Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Vanishing tails and a resilient mesophase: columnar liquid crystals in the limit of short tails†
Parikshit
Guragain
 *a,
Mitchell
Powers
*a,
Mitchell
Powers
 b,
John
Portman
b,
John
Portman
 b,
Brett
Ellman
b,
Brett
Ellman
 b and
Robert J.
Twieg
b and
Robert J.
Twieg
 a
a
aDepartment of Chemistry and Biochemistry, Kent State University, Kent, Ohio 44242, USA. E-mail: pguragai@kent.edu
bDepartment of Physics, Kent State University, Kent, Ohio 44242, USA
First published on 21st July 2023
Abstract
Discotic liquid crystals (DLCs) are disc shaped molecules, typically with a flat rigid aromatic core functionalized with long flexible tails about the periphery. Here two series of novel triphenylene based discotic liquid crystals are synthesized using strategies including the photocyclodehydrofluorination (PCDHF) reaction, the SNAr reaction and the Suzuki reaction. Some of these new partially fluorinated triphenylene compounds have significantly shorter tails than are usually found in DLCs. The first series comprises tetrafluorinated tetraalkoxy triphenylene compounds while the second series comprises difluorinated hexaalkoxy triphenylene compounds, with tail lengths also ranging from methoxy to hexyloxy for both series. This latter difluorinated hexaalkoxy series is directly comparable to the well-known HAT series. The tetrafluorinated compounds are of particular interest as they have retained their mesomorphic properties despite having significantly shorter tails than are typically found in DLCs, including methoxy and ethoxy tails.
Introduction
Discotic liquid crystals (DLCs) are mesogens which have a disc shaped planar, or nearly planar, rigid polyaromatic core that is almost invariably functionalized with multiple long flexible tails surrounding the molecular core. Such molecules form columns with strong cofacial alignment of the rigid cores while the flexible tails serve as a soft buffer between columns. Since their discovery in 1977 by Chandrashekar1 DLCs have attracted significant interest, particularly as potential electronic materials. The columnar structure leads to nearly one-dimensional charge transport via pi orbital overlap within the column, with the tails acting as an insulating sheath around the columns. This makes them of interest as organic semiconductors,2,3 organic photovoltaics (OPVs)4–6 organic field effect transistors (OFETs)7,8 organic light emitting diodes (OLEDs)9,10 as well as other devices.Of the various DLC cores suited for these purposes, triphenylenes are one of the most widely studied owing to their ease of synthesis and generally favourable temperature range. Hexaalkoxytriphenylenes (HAT)11–13 and hexaalkylthiotriphenylenes (HATT)14 are well known examples of discotic molecules which epitomize the triphenylene based DLCs. Numerous variations on these molecules have been made in order to study structure–function relationships and develop materials with more favourable properties.15–19 This includes compounds made with asymmetrically distributed tails20 as well as with additional substitutions either to the molecular core21 or to the tails themselves.22 These substitutions often include electronegative groups, including small substituents like fluorine and larger cyano or nitro groups which have been shown to dramatically affect the phase behaviour of the compounds.23–25 Fluorine in particular because of its small size, high polarity and high C–F bond strength ensures subtle but very significant modifications to the liquid crystal materials.26
Perhaps the best known DLCs are the HAT compounds, which are liquid crystalline when there are six –OC5H11 tails (n = 5) or longer tails at sites 2, 3, 6, 7, 10, 1111,25,27 (with plastic crystals being reported for n = 3 and 4). Asymmetrically substituted triphenylenes, such as the 2,3,6,7-tetraalkoxytriphenylenes, have not been as thoroughly studied, and those that have been synthesized are often non-mesogenic.24,28–30 This has led to the common belief amongst researchers that multiple long tails are a necessary to form a liquid crystal mesophase instead of a three dimensionally ordered crystal.31,32 This belief persists despite theoretical treatments33–35 and the existence of genuine tail free DLCs such as the halogenated indenes discovered by Barbera and coworkers;36 various mesogens that feature short trifluoromethyl groups instead of conventional tails37 and self-assembly of helical pores dendritic dipeptides by Percec.38 In this contribution we report a group of fluorinated alkoxytriphenylenes with tails varying from hexaalkoxy to mere methoxy groups. These compounds defy the conventional wisdom about the nature of the DLCs by retaining their mesophase even in the short-tailed limit.
These compounds were discovered in the course of development of the photocyclodehydrofluorination (PCDHF) reaction, a variant of the Mallory type photocyclization reaction39 which was reported by our group a few years back.40 This technique has proven to be especially useful for synthesizing selectively fluorinated triphenylene molecules.41,42 In some cases, the fluorine atoms are also latent reaction sites, as in the case of SNAr reactions which have been exploited here to add additional tails.
