Open Access Article
Open Access ArticleEncouraging tribomechanical and biological responses of hydroxyapatite coatings reinforced by various levels of niobium pentoxide particles†
Mir Saman
Safavi
*ab,
Jafar
Khalil-Allafi
 *a,
Amir
Motallebzadeh
c,
Cristina
Volpini
*a,
Amir
Motallebzadeh
c,
Cristina
Volpini
 b,
Vida
Khalili
d and
Livia
Visai
b,
Vida
Khalili
d and
Livia
Visai
 *be
*be
aResearch Center for Advanced Materials, Faculty of Materials Engineering, Sahand University of Technology, P.O. Box: 51335-1996, Tabriz, Iran. E-mail: samansafavi1992@gmail.com; jallafi@yahoo.de; Fax: +98 4133449454; Tel: +98 4133449454
bMolecular Medicine Department (DMM), Center for Health Technologies (CHT), UdR INSTM, University of Pavia, Via Taramelli 3/B, 27100 Pavia, Italy. E-mail: livia.visai@unipv.it
cKoç University Surface Science and Technology Center (KUYTAM), Koç University, İstanbul, Turkey
dInstitut für Werkstoffe, Ruhr-Universität Bochum, Universitätsstrasse 150, D-44780 Bochum, Germany
eMedicina Clinica-Specialistica, UOR5 Laboratorio di Nanotecnologie, ICS Maugeri, IRCCS, 27100 Pavia, Italy
First published on 24th October 2023
Abstract
The development of surface technologies to obtain improved tribomechanical and biological characteristics of synthetic NiTi implants is critical. A suitable match with the mechanical properties of the implanted biomaterial is necessary to reduce the risk of stress-shielding and implantation failure. The present contribution has attempted to assess the influence of the Nb2O5 particle level in an electrolyte, i.e., 0–1 g L−1, on the tribomechanical and biological performance of the HAp layers, which were obtained by galvanostatic pulse electrodeposition on NiTi. The surface characteristics of the electrodeposited layers were analyzed using GIXRD, FESEM, XPS, and contact angle measurements. Nanoindentation, nanoscratch, and pull-off assays were employed to study the tribomechanical properties of the films. The in vitro biocompatibility of the developed specimens was investigated using NIH3T3 fibroblast cells. The results illustrated that a more compact and hydrophilic surface is obtained with the incorporated particles. The Nb content and Ca/P molar ratio are increased from the outermost surface to the subsurface layers of the coatings. The composite coatings showed higher hardness, elastic modulus, bonding strength to the underlying NiTi, and lower COF compared to pure HAp layers. The elastic modulus of the Nb2O5-reinforced HAp films is close to that of cortical bone, reducing stress-shielding risk. The inclusion of Nb2O5 particles in the HAp matrix led to an improvement in cell functions, e.g., viability and proliferation, within the various cell culture durations. The adopted surface modification strategy in this work can create new opportunities for the successful use of NiTi in orthopedic applications.
1. Introduction
The surface properties of a material, as an interface between the material and the environment, decide its performance and service lifetime. Simply, the initial interactions between a material and the surrounding environment occur at its surface. It is important to adopt desirable surface modification approaches not only to tackle the challenges facing bulk materials but also to further increase their beneficial properties. Therefore, it is possible to tailor the surface properties of a material to simultaneously exploit the beneficial properties of bulk and the surface for a specific application.1–4 There are a variety of surface technologies, including electrochemical deposition, plasma spraying, magnetron sputtering, chemical vapor deposition, physical vapor deposition, and laser deposition, that have been developed to meet the requirements of various industrial fields. The coatings produced by vacuum-based and spraying technologies show high adhesion strength to the substrate; however, the electrodeposition engaged a specific position in the surface finishing industry of the synthetic biomaterials thanks to its simplicity and cost-effectiveness. The technique allows fabricating uniform layers and incorporating one or more reinforcing agents into the growing film.5–9NiTi shape memory alloys, falling under the category of metallic biomaterials, exhibit noticeable superelasticity, high corrosion resistance, suitable biocompatibility, and moderate elastic modulus (28–40 and 70 GPa in martensite and austenite phases, respectively). Thanks to its encouraging properties, NiTi is commonly used in various clinical branches, such as orthopedics, cardiovascular, and orthodontics.10–12 One potential challenge that threatens the success of orthopedic surgeries is the mismatch between the elastic modulus of the implanted material and the natural bone, which can cause a the stress-shielding phenomenon. The stress-shielding refers to the decrease in bone density due to the reduced/removed stress on the bone, as the synthetic implant with a higher elastic modulus takes the applied load. It may also lead to poor bone growth and cell death.13,14 NiTi has the lowest elastic modulus among the commercially available metallic biomaterials; however, it is necessary to minimize the mismatch between the elastic modulus values of the cortical bone (15–30 GPa) and NiTi (28–40 and 70 GPa in martensite and austenite phases, respectively). Moreover, an increase in the hardness of the implanted material is another milestone to achieve, which in turn, leads to more durability and wear resistance.15–17
The application of surface engineering techniques is found to be an effective strategy for either solving problems associated with NiTi or inducing new properties into its surface.18 To date, a variety of strategies, including magnetron sputtering, sol–gel, and spraying, have been utilized to modify the NiTi surface. Various ceramics, e.g., CaP and transition metal oxides (TiO2 and ZrO2), as well as polymers, such as chitosan, polylactic acid, and polypyrene, are potential coating materials used to attain superior properties or address the facing limitations of NiTi.19–22 The deposition of the CaP family layer on NiTi is a key solution to suppress Ni ion leaching, improve antibacterial performance, and increase bioactivity. On the other hand, there is a dire need to strengthen the brittle nature of CaP ceramics to obtain more tough coatings with the desired stiffness. Meanwhile, a new challenge, namely poor bonding strength, emerges when applying a ceramic coating on the metallic substrate.23–29 The recent progress in surface engineering offers the exploitation of constructive properties of the reinforcing agents to solve this problem. The reported results have demonstrated the positive role of ceramic reinforcing particles, e.g., ZnO and TiO2, in promoting the mechanical properties of the HAp electrodeposits.30,31 The type and concentration of the included reinforcing phase can seriously alter the mechanical properties of the HAp films. It is to be emphasized that the added phase should not degrade the biological performance of the films. Nb2O5 particles seem to be an appropriate candidate to promote the tribomechanical properties and biocompatibility of the electrodeposited HAp coatings due to their illustrious resistance against both wear and corrosion, high stability, magnificent mechanical strength (elastic modulus of over 100 GPa), and striking bioactivity and biocompatibility.32–36
A survey of the literature demonstrates that there is no empirical work focusing on the role of Nb2O5 reinforcing agents on the tribomechanical performance of HAp electrodeposits. Following the gap in previous studies, we have attempted to address the effect of the concentration of the dispersed Nb2O5 particles in the bath on tribomechanical performance and the in vitro biocompatibility of the HAp coatings. It is believed that the proposed strategy will be helpful in minimizing the stress-shielding of NiTi and improving HAp/NiTi adhesion strength without a fall in the biocompatibility of HAp-coated NiTi.
