The adjacent effect between Gd(III) and Cu(II) in layered double hydroxide nanoparticles synergistically enhances T1-weighted magnetic resonance imaging contrast†
Jianping
Liu
a,
Li
Li
 a,
Run
Zhang
a,
Run
Zhang
 *a and
Zhi Ping
Xu
*ab
*a and
Zhi Ping
Xu
*ab
aAustralian Institute for Bioengineering and Nanotechnology (AIBN), The University of Queensland, St Lucia, QLD 4072, Australia. E-mail: gordonxu@uq.edu.au; r.zhang@uq.edu.au; Fax: +61-7-33463973; Tel: +61-7-334 63809
bInstitute of Biomedical Health Technology and Engineering and Institute of Systems and Physical Biology, Shenzhen Bay Laboratory, Shenzhen, P. R. China 518107
First published on 22nd December 2022
Abstract
Magnetic resonance imaging (MRI) is one key technology in modern diagnostic medicine. However, the development of high-relaxivity contrast agents with favorable properties for imaging applications remains a challenging task. In this work, dual Gd(III) and Cu(II) doped-layered double hydroxide (GdCu-LDH) nanoparticles show significantly higher longitudinal relaxivity compared with sole-metal-based LDH (Gd-LDH and Cu-LDH) nanoparticles. This relaxation enhancement in GdCu-LDH is also much greater than the simple addition of the relaxivity rate of the two paramagnetic ions in Gd-LDH and Cu-LDH, presumably attributed to synergistic T1 shortening between adjacent Gd(III) and Cu(II) in the LDH host layers (adjacent effect). Moreover, our GdCu-LDH nanoparticles exhibit a pH-ultrasensitive property in MRI performance and show much clearer MR imaging for tumor tissues in mice than Gd-LDH and Cu-LDH at the equivalent doses. Thus, these novel Gd/Cu-co-doped LDH nanoparticles provide higher potential for accurate cancer diagnosis in clinic application. To the best of our knowledge, this is the first report that two paramagnetic metal ions in one nanoparticle synergistically improve the T1-MRI contrast.
New conceptsThe development of high-relaxivity contrast agents for T1-weighted magnetic resonance imaging (MRI) is a challenging task in the clinic. This research potentially enables dually-doped layered double hydroxide (LDH) nanoparticles as an outstanding MRI contrast agent. The prepared LDH nanoparticles contain two paramagnetic metal ions, Gd(III) and Cu(II), and show significantly higher longitudinal relaxivity compared with sole-metal-doped ones, presumably attributed to synergistic T1 shortening between adjacent Gd(III) and Cu(II) in the nanoparticle. This LDH nanoparticle also exhibits a pH-ultrasensitive MRI performance and much clearer MR imaging for tumor tissues in mice than Gd-LDH and Cu-LDH at the equivalent doses. To our knowledge, this is the first report that two paramagnetic metal ions in one nanoparticle synergistically improve the T1-MRI contrast and will motivate biomedical scientists to develop novel MRI contrast agents based on the current finding. |
Introduction
Magnetic resonance imaging (MRI), a technique with a superior spatial resolution and contrast, has been extensively applied in clinical diagnosis without using harmful high-energy radiation.1–5 The physical principles of MRI rely on the amount of water molecules in the examined area and finally the measurements show the spatial distribution of the relaxation of water protons.6 Hence, effective MRI contrast agents must accelerate proton relaxation processes to shorten the T1 (spin–lattice)/T2 (spin–spin) relaxation time and result in brighter/darker signals of the target tissues than the background.7Generally, the emphasis of nanoparticle-based MR imaging is to exploit the contrast agents with high performance via incorporating magnetic ions into nanoparticles with optimal structural features.8 Gadolinium(III) (Gd(III)) has a high magnetic moment and long electron spin relaxation time, and has become the most widely studied magnetic metal center for T1-MR imaging applications.9–11 Other metal ion-based agents, such as manganese(II) (Mn(II)) and copper(II) (Cu(II)), are becoming promising candidates for clinical T1-MRI practice.12–14 To improve MR imaging contrast, recent investigations have focused on embedding paramagnetic species (such as Gd(III), Mn(II), and Cu(II)) inside iron oxide (IO) nanoparticles to enable the spin of T1 contrast species to coordinate the local magnetic field induced by the T2-weighted IO nanoparticles.15,16 This strategy has led to the increase in both r1 and r2 values and simultaneous enhancement of positive-T1 and negative-T2 imaging.17–19 However, this strategy for enhanced dual-mode MRI is usually achieved by combining paramagnetic materials with T2 contrast capability. The additive or synergistic effect of dual paramagnetic ions in one nanoparticle on the longitudinal relaxation rate (T1) has not been reported yet.
Layered double hydroxide (LDH) nanoparticles, a kind of inorganic two-dimensional (2D) clay nanomaterial, have been widely explored in biomedical applications as a result of their unique physicochemical properties including controllable particle size, high specific surface area, high density of charge and superior capacity of cation exchange.20,21 Unlike most inorganic nanoparticles, LDH nanoparticles have negligible effects on the proliferation of vascular cells, the rupture of red blood cells (RBCs) and the activation of the innate immune system.22 Typically, clay nanoparticles are also able to be dissociate into ions in the acidic physiological environment (pH 5–6) for excretion.23 The LDH-based nanomedicine has thus attracted increasing attention for potential clinical translation in cancer diagnostics and treatments. LDH nanomaterials can be represented with a general chemical formula, i.e. [M(II)1−xM(III)x(OH)2](An−)x/n·mH2O, in which M(II) and M(III) refer to the divalent and trivalent metal cations, respectively, and An− the interlayer anions.24 Various metal cations are facilely incorporated into the hydroxide layers, among which Mg(II) and Al(III) are the typical examples. In connection with this, some functional metal cations can be incorporated by isomorphically substituting Mg(II) or Al(III) to endow LDH nanoparticles with some fascinating features.25,26 For example, Mn(II)/Cu(II)-based LDH nanosheets are found to be good T1-MRI contrast agents for cancer diagnosis, which can sensitively respond to acidic conditions (tumor microenvironment and cell endosome/lysosome) and show satisfactory imaging performance.27–29 Similarly, Gd(III) and Fe(III) are also incorporated into the LDH layers via isomorphic substitution of Al(III) ions to provide a magnetic relaxation function.30–32 An interesting issue that is never ever investigated is whether co-incorporation of two magnetic cations into the LDH layers enhances the T1-MRI performance.
Motivated by the superior paramagnetic properties of Gd(III) and Cu(II), in this work we integrate these two magnetic metal ions within one LDH nanoparticle step by step to achieve positive-contrast synergistical enhancement for tumor MRI diagnosis. As schematically shown (Scheme 1), the new T1-MRI contrast agents (named GdCu-LDH) were synthesized via a simple two-step method, i.e. first co-precipitation to get the Gd-LDH nanoparticles and then subsequent isomorphic substitution of partial Mg(II) with Cu(II) ions. As a result, the T1 relaxivity (r1) of GdCu-LDH was significantly increased in comparison with that of Gd-LDH (by 5–6 fold in pH 5.0–7.4 buffer for this example), demonstrating that there is a strong synergy between adjacent Gd(III) and Cu(II) in the LDH layers. Moreover, GdCu-LDH showed an ultra-sensitivity of T1-weighted MRI signals to the environment pH from 7.4 to 5.0. As a result, this LDH-based contrast agent showed superior T1-weighted MR imaging for tumor detection in vivo even at low doses (0.1 mg kg−1 of Gd and 0.5 mg kg−1 of Cu). To our knowledge, this is the first report to deeply study the adjacent effect between two paramagnetic metal ions in one nanoparticle in nanotechnology and nanomedicine. Synergistic T1 shortening between adjacent Gd(III) and Cu(II) in the nanoparticle demonstrates a new concept that could motivate scientists in the fields of materials science, nanotechnology, and biomedical science to develop novel MRI nano-contrast agents. The great contrast enhancement makes this novel GdCu-LDH nanoparticle a potential MRI contrast agent for clinical cancer diagnosis.
