Role of identified proteins in the proteome profiles of CDK4/6 inhibitor-resistant breast cancer cell lines†
Binayak
Kumar
a,
Peeyush
Prasad
b,
Ragini
Singh
a,
Ram Krishna
Sahu
a,
Ashutosh
Singh
 c,
Srikrishna Jayadev
Magani
*c and
Suresh
Hedau
c,
Srikrishna Jayadev
Magani
*c and
Suresh
Hedau
 *a
*a
aDivision of Molecular Oncology, ICMR-National Institute of Cancer Prevention and Research, I–7, Sector-39, Noida, Uttar Pradesh 201301, India. E-mail: suresh.hedau@gov.in; Tel: +91-0120-2446909
bDepartment of Research, Sir Ganga Ram Hospital, 110060, New Delhi, India
cDepartment of Life Sciences, Shiv Nadar University, NH-91, Tahsil-Dadri, Distt-Gautam Budhaa Nagar, Uttar Pradesh 201314, India. E-mail: jayadevmsk@snu.edu.in
First published on 7th March 2023
Abstract
Abemaciclib (Ab) and palbociclib (Pb) are CDK4/6 inhibitors used to cure advanced breast cancer (BC). However, acquired resistance is a major challenge. The molecular mechanisms and signature proteins of therapy resistance for Ab and Pb drugs need to be explored. Here we developed resistant cells for Ab and Pb drugs in MCF-7 cell lines and explored the mechanisms and signature proteins of therapy resistance in BC. Proteome profiling was performed using the label-free proteome-orbitrap-fusion-MS-MS technique. Gene ontology (GO)-terms, KEGG pathways and network analysis were performed for the proteome data. Drug-resistant cells showed increased drug tolerance, enhanced colony formation potential and an increased gap-healing tendency for the respective drug. Up-regulation of survival genes (BCL-2 and MCL-1) and down-regulation of apoptosis inducers were observed. Drug-resistance markers (MDR-1 and ABCG2 (BCRP)) along with ESR-1, CDK4, CDK6, and cyclin-D1 genes were up-regulated in resistant cells. A total of 237 and 239 proteins were found to be differentially expressed in the Ab and Pb-resistant cells, respectively. Down-regulated proteins induce apoptosis signalling and nucleotide metabolisms and restrict EGFR signalling; however, up-regulated proteins induce Erk, wnt-β-catenin, VEGFR-PI3K-AKT, glucose transportation, and hypoxia signalling pathways and regulate hydrogen peroxide signalling pathways. The panel of identified proteins associated with these pathways might have characteristics of molecular signature and new drug targets for overcoming drug resistance in breast cancer.
1. Introduction
Breast cancer (BC) is the second most commonly diagnosed cancer in the world, after lung cancer; however, it is the most prevalent in the Indian population.1–3 Breast cancers are classified by the presence or absence of surface receptors, that is, the estrogen receptor (ER) or the progesterone receptor (RP), and by the degree of expression of human epidermal growth factor receptor 2 (HER-2). Radiotherapy, chemotherapy, hormone therapy, and targeted therapy are all common treatment methods.4 To treat primary and metastatic breast cancer, chemotherapy drugs such as 5-fluorouracil, methotrexate, cyclophosphamide, doxorubicin, epirubicin and taxane (docetaxel) are used.5,6 An estrogen receptor modulator (tamoxifen), an aromatase inhibitor (letrozole), and a selective estrogen receptor degrader (fulvestrant) are used to treat ER-positive hormone-responsive breast cancer. Both the pharmacokinetics and the pharmacodynamics of these two medications share a lot of similarities. The selection of a specific CDK4/6 inhibitor for a given patient can indeed be critical with regard to disparities in the structure, therapeutic potency, IC50 values for CDK4 and CDK6, medication toxicity, and dosages. Tumor cells develop tolerance to drug cytotoxicity, resulting in a therapeutic resistant phenotype, which contributes to disease progression and recurrence.5,6 Tumor cells trigger other pathways essential for their survival in the process of acquiring the resistance trait.5,6 Investigation of the molecular mechanisms underlying therapeutic resistance in cancer care has resulted in a transition in drug development toward targeted therapy. By inhibiting certain proteins necessary for tumor cell proliferation and survival, targeted therapy prevents cancer progression. Proteins such as receptor tyrosine kinases, cytokine receptors, and intracellular serine–threonine kinases can be targeted.Abemaciclib (Ab) and palbociclib (Pb) are two synthetic (small-molecule) inhibitors of cyclin-dependent kinase 4 and 6 (CDK4 and CDK6).7 CDK 4 and 6 are intracellular, serine–threonine protein kinase enzymes associated with cell-cycle progression from the G1 to the S-phase. Inhibition of CDK4 and CDK6 will halt the G1 phase of the cells. The U.S. FDA approved Pb in 2016, while Ab was approved in 2017 to treat advanced breast cancer with ER and PR-positive and HER2-negative receptors.8,9 Deregulation of CDK4/6 causes uncontrolled cell proliferation, which may occur due to amplification of cyclin D1, a gain of CDK4/6, loss of p18, elevated Rb1, loss of p16, etc.10–13 CDK4/6 inhibitors have emerged as a breakthrough in breast cancer treatment. Recently, resistance to CDK4/6 inhibitors has been identified as a concern in the management of breast cancer.14–16 PI3K-AKT-mTOR signal-mediated resistance to CDK4/6 inhibitors is reported for a similar medicine ribociclib.17 However, the molecular signatures of drug resistance in Ab/Pb-treated breast cancer have not been reported.
This study aims to explore the molecular mechanisms and signatures in Ab and Pb drug-resistant breast cancer by developing a resistance model in the MCF-7 cell line for the respective drug.
