its photocatalytic activity, the double modification generated the synergistic effect, and the photocatalytic performance of C3N4–OH/YDH171 is the best. The kinetics of photocatalytic degradation of oily wastewater by all the catalysts conformed to the first-order reaction kinetics. After fitting the kinetic curve, the reaction rate constant is shown in Fig. 8. The reaction rate constants of g-C3N4, C3N4–OH, C3N4/YDH171 and C3N4–OH/YDH171 increased in turns, and were 0.01467 min−1, 0.01845 min−1, 0.02479 min−1 and 0.0395 min−1, respectively. The reaction rate of carbon nitride was increased by 2.7 times after the double modification of surface hydroxyl and coupling agent grafting.
Actives species trapping experiments were carried out to understand the photocatalytic mechanism. The concentration of capture agent is 0.01 mol L−1. In Fig. 9, the photocatalytic activity was mildly inhibited when isopropyl alcohol (IPA, a quencher of ˙OH) or benzoquinone (BQ, a quencher of ˙O2−) was added. That indicates that there have little ˙OH and ˙O2− reactive species in the degradation process. In contrast, the degradation efficiency decreased a lot by adding KI (a quencher of hole and ˙OH), with 73.2% of contaminants surplus after 30 min irradiation. Therefore, holes were the main reactive species to oxidize contaminants.
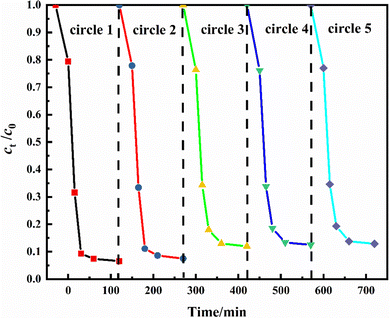
The regeneration and reusability of catalysts are very important in practical applications. The photocatalytic stability of C3N4–OH/YDH171 was reflected by measuring the rate of photocatalytic degradation of oily wastewater for five consecutive cycles. As shown in Fig. 10, the degradation ratio of fresh catalyst for oily wastewater was 93.4% at 120 min. In the second cycle, the photocatalytic activity changed a little, and the degradation ratio was 92.5% at 120 min. From the third cycle, the degradation rate changed slightly. In the third, fourth and fifth cycles, the degradation ratio of C3N4–OH/YDH171 was 88.0%, 87.5% and 87.1% respectively. It can be seen that the photocatalytic activity of C3N4–OH/YDH171 is very stable, which gives credit to the stable functional groups introduced on the surface of the catalyst. The catalyst after the cycle tests was characterized by SEM, as shown in Fig. 11(a). It can be seen that the catalyst after recycling is still a two-dimensional layered stacking structure, but there appear some holes on the surface, this is due to the strong stirring effect during the reactions, which has a certain stripping effect on the catalyst. The XRD characterization results before and after the cycle are shown in Fig. 11(b). It can be seen that the catalysts before and after the cycle tests have no obvious difference in peak strength or peak position, and the crystal structure remains basically unchanged.
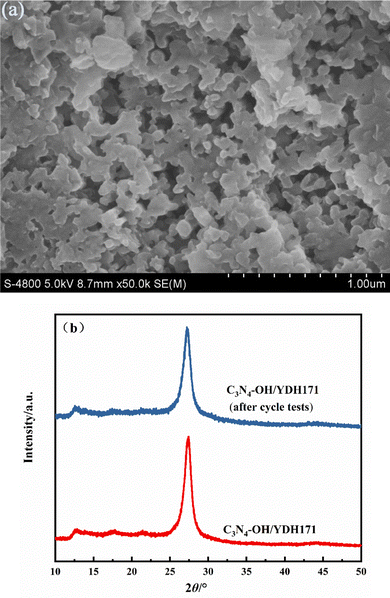 | ||
| Fig. 11 Structure characterization of catalyst after the cycle tests. (a) SEM image; (b) XRD pattern. | ||
3.4. Analysis of catalyst performance improvement
The light absorption ability of g-C3N4, C3N4–OH, C3N4/YDH171 and C3N4–OH/YDH171 for visible light was measured by UV-vis DRS, as shown in Fig. 12. The absorption sideband of all the catalysts appeared in the visible light region. Compared with g-C3N4, the light absorption capacity of the surface hydroxyl modified catalyst is improved and the visible light absorption range is also expanded. Compared with g-C3N4 and C3N4–OH, the light absorption ability of catalyst grafted with coupling agent was decreased. That is to say, the method of grafting on the surface of coupling agent is not conducive to the light absorption performance of the catalyst. It can be concluded that the improvement of the photocatalytic performance of the surface double modification is not due to the change of the light absorption performance.Photoluminescence spectroscopy is an effective method to estimate the separation efficiency of electrons and holes. Higher separation efficiency means that the lifetime of photogenerated electrons and holes is longer, which is also more favorable for photocatalytic reactions.52–54 The PL spectra of g-C3N4, C3N4–OH, C3N4/YDH171 and C3N4–OH/YDH171 are shown in Fig. 13. Compared with g-C3N4, the surface hydroxyl modification and grafting modification of the coupling agent can weaken the PL intensity, that is, the electron hole separation efficiency is improved.54,55 This is because the grafting of hydroxyl groups and organic groups on the surface of carbon nitride can induce the redistribution of local charges on the surface of carbon nitride, polarize the lone pair electrons of the two coordinated N atoms, make them deviate from the original orbit, promote the separation of local space charges, and thus inhibit the recombination of carriers.56,57 Among them, the fluorescence intensity of C3N4–OH/YDH171 is most obviously quenched by the synergistic effect of surface hydroxyl and coupling agent grafting, and the photogenerated carrier separation efficiency is the highest.
