Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The rational design of ARUK2007145, a dual inhibitor of the α and γ isoforms of the lipid kinase phosphatidylinositol 5-phosphate 4-kinase (PI5P4K)†
Gregory G.
Aldred‡
 ,
Timothy P. C.
Rooney‡
,
Timothy P. C.
Rooney‡
 ,
Henriette M. G.
Willems
,
Henriette M. G.
Willems
 ,
Helen K.
Boffey
,
Helen K.
Boffey
 ,
Christopher
Green§
,
Christopher
Green§
 ,
David
Winpenny
,
John
Skidmore
,
David
Winpenny
,
John
Skidmore
 ,
Jonathan H.
Clarke
,
Jonathan H.
Clarke
 and
Stephen P.
Andrews
and
Stephen P.
Andrews
 *
*
The ALBORADA Drug Discovery Institute, University of Cambridge, Island Research Building, Cambridge Biomedical Campus, Hills Road, Cambridge, CB2 0AH, UK. E-mail: spa26@cam.ac.uk
First published on 23rd August 2023
Abstract
The phosphatidylinositol 5-phosphate 4-kinases (PI5P4Ks) are therapeutic targets for diseases such as cancer, neurodegeneration and immunological disorders as they are key components in regulating cell signalling pathways. In an effort to make probe molecules available for further exploring these targets, we have previously reported PI5P4Kα-selective and PI5P4Kγ-selective ligands. Herein we report the rational design of PI5P4Kα/γ dual inhibitors, using knowledge gained during the development of selective inhibitors for these proteins. ARUK2007145 (39) is disclosed as a potent, cell-active probe molecule with ADMET properties amenable to conducting experiments in cells.
Introduction
Cellular phospholipids are structural components that delineate the outer boundary of the cell as well as all membrane-bound organelles and compartments. In addition to this structural role these lipids have also been shown to have various other functions, and the phosphoinositides, whilst a minor component of the total cellular phospholipid content, are prolific in their involvement in additional essential cellular processes such as membrane-trafficking, cell proliferation, cytoskeletal organisation, channel regulation and involvement in cell stress and death responses.1–4 The canonical route for phosphoinositide signalling results in the production of inositol trisphosphate (InsP3) from phosphatidylinositol (4,5) bisphosphate (PI(4,5)P2) as a result of lipase activity, to facilitate cellular calcium release.5 Subsequently each of the seven different phosphatidylinositol phosphate species (defined by the phosphorylation state (mono-, bis- or tris-) of positions 3–5 of the 6-carbon ring that constitutes the hydrophilic phosphatidylinositol headgroup1) have also been shown to have specific cellular functions, which can be location and cell-type specific.1,6 The interconversion of this phosphorylation state is controlled by a group of kinases and phosphatases that may act synergistically to remove or create localised pools of phosphoinositide that can directly (allosteric activation of proteins, regulation of membrane proteins such as ion channels) or indirectly (protein recruitment to membranes via specific phosphoinositide-binding or adapter proteins) result in downstream signalling events.1,2Phosphatidylinositol 5-phosphate (PI5P) is converted to PI(4,5)P2 by a family of phosphatidylinositol 5-phosphate 4-kinases (PI5P4Ks) consisting of three distinct isoforms; alpha, beta and gamma. Heterogeneity exists within the family; the alpha isoform, in vitro, is the most catalytically active,7 whereas the gamma isoform has very low intrinsic activity.8 The beta isoform is the only one to contain a recognised nuclear localisation sequence.9 Although all isoforms are generally ubiquitously expressed, tissue specific differences have been observed.8 Diverse cellular functions have also been associated with the different isoforms, for example PI5P4Kβ and PI5P4Kγ may have roles as GTP sensors,10 and both PI5P4Kα and PI5P4Kγ have been shown to associate to autophagic structures.11,12 Hence the different specific roles reported for each of the isoforms could be functions that are tissue or cell-type specific, or spatially or temporally differentiated in response to activation of different metabolic pathways. Structurally there are also differences between the isoforms9,13 but it is clear that each is able to heterodimerise,7,8 which leads to the intriguing possibility that isoforms are able to regulate the localisation, specific function or in vivo activity of each other.
The promiscuity of the phosphoinositides in cellular signalling pathways has implications for disease as enzyme dysfunction or misregulation may be causative in various developmental disorders, inflammation and infection.13–16 As oncology targets, PI5P4Ks have become increasingly associated with a number of diseases.17 Interestingly the involvement of PI5P4Kα alone has been documented in glioblastoma, acute myeloid leukemia (AML) and prostate cancer18–21 but other examples suggest that there may be a combinatorial role for the PI5P4Ks. Both PI5P4Kα and PI5P4Kβ are involved in p53-deficient breast cancer and soft tissue sarcomas22,23 and high expression of both PI5P4Kα and PI5P4Kγ associate with unfavourable clinical outcome in AML.24
Several recent reports detail inhibitors for PI5P4Ks, not only pan-specific25–29 but also isoform specific.30–34 Emerging tools are also being developed for dual-specific inhibitors for PI5P4Kα and PI5P4Kβ22,35 and methods have also been reported for removing protein completely in cells using PROTAC systems, such as JWZ-1-80 for PI5P4Kγ,36 which will be useful to interrogate non-catalytic roles. Here we present a new tool that can be used to investigate the impact of dual inhibition of both the PI5P4Kα and PI5P4Kγ isoforms.