2. Experimental
2.1. Reagents
Ab and Pb drugs were purchased from SelleckChem, USA. MCF-7 was bought from NCCS Pune, India. Cell culture media (Lonza), FBS (Gibco Life Technologies), plastic-wares for cell culture (Corning), 96-well plates for real-time-qPCR (Thermo Scientific), an RNA isolation kit (Qiagen), a cDNA synthesis kit (Takara), 2× SYBR Green-Master mix (Promega) and primers (Imperial Life Sciences Pvt. Ltd) were used. Both control and resistant cells were maintained in a high glucose DMEM supplemented with 10% FBS and 1% penicillin and streptomycin antibiotics in 5% CO2, humidified air and at 37 °C temperature. To develop resistance models, MCF-7 cells were treated with Ab and Pb drugs in separate T-25 flasks at initial 5 nm concentrations. The drug doses were gradually increased up to 200 nm and the cells were maintained at the same concentration.2.2. Cell-viability assay
MCF-7 cells and continued Ab-treated cells named Ab-R-MCF-7 and continued Pb-treated cells named Pb-R-MCF-7 were seeded in a 96-well plate with 2. 5 × 104 cell count in each well. After 24 h of incubation, cells were treated with the respective drug from 100 nm to 6 μM concentrations for drug-sensitive MCF-7 cells and up to 8 μM for drug-resistant MCF-7 cells for 48 and 72 h. 100 μl of 0.5 μg μl−1 MTT was added to each well and incubated for 2.5 h, and then the cells were lysed with a detergent (DMSO). The OD-value was measured at 595 nm wavelength using a micro-plate reader and the percent cell viability was calculated.2.3. PI-staining-microscopy
When cells undergo the death phase due to the drug effect, the cell membrane is compromised; hence propidium iodide (PI) can easily enter into the cells. PI-stain may be retained in the cytoplasm and enter into the nucleus and stain DNA. MCF-7, Ab-R-MCF-7, and Pb-R-MCF-7 cells were seeded (4 × 104 cells per well) in a 12-well plate and incubated in a CO2 incubator for 24 h. Cells were treated with Ab and Pb of 1.5 and 2.0 μM concentrations for 48 and 72 h, respectively. Cells were stained with 25 μg ml−1 PI for 15 min. PI-stained cells exposed to green monochromatic light and red-emission light were captured along with white light. To show the contrast in non-stained cells, the bright field images and PI-stained images were merged. Images were taken at 10× magnification using an inverted fluorescence microscope. During image capturing, the PI-stained cells were counted manually and represented as a graph against the number of PI-stained cells (Y-axis) and the category of cells (X-axis).2.4. Colony formation assay
Drug-sensitive and resistant cells were seeded in 6 well plates (1 × 103 cells per well), incubated for 24 h, treated with the Ab/Pb drug in respective wells for 48 h and then allowed to grow for two more days; cell colonies were fixed with 70% pre-chilled ethanol at −20 °C for 20 minutes. Cells were stained with 0.5% w/vol. crystal violet for 15 minutes, and the stained colonies were washed with water very carefully and dried on a plate at room temperature. ImageJ software was used to calculate the number of stained dots that represent a colony of cells. Images are opened in ImageJ tools maxima applying, prominence of >10 is used to count the number of stained dots in a selected area and edge maxima is then used to calculate the frequency of cells survival utilising| Plating efficiency (PE) = (No. of colonies formed/No. of seeded cells) × 100 |
| Survival frequency (SF) = (No. of colonies formed/No. of seeded cells) × PE |
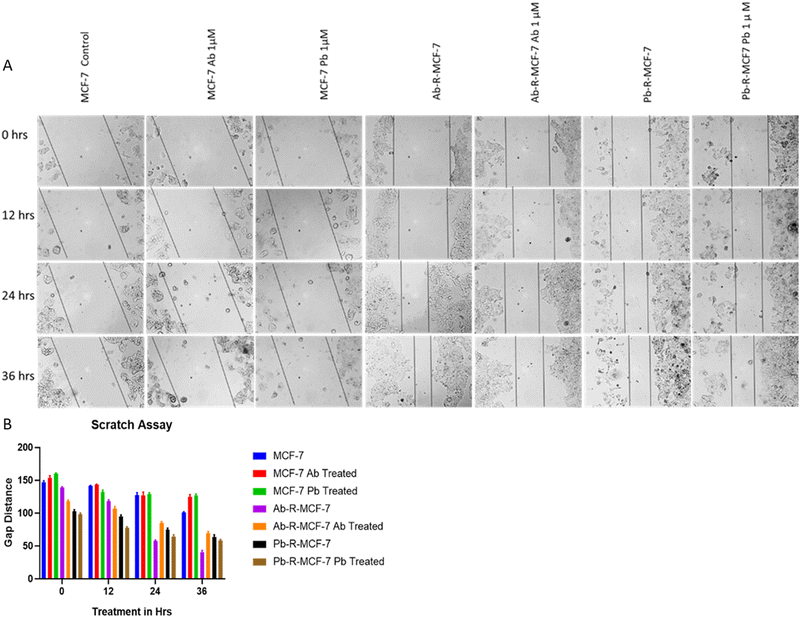
2.5. Wound healing assay
Drug-sensitive and resistant cells were seeded in 6 well plates (2 × 104 cells per well), incubated and allowed to grow up to 70% confluency, and a scratch is made in the well. Gap image at 10× magnification were acquired at 0, 12, 24, and 36 hours for the Ab/Pb-resistant cells treated with the respective drugs and the control. Using ImageJ, gaps were measured. A graph was plotted for gap vs. treatment duration using GraphPad Prism 8 analytical tools.2.6. Real-time-qPCR
MCF-7, Ab-R-MCF-7, and Pb-R-MCF-7 cells were seeded (7.5 × 104 cells per well) in 6-well plates and incubated in a CO2 incubator for 24 h. Cells were treated with Ab (0.25 μM) and Pb (2.0 μM) drugs for 48 h. Cells were detached and washed with cold PBS. Total RNA is extracted from the cell palette using a total RNA isolation kit following the kit protocols. The quantity and quality of total RNA were measured using Nano-Drop. 0.5 μg of total RNA was used for cDNA synthesis following the protocols mentioned in the cDNA synthesis kit. Real-time-qPCR was performed in triplicate using a Bio-Rad connect real-time-qPCR system using the 2× Master mix of SYBR Green (from Bio-Rad) and gene-specific primers.18S gene was selected as an internal control for normalization. The relative quantity (RQ) value for each gene was analysed for each sample from their Ct-value. The list of genes and primer sequences is provided in Table S1 (ESI†).2.7. Label free-proteome-orbitrap-fusion MS-MS
Samples were prepared for proteomics experiments of MCF-7 control, Ab-R-MCF-7 and Pb-R-MCF-7 cells. Cells were seeded (7.5 × 104 cells per well) in T-25 flasks and incubated in a CO2 incubator for 48 h; after that cells were harvested and lysed using a cell lysis buffer (6 M urea, 2M thiourea, 2% CHAPS and 0.5% SDS) containing 1% protease inhibitor cocktail. The cell samples were sonicated on ice for a 30-s pulse with 10 s gaps 5 times, the cell lysate was centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min at 4 °C and then the supernatant was collected. Proteins were estimated using a Bradford reagent (Sigma) and the OD value was measured using a microplate reader under exposure of 595 nm light. The protein quality was checked using Coomassie blue after running SDS-PAGE. The experiment was performed at the proteomics laboratory, MASSFIITB facility supported by DBT (BT/PR13114/INF/22/206/2015), IIT Bombay, Mumbai, India. The technique used is label free-proteome-orbitrap-fusion MS-MS. The protein search is performed against the Homo sapiens database downloaded from UniProt using Proteome Discoverer 2.218
000 rpm for 15 min at 4 °C and then the supernatant was collected. Proteins were estimated using a Bradford reagent (Sigma) and the OD value was measured using a microplate reader under exposure of 595 nm light. The protein quality was checked using Coomassie blue after running SDS-PAGE. The experiment was performed at the proteomics laboratory, MASSFIITB facility supported by DBT (BT/PR13114/INF/22/206/2015), IIT Bombay, Mumbai, India. The technique used is label free-proteome-orbitrap-fusion MS-MS. The protein search is performed against the Homo sapiens database downloaded from UniProt using Proteome Discoverer 2.218
2.8. Bioinformatics
Bioinformatics tools were used to analyse the proteomics data. The Venny tool is used to select common proteins in replicating data. The reactome pathway database was used to find the role of proteins in different reaction pathways. Gene ontology (GO) analysis was performed using the STRING database. A network analyst tool was used to find the protein hub. Generic PPI was analysed without any filter, but the subnetwork had maximum seeds. A miR-Net tool was used for miRNA–gene interaction analysis to explore the post-transcriptional regulation of gene expressions. Here, identified protein IDs were searched against the background of breast cancer tissues. The minimum network option was selected for the analysis.2.9. Statistical analysis