Results and discussion
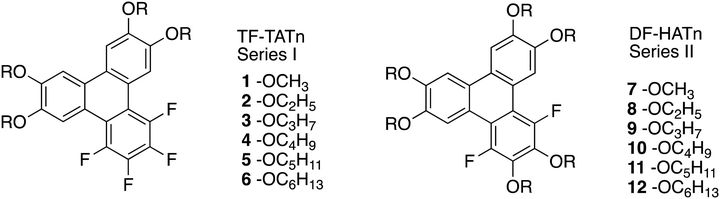
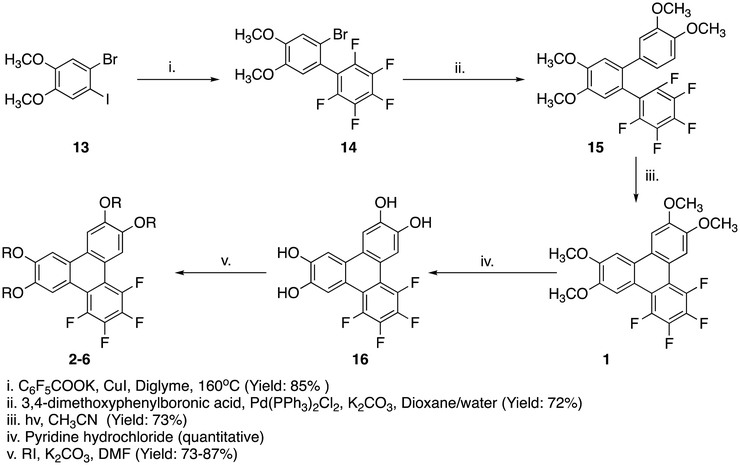
For this study two series of discotic compounds were synthesized, with the tail lengths varying within each series in order to study the relationship between the tails and potential mesomorphic properties. Specifically, the compounds are 1,2,3,4-tetrafluoro-6,7,10,11-tetra(n-alkoxy)triphenylenes and 1,4-difluoro-2,3,6,7,10,11-hexa(n-alkoxy)triphenylenes (Fig. 1). We refer to these compounds as TF-TATn and DF-HATn respectively, where n is the number of carbon atoms in each of the alkoxy tails. We have synthesized six TF-TATn compounds (Series I) and six DF-HATn compounds (Series II). We have previously described compounds 1, 5, 6, 11 and 12,41 and compound 1243 has previously been prepared by an alternative route.The PCDHF reaction was used in the synthesis of both series to obtain the targeted fluorinated triphenylenes. The overall synthetic route of the compounds found in Series I is found in Scheme 1. Starting with the simple substrate 1-bromo-2-iodo-4,5-dimethoxybenzene 13, a copper mediated decarboxylative coupling reaction44 was performed with the potassium salt of pentafluorobenzoic acid. This coupling reaction occurs with high specificity at the iodine atom. The resultant biphenyl 14 was subjected to a Suzuki coupling reaction with 3,4-dimethoxyphenylboronic acid to give the o-terphenyl 15 which on photoirradiation affords the 1,2,3,4-tetrafluoro-6,7,10,11-tetramethoxytriphenylene 1 which is the shortest tail entry amongst the target materials examined here.
In order to obtain the entries with longer tail lengths, all the methoxy groups of TF-TAT1 (1) were deprotected by heating in molten pyridine hydrochloride to obtain 1,2,3,4-tetrafluoro-6,7,10,11-tetrahydroxytriphenylene45 which was then subjected to realkylation reactions with different tail length alkyl iodides to produce the remaining entries in the set of target TF-TATn molecules. After full chemical characterization, phase studies were carried out with DSC and POM and, remarkably, all the compounds 1–6 were found to be mesogenic. This phase behaviour was subsequently confirmed using small angle X-ray scattering (SAXS). There is some reporting of 6,7,10,11-tetraalkoxytriphenylenes and variants with additional substituents and interestingly none of the reports shows those alkoxy triphenylenes in the shorter tail limits (even if there are a few they are non-mesogenic) however there are materials with five carbon tailed and higher which are mesogenic.24,28,30 The phase behaviour of this series of compounds is collected in Table 1 and representative phase textures and DSC scans of the Series I compounds are shown in Fig. 2.