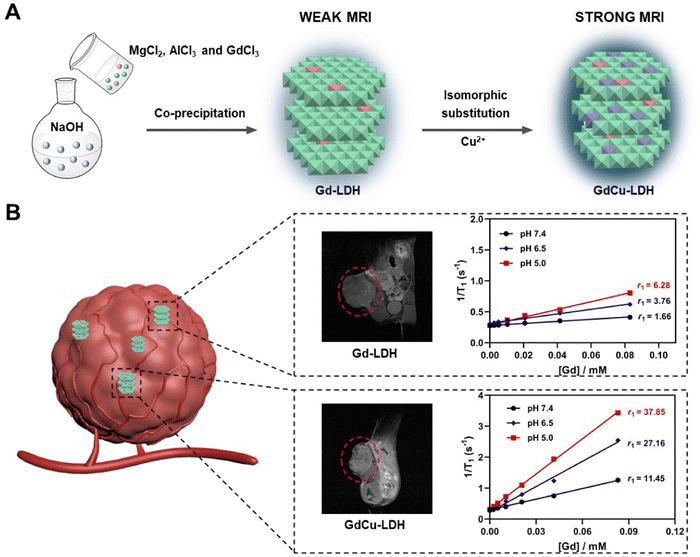 | ||
| Scheme 1 Schematic illustration of the (A) synthetic procedure and (B) in vivo MRI performance of Gd-LDH and GdCu-LDH nanoparticles. | ||
Results and discussion
T 1-weighted relaxivity of Gd- and Cu-doped LDH nanoparticles
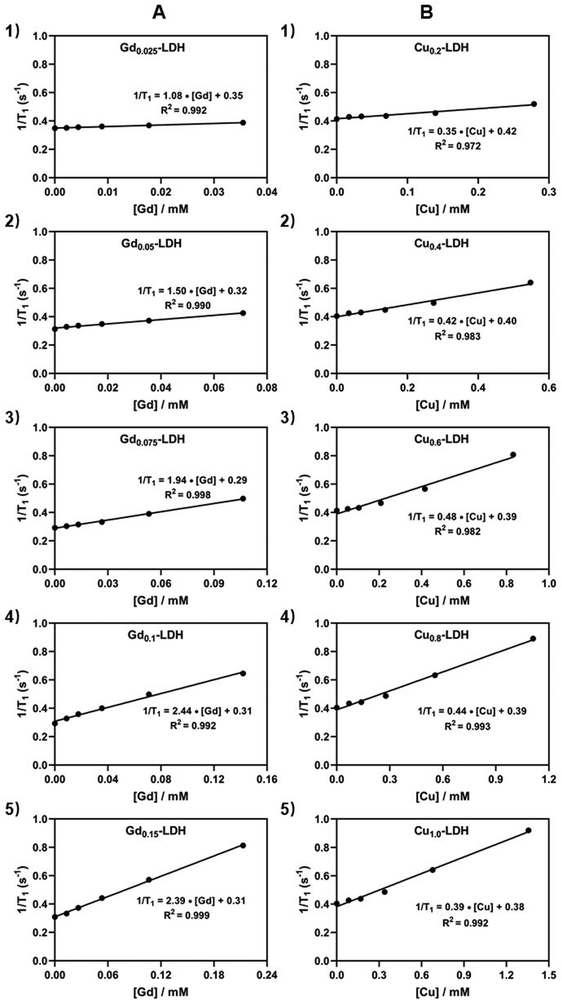
The Gdx-LDH (Mg3Al1−xGdx-LDH) nanoparticles were first prepared via a co-precipitation method (Scheme 1(A)), in which a fraction of Al(III) in MgAl-LDH was substituted by Gd(III) (x = 0.025, 0.05, 0.075, 0.1 and 0.15, i.e. the molar ratio x = Gd(III)/(Gd(III) + Al(III))). The elemental analysis using inductively coupled plasma optical emission spectrometry (ICP-OES) confirmed successful Gd(III) incorporation in MgAl-LDH, and the chemical formulae were estimated accordingly (Table S1, ESI†). All these Gdx-LDH nanoparticles showed the typical (003) and (006) reflections of LDH phase (Fig. S1, ESI†), which means that 2.5–15% of Al(III) was isomorphically substituted by Gd(III) within the very similar lattice. In Fig. S2 (ESI†), the Gdx-LDH nanoparticles with x from 0.025 to 0.1 exhibited a very similar particle size distribution (35–65 nm) and zeta potential (30–40 mV) (Table S1, ESI†). Gd0.15-LDH nanoparticles had a higher number-mean hydrodynamic diameter (94.2 nm) than other Gdx-LDH nanoparticles but a similar zeta potential (32.6 mV) (Table S1, ESI†).Gd(III) has seven unpaired electrons in the 4f orbitals and these electrons induce a fluctuating magnetic field under radio frequency perturbation to increase the relaxation rate of nearby water protons and display T1-MR imaging contrast.33,34 This is determined via evaluating the T1-weighted relaxivity of Gdx-LDH-containing aqueous suspensions (Fig. 1(A)). As expected, each Gd-LDH showed a linear relationship between 1/T1 and [Gd]. Specifically, the increase of the Gd(III) molar ratio from 0.025 to 0.1 gradually led to the increase of the longitudinal relaxivity (r1) value from 1.08 to 2.44 mM−1 s−1. An exception is Gd0.15-LDH, which had a r1 value (2.39 mM−1 s−1) similar to that of Gd0.1-LDH, presumably due to the adjacent effect (further discussed in the following sections).
 | ||
| Fig. 1 T 1-weighted relaxation of Gd-LDH and Cu-LDH nanoparticles. (A) Plot of 1/T1versus [Gd] of six Gdx-LDHs. (B) Plot of 1/T1versus [Cu] of six Cuy-LDHs. | ||
Paramagnetic cation Cu(II) with one unpaired electron has also been explored as a T1-MRI contrast agent thanks to its ability of affecting the relaxation rate of protons in nearby water molecules.35–37 Therefore, Cuy-LDH (CuyMg3−yAl-LDH) nanoparticles were also prepared in the method previously reported,38 in which some Mg(II) (y) in MgAl-LDH was substituted by Cu(II) (y = 0.2, 0.4, 0.6, 0.8 and 1.0). As shown in Fig. 1(B), Cuy-LDH possessed r1 values in the range of 0.35–0.48 mM−1 s−1. These r1 values are also similar to that of similar Cu-containing nanoparticles at pH 7.4, as previously reported.38 The T1-relaxivity of Cu-LDH nanoparticles is 3–6 fold lower than that of Gd-LDH, attributed to only one unpaired electron of Cu(II) ions compared with Gd(III) ions (seven unpaired electrons).