The association between the expressions of genes in the drug-resistant cells was analysed using the chi-square test. In addition, the statistical difference was calculated using GraphPad Prism 8 software by one-way analysis of variance (ANOVA).19P < 0.05 was considered statistically significant.3. Results and discussion
3.1. Increased tolerance to Ab and Pb in Ab-R-MCF-7 and Pb-R-MCF-7 cells
MTT forms formalin crystals in live cells that appear violet in colour whereas it fails to form crystals in the cells which are moving to the death phase. Based on crystal colour measurements, taking absorbance in a microplate reader gives an idea of the effect of xenobiotic compounds on cellular health and the cell viability is analyzed.20 MCF-7 cells were treated with Ab and Pb and the inhibitory concentration values were calculated. Cells treated with Ab exhibited an IC50 value of 1.5 μM at 48 h and cells treated with Pb showed an IC50 value of 3.0 μM after 72 h as shown in Fig. 1A and B respectively. Sensitive MCF-7 cells were treated continuously with an increasing concentration ranging from 0.1 to 8 μM. The cytotoxicity was assessed in the continuously drug-exposed cells and it was found that Ab-treated cells could tolerate the drug up to a concentration of 7.0 μM after 48 h of treatment and Pb-treated cells could tolerate up to 7.5 μM after 72 h as shown in Fig. 1A and B respectively. In Ab-R-MCF-7 cells 7.0-fold and in Pb-R-MCF-7 cells 2-fold higher drug tolerance capacities were observed, indicating that these cells acquired resistance to respective drugs.3.2. Reduced cell death in drug-resistant cells
The PI exclusion experiment was performed to measure the cytotoxic effect of the respective drug. Cells having a compromised cell membrane only allow PI to pass through them and it binds with DNA and emits fluorescence. Loss of membrane integrity was reported to be one of the hallmark features of cell death either by necrosis or by apoptosis.21 MCF-7 control and Ab/Pb-R-MCF-7 cells were treated with the respective drug for 48 h and stained with PI. MCF-7 control cells without drug treatment looked healthy and a few cells picked up the PI stain, whereas MCF-7 cells when treated with Ab and Pb exhibited a stressed phenotype and a great number of cells picked-up the PI stain. However, only a few Ab/Pb-R-MCF-7 cells picked up the PI stain and the rest of the cells looked healthier (Fig. 1C–E). This result re-confirmed the result of the cell viability assay that the Ab/Pb-R-MCF-7 cells acquired resistance against respective drugs.3.3. Enhanced colony formation potential of Ab/Pb-resistant cells
Colony formation assay was used to evaluate the drug-resistance characteristics via evaluation of cell survival and proliferation indicated by cell survival, the number of colonies formed and their growth.22 As shown in Fig. 2A and B, very few number of colonies were formed in Ab/Pb drug-treated sensitive MCF-7 cells as compared to the control. However, in Ab/Pb treated cells, the number of colonies formed are similar to the Ab/Pb-R-MCF-7 cells without the respective drug. This result indicates that resistant cells have elevated cell survival and proliferation potential even after drug treatment as shown in Fig. 2C and D.3.4. Enhanced wound healing potential of Ab/Pb-resistant cells
Here we have studied the tendency of cell migration, wound healing and proliferation using the wound healing assay.22 The results show that the gap made at 0 h in MCF-7 with the Ab/Pb drug as compared to MCF-7 without Ab/Pb drug treatment is not filled or slightly filled by cells at 12, 24 and 36 h indicating the high efficacy of drugs in the sensitive cells. However, the gaps made at 0 h in Ab-R-MCF-7 with the Ab/Pb drug as compared to Ab/Pb-R-MCF7 without the drug are significantly filled and almost the same under both conditions. This result indicated enhanced wound healing, cell migration and proliferation tendency of the cells in the resistant model even under the drug treated condition as shown in Fig. 3. A. Similar results are reflected in the graphical representation as shown in Fig. 3B.3.5. Up-regulation of the drug resistance marker MDR1 and ABCG2 genes
MDR-1 and ABCG-2 (BCRP) are xenobiotic transporters that help cells eliminate toxins. These proteins have been linked to the phenotype of drug resistance in various cancers. Increased MDR-1 and ABCG-2 expressions indicate drug resistance.23 The MDR1 expression was found to be identical in control and drug-treated responsive cells. It was shown to be increased by 3.0-fold and 8.2-fold in Ab-R-MCF-7 and Pb-R-MCF-7 cells, respectively. The expression of the ABCG2 gene was comparable in control and drug-treated responsive cells, but it was 4.0 and 5.9 times higher in Ab-RMCF-7 and Pb-R-MCF-7 cells, respectively (Fig. 4A)3.6. Up-regulation of pro-survival genes and down-regulation of apoptotic genes
Increased expressions of survival (Bcl-2 and MCL-1) genes and suppressed expressions of pro-apoptotic (BAX, BAD, Caspase-3, and PARP1) genes may lead to therapy resistance. Bcl-2 blocks the intrinsic apoptosis signal by inhibiting Bax and Bak interactions on the mitochondrial outer membrane. Drug-resistant cells have elevated levels of Bcl-2 and MCL-1.24,25 The qPCR results are shown in Fig. 4B. The expressions of pro-survival genes BCL-2 and MCL-1 were found to be up-regulated in drug-resistant cells. BCL-2 expression was enhanced by 3.13 and 1.60-fold in Ab-RMCF-7 and Pb-R-MCF-7 cells, respectively, whereas, MCL-1 showed 4.90 and 5.80-fold higher expressions in Ab-R-MCF-7 and Pb-R-MCF-7 cells respectively as compared to the control. Pro-apoptotic gene BAX, BAD, Casp-3 and PARP1 expressions in Ab-R-MCF-7 were similar to those of the control cells. They exhibited 1.04, 0.80, 1.14, and 1.28-fold changes, respectively. However, in Ab-treated drug-sensitive cells, these gene expressions were found to be 2.25, 2.17, 2.14, and 5.17-fold higher, respectively (Fig. 4C). BAX, BAD, Casp-3 and PARP1 expressions in Pb-RMCF-7 were 1.15, 0.95, 1.0, and 1.20-fold changed, respectively; however, in Pb-treated, drug-sensitive cells, these were 2.25, 2.15, 2.51, and 3.86-fold higher, respectively, compared to the control (Fig. 4D).3.7. Up-regulation of estrogen receptor-1 and CDK4 and 6 genes
Estrogen activates ER, which leads to DNA replication and cell division, as encoded by the ESR1 gene. Increased ESR1 expression suggests that it can contribute to therapy resistance.26 ESR1 genes are over-expressed in cancer cells, especially in breast cancer, and both Ab and Pb are CDK4/6 inhibitors. In this analysis, the expressions of ESR-1, CDK-4, and CDK-6 in the control and responsive cells were found to be identical, whereas the level of cyclin D1 decreased 0.55 fold. However, ESR-1, CDK-4, CDK-6, and cyclin D1 genes increased 11.9, 3.6, 15.5, and 4.1-fold in Ab-R-MCF-7 cells, respectively (Fig. 4E). In Pb-responsive cells, ESR-1, CDK-4, and CDK-6 gene expressions were unchanged compared to the controls, whereas, cyclin-D1 was 0.43-fold down-regulated. However, in Pb-resistant cells, ESR-1, CDK-4, CDK-6, and cyclin-D1 gene expressions were increased by 20.3, 2.8, 6.4, and 6.0-fold, respectively (Fig. 4F). CDK4 and CDK6 are targeted by Ab and Pb, which stimulates cyclin-D1 and promotes cell cycle progression. Increased CDK4/6 and cyclin D1 expression in our cells promote successful cell division, which is a hallmark of drug resistance.3.8. Differentially expressed proteins (DEPs) in global-profiling