| Compound | –OR | Phase behaviour (°C [kJ mol−1]) |
|---|---|---|
| a Enthalpies reported together. | ||
| 1 | –OCH3 | K 198 [19.5] Colh 310 [18.4] I 307 [7.7] Colh 182 [9.5] K |
| 2 | –OC2H5 | K 148 [22.4] Colh 242 [9.0] I 239 [7.7] Colh 128 [19.5] K |
| 3 | –OC3H7 | K 38.1 [−8.9] K′ 126 [30.2] Colh 230 [6.8] I 227 [6.6] Colh 46 K′ 38 [24.0]a K |
| 4 | –OC4H9 | K 109 [41.6] Colh 212 [5.4] I 209 [6.0] Colh 51 [21.0] K |
| 5 | –OC5H11 | K 110 [48.4] Colh 201 [4.7] I 198 [4.3] Colh 74 [45.8] K |
| 6 | –OC6H13 | K 98 [51.1] Colh 183 [4.9] I 180 [4.9] Colh 58 [48.7] K |
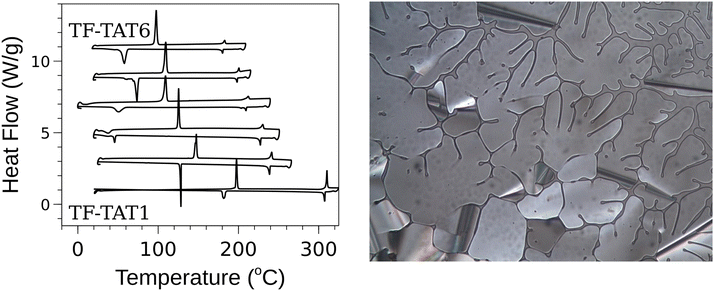 | ||
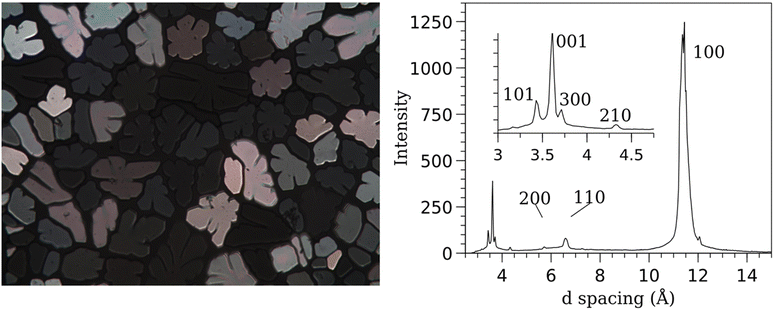
| Fig. 2 (Left) DSC curves for Series I compounds. (Right) Compound 2 at 229 °C while cooling from isotropic liquid viewed through crossed polarizers. | ||
Motivated by these results, we undertook further modifications of Series I compounds 1–6. Each of these materials was subjected to SNAr reactions with the appropriate aliphatic alcohol to provide the Series II 1,4-difluoro hexa(n-alkoxy) compounds (Scheme 2). The SNAr reactions are highly selective for the replacement of only the fluorine atoms at positions 2 and 3, with the initial substitution taking place faster than the second substitution.41 This process produced the target Series II materials in both useful yield and purity.
 | ||
| Scheme 2 Synthesis of Series II (DF-HATn) 1,4-difluoro-2,3,6,7,10,11-hexaalkoxytriphenylenes by SNAr reaction. | ||
An examination of the phase transitions of the Series II compounds revealed mesomorphic behaviour for only compounds with propyloxy or longer tails (Table 2). This is similar to the well-known HAT compounds and is in contrast to the tetrafluoro Series I compounds (Table 1).
| Compound | –OR | Phase behaviour (°C [kJ mol−1]) |
|---|---|---|
| 7 | –OCH3 | K 210 [21.9] I 207 [24.6] K |
| 8 | –OC2H5 | K 150 [31.4] I 131 [30.7] K |
| 9 | –OC3H7 | K 41 [2.6] K′ 141 [3.0] Colh 173 [11.7] I 170 [12.6] Colh 139 [3.1] K′ 37 [3.0] K |
| 10 | –OC4H9 | K 108 [2.3] Colh 165 [15.2] I 163 [17.4] Colh105 [2.3] K |
| 11 | –OC5H11 | K −12.5 [6.2] Colh 142 [10.1] I 139 [12.8] Colh −40 [4.3] K |
| 12 | –OC6H13 | Colh 116 [8.4] I 113 [10.2] Colh |
Interestingly, polarized microscopy on cooling shows compound 7 has a structure that is qualitatively similar to the other columnar mesogens. However, the material does not shear when subjected to mechanical perturbation. Its structure was examined using SAXS, which revealed a hexagonal structure with high order reflections including (hkl) 300 and 210, as well as 101 reflections. The 101 reflections indicate three-dimensional order stemming from correlations between molecules from adjacent columns. Together, these features indicate the presence of long-range three-dimensional order (Fig. 3), confirming that it is non-liquid crystalline.