Further analysis has suggested that the relaxivity of both Gdx-LDH and Cuy-LDH nanoparticles is related to the Gd and Cu fraction in a parabolic function. As shown in Fig. 2(A), r1 = 2.45–117(x − 0.135)2 for x = 0.025–0.15, demonstrating that Gd-LDH nanoparticles have a maximum relaxivity at x = 0.135, i.e. the highest adjacent effect or sensitivity in terms of the relaxivity. Similarly, r1 = 0.468–0.610 (y − 0.638)2, showing that there is an optimal Cu doping in LDH that leads to the highest adjacent effect (Fig. 2(B)). Below the optimal Gd and Cu doping, the adjacent effect increases with the doping amount (x, or y), as the chance for two paramagnetic cations to be close to each other increases. However, over the optimal doping, it is possible for three or more paramagnetic cations to affect one another, which may lead to just one adjacent effect (similar to that between two adjacent cations), thus reducing the overall relaxivity.
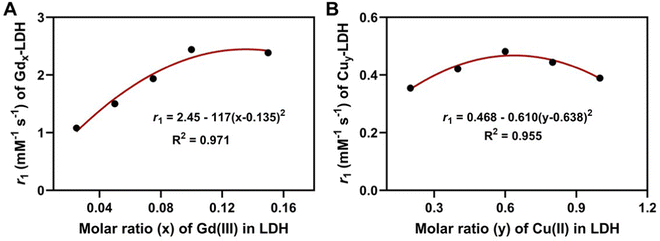 | ||
| Fig. 2 The relationship of relaxivity with the magnetic cation portion in LDH nanoparticles. (A) The relaxivity versus x of Gdx-LDHs. (B) The relaxivity versus y of Cuy-LDHs. | ||
T 1-weighted relaxivity of Gd/Cu-co-doped LDH nanoparticles
GdxCu0.6-LDH (x = 0.025–0.15) were then prepared and characterized (Table S2, ESI†). These samples displayed powder X-ray diffraction (XRD) patterns similar to that of Gdx-LDH nanoparticles, without significant change in the layered structure (Fig. 3(A)). The typical signals at 550 and 3400 cm−1 in the GdxCu0.6-LDH Raman spectra are related to M–O/M–OH and hydroxyl stretching modes, respectively (Fig. S3, ESI†). SEM images of GdxCu0.6-LDH further confirmed their sheet-like and layered structures (Fig. S4, ESI†). As shown in Fig. 3(B), no obvious difference in the average particle size (mostly around 50 to 60 nm) and zeta potentials (30–40 mV) was observed in GdxCu0.6-LDH (x = 0.025–0.1), except for Gd0.15Cu0.6-LDH. The as-prepared GdxCu0.6-LDH nanoparticles were irregularly shaped but relatively monodispersed in transmission electron microscopy (TEM) images (Fig. 3(C)), in agreement with other Gd-based LDH nanoparticles.39–42 Notably, the Gd0.15Cu0.6-LDH showed a larger hydrodynamic diameter (129 nm) than the others due to much more larger Gd ions incorporated, as also displayed in the TEM image (Fig. 3(C)).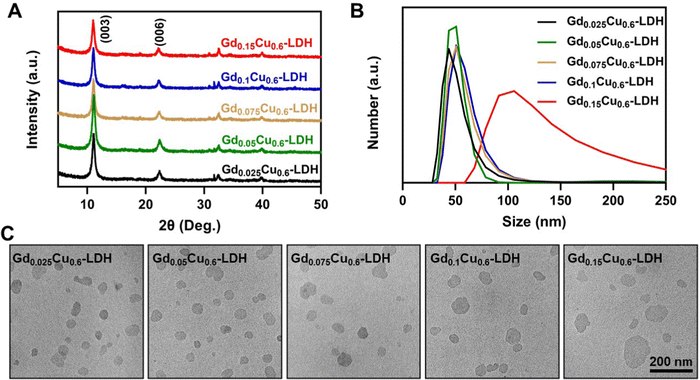 | ||
| Fig. 3 Characteristics of GdxCu0.6-LDH. (A) XRD patterns, (B) size distributions and (C) TEM images of a series of GdxCu0.6-LDH. | ||
To verify the effect of Cu(II) co-existence on MR imaging of Gd-LDH, the T1-weighted MRI performance of GdxCu0.6-LDH NPs was investigated. GdxCu0.6-LDH with x of 0.025–0.15 showed a remarkable increment in T1 relaxivity, with the r1 value ranging from 8–20 mM−1 s−1 in terms of the Gd(III) concentration (Fig. S5A–E, ESI†), 10–20 times higher than that of the corresponding Gdx-LDH nanoparticles. For example, 17-fold and 8-fold increments of r1 values in Gd0.025Cu0.6-LDH and Gd0.05Cu0.6-LDH were observed in comparison with that of Gd0.025-LDH and Gd0.05-LDH, respectively (Fig. 1(A)). Compared to 0.48 mM−1 s−1 of Cu0.6-LDH (Fig. 1(B3)), the r1 value in terms of the Cu concentration increased to 0.78–2.22 mM−1 s−1 with the increase of the Gd3+ content (Fig. S5F, ESI†), suggesting that the MRI ability of Cu(II) is in turn significantly enhanced by Gd(III) co-existence in the LDH nanoparticles.
Similarly, Gd0.1Cuy-LDH (y = 0.2–1.0) were further prepared and their MRI contrast property was examined. Compared with Cuy-LDH (Fig. 1(B)), the longitudinal relaxivity was increased by 3–8 fold (Fig. S6A–E, ESI†). In turn, the MRI relaxivity in terms of the Gd(II) concentration was also significantly increased (Fig. S6F, ESI†). Therefore, two paramagnetic metal ions (Gd(III) and Cu(II)) in one LDH nanoparticle seem to synergistically contribute to the T1-weighted MRI contrast performance, as clearly revealed in the following comparison.