Global proteome profiling was used to assess altered protein expressions in Ab and Pb-resistant MCF-7 cells.18 The proteome profile data showed the expression had changed for both the resistance cells as compared to control MCF-7 cells which represent the ratio of protein expression. A ratio value above 1.5 is up-regulated and that below 0.5 is down-regulated. Here, we used three biological replicates in which two biological sets were pooled down into equal quantities to make set 1 and the other was set 2 and the sample run on mass spec as duplicate. Proteome analysis was performed using “Proteome Discoverer 2.2” tools applying Protein FDR confidence: combined = high((FDR 1%) and exp. q-value (adjusted p-value)): combined <0.000. Here we compared the expressions of proteins in the proteome profile of drug (Ab/Pb) resistant-MCF-7 with MCF-7 and based on the expression, a ratio value of protein above 1.5 indicates differentially up-regulated proteins and that below 0.5 indicates differentially down-regulated proteins. In Ab-R-MCF-7 cells, 237 proteins were identified as DEPs, with 162 proteins being down-regulated and 75 being up-regulated. In Pb-R-MCF-7 cells, 239 proteins were identified as DEPs, with 153 proteins being down-regulated and 86 being up-regulated (Fig. 5A and B). Venn-diagram analysis showed common proteins among different sets of samples (Ab-RMCF- 7 vs. MCF-7 control (Set 1 F2/F1 and Set-2 F5/F4) and Pb-R-MCF-7 vs. MCF-7 control (Set-1 F3/F1 and Set-2 F6/F4)) (Fig. 3C and D). DEPs in Ab-R-MCF-7 and Pb-R-MCF-7 cells were manually curated from the PubMed literature search engine, and their cancer-related functions were revealed. Down-regulation of NUDT5, PEPD, ABAT, ATP1B1, GGCT, SELENBP1, M6PR, STOM, and ACTN1 proteins in Ab-R-MCF-7 cells has been identified as a prognostic indicator or a new drug target for breast cancer (Table S2, ESI† and Table 1). The up-regulation of SBSN, HSD17B10, CD9, PDIA3, PSMB4, SLC2A1, and VTN proteins in Ab-R-MCF-7 cells has been identified as a poor prognostic indicator or is involved in acquired drug resistance and has been proposed as a novel drug target for BC treatment (Table S3, ESI† and Table 1). NUDT5, PEPD, and GGCT proteins, which have been identified as prognosis or drug-sensitive markers were down-regulated in Pb-R-MCF-7 cells (Table S4, ESI† and Table 1). CD47, HIST1H2BN, LMNA, VTN, PSMB5, HBB, PSMA7, FLNB, PRDX4, VDAC1, GOT2, HSPA5, SERPINH1, EIF4A2, FTH1, and VIM proteins have been found to be up-regulated in Pb-R-MCF-7 cells, which are associated with poor prognosis or drug resistance markers (Table S5, ESI† and Table 1).| DEPs | Fold change | Molecular function | Earlier reports | Ref. |
|---|---|---|---|---|
| Ab-R-MCF-7 vs. MCF-7 | ||||
| SBSN | 3.45 | Upregulated SBSN enhances Wnt/β-catenin signalling and promotes proliferation and tumorigenicity | 37 | |
| PDIA3 | 3.1 | Catalyzes the rearrangement of –S–S-bonds in proteins | High expression of PDIA3 drives the production of secretory proteins and creates a favourable tumor microenvironment for invasion and metastasis | 40 |
| CD9 | 2.2 | Integral membrane protein associated with integrins, regulates platelet activation and aggregation, and cell adhesion | Tumor microenvironment facilitates CD9-mediated crosstalk between bone marrow-derived mesenchymal stem cells and breast cancer cells (via CCL5, CCR5, and CXCR12) that contributes to chemoresistance | 39 |
| SLC2A1 | 2.2 | Facilitative glucose transporter responsible for glucose uptake | Aggressive growth of breast cancer activates hypoxia-inducing factor HIF1 resulting in GLUT1 expression | 41 |
| HSD17B10 | 1.88 | Involved in fatty acid, branched-chain amino acid and steroid metabolism pathways | High level of HSD17B10 is an indicator of poor responders to chemotherapy in osteosarcoma | 38 |
| VTN | 1.8 | Cell adhesion and spreading factor | Downstream of VEGF/VEGFR and PI3K/AKT signalling induces cell migration and metastasis in breast cancer | 36 |
| PSMB4 | 1.59 | Proteolytic degradation of mostly intra-cellular proteins | PSMB4 overexpression enhances the cell growth and viability of breast cancer cells leading to poor prognosis | 33 |
| NUDT5 | 0.67 | Acts as ADP-sugar pyrophosphatase in the absence of diphosphate or catalyses the synthesis of ATP in the presence of diphosphate | Associated with low overall survival in clear cell renal cell carcinoma and poor prognosis | 27 |
| GGCT | 0.63 | Glutathione homeostasis, releases cyto-c from mitochondria, and induces apoptosis | Component of the GSH-pathway, its lower expression leads to chemotherapy resistance in breast cancer | 29 |
| SELENBP1 | 0.51 | Involved in intra-Golgi protein transport | ER +ve breast cancer patients with low SELENBP1 have poorer survival rates and resistance is induced toward the anti-proliferative effects of selenium | 32 |
| ATP1B1 | 0.48 | ATP hydrolysis coupled with Na/K ion exchange across the plasma membrane | Associated with metastasis is an important cell energy conversion system | 31 |
| ABAT | 0.15 | Catalyzes the conversion of gamma-amino butyrate and L-beta-aminoisobutyrate to succinate semialdehyde and methyl-malonate semialdehyde, respectively | Inverse-correlation between ABAT expression and therapy resistance in inflammatory breast cancer | 30 |
| PEPD | 0.14 | Collagen metabolism | Exogenous PEPD binds and inhibits Her-2 and EGFR signalling resulting in growth inhibition in cancer cells | 28 |
| Pb-R-MCF-7 vs. MCF-7 control | ||||
| VIM | 38.0 | Vimentins are class-III intermediate filaments found in various non-epithelial cells, especially mesenchymal cells | Activation of Erk-signalling promotes VIM over-expression whereas its expression is elevated in methotrexate and tamoxifen-resistant breast cancer | 54 |
| SERPINH1 | 36.5 | Chaperone protein involved in collagen folding | Showed a positive correlation between its expression and the aggressive phenotype of gastric cancer | 51 |
| HSPA5 | 20.3 | Endoplasmic reticulum chaperone that plays a key role in protein folding | HSPA5 was upregulated in lapatinib resistant breast cancer cells | 50 |
| FTH1 | 14.5 | Iron homeostasis | Up-regulated in doxorubicin-resistant breast cancer and promotes EMT | 53 |
| GOT2 | 11.1 | Phenylalanine metabolism and glucose metabolism | ZBRK1 and BRCA1 complex binds to the GOT2 promoter and regulates its expression. Impaired complex binding results in uncontrolled expression resulting in aspartate and α-ketoglutarate production leading to cell proliferation | 49 |
| VDAC1 | 9.6 | Present on the outer mitochondrial membrane regulating metabolite and ion exchange | ElVDAC1upregulation promotes cell proliferation, indicator of poor prognosis, involved in therapy resistance towards BRD inhibitors in breast cancer | 48 |