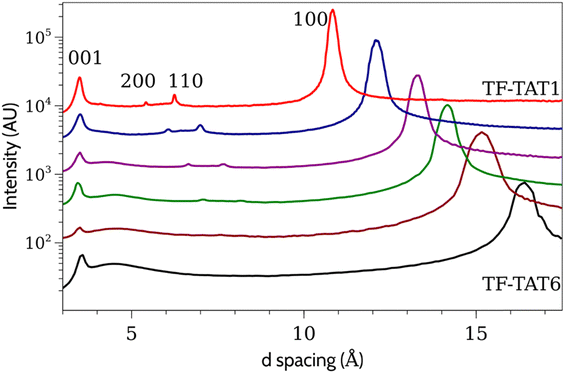
The TF-TATn compounds provide an interesting opportunity to analyze the role played by the tails in the mesophase as, to our knowledge, they are the only DLCs with alkoxy tails (or similar) that are mesogenic over a wide range of n starting with n = 1. We carried out SAXS studies of each of these compounds in order to see the evolution of the mesophase structure as the tails are shortened. Diffraction patterns for compounds 1–6 are collected in Fig. 4 (the structures of 5 and 6 have been reported elsewhere).41 As the tails shorten the intercolumn spacing (100 peak) decreases, as does the peak width (i.e., the correlation length increases), while the intracolumn spacing (001) is practically unchanged. Instead, the higher order reflections (110 and 200) become sharper as the hexagonal lattice becomes more ordered as tail length decreases. Unlike DF-HAT1, there is no 101 peak present in any of these compounds, so even as the two-dimensional order increases the columns retain the disorder characteristic of a discotic liquid crystal.
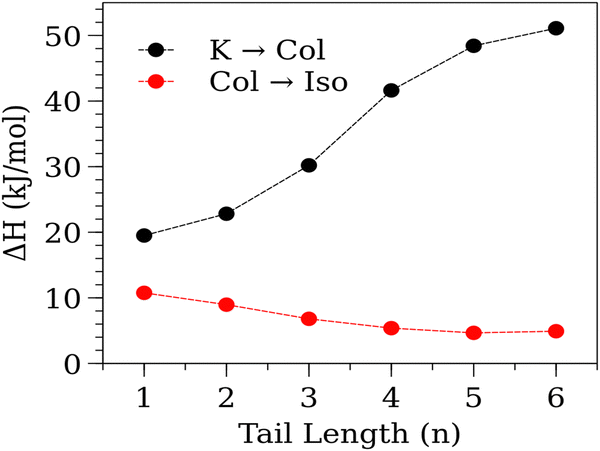
We can see further evidence of the increased two-dimensional ordering by examining the transition enthalpy of the TF-TATn compounds (Fig. 5). For all tail lengths, the enthalpy change at the isotropic transition is strictly smaller than at the crystal to liquid crystal transition. The liquid crystal transition is marked by the melting of the tails,46 and the conformational entropy of the tails can be substantial, resulting in a large enthalpy change. At the isotropic transition, the columns themselves melt, so the enthalpy change will be based on the cohesive interactions within and between columns being overcome and the columnar order being destroyed. The enthalpy of the isotropic transition is seen to be larger for short tails than for longer ones. This is another sign of the increased order that was apparent in the SAXS patterns. It is instructive to compare the Series I & II compounds against their nonfluorinated HAT counterparts (Fig. 6). In comparing these compounds, we can see consistent influence of increased tail lengths as well as trends that result from increased fluorination. With few exceptions (i.e., DF-HAT2), increasing the tail length reduces both the isotropic and crystalline transition temperatures. It is likely that the tails influence the transition temperatures through the combination of increased intercolumn distance and increased conformational entropy.
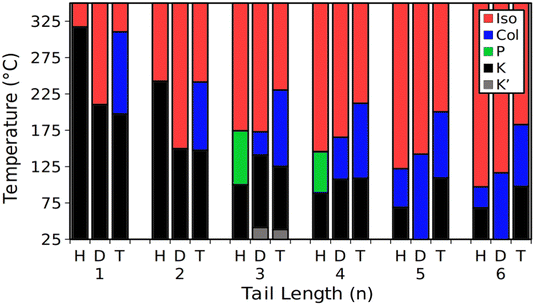 | ||
| Fig. 6 Phase transition temperatures for the HATn, DF-HATn and TF-TATn compounds (H, D and T respectively). HATn values shown here are from the literature11,25 and include a plastic crystal phase for HAT3 and HAT4. | ||
The effect of fluorination appears to be more complicated. In discussing this effect, it is useful to differentiate between two types of intermolecular interactions that are ubiquitous in the columnar mesophase: cofacial interactions between neighbouring molecules within a column, and edge-on interactions between molecules from neighbouring columns. The inter-columnar interactions are greatly influenced by the tail lengths. Columnar systems typically feature nanosegregation of the tails, and while this continues in the short-tailed limit,38 as the separation between molecules decreases the edge-edge interactions become increasingly sensitive to the specific electrostatic potential at the periphery of the molecular cores. Selective fluorination can significantly affect the character of these interactions, especially for short-tailed compounds. Conversely, the intra-columnar interactions are typically limited to the co-facial interactions between molecular cores and are unaffected by changing tail lengths.