We have previously reported PI5P4Kγ-selective inhibitors including ARUK2001607 (1) which was derived from virtual screening hit 2,30 as well as PI5P4Kα-selective inhibitors including tool compound ARUK2002821 (3) which was derived from virtual screening hit 4 (ref. 33) (Table 1). During the course of those studies, we also became interested in developing dual PI5P4Kα–PI5P4Kγ inhibitors as probe molecules. In particular, we had observed small amounts of PI5P4Kα inhibition in some analogues of 1 which we were able to further optimise through rational design. Herein we describe efforts to further increase the level of PI5P4Kα activity in the 1 chemotype towards the development PI5P4Kα/γ dual inhibitors with IC50 fold-selectivities within approximately 10-fold in either direction.
Results
Compound 5 was a lead in the development of our novel dual PI5P4K inhibitors. Compared to 1 and many of the other analogues previously explored in this chemical series,30,375 was differentiated by having a larger substituent at the 6-position of the thienylpyrimidine core (Table 1). This group gave a point of diversity for further SAR exploration and this compound appeared to be on the threshold of detection in the PI5P4Kα ADP-Glo assay, sometimes giving a partial inhibition curve leading to an inconsistent pIC50 with hints that the vector at the 6-position of the thienylpyrimidine core may be of value introducing further PI5P4Kα activity.As the phenyl group of 5 had an increased MW and X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P compared to 1 we also focussed on improving physicochemical properties during iterations of compound design. In particular, we were keen to introduce heteroatoms in this ring to modulate X
P compared to 1 we also focussed on improving physicochemical properties during iterations of compound design. In particular, we were keen to introduce heteroatoms in this ring to modulate X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P. The first synthesised compound of this type, 6, shows a marked decrease in X
P. The first synthesised compound of this type, 6, shows a marked decrease in X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P compared to 5 and was found to have increased potency for PI5P4Kα and PI5P4Kγ+ (Table 2).37 When the 8BQ4 crystal structure of PI5P4Kγ was later solved with 1 bound in the ATP binding site, docking of 6 suggested that its pyridyl N might interact with Lys216 of PI5P4Kγ (Fig. 1).
P compared to 5 and was found to have increased potency for PI5P4Kα and PI5P4Kγ+ (Table 2).37 When the 8BQ4 crystal structure of PI5P4Kγ was later solved with 1 bound in the ATP binding site, docking of 6 suggested that its pyridyl N might interact with Lys216 of PI5P4Kγ (Fig. 1).
| Inhibition of PI5P4K | Physicochemical properties | |||||
|---|---|---|---|---|---|---|
| R 1 | PI5P4Kα pIC50 | PI5P4Kγ+ pIC50 | Fold-selectivity (α IC50/γ IC50) | M W |
X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P P |
|
| a Inconsistent pIC50 measurements between replicates; value shown is upper limit. | ||||||
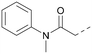
| 5 |
|
<5.3 | 7.4 | >130 | 381 | 4.5 |
| 6 |

|
5.9 | 7.7 | 63 | 382 | 3.1 |
| 7 |

|
5.9 | 7.8 | 79 | 371 | 3.7 |
| 8 |

|
5.2 | 7.4 | 160 | 385 | 3.1 |
| 9 |

|
5.3 | 7.5 | 130 | 382 | 3.1 |
| 10 |

|
6.2 | 7.9 | 50 | 396 | 3.4 |
| 11 |

|
5.6a | 7.5a | 79 | 412 | 3.1 |
| 12 |

|
<4.6 | 7.0 | >250 | 400 | 3.2 |
| 13 |
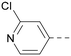
|
5.1a | 7.2 | 130 | 417 | 4.2 |
| 14 |

|
6.0 | 7.9 | 79 | 396 | 3.4 |
| 15 |

|
6.2 | 7.9 | 50 | 417 | 4.2 |
| 16 |

|
5.8a | 7.5 | 50 | 400 | 3.7 |
| 17 |

|
6.0 | 7.7 | 50 | 396 | 3.3 |
 | ||
| Fig. 1 Compound 6 (green) docked into the 8BQ4 structure of PI5P4Kγ–1 (cyan). | ||
Indeed, a range of heterocycles was tolerated at the 6-position of the thienylpyrimidine, with PI5P4Kγ+ activity being retained when compared to 5 and many showed some level of PI5P4Kα inhibition as well as a reduction in X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P (Table 2). Pyrazoles 7 and 8 retained good levels of PI5P4Kγ+ inhibition whilst showing some PI5P4Kα inhibition (Table 2). Pyridine 9 showed higher selectivity towards PI5P4Kγ+ than 6 (160 vs. 63-fold, respectively). The relative PI5P4Kα activity of 6 could be increased by methylation of the pyridyl 3-position (10) more effectively than by addition of OMe (11), or F (12) at the same position, or by chlorination at the pyridyl 2-position (13; Table 2). A range of small substituents was also trialled on template 6 and these were generally well tolerated (see 14–17). Compound 17 was a useful lead from this set with a good balance of activity at PI5P4Kα and γ+ as well as one of the lower X
P (Table 2). Pyrazoles 7 and 8 retained good levels of PI5P4Kγ+ inhibition whilst showing some PI5P4Kα inhibition (Table 2). Pyridine 9 showed higher selectivity towards PI5P4Kγ+ than 6 (160 vs. 63-fold, respectively). The relative PI5P4Kα activity of 6 could be increased by methylation of the pyridyl 3-position (10) more effectively than by addition of OMe (11), or F (12) at the same position, or by chlorination at the pyridyl 2-position (13; Table 2). A range of small substituents was also trialled on template 6 and these were generally well tolerated (see 14–17). Compound 17 was a useful lead from this set with a good balance of activity at PI5P4Kα and γ+ as well as one of the lower X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ps in this series. Compound 17 was therefore selected as a template for further exploration around the sulfone group.