| PRDX4 | 9.2 | Catalyses hydrogen peroxide and regulates hydrogen peroxide signalling | Elevated expression may lead to therapy resistance and tumor recurrence | 47 |
| HBB | 8.4 | Oxygen transport from the lung to the various peripheral tissues | Promote aggressiveness in breast cancer cells and poor prognosis | 45 |
| FLNB | 6.6 | Connects cell membrane constituents to the actin cytoskeleton | Induces EMT by releasing the transcription factor FOXC1 and the expression of EMT gene signature in tumorigenesis | 46 |
| LMNA | 5.2 | Nuclear lamina component, maintaining nuclear integrity | Lower expression is an indicator of poor prognosis and shorter outcome | 51 |
| EIF4A2 | 5.5 | ATP-dependent RNA helicase which is a subunit of the eIF4F complex involved in cap recognition and is required for mRNA binding to the ribosome | Targeting EIF4A2 by miR-5195-3p reverse chemoresistance in TNBC cells | 52 |
| PSMA7 | 4.9 | Proteolytic degradation of intracellular proteins | Elevated expression of PSMA7 in gastric cancer is associated with tumor invasion, metastasis, poor survival and having prognostic as well as diagnostic value | 35 |
| PSMB5 | 4.1 | Proteolytic degradation of intracellular proteins | High expression of PSMB5 indicates worse survival and can be severed as a novel drug target | 33 and 34 |
| VTN | 3.9 | Extracellular matrix (ECM) protein may be associated with cell adhesion and migration | VTN is downstream of VEGF/VEGFR and PI3K/AKT signalling induces cell migration and metastasis in breast cancer | 36 |
| HIST1H2BN | 2.7 | Core component of the nucleosome | Uncontrolled HIST1H2BN expression contributes to cancer initiation, progression, and indicator of poor prognosis in ovarian cancer | 44 |
| CD47 | 2.2 | Membrane transport and signal transduction | HIF-1 activates CD47 transcription under hypoxic conditions, CD47 maintains cancer stem cells, induces EMT and serves as an indicator of poor prognosis in breast cancer | 42 and 43 |
| GGCT | 0.61 | Glutathione homeostasis, induces the release of cytochrome c from mitochondria to induce apoptosis | Component of the GSH-pathway, its lower expression led to chemotherapy resistance in breast cancer | 29 |
| PEPD | 0.28 | Collagen metabolism | Exogenous PEPD binds and inhibits Her-2 and EGFR signalling resulting in growth inhibition in cancer cells | 28 |
| NUDT5 | 0.22 | Acts as ADP-sugar pyrophosphatase in the absence of diphosphate or catalyses the synthesis of ATP in the presence of diphosphate | Associated with low overall survival in clear cell renal cell carcinoma and poor prognosis | 27 |
The roles of proteins in therapy resistance in Ab-R-MCF-7 and Pb-R-MCF-7 cells are described in Tables S2–S5 (ESI†). DEPs are manually curated from the PubMed literature search engine to determine their molecular functions, roles in cancer progression, therapy resistance, and prognostic importance. NUDT5 is linked to a poor prognosis and low overall survival in clear cell renal cell carcinoma.27 PEPD binds to Her-2 and inhibits EGFR signaling, resulting in cancer cell growth inhibition.28 A lower expression of GGCT, a crucial component of the GSH pathway, has been linked to chemo-resistance.29 Inflammatory BC has an inverse association between ABAT expression and therapy resistance.30 A high level of ATP1B1 is linked to metastasis and it is an essential energy transfer system for cells.31 Low levels of SELENBP1 in ER + ve BC result in poor survival and selenium tolerance32 (Tables S2 and S4, ESI†).
PSMB is a group β-subunit of the 20S proteasome (PSMB4 and PSMB5), and the α-subunit PSMA7 is involved in the proteolytic degradation of intracellular proteins. The over-expression of PSMB4 promotes cell cycle progression from the G1 to the S phase and cell viability through NF-B signaling.33 A high PSMB5 level suggests a poor prognosis.34 PSMA7 levels in gastric cancer have been related to invasion, metastasis, and poor prognosis.35 VTN is a part of the extracellular matrix that promotes integrin signaling. VTN, which is downstream of VEGF/VEGFR and PI3K/AKT signaling, promotes cell migration and metastasis in breast cancer.36 SBSN is an onco-protein that promotes tumorigenicity by increasing Wnt/β-catenin signaling.37 The mitochondrial enzyme HSB17B10 is responsible for the oxidation of steroids, alcohols, and fatty acids. In osteosarcoma, a high level of HSD17B10 indicates a poor response to chemotherapy.38 Chemo-resistance is caused by the tumour microenvironment facilitating CD9-mediated crosstalk between mesenchymal stem cells and BC cells via CCL5, CCR5, and CXCR12.39 The expression of PDIA3 in the tumor microenvironment is high, which favours invasion and metastasis.40 GLUT1 proteins encoded by SLC2A1 aid glucose transport. Hypoxia-inducing factor-1(HIF-1) is activated as breast cancer grows aggressively. Due to hypoxic environments, HIF1 triggers GLUT1 expression41 under hypoxic conditions, and HIF-1 also activates CD47 transcription. In BC, CD47 retains stemness, induces EMT and leads to poor prognosis.42,43 HIST1H2BN is a part of the H2B protein family whose unregulated expression causes cancer and is a predictor of poor prognosis in ovarian cancer.44 Hemoglobin beta (HBB) is an oxygen transporter that promotes BC cell aggression and poor prognosis.45 By releasing the FOXC1 transcription factor, FLNB exon 30 skipping (gene splicing) induces EMT, and expression of the EMT gene signature induces tumorigenicity.46 Cancer stem cell survival and proliferation are influenced by redox control and oxidative stress. Peroxiredoxin 4 (PRDX4) catalyses hydrogen peroxide and regulates hydrogen peroxide signaling leading to tumor recurrence and therapy resistance.47 BRD4 is a downstream target of voltage-dependent anion channels (VDAC1), which are found on the outer mitochondrial membrane. VDAC1 over-expression causes breast cancer proliferation, is associated with a poor prognosis, and is linked to therapy resistance to BRD inhibitors in BC.48 The GOT2 promoter is regulated by the ZBRK1 and BRCA1 complex, which binds to it and regulates its expression. Impaired complex binding contributes to uncontrollable expression, which promotes cell proliferation.49 In lapatinib-resistant BC, a high level of HSPA5 was found.50 SERPINH1 is a chaperone protein, and its high expression has been linked to a more aggressive phenotype of gastric cancer, implying poor prognosis.37,51 miR-5195-3p targets EIF4A2, which can reverse chemoresistance in TNBC cells.52 FTH1 is a subunit of the ferritin complex that promotes EMT in doxorubicin-resistant breast cancer.53 Vimentin (VIM) is a member of the intermediate filament protein family that contributes to cell invasion, migration, and signaling. VIM expression is promoted by Erk signaling activation. Its increased expression has been identified in methotrexate and tamoxifen-resistant BC.54
3.9. GO-terms and KEGG pathway analysis of DEPs