2. Materials and methods
2.1. Electrolyte preparation and electroplating process
NiTi (Ni 50.2%–Ti 49.8%) disks with a diameter of 1 cm and thickness of 1 mm were used as working electrodes. The density, surface roughness, hardness, and elastic modulus of the used NiTi are 6.45 g cm−3, 10 nm, 3.3 GPa, and 38 GPa, respectively. The pre-treatment protocol of the NiTi substrates, electrolyte composition, and electrodeposition parameters have been provided in our previous work,37 schematically illustrated in Fig. 1.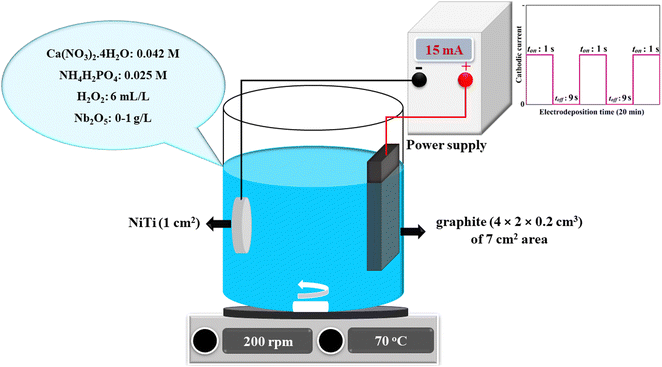 | ||
| Fig. 1 Schematic illustration of the electrolyte composition and electrodeposition parameters in the present work. | ||
The coatings produced by 0, 0.25, 0.50, and 1.0 g L−1 of Nb2O5 particles in the electrolyte will be labeled S1, S2, S3, and S4, respectively, in the following text (see Table 1). The Nb2O5 particles were added in the range of 0–1 g L−1. A further rise in the concentration of the particles in the electrolyte led to severe agglomeration of the particles in the microstructure of the layer and the deposition of an uneven coating. Moreover, it is difficult to maintain this high number of Nb2O5 particles suspended in the electrolyte due to their high density.
| Label | Nb2O5 level in the electrolyte (g L−1) |
|---|---|
| S1 | 0 |
| S2 | 0.25 |
| S3 | 0.50 |
| S4 | 1.0 |
2.2. Characterization
The phase composition of the electroplated coatings was evaluated by GIXRD using Cu-Kα radiation (Bruker D8 Advance, Germany). The patterns were collected at the incidence angle of 1° in the 2θ range of 5–85°. The crystallite size of the electrodeposited coatings was calculated through the Scherrer formula as follows:38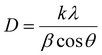 | (1) |
The surface and cross-sectional morphology of the produced coatings were assessed using a FESEM (MIRA3 TESCAN, Czech Republic).
The elemental composition and chemical states of the outermost and subsurface layers were studied via XPS (Thermo Fisher Scientific K-Alpha, USA) using an Al Kα source. To study the subsurface layers, a uniform etching process was carried out by a rastered Ar+ beam under the voltage of 4 keV at a rate of approx. 0.25 nm s−1 for 240 s. The hydrocarbon C 1s at a binding energy of 284.6 eV was selected as a reference in processing the XPS data.
The surface wettability was measured using the sessile drop method with a static/dynamic contact angle measurement device (KSV CAM200, KSV Instruments, Finland) at 25 ± 1 °C. The presented values are the average of three various measurements.
2.3. Mechanical behavior
The nanoindentation apparatus (Agilent G200, AGILENT Technologies Inc., CA, USA) with a Berkovich diamond tip was used to measure the hardness and elastic modulus of the electroplated coatings. The Oliver-Pharr method allowed automatic calculation of the hardness and elastic modulus value using the nanoindentation tester. The nanoindentation assay was performed in the load-control mode. The maximum load was 5 mN with a constant loading rate of 0.5 mN s−1. When it reached the maximum load, the loading was held for 5 s. The reported values are the mean of 15 separate measurements.The adhesion strength between the developed composite films and NiTi substrate was measured by the pull-off test according to the ASTM F1044-05 standard under a constant strain rate of 1 mm min−1 (a schematic of the pull-off test is presented in the ESI,† Fig. S1).39 The tensile strength of the used glue was 50 MPa.
The adhesion strength and tribological properties of the coatings were evaluated using the nanoscratch technique. The prepared coatings were subjected to scratching using the CSM nanoscratch tester (CSM Instruments, actual Anton Paar, Switzerland). A diamond tip with a diameter of 20 μm was employed to apply a normal load (FN) in the progressive mode. The scratch length was 1 mm, and the lateral scratch speed was maintained at 0.5 mm min−1. To determine the critical normal load of initial cracking (Lc), the following test parameters were employed: an initial load of 0.3 mN, a final load of 100 mN, and a loading/unloading rate of 49.85 mN min−1. The variations in normal force (Fn), frictional force (Ft), and frictional coefficient with the scratch distance were recorded using Scratch V4.52 software, along with the optical panorama image of the surface deformation after the scratch.
2.4. In vitro biocompatibility
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 342 (2 μg mL−1) was used for nuclei staining. To visualize the F-actin cytoskeleton organization, cells were stained with tetramethylrhodamine B isothiocyanate (TRITC) phalloidin conjugate solution (10 μg mL−1, EX/EM maxima ≈540/575, Sigma-Aldrich) in PBS for 40 min at RT. The CLSM images were obtained using a TCS SP8 confocal microscope (Leica Microsystems, Bensheim, Germany) equipped with a digital image capture system at 20× magnification.
342 (2 μg mL−1) was used for nuclei staining. To visualize the F-actin cytoskeleton organization, cells were stained with tetramethylrhodamine B isothiocyanate (TRITC) phalloidin conjugate solution (10 μg mL−1, EX/EM maxima ≈540/575, Sigma-Aldrich) in PBS for 40 min at RT. The CLSM images were obtained using a TCS SP8 confocal microscope (Leica Microsystems, Bensheim, Germany) equipped with a digital image capture system at 20× magnification.
2.5. Statistical analysis
Statistical analysis was carried out via GraphPad Prism version 9.0 (GraphPad, Inc., San Diego, CA, USA). Analysis was performed by one-way or two-way ANOVA analysis of variance (ANOVA), followed by the Bonferroni post hoc test (significance level of 0.05).3. Results and discussion
3.1. Phase structure
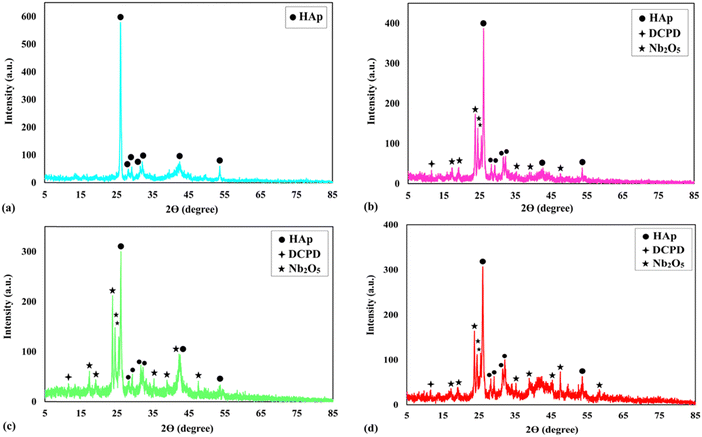
The GIXRD patterns of the studied specimens are illustrated in Fig. 2. While the phase composition of sample S1 consists of HAp diffraction (JCPDS card No. 09-0432), the phase structure of the S2, S3, and S4 coatings comprises HAp, Nb2O5 (JCPDS card No. 16-0053), and a trace amount of DCPD (JCPDS card No. 9-0077) phases. A weak peak emerged at 2θ ≈ 11° corresponding to DCPD, which is the precursor of the HAp phase.40 It has been shown that the presence of this phase, at low amounts, besides HAp, is beneficial for improved in vivo biomineralization and biocompatibility.32The incorporation of the particles into the growing layers led to the appearance of a new HAp peak, e.g., 2θ ≈ 42°, indicating that the included particles served as heterogeneous nucleation sites. A decrease in the crystallite size of the composite films, presented in Table 2, is another observation that approves the positive role of the included particles in providing extra nucleation sites. Moreover, the rapid nucleation rate during the deposition of the composite films, resulting from the presence of Nb2O5 particles, is responsible for the incomplete transformation of DCPD to HAp. The presence of enough hydroxyl ions together with enough time are two required factors for the mentioned transformation.32 The reason for the presence of strong Nb2O5 diffractions is using an X-ray beam with a low incident angle, which determines the phase structure of the outermost layers of the coatings.