It is perhaps not surprising, therefore, that the short-tailed (methoxy and ethoxy) compounds appear to behave differently than those compounds with longer alkoxy substituents. This is presumably due to the edge-on interactions between molecular cores, which is of increased significance for the short-tailed compounds. These materials feature high isotropic transition temperatures which is suppressed in the difluoro compounds, 7 and 8, relative to the analogous hydrocarbon and the tetrafluoro systems. The partial fluorination of the difluoro compounds reduces the isotropic transition temperature near to the mesophase transition temperature of the tetrafluoro compounds, so while the difluoro compounds melt, the tetrafluoro compounds develop a mesophase that persists to high temperatures. This stabilization of the mesophase following increased fluorination is reminiscent of the effect of other electronegative groups attached to triphenylene cores (e.g., HATn compounds with NO2 or another electronegative substitution at the 1-position) which have shown a similar tendency to extend the lower limit of the columnar mesophase.23 This suggests a mesophase which only becomes stable (and energetically favourable) relative to the isotropic phase after substantial electronegative substituents are added.
The compounds with longer tails, C3H7 and longer, behave more consistently, with a greater degree of fluorination increasing both isotropic and columnar transition temperatures for both the DF-HATn and TF-TATn compounds. Here, the cofacial core–core interactions, and the entropic contributions of the long, flexible tails, are presumed to dominate, with the edge-on interactions between molecules across columns playing a much more reduced role. The cofacial interactions are influenced by fluorination, while the entropic contributions from the tails greater for the DF-HATn compounds than for the TF-TATn compounds. The difluorinated DLC's show a particularly interesting feature, namely suppression of the crystal-columnar phase transition to very low temperatures (Table 2) for the C5H11 and C6H13 tails (well below the crystal-columnar transition temperatures of both the hydrocarbon and the tetrafluorinated systems). Again, this is reminiscent of nitro-substituted triphenylene compounds.23 A theoretical comparison of the interactions of singly nitro-substituted and di-fluorinated compounds on the one hand with tetra-fluorinated triphenylenes (which do not show this behaviour) would be interesting to better understand the core–core interactions and the role of tails in these systems.
The fact that all of the TF-TATn compounds support a columnar phase offers an unusual opportunity to study the effect of tails on the properties of the columnar mesophase over a broad length range of tail lengths. One exciting example, which we are pursuing, concerns charge transport in these materials.
Experimental
General information
The chemicals used in this research were purchased from chemical distributors including Combi-Blocks, TCI America, Acros Organics, and Matrix Scientific and were used directly without further purification. Column chromatography was done using silica gel (Silicycle SiliaFlash F60, 230–400 mesh), and for Thin Layer Chromatography (TLC) silica gel plates (Scientific Adsorbents Inc.) were used.The Rayonet photochemical reactor (16 × 254 nm lamps) was used for all the photochemical reactions. Solutions were irradiated in a quartz tube (approximately 40 cm × 2.5 cm with 24/40 ST joint).
A Bruker 400 NMR (400 MHz for 1H-NMR; 376 MHz for 19F-NMR and 101 MHz for 13C-NMR) and Agilent 500 NMR (500 MHz for 1H-NMR; 470 MHz for 19F-NMR and 126 MHz for 13C-NMR) was used for NMR data acquisition and plots were generated by MestreNova software.
GCMS was performed in the positive ionization mode with a scan range of 50–700 m/z, a mass resolving power setting of 140![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, and an automatic gain control (AGC) target value of 1 × 106 ions. The Xcalibur software (ver. 3.0, Thermo Scientific, San Jose, CA, USA) was used to collect and process mass spectral data.
000, and an automatic gain control (AGC) target value of 1 × 106 ions. The Xcalibur software (ver. 3.0, Thermo Scientific, San Jose, CA, USA) was used to collect and process mass spectral data.
Differential scanning Calorimetry (DSC) was run on a DSC 2920 from TA instruments (TA instruments Inc., Newcastle, DE, USA) and data were analyzed by using Thermal Advantage Software (version 1.1A, TA Instruments Inc., Newcastle, DE, USA).