Ps in this series. Compound 17 was therefore selected as a template for further exploration around the sulfone group.
Fig. 1 shows that the arylsulfone of dual inhibitor 6 is predicted to overlay well with the corresponding group of the PI5P4Kγ-selective molecule 1 in the PI5P4Kγ structure (8BQ4). A question remained as to whether the corresponding group of PI5P4Kα-selective 3 (i.e. 3,4-dimethylphenyl) would transpose onto this thienylpyrimidine template (Fig. 2). Using 17 as the optimal compound from Table 2, the dimethylphenyl group of 3 was transposed to provide compound 18, which was docked into the 3 structure (8C8C, Fig. 2).
 | ||
| Fig. 2 A) Compound 18 (orange) docked into the 8C8C structure of PI5P4Kα–3 (gold). The dimethylphenyl group of 3 overlays well with that of 18; B) 2D comparison of 3, 17 and 18. | ||
The rationally-designed compound 18 was found to have improved PI5P4Kα activity compared to 17 but diminished PI5P4Kγ+ activity (Table 3). An alternative potent PI5P4Kα inhibitor with a 2,4-dimethylphenyl group, “compound 25”,33 was also merged onto the thienylpyrimidine core to give the resulting compound 19. Both 18 and 19 are dual PI5P4Kα/γ+ inhibitors, as predicted. Indeed, the designed modifications increased the PI5P4Kα activity of this chemotype to the point that, relative to PI5P4Kγ-selective starting point 1, both 18 and 19 showed higher levels of PI5P4Kα than PI5P4Kγ+ inhibition (for reference, compound 1 showed no detectable inhibition of PI5P4Kα under the same assay conditions, Table 1).
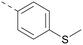
Lipophilic substituents at R2 have previously been found to be important for PI5P4Kα inhibition.33 As such, a variety of other lipophilic groups was tested at this position (Table 3). Compounds 20 and 21 were modest dual inhibitors and an interesting SAR trend was observed for the series of small aliphatic groups for 22–24, which increased in both PI5P4Kα and PI5P4Kγ+ activity with increasing size of the para substituent. In agreement with the hypothesis that the polar sulfone of 17 was not favourable for PI5P4Kα inhibition, apolar sulphide 25 was synthesised. This molecule showed an expected increase in PI5P4Kα inhibition with a modest reduction in PI5P4Kγ+ inhibition to afford a potent dual inhibitor.
Following on from this, combinations of groups which had been productive at R1 and R2 were explored (Table 4). Owing to the preference for lipophilic groups at R2, it was desirable to use polar heterocyclic groups at position R1, where possible, to help offset X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P. Nonetheless, these compounds were generally high in X
P. Nonetheless, these compounds were generally high in X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P, typically in the range 4–5 (compounds 26–30).
P, typically in the range 4–5 (compounds 26–30).
Substitution at the 5-position of the thienopyrimidine core was also investigated (Table 5). In general, this position was tolerant of a wide range of substituents from small lipophilic groups such as methyl (31) and chloro (32), to larger substituents such as a small ring (33) or a much larger group incorporating a polar amide moiety (34). A 5,6-disubstituted thiophene was also tolerated (35). However, these derivatives did not offer an advantage over 6-substituted thienylpyrimidines and were not pursued further.
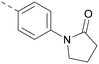
The thiophene was also ‘reversed’ from thieno[2,3-d]pyrimidines to afford thieno[3,2-d]pyrimidine derivatives such as 36, which is a direct analogue of 2 but with the sulfur atom moved within the thiophene ring (Table 6). Compound 36 shows a higher PI5P4Kγ+ pIC50 than 2 (7.3 vs. 6.5, respectively) and both compounds had undetectable levels of PI5P4Kα inhibition. Matched pairs of molecules in which the regiochemistry of the thienopyridine was alternated while the R groups were kept constant showed that thieno[3,2-d]pyrimidine was generally the more active regioisomer. For example, compound 38 forms a matched pair with 24 with the former being slightly more active at both isoforms (PI5P4Kα 7.0 vs. 6.6, respectively, and PI5P4Kγ+ 7.3 vs. 7.0). Similarly, 39 forms a matched pair with 28, with 39 being slightly more active at both PI5P4K isoforms. The thieno[3,2-d]pyrimidine template was also used to explore further alternative lipophilic groups at R2 (40, 41) and a group from potent PI5P4Kγ inhibitor ‘compound 7’ (42).30
A selection of the more active compounds identified to this point was profiled for PI5P4Kβ activity as well as PI5P4Kγ WT activity and cell penetration in a PI5P4K-WT InCELL Pulse cellular target engagement assay (Table 7). Both assays have previously been described for screening PI5P4Kγ inhibitors.38 All compounds tested in the PI5P4Kβ ADP-Glo assay returned IC50s > 10 μM (Table 7). Furthermore, there is a good correlation between the pIC50s obtained in the PI5P4Kγ+ ADP-Glo assay and the PI5P4K-WT InCELL Pulse assay, confirming that these compounds are bona fide inhibitors of the wild-type form of PI5P4Kγ. In general, there is a slight drop in pIC50 in the cellular assay, possibly owing to the requirement for cell permeability and/or the non-specific binding of the compounds to cellular components; however, these compounds such as 10 and 39 are potent PI5P4Kγ inhibitors in live cells.