GO-terms of down-regulated DEPs in Ab and Pb-resistant cells were as follows: biological processes were identified as apoptosis, cell adhesion, small molecule catabolic processes, cofactor metabolic processes, nucleotide metabolic processes, carbohydrate metabolic processes, etc. The cell surface, mitochondria, endoplasmic reticulum, extracellular matrix, and chaperone complex, among other cellular components, were established. Signaling pathways such as the estrogen metabolic pathway and pyruvate metabolism, gluconeogenesis, TCA-cycle, and MAPK-pathway were established (Fig. 4A). Up-regulated DEPs in Ab and Pb-resistant cells are linked to biological processes such as cell activation, generation of precursor metabolites, and energy, lung development, cellular response to chemical stimuli, response to hypoxia, cellular response to cytokine stimulus and small molecule metabolic processes. Cellular components are localized in the mitochondrial membrane, cytoplasmic vesicle, extracellular matrix, secretory vesicle and outer membrane. Signaling pathways include metabolic pathways, insulin signaling, choline metabolism in cancer, CGMP-PKG signalling, gluconeogenesis, ECM receptor interaction, MAPK signaling, NOTCH signaling, Wnt/β-catenin, PI3K/AKT/mTOR, JAK-STAT, and TGF-β signaling; these terms are more active in tumor progression, cancer recurrence and therapy resistance (Fig. 6 and 7).3.10. Protein–miRNA network analysis depicting miRNA targeting genes for DEPs in resistant cells
The miR-Net tool was used to analyse the miRNA–protein inverse correlation between miRNAs and their targeted DEPs. In Ab-R-MCF-7 cells, hsa-mir-1-3p, hsa-mir-155-5p, and hsa-mir-24-p with down-regulated proteins and hsa-mir-155-5p, hsa-mir-23b-3p, hsa-mir-124-3p, and hsa-mir-30a-5p with up-regulated DEPs showed an inverse relationship. Similarly,Hsa-mir-155-5p, hsa-mir-1-3p, hsa-mir-20a-5p, and hsa-mir-182-5p with down-regulated proteins and hsa-mir-130a-3p, hsa-mir-101-3p, hsa-mir-19a-3p, hsa-mir-34a-5p, and hsa-let-7a-5p with up-regulated proteins showed an inverse correlation in Pb-R-MCF-7 cells (Fig. 10 and Fig. S3, ESI†).4. Conclusions
To summarise, the increased drug tolerance potential, cell survival, wound healing, cell migration and colony formation tendency confirmed the Ab/Pb-resistant cell models. Increased expressions of MDR1 and ABCG2 proteins, which are already reported as drug resistance markers, also approved our Ab/Pb-resistant models. Increased expressions of MDR1 and ABCG2 proteins, which are already reported as drug resistance markers, also approved our Ab/Pb-resistant models. Both the drugs are known to target CDK4/6 genes but can act heterogeneously due to their molecular structure. The same was noticed in our proteome profiling where though both drugs target CDK4/6 they exhibited different sets of proteins involved in resistance development while sharing some common protein signatures such as NUDT5, PEPD, GGCT, PSMBs and VTN. The proteome profile of Ab-resistant cells showed differential expressions whereas NUDT5, PEPD, ABAT, ATP1B1, GGCT, SELENBP1, M6PR, STOM and ACTN1 are down-regulated and SBSN, HSD17B10, CD9, PDIA3, PSMB4, SLC2A1, and VTN proteins are up-regulated. These proteins are reported to target apoptosis, cell adhesion, estrogen signaling, metabolic pathways, pyruvate metabolism, gluconeogenesis, TCA-cycle, MAPK pathways, etc. The proteome profile of Pb-resistant cells exhibited differential expressions whereas NUDT5, PEPD, and GGCT are down-regulated and CD47, HIST1H2BN, LMNA, VTN, PSMB5, HBB, PSMA7, FLNB, PRDX4, VDAC1, GOT2, HSPA5, SERPINH1, EIF4A2, FTH, and VIM proteins are up-regulated. These proteins are involved in metabolic pathways, insulin signaling, choline metabolism in cancer, CGMP-PKG-signalling, gluconeogenesis, ECM–receptor interaction and MAPK signaling, NOTCH signaling, Wnt/β-catenin, PI3K/AKT/mTOR, JAK-STAT, TGF-β pathways, etc. Most of the differentially expressed proteins are reported to be involved in different pathways that help the cells in resistance development, metastasis and disease progression. These data hint the involvement of different pathways in resistance development but the exact role of each of all these differentially expressed proteins and miRNAs in resistance development needs further scientific evaluation.Abbreviations
| DEPs | Differentially expressed proteins |
| CDK | Cyclin-dependent kinase |
| PI | Propidium iodide |
| Ab-R-MCF-7 | Abemacilcib resistant MCF-7 |
| Pb-R-MCF-7 | Palbociclib resistant MCF-7 |
| Ab | Abemaciclib |
| Pb | Palbociclib |
| BC | Breast cancer |
Data availability
All data generated or analysed during this study are included in this article.Authors’ contributions
Conceptualization: Binayak Kumar and Suresh T. Hedau, data curation: Ram Krishna Sahu and Ragini Singh, funding acquisition: Binayak Kumar, project administration and supervision: Suresh T. Hedau, visualization: Ashutosh Singh and Peeyush Prasad, writing original draft: Binayak Kumar, and writing, reviewing and editing: Binayak Kumar, SrikrishnaJayadev M and Suresh T. Hedau.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work has been supported by the Indian Council of Medical Research, India under an ICMR-Postdoctoral fellowship Grant No. 3/1/3/PDF (17)/2017-HRD awarded to Binayak Kumar. The authors gratefully acknowledge the Indian Council of Medical Research for funding support. The authors acknowledge the National Institute of Cancer Prevention and Research for facilitating the work. The authors acknowledge the School of Life Sciences, Shiv Nadar University to conduct part of the work there. The authors acknowledge the MASSFIITB facility at IIT Bombay, Mumbai for conducting a non-labelled global proteome profiling experiment.References
- F. Bray, J. Ferlay, I. Soerjomataram, R. L. Siegel, L. A. Torre and A. Jemal, Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries, Ca-Cancer J. Clin., 2018, 68(6), 394–424 CrossRef PubMed.
- Cancer Incidence in Five Continents, ed. M. P. Curado, B. Edwards,H. R. Shin, H. Storm, J. Ferlay, M. Heanue, P. Boyle, IARC Scientific Publications No. 160, Lyon, IARC, 2007, vol. IX Search PubMed.
- D. Forman, F. Bray, D. H. Brewster, C. GombeMbalawa, B. Kohler, M. Piñeros, E. Steliarova- Foucher, R. Swaminathan and J. Ferlayeds, Cancer Incidence in Five Continents, Lyon, IARC, 2013, vol. X, electronic version Search PubMed.
- K. S. Saini, C. Taylor, A. J. Ramirez, C. Palmieri, U. Gunnarsson, H. J. Schmoll, S. M. Dolci, C. Ghenne, O. Metzger-Filho, M. Skrzypski, M. Paesmans, L. Ameye, M. J. Piccart-Gebhart and E. de Azambuja, Role of the multidisciplinary team in breast cancer management: results from a large international survey involving 39 countries, Ann. Oncol., 2012, 23(4), 853–859 CrossRef CAS PubMed.
- Y. Li, Y. Wang, H. Wang, L. Zhang, Y. Ding, S. Chen, Q. Yang and C. Chen, Effects of lncRNA RP11-770J1.3 and TMEM25 expression on paclitaxel resistance in human breast cancer cells, Zhejiang Da Xue Xue Bao Yi Xue Ban, 2017, 46(4), 364–370 Search PubMed.
- H. Zhou, Q. Lv and Z. Guo, Transcriptomic signature predicts the distant relapse in patients with ER+ breast cancer treated with tamoxifen for five years, Mol. Med. Rep., 2018, 17(2), 3152–3157 CAS.
- U. Asghar, A. K. Witkiewicz, N. C. Turner and E. S. Knudsen, The history and future of targeting cyclin-dependent kinases in cancer therapy, Nat. Rev. Drug Discovery, 2015, 14(2), 130–146 CrossRef CAS PubMed.