| Material type | Crystallite size (nm) |
|---|---|
| S1 | 91 |
| S2 | 54 |
| S3 | 51 |
| S4 | 56 |
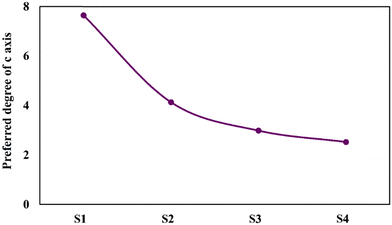
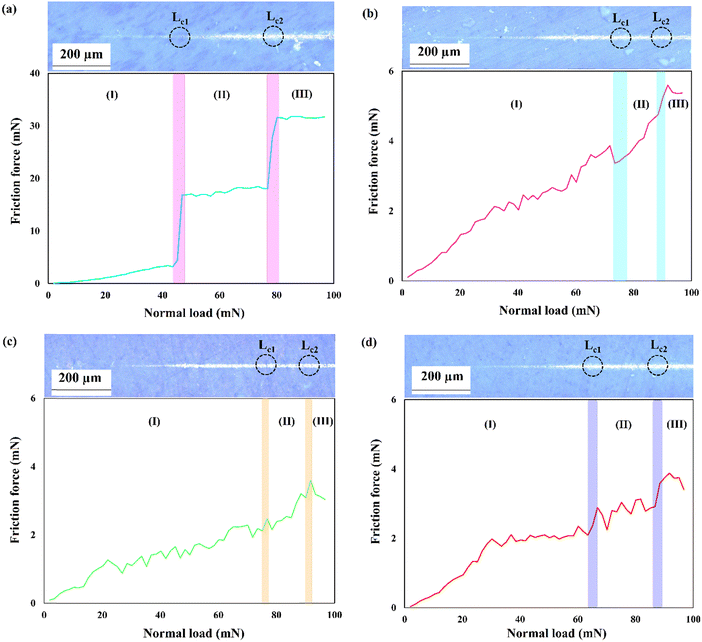
The strong HAp peak at 2θ ≈ 26° with the crystal face of (002) reveals that the crystals preferentially grow in the direction of the c-axis, namely perpendicular to the NiTi substrate. The intensity of the main HAp peak at 2θ ≈ 26° was significantly reduced for samples S2–S4. The following equations were used to quantify the preference of the crystals to grow along the c-axis41:
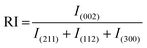 | (2) |
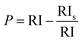 | (3) |
Overall, HAp crystals grow along the a-, b-, and c-axes. The growth units on the a- and b- axes are OH–Ca6 coordination cations, while the growth on the c-axis occurs on coordination anions Ca–P6O24. Ca–P6O24 is the most stable growth element, therefore, HAp crystals thermodynamically prefer to grow along the c-axis. The amount of OH− ions is the critical factor in increasing the tendency of the crystals to be grown along the c-axis. Since the included particles enhance the kinetics of the nucleation process, which consumes a higher concentration of the OH− ions, there are not sufficient ions that can support growth through the c-axis.43,44 Therefore, a descending trend is seen for the P value of the composite samples.
3.2. Surface characteristics
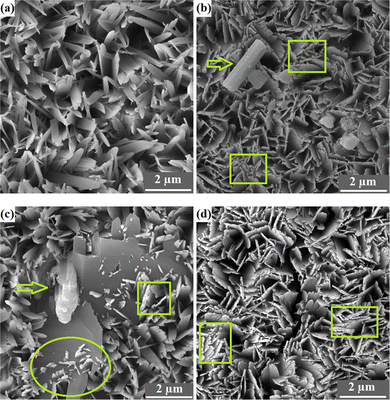
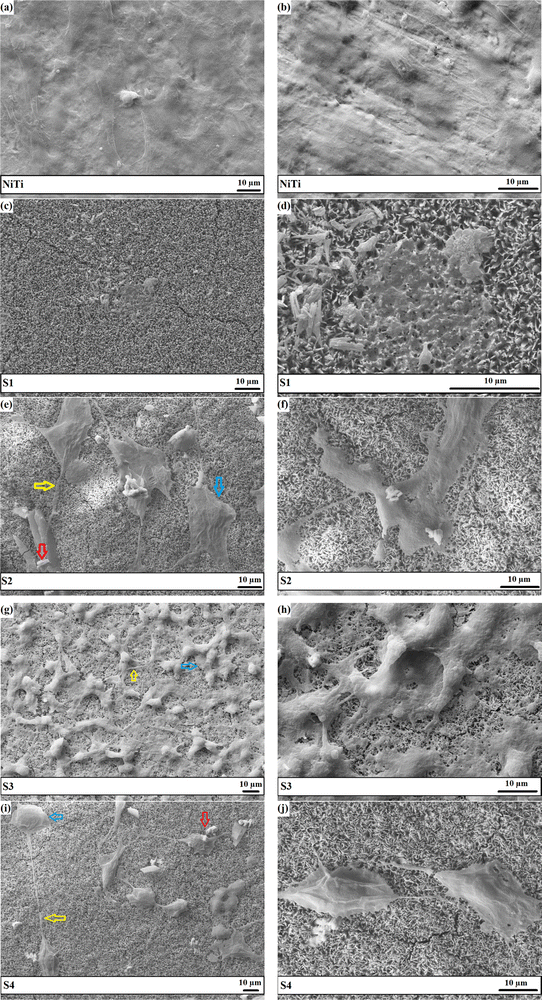
The FESEM top-view micrographs of the studied specimens are indicated in Fig. 4. The particle morphology of the porous S1 sample is needle-like. A change in the particle morphology from needle-like to plate-like is obvious with the inclusion of the particles in the HAp matrix. It is to be mentioned that there are still some needle-like crystals throughout the surface of the composite layers. The incorporated particles, shown by arrows, are both placed between the growing crystals and located on the surface. In general, a more compact surface was obtained with the addition of the reinforcing Nb2O5 particles, as shown by the rectangles in Fig. 4b–d. The S3 sample has the most compact surface.The nucleation of new HAp crystals over the existing HAp plates is indicated by the oval in Fig. 4c. The FESEM cross-sectional images of the samples obtained using both SE and BSE are exhibited in Fig. 5.
 | ||
| Fig. 5 The FESEM cross-sectional images of the samples obtained using both SE and BSE: (a) S1, (b) S2, (c) S3, and (d) S4. | ||
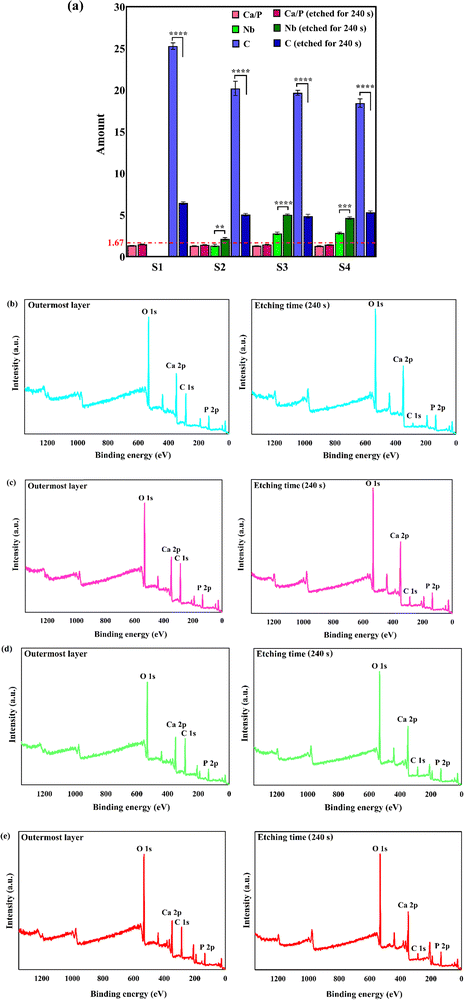
The images confirm the formation of an appropriate bond between the substrate and coatings without any flaws, cracks, or other defects. The thickness of the S1, S2, S3, and S4 coatings is 14.5, 11.5, 11, and 10 μm, respectively. A decrease in thickness with the inclusion of the reinforcing particles may be attributed to the formation of a more compact microstructure and the low preference for growth along the c-axis.45,46 The XPS survey spectra of the outermost and subsurface layers and the data extracted from the spectra are presented in Fig. 6.