| ADP-Glo pIC50 | InCELL Pulse pIC50 | |||
|---|---|---|---|---|
| PI5P4Kα | PI5P4Kβ | PI5P4Kγ+ | PI5P4Kγ-WT | |
| 5 | <5.3 | <4.6 | 7.4 | 6.6 |
| 8 | 5.2 | 4.9 | 7.4 | 6.7 |
| 10 | 6.2 | 4.9 | 7.9 | 7.7 |
| 15 | 6.2 | 4.9 | 7.9 | 7.1 |
| 17 | 6.0 | 4.7 | 7.7 | 7.3 |
| 37 | 6.5 | ND | 7.9 | 7.4 |
| 39 | 7.3 | ND | 8.1 | 7.3 |
Compounds with interesting profiles were selected for screening in further assays to assess ADMET properties with the aim of identifying potent dual inhibitors that would make suitable probes for applications in cellular experiments (Table 8). As such, cell permeability and efflux were measured in MDCK-MDR1 cells and aqueous solubility was measured at pH 7.4. To give an indication as to whether any of these probes may be suitable for in vivo testing, stability in mouse liver microsomes (MLMs) was also measured. In general, there was a wide range of values in each of the ADMET assays for this series of molecules. Efflux ratios varied from good (0.91) to very high (64). Cell permeabilities were generally good, ranging from low at 1.3 × 10−6 cm s−1 to high at 14.2 × 10−6 cm s−1. Solubilities ranged from low to moderate (1–30 μM). Half-lives in MLMs ranged from very poor to very good (0.94–383 minutes).
| Efflux ratioa | P app (A2B, 10−6 cm s−1) | Solubilityb (μM) | MLM t½c (min) | |
|---|---|---|---|---|
| a Permeability and efflux ratios determined in MDCK-MDR1 cells. b Aqueous solubility at pH 7.4. c Mouse liver microsome stability (half-life). d Recovery low A2B; possible solubility or binding issues; accuracy of result questionable. | ||||
| 5 | 1.3 | 6.23 | 1 | 54.5 |
| 8 | 26.4 | 2.88 | 30 | 73.3 |
| 10 | 25.2 | 3.13 | 30 | 9.23 |
| 14 | 34.1 | 2.45 | 30 | 8.2 |
| 15 | 15.7 | 4.73 | 3 | 383 |
| 17 | 64.3 | 1.33 | 10 | 35.5 |
| 26 | 1.1d | 3.7d | 3 | 0.94 |
| 32 | 3.7 | 14.2 | 10 | 51.4 |
| 37 | 31.8 | 1.33 | 10 | 70.8 |
| 38 | 0.91 | 9.95 | 3 | 1.94 |
| 39 | 0.98 | 12.9 | 10 | 0.44 |
| 42 | 27.4 | 3.15 | 10 | 5.39 |
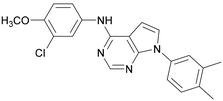
Compound 39 showed one of the most balanced profiles required for a cell-active probe: good permeability, no efflux and moderate solubility. This molecule shows good activity in the InCELL Pulse target engagement assay (PI5P4Kγ pIC50 = 7.3) and a good dual activity in the ADP-Glo assays showing similar activities for PI5P4Kγ+ and PI5P4Kα (fold-selectivity = 5). However, 39 has a very high turnover in MLMs and is unlikely to make a useful in vivo tool (MLM t½ = 0.44 min). Compound 39 (ARUK2007145) is therefore nominated as a PI5P4Kα/γ dual inhibitor probe molecule for cell experiments.
Other compounds of note are 32, which shows good ADMET properties in general, but is slightly less active than 39, particularly at PI5P4Kα, and the cluster of compounds 8, 15 and 37, which fall into the category of having a high MLM half-life and good PI5P4Kγ+ activity, but lower PI5P4Kα activity. All of these compounds have a 4-(methylsulfonyl)phenyl group at position R2. The polar methyl sulfonyl group is detrimental for PI5P4Kα activity but positively modulates microsomal stability, whereas more lipophilic groups in place of the methylsulfonyl group positively modulate PI5P4Kα activity but are metabolically liable. Nonetheless, compounds 8, 15 and 37, all have higher PI5P4Kα activities than previously reported compounds from this class30 and may make useful complementary probes.
Discussion
Rational design of dual PI5P4Kα–PI5P4Kγ inhibitors such as 39 began with the observation that 5 offers a new vector for SAR exploration. Using the novel vector provided by the aryl ring at position 6 of 5's core, further iterations of design led to PI5P4Kα-active compounds with improved physicochemical properties over 5, particularly X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P (Table 2). For example, 14 is a more polar derivative which docked well in the PI5P4Kγ structure, 8BQ4 (Fig. 3A). However, not all compounds docked well in this orientation, e.g.30 shows different docking pose in 8BQ4 (Fig. 3B). The thioether of 30 appears to prefer a conformation where the aniline has rotated 180 degrees from the 8BQ4 orientation (Fig. 3B), so that the NH is pointing outwards. Thus, it is possible that the nature of the substituent on the aniline may influence the conformational preference at the ψ dihedral angle, and therefore also impact on the predicted binding pose.