Synergistic adjacent effect of GdxCuy-LDH
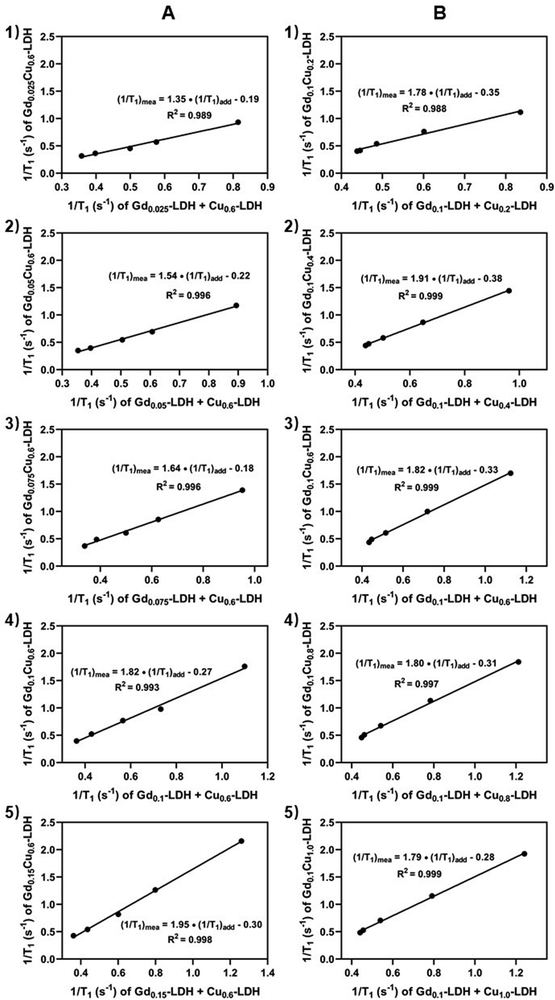
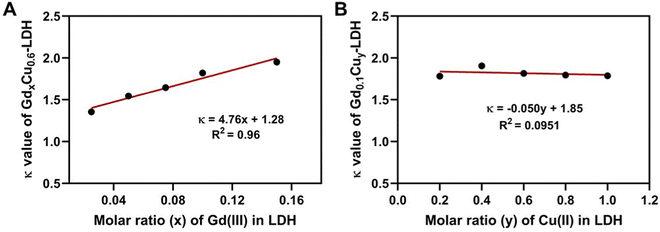
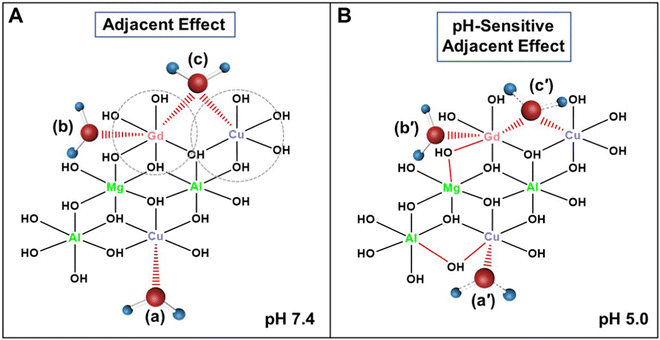
The further analysis has shown that the 1/T1 of GdxCu0.6-LDH is linear to the summative 1/T1 value of Gdx-LDH and Cu0.6-LDH (Fig. 4(A)). Moreover, the slope (κ) was all much greater than 1.0 (1.35–1.95, Fig. 5(A)). Presumably, the slope (κ) being 1 means that there is no adjacent effect between cations so the proton relaxation of GdxCu0.6-LDH corresponds to the additive relaxation of Gdx-LDH and Cu0.6-LDH. Thus the κ value being greater than 1 demonstrates that there is a synergistic adjacent effect between Gd(III) and Cu(II) in the GdxCu0.6-LDH layers and the synergy is enhanced by the Gd content increase (x = 0.025–0.15). Similarly, the analysis of T1 relaxivity has also revealed that the 1/T1 of Gd0.1Cuy-LDH is linear to the summative 1/T1 value of Gd0.1-LDH and Cuy-LDH (Fig. 4(B)) and the slope (κ) was all in 1.5–2.0 (Fig. 5(B)), further reflecting the synergistic adjacent effect between Gd(III) and Cu(II) in the Gd0.1Cuy-LDH layers. However, the slope (κ) seemed not obviously dependent on the Cu amount in the LDH layers.As schematically represented in Fig. 7(A), water molecules (a and b) are coordinated or associated with Cu(II) and Gd(III), respectively, and their proton relaxation is shortened by the micromagnetic field of Cu(II) and Gd(III) cations. Very specifically, water molecule c is coordinated with or affected by both adjacent Gd(III) and Cu(II) cations, and its proton relaxation is even more significantly shortened by the overlapping magnetic field produced by both cations. As presented in Fig. 2, the adjacent effect in Gdx-LDH and Cuy-LDH is also evidenced, which is reflected by the optimal x (0.135) and y (0.610). This optimal composition may indicate that two Gd(III) cations are able to affect the relaxation of water protons which are 0.8–1.0 nm away from both Gd(III) cations. In contrast, two Cu(II) cations shorten the relaxation of water protons within the range of 0.4–0.5 nm. This means that the two cations may not be really ‘adjacent’ in the LDH host layers, but nearby so that both magnetic cations can sense and affect the same surrounding water protons.
The adjacent effect in GdxCu0.6-LDH and Gd0.1Cuy-LDH nanoparticles has been reflected in Fig. 4, indicating that the Gd–Cu pair generates a much stronger adjacent effect (r1 up to 11.45 mM−1 s−1) than Gd–Gd (r1 up to 2.45 mM−1 s−1) and Cu-Cu pairs (r1 up to 0.468 mM−1 s−1) among these three adjacent pairs on the water proton relaxation in the vicinity of 0.4–0.8 nm (pH 7.4). This effective distance is slightly larger than the Gd–H2O coordination bond length (0.25–0.3 nm) in Gd–chelate complexes.43 However, this range seems to be similar to that in Gd3+-chelate co-complexing with Cu+/Cu2+, where the proton relaxivity of nearby water molecules is significantly enhanced.44,45 In one example, after Cu(II) coordination, the r1 of this Gd–DO3A complex-based molecular sensor exhibited 43% and 270% increase in the absence and presence of HSA, respectively. In this molecular sensor, the possible mechanism of r1 relaxivity increase is described as follows. Upon Cu(II) coordination to GdL1, a 43% increase of r1 relaxivity could be attributed to the alteration of the water exchange rate after a reduced interaction of anionic carboxyl groups (–COO–) on the bis-benzoic acid motif with Gd(III) ions. In the presence of HSA, the Cu(II) is then coordinated with both bis(benzoic acid)methylamine recognition motif and nitrogen donor atoms from HSA to form a stable ternary complex GdL1–Cu2+–HAS with slower molecular rotation of the GdL1 moiety, resulting in a 270% increase of r1 relaxivity. In our opinion, the adjacent effect may be also responsible for the enhanced proton relaxivity of these Gd3+/Cu-chelate complexes where the proton of water molecules coordinated with both Gd3+ or Cu+/Cu2+ may be 0.4–0.6 nm away from Cu+/Cu2+ or Gd3+ cations, respectively. Much different from this molecular Cu(II) sensor, the adjacent effect between Gd(III) and Cu(II) in GdCu-LDH is mainly based on interactions of both cations with the same H2O molecule.
pH-dependent relaxivity and high in vivo MR imaging of GdCu-LDH
Even though Gd–chelate complexes account for approximately 95% of current MRI application in the clinic,46 the inherent toxicity of Gd3+ ions is correlated with the prevalence of nephrogenic systemic fibrosis (NSF).47,48 Mohd et al. found that Gd3+ ions and Gd2O3 nanoparticles caused oxidative damage and toxicity to cells in a time- and concentration-dependent manner.49 Moreover, the redundant gadolinium in the body for a long period also poses potential risks to the host health.50 Therefore, we chose Gd0.05Cu0.6-LDH to minimize the dose of Gd(III) in the nanoparticles for the examination of pH sensitivity and in vivo tumor MRI. Gd-LDH, Cu-LDH and GdCu-LDH are hereafter referred to as Gd0.05-LDH, Cu0.6-LDH and Gd0.05Cu0.6-LDH, respectively, unless specifically mentioned otherwise.The pH dependence of T1-weighted MRI was examined in buffer solutions of pH 7.4, 6.5 and 5.0 to mimic the normal body fluid, tumor microenvironment and cell lysosome/endosome, respectively. A small r1 value (1.66 mM−1 s−1) was found in Gd-LDH soaked for 4 h in pH 7.4 buffer, while r1 significantly increased to 3.76 and 6.28 mM−1 s−1 in pH 6.5 and 5.0 buffers, respectively, 2–4 times that at pH 7.4 (Fig. 6(A)). As expected, this high pH-sensitive T1-MRI effect was also observed in GdCu-LDH in terms of the Gd(III) concentration (Fig. 6(B)). GdCu-LDH exhibited a relaxivity of 11.45 mM−1 s−1 in the neutral condition, several times that of Gd-LDH and commercial Gd(III)-diethylenetriaminepentaacetic acid (Gd-DTPA) complex (3.4 mM−1 s−1).51 Moreover, after 4 h incubation in pH 6.5 and 5.0 buffers, the r1 relaxivity was significantly increased to 27.16 and 37.85 mM−1 s−1, 2.5–3.5 times that at pH 7.4, and 6–8 times that of Gd-LDH in the same pH buffers, demonstrating the ultra-sensitivity of GdCu-LDH nanoparticle MRI performance to the environment pH. This high pH-sensitivity may mean that the dose of GdCu-LDH contrast agent can be 8–10 times lower than that of the commercially used one, e.g. Gd-DTPA. A similar phenomenon was also observed in terms of the Cu(II) concentration (Fig. S7, ESI†).