Polarizing Optical Microscope (POM) Nikon Eclipse E600 POL was employed to examine phase identity and transitions temperatures. Temperatures were controlled using a Mettler Toledo FP82HT Hot Stage and Mettler Toledo FP90 Central Processor temperature controller (Mettler Toledo, USA-Hightstown, NJ).
SAXS measurements were taken using a Rigaku Screen Machine with a Cu Kα source on samples contained in thin-walled quartz capillaries. Samples were heated into the isotropic with data collected on cooling, except for TF-TAT1, where the data was collected from a polycrystalline state on heating due to the high clearing temperature of the compound.
Synthesis
The remaining 1,2,3,4-tetrafluoro-6,7,10,11-tetraalkoxytriphenylenes 3–6 were synthesized using the same general procedure (specific details in the ESI†).
The remaining 1,4-difluoro-2,3,6,7,10,11-hexaalkoxyoxytriphenylenes 8–12 were synthesized using the similar general procedure (specific details in the ESI†).
Conclusions
Using PCDHF, a group of fluorinated polyalkoxytriphenylene DLCs were synthesized, including several that feature unusually short tails. Compounds like 1,2,3,4-tetrafluoro-6,7,10,11-tetra(n-alkoxy)triphenylene are particularly unusual as they are mesogenic despite featuring short tails. In these compounds the loss of the tails is compensated for through other, presumably energetic, contributions to the free energy. These compounds illustrate an alternate route to forming a mesophase, which offers unusual opportunities for studying the role of tails in stabilizing liquid crystalline phases, as well as on other (e.g., electronic) properties of columnar mesophases.42Conflicts of interest
There are no conflicts to declare.Acknowledgements
This material is based upon work supported by the National Science Foundation under Grant No. 1809536.Notes and references
- S. Chandrasekhar, B. K. Sadashiva and K. A. Suresh, Liquid Crystals of Disc-like Molecules, Pramana, 1977, 9, 471–480 CrossRef CAS.
- F. M. Mulder, J. Stride, S. J. Picken, P. H. J. Kouwer, M. P. De Haas, L. D. A. Siebbeles and G. J. Kearley, Dynamics of a Triphenylene Discotic Molecule, HAT6, in the Columnar and Isotropic Liquid Phases, J. Am. Chem. Soc., 2003, 125(13), 3860–3866 CrossRef CAS PubMed.
- R. J. Bushby and K. Kawata, Liquid Crystals That Affected the World: Discotic Liquid Crystals, Liq. Cryst., 2011, 38(11–12), 1415–1426 CrossRef CAS.
- C. J. Brabec, N. S. Sariciftci and J. C. Hummelen, Plastic Solar Cells, Adv. Funct. Mater., 2001, 11, 15–26 CrossRef CAS.
- H. C. Hesse, J. Weickert, M. Al-Hussein, L. Dössel, X. Feng, K. Müllen and L. Schmidt-Mende, Discotic Materials for Organic Solar Cells: Effects of Chemical Structure on Assembly and Performance, Sol. Energy Mater. Sol. Cells, 2010, 94(3), 560–567 CrossRef CAS.
- M. Kumar and S. Kumar, Liquid Crystals in Photovoltaics: A New Generation of Organic Photovoltaics, Polym. J., 2017, 49, 85–111 CrossRef CAS.
- Y. Kikuzawa, T. Mori and H. Takeuchi, Synthesis of 2,5,8,11,14,17-Hexafluoro-Hexa-Peri-Hexabenzocoronene for n-Type Organic Field-Effect Transistors, Org. Lett., 2007, 9(23), 4817–4820 CrossRef CAS PubMed.
- G. Horowitz, Organic Field-Effect Transistors, Adv. Mater., 1998, 10, 365–377 CrossRef CAS.
- G. L. Èssem and J. H. Wendorff, Liquid Crystalline Materials for Light-Emitting Diodes, Polym. Adv. Technol., 1998, 9, 443–460 CrossRef.
- J. De, R. A. K. Yadav, R. S. Yadav, S. P. Gupta, M. Joshi, A. Roy Choudhury, J. Jayakumar, J. H. Jou, C. H. Cheng and S. K. Pal, Molecular Engineering for the Development of a Discotic Nematic Mesophase and Solid-State Emitter in Deep-Blue OLEDs, J. Org. Chem., 2021, 86(10), 7256–7262 CrossRef CAS PubMed.
- C. Destrade, M. Mondon and J. Malthete, Hexasubstituted Triphenylenes: A New Mesomorphic Order, J. Phys., Colloq., 1979, 40, 17–21 CrossRef.
- D. Sahoo, E. Aqad, M. Peterca and V. Percec, A highly ordered 8/1 helical pyramidal column self-organized from the crown conformation of achiral hexa(butyloxy)triphenylene, Giant, 2023, 13, 100135 CrossRef CAS.