P (Table 2). For example, 14 is a more polar derivative which docked well in the PI5P4Kγ structure, 8BQ4 (Fig. 3A). However, not all compounds docked well in this orientation, e.g.30 shows different docking pose in 8BQ4 (Fig. 3B). The thioether of 30 appears to prefer a conformation where the aniline has rotated 180 degrees from the 8BQ4 orientation (Fig. 3B), so that the NH is pointing outwards. Thus, it is possible that the nature of the substituent on the aniline may influence the conformational preference at the ψ dihedral angle, and therefore also impact on the predicted binding pose.
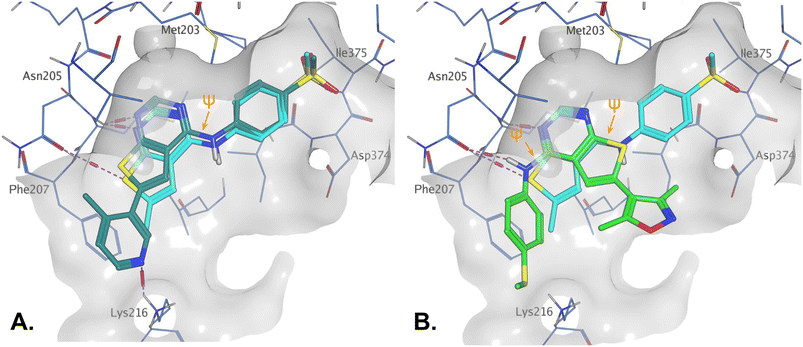 | ||
| Fig. 3 A) Compound 14 (teal); B) compound 30 (green) docked to PI5P4Kγ structure (8BQ4). Compound 1 shown for reference in both (cyan). The ψ dihedral angle is indicated in orange, this changes from 4° for 1 and 14 to 170° for 30. | ||
Later in the program, liganded PI5P4Kα crystal structures became available which allowed further structural assessment. The 8C8C PI5P4Kα structure shows ligand 3 in a binding mode in which, compared to 1 in the 8BQ4 PI5P4Kγ structure, has the aminopyrimidine flipped so that N2 rather than N4 is making an interaction with the hinge (Val199NH in PI5P4Kα, Met206 in PI5P4Kγ; Fig. 4). In the PI5P4Kα structure (8C8C) the aniline NH of 3 is also interacting with the hinge (Asn98OD1), whereas in the PI5P4Kγ structure (8BQ4) the aniline NH of 1 is not near the hinge residues. Retrospective docking suggested that our dual PI5P4Kα/PI5P4Kγ ligands 14 and 18 can interact with PI5P4Kα in two different poses, one is 1-like (Fig. 5A) and the other 3-like (Fig. 5B). For most of the dual inhibitors discussed here, the binding mode for 14 (shown in Fig. 5B) is only accessible in a protein conformation where Leu230 and Pro231 are not near the active site (seen, for example, in the PI5P4Kα structure solved with BAY297 bound, pdb 6YM4),31 as these two residues clash with the R1 extension in the 8C8C protein structure. Docking favours the pose shown in Fig. 5B for thieno[2,3-d] pyrimidines with an R1 3-pyridine and an acceptor atom in R2, e.g.9, 14, 15, 16 and 17. This appears to be driven by interactions with Lys145 and Lys209. In contrast, the thieno[3,2-d]pyrimidines (Table 6) all adopt the pose shown in Fig. 5A in preference. This may stem from a lower conformational energy for the extended conformation shown in Fig. 5A for this core, or perhaps the thiophene C–H offers a better interaction with the side chain of Asn198 than the sulphur.
 | ||
| Fig. 4 Compound 3 and PI5P4Kα protein (pdb 8C8C; gold) superimposed onto 1 and PI5P4Kγ protein (pdb 8BQ4; cyan), aligned using the hinge residues Thr196–Phe200 (numbering from PI5P4Kα sequence). This shows that the pyrimidine moiety does not bind in the same orientation in the PI5P4Kα and PI5P4Kγ ligands. PI5P4Kα residues are labelled in black and PI5P4Kγ residues in blue. | ||
 | ||
| Fig. 5 A) The dimethylphenyl group of 3 bound to PI5P4Kα (pdb 8C8C; gold) overlays well with that of 18 (orange) as positioned by docking to the 8C8C structure; B) 14 (brown) docked to alternative PI5P4Kα structure pdb 6YM4 (grey); 3 (pdb 8C8C; gold) was transposed onto the 6YM4 structure by alignment. | ||
Interestingly, all of the ligands with good PI5P4Kα activities (pIC50 > 6.5), with the exception of 42, have a much better docking score in the PI5P4Kα–3 structure (8C8C) than the PI5P4Kα–BAY297 (6YM4) structure (see ESI† Table S1).