The XPS survey spectrum of the S1 sample comprises Ca 2p, P 2p, and O 1s peaks, as the main precursors of HAp structure, along with a C 1s peak, originating from the dissolving of the atmospheric CO2 in the plating bath.47 Besides the mentioned regions, there is an additional Nb 3d region in the XPS spectra of the S2–S4 samples, which is assigned to the presence of Nb2O5 throughout the surface of the composite films. The binding energy values of Ca 2p3/2, P 2p3/2, O 1s, and Nb 3d5/2 peaks are 346.9, 132.5, 530.7, and 206.5 eV, respectively. The obtained binding energy values of Ca 2p3/2 and P 2p3/2 comply well with the energies of Ca–O and P–O bonds in standard HAp.48 Moreover, the binding energy value of O 1s matches the energy of hydroxyl ions existing in the HAp structure and oxide species.47 The XPS high-resolution spectra of Ca 2p, P 2p O 1s, and Nb 3d regions in the S3 sample are exhibited in the ESI,† Fig. S2. The data presented in Fig. 6a) reveal that the Ca/P molar ratio of the samples is in the range of 1.27–1.33. The Ca/P ratio in the precursor powders is 1.67; however, the Ca/P molar ratio of the outermost surface of the coatings is in the range of 1.27–1.33. A decrease in the Ca/P ratio can be attributed to the dissolution of the atmospheric carbon in the aqueous electrolyte. The application of the etching treatment led to an increase in the Ca/P, where the ratio is in the range of 1.42–1.46. The reported results in the literature confirm that the Ca/P ratio of the natural bone, measured by the XPS, is 1.42 ± 0.02,49 which is in agreement with the data presented in this work. It is to be mentioned that there is no obvious change in the binding energies of the various regions after the etching treatment. A statistically significant decrease in the C content of the coatings is observed with the etching process. Similar results have been reported by Ohtsu et al.50 On the other hand, the Nb content increases with the etching, which demonstrates the presence of the reinforcing phase throughout the thickness of the film. In summary, it can be concluded that the chemical composition of the outermost and subsurface layers is similar to that of the bone.
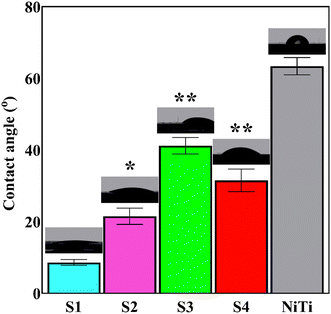
The results of contact angle measurements using SBF drop are presented in Fig. 7. The applied surface modification technique resulted in a more hydrophilic surface. The (i) polar OH groups of the top HAp layer and (ii) ionic character of the deposited ceramic coating led to an increase in the wettability of NiTi.32 The contact angle between the SBF drop and the coated samples increased with the inclusion of the Nb2O5 particles due to the fact that the particles regionally prevented the water molecules from penetrating the surface. Optimizing surface wettability is a key strategy to achieving the most appropriate biological responses. This means that moderate hydrophilicity, in the range of 20–40°, is needed for improved cell-material interactions.51,52 The composite samples provide a favorable substrate for the adhesion and proliferation of the eukaryotic cells in terms of surface hydrophilicity.
3.3. Tribomechanical performance
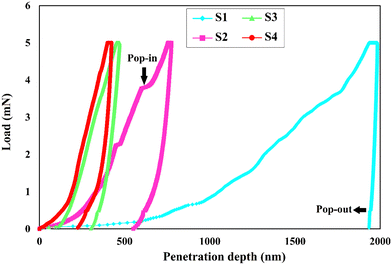
The mechanical properties of a synthetic implant, especially hardness and elastic modulus, are very important in orthopedics for load-bearing applications since the material may undergo friction and stresses in vivo. In fact, a poor mechanical behavior of the coatings leads to the rapid dissolution of the coating. For analyzing the mechanical properties of brittle ceramics, the nanoindentation test bears some advantages over the other characterization methods as the nanoindentation results are more reliable in the nanoscale, and it gives a perspective about the overall properties, such as elastic modulus, hardness, and mechanical strength.5,53 The load–displacement curves of the electrodeposited specimens are shown in Fig. 8. The extracted data from the curves are outlined in Table 3. The lower the indentation depth, the higher the hardness is.54 From the curves, it can be deduced that the S4 sample has the highest hardness. The reason why the load–displacement curve of the S1 sample shows a large plateau-like behavior in the range of 0–700 nm is attributed to the high amount of porosity over the surface so that the indenter runs throughout the surface to reach the maximum load.31 The presence of several discontinuities in the load–displacement curves, in particular for the S1 sample, can be interpreted by the crystals bending or cracking.55 It is not surprising to see the pop-in and pop-out in the load–displacement diagrams of the layers as they are made of brittle HAp ceramic. The pop-in phenomenon, where a sudden increase in indenter displacement occurs, is seen during the loading path of the porous or crack-containing materials. The load–displacement curve of the S1 sample has the highest number of pop-ins due to its high porosity and micro-sized pores. The pop-in events can be categorized into three types depending on their length: (i) micro, (ii) moderate, and (iii) extreme. While the pop-in events measured less than 10 nm and 100 nm in length fall under the micron and moderate classes, respectively, those with a length >100 nm are labeled extreme.56 In general, the observed pop-ins in this work are micron and moderate. For instance, there is a moderate pop-in with a length of 25 nm in the load–displacement curve of the S2 sample, shown by an arrow.| Specimen type | H (GPa) | E (GPa) | H/E | H 3/E2 |
|---|---|---|---|---|
| NiTi | 3.3 ± 0.13 | 38 ± 1.1 | 86.8 × 10−3 | 24.88 × 10−3 |
| S1 | 0.05 ± 0.001 | 17.1 ± 0.6 | 2.92 × 10−3 | 4.27 × 10−7 |
| S2 | 0.38 ± 0.01 | 17.3 ± 0.8 | 21.9 × 10−3 | 1.83 × 10−4 |
| S3 | 1.15 ± 0.04 | 26.8 ± 1.1 | 42.9 × 10−3 | 21.17 × 10−4 |
| S4 | 1.3 ± 0.06 | 43.9 ± 2.1 | 29.6 × 10−3 | 11.39 × 10−4 |
The elastic deformation and plastic deformation energies are obtained by calculation of the corresponding areas in the load–displacement curve (see the ESI,† Fig. S3). The area surrounded by the loading curve and displacement axis, and that enclosed by the unloading curve and displacement axis, are assigned to elastic deformation and plastic deformation energies, respectively. In all of the specimens, the area corresponding to plastic energy is much larger than that of elastic energy, illustrating that the irreversible deformation was dominant as a result of the applied load.
To avoid the effect of substrate on the measured mechanical properties by the nanoindentation, the penetration depth should not exceed 10% of the coating thickness.55 Bearing in mind the thickness of the composite layers, ranging from 10–11.5 μm, and the penetration depths, maximum 0.5 μm, it is obvious that the substrate has no influence on the measured properties. For the S1 sample, although the substrate may affect the properties, the obtained results confirm that the sample has a weaker mechanical performance than that of composite films, even with the contribution of the substrate. This also shows that the contribution of the substrate was negligible.
In general, a marked increase in the mechanical properties of the HAp coatings is obtained with the co-deposition of the Nb2O5 particles. There are potential factors, including smaller crystallite size, more compact surface, the high level of hardness and elastic modulus of the included Nb2O5 particles, favorable load-transfer between the reinforcing particles and the HAp matrix arising from the larger interface-volume ratio, and the plate-like particle morphology, governing the higher mechanical properties of the composite samples compared to the pure HAp one.53,57 It was found that the plate-like morphology renders higher mechanical properties than that of the needle-like one due to decreased porosity.53,55
The potential toughening mechanisms of the pure HAp layer with the included Nb2O5 reinforcing phase are crack deflection, crack branching, and crack bridging. Once the crack reaches the Nb2O5 particles, it has to be branched or deflected to find a new propagation path, which takes much more energy.58
The hardness and elastic modulus of the cortical bone are 0.52–0.74 and 15–30 GPa, respectively.31 The excellent match between the elastic modulus of the composite coatings, in particular S3 and the bone, along with the higher hardness of the coatings than that of bone, makes the developed coatings an excellent candidate for orthopedic applications. While the former lead to a decrease in implant loosening and an increase in stimulation of new bone formation, the latter eliminates the risk of coating demolition during the surgical procedure.59 Additionally, the high mechanical behavior and durability of the biomedical coatings guarantee their excellent corrosion protection efficiency. The published results on the improved corrosion performance of the HAp-Nb2O5 films by our research team approve such a claim.37 Fornell et al.55 measured the mechanical properties of electrodeposited HAp coatings from H2O2-containing electrolyte with a thickness of ≈14 μm using a nanoindentation test under the load of 5 mN and showed that the hardness and reduced elastic modulus of the coatings are 0.014 ± 0.004 and 3.1 ± 1.0 GPa, respectively.