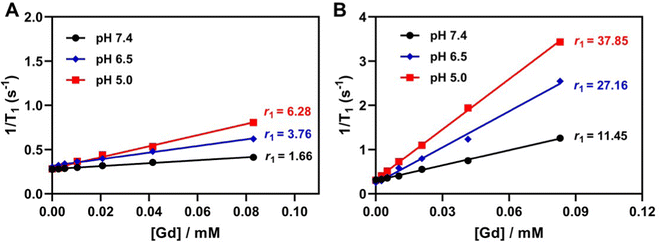 | ||
| Fig. 6 pH-dependent MRI performance. Plot of 1/T1versus [Gd] of (A) Gd-LDH and (B) GdCu-LDH (in terms of [Gd]) after co-incubation in different pH buffer solutions for 4 h. | ||
The ultrahigh pH-sensitivity of the T1 relaxivity of GdCu-LDH nanoparticles is presumably originated from the peculiar property of LDH materials. As explained previously,27 H+ in pH 6.5 and 5.0 buffers may protonate –OH groups in the hydroxide layer and form temporary ‘H2O’ molecules. As schematically represented in Fig. 7(B), water molecules a′ and c′ may be temporarily formed just after protonation of the relevant –OH groups. Clearly, temporary ‘H2O’ molecules will be much more prevalent in pH 6.5 and 5.0 buffers than in pH 7.4 buffer. Moreover, the metal-proton distance in these temporary Gd/Cu of ‘H2O’ complexes will be relatively shorter than in normal Gd/Cu-coordinated H2O, thus leading to significantly reduced longitudinal relaxation time of these protons as the relaxivity is inversely proportional to the 6th power of the metal-proton distance. In addition, the temporarily formed ‘H2O’ molecules also have stronger binding with Gd/Cu cations in comparison with normal Gd/Cu-coordinated H2O, resulting in a prolonged residence lifetime (τm) of water molecules, e.g. a slower exchange rate of coordinated H2O with free H2O molecules, which may further enhance the T1 relaxivity, in agreement with that for reported Mn-LDH nanoparticles.27
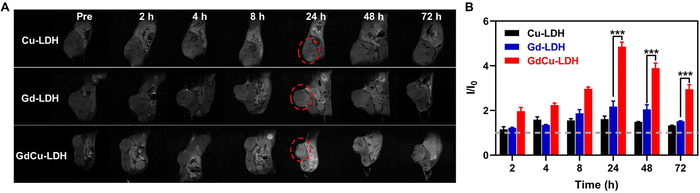
Taking advantage of the excellent MRI synergy between Gd(III) and Cu(II) and the outstanding pH sensitivity of GdCu-LDH, MR imaging of an in vivo tumor model was carried out to confirm the strong adjacent effect of GdCu-LDH after intravenous (i.v.) injection of 0.1 mg kg−1 Gd (0.64 μmol kg−1) and 0.5 mg kg−1 Cu. Prior to i.v. injection, all LDH-based nanoparticles were coated with bovine serum albumin (BSA) to colloidally stabilize the LDH nanoparticles in blood, e.g. decreasing adsorption of various biomolecules in blood and aggregation in physiological conditions, thus finally reducing the clearance by the reticuloendothelial system (RES), as reported previously.52 Fig. S8A (ESI†) demonstrates that the nanoparticles size did not change obviously but the zeta potential was significantly changed from 39.2 mV to −12.0 mV after BSA coating, which could effectively prevent particle aggregation in biological conditions.52 In addition, GdCu-LDH@BSA in the TEM image also exhibited similar morphology compared with bare GdCu-LDH (Fig. S8B, ESI†). The LDH crystal structure was also retained after BSA coating as demonstrated in our previous study.53 These data collectively verify that BSA coating did not change the size and structure of the LDH nanoparticles, thus did not disturb the in vivo MRI performance of GdCu-LDH. As shown in Fig. 8(A), the T1-weighted MR imaging signals (brightness) within the tumor area were enhanced in a time-dependent manner until 24 h post i.v. injection of Gd-LDH, Cu-LDH and GdCu-LDH, due to continuous infiltration of LDH nanoparticles into the tumor tissues via the enhanced permeability and retention (EPR) effect.54 The MR signals around the tumors were then decreased gradually and scarcely observed at 72 h, resulting from the metabolization of LDH nanoparticles in the acidic tumor microenvironment. The comparison of the relative MRI intensities at 24 h showed that GdCu-LDH injection led to a multi-fold MRI contrast in comparison with Gd-LDH and Cu-LDH (Fig. 8(B) and Fig. S9, ESI†). Clearly, the enhancement in the GdCu-LDH group is much higher than the additive enhancements in the Gd-LDH and Cu-LDH groups at the time point of 24 h (Fig. S9, ESI†), qualitatively consistent with the in vitro data. This MRI contrast increase benefits from high T1 relaxivity of GdCu-LDH even at this very low concentration. Compared with the recommended Gd-DTPA dose in clinical use (0.1 mmol kg−1), our Gd dose is >150 times lower (0.64 μmol kg−1).55 In addition, the i.v. administrated Cu is only half of our previously reported Cu-LDH dose.38 So low doses of Gd and Cu may significantly reduce their adverse effects. Notably, although pH-dependent contrast enhancement may not exist in the normal organs, the accumulation of LDH@BSA nanoparticles in the liver and spleen was 3–4 times that in the tumor tissues according to our published work53 and obvious T1-MRI signals were still observed in the normal organs at 24 h (Fig. 8(A)). In comparison, the signal intensity would be relatively lower than that in the tumor tissues due to the pH-sensitive contrast enhancement, similar to our previously reported Mn-LDH.27 Therefore, the development of LDH-based tumor targeted nanomedicine could promote the future translation of the clay nanoparticles for clinical applications.
Further note that the biocompatibility of GdCu-LDH nanoparticles was found to be good for the mouse melanoma skin cancer cell line (B16F0) and normal cells (DC 2.4, HUVEC, Raw 264.7). As shown in Fig. S10 (ESI†), the cell viabilities were all >90% upon GdCu-LDH nanoparticle treatment, even at the LDH concentration up to 400 μg mL−1 (corresponding to approximately 5–10 mg kg−1 of Gd(III) in vivo).