In addition to the PL technique, EIS and transient photocurrent experiments are also effective methods to investigate the separation efficiency of electron–hole pairs in a photocatalyst. Fig. 14 shows the EIS changes of g-C3N4, C3N4–OH, C3N4/YDH171 and C3N4–OH/YDH171 electrodes. It can be observed that the arc size of the three electrodes is C3N4–OH/YDH171 < C3N4/YDH171 < C3N4–OH < g-C3N4. Fig. 14 suggests that C3N4–OH/YDH171 has the highest efficiency in charge separation. The same result is also obtained by the transient photocurrent response experiment. As shown in Fig. 15, the photocurrent of C3N4–OH/YDH171 is the highest which holds the strongest ability in generating and transferring the photoexcited charge carrier under light irradiation.
The particle size distribution of g-C3N4, C3N4–OH, C3N4/YDH171 and C3N4–OH/YDH171 are shown in Fig. 16. The aromatic ring and two-dimensional planar structure of g-C3N4 lead to stacking in the reaction process. There are a certain number of active hydroxyl groups on the surface of g-C3N4 and C3N4–OH, which have strong hydrophilicity, making it extremely easy for the particles to form agglomerates, so they have larger particle size and wider distribution. After surface grafting with coupling agent YDH171, the steric repulsion force can overcome the attraction between particles, and it is not easy to form agglomerates.58 Good dispersion is conducive to the absorption of light by the catalyst particles, and is also conducive to contact with reactants, so as to promote the photocatalytic reaction.
Based on the above results, hydroxyl groups generated on the surface of carbon nitride by surface hydroxyl modification, and then organic groups were added on the surface of carbon nitride through condensation reaction between coupling agent and hydroxyl groups. The double modification process is simple, which can effectively improve the separation efficiency of photogenerated electrons and holes, improve the dispersion of carbon nitride in the reaction system, promote the absorption of light and strengthen the contact between the active site and the reactant, thereby improving the photocatalytic performance.
4. Conclusions
The double modification method of surface hydroxyl coordinated coupling agent grafting on carbon nitride was studied. It was confirmed that hydroxyl and coupling agent groups were successfully grafted on the surface of carbon nitride by FT-IR, XPS and contact angle measurements. The photocatalytic degradation of oily wastewater under visible light was studied. Compared with g-C3N4, the reaction rate of surface double modified carbon nitride was 2.7 times higher. It is confirmed by PL, EIS, photocurrent and particle size dispersion performance that the surface double modification method can significantly improve the separation efficiency of photogenerated electrons and holes of carbon nitride, and improve the dispersion in the reaction system, thus improving the photocatalytic performance of the catalyst.Author contributions
Jing Luo and Yanxiu Liu contributed equally as first authors. Jing Luo: catalyst preparation, data curation, writing original draft. Yanxiu Liu: conceptualization, writing review & editing. Jinqi Li: characterization. Hua Song: investigation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for the tireless efforts of our project team and the support of the youth scientific funds (2019QNL-25) of Northeast Petroleum University.References
- D. Vaya and P. K. Surolia, Environ. Technol. Innovation, 2020, 20, 101128 CrossRef CAS.
- D. S. Bhatkhande, V. G. Pangarkar and A. A. Beenackers, J. Chem. Technol. Biotechnol., 2001, 77, 102–116 CrossRef.
- K. Tanaka, K. Padermpole and T. Hisanaga, Water Res., 2000, 34(1), 327–333 CrossRef CAS.
- X. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, J. M. Carlsson, K. Domen and M. Antonnietti, Nat. Mater., 2009, 8(1), 76–80 CrossRef CAS PubMed.