- D. Sahoo, E. Aqad, M. Peterca and V. Percec, Molecular design principles of helical pyramidal chirality self-organized from achiral hexakis(alkyloxy)triphenylene, Giant, 2023, 13, 100138 CrossRef CAS.
- E. Fontes, P. A. Heiney and W. H. De, Jeu, Liquid-Crystalline and Helical Order in a Discotic Mesophase, Phys. Rev. Lett., 1988, 61, 1202–1205 CrossRef CAS PubMed.
- R. J. Bushby and O. R. Lozman, Discotic liquid crystals 25 years on, Curr. Opin. Colloid Interface Sci., 2002, 7, 343–354 CrossRef CAS.
- T. Wöhrle, I. Wurzbach, J. Kirres, A. Kostidou, N. Kapernaum, J. Litterscheidt, J. C. Haenle, P. Staffeld, A. Baro, F. Giesselmann and S. Laschat, Chem. Rev., 2016, 116, 1139–1241 CrossRef PubMed.
- V. Percec and D. Sahoo, Discotic liquid crystals 45 years later. Dendronized discs and crowns increase liquid crystal complexity to columnar from spheres, cubic Frank-Kasper, liquid quasicrystals and memory-effect induced columnar-bundles, Giant, 2022, 12, 100127 CrossRef CAS.
- D. Demus, Handbook of Liquid Crystals, Low Molecular Weight Liquid Crystals II, Wiley-VCH, Weinheim, 1998, vol. 2B Search PubMed.
- S. K. Pal, S. Setia, B. S. Avinash and S. Kumar, Triphenylene-based discotic liquid crystals: recent advances, Liq. Cryst., 2013, 40, 1769–1816 CrossRef CAS.
- P. J. Stackhouse and M. Hird, Influence of Branched Chains on the Mesomorphic Properties of Symmetrical and Unsymmetrical Triphenylene Discotic Liquid Crystals, Liq. Cryst., 2008, 35(5), 597–607 CrossRef CAS.
- K. Praefcke, A. Eckert and D. Blunk, Core-Halogenated, Helical-Chiral Triphenylene-Based Columnar Liquid Crystals, Liq. Cryst., 1997, 22(2), 113–119 CrossRef CAS.
- N. Boden, R. J. Bushby, P. S. Martin, S. D. Evans, R. W. Owens and D. A. Smith, Triphenylene-Based Discotic Liquid Crystals as Self-Assembled Monolayers, Langmuir, 1999, 15(11), 3790–3797 CrossRef CAS.
- N. Boden, R. J. Bushby, A. N. Cammidge, S. Duckworth and G. Headdock, α-Halogenation of triphenylene-based discotic liquid crystals: towards a chiral nucleus, J. Mater. Chem., 1997, 7(4), 601–605 RSC.
- M. Ichihara, H. Suzuki, B. Mohr and K. Ohta, Different Disk Structures in the Hexagonal Columnar Mesophases of 2,3-Dicyano-6,7,10,11-Tetraalkoxy-1,4-Diazatriphenylenes and 2,3-Dicyano-6,7,10,11-Tetraalkoxytriphenylenes, Liq. Cryst., 2007, 34(3), 401–410 CrossRef CAS.
- R. J. Bushby, N. Boden, C. A. Kilner, O. R. Lozman, Z. Lu, Q. Liu and M. A. Thornton-Pett, Helical Geometry and Liquid Crystalline Properties of 2,3,6,7,10,11-Hexaalkoxy-1-Nitrotriphenylenes, J. Mater. Chem., 2003, 13(3), 470–474 RSC.
- M. Hird, Fluorinated liquid crystals – properties and applications, Chem. Soc. Rev., 2007, 36, 2070–2095 RSC.
- B. Glusen, A. Kettner, J. Kopitzke and J. H. Wendorff, On the character of the glass transition in columnar discotics, J. Non-Cryst. Solids, 1998, 241, 113–120 CrossRef CAS.
- A. N. Cammidge and D. J. B. Jackson, The Effect of Size and Shape Variation in Discotic Liquid Crystals Based on Triphenylene Cores, Philos. Trans. R. Soc., A, 2006, 364(1847), 2697–2708 CrossRef CAS PubMed.
- M. Chu, A. N. Scioneaux and C. S. Hartley, Solution-Phase Dimerization of an Oblong Shape-Persistent Macrocycle, J. Org. Chem., 2014, 79(19), 9009–9017 CrossRef CAS PubMed.