No adjacent effect in other dual-magnetic-ion containing LDH (GdM-LDH)
We further investigated whether the synergistic adjacent effect exists in Gd(III) and other contrast metal cations that are co-doped into the LDH layers. As shown in Fig. S11 (ESI†), when Gd(III) was incorporated into LDH nanoparticles with other metal cations (such as Mn(II), Co(II), Ni(II), and Fe(II)) in similar conditions, these LDH-based nanoparticles (e.g. Gd0.1Mn0.6-LDH, Gd0.1Co0.6-LDH, Gd0.1Ni0.6-LDH and Gd0.1Fe0.6-LDH) exhibited limited MR signal enhancement compared with Gd0.1Cu0.6-LDH. As estimated in Fig. S12 (ESI†), these LDH-based nanoparticles had r1 values of 1.7–2.1 mM−1 s−1, which are very similar to that of Gd0.1-LDH (1.72 mM−1 s−1), suggesting that there is no synergy between Gd(III) and M(II) in the LDH layers for T1-MRI. This may be ascribed to the inappropriate molar ratio of Gd(III) to other metal cations in the LDH layers and more possibly, no synergy between Gd(III) and other metal cations in affecting the proton relaxation of nearby water molecules. In comparison, these r1 values are 4–5 fold lower than that of Gd0.1Cu0.6-LDH (8.57 mM−1 s−1), further showing the synergy between Gd(III) and Cu(II) in the LDH layers. This difference in T1-MRI synergy should be caused by the adjacent cation effect, and thus deep understanding warrants further investigation for developing new efficient MRI contrast agents, such as polymer and lipid nanoparticles.56,57Conclusions
In summary, we have for the first time found that there is a strong synergy between Gd(III) and Cu(II) in Gd-/Cu-co-incorporated LDH nanoparticles for excellent T1-MRI performance in vitro and in vivo. The synergy is presumably based on the adjacent effect between magnetic cations (Gd(III) and/or Cu(II)) in the LDH layers and there seems to be an optimal amount of magnetic ions in the Gd-LDH and Cu-LDH nanoparticles. Moreover, the GdCu-LDH nanoparticles show an ultra-sensitivity of the longitudinal relaxivity to the environment pH (7.4 to 5.0). In the in vivo imaging, the GdCu-LDH nanoparticles result in much stronger MRI signals of the tumor tissues than the corresponding Gd-LDH and Cu-LDH nanoparticles. This finding thus provides a way to develop excellent T1-MRI contrast agents that are capable of enhancing the positive contrast for efficient cancer diagnosis at low doses.Experimental section
Materials
MgCl2·6H2O, AlCl3·6H2O and NaOH were purchased from Chem-Supply. GdCl3·6H2O, CuCl2·2H2O, and bovine serum albumin (BSA) were purchased from Sigma-Aldrich. Dulbecco's Modified Eagle's Medium (DMEM), penicillin/streptomycin, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were acquired from Thermal Fisher SCIENTIFIC. Phosphate buffered saline (PBS, 10×) and fetal bovine serum (FBS) were purchased from BioWhitter. Milli-Q water was used throughout the experiments.Preparation of Gdx-LDH, Cuy-LDH and GdxCuy-LDH
Gdx-LDH was first synthesized via a simple co-precipitation method. 3 mmol of MgCl2·6H2O, (1 − x) mmol of AlCl3·6H2O and x mmol of GdCl3·6H2O (x = 0.025, 0.05, 0.075, 0.1, 0.15) were quickly added into 20 mL of 0.4 M NaOH solution under nitrogen protection. After vigorous stirring for 20 min, the resultant Gdx-LDH slurry was separated, washed and then re-dispersed in water. When the particle size distribution became stable, Cu2+ substitution was carried out in the second step. Briefly, y mmol of CuCl2·2H2O (y = 0.2, 0.4, 0.6, 0.8, 1.0) was added slowly into 2 mL of Gdx-LDH (8 mg mL−1) suspension with vigorous stirring for 6 h. Subsequently, GdxCuy-LDH was obtained via centrifugation (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 20 min) and re-dispersed in deionized water for further use.
000 rpm, 20 min) and re-dispersed in deionized water for further use.
MgAl-LDH was synthesized similar to Gdx-LDH preparation, except for the addition of GdCl3·6H2O. Then Cuy-LDH was prepared by substituting Cu2+ into MgAl-LDH as above.
Characterization
The amount of Gd, Cu, Mg and Al in the nanoparticles was determined by inductively coupled plasma-atomic emission spectrometry (ICP-AES). The size distributions were measured with dynamic light scattering (DLS) by photon correlation spectroscopy (PCS, Nanosizer, MALVERN instruments) at 25 °C. The nanoparticle morphology was recorded in transmission electron microscopy (TEM) on a HITACHI 7700A electron microscope operated at an acceleration voltage of 80 kV. Powder X-ray diffraction (XRD) patterns of all lyophilized samples were examined by a Rigaku Miniflex X-ray diffractometer with a variable slit width at a scanning rate of 2° min−1 with 2θ in the range between 5° and 50° using Cu Kα radiation (λ = 0.15406 nm). Raman measurements were conducted in a Horiba Jobin Yvon Modular Raman spectrometer using a stella pro Argon-ion laser at 514 nm. The surface morphology was acquired by field emission scanning electron microscopy (FE-SEM) using a FEI Quanta 650 FEG at an acceleration voltage of 20.0 kV.MRI evaluation
In order to assess the MRI performance of Gdx-LDH, Cuy-LDH and GdxCuy-LDH, T1-weighted MR imaging of Gdx-LDH, Cuy-LDH and GdxCuy-LDH solutions was transferred into SampleJet tubes (5.0 × 103.5 mm) and performed on a 16.4-T Bruker micro-imaging system (Bruker Biospin, Karlsruhe, Germany).For testing the longitudinal relaxivities (r1) in solutions with different pH values, Gdx-LDH, Cuy-LDH and GdxCuy-LDH nanoparticles were dispersed in buffer solutions with pH 7.4, 6.5 and 5.0 (citrate-phosphate buffer), respectively. After shaking (37 °C, 100 rpm) for 4 h, the heterogeneous buffers containing Gdx-LDH, Cuy-LDH or GdxCuy-LDH were transferred into SampleJet tubes for MRI scanning.
Cell viability assay
B16F0 mouse melanoma cells were cultured in complete Dulbecco's Modified Eagle's Medium (DMEM) complemented with 10% foetal bovine serum and 1% penicillin/streptomycin in a 37 °C incubator supplying 5% CO2. The cells were seeded in a 96-well plate at a density of 5 × 103 cells per well and incubated for 24 h to allow cell attachment. Then the cells were treated with GdCu-LDH nanoparticles and continuously incubated for 48 h. Finally, the cell viability was examined by the typical 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction assay.The cytotoxicity of GdCu-LDH in DC 2.4, HUVEC and Raw 264.7 cell lines was measured in a similar way.
In vivo MRI evaluation
In order to improve the stability of the LDH nanoparticles in physiological conditions, BSA was coated on the surface of Gd-LDH, Cu-LDH and GdCu-LDH, respectively. Taking Cu-LDH as an example, 5 mL of Cu-LDH suspension (4 mg mL−1) was dropwise added into 5 mL of BSA solution (10 mg mL−1) under stirring. After a further 30 min-continuous stirring, the homogenous BSA/Cu-LDH was diluted with PBS and used for in vivo characterization. BSA/Gd-LDH and BSA/GdCu-LDH were prepared via similar methods.The melanoma tumor model was built by implanting subcutaneously 1 × 105 B16F0 cells on the right back of each C57Bl/6 female mouse (8 weeks old). All animal operations were carried out in accordance with the guidelines of the Animal Ethics Committee of The University of Queensland. After the tumor diameter reached about 300 mm3, each mouse was injected through the tail-vein with 100 μL of BSA/Gd-LDH, BSA/Cu-LDH and BSA/GdCu-LDH, respectively. The equivalent doses of Gd(III) and Cu(II) in each mouse were 0.1 mg kg−1 and 0.5 mg kg−1. In vivo T1-weighted MR images were recorded at 0, 2, 4, 8 24, 48, and 72 h post injection using FOV (field of view) = 30 × 30 mm2, MTX (matrix size) = 128 × 128, TR/TE (reception time/echo time) = 400.0/5.5 ms, FA (flip angle) = 90°, and slice thickness = 1 mm.