Conclusions
The phosphatidylinositol 5-phosphate 4-kinases (PI5P4Ks) play a central role in regulating cell signalling pathways and, as such, have become therapeutic targets for diseases such as cancer, neurodegeneration and immunological disorders but useful probe molecules have only recently become available to further study these systems. To expand the tools available to the field, herein we disclose compound 39 and a series of related PI5P4Kα/γ dual inhibitors which were developed from a PI5P4Kγ-selective series, using rational approaches starting from PI5P4Kα-selective ligands and PI5P4Kγ-selective ligands. We anticipate that these tools will enable further discoveries in the field.Experimental details
Biochemical assays
Assays to determine kinase activity of PI5P4Kα and PI5P4Kγ+ in the presence of inhibitors using an ADP-Glo assay (Promega) were performed as described previously.38 Recombinant PI5P4K protein was prepared as described previously.8E. coli BL21(DE3) clones harbouring PI5P4Kα (PIP4K2A; UniGene 138363), PI5P4Kβ (PIP4K2B; UniGene 171988) or PI5P4Kγ (PIP4K2C; UniGene 6280511), cloned into pGEX6P plasmid (Cytiva), were used to overexpress these proteins. PI5P4Kγ protein (designated “PI5P4Kγ+”) was a genetically modified chimera of PI5P4Kγ with a specific activity close to that of the active PI5P4Kα isoform8 and carries a number of PI5P4Kα-like mutations; insertion of three amino acids (QAR) at 139 plus an additional 11 amino acid mutations: S132L, E133P, S134N, E135D, G136S, D141G, G142A, E156T, N198G, E199G and D200E. Cultures were induced with 0.4 mM IPTG overnight and probe-sonicated in the presence of protease inhibitors. GST fusion protein was harvested using a GSTrap FF affinity column (Cytiva) and the GST tag removed in situ with 50 U of PreScission protease (Cytiva) for 4 h at 4 °C. The cleaved protein was further purified by size-exclusion chromatography (ÄKTA Pure, Cytiva). The protein purity was confirmed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and the concentration was determined by colorimetric assay (Bio-Rad).Binding of compounds to PI5P4Kγ in intact cells was assessed using an InCELL Pulse thermal stabilisation assay (DiscoverX) as described previously.38 PI5P4Kγ was cloned into the pICP vector (DiscoverX) to allow overexpression of the ePL-tagged target. Hek293 cells stably expressing ePL-tagged PI5P4Kγ were incubated with 25 nL of test compound in 100% DMSO in a black skirted PCR plate for 60 minutes at 38 °C. After incubation for 3 minutes at 46 °C, followed by cooling for 3 minutes at room temperature, 12 μL of EA-3 reagent (prepared as per the manufactures guidelines) was added to each well. The plate was then incubated for 60 minutes in the dark prior to luminescence reading on a Pherastar FSX plate reader (BMG Labtech).
Data analysis
Statistical analysis was performed using nonparametric testing in Prism 8 (GraphPad). Activity pIC50 values and in vivo binding pEC50 values were estimated using a 4-parameter fit (Dotmatics).Computational modelling
The virtual screening procedure was performed as previously described.30 Dockings were performed using Glide SP (release 2022-3, Schrodinger, https://www.schrodinger.com). No constraints were used for docking. For 6YM4 docking the Lys209 conformation was changed to the rotamer that created the largest binding pocket using the MOE protein builder (release 22.02, Chemical Computing Group, https://www.chemcomp.com). Water molecules that are located in similar positions in 6YM4 and 8C8C were left in the site during docking. The structures shown in Fig. 1 and 3 resulted from minimization of the binding site residues after docking to allow H-bonds with lysines to form.Abbreviations
| ADME | Absorption, distribution, metabolism, excretion and toxicity |
| ATP | Adenosine triphosphate |
| GTP | Guanosine triphosphate |
| MDCK-MDR1 | Madin Darby canine kidney-multidrug resistance mutation 1 |
| MLM | Mouse liver microsomes |
| M W | Molecular weight |
| ND | Not determined |
| P app | Apparent permeability coefficient |
| PI5P4K | Phosphatidylinositol 5-phosphate 4-kinase |
| QM | Quantum mechanics |
| SAR | Structure–activity relationship |
| WT | Wild-type |
X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Log Log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P P | Log partition coefficient |
Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. Stephen Andrews and Jonathan Clarke were programme leaders, John Skidmore offered further project leadership; together all three designed the project plan. Gregory Aldred, Timothy Rooney and Helen Boffey designed and synthesised the compounds. Henriëtte Willems performed computational chemistry and docked compounds. David Winpenny and Christopher Green ran ADP-Glo and InCELL Pulse assays and screened the compounds.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was funded by Alzheimer's Research UK (grant: ARUK-2015DDI-CAM), with support from the ALBORADA Trust. The ALBORADA Drug Discovery Institute is core funded by Alzheimer's Research UK (registered charity No. 1077089 and SC042474). The authors wish to thank Professor David C. Rubinsztein for insightful discussions on the PI5P4K biology.References
- T. Balla, Physiol. Rev., 2013, 93, 1019–1137 CrossRef CAS PubMed.
- G. R. V. Hammond and J. E. Burke, Curr. Opin. Cell Biol., 2020, 63, 57–67 CrossRef CAS PubMed.
- Y. Posor, W. Jang and V. Haucke, Nat. Rev. Mol. Cell Biol., 2022, 23, 797–816 CrossRef CAS PubMed.
- M. C. Vidalle, B. Sheth, A. Fazio, M. V. Marvi, S. Leto, F.-D. Koufi, I. Neri, I. Casalin, G. Ramazzotti, M. Y. Follo, S. Ratti, L. Manzoli, S. Gehlot, N. Divecha and R. Fiume, Biomolecules, 2023, 13, 1049 CrossRef CAS PubMed.
- M. J. Berridge and R. F. Irvine, Nature, 1989, 341, 197–205 CrossRef CAS PubMed.
- G. Di Paolo and P. De Camilli, Nature, 2006, 443, 651–657 CrossRef CAS PubMed.
- Y. Bultsma, W. J. Keune and N. Divecha, Biochem. J., 2010, 430, 223–235 CrossRef CAS PubMed.