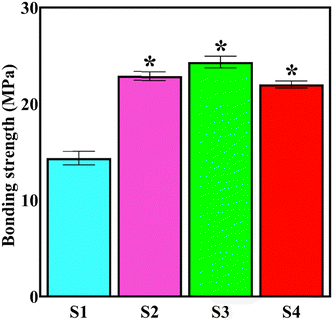
Strong adhesion between the coating and the substrate ensures the efficient performance and durability of the coated biomaterial during/after implantation since the applied stresses can cause detachment of the coating from the substrate. Poor implant/coating adhesion is one of the main challenges facing the successful use of the electrodeposition technique in the surface finishing of biomaterials.5,57Fig. 9 shows the adhesion strength values of the coatings. According to the ISO 13779-2 standard, the minimum implant/coating bonding strength should be 15 MPa so that the system is qualified for use in vivo.57 The results show that almost all of the developed systems meet the standard requirement. The NaOH alkali pre-treatment of NiTi, electrodeposition under pulsed current mode with a low duty cycle, and the application of moderate current density play a constructive role in achieving a strong bonding strength even for the S1 sample. The formation of a highly porous surface under high current densities due to the increased hydrogen evolution degrades the bonding strength, while at moderate current densities, the cohesive strength is dominant, leading to a failure at the interface of the coating/biomaterial.60 Similarly, there are fewer H2 bubbles generated during the pulsed current electroplating.61 The bonding strength between the HAp top layer and the underlying NiTi substrate is enhanced with the inclusion of the Nb2O5 phase.
The synergistic role of cohesive strength and adhesive strength determines the overall adhesion strength. While the cohesive strength depends on crystallinity, porosity, and crack extension, the top layer/substrate interlocking, the mismatch between the elastic modulus of coating and substrate, and residual stress affect the adhesive strength.62
An increase in the coating density, decrease in CTE mismatch between the coating and implant, grain refinement, higher mechanical interlocking, and enhancement in the elastic modulus of the coatings (getting closer to that of NiTi), obtained by the inclusion of the particles, are the factors that promote the adhesive and cohesive strength. The CTE of NiTi, HAp, and Nb2O5 is 6.6 × 10−6, 14 × 10−6, and 5.3–5.9 × 10−6 1/k, respectively.63,64
The adhesion strength between the S3 coating and the substrate is the highest, emphasizing the use of the optimum concentration of the reinforcements. Shojaee et al.65 reported that the bonding strength between electrodeposited HAp and 316L stainless steel increased from 11.6 to 20.8 MPa with co-deposition of the 10 g L−1 of ZrO2 particles; however, a further rise in the concentration up to 15 g L−1 degraded the strength.
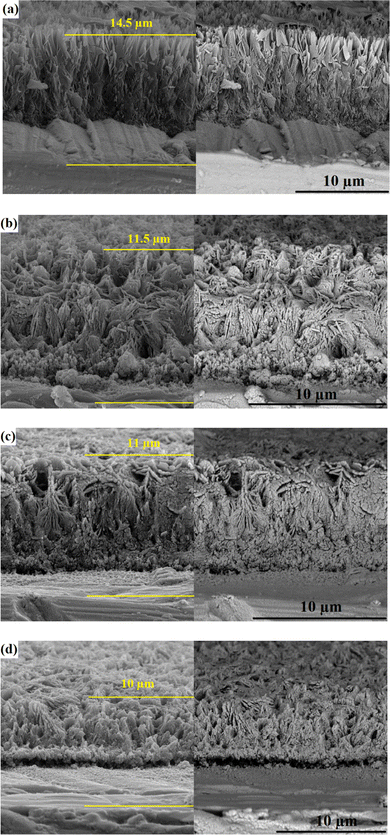
Nanoscratch is a novel standard technique, rendering fruitful information on the coating/substrate adhesion strength and tribological properties of the coatings. The technique claims several advantages over the pull-off method, including (i) its ability to assess the shear stresses, which are more likely to occur in the human body during daily activities than tensile stress, and (ii) giving more accurate data. While the pull-off results are the sum of cohesive failure, adhesive failure and glue failure, the nanoscratch results only reveal the coating/substrate bonding strength.31,66,67Fig. 10 shows the obtained curves from nanoscratch testing along with the corresponding panorama images of track traces. The curves are divided into three regions: In the first region, shown by (I), there are no sharp fluctuations in the curves, where the diamond tip only touches the surface of the coating and slides over it. In the second region, indicated by (II), some significant fluctuations are observable, which illustrate the penetration of the tip in the coating. The third region, shown by (III), appears when the load has reached a certain level, indicating the serious plastic deformation of the coating.66,68 Mokabber et al.66 reported the absence of sudden changes in region II of the scratch curves of the electrodeposited calcium phosphate films, similar to those observed in the present work, which depict that the cohesive failure occurs without brittle delamination.
 | ||
| Fig. 10 Obtained curves from nanoscratch testing along with the corresponding panorama images of track traces (at the magnification of 100×): (a) S1, (b) S2, (c) S3, and (d) S4. | ||
Critical load (Lc) is determined using typical scratch curves and/or panorama images of track traces. An abrupt increase in friction force and/or coating removal in scratch track corresponds to the critical load.69 As shown, there are two Lc values for the studied coatings, indicating the gradual spallation of the coatings rather than sudden delamination. The latter takes place when there is one Lc.70 The critical load values derived from the scratch test measurements are summarized in Table 4.
| Specimen | L c1 (mN) | L c2 (mN) |
|---|---|---|
| S1 | 45.1 | 76.7 |
| S2 | 73.4 | 88.4 |
| S3 | 76.7 | 90.0 |
| S4 | 66.81 | 86.7 |
L c1 and Lc2 refer to the cracking initiation and coating spallation, respectively.70 The higher Lc value correlates with a higher adhesion strength. The presented data in Table 4 reveal an improvement in the Lc value of the S1 sample with the inclusion of the Nb2O5 strengthening phase. The nanoscratch results comply with the results of the pull-off test, where S3 has the highest bonding strength due to its excellent surface and microstructural features. The published results demonstrate the increase in Lc value of HAp coating with the addition of the reinforcements. For instance, Mehrvarz et al.31 reported that the Lc of the HAp electrodeposit increases from 58 to 91 mN with the addition of 0.4 g L−1 ZnO nanoparticles to the HAp electrolyte.
The panorama images of the scratch track demonstrate that there are no visible fracture signs inside/at the border of the scratch track, which is a marker of appropriate coating/substrate bonding. In addition, the damage induced by the tip is limited to the contact area inside the coating.