- J. Liu, H. Wang and M. Antonietti, Chem. Soc. Rev., 2016, 45, 2308–2326 RSC.
- G. Mamba and A. K. Mishra, Appl. Catal., B, 2016, 198, 347–377 CrossRef CAS.
- S. Samanta, S. Martha and K. Parida, ChemCatChem, 2014, 6(5), 1453–1462 CAS.
- S. Patnaik, D. P. PravaSahoo and K. Parida, Carbon, 2021, 172, 682–711 CrossRef CAS.
- F. Su, S. Mathew, G. Lipner, X. Fu, M. Antonietti, S. Blechert and X. Wang, Communications, 2010, 132(46), 16299–16301 CAS.
- H. Katsumata, T. Sakai, T. Suzuki and S. Kaneco, Ind. Eng. Chem. Res., 2014, 53(19), 8018–8025 CrossRef CAS.
- J. Meng, X. Wang, Y. Liu, M. Ren, X. Zhang, X. Ding, Y. Guo and Y. Yang, Chem. Eng. J., 2021, 403, 126354 CrossRef CAS.
- X. Wang, W. Xu, R. Liu, H. Pan and S. Zhu, J. Inorg. Mater., 2022, 37(7), 731–740 CrossRef.
- M. Preeyanghaa, V. Vinesh and B. Neppolian, Chemosphere, 2022, 287, 132380 CrossRef CAS PubMed.
- G. Palanisamy, K. Bhuvaneswari, M. Srinivasan, S. Vignesh, N. Elavarasan, G. Venkatesh and P. Ramasamy, Diamond Relat. Mater., 2021, 118, 108540 CrossRef CAS.
- S. Gahlot, F. Dappozze, S. Mishra and C. Guillard, J. Environ. Chem. Eng., 2021, 9(4), 105587 CrossRef CAS.
- R. Rajendran, S. Vignesh, V. Raj, B. Palanivel, A. M. Ali, M. A. Sayed and M. Shkir, J. Alloys Compd., 2022, 894, 162498 CrossRef CAS.
- Z. Feng, L. Zeng, Q. Zhang, S. Ge, X. Zhao, H. Lin and Y. He, J. Environ. Sci., 2020, 87, 149–162 CrossRef CAS PubMed.
- Y. He, J. Cai, T. Li, Y. Wu, H. Lin, L. Zhao and M. Luo, Chem. Eng. J., 2013, 215–216, 721–730 CrossRef CAS.
- I. Benisti, F. Shaik, Z. Xing, A. Ben-refael, L. Amirav and Y. Paz, Appl. Surf. Sci., 2021, 542, 148432 CrossRef CAS.
- A. Nasri, B. Jaleh, Z. Nezafat, M. Nasrollahzadeh, S. Azizian, H. W. Jang and M. Shokouhimehr, Ceram. Int., 2021, 47(3), 3565–3572 CrossRef CAS.
- R. Saher, M. A. Hanif, A. Mansha, H. M. A. Javed, M. Zahid, N. Nadeem and O. Riaz, Int. J. Environ. Sci. Technol., 2021, 18(4), 927–938 CrossRef CAS.
- T. T. H. Nguyen, M. C. Le and N. N. Ha, Mol. Simul., 2021, 47(1), 10–17 CrossRef CAS.
- D. A. Tran, C. T. N. Pham, T. N. Ngoc, H. N. Phi, Q. T. H. Ta, D. H. Truong and V. Vo, J. Phys. Chem. Solids, 2021, 151, 109900 CrossRef CAS.
- W. D. Oh, L. W. Lok, A. Veksha, A. Giannis and T. T. Lim, Chem. Eng. J., 2018, 333, 739–749 CrossRef CAS.
- T. Mahvelati-Shamsabadi and B. K. Lee, Renewable Sustainable Energy Rev., 2020, 130, 109957 CrossRef CAS.
- N. Sagara, S. Kamimura, T. Tsubota and T. Ohno, Appl. Catal., B, 2016, 192, 193–198 CrossRef CAS.
- P. Chen, L. Chen, S. Ge, W. Zhang, M. Wu, P. Xing, T. Rotamond, H. Lin, Y. Wu and Y. He, Int. J. Hydrogen Energy, 2020, 45, 14354–14367 CrossRef CAS.
- Q. Zhang, P. Chen, L. Chen, M. Wu, X. Dai, P. Xing, H. Lin, L. Zhao and Y. He, J. Colloid Interface Sci., 2020, 568, 117–129 CrossRef CAS.