- J. A. Beaver, L. Amiri-Kordestani, R. Charlab, W. Chen, T. Palmby, A. Tilley, J. F. Zirkelbach, J. Yu, Q. Liu, L. Zhao, J. Crich, X. H. Chen, M. Hughes, E. Bloomquist, S. Tang, R. Sridhara, P. G. Kluetz, G. Kim, A. Ibrahim, R. Pazdur and P. Cortazar, FDA Approval: Palbociclib for the Treatment of Postmenopausal Patients with Estrogen Receptor-Positive, HER2-Negative Metastatic Breast Cancer, Clin. Cancer Res., 2015, 21(21), 4760–4766 CrossRef CAS PubMed.
- A. Patnaik, L. S. Rosen, S. M. Tolaney, A. W. Tolcher, J. W. Goldman, L. Gandhi, K. P. Papadopoulos, M. Beeram, D. W. Rasco, J. F. Hilton, A. Nasir, R. P. Beckmann, A. E. Schade, A. D. Fulford, T. S. Nguyen, R. Martinez, P. Kulanthaivel, L. Q. Li, M. Frenzel, D. M. Cronier, E. M. Chan, K. T. Flaherty, P. Y. Wheng and G. I. Shapiro, Efficacy and Safety of Abemaciclib, an Inhibitor of CDK4 and CDK6, for Patients with Breast Cancer, Non-Small Cell Lung Cancer, and Other Solid Tumors, Cancer Discovery, 2016, 6(7), 740–753 CrossRef CAS PubMed.
- D. L. Burkhart and J. Sage. Cellular mechanisms of tumor suppression by the retinoblastoma gene. Nat. Rev. Cancer 2008; 8(9):671–682 Search PubMed.
- E. A. Musgrove, C. E. Caldon, J. Barraclough, A. Stone and R. L. Sutherland, Cyclin D as a therapeutic target in cancer, Nat. Rev. Cancer, 2011, 11(8), 558–572 CrossRef CAS PubMed.
- D. C. Koboldt, Q. Zhang and D. E. Larson, et al., VarScan 2: somatic mutation and copy number alteration discovery in cancer by exome sequencing, Genome Res., 2012, 22(3), 568–576 CrossRef CAS PubMed.
- G. I. Shapiro, Cyclin-dependent kinase pathways as targets for cancer treatment, J. Clin. Oncol., 2006, 24(11), 1770–1783 CrossRef CAS PubMed.
- C. Guarducci, M. Bonechi and G. Boccalini, et al., Mechanisms of Resistance to CDK4/6 Inhibitors in Breast Cancer and Potential Biomarkers of Response, Breast Care, 2017, 12(5), 304–308 CrossRef PubMed.
- K. Pandey, H. J. An and S. K. Kim, et al., Molecular mechanisms of resistance to CDK4/6 inhibitors in breast cancer: A review, Int. J. Cancer, 2019, 145(5), 1179–1188 CrossRef CAS PubMed.
- N. Portman, S. Alexandrou, E. Carson, S. Wang, E. Lim and C. E. Caldon, Overcoming CDK4/6 inhibitor resistance in ER-positive breast cancer, Endocr.-Relat. Cancer, 2019, 26(1), R15–R30 CAS.
- V. M. Jansen, N. E. Bhola and J. A. Bauer, et al., Kinome-Wide RNA Interference Screen Reveals a Role for PDK1 in Acquired Resistance to CDK4/6 Inhibition in ER-Positive Breast Cancer, Cancer Res., 2017, 77(9), 2488–2499 CrossRef CAS PubMed.
- F. A. Solari, N. J. Mattheij, J. M. Burkhart, F. Swieringa, P. W. Collins, J. M. Cosemans, A. Sickmann, J. W. Heemskerk and R. P. Zahedi, Combined Quantification of the Global Proteome, Phosphoproteome, and Proteolytic Cleavage to Characterize Altered Platelet Functions in the Human Scott Syndrome, Mol. Cell. Proteomics, 2016, 15(10), 3154–3169 CrossRef CAS PubMed.
- J. M. Madden, K. L. Mueller, A. Bollig-Fischer, P. Stemmer, R. R. Mattingly and J. L. Boerner, Abrogating phosphorylation of eIF4B is required for EGFR and mTOR inhibitor synergy in triple-negative breast cancer, Breast Cancer Res. Treat., 2014, 147(2), 283–293 CrossRef PubMed.
- L. Jiang, Y. Zhang, L. Guo, C. Liu, P. Wang and W. Ren., Exosomal microRNA-107 reverses chemotherapeutic drug resistance of gastric cancer cells through HMGA2/mTOR/P-gp pathway, BMC Cancer, 2021, 21(1), 1290 CrossRef CAS PubMed.
- H. Lecoeur, Nuclear apoptosis detection by flow cytometry: influence of endogenous endonucleases”, Exp. Cell Res., 2002, 277(1), 1–14 CrossRef CAS PubMed.
- R. K. Sahu, B. Kumar, R. Singh, S. Kumari, S. Tandon, B. C. Das and S. T. Hedau, Schleicheraoleosa Seed Extract Reduced the Proliferation of Breast Cancer by Regulating the BRCA1 and p16 Genes, Asian Pac. J. Cancer Prev., 2022, 23(1), 151–160 CrossRef CAS PubMed.
- A. A. Kovalev, D. A. Tsvetaeva and T. V. Grudinskaja, Role of ABC-cassette transporters (MDR1, MRP1, BCRP) in the development of primary and acquired multiple drug resistance in patients with early and metastatic breast cancer, Exp. Oncol., 2013, 35(4), 287–290 CAS.
- M. Zhang, R. Guo, Y. Zhai and D. Yang, LIGHT sensitizes IFNgamma-mediated apoptosis of MDA-MB-231 breast cancer cells leading to down-regulation of anti-apoptosis Bcl-2 family members, Cancer Lett., 2003, 195(2), 201–210 CrossRef CAS PubMed.
- F. Lin, J. Luo, W. Gao, J. Wu, Z. Shao, Z. Wang, J. Meng, Z. Ou and G. Yang, COX-2 promotes breast cancer cell radioresistance via p38/MAPK-mediated cellular anti-apoptosis and invasiveness, Tumour Biol., 2013, 34(5), 2817–2826 CrossRef CAS PubMed.
- P. La Rosa, V. Pesiri, M. Marino and F. Acconcia, 17β-Estradiol-induced cell proliferation requires estrogen receptor (ER) α monoubiquitination, Cell Signaling, 2011, 23(7), 1128–1135 CrossRef CAS PubMed.
- Y. Wang, F. Wan, K. Chang, X. Lu, B. Dai and D. Ye, NUDT expression is predictive of prognosis in patients with clear cell renal cell carcinoma, Oncol. Lett., 2017, 14(5), 6121–6128 Search PubMed.
- L. Yang, Y. Li, A. Bhattacharya and Y. Zhang, A recombinant human protein targeting HER2 overcomes drug resistance in HER2-positive breast cancer, Sci. Transl. Med., 2019, 11(476), eaav1620 CrossRef CAS PubMed.
- Z. Wang, S. Liang and X. Lian, et al., Identification of proteins responsible for Adriamycin resistance in breast cancer cells using proteomics analysis, Sci. Rep., 2015, 5, 9301 CrossRef CAS PubMed.
- M. P. Jansen, L. Sas and A. M. Sieuwerts, et al., Decreased expression of ABAT and STC2 hallmarks ER-positive inflammatory breast cancer and endocrine therapy resistance in advanced disease, Mol. Oncol., 2015, 9(6), 1218–1233 CrossRef CAS PubMed.