The conventional pin-on-disk technique is not suitable for determining the tribological behavior of the brittle HAp coatings. The slope of the first region in the typical scratch curve, where the tip only slides over the surface, provides the COF values.31,71 The COF-load curves obtained from the scratch test data are exhibited in the ESI,† Fig. S4. The COF varies in the range of 0–0.1, showing the favorable tribological and wear properties of the electrodeposited films. The mean COF values of the S1, S2, S3, and S4 samples are 0.058, 0.055, 0.038, and 0.042, respectively. The included particles partially act as solid-lubricant. The lower the COF value, the higher the wear resistance is. Moreover, the enhanced hardness of the Nb2O5-reinforced layers contributes to the higher wear resistance, in accordance with Archard's law.72
3.4. In vitro biocompatibility
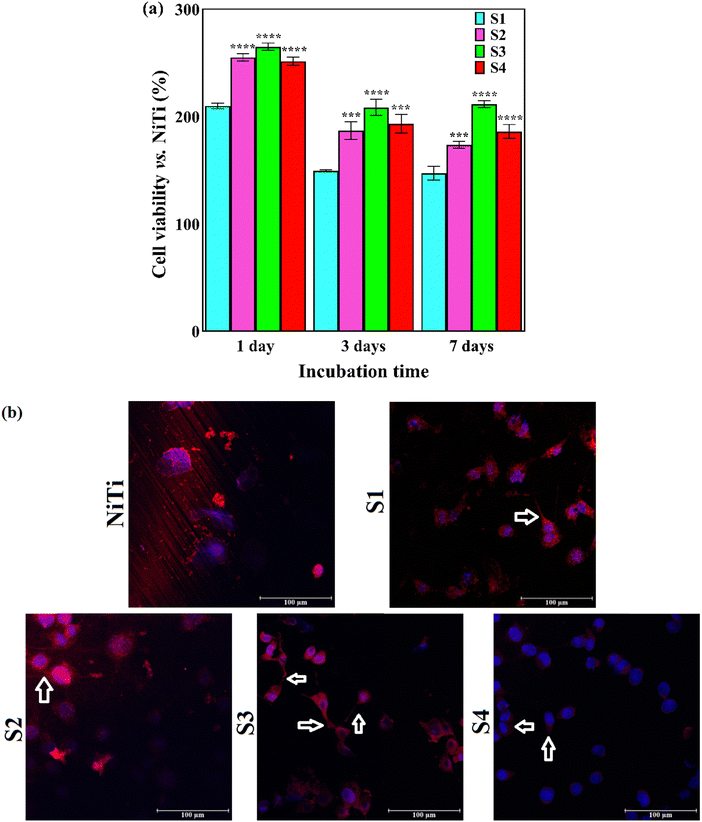
The application of embryonic fibroblast cell lines (NIH3T3) has garnered great attention in biomedical fields since the fibroblasts are mesenchymal cells of connective tissue, not only producing extracellular matrix components but also involved in cell cycle, tissue architecture, and wound and bone healing. These cells allow the assessment of the basal cytocompatibility of the synthetic implant from the viewpoint of cell viability, growth, proliferation, etc., on its surface. The fibroblasts are the first cells attached to the implanted material. They produce immature collagen, which precipitates on the material. Overall, fibroblasts and osteoblasts are two types of cells that are in direct connection with the surface of the orthopedic implants.73–76The outermost layer of the biomaterial decides its biological response in vivo since it is the first component that comes into contact with the surrounding cells and tissues. The beneficial role of the HAp-Nb2O5 composite layer on the in vitro biocompatibility of NiTi implants, determined by SAOS-2 osteoblastic cells, has been demonstrated in our previous work.32 The results of the MTT assay and CLSM images of cell nuclei and F-actin are illustrated in Fig. 11a and b, respectively. The applied surface modification strategy led to at least 200% increase in cell viability of the NIH3T3 cells within the first 24 h of incubation. Moreover, a significant difference in cell proliferation is observed for the composite coatings compared to the HAp one.
The adsorption of plasma proteins is the first stage in the formation of the protein layer, which serves as the interface for cell adhesion. The surface morphology, chemistry, wettability, and topography affect the protein adsorption rate.32,77 The optimum surface wettability, porous surface, and presence of Nb2O5 particles are the contributing parameters for enhanced cell adhesion. It seems that the favorable surface characteristics of the Nb2O5-containing coatings provided a better substrate for NIH3T3 cell adhesion. The results confirm that the added Nb2O5 particles not only induce no toxicity but also promote cell functions. The NIH3T3 cell numbers on the bare and coated NiTi samples and the cell viability versus TCP after various incubation periods are indicated in the ESI,† Fig. SV and SVI, respectively. The cell proliferation for all of the studied coatings markedly increased during the incubation period. A material rendering cell viability >75% is considered non-cytotoxic in accordance with ISO 10993-5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1999.78 All of the coated samples, in particular those reinforced by the Nb2O5 phase, provided higher viability than 75%, confirming their favorable biological responses.
1999.78 All of the coated samples, in particular those reinforced by the Nb2O5 phase, provided higher viability than 75%, confirming their favorable biological responses.
Favorable cell adhesion is observable for all of the coated samples and may be due to the strong focal adhesions. There is a higher number of cells adhered to composite coatings compared with bare NiTi and S1 coating. The morphology of the overwhelming fraction of cell nuclei on the coatings is polygonal. The filopodia projections from cells, shown by arrows, are observable in the CLSM images of the coatings. The presence of filopodia demonstrates the beginning of the cell interactions with the substrate and surrounding cells within the first 24 h of incubation. There are a high number of long filopodia along with well-developed actin filaments in the CLSM image of S3, approving the accuracy of the MTT quantitative results. It is not possible to focus at high magnification via this microscope due to the high light reflection and pore topology. The morphology of the NIH3T3 cells after 7 days of being cultured on the NiTi and coatings is presented in Fig. 12. It is seen that the cultured NIH3T3 cells have different morphologies on the coatings. Cell colonies consisting of small globules are formed on the S1 sample (see Fig. 12d), while well-attached cells that spread over the surface with typical elongated shapes can be seen in SEM images of the S2–S4 samples. The fusiform and polygonal cell morphologies can also be observed in Fig. 12e–j. Fig. 12g indicates the formation of a confluent layer of the fibroblast cells that extends throughout the surface of the S3. The presence of filopodia and lamellipodia protrusions, marked by yellow and blue arrows, respectively, confirms the excellent adhesion of the cells to the surface and cell–cell contacts.79 They are also responsible for cell migration and movement.80 In summary, the S3 sample, benefiting from the most uniform surface with favorable hydrophilicity, the optimum amount of Ca/P ratio and Nb2O5 particles, and the smallest crystallite size, offers the best in vitro compatibility with the NIH3T3 cells.
4. Conclusions
The present work has endeavored to evaluate the effect of Nb2O5 particle levels in the electrolyte, i.e., 0–1 g L−1, on the morphological, microstructural, tribomechanical, and biological performance of the HAp layers. The main results are drawn as follows:•The phase structure of the particle-free coating consists mainly of the HAp phase. The addition of the particles led to the formation of trace amounts of DCPD and limited the crystal growth along the c-axis.
•A more compact surface with a favorable wettability for cell adhesion, in the range of 20–40°, is obtained with the incorporation of the Nb2O5 particles into the HAp matrix. There were no defects throughout the coating/substrate interface.
•The elemental composition of HAp-Nb2O5 throughout the outermost and subsurface layers was close to that of natural bone. The Nb content in the coatings was enhanced with an increase in particles level in the plating electrolyte.
•The addition of the Nb2O5 particles greatly improved the hardness, elastic modulus, and toughness of the HAp coatings. The high hardness and the favorable elastic modulus close to that of cortical bone reduce the risk of implant loosening and stress-shielding, respectively.
•The coating/implant bonding strength, studied by pull-off and nanoscratch, was promoted with the co-deposition of the reinforcing phase. Moreover, the COF of the composite coatings was lower than that of HAp coating due to the beneficial contribution of the Nb2O5 phase.
•The included Nb2O5 particles not only rendered a favorable platform for cell adhesion but also gave rise to cell functions, such as growth and proliferation.
•The developed HAp-Nb2O5 coating systems, in particular those electrodeposited from a bath containing 0.50 g L−1 Nb2O5, provided the best tribomechanical and biological properties. These coatings are believed to be successfully used for load-bearing orthopedics purposes.