- J. H. Clarke and R. F. Irvine, Biochem. J., 2013, 454, 49–57 CrossRef CAS PubMed.
- V. D. Rao, S. Misra, I. V. Boronenkov, R. A. Anderson and J. H. Hurley, Cell, 1998, 94, 829–839 CrossRef CAS PubMed.
- K. Sumita, Y.-H. Lo, K. Takeuchi, M. Senda, S. Kofuji, Y. Ikeda, J. Terakawa, M. Sasaki, H. Yoshino, N. Majd, Y. Zheng, E. R. Kahoud, T. Yokota, B. M. Emerling, J. M. Asara, T. Ishida, J. W. Locasale, T. Daikoku, D. Anastasiou, T. Senda and A. T. Sasaki, Mol. Cell, 2016, 61, 187–198 CrossRef CAS PubMed.
- M. R. Lundquist, M. D. Goncalves, R. M. Loughran, E. Possik, T. Vijayaraghavan, A. Yang, C. Pauli, A. Ravi, A. Verma, Z. Yang, J. L. Johnson, J. C. Y. Wong, Y. Ma, K. S. K. Hwang, D. Weinkove, N. Divecha, J. M. Asara, O. Elemento, M. A. Rubin, A. C. Kimmelman, A. Pause, L. C. Cantley and B. M. Emerling, Mol. Cell, 2018, 70, 531–544.e9 CrossRef CAS PubMed.
- M. Vicinanza, V. I. Korolchuk, A. Ashkenazi, C. Puri, F. M. Menzies, J. H. Clarke and D. C. Rubinsztein, Mol. Cell, 2015, 57, 219–234 CrossRef CAS PubMed.
- J. E. Burke, Mol. Cell, 2018, 71, 653–673 CrossRef CAS PubMed.
- T. E. J. Phillips and E. Maguire, Front. Cell. Neurosci., 2021, 15, 652593 CrossRef CAS PubMed.
- A. Beziau, D. Brand and E. Piver, Viruses, 2020, 12, 1124 CrossRef CAS PubMed.
- M. D. Goncalves, B. D. Hopkins and L. C. Cantley, N. Engl. J. Med., 2018, 379, 2052–2062 CrossRef CAS PubMed.
- G. K. Arora, L. Palamiuc and B. M. Emerling, FEBS Lett., 2022, 596, 3–16 CrossRef CAS.
- Y. J. Shin, J. K. Sa, Y. Lee, D. Kim, N. Chang, H. J. Cho, M. Son, M. Y. T. Oh, K. Shin, J. K. Lee, J. Park, Y. K. Jo, M. Kim, P. J. Paddison, V. Tergaonkar, J. Lee and D. H. Nam, J. Exp. Med., 2019, 216, 1120–1134 CrossRef CAS PubMed.
- J. Triscott, M. Reist, L. Küng, F. C. Moselle, M. Lehner, J. Gallon, A. Ravi, G. K. Arora, S. de Brot, M. Lundquist, H. Gallart-Ayala, J. Ivanisevic, S. Piscuoglio, L. C. Cantley, B. M. Emerling and M. A. Rubin, Sci. Adv., 2023, 9, eade8641 CrossRef CAS PubMed.
- J. G. Jude, G. J. Spencer, X. Huang, T. D. D. Somerville, D. R. Jones, N. Divecha and T. C. P. Somervaille, Oncogene, 2015, 34, 1253–1262 CrossRef CAS PubMed.
- S. Zhang, Z. Li, X. Yan, L. Bao, Y. Deng, F. Zeng, P. Wang, J. Zhu, D. Yin, F. Liao, X. Zhou, D. Zhang, X. Xia, H. Wang, X. Yang, W. Zhang, H. Gao, W. Zhang, L. Yang, Q. Hou, H. Xu, Y. Zhang, Y. Shu and Y. Wang, Front. Genet., 2019, 9, 721 CrossRef PubMed.
- B. M. Emerling, J. B. Hurov, G. Poulogiannis, K. S. Tsukazawa, R. Choo-Wing, G. M. Wulf, E. L. Bell, H. S. Shim, K. A. Lamia, L. E. Rameh, G. Bellinger, A. T. Sasaki, J. M. Asara, X. Yuan, A. Bullock, G. M. Denicola, J. Song, V. Brown, S. Signoretti and L. C. Cantley, Cell, 2013, 155, 844–857 CrossRef CAS PubMed.
- A. Ravi, L. Palamiuc, R. M. Loughran, J. Triscott, G. K. Arora, A. Kumar, V. Tieu, C. Pauli, M. Reist, R. J. Lew, S. L. Houlihan, C. Fellmann, C. Metallo, M. A. Rubin and B. M. Emerling, Dev. Cell, 2021, 56, 1661–1676.e10 CrossRef CAS PubMed.
- K. Lima, J. L. Coelho-Silva, G. S. Kinker, D. A. Pereira-Martins, F. Traina, P. A. C. M. Fernandes, R. P. Markus, A. R. Lucena-Araujo and J. A. Machado-Neto, Cancer Genet., 2019, 233–234, 56–66 CrossRef CAS PubMed.