- K. Q. Zhao, J. Q. Du, X. H. Long, M. Jing, B. Q. Wang, P. Hu, H. Monobe, B. Henrich and B. Donnio, Design of Janus Triphenylene Mesogens: Facile Synthesis, Mesomorphism, Photoluminescence, and Semiconductivity, Dyes Pigm., 2017, 143, 252–260 CrossRef CAS.
- R. Termine and A. Golemme, Charge Mobility in Discotic Liquid Crystals, Int. J. Mol. Sci., 2021, 22(877), 1–51 Search PubMed.
- S. Laschat, A. Baro, N. Steinke, F. Giesselmann, C. Hägele, G. Scalia, R. Judele, E. Kapatsina, S. Sauer, A. Schreivogel and M. Tosoni, Discotic Liquid Crystals: From Tailor-Made Synthesis to Plastic Electronics, Angew. Chem., Int. Ed., 2007, 46, 4832–4887 CrossRef CAS PubMed.
- F. M. Van der Kooij, K. Kassapidou and H. N. W. Lekkerkerker, Liquid crystal phase transitions in suspensions of polydisperse plate-like particles, Lett. Nat., 2000, 406, 868–871 CrossRef CAS PubMed.
- M. A. Bates and G. R. Luckhurst, Computer Simulation Studies of Anisotropic Systems. XXVI. Monte Carlo Investigations of a Gay-Berne Discotic at Constant Pressure, J. Chem. Phys., 1996, 104(17), 6696–6709 CrossRef CAS.
- J. A. C. Veerman and D. Frenkel, Phase Behavior of Disklike Hard-Core Mesogens, Phys. Rev. A: At., Mol., Opt. Phys., 1992, 45(8), 5632–5648 CrossRef PubMed.
- J. Barbera, O. A. Rakitin, M. B. Ros and T. Â. S. Torroba, Breaking the Mold of Discotic Liquid Crystals, Angew. Chem., Int. Ed., 1998, 37(3), 296–299 CrossRef CAS PubMed.
- D. Pucci, I. Aiello, A. Aprea, A. Bellusci, A. Crispini and M. Ghedini, Unsuspected Mesomorphism in “Tail-Free” Cyclopalladated 3,5-Disubstituted-2-(2′-Pyridyl)Pyrroles, Chem. Commun., 2009, 1550–1552 RSC.
- V. Percec, A. E. Dulcey, M. Peterca, M. Ilies, S. Nummelin, M. J. Sienkowska and P. A. Heiney, Principles of self-assembly of helical pores from dendritic dipeptides, Proc. Natl. Acad. Sci. U. S. A., 2006, 103(8), 2518–2523 CrossRef CAS PubMed.
- F. B. Mallory and C. W. Mallory, Photocyclization of Stilbenes and Related Molecules, in Organic Reactions, 2005 DOI:10.1002/0471264180.or030.01.
- Z. Li and R. J. Twieg, Photocyclodehydrofluorination, Chem. – Eur. J., 2015, 21(44), 15534–15539 CrossRef CAS PubMed.
- Z. Li, M. Powers, K. Ivey, S. Adas, B. Ellman, S. D. Bunge and R. J. Twieg, Fluorinated Triphenylenes and a Path to Short Tailed Discotic Liquid Crystals: Synthesis, Structure and Transport Properties, Mater. Adv., 2022, 3(1), 534–546 RSC.
- M. Powers, R. J. Twieg, J. Portman and B. Ellman, Structure and dynamics of tail-free discotic liquid crystals: simulations of fluorinated triphenylene, J. Chem. Phys., 2022, 157, 134901 CrossRef CAS PubMed.
- N. Boden, R. J. Bushby, G. Cooke, O. R. Lozman and Z. Lu, CPI: A Recipe for Improving Applicable Properties of Discotic Liquid Crystals, J. Am. Chem. Soc., 2001, 123, 7915–7916 CrossRef CAS PubMed.
- R. Shang, Y. Fu, Y. Wang, Q. Xu, H. Z. Yu and L. Liu, Copper-Catalyzed Decarboxylative Cross-Coupling of Potassium Polyfluorobenzoates with Aryl Iodides and Bromides, Angew. Chem., Int. Ed., 2009, 48(49), 9350–9354 CrossRef CAS PubMed.
- P. P. Kulkarni, A. J. Kadam, R. B. Mane, U. V. Desai and P. P. Wadgaonkar, Demethylation of Methyl Aryl Ethers Using Pyridine Hydrochloride in Solvent-Free Conditions under Microwave Irradiation, J. Chem. Res., 1999, 394–395 CAS.
- M. Sorai and K. Saito, Alkyl Chains Acting as Entropy Reservoir in Liquid Crystalline Materials, Chem. Rec., 2003, 3(1), 29–39 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ma00281k |
| This journal is © The Royal Society of Chemistry 2023 |