Abbreviations
| BSE | Backscattered electron |
| CLSM | Confocal laser scanning microscopy |
| COF | Coefficient of friction |
| CTE | Coefficient of thermal expansion |
| DCPD | Dicalcium phosphate dihydrate |
| DMEM | Dulbecco's modified Eagle's medium |
| ECM | Extracellular matrix |
| FESEM | Field emission scanning electron microscopy |
| FWHM | Full width at half maximum |
| GIXRD | Grazing incidence X-ray diffraction |
| HAp | Hydroxyapatite |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide |
| OCP | Octacalcium phosphate |
| PBS | Phosphate-buffered saline |
| PFA | Paraformaldehyde |
| SBF | Simulated body fluid |
| SE | Secondary electron |
| SEM | Scanning electron microscopy |
| TCP | Tissue culture plate |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
L.V. acknowledges the grant of the Ministry of University and Research (MUR) to the Department of Molecular Medicine (DMM) of the University of Pavia under the initiative “Dipartimenti di Eccellenza (2018-2022) and (2023-2027)”. M.S.S., L.V., and E.R. acknowledge Patrizia Vaghi for the CLSM images (https://cgs.unipv.it/eng/), Giovanna Bruni (University of Pavia, Italy) for the SEM observations and Giacomo Dacarro for the contact angle measurements (University of Pavia, Italy).References
- M. Mozetič, Materials, 2019, 12, 441 CrossRef PubMed.
- R. Junker, A. Dimakis, M. Thoneick and J. A. Jansen, Clin. Oral. Implants Res., 2009, 20, 185–206 CrossRef PubMed.
- S. Mohandesnezhad, M. Etminanfar, S. Mahdavi and M. S. Safavi, Surf. Interfaces, 2022, 28, 101604 CrossRef CAS.
- S. Ahmadiyan, J. Khalil-Allafi, M. R. Etminanfar, M. S. Safavi and M. Hosseini, Trans. IMF, 2022, 100, 93–102 CrossRef CAS.
- M. S. Safavi, M. A. Surmeneva, R. A. Surmenev and J. Khalil-Allafi, Ceram. Int., 2021, 47, 3031–3053 CrossRef CAS.
- N. Shokri, M. S. Safavi, M. Etminanfar, F. C. Walsh and J. Khalil-Allafi, Mater. Chem. Phys., 2021, 259, 124041 CrossRef CAS.
- A. Menazea, S. A. Abdelbadie and M. Ahmed, Appl. Surf. Sci., 2020, 508, 145299 Search PubMed.
- P. Zhao, Y. Liu, L. Xiao, H. Deng, Y. Du and X. Shi, J. Mater. Chem. B, 2015, 3, 7577–7584 RSC.
- J. Khalil-Allafi, H. Daneshvar, M. S. Safavi and V. Khalili, Phys. B Condens. Matter, 2021, 615, 413086 CrossRef CAS.
- M. S. Safavi, A. Bordbar-Khiabani, J. Khalil-Allafi, M. Mozafari and L. Visai, J. Manuf. Mater. Process, 2022, 6, 65 CAS.
- M. S. Safavi, A. Bordbar-Khiabani, F. C. Walsh, M. Mozafari and J. Khalil-Allafi, Curr. Opin. Biomed. Eng., 2023, 25, 100429, DOI:10.1016/j.cobme.2022.100429.
- Y. Zhang, S. Attarilar, L. Wang, W. Lu, J. Yang and Y. Fu, Int. J. Bioprinting, 2021, 7, 340 CrossRef CAS PubMed.
- D. Savio and A. Bagno, When the total hip replacement fails: A review on the stress-shielding effect, Processes, 2022, 10, 612 CrossRef CAS.
- M. Zhang, T. Gregory, U. Hansen and C. K. Cheng, J. Orthop. Res., 2020, 38, 1566–1574 CrossRef PubMed.
- L. Wang, J. Wang, Y. Xu, J. Zhu and W. Zhang, Mech. Adv. Mater. Struct., 2022, 1–14, DOI:10.1080/15376494.2022.2121990.
- K. Kumar, A. Das and S. B. Prasad, Mater. Today: Proc., 2021, 44, 2038–2042 CAS.
- T. Alonso, D. Favier and G. Chagnon, J. Mater. Eng. Perform., 2019, 28, 4667–4679 Search PubMed.
- R. Asghari, M. S. Safavi and J. Khalil-Allafi, Trans. IMF, 2020, 98, 250–257 CrossRef CAS.
- R. A. Surmenev, M. A. Ryabtseva, E. V. Shesterikov, V. F. Pichugin, T. Peitsch and M. Epple, J. Mater. Sci.: Mater. Med., 2010, 21, 1233–1239 CrossRef CAS PubMed.
- D. Qiu, A. Wang and Y. Yin, Appl. Surf. Sci., 2010, 257, 1774–1778 CrossRef CAS.
- D. P. Aun, M. Houmard, M. Mermoux, L. Latu-Romain, J. C. Joud, G. Berthomé and V. T. Buono, Appl. Surf. Sci., 2016, 375, 42–49 CrossRef CAS.
- M. S. Safavi, J. Khalil-Allafi, E. Restivo, A. Ghalandarzadeh, M. Hosseini, G. Dacarro, L. Malavasi, A. Milella, A. Listorti and L. Visai, Sci. Rep., 2023, 13, 16045 CrossRef CAS PubMed.
- Y. Guo, Z. Xu, M. Liu, S. Zu, Y. Yang, Q. Wang, Z. Yu, Z. Zhang and L. Ren, J. Mater. Res. Technol., 2022, 17, 622–635 CrossRef CAS.
- Y. L. Lai, P. Y. Cheng, C. C. Yang and S. K. Yen, Thin Solid Films, 2018, 649, 192–201 CrossRef CAS.
- K. Dudek, M. Dulski, T. Goryczka and A. Gerle, Ceram. Int., 2018, 44, 11292–11300 CrossRef CAS.
- M. E. Aksoy, B. Aksakal, N. Aslan and B. Dikici, J. Mater. Eng. Perform., 2021, 30, 7365–7375 CrossRef CAS.
- Y. Say and B. Aksakal, J. Mater. Res. Technol., 2020, 9, 1742–1749 CrossRef CAS.
- E. Mohseni, E. Zalnezhad and A. R. Bushroa, Int. J. Adhes. Adhes., 2014, 48, 238–257 CrossRef CAS.
- W. S. Harun, R. I. Asri, J. Alias, F. H. Zulkifli, K. Kadirgama, S. A. Ghani and J. H. Shariffuddin, Ceram. Int., 2018, 44, 1250–1268 CrossRef CAS.
- D. Qiu, L. Yang, Y. Yin and A. Wang, Surf. Coat. Technol., 2011, 205, 3280–3284 CrossRef CAS.
- A. Mehrvarz, J. Khalil-Allafi, A. Motallebzadeh and V. Khalili, Ceram. Int., 2022, 48, 35039–35049 CrossRef CAS.
- M. S. Safavi, J. Khalil-Allafi and L. Visai, Biomater. Adv., 2023, 150, 213435, DOI:10.1016/j.bioadv.2023.213435.
- P. Amaravathy, S. Sowndarya, S. Sathyanarayanan and N. Rajendran, Surf. Coat. Technol., 2014, 244, 131–141 CrossRef CAS.
- Y. Li, K. S. Munir, J. Lin and C. Wen, Bioact. Mater., 2016, 1, 127–131 CrossRef PubMed.
- M. S. Safavi, F. C. Walsh, L. Visai and J. Khalil-Allafi, ACS Omega, 2022, 7, 9088–9107 CrossRef CAS PubMed.
- O. Shcherbina, M. Palatnikov and V. Efremov, Inorg. Mater., 2012, 48, 433–438 CrossRef CAS.
- M. S. Safavi, J. Khalil-Allafi, I. Ahadzadeh, F. C. Walsh and L. Visai, Surf. Coat. Technol., 2023, 470, 129822 CrossRef CAS.
- T. C. Paul and J. Podder, Appl. Phys. A: Mater. Sci. Process., 2019, 125, 818 CrossRef CAS.
- M. Hosseini, J. Khalil-Allafi, M. Etminanfar, M. S. Safavi, N. Bloise and A. Ghalandarzadeh, Mater. Chem. Phys., 2023, 293, 126899 CrossRef CAS.
- X. Chu, W. Jiang, Z. Zhang, Y. Yan, H. Pan, X. Xu and R. Tang, J. Phys. Chem. B, 2011, 115, 1151–1157 CrossRef CAS PubMed.