- Y. Y. Qi, K. Liu, J. Zhang, K. Li, J. J. Ren and P. Lin, Aizheng, 2009, 28(8), 861–867 CAS.
- Y. Wang, W. Fang and Y. Huang, et al., Reduction of selenium-binding protein 1 sensitizes cancer cells to selenite via elevating extracellular glutathione: a novel mechanism of cancer-specific cytotoxicity of selenite, Free Radical Biol. Med., 2015, 79, 186–196 CrossRef CAS PubMed.
- H. Wang, Z. He and L. Xia, et al., PSMB4 overexpression enhances the cell growth and viability of breast cancer cells leading to a poor prognosis, Oncol. Rep., 2018, 40(4), 2343–2352 CAS.
- W. Wei, Y. Zou and Q. Jiang, et al., PSMB5 is associated with proliferation and drug resistance in triple-negative breast cancer, Int. J. Biol. Markers, 2018, 33(1), 102–108 CrossRef CAS PubMed.
- S. Xia, Q. Tang, X. Wang, L. Zhang, L. Jia, D. Wu, P. Xu, X. Zhang, G. Tang, T. Yang, Z. Feng and L. Lu, Overexpression of PSMA7 predicts poor prognosis in patients with gastric cancer, Oncol. Lett., 2019, 18(5), 5341–5349 CAS.
- A. Bera, M. Subramanian, J. Karaian, M. Eklund, S. Radhakrishnan, N. Gana, S. Rothwell, H. Pollard, H. Hu, C. D. Shriver and M. Srivastava, Functional role of vitronectin in breast cancer, PLoS One, 2020, 15(11), e0242141 CrossRef CAS PubMed.
- S. Hubackova, M. Pribyl and L. Kyjacova, et al., Interferon-regulated suprabasin is essential for stress-induced stem-like cell conversion and therapy resistance of human malignancies, Mol. Oncol., 2019, 13(7), 1467–1489 CrossRef CAS PubMed.
- S. Salas, P. Jézéquel and L. Campion, et al., Molecular characterization of the response to chemotherapy in conventional osteosarcomas: predictive value of HSD17B10 and IFITM2, Int. J. Cancer, 2009, 125(4), 851–860 CrossRef CAS PubMed.
- M. Ullah, A. Akbar, N. N. Ng, W. Concepcion and A. S. Thakor, Mesenchymal stem cells confer chemoresistance in breast cancer via a CD9-dependent mechanism, Oncotarget, 2019, 10(37), 3435–3450 CrossRef PubMed.
- F. S. Ramos, L. T. Serino, C. M. Carvalho, R. S. Lima, C. A. Urban, I. J. Cavalli and E. M. Ribeiro, PDIA3 and PDIA6 gene expression as an aggressiveness marker in primary ductal breast cancer, Genet. Mol. Res., 2015, 14(2), 6960–6967 CrossRef CAS PubMed.
- I. Hamann, D. Krys, D. Glubrecht, V. Bouvet, A. Marshall, L. Vos, J. R. Mackey, M. Wuest and F. Wuest, Expression and function of hexose transporters GLUT1, GLUT2, and GLUT5 in breast cancer-effects of hypoxia, FASEB J., 2018, 32(9), 5104–5118 CrossRef CAS PubMed.
- H. Zhang, H. Lu, L. Xiang, J. W. Bullen, C. Zhang, D. Samanta, D. M. Gilkes, J. He and G. L. Semenza, HIF-1 regulates CD47 expression in breast cancer cells to promote evasion of phagocytosis and maintenance of cancer stem cells, Proc. Natl. Acad. Sci. U. S. A., 2015, 112(45), E6215–E6223 CAS.
- J. Yuan, X. Shi and C. Chen, et al., High expression of CD47 in triple negative breast cancer is associated with epithelial-mesenchymal transition and poor prognosis, Oncol. Lett., 2019, 18(3), 3249–3255 CAS.
- Y. P. Liao, L. Y. Chen and R. L. Huang, et al., Hypomethylation signature of tumor-initiating cells predicts poor prognosis of ovarian cancer patients, Hum. Mol. Genet., 2014, 23(7), 1894–1906 CrossRef CAS PubMed.
- M. Ponzetti, M. Capulli and A. Angelucci, et al., Non-conventional role of haemoglobin beta in breast malignancy, Br. J. Cancer, 2017, 117(7), 994–1006 CrossRef CAS PubMed.
- J. Li, P. S. Choi, C. L. Chaffer, K. Labella, J. H. Hwang, A. O. Giacomelli, J. W. Kim, N. Ilic, J. G. Doench, S. H. Ly, C. Dai, K. Hagel, A. L. Hong, O. Gjoerup, S. Goel, J. Y. Ge, D. E. Root, J. J. Zhao, A. N. Brooks, R. A. Weinberg and W. C. Hahn, An alternative splicing switch in FLNB promotes the mesenchymal cell state in human breast cancer, eLife, 2018, 7, e37184 CrossRef PubMed.
- W. Jia, P. Chen and Y. Cheng, PRDX4, and Its Roles in Various Cancers, Technol. Cancer Res. Treat., 2019, 18, 1533033819864313 CAS.
- G. Yang, D. Zhou and J. Li, et al., VDAC1 is regulated by BRD4 and contributes to JQ1 resistance in breast cancer, Oncol. Lett., 2019, 18(3), 2340–2347 CAS.
- R. Hong, W. Zhang, X. Xia, K. Zhang, Y. Wang, M. Wu, J. Fan, J. Li, W. Xia, F. Xu, J. Chen, S. Wang and Q. Zhan, Preventing BRCA1/ZBRK1 repressor complex binding to the GOT2 promoter results in accelerated aspartate biosynthesis and promotion of cell proliferation, Mol. Oncol., 2019, 13(4), 959–977 CrossRef CAS PubMed.
- L. Zhang, Y. Huang, W. Zhuo, Y. Zhu, B. Zhu and Z. Chen, Identification, and characterization of biomarkers and their functions for Lapatinib-resistant breast cancer, Med. Oncol., 2017, 34(5), 89 CrossRef PubMed.
- K. Kawagoe, M. Wada, T. Idichi, R. Okada, Y. Yamada, S. Moriya, K. Okubo, D. Matsushita, T. Arigami, H. Kurahara, K. Maemura, S. Natsugoe and N. Seki, Regulation of aberrantly expressed SERPINH1 by antitumor miR-148a-5p inhibits cancer cell aggressiveness in gastric cancer, J. Hum. Genet., 2020, 65(8), 647–656 CrossRef PubMed.
- M. Liu, C. Gong, R. Xu, Y. Chen and X. Wang, MicroRNA-5195-3p enhances the chemosensitivity of triple-negative breast cancer to paclitaxel by downregulating mEIF4A2, Cell. Mol. Biol. Lett., 2019, 24, 47 CrossRef PubMed.
- E. Bigagli, L. Cinci, M. D'Ambrosio and C. Luceri, Transcriptomic Characterization, Chemosensitivity and Regulatory Effects of Exosomes in Spontaneous EMT/MET Transitions of Breast Cancer Cells, Cancer Genomics Proteomics, 2019, 16(3), 163–173 CrossRef CAS PubMed.
- S. Chen, J. Cai, W. Zhang, X. Zheng, S. Hu, J. Lu, J. Xing and Y. Dong, Proteomic identification of differentially expressed proteins associated with the multiple drug resistance in methotrexate-resistant human breast cancer cells, Int. J. Oncol., 2014, 45(1), 448–458 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2mo00285j |
| This journal is © The Royal Society of Chemistry 2023 |