Statistical analysis
All experiments were conducted at least in triplicate with the data expressed as mean ± standard error of the mean (SEM). The Student's t test was used to test the significant differences between the experimental groups. NS: no significant difference when p > 0.05; *: p < 0.05; **: p < 0.01; ***: p < 0.001; and ****: p < 0.0001.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research is financially supported by the Australian Research Council (ARC) Discovery Project (DP190103486). R. Zhang wishes to acknowledge Emerging Leadership of the National Health and Medical Research Council (APP1175808). J. Liu acknowledges the Australian Government Research Training Program Scholarship (RTP). The authors further appreciate the facilities and technical assistance of the Australian Microscopy & Microanalysis Research Facility at the Centre for Microscopy and Microanalysis (CMM), Australian National Fabrication Facility (ANFF) and Centre of Advance Imaging (CAI), The University of Queensland.References
- H. H. Chen, Z. Khatun, L. Wei, C. Mekkaoui, D. Patel, S. J. W. Kim, A. Boukhalfa, E. Enoma, L. Meng, Y. I. Chen, L. Kaikkonen, G. Li, D. E. Capen, P. Sahu, A. T. N. Kumar, R. M. Blanton, H. Yuan, S. Das, L. Josephson and D. E. Sosnovik, Nat. Biomed. Eng., 2022, 6, 1045–1056 CrossRef CAS PubMed.
- Y. Zhang, X. Jiang, J. Zhang, H. Zhang and Y. Li, Biosens. Bioelectron., 2019, 130, 315–321 CrossRef CAS PubMed.
- L. Zhang, K. Khan, J. Zou, H. Zhang and Y. Li, Adv. Mater. Interfaces, 2019, 6, 1901329 CrossRef.
- T. Xue, S. R. Bongu, H. Huang, W. Liang, Y. Wang, F. Zhang, Z. Liu, Y. Zhang, H. Zhang and X. Cui, Chem. Commun., 2020, 56, 7041–7044 RSC.
- Z. Chen, J. Li, T. Li, T. Fan, C. Meng, C. Li, J. Kang, L. Chai, Y. Hao, Y. Tang, O. A. Al-Hartomy, S. Wageh, A. G. Al-Sehemi, Z. Luo, J. Yu, Y. Shao, D. Li, S. Feng, W. J. Liu, Y. He, X. Ma, Z. Xie and H. Zhang, Natl. Sci. Rev., 2022, 9, nwac104 CrossRef PubMed.
- Z. Wang, X. Xue, H. Lu, Y. He, Z. Lu, Z. Chen, Y. Yuan, N. Tang, C. A. Dreyer, L. Quigley, N. Curro, K. S. Lam, J. H. Walton, T.-Y. Lin, A. Y. Louie, D. A. Gilbert, K. Liu, K. W. Ferrara and Y. Li, Nat. Nanotechnol., 2020, 15, 482–490 CrossRef CAS PubMed.
- O. Perlman, H. Ito, K. Herz, N. Shono, H. Nakashima, M. Zaiss, E. A. Chiocca, O. Cohen, M. S. Rosen and C. T. Farrar, Nat. Biomed. Eng., 2022, 6, 648–657 CrossRef CAS PubMed.
- Z. Zhou, L. Yang, J. Gao and X. Chen, Adv. Mater., 2019, 31, 1804567 CrossRef PubMed.
- Z. Shen, T. Liu, Z. Yang, Z. Zhou, W. Tang, W. Fan, Y. Liu, J. Mu, L. Li, V. I. Bregadze, S. K. Mandal, A. A. Druzina, Z. Wei, X. Qiu, A. Wu and X. Chen, Biomaterials, 2020, 235, 119783 CrossRef CAS PubMed.
- K. Liu, Z. Cai, X. Chi, B. Kang, S. Fu, X. Luo, Z.-W. Lin, H. Ai, J. Gao and H. Lin, Nano Lett., 2022, 22, 3219–3227 CrossRef CAS PubMed.
- Z. Zhou, H. Deng, W. Yang, Z. Wang, L. Lin, J. Munasinghe, O. Jacobson, Y. Liu, L. Tang, Q. Ni, F. Kang, Y. Liu, G. Niu, R. Bai, C. Qian, J. Song and X. Chen, Nat. Commun., 2020, 11, 3032 CrossRef CAS PubMed.
- W. Xu, X. Qing, S. Liu, D. Yang, X. Dong and Y. Zhang, Small, 2022, 18, 2106511 CrossRef CAS PubMed.
- Y. Bian, B. Liu, S. Liang, B. Ding, Y. Zhao, F. Jiang, Z. Cheng, A. A. A. Kheraif, P. A. Ma and J. Lin, Chem. Eng. J., 2022, 435, 135046 CrossRef CAS.
- P. Mi, D. Kokuryo, H. Cabral, H. Wu, Y. Terada, T. Saga, I. Aoki, N. Nishiyama and K. Kataoka, Nat. Nanotechnol., 2016, 11, 724–730 CrossRef CAS PubMed.
- M. Zhang, Y. Cao, L. Wang, Y. Ma, X. Tu and Z. Zhang, ACS Appl. Mater. Interfaces, 2015, 7, 4650–4658 CrossRef CAS PubMed.
- Z. Zhou, L. Wang, X. Chi, J. Bao, L. Yang, W. Zhao, Z. Chen, X. Wang, X. Chen and J. Gao, ACS Nano, 2013, 7, 3287–3296 CrossRef CAS PubMed.
- G. Huang, H. Li, J. Chen, Z. Zhao, L. Yang, X. Chi, Z. Chen, X. Wang and J. Gao, Nanoscale, 2014, 6, 10404–10412 RSC.
- Z. Zhou, D. Huang, J. Bao, Q. Chen, G. Liu, Z. Chen, X. Chen and J. Gao, Adv. Mater., 2012, 24, 6223–6228 CrossRef CAS PubMed.
- Y. Si, G. Zhang, D. Wang, C. Zhang, C. Yang, G. Bai, J. Qian, Q. Chen, Z. Zhang, Z. Wu, Y. Xu and D. Zou, Chem. Eng. J., 2019, 360, 289–298 CrossRef CAS.
- Z. Gu, J. J. Atherton and Z. P. Xu, Chem. Commun., 2015, 51, 3024–3036 RSC.
- Z. Cao, B. Li, L. Sun, L. Li and Z. Gu, Small Methods, 2019, 4, 1900343 CrossRef.
- Z. Gu, S. Yan, S. Cheong, Z. Cao, H. Zuo, A. C. Thomas, B. E. Rolfe and Z. P. Xu, J. Colloid Interface Sci., 2018, 512, 404–410 CrossRef CAS PubMed.