- D. He, X. Zhang, P. Liu, X. Liu, X. Chen, F. Ma, W. Li, K. Zhang and H. Zhou, Surf. Coat. Technol., 2021, 406, 126656 CrossRef CAS.
- M. C. Barbosa, N. R. Messmer, T. R. Brazil, F. R. Marciano and A. O. Lobo, Mater. Sci. Eng. C, 2013, 33, 2620–2625 CrossRef CAS PubMed.
- Y. Zhu, L. Xu, C. Liu, C. Zhang and N. Wu, AIP Adv., 2018, 8, 085221 CrossRef.
- P. Wang, C. Li, H. Gong, X. Jiang, H. Wang and K. Li, Powder Technol., 2010, 203, 315–321 CrossRef CAS.
- T. T. Nguyen, N. T. Pham, T. T. Dinh, T. T. Vu, H. S. Nguyen and L. D. Tran, Adv. Polym. Technol., 2020, 1–10, DOI:10.1155/2020/8639687.
- N. T. Thom, P. T. Nam, N. T. Phuong, C. T. Hong, N. Van Trang, N. T. Xuyen and D. T. Thanh, Vietnam J. Sci. Technol., 2017, 55, 706–715 CrossRef.
- M. E. El-Naggar, O. A. Ali, D. I. Saleh, M. A. Abu-Saied, M. K. Ahmed, E. Abdel-Fattah and S. F. Mansour, J. Inorg. Organometallic Polym. Mater., 2022, 32, 399–411 CrossRef CAS.
- N. Lowry, M. Brolly, Y. Han, S. McKillop, B. J. Meenan and A. R. Boyd, Ceram. Int., 2018, 44, 7761–7770 CrossRef CAS.
- H. B. Lu, C. T. Campbell, D. J. Graham and B. D. Ratner, Anal. Chem., 2000, 72, 2886–2894 CrossRef CAS PubMed.
- N. Ohtsu, S. Hiromoto, M. Yamane, K. Satoh and M. Tomozawa, Surf. Coat. Technol., 2013, 218, 114–118 CrossRef CAS.
- M. Steinerova, R. Matejka, J. Stepanovska, E. Filova, L. Stankova, M. Rysova, L. Martinova, H. Dragounova, M. Domonkos, A. Artemenko and O. Babchenko, Mater. Sci. Eng. C, 2021, 121, 111792 CrossRef CAS PubMed.
- S. Tang, B. Tian, Y. J. Guo, Z. A. Zhu and Y. P. Guo, Surf. Coat. Technol., 2014, 251, 210–216 CrossRef CAS.
- M. Bartmanski, A. Zielinski, B. Majkowska-Marzec and G. Strugala, Ceram. Int., 2018, 44, 19236–19246 CrossRef CAS.
- M. R. Hosseini, M. Ahangari, M. H. Johar and S. R. Allahkaram, Mater. Lett., 2021, 285, 129097 CrossRef CAS.
- J. Fornell, Y. P. Feng, E. Pellicer, S. Suriñach, M. D. Baró and J. Sort, J. Alloys Compd., 2017, 729, 231–239 CrossRef CAS.
- S. S. Kasyap and K. Senetakis, Materials, 2021, 14, 4579 CrossRef CAS PubMed.
- M. S. Safavi, F. C. Walsh, M. A. Surmeneva, R. A. Surmenev and J. Khalil-Allafi, Coatings, 2021, 11, 110 CrossRef CAS.
- A. Janković, S. Eraković, M. Mitrić, I. Z. Matić, Z. D. Juranić, G. C. Tsui, C. Y. Tang, V. Mišković-Stanković, K. Y. Rhee and S. J. Park, J. Alloys Compd., 2015, 624, 148–157 CrossRef.
- A. Fomin, M. Fomina, V. Koshuro, I. Rodionov, A. Zakharevich and A. Skaptsov, Ceram. Int., 2017, 43, 11189–11196 CrossRef CAS.
- Y. Han, T. Fu, J. Lu and K. Xu, J. Biomed. Mater. Res., 2001, 54, 96–101 CrossRef CAS PubMed.
- D. Khazeni, M. Saremi and R. Soltani, Ceram. Int., 2019, 45, 11174–11185 CrossRef CAS.
- D. Gopi, E. Shinyjoy, M. Sekar, M. Surendiran, L. Kavitha and T. S. Kumar, Corros. Sci., 2013, 73, 321–330 CrossRef CAS.
- S. Saber-Samandari, C. C. Berndt and K. A. Gross, Acta Biomater., 2011, 7, 874–881 CrossRef CAS PubMed.
- A. Hassan, S. Naga and M. Awaad, Int. J. Refract. Met. Hard Mater., 2015, 48, 338–345 CrossRef CAS.
- P. Shojaee and A. Afshar, Surf. Coat. Technol., 2015, 262, 166–172 CrossRef CAS.
- T. Mokabber, Q. Zhou, A. I. Vakis, P. Van Rijn and Y. T. Pei, Mater. Sci. Eng., C, 2019, 100, 475–484 CrossRef CAS PubMed.
- M. Bartmański, L. Pawłowski, A. Mielewczyk-Gryń, G. Strugała, K. Rokosz, S. Gaiaschi, P. Chapon, S. Raaen and A. Zieliński, Materials, 2021, 14, 1638 CrossRef PubMed.
- S. Liu, B. Li, C. Liang, H. Wang and Z. Qiao, Appl. Surf. Sci., 2016, 362, 109–114 CrossRef CAS.
- K. Khlifi, H. Dhiflaoui, A. Ben Rhouma, J. Faure, H. Benhayoune and A. Ben Cheikh Laarbi, Coatings, 2021, 11, 477 CrossRef CAS.
- R. Drevet, N. B. Jaber, J. Fauré, A. Tara, A. B. Larbi and H. Benhayoune, Surf. Coat. Technol., 2016, 301, 94–99 CrossRef CAS.
- K. Cheng, C. Ren, W. Weng, P. Du, G. Shen, G. Han and S. Zhang, Thin Solid Films, 2009, 517, 5361–5364 CrossRef CAS.
- Y. C. Li, C. Zhang, W. Xing, S. F. Guo and L. Liu, ACS Appl. Mater. Interfaces, 2018, 10, 43144–43155 CrossRef CAS PubMed.
- A. M. Rahimi, M. Cai and S. Hoyer-Fender, Cells, 2022, 11, 2677 CrossRef CAS PubMed.
- A. Turlybekuly, A. D. Pogrebnjak, L. F. Sukhodub, L. B. Sukhodub, A. S. Kistaubayeva, I. S. Savitskaya, D. H. Shokatayeva, O. V. Bondar, Z. K. Shaimardanov, S. V. Plotnikov and B. H. Shaimardanova, Mater. Sci. Eng., C, 2019, 104, 109965 CrossRef CAS PubMed.
- K. M. Nuss and B. von Rechenberg, Open Orthop. J., 2008, 2, 66 CrossRef PubMed.
- L. Trentani, F. Pelillo, F. C. Pavesi, L. Ceciliani, G. Cetta and A. Forlino, Biomaterials, 2002, 23, 2863–2869 CrossRef CAS PubMed.
- Q. Zhang, J. Dong, M. Peng, Z. Yang, Y. Wan, F. Yao, J. Zhou, C. Ouyang, X. Deng and H. Luo, Mater. Sci. Eng., C, 2020, 111, 110847 CrossRef CAS PubMed.
- S. Saraiva, P. Pereira, C. T. Paula, R. C. Rebelo, J. F. Coelho, A. C. Serra and A. C. Fonseca, Mater. Sci. Eng., C, 2021, 131, 112498 CrossRef CAS PubMed.
- S. Datta, M. Das, V. K. Balla, S. Bodhak and V. K. Murugesan, Surf. Coat. Technol., 2018, 344, 214–222 CrossRef CAS.
- P. K. Mattila and P. Lappalainen, Nat. Rev. Mol. Cell Biol., 2008, 9, 446–454 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ma00704a |
| This journal is © The Royal Society of Chemistry 2023 |