- K. Lima, D. A. Pereira-Martins, L. B. L. de Miranda, J. L. Coelho-Silva, G. da S. Leandro, I. Weinhäuser, R. de C. Cavaglieri, A. de M. Leal, W. F. da Silva, A. P. A. de L. Lange, E. D. R. P. Velloso, E. Griessinger, J. R. Hilberink, E. Ammatuna, G. Huls, J. J. Schuringa, E. M. Rego and J. A. Machado-Neto, Blood Cancer J., 2022, 12, 151 CrossRef PubMed.
- S. C. Sivakumaren, H. Shim, T. Zhang, F. M. Ferguson, M. R. Lundquist, C. M. Browne, H. S. Seo, M. N. Paddock, T. D. Manz, B. Jiang, M. F. Hao, P. Krishnan, D. G. Wang, T. J. Yang, N. P. Kwiatkowski, S. B. Ficarro, J. M. Cunningham, J. A. Marto, S. Dhe-Paganon, L. C. Cantley and N. S. Gray, Cell Chem. Biol., 2020, 27, 525–537.e6 CrossRef CAS PubMed.
- M. Kitagawa, P. J. Liao, K. H. Lee, J. Wong, S. C. Shang, N. Minami, O. Sampetrean, H. Saya, D. Lingyun, N. Prabhu, G. K. Diam, R. Sobota, A. Larsson, P. Nordlund, F. McCormick, S. Ghosh, D. M. Epstein, B. W. Dymock and S. H. Lee, Nat. Commun., 2017, 8, 2200 CrossRef PubMed.
- T. D. Manz, S. C. Sivakumaren, F. M. Ferguson, T. Zhang, A. Yasgar, H. S. Seo, S. B. Ficarro, J. D. Card, H. Shim, C. V. Miduturu, A. Simeonov, M. Shen, J. A. Marto, S. Dhe-Paganon, M. D. Hall, L. C. Cantley and N. S. Gray, J. Med. Chem., 2020, 63, 4880–4895 CrossRef CAS PubMed.
- T. D. Manz, S. C. Sivakumaren, A. Yasgar, M. D. Hall, M. I. Davis, H. S. Seo, J. D. Card, S. B. Ficarro, H. Shim, J. A. Marto, S. Dhe-Paganon, A. T. Sasaki, M. B. Boxer, A. Simeonov, L. C. Cantley, M. Shen, T. Zhang, F. M. Ferguson and N. S. Gray, ACS Med. Chem. Lett., 2020, 11, 346–352 CrossRef CAS PubMed.
- T. P. C. Rooney, G. G. Aldred, H. K. Boffey, H. M. G. Willems, S. Edwards, S. J. Chawner, D. E. Scott, C. Green, D. Winpenny, J. Skidmore, J. H. Clarke and S. P. Andrews, J. Med. Chem., 2023, 66, 804–821 CrossRef CAS PubMed.
- L. Wortmann, N. Bräuer, S. J. Holton, H. Irlbacher, J. Weiske, C. Lechner, R. Meier, J. Karén, C. B. Siöberg, V. Pütter, C. D. Christ, A. Ter Laak, P. Lienau, R. Lesche, B. Nicke, S. H. Cheung, M. Bauser, A. Haegebarth, F. Von Nussbaum, D. Mumberg and C. Lemos, J. Med. Chem., 2021, 64, 15883–15911 CrossRef CAS PubMed.
- D. H. Drewry, F. M. Potjewyd, J. L. Smith, S. Howell and A. D. Axtman, Curr. Res. Chem. Biol., 2023, 3, 100036 CrossRef CAS.
- H. M. G. Willems, S. Edwards, H. K. Boffey, S. J. Chawner, C. Green, T. Romero, D. Winpenny, J. Skidmore, J. H. Clarke and S. P. Andrews, RSC Med. Chem., 2023, 14, 934–946 RSC.
- I. Al-Ramahi, S. S. P. Giridharan, Y.-C. Chen, S. Patnaik, N. Safren, J. Hasegawa, M. de Haro, A. K. W. Gee, S. A. Titus, H. Jeong, J. Clarke, D. Krainc, W. Zheng, R. F. Irvine, S. Barmada, M. Ferrer, N. Southall, L. S. Weisman, J. Botas and J. J. Marugan, elife, 2017, 6, e29123 CrossRef PubMed.
- S. Chen, C. C. Tjin, X. Gao, Y. Xue, H. Jiao, R. Zhang, M. Wu, Z. He, J. Ellman and Y. Ha, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2002486118 CrossRef CAS PubMed.
- W. Ji, E. S. Wang, T. D. Manz, J. Jiang, K. A. Donovan, X. Abulaiti, E. S. Fischer, L. C. Cantley, T. Zhang and N. S. Gray, Eur. J. Med. Chem., 2023, 247, 115027 CrossRef CAS PubMed.
- Note that, as previously reported in ref. 30 and 38 a “PI5P4Kγ+” ADP-Glo assay is used for routine screening and a PI5P4Kγ wild-type InCELL Pulse assay is later used to verify PI5P4Kγ activity with series exemplars.
- H. K. Boffey, T. P. C. Rooney, H. M. G. Willems, S. Edwards, C. Green, T. Howard, D. Ogg, T. Romero, D. E. Scott, D. Winpenny, J. Duce, J. Skidmore, J. H. Clarke and S. P. Andrews, J. Med. Chem., 2022, 65, 3359–3370 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3md00355h |
| ‡ These authors contributed equally. |
| § Present address: UK Dementia Research Institute, University of Cambridge, Island Research Building, Cambridge Biomedical Campus, Hills Road, Cambridge, CB2 0AH, UK. |
| This journal is © The Royal Society of Chemistry 2023 |