- M. L. Parello, R. Rojas and C. E. Giacomelli, J. Colloid Interface Sci., 2010, 351, 134–139 CrossRef CAS PubMed.
- Y. Wang, S. Shen, T. Hu, G. R. Williams, Y. Bian, B. Feng, R. Liang and X. Weng, ACS Nano, 2021, 15, 9732–9745 CrossRef PubMed.
- W. Shen, T. Hu, X. Liu, J. Zha, F. Meng, Z. Wu, Z. Cui, Y. Yang, H. Li, Q. Zhang, L. Gu, R. Liang and C. Tan, Nat. Commun., 2022, 13, 3384 CrossRef CAS PubMed.
- H. Fu, L. Wang, Q. Bao, D. Ni, P. Hu and J. Shi, J. Am. Chem. Soc., 2022, 144, 8987–8999 CrossRef CAS PubMed.
- B. Li, Z. Gu, N. Kurniawan, W. Chen and Z. P. Xu, Adv. Mater., 2017, 29, 1700373 CrossRef PubMed.
- C. Zhang, L. Li, F. Y. Han, X. Yu, X. Tan, C. Fu, Z. P. Xu and A. K. Whittaker, Small, 2019, 15, 1902309 CrossRef PubMed.
- W. Xie, Z. Guo, Q. Gao, D. Wang, K. Liang, Z. Gu and L. Y. Zhao, ACS Appl. Bio Mater., 2020, 3, 5845–5855 CrossRef CAS PubMed.
- M. S. Usman, M. Z. Hussein, A. U. Kura, S. Fakurazi, M. J. Masarudin and F. F. Ahmad Saad, Mater. Chem. Phys., 2020, 240, 122232 CrossRef CAS.
- L. Wang, S.-M. Xu, X. Yang, S. He, S. Guan, G. I. N. Waterhouse and S. Zhou, ACS Appl. Mater. Interfaces, 2020, 12, 54916–54926 CrossRef CAS PubMed.
- L. Peng, X. Mei, J. He, J. Xu, W. Zhang, R. Liang, M. Wei, D. G. Evans and X. Duan, Adv. Mater., 2018, 30, 1707389 CrossRef PubMed.
- W. Zhang, L. Liu, H. Chen, K. Hu, I. Delahunty, S. Gao and J. Xie, Theranostics, 2018, 8, 2521–2548 CrossRef CAS PubMed.
- S. Wang, Z. Zhou, Z. Wang, Y. Liu, O. Jacobson, Z. Shen, X. Fu, Z.-Y. Chen and X. Chen, Small, 2019, 15, 1900691 CrossRef PubMed.
- G. Zhang, W. Xie, Z. Xu, Y. Si, Q. Li, X. Qi, Y. Gan, Z. Wu and G. Tian, Mater. Horiz., 2021, 8, 1017–1028 RSC.
- R. Ge, M. Lin, X. Li, S. Liu, W. Wang, S. Li, X. Zhang, Y. Liu, L. Liu, F. Shi, H. Sun, H. Zhang and B. Yang, ACS Appl. Mater. Interfaces, 2017, 9, 19706–19716 CrossRef CAS PubMed.
- Y. Liu, J. Wu, Y. Jin, W. Zhen, Y. Wang, J. Liu, L. Jin, S. Zhang, Y. Zhao, S. Song, Y. Yang and H. Zhang, Adv. Funct. Mater., 2019, 29, 1904678 CrossRef CAS.
- B. Li, J. Tang, W. Chen, G. Hao, N. Kurniawan, Z. Gu and Z. P. Xu, Biomaterials, 2018, 177, 40–51 CrossRef CAS PubMed.
- S. Guan, R. Liang, C. Li and M. Wei, Talanta, 2017, 165, 297–303 CrossRef CAS PubMed.
- L. Wang, H. Xing, S. Zhang, Q. Ren, L. Pan, K. Zhang, W. Bu, X. Zheng, L. Zhou, W. Peng, Y. Hua and J. Shi, Biomaterials, 2013, 34, 3390–3401 CrossRef CAS PubMed.
- X. Mei, J. Ma, X. Bai, X. Zhang, S. Zhang, R. Liang, M. Wei, D. G. Evans and X. Duan, Chem. Sci., 2018, 9, 5630–5639 RSC.
- X. Mei, W. Wang, L. Yan, T. Hu, R. Liang, D. Yan, M. Wei, D. G. Evans and X. Duan, Biomaterials, 2018, 165, 14–24 CrossRef CAS PubMed.
- H. Li and T. J. Meade, J. Am. Chem. Soc., 2019, 141, 17025–17041 CrossRef CAS PubMed.
- E. L. Que, E. Gianolio, S. L. Baker, A. P. Wong, S. Aime and C. J. Chang, J. Am. Chem. Soc., 2009, 131, 8527–8536 CrossRef CAS PubMed.
- N. N. Paranawithana, A. F. Martins, V. Clavijo Jordan, P. Zhao, S. Chirayil, G. Meloni and A. D. Sherry, J. Am. Chem. Soc., 2019, 141, 11009–11018 CrossRef CAS PubMed.
- B. A. Bony, J. S. Baeck, Y. Chang, J. E. Bae, K. S. Chae and G. H. Lee, Bull. Korean Chem. Soc., 2015, 36, 1203–1208 CAS.
- T. Grobner, Nephrol., Dial., Transplant., 2006, 21, 1104–1108 CrossRef CAS PubMed.
- A. Z. Khawaja, D. B. Cassidy, J. Al Shakarchi, D. G. McGrogan, N. G. Inston and R. G. Jones, Insights Imaging, 2015, 6, 553–558 CrossRef PubMed.
- M. J. Akhtar, M. Ahamed, H. Alhadlaq and S. Alrokayan, Curr. Drug Metab., 2019, 20, 907–917 CrossRef CAS PubMed.
- J. Wahsner, E. M. Gale, A. Rodríguez-Rodríguez and P. Caravan, Chem. Rev., 2019, 119, 957–1057 CrossRef CAS PubMed.
- K. M. Donahue, D. Burstein, W. J. Manning and M. L. Gray, Magn. Reson. Med., 1994, 32, 66–76 CrossRef CAS PubMed.
- Z. Gu, H. Zuo, L. Li, A. Wu and Z. P. Xu, J. Mater. Chem. B, 2015, 3, 3331–3339 RSC.
- J. Liu, Y. Wu, C. Fu, B. Li, L. Li, R. Zhang, T. Xu and Z. P. Xu, Small, 2020, 16, 2002115 CrossRef CAS PubMed.
- L. Qin, M. Xue, W. Wang, R. Zhu, S. Wang, J. Sun, R. Zhang and X. Sun, Int. J. Pharm., 2010, 388, 223–230 CrossRef CAS PubMed.
- M. Filippi, R. Capra, A. Campi, B. Colombo, F. Prandini, N. Marcianò, R. Gasparotti and G. Comi, J. Neurol., Neurosurg. Psychiatry, 1996, 60, 526–530 CrossRef CAS PubMed.
- Y. Zhang, M. Cao, B. Yuan, T. Guo and W. Zhang, Polym. Chem., 2017, 8, 7325–7332 RSC.
- M. N. Vu, H. G. Kelly, A. K. Wheatley, S. Peng, E. H. Pilkington, N. A. Veldhuis, T. P. Davis, S. J. Kent and N. P. Truong, Small, 2020, 16, e2002861 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2nh00478j |
| This journal is © The Royal Society of Chemistry 2023 |