- S. Wan, J. Xu, S. Cao and J. Yu, Interdiscip. Mater., 2022, 1, 294–308 CrossRef.
- C. Yang, S. Wan, B. Zhu, J. Yu and S. Cao, Angew. Chem., Int. Ed., 2022, 61, e202208438 CAS.
- N. Iqbal, A. Afzal, I. Khan, M. S. Khan and A. Qurashi, Sci. Rep., 2021, 11(1), 1–12 CrossRef.
- H. Singh and J. K. Rajput, Food Chem., 2021, 343, 128451 CrossRef.
- Z. Mo, X. Jing, R. Zong and Y. Zhu, Appl. Catal., B, 2014, 147(8), 229 Search PubMed.
- S. Yu, J. Li, Y. Zhang, M. Li, F. Dong, T. Zhang and H. Huang, Nano Energy, 2018, 50, 383–392 CrossRef CAS.
- M. Majdoub, Z. Anfar and A. Amedlous, ACS Nano, 2020, 14(10), 12390–12469 CrossRef CAS PubMed.
- A. Ahadi, H. Alamgholiloo, S. Rostamnia, X. Liu, O. Shokouhimehr, D. A. Alonso and R. Luque, ChemCatChem, 2019, 11, 4803–4809 CrossRef CAS.
- Q. Chen and N. L. Yakovlev, Appl. Surf. Sci., 2010, 257, 1395–1400 CrossRef CAS.
- J. Zhao, M. Milanova, M. M. C. G. Warmoeskerken and V. Dutschk, Colloids Surf., A, 2012, 413, 273–279 CrossRef CAS.
- Y. Liu, H. Song, Q. Zhang and Z. Ding, Ind. Eng. Chem. Res., 2012, 51, 4779–4782 CrossRef CAS.
- Y. Zheng, Z. Zhang, C. Li and S. Proulx, Mater. Res. Bull., 2016, 84, 46–56 CrossRef CAS.
- H. Dai, A. Tian, X. Huang, N. Liu and G. Gao, Mod. Chem. Ind., 2017, 37(7), 145–148 Search PubMed.
- Q. Zhang, Y. Xia and S. Cao, Chin. J. Catal., 2021, 42, 1667–1676 CrossRef CAS.
- C. Liu, J. Tang, H. Chen, B. Liu and P. Yang, Nano Lett., 2013, 13(6), 2989–2992 CrossRef CAS.
- J. Yu, S. Wang, J. Low and X. Wei, Phys. Chem. Chem. Phys., 2013, 15(39), 16883–16890 RSC.
- K. Muniandy, H. Ismail and N. Othman, BioResources, 2012, 7(1), 957–971 CAS.
- Y. Lan and W. Wang, Adv. Polym. Technol., 2018, 37(6), 1979–1986 CrossRef CAS.
- J. Qi, T. Zhang, J. Xiao, Q. Zhang and C. Xiong, New J. Chem., 2013, 00, 1–3 Search PubMed.
- F. Dong, L. Wu, Y. Sun, M. Fu, Z. Wu and S. C. Lee, J. Mater. Chem., 2011, 21(39), 15171–15174 RSC.
- G. Dong, Y. Zhang, Q. Pan and J. Qiu, J. Photochem. Photobiol., C, 2014, 20(1), 33–50 CrossRef CAS.
- Z. Yu and Z. Jianqiang, J. Shanxi Ins. Energy, 2019, 32(5), 89–92 Search PubMed.
- B. Guo, X. Li and L. Shen, Paint Coat. Ind., 2007, 37(10), 21–24 Search PubMed.
- Y. Song, L. Huang, Q. Li and L. Chen, Chem. J. Chin. Univ., 2022, 43(6), 20220126 Search PubMed.
- X. Li, H. Xu, Y. Wang, Y. Song and Y. Cui, Chin. J. Inorg. Chem., 2022, 38(4), 654–664 Search PubMed.
- K. Xiao, W. Li, L. Zhou, L. Xie and X. Chai, Funct. Mater., 2022, 53(1), 01090–01095 CAS.
- C. Liu, F. Liu, F. Huang and X. Wang, Chin. J. Inorg. Chem., 2021, 36(11), 1154–1161 Search PubMed.
- S. Yu, J. Li, Y. Zhang, M. Li, F. Dong, T. Zhang and H. Huang, Nano Energy, 2018, 50, 383–392 CrossRef CAS.
- N. Meng, W. Zhou, Y. Yu, Y. Liu and B. Zhang, ACS Catal., 2019, 9, 10983–10989 CrossRef CAS.
- C. Yin, X. Wei and J. Li, J. Magn. Mater. Devices, 2013, 44(4), 24–27 CAS.
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2023 |